Abstract
Purpose
Although it is known that peripheral arterial disease (PAD) is associated with chronic myopathies, the acute muscular responses to exercise in this population are less clear. This study used diffusion tensor imaging (DTI) to compare acute exercise-related muscle damage between PAD patients and healthy controls.
Methods
Eight PAD patients and seven healthy controls performed graded plantar flexion in the bore of a 3T MRI scanner. Exercise began at 2 kg and increased by 2 kg every 2 min until failure, or completion of 10 min of exercise. DTI images were acquired from the lower leg pre- and post-exercise, and were analyzed for mean diffusivity, fractional anisotropy (FA), and eigenvalues 1–3 (λ1–3) of the medial gastrocnemius (MG) and tibialis anterior (TA).
Results
Results indicated a significant leg by time interaction for mean diffusivity, explained by a significantly greater increase in diffusivity of the MG in the most affected legs of PAD patients (11.1 × 10–4 ± 0.5 × 10–4 mm2/s vs. 12.7 × 10–4 ± 1.2 × 10–4 mm2/s at pre and post, respectively, P = 0.02) compared to healthy control subjects (10.8 × 10–4 ± 0.3 × 10–4 mm2/s vs. 11.2 × 10–4 ± 0.5 × 10–4 mm2/s at pre and post, respectively, P = 1.0). No significant differences were observed for the TA, or λ1–3 (all P ≥ 0.06). Moreover, no reciprocal changes were observed for FA in either group (all P ≥ 0.29).
Conclusion
These data suggest that calf muscle diffusivity increases more in PAD patients compared to controls after exercise. These findings are consistent with the notion that acute exercise results in increased muscle damage in PAD.
Keywords: Skeletal muscle, Nuclear magnetic resonance, Diffusion weighted imaging, Occlusive disease
Introduction
Peripheral arterial disease (PAD) is a systemic atherosclerotic vascular disease that affects over 200 million people worldwide (Benjamin et al. 2017; Shu and Santulli 2018). While disease severity can range from patients being asymptomatic to having critical limb ischemia, the most common manifestation of PAD is pain in the lower legs during light to moderate intensity work. This classic symptom is termed intermittent claudication. Over time, the progression of PAD leads to skeletal muscle atrophy (Regensteiner et al. 1993), myopathy (Ha et al. 2016; Makitie and Teravainen 1977; Mitchell et al. 2007), and mitochondrial dysfunction (AlGhatrif et al. 2017; Pipinos et al. 2003). While it may seem logical that this would be caused by the repeated ischemic insults associated with chronic PAD (Gratl et al. 2020; Signolet et al. 2018), there is stronger evidence linking skeletal muscle atrophy (Belavy et al. 2017; Dirks et al. 2016; Reimers et al. 1998) and mitochondrial dysfunction (Distefano et al. 2018) to reductions in physical activity and exercise tolerance. Indeed, many of the functional impairments associated with PAD are not entirely explained by limited blood flow delivery (Gardner and Killewich 2001). For example, maximal torque production, which is not dependent on convective oxygen transport, is reduced in the plantar flexors of PAD patients (Schieber et al. 2017). Moreover, the mitochondrial impairments that are observed in simulated PAD models persist in ex vivo preparations (Bhat et al. 1999; Schmidt et al. 2017), where in vivo perfusion limitations are no longer a contributing factor. Taken together, these findings illustrate the important role of skeletal muscle physiology in the pathophysiology and progression of PAD.
While the chronic myopathies associated with PAD are well established, the effects of acute exercise on skeletal muscle in this population are less clear. In healthy adults, moderate to high-intensity exercise (particularly eccentric muscle actions) often results in acute damage to skeletal muscle (Beaton et al. 2002; Burt et al. 2014; Chen et al. 2014), which is primarily attributed to dissolution of sarcomeres and disruption of the muscle cell’s plasma membrane (Friden and Lieber 1992; McHugh et al. 1999; Morgan 1990; Saxton and Donnelly 1996). While the term ‘damage’ may be most commonly associated with a detrimental effect, acute exercise-related skeletal muscle damage has been associated with hypertrophy and strength adaptations to resistance training in humans (Douglas et al. 2017). This occurs in conjunction with an acute immune response, characterized by transient increases in recruitment of neutrophils (Nieman et al. 2005) and cytokines (Jajtner et al. 2016, 2018). Considering the limited exercise tolerance and recurrent exposure to ischemic insults during lower body activity (i.e. locomotion) in PAD patients, combined with a well-documented impairment in skeletal muscle function (Regensteiner et al. 1993; Gardner et al. 1992; Schieber et al. 2017; McDermott et al. 2004), it would be reasonable to suspect that this skeletal muscle damage response would be augmented after acute exercise of a matched workload (and intensity) in PAD patients compared to their healthy counterparts. However, to our knowledge, no studies have directly examined this issue. This information would be particularly useful for prescribing exercise intensity in this population, which is an area of significant debate among researchers and clinicians (Haas et al. 2012; Murrow et al. 2019; Parmenter et al. 2015).
A common method of evaluating skeletal muscle fiber structure and integrity is by excising tissue with a muscle biopsy (Becker et al. 2017; Lindegaard Pedersen et al. 2017). While this procedure is useful for acquiring tissue samples from live subjects, it is also invasive and relies on the interpretation of a very small sample of tissue, which may not always represent changes observed across the entire muscle (Dwyer et al. 1999). Therefore, in lieu of muscle biopsies, diffusion tensor imaging (DTI) is a valuable method of assessing skeletal muscle in vivo, and across the entire muscle (or limb, for that matter). DTI works by exploiting the diffusive properties of cellular structures, such as neurons (Li et al. 2016) and skeletal muscle fibers (Schenk et al. 2013). This technique can be used to evaluate the threedimensional organization of skeletal muscle (tractography), or to quantify changes in skeletal muscle integrity. Specifically, increases in mean diffusivity (and reciprocal decreases in fractional anisotropy) of skeletal muscle has been linked to muscle damage and inflammation (Esposito et al. 2013; Fan and Does 2008; Yanagisawa et al. 2011), an effect that appears to be augmented when exercise is performed under ischemic conditions (Heemskerk et al. 2006). DTI is also useful for quantifying changes in the cross-sectional geometry (ellipticity) of muscle fibers (Karampinos et al. 2009), which can provide valuable insight regarding myocyte integrity (Andersen 2003). The aim of this study was to use DTI to compare acute exercise-related muscle damage between patients with PAD and healthy controls. We expected that a single bout of exercise would elicit significant increases in mean diffusivity of the active muscle in PAD patients compared to healthy controls. In contrast, we expected factional anisotropy to significantly decrease in response to exercise in PAD patients compared to healthy controls.
Methods
Participants and study design
All data were collected at the Penn State Hershey Center for Nuclear Magnetic Resonance Research and the Penn State Hershey Clinical Research Center in the Clinical and Translational Science Institute, and all experiments were approved by the Penn State Hershey College of Medicine Institutional Review Board. A total of 15 participants completed this study, including 8 PAD patients (defined by an ankle brachial index [ABI] < 0.9) and 7 healthy controls (CON). Participant demographics are presented in Table 1. Data from 14 of these participants (7 PAD and all controls) were included in a recent publication (Stavres et al. 2019) that examined oxygen transport during exercise, but did not report DTI data. A preliminary analysis of these data was also prepared for presentation at the 2020 American College of Sports Medicine Conference (Stavres et al. 2020).
Table 1.
Participant characteristics compared between groups
| CON | PAD | |
|---|---|---|
| Male/female (n) | 6/1 | 7/1 |
| Age (years) | 64 ± 7 | 66 ± 6 |
| Height (cm) | 174.8 ± 7.6 | 173.3 ± 9.6 |
| Weight (kg) | 81.7 ± 11.5 | 85.9 ± 9.4 |
| BMI (kg/m2) | 26.6 ± 2.2 | 28.5 ± 2.0 |
| ABI-right | 1.10 ± 0.09 | 0.75 ± 0.20* |
| ABI-left | 1.05 ± 0.10 | 0.62 ± 0.24* |
| Medications (n) | ||
| Aspirin | 1 | 6 |
| Statins | – | 8 |
| Plavix | – | 2 |
| Antihypertensives | – | 4 |
| β-blockers | – | 2 |
| Cilostazol/Pentoxyfiline | – | 3 |
| Interventions (n) | ||
| Angioplasty | – | 3 |
| Stent placement | – | 3 |
| Femoral bypass | – | 3 |
P < 0.05
Participation in this study involved a single visit to the research lab, with the exception of bilaterally diseased patients, who participated in two research visits (one for each diseased leg). Each visit consisted of two bouts of exercise, and the imaging coil remained on the same leg throughout the duration of the visit. In the first bout, the leg that was imaged performed exercise. Afterwards, the plantar flexion device was moved to the opposite leg, while the imaging coil remained on the resting leg. The order of visits was also counterbalanced between imaging of the most symptomatic and least symptomatic legs for bilaterally diseased patients (n = 5). The most symptomatic leg was identified as the leg which patients described as experiencing more pain during typical daily activities and being most prone to claudication related discomfort. In cases where patients were unable to distinguish a most symptomatic leg, the leg with the lowest ABI was recorded as the most symptomatic leg. Participants were not instructed to withhold medications prior to participation (Table 1), which included assessment of resting ABI and blood pressure.
