Abstract
Objectives:
Carcinosarcomas are highly aggressive gynecologic malignancies containing both carcinomatous and sarcomatous elements with heterogeneous HER2/neu expression and limited therapeutic options. We compared the efficacy of trastuzumab deruxtecan (DS-8201a), a novel HER2/neu-targeting antibody-drug conjugate (ADC) to an ADC isotype control (MAAA-9199) against primary uterine and ovarian carcinosarcomas in vitro and in vivo.
Methods:
Twelve primary carcinosarcoma (CS) cell lines were evaluated for HER2/neu surface expression by immunohistochemistry (IHC) and by flow cytometry, and gene amplification by fluorescence in situ hybridization (FISH) assays. The in vitro experiments included cytotoxicity and bystander killing effect assays on three cell lines of variable HER2/neu expression. In vivo activity was studied in a mouse CS xenograft model of 3+ HER2/neu uterine CS.
Results:
In vitro studies showed that DS-8201a was highly effective against uterine and ovarian CS cell lines demonstrating 3+ HER2/neu expression compared to MAAA-9199 control; there was no significant improvement in the 0 HER2/neu CS cell line. However, DS-8201a induced efficient bystander killing of 0 HER2/neu tumor cells when admixed with 3+ HER2/neu cells. In vivo studies confirmed that DS-8201a was more effective than MAAA-9199 in 3+ HER2/neu-expressing CS xenografts.
Conclusion:
DS-8201a may represent a novel and highly effective ADC against HER2/neu-expressing CS.
Keywords: Trastuzumab deruxtecan, DS-8201a, Uterine Carcinosarcoma, Ovarian Carcinosarcoma, HER2/neu, Antibody-drug conjugate
INTRODUCTION
Carcinosarcomas (CS), previously known as mixed malignant Müllerian tumors (MMMT), are rare but extremely aggressive gynecologic malignancies that can arise in the uterus, ovary, or cervix. They account for less than 6% of all gynecologic cancers [1,2]. Five-year survival rates of uterine and ovarian CS are significantly lower than their serous or carcinomatous counterparts [3–5]. Finding targeted therapies for CS has proven difficult, and given its low incidence, there is a paucity of randomized control trial evidence to guide adjuvant and salvage treatment.
Many CS have a high-grade serous carcinomatous component [6], a histology known to have HER2/neu protein overexpression from studies of uterine serous carcinomas [7,8]. Given this, it has been posited that HER2/neu may serve as an attractive target for anticancer therapy for CS [9]. HER2/neu gene amplification and/or protein overexpression have been found in a variable proportion of uterine CS, ranging between 6% and 56% in the literature [2,10]. One recent study reported HER2/neu gene amplification and/or protein overexpression in 16% of uterine CS and 13% of ovarian CS [2]. The effectiveness of trastuzumab against HER2/neu positive uterine and ovarian CS has been demonstrated in vitro showing high-level antibody-dependent cellular cytotoxicity [7], and improves progression-free survival (PFS) and overall survival (OS) when added to chemotherapy in the treatment of advanced/recurrent HER2/neu-positive uterine serous carcinoma patients [11]. With the exception of an abstract presentation [12], to our knowledge there is no published peer reviewed clinical data on HER2/neu-targeting therapies in uterine and ovarian CS. Notably, an NRG Oncology study currently recruiting is looking to evaluate the addition of trastuzumab to usual chemotherapy in chemo-naïve patients with HER2 positive endometrial carcinosarcoma (NCT05256225).
Antibody-drug conjugates (ADCs) are monoclonal antibodies that are covalently linked to small-molecule cytotoxic drugs and further improve the cytotoxic effects of monoclonal antibody therapies. ADCs also confer higher tumor selectivity, leading to better tolerability and decreased systemic exposure in patients. In uterine and ovarian CS overexpressing HER2/neu, the ADC trastuzumab-emtansine (T-DM1) has shown improved inhibition of cancer cell proliferation in vitro and reduced tumor formation in CS xenografts compared to trastuzumab alone [13]. Subsequent in vitro and in vivo studies of a duocarmycin-based ADC, trastuzumab duocarmazine (SYD985), demonstrated additional effectivity against CS that had low or heterogeneous HER2/neu expression, with improved effectivity compared to T-DM1 [14]. Targeted ADC therapies are a promising frontier in treatment of ovarian and uterine CS, however those currently available or in Phase I trials have been found to have several dose-limiting toxicities including thrombocytopenia, neutropenia, and neuropathy, thought to be due to unbound cytotoxic drugs in circulation [15].
Trastuzumab deruxtecan (DS-8201a) is a novel HER2-directed ADC. The payload of T-DXd, DXd, binds to topoisomerase I and DNA complexes, leading to double-stranded DNA damage and cell apoptosis. DS-8201a has a tetrapeptide-based linker (maleimide glycine-phenylalanine-glycine (GGFG) peptide) that ensures stability in the patient’s systemic circulation and limits systemic toxicity [16]. This tetrapeptide linker is designed to be cleaved by lysosomal enzymes such as cathepsins B and L, which are overexpressed in tumor cells [17]. The cleavable linker and physicochemical properties of the payload allows the released payload to permeate through the cell membrane of target cells to neighboring cells, providing potential for bystander killing and improved clinical efficacy [18]. Preclinical studies demonstrated that DS-8201a has improved potency compared to T-DM1, and maintains anti-tumor impact on tumors with low HER2 expression [16]. DS-8201a is now approved by the United States Federal Drug Administration (FDA) for use in patients with metastatic HER2-positive or HER2-low breast cancer, as well as HER2-mutant non-small cell lung cancer, and metastatic gastric or gastroesophageal junction adenocarcinoma [19–21].
The objective of this study was to compare the preclinical efficacy of the novel ADC, trastuzumab deruxtecan (DS-8201a), to the ADC isotype control MAAA-9199 against primary uterine and ovarian CS cell lines with different HER2/neu expression status. We report preclinical data that DS-8201a is significantly more potent than the ADC isotype control against HER2/neu-positive CS cell lines in vitro and in vivo experiments. Our results also demonstrate that DS-8201a was able to induce a significant bystander killing effect against tumor cells with low/negligible HER2/neu expression when admixed with cells with strong membranous HER2/neu expression, suggesting potential clinical activity against not only the HER2/neu positive epithelial carcinomatous component of CS but also to the HER2/neu negative sarcomatous components of these cancers.
MATERIALS AND METHODS
Establishment of Cell Lines
Study approval was obtained from the Institutional Review Board at Yale University, and all patients signed consent prior to tissue collection according to the institutional guidelines. Twelve primary CS cell lines were established from chemotherapy-naïve patients at the time of primary staging surgery after sterile processing of fresh tumor biopsy samples, as previously described [6,7]. Cell sample characteristics and patient demographics are described in Table 1. Tumors were staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system. All revived cells were used within fifty passages and cultured for less than six months. Primary uterine and ovarian cell lines with limited passages were used in the experiments listed below and corresponding cell blocks were analyzed for HER2/neu surface expression by immunohistochemistry (IHC) and by flow cytometry, and for HER2/neu gene amplification by fluorescent in situ hybridization (FISH).
Table 1.
