Abstract
Objectives
To examine the clinical characteristics of patients with non-alcoholic steatohepatitis (NASH) and associated comorbidities.
Design
A case–control study using the national health insurance and the long-term elderly health insurance claims database.
Setting
Eligible patients diagnosed with NASH (ICD-10 K-75.8, other inflammatory liver disease or K-76.0, other fatty liver) between April 2015 and March 2020 were included.
Participants
Patients who met the diagnostic definitions for NASH (n=545) were matched with non-NASH controls (n=185 264) and randomly selected according to sex, birth year and residential area.
Interventions
No interventions were made.
Primary and secondary outcome measures
ORs were estimated for the relationship between patient background, such as age and sex, body mass index (BMI), NASH-related comorbidities and lifestyle-related diseases.
Results
In total, 545 patients with NASH (38.3% men) and 185 264 non-NASH controls (43.2% men) were identified, with median ages of 68 (IQR 63.0–75.0) and 65 (IQR 44.0–74.0) years, respectively. BMI was significantly higher in patients with NASH than in controls (25.8 kg/m2 vs 22.9 kg/m2, p<0.001). The proportions of women, patients with hypertension, patients with dyslipidaemia and patients with type 2 diabetes were higher in the NASH group. In addition, NASH was associated with an increased risk of hepatic cirrhosis (OR 28.81 (95% CI 21.79 to 38.08)), followed by liver cancer (OR 18.38 (95% CI 12.56 to 26.89)). There was no significant association between NASH and risk for depression (OR 1.11 (95% CI 0.87 to 1.41)), insomnia (OR 1.12 (95% CI 0.94 to 1.34)) or chronic kidney diseases (OR 0.81 (95% CI 0.58 to 1.12)).
Conclusions
In the daily medical care of patients, it is necessary to consider sex and age differences and to pay close attention to the risk of liver cancer, as well as other lifestyle-related comorbidities associated with NASH.
Keywords: PUBLIC HEALTH, Hepatobiliary disease, Case-Control Studies
Strengths and limitations of this study.
In this study, analysis was performed using claims data covering a wide range of age groups, including elderly patients.
Data extraction was limited to patients with a history of liver biopsies, which may have been considered to include a group with more severe non-alcoholic steatohepatitis.
A long-term observation period of 5 years was established.
Japan has several public health insurance systems, but the data used in this study were obtained from the National Health Insurance claims database and therefore did not cover the whole of Japan.
Secondary data were used in this study, and some of them were missing.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common type of liver disease, affecting approximately 20%–30% of the global population.1 NAFLD includes non-alcoholic fatty liver (NAFL), which is pathologically pure steatosis alone or a situation in which steatosis is accompanied by inflammatory cell infiltration, and non-alcoholic steatohepatitis (NASH), which is accompanied by hepatic steatosis, inflammatory cell infiltration, ballooning (hepatocellular ballooning) and hepatic fibrosis.2
Liver tissue biopsy is the gold standard for diagnosing NAFLD,3–6 which is consistent with guidelines published overseas. However, in clinical practice, performing a liver biopsy with bleeding or pain in all patients with NAFLD is not feasible. Therefore, the proportion of patients undergoing liver biopsy for a NASH diagnosis in clinical practice is not fully understood. According to Rinella and Sanyal,4 the biopsy rate for NASH diagnosis and the therapeutic drugs prescription rate recommended by the guidelines are low, and NASH is underdiagnosed.6 According to an estimate based on a Markov model of the number of patients with NAFL and NASH worldwide, the number of patients with fibrotic NASH at stage III or higher in Japan was predicted to increase to 660 000 in 2016 and 990 000 by 2030.7 Moreover, although NASH prevalence has been estimated to be approximately 3%–5% of the population,8 9 there is insufficient evidence for NASH prevalence in the general population due to selection bias in liver biopsies and diagnostic difficulties.
NASH is strongly associated with metabolic syndrome, obesity, diabetes mellitus (DM), hypertension and dyslipidaemia,1 and the major causes of death are cardiovascular and liver disease-related events.6 Obesity and DM are risk factors for cardiovascular and liver disease-related events, including decompensated cirrhosis and liver cancer. Overseas guidelines propose evaluating liver function by abdominal echography and blood tests in patients with obesity or DM.3 In the Evidence-based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Diseases/Nonalcoholic Steatohepatitis 2020 (2nd edition) of Japan (the NASH/NAFLD guideline),10 it is recommended that primary care physicians assess liver function in patients with risk factors including obesity, DM, dyslipidaemia and hypertension, and identify all cases of NAFLD fibrosis progression (as primary screening). As NASH is often asymptomatic and cirrhosis may already be present at the time of diagnosis, efficient screening and timing of referral to a gastroenterologist are important. In NASH, liver fibrosis progresses by one stage in approximately 7 years and progresses faster in patients with comorbid metabolic diseases such as obesity and DM.11 Therefore, it is recommended that the degree of fibrosis in the NASH group should be regularly evaluated, and, depending on the results, follow-up observation or screening should be performed for liver-related diseases such as liver failure and liver cancer, and non-liver-related diseases such as cerebrocardiovascular events and cancers of other organs.2
Japan, with a universal health insurance system, has almost all residents covered by medical insurance. Understanding the medical situation of local residents is possible by investigating the claims data of medical insurance provided by the administration.12 Each municipality serves as a payer of the National Health Insurance (NHI), and the municipalities jointly established the ‘Federation of National Health Insurance Organizations’ to provide insurance services. Each prefecture has one payer.
