Abstract
Background:
The effectiveness of internal limiting membrane (ILM) peeling in the surgical treatment of tractional diabetic macular edema (DME), although widely examined, remains controversial. This study aimed to assess the efficacy of pars plana vitrectomy (PPV) in the management of tractional DME and to highlight any benefits of additional ILM peeling.
Methods:
This was an open-label, prospective, comparative, and interventional study that enrolled 50 eyes with tractional DME that underwent PPV and allocated each to one of two groups: group A consisted of 25 eyes that had no ILM peeling and group B consisted of 25 eyes that underwent ILM peeling. Postoperative assessments of best-corrected distance visual acuity (BCDVA) in the logarithm of minimal angle of resolution (logMAR) notation and central macular thickness (CMT) were performed at 1, 3, and 6 months postoperatively.
Results:
At baseline, the two groups were comparable in terms of sex ratios, phakic status, insulin use, coexistence of hypertension, and mean (standard deviation [SD]) age, BCDVA, CMT, duration of diabetes mellitus, and glycosylated hemoglobin (HbA1c) levels. In group A, the mean (SD) BCDVA improved significantly from 0.89 (0.12) logMAR preoperatively to 0.64 (0.24) logMAR (P < 0.001), and the mean (SD) CMT declined significantly from 471.28 (80.83) µm to 228.20 (26.45) µm (P < 0.001), at the 6-month postoperative assessment. Likewise, in group B, the mean (SD) BCDVA improved significantly from 0.83 (0.10) logMAR preoperatively to 0.58 (0.24) logMAR (P < 0.001), and the mean (SD) CMT decreased significantly from 496.84 (89.82) µm to 226.20 (18.04) µm (P < 0.001), after 6 months. There were no significant differences between groups A and B in the changes in BCDVA (Delta BCDVA) or CMT (Delta CMT) at 1, 3, and 6 months postoperatively with respect to the baseline values (all P > 0.05). Postoperative complications were comparable between the two groups. A significant negative correlation was detected between the preoperative HbA1c level and BCDVA improvement in all participants (r = - 0.82; P < 0.001).
Conclusions:
PPV is an effective treatment for tractional DME. Additional ILM peeling was not significantly associated with functional and anatomical benefits over a short period. Long-term glycemic control plays a role in vision gain after vitrectomy in patients with diabetes. Further long-term studies are required to verify our findings.
Key Words: vitrectomy, edema, macular, macular edema, visual acuities, internal limiting membrane, optical coherence tomography, glycosylated hemoglobin A, diabetes mellitus
INTRODUCTION
Diabetes mellitus (DM) is presently one of the most challenging public health issues in almost all communities [1]. In the year 2010, DM affected approximately 285 million individuals. The International Diabetes Federation anticipates a jump in the number of diabetic patients to approximately 550 million by 2030 [2]. Diabetic retinopathy (DR) is one of the leading causes of vision loss worldwide [3]. In 2010, DR affected more than 93 million individuals worldwide, 28 million of whom experienced vision-threatening DR (VTDR). VTDR is defined as severe non-proliferative DR (NPDR), proliferative DR (PDR), or diabetic macular edema (DME). The prevalence of DR increases from nearly 20% to approximately 50% when glycosylated hemoglobin (HbA1c) levels rise from ≤ 7.0% to > 9.0% [4].
Eyes with DME and complete posterior vitreous detachment (PVD) are less likely to require treatment during follow-up than eyes with DME and without complete PVD [5]. Several authors have confirmed the benefits of pars plana vitrectomy (PPV) in DME combined with tangential hyaloid traction caused by taut thickened posterior hyaloid [6-8].
The internal limiting membrane (ILM) is the Müller cells’ basement membrane, which is stiffer than the underlying retinal layers and can easily bend and change its shape [9]. Müller cells may move to the inner side of the ILM, forming a membranous layer that can contract tangentially [10]. The ILM is an optimal medium for fibrocellular proliferation, which is usually entangled in the vitreomacular interface disorders [11]. Many studies have examined the value of ILM peeling during surgical treatment of DME. Some studies have shown that ILM removal has additional anatomical and visual advantages that prevent recrudescence of the epi-macular membrane (EMM) [12-14]. In contrast, others reported that ILM peeling did not lead to visual improvement or differences in anatomical and functional outcomes [15-17].
This study aimed to assess the efficacy of PPV in treating tractional DME and to highlight any benefits of additional ILM peeling.
METHODS
This open-label, prospective, interventional, and comparative study included 50 eyes of Egyptian patients who underwent PPV for tractional DME between January 2018 and May 2022 in the Ophthalmology Department at Al-Azhar University Hospitals, Cairo, Egypt. The study was approved by the Al-Azhar Medical Research Ethical Committee, observed the tenets of the Helsinki Declaration, and was presented to all patients before surgery. All participants provided informed consent after receiving an explanation of the study procedures.
