Abstract
Multiple sclerosis is a chronic autoimmune demyelinating disease of the central nervous system and is difficult to diagnose in early stages. Without homeostatic control, urine was reported to have the ability to accumulate early changes in the body. We expect that urinary proteome can reflect early changes in the nervous system. The early urinary proteome changes in a most employed multiple sclerosis rat model (experimental autoimmune encephalomyelitis) were analysed to explore early urinary candidate biomarkers, and early treatment of methylprednisolone was used to evaluate the therapeutic effect. Twenty-five urinary proteins were altered at day 7 when there were no clinical symptoms and obvious histological changes. Fourteen were reported to be differently expressed in the serum/cerebrospinal fluid/brain tissues of multiple sclerosis patients or animals such as angiotensinogen and matrix metallopeptidase 8. Functional analysis showed that the dysregulated proteins were associated with asparagine degradation, neuroinflammation and lipid metabolism. After the early treatment of methylprednisolone, the incidence of encephalomyelitis in the intervention group was only 1/13. This study demonstrates that urine may be a good source of biomarkers for the early detection of multiple sclerosis. These findings may provide important information for early diagnosis and intervention of multiple sclerosis in the future.
Keywords: urine proteome, multiple sclerosis, biomarker
1. Introduction
Multiple sclerosis is a chronic autoimmune demyelinating disease of the central nervous system that is characterized by both inflammatory components and neurodegeneration [1]. It contributes to a significant proportion of neurologic morbidity in young adults [2], but the causes of multiple sclerosis are not fully understood. Magnetic resonance imaging (MRI) is the most common diagnostic tool for multiple sclerosis [3]. Additionally, some clinical features may help to diagnose the disease. However, few clinical manifestations are specific to multiple sclerosis, and MRI lacks specificity for the early stages of the disease [4]. In addition, when clinical symptoms develop, medical treatment is not always timely enough. Furthermore, the early intervention of multiple sclerosis is still a matter of concern. Early treatment to slow or reverse inflammatory lesion formation is advocated as an effective way to prevent accumulation of disability [5].
Without homeostatic control, urine can reflect most changes in the body and thus can be a better source of biomarkers [6]. Numerous biomarkers of various diseases can be detected in urine, and some of these biomarkers perform even better than plasma biomarkers [7]. Compared with blood or cerebrospinal fluid (CSF), urine samples have several advantages in central nervous system (CNS) analysis mainly including: (1) urine collection is non-invasive and simple, which is extremely suitable for time observation; (2) the changed information in blood or CSF might not been preserved for a long time, but the important information can be accumulated in urine; (3) recent studies have elucidated the potential of urine as a useful biomarker source for CNS diseases such as Alzheimer's disease and Parkinson's disease [8–10]. As far as we know, there have never been any reports of early urinary proteome changes associated with multiple sclerosis. Advancements in mass spectrometry (MS) have made it possible to uncover new distinct molecular components. During the past few years, a few proteomics approaches have been used to investigate changes in urinary proteins/metabolites of multiple sclerosis patients [11]. For example, proteins such as trefoil factor 3 and lysosome-associated membrane protein 2, both of which are related to immune responses, were differentially expressed in two phases (the third trimester of pregnancy and the postpartum period) of multiple sclerosis patients [12]. Multiple sclerosis shares many overlapping clinical features with neuromyelitis optica spectrum disorders, but the treatment strategies differ substantially for these two diseases, and thus several urinary proteomic/metabolome studies have been conducted to differentiate these two diseases [13,14].
Experimental autoimmune encephalomyelitis (EAE) is the most commonly employed model for multiple sclerosis [15], and it has been a powerful tool for studying relevant mechanisms in multiple sclerosis as well as for translating the findings into clinically meaningful therapeutic approaches [16]. The clinicopathologic characteristics of the EAE model, including inflammation and demyelination of the central nervous system, are like those of multiple sclerosis [17]. Thus, studies using the EAE model have provided new insights into the pathogenesis and pathophysiology of multiple sclerosis. Because multiple sclerosis is difficult to diagnose at early stages in clinical practice, in this study, the EAE model was used for the discovery of early urinary biomarkers of multiple sclerosis. And for animal models, it is convenient to limit confounding factors to understand the onset of diseases [18].
In the present study, we used two-dimensional high-resolution MS to investigate changes in the urinary proteome during the early stages of multiple sclerosis in EAE rat models. On this basis, we explored the effect of early intervention with methylprednisolone. Urine samples were analysed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) before the onset of disease symptoms (day 7). The changed proteins were then correlated to neurological functions by network and canonical pathway analysis. The study showed that the early diagnosis and intervention of multiple sclerosis are important, and urine proteome could reflect early changes of EAE models.
2. Material and methods
2.1. Experimental rats
Fifty-six male Lewis rats (8 weeks old) were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Science & Peking Union Medical College. The experiment was approved by the Institute of Basic Medical Sciences Animal Ethics Committee, Peking Union Medical College (Animal Welfare Assurance Number: ACUC-A02-2014-007). The study was performed according to guidelines developed by the Institutional Animal Care and Use Committee of Peking Union Medical College. The rats were housed individually on a 12 h/12 h light–dark cycle, with food and water ad libitum provided; room humidity (40 ± 5%) and temperature (22 ± 1°C) were kept constant.
2.2. EAE rat models for early diagnosis
Thirty rats were randomly divided into two groups, namely, the EAE group (n = 15) and the control group (n = 15). EAE was induced in Lewis rats with myelin basic protein (MBP) as previously described [19]. Rats in the EAE group were immunized with subcutaneous injections of 100 µg MBP (Sigma) emulsified in 5 mg ml−1 complete Freund's adjuvant (CFA) containing Mycobacterium butyricum (Sigma). Rats in the control group were administered with CFA and infused with an equal amount of saline. On day 7, all rats were placed in metabolic cages to collect urine. Three pairs of rats in the two groups were sacrificed at each timepoint (day 0, 7, 14 and 21), and tissues were collected for histological analyses. Body weight and neurological impairment scores were evaluated daily. The progression of EAE was measured daily based on neurological impairment and scored from 0 to 5 as follows [20]: grade 0, no symptoms; 0.5, mild floppy tail; 1, floppy tail; 2, hindlimb weakness; 3, severe paraparesis; 4, tetraparesis; and 5, moribund.