The University of Occupational and Environmental Health, Japan has used health insurance claims data by closely cooperating with payers in the NHI; the data enables the understanding of the disease information of NHI beneficiaries every month. Eguchi et al13 showed that age-specific NAFLD prevalence was higher in middle-aged men and older women, with differences in the age distribution of NAFLD onset between both sexes. To obtain data on the late-stage elderly, in this study, we matched the NHI claims and the health insurance database for persons aged ≥75 years individually and constructed an original database.
Methods
Study design and data source
Japan’s health insurance system is commonly divided into three types: company health insurance for those employed in a business, NHI for residents of each region and long-term elderly health insurance (LEHI) for those aged ≥75 years. NHI is a mutual assistance programme in which enrolled members pay premiums to a financial pool to which the national government and local municipalities add funds. This case–control study was analysed using the NHI and LEHI claims databases, comprising inpatient, outpatient and dispensing service data from domestic payers over April 2015 (through March 2020), provided by the public institution in Japan.
The data included the age and sex of each beneficiary, the type of service used, the month during which the service was used, monthly expenditures on the use of the services and exit information (death or move-out). We prepared a panel database combining basic medical check-up data and claims databases conducted on a patient-by-patient basis to examine the clinical characteristics of patients with NASH.
Study population and eligibility criteria
The inclusion criterion was patients of any age with a record of at least one episode of NASH during the study period from April 2015 to March 2020. An episode of NASH was defined as NASH diagnosis (ICD-10 K-75.8; other inflammatory liver diseases or K-76.0; other fatty liver). Furthermore, patients whose disease name string could be confirmed as ‘hepatitis’, ‘non-alcoholic’ and ‘NASH’ in the claims data were also included in the analysis. Using ICD-10; K-75.8 and K-76.0, we have learned that differentiating patients with NASH from patients with NAFLD is extremely difficult. Since a definitive diagnosis of NASH is histopathological diagnosis by liver biopsy and it is essential to confirm pathologically characteristic finding, patients diagnosed with NASH after liver biopsy (percutaneous needle biopsy, endoscopic biopsy and endoscopic ultrasound-guided fine-needle aspiration biopsy) indication were selected. Controls that never had a claim associated with NASH were randomly selected from patients who visited a medical facility at least once between April 2015 and March 2020.
The exclusion criterion was claims for any of the following conditions at any time: hepatitis B virus, hepatitis C virus, HIV, alcoholic liver disease, toxic liver injury, copper metabolism disorder, autoimmune hepatitis, Gaucher’s disease, lysosomal acid lipase deficiency, biliary cirrhosis, cholangitis or iron metabolism disorder. ICD-10 codes were used to identify the patients with these diseases. It should be noted that, given the expert opinion that a liver biopsy may be performed for a definitive diagnosis of autoimmune hepatitis to extract a purer sample in patients with NASH, patients with autoimmune hepatitis were excluded.
A patient was defined as having a comorbidity if they had at least one claim for the relevant ICD-10 code during the analysis period. Fourteen comorbidities of interest identified using ICD-10 diagnosis codes (online supplemental table 1) were prespecified: hypertension, dyslipidaemia, type 2 diabetes (T2D), osteoporosis, insomnia, depression, hepatic cirrhosis, liver cancer, cancer, colorectal adenomas, chronic kidney disease (CKD), gastro-oesophageal reflux disease (GERD), cardiovascular disease (CVD) and sleep apnoea syndrome (SAS). The prevalence of these predefined comorbidities has been reported in all patients with NASH and non-NASH comparators.
bmjopen-2023-074851supp001.pdf (222.6KB, pdf)
According to our definition, each patient classified as having NASH was compared with non-NASH comparators randomly selected from the original database by sex, birth year and residential area (online supplemental figure 1).
Data collection
Baseline data on all patient characteristics (age, sex), date of death (if data were recorded), prescribed drugs for treating NASH, and NASH-related comorbidities were collected. Age and sex were obtained as of April 2015. The dates of death and prescribed drugs for treating NASH-related comorbidities were obtained at any time during the study period. Height, weight and laboratory test values were also obtained from patients’ available data at any time during the study period. Body mass index (BMI) was calculated from the data of height and weight recorded.