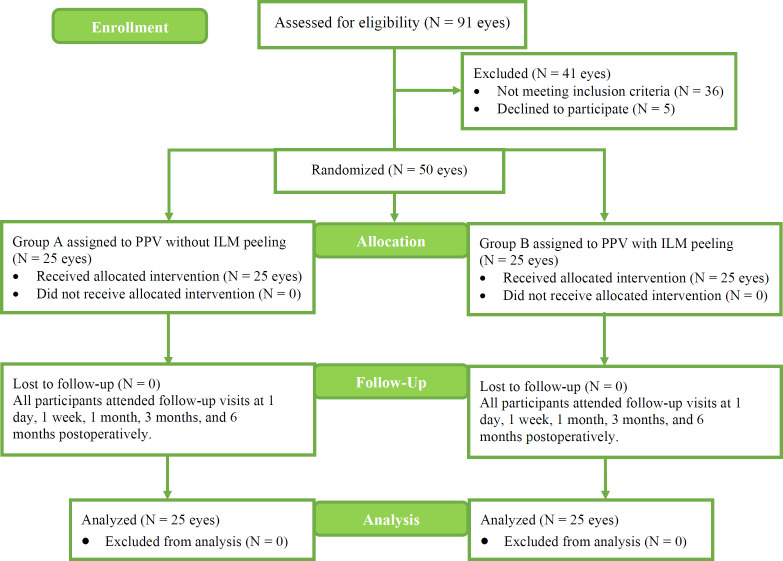
Preoperatively, we randomly assigned eyes to group A or group B using the random number generator within Microsoft Excel (Microsoft Corp., Redmond, WA, USA) (Figure 1). Group A included 25 eyes with no additional ILM peeling, and group B comprised the remaining 25 eyes, all of which underwent ILM peeling. The inclusion criteria were (1) presence of type 1 or 2 DM; (2) presence of PDR; (3) age ≥ 40 years; (4) Snellen chart best-corrected distance visual acuity (BCDVA) better than 20/300 in the study eye; and (5) EMM causing tangential traction with resultant tractional DME detected by spectral domain optical coherence tomography (SD-OCT). The exclusion criteria included tractional retinal detachment; macular holes; angiographic evidence of macular ischemia; history of macular photocoagulation, intravitreal corticosteroid treatment, or anti-vascular endothelial growth factor injection within 3 months before PPV; cataract surgery within 3 months before PPV; previous PPV or glaucoma surgery; presence of iris or angle neovascularization; history of trauma or uveitis; presence of corneal opacification; dense cataract or vitreous hemorrhage preventing imaging of the retina; and uncontrolled hypertension (blood pressure > 190/100 mmHg).
Figure 1.
Patient allocation into group A (non-ILM peeling) or group B (ILM peeling). Abbreviations: N, number of eyes; PPV, pars plana vitrectomy; ILM, internal limiting membrane
We collected preoperative patient data, including detailed medical history, Snellen BCDVA (auto chart projector CP 670; Nidek Co., Ltd., Gamagori, Japan) and converted to approximate logarithm of minimal angle of resolution (logMAR) equivalents for statistical calculations, detailed slit-lamp examination (Photo-Slit Lamp BX 900; Haag-Streit, Koeniz, Switzerland) of the anterior segment, intraocular pressure (IOP) measurement using the Goldmann applanation tonometer (AT900, Haag-Streit, Koeniz, Switzerland), gonioscopy using the Goldmann 3-mirror lens (Volk Optical, Mentor, OH, USA) to ensure the absence of iris neovascularization, and fundus examination using a slit-lamp with a + 78 D indirect lens (Volk Optical). All examinations were performed by a single experienced ophthalmologist who was blinded to the group assignments.
We performed fluorescein angiography using a Topcon TRC-50DX retinal fundus camera (Topcon Co., Tokyo, Japan) to exclude macular ischemia and to assess PDR. We informed the patients of the potential risks associated with the procedure before dye injection.
We performed SD-OCT (Heidelberg Engineering, Heidelberg, Germany) to document central macular thickness (CMT), the configuration of tractional macular edema, and the EMM. We defined CMT as the distance between the initial signal recorded from the vitreoretinal interface and the signal received from the outer border of the retinal pigment epithelium at the central fovea [18]. OCT images were generated using a fast-volume scan: 20°×20° (6×6 mm) raster scans consisting of 25 horizontal slices. All imaging procedures were performed by a single technician who was blinded to the patient group assignments.
We requested consultation from internal medicine physicians and performed preoperative laboratory tests (complete blood count, bleeding profile, liver and kidney functions, random blood glucose, and HbA1c).
