Abstract
Introduction
With longer life expectancy in people living with HIV (PLWH) on antiretroviral therapy, cardiovascular disease (CVD) has become a common cause of mortality among them. Abacavir has been associated with an increased risk of myocardial infarction, but the mechanism is unknown. Additionally, abacavir may be obesogenic which could mediate an additional risk factor of CVD. We aim to investigate if discontinuation of abacavir will have a favourable impact on body weight and cardiac parameters in PLWH.
Methods and analysis
Randomised, controlled, superiority trial of virologically suppressed PLWH on dolutegravir, abacavir and lamivudine (DTG/ABC/3TC) for ≥6 months. In total, 70 PLWH will be randomised 1:2 to either continue DTG/ABC/3TC or to switch to dolutegravir and lamivudine (DTG/3TC) providing the power of 80% at alpha 5% to detect a mean difference in weight change of 2 kg (Δ) given an SD of 2.7 kg. Follow-up will be 48 weeks. Data will be collected at baseline and week 48. Primary outcome will be change in mean body weight from baseline to week 24 and 48 evaluated in a linear mixed model. Secondary outcomes will be changes in cardiac, inflammatory and metabolic parameters, fat distribution, coagulation, endothelial, platelet function, quality of life and virological control from baseline to week 48. Measurements include CT of thorax and abdomen, external carotid artery ultrasound, liver elastography and dual energy X-ray absorptiometry and blood analysis. Plasma HIV RNA will be measured at baseline, week 4, 24 and 48. Forty participants (20 from each arm) will be included in a substudy involving cardiac MRI at baseline and week 48. Twenty non-HIV-infected controls will be included with a single scan to compare with baseline scan data.
Ethics and dissemination
Result from this study will lead to a better understanding of the association between antiretroviral therapy and the impact on weight and risk of CVD. Findings will be useful for both clinicians and PLWH in the guidance of a more individualised HIV treatment. Results from the main study and the substudies will be submitted for publication in a peer-reviewed journal(s). The AVERTAS study is approved by the Ethics Committee of the Capital Region, Denmark (H-20011433), Danish Medicines Agency (EudraCT no. 2019-004999-19) and Regional Data Protection Centre (P-2020–207).
Trial registration number
Pre-results registration at ClinicalTrials.gov Identifier: NCT04904406, registered 27 May 2021. Protocol version: Protocol version 9.0, 4 April 2023, approved 10-05-2023 by Ethics Committee of the Capital Region, Denmark (H-20011433). Danish Medicines Agency (EudraCT no. 2019-004999-19). Regional Data Protection Centre (P-2020–207) ClinicalTrials.gov.
Keywords: obesity; infectious diseases; virology; HIV & AIDS; other metabolic, e.g. iron, porphyria
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Study design is randomised, controlled, multicentre trial, which limits confounding.
The study is limited by the unblinded setting which enables bias.
The study is carried out in outpatient clinics, where participants receive their usual care. This might enhance adherence to study visits.
Introduction
Background and rationale
The introduction of antiretroviral treatment (ART) with ≥3 drugs changed the prognosis of HIV infection dramatically. Today, people living with HIV (PLWH) treated with ART have a life expectancy close to that of the HIV-uninfected population.1 However, this longer life expectancy has led to higher rates of serious non-AIDS events such as cardiovascular disease (CVD) among PLWH.2–4 Today, CVD is the main cause of mortality in PLWH.5 The mechanism is thought to be a multifactorial interplay between traditional CVD risk factors, HIV specific factors and ART. Studies have shown a correlation between the use of the nucleoside reverse transcriptase inhibitor (NRTI) abacavir and myocardial infarction.6–10 The underlying mechanism remains largely unknown but may include endothelial dysfunction, increased inflammation, platelet activation and platelet/collagen interactions, all of which can modify CVD risk.11
In recent years, dual therapy as an alternative to traditional 3-drug-regimen (3DR) has emerged as an HIV treatment option. The combination of XTC/DTG is one of several 2-drug regimens (2DR) now recommended in the European AIDS Clinical Society (EACS) guidelines (V.11.1) and the International Antiviral Society-USA (IAS-USA) for either initiating ART in ART-naïve adults with plasma HIV-RNA<500 000 copies/mL and without Hepatits B virus (HBV) infection or as a switch option for individuals with viral suppression (plasma HIV-RNA<50 copies/mL for the past 6 months).12 13 These recommendations were supported by data from randomised controlled trials (RCT) reporting non-inferiority in achieving or maintaining viral suppression (plasma HIV-RNA 50 copies/mL) in ART naïve and virally suppressed PLWH, respectively.14–17
