Abstract
In recent years, because of the growing desire to improve the noninvasiveness and safety of tumor treatments, sonodynamic therapy has gradually become a popular research topic. However, due to the complexity of the therapeutic process, the relevant mechanisms have not yet been fully elucidated. One of the widely accepted possibilities involves the effect of reactive oxygen species. In this review, the mechanism of reactive oxygen species production by sonodynamic therapy (SDT) and ways to enhance the sonodynamic production of reactive oxygen species are reviewed. Then, the clinical application and limitations of SDT are discussed. In conclusion, current research on sonodynamic therapy should focus on the development of sonosensitizers that efficiently produce active oxygen, exhibit biological safety, and promote the clinical transformation of sonodynamic therapy.
Keywords: Sonodynamic therapy, Reactive oxygen species, Hypoxic, Tumor Microenvironment, Sonosensitizer
Core Tip: This review mainly describes the mechanism of reactive oxygen species generation by sonodynamic therapy and enhances the efficiency of reactive oxygen species generation by improving hypoxia to increase the efficacy of sonodynamic therapy on tumor, and finally summarizes the clinical applications and prospects of sonodynamic therapy.
INTRODUCTION
According to the latest statistics from Cancer Statistics, 2023, it is estimated that there will be 1958310 new cancer cases and 609820 cancer deaths in the United States in 2023[1]. Likewise, the cancer situation in China remains critical, with 4.064 million new cases and approximately 2.41 million deaths, according to data released by the National Cancer Centre in 2023[2]. Thus, cancer has become one of the major global threats to human health. Surgery, radiotherapy and chemotherapy are still the main treatment modalities for most malignancies. For example, the standard treatment for ovarian cancer, a common malignancy in women, is extensive tumor reduction surgery in combination with platinum or paclitaxel-based drugs, with or without angiogenesis inhibitors such as bevacizumab[3,4]. Despite the clinical benefits of combining multiple modalities for cancer, the mortality rate of cancer patients unfortunately continues to rise each year: late detection because early symptoms of malignant tumors are atypical, tumor recurrence and metastasis, resistance to therapeutic agents, and the systemic toxicity of treatment are important causes of failure of cancer treatment[5-8]. Therefore, exploring novel cancer therapeutics with higher efficacy, lower toxicity and fewer adverse reactions has become an urgent challenge.
Noninvasive therapies such as high-intensity focused ultrasound (HIFU)[9], photodynamic therapy (PDT)[10], sonodynamic therapy (SDT)[11], and photothermal therapy (PTT)[12] have been widely used in clinical practice and have achieved good therapeutic effects. PDT is a treatment based on reactive oxygen species (ROS) that utilizes a photosensitizer (PS) combined with a specific light source to exert cytotoxic activity on tumor cells[13]. The PS, light and oxygen are the three key factors in PDT, and the combination of the three factors can generate ROS. The antitumor effect of PDT comes from three interrelated mechanisms—the direct cytotoxic effect on tumor cells; the destruction of tumor blood vessels, resulting in the deprivation of nutrients needed for tumors to survive[14]; and the release of cytokines and exosomes by tumor cells, which stimulate the recruitment of immune cells into tumor tissues and promote the antitumor immune response, reducing the mobility and invasion ability of tumor cells[10,15,16]. However, due to adverse factors such as the phototoxicity of PSs, the lack of specific accumulation in malignant tissues, the lack of endogenous oxygen in tumors, and limited light penetration depth (depth < 0.5 cm), PDT has unsatisfactory therapeutic effects on deep tumors, impeding its practical application[17]. Ultrasound has great preclinical and clinical potential due to its noninvasive nature, low energy attenuation and deep tissue penetration[18]. Yumita et al[19] overcame the disadvantages of, such as shallow tissue penetration (depth < 0.5 cm) and phototoxicity, by first proposing SDT to treat solid tumors. SDT is a noninvasive therapeutic modality that synergizes low-intensity and low-frequency ultrasound (0.5-3 W/cm2, 1.0-2.0 MHz) with a sonosensitizer. Its main principle is to irradiate tumor sites with ultrasound under aerobic conditions to achieve the directional activation of sensitizers and a series of sonochemical reactions to kill tumor cells and achieve a therapeutic effect[20]. As an advanced treatment method of low-intensity ultrasound combined with an acoustic sensitizer, SDT has the advantages of high tissue penetration (> 10 cm), high long-range space-time selectivity, and noninvasiveness. It can treat deep lesions that are difficult to access by photodynamic therapy (PDT) and therefore has broad clinical application prospects[18,21].
The therapeutic effect of SDT depends on ROS-mediated oxidative stress. However, the production of ROS is low, and the overexpression of the antioxidant glutathione in tumor tissues leads to high ROS consumption, which significantly reduces the therapeutic effect of SDT[22,23]. Therefore, improving the production capacity of ROS and reducing their consumption are the main strategies to improve the therapeutic effect of SDT[24].
ROS AND THEIR PRODUCTION MECHANISM IN SDT
ROS are a class of oxygen-containing, chemically active substances formed by the incomplete reduction of O2. There are two types of ROS: Free radical ROS and non-free radical ROS. Common ROS include hydrogen peroxide (H2O2), hypochlorous acid, singlet oxygen (1O2), superoxide anion (O2•-), and the hydroxyl group (•OH)[25].
