Abstract
Background:
Approximately 50% of older adults with cognitive impairment suffer from insomnia. When untreated, pre-existing cognitive problems may be exacerbated and potentially contribute to further cognitive decline. One promising approach to maintain cognitive health is to improve sleep quantity and quality.
Objective:
To determine feasibility, acceptability, and preliminary efficacy of Sleep Health Using the Internet for Older Adult Sufferers of Insomnia and Sleeplessness (SHUTi OASIS), an Internet-delivered cognitive behavioral therapy for insomnia (CBT-I) program in older adults with mild cognitive impairment (MCI).
Methods:
Older adults with MCI and insomnia were recruited from hospital-based memory and sleep disorders clinics and enrolled in a single-arm pilot study. Participants completed the six cores of SHUTi OASIS, over nine weeks with two-week baseline and post-assessments using self-reported sleep diaries. Feasibility and acceptability were informed by usage statistics and qualitative interviews; preliminary efficacy was informed by patient-generated sleep data.
Results:
Twelve participants enrolled and, on average, were 75.8 years of age. Ten participants completed the study and logged in most days. Most participants reported a positive overall experience, and interviews revealed successful and independent program management and completion. There were significant changes on all baseline to post-assessment sleep measures, including clinically meaningful improvements on the Insomnia Severity Index (13.5 to 8.3, p < 0.01), sleep efficiency, wake after sleep onset, and sleep onset latency (ps < 0.02). There was no statistically significant change in cognitive measures (p > 0.05).
Conclusion:
This study supports that older adults with cognitive impairment can independently complete CBT-I via the Internet and achieve clinical sleep improvements.
Keywords: Aged, behavioral intervention, cognitive dysfunction, insomnia, Internet, mild cognitive impairment, research
INTRODUCTION
Insomnia is characterized by difficulty falling or maintaining sleep coupled with impaired functioning during waking hours [1]. Approximately 25–35% of adults over the age of 65 have insomnia, and up to 50% of older adults report some symptoms of insomnia impacting daytime functioning [1, 2]. However, fewer than 15% of adults with insomnia receive specialized treatment [3]. Individuals with cognitive impairment experience more sleep disorders than those without cognitive concerns [4–7], with up to 50% of older adults with cognitive impairment living with insomnia [8]. The prevalence of mild cognitive impairment (MCI) is estimated to be as high as 19% in older adults [9], with 8–15% of individuals with MCI progressing to dementia each year [10]. With greater risk for further cognitive decline [4], but no known cure for MCI, care is focused on preventing further deterioration of cognitive function and cognitive and behavioral symptom management. A promising approach to maintain cognitive health in individuals with MCI may lie in the improvement in sleep.
Insomnia is typically treated using pharmacotherapy which has been shown to provide short-term benefits [11]. However, there are unique challenges to using sleep medications with older adults, including the increased potential to experience age-related pharmacokinetic and pharmacodynamic changes [12], consequences of potentially inappropriate medications [13], and negative side-effects of polypharmacy [14]. The gold standard non-pharmacological intervention is cognitive behavioral therapy for insomnia (CBT-I) [15, 16]. CBT-I is traditionally delivered by a clinician and targets the thoughts, feelings, and behaviors that contribute to sleep impairment. Although effective, CBT-I may be challenging to access due to limited trained therapists’ availability, travel, and direct costs [17]. To overcome these barriers, Internet-delivered CBT-I has emerged as a feasible, effective, and more accessible solution. CBT-I has demonstrated positive results in a variety of populations [18, 19], including a subset of healthy older adults [20]. However, the use of an Internet-delivered program in individuals with cognitive concerns had not been explored prior to this study. Thus, the purpose of this pilot study was to determine feasibility, acceptability, and preliminary efficacy of Internet-delivered CBT-I in an MCI population with insomnia.
METHODS
We conducted a multi-site, single-arm intervention study from December 2018 to June 2020. Total study participation lasted approximately 5–6 months. The study intervention period lasted approximately 3.5 months; in addition, two study-related visits were also conducted. Patients with MCI and their care partners were recruited from three recruitment sites: a rural-based, MCI-specific, University-hospital clinic; a University hospital aging-care clinic; and a University hospital sleep clinic. Inclusion criteria were: ≥ 55 years of age; English literacy; a diagnosis of MCI based on formal neurocognitive testing (operationalized by Peterson et al. [10]); Internet access; and an estimated average of ≤6.5 h of sleep each night over the past three months. These criteria paralleled previous efficacy studies using the same Internet-based insomnia intervention [19]. Eligibility was not limited by MCI type. Exclusion criteria included current psychological treatment for insomnia; initiation of psychological or psychiatric treatment within the past 3 months; other untreated sleep disorders; irregular sleep schedule (defined by schedule resulting in usual bedtime earlier than 8PM or later than 2AM, arising time earlier than 4AM or later than 10AM); Huntington’s or Parkinson’s diseases; hyperthyroidism; current chemotherapy; presence of asthma or respiratory concerns with night treatment; chronic pain treated with opioids; epilepsy; taking medications at night that interfere with sleep; severe depression; bipolar disorder; current alcohol dependence or abuse; current substance dependence or abuse; current suicidality; and inability to provide informed consent. Major psychiatric disorders were assessed using the Mini-International Neuropsychiatric Interview (MINI) [21] and the Quick Inventory of Depressive Symptoms (QIDS) [22] for severe depression. If potential participants endorsed current alcohol dependence or abuse, current substance dependence or abuse, current manic or hypomanic episode (in the past 10 years), or current suicidality on the MINI screen, additional condition-specific MINI questions were administered; with the exception that a Quick Inventory of Depressive Symptoms-Clinician rated (QIDS-C16) was conducted if a potential participant met criteria for Current Major Depressive Episode on the MINI. All were administered in-person by a Registered Nurse on the study team.
Care partners were invited to enroll at the same time as the participant with MCI to join a one-time, end-of-study interview about their role and perspectives on intervention feasibility and acceptability. Inclusion criteria for care partners were ≥18 years old, reside with participant ≥ 3 nights/week, ≥ and English literacy.
