This post hoc analysis of a randomized clinical trial investigates the effect of vitamin D supplementation on risk of relapse or death in a subgroup of patients with digestive tract cancer who were p53 immunoreactive.
Key Points
Question
Can vitamin D supplementation reduce the risk of relapse or death in a subgroup of patients with digestive tract cancer who were p53 immunoreactive, defined by positivity for anti-p53 antibodies in serum and p53 protein in more than 99% of cancer cells?
Findings
In this post hoc analysis of a randomized clinical trial including 392 patients with digestive tract cancer, 5-year relapse-free survival was significantly higher in the vitamin D group (80.9%) than the placebo group (30.6%) among patients in the p53-immunoreactive subgroup but not in the non–p53-immunoreactive subgroup.
Meaning
These findings suggest that vitamin D supplementation may reduce the risk of relapse or death in this subgroup of patients.
Abstract
Importance
Recent meta-analyses of randomized clinical trials found that daily vitamin D3 supplementation had beneficial effects on cancer mortality, although the results are still controversial.
Objective
To examine whether vitamin D supplementation reduces the risk of relapse or death in a supgroup of patients with digestive tract cancer who were p53 immunoreactive.
Design, Setting, and Participants
This was a post hoc subgroup analysis of the AMATERASU randomized, double-blind, placebo-controlled clinical trial. This trial included patients at a single university hospital in Japan with digestive tract cancers between January 2010 and February 2018 followed up for a median (IQR) of 3.5 (2.5-5.3) years to compare the effects of vitamin D supplementation with placebo and was reported in 2019. Patients from among 417 participants in the AMATERASU trial whose residual serum samples were available were included. Data were analyzed from October 20 to November 24, 2022.
Interventions
Vitamin D3 (2000 IU/d) supplementation or placebo.
Main Outcomes and Measures
The primary outcome was 5-year relapse or death. The subgroup of patients who were p53 immunoreactive was defined by positivity for anti-p53 antibodies in serum and nuclear accumulation of p53 oncosuppressor protein in more than 99% of cancer cells, which is considered a biomarker for p53 missense mutations. Anti-p53 antibody levels were measured using chemiluminescent enzyme immune assay. Immunohistochemical staining data of p53 protein in cancer tissue in pathologic specimens were obtained from a previous study and divided into 4 grades.
Results
Among 392 patients with digestive tract cancer (mean [SD] age, 66 [10.7] years; 260 males [66.3%]), there were 37 patients with esophageal cancer (9.4%), 170 patients with gastric cancer (43.4%), 2 patients with small bowel cancer (0.5%), and 183 patients with colorectal cancer (46.7%). Serum anti-p53 antibody was detectable in 142 patients (36.2%), and p53-immunohistochemistry grade showed a positive association with serum anti-p53 antibody levels (coefficient = 0.19; P < .001). In the p53-immunoreactive subgroup (80 patients), relapse or death occurred in 9 of 54 patients (16.7%) in the vitamin D group and 14 of 26 patients (53.8%) in the placebo group; 5-year relapse-free survival (RFS) was significantly higher in the vitamin D group (13 patients [80.9%]) than the placebo group (1 patient [30.6%]; hazard ratio [HR], 0.27; 95% CI, 0.11-0.61; P = .002). This was significantly different from 272 patients in the non–p53 immunoreactive subgroup, in which vitamin D had no effect on 5-year RFS (vitamin D: 35 of 158 patients [22.2%] vs placebo: 24 of 114 patients [21.1%]; HR, 1.09; 95% CI, 0.65-1.84) (P for interaction = .005).
Conclusions and Relevance
This study found that vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients with digestive tract cancer who were p53 immunoreactive.
Trial Registration
Identifier: UMIN000001977
Introduction
Worldwide, an estimated 10.0 million cancer deaths occurred in 2020,1 and this number is projected to reach 16.3 million deaths by 2040.2 Faced with this alarming trend, researchers have intensified their efforts to identify interventions that can effectively reduce all kinds of cancer mortality. Notably, in the Vitamin D and Omega-3 Trial (VITAL) study,3 2000 IU of vitamin D3 administered daily reduced all cancer mortality by 25% when the first 2 years of observation were excluded. Moreover, a 2023 meta-analysis4 of randomized clinical trials (RCTs) found that daily vitamin D3 supplementation was associated with improved cancer mortality in the general population and survival in patients with cancer, although the results are still controversial.5 Beneficial effects of vitamin D have been reported for cancers at various sites, and p53 oncosuppressor is the most frequently mutated gene in approximately half of cancers and relatively common across cancers at all primary sites. These findings suggest that p53 and its related molecules may be plausible targets of vitamin D’s anticancer effects.6,7 Indeed, in our previous post hoc study of the AMATERASU RCT, which enrolled patients with digestive tract cancer,8 vitamin D supplementation was found to improve relapse-free survival (RFS) in a patient subgroup with nuclear accumulation of p53 oncosuppressor protein, as seen by immunohistochemistry (IHC) staining of pathological specimens.9 Strong positivity in the IHC staining was reported as a convenient biomarker for detecting p53 missense mutations with high sensitivity and specificity.10,11 Additionally, mutant p53 proteins that accumulated in cancer cells were shown to be immunogenic, leading to generation of anti-p53 antibody (p53-Ab) in vitro12,13,14 and have previously been detected in the serum of patients with a variety of cancers.15,16,17,18,19 It is assumed that the immune system responds to the abnormal p53 protein as a cancer-specific antigen.
