Abstract
We have previously demonstrated that interaction of infected thymocytes with autologous thymic epithelial cells (TEC) is a prerequisite for a high level of human immunodeficiency virus type 1 (HIV-1) replication in thymocytes (M. Rothe, L. Chêne, M. Nugeyre, F. Barré-Sinoussi, and N. Israël, J. Virol. 72:5852–5861, 1998). We report here that this activation of HIV replication takes place at the transcriptional level through activation of the Rel/NF-κB transcription factors. We first demonstrate that an HIV-1 provirus (SF-2 strain) very effectively replicates in thymocytes cocultured with TEC whereas this provirus, with κB sites deleted, fails to replicate. We provide evidence that several NF-κB complexes are constitutively found in the nuclei of thymocytes either freshly isolated from the thymus or maintained in coculture with autologous or heterologous TEC. The prevalent complex is the heterodimer p50-p65. NF-κB activity is tightly correlated with the transcriptional activity of a long terminal repeat (LTR) of HIV-1 transfected in thymocytes. The cotransfection of this LTR with a mutated IκBα molecule formally demonstrates that LTR transactivation is regulated by members of the Rel/NF-κB family in thymocytes. We also showed that tumor necrosis factor (TNF) and to a lesser extent interleukin-1 (IL-1), secreted within the coculture, induce NF-κB activity and a correlative LTR transactivation. However IL-7, a crucial factor for thymopoiesis that is secreted mainly by TEC, is a necessary cofactor for NF-κB activation elicited by TNF or IL-1. Together, these data indicate that NF-κB activation, required for a high level of HIV replication in thymocytes, is regulated in a specific manner in the thymic microenvironment which provides the necessary cytokines: TNF, IL-1, and IL-7.
One explanation (24, 61) for the depletion in the number of CD4+ T lymphocytes, which is the hallmark of human immunodeficiency virus (HIV) infection, is the exhaustion of the T-cell turnover, which has to occur at a very high rate to replace the cells permanently destroyed by the virus (9). This high-turnover model does not take into account the possible impairment of T-cell renewal caused by HIV infection of primary lymphoid organs (20, 40). A number of studies suggested a correlation between the rapid progression toward AIDS of some pediatric seropositive patients and thymic alterations including a profound disorganization of the epithelial network (28, 45, 47) and thymocyte depletion. Furthermore, antiretroviral treatment applied to HIV-infected SCID-hu mice did not lead to a complete renewal of the mature thymocyte population, underlining the possibility of an impairment either of progenitor thymocyte function or of stromal cell function (65). To better understand the pathogenesis of HIV infection in the thymus, it is important to identify the stimuli and mechanisms controlling the virus replication and spreading within this organ. Most thymocytes are susceptible to HIV infection in vitro (58–60) and in the SCID-hu mouse model (2, 32, 54, 56). We previously determined the factors physiologically involved in the control of HIV replication in thymocytes. We showed that interaction of infected thymocytes with thymic epithelial cells (TEC) is a prerequisite for a high level of HIV replication in thymocytes. We determined that this cell-to-cell contact, while necessary, is not per se the effector of HIV replication but, rather, that soluble factors present in the coculture synergize to induce HIV replication (48). Two types of factors were identified according to their role, either in cell activation (such as tumor necrosis factor [TNF], interleukin-1 [IL-1], and IL-6) (67) or in cell proliferation or protection against apoptosis (such as granulocyte-macrophage colony-stimulating factor [GM-CSF]) (3, 27, 31).
The requirement for TNF and to a lesser extent for IL-1 in HIV replication during the coculture of thymocytes and TEC suggests that a major role of this interaction might be the induction of NF-κB activation, which in turn would activate transcription from the two κB sites present in the HIV-1 long terminal repeat (LTR). NF-κB is composed of homodimers and heterodimers of members of the Rel/NF-κB family. To date, five proteins belonging to the NF-κB family have been identified: p65, c-Rel, RelB, p50/p105, and p52/p100 (5, 33, 39). NF-κB dimers are sequestered in the cytosol of unstimulated cells via interactions with a family of inhibitory proteins called IκBs (IκBα, IκBβ, and IκBɛ) (64). Following activation by various immune system and inflammatory stimuli, IκB molecules are degraded through the ubiquitin-proteasome pathway, thus allowing nuclear translocation of NF-κB and activation of its target genes (39).
To verify the hypothesis that thymic microenvironment controls HIV replication at the transcriptional level, we used autologous mixed culture of TEC and thymocytes as previously described (48). We first demonstrated that NF-κB activation is a prerequisite for a high level of HIV replication in thymocytes, since a provirus with its two κB sites deleted failed to replicate even in coculture with TEC. We then dissected the mechanism underlying this activation process. We first demonstrated that coculture with TEC led to the induction of nuclear NF-κB complexes in thymocytes. The relevance of these NF-κB complexes was demonstrated by specific inhibition of LTR transactivation by a mutated form of the IκBα constitutively inhibiting NF-κB.
We also showed that TNF could not replace the activating effect of the coculture and that IL-7, a TEC-secreted factor crucial for thymopoiesis (21, 22, 46), was required to obtain a strong and prolonged TNF-induced NF-κB activity. We provide correlative evidence that the association of these two cytokines is sufficient to induce the transactivation of the NF-κB-dependent HIV LTR.
Taking the results together, we show here that cytokines provided by different cell types within the thymus combine their effects to sustain a high level of HIV transcription in thymocytes through NF-κB activation.
MATERIALS AND METHODS
Reagents. (i) Antibodies.
To characterize both TEC and thymocytes populations, the monoclonal antibodies (MAbs) used were against CD3 (X35), CD7 (8H8,1), CD14 (RM052), CD83 (HB15A), cytokeratin (KL1), and vimentin (vim3B4). Immunoglobulin G1 (IgG1) (MARK1) and IgG2a (U7.27) were used as negative controls. All these antibodies were purchased from Immunotech (Marseilles, France), except vim3B4, which was purchased from Boehringer Mannheim (Meylan, France). Conjugated MAbs, including CD4-phycoerythrin (PE) (13B8.2), CD8-fluorescein isothicyanate (FITC) (B9.11), control IgG1-PE (679.1Mc7) and IgG1-FITC (679.1Mc7), goat-anti-rabbit-FITC, and goat-anti-mouse-FITC, were purchased from Immunotech.
Rabbit polyclonal serum against TNF was kindly provided by J.-M. Cavaillon, Pasteur Institute (Paris, France). IL-1 receptor antagonist (IL-1ra) and mouse MAb against IL-7 were purchased from R&D Systems (Minneapolis, Minn.). MAb against IL-1β (B-A15) was purchased from Diaclone Research (Besançon, France). Mouse MAb against IL-6 (AH65) and mouse MAb against the α chain of the IL-7 receptor (IL-7Rα) CDw127 (R34.34) were purchased from Immunotech.
(ii) Cytokines.
Human recombinant cytokines GM-CSF, IL-1β, and TNFα were purchased from Genzyme Corp. (Cambridge, Mass.). IL-6 and IL-7 were purchased from R&D Systems.
Plasmids.
All reporter constructs and plasmids used in this work have been previously described. Briefly, the LTR-luc construct carries the luciferase reporter gene under the control of the U3R (BglII-HindIII fragment) of the HIV-1B-LAI LTR (19). The 3Enh-TK-luc construct contains three copies of a synthetic oligonucleotide encompassing the two 10-bp repeats of the HIV-1B-LAI enhancer (−107 to −76) cloned upstream of the truncated thymidine kinase (TK) promoter (−105 to +51) of herpes simplex virus in a TK-luc expression vector (19). LTRΔκB was obtained by insertion into the pC-luciferase plasmid (52) of the XhoI-HindIII fragment of the HIV-1B-LAI LTR from the original construct of Leonard et al. with the two κB motifs deleted (35). The CMV-tat plasmid carries the tat cDNA inserted downstream of the human cytomegalovirus (CMV) immediate-early enhancer/promoter (19). The EF1-βgal construct, containing the β-galactosidase reporter gene under the control of the basal promoter of elongation factor EF1α, was kindly provided by S. Mémet (Pasteur Institute, Paris, France). The construct IκBα-DN, carrying the cDNA of IκBα protein mutated on serines 32 and 36 under the control of the CMV early promoter, was kindly provided by N. Rice (63). RcCMV (Invitrogen BV) was the corresponding control vector which contains no cDNA sequence.