Initiating ART per se is associated with weight gain.18–20 Some of this weight gain is thought to be related to a ‘return-to-health’ phenomenon where initiation of ART causes a return to baseline weight after HIV induced wasting. This mechanism is not fully understood and seems to be only partly responsible for the observed weight gain. A meta-analysis of eight RCTs reported that weight increased more in ART-naïve PLWH initiating treatment with an integrase strand transfer inhibitor (INSTI)-based regimen as compared with a non-NRTI or a protease inhibitor-based regimen. Among NRTIs, abacavir and tenofovir alafenamide (TAF), were associated with more weight gain than tenofovir disoproxil fumarate (TDF) or zidovudine.21 Weight gain after ART switch is modest and it remains uncertain whether this is due to the loss of a weight suppressive effect of prior regimens with older agent such as TDF or efavirenz or a weight gain effect of the newer regimens especially TAF and/or INSTI, or both.22 Overweight and obesity are associated with an increased risk of metabolic syndrome, diabetes, hypertension and dyslipidaemia that converge as risk factors for CVD including myocardial infarction.23 Thus, it is conceivable that there could be synergistic effects and a higher overall risk of CVD using abacavir and dolutegravir in combination. We want to investigate the effect on weight change and cardiac function after discontinuation of abacavir in PLWH treated with abacavir, lamivudine and dolutegravir.
Objectives
The aim of this study is to investigate if discontinuing abacavir by switching from a 3DR with dolutegravir, abacavir and lamivudine (DTG/ABC/3TC) to a 2DR with dolutegravir and lamivudine (DTG/3TC) will decrease weight and improve metabolic and cardiac parameters in PLWH.
Trial design
This study is a randomised, controlled, parallel, open-label, phase 4, interventional trial.
Methods: participant, interventions and outcomes
Study design
Participants will be recruited from outpatient clinics at the departments of infectious diseases at two hospitals: Copenhagen University Hospital—Amager and Hvidovre; and Copenhagen University Hospital—Rigshospitalet. Both are located in the Capital Region of Denmark. Baseline and follow-up study visits and data collection will be performed at Copenhagen University Hospital—Amager and Hvidovre.
Eligibility criteria
Inclusion and exclusion criteria are listed in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|
|
Participants will be eligible for an MRI substudy participation, if they comply with standard MRI safety guidelines.
*Existing genotypic resistance test results will be screened prior to inclusion.
DTG/ABC/3TC, dolutegravir/abacavir/lamivudine; HBV, hepatitis B virus.
Interventions
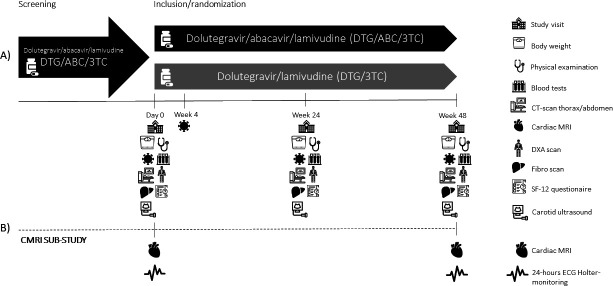
At day 0 patients will be randomised to either continued therapy with co-formulated dolutegravir 50 mg, abacavir 600 mg and lamivudine 300 mg (Triumeq, control arm) or switch to co-formulated dolutegravir 50 mg and lamivudine 300 mg (Dovato, intervention arm) for 48 weeks. See figure 1 for participant timeline.
Figure 1.
Participants timeline and data collection. (A) Eligible participants on abacavir/dolutegravir/lamivudine (ABC/DTG/3TC) for HIV infection for more than 6 months will be enrolled. Inclusion, randomisation, and data collection will be performed at a baseline visit (day 0). Participants will be randomised to either continuation of a 3-drug regimen with ABC/DTG/3TC (control) or a 2-drug regimen with DTG/3TC (intervention). Data will be collected at follow-up visits at week 4 (w4), week 24 (w24) and week 48 (w48). At study visits w1, w24 and w48 the following tests will be performed: Physical examination by clinician; blood tests including HIV-RNA and CD4 cell count; CT scan of thorax and the upper abdomen, dual-energy X-ray absorptiometry (DEXA) scan; 12-Item Short Form Survey (SF-12); liver elastography; external carotid artery ultrasound. Participants in the intervention arm will have plasma HIV RNA determined at week 4 for safety reasons. (B) Participants enrolled in the cardiac MRI (CMRI) substudy will additionally receive CMRI and 24 hours Holter ECG monitoring at visits w1 and w48.