ACOUSTIC CAVITATION
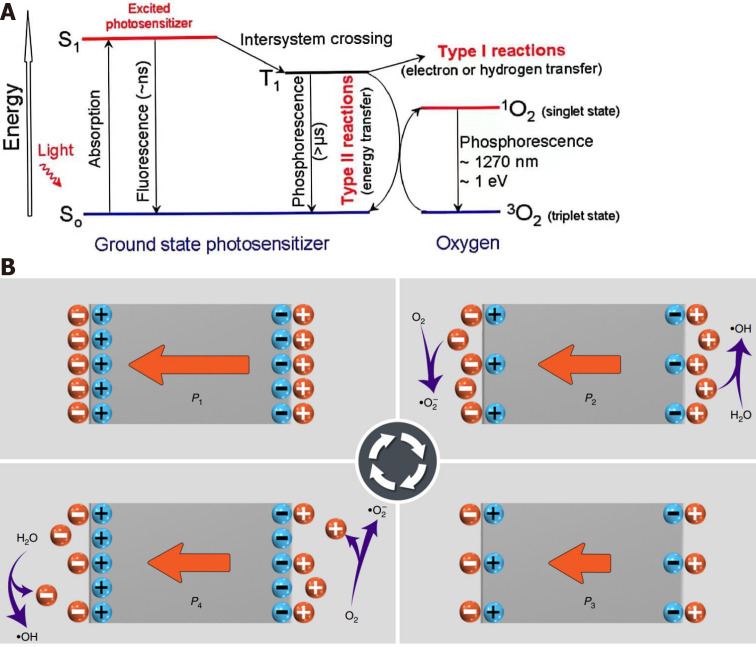
In the 1890s, the British Army found that the propeller of a warship could produce a large number of bubbles during operation, resulting in serious damage to the propeller, which was the first historical description of the cavitation effect[26]. Since then, scientists have performed extensive research on the cavitation effect. It is a special physical phenomenon of ultrasound propagation in liquid[27], referring to a series of dynamic processes including the nucleation, growth and oscillation of microbubbles (cavitation nuclei) in liquid under the action of ultrasound[20]. It can be divided into inertial cavitation and noninertial cavitation. Noninertial cavitation means that under low sound pressure, microbubbles contract under ultrasonic positive pressure and expand under negative pressure, and the bubble diameter remains relatively constant without rupture; inertial cavitation occurs when the sound pressure exceeds the threshold, and microbubbles cannot maintain structural stability in the process of contraction and expansion: therefore, they collapse and implode in the compression stage and produce local high temperature (4000-25000 K) and high pressure (81 MPa) instantaneously, accompanied by high-speed shockwaves, microjets and sonochemical effects[20]. SDT produces ROS through sonoluminescence and pyrolysis[28]. The mechanism of ROS production by sonoluminescence is similar to that of PTD, which stimulates the production of active oxygen by sonosensitizers through type I and type II reactions (Figure 1A)[29]. In the type I reaction, under the action of ultrasound, the sonosensitizer is excited from the ground state S0 to the excited state S1 and then changes to the excited triplet state (T1) through internal conversion. The T1 sonosensitizer reacts with the intracellular matrix to produce ROS (H2O2, 1O2,•OH)[30]. The energy generated by the cavitation effect in the type II reaction is directly transferred to excited 3O2 to produce 1O2[30,31]. The mechanism by which pyrolysis produces ROS includes the implosion of transient cavitation microbubbles to generate high temperature instantaneously: this high temperature directly decomposes H2O to produce •H and •OH, which can react with the sonosensitizer to produce ROS with a longer half-life[32]. In addition, the sonosensitizer can directly decompose at high temperature to generate free radicals, which further react with endogenous substances to generate other active forms of oxygen (Figure 1B)[32].
Figure 1.
Schematic diagram of the different mechanisms of reactive oxygen species generation. A: Schematic diagram of the principle of sonodynamic therapy. Citation: Greenwald BD. Photodynamic therapy for esophageal cancer. Update. Chest Surg Clin N Am 2000; 10(3): 625-637. Copyright © 2011 American Cancer Society, Inc. Published by American Cancer Society, Inc; B: ROS generation by active piezoelectric catalysis. Piezoelectric materials in the electrostatic balance state release charge when under compressive stress and react with H2O to produce •OH, and O2 obtains negative charge to generate •O2−. Then Piezoelectric materials adsorb the charges from the surrounding electrolyte under reduced compressive stress, H2O loses negative electrons to generate ·OH, and O2 releases positive charge to generate •O2−. Citation: Wang Y, Wen X, Jia Y, Huang M, Wang F, Zhang X, Bai Y, Yuan G, Wang Y. Piezo-catalysis for nondestructive tooth whitening. Nat Commun 2020; 11(1): 1328. Copyright © The Author(s) 2020. Published by Springer Nature Limited.
PIEZOCATALYSIS
Piezoelectric catalysis is an emerging active ROS generation method, especially in SDT, that can directionally trigger the generation of in situ ROS in particular[33]. When not mechanically stimulated, piezoelectric materials are in electrostatic equilibrium[34]. When ultrasound acts on piezoelectric materials, they are subjected to rapid periodic mechanical stimulation[33], and the polarization amplitude oscillates rapidly with the piezoelectric force field, resulting in the continuous separation of electrons and holes in piezoelectric materials and establishing a built-in electric field in piezocatalysts. This in turn catalyzes the generation of toxic •OH and •O2− (Figure 1B)[34-36].
METHODS TO IMPROVE THE GENERATION OF ROS
Improving the hypoxic tumor microenvironment
The tumor microenvironment (TME) refers to the cellular environment in which tumors or tumor stem cells exist[37], including cancer-associated fibroblasts, vascular endothelial cells, and extracellular matrix[38], characterized by low pH, low oxygen (PO2 ≤ 2.5 mmHg), high H2O2 (50-100 × 10-6 mmol/L), glucose deprivation, and so on[39-41]. Hypoxia is one of the common and important characteristics of malignant tumors and is mainly caused by the imbalance between oxygen supply and consumption in tumor tissues[38]. The relevant mechanisms include oxygen perfusion limitation, oxygen diffusion limitation and anemic hypoxia[42]. Based on the dynamic changes in the TME, hypoxia is divided into acute hypoxia and chronic hypoxia. Acute hypoxia is related to perfusion and is caused by the instability of red blood cell flux in the tumor microvascular network, while chronic hypoxia is long-term or irreversible and is related to oxygen diffusion limitation[38].
Hypoxia inducible factor is activated by hypoxia and plays an important role in tumor progression, metastasis and immune escape, thus making tumors more resistant to many current treatments[43]. An increasing volume of data indicates that tumor hypoxia is the main reason for the failure of many current therapies. As the substrate for SDT to produce ROS, tumor hypoxia will inevitably affect the production efficiency of ROS and reduce the efficacy of SDT. Therefore, adding in situ oxygen-generating materials or oxygen carriers to sonosensitizers to increase the oxygen content in the TME and increase the efficacy of SDT has become a new tumor treatment approach[44-46].