One surgeon (M. M. A.) performed the standard 23-gauge PPV using a DORC vitrectomy machine (DORC International, The Netherlands) for all included eyes. The BIOM system (Oculus Insight BIOM, Oculus Inc., Wetzlar, Germany) attached to a surgical microscope (Leica M844, Leica Microsystems, Wetzlar, Germany) was used to allow visualization of the posterior segment without contact lenses. Membrane-Blue-Dual (DORC International) was used to visualize and ensure complete removal of the posterior hyaloid and EMM. Brilliant Blue G dye (DORC International) was injected to stain the ILM. In group A, the ILM was left without peeling after staining, whereas in group B, ILM peeling was circularly achieved by laminorrhexis using intraocular end-gripping ILM forceps. The ILM peeling area was nearly two discs in diameter, extending from the upper to the lower arcades centered on the fovea. Endo-laser pan-retinal photocoagulation was performed in all eyes. Intraoperatively, all eyes required surgically induced PVD, as a taut posterior hyaloid was present. The vitreous cavity was left fluid-filled. In the presence of clinically significant cataracts, phacoemulsification and intraocular lens implantation were performed prior to PPV. Combined phacovitrectomy was performed in 13 eyes: 7 in group A and 6 in group B.
On the first postoperative day, we performed a detailed slit-lamp examination to rule out early complications. We then examined the patients at 1 week, 1 month, 3 months, and 6 months after surgery. The procedures included anterior segment examination, fundus examination, intraocular pressure measurement, and Snellen chart BCDVA assessment. We measured CMT using SD-OCT at the first, third, and sixth months after surgery.
A specialized statistician performed statistical analyses using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 23.0; IBM Corp., Armonk, NY, USA). Kolmogorov–Smirnov and Shapiro–Wilk tests were used to investigate the normality of data distribution. Depending on the normality of distribution, parametric or non-parametric statistical tests were used for data analyses. Quantitative variables are presented as mean (standard deviation [SD]) and ranges, and qualitative variables as numbers and percentages. Furthermore, we measured and compared changes in BCDVA values (Delta BCDVA) and changes in CMT (Delta CMT) between groups A and B at 1, 3, and 6 months postoperatively with respect to their corresponding baseline values. The Pearson product-moment correlation coefficient was used to determine the correlation between BCDVA at the final follow-up and baseline HbA1c. P-values < 0.05 were considered significant, and P-values < 0.001 were considered highly significant.
RESULTS
We performed surgeries in 50 eyes with tractional DME. Table 1 shows the preoperative demographic and baseline characteristics of the participants, which were comparable between the two groups.
Table 1.
Comparison of demographic and baseline data between study groups
| Characteristics | Group A (n = 25) | Group B (n = 25) | P -value |
|---|---|---|---|
| Sex (Male / Female), n (%) | 10 (40.0) / 15 (60.0) | 9 (36.0) / 16 (64.0) | 0.771* |
| Age (y), Mean ± SD (Range) | 55.12 ± 4.27 (48 to 62) | 54.00 ± 4.97 (46 to 62) | 0.397** |
| Phakic status (Pseudophakic / Phakic), n (%) | 7 (28) / 18 (72) | 9 (36) / 16 (64) | 0.548* |
| Duration of DM (y), Mean ± SD (Range) | 8.32 ± 2.08 (5 to 12) | 9.12 ± 2.37 (5 to 13) | 0.229*** |
| Insulin use (Yes / NO), n (%) | 13 (52.0) / 12 (48.0) | 16 (64.0) / 9 (36.0) | 0.390* |
| HbA1c level (%), Mean ± SD (Range) | 7.51 ± 0.57 (6.1 to 8.8) | 7.76 ± 0.66 (6.1 to 8.7) | 0.158** |
| Coexistent hypertension (Yes / NO), n (%) | 11 (44.0) / 14 (56.0) | 12 (48.0) / 13 (52.0) | 0.777* |
Abbreviations: n, numbers; %, percentage; y, years; SD, standard deviation; DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; PPV, pars plana vitrectomy; ILM, internal limiting membrane. Notes: Group A, eyes with tractional diabetic macular edema that underwent PPV without ILM peeling; Group B, eyes with tractional diabetic macular edema that underwent PPV with ILM peeling (*the chi-square test was used; **the independent sample t-test was used; ***the Mann–Whitney U test was used).
Preoperatively, 13 eyes had significant cataracts and underwent combined phacovitrectomy (7 eyes in group A and 6 eyes in group B) (P = 0.749).