2.3. EAE rat models for methylprednisolone early intervention
Twenty-six rats were randomly divided into three groups: the early treatment group (n = 13), the late treatment group (n = 7), and the control group (n = 6). EAE models were induced in all the rats as described before. According to the results of the EAE rat models for early diagnosis as above, the early treatment group received intraperitoneal injection of methylprednisolone (Pfizer) on the 9th day after modelling, and the late treatment group received intraperitoneal injection of methylprednisolone after disease progression (day 10, 11 and 12). Methylprednisolone was injected at a dose of 30 mg kg−1 for 5 consecutive days. The control group was injected with the same amount of normal saline after onset. Body weight and neurological impairment scores were evaluated daily as mentioned above. Statistical analysis was performed with GraphPad Prism 9.0.1 (GraphPad, San Diego, CA, USA). Data were tested for statistical significance using the paired Student's t-test.
2.4. Histological analysis
On days 7, 14 and 21, spinal cord samples were harvested from both EAE and control groups and dissected after blood withdrawal. After being fixed in 4% paraformaldehyde, the samples were embedded in paraffin. Sections of paraffin-embedded spinal cord samples were stained with hematoxylin and eosin (H&E) for the evaluation of inflammatory foci. The sections were examined with a transmission electron microscope (Talos L120C G2, 120 kV, Thermo Fisher Scientific).
2.5. Urine sample preparation
After collection, urine was immediately centrifuged at 2000g to remove pellets. Urinary proteins were extracted by adding three volumes of acetone. After centrifugation, proteins were dissolved in lysis buffer (8 M urea, 2 M thiourea, 25 mM dithiothreitol and 50 mM Tris). The urinary proteins were then denatured with dithiothreitol, alkylated with iodoacetamide, and digested with trypsin (Promega) (1 : 50) at 37°C overnight using filter-aided sample preparation methods as previously described [12]. The digested peptides were desalted using Oasis HLB cartridges (Waters, USA).
Urine samples (from fifteen rats with EAE and fifteen control rats) collected on day 7 were used for MS analysis. As the tandem mass tag (TMT) reagents (Thermo Fisher Scientific) have six channels, the fifteen EAE samples were randomly divided into three TMT channels and the other controls samples were divided into the other TMT channels (total six group). Peptides in each sample were labelled with 126, 127, 128, 129, 130 and 131 according to the manufacturer's instructions. The labeled peptides were mixed and then analysed with two-dimensional LC-MS/MS.
2.6. Reverse-phase liquid chromatography separation
TMT-labelled peptides were fractionated using offline high-pH reverse-phase liquid chromatography (RPLC) columns (XBridge, C18, 3.5 μm, 4.6 mm × 250 mm, Waters). Peptides were diluted in buffer A1 (10 mM ammonium formate in H2O, pH = 10) and then loaded onto the RPLC column. The elution buffer consisted of 10 mM ammonium formate in 90% acetonitrile (pH = 10; flow rate = 1 ml min−1; 60 min). The eluted peptides were collected at a rate of one fraction per minute. After lyophilization, 60 dried fractions were resuspended in 0.1% formic acid and combined into 15 fractions; for example, fractions 1, 16 and 31 were combined with fraction 46.
2.7. LC-MS/MS analysis
Each fraction was analysed in duplicate using a reverse-phase C18 (3 µm, Dr. Maisch, Germany) self-packed capillary LC column (75 µm × 120 mm). The elution gradient was 5–30% buffer B (0.1% formic acid in acetonitrile: flow rate 0.3 µl min−1) for 60 min. A TripleTOF 5600 MS system was used to analyse the eluted peptides. The MS data were acquired using data-dependent acquisition mode with the following parameters: 30 data-dependent MS/MS scans per full scan; acquisition of full scans at a resolution of 40 000, and acquisition of MS/MS scans at a resolution of 20 000; rolling collision energy; charge state screening (including precursors with +2 to +4 charge states); dynamic exclusion (exclusion duration 15 s); an MS/MS scan range of 250–1800 m/z; and a scan time of 50 ms.
2.8. Data analysis
All MS/MS spectra were analysed using the Mascot search engine (version 2.4.1, Matrix Science), and proteins were searched against the SwissProt_2014_07 database (taxonomy: Rattus, containing 7906 sequences). Carbamidomethylation of cysteines was set as fixed modifications, the precursor mass tolerance and the fragment mass tolerance were set to 0.05 Da, and two missed trypsin cleavage sites were allowed. To obtain convincing results, proteins were filtered using the decoy database method in Scaffold (v. 4.3.2, Proteome Software Inc., Portland). Only peptides identified with strict spectral FDR of <1% (q-value < 0.01) were retained. And each protein contained at least two unique peptides. The samples were analysed by LC-MS/MS for two technical replicates. And only proteins with CV less than 20% were retained for further analysis. Scaffold Q+ software was employed for the quantification of TMT labelling. The statistical test used in Scaffold Q+ was permutation; the changed proteins were defined based on a fold change >1.5 and Benjamini–Hochberg-corrected p-value < 0.05. The reported fold change (EAE/control ratio) for each protein was computed automatically by dividing the EAE protein intensity area by the control protein intensity area (both calculated in Scaffold Q+ software).
2.9. Functional analysis
For functional analysis, deregulated urinary proteins identified by MS were further annotated by Ingenuity Pathway Analysis (IPA) software, Gene Ontology (GO) and STRING database. After importing the information on the deregulated proteins and their fold changes into the Ingenuity website, we analysed the affected canonical pathway, networks and related diseases. Biological functions assigned to each canonical pathway were ranked according to the significance of that biological function in the pathway.