IN SITU O2 GENERATION
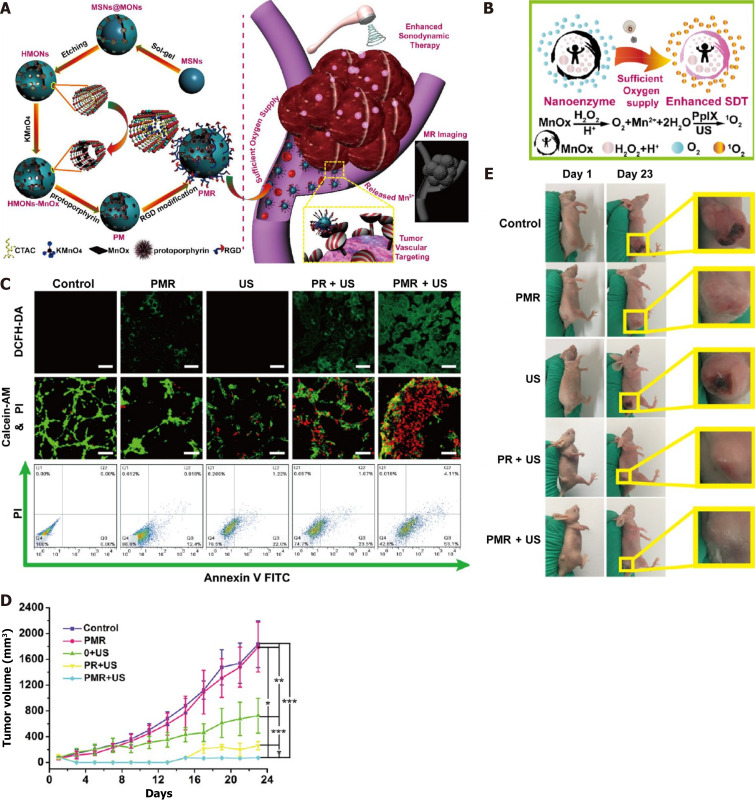
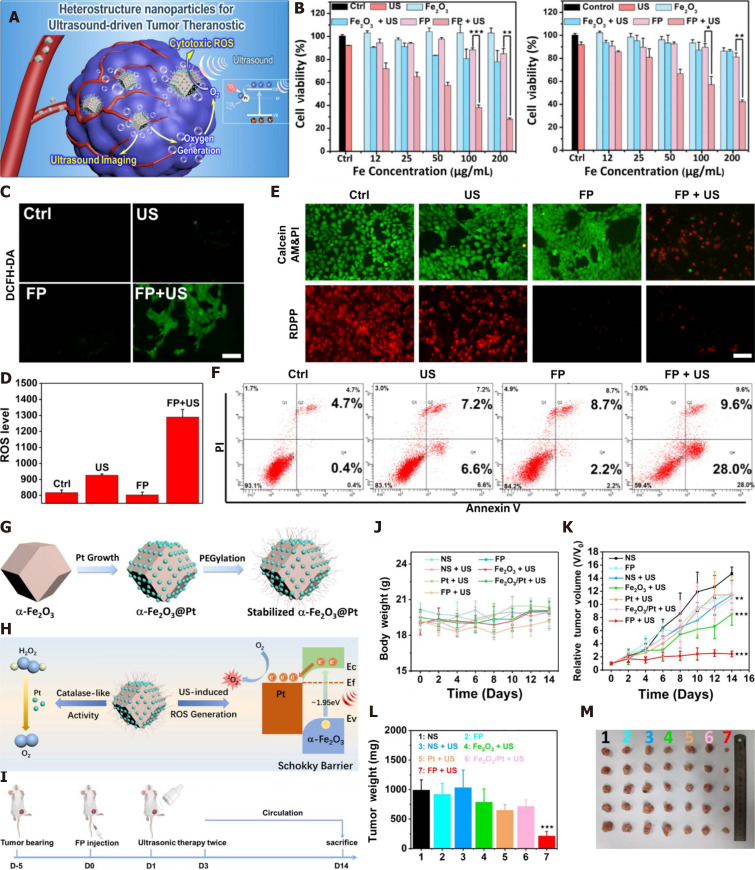
Catalase (CAT) can catalyze endogenous H2O2 in tumors to generate O2, which is an effective method to overcome tumor hypoxia. One researcher designed a thermotriggered in situ hydrogel system (TCCP-CAT CS/GP) based on the catalytic properties of CAT, which couples meso-tetra (4-carboxyphenyl) porphine (TCCP) with CAT and then mixes it with chitosan (CS) and disodium β-glycerophosphate (GP) to form a solution. At mouse body temperature (37 °C), this acoustic sensitizer was injected into the tumor site and was able to undergo sol-gel conversion, leaving the acoustic sensitizer at the tumor site. CAT catalyzed the original hydrogen peroxide to produce O2, continuously relieving tumor hypoxia, promoting TCCP to produce large amounts of ROS, and exerting a tumor suppression effect[47]. Since the first report of Fe3O4 magnetic nanoparticles with intrinsic peroxidase-like activity in 2007[48], several O2-releasing nanosystems (e.g., manganese dioxide nanoparticles[49,50], gold nanoclusters, and Fe3+-doped structural units) have been shown to be transported to tumor sites to catalyze the conversion of endogenous H2O2 to O2 and alleviate tumor hypoxia. For instance, manganese dioxide (MnO2) nanoparticles have been reported to possess catalase properties, high reactivity with H2O2, and the ability to continuously produce O2 and were shown to effectively alleviate tumor hypoxia. Piao Zhu et al. introduced MnOx into hollow mesoporous organosilica nanoparticles (HMONs) in situ through a simple redox reaction, anchored PpIX with HMONs, MnOx, and cyclic arginine-glycine-aspartic pentapeptide (RGD, as a targeting peptide) to modify the surface of nanoparticles, and finally constructed a multifunctional nanosonosensitizer (PpIX@HMON-MnOx-RGD) (Figure 2A). Both in vivo and in vitro experiments showed that MnOx can act as a nanoenzyme, catalyze the decomposition of excessive H2O2 in tumors, produce oxygen (Figure 2B), alleviate tumor hypoxia, provide a sufficient oxygen source for SDT, promote the production of reactive oxygen species (Figure 2C), and improve the efficacy of SDT (Figure 2D and E)[51]. In addition, platinum (Pt) nanocrystals can be used as nanoenzymes to decompose H2O2 and produce a large amount of O2. Based on the characteristics of Pt nanocrystals, Tian Zhang et al. constructed a Pt nanocrystal-encapsulated sonosensitizer (α-Fe2O3@Pt), and the pairing of α-Fe2O3 and Pt formed an effective electron capture trap to prevent the recombination of electrons and holes and promote the generation of 1O2 (Figure 3). At the same time, Pt, as a nanoenzyme, can decompose H2O2 to generate a large amount of O2, which is a more effective means of O2 generation for SDT and the effective inhibition of tumor growth[52]. Nevertheless, the methods mentioned above inevitably consume H2O2, resulting in an insufficient supply of H2O2 in the SDT process. Based on this shortcoming, Jiang et al[53] developed an H2O2 economizer, namely, membrane-coated Fe-PDAP/Ce6 (MFC) coated with cancer cell membrane. Prior to ultrasound irradiation, the cancer cell membrane coated with the acoustic sensitizer could prevent the early release of catalase-like nanoenzyme Fe-PDAP and reduce the unnecessary consumption of H2O2 in the TME. After ultrasonic irradiation, MFC could be selectively dismantled to release Fe-PDAP, which could catalyze H2O2 to generate O2 and more effectively produce ROS.
Figure 2.
Schematic representation of construction of PMR nanosonosensitizers and catalytic oxygen generation-enhanced sonodynamic therapy against cancer. A: Detailed steps for preparation of PMR nanosonosensitizers; B: Scheme of MnOx was used as the catalase-like nanoenzyme for the generation of O2 and further generation of 1O2 under ultrasonic irradiation; C: Confocal laser scanning microscope observation of the production of ROS after various treatment and flow cytometry analysis of cancer cells apoptosis after various treatments; D: Tumor-volume changes after varied treatments; E: Corresponding photographic images of tumor at the end of different treatments. Citation: Zhu P, Chen Y, Shi J. Nanoenzyme-Augmented Cancer Sonodynamic Therapy by Catalytic Tumor Oxygenation. ACS Nano 2018; 12(4): 3780-3795. Copyright © 2018, American Chemical Society. Published by ACS Publication. SDT: Sonodynamic therapy; MnOx: Manganese oxide; H2O2: Hydrogen peroxide; US: Ultrasound; 1O2: Singlet oxygen; O2: Oxygen; PpIX: Protoporphyrin; PMR: PpIX@HMONs-MnOx-RGD; PR: Protoporphyrin.
Figure 3.