Information on the pathological classification of NASH could not be obtained due to the unavailability of medical examination test results in the NHI and LEHI claims databases.
Statistical analyses
We designed a case–control study to compare the occurrence of comorbidities between the NASH and non-NASH groups during the analysis period and to assess the relationship between NASH and comorbidities. ORs for age, sex, life-related diseases (hypertension, dyslipidaemia, T2D) and NASH-related comorbidities were evaluated. All analyses were conducted for the two groups: the NASH group, in which patients had at least one record of being diagnosed with NASH, and the non-NASH group, in which the patients had no record of being diagnosed.
Descriptive statistics were conducted using logistic regression models to analyse the relationship of NASH with sex, age, lifestyle-related diseases, death and comorbidities, along with their ORs and 95% CIs. Differences between the NASH and non-NASH groups were evaluated using Pearson’s χ2 test for categorical variables and independent t-tests for continuous variables. Statistical significance was set at p<0.05.
All statistical analyses were performed using Stata V.17.0 released in April 2021 (Stata Corp).
Patient and public involvement
Patients and members of the public were not involved in the conduct of the study.
Results
Patient characteristics
Patient background characteristics are shown in online supplemental table 2. In total, 545 patients with NASH (209 men and 336 women) were selected from the claims databases, and 185 264 non-NASH controls (80 051 men and 105 213 women) were identified, with median (IQR) ages of 68 (63.0–75.0) and 65 (44.0–74.0) years, respectively. Among the NASH group, the most frequently prescribed agents were statins (53.2%), followed by angiotensin receptor blockers (ARBs) (45.9%), and vitamin E (12.1%), and among the non-NASH group, they were ARBs (22.2%), statins (21.4%) and ACE inhibitor (2.8%).
Table 1 summarises the height, weight, BMI and blood test values of patients whose data could be extracted for each group. In total, 220 patients were identified in the NASH group, and 44 913 patients were identified in the non-NASH group. BMI was significantly higher than in the NASH versus non-NASH group (25.8 kg/m2 vs 22.9 kg/m2, p<0.001). The laboratory test value (>5%) was also higher than in the NASH versus non-NASH group, except for high-density cholesterol (54.0 mg/dL and 61.0 mg/dL, respectively) and low-density lipoprotein cholesterol (109.0 mg/dL and 117.0 md/dL, respectively).
Table 1.
Characteristics of the patients with specific health examination data in analysed NASH and non-NASH groups
| NASH group | Non-NASH group | P value | |||
| N=220 | N=44 913 | ||||
| Degree of obesity; median (IQR) | |||||
| Body weight, kg | 63.9 | (55.4–71.5) | 56.0 | (48.9–64.0) | <0.001 |
| Height, cm | 156.8 | (150.8–163.8) | 156.0 | (149.8–163.4) | 0.400 |
| Body mass index, kg/m2 | 25.8 | (23.4–28.1) | 22.9 | (20.8–25.2) | <0.001 |
| Laboratory test values; median (IQR) | |||||
| AST, U/L | 31.0 | (23.0–52.0) | 22.0 | (19.0–26.0) | <0.001 |
| ALT, U/L | 31.5 | (21.0–55.5) | 17.0 | (13.0–23.0) | <0.001 |
| γ-GTP, U/L | 42.0 | (27.0–77.0) | 22.0 | (16.0–34.0) | <0.001 |
| SBP, mm Hg | 130.0 | (121.0–140.0) | 129.0 | (119.0–140.0) | 0.220 |
| DBP, mm Hg | 75.0 | (70.0–81.5) | 74.0 | (67.0–80.0) | 0.020 |
| HbA1c, % | 6.0 | (5.7–6.5) | 5.7 | (5.4–6.0) | <0.001 |
| TG, mg/dL | 127.5 | (90.5–171.0) | 95.0 | (70.0–134.0) | <0.001 |
| HDL, mg/dL | 54.0 | (44.5–63.0) | 61.0 | (51.0–73.0) | <0.001 |
| LDL, mg/dL | 109.0 | (91.0–127.5) | 117.0 | (99.0–138.0) | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein cholesteryl; LDL, low-density lipoprotein cholesterol; NASH, non-alcoholic steatohepatitis; SBT, systolic blood pressure; TG, triglyceride; γ-GTP, γ-guanosine triphosphate.