3. Results and discussion
3.1. Workflow for quantitative proteomics analysis of EAE rats
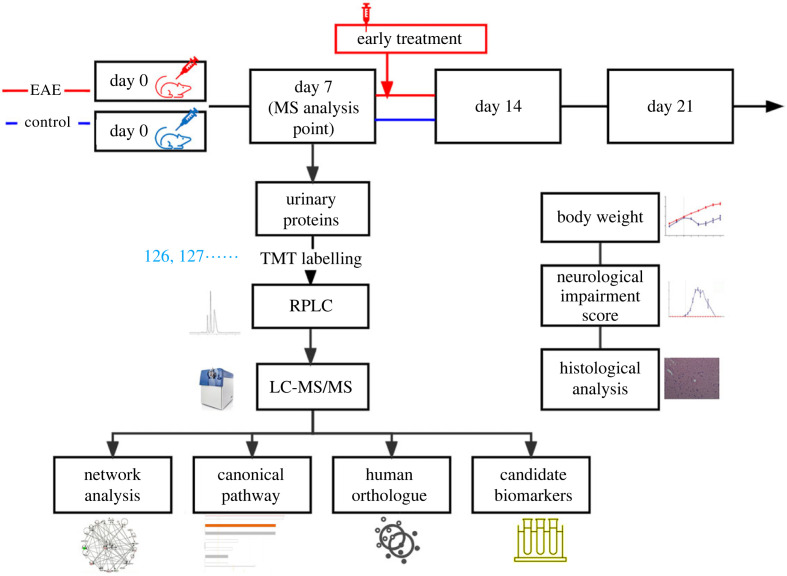
To determine the urinary candidate biomarkers of EAE, we immunized rats with MBP to establish an EAE model. Body weight indexes, neurological impairment scores and histopathological characterization were used to evaluate disease progression. On day 7 after immunization, few rats with EAE display clinical symptoms, and this phenomenon was identified as the time point to study early candidate biomarkers. To illustrate the importance of early diagnosis, early intervention was administrated at the onset of symptoms on day 9 and late intervention was administrated as the disease progressed in some rats. For comprehensive and comparative analyses, the urinary peptides were labelled with TMT. The TMT-labelled samples were separated into 15 fractions by offline RPLC, and each fraction was then analysed in duplicate by LC-MS/MS. The workflow for the quantitative proteomics analysis is shown in figure 1.
Figure 1.
Workflow of the study. The study was divided to two parts, the early biomarkers discovery and the early intervention study. Urinary proteins at day 7 were identified by TMT labelling followed by LC-MS/MS. For the intervention study, EAE rats received intraperitoneal injection of methylprednisolone on the 9th day after modelling in the early treatment group.
Raw data were searched against the SwissProt database for the taxonomy Rattus and then imported into Scaffold Q+ software for protein identification and quantification. If the identified peptides were shared between two proteins and could not be separated based on the MS data, the two proteins were grouped and annotated as one protein group. In total, 613 proteins that consisted of at least two peptides were identified at a 1% FDR at the protein level. Among these proteins, 566 high-confidence proteins were quantified by TMT labelling analysis in duplicate (electronic supplementary material, table S1).
3.2. Body weight and neurological impairment characterization
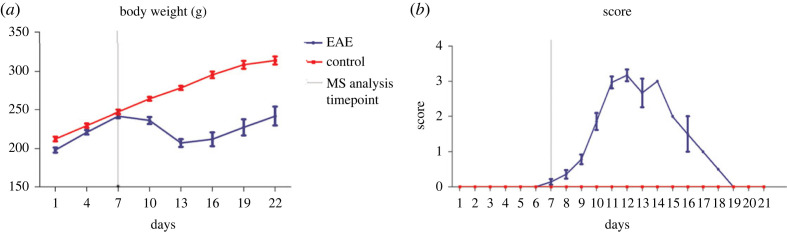
Parameters of the EAE and control groups were evaluated from day 0 to day 21. Both MBP-immunized Lewis rats and control rats exhibited a consistent 10 to 15% increase in body weight from the first day to the seventh day. In the first week, no significant difference in body weight was observed. From the eighth day onward, the weight of rats in the control group remained on the rise and was higher than that of rats with EAE. From day 11 onward, there was a marked decrease in the weight of the EAE group (225.1 ± 16.9 g), whereas the weight of the normal group was 268.1 ± 10.1 g. From day 13 to day 14, the weight of the EAE group stopped decreasing and remained unchanged. By day 21, the last time point examined in the study, the weight of the EAE group returned to the value on day 7. As shown in figure 2a, the difference in body weight between the EAE and control groups was significant, indicating possible impairment due to MBP immunization. Additionally, Lewis rats with EAE displayed a monophasic clinical course and spontaneous recovery, both of which are also consistent with previous results [21].
Figure 2.
Changes in (a) body weight and (b) neurological damage score during the experiment. The red line indicates the control group, and the purple line indicates the EAE group. All data are shown as mean ± standard deviation.
In the present study, no rats died or reached grade 5 in disease severity. From day 0 to day 7, consistent with the lack of negative changes in body weight, neither the MBP-immunized Lewis rats nor the control rats displayed any abnormal clinical symptoms. All rats immunized with MBP demonstrated typical clinical manifestations of neurological impairment lasting from 8 to 21 days post-immunization. On the eighth day, which is also the time when the weight began to decrease in rats with EAE, 4/15 MBP-immunized rats developed mildly flaccid tails, and this symptom became obvious in nearly all rats with EAE by the ninth day. From day 12 to day 14, all rats in the EAE group exhibited progressive bilateral hindlimb paralysis (grades 2 to 3), which was also the most severe symptom observed in this study. Then, the rats recovered from paralysis. By the end of the experiment, these effects disappeared (figure 2b).
3.3. Histopathological findings during the progression of EAE
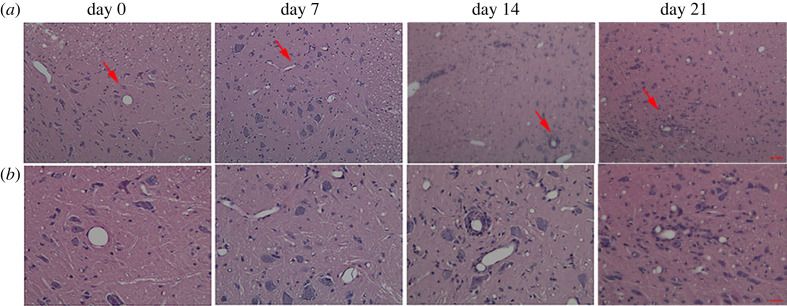
Histopathological examinations were performed by H&E staining of spinal cord sections from rats with EAE to evaluate the severity of disease (figure 3). During the early stages after initial immunization, very few infiltrated inflammatory cells were observed. On day 14, H&E staining revealed the presence of numerous inflammatory infiltrates in the parenchyma of the spinal cord and perivascular area. On day 21, also known as the recovery stage, some inflammatory lesions had disappeared; nevertheless, unlike the absence of inflammatory cell infiltration in the control samples, several inflammatory cells were still detected in the EAE samples. These pathological changes demonstrated the successful induction of EAE in Lewis rats.
Figure 3.
Histological characterization of the spinal cord in rats with EAE. The red arrows indicate the portion that has been magnified under high-power microscope. (a) Histological changes in the four stages in rats with EAE (H&E staining; magnification 10× objective; scale bar, 100 µm). (b) Histological changes in the four stages in rats with EAE (H&E staining; magnification 40× objective; scale bar, 25 µm).