Schematic representation of construction ofα-Fe2O3@Pt nanosonosensitizers and catalytic oxygen generation-enhanced SDT against cancer. A, G: Schematic diagram of action mechanism of α-Fe2O3@Pt nanoparticles and synthetic method ofα- Fe2O3@Pt; B: The relative cell viability of Fe2O3 and α- Fe2O3@Pt with or without ultrasound under normoxic and hypoxic conditions; C and D: Qualitative and quantitative analysis of ROS by flow cytometer produced by α- Fe2O3@Pt; E: Fluorescence image stained with calcein AM (green, live cells) and PI (Propidium iodide, red, dead cells); F: The flow cytometer apoptosis assay staining with PI and Annexin-FTIC; H: Mechanism diagram of O2 and ROS produced by α- Fe2O3@Pt; I: Flow chart of in vivo study experiment; J-M: The variations of d body weight,relative tumor volume, tumor weight and tumor images of mice from different groups after sacrificing the mice on the 14th day. Citation: Zhang T, Zheng Q, Fu Y, Xie C, Fan G, Wang Y, Wu Y, Cai X, Han G, Li X. α-Fe2O3@Pt heterostructure particles to enable sonodynamic therapy with self-supplied O2 and imaging-guidance. J Nanobiotechnology 2021; 19(1): 358. Copyright © The Author(s) 2021. Published by BioMed Central Ltd. US: Ultrasound; Fe2O3: Ferric oxide; Pt: Platinum; FP:α-Fe2O3@Pt; 1O2: Singlet oxygen; O2: Oxygen; H2O2: Hydrogen peroxide; ROS: Reactive oxygen species.
EXOGENOUS OXYGEN TRANSPORT
Due to the limited H2O2 available in the TME and the inability to continuously provide O2[54], oxygen carriers (hemoglobin[55], microbubbles[56], fluorocarbon[57]) and sonosensitizers can better alleviate tumor hypoxia.
Hemoglobin (Hb), as a natural oxygen carrier, can bind four oxygen atoms per Hb unit. At the same time, the generation of ultrasonic-induced active ROS provides a rich source of oxygen. In one study, a pH-sensitive zeolitic imidazolate framework (ZIF-8) was used as the carrier to encapsulate Hb and synthesize Hb@ZIF-8 (HZ) to achieve the release of pH-responsive Hb/O2 at the tumor site[58]. In vitro experiments showed that adjusting the pH 5.5 could release a large amount of O2, alleviate tumor hypoxia, generate a large amount of ROS under ultrasonic response, activate the mitochondrial apoptosis pathway, and effectively inhibit the growth of tumor cells. In vivo experiments revealed that nanoparticles irradiated by ultrasound can not only inhibit the growth of subcutaneous tumors but also control the growth of deep tumors, revealing that SDT can be applied at different depths.
Furthermore, perfluorocarbons (perfluorobutane, perfluoropentane and the like) are widely used for the delivery of chemotherapeutic drugs, genes, oxygen, or contrast agents due to their high oxygen solubility and biocompatibility[59,60]. Studies have shown that perfluorocarbon carries oxygen more efficiently than Hb, with 100 mL of perfluorocarbon carrying approximately 40 to 50 mL of O2 at 25℃, whereas the same volume of Hb carries only 20 mL of O2[61,62]. Chen et al[57] successfully developed the fluorocarbon-chain-media oxygen-self-produced nanoplatform (IR780@O2-FHMON). Both cellular and in vivo experiments have shown that the nanoplatform can better accumulate in tumors, accelerate the release of O2, permanently reverse hypoxia, and generate more ROS to achieve the high-efficiency treatment of PANC-1 pancreatic cancer by SDT. Yang et al[63] also reported a hierarchical nanoformulation (PFCE@THPPpf-COPs) that can effectively alleviate hypoxia ub prostate cancer, generate a large amount of ROS, improve the curative effect of SDT, and achieve tumor eradication through the high-loading oxygen carrier perfluoropolyether.
REDUCING THE ROS SCAVENGING CAPACITY
Glutathione (GSH), an important nonprotein mercaptan that contains thiols and amide linkages, is a major intracellular antioxidant that plays a key role in many physiological and pathological processes[64,65]. In addition to hypoxia, the overexpression of glutathione (1-10 × 10-3 mmol/L) is also an important feature of the TME[66]. High levels of glutathione can protect cancer cells from ROS-induced oxidative damage. Therefore, as SDT is an active oxygen-based therapy, excessive glutathione in the TME can negatively affect the efficacy[64]. At present, the intracellular glutathione level is mainly reduced through two pathways, namely, the upstream and downstream pathways of glutathione[67]. The glutathione upstream pathway refers to the inhibition of glutathione synthesis by cancer cells through the use of glutathione biosynthesis inhibitors, such as L-butynyl sulfoxide amine (BSO) and γ-glutamyl cysteine synthetase[68]. For example, a study successfully synthesized BSO-TCPP-Fe@CaCO3-PEG nanoparticles, which amplified the oxidative stress of tumors through Ca2+ overload-induced ROS generation, BSO-mediated GSH synthesis inhibition and meso-tetra-(4-carboxyphenyl) porphine (TCPP)-mediated sonodynamic effects, leading to significant cancer cell death and overall effective inhibition of tumor growth to enhance the therapeutic effect of SDT[68]. The downstream pathway refers to the conversion of GSH to glutathione disulfide through a redox reaction between glutathione and some reducing agents. For example, Huang et al[64] designed GSH-depleting nanoplatelets consisting of cinnamic aldehyde (CA) and IR780-supported mesoporous silica nanoparticles (MSNs) coated with a platelet membrane called PV-coated MSN-CA/IR780 (PSCI). CA, serving as an oxidative stress amplification agent, consumes excessive glutathione in the TME, weakens the ability of tumor cells to produce active oxygen by eliminating SDT through glutathione, increases the content of active oxygen, and further enhances the therapeutic effect of SDT to effectively inhibit tumor growth.
CLINICAL APPLICATION AND LIMITATIONS OF SDT
Although good progress has been made in preclinical studies on SDT (Table 1), no large-scale clinical studies have been executed, and only a few cases have been reported. All the existing studies have combined SDT with other treatments (chemotherapy, hormone therapy, and immunotherapy)[69,70]. For example, Wang et al[71] reported the clinical results of SDT combined with photodynamic therapy (SPDT) after treating 3 patients with advanced refractory breast cancer. After sublingual absorption of the acoustic sensitizer SF1 for 2 to 3 d, the tumor area or the whole body was irradiated with a red LED lamp (wavelength: 630 nm, power: 20 Mw/cm2) for 30 min, and then the tumor area was irradiated with a portable ultrasound device (frequency: 1 MHz, power: 2.0 W/cm2) for 20 min for a continuous period of 3 d. After treatment, the tumors in all three patients were significantly reduced, and there was no significant effect of SPDT on vital organs throughout the body. Subsequently, Inui et al[72] used SDT in combination with immunotherapy to treat a patient with advanced breast cancer (invasive ductal carcinoma, grade 3, ER+, PR+, HER2+, right axillary, spinal, and pleural metastases). After 19 sessions of sonodynamic therapy with SDT (5-ALA (10 mg/kg)-modified Ce 6 (25 mg) in combination with exemestane (25 mg/d), the tumor in the right axilla and pleura completely disappeared, and tumor markers rapidly declined without serious side effects.