Experimental protocol
In each visit, participants performed dynamic graded plantar flexion in the bore of a 3T MRI scanner (Magnetom PrismaFit, Siemens Healthineers, Erlangen, Germany). This was achieved using a custom-built non-ferromagnetic plantar flexion device, as previously described (Stavres et al. 2019). In brief, this plantar flexion device allows for dynamic contractions, which is achieved via a wooden lever system affixed to a bin. The resistance is applied by adding weighted sandbags to this bin, and the force required is calibrated at the footplate. This device permits participants to perform exercise within a comfortable range of motion at a 1:1 work-rest duty cycle. Each condition began with a baseline DTI scan, followed by 2 min of perfusion imaging (described in the “Measurements” section). Perfusion data were collected pre- and post-exercise to account for any potential influence of pseudodiffusion on DTI data, which may influence DTI parameters. After baseline data were collected, participants performed plantar flexion beginning at 2 kg, and increasing by 2 kg every 2 min until failure (identified by voluntary termination), or until the end of 10 kg. A metronome was also used to maintain a contraction frequency of 30 contractions per minute. To limit any potential carryover effects, ~ 10 min of rest separated the exercising leg and resting leg conditions within each session. Perfusion data were collected again immediately after exercise, during which time leukocyte infiltration has been reported to be acutely elevated (Fortunato et al. 2018). This perfusion scan was followed by a second (post) DTI scan.
Measurements
In the first visit, resting hemodynamic and anthropometric data were recorded by clinical staff at the Penn State Hershey Clinical Research Center. This included height, weight, resting blood pressure, heart rate, and bilateral ABI. Leg pain was also recorded immediately following exercise using the Numeric Rating Scale (Williamson and Hoggart 2005).
DTI images were collected at baseline and 5 min after the end of exercise with a 15-channel transmit–receive coil. A small cross-shaped fiducial marker made of fish oil was placed on the anterior of the tibia to indicate the position of the thickest part of the calf (isocenter). DTI images were acquired using a spin-echo echo-planar DTI sequence with repetition time = 2.8 s, echo time = 70.40 ms, flip angle = 90°, 32 4-mm-thick transverse slices, field of view = 204 mm × 171 mm, scan matrix = 112 × 94, image resolution = 1.8 mm × 1.8 mm, multi-band acceleration factor = 2, b-value = 0, 200, 400, 600 s/mm2, 30 angular diffusion gradient directions, four b0 images interleaved with the diffusion-weighted images with 1 b0 image per 10 diffusion-weighted images.
The DTI data were processed with Diffusion Toolkit 0.6.4.1 and TrackVis 0.6.1 (http://trackvis.org/dtk). For quality control of the motion artifact, the four b0 images acquired from the beginning to the end of each DTI data acquisition were realigned using SPM12 (The Wellcome Centre for Human Neuroimaging, University College London) with a threshold of movement < 1 mm and rotation < 1°. The majority of the medial gastrocnemius (MG) and tibialis anterior (TA) at the level of the fiducial marker were manually labeled as the seed for fiber-tracking of the muscle fibers for each participant. Diffusion parameters, including mean diffusivity (MD), fractional anisotropy (FA), and eigenvalues 1 (λ1) 2 (λ2), and 3 (λ3) of the muscle fiber bundle were recorded.
Perfusion images were also acquired immediately post-exercise using a modified version of the PIVOT sequence (Englund et al. 2013). These data were collected to account for the potentially confounding influence of capillary perfusion on measures of skeletal muscle diffusivity, termed “pseudodiffusion” (Le Bihan et al. 1986; Oudeman et al. 2016; Yanagisawa et al. 2009). Specifically, by comparing perfusion between groups immediately prior to collection of DTI data, we were able to account for any potential differences in muscle perfusion. Arterial spin labeling images were acquired with a single-shot echo-planar imaging readout, with echo time = 9.6 ms, scan matrix = 64 × 64, resolution = 2.75 mm × 2.75 mm, slice thickness = 8.0 mm, partial Fourier factor 5/8, excitation flip angle = 90°, and a spectral-spatial pulse (Meyer et al. 1990) for fat suppression. The entire sequence repetition time was 3.25 s, and also included multi-echo gradient-echo acquisition, which was interleaved into the post-labeling delay of the arterial spin labeling sequence. However, those data were not included in this analysis. After all DTI and perfusion data were collected, regions of interest were used to isolate the MG and TA of each participant. These specific muscles were selected due to their roles as the primary agonist (MG) and antagonist (TA) to dynamic plantar flexion.
Statistical approach
Based on an estimated effect size of 0.45 (calculated from pilot data comparing DTI responses pre- and post-exercise), a power analysis [G*Power 3.1 (Faul et al. 2007)] indicated that 12 participants (6 per group) would be required to reach statistical significance at a desired power of 0.8. After all data were collected, MD, FA, λ1–3 were compared across time (pre-post) and between legs (most symptomatic leg vs. least symptomatic leg vs. healthy control leg) for each muscle using a linear mixed models’ approach. This analysis was performed for each condition (exercising leg and resting leg). Also, to account for the potentially confounding influence of pseudodiffusion, a Pearson’s correlation analysis was used to examine the relationships between muscle perfusion (pre- and post-exercise) and all DTI parameters. This analysis indicated that perfusion was significantly correlated to MD and λ1–3 in the MG of the exercising leg (Table 2). Based on this, perfusion was included as a covariate in each linear mixed models’ analysis for MD and λ1–3 in both the MG and the TA. Any significant interactions or main effects of time were further analyzed using a Sidak correction. Lastly, one-way analyses of variance (ANOVA) were used to compare limb-specific exercise data (end-exercise leg pain, rating of perceived exertion [RPE], time of exercise in seconds, and peak workload) of the most symptomatic legs to the least symptomatic legs of PAD patients, as well as healthy control legs. Significance was accepted at P < 0 0.05, and all data are presented as mean ± standard deviation (SD). All statistical analyses were performed using SPSS version 27 (IBM Corp., Armonk, NY).
Table 2.
Pearson’s correlation analyses comparing the linear relationships between perfusion, fractional anisotropy, mean diffusivity, and each individual eigenvalue (λ1–3)
| FA (AU) | MD (mm2/s) | λ1 (mm2/s) | λ2 (mm2/s) | λ3 (mm2/s) | |
|---|---|---|---|---|---|
| Perfusion (mL/100 g/min) | |||||
| Exercising leg | |||||
| MG | r = 0.07, P = 0.64 | r = 0.69, P < 0.01 | r = 0.61, P < 0.01 | r = 0.69, P < 0.01 | r = 0.71, P < 0.01 |
| TA | r = 0.28, P = 0.07 | r = 0.53, P < 0.01 | r = 0.24, P = 0.12 | r = − 0.21, P = 0.18 | r = 0.09, P = 0.56 |
| Resting leg | |||||
| MG | r = 0.27, P = 0.08 | r = 0.53 P = < 0.01 | r = 0.19, P = 0.24 | r = − 0.14, P = 0.36 | r = 0.34, P = 0.02 |
| TA | r = 0.10, P = 0.51 | r = 0.47, P < 0.01 | r = − 0.10, P = 0.50 | r = − 0.36, P = 0.02 | r = − 0.04, P = 0.79 |
Sci. Not. scientific notation, MD mean diffusivity (mm2/s), FA fractional anisotropy (AU), λ1–3 eigenvalues 1–3 (mm2/s), Perf perfusion (mL/100g/min)
Results
Exercising leg
After including perfusion as a covariate, results indicated a significant leg (most symptomatic vs. least symptomatic vs. healthy control leg) by time (pre and post-exercise) interaction for MD (f(1,23) = 3.4, P = 0.04) of the MG. When analyzed further, MD of the medial gastroc (MG) significantly increased in response to exercise in the most symptomatic legs of PAD patients (11.1 × 10–4 ± 0.5 × 10–4 mm2/s vs. 12.7 × 10–4 ± 1.2 × 10–4 mm2/s at pre and post, respectively, P = 0.02), which was not observed in either the least symptomatic legs of PAD patients or healthy control legs (all P ≥ 1.0; Fig. 1). In contrast, no significant leg by time interactions were observed for any of the individual eigenvalues (λ1–3) when perfusion was included as a covariate (All P ≥ 0.09; Fig. 2). However, significant effects of time were observed for each of the individual eigenvalues (All P ≤ 0.02; Fig. 2), and posthoc analyses indicated that this was explained by a significant increase in λ1–3 in the most affected legs of PAD patients (P ≤ 0.02; Fig. 2). Significant effects of leg were also observed for λ1 (f(2,17) = 4.5, P = 0.02) and λ2 (f(2,30) = , P = 0.01; Fig. 2) in the MG of the exercising leg. Contrary to our original hypothesis, no significant interactions or main effects leg or time were observed for FA of the MG (22.4 × 10–2 ± 1.5 × 10–2 AU vs. 22.4 × 10–2 ± 5.3 × 10–2 AU in the symptomatic legs of PAD patients, 21.4 × 10–2 ± 1.0 × 10–2 AU vs. 21.3 × 10–2 ± 3.2 × 10–2 AU in the least symptomatic legs of PAD patients, and 21.4 × 10–2 ± 0.8 × 10–2 vs. 19.6 × 10–2 ± 0.6 × 10–2 AU in the healthy control legs at pre and post, respectively, all P ≥ 0.29).