3.4. Early intervention in EAE models by methylprednisolone
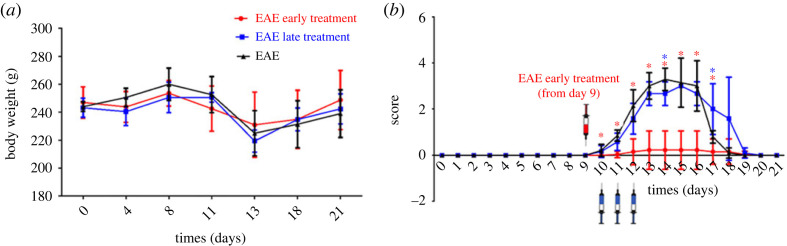
To explore the effect of early intervention, we established another EAE rat model injected with methylprednisolone at different time points to observe the incidence of the rats. Methylprednisolone is an effective drug in the treatment of autoimmune disease, such as multiple sclerosis, due to long-acting anti-inflammatory, antiallergic and immunosuppressant effects. Several studies have shown that methylprednisolone remarkably improved the clinical score of EAE and ameliorated the spinal cord inflammation and demyelination [22]. For the early intervention group, all the EAE rats received intraperitoneal injection of methylprednisolone on the 9th day after modelling for 5 consecutive days. The choices of treatment times were based on the actual onset time of the rats. The early treatment time was chosen before the EAE rats had no symptoms of disease onset, and the late treatment time was after the onset of symptoms. Only one out of the thirteen rats had onset of illness on day 11; the rest of the rats showed no obvious signs of nerve damage throughout the trial. For the late intervention group, the rats received intraperitoneal injection of methylprednisolone for 5 consecutive days after symptom onset. On day 10, 2/7 rats developed symptoms of nerve damage. All rats showed varying degrees of symptoms until day 12. For the EAE control group injected with normal saline after onset, 3/6 rats had onset of illness on day 10. Other rats showed varying degrees of symptoms on day 11 and day 12. One out of 7 rats in the late treatment group and 2/6 in the control group died during the experiment due to severe disease.
The changes in body weight and neural scores of the three groups are shown in figure 4. From the 8th day, the body weight of the three groups decreased to varying degrees. After the nerve damage symptoms recovered on the 18th day, the body weight of all rats continued to increase. The experimental results showed that when EAE rats had onset of illness, whether or not methylprednisolone was used for treatment, the neurological damage symptoms of the rats gradually improved after the peak on day 15, which may be related to the spontaneous recovery of the model [23]. Comparing the incidence of the three groups, the incidence in the early intervention group was only 1/13. The results indicate that early intervention can significantly reduce morbidity.
Figure 4.
Changes in body weights and neurological damage score during the intervention experiment. (a) Change in body weight of rats after modelling. (b) The neurological injury score of rats after modelling. *p-value less than 0.05. All data are shown as mean ± standard deviation.
3.5. Early changes in urinary proteins in EAE models
As indicated above, on day 7, the clinical scores in the EAE group were ‘0’ and were like the scores in the control group; no obvious histological changes were observed. Therefore, urine samples collected on day 7 after immunization were used for early biomarker detection. All identified proteins were quantitated by Scaffold Q+ software. The quantitative data are listed in electronic supplementary material, table S1. The median CV of retained proteins was 7.7% after using the Scaffold Q+ software normalized the MS/MS data The altered proteins were defined based on the following parameters: Benjamini–Hochberg-corrected p-value < 0.05 and fold change ratio >1.5. Statistical analyses indicated that 25 proteins were significantly affected by MBP immunization (14 up- and 11 downregulated proteins). The proteins are listed in table 1.
Table 1.
The early dysregulated urinary proteins in EAE models.
| ID | protein name | MW (kDa) | human orthologues | fold change E/N | p value | type(s) | reported in multiple sclerosis patients or animal model before |
|---|---|---|---|---|---|---|---|
| P55054 | fatty acid binding protein 9 | 15 | yes | 6.17 | 3.32×10−2 | transporter | serum [24] |
| P12020 | cysteine-rich secretory protein 1 | 28 | no | 3.18 | 1.28×10−6 | other | |
| P01048 | T-kininogen 1 | 48 | yes | 3.18 | 3.87×10−3 | other | CSF [25] |
| Q62714 | defensin RatNP-3 precursor | 10 | yes | 2.73 | 1.50×10−2 | other | |
| P02764 | orosomucoid 1 | 24 | yes | 2.32 | 4.77×10−2 | other | CSF [25] |
| O88766 | matrix metallopeptidase 8 | 53 | yes | 2.30 | 6.20×10−3 | peptidase | tissue/plasma [26] |
| P01015 | angiotensinogen | 52 | yes | 2.16 | 2.66×10−3 | growth factor | serum/CSF [27] |
| P20059 | hemopexin | 51 | yes | 1.82 | 2.29×10−3 | transporter | CSF [25] |
| Q8VI04 | asparaginase like 1 | 34 | yes | 1.78 | 4.00×10−2 | enzyme | |
| Q62930 | complement C9 | 62 | yes | 1.73 | 2.27×10−3 | other | CSF [25] |
| P08649 | complement C4B | 192 | yes | 1.66 | 1.08×10−2 | peptidase | CSF [25] |
| P13635 | ceruloplasmin | 121 | yes | 1.52 | 7.50×10−3 | enzyme | CSF [25] |
| Q9EQV9 | carboxypeptidase B2 | 49 | yes | 1.51 | 1.58×10−3 | peptidase | CSF [28] |
| P70490 | milk fat globule-EGF factor 8 protein | 47 | yes | 1.50 | 1.79×10−2 | other | |
| P47853 | biglycan | 42 | yes | 0.66 | 2.61×10−2 | other | |
| P10959 | carboxylesterase 1C | 60 | no | 0.66 | 1.77×10−2 | enzyme | |