The mean (SD) preoperative BCDVAs in the total of 50 eyes, group A, and group B were 0.86 (0.11) logMAR, 0.89 (0.12) logMAR, and 0.83 (0.10) logMAR, respectively (group A versus group B, P = 0.243). At 1 month postoperatively, the mean (SD) BCDVAs in the total of 50 eyes, group A, and group B were significantly improved compared to those at baseline (P < 0.05), measuring 0.74 (0.14) logMAR, 0.76 (0.16) logMAR, and 0.72 (0.12) logMAR, respectively (group A versus group B, P = 0.411). At 3 months, the mean (SD) BCDVAs displayed significant improvement compared to baseline measurements (P < 0.05) in the total of 50 eyes, group A, and group B, reaching 0.65 (0.20) logMAR, 0.68 (0.19) logMAR, and 0.62 (0.20) logMAR, respectively (group A versus group B, P = 0.198). Likewise, at 6 months, the mean (SD) BCDVAs displayed highly significant improvement compared to baseline measurements (P < 0.001) in the total of 50 eyes, group A, and group B, reaching 0.61 (0.24) logMAR, 0.64 (0.24) logMAR, and 0.58 (0.24) logMAR, respectively (group A versus group B, P = 0.204). This improvement was comparable between the two groups at all time points (Table 2 and Figure 2).
Table 2.
Comparison of functional and anatomical outcomes in all 50 included eyes over the follow-up periods
| Variable |
Follow-up points
|
|||
|---|---|---|---|---|
| Pre-op | 1-month post-op | 3-month post-op | 6-month post-op | |
| BCDVA (logMAR), Mean ± SD | 0.86 ± 0.11 | 0.74 ± 0.14 | 0.65 ± 0.20 | 0.61 ± 0.24 |
| P 0 | P = 0.007 | P < 0.001 | P < 0.001 | |
| Significance between periods | - | P 1 = 0.011, P2 = 0.001, P3 = 0.368 | ||
| CMT (µm), Mean ± SD | 484.06 ± 85.55 | 306.36 ± 57.18 | 254.80 ± 26.73 | 227.20 ± 22.43 |
| P 0 | - | P <0.001 | P <0.001 | P < 0.001 |
| Significance between periods | - | P 1 <0.001, P 2 <0.001, P 3 < 0.001 | ||
Abbreviations: Pre-op, preoperative; post-op, postoperative; BCDVA, best-corrected distance visual acuity; logMAR, logarithm of minimal angle of resolution; SD, standard deviation; CMT, central macular thickness, µm, micrometer. Notes: P-values < 0.05 are shown in bold (the paired t-test was used). P0, P-value for comparison between baseline and each postoperative measurement; P1, P-value between 1-month and 3-month postoperative measurements; P2, P-value between 1-month and 6-month postoperative measurements; P3, P-value between 3-month and 6-month postoperative measurements.
Figure 2.
Mean BCDVA (logMAR) before PPV and at 1 month, 3 months, and 6 months after the operation in the two groups. Abbreviations: BCDVA, best-corrected distance visual acuity; logMAR, logarithm of minimal angle of resolution; PPV, pars plana vitrectomy; ILM, internal limiting membrane. Note: Group A, eyes with tractional diabetic macular edema that underwent PPV without ILM peeling; Group B, eyes with tractional diabetic macular edema that underwent PPV with ILM peeling. A highly significant improvement was seen in group A after 1 month (P < 0.001) along with a significant improvement in group B (P = 0.047). A highly significant improvement was seen in group A after 3 months (P = 0.001) along with a significant improvement in group B (P = 0.024). The improvement in BCDVA in both groups after 6 months was highly significant (P < 0.001( (the Wilcoxon signed-rank test was used)
The functional outcomes were comparable between the two groups at 1, 3, and 6 months postoperatively (all P > 0.05) (Table 3).
Table 3.
Comparison of groups A and B according to Delta BCDVA and Delta CMT
| Change between | Group A (n = 25) | Group B (n = 25) | P -value * |
|---|---|---|---|
|
Baseline-1month BCDVA (logMAR),
Mean ± SD (Delta) |
-0.13 ± 0.02 (-14.61) | -0.11 ± 0.03 (-13.3) | 0.082 |
|
Baseline-3months BCDVA (logMAR),
Mean ± SD (Delta) |
-0.21 ± 0.03 (-23.6) | -0.21 ± 0.04 (-25.3) | 0.142 |
|
Baseline-6months BCDVA (logMAR),
Mean ± SD (Delta) |
-0.25 ± 0.04 (-28.09) | -0.25 ± 0.05 (-30.12) | 0.256 |
|
Baseline-1month CMT (µm),
Mean ± SD (Delta) |
-178.84 ± 54.94 (-37.9) | -176.56 ± 54.82 (-35.5) | 0.816 |
|
Baseline-3months CMT (µm),
Mean ± SD (Delta) |
-223.88 ± 64.59 (-47.5) | -234.64 ± 81.47 (-47.2) | 0.712 |
|
Baseline-6months CMT (µm),
Mean ± SD (Delta) |
-243.08 ± 66.64 (-51.6) | -270.64 ± 87.00 (-54.5) | 0.282 |
Abbreviations: n, number of eyes; BCDVA, best-corrected distance visual acuity; logMAR, logarithm of minimal angle of resolution; SD, standard deviation; CMT, central macular thickness; µm, micrometer; PPV, pars plana vitrectomy; ILM, internal limiting membrane. Notes: Delta, is a change in BCDVA or CMT of each postoperative measurement with respect to its corresponding baseline value. Group A, eyes with tractional diabetic macular edema that underwent PPV without ILM peeling; Group B, eyes with tractional diabetic macular edema that underwent PPV with ILM peeling (*the Mann–Whitney U test was used).