Fig. 1.
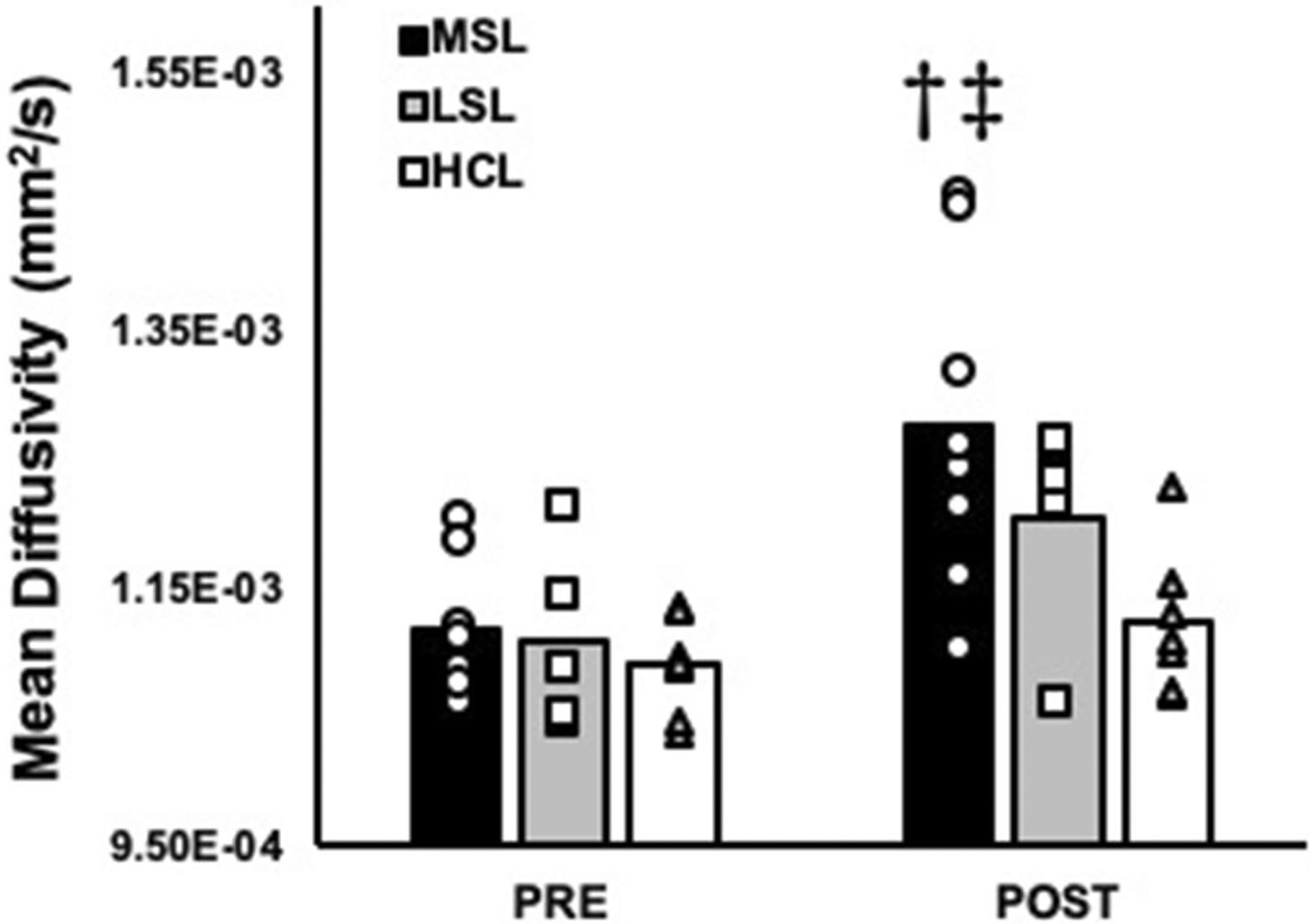
Mean diffusivity (MD) of the medial gastrocnemius compared between baseline and post-exercise in the most symptomatic legs (MSL; n = 8) and least symptomatic legs (LSL; n = 5) of PAD patients, as well as healthy control legs (HCL; n = 7). †Significant change compared to baseline, and ‡significant interaction across time compared to the healthy control legs. Significance accepted at P < 0.05
Fig. 2.
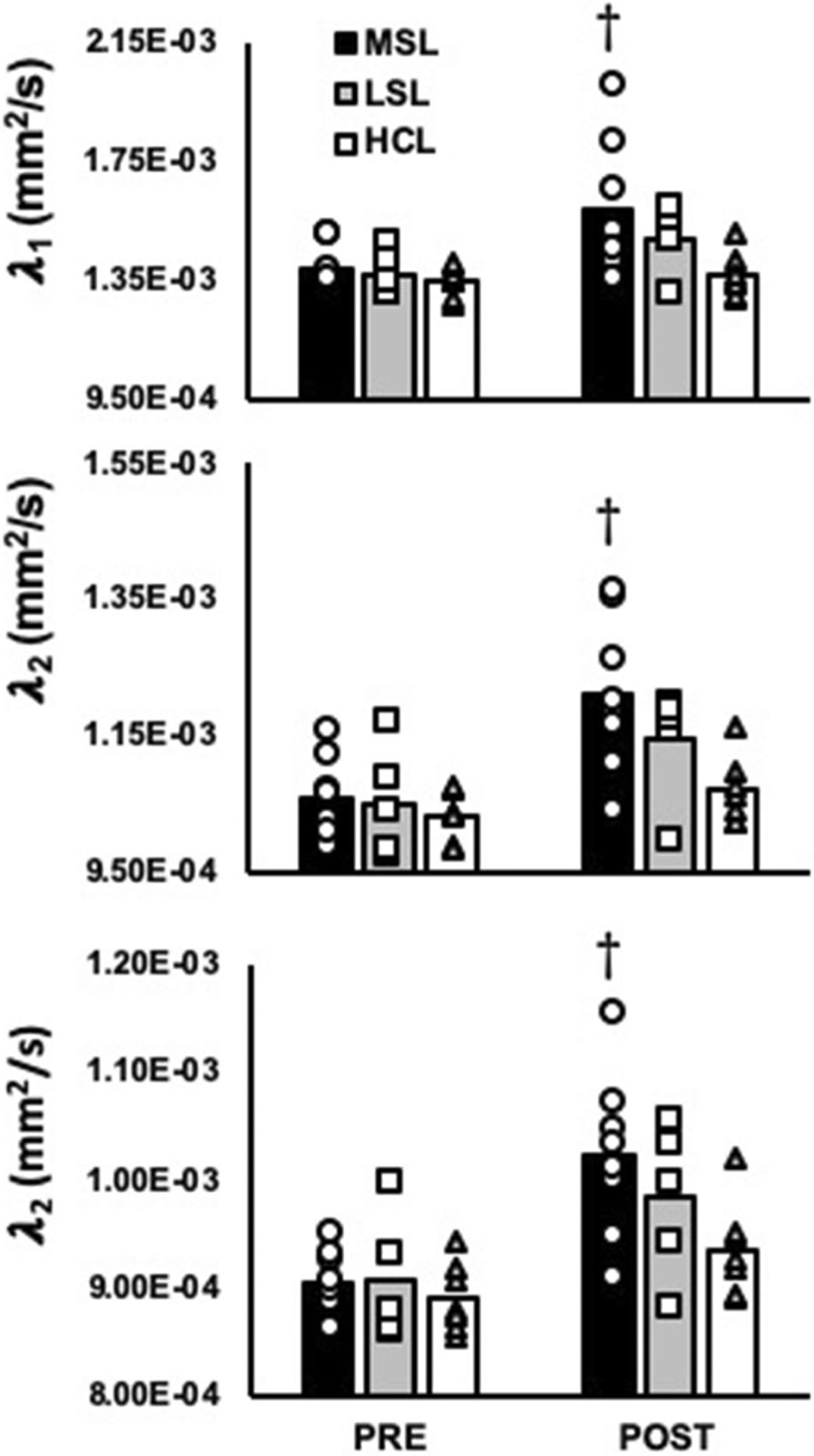
Eigenvalues λ1, λ2, and λ3 of the medial gastrocnemius (MG) in the most symptomatic legs (MSL; n = 8) and least symptomatic legs (LSL; n = 5) of PAD patients, as well as healthy control legs (HCL; n = 7). †Significant change compared to baseline. Significance accepted at P < 0.05
In contrast to the MG, no significant leg by time interactions were observed for mean diffusivity of the TA when perfusion was included as a covariate, nor did we observe any significant main effects of leg or time (all P ≥ 0.17; Fig. 3). Likewise, no significant differences were observed for FA (26.2 × 10–2 ± 1.9 × 10–2 AU vs. 27.0 × 10–2 ± 3.2 × 10–2 AU in the most symptomatic legs of PAD patients, 24.5 × 10–2 ± 9.8 × 10–2 AU vs. 25.8 × 10–2 ± 1.4 × 10–2 AU in the least symptomatic legs of PAD patients, and 26.1 × 10–2 ± 1.8 × 10–2 AU vs. 26.4 × 10–2 ± 2.1 × 10–2 AU in the legs of healthy control subjects at pre- and post-exercise, respectively, all P ≥ 0.13) or for any of the eigenvalues (all P ≥ 0.06; Fig. 4) in the TA during the exercising leg condition.