Procedure
This study was approved by the University Institutional Review Board. Patients with MCI or insomnia diagnoses were identified as potential participants from individual-site clinic schedules or electronic medical record review (see Fig. 1 for detailed study flow). Once potential participants were identified, a study team member reviewed the medical chart for additional eligibility criteria. If seemingly eligible, clinic staff contacted the patient, provided study details, and administered a short pre-screen questionnaire in the clinic or by phone. If no exclusion criteria were identified during the pre-screening, an in-person visit (Visit 1) was scheduled to obtain informed consent, administer the full screening questionnaire, and complete the Montreal Cognitive Assessment (MoCA).
Fig. 1.
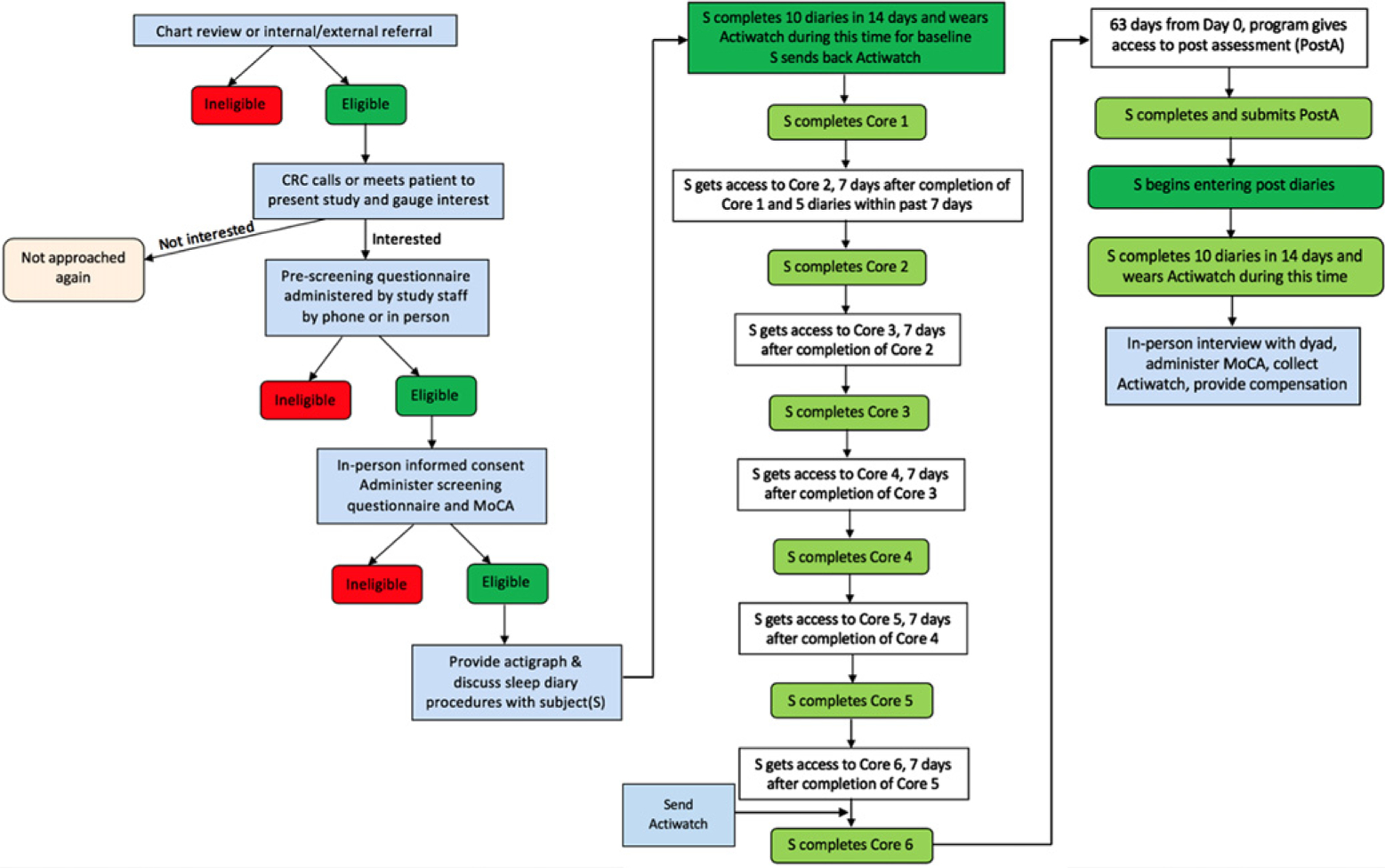
SHUTi in MCI Study Flow.
Once enrolled in the study, participants were provided log-in information and instructions for Sleep Healthy Using the Internet for Older Adult Sufferers of Insomnia and Sleeplessness (SHUTi OASIS), an online CBT-I intervention for older adults with insomnia. Once participants logged in to the online study platform, they were directed to a 45-min, baseline assessment battery, which included the Insomnia Severity Index (ISI), through Qualtrics (Provo, UT). Upon completion, participants were asked to begin entering daily sleep diaries in SHUTi OASIS. Ten sleep diaries, each comprised of the same 10 questions, were required over 14 days. Questions included information about bedtime, sleep onset latency, number of awakenings, the total length of awakenings, wake time, arising time, daytime naps, rating of the soundness of previous night’s sleep, rating of refreshed feeling upon morning awakening, and information about sleep aids (medication and/or alcohol use details). Once 10 diaries were entered within a 14-day period, participants were immediately and automatically moved forward in the system and able to begin using SHUTi OASIS.
SHUTi OASIS is a fully automated, interactive, and tailored Internet-delivered CBT-I program [19]. A series of six cores, comprised of the key tenets of CBT-I, are metered out over time (see [24] for additional details). The core intervention content is enhanced through interactive features such as animations/illustrations, patient vignettes, and video-based expert explanations. Users continue to enter sleep diaries throughout the intervention, and the program uses the online sleep diaries to track progress and tailor intervention recommendations. To encourage program adherence, automated emails are sent to participants to both remind them to enter diaries as well as helping guide them through the use of the program. SHUTi OASIS is a version of SHUTi with unique content and modified algorithms [19, 20, 24] built specifically for older adults (ages 55+) as part of an NIH-funded trial (R01AG047885). SHUTi OASIS was used in an unmodified state for this trial. Participants were given nine weeks to complete the intervention; however, it could be completed as quickly as six weeks based on program progress, with each new core available as early as seven days from the completion of the previous core.