There was a highly significant negative correlation between BCDVA improvement after 6 months and preoperative HbA1c level in all study participants (r = -0.824; P < 0.001). By the end of the study, BCDVA had improved (two or more lines in the Snellen chart) in 17 eyes (68%) in group A compared with 19 eyes (76%) in group B. Four eyes (16%) in group A had no improvement in BCDVA, or had less than two lines of improvement, compared to three eyes (12%) in group B. Four eyes (16%) had worsened BCDVA in group A, compared to three eyes (12%) in group B (P = 0.820).
The mean (SD) preoperative CMTs in the total of 50 eyes, group A, and group B were 484.06 (85.55) µm, 471.28 (80.83) µm, and 496.84 (89.82) µm, respectively (group A versus group B, P = 0.296). At 1 month postoperatively, the mean (SD) CMTs in the total of 50 eyes, group A, and group B showed highly significant decreases compared to baseline values (P < 0.001), measuring 306.36 (57.18) µm, 292.44 (61.34) µm, and 320.28 (50.09) µm, respectively (group A versus group B, P = 0.085). After 3 months, the mean (SD) CMTs in the total of 50 eyes, group A, and group B were 254.80 (26.73) µm, 247.40 (28.35) µm, and 262.20 (23.25) µm, respectively (group A versus group B, P = 0.049). After 6 months, the mean CMTs were highly significantly decreased compared to baseline values (P < 0.001); in the total of 50 eyes, group A, and group B, measurements were 227.20 (22.43) µm, 228.20 (26.45) µm, and 226.20 (18.04) µm, respectively (group A versus group B, P = 0.756). This decrease in CMT was comparable between the two groups at all time points (Table 2 and Figure 3).
Figure 3.
CMT (μm) before PPV and at 1 month, 3 months, and 6 months after surgery in the two groups. Abbreviations: CMT, central macular thickness, µm, micrometer; PPV, pars plana vitrectomy; ILM, internal limiting membrane. Note: Group A, eyes with tractional diabetic macular edema that underwent PPV without ILM peeling; Group B, eyes with tractional diabetic macular edema that underwent PPV with ILM peeling. There was a highly significant reduction in CMT at all postoperative time points in both groups (all P < 0.001, paired t-test)
According to Delta CMT, anatomical outcomes were comparable between the two groups at 1, 3, and 6 months postoperatively (all P > 0.05) (Table 3).
By the end of the study, 17 eyes (68%) in group A had a reduction in CMT of much more than one-half compared with 23 eyes (92%) in group B. A CMT reduction of less than 50% was detected in 8 eyes (32%) in group A and 2 eyes (8%) in group B (P = 0.321). Only 1 eye in group A and 2 eyes in group B had an iatrogenic break (P = 0.556). These eyes were treated intraoperatively using endolaser photocoagulation. Complications were detected in 16 eyes but did not prevent follow-up and OCT imaging; 7 eyes were in group A and 9 eyes were in group B (P = 0.762) (Table 4).
Table 4.
Comparison of postoperative complications between group A and group B
| Postoperative Complications | Total (n = 50) | Group A (n = 25) | Group B (n = 25) | P -value * |
|---|---|---|---|---|
| Complications (Yes / NO), n (%) | 16 (32.0) / 34 (68.0) | 7 (28.0) / 18 (72.0) | 9 (36.0) / 16 (64.0) | 0.762 |
| Cataract, n (%) | 5 out of 21 phakic eyes (23.8) | 2 out of 11 phakic eyes (18.2) | 3 out of 10 phakic eyes (30.0) | 0.641 |
| EMM, n (%) | 1 (2.0) | 1 (4.0) | 0 (0.0) | 0.317 |
| Glaucoma, n (%) | 1 (2.0) | 0 (0.0) | 1 (4.0) | 0.317 |
| Vitreous hemorrhage, n (%) | 6 (12.0) | 2 (8.0) | 4 (16.0) | 0.389 |
| Iridocyclitis, n (%) | 3 (6.0) | 2 (8.0) | 1 (4.0) | 0.556 |
Abbreviations: n, number of eyes; %, percentage; EMM, epi-macular membrane; PPV, pars plana vitrectomy; ILM, internal limiting membrane. Notes: Group A, eyes with tractional diabetic macular edema that underwent PPV without ILM peeling; Group B, eyes with tractional diabetic macular edema that underwent PPV with ILM peeling (*the chi-square test was used).