| P09656 | serine protease inhibitor Kazal-type 1-like | 9 | yes | 0.65 | 3.35×10−2 | other | |
| P02631 | oncomodulin | 12 | yes | 0.61 | 2.64×10−3 | other | tissue [29] |
| P10760 | adenosylhomocysteinase | 48 | yes | 0.60 | 1.85×10−2 | enzyme | |
| P11232 | thioredoxin | 12 | yes | 0.60 | 2.08×10−3 | enzyme | serum [30] |
| O88989 | malate dehydrogenase 1 | 36 | yes | 0.59 | 7.12×10−3 | enzyme | |
| Q9QZ76 | myoglobin | 17 | yes | 0.56 | 1.76×10−2 | transporter | |
| P10111 | peptidylprolyl isomerase A | 18 | yes | 0.56 | 1.77×10−2 | enzyme | |
| P07092 | serpin family E member 2 | 44 | yes | 0.51 | 8.29×10−5 | other | CSF [31] |
| P50116 | S100 calcium binding protein A9 | 13 | yes | 0.37 | 1.60×10−2 | other | plasma [32] |
3.6. Canonical pathway and proteomic interactomes of the changed proteins
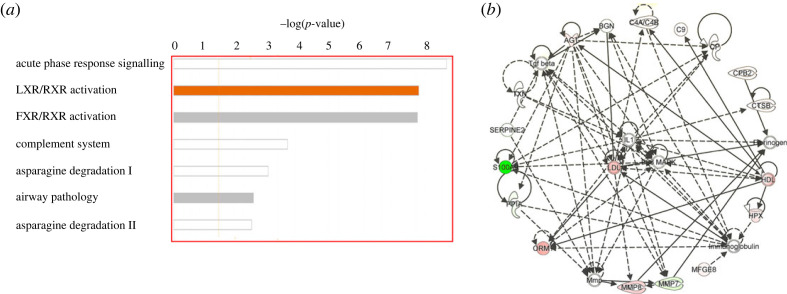
The canonical pathways in which the changed proteins were involved were generated by IPA software. The most significant pathways are acute phase response signalling, LXR/RXR activation, FXR/RXR activation, complement system and asparagine degradation (figure 5a). The other pathways are mostly related to the acute response except for asparagine pathway. Though its role in multiple sclerosis is unclear, asparagine may have roles in proper immune functioning and asparagine biosynthesis may be one of the main canonical pathways involved in multiple sclerosis [33]. The inhibition of asparagine endopeptidase greatly enhanced presentation of the myelin basic protein [34], which plays a key role in pathogenesis of multiple sclerosis. As a result, the asparagine degradation pathway may play important roles in the disease onset and progression.
Figure 5.
Functional analysis of changed proteins by IPA software. (a) The top canonical pathways generated from the software. (b) Network related to neurological functions annotated by IPA.
To investigate the analytical underpinning of this urinary proteomics study, the proteomic interactome of altered proteins after MBP immunization was determined. The IPA software highlights interactions among specific molecules and demonstrates how they might work together at the molecular level. Thus, these networks might represent efficient and unified interactions among the deregulated proteins and highlight potential causal molecules that may be used as targets for preventing multiple sclerosis progression. As shown in figure 5b, we uploaded 25 deregulated proteins into IPA and determined their interactions with network proteins associated with ‘neurological diseases'. A total of 13 deregulated molecules were identified in this functional interaction. Specific peptidases (complement C4, carboxypeptidase B and matrix metallopeptidase 8), enzymes (ceruloplasmin, peptidylprolyl isomerase A and thioredoxin), transporter (hemopexin), growth factor (angiotensinogen) and others (biglycan, complement C9, orosomucoid 1, S100 calcium binding protein A9 and serpin family E member 2) were identified as being altered in MBP-induced EAE. Within these networks, highly interconnected hub molecules are more likely to have important biological functions. The hub proteins identified in the neurological disease networks, namely, IL-1, LDL and P38 MAPK, are in the extracellular space, plasma membrane and cytoplasm, respectively. The upregulation of P38 MAPK is closely related to 4-1BB signaling in T cells and is consistent with the induction of EAE, because EAE is initiated by immunization with autoantigens presented to MHC class II-restricted CD4+ T helper cells [15]. Additionally, the inhibition of active mouse p38 MAPK in CD4+ T cells was shown to decrease the severity of EAE in mice [35]. IL-1 is one of the commonly used inflammatory factors. Although it is rarely detected in the normal brain, IL-1 is significantly upregulated and plays a central role in neuroinflammation, especially under neurodegenerative conditions [36]. Therefore, the proteins dysregulated in response to MBP immunization include interactors that preferentially function in CD4+ T cells, neuroinflammation and lipid metabolism and hence may affect neurological functions.
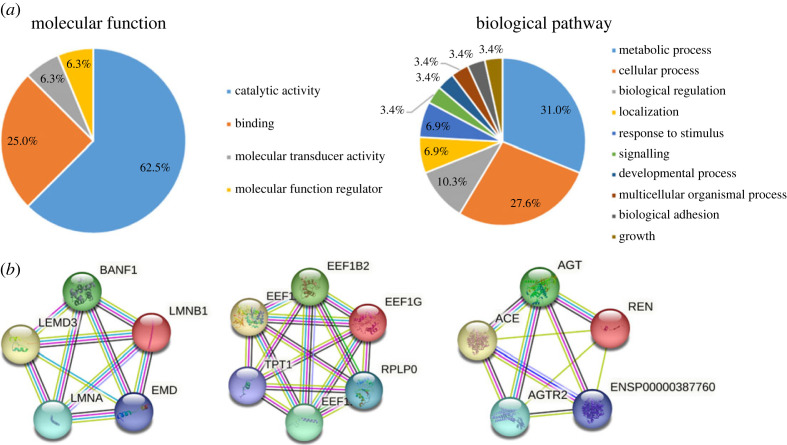
Then molecular functions and biological pathways of the altered proteins were determined by Panther Gene Ontology database (figure 6a). The study identified 25 dysregulated proteins in the multiple sclerosis rat models. The majority of these proteins, as determined by their molecular functions in Panther database, are involved in catalytic activities, which is consistent with the findings of the IPA that has revealed the presence of several proteins that have been annotated as enzymes and peptidases. Another significant molecular function is the binding function. Upon further inspection of the biological pathways, it has been found that the most prominent pathways, as determined by the GO database, include metabolic processes, cellular processes and biological regulation.
Figure 6.
GO annotation and network analysis. (a) Molecular function and biological pathway of dysregulated proteins annotated by Panther database. (b) The top three protein interaction networks in strings: progeria, eukaryotic translation elongation factor 1 complex and G protein-coupled receptor signalling.