Cells and culture conditions. (i) Isolation of enriched human populations of TEC and thymocytes.
Fresh thymus fragments were obtained during elective cardiac surgery on HIV-1-seronegative children (aged 6 days to 24 months). The enrichment procedures for thymocytes and TEC have been described previously (48). Cells were then characterized at day 3 after thymus excision, using different selective markers (with the various antibodies listed above), indicating that these populations of TEC and thymocytes were respectively purified at 95 and 99% (48). Prior to mixed culture, TEC were maintained in a selective medium (McCoy’s 5A [GIBCO] containing 10% fetal calf serum [FCS], 1 mM l-Glu, 50 μg of penicillin-streptomycin per ml, 100 μg of neomycin per ml, 0.25 μg of Fungizone per ml, 20 ng of epidermal growth factor per ml, 5 × 10−9 M cholera toxin, 500 ng of hydrocortisone per ml, and 5 × 10−5 M β-mercaptoethanol). Thymocytes were stored at +4°C in McCoy’s 5A supplemented with antibiotics, l-glutamine, 10 mM HEPES, and 20% FCS.
(ii) Mixed culture of TEC and thymocytes.
Autologous or heterologous cocultures of TEC and infected or control thymocytes were carried out 3 days after thymic excision either under conditions permitting cell-to-cell contact or in transwell chambers (0.45-μm pore diameter). The ratio of TEC to thymocytes was 1/25. The coculture medium was McCoy’s 5A supplemented with antibiotics, 1 mM l-glutamine, 10 mM HEPES, and 10% FCS. IL-6 and GM-CSF were used at 10 ng/ml, TNF-α and IL-1β were used at 5 ng/ml, and IL-7 was used at 1 ng/ml. MAb against IL-6 was used at 20 μg/ml, and polyclonal antibody against TNF was used at a serum dilution of 1/300. IL-1ra was used at 20 μg/ml. Cytokines or cytokine inhibitors were added at the start of the coculture, except antibody against IL-7Rα. The thymocytes were preincubated with the antibody against IL-7Rα (5 μg/ml per 8 × 106 thymocytes) for 6 h at 4°C before their coculture with TEC. In some experiments, the thymocyte culture was supplemented with 50% (vol/vol) noninfected TEC conditioned medium (CM) obtained after 72 h of TEC culture and then centrifuged and filtered through a 0.45-μm-pore-size membrane. In experiments with IL-7 antibody, TEC CM was preincubated for 6 h at 4°C with 10 μg of the antibody per ml before the onset of thymocyte culture.
Immunostaining and cytofluorometry.
Indirect immunostaining with antibodies against cytokeratin and vimentin was performed in Lab-Tech chambers (Corning Costar Corp., Cambridge, Mass.) on cells permeabilized by acetone-methanol treatment (6). Other direct or indirect immunostaining was analyzed by cytofluorometry with an EPICS Profile II fluorometer (Coulter).
Virus and infection procedure.
HIV-1wt and HIV-1κB-mut were obtained after transfection of Cos-7 cells with the corresponding provirus constructs (4) by using standard methods with Lipofectamine (GIBCO). HIV-1wt was obtained after transfection of the entire sequence (5′-3′) of the molecular clone HIV-1 SF2 (48). HIVκB-mut, obtained in the same way, differed from the wild-type construct by having a 5-bp substitution in the enhancer region of the viral LTR (17). The p24gag protein was determined in cell-free supernatants by an enzyme-linked immunosorbent assay (ELAVIA; Diagnostics Pasteur) and was used in the infection procedure immediately. All infections were standardized to the p24gag levels: 10 ng of p24gag was used to infect 106 thymocytes. Briefly, thymocytes were washed in RPMI and exposed either to HIV-1wt or to HIVκB-mut for 1 h at 37°C. After infection, the thymocytes were washed three times and cultured at a density of 8 × 106 per well (12-well plates) in 2 ml of medium (McCoy’s 5A supplemented with antibiotics, 10% FCS, and 1 mM l-Glu) or cocultured with 5 × 104 TEC. On days 6, 8, 11, 13, 15, and 18 after the start of the coculture, 300 μl of supernatant were collected to determine the p24gag concentration and was replaced by the same volume of fresh medium.
Transfection procedure.
Thymocytes were maintained for 0 to 48 h in different culture or coculture conditions prior to transfection. They were then transfected with 20 to 25 μg of plasmid(s) by electroporation in MacCoy’s 5A medium with 20% FCS. A single pulse at 875 V/cm and 960 μF was performed. Expression vectors of the luciferase reporter gene were used, except for EF1-βgal, expressing β-galactosidase, which was used for standardization of transfections. After transfection, the thymocytes were washed and were then returned for an additional 20 h to the previous dishes containing the conditioned medium of 48 h of culture. At 20 h posttransfection, the luciferase and β-galactosidase activities were detected in the cell lysates. Luciferase activity was measured by standard procedures, as previously described (52), and β-galactosidase activity was measured by using the luminescent β-galactosidase genetic reporter system II (Clontech, Palo Alto, Calif.). Normalized luciferase activity represents the ratio of luciferase to β-galactosidase activities.
Electrophoretic mobility shift assay (EMSA).
Total extracts were performed as previously described (26). Briefly, after 5 × 106 to 10 × 106 thymocytes cultured under different conditions were harvested, they were washed once in PBS 1×; 30 μl of lysis buffer was added, centrifugation (13,220 × g for 10 min) was performed 10 min later, and the protein concentration in the supernatant was quantified by using the Bradford reagent (Bio-Rad Laboratories, Ivry sur Seine, France). For the band shift assay, the binding-reaction mixture was prepared by adding, in the following order, binding buffer (20 mM HEPES, 2 mM dithiothreitol, 60 mM KCl, 0.01% Nonidet P-40, 0.1 mg of bovine serum albumin/ml, 4% Ficoll), 0.4 μg of sonicated salmon sperm, 10 μg of protein extract, and 30,000 cpm of 32P-labelled DNA probe corresponding to 0.25 ng of probe. The sequence of the oligonucleotide used is:
5′ CTAGACGGGGATTTCCGAGAGGT TGCCCCTAAAGGCTCTCCAGATC 5′
The NF-κB consensus binding sequence is in boldface type.
Specific binding was controlled by competition with a 40-fold (10-ng) excess of the same nonlabeled oligonucleotide, added to the protein extract before the binding reaction was started. To identify the subunits constituting NF-κB complexes, specific antibodies against p50, p52, p65, c-Rel, and RelB were used. The p50, p52, p65, and c-Rel antibodies were a kind gift from A. Israël (Pasteur Institute, Paris, France), and anti-RelB was provided by Santa Cruz Biotechnology (Santa Cruz, Calif.). Antibodies were added to the protein extract, and the mixture was allowed to stand for 5 min at 4°C before incubation with the radiolabeled probe.
RESULTS
Coculture-induced activation of HIV replication requires the two κB sites within the HIV-1 LTR.
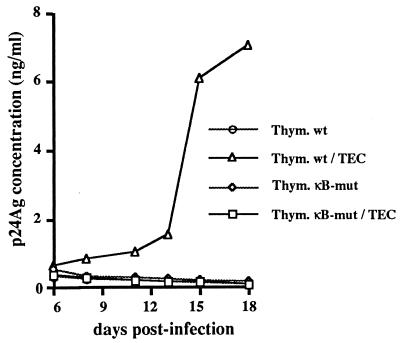
In an attempt to evaluate the relevance of NF-κB in the activation of HIV-1 replication in thymocytes, we compared the replication capacity of a virus mutated in the two κB sites within the LTR (4, 18) with that of the corresponding wild-type virus. Wild-type and mutated (ΔκB) viruses were obtained from the supernatant of Cos-7 cells transfected with the corresponding provirus constructs. We then carried out infection of thymocytes and cocultured them with TEC under conditions previously described by Rothe et al. (48). As shown in Fig. 1, the ΔκB virus is unable to replicate in thymocytes even in the presence of TEC, in contrast to the wild-type.
FIG. 1.
Coculture-induced activation of HIV replication requires the two κB sites within the HIV LTR. Freshly isolated thymocytes were infected with viral supernatants produced after 48 h of culture of either wild-type (wt) or κB-mut proviral DNA-transfected Cos-7 cells. Infected thymocytes were cultured alone (Thym. wt or Thym. κB-mut) or cocultured with TEC (Thym. wt/TEC or Thym. κB-mut/TEC). Viral replication was determined at various times postinfection by the detection of p24 antigen in the thymocyte culture or in the coculture medium. The data shown here are representative of three independent experiments, each carried out on a different three thymus.