Vitamin D was previously demonstrated to upregulate innate and adaptive immunity.20,21 A meta-analysis22 of RCTs showed that vitamin D supplementation reduced the incidence of acute respiratory tract infection compared with placebo. In addition, vitamin D supplementation reduced the risk of relapse in the subgroup of patients who had sufficient infiltration of immune cells into the tumor microenvironment in our previous research.23 We therefore hypothesized that vitamin D supplements may reduce the risk of relapse or death by enhancing anticancer immunoreaction, indicated by the presence of antibodies against mutated p53 proteins as cancer-specific antigens that are distinct from normal p53. In this post hoc analysis of the AMATERASU RCT,8 we explored whether serum levels of p53-Ab were higher in patients with abnormally expressed p53 protein in cancer tissue than those without abnormal expression. We also aimed to examine whether vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients who were p53 immunoreactive, defined by positivity for p53 protein in cancer cells and p53-Ab in serum.
Methods
The trial protocol (Supplement 1) for this post hoc analysis of an RCT was approved by the ethics committee of the International University of Health and Welfare Hospital, as well as the Jikei University School of Medicine (Nishi-shimbashi, Tokyo, Japan). Written informed consent was obtained from each participating patient. The ethics committee and consent statements apply to the original and current study. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Trial Design
This was a post hoc analysis of parent trial participants from the AMATERASU RCT8 focusing on a subgroup of patients who were positive for p53-Ab in serum and p53 protein in pathological specimens. Briefly, the parent trial enrolled 417 patients with stage I to III cancer of the digestive tract from the esophagus to the rectum who underwent curative surgery and participated in a randomized, double-blind, placebo-controlled clinical trial to compare the effects of postoperative daily oral vitamin D3 supplementation (2000 IU/d) vs placebo on relapse or death. Participants were allocated at a ratio of 3:2, and the study was conducted at the International University of Health and Welfare Hospital (Otawara, Tochigi, Japan) between January 2010 and February 2018. Participants were asked to continue supplements until the end of the trial.
Participants
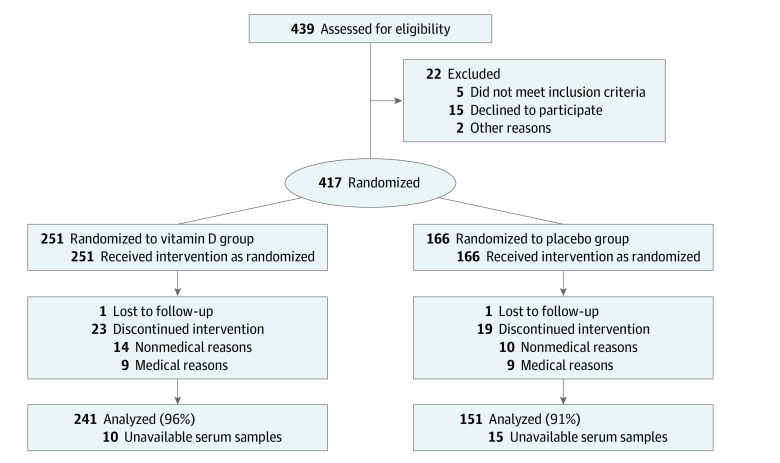
Of 417 patients with digestive tract cancers who were randomly assigned to receive vitamin D supplements (251 patients [60.2%]) or placebo (166 patients [39.8%]) and who were followed up for a median (IQR; maximum) period of 3.5 (2.5-5.3; 7.6) years, 392 participants (94.0%) had available serum samples and were eligible for this post hoc analysis. Serum samples were not obtained for 25 patients (6.0%). Details of inclusion and exclusion criteria are described in the original report.8
Outcome
The outcome was prespecified as relapse or all-cause death. Elapsed time was defined as the time from starting the supplement to the date the outcome occurred or the patient was censored.