Table 1.
Application of sonodynamic therapy in different tumors
|
Cancer type
|
Sonosensitizer
|
Therapeutic parameters
|
Result
|
Ref.
|
| Glioma | Ce6 | 0.6 W/cm2, 60 s | SDT inhibits xenograft tumor growth by inducing apoptosis and inhibiting mitochondrial autophagy | [80] |
| Breast cancer | Mn-MOF | 1.0 MHz, 0.9 W/cm2, 30% duty cycle | Mn-MOF catalyzes the in situ production of O2 to alleviate tumor hypoxia and reduce GSH and GPX4, which contributes to ROS formation and iron death, thereby killing cancer cells | [81] |
| Melanoma | Ce6 | 2.0 MHz, 2.0 W/cm2, 20% duty cycle | The combination of SDT and aPD-L1 immunotherapy effectively inhibits tumor infiltration and promotes activation of cytotoxic T cells, resulting in strong anticancer immunity and long-term immune memory, effectively inhibiting melanoma growth | [82] |
| Pancreatic cancer | Hematoporphyrin | 1.0 MHz, 3.0 W/cm2, 50% duty cycle | SDT exerts antitumor effects by suppressing the expression of immunosuppressive T-cell phenotypes | [83] |
| Cervical cancer | IR780 | 2.5W/cm2, 20 s | IR780 selectively positions the nanoparticles into the mitochondria of cancer cells, and generates the acoustic droplet vaporization effect after perfluorohexane phase transition to achieve the synergistic treatment of tumors | [84] |
| Ovarian cancer | ICG | 1.0 W/cm2, 1 min | SDT in combination with PDT and oxaliplatin can increase antitumor effects, enhance immunological potency and improve dual-mode imaging | [85] |
| Prostate cancer | hematoporphyrin | 1.0 MHz, 3.5 W/cm2, 30% duty cycle, 3.5 mim | pH- and histone B-responsive nanoparticles combined with SDT have a significant induced cytotoxic effect on prostate cancer cells and can effectively treat cancer | [86] |
| Gastric cancer | Pyropheophorbide-lipid | 1.0 MHz, 1.0 W/cm2, 50% duty cycle, 3 min | Construction of an ultrasound microbubble using pyrophosphorylated lipids in combination with trastuzumab for the synergistic treatment of HER2-positive gastric cancer with sonodynamic therapy and antibody therapy | [87] |
| Lung cancer | DVDMS | 0.5MHz, 0.5 W/cm2, 10% duty cycle, 5 min | DVDMS in combination with SDT exerts antitumor effects via the mitochondria-mediated apoptosis signaling pathway and the extrinsic apoptosis pathway | [88] |
Ce6: Chlorin e6; Mn-MOF: Manganese metal-organic framework; GSH: Glutathione; GPX4: Glutathione peroxidase 4; ROS: Reactive oxygen species; SDT: Sonodynamic therapy; PDT: Photodynamic therapy; aPD-L1: anti-Programmed cell death 1 ligand 1 antibody; ICG: Indocyanine Green; HER2: Human epidermal growth factor receptor 2; DVDMS: Dinoporphyrin sodium.
In the abovementioned examples, SDT combined with other treatments showed good therapeutic effects, but it is difficult to quantify the role of SDT in treatment success[70]. Most acoustic sensitizers have low biosafety and ROS-producing ability, resulting in insufficient efficacy to replace traditional antitumor therapy. Therefore, SDT has not become widespread in clinical practice. The following problems exist regarding SDT: (1) The therapeutic mechanism of SDT has not been fully elucidated[73]; (2) there are relatively few ultrasonic treatment devices suitable for clinical application[74]; (3) for different types of tumors, more detailed studies are needed on the key parameters of ultrasonic frequency, intensity and irradiation time[11,75]; (4) further research is needed on sound-sensitive agents with good photoacoustic dynamic effects and biocompatibility[76]; and (5) the biosafety of various kinds of sonosensitizers needs to be systematically studied in vivo and in vitro. In particular, inorganic sonosensitizers have poor biodegradability and are not easily metabolized[77]. Currently, the sonosensitizers approved by the Food and Drug Administration of the United States are mainly organic acoustic sonosensitizers[11], such as indocyanine green[44], sodium warfarin (DVDMS)[78], chlorin e6[60] and 5- aminolevulinic acid[79].
CONCLUSION
SDT, which relies on the strong penetration of ultrasound and the tumor-specific accumulation of sonosensitizers, has been proven to be an effective, low-cost and safe antitumor treatment technique with good clinical application prospects[80]. SDT mainly relies on the research and development of sonosensitizers and the alleviation of the tumor hypoxic microenvironment to promote the efficient production of reactive oxygen species by sonosensitizers. Therefore, the development of sonosensitizers with strong ROS generation ability and good biodegradability will help SDT to obtain better clinical application prospects. In short, SDT has been proven to have good therapeutic effects on tumors, but most of these effects are based on preclinical research. In the future, more research efforts are needed to promote the clinical transformation of SDT[21,32].
ACKNOWLEDGEMENTS
Many thanks to Professor Jiang Zhu for her careful guidance and many valuable comments on the thesis, and to Minyan Wang and Xiaofeng Fu for his contribution in collecting the relevant materials.
Footnotes
Conflict-of-interest statement: All the authors have no conflict-of-interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 23, 2023
First decision: April 19, 2023
Article in press: May 22, 2023
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Gaman MA, Romania S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
Contributor Information
He-Qin Dong, School of Medicine, Shaoxing University, Shaoxin 312000, Zhejiang Province, China.
