Abstract
Background
Chronic idiopathic thrombocytopenic purpura (ITP) is an acquired autoimmune disorder that is characterized predominantly by a low platelet count. Thrombopoietin (TPO) receptor agonists increase production of platelets by stimulating the TPO receptor in people with chronic ITP.
Objectives
To determine the efficacy and safety of TPO receptor agonists in chronic ITP patients.
Search methods
We searched MEDLINE (from 1950 to March 2011), EMBASE (from 1974 to March 2011), and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3) to identify all randomized trials in chronic ITP. We also contacted authors of included studies and TPO receptor agonists manufacturers.
Selection criteria
Randomized controlled trials (RCTs) comparing TPO receptor agonists alone, or in combination with other drugs, to placebo, no treatment, other drugs, splenectomy or another TPO receptor agonist in patients with chronic ITP.
Data collection and analysis
Two review authors independently screened papers, extracted data and assessed the risk of bias in the included studies.
Main results
Six trials with 808 patients were included. Five studies compared TPO receptor agonists with placebo (romiplostim: 100, eltrombopag: 299, placebo: 175); one study compared TPO receptor agonists with standard of care (SOC) (romiplostim: 157; SOC: 77). SOC included a variety of therapies, such as glucocorticoid, anti‐D immune globulin, intravenous immune globulin, rituximab, azathioprine, and so on. Overall survival, one of our primary outcomes, was not studied by these RCTs and we could not estimate number needed to treat (NNT). Another primary outcome, improving significant bleeding events, did not reveal any significant differences between the TPO receptor agonists group and the control group (placebo or SOC) (versus placebo risk ratio (RR) 0.48, 95% confidence interval (CI) 0.20 to 1.15; versus SOC RR 0.49, 95% CI 0.15 to 1.63).
For secondary outcomes, TPO receptor agonists statistically significantly improved overall platelet response (versus placebo RR 4.06, 95% CI 2.93 to 5.63; versus SOC RR 1.81, 95% CI 1.37 to 2.37), complete response (versus placebo RR 9.29, 95% CI 2.32 to 37.15) and durable response (versus placebo RR 14.16, 95% CI 2.91 to 69.01). There was a significant reduction in overall bleeding events (WHO grades 1 to 4) when compared to placebo (RR 0.78, 95% CI 0.68 to 0.89), but not when compared to SOC(RR 0.97, 95% CI 0.75 to 1.26).
Total adverse events (Grades 1 to 5) were not statistically significantly different between the treatment and control groups(both placebo and SOC) (versus placebo RR 1.04, 95% CI 0.95 to 1.15; versus SOC RR 0.97, 95% CI 0.75 to 1.26). Total serious adverse events (Grade 3 and higher adverse events) were increased when patients receiving treatment with SOC (RR 0.61, 95% CI 0.40 to 0.92), but not receiving treatment with placebo (RR 0.92, 95% CI 0.61 to 1.38).
There are selective and performance biases because of open‐label and inadequate allocation.
Authors' conclusions
There was currently no evidence to support that TPO receptor agonists are effective in chronic ITP. Compared to placebo or SOC, despite significantly increased platelet response, there was no evidence to demonstrate that TPO receptor agonists did improve significant bleeding events in chronic ITP. The effect on overall survival awaits further analysis. Although long‐term studies are lacking, current data demonstrated adverse effects of TPO receptor agonists were similar to that of placebo and SOC.
Plain language summary
TPO receptor agonists for treating chronic idiopathic thrombocytopenic purpura
Chronic idiopathic thrombocytopenic purpura (ITP) is an acquired autoimmune disorder characterized by low platelet counts. To date, the therapies that primarily aim to reduce platelet destruction, such as corticosteroids, intravenous immunoglobulins and splenectomy, have been the mainstay of treatment in ITP. However, TPO receptor agonists such as romiplostim and eltrombopag, which aim to enhance platelet production, are novel drugs that have been suggested to be more effective. This review included six trials with 808 patients and compared TPO receptor agonists with placebo or standard of care (SOC).
None of the studies included overall survival, therefore we could not confirm whether TPO receptor agonists help to prolong life. There is uncertainty as to whether TPO receptor agonists reduce the risk of significant bleeding events in chronic ITP. This review confirms the greater platelet response by using TPO receptor agonists. Adverse effects of TPO receptor agonists were similar to that of placebo and SOC. More research is needed to explore the role of TPO receptor agonists in the treatment of chronic ITP more fully.
Background
Description of the condition
Chronic idiopathic thrombocytopenic purpura (ITP) is an acquired autoimmune disorder that is characterized predominantly by a low platelet count secondary to accelerated platelet destruction by antiplatelet antibodies (Karpaktin 1997). In addition, platelet production can be impaired because the antiplatelet antibody can also damage megakaryocytes (Chang 2003; McMillan 2005). ITP is a diagnosis of exclusion, the diagnosis of ITP requires decreased platelets on the blood film and the exclusion of other causes of thrombocytopenia. Normal or increased numbers of marrow megakaryocytes are found in the majority of patients (George 1998).
No firm data are available on the incidence and prevalence of ITP. The estimated incidence is 100 cases per one million persons per year, with about half occurring in children and half in adults (Cines 2002).
ITP can be classified based on patient age (adult or childhood ITP), and duration of thrombocytopenia (acute or chronic). Acute ITP is defined as thrombocytopenia occurring for less than six months and usually resolving spontaneously, while chronic ITP lasts more than six months and requires therapy.
Adult‐onset and childhood‐onset immune thrombocytopenic purpura are strikingly different. In children, the course of the disease is usually acute, with an abrupt and symptomatic onset, often following viral infection, with a high rate of spontaneous remission (within six months from diagnosis). Only about one‐third of cases evolve into chronic ITP; moreover, boys and girls are equally affected. By contrast, in adult, ITP is generally an insidious disease, with a frequent chronic evolution and is two to three times as common in women as in men (female:male, 3:1) (Cines 2002; Provan 2003).
Although the thrombocytopenia of chronic ITP can be severe, signs of bleeding are usually only minor. In general, the severity and frequency of hemorrhagic manifestations correlate with the platelet count.
Management is predicated primarily on the severity of thrombocytopenia and bleeding.
Those with platelet counts more than 50 × 109/l are not treated.
Patients with platelet counts between 20 × 109/l and 50 × 109/l, in the absence of bleeding or predisposing comorbid conditions such as uncontrolled hypertension, active peptic ulcer disease, anticoagulation, recent surgery or head trauma, do no't require immediate therapy.
Treatment is indicated in all patients who present with significant bleeding and those with platelet counts less than 20 × 109/l (Cines 2005).
Description of the intervention
Currently available therapies for patients with chronic ITP are primarily aimed at reducing platelet destruction, and include corticosteroids, intravenous immunoglobulins, splenectomy, rituximab, azathioprine, cyclophosphamide, danazol and, cyclosporine (Cines 2002; George 2000). Although these treatments are often useful, not all patients respond to them (Arnold 2007; Cines 2005; Kojouri 2004; Vesely 2004).
Recently, the evidence that platelet production in ITP is often suboptimal has lead to the use of treatments aimed at enhancing thrombopoiesis (Jenkins 2007; Kuter 2007; Kuter 2008d; Newland 2006). This approach has focused on thrombopoietic platelet growth factors that increase production of platelets by stimulating the thrombopoietin (TPO) receptor. In previous studies, a recombinant human thrombopoietin (rHuTPO) and pegylated recombinant human megakaryocyte growth and development factor (PEG‐rHuMGDF), as the first‐generation thrombopoietic growth factors, increased platelet counts in healthy patients as well as those with thrombocytopenic disorders. However, the antibody formation to pegylated recombinant human megakaryocyte growth and development factor (PEG‐rHuMGDF) and the antibodies cross‐reaction with endogenous thrombopoietin in the patients, caused severe, persistent thrombocytopenia. As a result, the clinical development of human recombinant TPOs was suspended in 1998 (Kuter 2007). Despite the risk of antigenicity, the success of recombinant TPOs in increasing platelet counts provided a stimulus for continuing the search and development of new thrombopoietic platelet growth factors. Currently, at least seven different thrombopoietin receptor agonists are in various stages of preclinical and clinical development. The second‐generation thrombopoietic agonists can be divided into three categories: TPO peptide mimetics (Fab59, Peg‐TPOmp, romiplostim), TPO non‐peptide mimetics (AKR‐501, eltrombopag), and TPO agonist antibodies (Minibodies, MA01G4G344). Among these second‐generation thrombopoietic growth factors, two have completed extensive phase III studies in patients with ITP: romiplostim and eltrombopag (Kuter 2008d).
Romiplostim is currently being administered as a subcutaneous injection every week for 24 weeks and doses from 0.1 to 2.0 g/kg demonstrated a dose‐dependent rise in platelet count, starting at day five and peaking at days 12 to 15. Eltrombopag is administered as a once‐daily oral pill taken on an empty stomach for six weeks and a dose‐dependent rise in platelet count was seen at doses of 30 mg, 50 mg, and 75 mg. At the 75 mg dose, the platelet count rise started on day seven and peaked at day 16. Adverse effects were similar in patients given romiplostim or eltrombopag and placebo, such as bleeding, headache, nasopharyngitis, nausea, diarrhoea, vomiting, arthralgia, fatigue, myalgia, etc. In general, both eltrombopag and romiplostim have been well‐tolerated (Kuter 2007).
How the intervention might work
Preclinical and clinical studies have shown the efficacy and safety of TPO receptor agonists in managing chronic ITP. They bind to and activate the human thrombopoietin receptor, called c‐Mpl, which is expressed in pluripotent hematopoietic stem cells (Solar 1998) and in megakaryocyte progenitors and megakaryocyte (Debili 1995). C‐Mpl's extra‐cellular region contains two cytokine receptor homology modules (CRMs) (Kuter 2007). TPO binds only the distal CRM and thereby initiates signal transduction. The resulting signal transduction cascade through the JAK/STAT, Ras, and mitogen‐activated protein kinase pathways leads to megakaryocyte differentiation and platelet production (Kuter 2008d).
Romiplostim (AMG 531, Nplate) is a subcutaneously administered, peptide mimetic thrombopoietin‐receptor agonist. It is a 60 kDa molecule that consists of disulfide‐bonded human IgG1 heavy chain and Kappa light‐chain constant regions (an Fc fragment) with two identical peptide sequences linked covalently through a polyglycine bridge atresidue 228 of each of the heavy chains (Wang 2004). The peptide portion was selected by screening libraries of peptides that have no sequence homology with human thrombopoietin to find one with a tertiary structure that would allow it to bind to and activate the human thrombopoietin receptor, then stimulate megakaryopoiesis and induce increases in platelet count. Romiplostim did not induce neutralizing or cross‐reacting antibodies against TPO.
Eltrombopag (Promacta, SB‐497115) is an orally bioavailable, non‐peptide mimetic thrombopoietin‐receptor agonist. It is a hydrazone small molecule with a molecular weight of 564 kDa (Erickson‐Miller 2005). Eltrombopag initiates the JAK‐STAT pathway by interacting with the transmembrane domain of TPO receptor, thereby promoting megakaryocyte differentiation and proliferation of human bone marrow cells in the megakaryocytic lineage (Erickson‐Miller 2005). Eltrombopag shows species specificity by binding only to a histidine in position 499 in the human and chimpanzee transmembrane domain of TPO receptor, whereas all the other species have a leucine (Erickson‐Miller 2004). In a word, Eltrombopag activates the TPO receptor by a different way than rHuTPO, and its biological effects are additive to the endogenous TPO (Panzer 2009).
Why it is important to do this review
These TPO receptor agonists have been used in chronic ITP in attempt to stimulate the production of platelets and increase platelet counts. Several randomized trials investigating the use of romiplostim and eltrombopag in chronic ITP have been completed and these agents have been found to be highly effective and apparently safe. However, the evidence on the effects of romiplostim and eltrombopag has not been systematically assessed. Considering the lack of conclusive evidence on the role of TPO receptor agonist in chronic ITP, a systematic review and meta‐analysis combining data from several randomized controlled trials is needed to critically assess the current evidence on the role of TPO receptor agonists in chronic ITP.