At week ten of the trial, regardless of progress, participants were instructed to complete the online post-assessment battery, begin the two-week, post-intervention period of daily sleep diaries. A study team member also reached out by phone or email to schedule the end-of-study meeting (Visit 2) at this time. Visit 2 occurred either in the participants’ home or by phone or video conference (due to state-mandated stay-at-home orders in response to the COVID-19 pandemic) after the completion of the two-week post-intervention sleep diaries and post-assessment battery, which included the Insomnia Severity Index (ISI). Visit 2 included an interview with the participant and care partner (if consent had been provided during the enrollment of the participant with MCI), administration of the MoCA, and provision of participant payment.
Participants were also instructed to wear a wrist-worn Actiwatch Spectrum Plus (©Koninklijke Philips N.V., 2004–2020) while completing baseline and post-intervention assessment sleep diaries. However, Actiwatch usage has been reported elsewhere [23] and is not further presented here.
Measures
Measures were administered online and in-person to assess feasibility, acceptability, and preliminary efficacy for SHUTi OASIS for older adults with MCI. Data collected from care partners included demographics and an end-of-study dyadic interview. Intervention engagement was measured by SHUTi OASIS core completion and log-in count. Study engagement was assessed using qualitative interviews. Preliminary efficacy was determined by the change in Insomnia Severity Index from baseline to post-assessment. Change in cognition was measured as the change in Montreal Cognitive Assessment score from baseline to post-assessment.
Core completion –
The number of cores completed was used as a metric of engagement, where 0 indicates a participant who completed the baseline assessment, but did not complete the first core, and 6 describes a participant who completed the entire intervention.
Log-in count –
The number of times a participant logged into SHUTi OASIS during the intervention period was defined as participant log-in to the SHUTi OASIS program. Log-ins within 5 min of a previous log-in were not considered unique sessions and were omitted.
Qualitative interviews –
Semi-structured qualitative interviews were conducted with each participant or the participant-care partner dyad at the end of the study to capture study experiences, including study successes or challenges. Care partners were encouraged to participate because of the possible influence of dyad dynamics on adherence and efficacy of the intervention in this population. Open-ended questions were used to draw out descriptions of the study experience, consisting of six lead questions focusing on the experience of using the intervention, barriers, and facilitators to using the intervention, as well as care partner involvement in the intervention. The lead investigator (MM) conducted all qualitative interviews, which were audio recorded and transcribed verbatim for iterative analysis.
Insomnia Severity Index [25] –
The seven-item ISI provided a quantitative index of overall insomnia severity, as reported by participants in the baseline and post-intervention questionnaire. Participants rated the severity of problems with sleep onset, sleep maintenance, and early morning awakening; interference with daytime functioning; how noticeable the impairment is to others; distress or concern caused by the sleep problem; and satisfaction with the current sleep pattern on a 5-point Likert scale. Scores ranged from 0 to 28, with higher scores indicating more severe insomnia [26, 27]. The ISI is a valid and reliable measure [25] when delivered via the Internet [27] that is sensitive to changes in treatment studies [19].
Montreal Cognitive Assessment [28] –
The MoCA is a brief cognitive screener used to describe patient’s global cognitive functioning that was administered at Visit 1 (v.8.1) and Visit 2 (v.8.3). In March 2020, during the COVID-19 pandemic, the MoCA website sent an e-mail stating that the Blind MoCA had been validated for remote testing in dementia participants. Because of COVID-19 pandemic-mandated stay-at-home orders, the Blind MoCA v.7.1 ©Nasreddine [29, 30] was administered by phone at Visit 2 for two participants. The original MoCA scores range from 0–30, while the Blind MoCA scores ranged from 0–22 due to omission of the visuospatial/ executive and naming sections of the test.
Analysis
All quantitative data were analyzed in SPSS® Statistics (v.26, IBM Corp., Armonk, NY). Participant descriptive statistics were computed for age, sex, marital status, education, comfort with using the Internet, history of sleep difficulties, nights per week experiencing sleep difficulties, and feasibility and acceptability measures (core completion and log-in count). Paired t-tests for repeated ISI and sleep diary variables were used to calculate preliminary efficacy of the SHUTiOASIS intervention. Paired t-tests were conducted for each participant’s baseline and post-intervention assessment MoCA scores (adjusted to reflect the 0–22 scale of the Blind MoCA) for all completers.
Qualitative interview transcripts were analyzed using line-by-line coding and content analysis. Qualitative content analysis was performed by two independent investigators (MM and ME) beginning with line-by-line coding. Categories of shared or similar experiences organized findings. Category similarities, differences, and counter examples were discussed among investigators until findings were verified by consensus and convergence [31]. An additional investigator (RP) was brought in to serve as a tiebreaker for dissonant findings.
RESULTS
Twelve participants enrolled with an average age of 75.8 years and reported 7.5 years of sleep difficulties. The majority of participants were female (n = 7, 58.3%), married or living with a partner (n = 12, 100%), white (n = 12, 100%), and enrolled with a care partner (n = 7, 58.3%). Care partners (n = 7) were, on average, 75.6 years of age, and more likely to be female (n = 5, 71.4%). All care partners who enrolled were white (n = 7, 100%) and identified as a spouse or partner. No potential participants screened positive for substance or alcohol abuse on the MINI screen. Of the 11 participants who completed the baseline questionnaires, most reported using the Internet several times a day (n = 6, 54.5%), owned a cell phone (n = 11, 100%), sent or received 1–50 text messages on an average day on a cell or mobile phone (n = 8, 72.7%), and owned a tablet (n = 8, 72.7%). Most (n = 10) were “very comfortable” or “somewhat comfortable” using the Internet. See Table 1 for detailed descriptive characteristics.
Table 1.