Fig. 3.
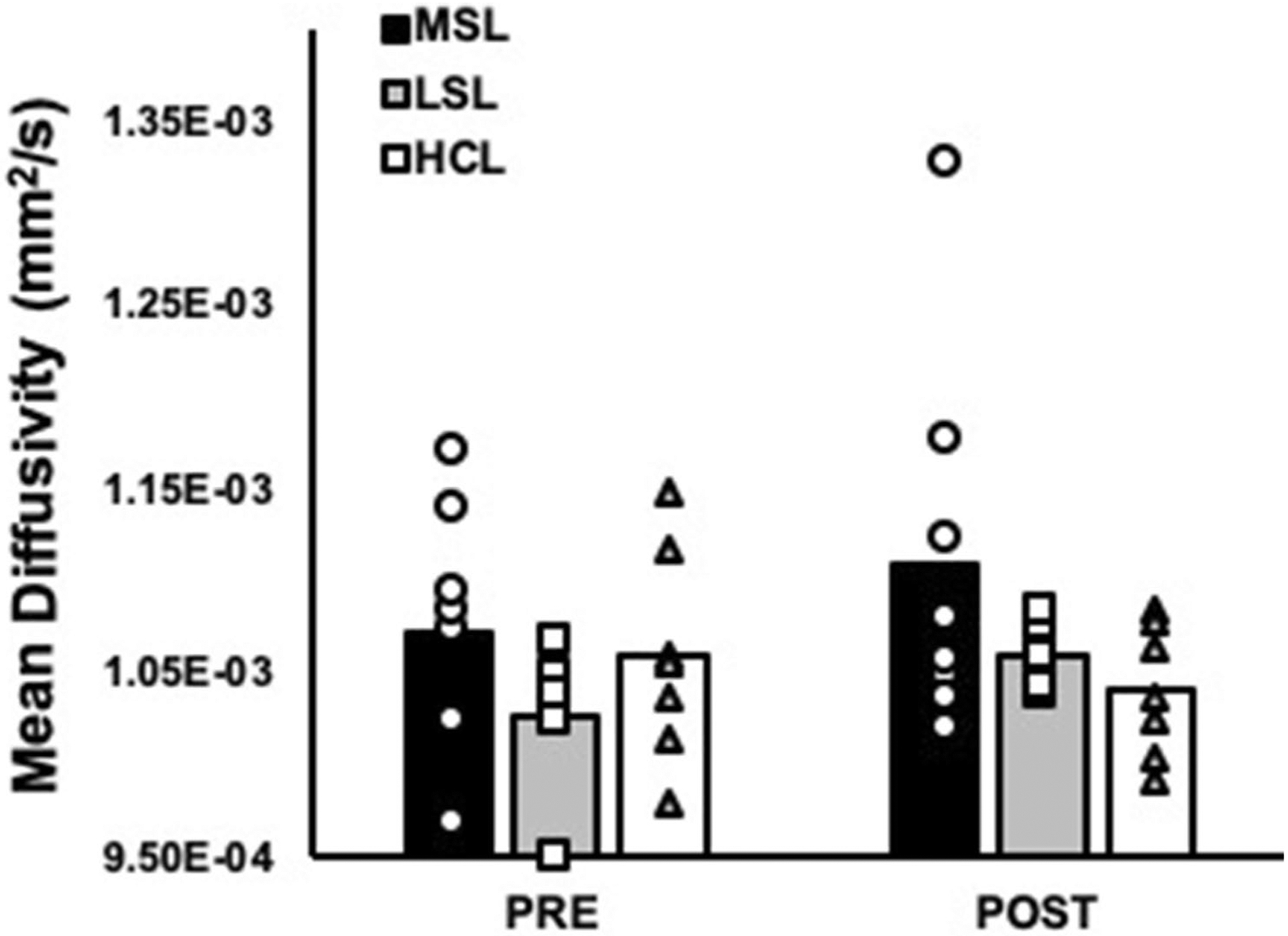
Mean diffusivity (MD) of the tibialis anterior (TA) compared between baseline and post-exercise in the most symptomatic legs (MSL; n = 8) and least symptomatic legs (LSL; n = 5) of PAD patients, as well as healthy control legs (HCL; n = 7). No significant differences were observed for mean diffusivity of the TA
Fig. 4.
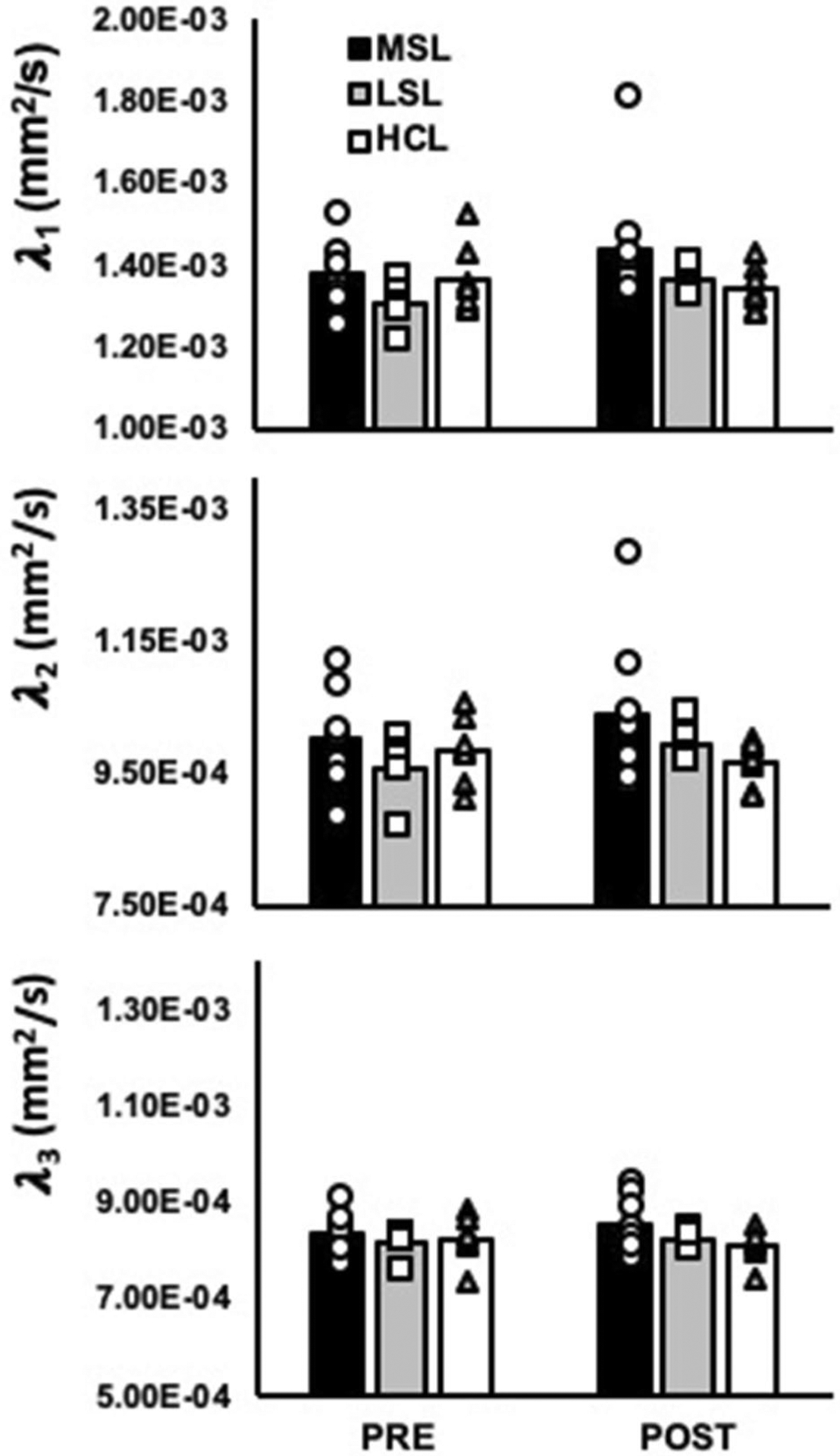
Eigenvalues λ1, λ2, and λ3 of the medial gastrocnemius (MG) in the most symptomatic legs (MSL; n = 8) and least symptomatic legs (LSL; n = 5) of PAD patients, as well as healthy control legs (HCL; n = 7). No significant differences were observed for λ1, λ2, or λ3 of the TA
Lastly, plantar flexion exercise elicited an augmented pain response in the symptomatic legs of PAD patients compared to controls (6 ± 2 [on a 10-point scale] vs. 0 ± 0, respectively, P = 0.001) in the exercising leg, whereas no significant differences were observed between the most and least symptomatic legs of PAD patients (P = 0.57; Table 3). All healthy participants achieved 720 s of continuous exercise, ending with a peak workload of 10 kg, while two patients in the PAD group failed reach the final 720 s of exercise. Surprisingly, RPE was not different between the most symptomatic legs of PAD patients, the least symptomatic legs of PAD patients, or the healthy control legs (15 ± 1, 13 ± 3, and 12 + 1, respectively, P = 0.08). All exercise data are presented in Table 3.