Xiao-Feng Fu, Department of Ultrasound, Women's Hospital, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Min-Yan Wang, Department of Ultrasound, Women's Hospital, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Jiang Zhu, Department of Ultrasound, Women's Hospital, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China. zhujiang1046@zju.edu.cn.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J. [Cancer statistics in China, 2016] Zhonghua Zhong Liu Za Zhi. 2023;45:212–220. doi: 10.3760/cma.j.cn112152-20220922-00647. [DOI] [PubMed] [Google Scholar]
- 3.Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. doi: 10.1136/bmj.m3773. [DOI] [PubMed] [Google Scholar]
- 4.Marchetti C, Muzii L, Romito A, Benedetti Panici P. First-line treatment of women with advanced ovarian cancer: focus on bevacizumab. Onco Targets Ther. 2019;12:1095–1103. doi: 10.2147/OTT.S155425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik D, Mahendiratta S, Kaur H, Medhi B. Futuristic approach to cancer treatment. Gene. 2021;805:145906. doi: 10.1016/j.gene.2021.145906. [DOI] [PubMed] [Google Scholar]
- 6.Sato H, Yoshida R, Yasui K, Umeda Y, Yoshida K, Fuji T, Kumano K, Takagi K, Yagi T, Fujiwara T. Feasibility of local therapy for recurrent pancreatic cancer. Pancreatology. 2022;22:774–781. doi: 10.1016/j.pan.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, Jin WL. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front Immunol. 2020;11:577869. doi: 10.3389/fimmu.2020.577869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papież MA, Krzyściak W. Biological Therapies in the Treatment of Cancer-Update and New Directions. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachu VS, Kedda J, Suk I, Green JJ, Tyler B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann Biomed Eng. 2021;49:1975–1991. doi: 10.1007/s10439-021-02833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansoori B, Mohammadi A, Amin Doustvandi M, Mohammadnejad F, Kamari F, Gjerstorff MF, Baradaran B, Hamblin MR. Photodynamic therapy for cancer: Role of natural products. Photodiagnosis Photodyn Ther. 26:395–404.. doi: 10.1016/j.pdpdt.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan X, Wang H, Wang S, Sun X, Wang L, Wang W, Shen H, Liu H. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61:415–426. doi: 10.1007/s11427-017-9262-x. [DOI] [PubMed] [Google Scholar]
- 12.Ren Y, Yan Y, Qi H. Photothermal conversion and transfer in photothermal therapy: From macroscale to nanoscale. Adv Colloid Interface Sci. 2022;308:102753. doi: 10.1016/j.cis.2022.102753. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 14.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn Ther. 2005;2:1–23. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun B, Bte Rahmat J N and Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials . 2022;291:.121875. doi: 10.1016/j.biomaterials.2022.121875. [DOI] [PubMed] [Google Scholar]
- 16.Mkhobongo B, Chandran R, Abrahamse H. The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22189726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan K, Zhang Y, Mu C, Xu Q, Jing X, Wang D, Dang D, Meng L, Ma J. Versatile Nanoplatforms with enhanced Photodynamic Therapy: Designs and Applications. Theranostics. 2020;10:7287–7318. doi: 10.7150/thno.46288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Z, Dai Z. Design and Challenges of Sonodynamic Therapy System for Cancer Theranostics: From Equipment to Sensitizers. Adv Sci (Weinh) 2021;8:2002178. doi: 10.1002/advs.202002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res. 1989;80:219–222. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang S, Deng X, Ma P, Cheng Z, Lin J. Recent Advances in Nanomaterial-Assisted Combinational Sonodynamic Cancer Therapy. Adv Mater. 2020;32:e2003214. doi: 10.1002/adma.202003214. [DOI] [PubMed] [Google Scholar]
- 21.Yan P, Liu LH, Wang P. Sonodynamic Therapy (SDT) for Cancer Treatment: Advanced Sensitizers by Ultrasound Activation to Injury Tumor. ACS Appl Bio Mater. 2020;3:3456–3475. doi: 10.1021/acsabm.0c00156. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Shi L, Liao Y, Cao X, Liu X, Yu Y, Wang Z, Lu X, Wang J. Ultrathin-FeOOH-Coated MnO(2) Sonosensitizers with Boosted Reactive Oxygen Species Yield and Remodeled Tumor Microenvironment for Efficient Cancer Therapy. Adv Sci (Weinh) 2022;9:e2200005. doi: 10.1002/advs.202200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Um W, E K PK, Lee J, Kim CH, You DG, Park JH. Recent advances in nanomaterial-based augmented sonodynamic therapy of cancer. Chem Commun (Camb) 2021;57:2854–2866. doi: 10.1039/d0cc07750j. [DOI] [PubMed] [Google Scholar]
- 24.Dong Y, Dong S, Liu B, Yu C, Liu J, Yang D, Yang P, Lin J. 2D Piezoelectric Bi2 MoO6 Nanoribbons for GSH-Enhanced Sonodynamic Therapy. Adv Mater. 2021;33:e2106838. doi: 10.1002/adma.202106838. [DOI] [PubMed] [Google Scholar]
- 25.Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J Control Release. 2006;114:89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114:10869–10939. doi: 10.1021/cr400532z. [DOI] [PubMed] [Google Scholar]
- 28.Wu Q, Zhang F, Pan X, Huang Z, Zeng Z, Wang H, Jiao J, Xiong X, Bai L, Zhou D, Liu H. Surface Wettability of Nanoparticle Modulated Sonothrombolysis. Adv Mater. 2021;33:e2007073. doi: 10.1002/adma.202007073. [DOI] [PubMed] [Google Scholar]
- 29.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K, Kulbacka J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 31.Debele TA, Peng S, Tsai HC. Drug Carrier for Photodynamic Cancer Therapy. Int J Mol Sci. 2015;16:22094–22136. doi: 10.3390/ijms160922094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhang X, Yang H, Yu L, Xu Y, Sharma A, Yin P, Li X, Kim JS, Sun Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem Soc Rev. 2021;50:11227–11248. doi: 10.1039/d1cs00403d. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Xu Y, Dong S, Wang P, Chen W, Lu Z, Ye D, Pan B, Wu D, Vecitis CD, Gao G. Ultrasonic activation of inert poly(tetrafluoroethylene) enables piezocatalytic generation of reactive oxygen species. Nat Commun. 2021;12:3508. doi: 10.1038/s41467-021-23921-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Wen X, Jia Y, Huang M, Wang F, Zhang X, Bai Y, Yuan G, Wang Y. Piezo-catalysis for nondestructive tooth whitening. Nat Commun. 2020;11:1328. doi: 10.1038/s41467-020-15015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu P, Chen Y, Shi J. Piezocatalytic Tumor Therapy by Ultrasound-Triggered and BaTiO(3) -Mediated Piezoelectricity. Adv Mater. 2020;32:e2001976. doi: 10.1002/adma.202001976. [DOI] [PubMed] [Google Scholar]