Comorbidities
Table 2 summarises NASH-related comorbidities of patients in each group. The prevalence rates of all NASH-related comorbidities were significantly higher in the NASH versus the non-NASH group, except that of autoimmune hepatitis. The five most prevalent comorbidities had rates above 50% in the NASH group: dyslipidaemia (82.6 %), hypertension (78.7 %), GERD (69.9%), T2D (62.2%) and CVD (56.0%). In the non-NASH group, the rates were not higher than 50%: hypertension (46.5%) being the most common comorbidity, followed by dyslipidaemia (36.4%).
Table 2.
NASH-related comorbidities identified in the analysed population
| Combination or disease, n (%) | NASH group | Non-NASH group | P value | ||
| N=545 | N=185 264 | ||||
| Dyslipidaemia | 450 | (82.6%) | 67 463 | (36.4%) | <0.001 |
| Hypertension | 429 | (78.7%) | 86 101 | (46.5%) | <0.001 |
| Gastro-oesophageal reflux disease | 381 | (69.9%) | 53 156 | (28.7%) | <0.001 |
| Type 2 diabetes | 339 | (62.2%) | 26 732 | (14.4%) | <0.001 |
| Cardiovascular disease | 305 | (56.0%) | 54 293 | (29.3%) | <0.001 |
| Insomnia | 225 | (41.3%) | 48 487 | (26.2%) | <0.001 |
| Osteoporosis | 188 | (34.5%) | 38 149 | (20.6%) | <0.001 |
| Cancer | 166 | (30.5%) | 22 310 | (12.0%) | <0.001 |
| Hepatic cirrhosis | 71 | (13.0%) | 632 | (0.3%) | <0.001 |
| Depression | 80 | (14.7%) | 18 038 | (9.7%) | <0.001 |
| Chronic renal failure | 42 | (7.7%) | 9136 | (4.9%) | 0.005 |
| Liver cancer | 34 | (6.2%) | 428 | (0.2%) | <0.001 |
| Sleep apnoea syndrome | 24 | (4.4%) | 2368 | (1.3%) | <0.001 |
| Colorectal adenomas | 8 | (1.5%) | 634 | (0.3%) | <0.001 |
NASH, non-alcoholic steatohepatitis.
Age, sex and life-related disease as risk factors for NASH
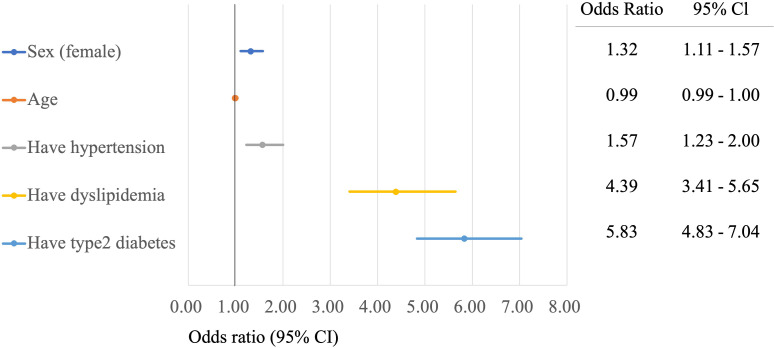
The influence of sex, age and life-rated disease on NASH has been reported previously.14 Multiple logistic regression models were used to assess the effect of sex, age and lifestyle-related diseases on NASH prevalence. Figure 1 shows the ORs for factors associated with NASH prevalence. Significantly higher risks were observed among women, patients with hypertension, patients with dyslipidaemia and patients with T2D, compared with the non-NASH group; the ORs for dyslipidaemia and T2D were very high (4.39 and 5.83, respectively). The association with age was insignificant, compared with that in the non-NASH group.
Figure 1.
Forest plot of risk adjusted ORs of diagnosed with non-alcoholic steatohepatitis according to patients’ background and life-related diseased.
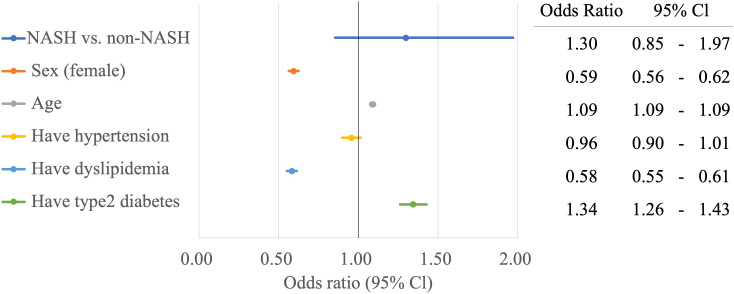
In a separate multiple logistic regression model examining the association between mortality due to NASH and each risk factor, adjusted for sex, age and lifestyle-related diseases, the ORs for age and T2D were significantly higher than those in the non-NASH group (figure 2).
Figure 2.
Forest plot of risk adjusted ORs for mortality based on patients’ background and life-related diseases. NASH, non-alcoholic steatohepatitis.