Objectives
To determine the efficacy and safety of TPO receptor agonists in chronic ITP patients.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled studies. We excluded other types of studies, such as quasi‐randomized trials and cross‐over trials, which might increase bias‐related issues.
Types of participants
Adults and children, (male and female), with a clinical diagnosis of chronic ITP and a platelet count less than 30 × 109/l, irrespective of prior treatment.
Types of interventions
TPO receptor agonists versus placebo or no treatment..
TPO receptor agonists plus other drugs versus the other drugs only.
TPO receptor agonists versus other drugs.
TPO receptor agonists versus splenectomy.
TPO receptor agonists versus another TPO receptor agonists (different types or dosages).
Types of outcome measures
Primary outcomes
Overall survival.
Incidence of severe bleeding (WHO grade III and IV) according to the World Health Organization (WHO bleeding scale):
grade 0, no bleeding;
grade 1, petechiae;
grade 2, mild blood loss;
grade 3, gross blood loss;
grade 4, debilitating blood loss.
Secondary outcomes
Overall platelet response (durable plus transient rates of platelet response).
All subtypes of responses (we defined a complete response (CR) as an increase in platelet counts to > 150 × 109/l; we defined a partial response (PR) as an increase in the platelet count to between 50 and 150 × 109/l) (Cooper 2004).
Durable response (platelet count ≥ 50 × 109/L during six or more of the last eight weeks of treatment) (Kuter 2008c).
Time to platelet increase from the start of therapy (we defined platelet increase as a platelet count of 50 × 109/l or more).
Incidence and severity of bleeding.
Incidence of adverse effects.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 3.0.
grade 1, mild;
grade 2, moderate;
grade 3, severe;
grade 4, life threatening;
grade 5, fatal.
Search methods for identification of studies
Electronic searches
We adapted the search strategies from those suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2009) and the Cochrane Haematological Malignancies Group (CHMG), without any restriction on language.
Electronic searches
Bibliographic databases
MEDLINE (OVID) (1950 to March 2011) (see Appendix 1).
EMBASE (1974 to March 2011) (see Appendix 2).
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3) (see Appendix 3).
Conference proceedings
We searched conference proceedings of relevant conferences of the following societies (up to the end of December 2010) for the years that are not included in CENTRAL:
American Society of Hematology (ASH): Blood (2001 to December 2010 electronically).
Electronic searches in databases of ongoing trials
The National Research Register http://www.nihr.ac.uk/Pages/NRRArchiveSearch.aspx).
Clinical Trials (http://www.clinicaltrials.gov).
Chinese Clinical Trial Register (http://www.chictr.org).
Australian New Zealand Clinical Trial Registry (http://www.anzctr.org.au/).
WHO ICTRP Search Portal (http://www.who.int/ictrp/network/en/index.html).
The metaRegister (http://www.controlled‐trials.com/mrct/).
Searching other resources
Handsearch of references
We checked references of all identified trials, relevant review articles and current treatment guidelines for further literature.
Institutions
We searched the websites of relevant institutions, agencies (for example the Food and Drug Administration (FDA)), organizations (for example ITP study groups), and societies (pharmaceutical companies) for completed and ongoing studies.
Personal contacts
We contacted the authors of relevant studies, study groups, experts, and investigators from pharmaceutical companies worldwide who are known to be active in the field for unpublished material or further information on ongoing studies.
Data collection and analysis
Selection of studies
Two review authors (XD, XN) scanned the titles and abstracts of studies for eligibility; moreover, we obtained a full‐text version for assessment for all papers where study eligibility was uncertain. Disagreement between these review authors was resolved by discussion. Two review authors (XD and XN) reviewed full‐text articles of studies identified, to decide their inclusion in the meta‐analysis, following an inclusion criteria form. The criteria for eligibility were:
randomized controlled trials, including adults and children;
platelet counts below 30 × 109/l;
diagnosis of chronic ITP; and
comparing TPOs with one of the comparators listed above
Any disagreement between the two review authors was resolved by a third review author (YZ), with an adjudicating role.
Data extraction and management
Two review authors (YZ and XD) independently extracted information from the included trials using a standardized data collection form. Any differences in data extraction were resolved through discussion and, where necessary, in consultation with a third author (JX). We compared data extractions and discussed inconsistencies until consensus was reached.
This form included the following items:
General information: title, authors, journal/source, contact address, country of origin, language, publication type, year of publication, setting of trial.
Trial characteristics including design, sample size, setting, location of trial, randomization method, concealment of allocation, blinding of patients and clinicians, withdrawals, median length of follow up, funding, conflict of interest statement.
Study interventions basics: disease(s)/stage(s) studied, category of treatment investigated, inclusion criteria, exclusion criteria, experimental intervention, control intervention, type of control, additional treatment, compliance, outcomes assessed, subgroup evaluated, confounders.
Baseline characteristics of patients: number of patients, age, ethnicity, gender, diagnosis, definition of diagnosis, extent of disease, organ involvement, additional diagnoses in group, stage, previous treatment, concurrent conditions.
Treatment details according to study protocol: primary intervention (medication, dosage, administration).
Outcomes: definition of outcome, timing of assessment, statistics, length of follow up, reasons for dropout, reasons for exclusion, source of information.
If necessary, we contacted the article's principal author to clarify data and obtain additional information about the extracted data.
Assessment of risk of bias in included studies
Two review authors (XD, XN) assessed the quality of eligible studies independently. Any disagreements were discussed together with ZY until consensus was obtained. We evaluated quality using an assessment form designed for the topic of this review. The following criteria were considered:
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated intervention adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a high risk of bias?
For any criterion that was unclear, we contacted the corresponding author of the study. We explored the influence of study quality on the treatment effect in sensitivity analyses.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI). We also calculated and reported numbers needed to treat (NNT) for outcomes where the RR was statistically significant. For unwanted effects (e.g. adverse events), this became the number needed to harm (NNH).
Continuous data
We used the mean difference if outcomes were measured in the same way between studies. We used the standardized mean difference to combine studies that measured the same outcome, but used different methods.
Time‐to‐event data would have been analyzed if information had been available. For future updates of this review with long‐term follow‐up data available, we will include survival data in our analyses and calculate hazard ratios (HR) using the methods suggested by Tierney (Tierney 2007).
Dealing with missing data
We dealt with missing data as follows (Higgins 2009).
Contacted the original investigators to request missing data whenever possible.
Made explicit the assumptions of any methods used to cope with missing data. The missing data were imputed with replacement values, and these treated as if they were observed (e.g. last observation carried forward, imputing an assumed outcome such as assuming all were poor outcomes, imputing the mean, imputing based on predicted values from a regression analysis).
Performed sensitivity analyses to assess how sensitive results are to reasonable changes in the assumptions that are made.
Addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We tested heterogeneity between studies with RevMan software, version 5 (RevMan 2008) based on the results of the respective risk of bias questions, using the I² statistic and the Chi² test, with significance being set at P < 0.05. We explored causes of heterogeneity by subgroup analysis.
Assessment of reporting biases
We would have assessed publication bias by a funnel plot if the number of studies included was equal to or more than 10.
Data synthesis
If data were sufficiently similar, we carried out meta‐analysis using Review Manager 5.0. We used the fixed‐effect model for pooled analysis of data, while we used the random‐effects model as a sensitivity analysis. We did not intend to combine results of trials with different comparator drugs (Elbourne 2002).
Subgroup analysis and investigation of heterogeneity
We planned to explore the following potential sources of heterogeneity using subgroup analyses or meta‐regression (Deeks 2009):
Different initial platelet count (less than 20 × 109/l versus between 20 to 30 × 109/l);
Age;
Pretreated versus not pretreated patients;
Dosage;
Duration of treatment; and
Patients requiring treatment according to guidelines or not (or proportion of patients requiring treatment).
For this review, we did not carry out the subgroup analyses mentioned above because there was no statistically significant heterogeneity among the trials according to the results.
Sensitivity analysis
We planned the following sensitivity analyses in order to explore effect size differences and the robustness of conclusions:
Exclusion of studies with inadequate concealment of allocation;
Exclusion of studies in which blinding was not adequate;
Comparing the difference of pooling analysis results by using fixed‐effect model and random‐effect model. Robust evidence should be conversed by changing the effect model);
Exclusion of studies with inadequately generating allocation sequence;
Exclusion of studies with inadequately preventing allocated intervention; and
Exclusion of studies with other problems that could put it at a high risk of bias.
Results
Description of studies
Results of the search
The search of electronic databases in March 2011 resulted in 656 records. Handsearching of the American Society of Hematology (ASH) 2010 conference abstracts resulted in 74 additional records. We excluded 720 based on title or abstract. We retrieved ten full‐text articles. We excluded two articles after reviewing the full text (see 'Characteristics of excluded studies' for further details).
Included studies
A total of six studies met the inclusion criteria (Bussel 2006; Bussel 2009a; Cheng 2011; Kuter 2008a; Kuter 2008b; Kuter 2010). Bussel 2006 referred to the clinical trial: NCT00111475; Bussel 2009a referred to the clinical trial: NCT00102739; Cheng 2011 referred to the clinical trial: NCT00370331; Kuter 2008a referred to the clinical trial: NCT00102323; Kuter 2008b referred to the clinical trial: NCT00102336; and Kuter 2010 referred to the clinical trial: NCT00415532. (all registered in http://www.ClinicalTrials.gov). None of the eligible trials studied our primary outcome of overall survival.
Details of the methods, participants, interventions and outcome measures of individual studies are provided in the 'Characteristics of included studies'.
Design
All included studies except Kuter 2010 were reported to be randomized, double‐blind, placebo‐controlled trials. Bussel 2006 reported a ratio of romiplostim to placebo of 4:1. Bussel 2009a was conducted in two parts (Part A and Part B); Part A (Bussel 2007) reported that patients were randomly assigned 1:1:1:1 to receive placebo or 30 mg, 50 mg, or 75 mg of eltrombopag and Part B (Bussel 2009) reported a randomization ratio of 2:1 for eltrombopag to placebo. Cheng 2011 reported a ratio of eltrombopag to placebo of 2:1. Kuter 2008a and Kuter 2008b reported a randomization ratio of 2:1 for romiplostim to placebo. Furthermore, randomization was stratified by concomitant ITP medication (yes or no), splenectomy (yes or no), and the baseline platelet count (> 15,000 per cubic millimeter versus ≤ 15,000 per cubic millimeter) in Bussel 2009a and stratified by concurrent ITP therapy (yes or no) in Kuter 2008a and Kuter 2008b. Kuter 2010 was reported to be a randomized, open‐label, medical standard of care (SOC)‐controlled trial, and Kuter 2010 reported that patients were randomly assigned 2:1 to receive romiplostim or the medical standard of care and were stratified on the basis of geographic region.
Sample sizes
Sample sizes ranged from 21 patients in Bussel 2006 to 234 patients in Kuter 2010.
Setting
All included studies were reported as 'multicenter' trials. Bussel 2006 reported a multicenter study conducted in nine US centers; Bussel 2009a reported that the study was conducted at 90 sites in 23 countries; Cheng 2011 reported that the study was conducted at 75 sites in 23 countries; Kuter 2008a and Kuter 2008b reported a multicenter study conducted in 35 centers in the USA and Europe; and Kuter 2010 reported a multicenter study conducted in 85 sites in North America, Europe and Australia.