Baseline Descriptive Characteristics of Participants with Mild Cognitive Impairment (N = 12)
| Variable | Mean ± standard deviation (range) or n (%) |
|---|---|
| Age, M ± SD (range) y | 75.8 ± 7.7 (64–90) |
| Sex, n (%) female | 7 (58.3) |
| Race, n (%) white | 12 (100) |
| Average sleep time, M ± SD (range) min | 392.5 ± 45.2 (300–450) |
| Length of sleep difficulties, M ± SD (range) months | 146.2 ± 151.5 (9–468) |
| Highest degree,* n (%) | |
| High school | 3 (27.3) |
| Associate’s | 1 (9.1) |
| Bachelor’s | 4 (36.4) |
| Master’s | 3 (27.3) |
| Income,* n (%) | |
| $35,000–49,999 | 2 (18.2) |
| $50,000–74,999 | 3 (27.3) |
| $75,000–99,999 | 1 (9.1) |
| $100,000–199,999 | 2 (18.2) |
| $200,000 or more | 1 (9.1) |
| Don’t know/not sure | 2 (18.2) |
| Internet use,* n (%) | |
| 1–2 days/week | 2 (18.2) |
| 3–5 days/week | 1 (9.1) |
| Once a day | 2 (18.2) |
| Several times per day | 6 (54.5) |
| Internet comfort,* n (%) | |
| Very comfortable | 5 (45.5) |
| Somewhat comfortable | 4 (36.4) |
| Neither comfortable nor uncomfortable | 2 (18.2) |
| Number of daily emails,* n (%) | |
| 1–10 | 6 (54.5) |
| 11–20 | 5 (45.5) |
| Smartphone,* n (%) | |
| Yes | 8(72.7) |
| No | 2 (18.2) |
| Not sure/don’t know | 1 (9.1) |
| Number of texts,* n (%) | |
| None | 3 (27.3) |
| 1–10 | 3 (27.3) |
| 11–20 | 4 (36.4) |
| 21–50 | 1 (9.1) |
| Device use,* n (%) | |
| Computer or laptop | 6 (54.5) |
| Cell or smartphone | 2 (18.2) |
| Tablet or e-reader | 3 (27.3) |
n = 11
Of the 12 participants enrolled, 10 participants completed the study. One participant, also the oldest enrollee (>90 years of age), withdrew due to reported difficulties completing the Internet-delivered baseline survey citing it was “too hard to stay focused.” The other participant withdrew during the intervention period for self-reported cognitive decline. Both withdrawals also enrolled with a care partner. All participants who completed the Internet-delivered baseline survey were included in the core completion (Table 2 presents individual core descriptions) and log-in analyses. Of the six total cores, average completion was 5.2 ± 1.3 (standard deviation [SD], Range: 2–6, n = 11). Across the intervention period, 49.6 ± 15.0 (mean ± SD) unique log-ins were recorded (median = 53.5, range: 27–65).
Table 2.
Core Focus and Participant Core Completion (n = 11*)
| Core | Focus | Participants Completed (#) |
|---|---|---|
| 1 | “Getting Ready” | 11 |
| 2 | “Sleep Scheduling” | 11 |
| 3 | “Sleep Practices” | 9 |
| 4 | “Thinking Differently” | 9 |
| 5 | “Sleep Hygiene” | 8 |
| 6 | “Moving On” | 6 |
Includes one participant who withdrew during the intervention period (completed through core 2).
Interviews with participants (n = 10) and care partners (n = 5) provided a rich understanding of how the intervention fit with daily-life activities and how it might be improved in future studies (categories and frequencies presented Table 3). Most participants endorsed a positive overall experience (n = 6), three participants provided mixed responses, and one reported a negative experience. Participants with mixed experiences identified challenges with initiating sleep restriction, implementing structured sleep windows, and remembering sleep details of a previous night (e.g., time to sleep, number of awakenings). The primary reason supporting the negative experience was trouble with personal computer delays; for example, “often the computer got hung up or I had to repeat some things.” All participants stated the program was acceptable. Eight participants stated they used the program every day throughout the study and two said they used it “most days.” Participants reported challenges with correctly remembering and accurately entering times of falling asleep and waking in the morning (n = 6) and others related concerns about the sleep window (n = 3).
Table 3.
Qualitative Categories
| Categories | Total count | Sub-category | Count | Exemplary quotes | Additional notes |
|---|---|---|---|---|---|
| Overall experience | N = 10 | Positive | 6 | “simple” “no problem with it” | |
| Mixed | 3 | “It was horrendous for a while at the beginning when I had to wait too long to go to bed.” | |||
| Negative | 1 | “frustrating overall because often the computer got hung up or I had to repeat some things when I was rushing through” | |||
| SHUTi OASIS acceptability | N = 10 | Acceptable | 10 | “good” “easy” “okay” | |
| SHUTi OASIS access location | n = 9 | In bedroom | 1 | ||
| Outside bedroom | 8 | Living room (n = 3) Office/study (n = 4) Kitchen (n = 1) |
|||
| Device used to access SHUTi OASIS | N = 10 | Tablet | 1 | “I could not have filled this form out on my phone” | Laptop (n = 4) Desktop (n = 5) |
| Computer | 8 | ||||
| Cell phone | 1 | One subject changed device | |||
| Program use frequency | N = 10 | Every day | 8 | ||
| Most days | 2 | ||||
| Concerns or difficulties with SHUTi OASIS | N = 10 | Accuracy of sleep window | 9 | ||
| Content repetitious or boring | 2 | ||||
| None | 1 | ||||
| Self-perceived sleep change over time | N = 10 | Sleep improved | 7 | ||
| Sleep improved, then regressed | 1 | ||||
| No change | 1 | ||||
| Sleep worsened | 1 | Pt noted that worsening sleep was non-study-related stress | |||
| Self-perceived change in cognition or memory over time | N = 10 | Yes | 3 | “thinking was clearer” improved short-term memory (n = 2) |
|
| No | 3 | ||||
| Unsure/maybe/don’t remember | 4 | “maybe better long-term memory” “sometimes, sometimes not” |
|||
| Care partner study-related activities | N = 10 | Sleep diary entry | 4 | Sleep-diary related calculations | |
| Tech support | 3 | Help with printing, device-related issues | |||
| Emotional support | 1 | Discuss study difficulties | |||
| Sleep window support | 1 | Wake-up subject to maintain sleep window | |||
| Care partner not enrolled | 5 |
Some participants believed that SHUTi OASIS provided structure to their daily routine (n = 3), requiring discipline to enter daily sleep dairies, leading to feeling “empowered.” However, one participant suggested that sleep diary entry interfered with the morning routine, specifically during COVID-19. Participants also reported that their cognitive impair ment impacted participation and sleep improvement; for example, “I do have a memory problem that’s already been identified by a neurologist, that was difficult for me,” and “ … the problems I’m having most likely ha[ve] to do with my memory issues.”