Patient characteristics and HER2/Neu expression in tumors.
| Histology |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (yrs) | Race | FIGOa stage | Primary tumor site | Carcinoma component | Sarcoma component | IHC cell block | FISH | Flow MFI |
|
| |||||||||
| SARARK-1 | 70 | Black | IC | Uterus | Endometrioid + clear cell | Homologous | 0 | Not amplified | 17.2 |
| SARARK-3 | 74 | White | IIIC | Ovary | Serous | Heterologous; CDRS | 0 | Not tested | 22.8 |
| SARARK-4 | 77 | White | IIIC | Ovary | Serous | Heterologous; CDRS | 0 | Not amplified | 32.36 |
| SARARK-6 | 78 | White | IV | Ovary | Serous | Homologous | 3+ | Amplified | 508.91 |
| SARARK-7 | 55 | White | IV | Ovary | Serous + clear cell | Heterologous; CDRS | 1+ | Not amplified | 38.35 |
| SARARK-8 | 46 | White | IIB | Uterus | Undifferentiated | Homologous | 0 | Not amplified | 12.06 |
| SARARK-9 | 66 | White | IIIC2 | Uterus | Serous | Homologous | 3+ | Amplified | 300.74 |
| SARARK-10 | 63 | White | IVB | Uterus | Serous | Homologous | 1+ | Not tested | 23.05 |
| SARARK-11 | 67 | White | IIIC1 | Uterus | Endometroid | Heterologous; CDRS | 0 | Not tested | 8.24 |
| SARARK-12 | 39 | White | IVB | Uterus | Serous | Homologous | 0 | Not tested | 7.94 |
| SARARK-13 | 71 | White | IVB | Uterus | Serous | Heterologous; CDRS | 0 | Not tested | 13.77 |
| SARARK-14 | 59 | Black | IVB | Uterus | Serous + endometrioid | Homologous | 1+ | Not tested | 6.99 |
Abbreviations: CDR, chondroid; CDRS, chondrosarcoma; ESS, endometrial stromal sarcoma; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; MFI, mean fluorescence intensity
FIGO, International Federation of Gynecology and Obstetrics
DS-8201a and MAAA-9199
DS-8201a was donated by the Daiichi Sankyo Co., Ltd (Tokyo, Japan). DS-8201a is a next-generation HER2-targeting ADC that has been previously described [16]. The average drug-to-antibody ratio (DAR) of DS-8201a is 7 to 8, which is higher than that of most ADCs, including T-DM1 (DAR: 3.5) [22] and SYD985 (DAR: 2.8) [23,24]. This high DAR enables the delivery of greater payload concentrations to cancer cells, providing the rationale for a more potent antitumor activity than other ADCs [17]; additionally, the payload itself, DXd, has 10-fold potency compared to irinotecan as a topoisomerase I inhibitor [25]. MAAA-9199 is an isotype control non-binding ADC, also donated by Daiichi Sankyo Co., Ltd.
Immunostaining of Cell Blocks of Primary Carcinosarcoma
Cell blocks from all 12 CS cell lines were reviewed by a gynecologic surgical pathologist to confirm the presence of CS cells; the cell lines and patients are described in Table 1. HER2/neu immunohistochemical staining was performed on paraffin-embedded 5 μm sections of cell blocks after deparaffinization and rehydration, using the c-erbB-2 antibody (Thermo Fisher Scientific, Fremont, CA) at 1:800 dilution. HER2/neu staining was scored using the recently proposed endometrial serous carcinoma-specific criteria [26]. Appropriate positive and negative controls were used for each case.
Fluorescent In Situ Hybridization (FISH) of Cell Blocks from Primary Carcinosarcoma
FISH analysis was performed using the PathVysion HER-2 DNA FISH Kit (Abbott Molecular Inc.) according to the manufacturer’s instructions. Cell block sections of 5 μm were deparaffinized and rehydrated, followed by acid pretreatment and proteinase K digestion. A probe mix containing an orange probe directed against the HER2/neu gene (Vysis LSI HER-2) and a green probe directed against the pericentromeric region of chromosome 17 (Vysis CEP 17) were added and specimens were denatured for 5 minutes at 73°C. Slides were then incubated overnight in a humidified chamber at 37°C and washed the day after when a fluorescence mounting medium, containing 4, 6-diamidino-2-phenylindole (DAPI), was applied. Fluorescent signals in at least 30 nonoverlapping interphase nuclei with intact morphology were scored using a Zeiss Axioplan 2 microscope (Carl Zeiss Meditec, Inc.) with a 100× planar objective, using a triple band-pass filter that permits simultaneous blue, green, and red colors. Tumor cells were scored for the number of orange (HER2/neu) and green (chromosome 17) signals. A case was scored as amplified when the ratio of the number of fluorescent signals of HER2/neu to chromosome 17 was ≥2.0.
Cell Viability Assay
CS cell lines were plated at log phase of growth in 6-well tissue culture plates at a density of 70,000–80,000 cells in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Mediatech, Manassas, VA), and 0.3% fungizone (Life Technologies, Carlsbad, CA). Cells were incubated at 37°C, 5% CO2. After 24 hours of incubation, cells were treated with DS-8201a and ADC isotype control (MAAA-9199). DS-8201a and MAAA-9199 were used at scalar concentrations of 0.005 μg/mL, 0.05 μg/mL, 0.2 μg/mL, 0.5 μg/mL, and 2.0 μg/mL. Three days after drug treatment, cells were harvested in their entirety, centrifuged, and stained with propidium iodide (2μl of 500 μg/ml stock solution in phosphate-buffered saline (PBS)). Analysis was performed using a flow cytometry-based assay to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. Half-maximal inhibitory concentration, IC50, was then calculated for each cell line. A minimum of two independent experiments per cell line were performed.
Bystander killing
Briefly, HER2/neu negative uterine serous carcinoma (USC) cells (i.e., USC ARK-4) that were stably transfected with a Green Fluorescent Protein (GFP) plasmid (pCDH-CMV-MCSEF1-copGFP, donated by Dr. Simona Colla, MD Anderson Cancer Center, Houston, Texas) and 3+ HER2/neu positive CS cells (i.e., SARARK-9) were mixed in a 1:1 ratio (i.e., 20,000 cells/well of each cell type) and plated in 6-well plates (3 mL/well). After an overnight incubation, DS-8201a or ADC isotype control (MAAA-9199) at a concentration of 0.05 μg/mL or vehicle were added. After 72-hour incubation, cells were harvested in their entirety, centrifuged, and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS) to facilitate the identification of dead cells. Analysis of cell viability after treatment was performed using a flow cytometry-based assay, which allowed us to quantify the fluorescently tagged viable USC ARK-4 cells versus the non-tagged SARARK-9 cells. Using the cell viability data acquired through propidium iodide staining, we were then able to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls for both SARARK-9 and USC ARK-4 cell lines. A minimum of two independent experiments were performed.
Tests for Antibody Dependent Cell Cytotoxicity (ADCC)
Standard 4-hour chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque-separated PBLs from several healthy donors in combination with trastuzumab, DS-8201a or ADC isotype control (MAAA-9199) against 51Cr-labeled primary CS target cell lines at effector to target ratios (E:T) of 20:1 and 40:1. The release of 51Cr from target cells was measured as evidence of tumor cell lysis after exposure of the tumor cells to 2.5 μg/ml of trastuzumab, rituximab (anti-CD20), DS-8201a or ADC isotype control. As a positive control condition, 0.1% sodium dodecyl sulfate (SDS) was used to achieve complete lysis of target cells. Chimeric anti-CD20 mAb rituximab 2.5 μg/ml was used as the negative control for trastuzumab, DS-8201a or ADC isotype control in all bioassays. The percentage cytotoxicity of trastuzumab or DS-8201a was calculated by the following formula: % cytotoxicity = 100 × (E-S)/(T-S), where E is the experimental release, S is the spontaneous release by target cells, T is the maximum release by target cells lysed with 0.1% SDS.