Table 3.
Individual exercise data compared between PAD patients and healthy controls
| Exercising leg condition | Resting leg condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Imaged leg | Total time (sec) | Peak workload (kg) | Pain | RPE | Total time (sec) | Peak workload (kg) | Pain | RPE |
| Most symptomatic leg | |||||||||
| S1 | R | 720 | 10 | 4 | 13 | 720 | 10 | 2 | 7 |
| S2 | L | 720 | 10 | 7 | 14 | 720 | 10 | 6 | 14 |
| S8 | R | 420 | 8 | 9 | 18 | 415 | 6 | 8 | 18 |
| S9 | L | 720 | 10 | 8 | 17 | 480 | 8 | 7 | 17 |
| S12 | R | 500 | 8 | 8 | 14 | 626 | 10 | 6 | 15 |
| S14 | R | 720 | 10 | 0 | 13 | 720 | 10 | 0 | 13 |
| S15 | L | 720 | 10 | 7 | 16 | 720 | 10 | 5 | 15 |
| S16 | L | 720 | 10 | 5 | 15 | 720 | 10 | 1 | 13 |
| Least symptomatic leg | |||||||||
| S2 | R | 720 | 10 | 0 | 8 | 720 | 10 | 6 | 13 |
| S8 | L | 360 | 6 | 7 | 15 | 427 | 6 | 7 | 15 |
| S9 | R | 720 | 10 | 7 | 17 | 583 | 8 | 9 | 18 |
| S12 | L | 662 | 10 | 7 | 13 | 593 | 8 | 7 | 15 |
| S14 | L | 720 | 10 | 0 | 13 | 720 | 10 | 0 | 13 |
| Healthy control leg | |||||||||
| S5 | L | 720 | 10 | 0 | 15 | 720 | 10 | 0 | 16 |
| S3 | R | 720 | 10 | 0 | 13 | 720 | 10 | 0 | 13 |
| S7 | L | 720 | 10 | 0 | 12 | 720 | 10 | 0 | 12 |
| S10 | L | 720 | 10 | 0 | 12 | 720 | 10 | 4 | 13 |
| S11 | L | 720 | 10 | 0 | 12 | 720 | 10 | 0 | 14 |
| S13 | L | 720 | 10 | 0 | 12 | 720 | 10 | 0 | 14 |
| S17 | L | 720 | 10 | 0 | 11 | 720 | 10 | 0 | 13 |
| ANOVA | F = 1.03, P = 0.37 | F = 0.90, P = 0.42 | F = 7.88, P < 0.01 | F = 2.81, P = 0.08 | F = 2.11, P = 0.15 | F = 2.38, P = 0.12 | F = 6.41, P < 0.01 | F = 0.36, P = 0.70 | |
Resting leg
Contrary to the exercising leg condition, no significant main effects or leg by time interactions were observed for FA (all P ≥ 0.11), MD (all P ≥ 0.11), λ1 (all P ≥ 0.24), or λ2 (all P ≥ 0.052) of the MG or TA in the resting leg condition. Significant effects of time were observed for λ3 in both the MG (9.0 × 10–4 ± 0.6 × 10–4 mm2/s vs. 8.9 × 10–4 ± 0.4 × 10–4 mm2/s in the most symptomatic legs of PAD patients, 9.2 × 10–4 ± 0.5 × 10–4 mm2/s vs. 9.0 × 10–4 ± 0.4 × 10–4 mm2/s in the least symptomatic legs of PAD patients, and 8.8 × 10–4 ± 0.5 × 10–4 mm2/s vs. 8.8 × 10–4 ± 0.5 × 10–4 mm2/s in the legs of healthy control subjects at pre and post, respectively, [main effect of time] P = 0.02) and the TA (8.3 × 10–4 ± 0.5 × 10–4 mm2/s vs. 8.1 × 10–4 ± 0.5 × 10–4 mm2/s in the most symptomatic legs of PAD patients, 8.2 × 10–4 ± 0.1 × 10–4 mm2/s vs. 8.1 × 10–4 ± 0.2 × 10–4 mm2/s in the least symptomatic legs of PAD patients, and 8.2 × 10–4 ± 0. × 10–4 mm2/s vs. 8.0 × 10–4 ± 0.4 × 10–4 mm2/s in the legs of healthy control subjects at pre and post, respectively, [main effect of time] P = 0.02). Despite this, no significant leg by time interactions were observed for λ3 in either the MG or TA during the resting leg condition (all P ≥ 0.37).
Exercise in the resting leg condition elicited a similarly augmented pain response in PAD patients, characterized by significantly elevated pain responses in the both legs of PAD patients compared to healthy controls (4 ± 2 [on a 10-point scale] in the most symptomatic legs of PAD patients, 5 ± 3 in the least symptomatic legs of PAD patients, and 0 ± 0 in the legs of healthy control subjects, P ≤ 0.04). This is notable, as the mean pain response in the least symptomatic legs of PAD patients appears to be higher than the mean pain response in the legs classified as most symptomatic. However, this mean difference is not statistically significant (P = 0.74), and is likely reflective of the variability of symptomology within our sample of PAD patients. In contrast to the pain response, no differences were observed for RPE, time of exercise, or peak workload between the most and least symptomatic legs of PAD patients or the legs of healthy control subjects in the resting leg condition (Table 3).
Discussion
The data presented here collectively suggest that markers of acute exercise-related muscle damage are augmented in PAD patients. Specifically, mean diffusivity of the active muscle increased more in the most symptomatic leg of PAD patients compared to healthy controls in response to plantar flexion (Fig. 1), and this response was not observed during opposite leg exercise (Fig. 3). This is also depicted in Figs. 5 and 6, which illustrate the exercise-related MD changes in the active and inactive legs of a representative PAD patient (S12). These findings are consistent with prior studies that report significant increases in muscle diffusivity after sterile muscle trauma (Esposito et al. 2013) and edema (Fan and Does 2008). Notably, we did not observe any changes in FA. This does not support our initial hypothesis, and is not consistent with the typical observations of reciprocal decreases in FA with increases in MD. However, non-inverse and dissociated changes in MD and FA have been reported elsewhere. Bryant and colleagues (Bryant et al. 2014) reported significant increases in MD but not FA after λ-carrageenan induced muscle inflammation in mice, and McMillan and colleagues (McMillan et al. 2011) reported concurrent increases in MD and FA after muscle damaging eccentric exercise in mice. Considering our present findings, it seems likely that the typical inverse relationship between MD and FA is fundamentally altered by exercise-related muscle damage.
Fig. 5.