For those who completed the intervention (n = 10, see Table 1), statistically significant changes were found on all baseline-post-assessment ISI and sleep measures obtained via self-report questionnaire and sleep diaries. Most notable, there were clinically meaningful improvements in ISI (13.5 to 8.3, p < 0.01), sleep efficiency (SE, 77.3% to 86.5%, p < 0.001), wake after sleep onset (WASO, 71.2 to 44.4, p = 0.001), and sleep onset latency (SOL, 36.0 to 18.3, p < 0.001). 40% (n = 4 of 10) of the sample can be classified as remitters, participants who reported a change in ISI to < 8 (“no insomnia symptoms”) at post assessment. 30% (n = 3 of 10) of the sample are responders, those who reported a decrease of more than 7 points on the ISI. Qualitative interviews indicate that the majority of participants endorsed improvement in their sleep over the course of the study (n = 7), while one person reported her sleep improved and regressed, one experienced no change, and one participant believed her sleep had worsened due to COVID-related stressors.
Complete MoCA scores (30-point) from baseline and post-intervention assessments were unavailable for two participants due to COVID-19 limitations. Using baseline and post-Blind MoCA scores, there was no statistically significant change in blind MoCA scores over time (15.9 ± 7.3 to 15.6 ± 2.2, p = 0.71). If baseline MoCA scores were carried forward for missing MoCA data (n = 2), there remained no statistically significant change in outcome. When asked during the qualitative interview about changes in cognition or memory over the course of the study, three participants reported that their cognition or memory improved, one person thought her “thinking was clearer,” and two endorsed improvements in short-term memory. Most participants said they were “unsure” or “don’t remember” if their memory changed since the start of the study.
COVID-19 considerations
COVID-19 mandated stay-at-home orders from March to June 2020 impacted in-person post-intervention cognitive assessments and interviews. As an exploratory study, the four participants completing the post-assessment remotely were asked about the impact of COVID-19 on their study par ticipation and sleep. The four participants stated that COVID-19 did not impact study participation (n = 4); however, two participants stated that their sleep was impacted due to stress related to COVID-19. One participant stated, “the virus hit, and I had to take way more time to do anything …”
DISCUSSION
The current study demonstrates feasibility, acceptability, and preliminary efficacy of a non-phar macological, Internet-delivered CBT-I program in older adults with MCI. Significant sleep changes are especially meaningful as improving sleep in individuals with MCI may serve as a first step in delaying progression to dementia [32]. Given that sleep may mediate cognitive functioning in individuals with MCI [33], improving sleep may ultimately delay progression to dementia.
Participants with MCI and insomnia demonstrated feasibility of Internet-delivered CBT-I with consistent, frequent program use. Most enrolled participants accessed the intervention daily, pointing to a consistent routine incorporated into their day. Interestingly, participants with MCI were most concerned about possible inaccuracies in the sleep windows due to inaccurate data input by the participant. In the future, it may be beneficial to provide additional information in the SHUTi OASIS program about how sleep diary data is used to create the sleep window and/or incorporate more passive ways to collect sleep diary data assuming it will not significantly impact the positive outcomes when using the intervention.
Similar to previous Alzheimer’s disease trials [34], seven of the twelve care partners enrolled in the study. No instructions or suggestions were provided to the care partners about their involvement in intervention use. We were most interested in learning about the care partner’s description of the experience by the participant with MCI and if the care partner felt engaged or played a role in use of the program. As MCI is considered a risk state for AD, individuals with MCI, and likely their care partners, are more likely to initiate changes in habits and routines to accommodate MCI symptoms [35, 36]. This proactive approach and dyadic support system can be leveraged to promote positive cognitive and behavioral outcomes in intervention research. Although we observed no notable differences between participant with and without care partners, the two individuals who withdrew enrolled with care partners. Although the small sample size precludes making meaningful generalizations, future studies may want to consider the potential impact of care partners on retention, adherence and participation.
Our findings of statistically and clinically meaningful changes in sleep efficiency, wake after sleep onset, sleep onset latency, and insomnia severity index are compelling. Specifically, for ISI, 40% of participants reported experiencing little to no insomnia at post-assessment as demonstrated by a change in ISI from subthreshold/clinical insomnia (score 8–28) to “no clinically significant insomnia,” (score 0–7), [25] known as remitters. And 30% experienced clinically meaningful improvement in their insomnia by reporting a change in ISI of more than seven points, known as responders. In an RCT examining the efficacy of the original SHUTi intervention, 57% were considered remitters, and 70% considered responders at one year follow up [19]. Responder and remitter rates for older adults are promising but limited due to our study’s small sample size and short study duration.
The significant changes in sleep variables over time found in this trial are consistent with findings from other SHUTi studies [19, 37] even though participants in this study enrolled with less disturbed overall sleep and fewer sleep issues at onset. At baseline, SOL was 36.0 min in this study compared to 43.7 min in the pivotal SHUTi efficacy trial [19]; SE was better at 77.3% compared to 73.2%; and total sleep time of 6.2 h compared to 5.8 h [19]. Similarly, there was a relatively low baseline ISI score observed in this study (13.5) compared to other SHUTi trials (mean baseline ISI score for the pivotal SHUTi efficacy trial was 17.0 [19] and 15.9 in the SHUTi depression prevention trial [35]). Interestingly, the ISI score change for intervention groups was similar across the three studies at post assessment: SHUTi in MCI: 13.5 to 8.3; pivotal SHUTi efficacy: 15.9 to 9.3 [19]; SHUTi for depression: 15.9 to 7.3 [37]. We anticipated that age-related sleep changes, such as decreased total sleep duration and sleep efficiency, may significantly impact the preliminary efficacy of our study, but as demonstrated with the superior baseline sleep characteristics of our older sample compared to the younger SHUTi efficacy sample (43.75 years of age), this was not the case.