Comorbidities as risk factors for NASH
The ORs for NASH and NASH-related comorbidities, adjusted for sex, age and lifestyle-related diseases, are shown in online supplemental table 3. In a multiple logistic regression model examining the association between NASH and developing comorbidities, compared with non-NASH, the risk of developing hepatic cirrhosis was the greatest (OR 28.81, 95% CI 21.79 to 38.08), followed by that for liver cancer (OR 18.38, 95% CI 12.56 to 26.89), GERD (OR 3.08, 95% CI 2.53 to 3.73), colorectal adenomas (OR 2.54, 95% CI 1.25 to 5.16), colon cancer (OR 2.36, 95% CI 1.70 to 3.28), cancer (OR 2.16, 95% CI 1.79 to 2.62), SAS (OR 1.82, 95% CI 1.20 to 2.76), CVD (OR 1.40, 95% CI 1.16 to 1.69) and osteoporosis (OR 1.25, 95% CI 1.02 to 1.53). No significant difference in comorbidities was observed for depression (OR 1.11, 95% CI 0.87 to 1.41), insomnia (OR 1.12, 95% CI 0.94 to 1.34) and CKD (OR 0.81, 95% CI 0.58 to 1.12).
There was no significant difference in the OR for osteoporosis (OR 1.03, 95% CI 0.94 to 1.13) between the NASH and non-NASH groups; however, the OR significantly increased to 6.68 (95% CI 6.47 to 6.91) in women. The OR for CKD was less <1, and it was not significantly elevated in the NASH group. However, when patients with NASH had a history of hypertension, dyslipidaemia or T2D, the ORs increased significantly to 4.33 (95% CI 4.00 to 4.68), 1.35 (95% CI 1.29 to 1.41) and 2.08 (95% CI 1.99 to 2.18), respectively, which has been shown to increase the risk of developing CKD.
Discussion
Using the NHI and LEHI claims databases, we constructed an original database and examined the clinical characteristics of patients with NASH for 5 years from April 2015 to March 2020. It has been reported that NAFLD/NASH prevalence varies by age and sex.13 15 16 In a cross-sectional study13 conducted among 8352 participants who underwent health checkups from 2009 to 2010 at three health centres in Japan, NAFLD prevalence was 29.7% overall, more than 30% in men aged 30–60 years, and increased with age in women aged 30–60 years old. It is considered that decreased oestrogen levels due to ageing and menopause affect NAFLD progression in women.14 Similar to that of NAFLD prevalence, there are more middle-aged men and older women in the age distribution of NASH prevalence. In this study, the median age of the NASH group was 68 years (IQR, 63.0–75.0), showing an older age and a higher proportion of women than men (38.3% vs 61.7%). This finding also suggests that NASH prevalence is higher in older women.
NAFLD or NASH is strongly associated with obesity.7 13 15 This study showed that BMI was significantly higher in the NASH group than in the non-NASH group (25.8 kg/m2 vs 22.9 kg/m2, p<0.001). Obesity is the most common manifestation of metabolic syndrome and the most important risk factor for NAFLD/NASH, which can also be regarded as a liver lesion.17 The WHO diagnostic criteria define BMI ≥25 kg/m2 as overweight and BMI ≥30 kg/m2 as obesity. In Japan, the definition of obesity as judged by the Japan Society for Study of Obesity is BMI ≥25 kg/m2, which is lower than the WHO value. This is because Japanese people are more likely to develop fatty liver if their BMI is less than 25 kg/m2 and develop fatty liver at a high rate after their BMI exceeds 25 kg/m2. A previous study reported NAFLD/NASH prevalence in non-obese participants (BMI <23 kg/m2) to be ≤10% and that in highly obese participants (BMI >30 kg/m2) to be approximately 80%.10 It has also been reported body weight loss ≥7% led to a decrease in the prevalence rates of hepatic steatosis, inflammatory cell infiltration, and ballooning decreased by 7% or more of body weight loss, and improved NAFLD activity score.18 The present study also showed that the median BMI was >25 kg/m2 in the NASH group, suggesting the importance of liver lesions and active lifestyle interventions in daily medical practice for NAFLD/NASH. However, it is essential to improve the consciousness of the patients for lifestyle interventions, and maintenance of the target achievement rate and adherence may become an issue. Similar to previous research,19 20 the results of the present study may support NAFLD/NASH association with several metabolic comorbidities, including T2D, dyslipidaemia, hypertension and CVD. Regarding CKD, patients with NASH were shown to have a higher risk of complications if they had hypertension or T2D. Management of these conditions may complicate the treatment of NASH, impacting clinical care outcomes.