Participants
Six studies with 808 patients were included. Of those 556 patients were randomized to TPO receptor agonists (romiplostim: 257 and eltrombopag: 299), 175 to placebo and 77 to medical standard of care. The majority of patients were white women. The median age of participants in all the studies was early to mid‐50s. To be eligible for the studies, patients had to have had chronic ITP for at least six months with a platelet count (PLT) ≤ 30 × 109/l, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids, must have been stable for at least one month, and patients had to be more than 18 years old. Moreover, eligible patients in Kuter 2008a had to have had a splenectomy for the treatment for ITP four weeks before screening, while patients in Kuter 2008b and Kuter 2010 must have not received splenectomy. (Table 1)
1. Patients’ characteristics.
| Study ID | Number of patients | Median age (range) |
Race white |
Race other |
Female | Male | Baseline platelet count | Previous ITP treatments ‐ no. (%) ≥ 3 | Concomitant ITP medication ‐ no. (%) | Splenectomy ‐ no. (%) |
| Bussel 2006 | 21 | 46 (19 to 64) | 14 (67%) | 7 (33%) | 15 (71%) | 6 (29%) | 16,000/m3 | 12 (57%) | 7 (33%) | 14 (67%) |
| Bussel 2009a | 231 | 49 (18 to 85) | 177 (77%) | 54 (23%) | 143 (62%) | 88 (38%) | 15,000/m3 | 118 (51%) | 87 (38%) | 100 (43%) |
| Cheng 2011 | 197 | 49.5 (34 to 63) | 145 (74%) | 52 (26%) | 136 (69%) | 61 (31%) | 16,000/m3 | 107 (54%) | 94 (48%) | 71 (36%) |
| Kuter 2008a | 63 | 53 (26 to 88) | 53 (84%) | 10 (16%) | 38 (60%) | 25 (40%) | 14,500/m3 | 59 (94%) | 18 (29%) | 63 (100%) |
| Kuter 2008b | 62 | 49 (21 to 88) | 49 (79%) | 13 (21%) | 43 (69%) | 19 (31%) | 19,000/m3 | 20 (32%) | 21 (34%) | 0 (0) |
| Kuter 2010 | 234 | 57 (18 to 90) | 206 (88%) | 28 (12%) | 131 (56%) | 103 (44%) | 29,000/m3 | not mentioned | not mentioned | 0 (0) |
ITP: idiopathic thrombocytopenic purpura PLT: platelet count
Intervention
Romiplostim was administered by subcutaneous (SC) injection once per week in Bussel 2006; Kuter 2008a,Kuter 2008b and Kuter 2010. The duration of studies ranged from six weeks (Bussel 2006) to 52 weeks (Kuter 2010). Bussel 2006 had three dosages of romiplostim (1, 3, 6 μg/kg) and the 6 μg cohort were eliminated later. In Kuter 2008a and Kuter 2008b, the starting dose was 1 μg/kg and doses could be increased according to the following algorithm: 2 μg/kg every week if the count was 10 × 109/L or less and 2 μg/kg every 2 weeks if 11 × 109/L to 50 × 109/L. Once platelets reached more than 50 × 109/L, the maintenance algorithm was used: dose was increased by 1 μg/kg every week if 10 × 109/L or less; increased by 1 μg/kg after 2 weeks if 11 × 109/L to 50 × 109/L; reduced by 1 μg/kg after two consecutive weeks at 201 × 109/L to 400 × 109/L; withheld if more than 400 × 109/L and subsequent doses reduced by 1 μg/kg and given after the count was less than 200 × 10/L. The maximum allowed dose was 15 μg/kg.
In Kuter 2010, the starting dose of 3 μg/kg and dose adjustments were made according to the following guidelines: dose was increased by 1 μg/kg every week if the count was less than 30×109/L; dose may be adjusted (increased or decreased) in increments of 1 μg/kg at the investigator's discretion no more frequently than every two weeks when the count was between 30 and 450 × 109/L; dose was held if the count was more than 450 × 109/L, the dose was reduced by 1 μg/kg at the next scheduled dosing day when platelet count had fallen to < 200 × 109/L. The maximum permitted dose of AMG 531 was 10 μg/kg.
Eltrombopag was administere orally once daily in Bussel 2009a and Cheng 2011. The duration of studies ranged from 6 weeks (Bussel 2009a) to 6 months (Cheng 2011). In part A of Bussel 2009a, subjects receiving placebo or active drug (30, 50, and 75 mg) per day respectively (Bussel 2007). In Part B of Bussel 2009a and in Cheng 2011, subjects started on placebo or active drug (50 mg) and may have a dose increase to 75 mg based upon their platelet count at day 22 (Bussel 2009; Cheng 2011).
All included studies except Kuter 2010 compared TPO receptor agonists with one placebo. Kuter 2010 used medical SOC treatments as control intervention. Since no single drug has been established as the standard treatment for immune thrombocytopenia, investigators compared romiplostim with a variety of standard‐of‐care therapies, such as glucocorticoid, anti‐D immune globulin, intravenous immune globulin, rituximab, azathioprine, and so on. Medical SOC treatments were selected and prescribed by the investigators according to standard institutional practices or therapeutic guidelines. Dose adjustment of medical SOC for ITP was allowed throughout the study, and medical SOC treatments was obtained through the usual commercial routes.
Outcomes
All studies assessed overall platelet response, incidence and severity of bleeding and incidence of adverse events as primary outcomes or secondary outcomes. Moreover, Kuter 2008a and Kuter 2008b reported incidence of durable platelet response as the primary outcome; Kuter 2010 reported incidence of treatment failure and incidence of splenectomy as the primary outcome. The outcomes and their definitions are specified in the table 'Characteristics of included studies'.
Bussel 2006 and Bussel 2009a reported the timing of the primary outcome at six weeks, Cheng 2011 reported the timing of the primary outcome at six months, Kuter 2008a and Kuter 2008b measured the primary outcome at 24 weeks, and Kuter 2010 reported the timing of the primary outcome at 52 weeks.
Excluded studies
We excluded two publications (Bussel 2009b; Kuter 2009) because they were non‐randomized trials or included non‐randomized data (see 'Characteristics of excluded studies'). Three studies fulfilled the inclusion criteria, however, currently these studies are ongoing and no results are reported (Amgen 2007; Amgen 2010; GlaxoSmithKline 2009).
Risk of bias in included studies
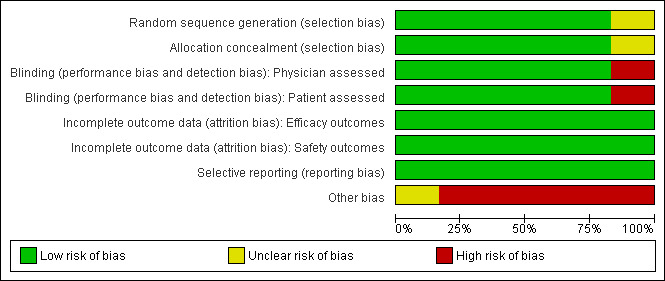
We contacted study authors, Amgen and GlaxoSmithKline and asked them to provide further details about the methods of concealing allocation and blinding in those studies where this was not clear from the published study report. The risk of bias of the included studies (Bussel 2006; Bussel 2009a; Cheng 2011; Kuter 2008a; Kuter 2008b; Kuter 2010) is summarized in Figure 1 and Figure 2.
1.
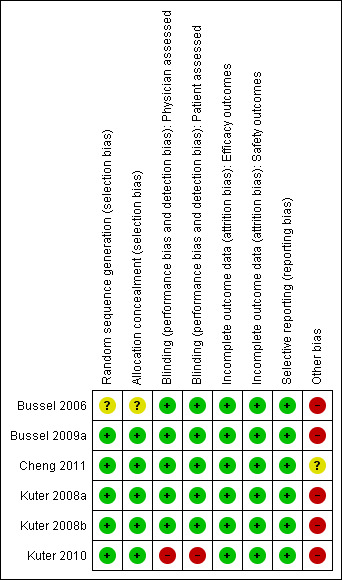
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
2.
Allocation
Five studies (Bussel 2009a; Cheng 2011; Kuter 2008b; Kuter 2008a; Kuter 2010) were judged as having adequate allocation concealment, and one study (Bussel 2006) as being unclear.
Blinding
All studies except Kuter 2010 were reported as 'double‐blind'. However, Bussel 2006; Bussel 2009a; Cheng 2011; Kuter 2008a and Kuter 2008b did not give any further details and additional information was not available from the authors or drug company. Kuter 2010 was reported as 'open‐label'.
Incomplete outcome data
In judging the risk of bias for this item, we considered a less than 80% completion rate in the treatment group as a high risk of bias. We also assessed whether missing data were imputed appropriately and whether an intention‐to‐treat analysis was reported for the primary outcome.
Kuter 2008a and Kuter 2008b included all subjects into the full, efficacy, and safety analysis datasets. In Bussel 2006 and Bussel 2009a, there were 4.8% and 5.2% patients excluded from efficacy analysis, respectively, and these two studies did not meet the standards required for an intention‐to‐treat (ITT) analysis. Moreover, these studies (Bussel 2006; Bussel 2009a) excluded patients from efficacy analyses but included them in safety analyses, therefore these studies may have a higher risk of bias for efficacy outcomes compared to safety outcomes. Cheng 2011 included all subjects in efficacy analysis and excluded 0.5% patients from safety analysis, and Kuter 2010 included all subjects in efficacy analysis and excluded 3.0% patients from safety analysis, therefore Cheng 2011 and Kuter 2010 may have a higher risk of bias for safety outcomes compared to efficacy outcomes.
Selective reporting
All studies reported a primary outcome measure. The time points reported in all of the studies were reasonable. There is little risk of bias due to selective reporting in these studies. See 'Characteristics of included studies' for further details.
Other potential sources of bias
The studies on eltrombopag (Bussel 2009a; Cheng 2011) were sponsored by GlaxoSmithKline, the manufacturer of eltrombopag, and the studies on romiplostim (Bussel 2006; Kuter 2008a; Kuter 2008b; Kuter 2010) were sponsored by Amgen, the manufacturer of romiplostim.
Effects of interventions
Six studies with 808 patients were included. Five trials (Bussel 2006; Bussel 2009a; Cheng 2011; Kuter 2008a; Kuter 2008b) compared TPO receptor agonists versus placebo (romiplostim: 100, eltrombopag: 299, placebo: 175). One trial (Kuter 2010) compared TPO receptor agonists versus SOC (romiplostim: 157; SOC: 77). SOC therapies included glucocorticoid, anti‐D immune globulin, intravenous immune globulin, rituximab, azathioprine, vincristine, cyclosporine, tranexamic acid, ascorbic acid, calcium, ethamsylate, pantoprazole, Expasyl and platelet transfusions. Medical SOC treatments were selected and prescribed by the investigators according to standard institutional practices or therapeutic guidelines.
Overall survival (primary outcome)
None of the six included studies studied overall survival.
TPO receptor agonists versus placebo (five trials)
Incidence of significant bleeding events (those rated severe, life‐threatening, or fatal) (primary outcome)
Cheng 2011, Kuter 2008a and Kuter 2008b provided data on incidence of significant bleeding events (those rated severe, life‐threatening, or fatal) and included 321 patients. Treatment with TPO receptor agonists results in a similar incidence of significant bleeding events compared to placebo (RR 0.48, 95% CI 0.20 to 1.15, P=0.10). We did not estimate NNH due to the absence of statistically significant results. There was no significant statistical heterogeneity detected among these studies (Chi2=0.55, degrees of freedom (df )=2, P=0.76). (Figure 3).
3.
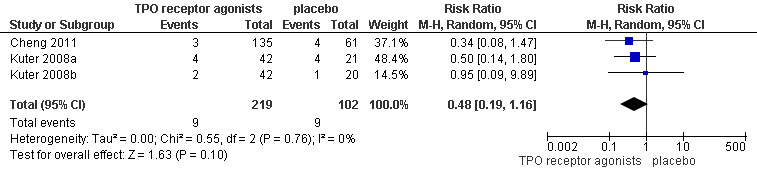
Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.1 Incidence of significant bleeding events (severe, life‐threatening or fatal).
Overall platelet response
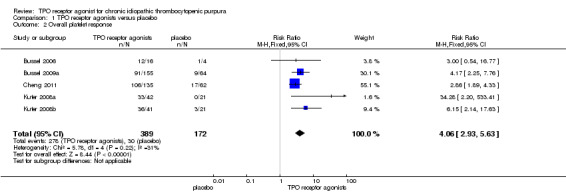
Data on overall platelet response were available for five studies, both on romiplostim and eltrombopag including 561 patients (Bussel 2006; Bussel 2009a; Cheng 2011; Kuter 2008a; Kuter 2008b). The response rates were significantly higher with the use of TPO receptor agonists compared with placebo (RR 4.06, 95% CI 2.93 to 5.63, P<0.00001). Given the overall platelet response rate of 17.4% with placebo, two patients would have to be treated with TPO receptor agonists to achieve one additional overall platelet response (NNT=1.88, 95% CI 1.24 to 2.98). There was no significant statistical heterogeneity detected among these studies (Chi2=5.78, df =4, P=0.22). (Figure 4).