Participants completed a mean of 5.2 of the total six cores, similar to the average core completions in the pivotal SHUTi efficacy trial of 4.9 cores [19]. Participants in our study had more frequent log-ins than other SHUTi studies (median = 53.5 compared to 25 in SHUTi efficacy). Adherence rates are encouraging for future studies in this MCI population. Another interesting finding is that older adults with MCI notably increased their nap length over the course of the study. This may be due to core content that was modified for older adults that did not discourage short, afternoon naps and explained how best to implement naps if needed.
Qualitative interviews provided valuable descriptions of the participant experience, and care partners provided critical considerations for future studies. Interviews confirmed that participants were proactive in seeking out and implementing ways to improve their sleep. This was not surprising as interest and enrollment in the research study required both a time commitment and general desire to improve cognition and sleep. Dyadic interviews provided the opportunity to understand a care partner’s role in participant adherence and symptom resolution, such as reminders provided by the care partners to participants to complete sleep diaries. We had anticipated that there might be a difference in program use and adherence between participants who enrolled with and without care partners, but this was not observed. The lack of difference is likely due to the small sample size, and a larger trial is needed to determine if care partner enrollment is critical to the intervention’s efficacy.
There were no statistically significant differences found in baseline and post-MoCA or Blind MoCA scores. The Blind MoCA is limited, however, as it is not a comprehensive neuropsychological assessment; and, although it had been validated among individuals with dementia, it is yet to be validated in individuals with MCI [29]. In the future, comprehensive neuropsychological testing is imperative, and the use of other neurocognitive assessment tools should be considered, with an emphasis on those that are validated when administered remotely.
Limitations
The study’s primary purpose was to determine feasibility and acceptability; however, we also present preliminary efficacy findings although the sample is small, limiting the generalizability of findings. Other limitations to this study include the single-arm design and pre-post assessment, both chosen due to the study’s focus on feasibility and acceptability of the program [38]. There is also a potential selection bias such that the ten participants who completed the study were highly motivated and, therefore, most likely to benefit from the intervention. In addition, the study duration was short, limiting the ability to determine whether there are changes over time. In particular, given that only 8–15% of individuals with MCI convert to AD each year [10], we did not anticipate a significant change in cognitive from baseline to post-assessment. However, the findings of no significant change in cognition over the three-month study period are promising. Future studies should examine the impact of online CBT-I in individuals with cognitive impairment over a longer period of time to determine whether improved sleep might delay cognitive decline.
Another consideration for future studies is the low enrollment rate of eligible patients. Seventy-four of the 173 potential participants (42.8%) chose not to participate for reasons including not interested in study participation, maximum contact attempts reached, and concerns about logging in (see Fig. 2). Twelve of 173 potential participants approached for this study enrolled (6.9%), similar to less than 4% of patients with dementia participating in clinical research studies when approached in person [41]. Understanding and overcoming study recruitment barriers are critical to ensuring full representation of older adults with mild cognitive impairment.
Fig. 2.
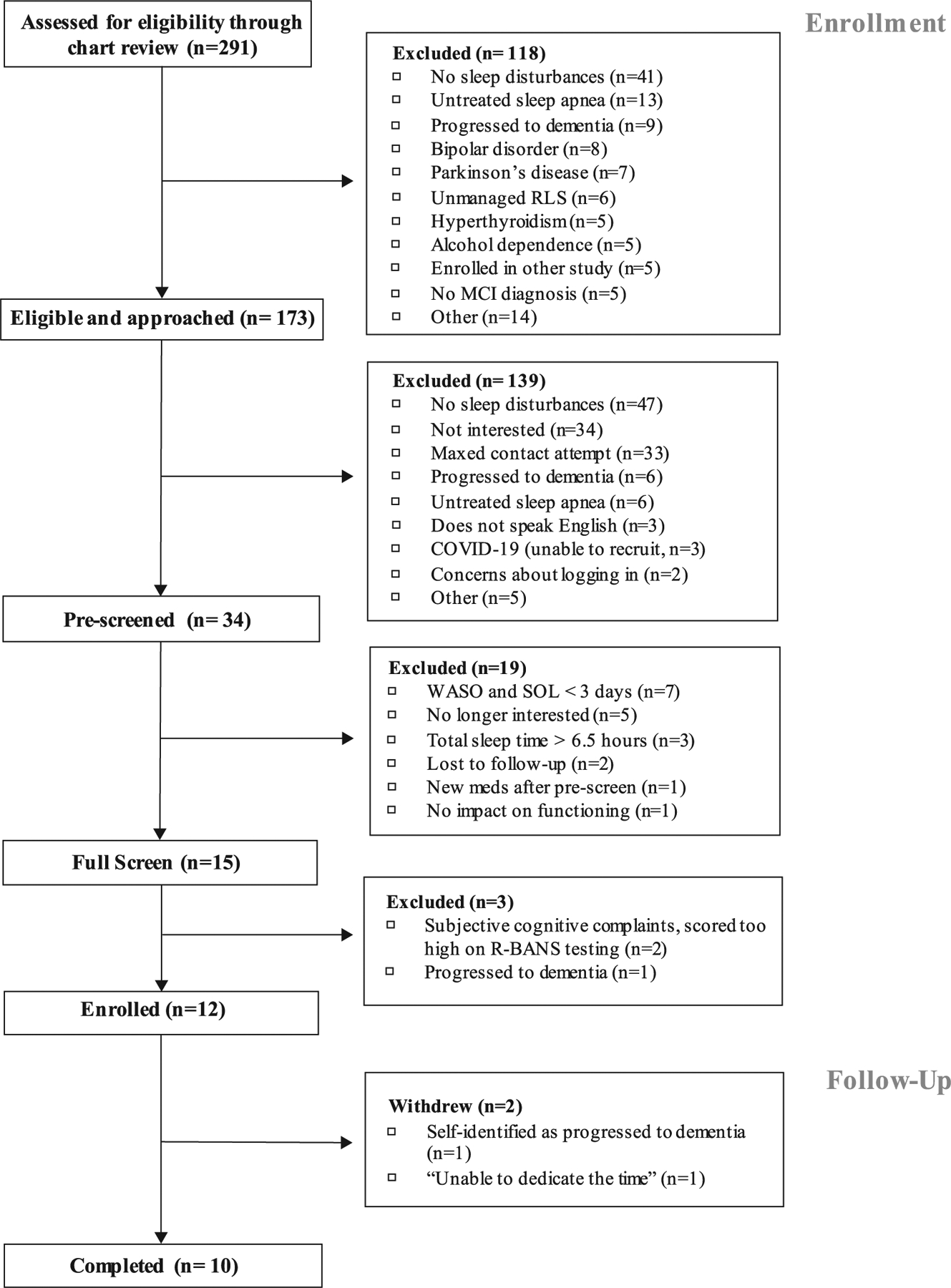
CONSORT: Enrollment and Follow-up.