Detection of p53 Protein in Pathological Specimens
Immunohistochemical staining data of p53 in cancer tissue in pathology specimens was obtained from our previous study.9 Briefly, patients were divided into 4 grades based on p53 protein expression patterns in pathological specimens by IHC: (1) overexpressed p53 (p53-IHC [3+]): more than 99% of cellular nuclei in the cancerous region overexpressed p53 protein; (2) strongly expressed p53 (p53-IHC [2+]): some (>10% and ≤99%) cellular nuclei in the cancerous region showed strong p53 positivity; (3) faintly expressed p53 (p53-IHC [1+]): some cellular nuclei in the cancerous region showed faint p53 positivity, and a few cells (≥1% and ≤10%) showed strong p53 nuclear accumulation; (4) p53 not expressed (p53-IHC [0]): strong or faint p53 cells were rarely found in the cancerous region (<1%). To conduct a sensitivity analysis, the study population was also divided into 2 subgroups: with more than 10% (p53-IHC [+]) and 10% or less (p53-IHC [−]) of cellular nuclei in the cancer showing any p53 positivity. The cutoff point of 10% was used in this study because this was found to be the most commonly chosen cutoff point in a meta-analysis of 36 studies on p53 IHC for p53 gene mutations.10,11 Details are described in the original report.9
Serum Anti-p53 Antibody Level Measurement
Patient serum p53-Ab levels at approximately 2 weeks after operation and just before starting trial vitamin D supplementation (ie, at randomization), were measured by SRL, Inc (Shinjuku, Tokyo, Japan) using chemiluminescent enzyme immune assay (CLEIA). The lowest measurable serum p53-Ab level is 0.4 U/mL. The study population was divided into 2 subgroups based on serum p53-Ab levels, with patients having levels of 0.4 U/mL or higher designated as p53-Ab (+) and those having levels less than 0.4 U/mL designated as p53-Ab (−).
Statistical Analysis
Patients who underwent randomization and for whom residual serum samples were available for measurement of p53-Ab levels were included in this analysis. Relapse and death-related outcomes were assessed according to randomization group (ie, whether supplements were taken) as an intention-to-treat analysis. Effects of vitamin D and placebo on the risk of relapse or death were estimated using Nelson-Aalen cumulative hazard curves. A Cox proportional hazards model was used to determine hazard ratios (HRs) and 95% CIs. The p53-immunoreactive subgroup included patients who were p53-Ab (+) in serum and p53-IHC [3+] in cancer cells. To clarify whether vitamin D supplementation affected outcomes in the p53-immunoreactive subgroup but not in the non–p53-immunoreactive subgroup, P values for the interaction were analyzed based on a Cox regression model. The model included treatment allocation, the p53-immunoreactive subgroup, and both factors multiplied together as an interaction variable and analyzed the p53-immunoreactive group by 2-way interaction tests compared with the non-p53-immunoreactive subgroup. Because the P value was assessed 5 times, a 2-sided P < .01 was considered statistically significant with application of the Bonferroni correction (ie, .05/5 = .01, considering the potential for a type I error due to multiple comparisons). Data were analyzed using Stata statistical software version 17.0 from October 20 to November 24, 2022.
Results
Study Population
The study population consisted of 392 patients with levels of p53-Ab measured (260 males [66.3%]; mean [SD; range] age, 66 [10.7; 35-90] years), including 241 patients in the vitamin D group and 151 patients in the placebo group (Figure 1). The distribution of cancer types and stages were as follows: 37 patients with esophageal cancer (9.4%), 170 patients with gastric cancer (43.4%), 2 patients with small bowel cancer (0.5%), 183 patients with colorectal cancer (46.7%), and 173 patients with stage I (44.1%), 104 patients with stage II (26.5%), and 115 patients with stage III (29.4%) cancer. There were 40 patients (10.2%) with missing histopathology specimens.
Figure 1. Study Flowchart.
Patient Characteristics
There was detectable p53-Ab in the serum of 142 patients (36.2%), constituting the p53-Ab (+) group, with levels ranging from 0.4 to 1890.0 U/mL, but not in the remaining 250 patients (63.8%), constituting the p53-Ab (−) group. Characteristics of participants in these groups are shown in the Table. Patients in the p53-Ab (+) group more frequently had squamous cell cancer (21 patients [14.8%] vs 14 patients [5.6%]) and less frequently had adenocarcinoma (120 patients [84.5%] vs 232 patients [92.8%]) compared with those in the p53-Ab (−) group. Other characteristics, including intervention group, serum 25-hydroxy vitamin D level, sex, age, and body mass index (calculated as weight in kilograms divided by height in meters squared), were not different between groups.
Table. Patient Characteristics.