These data suggest that NF-κB activity is required for HIV replication in thymocytes and that this activation is provided by their interaction with TEC.
Interaction with TEC permanently induces NF-κB activity in thymocytes.
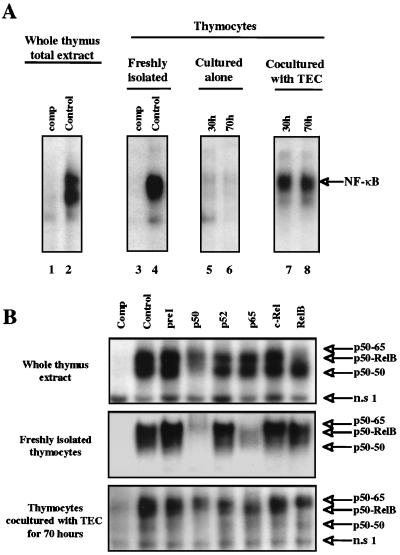
We then determined whether NF-κB activity was present in the human thymus as a whole by performing EMSAs with protein extract from a thymus fragment immediately crushed after surgery and a probe representing the HIV-1 LTR κB-derived motifs. As shown in Fig. 2A (lane 2 [control]), we observed a specific binding activity of NF-κB complexes in this whole-cell extract. This activity was also observed in whole-cell extracts from freshly isolated thymocytes (lane 4 [control]). The NF-κB activity, observed in freshly isolated thymocytes, was very probably present in these cells within the thymus (since it was also detected in the thymus as a whole) and was not induced by the enrichment procedure used for these cells. The NF-κB activity represented specific DNA binding activities since they were competed by an excess of a nonradiolabeled κB probe (lanes 1 and 3).
FIG. 2.
Interaction of thymocytes with TEC leads to a permanent induction of NF-κB complexes in thymocytes. (A) Protein extracts were obtained from a frozen and crushed piece of thymus (lanes 1 and 2) or from thymocytes freshly isolated (lanes 3 and 4), cultured for 30 h (lane 5) to 70 h (lane 6), or cocultured with TEC for 30 h (lane 7) or 70 h (lane 8). These extracts were incubated with a 32P-labeled oligonucleotide representing the HIV LTR-derived κB motif. Competition (comp, lanes 1 and 3) with a 40-fold molar excess of unlabeled oligonucleotide was used to confirm the specificity of the DNA binding activity detected. “NF-κB” refers to several NF-κB complexes, which are further analyzed in panel B. This experiment is representative of 3 independent experiments carried out with autologous TEC on three thymuses and 10 independent experiments carried out with heterologous TEC. (B) Identification by immunoreactivity of the distinct NF-κB complexes present in the thymocytes. Protein extracts were obtained either from a frozen and crushed piece of thymus or from whole-cell extracts of freshly isolated thymocytes or of thymocytes cultured for 70 h in the presence of TEC. These extracts were preincubated with preimmune serum (preI) or with antisera raised against distinct subunits of NF-κB complexes: p50, p52, p65, c-Rel, or RelB (as indicated above the lanes) before incubation with the labeled probe. The composition of the complexes is indicated on the right. This experiment is representative of three independent experiments, each carried out on a different thymus. n.s 1 refers to a nonspecific binding activity since it was not competed by an excess of nonradiolabeled κB probe.
This NF-κB activity decreased progressively when thymocytes were cultured alone (Fig. 2A, lanes 5 and 6). The kinetics of this decrease in culture varies according to the different preparations of thymocytes obtained from different thymuses. The minimal levels were observed at between 24 and 48 h of culture. In any case, this activity was maintained when thymocytes were cocultivated with autologous TEC (lanes 7 and 8). The same result was obtained with either autologous (from 3 thymuses) or heterologous (from 10 thymuses) TEC, indicating that induction of NF-κB activity did not result from cellular activation caused by a mixed lymphocyte reaction but was due to physiological interactions between thymocytes and TEC. These results demonstrate that coculture with TEC induces a sustained NF-κB activation in thymocytes.
We then characterized the NF-κB complexes which bind to the HIV enhancer element by using antibodies against each subunit of the NF-κB family (p50, p52, p65, RelB, and c-Rel). As shown in Fig. 2B, in the whole-thymus extract, an antibody against the p50 subunit abolished the binding of most of the complexes observed, an antibody against p65 abolished the very faint band corresponding to the upper complex, and an antibody against RelB abolished the binding of the upper and intermediate complexes. The antibody against c-Rel did not significantly modify the binding of any of these complexes. These data suggest that the lowest complex is p50-p50, the intermediate and major complex is p50-RelB, and the upper complex probably contains p50, p52 (as a minor component), and p65. As also shown in Fig. 2B, in freshly isolated thymocytes p50-p65 was the prevalent complex, and coculture with TEC maintained this complex and to a lesser extent the p50-RelB one.
The presence of NF-κB binding activity in freshly isolated thymocytes or in thymocytes cultured with TEC is responsible for a high HIV-1 LTR activity.
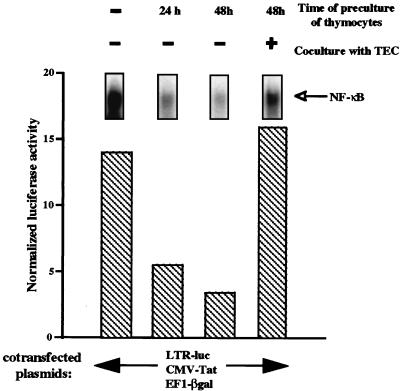
We then determined whether the sustained NF-κB activity observed in thymocytes, either freshly isolated or cocultured with TEC, correlated with the level of LTR activity in thymocytes. For this purpose, we determined, in parallel experiments, NF-κB DNA binding activity and the level of luciferase activity of the LTR-luc construct transfected in thymocytes. A CMV-tat construct was cotransfected with LTR-luc since expression of the powerful viral transactivator Tat emphasizes the magnitude of NF-κB-dependent transactivation of the LTR (15). EF1-βgal plasmid containing the reporter gene β-galactosidase under the control of the basal promoter of the elongation factor EF1α was also cotransfected to normalize the transfection efficiencies.
Thymocytes were cultured alone or cocultured with TEC for 48 h prior to cotransfection by electroporation with LTR-luc, CMV-tat, and EF1-βgal. The thymocytes were collected from the culture dishes (then containing a 48-h conditioned medium) for the time of electroporation and were then returned to this conditioned medium for an additional 20 h. LTR activity, expressed as normalized luciferase activity, was then determined. As shown in Fig. 3, LTR activity correlated with the level of NF-κB binding activity. LTR activity was high in freshly isolated thymocytes and progressively decreased (for 24 or 48 h) when these cells were cultured alone, whereas it was maintained at a high level in those cocultivated with TEC (for 48 h). However, whereas NF-κB binding activity was higher in freshly isolated thymocytes than in thymocytes cocultivated with TEC, LTR activity was comparable in the two cell systems. This can be explained by the fact that NF-κB activity was determined just after thymocyte isolation whereas the luciferase assay was performed 20 h after transfection. This interval is sufficient to allow a significant decrease of NF-κB binding activity in freshly isolated thymocytes cultured in the absence of TEC.
FIG. 3.
The presence of NF-κB binding activity in thymocytes correlates with the level of HIV-LTR activity. NF-κB activity was determined as described in the legend to Fig. 2 in whole-cell extracts from freshly isolated thymocytes or thymocytes cultured alone for 24 or 48 h or cocultured with TEC for 48 h. In parallel, freshly isolated thymocytes or thymocytes precultured for 24 and 48 h alone or with TEC were cotransfected by electroporation with the constructs LTR-luc, CMV-Tat, EF1-βgal (respectively used at 10, 2.5, and 10 μg per 107 cells). The transfected thymocytes were then returned to the previous culture conditions for 20 h. Luciferase and β-galactosidase activities were determined 20 h after transfection in the thymocyte lysates. LTR activity was expressed as normalized luciferase activity corresponding to the ratio of luciferase to β-galactosidase activity per 106 cells. This experiment is representative of four independent experiments, each carried out on a different thymus.