In vivo treatment
The in vivo antitumor activity of DS-8201a and MAAA-9199 (ADC isotype control) was tested in xenograft models with 3+ HER2/neu expression established from a primary uterine CS cell line (SARARK-9). Specimen collection and all animal experiments were approved by the institutional ethical committee (HIC) and Institutional Animal Care and Use Committee (IACUC) of Yale University. Briefly, six- to eight-week-old CB-17/SCID mice were given a single subcutaneous injection of 8 × 106 3+ HER2/neu uterine CS cells (SARARK-9) in approximately 200 μL of a 1:1 solution of sterile PBS containing cells and Matrigel (Corning Life Sciences). The size of the implanted tumor was checked 2 to 3 times per week using Vernier calipers. When the implanted tissue was palpable, the volume was calculated as length × (width)2/2. Once the tumor volume was approximately 0.2 cm3, the mice were randomized into three treatment groups (n=5): those treated with DS-8201a (4 mg/kg), ADC isotype control (MAAA-9199) (4 mg/kg), and PBS. Drug dosages were chosen according to previous publications demonstrating therapeutic activity in the dose range of 3 to 10 mg/kg [16]. All treatment drugs were given as a single retro-orbital injection. Mice were observed for overall survival (OS) as the primary outcome measure. Tumor measurements were recorded twice weekly. Mice were sacrificed if tumor volume reached 1.0 cm3. At the conclusion of the study on day 52, the surviving mice were censored. Animal care and euthanasia were carried out according to the rules and regulations as set forth by the IACUC.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, Inc. San Diego, CA). Dose response curves for different CS cell lines after exposure to DS-8201a and ADC isotope control were evaluated by one-way analysis of variance (ANOVA). OS data were analyzed and plotted using the Kaplan–Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered statistically significant at p < 0.05.
RESULTS
HER2/neu expression in primary Carcinosarcoma cell lines
We evaluated HER2/neu gene amplification by FISH, and surface HER2/neu protein expression by IHC and flow cytometry in twelve primary CS cell lines (8 uterine, 4 ovarian). High levels (3+) of HER2/neu protein expression by IHC were detected in two out of twelve (16.7%) of the CS cell lines (Table 1). Three cell lines (25%) had low (1+), and seven (58.3%) had negligible (0) HER2/neu expression on IHC. Both cell lines (SARARK-6 and SARARK-9) which overexpressed (3+) HER2/neu showed HER2/neu gene amplification by FISH. Among the uterine CS cell lines, 12.5% (1/8), 25% (2/8) and 62.5% (5/8) had 3+ HER2/neu, 1+/2+ HER2/neu and 0 HER2/neu respectively by IHC. Among the ovarian CS cell lines, 25% (1/4), 25% (1/4) and 50% (2/4) had 3+ HER2/neu, 1+ HER2/neu and 0 HER2/neu, respectively by IHC. The two cell lines (SARARK-6 and SARARK-9) that overexpressed (3+) HER2/neu and had positive HER2/neu gene amplification by FISH were verified to have very elevated (508.91 and 300.74) mean HER2/neu fluorescence intensities by flow cytometry (Table 1). Based on these results, we selected one primary ovarian CS cell line (SARARK-6) and one primary uterine CS cell line (SARARK-9) which overexpressed HER2/neu (3+), and one primary uterine CS cell line (SARARK-1) which was a non-expressor of HER2/neu for the additional in vitro and in vivo experiments described below.
Cell viability experiments with DS-8201a in vitro
We exposed the selected cell lines with different HER2/neu expression (Table 1) to scalar concentrations of DS-8201a and of MAAA-9199 for a total of 3 days. DS-8201 was significantly more potent in inducing cell death than ADC isotope control (MAAA-9199) in both CS cell lines overexpressing (3+) HER2/neu, (Figures 1, A and B). In the 3+ HER2/neu uterine CS cell line (SARARK-9, Figure 1A), there was a statistically significant difference in the mean IC50 values between DS-8201a and the isotope control ADC (MAAA-9199), 0.03 μM and 9.75 μM, respectively (p<0.0001). The same statistically significant difference was seen in the 3+ HER2/neu ovarian CS cell line (SARARK-6, Figure 1B), DS-8201a exhibited a mean IC50 of 0.03 μM while MAAA-9199 exhibited a mean IC50 of 9.12 μM (p<0.0001). As for the representative HER2/neu non-expressor cell line (SARARK-1, Figure 1C), DS-8201a exhibited a mean IC50 of 20.02 μM while MAAA-9199 showed a mean IC50 of 11.96 μM. This difference was not statistically significant (p=0.91).
Figure 1.
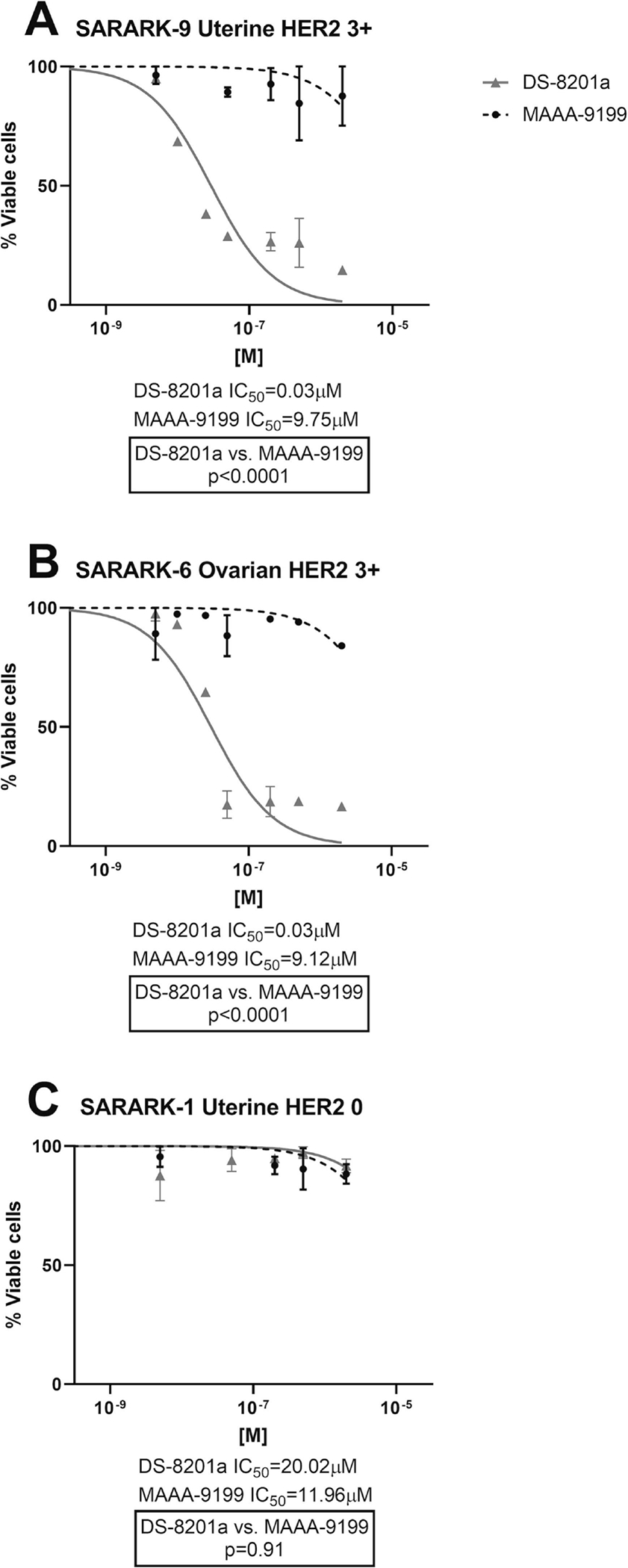
IC50 dose-response curves of DS-8201a and ADC isotype control (MAAA-9199) in: A) 3+ HER2/neu uterine CS SARARK-9; B) 3+ HER2/neu ovarian CS SARARK-6; C) HER2/neu 0 uterine CS SARARK-1.