| Characteristic | Patients, No. (%) (N = 392) | |
|---|---|---|
| p53-Ab (+) (n = 142)a | p53-Ab (−) (n = 250)b | |
| Intervention | ||
| Vitamin D | 86 (60.6) | 155 (62.0) |
| Placebo | 56 (39.4) | 95 (38.0) |
| 25(OH)D serum level, ng/mL | ||
| Low: <20 | 56 (39.4) | 105 (42.0) |
| Middle: ≥20 and ≤40 | 82 (57.7) | 141 (56.4) |
| High: >40 | 3 (2.1) | 2 (0.8) |
| Median (IQR) | 21 (16-26) | 21 (16-28) |
| Sex | ||
| Male | 96 (67.6) | 164 (65.6) |
| Female | 46 (32.4) | 86 (34.4) |
| Age quartile, y | ||
| Q1, 35-59 | 40 (28.2) | 58 (23.2) |
| Q2, 60-65 | 27 (19.0) | 61 (24.4) |
| Q3, 66-73 | 34 (23.9) | 68 (27.2) |
| Q4, 74-90 | 41 (28.9) | 63 (25.2) |
| BMI quartile | ||
| Q1, 15.0-19.7 | 37 (26.1) | 56 (22.4) |
| Q2, 19.8-21.8 | 36 (25.4) | 63 (25.2) |
| Q3, 21.9-23.7 | 36 (25.4) | 64 (25.6) |
| Q4, 23.8-37.3 | 32 (22.5) | 65 (26.0) |
| History of other cancer | 5 (3.5) | 11 (4.4) |
| Comorbidity | ||
| Hypertension | 46 (32.4) | 105 (42.0) |
| Diabetes | 25 (17.6) | 38 (15.2) |
| Endocrine disease | 6 (4.2) | 42 (16.8) |
| Cardiovascular disease | 9 (6.3) | 20 (8.0) |
| Chronic kidney disease | 5 (3.5) | 0 |
| Asthma | 1 (0.7) | 2 (0.8) |
| Orthopedic disease | 0 | 2 (0.8) |
| Site of cancer | ||
| Esophageal | 21 (14.8) | 16 (6.4) |
| Stomach | 52 (36.6) | 118 (47.2) |
| Small bowel | 0 | 2 (0.8) |
| Colorectal | 69 (48.6) | 114 (45.6) |
| Stage | ||
| I | 51 (35.9) | 122 (48.8) |
| II | 44 (31.0) | 60 (24.0) |
| III | 47 (33.1) | 68 (27.2) |
| Pathology | ||
| Adenocarcinoma | 120 (84.5) | 232 (92.8) |
| Squamous cell carcinoma | 21 (14.8) | 14 (5.6) |
| Other | 1 (0.7) | 4 (1.6) |
| Adjuvant chemotherapy | 59 (41.5) | 78 (31.2) |
Abbreviations: 25(OH)D, 25-hydroxy vitamin D; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); p53-Ab, anti-p53 antibody.
≥0.4 U/mL.
<0.4 U/mL.
Characteristics of patients who were p53-Ab (+) stratified by intervention are shown in the eTable in Supplement 2. The vitamin D group included more older patients and patients with cardiovascular diseases than the placebo group. Other characteristics did not differ between groups.
Subgroup Analysis of Patients With and Without p53-Ab in Serum
In the p53-Ab (+) group, relapse or death occurred in 17 of 86 patients (19.8%) in the vitamin D group and 19 of 56 patients (33.9%) in the placebo group; 5-year RFS was 26 patients (77.2%) in the vitamin D group and 10 patients (60.0%) in the placebo group, indicating no significant difference (HR, 0.57; 95% CI, 0.30-1.10) (Figure 2A). The difference was significant when adjusted for age and a history of cardiovascular diseases (HR, 0.47; 95% CI, 0.23-0.95). In the p53-Ab (−) group, relapse or death occurred in 33 of 155 patients (21.3%) in the vitamin D group and 21 of 95 patients (22.1%) in the placebo group; the 5-year RFS was 37 patients (75.9%) in the vitamin D group and 20 patients (72.5%) in the placebo group, which were not significantly different (HR, 0.98; 95% CI, 0.56-1.69) (Figure 2B), with no 2-way interaction between vitamin D supplementation and the subgroup of patients who were p53-Ab (+).
Figure 2. Subgroup Analysis of Patients With and Without Serum Anti-p53 Antibody (p53-Ab).