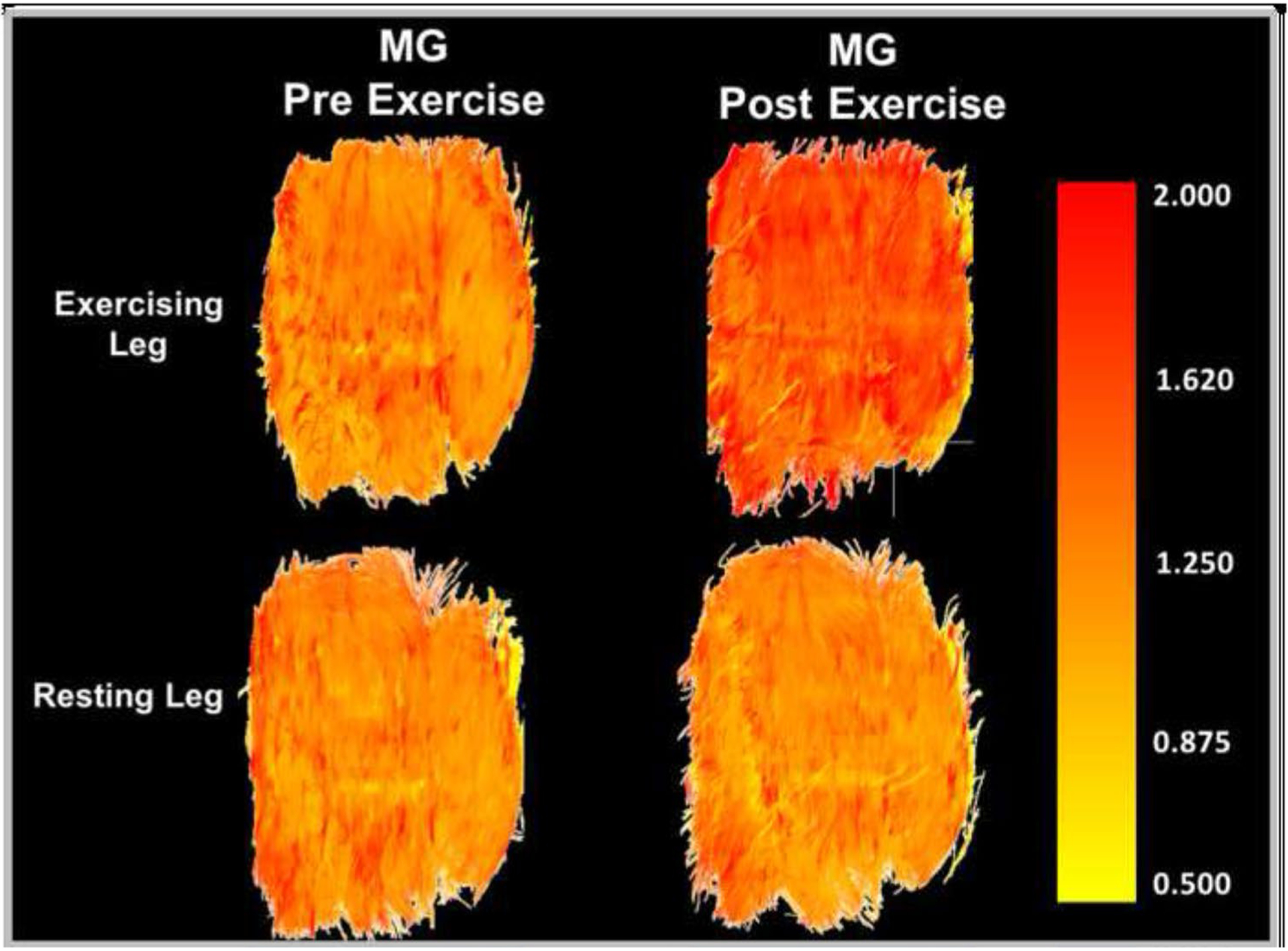
Mean diffusivity (MD) of the medial gastrocnemius (MG) compared between the exercising and resting legs of a single representative PAD patient (S12) before and after exercise
Fig. 6.
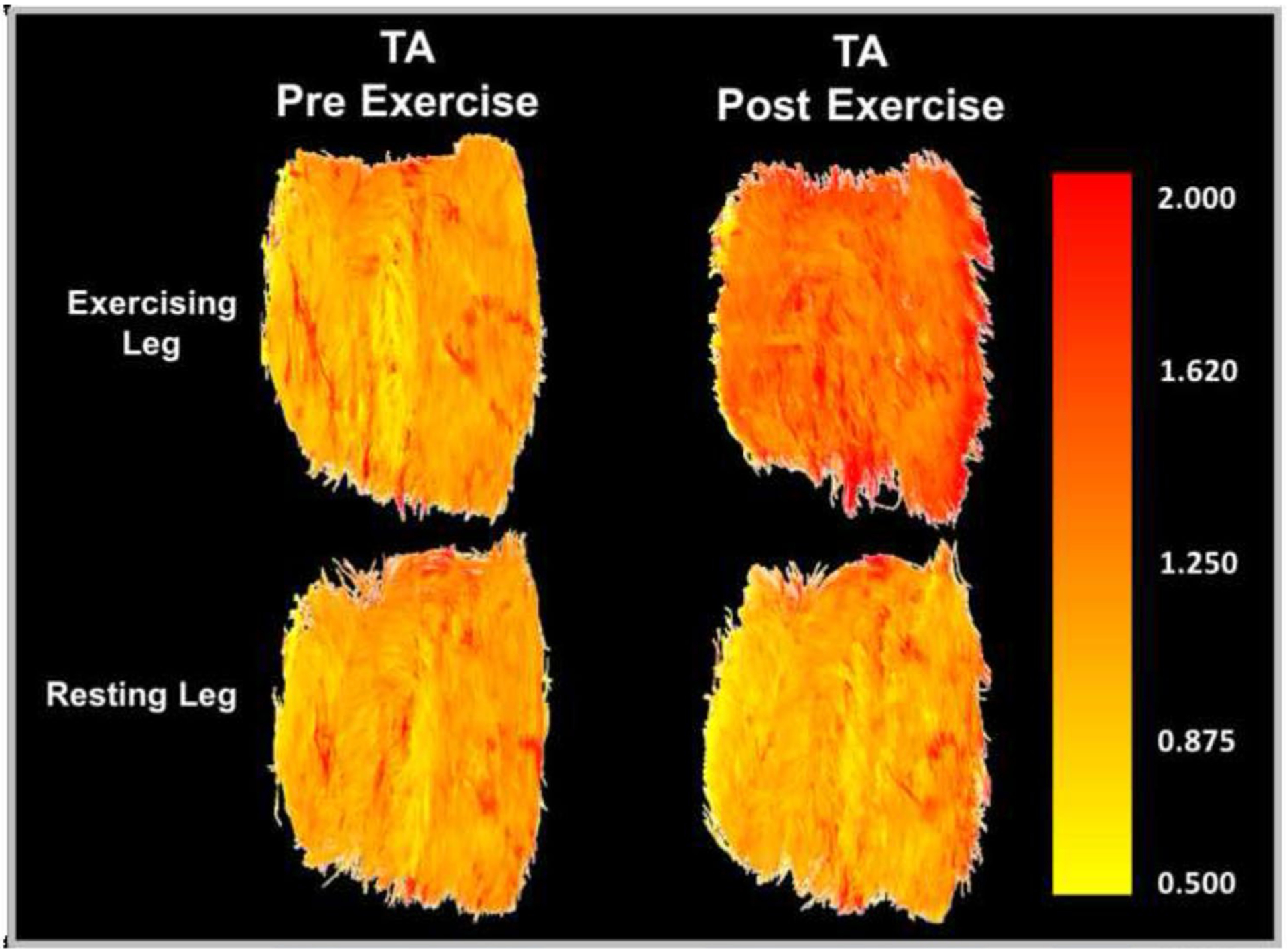
Mean diffusivity (MD) of the tibialis anterior (TA) compared between the exercising and resting legs of a single representative PAD patient (S12) before and after exercise
There are a number of potential mechanisms that could explain this observed increase in markers of exercise-related skeletal muscle damage in PAD patients. For instance, these patients are exposed to demand ischemia during light to moderate intensity exercise. As noted in the introduction, muscular contractions performed in ischemic conditions have been reported to evoke significant increases in MD. Heemskerk and colleagues (Heemskerk et al. 2006) demonstrated this by comparing changes in DTI parameters in the hind limbs of mice (MD, λ1–3, and FA) in response to ischemic insults with and without electrically stimulated dorsiflexion. The authors reported that the mean diffusivity and T2 signal intensity of the tibialis anterior (the primary dorsiflexor) were augmented immediately following stimulated contractions performed under ischemic conditions as compared to non-stimulated muscle groups, and these increases persisted for 24 h after the experiment. The authors also reported significant relationships between histological indices of muscle damage and changes in λ2, λ3, MD, FA, and T2 signal intensity. Based on these findings, it is reasonable to suspect that the ischemia associated with PAD would augment acute exercise-related muscle damage in this population. However, it is also worth noting that there were no significant differences observed between the most symptomatic and least symptomatic legs of PAD patients. Therefore, more research is needed to better understand the influence of ischemia on markers of acute muscle damage in PAD patients.
In addition to demand ischemia, the chronic skeletal muscle myopathies associated with PAD are also likely to contribute to an augmentation of acute exercise-related muscle damage. One of the proposed mechanisms for exercise-induced sarcomere dissolution in a healthy adult is “popping” of the contractile element (Morgan 1990). This is explained by a complete decoupling of the actin and myosin myofilaments during a lengthening contraction, leading to Z-band disruption (Friden and Lieber 1992; McHugh et al. 1999). Considering the chronic muscle atrophy associated with PAD, it is likely that the number of “popped” sarcomeres is higher at the same absolute workload in these patients compared to heathy controls, especially as the relative intensity at the same absolute workload is likely to be augmented in PAD patients. This is supported by evidence of increased sarcomere disorganization (including Z-band disorganization) at rest in the affected muscles of PAD patients (Makitie and Teravainen 1977; Pipinos et al. 2007).
Lastly, it is notable that neither total exercise time, peak workload nor RPE were significantly different between the most and least symptomatic legs of PAD patients or between the most symptomatic legs of PAD patients and healthy controls, despite significant differences in the pain response. This contrast could be related to an increased familiarity with exercise-related muscle pain in PAD patients, allowing many (6/8) of the patients in this cohort to complete the entire plantar flexion protocol with their most symptommatic leg (Table 3). Future studies may consider extending the workload through fatigue or failure in all subjects to determine if these observations (augmented muscle-specific diffusivity in PAD patients compared to healthy controls) extend to maximal exercise.