Bystander killing in vitro
We then evaluated the ability of DS-8201a and MAAA-9199 to induce bystander cytotoxicity on nearby cancer cells with 0 HER2/neu expression (i.e., GFP-ARK-4) when admixed with 3+ HER2/neu uterine CS cells (SARARK-9) for 96 hours. When treated with DS-8201a, SARARK-9/ARK-4 co-cultures yielded a significant increase (i.e., 52%, p= 0.003) in the amount of bystander killing of 0 HER2/neu ARK-4 cells when compared to ARK-4 alone (Figure 2A). In contrast, minimal, non-significant bystander cytotoxicity was detected in 0 HER2/neu ARK-4 cells when SARARK-9/ARK-4 co-cultures were challenged with MAAA-9199 (i.e., 8 %, p = 0.189), (Figure 2B).
Figure 2.
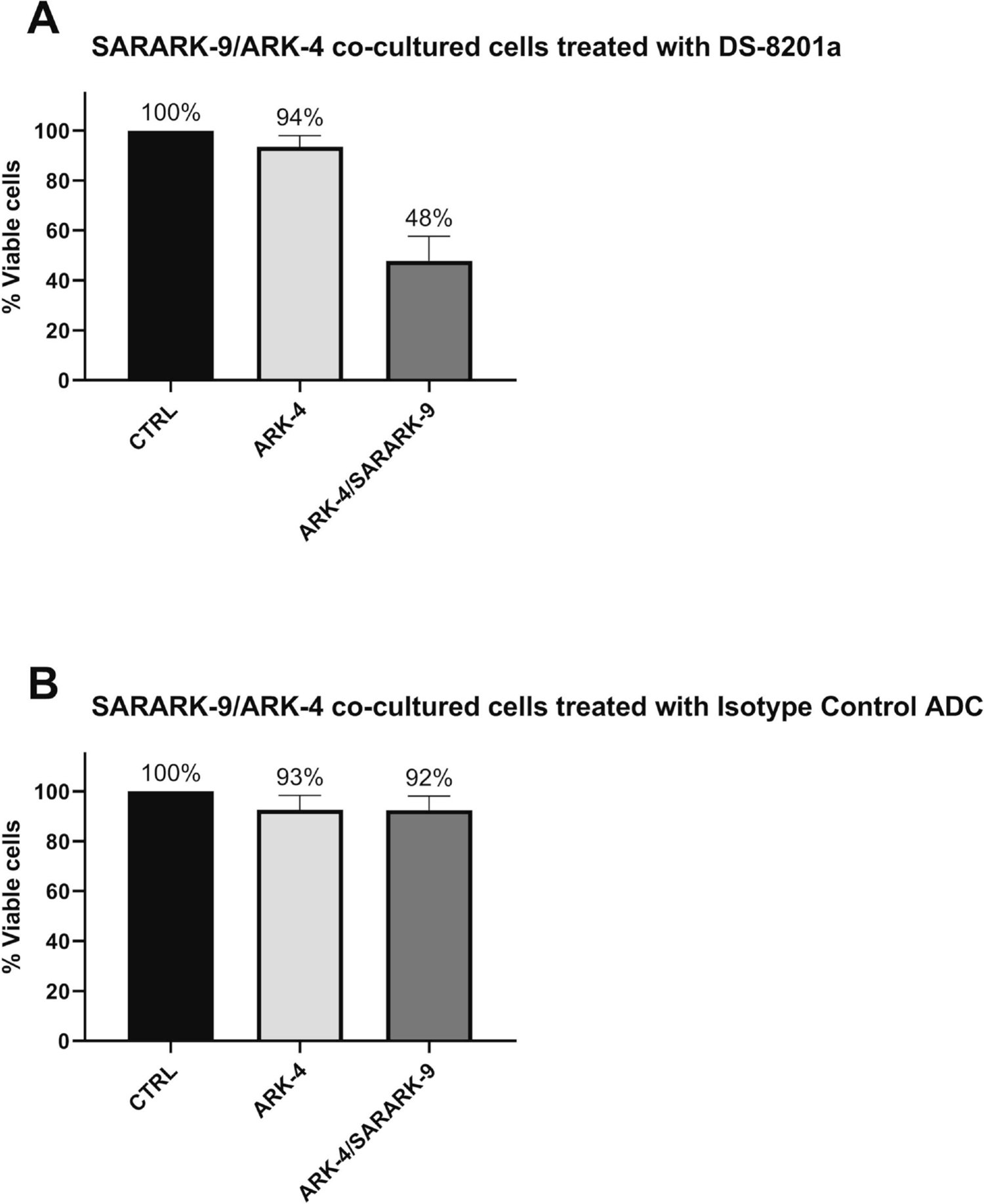
A) Cytotoxicity induced on HER2/neu 0 USC cells (ARK-4) and 3+ HER2/neu uterine CS SARARK-9 co-cultured with ARK-4 with 0.05μg/mL of DS-8201a vs. untreated controls. B) Cytotoxicity induced on HER2/neu 0 USC cells (ARK-4) and 3+ HER2/neu uterine CS SARARK-9 co-cultured with ARK-4 with 0.05μg/mL of MAAA-9199 (ADC isotype control) vs. untreated controls. No significant increase in the amount of killing of 0 HER2/neu USC cells was detected after treatment with ADC isotype control.
DS-8201a and trastuzumab mediated ADCC against HER2-positive primary carcinosarcoma
Two representative primary CS cell lines (i.e., HER2/neu score 0 and HER2/neu score 3+) were tested for their sensitivity to PBL-mediated cytotoxicity when challenged with heterologous PBLs collected from several healthy donors in standard 4-h 51Cr release assays. CS cell lines were consistently found to be resistant to PBL-mediated cytotoxicity when combined with PBLs and isotype control ADC MAAA-9199, as well as PBLs and Rituximab (2.5 μg/mL) at E:T ratios of 5:1 and 10:1 (Figure 3). We then investigated the sensitivity of CS cell lines to heterologous PBLs in the presence of trastuzumab and DS-8201a at 2.5 μg/mL (Figure 3). DS-8201a and trastuzumab were similarly effective in inducing strong ADCC against primary CS cell lines expressing HER2/neu at high levels (i.e., SARARK-9, Figure 3B) with mean cytotoxicity ± SEM = 56.57 ± 7.48% for DS-8201a and 69.18 ± 7.77% for trastuzumab. In contrast, low/negligible killing was observed after DS-8201a and trastuzumab exposure of HER2/neu low/negative CS cell line (i.e., SARARK-12, Figure 3A).
Figure 3.