DISCUSSION
In this study, we observed functional and anatomical improvement (decrease in CMT and improvement in BCDVA) in the two groups at 1, 3, and 6 months after PPV. However, we found no significant difference in these changes (Delta BCDVA or Delta CMT) attributable to ILM peeling.
For DME caused by tractional EMM, PPV with or without ILM peeling remains the mainstay of therapies to relieve vitreomacular abnormalities and restore normal central retinal contour [16], as we found in the current study. Additional ILM peeling may have positive effects on vision and macular thickness. The ILM may form a scaffold that promotes formation of the EMM [13]. In diabetic eyes, abnormal fibril cross-links cause strong connections between the posterior hyaloid and the ILM. The ILM contributes to DME because of its rigidity, and removing it releases the tangential tractional forces [19]. The dense ILM found in diabetic patients also retards oxygen diffusion from the vitreous into the retinal tissue [20]. The main goal of surgery is to isolate the posterior hyaloid from the macula, and ILM peeling ensures that all hyaloidal elements are released [21]. Some researchers believe that ILM peeling does not add additional benefits to PPV for DME treatment. Others believe that ILM peeling can cause retinal damage, especially in pathological retinas [22]. Our study did not detect functional or anatomical benefits when ILM peeling was added to PPV in the management of tractional DME.
In the current study, the mean BCDVAs improved by approximately 14%, 24%, and 29% after 1, 3, and 6 months, respectively, after PPV with or without ILM peeling in all the included eyes. Several studies have shown that PPV in the presence of tractional DME improves BCDVA in more than half of cases. The degree of BCDVA improvement in our study was comparable with those of Pendergast et al., who found an improvement of nearly 33% [23]; Yamamoto et al., who found approximately 57.5% improvement in group A with eyes having PVD and EMM and approximately 53.5% improvement in group C with eyes having EMM without PVD [24]; the Diabetic Retinopathy Clinical Research Network (DRCR.net) vitrectomy study results, which reported improvement ranging from 28% to 49% [7]; Kim et al., who found a nearly 24% improvement after 3 months, approximately 30% after 6 months, and 42% after 12 months [25]; and Someya et al., who found approximately 37% improvement after 6 months [26]. Jackson et al. studied PPV for vitreomacular traction and reported similar results. The diabetic group analysis showed that the median BCDVA improved from 0.7 logMAR at the time of surgery to 0.5 logMAR at 6 to 12 months post-surgery, with a 33% improvement in BCDVA and at least 0.3 logMAR (approximately two Snellen lines) [27].
In our study, the mean CMT in the total of 50 eyes decreased by approximately 37%, 47%, and 53% after 1, 3, and 6 months, respectively, after PPV with or without ILM peeling. According to several studies, PPV can lead to a significant decrease in CMT in most cases. Pendergast et al. found a decrease in CMT in 81.8% of cases, and DME exhibited complete resolution over 4.5 months [23]. Yamamoto et al. found decreases in CMT of approximately 46.9% in group A and 50% in group C [24]. Kim et al. found a decrease in CMT of approximately 28% after 12 months in a tractional DME group [25]. Someya et al. found a nearly 45% decrease in CMT after 12 months in a tractional DME group [26].
Improvement in BCDVA and reduction in CMT were greater in group A, without ILM peeling, at the beginning of follow-up (1 month after PPV); however, the differences between the two groups were not statistically significant. The improvement was nearly the same after 3 months, and group B, with ILM peeling, improved after 6 months. This may be related to damage [22] caused by the ILM gripping forceps and slightly more time for peeling.
In a meta-analysis of diabetic eyes that underwent PPV and ILM peeling versus eyes with PPV only, Rinaldi et al. [28] found no significant differences in postoperative BCDVA, BCDVA change, CMT, or CMT change between the two groups. They concluded that additional ILM peeling in PPV did not significantly improve the visual or anatomical results [28]. In a systematic review and meta-analysis, Nakajima et al. [17] examined the impact of combining ILM peeling with PPV in DME. Compared to the non-peeling group, the postoperative BCDVA in the ILM peeling group was superior by 0.04 logMAR, and the BCDVA change was enhanced by 0.04 logMAR, yet both results were statistically insignificant. There was no discernible difference in postoperative CMT or CMT reduction between the two groups. They concluded that there was no significant difference in visual acuity results between PPV with ILM peeling and without ILM peeling [17]. Likewise, we found no significant difference in visual or anatomical outcomes between eyes undergoing PPV with and without ILM peeling.