CONCLUSION
Participants with MCI demonstrated the feasibility, acceptability, and preliminary efficacy of an Internet-delivered, cognitive behavioral therapy for insomnia intervention. Results suggest that older adults with cognitive impairment can independently complete CBT-I via the Internet and can experience clinically improved sleep. Behavioral interventions provide the opportunity to include and engage patients to improve their health without a pharmacological additive, and delivering them using the Internet makes them more available. Both qualitative and quantitative findings from this study should inform potential large-scale trials in this population. Future studies should consider the possibilities of intervening at the crossroads of early cognitive changes and insomnia to influence long-term cognitive health.
Table 4.
Preliminary Efficacy of SHUTi OASIS in Participants with MCI (n = 10)
| Variable | Baseline* | Post-Intervention* | Test statistic | p |
|---|---|---|---|---|
| Montreal Cognitive Assessment (Range 0–30), n = 8 | 21.9 ± 2.8 (18–27) |
21.3 ± 4.5 (14–28) |
t(9) = 0.50 | 0.63 |
| Blind Montreal Cognitive Assessment (Range 0–22) | 15.9 ± 2.3 (13–20) |
15.6 ± 2.2 (13–20) |
t(9) = 0.38 | 0.71 |
| Insomnia Severity Index (Range 0–28) | 13.5 ± 4.8 (5–21) |
8.3 ± 5.3 (0–19) |
t(9) = 4.0 | <0.01 |
| Sleep efficiency, % | 77.3 ± 18.8 (21–99) |
86.5 ± 13.0 (31–99) |
t(99) = −4.8 | <0.001 |
| Total sleep time, min | 371.7 ± 114.9 (120–620) |
396.8 ± 100.4 (120–695) |
t(99) = −2.2 | 0.03 |
| Time in bed, min | 478.9 ± 76.6 (280–665) |
459.5 ± 91.6 (300–715) |
t(99) = 2.2 | 0.03 |
| Total nap duration, min | 10.6 ± 21.4 (0–90) |
23.8 ± 61.8 (0–240) |
t(99) = −2.5 | 0.02 |
| Sleep onset latency, min | 36.0 ± 40.3 (5–180) |
18.3 ± 26.3 (5–150) |
t(99) = 4.3 | <0.001 |
| Night awakenings, # | 1.5 ± 1.5 (0–8) |
1.3 ± 1.1 (0–5) |
t(99) = 1.3 | 0.20 |
| Wake after sleep onset,** min | 71.2 ± 80.6 (0–360) |
44.4 ± 54.5 (0–285) |
t(99) = 3.3 | 0.001 |
mean ± standard deviation (range).
wake after sleep onset includes early morning awakening.
ACKNOWLEDGMENTS
This work is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016; National Institute of Aging 5R01AG047885 (PI: Ritterband). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We want to acknowledge Christina Frederick, Jacqueline Le, Gabriel Heath, and the rest of the UVA Center for Behavioral Health and Technology team for their significant contributions to this study. We also want to recognize Reanna Panagides for her contributions to the qualitative analysis for this work.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0657r2).