Administration
The investigational medicinal products (IMP) will be self-administered in original pharmaceutical packaging by participants once daily. Double controlled administration of the IMP and IMP-log registration will be performed by two designated and Good Clinical Practice (GCP)-trained study personnel. The study IMP will be distributed at/day 0 and week 24 in original wrapping, with a study specific label. All IMPs are authorised, registered and marketed by the Danish Medicines Agency and the European Medicines Agency. Dosage and administration frequency are assigned according to treatment guidelines.
Withdrawal from study
Participants will be withdrawn from the assigned study treatment in case of viral rebound or if any serious adverse reaction (SAE) or suspected unexpected serious adverse reaction (SUSAR) is considered to compromise participants safety as assessed by the investigator. Any case of withdrawal will result in an immediate visit, where the study participant will be offered resistance test and be assigned to a new suppressive HIV regimen. This will be managed by the study team and the participants’ primary healthcare provider, who will be involved in the decision and further monitoring of viral load and participants. Participants withdrawn from the study will be included in the intention-to-treat (ITT) analysis.
Adherence
Adherence will be monitored by measurements of plasma HIV-RNA viral load at baseline, weeks 4 (2DR group only), 24 and 48. Virological failure defined as two consecutive viral loads >50 copies/mL with an interval of 14–30 days will lead to immediate withdrawal from the study.
If participants dropout or are withdrawn, an immediate follow-up meeting will be arranged to make sure patients return to their usual treatment an clinical control. A resistance test will be performed, and their regular physician will be informed. Data from withdrawn participants or dropouts will contribute to the ITT analysis with the patient’s acceptance.
Outcomes
Primary outcome
Change in body weight (continous) from baseline to week 48 measured as difference between means in the two study arms.
Secondary outcomes
Development of metabolic syndrome at week 48.24
Development of type 2 diabetes at week 48.25
Impaired insulin resistance and/or β-cell function determined by changes in Homeostatic Model Assessment of Insulin Resistance at week 48.26
Virological control at week 48 as defined by a plasma HIV-RNA<50 copies/mL.
Changes from baseline to week 48 in:
Self-rated health evaluated by 12-Item Short Form Survey.
Framingham Risk Score.27 28
D:A:D (Data Collection on Adverse Effects of Anti-HIV Drugs Cohort) CVD risk score.29
Blood glycated haemoglobin.
Total plasma cholesterol.
Plasma high-density lipoprotein cholesterol.
Plasma low-density lipoprotein cholesterol.
Plasma very low-density lipoprotein cholesterol.
Plasma triglycerides.
Visceral adipose tissue and subcutaneous adipose tissue ratio (abdominal CT).
Fat distribution in trunk, limb and extremities measured by dual-energy X-ray absorption (DEXA).
Development of liver fibrosis or progression of existing liver fibrosis. Significant liver fibrosis as Fibroscan Liver Stiffness (LSM)≥7.6 kPa.
-
Fatty infiltration of the liver evaluated as:
Development of steatosis or increase in existing steatosis from baseline (CT liver and Controlled Attenuated Parameter (FibroScan).
Blood pressure.
Cardiac MRI.
Carotid artery intima-media thickness measured by ultrasound.
Coronary artery calcium score.
Plasma N-terminal pro-B-type natriuretic peptide.
Plasma troponin T.
-
Inflammation:
Plasma high-sensitive C reactive protein.
Plasma interleukin 1β.
Plasma interleukin 6.
-
Endothelial function:
Plasma vascular cell adhesion molecule 1.
Plasma intercellular adhesion molecule 1.
-
Platelet function:
Plasma soluble P-selectin.
Plasma soluble glycoprotein VI.
Coagulation:
Plasma D-dimer.
Plasma coagulations factor 2, 7 and 10 (extrinsic pathway).
Plasma fibrinogen.
Blood haemoglobin, leucocyte count and platelet count.
Plasma creatinine, urea, sodium, potassium, bilirubin and alanine aminotransferase.
Outcomes MRI substudy
Primary outcome
In the cardiac MRI (CMRI) substudy the outcome is a composite endpoint consisting of any abnormalities in:
Extracellular myocardial volume from baseline to week 48.