Flaxel et al. collected prospective data from 241 eyes that underwent PPV for DME. They reported that ILM peeling showed superior anatomical outcomes but was not associated with enhanced BCDVA improvement after PPV [15]. In contrast, Hu et al., in a meta-analysis of 14 studies involving 857 eyes, suggested that PPV was successful for DME and could be improved by peeling the ILM, without increasing the incidence of complications [14]. In the current study, according to the Delta BCDVA and Delta CMT, functional and anatomical outcomes were comparable between the ILM peeling group and the non-peeling group at 1, 3, and 6 months postoperatively. We found no significant difference between groups A and B in these changes attributable to ILM peeling.
We found a significant negative correlation between BCDVA improvement and long-term blood glycemic control. This finding is consistent with that of Kumagai et al., who studied the long-term results of PPV for DME and reported a negative correlation between preoperative HbA1c level and postoperative BCDVA [29]. Yamada et al. also studied the relationship between preoperative systemic or ocular factors and BCDVA or CMT, before and 6 months after the surgical procedure, in 44 eyes that underwent PPV with ILM peeling. Although BCDVA and CMT were related to ocular factors before surgery, they were also related to long-term glycemic control after surgery [30].
Our study was limited by its relatively old argument. However, this issue has not yet been fully resolved. The study failed to assess the extent and intensity of macular edema and the size of the foveolar avascular zone at baseline and during follow-up. The points of strength in the study were that it was conducted at the same location within the same ethnic group, and one surgeon performed all operations with uniform steps, except for the ILM peeling in group B. All vitreous cavities were left fluid-filled. The surgeon did not administer intravitreal steroids during any PPV. Instead, he used a double-staining technique with vital dyes. Further double-blinded, multicenter, clinical trials involving more patients and longer follow-up periods are necessary to address the actual benefit of additional ILM peeling during PPV in eyes with tractional DME.
CONCLUSIONS
Vision significantly improved in both groups, with no significant anatomical or functional differences attributable to ILM peeling. Postoperative BCDVA was poorer in patients with inadequate glycemic control and high preoperative HbA1c levels. Further multicenter, randomized, controlled trials with long-term follow-up are needed to verify or disprove our findings.
ETHICAL DECLARATIONS
Ethical approval:
The study was approved by the Al-Azhar Medical Research Ethical Committee, observed the tenets of the Helsinki Declaration, and was presented to all patients before surgery. All participants provided informed consent after receiving an explanation of the study procedures.
Conflict of interest:
None.
FUNDING
None.
ACKNOWLEDGMENTS
We thank our colleagues at the Al-Azhar University Hospitals.
References
- 1.Workneh MH, Bjune GA, Yimer SA. Assessment of health system challenges and opportunities for possible integration of diabetes mellitus and tuberculosis services in South-Eastern Amhara Region, Ethiopia: a qualitative study. BMC Health Serv Res. 2016;16:135 . doi: 10.1186/s12913-016-1378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17 . doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson W, Piggott K, Bao YK, Pham H, Kavali S, Rajagopal R. Complete Posterior Vitreous Detachment Reduces the Need for Treatment of Diabetic Macular Edema. Ophthalmic Surg Lasers Imaging Retina. 2019;50(11):e266–e273. doi: 10.3928/23258160-20191031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta V, Arevalo JF. Surgical management of diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20(4):283–92. doi: 10.4103/0974-9233.120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, Browning DJ, et al. Diabetic Retinopathy Clinical Research Network Writing Committee. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117(6):1087–1093. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capone A Jr, Panozzo G. Vitrectomy for refractory diabetic macular edema. Semin Ophthalmol. 2000;15(2):78–80. doi: 10.3109/08820530009039996. [DOI] [PubMed] [Google Scholar]
- 9.Semeraro F, Morescalchi F, Duse S, Gambicorti E, Russo A, Costagliola C. Current Trends about Inner Limiting Membrane Peeling in Surgery for Epiretinal Membranes. J Ophthalmol. 2015;2015:671905. doi: 10.1155/2015/671905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichi F, Lembo A, Morara M, Veronese C, Alkabes M, Nucci P, et al. Early and late inner retinal changes after inner limiting membrane peeling. Int Ophthalmol. 2014;34(2):437–46. doi: 10.1007/s10792-013-9831-6. [DOI] [PubMed] [Google Scholar]