The interaction of the 25 proteins was evaluated by using the search tool STRING. This approach furnished a network of protein–protein interactions encompassing 53 nodes and 103 edges, featuring a mean node degree of 4.11 and a clustering coefficient of 0.59. The expected number of edges was 63 and the p-value of the PPI enrichment was 7.8 × 10−8. The analysis identified three prominent string networks, which include progeria and osteopoikilosis, eukaryotic translation elongation factor 1 complex and nephrosclerosis, and G protein-coupled receptor signalling (figure 6b). Among them, the eukaryotic translation elongation factor families play a central role in the protein's biosynthesis during the elongation step of translation, and was reported to be related to multiple sclerosis [37]. Furthermore, numerous evidences from animal and clinical studies indicate that the G protein-coupled receptors play a significant role in several aspects of multiple sclerosis pathogenesis, including antigen presentation, cytokine production, T-cell differentiation and proliferation [38].
3.7. Human homologues of the altered urinary proteins
Altered proteins that are homologous to human proteins are potentially useful in clinical practice and may be candidate biomarkers of multiple sclerosis. When we imported the 25 altered proteins into the InParanoid database [39] to search for human homologues, 23 of the 25 proteins were found to have human counterparts (table 1). Because these 23 proteins may be useful for clinical practice, we will discuss these proteins that have human homologues in the following part of the paper.
Among the twenty-three urinary proteins that were affected and had a relatively significant fold change compared with the control group, fourteen were reported to be differently expressed in the serum/CSF/brain tissues of multiple sclerosis (table 1). For example, plasma thioredoxin was thought to be dysregulated in multiple sclerosis patients exposed and non-exposed to treatment [30] and serpin family in CSF had a significant and reproducible correlation with multiple sclerosis severity [31]. And these dysregulated proteins can be detected in the current urine studies. Previous studies have also identified biomarkers for the diagnosis and staging of multiple sclerosis in urine. The urinary neopterin appeared as a potential marker that could differentiate multiple sclerosis from other demyelinating patient groups such as myelin oligodendrocyte glycoprotein antibody-associated disorder and neuromyelitis optica spectrum disorders [40]. And urine interferon-gamma-inducible protein-10, and macrophage inflammatory protein-1β were associated with poorer social–cognitive abilities in multiple sclerosis patients [41]. Also, urinary toll-like receptor 2-stimulant levels are significantly elevated in multiple sclerosis patients [42]. Although the urinary biomarkers discovered in this experiment do not overlap with those found in previous studies, it demonstrates that urine may be a promising source for biomarker discovery.
The dysregulated proteins in urine are consistent with the previous studies in CSF and plasma. For example, both kininogen (a precursor for kinin) and complement component 9 are mediators of inflammation and play important roles in response to inflammatory injury. Elevated levels of kininogen and complement 9 in the CSF have been reported in rats that have EAE [25]. Additionally, expression of the kinin B1 receptor mRNA on peripheral blood mononuclear cells can serve as an index of disease activity in multiple sclerosis [43]. Therefore, in EAE or multiple sclerosis, the expression of kininogen is upregulated in the plasma, CSF, and urine. Protease family members, including metalloproteases, serine proteases, and cysteine proteases, can be markers of disease activity in multiple sclerosis [26]. Serum fatty acid binding protein (FABP) is thought to distinguish subtypes of multiple sclerosis, because it is expressed at the highest level in secondary progressive multiple sclerosis and increased during early stages of pediatric-onset multiple sclerosis [24]. In the current study, urinary FABP 9 level was also increased in the early stages of disease. Oncomodulin, a factor produced by macrophages, promotes axon growth in neurons and is an indicator of central nervous system injury [29], including multiple sclerosis. In the early stages of EAE, the levels of oncomodulin are reduced, which may partly be due to axonal injury. Matrix metallopeptidase 8 (MMP-8), a metalloprotease, has been shown to increase in the central nervous system in response to EAE and is correlated with symptom severity [26]. Compared with MMP-8 in the central nervous system, MMP-8 in urine is upregulated by as much as 1.5-fold during the early stages of EAE and can further increase with disease progression. Angiotensinogen is involved in maintaining blood pressure and in the pathogenesis of essential hypertension and preeclampsia. Interestingly, the upregulation of serum angiotensin-converting enzymes is related to disease activity in longitudinal analysis [44], while reduced levels of intrathecal angiotensin II in the CSF are indicators of neural damage and repair processes in multiple sclerosis [45]. Consistent with plasma angiotensinogen, urinary angiotensinogen was also increased after immunization in rats that have EAE.
As the significantly altered proteins in this study might be related to multiple sclerosis, this finding may provide useful information for multiple sclerosis research. This study may further illustrate the clinical value of urinary proteome in studying the disease in central nervous system. These differential proteins that have not been confirmed in the literature still need to be further evaluated for their diagnostic value. As far as we know, there have never been any reports of early urinary protein changes associated with experimental autoimmune encephalomyelitis rat models prior to the absence of symptoms. The early urinary protein biomarker panel changes of multiple sclerosis in this study are unique. It was the first time to report that the protein panel in urine is changed in such an early time of experimental autoimmune encephalomyelitis animal models even before the symptom onset. The changes of neuronal damage could be detected in the urine, which is a new insight of the neuronal damage. Urine biomarkers of neuronal damage could be used and more as convenient for clinical application if proved to be reliable in future clinical practice.
3.8. Limitations
Several limitations should be taken into account regarding this study. Firstly, the altered urinary proteins identified in the initial investigation require validation in a larger cohort by a targeted approach with authentic standards. Secondly, it is important to note that this is an animal experiment and that the biomarkers necessitate further validation in clinical settings. It is necessary to conduct tests to detect these potential biomarkers in the urine of patients with multiple sclerosis. Lastly, while the study successfully identified altered proteins in animal models of multiple sclerosis, the precise effects of these proteins/genes remain unclear. Therefore, further investigations involving functional analysis are required.
4. Conclusion
In this study, early candidate urinary biomarkers of multiple sclerosis were identified in a rat model of EAE before histological changes and clinical symptom onset. More than half of the differentially regulated proteins were identified as participants in neurological functions and reported to be related to multiple sclerosis. Additionally, intervention at the early time points when urinary protein has changed can effectively reduce the morbidity of EAE models. In conclusion, this study showed that urine may be a good source of multiple sclerosis early biomarkers such as angiotensinogen and matrix metallopeptidase 8. These findings may provide important information for early diagnosis and intervention of multiple sclerosis in the future.
Ethics
The experiment was approved by the Institute of Basic Medical Sciences Animal Ethics Committee, Peking Union Medical College (Animal Welfare Assurance Number: ACUC-A02-2014-007). The study was performed according to guidelines developed by the Institutional Animal Care and Use Committee of Peking Union Medical College.
Data accessibility
The raw data can be publicly accessed at ProteomeXchange Dataset (PXD043037: https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD043037).