- 36.Tang Q, Sun S, Wang P, Sun L, Wang Y, Zhang L, Xu M, Chen J, Wu R, Zhang J, Gong M, Chen Q, Liang X. Genetically Engineering Cell Membrane-Coated BTO Nanoparticles for MMP2-Activated Piezocatalysis-Immunotherapy. Adv Mater. e2300964:.. doi: 10.1002/adma.202300964. [DOI] [PubMed] [Google Scholar]
- 37.Arneth B. Tumor Microenvironment. Medicina (Kaunas) 2019;56 doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Zhao L, Li XF. Hypoxia and the Tumor Microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304. doi: 10.1177/15330338211036304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang G, Ji J, Liu Z. Multifunctional MnO(2) nanoparticles for tumor microenvironment modulation and cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1720. doi: 10.1002/wnan.1720. [DOI] [PubMed] [Google Scholar]
- 40.Dong S, Dong Y, Jia T, Liu S, Liu J, Yang D, He F, Gai S, Yang P, Lin J. GSH-Depleted Nanozymes with Hyperthermia-Enhanced Dual Enzyme-Mimic Activities for Tumor Nanocatalytic Therapy. Adv Mater. 2020;32:e2002439. doi: 10.1002/adma.202002439. [DOI] [PubMed] [Google Scholar]
- 41.Wang P, Tang Q, Zhang L, Xu M, Sun L, Sun S, Zhang J, Wang S, Liang X. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. ACS Nano. 2021;15:11326–11340. doi: 10.1021/acsnano.1c00616. [DOI] [PubMed] [Google Scholar]
- 42.Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomedicine. 2018;13:6049–6058. doi: 10.2147/IJN.S140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, Wu Q, Kuca K. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2021;41:1622–1643. doi: 10.1002/med.21771. [DOI] [PubMed] [Google Scholar]
- 44.Wu T, Liu Y, Cao Y, Liu Z. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv Mater. 2022;34:e2110364. doi: 10.1002/adma.202110364. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Wu M, Li H, Jiang J, Zhou S, Chen W, Xie C, Zhen X, Jiang X. Enhancing Penetration Ability of Semiconducting Polymer Nanoparticles for Sonodynamic Therapy of Large Solid Tumor. Adv Sci (Weinh) 2022;9:e2104125. doi: 10.1002/advs.202104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao X, Xue L, Huang H, Dai X, Chen Y, Ding H. Engineering defected 2D Pd/H-TiO(2) nanosonosensitizers for hypoxia alleviation and enhanced sono-chemodynamic cancer nanotherapy. J Nanobiotechnology. 2022;20:186. doi: 10.1186/s12951-022-01398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.She J, Zhou X, Zhang Y, Zhang R, Li Q, Zhu W, Meng Z, Liu Z. Thermo-Triggered In Situ Chitosan-Based Gelation System for Repeated and Enhanced Sonodynamic Therapy Post a Single Injection. Adv Healthc Mater. 2021;10:e2001208. doi: 10.1002/adhm.202001208. [DOI] [PubMed] [Google Scholar]
- 48.Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Wang H, Jia X, Du S, Yin Y, Zhang X. Cascade catalytic nanoplatform for enhanced starvation and sonodynamic therapy. J Drug Target. 2020;28:195–203. doi: 10.1080/1061186X.2019.1641507. [DOI] [PubMed] [Google Scholar]
- 50.Chen T, Zeng W, Tie C, Yu M, Hao H, Deng Y, Li Q, Zheng H, Wu M, Mei L. Engineered gold/black phosphorus nanoplatforms with remodeling tumor microenvironment for sonoactivated catalytic tumor theranostics. Bioact Mater. 2022;10:515–525. doi: 10.1016/j.bioactmat.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu P, Chen Y, Shi J. Nanoenzyme-Augmented Cancer Sonodynamic Therapy by Catalytic Tumor Oxygenation. ACS Nano. 2018;12:3780–3795. doi: 10.1021/acsnano.8b00999. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T, Zheng Q, Fu Y, Xie C, Fan G, Wang Y, Wu Y, Cai X, Han G, Li X. α-Fe(2)O(3)@Pt heterostructure particles to enable sonodynamic therapy with self-supplied O(2) and imaging-guidance. J Nanobiotechnology. 2021;19:358. doi: 10.1186/s12951-021-01105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Q, Qiao B, Lin X, Cao J, Zhang N, Guo H, Liu W, Zhu L, Xie X, Wan L, Tang R, Liang B, Wang D, Wang Z, Zhou Y, Ran H, Li P. A hydrogen peroxide economizer for on-demand oxygen production-assisted robust sonodynamic immunotherapy. Theranostics. 2022;12:59–75. doi: 10.7150/thno.64862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu H, Yan X, Wang H, Tanaka J, Wang M, You W, Li Z. Perfluorocarbon-based O(2) nanocarrier for efficient photodynamic therapy. J Mater Chem B. 2019;7:1116–1123. doi: 10.1039/c8tb01844h. [DOI] [PubMed] [Google Scholar]
- 55.Yin T, Yin J, Ran H, Ren Y, Lu C, Liu L, Shi Q, Qiu Y, Pan H, Ma A. Hypoxia-alleviated sonodynamic therapy based on a hybrid protein oxygen carrier to enhance tumor inhibition. Biomater Sci. 2021;10:294–305. doi: 10.1039/d1bm01710a. [DOI] [PubMed] [Google Scholar]
- 56.McEwan C, Kamila S, Owen J, Nesbitt H, Callan B, Borden M, Nomikou N, Hamoudi RA, Taylor MA, Stride E, McHale AP, Callan JF. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials. 2016;80:20–32. doi: 10.1016/j.biomaterials.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Luo H, Liu Y, Zhang W, Li H, Luo T, Zhang K, Zhao Y, Liu J. Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano. 2017;11:12849–12862. doi: 10.1021/acsnano.7b08225. [DOI] [PubMed] [Google Scholar]
- 58.Yuan M, Liang S, Zhou Y, Xiao X, Liu B, Yang C, Ma P, Cheng Z, Lin J. A Robust Oxygen-Carrying Hemoglobin-Based Natural Sonosensitizer for Sonodynamic Cancer Therapy. Nano Lett. 2021;21:6042–6050. doi: 10.1021/acs.nanolett.1c01220. [DOI] [PubMed] [Google Scholar]
- 59.Zeng Q, Qiao L, Cheng L, Li C, Cao Z, Chen Z, Wang Y, Liu J. Perfluorohexane-Loaded Polymeric Nanovesicles with Oxygen Supply for Enhanced Sonodynamic Therapy. ACS Biomater Sci Eng. 2020;6:2956–2969. doi: 10.1021/acsbiomaterials.0c00407. [DOI] [PubMed] [Google Scholar]
- 60.Hong L, Pliss AM, Zhan Y, Zheng W, Xia J, Liu L, Qu J, Prasad PN. Perfluoropolyether Nanoemulsion Encapsulating Chlorin e6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor. Nanomaterials (Basel) 2020;10 doi: 10.3390/nano10102058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew Chem Int Ed Engl. 2018;57:11522–11531. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 63.Yang Z, Tao D, Zhong W, Liu Z, Feng L, Chen M. Perfluorocarbon loaded fluorinated covalent organic polymers with effective sonosensitization and tumor hypoxia relief enable synergistic sonodynamic-immunotherapy. Biomaterials. 2022;280:121250. doi: 10.1016/j.biomaterials.2021.121250. [DOI] [PubMed] [Google Scholar]
- 64.Huang C, Ding S, Jiang W, Wang FB. Glutathione-depleting nanoplatelets for enhanced sonodynamic cancer therapy. Nanoscale. 2021;13:4512–4518. doi: 10.1039/d0nr08440a. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Xue Y, Zhao W, Ye D. Orange-red emitting copper nanoclusters for endogenous GSH, temperature sensing, and cellular imaging. Analyst. 2020;145:7063–7070. doi: 10.1039/d0an01535k. [DOI] [PubMed] [Google Scholar]