4.

Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.2 Overall platelet response.
Durable response
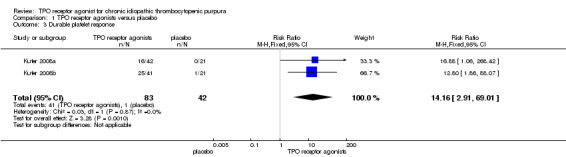
Data on duration of response were available for two parallel studies on romiplostim, including 125 patients (Kuter 2008a; Kuter 2008b). The duration response rates were significantly higher with the use of TPO receptor agonists compared with placebo (RR 14.16, 95% CI 2.91 to 69.01, P = 0.001). Given the duration response rate of 2% with placebo, four patients would have to be treated with TPO receptor agonists to achieve one additional duration response (NNT = 3.80, 95% CI 0.735 to 26.18). There was no significant statistical heterogeneity detected among these studies (Chi2 = 0.03, df = 1, P = 0.87) (Figure 5).
5.

Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.3 Durable platelet response.
Complete response
Data on complete response were available for the Bussel 2009a study on eltrombopag, including 219 patients. The complete response rates were statistically significantly higher with the use of TPO receptor agonists compared with placebo (RR 9.29, 95% CI 2.32 to 37.15, P = 0.002). Given the complete response rate of 3.1% with placebo, four patients would have to be treated with TPO receptor agonists to achieve one additional complete response (NNT = 3.89, 95% CI 0.89 to 24.44). (Figure 6).
6.
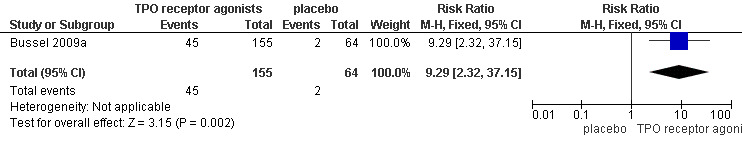
Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.4 Complete response.
Number of weeks with platelet response
Data on number of weeks with platelet response were available for two studies on romiplostim (Kuter 2008a; Kuter 2008b). The number of weeks with platelet response was significantly longer with the use of romiplostim compared with placebo (MD 12.88, 95% CI 11.08 to 14.69, P < 0.00001) (Figure 7).
7.
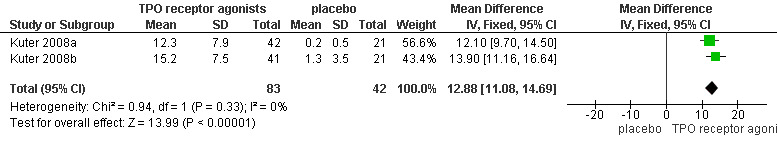
Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.5 Number of weeks with platelet response.
Incidence of bleeding events (WHO grades1 to 4)
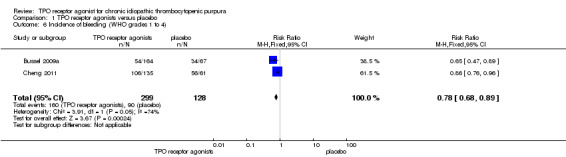
Bussel 2009a and Cheng 2011 provided data on incidence of bleeding events (WHO grades 1 to 4) and included 427 patients. Treatment with TPO receptor agonists results in a lower incidence of bleeding events (WHO grades1 to 4) compared to placebo (RR 0.78, 95% CI 0.68 to 0.89, P=0.0002). Given the incidence of bleeding events of 70% with placebo, seven patients would have to be treated with TPO receptor agonists to avoid one additional bleeding events (NNT=6.49, 95% CI 4.46 to 12.99). (Figure 8).
8.
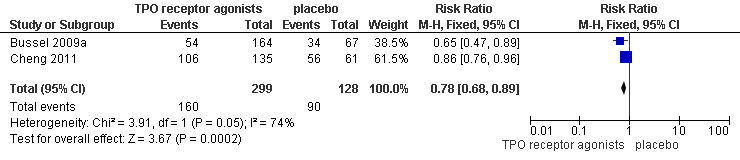
Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.6 Incidence of bleeding (WHO grades 1 to 4).
Total adverse events (Grades1 to 5)
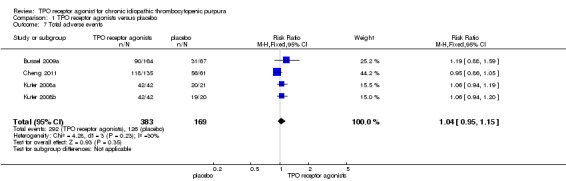
Four trials (Bussel 2009a; Cheng 2011; Kuter 2008a; Kuter 2008b) reported total adverse events. Almost all adverse events were rated as mild to moderate, such as dizziness, insomnia, myalgia, extremity pain, abdominal pain, vomiting and nausea. The TPO receptor agonists and placebo groups had similar frequencies of total adverse events (RR 1.04, 95% CI 0.95 to 1.15, P=0.35) and there was no significant statistical heterogeneity detected among these studies (Chi2=4.28, df =3, P=0.23). (Figure 9).
9.
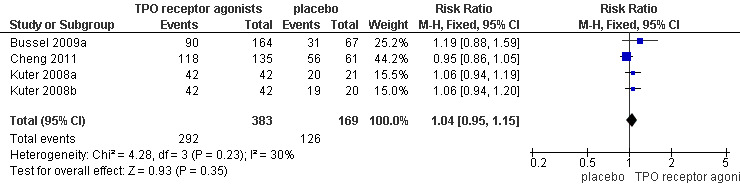
Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.7 Total adverse events.
Total serious adverse events (Grades 3 to 5)
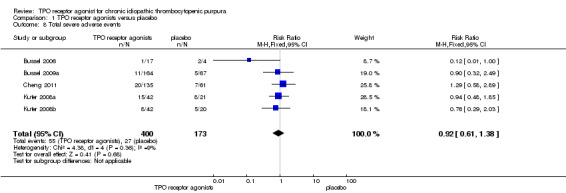
Five trials (Bussel 2006; Bussel 2009a; Cheng 2011; Kuter 2008a; Kuter 2008b) reported total serious adverse events (Grade 3 and higher adverse events). Treatment with TPO receptor agonists results in similar frequencies of total serious adverse events compared to placebo (RR 0.92, 95% CI 0.61 to 1.38, P=0.68). There was no significant statistical heterogeneity detected among these studies (Chi2=4.38, df =4, P=0.36). (Figure 10).
10.
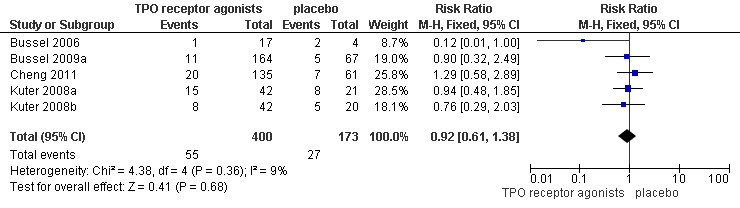
Forest plot of comparison: 1 TPO receptor agonists versus placebo, outcome: 1.8 Total severe adverse events.
Three uncommon, serious adverse effects were reported: reversible bone marrow reticulin formation occurred in two patients treated with romiplostim (Kuter 2008a; Kuter 2008b); thromboembolic events occurred in two patients who received romiplostim, three patients received eltrombopag, and one patient who received placebo (Bussel 2006; Cheng 2011; Kuter 2008a; Kuter 2008b); 11 patients in the eltrombopag group and four patients in the placebo group had reports of cataract (Bussel 2009a; Cheng 2011).
TPO receptor agonists versus standard of care (SOC) (one trial)
Incidence of significant bleeding events (those rated severe, life‐threatening, or fatal) (primary outcome)
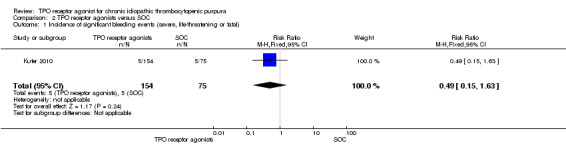
Kuter 2010 provided data on the incidence of significant bleeding events (those rated severe, life‐threatening, or fatal) and included 229 patients. The TPO receptor agonists and SOC had similar frequencies of incidence of significant bleeding events (RR 0.49, 95% CI 0.15 to 1.63, P = 0.24). We did not estimate the NNH due to the absence of statistically significant results. (Figure 11).
11.
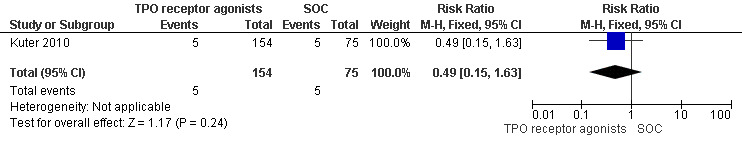
Forest plot of comparison: 2 TPO receptor agonists versus SOC, outcome: 2.1 Incidence of significant bleeding events (severe, life‐threatening or fatal).
Overall platelet response
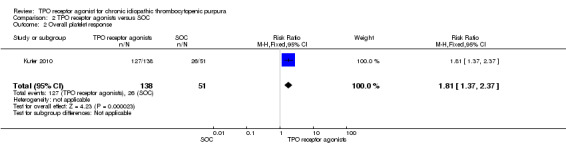
Data on overall platelet response were available for the Kuter 2010 study on romiplostim at 52 weeks including 189 patients.
The platelet response rates were significantly higher with the use of TPO receptor agonists compared with SOC (RR 1.81, 95% CI 1.37 to 2.37, P < 0.0001). Given the overall platelet response rate of 51% with SOC, three patient would have to be treated with TPO receptor agonists to achieve one additional overall platelet responses (NNT = 2.42, 95% CI 1.43 to 5.3) (Figure 12).
12.

Forest plot of comparison: 2 TPO receptor agonists versus SOC, outcome: 2.2 Overall platelet response.
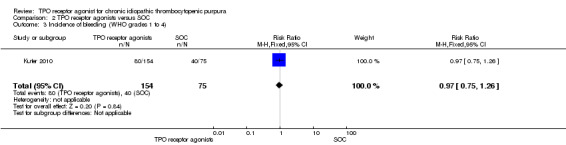
Incidence of bleeding events (WHO grades1 to 4)
Kuter 2010 also provided data on incidence of bleeding events (WHO grades1 to 4) and included 229 patients. Treatment with TPO receptor agonists (romiplostim) results in a similar incidence of significant bleeding events compared to SOC (RR 0.97, 95% CI 0.75 to 1.26, P = 0.84). (Figure 13).
13.
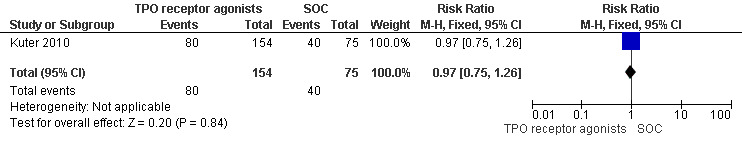
Forest plot of comparison: 2 TPO receptor agonists versus SOC, outcome: 2.3 Incidence of bleeding (WHO grades 1 to 4).
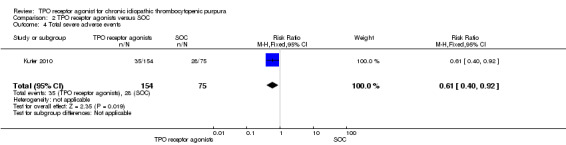
Total serious adverse events (Grades 3 to 5)
Kuter 2010 provided data on incidence of total serious adverse events (Grade 3 and higher adverse events) and included 229 patients. The total serious adverse events were significantly lower with the use of TPO receptor agonists compared with SOC (RR 0.61, 95% CI 0.40 to 0.92, P = 0.02). Given the incidence of total serious adverse events rate of 37.3% with SOC, seven patients would have to be treated with TPO receptor agonists to avoid one additional severe adverse event (NNT = 6.90, 95% CI 4.47 to 33.5). (Figure 14).
14.