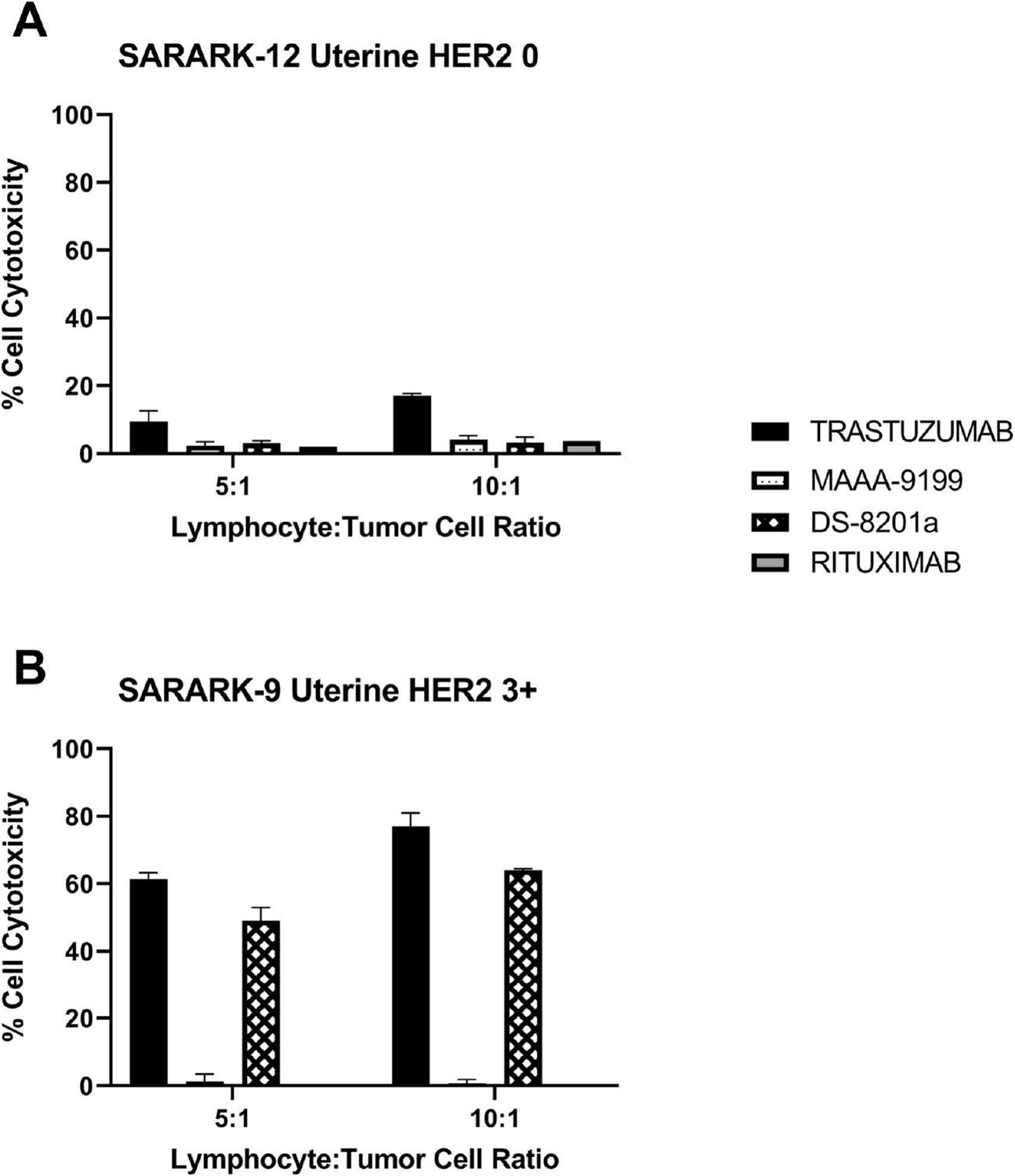
ADCC results of DS-8201a, trastuzumab, rituximab, and ADC isotype control MAAA-9199 in a representative A) uterine HER2 0 expressing cell line SARARK-12 and B) uterine HER2 3+ expressing cell line SARARK-9.
In vivo antitumor activity of DS-8201 versus MAAA-9199
The in vivo effects of DS-8201a were determined by establishing xenografts from primary CS cell lines with 3+ HER2/neu expression (SARARK-9). After the tumors had reached the goal size, animals were randomized into treatment groups and treated as described in the above Methods. Tumors were assessed and measured twice weekly, and mice were sacrificed if tumors became necrotic, reached a volume of 1.0 cm3, or mice appeared to be in poor health. Treatment with a single injection of DS-8201a showed remarkable inhibition of tumor growth in mice harboring xenografts with 3+ HER2/neu expression (Figure 4). On day 8, we detected a significant difference in tumor growth inhibition between mice treated with DS-8201a when compared to ADC isotope control and vehicle (PBS) control with mean tumor volumes of 0.271 cm3 versus 0.859 cm3 (p = 0.0011) and 0.804 cm3 (p = 0.0047), respectively. On day 11, where the DS-8201a mice continued to have significant tumor size reduction, the mice treated with ADC isotope control and the mice that received vehicle reached the cut-off tumor volume (1 cm3), 0.179 cm3 versus 1.166 cm3 (p <0.0001) and 1.151 cm3 (p <0.0001), correspondingly, (Figure 4A). The five DS-8201a-treated mice showed continuous reduction in mean volume to 0.023 cm3, (Figure 4B) at 39 days. From day 32 to day 45, 4 out of five mice (80%) had no measurable tumor. One mouse had a nadir tumor volume of 0.026cm3 (from starting volume of 0.493cm3) at day 24, and surpassed it’s starting volume at day 52 after only a single infusion. Accordingly, a significant survival advantage was seen in DS-8201a-treated xenografts, (Figure 5A). The median survival for MAAA-9199 was 22 days, and the median survival for the vehicle control was 18 days. The median survival for DS-8201a was not met at the conclusion of the study on day 52 as all 5 mice were still alive. The difference in OS curves between DS-8201a and controls was statistically significant (DS-8201a vs. MAAA-9199 p=0.003, and DS-8201a vs. Vehicle p=0.002). Notably, 5 out of 5 (100%) of the mice were alive, and 3 out of 5 (60%) were disease-free at 52 days after a single injection of DS-8201a at a dose of 4 mg/kg, (Figure 4A and B). Evaluation of weight loss is a common and straightforward relevant measure of dose-limiting toxicity in in vivo chemotherapeutic testing. Accordingly, the weight of the three mice cohorts were obtained twice a week. There was no significant difference (p=0.149 using 2-way ANOVA) in mean body weight change in xenografted animals treated with DS-8201 (mean weight change ± SEM: 1.01 ± 0.75) versus treated MAAA-9199 (−1.46% ± 0.9), or vehicle (mean weight loss 0.48% ± 2.25). None of the three groups had a mean weight loss of 10% or more, (Figure 5B).
Figure 4.