Left atrial volume from baseline to week 48.
Diastolic function from baseline to week 48.
Myocardial mass from baseline to week 48.
Secondary outcomes
Changes in:
Left ventricular ejection fraction.
Myocardial perfusion.
Myocardial oedema/inflammation.
Myocardial fibrosis.
Myocardial lipid-water profile.
Participant timeline
Participant timeline is illustrated in figure 1.
Sample size
The hypothesis on weight change in this study relies on a meta-analysis with pooled weight data from eight RCTs of treatment naïve PLWH. In the meta-analysis mean weight gain with abacavir (ABC) was 3.08 kg (95% CI, 2.36 to 3.81) in 96 weeks. In the same meta-analysis dolutegravir lead to a mean weight gain on DTG, 4.07 kg (95% CI, 3.51 to 4.62).21
Since dolutegravir treatment continues in both study arms, we hypothesise the possible contribution from dolutegravir to weight gain will be equal in the groups. We speculate that the absence of ABC in the intervention (2DR) group can lead to a small weight loss in. Anticipated annual weight change in the two groups are: DTG/3TC+0 to −1 kg and DTG/ABC/3TC+2 kg. Sample size is estimated by Student’s unpaired t-test. Patients will be randomised 2:1 to intervention or control.
Assuming a 2:1 randomisation ratio for the intervention and control arm, a significance level (α) of 5%, a power (β) of 80%, mean difference in weight between the two arms of 2 kg (Δ) and an SD of 2.7 kg the estimated sample size required will be 44 randomised individuals in the intervention group and 22 randomised individuals in the control group. To account for a 5% withdrawal or dropout rate, the sample size will be 70 patients in total.
Recruitment
Eligible patients will be identified by treating physicians at outpatient clinics at the involved sites. Information on eligible patients will be disclosed to the responsible investigator for the purpose of recruitment. Eligible patients will receive verbal and written study information, and subsequently be offered participation. All participants must provide written informed consent.
Study status
The first participant was included on 20 October 2020. Recruitment is expected to be completed by November 2023.
Methods: assignment of interventions
Allocation
Eligible participants will be randomised in two parallel arms with an allocation ratio of 2:1 to switch to 2DR therapy with DTG/3TC or to continue 3DR treatment with DTG/ABC/3TC (control). Key variables will be entered into a secure web-based programme (Research Electronic Data Capture (REDCap)) to randomise patients into two parallel arms on inclusion. The randomisation list will be centrally generated in REDCap using random blocks stratified by study centre and sex. Additionally, 40 participants (20 control and 20 intervention) will be invited to participate in the CMRI substudy. The study will be open label, both participants and the study personnel will be unblinded to randomisation.
Methods: data collection, management and analysis
Data collection methods
Study visits and data collection will be performed at Copenhagen University Hospital—Amager and Hvidovre and participants will be examined by trained clinicians or study nurses. At the inclusion visit (day 0) baseline data will be collected, and participants will be randomised. Subsequently a safety blood test will be performed at week 4 (for the 2DR arm), and follow-up data will be collected at study visits at week 24 (range 20–28) and finally at week 48 (range 46–52). An overview data collection is listed in tables 2 and 3. Blood testing is listed in table 4. Detailed description on radiological tests are included in online supplemental appendix 1.
Table 2.
Data collection
| Day 0 | Week 4 | Week 24 | Week 48 | |
| Informed consent | x | |||
| Randomisation | x | |||
| Demographics* | x | |||
| Framingham Risk Score | x | x | x | |
| SF-12† | x | x | x | |
| Body weight | x | x | x | |
| Vital signs | x | x | x | |
| Blood tests‡ | x | x | x | |
| HIV safety blood tests§ | x | x | x | x |
| Transient elastography/Controlled Attenuated Parameter | x | x | x | |
| CT¶ | (x) | (x) | (x) | |
| DEXA** | x | x | x | |
| cIMT†† | x | x | x | |
| MRI substudy | ||||
| Cardiac MRI‡‡ | x | x | ||
| 24 hours ECG Holter monitoring | x | x |
*Age, gender, tobacco use, alcohol consumption, medication, medical history, nursing home residency and activities of daily living.
†12-Item Short-Form Health Survey (SF-12).
‡HIV safety blood tests: Plasma HIV-RNA and CD4 count.