The data are provided in electronic supplementary material [46].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
M.Z.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft; Z.Y.: methodology, writing—review and editing; J.W.: methodology; X.L.: conceptualization, investigation, methodology; Y.G.: conceptualization, funding acquisition, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare that they have no competing interests.
Funding
This work was supported by National Key Research and Development Program of China (grant no. 2018YFC0910202), the Fundamental Research Funds for the Central Universities (grant no. 2020KJZX002), and Beijing Normal University (grant no. 11100704).
Reference
- 1.Compston A, Coles A. 2008. Multiple sclerosis. Lancet 372, 1502-1517. ( 10.1016/S0140-6736(08)61620-7) [DOI] [PubMed] [Google Scholar]
- 2.O'Connell K, Kelly SB, Fogarty E, Duggan M, Buckley L, Hutchinson M, McGuigan C, Tubridy N. 2014. Economic costs associated with an MS relapse. Mult. Scler. Relat. Disord. 3, 678-683. ( 10.1016/j.msard.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 3.Hemond CC, Bakshi R. 2018. Magnetic resonance imaging in multiple sclerosis. Cold Spring Harb. Perspect. Med. 8, a028969. ( 10.1101/cshperspect.a028969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavrova E, Lommers E, Woodruff HC, Chatterjee A, Maquet P, Salmon E, Lambin P, Phillips C. 2021. Exploratory radiomic analysis of conventional vs. quantitative brain MRI: toward automatic diagnosis of early multiple sclerosis. Front. Neurosci. 15, 679941. ( 10.3389/fnins.2021.679941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart FM, Bainbridge J. 2016. Current and emerging treatment of multiple sclerosis. Am. J. Manag. Care. 22, s159-s170. [PubMed] [Google Scholar]
- 6.Gao Y. 2013. Urine—an untapped goldmine for biomarker discovery? Sci. China Life Sci. 56, 1145-1146. ( 10.1007/s11427-013-4574-1) [DOI] [PubMed] [Google Scholar]
- 7.Wu T, et al. 2013. Urinary angiostatin—a novel putative marker of renal pathology chronicity in lupus nephritis. Mol. Cell. Proteomics 12, 1170-1179. ( 10.1074/mcp.M112.021667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho DH, Yi S, Seo H, Son I, Seol W. 2014. Increased DJ-1 in urine exosome of Korean males with Parkinson's disease. BioMed Res. Int. 2014, 704678. ( 10.1155/2014/704678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, et al. 2015. Combining serum and urine biomarkers in the early diagnosis of mild cognitive impairment that evolves into Alzheimer's disease in patients with the apolipoprotein E ɛ4 genotype. Biomarkers 20, 84-88. ( 10.3109/1354750X.2014.994036) [DOI] [PubMed] [Google Scholar]
- 10.Nam D, Lee JY, Lee M, Kim J, Seol W, Son I, Ho DH. 2020. Detection and assessment of alpha-synuclein oligomers in the urine of Parkinson's disease patients. J. Parkinson's Dis. 10, 981-991. ( 10.3233/JPD-201983) [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Cerghet M, Poisson LM, Datta I, Labuzek K, Suhail H, Rattan R, Giri S. 2019. Urinary and plasma metabolomics identify the distinct metabolic profile of disease state in chronic mouse model of multiple sclerosis. J. Neuroimmune Pharmacol. 14, 241-250. ( 10.1007/s11481-018-9815-4) [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Stingl C, Stoop MP, Zeneyedpour L, Neuteboom RF, Smitt PS, Hintzen RQ, Luider TM. 2015. Proteomics urine analysis of pregnant women suffering from multiple sclerosis. J. Proteome Res. 14, 2065-2073. ( 10.1021/pr501162w) [DOI] [PubMed] [Google Scholar]
- 13.Nielsen HH, et al. 2015. The urine proteome profile is different in neuromyelitis optica compared to multiple sclerosis: a clinical proteome study. PLoS ONE 10, e0139659. ( 10.1371/journal.pone.0139659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebregiworgis T, Nielsen HH, Massilamany C, Gangaplara A, Reddy J, Illes Z, Powers R. 2016. A urinary metabolic signature for multiple sclerosis and neuromyelitis optica. J. Proteome Res. 15, 659-666. ( 10.1021/acs.jproteome.5b01111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson AP, Harp CT, Noronha A, Miller SD. 2014. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 122, 173-189. ( 10.1016/B978-0-444-52001-2.00008-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H. 2014. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J. Autoimmun. 54, 33-50. ( 10.1016/j.jaut.2014.06.004) [DOI] [PubMed] [Google Scholar]
- 17.Merkler D, Ernsting T, Kerschensteiner M, Bruck W, Stadelmann C. 2006. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain 129, 1972-1983. ( 10.1093/brain/awl135) [DOI] [PubMed] [Google Scholar]
- 18.Gao Y. 2014. Roadmap to the urine biomarker era. MOJ Proteomics Bioinform. 1, 18. [Google Scholar]
- 19.Mannie MD, Paterson PY, U'Prichard DC, Flouret G. 1985. Induction of experimental allergic encephalomyelitis in Lewis rats with purified synthetic peptides: delineation of antigenic determinants for encephalitogenicity, in vitro activation of cellular transfer, and proliferation of lymphocytes. Proc. Natl Acad. Sci. USA 82, 5515-5519. ( 10.1073/pnas.82.16.5515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn M, Yang W, Kim H, Jin JK, Moon C, Shin T. 2012. Immunohistochemical study of arginase-1 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 1453, 77-86. ( 10.1016/j.brainres.2012.03.023) [DOI] [PubMed] [Google Scholar]
- 21.Schneider C, Schuetz G, Zollner TM. 2009. Acute neuroinflammation in Lewis rats: a model for acute multiple sclerosis relapses. J. Neuroimmunol. 213, 84-90. ( 10.1016/j.jneuroim.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 22.Rassy D, et al. 2020. Intranasal methylprednisolone effectively reduces neuroinflammation in mice with experimental autoimmune encephalitis. J. Neuropathol. Exp. Neurol. 79, 226-237. ( 10.1093/jnen/nlz128) [DOI] [PubMed] [Google Scholar]
- 23.Swanborg RH. 2001. Experimental autoimmune encephalomyelitis in the rat: lessons in T-cell immunology and autoreactivity. Immunol. Rev. 184, 129-135. ( 10.1034/j.1600-065x.2001.1840112.x) [DOI] [PubMed] [Google Scholar]