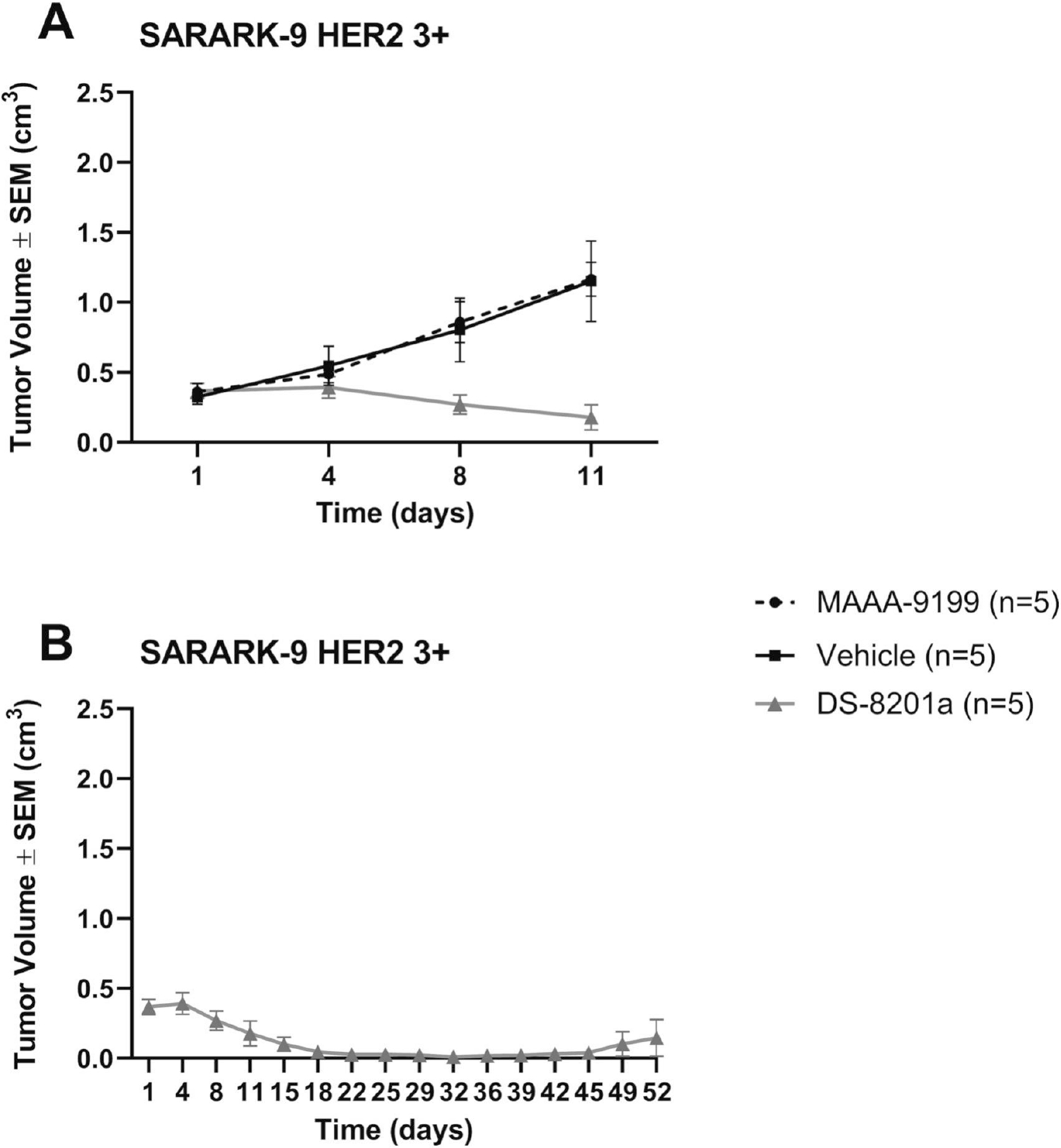
Antitumor activity in mice inoculated with CS xenograft tumor model of HER2/neu 3+ (SARARK-9) after treatment with DS-8201a compared with MAAA-9199 isotype control and vehicle control. A) DS-8201a demonstrates significant tumor growth inhibition compared to isotype and vehicle control. 5 mice contribute to means in all treatment arms at all timepoints except Vehicle has only 4 mice from day 4–11. B) DS-8201a treated mice demonstrate reduction in mean tumor volumes up to day 52.
Figure 5.
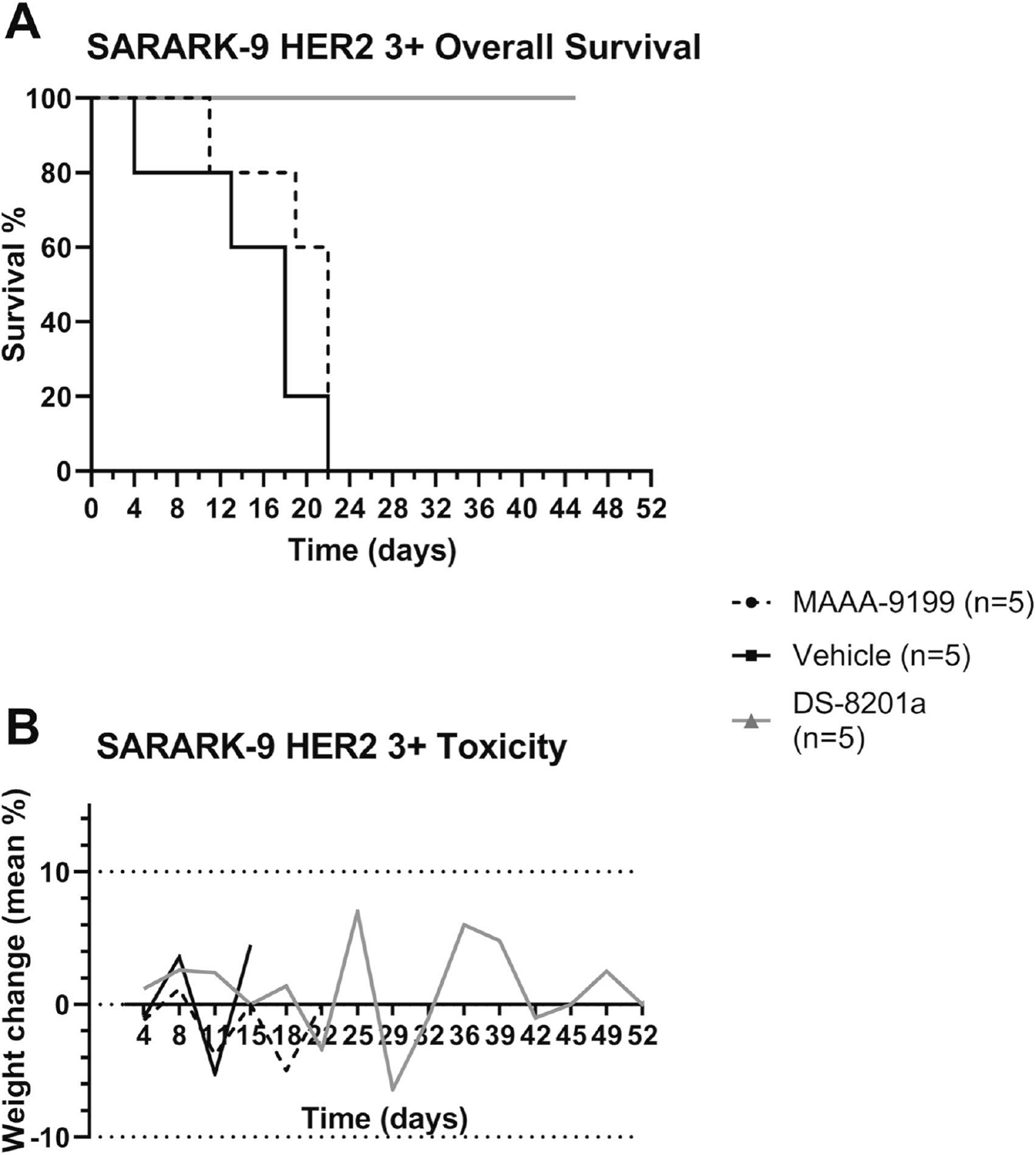
A) Overall survival in mice inoculated with CS xenograft tumor model of HER2/neu 3+ (SARARK-9) after treatment with DS-8201a compared with MAAA-9199 isotype control and vehicle control. Overall survival was significantly prolonged among the group treated with DS-8201a compared to other treatment groups. B) Xenograft mice weight changes by treatment group.