§Blood tests: leucocytes, platelets, haemoglobin, creatinine, urea, sodium, potassium, bilirubin, alanine aminotransferase, lactate dehydrogenase, erythrocyte fraction. Metabolism: Fasting p-glucose, insulin, glycated haemoglobin, total cholesterol, high-density lipoprotein, low-density lipoprotein, very low-density lipoprotein, triglyceride. Inflammation: High-sensitive C reactive protein, interleukin 1 and 6. Coagulation: D-dimer, factor 2, 7 and 10, fibrinogen. Platelet function: soluble P-selectin, soluble glycoprotein VI. Endothelial function: Vascular cell adhesion molecule 1, intercellular adhesion molecule 1, cardiac: N-terminal pro-B-type natriuretic peptide, troponin T.
¶Low dose CT of thorax and abdomen to determine coronary artery calcium score, visceral adipose tissue, subcutaneous adipose tissue and liver steatosis.
**Dual energy X-ray absorptiometry (DEXA), optional.
††Carotid intima-media thickness (cIMT) determined by ultrasound.
‡‡Cardiac MRI substudy (optional).
§§Blood pressure, heart rate
Table 3.
Clinical measurements and methods
| Measurements | Description |
| Body weight | Measured after minimum 6 hours of fasting and without cloth Scale: Seca 701 7021099 |
| Blood tests (fasting) | Peripheral venous blood |
| Transient liver elastography/Controlled Attenuated Parameter | FibroScan, M-XL probe (Echosens, Paris, France) |
| Carotid intima-media thickness | Sonosite M-Turbo (Sonosite, Bothell, Washington, USA), analysed with a 13.6 MHz linear transducer and automated software (SonoCalc IMT 5.0; Sonosite) |
| CT scans | CT chest, CT upper abdomen and coronary calcium scan Aquillion ONE scanner, Toshiba Medical Systems, (Otawara-shi, Tochigi-ken, Japan) |
| DEXA scan | Dual energy X-ray absorptiometry (DEXA) Whole-body DEXA scanning Hologic QDR-2000 W (Bedford, Massachusetts, USA) |
| Cardiac MRI | MAGNETOM Prisma 3 Tesla scan Siemens Healthineers (Erlangen Germany) |
| Metabolic syndrome* | Central obesity: waist circumference ≥94 cm for men and ≥80 cm for women (Europids), or body mass index >30 kg/m², plus any two of the following four factors:
|
| Insulin resistance and β-cell function | Homeostatic Model Assessment of Insulin Resistance (HOMA-IR): |
| Type 2 diabetes† |
|
Cardiovascular disease risk scores:
|
Online calculation tool, CHIP Centre of Excellence for Health and Framingham Heart Study |
| Survey |
|
*Metabolic syndrome defined in accordance with The International Diabetes Federations definition.
†WHO definition.
CHIP, Centre of Excellence for Health, Immunity and Infections; D:A:D, Data Collection on Adverse Effects of Anti-HIV Drugs Cohort.
Table 4.
Blood and plasma analysis
| Category | Blood and plasma analysis |
| Haematology | Leucocyte count and differential count, haemoglobin, haematocrit |
| Electrolytes | Sodium, potassium |
| Renal | Creatinine, urea, albumin |
| Liver | Alanine aminotransferase, aspartate transaminase, bilirubin, lactate dehydrogenase |
| HIV-safety | HIV-RNA*, CD4 cell count* |
| Metabolism | Glucose, insulin, blood glycated haemoglobin, total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, very low-density lipoprotein-cholesterol (calculated) |
| Inflammation | High sensitivity C reactive protein, interleukin 1β and 6* |
| Coagulation | D-dimer, factor II+VII+X, fibrinogen, platelet count |
| Platelet function | Soluble P-selectin*, soluble glycoprotein VI* |
| Endothelial function | Vascular cell adhesion molecule 1*, intercellular adhesion, molecule 1* |
| Cardiac | N-terminal pro-B-type natriuretic peptide, troponin T |
Blood tests will be performed at visits day 0 week 24 and week 48. Participants will be fasting for a minimum of 6 hours prior to blood tests. Non-HIV-infected controls will have blood tests prior to or at the day of cardiac MRI and will not be fasting. Blood analysis with * will be performed only in people living with HIV.
bmjopen-2023-075673supp001.pdf (156.3KB, pdf)
Participants must be fasting for at least 6 hours prior to visits at day 0, 24 and 48, to ensure fasting measurements of weight, blood tests, transient liver elastography and DEXA.