According to the NASH/NAFLD guidelines,10 some therapeutic drugs for dyslipidaemia, hypertension and DM have been suggested to be effective for NASH, and aggressive treatment of patients with complications of these lifestyle-related diseases is recommended. Therefore, this survey investigated the proportion of prescriptions for antihyperlipidaemic, hypertensive and antidiabetic drugs. As a result, the proportion of prescriptions was 53.2% for statins and 45.9% for ARBs in the NASH group, which was significantly higher than 21.4% and 22.2%, respectively, in the non-NASH group, less than 50%. Premature mortality in NASH is related to both hepatic (cirrhosis and hepatocellular carcinoma) and extra-hepatic complications, largely CVD. Many therapeutic agents have been tested but are still non-approved, specifically for NASH. Moreover, presently, there is no drug with sufficient evidence of improving fibrosis in patients with NASH. Many clinical studies on drug therapy and development for NASH are expected to be conducted in the future.
The prevalence of lifestyle-related diseases in NAFLD in Japan is reported to be approximately 60%–80% for dyslipidaemia, 40% for hypertension and 20%–50% for DM.10 The results of this study focusing on NASH also showed that the complication rates of dyslipidaemia, hypertension, GERD and T2D were significantly higher in the NASH group than in the non-NASH group (82.6% vs 36.4 %, p<0.001; 78.7% vs 46.5%, p<0.001; 69.9% vs 28.7%, p<0.001; 62.2% vs 14.4%, p<0.001), higher than those of lifestyle-related diseases in NAFLD. In a study by Terai et al,21 who estimated complications in patients with NAFLD/NASH using the Medical Data Vision claims database, dyslipidaemia prevalence in the 67–74 years group was 57.9%, hypertension prevalence was 57.2% and T2D prevalence was 32.5%, lower than the rates reported in the present study (82.6%, 78.7% and 62.2%, respectively). However, CVD prevalence was 75.8%, higher than that in the present study (56.0%). This is because the database used by Terai et al21 summarises the health insurance data for acute-care hospitals in Japan but does not include information on health insurance data for general practitioners and core hospitals. This may have contributed to the higher proportion of CVD cases requiring surgery. In the present study, a multivariate analysis was performed for sex, age and risk factors for lifestyle-related diseases in NASH. The OR was ≥1 for factors other than age. Among them, dyslipidaemia (OR 4.39, 95% CI 3.41 to 5.65) and T2D (OR 5.83, 95% CI 4.83 to 7.04) showed an OR ≥4, indicating that these are major risk factors for NASH. In addition, T2D increased the risk of death from NASH (OR 1.34, 95% CI 1.26 to 1.43). These results suggest that more aggressive interventions are needed for patients with dyslipidaemia and T2D.
NAFLD should be considered a systemic disease that presents with many comorbidities and other lifestyle-related diseases.22 A multivariate analysis was performed for risk factors for comorbidity of NASH (risk of onset). Cirrhosis (OR 28.81, 95% CI 21.79 to 38.08) and liver cancer (OR 18.38, 95% CI 12.56 to 26.89) were significant and major risk factors for comorbidity. Cancer development from NAFLD occurs at an annual rate of approximately 0.04%, while hepatocarcinogenesis from NASH cirrhosis occurs at an annual rate of approximately 2%–3%.22 Our analysis showed a higher risk factor than that reported in previous studies. In a hospital-based study,23 68 patients with NASH cirrhosis (mean age, 63 years; 57% men) were observed for an average of 3.4 years, of whom seven patients developed cancer. Furthermore, the 5-year cumulative rate of hepatocellular carcinoma among patients with NASH was 11.3%, which was lower than the 30.5% rate of hepatitis C virus cirrhosis in the study group.24 In a median 3.2-year observational study of 195 patients with NASH cirrhosis (mean age 56.6 years, 44.1% men), 25 (12.8%) participants developed hepatocarcinogenesis, a lower rate than 20.3% of hepatitis C virus cirrhosis evident for the control group. In NASH, liver fibrosis progresses by one step every 7 years.11 The observation period of this study was 5 years, whereas the observation period of Yatsuji et al23 and Ascha et al25 was approximately 3 years, which may have been related to the difference in cancer incidence; thus, our results are acceptable. The prognosis of NASH cirrhosis worsens with increasing degrees of fibrosis and severity of cirrhosis.26 Since liver cancer is the most important vital prognostic factor in patients with cirrhosis, it is important to monitor its course in consideration of carcinogenesis.
After liver cancer and cirrhosis, GERD showed the highest OR. In the present study, the complication rate of GERD in the NASH group was as high as 69.9% and was also a high-risk factor. Several cross-sectional and cohort studies have investigated the association between NAFLD and GERD risk.27–35 However, their results have been conflicting so far. Some studies have shown a higher prevalence of GERD among patients with NAFLD, compared with the general population, while other studies have failed to find a significant association between NAFLD and GERD risk. Obesity is a potential confounding factor in clinical studies on the association between NAFLD and GERD, as it has been established as a common risk factor for both diseases.33 36 A systematic review and meta-analysis of observation studies of NAFLD patients with and without obesity in the development of GERD by Xue et al37 showed a significant association between NAFLD and GERD risk. However, to our knowledge, no study has defined a temporal or causal relationship between NAFLD and GERD. As NASH is advanced from NAFLD, GERD risk should be considered in clinical practice.