Implications
While it may not be surprising that PAD patients express elevated markers of acute exercise-related muscle damage, this information has important implications for the mechanisms of accelerated muscle myopathies in PAD patients, as well as optimizing exercise prescription in this population. Specifically, our data would suggest that PAD patients are more susceptible to exercise-related muscle damage compared to healthy controls. While regular eccentric exercise is known to elicit improvements in muscle strength and hypertrophy in the absence of muscle pathology (Douglas et al. 2017), this increased exposure to skeletal muscle damage should be investigated further within the context of PAD patients. More research is needed to understand the influence of acute exercise on muscle function in PAD.
Limitations
The ASL method of measuring skeletal muscle perfusion is subject to a “low-flow” limitation. That is, this technique requires an increase in perfusion to detect a measurable value. This means that baseline perfusion values may have been different between groups. Despite this, pseudodiffusion would still not account for the observed group by time interactions, as perfusion was included as a covariate in the primary statistical analyses. Therefore, we remain confident that this had very little, if any effect on our final outcomes. We should also note that the exercise modality used in this study was not relative to each participant’s functional capacity, but rather, was based on absolute workloads. Because of this, the relative intensities (% of maximum work) were likely much greater in the PAD group. While this does not detract from our current findings, differences in relative exercise intensity may help explain any differences in DTI markers of acute muscle damage. This would be a valuable focus for future research. Lastly, the present study only focuses on acute responses to a single bout of plantar flexion exercise. It does not provide insight into the chronic adaptations to exercise training or chronic walking protocols for PAD patients. Understanding how regular exercise training influences these acute muscular responses would have significant implications for treatment and rehabilitation.
Future directions
As noted above, the present study is limited to describing the acute muscular responses to exercise. Future investigations may consider performing follow-up studies at 24 and 48 h, during which time delayed onset muscle soreness (DOMS) typically peaks (Gleeson et al. 1995). Likewise, future studies may consider testing the effects of chronic exercise training on these acute muscular responses to exercise. It would also be valuable to know which muscle fibers, fast glycolytic (Type IIx) or slow oxidative (Type I), are more susceptible to acute muscle damage in these patients. DTI could potentially be used to segment muscle fibers on the basis of fiber diameter (smallest diameter = Type I and largest diameter = Type IIx), and the exercise-related changes in diffusivity and geometry could be compared between these fiber types.
Conclusions
In conclusion, our data suggest that markers of acute exercise-related muscle damage are augmented in PAD patients compared to healthy controls. This is driven by a greater increase in the mean diffusivity of the active skeletal muscle in PAD patients, without a reciprocal decrease in fractional anisotropy. Future studies should evaluate the effects of chronic exercise training on acute skeletal muscle response to exercise in the affected muscles of PAD patients.
Acknowledgements
The authors would like to thank all of the clinical staff at the Penn State Hershey Heart and Vascular Institute, as well as Kris Gray and Jen Stoner for their technical and administrative support.
Funding
This project was supported by the NIH P01 HL134609 (Sinoway) and UL1 TR002014 (Sinoway).
Abbreviations
- ABI
Ankle brachial index
- CON
Control group
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- HR
Heart rate
- MD
Mean diffusivity
- MG
Medial gastrocnemius
- MRI
Magnetic resonance imaging
- PAD
Peripheral arterial disease
- TA
Tibialis anterior
- λ 1–3
Eigenvalues 1, 2, and 3
Footnotes
Conflict of interest No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval This study was approved by the Penn State Hershey/Penn State College of Medicine Institutional Review Board (#5331).
Consent to participate All participants provided written informed consent for participation in this study.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to data sharing policies of the host institution. However, data may be made available by the corresponding author upon reasonable request, and with the permission of the Pennsylvania State University.
References
- AlGhatrif M, Zane A, Oberdier M, Canepa M, Studenski S, Simonsick E, Spencer RG, Fishbein K, Reiter D, Lakatta EG, McDermott MM, Ferrucci L (2017) Lower mitochondrial energy production of the thigh muscles in patients with low-normal ankle-brachial index. J Am Heart Assoc. 10.1161/JAHA.117.006604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL (2003) Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13(1):40–47. 10.1034/j.1600-0838.2003.00299.x [DOI] [PubMed] [Google Scholar]
- Beaton LJ, Tarnopolsky MA, Phillips SM (2002) Variability in estimating eccentric contraction-induced muscle damage and inflammation in humans. Can J Appl Physiol 27(5):516–526 [DOI] [PubMed] [Google Scholar]
- Becker RA, Cluff K, Duraisamy N, Casale GP, Pipinos II (2017) Analysis of ischemic muscle in patients with peripheral artery disease using X-ray spectroscopy. J Surg Res 220:79–87. 10.1016/j.jss.2017.06.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belavy DL, Gast U, Felsenberg D (2017) Exercise and transversus abdominis muscle atrophy after 60-d bed rest. Med Sci Sports Exerc 49(2):238–246. 10.1249/MSS.0000000000001096 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135(10):e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat HK, Hiatt WR, Hoppel CL, Brass EP (1999) Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 99(6):807–812 [DOI] [PubMed] [Google Scholar]
- Bryant ND, Li K, Does MD, Barnes S, Gochberg DF, Yankeelov TE, Park JH, Damon BM (2014) Multi-parametric MRI characterization of inflammation in murine skeletal muscle. NMR Biomed 27(6):716–725. 10.1002/nbm.3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DG, Lamb K, Nicholas C, Twist C (2014) Effects of exercise-induced muscle damage on resting metabolic rate, sub-maximal running and post-exercise oxygen consumption. Eur J Sport Sci 14(4):337–344. 10.1080/17461391.2013.783628 [DOI] [PubMed] [Google Scholar]
- Chen TC, Chen HL, Liu YC, Nosaka K (2014) Eccentric exercise-induced muscle damage of pre-adolescent and adolescent boys in comparison to young men. Eur J Appl Physiol 114(6):1183–1195. 10.1007/s00421-014-2848-3 [DOI] [PubMed] [Google Scholar]
- Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ (2016) One week of bed rest leads to substantial muscle atrophy and induces wholebody insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 65(10):2862–2875. 10.2337/db15-1661 [DOI] [PubMed] [Google Scholar]
- Distefano G, Standley RA, Zhang X, Carnero EA, Yi F, Cornnell HH, Coen PM (2018) Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle 9(2):279–294. 10.1002/jcsm.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J, Pearson S, Ross A, McGuigan M (2017) Chronic adaptations to eccentric training: a systematic review. Sports Med 47(5):917–941. 10.1007/s40279-016-0628-4 [DOI] [PubMed] [Google Scholar]
- Dwyer D, Browning J, Weinstein S (1999) The reliability of muscle biopsies taken from vastus lateralis. J Sci Med Sport 2(4):333–340 [DOI] [PubMed] [Google Scholar]
- Englund EK, Langham MC, Li C, Rodgers ZB, Floyd TF, Mohler ER, Wehrli FW (2013) Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. J Cardiovasc Magn Reson 15:70. 10.1186/1532-429X-15-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito A, Campana L, Palmisano A, De Cobelli F, Canu T, Santarella F, Colantoni C, Monno A, Vezzoli M, Pezzetti G, Manfredi AA, Rovere-Querini P, Del Maschio A (2013) Magnetic resonance imaging at 7T reveals common events in age-related sarcopenia and in the homeostatic response to muscle sterile injury. PLoS ONE 8(3):e59308. 10.1371/journal.pone.0059308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan RH, Does MD (2008) Compartmental relaxation and diffusion tensor imaging measurements in vivo in lambda-carrageenan-induced edema in rat skeletal muscle. NMR Biomed 21(6):566–573. 10.1002/nbm.1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191 [DOI] [PubMed] [Google Scholar]
- Fortunato AK, Pontes WM, De Souza DMS, Prazeres JSF, Marcucci-Barbosa LS, Santos JMM, Veira ELM, Bearzoti E, Pinto KMC, Talvani A, Da Silva AN (2018) Strength training session induces important changes on physiological, immunological, and inflammatory biomarkers. J Immunol Res 2018:9675216. 10.1155/2018/9675216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden J, Lieber RL (1992) Structural and mechanical basis of exercise-induced muscle injury. Med Sci Sports Exerc 24(5):521–530 [PubMed] [Google Scholar]
- Gardner AW, Killewich LA (2001) Lack of functional benefits following infrainguinal bypass in peripheral arterial occlusive disease patients. Vasc Med 6(1):9–14 [PubMed] [Google Scholar]