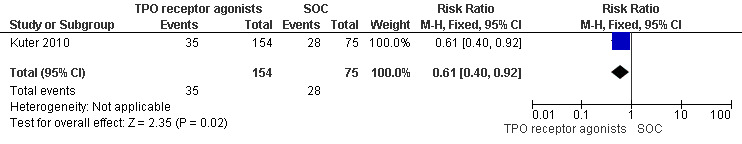
Forest plot of comparison: 2 TPO receptor agonists versus SOC, outcome: 2.4 Total severe adverse events.
Two uncommon, serious adverse effects were reported: reversible bone marrow reticulin formation occurred in one patient treated with romiplostim; thromboembolic events occurred in six patients who received romiplostim and two patients who received SOC. (Kuter 2010)
Sensitivity analyses
Except Bussel 2006 (unclear allocation concealment), the other three studies comparing TPO receptor agonists with placebo reported adequate allocation concealment. Excluding Bussel 2006 from the overall platelet response did not significantly change the result (with Bussel 2006, overall platelet response (RR 4.06, 95% CI 2.93 to 5.63; excluding Bussel 2006 overall platelet response (RR 4.11, 95% CI 2.95 to 5.72)). Excluding Bussel 2006 from the total severe adverse events also did not significantly change the result (with Bussel 2006, total severe adverse events (RR 0.92, 95% CI 0.61 to 1.38); excluding Bussel 2006 total severe adverse events (RR 0.99, 95% CI 0.65 to 1.52)).
We additionally performed sensitivity analyses by switching from fixed‐effect to random‐effects models. Due to the lack of important heterogeneity the fixed and random‐effects models gave the same results for all the outcomes.
Discussion
Summary of main results
Compared to placebo or SOC, there was no evidence to demonstrated that thrombopoietin (TPO) receptor agonists did improve significant bleeding events (those rated as severe, life‐threatening, or fatal) in chronic ITP.
Due to the absence of data available from the included studies we could not assess the effect of TPO receptor agonists on overall survival.
Both romiplostim and eltrombopag statistically significantly improve overall platelet response. In 24‐week duration studies romiplostim has shown more efficacy for achieving durable response, while in six‐week duration studies eltrombopag has shown efficacy for achieving complete response.
There was a significant reduction in overall bleeding events (WHO grades 1 to 4) when TPO receptor agonists were compared to placebo, but no statistical significant difference between TPO and SOC
TPO receptor agonists were well‐tolerated. We found no statistically significant difference in risk of total adverse events (Grades1 to 5) in TPO receptor agonists group and control group (both placebo and SOC). The incidence of serious adverse events (Grade 3 and higher adverse events) in the patients receiving treatment with TPO receptor agonists was similar to patients receiving treatment with placebo, but was significantly lower than in patients receiving treatment with SOC. The risks of developing bone marrow reticulin, thromboembolic events with romiplostim, the risk of developing cataracts with eltrombopag, and the risk of developing more severe thrombocytopenia than at pretreatment after drug discontinuation need to be defined.
Overall completeness and applicability of evidence
Six studies addressed the use of TPO receptor agonists for chronic ITP. Participants in the majority of the included studies had refractory ITP of at least six months duration.
The included studies generally did not enroll patients with previously untreated chronic ITP, so it is not clear how efficacious TPO receptor agonists may be in people with previously untreated chronic ITP.
Quality of the evidence
All the included trials except Kuter 2010 were reported as randomized and double‐blind. Kuter 2010 was reported as open‐label. All the included trials except Bussel 2006 reported adequate allocation concealment. The open‐label and inadequate allocation concealment could lead to the existence of selective and performance biases. Overall, we consider that there is 'moderate' level evidence based on observational data.
Potential biases in the review process
We are not aware of any obvious flaws in our review process.
Agreements and disagreements with other studies or reviews
There are no other reviews to compare with this Cochrane Review.
Authors' conclusions
Implications for practice.
There was currently no evidence to support that TPO receptor agonists are effective in chronic ITP. Compared to placebo or SOC, despite significantly increased platelet response, there was no evidence to demonstrate that TPO receptor agonists did improve significant bleeding events in chronic ITP. The effect on overall survival awaits further analysis. Although long‐term studies are lacking, current data demonstrated adverse effects of TPO receptor agonists were similar to that of placebo and SOC.
Implications for research.
Published studies should follow the CONSORT statement (Schulz 2010) for reporting controlled studies. Uncertainties regarding the methodology employed in the studies in this review may have been avoided had the CONSORT checklist been adhered to. The studies should include overall survival, which may be particularly important in situations where platelet response rates are not improved. Further long‐term studies are needed to know more about TPO receptor agonists's relative efficacy and tolerability.
Acknowledgements
Many thanks to the Cochrane Haematological Malignancies Group for their help, and to Nicole Skoetz for her expertise and technical support.
Appendices
Appendix 1. MEDLINE search strategy
| 1 | exp PURPURA, THROMBOCYTOPENIC/ |
| 2 | ((purpura$ or porpor$) adj5 (thrombocytopenic$ or thrombocytopaenic$ or trombocitopenic$)).tw,kf,ot. |
| 3 | ((purpura$ or porpor$) adj5 (thrombopenic$ or thrombopaenic$ or trombopenic$ or trombotic$)).tw,kf,ot. |
| 4 | PURPURA, THROMBOCYTOPENIC, IDIOPATHIC/ |
| 5 | (autoimmun$ adj5 (thrombocytopenic$ or thrombocytopaenic$ or trombocitopenic$ or thrombopenic$ or thrombopaenic$ or trombopenic$ or trombotic$)).tw,kf,ot. |
| 6 | itp.tw,kf,ot. |
| 7 | aitp.tw,kf,ot. |
| 8 | werlhof$.tw,kf,ot. |
| 9 | or/1‐8 |
| 10 | THROMBOPOIESIS/ |
| 11 | thrombopoie$.tw,kf,ot. |
| 12 | thrombocytopoie$.tw,kf,ot. |
| 13 | megakaryocytopoies$.tw,kf,ot. |
| 14 | megakaryocyte$.tw,kf,ot. |
| 15 | MEGAKARYOCYTES/ |
| 16 | THROMBOPOIETIN RECEPTOR/ |
| 17 | eltrombopag$.tw,kf,ot,nm. |
| 18 | (romiplastin$ or romiplostim$).tw,kf,ot,nm. |
| 19 | (amg531 or amg 531 or amg‐531).tw,kf,ot,nm. |
| 20 | (sb497115 or sb‐497115) .tw,kf,ot,nm. |
| 21 | tpo.tw,kf,ot. |
| 22 | (thrombopoie$ and agent$).tw,kf,ot. |
| 23 | or/10‐22 |
| 24 | 9 and 23 |
Appendix 2. EMBASE search strategy
| 1 | exp THROMBOCYTOPENIC PURPURA/ |
| 2 | ((purpura$ or porpor$) adj5 (thrombocytopenic$ or thrombocytopaenic$ or trombocitopenic$)).tw. |
| 3 | ((purpura$ or porpor$) adj5 (thrombopenic$ or thrombopaenic$ or trombopenic$ or trombotic$)).tw. |
| 4 | IDIOPATHIC THROMBOCYTOPENIC PURPURA/ |
| 5 | (autoimmun$ adj5 (thrombocytopenic$ or thrombocytopaenic$ or trombocitopenic$ or thrombopenic$ or thrombopaenic$ or trombopenic$ or trombotic$)).tw. |
| 6 | itp.tw. |
| 7 | aitp.tw. |
| 8 | werlhof$.tw. |
| 9 | or/1‐8 |
| 10 | THROMBOCYTOPOIESIS/ |
| 11 | thrombopoie$.tw. |
| 12 | thrombocytopoie$.tw. |
| 13 | megakaryocytopoies$.tw. |
| 14 | megakaryocyte$.tw. |
| 15 | MEGAKARYOCYTE/ |
| 16 | THROMBOPOIETIN RECEPTOR/ |
| 17 | eltrombopag$.tw. |
| 18 | (romiplastin$ or romiplostim$).tw. |
| 19 | (amg531 or amg 531 or amg‐531).tw. |
| 20 | (sb497115 or sb‐497115).tw. |
| 21 | tpo.tw. |
| 22 | (thrombopoie$ and agent$).tw. |
| 23 | or/10‐22 |
| 24 | 9 and 23 |
| 25 | CLINICAL TRIAL/ |
| 26 | RANDOMIZED CONTROLLED TRIAL/ |
| 27 | RANDOMIZATION/ |
| 28 | SINGLE BLIND PROCEDURE/ |
| 29 | PLACEBO/ |
| 30 | randomi?ed controlled trial$.tw. |
| 31 | rct.tw. |
| 32 | random allocation.tw. |
| 33 | randomly allocated.tw. |
| 34 | allocated randomly.tw. |
| 35 | (allocated adj2 random).tw. |
| 36 | single blind$.tw. |
| 37 | double blind$.tw. |
| 38 | ((treble or triple) adj (blind$).tw. |
| 39 | placebo$.tw. |
| 40 | PROSPECTIVE STUDY/ |
| 41 | or/ 25‐40 |
| 42 | CASE STUDY/ |
| 43 | case report.tw. |
| 44 | ABSTRACT REPORT/ or LETTER/ |
| 45 | or/ 42‐44 |
| 46 | 41 not 45 |
| 47 | ANIMAL/ |
| 48 | HUMAN/ |
| 49 | 47 not 48 |
| 50 | 46 not 49 |
| 51 | 24 and 50 |
Appendix 3. CENTRAL search strategy
| #1 | MeSH descriptor Purpura, Thrombocytopenic explode all trees |
| #2 | ( (purpura* in All Text near/5 thrombocytopenic* in All Text) or (purpura* in All Text near/5 thrombocytopaenic* in All Text) or (porpor* in All Text near/5 trombocitopenic* in All Text) ) |
| #3 | ( (purpura* in All Text near/5 thrombocytopenic* in All Text) or (purpura* in All Text near/5 thrombocytopaenic* in All Text) or (purpura* in All Text near/5 trombocitopenic* in All Text) or (porpor* in All Text near/5 trombotic* in All Text) ) |
| #4 | MeSH descriptor Purpura, Thrombocytopenic, Idiopathic explode all trees |
| #5 | ( (autoimmun* in All Text near/5 thrombocytopenic* in All Text) or (autoimmun* in All Text near/5 thrombocytopaenic* in All Text) or (autoimmun* in All Text near/5 trombocitopenic* in All Text) or (autoimmun* in All Text near/5 thrombopenic* in All Text) or (autoimmun* in All Text near/5 thrombopaenic* in All Text) ) |
| #6 | ( (autoimmun* in All Text near/5 trombopenic* in All Text) or (autoimmun* in All Text near/5 trombotic* in All Text) ) |
| #7 | itp in All Text |
| #8 | aitp in All Text |
| #9 | werlhof* in All Text |
| #10 | (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) |
| #11 | MeSH descriptor Thrombopoiesis explode all trees |
| #12 | thrombopoie* in All Text |
| #13 | thrombocytopoie* in All Text |
| #14 | megakaryocytopoies* in All Text |
| #15 | megakaryocyte in All Text |
| #16 | MeSH descriptor Megakaryocytes explode all trees |
| #17 | MeSH descriptor Receptors, Thrombopoietin explode all trees |
| #18 | eltrombopag* in All Text |
| #19 | (romiplastin* in All Text or romiplostim* in All Text) |
| #20 | (amg531 in All Text or (amg in All Text and 531 in All Text) or amg‐531 in All Text) |
| #21 | (sb497115 in All Text or sb‐497115 in All Text) |
| #22 | tpo in All Text |
| #23 | (thrombopoie* in All Text and agent* in All Text) |
| #24 | (#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23) |
| #25 | (#10 and #24) |
Data and analyses
Comparison 1. TPO receptor agonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of significant bleeding events (severe, life‐threatening or fatal) | 3 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.19, 1.16] |
| 2 Overall platelet response | 5 | 561 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.06 [2.93, 5.63] |
| 3 Durable platelet response | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.16 [2.91, 69.01] |
| 4 Complete response | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.29 [2.32, 37.15] |
| 5 Number of weeks with platelet response | 2 | 125 | Mean Difference (IV, Fixed, 95% CI) | 12.88 [11.08, 14.69] |
| 6 Incidence of bleeding (WHO grades 1 to 4) | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.89] |
| 7 Total adverse events | 4 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.95, 1.15] |
| 8 Total severe adverse events | 5 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.61, 1.38] |
1.1. Analysis.

Comparison 1 TPO receptor agonists versus placebo, Outcome 1 Incidence of significant bleeding events (severe, life‐threatening or fatal).