Limitations
First, our results may not be generalisable to all patients with NASH in Japan. Japan has several public health insurance systems; however, the database used only contained data from the NHI and did not cover the whole of Japan. Moreover, missing records and insufficient data entries were inevitable. The NHI covers self-employed, unemployed and retired persons aged <75 years. Therefore, to obtain data for the oldest of the older population, we added data on health insurance for persons aged <75 years. However, each patient’s medical record may not trace the patient’s full medical history if the patient moved or switched to employer-based health insurance.
Second, the lack of information must be acknowledged. NASH and its comorbidities were categorised based on ICD-10 three-character code block categories. A stable version of the ICD-11 was released in 2018 and officially endorsed by all WHO members during the 72nd World Health Assembly in 2019. The original code for NASH in ICD-11 is given but has not yet been officially enforced in Japan. Third, the current state of NASH diagnosis in Japan has not been clarified, and it was difficult to accurately extract NASH cases from actual medical care data in Japan. Furthermore, the study results suggest that the prevalence of NASH is higher in older women. However, there is a possibility of selection bias (eg, those who visited a healthcare provider, had a blood test or agreed to undergo a liver biopsy). There are also limitations in drawing firm conclusions about the exact age and sex distributions, given that they are not necessarily representative of all Japanese patients.
This study showed that NASH is significantly involved in the development of intrahepatic lesions such as cirrhosis and liver cancer. To better understand the complex aetiology of NASH, it may be necessary to investigate its relationship with extrahepatic primary cancers, such as extra-hepatic cancer.
Conclusions
The database we developed combines a large health claims database with specific medical examination data. Therefore, our study is the first to include an overview of NASH-attributable patients in Japan. NASH is expected to become an increasingly common health disorder from social and epidemiological perspectives because of the recent increase in prevalence and the diversity of diseases and conditions. The results of this study indicated that NASH is associated with high risks of complications of liver cancer and cirrhosis, and that coexisting lifestyle-related diseases increase the risk of death and the risk of complications of GERD. In the daily medical care of patients with NASH, it is necessary to consider sex and age and pay close attention to liver lesions and various other lifestyle-related neoplasms.
Supplementary Material
Footnotes
Contributors: All authors contributed significantly. Substantial contribution to study conception, design and planning of the study: KT, SK, KI, SMinami, KF, KM, SMatsuda; substantial contributions to analysis and interpretation of the data: KT, SK, SMinami; drafting the article or revising it critically for important intellectual content: KI, KT, SMinami; interpretation of results and supervised the project: SMatsuda. All the authors have read and agreed to the published version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The NHI data is not permitted to use except for those who are authorised.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the ethics committee of the University of Occupational and Environmental Health, Japan (R4-026).
References
- 1.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 2020;158:1851–64. 10.1053/j.gastro.2020.01.052 [DOI] [PubMed] [Google Scholar]
- 2.Tokushige K, Ikejima K, Ono M, et al. Evidence-based clinical practice guidelines for nanalchoholic fatty liver disease/nonalcholic steatophepatitis 2020 (2ND edition). J Gastroenterol 2021;56:951–63. 10.1007/s00535-021-01796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol 2016;13:196–205. 10.1038/nrgastro.2016.3 [DOI] [PubMed] [Google Scholar]
- 5.Wong VW-S, Chan W-K, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70–85. 10.1111/jgh.13857 [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896–904. 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 8.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. 10.1053/j.gastro.2010.09.038 [DOI] [PubMed] [Google Scholar]
- 9.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic Steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. 10.1053/j.gastro.2010.09.038 [DOI] [PubMed] [Google Scholar]
- 10.Tokushige K, Ikejima K, Ono M, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol 2021;56:951–63. 10.1007/s00535-021-01796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–54. 10.1016/j.cgh.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamaki J, Fujimori K, Ikehara S, et al. Estimates of hip fracture incidence in Japan using the national health insurance claim database in 2012-2015. Osteoporos Int 2019;30:975–83. 10.1007/s00198-019-04844-8 [DOI] [PubMed] [Google Scholar]