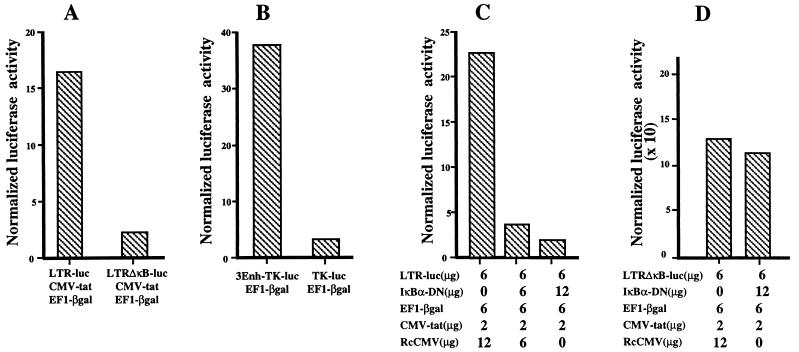
We then confirmed that the κB sites were required for the high transcriptional activity of the LTR in freshly isolated thymocytes or in thymocytes in coculture with TEC. As shown in Fig. 4A, the activity of an LTR-ΔκB mutated in the two κB sites was 5- to 10-fold lower than that observed with the wild-type LTR construct. The role of the κB-responsive element was further confirmed by comparing the luciferase activity obtained with the 3Enh-TK-luc construct to that obtained with the control vector TK-luc (Fig. 4B). In this case, a 10- to 15-fold difference was observed between the two constructs.
FIG. 4.
NF-κB activity is responsible for the high transcriptional activity of the LTR in thymocytes freshly isolated or cocultured with TEC. Freshly isolated thymocytes were cotransfected by electroporation with the following constructs: LTRluc, CMV-tat, and EF1-βgal (respectively used at 10, 5, and 10 μg per 107 cells) or LTR-ΔκB-luc, CMV-tat, and EF1-βgal (used at 10, 5, and 10 μg per 107 cells) (A); 3Enh-TK-luc and EF1-βgal (each used at 10μg per 107 cells) or TK-luc and EF1-βgal (used at 10 and 10 μg per 107 cells) (B); LTRluc, CMV-tat, and EF1-βgal (used at 6, 2, and 6 μg per 107 cells) and increasing amounts of IκBα-DN expression vector as indicated (C); and LTR-ΔκB-luc, CMV-tat, and EF1-βgal (used at 6, 2, and 6 μg per 107 cells) and 12 μg of IκBα-DN expression vector as indicated (D). Rc-CMV was added, as indicated, to normalize the amount of transfected DNA. Luciferase and β-galactosidase activities were determined 20 h after transfection in the thymocyte lysates. LTR activity was expressed as normalized luciferase activity. This experiment is representative of three independent experiments, each carried out on a different thymus.
In addition, we formally demonstrated that NF-κB was specifically responsible for transactivation of the HIV-1 LTR by cotransfecting freshly isolated thymocytes with LTR-luc, CMV-tat, and an expression vector encoding the IκBα inhibitory protein mutated on serines 32 and 36 (IκBα-DN) (63). These mutations prevent phosphorylation and subsequent degradation of IκBα. IκBα-DN behaves as a specific and constitutive inhibitor of NF-κB activity. As a control, we replaced IκBα-DN by the vector RcCMV. Indeed, cotransfection of increasing amounts of this mutant progressively reduced the transcriptional activity of LTR to a value comparable to that obtained with the LTR-ΔκB in freshly isolated thymocytes (Fig. 4C) as well as in thymocytes cocultured with TEC (data not shown). As expected, IκBα-DN did not modify the transcriptional activity of the LTR-ΔκB promoter (Fig. 4D), confirming against that it specifically targets NF-κB on the wild-type LTR.
Taken together, these experiments provide evidence of a permanent induction of NF-κB activity leading to a permanent LTR transactivation in freshly isolated thymocytes or in thymocytes cocultured with TEC.
NF-κB activation induced by the interaction of thymocytes with TEC is mediated mainly by TNF and to a lesser extent by IL-1, but NF-κB inducibility requires IL-7.
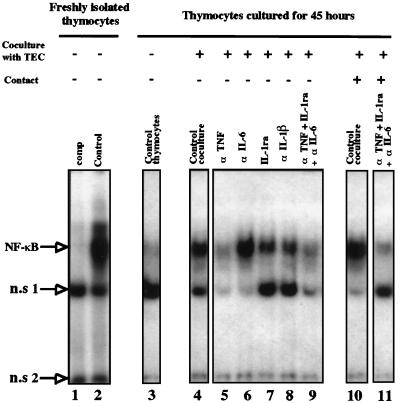
TNF and IL-1 are well-known triggers of NF-κB activation (10, 26, 44). We have recently shown that the strong activation of HIV replication in thymocytes, elicited by their interaction with TEC, is mediated mainly by soluble factors including IL-1, TNF, IL-6, and GM-CSF (48). Therefore, we first evaluated whether these cytokines, particularly IL-1 and TNF, were involved in the permanent induction of NF-κB observed in thymocytes cocultured with TEC. For this purpose, we performed cocultures in transwell chambers to prevent cell-to-cell contact but to allow the exchange of soluble factors. At the start of the coculture, neutralizing antibodies against IL-1 and TNF were added to test their ability to block NF-κB activation. An antagonist of the IL-1 receptor (IL-1ra) was also used. A neutralizing antibody against IL-6 was used as a negative control, since IL-6 is not an NF-κB inducer.
As shown in Fig. 5 (lane 4), NF-κB activation was sustained by the coculture even in the absence of cell-to-cell contact, indicating that soluble factors were mainly involved. As expected, antibody against IL-6 did not reduce NF-κB activation (lane 6). The effects of IL-1ra or antibody against IL-1β were very modest (lanes 7 and 8). In contrast, anti-TNF almost completely abolished NF-κB activity in thymocytes (lane 5). The fact that the extent of inhibition was about the same with anti-TNF as with the combination of anti-TNF, IL-1ra, and anti-IL-6 in the presence or absence of cell-to-cell contact (lanes 9 and 11) strongly suggests that TNF is the major inducer of NF-κB activation within the coculture.
FIG. 5.
TNF and, to a lesser extent, IL-1 are involved in the permanently induced NF-κB activity present in thymocytes during the coculture with TEC. Thymocytes were freshly isolated (lanes 1 and 2) or cultured for 45 h (lanes 3 to 11) either alone (lane 3 [Control thymocytes]) or in the presence of TEC under conditions avoiding contact (lanes 4 to 9) or allowing contact (lanes 10 and 11). During the coculture, the cells were either left untreated (lane 4 [Control coculture, 45 hours] and lane 10 [Control coculture]) or were treated at the start of the coculture with antibodies respectively raised against TNF (lane 5), IL-6 (lane 6), and IL-1β (lane 8), with the antagonist of the IL-1 receptor (lane 7), or with αTNF plus IL-1 ra plus αIL-6 (lanes 9 and 11). Whole-cell extracts from these various samples were incubated with a 32P-labeled oligonucleotide representing the HIV LTR-derived κB motif. Competition (comp [lane 1]) with a 40-fold molar excess of unlabeled oligonucleotide was used to confirm the specificity of the DNA-binding activity detected. NF-κB activity is indicated. The positions of two nonspecific (n.s 1 and n.s 2) binding activities are indicated. The positions of two nonspecific (n.s 1 and n.s 2) binding activities are indicated. This experiment is representative of three independent experiments, each carried out on a different thymus.