REFERENCES
- [1].Ancoli-Israel S, Cooke JR (2005) Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc 53, 264–271. [DOI] [PubMed] [Google Scholar]
- [2].Foley D, Monjan A, Brown S, Simonsick E, Wallace R, DG DB (1995) Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep 18, 425–32. [DOI] [PubMed] [Google Scholar]
- [3].Mellinger G, Balter M, Uhlenhuth E (1985) Insomnia and its treatment: Prevalence and correlates. Arch Gen Psychiatry 42, 225–232. [DOI] [PubMed] [Google Scholar]
- [4].Spira A, Chen-Edinboro L, Wu M, Yaffe K (2014) Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry 27, 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bokenberger K, Ström P, Dahl Aslan AK, Johansson AL, Gatz M, Pedersen NL, Åkerstedt T (2017) Association between sleep characteristics and incident dementia accounting for baseline cognitive status: A prospective population-based study. J Gerontol A Biol Sci Med Sci 72, 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naismith SL, Mowszowski L (2018) Sleep disturbance in mild cognitive impairment: A systematic review of recent findings. Curr Opin Psychiatry 31, 153–159. [DOI] [PubMed] [Google Scholar]
- [7].da Silva R (2015) Sleep disturbances and mild cognitive impairment: A review. Sleep Sci 8, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino FII, Ferrara S, Fermi S, Ferri R, Gelosa G, Lombardi G, Mazzei D, Mearelli S, Morrone E, Murri L, Nobili FM, Passero S, Perri R, Rocchi R, Sucapane P, Tognoni G, Zabberoni S, Sorbi S (2012) Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: A multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 33, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Leon M De, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B (2006) Mild cognitive impairment. Lancet 367, 1262–1270. [DOI] [PubMed] [Google Scholar]
- [10].Petersen RC (2016) Mild cognitive impairment. Clin Geriatr Med 33, 325–337. [DOI] [PubMed] [Google Scholar]
- [11].Krystal A (2009) A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: The empirical basis for U.S. clinical practice. Sleep Med Rev 13, 265–274. [DOI] [PubMed] [Google Scholar]
- [12].Mangoni AA, Jackson SHD (2004) Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br J Clin Pharmacol 57, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, Hanlon JT (2007) Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 370, 173–184. [DOI] [PubMed] [Google Scholar]
- [14].Maher RL, Hanlon J, Hajjar ER (2014) Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 13, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, Barry MJ, Boyd C, Chow RD, Fitterman N, Harris RP, Humphrey LL, Manaker S, McLean R, Mir TP, Schünemann HJ, Vijan S, Wilt T (2016) Management of chronic insomnia disorder in adults: A clinical practice guideline from the American college of physicians. Ann Intern Med 165, 125–133. [DOI] [PubMed] [Google Scholar]
- [16].Schutte-Rodin SL, Broch L, Buysee D, Dorsey C, Sateia M (2008) Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4, 487–504. [PMC free article] [PubMed] [Google Scholar]
- [17].Perlis ML, Smith MT (2008) How can we make CBT-I and other BSM services widely available? J Clin Sleep Med 4, 11–13. [PMC free article] [PubMed] [Google Scholar]
- [18].Zachariae R, Amidi A, Damholdt MF, Clausen CDR, Dahlgaard J, Lord H, Thorndike FP, Ritterband LM (2018) Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: A randomized controlled trial. J Natl Cancer Inst 110, 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ritterband LM, Thorndike FP, Ingersoll KS, Lord HR, Gonder-Frederick L, Frederick C, Quigg MS, Cohn WF, Morin CM (2017) Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: A randomized clinical trial. JAMA Psychiatry 74, 68–75. [DOI] [PubMed] [Google Scholar]
- [20].Ritterband LM, Frederick C, Hilgart M, Murphy B, Johnson S, Heath G, Floryan M, Ingersoll K, Thorndike F Development of an optimized version of SHUTi for older adults. In 10th International Conference of the International Society for Research on Internet Interventions, Auckland, New Zealand. [Google Scholar]
- [21].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neurpsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20), 22–33; quiz 34–57. [PubMed] [Google Scholar]
- [22].Rush A, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Depression 54, 573–583. [DOI] [PubMed] [Google Scholar]
- [23].Mattos MK, Barnes L, Davis EM, Manning CA, Mostafavi M, Quigg MS, Perez H, Sollinger A, Ritterband LM (2020) Preliminary feasibility of technology use in an Internet-delivered intervention: Improving sleep in older adults with mild cognitive impairment. Alzheimers Dement 16 (Suppl 7), e038831. [Google Scholar]
- [24].Thorndike FP, Saylor DK, Bailey E, Gonder-Frederick L, Morin C, Ritterband L (2008) Development and perceived utility and impact of an internet intervention for insomnia. E J Appl Psychol 4, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morin CM, Belleville G, Bélanger L, Ivers H (2011) The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Savard MH, Simard S, Ivers H (2005) Empirical validation of the insomnia severity index in cancer patients. Psychooncology 14, 429–441. [DOI] [PubMed] [Google Scholar]
- [27].Thorndike F, Ritterband L, Saylor D, Magee J, Gonder-Frederick L, Morin C (2011) Validation of the insomnia severity index as a web-based measure. Behav Sleep Med 9, 216–223. [DOI] [PubMed] [Google Scholar]
- [28].Nasreddine Z, Phillips N (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [29].Dawes P, Pye A, Reeves D, Yeung WK, Sheikh S, Thodi C, Charalambous AP, Gallant K, Nasreddine Z, Leroi I (2019) Protocol for the development of versions of the Montreal Cognitive Assessment (MoCA) for people with hearing or vision impairment. BMJ Open 9, e026246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Montreal Cognitive Assessment/ MOCA-Blind, Version 7.1, Last updated 2019, Accessed on 2019.
- [31].Huberman A, Miles M (1994) Data Management and Analysis Methods, Sage Publishing, Inc., Thousand Oaks. [Google Scholar]
- [32].About Alzheimer’s Disease: Treatment | National Institute on Aging. [Google Scholar]
- [33].Atienza M, Ziontz J, Cantero JL (2018) Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep Med Rev 42, 171–183. [DOI] [PubMed] [Google Scholar]
- [34].Grill JD, Raman R, Ernstrom K, Aisen P, Karlawish J (2013) Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology 80, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mattos M, Burke L, Baernholdt M, Hu L, Nilsen M, Lingler J (2019) Perceived social determinants of health among older, rural-dwelling adults with early-stage cognitive impairment. Dementia (London) 18, 920–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lingler JH, Terhorst L, Schulz R, Gentry A, Lopez O (2016) Dyadic analysis of illness perceptions among persons with mild cognitive impairment and their family members. Gerontologist 56, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Christensen H, Batterham PJ, Gosling JA, Ritterband LM, Griffiths KM, Thorndike FP, Glozier N, O’Dea B, Hickie IB, Mackinnon AJ (2016) Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): A randomised controlled trial. Lancet Psychiatry 3, 333–341. [DOI] [PubMed] [Google Scholar]
- [38].Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Bakken S, Kaplan CP, Squiers L, Fabrizio C, Fernandez M (2009) How we design feasibility studies. Am J Prev Med 36, 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Internet/ Broadband Fact Sheet, Last updated 2017, Accessed on 2017.
- [40].Internet Fact Sheet: Who has home broadband, Last updated 2021, Accessed on 2021.
- [41].Alzheimer’s Association (2014) 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10, e47–e92. [DOI] [PubMed] [Google Scholar]
- [42].Nuño MM, Gillen DL, Dosanjh KK, Brook J, Elashoff D, Ringman JM, Grill JD (2017) Attitudes toward clinical trials across the Alzheimer’s disease spectrum. Alzheimers Res Ther 9, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]