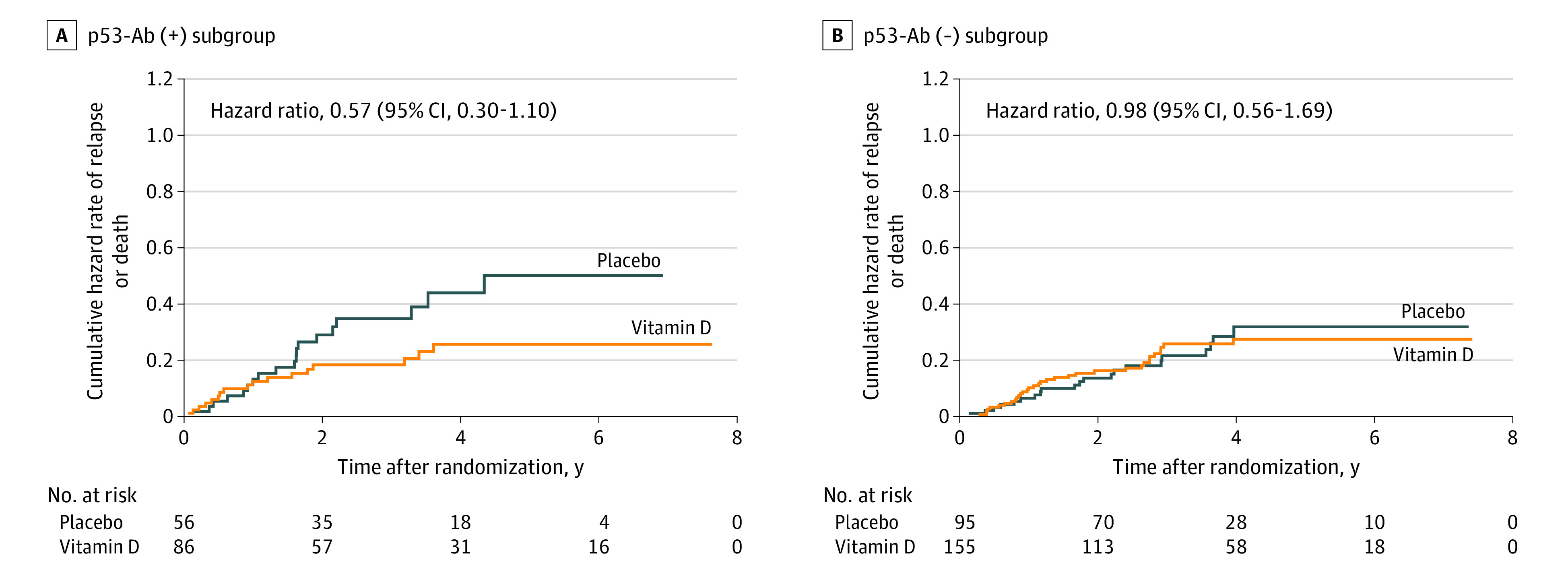
Nelson-Aalen cumulative hazard curves for relapse or death in p53-Ab (+) and p53-Ab (−) groups, assessing patients taking vitamin D vs those taking placebo, were compared using hazard ratios and 95% CIs.
Association Between Expression of p53 Protein and Serum p53-Ab Levels
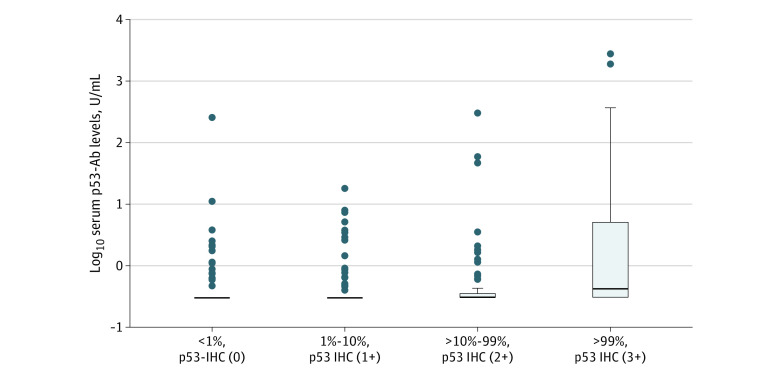
Figure 3 shows log-transformed serum p53-Ab levels according to p53 protein expression (ie, p53-IHC [0], p53-IHC [1+], p53-IHC [2+], and p53-IHC [3+]). There was a positive association between p53-IHC grades and serum p53-Ab levels in linear regression analysis (coefficient = 0.19; P < .001). Similarly, mean (SD) serum p53-Ab levels were significantly higher in patients who were p53-IHC (+) (32.9 [227.1] U/mL) than in those who were p53-IHC (−) (2.7 [21.5] U/mL) (Mann-Whitney test; P < .0001).
Figure 3. Serum Anti-p53 Antibody (p53-Ab) Levels by p53 Immunohistochemistry (IHC) Protein Expression.
IHC levels were 0 (<1% strong or faint p53 cells in cancerous region; p53-IHC [0]), 1 (1%-10% strong p53 cells in cancerous region; p53 IHC [1+]), 2 (>10%-99% strong p53 cells in cancerous region; p53 IHC [2+]), and 3 (>99% overexpressed p53 cells in cancerous region; p53 IHC [3+]).
Analyses in p53-Immunoreactive Subgroup
In 80 patients in the p53-immunoreactive subgroup (ie, patients who were p53-Ab [+] and p53-IHC [3+]), relapse or death occurred in 9 of 54 patients (16.7%) in the vitamin D group and 14 of 26 patients (53.8%) in the placebo group; the 5-year RFS was significantly higher in the vitamin D group (13 patients [80.9%]) than the placebo group (1 patient [30.6%]; HR, 0.27; 95% CI, 0.11-0.61; P = .002; estimated power > 0.99) (Figure 4A). The difference remained significant after adjustment for age and history of cardiovascular disease (HR, 0.20; 95% CI, 0.08-0.50). In 272 patients in the non–p53-immunoreactive group (ie, patients who were p53-Ab [−] or p53-IHC [2+, 1+, or 0] negative), relapse or death occurred in 35 of 158 patients (22.2%) in the vitamin D group and 24 of 114 patients (21.1%) in the placebo group; the 5-year RFS was 37 patients (74.7%) in the vitamin D group and 24 patients (74.1%) in the placebo group, indicating no significant difference (HR, 1.09; 95% CI, 0.65-1.84) (Figure 4B), which was significantly different from the effect of vitamin D in the p53-immunoreactive subgroup (P for interaction = 0.005).