- 24.Messina S, et al. 2013. Increased leptin and A-FABP levels in relapsing and progressive forms of MS. BMC Neurol. 13, 172. ( 10.1186/1471-2377-13-172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenling T, et al. 2012. Profiling and identification of cerebrospinal fluid proteins in a rat EAE model of multiple sclerosis. J. Proteome Res. 11, 2048-2060. ( 10.1021/pr201244t) [DOI] [PubMed] [Google Scholar]
- 26.Scarisbrick IA. 2008. The multiple sclerosis degradome: enzymatic cascades in development and progression of central nervous system inflammatory disease. Curr. Top. Microbiol. Immunol. 318, 133-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawajiri M, et al. 2009. Angiotensin-converting enzyme (ACE) and ACE2 levels in the cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. 15, 262-265. ( 10.1177/1352458508097923) [DOI] [PubMed] [Google Scholar]
- 28.Stoop MP, et al. 2012. Minocycline effects on the cerebrospinal fluid proteome of experimental autoimmune encephalomyelitis rats. J. Proteome Res. 11, 4315-4325. ( 10.1021/pr300428e) [DOI] [PubMed] [Google Scholar]
- 29.Flanders KC, Ren RF, Lippa CF. 1998. Transforming growth factor-betas in neurodegenerative disease. Prog. Neurobiol. 54, 71-85. ( 10.1016/S0301-0082(97)00066-X) [DOI] [PubMed] [Google Scholar]
- 30.Mahmoudian E, Khalilnezhad A, Gharagozli K, Amani D. 2017. Thioredoxin-1, redox factor-1 and thioredoxin-interacting protein, mRNAs are differentially expressed in multiple sclerosis patients exposed and non-exposed to interferon and immunosuppressive treatments. Gene 634, 29-36. ( 10.1016/j.gene.2017.08.021) [DOI] [PubMed] [Google Scholar]
- 31.Masvekar R, Wu T, Kosa P, Barbour C, Fossati V, Bielekova B. 2019. Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity. Mult. Scler. Relat. Disord. 28, 34-43. ( 10.1016/j.msard.2018.11.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremlett H, et al. 2015. Serum proteomics in multiple sclerosis disease progression. J. Proteomics 118, 2-11. ( 10.1016/j.jprot.2015.02.018) [DOI] [PubMed] [Google Scholar]
- 33.Yeo T, et al. 2021. Objective biomarkers for clinical relapse in multiple sclerosis: a metabolomics approach. Brain Commun. 3, fcab240. ( 10.1093/braincomms/fcab240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, Mazza G, Wraith DC, Watts C. 2002. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat. Immunol. 3, 169-174. ( 10.1038/ni754) [DOI] [PubMed] [Google Scholar]
- 35.Noubade R, et al. 2011. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 118, 3290-3300. ( 10.1182/blood-2011-02-336552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spulber S, Bartfai T, Schultzberg M. 2009. IL-1/IL-1ra balance in the brain revisited: evidence from transgenic mouse models. Brain Behav. Immun. 23, 573-579. ( 10.1016/j.bbi.2009.02.015) [DOI] [PubMed] [Google Scholar]
- 37.Cristiano L. 2022. The pseudogenes of eukaryotic translation elongation factors (EEFs): role in cancer and other human diseases. Genes Dis. 9, 941-958. ( 10.1016/j.gendis.2021.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du C, Xie X. 2012. G protein-coupled receptors as therapeutic targets for multiple sclerosis. Cell Res. 22, 1108-1128. ( 10.1038/cr.2012.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Brien KP, Remm M, Sonnhammer EL. 2005. Inparanoid: a comprehensive database of eukaryotic orthologs. Nucleic Acids Res. 33, D476-D480. ( 10.1093/nar/gki107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayaoglu MY, Girgin G, Solmaz I, Baydar T, Anlar B. 2022. Urine neopterin in childhood acute demyelinating diseases: potential for differential diagnosis. Mult. Scler. Relat. Disord. 59, 103662. ( 10.1016/j.msard.2022.103662) [DOI] [PubMed] [Google Scholar]
- 41.Turner JA, Padgett C, McDonald S, Ahuja KDK, Francis HM, Lim CK, Honan CA. 2021. Innate immunity impacts social-cognitive functioning in people with multiple sclerosis and healthy individuals: implications for IL-1ra and urinary immune markers. Brain Behav. Immun. Health 14, 100254. ( 10.1016/j.bbih.2021.100254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hossain MJ, Morandi E, Tanasescu R, Frakich N, Caldano M, Onion D, Faraj TA, Erridge C, Gran B. 2018. The soluble form of Toll-like receptor 2 is elevated in serum of multiple sclerosis patients: a novel potential disease biomarker. Front. Immunol. 9, 457. ( 10.3389/fimmu.2018.00457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prat A, Biernacki K, Saroli T, Orav JE, Guttmann CR, Weiner HL, Khoury SJ, Antel JP. 2005. Kinin B1 receptor expression on multiple sclerosis mononuclear cells: correlation with magnetic resonance imaging T2-weighted lesion volume and clinical disability. Arch. Neurol. 62, 795-800. ( 10.1001/archneur.62.5.795) [DOI] [PubMed] [Google Scholar]
- 44.Constantinescu CS, Goodman DB, Grossman RI, Mannon LJ, Cohen JA. 1997. Serum angiotensin-converting enzyme in multiple sclerosis. Arch. Neurol. 54, 1012-1015. ( 10.1001/archneur.1997.00550200068012) [DOI] [PubMed] [Google Scholar]
- 45.Kawajiri M, Mogi M, Osoegawa M, Matsuoka T, Tsukuda K, Kohara K, Horiuchi M, Miki T, Kira JI. 2008. Reduction of angiotensin II in the cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. 14, 557-560. ( 10.1177/1352458507085760) [DOI] [PubMed] [Google Scholar]
- 46.Zhao M, Zhang Y, Wu J, Li X, Gao Y. 2023. Early urinary candidate biomarkers and clinical outcomes of intervention in a rat model of experimental autoimmune encephalomyelitis. Figshare. ( 10.6084/m9.figshare.c.6781091) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhao M, Zhang Y, Wu J, Li X, Gao Y. 2023. Early urinary candidate biomarkers and clinical outcomes of intervention in a rat model of experimental autoimmune encephalomyelitis. Figshare. ( 10.6084/m9.figshare.c.6781091) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The raw data can be publicly accessed at ProteomeXchange Dataset (PXD043037: https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD043037).
The data are provided in electronic supplementary material [46].