Cardiac MRI substudy
Forty participants from the main study, 20 from the 2DR-group and 20 from the 3DR-group, will be included in the CMRI substudy. CMRI and ECG-monitoring will be performed on the substudy population at baseline and week 48. Further, 20 non-HIV-infected controls will be recruited for a single CMRI to compare baseline data. CMRI technical details and scan protocol are elaborated in online supplemental appendix 1.
The following clinical information will be obtained from healthy controls or from their patient records: Medicine use, known medical conditions, cardiac risk (smoking status, alcohol consumption, family CVD history), height and weight. Blood pressure and heart rate will be measured on the day of CMRI. Blood tests will be performed prior to or at the day of CMRI. See table 4 for blood and plasma testing details.
Data management
The participant data including demographics, medical history, laboratory and investigational results will be recorded in digital electronic Case Report Form (eCRFs) in REDCap, a secure web application for administration of databases in non-commercial clinical research. Investigators or appointed research nurses will manually enter the data. Only authorised personnel such as the sponsor, investigator, subinvestigator or study nurses will have encoded access via personal user ID and password. All data will be handled confidentially, in accordance with ‘The Danish Act on Processing of Personal Data and General Data Protection Regulation’ (GDPR). Study data will be published in pseudonymous form only after being extracted by the primary investigator at study termination.
Statistical methods
Intention-to-treat analysis
The ITT analysis will include all participants who were randomised, regardless of their adherence to the assigned treatment or whether they discontinued the treatment. This analysis will also include participants who were lost to follow-up.
Per-protocol analysis
The per-protocol (PP) analysis will only include randomised participants who completed the entire study and the last follow-up. If participants did not adhere to the assigned treatment but adhered to one of the two treatment arms, they will be included in the PP analysis with their actual treatment. However, if participants discontinued the treatment or started a treatment regimen outside the study protocol, they will only be included in the ITT analysis.
Descriptive statistics
Baseline characteristics will be presented in table 1 grouped by treatment arm. Continuous variables will be presented as median (IQR) or mean and SD and compared by unpaired t-test or Mann-Whitney U test depending on distribution of data. Categorical variables will be presented as number and percentage, groups will be compared with χ2 test or Fisher’s exact test. CMRI substudy data will be presented similarly.
Analysis set
Absolute change in mean fasting weight from baseline to week 24 and from baseline to week 48 will be included in a linear mixed model for analysis of the primary outcome. Continuous secondary endpoints will be analysed and presented similarly to the primary analysis of weight change. Categorical variables will be compared with χ2 test or Fisher’s exact test. No interim analysis is planned.
Missing data
Missing data will be analysed with the assumption that data are ‘missing at random’ (MAR) and analysed using multiple imputation. At study termination an analysis will be performed to check for patterns of missing data and association between missing and observed data will be performed to ensure missing data is MAR.
Loss to follow-up
Participants who are lost to follow-up including dropouts withdrawing their consent or unreachable participants will be included in the ITT analysis. The participant’s primary healthcare provider will be informed to ensure that regular monitoring and treatment will be resumed.
Methods: monitoring
Data monitoring
External data monitoring will be performed according to ICH-GCP (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use - Good Clinical Practice) by the public ‘Danish GCP Units, Copenhagen’. Data collection and handling will be conducted according to a monitoring plan and written standard procedures and in accordance with GCP regulations and requirements. There are no planned interim analyses in the study.
Harms
SAEs and SUSARs will be evaluated and documented by the primary investigator and reported to the sponsor in accordance with GCP and Danish Medicines Agency regulatory. A yearly report including all emerging SAEs and SUSARs and comments on general safety in the study will be reported to the Danish Medicines Agency. In case of death or life-threatening disease, the sponsor will report the SUSAR to the Danish Medicines Agency and the regional Research Health Ethics Committee within 7 days of the sponsor’s knowledge of the event. A final report of registered events will be generated at study terminations and reported to the Danish Medicines Agency and Health Research Ethics committee of the Capital Region of Denmark.
Patient and public involvement statement
Patients/public were not involved until initiation of inclusion in the trial. The research question was developed in the clinic by clinicians treating the involved patient group. The patients/public were not involved in the design, conduct or recruitment of the study, but the study was designed to make the participation as accessible and effortless as possible for patients. Participants will be informed about the study results by letter at study termination unless they opt this out in the consent form.