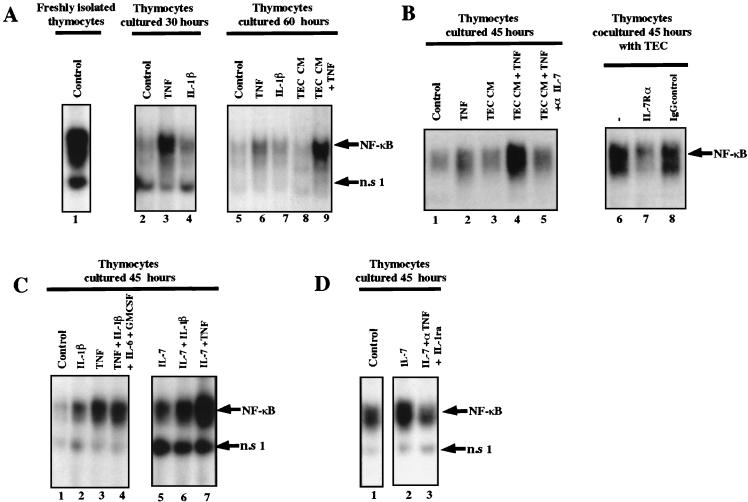
We then tested whether TNF or IL-1β was sufficient to induce NF-κB binding activity in thymocytes cultured alone. As shown in Fig. 6, IL-1β had a weak stimulating effect on NF-κB activity in thymocytes (lane 4), in contrast to TNF, which was able by itself to sustain a high NF-κB binding activity in thymocytes cultured for 30 h (lane 3). However, the level of NF-κB activity obtained with TNF was lower than that observed in freshly isolated thymocytes (compare lane 3 to lane 1). In addition, TNF was not efficient in maintaining long-term (for instance, 60-h) NF-κB activity (lane 6). This suggested that other factors within the microenvironment might be required to maintain NF-κB activity in thymocytes. This hypothesis was confirmed by the fact that TEC CM, when supplemented with TNF, was able to maintain NF-κB activity for 60 h (lane 9) whereas TEC CM alone had no effect (lane 8). We conclude that TNF is mainly required to maintain NF-κB activity in thymocytes but that its effect requires another factor(s) secreted by TEC.
FIG. 6.
TNF and, to a lesser extent, IL-1 induce NF-κB activity in thymocytes, and IL-7 is required for this effect. Whole-cell extracts were prepared from freshly isolated thymocytes (panel A, lane 1) or from thymocytes cultivated for 30, 60, or 45 h. (A) During the indicated culture times, thymocytes were either left untreated (lane 1, control for freshly isolated; lane 2, control 30 h; and lane 5, control 60 h) or were stimulated with either TNF (lanes 3 and 6), IL-1β (lanes 4 and 7), or TNF in the presence of TEC CM (TEC CM + TNF) (lane 9). TEC conditioned medium was also added alone (lane 8 [TEC CM]). The data shown here are representative of three independent experiments carried out on three thymuses. (B) Thymocytes were cultured for 45 h either untreated (lane 1, control 45 h), with TNF (lane 2), with TEC CM (lane 3), or with TNF in the presence of TEC CM (TEC CM + TNF) preincubated (lane 5) or not (lane 4) with antibody against IL-7. In lanes 6 to 8, the thymocytes were left untreated for 6 h (lane 6) or incubated with an antibody against IL-7Rα (lane 7) or with an IgG1 control serum (lane 8) before being cocultured with TEC for 45 h. The data shown here are representative of two independent experiments carried out on two thymuses. (C) Cells were left untreated for the 45 h of the culture (lane 1, control 45 h) or treated with IL-1β (lane 2), TNF (lane 3), IL-1β plus TNF plus IL-6 plus GM-CSF (lane 4), IL-7 (lane 5), IL-7 plus IL-1β (lane 6), or IL-7 plus TNF (lane 7). This experiment is representative of three independent experiments carried out on three thymuses. (D) Thymocytes were left untreated (lane 1, control) or stimulated with IL-7 (lane 2) or IL-7 in presence of anti-TNF and Il-1ra (lane 3). Whole-cell extracts were incubated with a 32P-labeled oligonucleotide representing κB motif. NF-κB activity is indicated. This experiment is representative of two independent experiments, each carried out on a different thymus.
We tested the possibility that IL-7, a cytokine expressed by TEC which plays a crucial role in T-cell development (41, 43, 45, 49), was this cofactor. We first showed that TEC CM obtained after 3 days contains between 5 and 10 pg of IL-7/ml per 105 cells. We then demonstrated (Fig. 6B) that the costimulatory effect of the TEC CM was abrogated when this medium was depleted of IL-7 by using an antibody against IL-7 (lane 5). In parallel, TEC failed to sustain this activity when thymocytes were preincubated with an antibody against the specific α chain of the IL-7 receptor before coculture. The role of IL-7 was also confirmed by the fact that IL-7 elicited a strong costimulatory effect with TNF and to a lesser extent with IL-1 (Fig. 6C, lanes 6 and 7) whereas IL-6 and GM-CSF, which are secreted by TEC (34) and involved in HIV replication, did not modify the NF-κB binding activity induced by IL-1 or TNF (lane 4).
NF-κB activation was also observed when thymocytes were treated with IL-7 alone (Fig. 6C, lane 5, and Fig. 6D, lane 2). However, antibodies against TNF and IL-1, added simultaneously with IL-7 at the start of the culture (Fig. 6D, lane 3), prevented this induction, again indicating that IL-7 plays the role of a cofactor which amplifies the effect of low levels of TNF (and IL-1) secreted by thymocytes.
Together, these data argue for a major role of TNF in maintaining NF-κB activation in thymocytes, but this activation requires IL-7, which is secreted mainly by TEC.
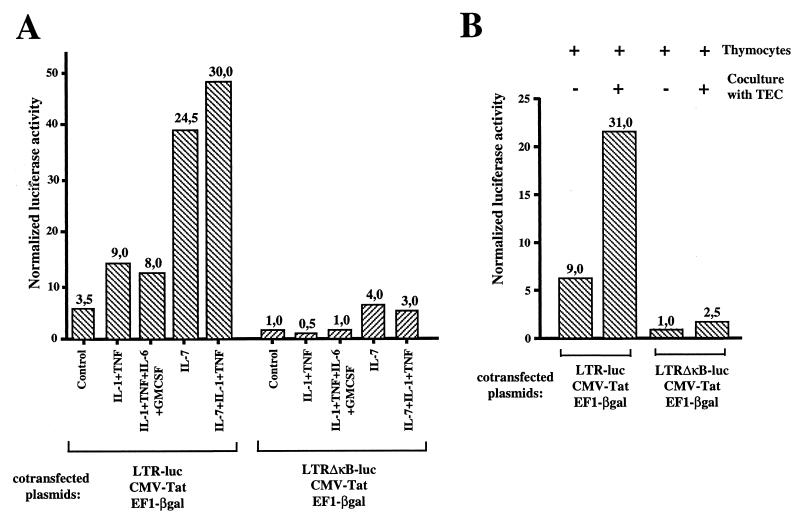
We then determined whether HIV LTR transactivation in thymocytes was also dependent upon the stimulation by these cytokines. To emphasize the NF-κB-dependent transactivation, we used a combination of IL-1 and TNF (termed IL-1–TNF), since their pathways of NF-κB activation are known to be synergistic (44). Other combinations of cytokines were also used, as shown in Fig. 7. Thymocytes were maintained in culture either unstimulated or treated with the various cytokines for 48 h prior to transfection. Cotransfection was then performed with CMV-tat, EF1-βgal, and either LTR-luc or LTRΔκB-luc. The transfected thymocytes were then returned for an additional 20 h to the previous conditioned medium prior to the determination of reporter gene activities. The activities of the two LTRs, wild type and ΔκB, were of the same level in nonactivated Jurkat cells (data not shown) whereas the activity of the wild-type LTR was 3.5-fold higher than that of LTRΔκB in thymocytes (considered the basal activity of the LTR, independent of NF-κB activation) (Fig. 7A). These data confirmed that thymocytes left unstimulated even for 48 h were not completely devoid of NF-κB activity (see also the LTR and NF-κB activity [Fig. 3]). IL-1–TNF stimulation further enhanced this level (ninefold). The transactivation level observed with IL-1–TNF was not further modified by addition of IL-6 and GM-CSF. Only addition of IL-7 to IL-1–TNF led to a dramatic increase in LTR transactivation (30-fold). However, it should be noted that IL-7 alone also increased HIV-1 LTR activity (24-fold). This is in agreement with the significant NF-κB activity elicited by IL-7 acting as a cofactor of low levels of TNF (and IL-1) constitutively produced by thymocytes (Fig. 6C, lanes 5 through 7). As expected, the effect of TNF–IL-1 required κB sites, since the LTRΔκB was unresponsive. In contrast, IL-7 triggered LTRΔκB transactivation, although less efficiently than that of the wild-type LTR, indicating that this cytokine acts through both NF-κB-dependent and NF-κB-independent pathways.
FIG. 7.