- 11.Schumann RG, Schaumberger MM, Rohleder M, Haritoglou C, Kampik A, Gandorfer A. Ultrastructure of the vitreomacular interface in full-thickness idiopathic macular holes: a consecutive analysis of 100 cases. Am J Ophthalmol. 2006;141(6):1112–1119. doi: 10.1016/j.ajo.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 12.Hoerauf H, Brüggemann A, Muecke M, Lüke J, Müller M, Stefánsson E, et al. Pars plana vitrectomy for diabetic macular edema nternal limiting membrane delamination vs posterior hyaloid removal A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):997–1008. doi: 10.1007/s00417-010-1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe S, Yamamoto T, Kashiwagi Y, Kirii E, Goto S, Yamashita H. Three-dimensional imaging of the inner limiting membrane folding on the vitreomacular interface in diabetic macular edema. Jpn J Ophthalmol. 2013;57(6):553–62. doi: 10.1007/s10384-013-0275-3. [DOI] [PubMed] [Google Scholar]
- 14.Hu XY, Liu H, Wang LN, Ding YZ, Luan J. Efficacy and safety of vitrectomy with internal limiting membrane peeling for diabetic macular edema: a Meta-analysis. Int J Ophthalmol. 2018;11(11):1848–1855. doi: 10.18240/ijo.2018.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaxel CJ, Edwards AR, Aiello LP, Arrigg PG, Beck RW, Bressler NM, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30(9):1488–95. doi: 10.1097/IAE.0b013e3181e7974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai K, Hangai M, Ogino N, Larson E. Effect of Internal Limiting Membrane Peeling on Long-Term Visual Outcomes for Diabetic Macular Edema. Retina. 2015;35(7):1422–8. doi: 10.1097/IAE.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima T, Roggia MF, Noda Y, Ueta T. Effect of Internal Limiting Membrane Peeling During Vitrectomy for Diabetic Macular Edema: Systematic Review and Meta-analysis. Retina. 2015;35(9):1719–25. doi: 10.1097/IAE.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 18.Leung CK, Chan WM, Chong KK, Chan KC, Yung WH, Tsang MK, et al. Alignment artifacts in optical coherence tomography analyzed images. Ophthalmology. 2007;114(2):263–70. doi: 10.1016/j.ophtha.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 19.Domalpally A, Gangaputra S, Danis RP. ‘Anatomy and Physiology of the Vitreo-macular Interface’. In: Girach, A, de Smet, M, editors. Diseases of the Vitreo-Macular Interface. Essentials in Ophthalmology . Vol. 10. Berlin, Heidelberg: Springer; 2014. [Google Scholar]
- 20.Stefánsson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):147–63. doi: 10.1007/s00417-008-0980-7. [DOI] [PubMed] [Google Scholar]
- 21.Mikhail M, Hassan TS. ‘Surgical Management of Diabetic Macular Edema’. In: Jain, A, Natarajan, S, Saxena, S, editors. Cutting-edge Vitreoretinal Surgery. Singapore: Springer; 2021. [Google Scholar]
- 22.Romano MR, Romano V, Vallejo-Garcia JL, Vinciguerra R, Romano M, Cereda M, et al. Macular hypotrophy after internal limiting membrane removal for diabetic macular edema. Retina. 2014;34(6):1182–9. doi: 10.1097/IAE.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 23.Pendergast SD, Hassan TS, Williams GA, Cox MS, Margherio RR, Ferrone PJ, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130(2):178–86. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132(3):369–77. doi: 10.1016/s0002-9394(01)01050-9. [DOI] [PubMed] [Google Scholar]
- 25.Kim KT, Jang JW, Kang SW, Chae JB, Cho K, Bae K. Vitrectomy Combined with Intraoperative Dexamethasone Implant for the Management of Refractory Diabetic Macular Edema. Korean J Ophthalmol. 2019;33(3):249–258. doi: 10.3341/kjo.2018.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Someya H, Takayama K, Takeuchi M, Yokoyama H, Kimura T, Morioka M, et al. Outcomes of 25-Gauge Vitrectomy for Tractional and Nontractional Diabetic Macular Edema with Proliferative Diabetic Retinopathy. J Ophthalmol. 2019;2019:5304524. doi: 10.1155/2019/5304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson TL, Donachie PH, Johnston RL. Electronic Medical Record Database Study of Vitrectomy And Observation for Vitreomacular Traction. Retina. 2016;36(10):1897–905. doi: 10.1097/IAE.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 28.Rinaldi M, dell’Omo R, Morescalchi F, Semeraro F, Gambicorti E, Cacciatore F, et al. ILM peeling in nontractional diabetic macular edema: review and metanalysis. Int Ophthalmol. 2018;38(6):2709–2714. doi: 10.1007/s10792-017-0761-6. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai K, Furukawa M, Ogino N, Larson E, Iwaki M, Tachi N. Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2009;29(4):464–72. doi: 10.1097/IAE.0b013e31819c632f. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Suzuma K, Ryu M, Tsuiki E, Fujikawa A, Kitaoka T. Systemic factors influence the prognosis of diabetic macular edema after pars plana vitrectomy with internal limiting membrane peeling. Curr Eye Res. 2013;38(12):1261–5. doi: 10.3109/02713683.2013.820327. [DOI] [PubMed] [Google Scholar]