- 66.Lin LS, Song J, Song L, Ke K, Liu Y, Zhou Z, Shen Z, Li J, Yang Z, Tang W, Niu G, Yang HH, Chen X. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO(2) -Based Nanoagent to Enhance Chemodynamic Therapy. Angew Chem Int Ed Engl. 2018;57:4902–4906. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 67.Geng P, Yu N, Zhang J, Jin Z, Wen M, Jiang Q, Kang L, Peng C, Li M, Zhang H, Zhu M, Chen Z. One Responsive Stone, Three Birds: Mn(III)-Hemoporfin Frameworks with Glutathione-Enhanced Degradation, MRI, and Sonodynamic Therapy. Adv Healthc Mater. 2021;10:e2001463. doi: 10.1002/adhm.202001463. [DOI] [PubMed] [Google Scholar]
- 68.Dong Z, Feng L, Hao Y, Li Q, Chen M, Yang Z, Zhao H, Liu Z. Synthesis of CaCO 3 -Based Nanomedicine for Enhanced Sonodynamic Therapy via Amplification of Tumor Oxidative Stress. Chem . 2020;6:1391–1407. [Google Scholar]
- 69.Lafond M, Yoshizawa S, Umemura SI. Sonodynamic Therapy: Advances and Challenges in Clinical Translation. J Ultrasound Med. 2019;38:567–580. doi: 10.1002/jum.14733. [DOI] [PubMed] [Google Scholar]
- 70.Costley D, Mc Ewan C, Fowley C, McHale AP, Atchison J, Nomikou N, Callan JF. Treating cancer with sonodynamic therapy: a review. Int J Hyperthermia. 2015;31:107–117. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Zhang W, Xu Z, Luo Y, Mitchell D, Moss RW. Sonodynamic and photodynamic therapy in advanced breast carcinoma: a report of 3 cases. Integr Cancer Ther. 2009;8:283–287. doi: 10.1177/1534735409343693. [DOI] [PubMed] [Google Scholar]
- 72.Inui T, Makita K, Miura H, Matsuda A, Kuchiike D, Kubo K, Mette M, Uto Y, Nishikata T, Hori H, Sakamoto N. Case report: A breast cancer patient treated with GcMAF, sonodynamic therapy and hormone therapy. Anticancer Res. 2014;34:4589–4593. [PubMed] [Google Scholar]
- 73.Lin X, Song J, Chen X, Yang H. Ultrasound-Activated Sensitizers and Applications. Angew Chem Int Ed Engl. 2020;59:14212–14233. doi: 10.1002/anie.201906823. [DOI] [PubMed] [Google Scholar]
- 74.Araújo Martins Y, Zeferino Pavan T, Fonseca Vianna Lopez R. Sonodynamic therapy: Ultrasound parameters and in vitro experimental configurations. Int J Pharm. 2021;610:121243. doi: 10.1016/j.ijpharm.2021.121243. [DOI] [PubMed] [Google Scholar]
- 75.Zhao P, Deng Y, Xiang G, Liu Y. Nanoparticle-Assisted Sonosensitizers and Their Biomedical Applications. Int J Nanomedicine. 2021;16:4615–4630. doi: 10.2147/IJN.S307885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Truong Hoang Q, Kim M, Kim BC, Lee CY, Shim MS. Pro-oxidant drug-loaded porphyrinic zirconium metal-organic-frameworks for cancer-specific sonodynamic therapy. Colloids Surf B Biointerfaces. 2022;209:112189. doi: 10.1016/j.colsurfb.2021.112189. [DOI] [PubMed] [Google Scholar]
- 77.Son S, Kim JH, Wang X, Zhang C, Yoon SA, Shin J, Sharma A, Lee MH, Cheng L, Wu J, Kim JS. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49:3244–3261. doi: 10.1039/c9cs00648f. [DOI] [PubMed] [Google Scholar]
- 78.Mai B, Wang X, Liu Q, Zhang K, Wang P. The Application of DVDMS as a Sensitizing Agent for Sono-/Photo-Therapy. Front Pharmacol. 2020;11:19. doi: 10.3389/fphar.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raspagliesi L, D'Ammando A, Gionso M, Sheybani ND, Lopes MB, Moore D, Allen S, Gatesman J, Porto E, Timbie K, Franzini A, Di Meco F, Sheehan J, Xu Z, Prada F. Intracranial Sonodynamic Therapy With 5-Aminolevulinic Acid and Sodium Fluorescein: Safety Study in a Porcine Model. Front Oncol. 2021;11:679989. doi: 10.3389/fonc.2021.679989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qu F, Wang P, Zhang K, Shi Y, Li Y, Li C, Lu J, Liu Q, Wang X. Manipulation of Mitophagy by "All-in-One" nanosensitizer augments sonodynamic glioma therapy. Autophagy. 2020;16:1413–1435. doi: 10.1080/15548627.2019.1687210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Q, Zhan G, Zhang Z, Yong T, Yang X, Gan L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics. 2021;11:1937–1952. doi: 10.7150/thno.45511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang J, Xiao Z, An Y, Han S, Wu W, Wang Y, Guo Y, Shuai X. Nanodrug with dual-sensitivity to tumor microenvironment for immuno-sonodynamic anti-cancer therapy. Biomaterials. 2021;269:120636. doi: 10.1016/j.biomaterials.2020.120636. [DOI] [PubMed] [Google Scholar]
- 83.Hadi MM, Farrell S, Nesbitt H, Thomas K, Kubajewska I, Ng A, Masood H, Patel S, Sciscione F, Davidson B, Callan JF, MacRobert AJ, McHale AP, Nomikou N. Nanotechnology-augmented sonodynamic therapy and associated immune-mediated effects for the treatment of pancreatic ductal adenocarcinoma. J Cancer Res Clin Oncol. 2022 doi: 10.1007/s00432-022-04418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J, Hou J, Liu S, Xu J, Luo Y, Zheng J, Li X, Wang Z, Ran H, Guo D. Theranostic Nanoplatform with Sequential SDT and ADV Effects in Response to Well-Programmed LIFU Irradiation for Cervical Cancer. Int J Nanomedicine. 2021;16:7995–8012. doi: 10.2147/IJN.S339257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie W, Zhu S, Yang B, Chen C, Chen S, Liu Y, Nie X, Hao L, Wang Z, Sun J, Chang S. The Destruction Of Laser-Induced Phase-Transition Nanoparticles Triggered By Low-Intensity Ultrasound: An Innovative Modality To Enhance The Immunological Treatment Of Ovarian Cancer Cells. Int J Nanomedicine. 2019;14:9377–9393. doi: 10.2147/IJN.S208404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadi MM, Nesbitt H, Masood H, Sciscione F, Patel S, Ramesh BS, Emberton M, Callan JF, MacRobert A, McHale AP, Nomikou N. Investigating the performance of a novel pH and cathepsin B sensitive, stimulus-responsive nanoparticle for optimised sonodynamic therapy in prostate cancer. J Control Release. 2021;329:76–86. doi: 10.1016/j.jconrel.2020.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun L, Zhang J, Xu M, Zhang L, Tang Q, Chen J, Gong M, Sun S, Ge H, Wang S, Liang X, Cui L. Ultrasound Microbubbles Mediated Sonosensitizer and Antibody Co-delivery for Highly Efficient Synergistic Therapy on HER2-Positive Gastric Cancer. ACS Appl Mater Interfaces. 2022;14:452–463. doi: 10.1021/acsami.1c21924. [DOI] [PubMed] [Google Scholar]
- 88.Shen J, Cao S, Sun X, Pan B, Cao J, Che D, Jin S, Cao Y, Tian Y, Yu Y. Sinoporphyrin Sodium-Mediated Sonodynamic Therapy Inhibits RIP3 Expression and Induces Apoptosis in the H446 Small Cell Lung Cancer Cell Line. Cell Physiol Biochem. 2018;51:2938–2954. doi: 10.1159/000496045. [DOI] [PubMed] [Google Scholar]