DISCUSSION
Our experiments showed that DS-8201a is a highly effective ADC against HER2/neu overexpressing uterine and ovarian CS cell lines. DS-8201a was significantly more potent than ADC isotype control (MAAA-9199) in both uterine and ovarian cell lines with HER2/neu overexpression (p<0.0001). DS-8201a also showed significant bystander cytotoxicity of low/negligible HER2/neu expressing cells when admixed with cells strongly overexpressing HER2/neu.
Mouse models harboring CS xenografts with 3+ HER2/neu expression confirmed the in vitro results of the high efficacy of DS-8201a. A single injection of DS-8201a was sufficient to demonstrate a significant difference in tumor growth inhibition compared to mice treated with ADC isotope control or vehicle as early as day 8. Even after 52 days, the DS-8201a-treated mice showed a dramatic reduction in mean volume (i.e., 0.023 cm3) when compared to control-treated mice. Importantly, three mice (60%) had a complete resolution of their tumors at the completion of the study. A preclinical study by Menderes et al. directly compared the antitumor activity of two other ADCs, T-DM1 and SYD985, in CS PDX mouse models [14]. Their findings demonstrated that SYD985 was the preferable ADC; it had a prolonged and superior impact on CS PDX mouse model tumor volumes as well as prolonged overall survival compared to T-DM1 on the same PDX mouse models. While inter-study comparison is fraught, DS-8201a seems to have similar to improved in vivo effectivity compared to T-DM1 and SYD985. For example, while Menderes et al. demonstrate that SYD985 showed tumor inhibition to day 29, we found that DS-8201a provided similar levels of tumor inhibition for 45 days in a similar CS PDX mouse model. Median OS appear to be similar for SYD985 and DS-8201a, both of which appear to have significant survival advantages compared to T-DM1 [13,14].
Collectively, our results on cytotoxic activity, bystander killing, and tumoricidal effects strongly suggest that DS-8201a may represent a highly effective novel therapeutic tool in HER2/neu-expressing CS. Unlike prior targeted ADC therapies, DS-8201a has the advantage of a cleavable linker and physicochemical properties of the payload that allows the released cytotoxic payload to permeate through the cell membrane of target cells to neighboring cells. This effect supports the idea that DS-8201a may not only be effective against CS cells with strong HER2/neu expression, but also against cells with moderate, low and negligible HER2/neu expression such as the sarcomatous cell components of CS, due to its untargeted release into the vicinity of HER2/neu high tumor cells. Of course, in HER2/neu low expressing cells, effectivity of DS-8201a may also be due to a direct on-target effect. Recently published data on the effectivity of DS-8201a in patients with previously treated HER2-low breast cancer confirms this concept.[27] Further studies are warranted in HER2-low expressing gynecologic malignancies.
Our study has a few important limitations. Due to the rarity of carcinosarcoma, we were only able to examine two cell lines with 3+ HER2/neu expression. Additionally, our in vivo experiments were only able to include cells from one uterine 3+ HER2/neu cell line, and our two HER2/neu 3+ cell lines were both from white patients. However, the inclusion of both uterine and ovarian 3+ HER2/neu cell lines in our in vitro experiments is a strength and increases the generalizability of our findings. We note that the rate of HER2/neu positivity in the collection of CS cell lines described here was only 16%. While the true incidence of HER2/neu overexpressing CS has not been defined, rates in our sample is consistent with other reports [2,10].
DS-8201a has been approved for HER2-positive or HER2-low recurrent breast cancer, HER2-mutant non-small cell lung cancer, and metastatic gastric or gastroesophageal junction adenocarcinoma. Our study’s DS-8201a dose of 4mg/kg is within the dose range of ongoing clinical trials. To date, there are over forty clinical trials on DS-8201a, as a single agent or in combination with other drugs, most of them focused on metastatic and recurrent breast cancer. Several incorporate other solid tumors including ovarian and uterine cancers (ClinicalTrials.gov Identifier: NCT02564900, NCT03383692, NCT04294628, NCT04639219, NCT04704661). One trial has specific arms for cervical, uterine, and ovarian cancers and does not exclude CS (ClinicalTrials.gov Identifier: NCT04482309), and one trial is specific for uterine serous carcinoma using combination olaparib and DS-8201a,but allows for CS in two out of three arms (ClinicalTrials.gov Identifier: NCT04585958). The STATICE trial (abstract presented at ESMO 2021) recently evaluated effectivity of DS-8201a in patients with unresectable and standard chemotherapy-refractory HER2-expressing uterine carcinosarcomas in Japan. In patients with HER2 2+/3+, overall response rate was found to be 55%, and 45% of patients maintained stable disease through the study period. They also showed partial response in 7/10 (70%) of patients enrolled with HER2 1+ disease [28]. These are promising results that are consistent with our findings presented here.
In conclusion, we have demonstrated that DS-8201a is a novel ADC with remarkable preclinical activity against uterine and ovarian CS with strong (3+) HER2/neu expression. In vitro studies showed significant bystander toxicity of low/minimal HER2/neu-expressing CS cells while DS-8201a confirmed strong tumoricidal activity in our animal CS models. On the basis of these encouraging preclinical results, further clinical trials with DS-8201a in CS patients are warranted.
DS-8201a exhibited marked cytotoxicity in vitro among HER2/neu expressing cell lines
0 HER2/neu cells are receptive to bystander killing effect from DS-8201a when mixed with 3+ HER2/neu-expressing cells
DS-8201a inhibits HER2/neu expressing carcinosarcoma tumor growth in patient-derived xenografts
DS-8201a is an effective antibody drug conjugate against HER2/neu-expressing uterine and ovarian carcinosarcomas
Financial support:
This work was supported in part by grants from NIH U01 CA176067–01A1, the Deborah Bunn Alley Foundation, the Domenic Cicchetti, the Discovery to Cure Foundation, the Guido Berlucchi Foundation to A.D.S., and Gilead Sciences Inc., Foster City, CA. This investigation was also supported by NIH Research Grant CA-16359 from NCI and Standup-to-cancer (SU2C) convergence grant 2.0 to Alessandro Santin.