Figure 4. Analysis of p53-Immunoreactive Subgroup.
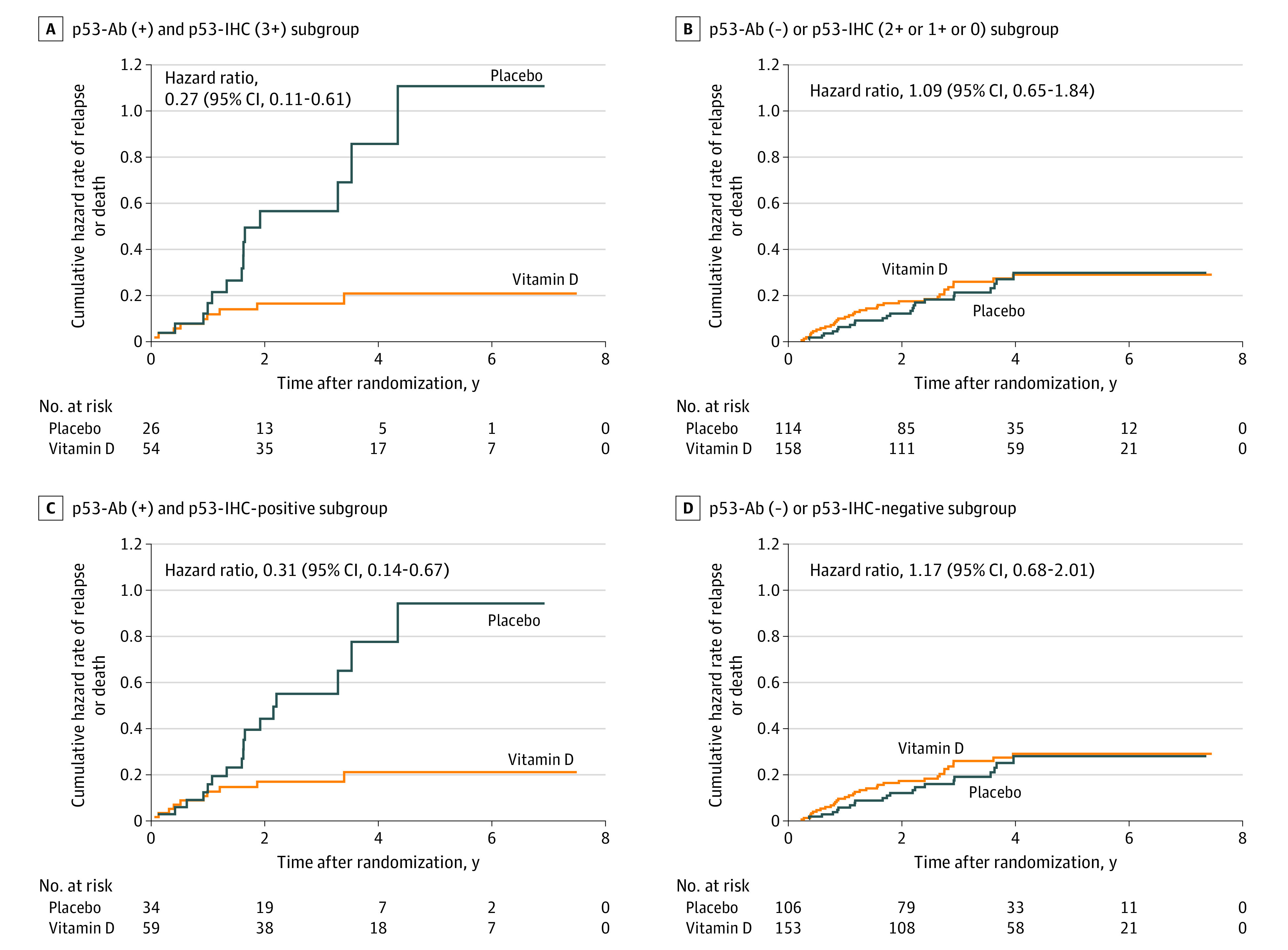
IHC indicates immunohistochemistry; p53-Ab, anti-p53 antibody. Nelson-Aalen cumulative hazard curves are presented in the p53-Ab (+) and p53-IHC (3+) subgroup, p53-Ab (−) or p53-IHC (2+, 1+, or 0) subgroup, p53-Ab (+) and p53-IHC-positive subgroup, and p53-Ab (−) or p53-IHC-negative subgroup. Hazard curves for relapse or death in patients taking vitamin D were compared with those taking placebo.