Ethics and dissemination
Protocol amendments
Any possible protocol modification will be reported in a protocol amendment to relevant public authorities.
Consent or assent
All participants will be informed of the study by the means of oral and written information, per usual ICH standards, with full details of the study, including risk and benefits, before enrolment. Participants will be informed of the right to obtain an assessor. Only the principal investigator or co-investigators will be allowed to obtain informed consent from participants. Online supplemental appendix 2 shows the informed consent form.
An additional consent will be retrieved for a project specific plasma biobank for subsequent analyses (project specific biobank) and a biobank for future research. Biobank details are listed in online supplemental appendix 3.
Confidentiality
Sponsor and investigators are obliged to handle all data on trial participants confidentially in accordance with the Act on Processing of Personal Data. At the end of the study, the primary investigator will extract data from the electronic database REDCap to perform the planned analyses on primary and secondary outcomes. Data will be processed and analysed in the free statistical software RStudio. Study data will subsequently be published only in anonymous form. Data will be handled based on Danish law of data protection and Danish data protection regulation.
Data access
The study is registered at ClinicalTrials.gov (registered 27 May 2021). Access to final data will be limited to sponsor, primary investigator and personnel involved in the analysis of data, co-investigator and statisticians. The data that support the findings of this study are available on reasonable request. The data are not publicly available due to Danish legislation regarding GDPR.
Ancillary and post-trial care
All areas of the Danish healthcare system are covered by a publicly funded compensation scheme. The scheme covers if a participant is injured in connection with treatment at a public hospital. The scheme covers medicinal product injuries. This also applies for patients involved in research. At inclusion, the participants will be informed of the compensation and report avenues in case a drug injury occurs, which is in adherence to Danish law.
Dissemination policy: trial results
On the completion of the trial, the data collected from all participating sites will be pooled and analysed together. Researchers involved in the trial will not be permitted to publish data until after the main study publication is released. The results of the primary study will be featured in a peer-reviewed journal, with the primary investigator as the first author, the sponsor as the senior author and the participating investigators as coauthors based on their work and involvement in the study. All findings, whether positive, negative or inconclusive, will be published. The CMRI substudy data will be reported separately.
Limitations
The main limitation in this study is non-blinding of the intervention which introduces both observer and performer bias. We try to minimise the performance bias by not informing the participants what to expect in terms of weight change with their assigned treatment. Further we limit this bias with a 48-week follow-up as a long-term study intervention may decrease the probability to adhere to other weight interventions such as diets or excessive training.
We expect high adherence to the study and to the randomisation. We only include virally suppressed PLWH. PLWH are used to adhere to prescribed medication, and they are all treated with the drugs used at study entry (they either continue three drugs and switch to a regimen consisting of two of the three drugs), thus we expect very few adverse events. The study set-up an expected low Adverse Event (AE) rate that is thought to limit dropout. The randomised controlled design of the study will contribute to even distribution of bias, non-adherence, and loss to follow-up in the groups.
Supplementary Material
Footnotes
Contributors: The authors listed have all made contributions to this paper in accordance with the recommendations of the International Committee of Medical Journal Editors (ICMJE). TB, JG, AK and KBHP contributed to the study design. JDH, HRS and SM contributed specifically to the radiological aspects of the protocol, including technical details, setup and access to all radiological examinations. All authors will be involved in the analysis and interpretation of data upon study termination. The primary drafting work was conducted and organised by the corresponding author, KBHP, and the study sponsor, TB. JDH and HSR drafted the CMRI-scan protocol. All authors participated in reviewing the manuscript and providing intellectual input. The authors listed have approved the final version of the paper and are willing to participate in any future revisions. All authors (KBHP, AK, SM, HRS, JDH, JG and TB) take responsibility for the accuracy and integrity of the work and are committed to addressing any concerns that may arise. Artificial intelligence (AI)-Assisted Technology, ChatGBT from OpenAI, was used solely for grammatical and linguistic proofreading of the manuscript. No AI was involved in the study design, data analysis, interpretation or substantial writing and reviewing of the manuscript.