- Gardner AW, Skinner JS, Cantwell BW, Smith LK (1992) Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc 24(2):163–170 [PubMed] [Google Scholar]
- Gleeson M, Almey J, Brooks S, Cave R, Lewis A, Griffiths H (1995) Haematological and acute-phase responses associated with delayed-onset muscle soreness in humans. Eur J Appl Physiol Occup Physiol 71(2–3):137–142. 10.1007/BF00854970 [DOI] [PubMed] [Google Scholar]
- Gratl A, Frese J, Speichinger F, Pesta D, Frech A, Omran S, Greiner A (2020) Regeneration of mitochondrial function in gastrocnemius muscle in peripheral arterial disease after successful revascularisation. Eur J Vasc Endovasc Surg 59(1):109–115. 10.1016/j.ejvs.2019.08.011 [DOI] [PubMed] [Google Scholar]
- Ha DM, Carpenter LC, Koutakis P, Swanson SA, Zhu Z, Hanna M, DeSpiegelaere HK, Pipinos II, Casale GP (2016) Transforming growth factor-beta 1 produced by vascular smooth muscle cells predicts fibrosis in the gastrocnemius of patients with peripheral artery disease. J Transl Med 14:39. 10.1186/s12967-016-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Lloyd PG, Yang HT, Terjung RL (2012) Exercise training and peripheral arterial disease. Compr Physiol 2(4):2933–3017. 10.1002/cphy.c110065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk AM, Drost MR, van Bochove GS, van Oosterhout MF, Nicolay K, Strijkers GJ (2006) DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magn Reson Med 56(2):272–281. 10.1002/mrm.20953 [DOI] [PubMed] [Google Scholar]
- Jajtner AR, Hoffman JR, Townsend JR, Beyer KS, Varanoske AN, Church DD, Oliveira LP, Herrlinger KA, Radom-Aizik S, Fukuda DH, Stout JR (2016) The effect of polyphenols on cytokine and granulocyte response to resistance exercise. Physiol Rep. 10.14814/phy2.13058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajtner AR, Townsend JR, Beyer KS, Varanoske AN, Church DD, Oliveira LP, Herrlinger KA, Radom-Aizik S, Fukuda DH, Stout JR, Hoffman JR (2018) Resistance exercise selectively mobilizes monocyte subsets: role of polyphenols. Med Sci Sports Exerc 50(11):2231–2241. 10.1249/MSS.0000000000001703 [DOI] [PubMed] [Google Scholar]
- Karampinos DC, King KF, Sutton BP, Georgiadis JG (2009) Myofiber ellipticity as an explanation for transverse asymmetry of skeletal muscle diffusion MRI in vivo signal. Ann Biomed Eng 37(12):2532–2546. 10.1007/s10439-009-9783-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161(2):401–407. 10.1148/radiology.161.2.3763909 [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Wang Y, Lv Y, Ma L (2016) Study on lumbosacral nerve root compression using DTI. Biomed Rep 5(3):353–356. 10.3892/br.2016.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard Pedersen B, Baekgaard N, Quistorff B (2017) Mitochondrial dysfunction in calf muscles of patients with combined peripheral arterial disease and diabetes type 2. Int Angiol 36(5):482–495. 10.23736/S0392-9590.17.03824-X [DOI] [PubMed] [Google Scholar]
- Makitie J, Teravainen H (1977) Histochemical changes in striated muscle in patients with intermittent claudication. Arch Pathol Lab Med 101(12):658–663 [PubMed] [Google Scholar]
- McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L (2004) Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc 52(3):405–410. 10.1111/j.1532-5415.2004.52113.x [DOI] [PubMed] [Google Scholar]
- McHugh MP, Connolly DA, Eston RG, Gleim GW (1999) Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med 27(3):157–170. 10.2165/00007256-199927030-00002 [DOI] [PubMed] [Google Scholar]
- McMillan AB, Shi D, Pratt SJP, Lovering RM (2011) Diffusion tensor MRI to assess damage in healthy and dystrophic skeletal muscle after lengthening contractions. J Biomed Biotechnol 2011:970726. 10.1155/2011/970726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CH, Pauly JM, Macovski A, Nishimura DG (1990) Simultaneous spatial and spectral selective excitation. Magn Reson Med 15(2):287–304 [DOI] [PubMed] [Google Scholar]
- Mitchell RG, Duscha BD, Robbins JL, Redfern SI, Chung J, Bensimhon DR, Kraus WE, Hiatt WR, Regensteiner JG, Annex BH (2007) Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med 12(4):285–290. 10.1177/1358863X07084858 [DOI] [PubMed] [Google Scholar]
- Morgan DL (1990) New insights into the behavior of muscle during active lengthening. Biophys J 57(2):209–221. 10.1016/S0006-3495(90)82524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrow JR, Brizendine JT, Djire B, Young HJ, Rathbun S, Nilsson KR Jr, McCully KK (2019) Near infrared spectroscopy-guided exercise training for claudication in peripheral arterial disease. Eur J Prev Cardiol 26(5):471–480. 10.1177/2047487318795192 [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Austin MD, Brown VA (2005) Immune response to a 30-minute walk. Med Sci Sports Exerc 37(1):57–62. 10.1249/01.mss.0000149808.38194.21 [DOI] [PubMed] [Google Scholar]
- Oudeman J, Nederveen AJ, Strijkers GJ, Maas M, Luijten PR, Froeling M (2016) Techniques and applications of skeletal muscle diffusion tensor imaging: a review. J Magn Reson Imaging 43(4):773–788. 10.1002/jmri.25016 [DOI] [PubMed] [Google Scholar]
- Parmenter BJ, Dieberg G, Smart NA (2015) Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med 45(2):231–244. 10.1007/s40279-014-0261-z [DOI] [PubMed] [Google Scholar]
- Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN (2003) Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg 38(4):827–832 [DOI] [PubMed] [Google Scholar]
- Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL (2007) The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg 41(6):481–489. 10.1177/1538574407311106 [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Wolfel EE, Brass EP, Carry MR, Ringel SP, Hargarten ME, Stamm ER, Hiatt WR (1993) Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation 87(2):413–421. 10.1161/01.cir.87.2.413 [DOI] [PubMed] [Google Scholar]
- Reimers CD, Harder T, Saxe H (1998) Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. J Neurol Sci 159(1):60–66. 10.1016/s0022-510x(98)00134-8 [DOI] [PubMed] [Google Scholar]
- Saxton JM, Donnelly AE (1996) Length-specific impairment of skeletal muscle contractile function after eccentric muscle actions in man. Clin Sci (lond) 90(2):119–125. 10.1042/cs0900119 [DOI] [PubMed] [Google Scholar]
- Schenk P, Siebert T, Hiepe P, Gullmar D, Reichenbach JR, Wick C, Blickhan R, Bol M (2013) Determination of three-dimensional muscle architectures: validation of the DTI-based fiber tractography method by manual digitization. J Anat 223(1):61–68. 10.1111/joa.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MN, Hasenkamp RM, Pipinos II, Johanning JM, Stergiou N, DeSpiegelaere HK, Chien JH, Myers SA (2017) Muscle strength and control characteristics are altered by peripheral artery disease. J Vasc Surg 66(1):178–186 e112. 10.1016/j.jvs.2017.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CA, Ryan TE, Lin CT, Inigo MMR, Green TD, Brault JJ, Spangenburg EE, McClung JM (2017) Diminished force production and mitochondrial respiratory deficits are strain-dependent myopathies of subacute limb ischemia. J Vasc Surg 65(5):1504–1514.e1511. 10.1016/j.jvs.2016.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J, Santulli G (2018) Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 275:379–381. 10.1016/j.atherosclerosis.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signolet I, Abraham P, Chupin S, Ammi M, Gueguen N, Letournel F, Picquet J, Baufreton C, Daligault M, Procaccio V, Reynier P, Henni S (2018) Mitochondrial complex I defect resulting from exercise-induced lower limb ischemia in patients with peripheral arterial disease. J Appl Physiol (1985) 125(3):938–946. 10.1152/japplphysiol.00059.2018 [DOI] [PubMed] [Google Scholar]
- Stavres J, Sica CT, Blaha C, Herr M, Wang J, Pai S, Cauffman A, Vesek J, Yang QX, Sinoway LI (2019) The exercise pressor reflex and active O2 transport in peripheral arterial disease. Physiol Rep 7(20):e14243. 10.14814/phy2.14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavres J, Wang J, Sica CT, Blaha C, Herr M, Pai S, Cauffman A, Vesek J, Luck JC, Yang QX, Sinoway LI (2020) Acute muscle damage is augmented after exercise in PAD patients: evidence from diffusion tensor imaging: 2883 board #344May 29 10:30 AM-12:00 PM. Med Sci Sports Exerc 52(7S):802. 10.1249/01.mss.0000683972.21824.28 [DOI] [Google Scholar]
- Williamson A, Hoggart B (2005) Pain: a review of three commonly used pain rating scales. J Clin Nurs 14(7):798–804. 10.1111/j.1365-2702.2005.01121.x [DOI] [PubMed] [Google Scholar]
- Yanagisawa O, Shimao D, Maruyama K, Nielsen M, Irie T, Niitsu M (2009) Diffusion-weighted magnetic resonance imaging of human skeletal muscles: gender-, age- and muscle-related differences in apparent diffusion coefficient. Magn Reson Imaging 27(1):69–78. 10.1016/j.mri.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Yanagisawa O, Kurihara T, Kobayashi N, Fukubayashi T (2011) Strenuous resistance exercise effects on magnetic resonance diffusion parameters and muscle-tendon function in human skeletal muscle. J Magn Reson Imaging 34(4):887–894. 10.1002/jmri.22668 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to data sharing policies of the host institution. However, data may be made available by the corresponding author upon reasonable request, and with the permission of the Pennsylvania State University.