(A) LTR transactivation in thymocytes is triggered by TNF and IL-1 in the presence of IL-7. Freshly isolated thymocytes were precultured for 48 h alone or with a combination of cytokines as indicated prior to cotransfection with either LTR-luc, CMV-tat, and EF1-βgal or LTRΔκB-luc, CMV-tat, and EF1-βgal. The plasmids were transfected by electroporation at the respective amounts of 10, 2.5, and 10 μg per 107 cells. The transfected thymocytes were then returned for an additional 20 h under the previous culture conditions prior to determination of luciferase and β-galactosidase activities. LTR activity was expressed as normalized luciferase activity. Numbers at the top of the columns represent fold amplification relative to luciferase expression in unstimulated thymocytes transfected with LTRΔκB-luc (level of NF-κB-independent activity). This experiment is representative of three independent experiments carried out on three thymuses. (B) Interaction of thymocytes with TEC also induces an NF-κB independent LTR transactivation in thymocytes. Freshly isolated thymocytes were precultured for 48 h alone or in the presence of TEC prior to cotransfection with either LTR-luc, CMV-tat, and EF1-βgal or LTRΔκB-luc, CMV-tat, and EF1-βgal as described in the legend to panel A. This experiment is representative of four independent experiments, each carried out on a different thymus.
In Fig. 7B we show that the NF-κB-independent transactivation induced by IL-7 correlates with the weak induction of transcriptional activity of LTRΔκB in thymocytes during their interaction with TEC and suggests that secretion of IL-7 by TEC is mainly responsible for this effect. However, the induction of the transcriptional activity of the wild-type LTR by TEC is much more efficient than that of the LTRΔκB.
DISCUSSION
We report here that NF-κB activation is a major prerequisite for a high level of HIV-1 replication in thymocytes and that the interaction of infected thymocytes with TEC is necessary for such an induction. This conclusion was drawn from the fact that an HIV-1 provirus with its κB sites deleted failed to replicate in thymocytes whereas the wild-type provirus exhibited a high level of replication, although only in the presence of TEC. The active HIV replication observed within the thymus (58–60) thus suggested that NF-κB was permanently induced in the thymic microenvironment. We confirmed that a high level of NF-κB binding activity was present within this organ, taken as a whole. This is in agreement with the detection of nuclear NF-κB complexes in vivo in human thymus by in situ immunostaining (11). We also detected NF-κB binding activity in freshly isolated thymocytes, as assessed by the presence of distinct NF-κB complexes. p50-p65 was the prevalent complex, but p50-p50 and p50-RelB were also present. p52-containing complexes, although minor, were also detected. The difference between whole thymus and thymocytes suggests that the major RelB complexes detected in whole thymus were mainly derived from TEC, confirming previous reports of results obtained with murine thymus (7, 37, 62).
We then showed that NF-κB activity observed in freshly isolated thymocytes was the result of specific signals delivered within the microenvironment. This activity, which is mainly due to p50-p65 complexes, decreased when thymocytes were cultured alone. Furthermore, we demonstrated that at least part of the activation signals comes from the interaction with TEC, since addition of TEC to thymocyte culture prevented this progressive loss of activation. The same effect was observed with autologous and heterologous TEC, excluding the potential involvement of a mixed lymphocyte reaction. However, compared to that observed in freshly isolated thymocytes, the maintenance of NF-κB activity during coculture with TEC was less efficient, but the nature and ratio of the complexes were similar. Therefore, we could not exclude the possible role of other cells or factors within the microenvironment in strengthening NF-κB activity. We can also argue that disruption of thymocyte-TEC interaction during thymus preparation might not be immediately and efficiently restored by mixing these two cell subpopulations in the same culture dish, particularly with regard to the necessary accumulation of soluble factors.
The high level of active NF-κB complexes, including p50-p65, in freshly isolated thymocytes as well as in thymocytes cocultured with TEC was postulated to correlate with a strong activation of the HIV LTR. By using transfection assays, we showed that the decrease of NF-κB activity in thymocytes in culture was accompanied by a decrease of LTR activity, except in the presence of TEC, which maintained a high level of both NF-κB and LTR activity. We also confirmed that this high activity of the LTR was dependent upon κB binding sites since it was strongly decreased by deletion of these sites (LTRΔκB-luc). The involvement of the κB responsive element was further confirmed by comparing the luciferase activity obtained with the 3Enh-TK-luc construct with that obtained with the control vector TK-luc.
It should be noted that the migration of the activity indicated as n.s 1 in Fig. 5, observed in EMSA, was not significantly modified by competition with an excess of the unlabeled probe. In consequence, this activity was indicated as nonspecific. However, it was shown to be modulated by certain cytokines of the coculture (Fig. 5 and 6). This activity is likely to be KBF2/RBP, which has been shown to bind κB sites (25), albeit with an affinity much lower than that of NF-κB (25a).
Since certain nucleotides of the κB element within the LTR were found to participate in the binding of other transcription factors such as RBP/KBF2 (25), NF-AT (29), ets (53), and GABP (13), it was necessary to formally demonstrate that LTR transactivation was regulated by members of the Rel/NF-κB family. We therefore cotransfected a mutated IκBα molecule which cannot be phosphorylated and degraded (63) together with an HIV LTR. Overexpression of this mutated form of IκBα, acting in competition with endogenous IκBα, led to a dose-dependent decrease in LTR activity. The highest concentration of IκBα-DN decreased the activity of the wild-type LTR to a value comparable to that obtained with the LTR-ΔκB. These data confirm that enhanced transactivation of the LTR is triggered by the Rel/NF-κB family and that NF-κB activity in thymocytes is not constitutive but results from a permanent activation of NF-κB.
The major role of soluble factors in NF-κB activation was first demonstrated by the fact that thymocyte-TEC interaction was efficient even in absence of cell-to-cell contact. This does not exclude a role of the contact in sustaining the levels of these cytokines. We showed that TNF, secreted mainly by thymocytes, represented a major requirement for NF-κB activation, since antibodies against TNF abrogated the inductive effect of the coculture. IL-1, produced by both TEC and thymocytes (41, 66), modestly participated in this induction. Unexpectedly, these two cytokines were not sufficient to maintain a long-term activation of NF-κB in culture. We then demonstrated that IL-7, secreted mainly by TEC (42, 49), induced an NF-κB activity in thymocytes by acting as a cofactor of low levels of TNF and IL-1 constitutively produced by thymocytes (12, 16, 66). This was confirmed by the fact that the IL-7-induced NF-κB activity could be abolished by antibodies against IL-1 and TNF. Together, these data lead to the conclusion that TNF is the major inducer of NF-κB activity in thymocytes and that IL-1 has a modest synergistic effect. IL-7 is required for the maintenance of this activity, acting as an important cofactor. It is at first sight surprising that in our previous study (48), thymocytes were capable of replicating the virus in vitro in the absence of IL-7 addition (in the presence of TNF, IL-1, GM-CSF, and IL-6). The possible explanation is that IL-7 was expressed at low levels in the thymocyte culture. IL-7 might be provided either by the thymocytes themselves, which express at least detectable IL-7 mRNA (41, 66), or by stromal cells (42, 49), which might remain at a low level in the thymocyte preparation, although the purity of the thymocyte population was estimated to be 99%. This low level of IL-7 might then be sufficient to permit NF-κB activity in the presence of saturating concentrations of TNF and IL-1 added to the thymocyte culture medium. This hypothesis is in agreement with the fact that HIV-1 replication in thymocytes cultured with IL-1, IL-6, TNF, and GM-CSF or cocultured with TEC was delayed when an anti-human IL-7 antiserum was added at the start of the culture (data not shown).
Nevertheless, the level of replication obtained in thymocytes in vitro when IL-7 was used with TNF, IL-1, GM-CSF, and IL-6 was much higher than in absence of IL-7 (data not shown). This is in agreement with the transfection experiments performed on thymocytes after 48 h in culture, showing that the addition of IL-7 to IL-1-TNF led to a dramatic increase in LTR transactivation. Several hypotheses might be proposed to explain the role of IL-7 in the NF-κB-dependent pathway. IL-7 increases the activity of the AP1 transcription factor (17), which is able to physically interact and synergize with NF-κB (55). The increase in AP1 activity could also directly upregulate the expression of the TNF receptor p75, since an AP1-responsive element is present in the 5′ regulatory region of its gene (51). This last hypothesis is difficult to demonstrate by using the total freshly isolated thymocyte population, in which the number of thymocytes expressing this receptor never exceeded 3% by cytofluorometric analysis (data not shown). Indeed, IL-7 receptor is restricted to certain subsets of thymocytes (57). Work to identify the subpopulations of thymocytes responsible for the high level of HIV replication is in progress in our laboratory. Use of these subpopulations, which might be characterized by the expression of both IL-7 and TNF receptors (and to a lesser extent IL-1 receptor), will allow us to determine whether IL-7 modulates the expression of the TNF p75 receptor.