1.2. Analysis.
Comparison 1 TPO receptor agonists versus placebo, Outcome 2 Overall platelet response.
1.3. Analysis.
Comparison 1 TPO receptor agonists versus placebo, Outcome 3 Durable platelet response.
1.4. Analysis.

Comparison 1 TPO receptor agonists versus placebo, Outcome 4 Complete response.
1.5. Analysis.

Comparison 1 TPO receptor agonists versus placebo, Outcome 5 Number of weeks with platelet response.
1.6. Analysis.
Comparison 1 TPO receptor agonists versus placebo, Outcome 6 Incidence of bleeding (WHO grades 1 to 4).
1.7. Analysis.
Comparison 1 TPO receptor agonists versus placebo, Outcome 7 Total adverse events.
1.8. Analysis.
Comparison 1 TPO receptor agonists versus placebo, Outcome 8 Total severe adverse events.
Comparison 2. TPO receptor agonists versus SOC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of significant bleeding events (severe, life‐threatening or fatal) | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.15, 1.63] |
| 2 Overall platelet response | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.37, 2.37] |
| 3 Incidence of bleeding (WHO grades 1 to 4) | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.75, 1.26] |
| 4 Total severe adverse events | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.92] |
2.1. Analysis.
Comparison 2 TPO receptor agonists versus SOC, Outcome 1 Incidence of significant bleeding events (severe, life‐threatening or fatal).
2.2. Analysis.
Comparison 2 TPO receptor agonists versus SOC, Outcome 2 Overall platelet response.
2.3. Analysis.
Comparison 2 TPO receptor agonists versus SOC, Outcome 3 Incidence of bleeding (WHO grades 1 to 4).
2.4. Analysis.
Comparison 2 TPO receptor agonists versus SOC, Outcome 4 Total severe adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bussel 2006.
| Methods | Randomized, double‐blind, placebo‐controlled trial. Phase II. 6‐week study. Multicenter (9 sites in USA). | |
| Participants | Chronic ITP, a history of ITP for at least 3 months, PLT ≤ 30 × 109/l, one or more prior treatments for ITP, age 18 to 65 years, n = 21 | |
| Interventions | Romiplostim 1, 3, 6 ug ih qw for 6 weeks versus placebo | |
| Outcomes | Primary: incidence and severity of all adverse events and evaluation of antibody status Secondary: overall platelet response; peak platelet count; absolute change and fold change from baseline to peak platelet count; time to peak platelet count; duration within the targeted therapeutic platelet range Endpoints: Primary: incidence and severity of all adverse events and evaluation of antibody status Secondary: • Proportion of subjects who achieved the targeted therapeutic platelet level (a doubling of baseline platelet counts and within the range of ≥ 50 × 109/L and ≤ 450 × 109/L) • Proportion of subjects with an increase in platelet count of ≥ 20 × 109/L over baseline • Proportion of subjects with a peak platelet count of ≥ 100 × 109/L and > 450 × 109/L • Peak platelet count; absolute change and fold change from baseline to peak platelet count • Time to peak platelet count • Duration within the targeted therapeutic platelet range |
|
| Notes | Trial closed November 2008 Follow up period: 78 days Study supported by Amgen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in published article |
| Allocation concealment (selection bias) | Unclear risk | Not stated in published article |
| Blinding (performance bias and detection bias) Physician assessed | Low risk | Quote: "Double blind"; "The placebo consisted of the excipients of the AMG 531 formulation." |
| Blinding (performance bias and detection bias) Patient assessed | Low risk | Quote: "Double blind"; "The placebo consisted of the excipients of the AMG 531 formulation." |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 94% romiplostim group and 100% placebo group completed the trial. The withdrawals were accounted for. Quote: "the patient had received the 6‐μg dose and that the platelet count exceeded the upper limit of the targeted range." Judged a low risk of bias given the > 80% completion rate in the treatment group. |
| Incomplete outcome data (attrition bias) Safety outcomes | Low risk | 94% romiplostim group and 100% placebo group completed the trial. Judged a low risk of bias as safety analysis included all patients treated. |
| Selective reporting (reporting bias) | Low risk | Quote: "The primary objective was to assess the safety and tolerability of two injections of AMG 531 in patients with ITP. Secondary objectives were to determine the dose that would result in a platelet count that was within the targeted range (50,000 to 450,000 per cubic millimeter) and that was at least twice the baseline count and to determine the adequacy of two AMG 531 injections given within 2 to 3 weeks for achieving this range." |
| Other bias | High risk | Funded by drug company. There is evidence that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004). |
Bussel 2009a.
| Methods | Randomized, double‐blind, placebo‐controlled trial. Phase III. 6‐week study. Multicenter (90 sites in 23 countries). Stratification by concomitant ITP medication (yes or no), splenectomy (yes or no), and the baseline platelet count (> 15,000 per cubic millimeter versus ≤15,000 per cubic millimeter). | |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT≤ 30 × 109/l, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids, must have been stable for at least 1 month, age ≥ 18 years, n = 231 | |
| Interventions | Eltrombopag 30 mg, 50 mg or 75 mg p.o qd for 6 weeks versus placebo | |
| Outcomes | Primary: overall platelet response
Secondary: platelet counts, the odds of responding during weeks 2 to 6, proportion of patients with platelet counts 50,000 per μL or more and at least twice the baseline amount, incidence of bleeding symptoms, safety, and tolerability. |
|
| Notes | Trial closed May 2009 Followup period: 12 weeks Study supported by GlaxoSmithKline GlaxoSmithKline and the academic principal investigator (JBB) were jointly responsible for the study design and development of the study protocol. Decisions related to the content of the report were made by the principal investigator in consultation with all authors. All authors had access to the primary data, assumed responsibility for the completeness of the data reported, and contributed to the writing of the report. JBB had final responsibility for the decision to submit the report for publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate. Quote: "Patients were randomly assigned by an in‐house validated randomisation system (RANDALL)." |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Quote: "Patients were randomly assigned by an in‐house validated randomisation system (RANDALL)." |
| Blinding (performance bias and detection bias) Physician assessed | Low risk | Quote: "Double blind". "Patients were randomly assigned 2:1 to receive standard of care and either eltrombopag (GlaxoSmithKline) or placebo (GlaxoSmithKline)" |
| Blinding (performance bias and detection bias) Patient assessed | Low risk | Quote: "Double blind". "Patients were randomly assigned 2:1 to receive standard of care and either eltrombopag (GlaxoSmithKline) or placebo (GlaxoSmithKline)" |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 68.4% eltrombopag group and 79% placebo group completed the trial. Judged a low risk of bias as efficacy analysis included 98% patients treated. |
| Incomplete outcome data (attrition bias) Safety outcomes | Low risk | 68.4% eltrombopag group and 79% placebo group completed the trial. Judged a low risk of bias as safety analysis included all patients treated. |
| Selective reporting (reporting bias) | Low risk | Quote: "The primary study endpoint was the proportion of responders, defined as patients who had an increase in platelet counts to 50,000 per μL or more at day 43 (i.e., 6 weeks after the start of treatment); patients who withdrew prematurely because of a platelet count greater than 200,000 per μL were considered responders. Patients who discontinued treatment for any other reason (e.g., patient decision, lack of efficacy, adverse event) were considered non‐responders irrespective of their platelet count. Secondary endpoints included platelet counts, the odds of responding during weeks 2–6, proportion of patients with platelet counts 50,000 per μL or more and at least twice the baseline amount, incidence of bleeding symptoms, safety, and tolerability. The incidence and severity of bleeding symptoms were assessed at every study visit with the WHO bleeding scale (grade 0, no bleeding; grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; and grade 4, debilitating blood loss). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0)." |
| Other bias | High risk | Funded by drug company. There is evidence that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004). |
Cheng 2011.
| Methods | Randomised, double‐blind, placebo controlled trials. Phase III. 6‐week study. Multicenter (75 sites in 23 countries). Stratification by concomitant ITP medication (yes or no), splenectomy (yes or no), and the baseline platelet count (>15,000 per cubic millimeter versus ≤ 15,000 per cubic millimeter). | |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT≤ 30 × 109/l, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids, must have been stable for at least 1 month, age ≥ 18 years, n = 197 | |
| Interventions | Eltrombopag 50 mg p.o qd for 6 months versus placebo | |
| Outcomes | Primary: percentage of responders Secondary: median platelet counts, the proportion of patients who responded at 75% or more of assessments, mean cumulative weeks of response, mean maximum weeks of continuous response, bleeding symptoms, reduction of baseline treatment for immune thrombocytopenia, and use of rescue treatment; HR‐QoL Instrument and Domain Scores; adverse event | |
| Notes | Results first received: July/2009 Followed‐up period: 6 months Study supported by GlaxoSmithKline The protocol was developed by the principal investigators and employees of the sponsor. Data were collected and analysed by the sponsor. All authors had access to the primary data and vouch for the completeness and accuracy of the data and analyses. Interpretation of the data and decisions related to the content of the report were made through collaboration among all authors. The corresponding author had final responsibility for the decision to submit for publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate. Quote: "The randomisation schedule was computer generated with an in‐house validated randomisation system, and was generated by a member of the GlaxoSmithKline statistics and programming group, who held a statistical consultation role for the rest of the trial." |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Quote: "All randomisations were done with RAMOS, an automated interactive voice recognition telephone randomisation and drug ordering system." |
| Blinding (performance bias and detection bias) Physician assessed | Low risk | Quote: "Double blind". "Patients, investigators, and those assessing the data were masked to allocation." |
| Blinding (performance bias and detection bias) Patient assessed | Low risk | Quote: "Double blind". "Patients, investigators, and those assessing the data were masked to allocation." |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 83% eltrombopag group and 89% placebo group completed the trial. Judged a low risk of bias as efficacy analysis included all patients treated. |
| Incomplete outcome data (attrition bias) Safety outcomes | Low risk | 83% eltrombopag group and 89% placebo group completed the trial. Judged a low risk of bias as safety analysis included 99.5% patients treated. |
| Selective reporting (reporting bias) | Low risk | Quote: "Each patient was assessed for response (defined as a platelet count of 50000 ‐ 400000 per μL) at each assessment during the 6‐month treatment period; the primary endpoint was the odds of response to eltrombopag versus placebo during this period. As described above, any assessment, irrespective of platelet count, was regarded as a non‐response if it occurred during a period of rescue treatment. Bleeding was assessed with the WHO bleeding scale (grade 0, no bleeding; grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; grade 4, debilitating blood loss). For the secondary endpoint of median platelet counts, all platelet counts were included in the analysis irrespective of whether the patient received rescue treatment. Rescue treatment was defined as new treatment for chronic immune thrombocytopenia, an increased dose of baseline treatment, platelet transfusion, or splenectomy. The acute recall version of the short form‐36, version 2 (SF‐36v2) questionnaire was used to measure health‐related quality of life at baseline and at weeks 6, 14, and 26 or on discontinuation of study drug. Measurement of fatigue with the vitality domain of the SF‐36v2 was supplemented by use of the fatigue subscale of the functional assessment of chronic illness therapy (FACIT)‐fatigue questionnaire. The e? ect of bleeding on quality of life was assessed with a six‐item subset from the functional assessment of cancer therapy‐thrombocytopenia (FACT‐Th6) questionnaire. Changes to physical and mental energy and social motivation were assessed with the short form of the motivation and energy inventory (MEI‐SF) questionnaire. Adverse event reports (graded with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0) and laboratory assessments were completed at each on‐treatment and post‐treatment visit. On the basis of preclinical findings, ocular examinations were done at baseline and regularly during the study." |
| Other bias | Unclear risk | Funded by drug company. There is evidence that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004) |
Kuter 2008a.