Funding: The study is supported by the Simonsen’s Foundation (No grand number) and from Amager and Hvidovre Hospital’s Research Foundation (grand number: 2021-533). The Funders did not contribute to the design of the study, the decision to publish the findings or the preparation of the manuscript. The study is a Sponsor-Investigator trial. The sponsor, Thomas Benfield has no conflicts of interest or commercial interest in the study. Sponsor and investigators are independent of economic or competing interests. The grant is held in a foundation account managed by sponsor. Participants will not be financially compensated. Study results will be utilized only for scientific and public purpose and do not hold any commercial significance.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lohse N, Obel N. Update of survival for persons with HIV infection in Denmark. Ann Intern Med 2016;165:749–50. 10.7326/L16-0091 [DOI] [PubMed] [Google Scholar]
- 2.Belloso WH, Orellana LC, Grinsztejn B, et al. Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Med 2010;11:554–64. 10.1111/j.1468-1293.2010.00824.x [DOI] [PubMed] [Google Scholar]
- 3.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a Multicohort collaboration. Lancet 2014;384:241–8. 10.1016/S0140-6736(14)60604-8 [DOI] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–22. 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016;117:214–20. 10.1016/j.amjcard.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabin CA, Reiss P, Ryom L, et al. Is there continued evidence for an association between Abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med 2016;14:61. 10.1186/s12916-016-0588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010;201:318–30. 10.1086/649897 [DOI] [PubMed] [Google Scholar]
- 8.Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. The Lancet 2008;371:1417–26. 10.1016/S0140-6736(08)60423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi AI, Vittinghoff E, Deeks SG, et al. Cardiovascular risks associated with Abacavir and tenofovir exposure in HIV-infected persons. AIDS 2011;25:1289–98. 10.1097/QAD.0b013e328347fa16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai M, Joyce V, Bendavid E, et al. Risk of cardiovascular events associated with current exposure to HIV antiretroviral therapies in a US veteran population. Clin Infect Dis 2015;61:445–52. 10.1093/cid/civ316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Halloran JA, Dunne E, Tinago W, et al. Switching from Abacavir to tenofovir disoproxil fumarate is associated with rises in soluble glycoprotein VI, suggesting changes in platelet-collagen interactions. AIDS 2018;32:861–6. 10.1097/QAD.0000000000001783 [DOI] [PubMed] [Google Scholar]
- 12.EACSociety . EACS guidelines. 2019. Available: https:/www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
- 13.Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International antiviral society-USA panel. JAMA 2023;329:63–84. 10.1001/jama.2022.22246 [DOI] [PubMed] [Google Scholar]
- 14.Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus Dolutegravir plus tenofovir disoproxil fumarate and Emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two Multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019;393:143–55. 10.1016/S0140-6736(18)32462-0 [DOI] [PubMed] [Google Scholar]
- 15.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to Dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir Alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1. Clin Infect Dis 2020;71:1920–9. 10.1093/cid/ciz1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llibre JM, Brites C, Cheng C-Y, et al. Efficacy and safety of switching to the 2-drug regimen Dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1. Clinical Infectious Diseases 2023;76:720–9. 10.1093/cid/ciac130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to Dolutegravir/lamivudine versus continuing a tenofovir Alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1. Clinical Infectious Diseases 2022;75:975–86. 10.1093/cid/ciac036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Research and Human Retroviruses 2016;32:50–8. 10.1089/aid.2015.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother 2018;73:2177–85. 10.1093/jac/dky145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity Journal of Virus Eradication 2019;5:41–3. 10.1016/S2055-6640(20)30277-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020;71:1379–89. 10.1093/cid/ciz999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont E, Yombi JC. Antiretroviral therapy and weight gain in antiretroviral treatment-experienced HIV patients: A review. AIDS Rev 2023;25:54–64. 10.24875/AIDSRev.22000026 [DOI] [PubMed] [Google Scholar]
- 23.Thompson D, Edelsberg J, Colditz GA, et al. Lifetime health and economic consequences of obesity. Arch Intern Med 1999;159:2177–83. 10.1001/archinte.159.18.2177 [DOI] [PubMed] [Google Scholar]
- 24.Consensus statements. n.d. Available: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html
- 25.HEARTS D: diagnosis and management of type 2 diabetes. n.d. Available: https://www.who.int/publications-detail-redirect/who-ucn-ncd-20.1
- 26.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 27.2018 prevention guidelines tool CV risk Calculator. n.d. Available: http://static.heart.org/riskcalc/app/index.html#!/baseline-risk
- 28.Framingham heart study. 2019. Available: https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
- 29.CHIP . Centre of excellence for health, immunity and infections. n.d. Available: https://chip.dk/Tools-Standards/Clinical-risk-scores
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075673supp001.pdf (156.3KB, pdf)