Interestingly, IL-7 also increases the activity of the LTRΔκB construct, suggesting that this cytokine is also involved in an NF-κB-independent pathway. In addition to AP1, IL-7 activates several other transcription factors including NF-AT (17), Stat 3, and Stat 5 (36, 38). By systematic mutagenesis within the LTR, we are in the process of identifying the sequence responsible for this effect. The magnitude of transactivation through this pathway is much lower than that observed through the NF-κB one. This is very possibly the reason why no virus replication was detectable in the absence of κB sites. Together, these data suggest that NF-κB activity is not strictly necessary to induce LTR transactivation but is necessary to attain a high level of HIV replication. Similar conclusions have been reached concerning the involvement of NF-κB in HIV replication in T lymphocytes (8). It is worth noting that the regulation of NF-κB-dependent transactivation of the LTR is highly dependent on the NF-κB complexes present in a given cell and in its microenvironment. For instance, in peripheral resting T lymphocytes, an inactive p50 homodimer constitutively occupies the κB sites, preventing LTR activation by p50-p65 induced by TNF or IL-1. The major stimulus responsible for an NF-κB-dependent transactivation is the recognition of a specific antigen (19) permitting an induction of Bcl-3, a coactivator (14) which generates, with p50 homodimers, an active trimer on the HIV-LTR (unpublished data). In contrast, in thymocytes p50 homodimers are underrepresented with respect to p50-p65 heterodimers, which are permanently activated by TNF and IL-1 within the thymic microenvironment. Under these conditions, TNF and IL-1 induce LTR transactivation. Another specificity of the thymocytes and the thymic microenvironment is that these cytokines cannot efficiently function without IL-7. Together with our previous report, we demonstrate here that to sustain efficient HIV replication, thymocytes require cytokines responsible for their activation through NF-κB (TNF and IL-1) and for their survival (GM-CSF) (27, 30), as well as a cytokine which seems to fulfill both functions (IL-7) (1, 23). Through these cytokines, the thymus behaves as a virus reservoir, since it favors the maintenance of a high viral load in certain subpopulations of thymocytes (8a), as well as a prolonged life span of these thymocytes.
ACKNOWLEDGMENTS
We thank Sonia Berrih-Aknin and Claude Planché (hospital Marie Lannelongue, Le Plessis-Robinson, France) for providing us with thymuses from infants undergoing cardiac surgery. We thank A. Israël for providing us with antibodies against NF-κB subunits and for a review of the manuscript. We thank N. Rice for providing us with IκBα-DN expression vector, and we thank S. Mémet for the EF1α-βgal vector.
This work was supported by the Agence Nationale pour la Recherche sur le SIDA (ANRS). L. Chêne is a fellow of the French Ministry of Education and Research (MENESR).
REFERENCES
- 1.Akashi K, Kondo M, von Freeden U, Jeffry U, Murray R, Weissman I L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Guiet P, Raphael M, Grandadam M, Agut H, Candotti D, Grenot P, Puech F, Debré P, Cesbron J-Y. Thymocytes and thymic microenvironment alterations during a systemic HIV infection in a severe combined immunodeficient mouse model. AIDS. 1996;10:717–727. doi: 10.1097/00002030-199606001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Bach M K, Brashler J R. Evidence that granulocyte/macrophage-colony-stimulating factor and interferon-gamma maintain the viability of human peripheral blood monocytes in part by their suppression of IL-10 production. Int Arch Allergy Immunol. 1995;107:90–92. doi: 10.1159/000236940. [DOI] [PubMed] [Google Scholar]
- 4.Bachelerie F, Rodriguez M S, Dargemont C, Rousset D, Thomas D, Virelizier J L, Arenzana-Seisdedos F. Nuclear export signal of IκBα interferes with the Rev-dependent posttranscriptional regulation of human immunodeficiency virus type I. J Cell Sci. 1997;110:2883–2893. doi: 10.1242/jcs.110.22.2883. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin A S. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Braun J, Valentin H, Nugeyre M T, Ohayon H, Gounon P, Barré-Sinoussi S F. Productive and persistent infection of human thymic epithelial cells in vitro with HIV-1. Virology. 1996;225:413–418. doi: 10.1006/viro.1996.0617. [DOI] [PubMed] [Google Scholar]
- 7.Burkly L, Hession C, Ogata L, Reilly C, Marconi L A, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen B K, Feinberg M B, Baltimore D. The κB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J Virol. 1997;71:5495–5504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Chêne, L., et al. Unpublished data.
- 9.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 10.Duh E J, Maury W J, Folks T M, Fauci A S, Rabson A B. Tumor necrosis factor α activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-κB sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuillard J, Dargemont C, Ferreira V, Tarantino N, Debre P, Raphael M, Korner M. Nuclear Rel-A and c-Rel protein complexes are differentially distributed within human thymocytes. J Immunol. 1997;158:2585–2591. [PubMed] [Google Scholar]
- 12.Fisher M, MacNeil I, Suda T, Cupp J, Shortman K, Zlotnik A. Cytokines production by mature and immature thymocytes. J Immunol. 1991;146:3452–3456. [PubMed] [Google Scholar]
- 13.Flory E, Hoffmeyer A, Smola U, Rapp U R, Bruder J T. Raf-1 kinase targets GA-binding protein in transcriptional regulation of the human immunodeficiency virus type 1 promoter. J Virol. 1996;70:2260–2268. doi: 10.1128/jvi.70.4.2260-2268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita T, Nolan G P, Liou H C, Scott M L, Baltimore D. The candidate proto oncogene Bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 15.Gaynor R B. Regulation of HIV-1 gene expression by the transactivator protein Tat. Curr Top Microbiol Immunol. 1995;193:51–77. doi: 10.1007/978-3-642-78929-8_3. [DOI] [PubMed] [Google Scholar]
- 16.Giroir B P, Brown T, Beutler B. Constitutive synthesis of tumor necrosis factor in the thymus. Proc Natl Acad Sci USA. 1992;89:4864–4868. doi: 10.1073/pnas.89.11.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gringhuis S I, Deleij L, Verschuren E W, Borger P, Vellenga E. Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1. Blood. 1997;90:2690–2700. [PubMed] [Google Scholar]
- 18.Harrich D, Garcia J, Mitsuyasu R, Gaynor R. TAR independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 1990;9:4417–4423. doi: 10.1002/j.1460-2075.1990.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazan U, Thomas D, Alcami J, Bachelerie F, Israël N, Yssel H, Virelizier J-L, Arenzana-Seisdedos F. Stimulation of a human T-cell clone with anti-CD3 or tumor necrosis factor induces NF-κB translocation but not human immunodeficiency virus 1 enhancer-dependent transcription. Proc Natl Acad Sci USA. 1990;87:7861–7865. doi: 10.1073/pnas.87.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellerstein M K, McCune J M. T cell turnover in HIV-1 disease. Immunity. 1997;7:583–589. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 21.Herbelin A, Machavoine F, Schneider E, Papiernik M, Dy M. IL-7 is requisite for IL-1-induced thymocyte proliferation. Involvement of IL-7 in the synergistic effects of granulocyte-macrophage colony-stimulating factor or tumor necrosis factor with IL-1. J Immunol. 1992;148:99–105. [PubMed] [Google Scholar]
- 22.Herbelin A, Machavoine F, Vicari A, Schneider E, Papiernik M, Ziltener H, Penit C, Dy M. Endogenous granulocyte-macrophage colony-stimulating factor is involved in IL-1- and IL-7-induced murine thymocyte proliferation. J Immunol. 1994;153:1973–1981. [PubMed] [Google Scholar]
- 23.Hernandez-Caselles T, Martinez-Esparza M, Sancho D, Rubio G, Aparicio P. Interleukin-7 rescues human activated T lymphocytes from apoptosis induced by glucocorticosteroids and regulates bcl-2 and CD25 expression. Hum Immunol. 1995;43:181–189. doi: 10.1016/0198-8859(94)00168-p. [DOI] [PubMed] [Google Scholar]
- 24.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 25.Israël A, Yano O, Logeat F, Kieran M, Kourilsky P. Two purified factors bind to the same sequence in the enhancer of mouse MHC class I genes: one of them is a positive regulator induced upon differentiation of teratocarcinoma cells. Nucleic Acids Res. 1989;17:5245–5257. doi: 10.1093/nar/17.13.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Israël, A. Personal communication.