| Methods | Randomized, double‐blind, placebo‐controlled trial. Phase III. 6‐month study. Multicenter (35 sites in the USA and Europe). | |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT≤ 30 × 109/l, had a splenectomy for the treatment of ITP greater than or equal to 24 weeks prior to study entry, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids must have been stable for at least 1 month, age ≥ 18 years, n = 63 | |
| Interventions | Romiplostim versus placebo The starting dose (romiplostim or placebo) was 1 μg/kg. To achieve the target platelet count of 50 × 10/L to 200 × 10/L, doses could be increased according to the following algorithm: 2 μg/kg every week if the count was 10 × 10/L or less and 2 μg/kg every 2 weeks if 11 × 10/L to 50 × 10/L. Once platelets reached more than 50 × 10/L, the maintenance algorithm was used: dose was increased by 1 μg/kg every week if 10 × 10/L or less; increased by 1 μg/kg after 2 weeks if 11 × 10/L to 50 × 10/L; reduced by 1 μg/kg after 2 consecutive weeks at 201 × 10/L to 400 × 10/L; withheld if more than 400 × 10/L and subsequent doses reduced by 1 μg/kg and given after count was less than 200 × 10/L. The maximum allowed dose was 15 μg/kg. |
|
| Outcomes | Primary: durable platelet response Key secondary endpoints: overall platelet response; number of weeks with platelet response; proportion of subjects requiring rescue medication; incidence of durable platelet response with stable dose Descriptive secondary endpoints: adverse events; proportion of subjects able to reduce or discontinue concurrent ITP therapies during the first 12 weeks of treatment; health‐related quality of life (HRQoL) | |
| Notes | Trial closed May 2009 Study supported by Amgen Amgen designed the study, did statistical analyses and interpreted the data, which Amgen holds. Amgen collected the data and representatives (JLN and DMG) participated in the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Study was also reported in George 2009 and Pullarkat 2009 (see Kuter 2008a for references) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate. Quote: "The random allocation sequence was generated by Amgen Inc with the blocked randomisation method". |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Quote: "Clinphone was used to randomly assign patients into the study with the interactive voice response system (IVRS)". |
| Blinding (performance bias and detection bias) Physician assessed | Low risk | Quote: "Double blind (subject, investigator)". "Romiplostim and placebo were supplied in identical vials containing a lyophilised powder that was reconstituted with sterile water for subcutaneous injection." |
| Blinding (performance bias and detection bias) Patient assessed | Low risk | Quote: "Double blind (subject, investigator)". "Romiplostim and placebo were supplied in identical vials containing a lyophilised powder that was reconstituted with sterile water for subcutaneous injection." |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 95% romiplostim group and 90% placebo group completed the trial. Judged a low risk of bias as efficacy analysis included all patients treated. |
| Incomplete outcome data (attrition bias) Safety outcomes | Low risk | 95% romiplostim group and 90% placebo group completed the trial. Judged a low risk of bias as safety analysis included all patients treated. |
| Selective reporting (reporting bias) | Low risk | Quote: "All outcome measures were prospectively defined before the start of the studies. A weekly platelet response was defined as a platelet count of 50 × 10/L or more at a weekly study visit. Unless otherwise noted, platelet responses that occurred within 8 weeks after rescue drugs were used were not included in the efficacy analyses or in the determination of any other measures for platelet outcome. Rescue medication was defined as an increased dose of concurrent ITP therapy, or the use of any new drug to increase platelet counts. A durable platelet response (primary efficacy measure) was defined as weekly platelet responses during 6 or more weeks of the last 8 weeks of treatment. Patients who received rescue medication at any time during the study could not be counted as having a durable response. A transient platelet response was defIned as four or more weekly platelet responses without a durable platelet response from week 2 to 25. Additional secondary endpoints were the frequency of overall platelet response (durable plus transient rates of platelet response), the number of weekly platelet responses, the proportion of patients needing rescue drugs, and the frequency of durable platelet response with a stable dose (dose maintained within 1 μg/kg during the last 8 weeks of treatment). We also assessed changes in concurrent ITP therapies." |
| Other bias | High risk | Funded by drug company. There is evidence that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004). |
Kuter 2008b.
| Methods | Randomized, double‐blind, placebo‐controlled trial. Phase III. 6‐month study. Multicenter (35 sites in the USA and Europe). | |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT≤ 30 × 109/l, non‐splenectomized status, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids, must have been stable for at least 1 month, age ≥ 18 years, n = 62 | |
| Interventions | Romiplostim versus placebo The starting dose of study drug (romiplostim or placebo) was 1 μg/kg. To achieve the target platelet count of 50 × 10/L to 200 × 10/L, doses could be increased according to the following algorithm: 2 μg/kg every week if the count was 10 × 10/L or less and 2 μg/kg every 2 weeks if 11 × 10/L to 50 × 10/L. Once platelets reached more than 50 × 10/L, the maintenance algorithm was used: dose was increased by 1 μg/kg every week if 10 × 10/L or less; increased by 1 μg/kg after 2 weeks if 11 × 10/L to 50 × 10/L; reduced by 1 μg/kg after 2 consecutive weeks at 201 × 10/L to 400 × 10/L; withheld if more than 400 × 10/L and subsequent doses reduced by 1 μg/kg and given after count was less than 200 × 10/L. The maximum allowed dose was 15 μg/kg. |
|
| Outcomes | Primary: durable platelet response Key secondary endpoints: overall platelet response; number of weeks with platelet response; proportion of subjects requiring rescue medication; incidence of durable platelet response with stable dose Descriptive secondary endpoints: adverse events; proportion of subjects able to reduce or discontinue concurrent ITP therapies during the first 12 weeks of treatment; health‐related quality of life (HRQoL) | |
| Notes | Trial closed May 2009 Study supported by Amgen Amgen designed the study, did statistical analyses and interpreted the data, which Amgen holds. Amgen collected the data and representatives (JLN and DMG) participated in the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Study was also reported in George 2009 and Pullarkat 2009 (see Kuter 2008b for references) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate. Quote: "The random allocation sequence was generated by Amgen Inc with the blocked randomisation method." |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Quote: "Clinphone was used to randomly assign patients into the study with the interactive voice response system (IVRS)." |
| Blinding (performance bias and detection bias) Physician assessed | Low risk | Quote: "Double blind (subject, investigator)". "Romiplostim and placebo were supplied in identical vials containing a lyophilised powder that was reconstituted with sterile water for subcutaneous injection." |
| Blinding (performance bias and detection bias) Patient assessed | Low risk | Quote: "Double blind (subject, investigator)". "Romiplostim and placebo were supplied in identical vials containing a lyophilised powder that was reconstituted with sterile water for subcutaneous injection." |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 95% romiplostim group and 81% placebo group completed the trial. Judged a low risk of bias as efficacy analysis included all patients treated. |
| Incomplete outcome data (attrition bias) Safety outcomes | Low risk | 95% romiplostim group and 81% placebo group completed the trial. Judged a low risk of bias as safety analysis included all patients treated. |
| Selective reporting (reporting bias) | Low risk | Quote: "All outcome measures were prospectively defined before the start of the studies. A weekly platelet response was defined as a platelet count of 50 × 10/L or more at a weekly study visit. Unless otherwise noted, platelet responses that occurred within 8 weeks after rescue drugs were used were not included in the efficacy analyses or in the determination of any other measures for platelet outcome. Rescue medication was defined as an increased dose of concurrent ITP therapy, or the use of any new drug to increase platelet counts. A durable platelet response (primary efficacy measure) was defined as weekly platelet responses during 6 or more weeks of the last 8 weeks of treatment. Patients who received rescue medication at any time during the study could not be counted as having a durable response. A transient platelet response was defined as four or more weekly platelet responses without a durable platelet response from week 2 to 25. Additional secondary endpoints were the frequency of overall platelet response (durable plus transient rates of platelet response), the number of weekly platelet responses, the proportion of patients needing rescue drugs, and the frequency of durable platelet response with a stable dose (dose maintained within 1 μg/kg during the last 8 weeks of treatment). We also assessed changes in concurrent ITP therapies." |
| Other bias | High risk | Funded by drug company. There is evidence that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004). |
Kuter 2010.
| Methods | Randomized, open‐label, standard of care (SOC) controlled trial. Phase III. 52‐week study. Multicenter (85 sites in North America, Europe and Australia). Patient randomization was stratified based on the geographic region of the investigational site: North America, European Union, or Australia. | |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT ≤ 30 × 109/l, non‐splenectomized status, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, age ≥ 18 years, n = 234 | |
| Interventions | Romiplostim ih qw for 52 weeks versus medical SOC AMG 531 was administered weekly by SC injection for 52 weeks at a starting dose of 3 μg/kg. Dose adjustments were made according to the following guidelines: dose was increased by 1 μg/kg every week if the count was less than 30 × 109/L; dose may be adjusted (increased or decreased) in increments of 1 μg/kg at the investigator's discretion no more frequently than every 2 weeks when the count was between 30 and 450 × 109/L; dose was held if the count was more than 450 × 109/L, the dose was reduced by 1 μg/kg at the next scheduled dosing day when platelet count had fallen to < 200 × 109/L. The maximum permitted dose of AMG 531 was 10 μg/kg. If the platelet count was 20 × 109 per liter or less for 4 consecutive weeks while the patient was receiving a stable romiplostim dose of 10 μg/kg, romiplostim treatment was discontinued. Medical SOC treatments were to be selected and prescribed by the investigator according to standard institutional practices or therapeutic guidelines. Medical standard of care treatments were obtained through the usual commercial routes and were not provided by Amgen. These drugs were formulated, packaged, labeled, and stored according to local manufacturer, supplier, and institutional procedures. The investigator was responsible for obtaining supplies of these medications. |
|
| Outcomes | Primary outcome measures: incidence of treatment failure; incidence of splenectomy Secondary outcome measures: time to splenectomy; platelet response; change in the ITP‐PAQ (patient‐reported outcome scale) physical health domains. | |
| Notes | Trial closed July 2009 Follow up period: 6 months Study supported by Amgen Amgen (the sponsor) and the academic principal investigators jointly designed the study, gathered the data, conducted the statistical analyses, and interpreted the results. All authors participated in the design or execution of the study, contributed to interpretation of the data, approved the final draft of the manuscript, and made the decision to submit it for publication. The authors had unrestricted access to the primary data and were not limited by Amgen in the writing of this article. The principal (academic) investigator wrote the initial draft of the manuscript; subsequently, professional writing assistance was provided through Amgen. The academic authors vouch for the accuracy and completeness of the reported data and analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate. Quote: "Treatment assignment will be provided by the Interactive Voice Response System (IVRS) according to a pre‐determined randomization scheme, as provided by Amgen or designee. " |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Quote: "Treatment assignment will be provided by the Interactive Voice Response System (IVRS) according to a pre‐determined randomization scheme, as provided by Amgen or designee. " |
| Blinding (performance bias and detection bias) Physician assessed | High risk | Quote: "open label"; "No blinding of AMG 531 or medical SOC for ITP will be employed." |
| Blinding (performance bias and detection bias) Patient assessed | High risk | Quote: "open label"; "No blinding of AMG 531 or medical SOC for ITP will be employed." |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 92% romiplostim group and 81% SOC group completed the trial. Judged a low risk of bias as efficacy analysis included all patients treated. |
| Incomplete outcome data (attrition bias) Safety outcomes | Low risk | 92% romiplostim group and 81% SOC group completed the trial. Judged a low risk of bias as safety analysis included all patients treated. |
| Selective reporting (reporting bias) | Low risk | The first primary endpoint was the number of subjects undergoing a splenectomy by treatment group during the 52‐week treatment period. The second primary endpoint was the number of subjects with a treatment failure during the 52‐week treatment period. Treatment failure was defined as: a lack of efficacy defined as platelet count ≤ 20 × 109/L for 4 consecutive weeks at the highest recommended dose and schedule AMG 531‐10 μg/kg weekly; medical SOC for ITP‐apply standard institutional practices or therapeutic guidelines); or a major bleeding event; or a change in therapy due to an intolerable side effect or bleeding symptoms (including a minor bleeding event). A minor bleeding event is defined as “dry” or “wet” purpura (e.g., petechiae, mucosal bleeding from the gums or nose). A major bleeding event is defined as a hemorrhage (e.g., upper or lower gastrointestinal tract, genitourinary tract or central nervous system). The secondary endpoints were the following: time to splenectomy; platelet response, defined as the number of monthly platelet counts > 50 × 109/L; change in the ITP‐PAQ physical health domains of symptoms, bother, activity, and fatigue |
| Other bias | High risk | Funded by drug company. There is evidence that industry‐sponsored trials may overestimate the treatment effect (Bhandari 2004). |
ITP: idiopathic thrombocytopenic purpura PLT: platelet count
SC: subcutaneous SOC: standard of care
ih: hypodermic injection
p.o.: oral administration
qd: once daily
qw: once weekly
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bussel 2009b | Long‐term extension of Kuter 2008d; non‐randomized controlled trial. |
| Kuter 2009 | Non‐randomized data included in published analysis. |
Characteristics of ongoing studies [ordered by study ID]
Amgen 2007.