- 13.Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of Nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol 2012;47:586–95. 10.1007/s00535-012-0533-z [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol 2011;46:63–9. 10.1007/s00535-010-0311-8 [DOI] [PubMed] [Google Scholar]
- 15.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722–8. 10.7326/0003-4819-143-10-200511150-00009 [DOI] [PubMed] [Google Scholar]
- 16.Tobari M, Hashimoto E. Characteristic features of nonalcoholic fatty liver disease in Japan with a focus on the roles of age, sex and body mass index. Gut Liver 2020;14:537–45. 10.5009/gnl19236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan J-G, Kim S-U, Wong V-S. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67:862–73. 10.1016/j.jhep.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121–9. 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 20.Yoneda M, Yamamoto T, Honda Y, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol 2021;56:1022–32. 10.1007/s00535-021-01828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terai S, Buchanan-Hughes A, Ng A, et al. Comorbidities and healthcare costs and resource use of patients with nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) in the Japan medical data vision database. J Gastroenterol 2021;56:274–84. 10.1007/s00535-021-01759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol 2016;22:4079–90. 10.3748/wjg.v22.i16.4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yatsuji S, Hashimoto E, Tobari M, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol 2009;24:248–54. 10.1111/j.1440-1746.2008.05640.x [DOI] [PubMed] [Google Scholar]
- 24.Tokushige K, Hyogo H, Nakajima T, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: multicenter survey. J Gastroenterol 2016;51:586–96. 10.1007/s00535-015-1129-1 [DOI] [PubMed] [Google Scholar]
- 25.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–8. 10.1002/hep.23527 [DOI] [PubMed] [Google Scholar]
- 26.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 2018;155:443–57. 10.1053/j.gastro.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 27.Kimura S, Tanaka M. The relationship between non-alcoholic fatty liver disease and reflux esophagitis in Japanese subjects. Gastrointestinal Endoscopy 2007;65:AB144. 10.1016/j.gie.2007.03.188 [DOI] [Google Scholar]
- 28.Fujikawa Y, Tominaga K, Fujii H, et al. High prevalence of gastroesophageal reflux symptoms in patients with non-alcoholic fatty liver disease associated with serum levels of triglyceride and cholesterol but not simple visceral obesity. Digestion 2012;86:228–37. 10.1159/000341418 [DOI] [PubMed] [Google Scholar]
- 29.Miele L, Cammarota G, Vero V, et al. Non-alcoholic fatty liver disease is associated with high prevalence of gastro-oesophageal reflux symptoms. Dig Liver Dis 2012;44:1032–6. 10.1016/j.dld.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 30.Catanzaro R, Calabrese F, Occhipinti S, et al. Nonalcoholic fatty liver disease increases risk for gastroesophageal reflux symptoms. Dig Dis Sci 2014;59:1939–45. 10.1007/s10620-014-3113-7 [DOI] [PubMed] [Google Scholar]
- 31.Hung W-C, Wu J-S, Yang Y-C, et al. Nonalcoholic fatty liver disease vs. obesity on the risk of erosive oesophagitis. Eur J Clin Invest 2014;44:1143–9. 10.1111/eci.12348 [DOI] [PubMed] [Google Scholar]
- 32.Wijarnpreecha K, Panjawatanan P, Thongprayoon C, et al. Association between gastroesophageal reflux disease and nonalcoholic fatty liver disease: a meta-analysis. Saudi J Gastroenterol 2017;23:311–7. 10.4103/sjg.SJG_161_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min YW, Kim Y, Gwak G-Y, et al. Non-alcoholic fatty liver disease and the development of reflux esophagitis: a cohort study. J Gastroenterol Hepatol 2018;33:1053–8. 10.1111/jgh.14042 [DOI] [PubMed] [Google Scholar]
- 34.Bang KB, Shin JE, Shin HD, et al. Sa1103 - non-obese non-alcoholic fatty liver disease is associted with erosive esophagitis. Gastroenterology 2018;154:S241. 10.1016/S0016-5085(18)31184-3 [DOI] [Google Scholar]
- 35.Yang H-J, Chang Y, Park S-K, et al. Nonalcoholic fatty liver disease is associated with increased risk of reflux esophagitis. Dig Dis Sci 2017;62:3605–13. 10.1007/s10620-017-4805-6 [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara M, Eguchi Y, Fukumori N, et al. The symptoms of gastroesophageal reflux disease correlate with high body mass index, the aspartate aminotransferase/alanine aminotransferase ratio and insulin resistance in Japanese patients with non-alcoholic fatty liver disease. Intern Med 2015;54:3099–104. 10.2169/internalmedicine.54.4297 [DOI] [PubMed] [Google Scholar]
- 37.Xue J, Xin H, Ren N, et al. Nonalcoholic fatty liver disease increases the risk of gastroesophageal reflux disease: a systematic review and meta-analysis. Eur J Clin Invest 2019;49:e13158. 10.1111/eci.13158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074851supp001.pdf (222.6KB, pdf)
Data Availability Statement
No data are available. The NHI data is not permitted to use except for those who are authorised.