- 26.Israël N, Hazan U, Alcami J, Munier A, Arenzana-Seisdedos F, Bachelerie F, Israël A, Virelizier J L. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956–3960. [PubMed] [Google Scholar]
- 27.Iversen P O, To L B, Lopez A F. Apoptosis of hemopoietic cells by the human granulocyte-macrophage colony-stimulating factor mutant E21R. Proc Natl Acad Sci USA. 1996;93:2785–2789. doi: 10.1073/pnas.93.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi V V, Oleske J M, Saad S, Gadol C, Connor E, Bobila R, Minnefor A B. Thymus biopsy in children with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110:837–842. [PubMed] [Google Scholar]
- 29.Kinoshita S, Su L S, Amano M, Timmerman L A, Kaneshima H, Nolan G P. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita T, Yokoda T, Arai K, Miyajima A. Regulation of Bcl-2 expression by oncogenic Ras protein in hematopoetic cells. Oncogene. 1995;10:2207–2212. [PubMed] [Google Scholar]
- 31.Kitano K, Abboud C N, Ryan D H, Quan S G, Baldwin G C, Golde D W. Macrophage-active colony-stimulating factors enhance human immunodeficiency virus type 1 infection in bone marrow stem cells. Blood. 1991;77:1699–1705. [PubMed] [Google Scholar]
- 32.Kitchen S G, Uittenbogaart C H, Zack J A. Mechanism of human immunodeficiency virus type 1 localization in CD4-negative thymocytes—differentiation from a CD4-positive precursor allows productive infection. J Virol. 1997;71:5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp E B, Ghosh S. NF-kappa B and rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 34.Le P T, Lazorick S, Whichard L P, Yang Y-C, Clark S C, Haynes B F, Singer K H. Human thymic epithelial cells produce IL-6, granulocyte-monocyte-CSF, and leukemia inhibitory factor. J Immunol. 1990;145:3310–3315. [PubMed] [Google Scholar]
- 35.Leonard J, Parrott C, Buckler W A, Turner W, Ross E K, Martin M A, Rabson A B. The NF-kappa B binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J Virol. 1989;63:4919–4924. doi: 10.1128/jvi.63.11.4919-4924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard W J, Shores E W, Love P E. Role of the common cytokine receptor gamma chain in cytokine signaling and lymphoid development. Immunol Rev. 1995;148:97–114. doi: 10.1111/j.1600-065x.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 37.Lernbecher T, Muller U, Wirth T. Distinct NF-kappa B/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993;365:767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- 38.Lin J X, Migone T S, Tsang M, Friedmann M, Weatherbee J A, Zhou L, Yamauchi A, Bloom E T, Mietz J, John S, Leonard W J. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 39.May M J, Ghosh S. Rel/NF-kappa B and I kappa B proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 40.McCune J M. Thymic function in HIV-1 disease. Semin Immunol. 1997;9:397–404. doi: 10.1006/smim.1997.0098. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery R A, Dallman M J. Semi-quantitative polymerase chain reaction analysis of cytokine and cytokine receptor gene expression during thymic ontogeny. Cytokine. 1997;9:717–726. doi: 10.1006/cyto.1997.0227. [DOI] [PubMed] [Google Scholar]
- 42.Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;1:526–531. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- 43.Okazaki H, Ito M, Sudo T, Hattori M, Kano S, Katsura Y, Minato N. IL-7 promoters thymocyte proliferation and maintains immunocompetent thymocytes bearing alpha beta or gamma delta T-cell receptors in vitro: synergism with IL-2. J Immunol. 1989;143:2917–2922. [PubMed] [Google Scholar]
- 44.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papiernik M, Brossard Y, Mulliez N, Roume J, Brechot C, Barin F, Goudeau A, Bach J F, Griscelli C, Henrion R, Vazeux R. Thymic abnormalities in fetuses aborted from human immunodeficiency virus type 1 seropositive women. Pediatrics. 1992;89:297–301. [PubMed] [Google Scholar]
- 46.Plum J, De S M, Leclercq G, Verhasselt B, Vandekerckhove B. Interleukin-7 is a critical growth factor in early human T-cell development. Blood. 1996;88:4239–4245. [PubMed] [Google Scholar]
- 47.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Rothe M, Chêne L, Nugeyre M, Barré-Sinoussi F, Israël N. Contact with thymic epithelial cells as a prerequisite for cytokines enhanced HIV-1 replication in thymocytes. J Virol. 1998;72:5852–5861. doi: 10.1128/jvi.72.7.5852-5861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakata T, Iwagami S, Tsuruta Y, Teraoka H, Tatsumi Y, Kita Y, Nishikawa S, Takai Y, Fujiwara H. Constitutive expression of IL-7 mRNA and production of IL-7 by a cloned thymic stromal cell line. J Leukoc Biol. 1990;48:205–209. doi: 10.1002/jlb.48.3.205. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Pescador R, Power M, Barr P, Steimer K, Stempien M, Brown-Shimer S, Gee W, Renard A, Randolph A, Levy J. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2) Science. 1985;227:484–494. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- 51.Santee S M, Owen S L. Human tumor necrosis factor receptor p75/80 (CD120b) gene structure and promoter characterization. J Biol Chem. 1996;271:21151–21159. doi: 10.1074/jbc.271.35.21151. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz O, Virelizier J, Montagnier L, Hazan U. A microtransfection method using the luciferase encoding reporter gene for the assay of HIV LTR promoter activity. Gene. 1990;88:197–205. doi: 10.1016/0378-1119(90)90032-m. [DOI] [PubMed] [Google Scholar]
- 53.Seth A, Hodge D R, Thompson D M, Robinson L, Panayiotakis A, Watson D K, Papas T S. ETS family proteins activate transcription from HIV-1 long terminal repeat. AIDS Res Hum Retroviruses. 1993;9:1017–1023. doi: 10.1089/aid.1993.9.1017. [DOI] [PubMed] [Google Scholar]
- 54.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein B, Baldwin A S, Ballard D W, Greene W C, Angel P, Herrlich P. Cross-coupling of the NF-kappa-B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune J M. HIV-1-induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 57.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa S. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka K E, Hatch W C, Kress Y, Soeiro R, Calvelli T, Rashbaum W K, Rubinstein A, Lyman W D. HIV-1 infection of human fetal thymocytes. J Acquired Immune Defic Syndr. 1992;5:94–101. [PubMed] [Google Scholar]
- 59.Uittenbogaart C H, Anisman D J, Jamieson B D, Kitchen S, Schmid I, Zack J A, Hays E F. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS. 1996;10:9–16. doi: 10.1097/00002030-199606001-00001. [DOI] [PubMed] [Google Scholar]
- 60.Valentin H, Nugeyre M-T, Vuillier F, Boumsell L, Schmid M, Barré-Sinoussi F, Pereira R. Two subpopulations of human triple-negative thymic cells are susceptible to infection by human immunodeficiency virus type 1 in vitro. J Virol. 1994;68:3041–3050. doi: 10.1128/jvi.68.5.3041-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoefer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 62.Weih F, Carrasco D, Bravo R. Constitutive and inducible Rel/NF-kappa B activities in mouse thymus and spleen. Oncogene. 1994;9:3289–3297. [PubMed] [Google Scholar]
- 63.Whiteside S, Ernst M, Lebail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-kappa B activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whiteside S T, Israël A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 65.Withersward E S, Amado R G, Koka P S, Jamieson B D, Kaplan A H, Chen I, Zack J A. Transient renewal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 66.Wolf S S, Cohen A. Expression of cytokines and their receptors by human thymocytes and thymic stromal cells. Immunology. 1992;77:362–368. [PMC free article] [PubMed] [Google Scholar]
- 67.Zuniga-Pflücker J C, Jiang D, Lenardo M J. Requirement for TNF-α and IL-1α in fetal thymocyte commitment and differentiation. Science. 1995;268:1906–1909. doi: 10.1126/science.7541554. [DOI] [PubMed] [Google Scholar]