| Trial name or title | Safety and efficacy study of romiplostim to treat ITP in pediatric subjects |
| Methods | Phase II, multi‐center, randomized, double‐blind, placebo‐controlled study |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT ≤ 30 × 109/l, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids must have been stable for at least 1 month, between 1 year and < 18 years of age |
| Interventions | Romiplostim versus placebo |
| Outcomes | Primary outcome measures: incidence of adverse events Secondary outcome measures: overall platelet response, incidence of bleeding, incidence of requiring rescue therapy |
| Starting date | July 2007 |
| Contact information | ClinicalTrials.gov identifier: NCT00515203 |
| Notes | Estimated study completion date: June 2009 Study supported by Amgen |
Amgen 2010.
| Trial name or title | P3 study to evaluate efficacy and safety of AMG 531 in thrombocytopenic Japanese subjects with immune (idiopathic) thrombocytopenic purpura |
| Methods | Phase III, randomized, double‐blind, placebo‐controlled study |
| Participants | Chronic ITP of Japanese, a history of ITP for at least 6 months, PLT ≤ 30 × 109/l, one or more prior treatments for ITP, age ≥ 20 years |
| Interventions | Romiplostim versus placebo |
| Outcomes | Primary outcome measure: weeks with weekly platelet response. Secondary outcome measures: participants with increased platelet count from baseline of at least 20 × 109/L; change from baseline in mean of last 4 weekly platelet counts; weeks with platelet count between 50 and 200; participants requiring rescue medications. |
| Starting date | October 2007 |
| Contact information | ClinicalTrials.gov Identifier: NCT00603642 |
| Notes | Estimated study completion date: April 2009 Study supported by Amgen |
GlaxoSmithKline 2009.
| Trial name or title | Efficacy and safety study of eltrombopag in pediatric patients with thrombocytopenia from chronic ITP (PETIT) |
| Methods | Phase II, multi‐center, 3‐part, staggered cohort, open‐label and double‐blind, randomized, placebo‐controlled study |
| Participants | Chronic ITP, a history of ITP for at least 6 months, PLT ≤ 30 × 109/l, one or more prior treatments for ITP, the dose of maintenance immunosuppressive regimens, primarily glucocorticoids must have been stable for at least 1 month, between 1 year and < 18 years of age |
| Interventions | Eltrombopag versus placebo |
| Outcomes | Primary outcome measure: overall platelet response Secondary outcome measures: incidence of adverse events, incidence of bleeding, incidence of requiring rescue therapy |
| Starting date | September 2009 |
| Contact information | ClinicalTrials.gov identifier: NCT00908037 |
| Notes | Study supported by GlaxoSmithKline |
ITP: idiopathic thrombocytopenic purpura PLT: platelet count
Differences between protocol and review
None.
Contributions of authors
Yan Zeng: co‐ordinating the review, protocol development, searching for trials, eligibility and quality assessment, data extraction and analysis, drafting of final review.
Xin Duan: protocol development, providing general advice on the review, drafting of final review, securing funding.
Xun Ni: protocol development, searching for trials, eligibility and quality assessment, data extraction and analysis, drafting of final review.
Jiajun Xu: data management, statistical analysis, extraction of time‐to‐event data,data extraction and analysis.
Yan Zeng and Xin Duan contributed equally to this review.
Sources of support
Internal sources
No sources of support supplied
External sources
Department of Hematology, West China Hospital, China.
Declarations of interest
None known.
Yan Zeng and Xin Duan contributed equally to this review. Yan Zeng is co‐contact author to this review.
New
References
References to studies included in this review
Bussel 2006 {published data only}
- Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis‐stimulating protein, for chronic ITP. New England Journal of Medicine 2006;355:1672‐81. [DOI] [PubMed] [Google Scholar]
Bussel 2009a {published data only}
- Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine 2007;357:2237‐47. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double‐blind, placebo‐controlled trial. Lancet 2009;373:641‐8. [DOI] [PubMed] [Google Scholar]
Cheng 2011 {published data only}
- Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6‐month, randomised, phase 3 study. Lancet 2011;377:393‐402. [DOI] [PubMed] [Google Scholar]
Kuter 2008a {published data only}
- George JN, Mathias SD, Go RS, Guo M, Henry DH, Lyons R, et al. Improved quality of life for romiplostim‐treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo‐controlled trials. British Journal of Haematology 2009;144(3):409‐15. [DOI] [PubMed] [Google Scholar]
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet 2008;371:395‐403. [DOI] [PubMed] [Google Scholar]
- Pullarkat VA, Gernsheimer TB, Wasser JS, Newland A, Guthrie TH, Joost Th. M. de Wolf, et al. Quantifying the reduction in immunoglobulin (Ig) use over time in patients with chronic immune thrombocytopenic purpura (ITP) receiving romiplostim (AMG531). American Journal of Hematology 2009;84:538‐40. [DOI] [PubMed] [Google Scholar]
Kuter 2008b {published data only}
- George JN, Mathias SD, Go RS, Guo M, Henry DH, Lyons R, et al. Improved quality of life for romiplostim‐treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo‐controlled trials. British Journal of Haematology 2009;144(3):409‐15. [DOI] [PubMed] [Google Scholar]
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet 2008;371:395‐403. [DOI] [PubMed] [Google Scholar]
- Pullarkat VA, Gernsheimer TB, Wasser JS, Newland A, Guthrie TH, Joost Th. M. de Wolf, et al. Quantifying the reduction in immunoglobulin (Ig) use over time in patients withchronic immune thrombocytopenic purpura (ITP) receiving romiplostim (AMG531). American Journal of Hematology 2009;84:538‐40. [DOI] [PubMed] [Google Scholar]
Kuter 2010 {published data only}
- Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. New England Journal of Medicine 2010;363:1889‐99. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bussel 2009b {published data only}
- Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long‐term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009;113(10):2161‐71. [DOI] [PubMed] [Google Scholar]
Kuter 2009 {published data only}
- Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood 2009;114(18):3748‐56. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Amgen 2007 {published data only}
- Safety and efficacy study of romiplostim to treat ITP in pediatric subjects. Ongoing study July 2007.
Amgen 2010 {published data only}
- P3 study to evaluate efficacy and safety of AMG 531 in thrombocytopenic Japanese subjects with immune (idiopathic) thrombocytopenic purpura. Ongoing study October 2007.
GlaxoSmithKline 2009 {published data only}
- Efficacy and safety study of eltrombopag in pediatric patients with thrombocytopenia from chronic ITP (PETIT). Ongoing study September 2009.
Additional references
Arnold 2007
- Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Annals of Internal Medicine 2007;146:25‐33. [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [PMC free article] [PubMed] [Google Scholar]
Bussel 2007
- Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine 2007;357:2237‐47. [DOI] [PubMed] [Google Scholar]
Bussel 2009
- Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double‐blind, placebo‐controlled trial. Lancet 2009;373:641‐8. [DOI] [PubMed] [Google Scholar]
Chang 2003
- Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood 2003;102:887‐95. [DOI] [PubMed] [Google Scholar]
Cines 2002
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. New England Journal of Medicine 2002;346:995‐1008. [DOI] [PubMed] [Google Scholar]
Cines 2005
- Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura(ITP). Blood 2005;106:2244‐51. [DOI] [PubMed] [Google Scholar]
Cooper 2004
- Cooper N, Stasi R, Cunningham‐Rundles, Feuerstein MA, Leonard JP, Amadori S, et al. The efficacy and safety of B‐cell depletion with anti‐CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. British Journal of Haematology 2004;125:232‐9. [DOI] [PubMed] [Google Scholar]
Debili 1995
- Debili N, Wendling F, Cosman D, Titeux M, Florindo C, Dusanter‐Fourt I, et al. The Mpl receptor is expressed in the megakaryocytic lineage from late progenitors to platelets. Blood 1995;85:391‐401. [PubMed] [Google Scholar]
Deeks 2009
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009).The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Elbourne 2002
- Elbourne DR, Altmanb DG, Higginsc JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Erickson‐Miller 2004
- Erickson‐Miller C, Delorme E, Iskander M, Giampa L, Hopson CB, Luengo JI, et al. Species specificity and receptor domain interaction of a small molecule TPO receptor agonist. Blood 2004;104:abstract 2909. [Google Scholar]
Erickson‐Miller 2005
- Erickson‐Miller CL, Delorme E, Tian SS, Hopson CB, Stark K, Giampa L, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Experimental Hematology 2005;33:85‐93. [DOI] [PubMed] [Google Scholar]
George 1998
- George JN, Raskob GE. Idiopathic thrombocytopenic purpura: diagnosis and management. American Journal of the Medical Sciences 1998;316:87. [PubMed] [Google Scholar]
George 2000
- George JN. Treatment options for chronic idiopathic (immune) thrombocytopenic purpura. Seminars in Hematology 2000;37:31‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009).The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Jenkins 2007
- Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood 2007;109:4739‐41. [DOI] [PubMed] [Google Scholar]
Karpaktin 1997
- Karpaktin S. Autoimmune (idiopathic) thrombocytopenic purpura. Lancet 1997;349:1531‐6. [DOI] [PubMed] [Google Scholar]
Kojouri 2004
- Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long‐term platelet count responses, prediction of response, and surgical complications. Blood 2004;104(9):2623‐34. [DOI] [PubMed] [Google Scholar]
Kuter 2007
- Kuter DJ. New thrombopoietic growth factors. Blood 2007;109:4607‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuter 2008c
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet 2008;371:395‐403. [DOI] [PubMed] [Google Scholar]
Kuter 2008d
- Kuter DJ. New drugs for familiar therapeutic targets: thrombopoietin receptor agonists and immune thrombocytopenic purpura. European Journal of Haematology 2008;80 (Suppl 69):9‐18. [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009).The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
McMillan 2005
- McMillan R, Nugent D. The effect of antiplatelet autoantibodies on megakaryocytopoiesis. International Journal of Hematology 2005;91:94‐9. [DOI] [PubMed] [Google Scholar]
Newland 2006
- Newland A, Caulier MT, Kappers‐Klune M, Schipperus MR, Lefrere F, Zwagingu JJ, et al. An open‐label, unit dose‐finding study of AMG 531 a novel, thrombopoiesis stimulating peptidbody, in patients with immune thrombocytopenic purpura. British Journal of Haematology 2006;135:547‐53. [DOI] [PubMed] [Google Scholar]
Panzer 2009
- Panzer S. Novel approaches in management of immune thrombocytopenia. Thrombosis Research 2009;123(Suppl 2):S51‐5. [DOI] [PubMed] [Google Scholar]
Provan 2003
- Provan D, Newland A, Norfolk D, Bolton‐Maggs P, Lilleyman J, Greer I, et al. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. British Journal of Haematology 2003;120(4):574‐96. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Lancet 2010;152:726. [DOI] [PubMed] [Google Scholar]
Solar 1998
- Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, Sauvage FJ, et al. Role of c‐Mpl in early hematopoiesis. Blood 1998;92:4‐10. [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vesely 2004
- Vesely SK, Perdue JJ, Rizvi MA, Terrell DR, George JN. Management of adult patients with persistent idiopathic thrombocytopenic purpura following splenectomy: a systematic review. Annals of Internal Medicine 2004;140:112‐20. [DOI] [PubMed] [Google Scholar]
Wang 2004
- Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clinical Pharmacology & Therapeutics 2004;76:628‐38. [DOI] [PubMed] [Google Scholar]