Footnotes
Conflict of Interest Statement
A.D.S. reports grants from PUMA, grants from IMMUNOMEDICS, grants from GILEAD, grants from SYNTHON, grants and personal fees from MERCK, grants from BOEHINGER-INGELHEIM, grants from GENENTECH, grants and personal fees from TESARO and grants and personal fees from EISAI and R-Pharm USA. The other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Arend R, Doneza JA, Wright JD, Uterine carcinosarcoma, Curr. Opin. Oncol. 23 (2011) 531–536. [DOI] [PubMed] [Google Scholar]
- [2].Rottmann D, Snir OL, Wu X, Wong S, Hui P, Santin AD, Buza N, HER2 testing of gynecologic carcinosarcomas: tumor stratification for potential targeted therapy, Mod. Pathol. 33 (2020) 118–127. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, Keeney GL, The impact of multi-modal therapy on survival for uterine carcinosarcomas, Gynecol. Oncol. 116 (2010) 419–423. [DOI] [PubMed] [Google Scholar]
- [4].Rauh-Hain JA, Diver EJ, Clemmer JT, Bradford LS, Clark RM, Growdon WB, Goodman AK, Boruta DM 2nd, J.O. Schorge, M.G. del Carmen, Carcinosarcoma of the ovary compared to papillary serous ovarian carcinoma: a SEER analysis, Gynecol. Oncol. 131 (2013) 46–51. [DOI] [PubMed] [Google Scholar]
- [5].George EM, Herzog TJ, Neugut AI, Lu Y-S, Burke WM, Lewin SN, Hershman DL, Wright JD, Carcinosarcoma of the ovary: natural history, patterns of treatment, and outcome, Gynecol. Oncol. 131 (2013) 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao S, Bellone S, Lopez S, Thakral D, Schwab C, English DP, Black J, Cocco E, Choi J, Zammataro L, Predolini F, Bonazzoli E, Bi M, Buza N, Hui P, Wong S, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Odicino F, Pecorelli S, Donzelli C, Ardighieri L, Facchetti F, Falchetti M, Silasi D-A, Ratner E, Azodi M, Schwartz PE, Mane S, Angioli R, Terranova C, Quick CM, Edraki B, Bilgüvar K, Lee M, Choi M, Stiegler AL, Boggon TJ, Schlessinger J, Lifton RP, Santin AD, Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 12238–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, Guzzo F, English DP, Varughese J, Gasparrini S, Bortolomai I, Buza N, Hui P, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Carrara L, Pecorelli S, Silasi D-A, Ratner E, Azodi M, Schwartz PE, Rutherford TJ, Stiegler AL, Mane S, Boggon TJ, Schlessinger J, Lifton RP, Santin AD, Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 2916–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buza N, English DP, Santin AD, Hui P, Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice, Mod. Pathol. 26 (2013) 1605–1612. [DOI] [PubMed] [Google Scholar]
- [9].Guzzo F, Bellone S, Buza N, Hui P, Carrara L, Varughese J, Cocco E, Betti M, Todeschini P, Gasparrini S, Schwartz PE, Rutherford TJ, Angioli R, Pecorelli S, Santin AD, HER2/neu as a potential target for immunotherapy in gynecologic carcinosarcomas, Int. J. Gynecol. Pathol. 31 (2012) 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jenkins TM, Cantrell LA, Stoler MH, Mills AM, HER2 Overexpression and Amplification in Uterine Carcinosarcomas With Serous Morphology, Am. J. Surg. Pathol. 46 (2022) 435–442. [DOI] [PubMed] [Google Scholar]
- [11].Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, Chambers S, Secord AA, Havrilesky L, O’Malley DM, Backes FJ, Nevadunsky N, Edraki B, Pikaart D, Lowery W, ElSahwi K, Celano P, Bellone S, Azodi M, Litkouhi B, Ratner E, Silasi D-A, Schwartz PE, Santin AD, Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis, Clin. Cancer Res. 26 (2020) 3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hasegawa K, Nishikawa T, Hirakawa A et al. Efficacy and safety of trastuzumab deruxtecan in HER2-expressing uterine carcinosarcoma (STATICE TRIAL, NCCH1615): A MULTICENTER, PHASE 2 CLINICAL TRIAL. Poster presented at: European Society for Medical Oncology; September 16-21, 2021. [Google Scholar]
- [13].Nicoletti R, Lopez S, Bellone S, Cocco E, Schwab CL, Black JD, Centritto F, Zhu L, Bonazzoli E, Buza N, Hui P, Mezzanzanica D, Canevari S, Schwartz PE, Rutherford TJ, Santin AD, T-DM1, a novel antibody-drug conjugate, is highly effective against uterine and ovarian carcinosarcomas overexpressing HER2, Clin. Exp. Metastasis. 32 (2015) 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Menderes G, Bonazzoli E, Bellone S, Black J, Predolini F, Pettinella F, Masserdotti A, Zammataro L, Altwerger G, Buza N, Hui P, Wong S, Litkouhi B, Ratner E, Silasi D-A, Azodi M, Schwartz PE, Santin AD, SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine and Ovarian Carcinosarcoma with HER2/Neu Expression, Clin. Cancer Res. 23 (2017) 5836–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hinrichs MJM, Dixit R, Antibody Drug Conjugates: Nonclinical Safety Considerations, AAPS J. 17 (2015) 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T, DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1, Clin. Cancer Res. 22 (2016) 5097–5108. [DOI] [PubMed] [Google Scholar]
- [17].Andrikopoulou A, Zografos E, Liontos M, Koutsoukos K, Dimopoulos M-A, Zagouri F, Trastuzumab Deruxtecan (DS-8201a): The Latest Research and Advances in Breast Cancer, Clin. Breast Cancer. 21 (2021) e212–e219. [DOI] [PubMed] [Google Scholar]
- [18].Xu Z, Guo D, Jiang Z, Tong R, Jiang P, Bai L, Chen L, Zhu Y, Guo C, Shi J, Yu D, Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985), Eur. J. Med. Chem. 183 (2019) 111682. [DOI] [PubMed] [Google Scholar]
- [19].Yu J, Fang T, Yun C, Liu X, Cai X, Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers, Front Mol Biosci. 9 (2022) 847835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Office of the Commissioner, FDA Approves First Targeted Therapy for HER2-Low Breast Cancer, U.S. Food and Drug Administration. (n.d.). https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer (accessed October 5, 2022). [Google Scholar]
- [21].Center for Drug Evaluation, Research, FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer, U.S. Food and Drug Administration. (n.d.). https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung (accessed October 5, 2022). [Google Scholar]
- [22].Wu G, Gao Y, Liu D, Tan X, Hu L, Qiu Z, Liu J, He H, Liu Y, Study on the Heterogeneity of T-DM1 and the Analysis of the Unconjugated Linker Structure under a Stable Conjugation Process, ACS Omega. 4 (2019) 8834–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van der Lee MMC, Groothuis PG, Ubink R, van der Vleuten MAJ, van Achterberg TA, Loosveld EM, Damming D, Jacobs DCH, Rouwette M, Egging DF, van den Dobbelsteen D, Beusker PH, Goedings P, Verheijden GFM, Lemmens JM, Timmers M, Dokter WHA, The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers, Mol. Cancer Ther. 14 (2015) 692–703. [DOI] [PubMed] [Google Scholar]
- [24].Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, Loosveld E, van den Dobbelsteen D, Egging D, Mattaar E, Groothuis P, Beusker P, Coumans R, Elgersma R, Menge W, Joosten J, Spijker H, Huijbregts T, de Groot V, Eppink M, de Roo G, Verheijden G, Timmers M, Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform, Mol. Cancer Ther. 13 (2014) 2618–2629. [DOI] [PubMed] [Google Scholar]
- [25].Kotani D, Shitara K, Trastuzumab deruxtecan for the treatment of patients with HER2-positive gastric cancer, Ther. Adv. Med. Oncol. 13 (2021) 1758835920986518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buza N, HER2 Testing in Endometrial Serous Carcinoma: Time for Standardized Pathology Practice to Meet the Clinical Demand, Arch. Pathol. Lab. Med. 145 (2021) 687–691. [DOI] [PubMed] [Google Scholar]
- [27].Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga J-Y, Im S-A, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim S-B, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA, DESTINY-Breast04 Trial Investigators, Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer, N. Engl. J. Med. 387 (2022) 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hasegawa K, Nishikawa T, Hirakawa A, Kawasaki M, Tomatsuri S, Nagasaka Y, Nakamura K, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Kato T, Yagishita S, Hamada A, Yoshida H, Yonemori K, 813P Efficacy and safety of trastuzumab deruxtecan in HER2-expressing uterine carcinosarcoma (STATICE trial, NCCH1615): A multicenter, phase II clinical trial, Ann. Oncol. 32 (2021) S767. [Google Scholar]