To conduct a sensitivity analysis using a cutoff p53-IHC value of 10% instead of 99%, patients were divided into 2 groups of p53-IHC (+), or greater than 10%, and p53-IHC (−), or 10% or less. In the p53-Ab (+) and p53-IHC (+) group, relapse or death occurred in 10 of 59 patients (16.9%) in the vitamin D group and 17 of 34 patients (50.0%) in the placebo group; the 5-year RFS was significantly higher in the vitamin D group (14 patients [80.7%]) than the placebo group (2 patients [37.4%]; HR, 0.31; 95% CI, 0.14-0.67; estimated power > 0.99) (Figure 4C). The difference remained significant after adjustment for age and history of cardiovascular disease (HR, 0.24; 95% CI, 0.10-0.57). In the p53-Ab (−) or p53-IHC (−) group, relapse or death occurred in 34 of 153 patients (22.2%) in the vitamin D group and 21 of 106 patients (19.8%) in the placebo group; the 5-year RFS was 36 patients (74.6%) in the vitamin D group and 23 patients (75.4%) in the placebo group, indicating no significant difference (HR, 1.17; 95% CI, 0.68-2.01) (Figure 4D). This was significantly different from the effect of vitamin D in the p53-immunoreactive subgroup (P for interaction = .006).
Discussion
In this post hoc analysis of the AMATERASU RCT, p53-IHC grades showed a positive association with serum p53-Ab levels. Additionally, p53-Ab levels were significantly higher in patients who were p53-IHC (+) than those who were p53-IHC (−). Previously, mutant p53 protein that accumulated in cancer cells was reported to be immunogenic and to lead to the generation of p53-Ab in vitro.12,13,14 These results suggest the possibility that patient immune systems may recognize the abnormally expressed p53 protein as a cancer-specific antigen.
The main findings of this study were that daily supplementation of 2000 IU of vitamin D reduced the risk of relapse or death by 27% compared with placebo in the p53-immunoreactive subgroup, defined by positivity for p53-Ab in serum and p53 protein in more than 99% of cancer cells. Results indicated interactions between vitamin D supplementation and p53-immunoreactive subgroup even after Bonferroni correction. Moreover, results showed a similar risk reduction by shifting the cutoff point of p53-IHC from more than 99% to 10% for sensitivity analysis. As preliminary evidence, we previously reported that vitamin D reduced the risk of relapse or death by 52% compared with the placebo group in the p53-IHC (+) cancer subgroup in a post hoc analysis of the same AMATERASU trial.9 Building on the previous study, this study’s results additionally suggest that vitamin D supplementation may be more effective in improving prognosis in patients who are p53-Ab (+) in serum and p53-IHC (+) in cancer tissue compared with patients who are only p53-IHC (+) independent of p53-Ab status. Another previous study of a murine model24 showed that p53-Ab treatment could prevent metastasis of colon cancer cells to the liver. Although further research is needed, these results suggest that cancer immunotherapy targeting mutated p53 proteins should be developed.
Limitations
This study has several limitations. First, this was a post hoc analysis of the AMATERASU RCT, and the number of patients in the p53-immunoreactive subgroup was very small. Second, because analyses assessed a post hoc hypothesis, observer error or bias could have influenced results. Thus, the findings must be considered exploratory and interpreted with caution, although the main results of this study were remarkable and remained significant even after Bonferroni correction. Third, mutations of the p53 gene were not directly sequenced. Thus, observation of abnormal expression of the p53 protein by IHC may not always imply mutations in the p53 gene. Fourth, because serum levels of p53-Ab were measured at the first outpatient visit at approximately 2 weeks after tumor resection by operation, their values may have been lower than before the operation. Fifth, the number of patients analyzed was lower than in the original study because serum samples were not obtained for 6.0% of them. However, patient characteristics in this post hoc study did not substantially differ from those in the original trial. Sixth, because the AMATERASU trial was conducted in Japan, all patients were Asian, most esophageal cancers were squamous cell carcinomas, the incidence of gastric cancer was relatively high, and the bioavailability of 25-hydroxy vitamin D could be different from that in other population groups.25,26 Seventh, 40 of 392 patients (10.2%) in this study had missing histopathology specimens, which may have biased results of analyses toward the null hypothesis. Eighth, it is uncertain in digestive tract cancers whether squamous cell carcinoma and adenocarcinoma with different characteristics can be analyzed together. Thus, results of this study may not necessarily be generalizable to other populations.
Conclusions
This post hoc analysis of an RCT found that vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients with digestive tract cancer who were p53 immunoreactive. Findings suggest the importance of developing cancer immunotherapy targeting mutated p53 proteins.
Trial Protocol and Statistical Analysis Plan
eTable. Characteristics of Patients Who Were Anti-p53 Antibody Positive by Intervention
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer . Estimated number of new cases from 2020. to 2040, both sexes, age [0-85+]. Accessed January 7, 2023. https://gco.iarc.fr/tomorrow/en/dataviz/isotype
- 3.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuznia S, Zhu A, Akutsu T, et al. Efficacy of vitamin D3 supplementation on cancer mortality: systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res Rev. 2023;87:101923. doi: 10.1016/j.arr.2023.101923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Zhang Y, Liu Z, et al. Association between vitamin D supplementation and cancer mortality: a systematic review and meta-analysis. Cancers (Basel). 2022;14(15):3717. doi: 10.3390/cancers14153717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307-310. doi: 10.1038/35042675 [DOI] [PubMed] [Google Scholar]
- 7.Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database issue):D945-D950. doi: 10.1093/nar/gkq929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urashima M, Ohdaira H, Akutsu T, et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. 2019;321(14):1361-1369. doi: 10.1001/jama.2019.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akutsu T, Okada S, Hirooka S, et al. Effect of vitamin D on relapse-free survival in a subgroup of patients with p53 protein-positive digestive tract cancer: a post hoc analysis of the AMATERASU trial. Cancer Epidemiol Biomarkers Prev. 2020;29(2):406-413. doi: 10.1158/1055-9965.EPI-19-0986 [DOI] [PubMed] [Google Scholar]
- 10.Guedes LB, Almutairi F, Haffner MC, et al. Analytic, preanalytic, and clinical validation of p53 IHC for detection of TP53 missense mutation in prostate cancer. Clin Cancer Res. 2017;23(16):4693-4703. doi: 10.1158/1078-0432.CCR-17-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Li W, Deng M, Liu D, Ma Q, Feng X. Immunohistochemical determination of p53 protein overexpression for predicting p53 gene mutations in hepatocellular carcinoma: a meta-analysis. PLoS One. 2016;11(7):e0159636. doi: 10.1371/journal.pone.0159636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legros Y, Meyer A, Ory K, Soussi T. Mutations in p53 produce a common conformational effect that can be detected with a panel of monoclonal antibodies directed toward the central part of the p53 protein. Oncogene. 1994;9(12):3689-3694. [PubMed] [Google Scholar]
- 13.Lubin R, Schlichtholz B, Bengoufa D, et al. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: distribution on primary structure and exposure on protein surface. Cancer Res. 1993;53(24):5872-5876. [PubMed] [Google Scholar]
- 14.Schlichtholz B, Trédaniel J, Lubin R, Zalcman G, Hirsch A, Soussi T. Analyses of p53 antibodies in sera of patients with lung carcinoma define immunodominant regions in the p53 protein. Br J Cancer. 1994;69(5):809-816. doi: 10.1038/bjc.1994.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30(4):403-408. doi: 10.1002/ijc.2910300404 [DOI] [PubMed] [Google Scholar]
- 16.Angelopoulou K, Diamandis EP, Sutherland DJ, Kellen JA, Bunting PS. Prevalence of serum antibodies against the p53 tumor suppressor gene protein in various cancers. Int J Cancer. 1994;58(4):480-487. doi: 10.1002/ijc.2910580404 [DOI] [PubMed] [Google Scholar]
- 17.Tang R, Ko MC, Wang JY, et al. Humoral response to p53 in human colorectal tumors: a prospective study of 1,209 patients. Int J Cancer. 2001;94(6):859-863. doi: 10.1002/ijc.1541 [DOI] [PubMed] [Google Scholar]
- 18.Shimada H, Ochiai T, Nomura F; Japan p53 Antibody Research Group . Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer. 2003;97(3):682-689. doi: 10.1002/cncr.11092 [DOI] [PubMed] [Google Scholar]
- 19.Harlid S, Gunter MJ, Van Guelpen B. Risk-predictive and diagnostic biomarkers for colorectal cancer; a systematic review of studies using pre-diagnostic blood samples collected in prospective cohorts and screening settings. Cancers (Basel). 2021;13(17):4406. doi: 10.3390/cancers13174406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 21.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502-2521. doi: 10.3390/nu5072502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akutsu T, Kanno K, Okada S, Ohdaira H, Suzuki Y, Urashima M. Effect of vitamin D supplements on relapse of digestive tract cancer with tumor stromal immune response: a secondary analysis of the AMATERASU randomized clinical trial. Cancers (Basel). 2021;13(18):4708. doi: 10.3390/cancers13184708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen JE, Fischer LK, Chan G, et al. Antibody-mediated p53 protein therapy prevents liver metastasis in vivo. Cancer Res. 2007;67(4):1769-1774. doi: 10.1158/0008-5472.CAN-06-3783 [DOI] [PubMed] [Google Scholar]
- 25.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of Black Americans and White Americans. N Engl J Med. 2013;369(21):1991-2000. doi: 10.1056/NEJMoa1306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urashima M, Okuyama M, Akutsu T, Ohdaira H, Kaji M, Suzuki Y. Effect of vitamin D supplementation on survival of digestive tract cancer patients with low bioavailable 25-hydroxyvitamin D levels: a post hoc analysis of the AMATERASU randomized clinical trial. Cancers (Basel). 2020;12(2):347. doi: 10.3390/cancers12020347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable. Characteristics of Patients Who Were Anti-p53 Antibody Positive by Intervention
Data Sharing Statement