Synopsis
Aedes aegypti, the yellow fever mosquito, presents a major threat to human health across the globe as a vector of disease-causing pathogens. Females of this species generally mate only once. From this single mating event, the female stores sufficient sperm to fertilize the multiple clutches of eggs produced during her lifetime. Mating causes dramatic changes in the female’s behavior and physiology, including a lifetime suppression of her mating receptivity. Female rejection behaviors include male avoidance, abdominal twisting, wing-flicking, kicking, and not opening vaginal plates or extruding the ovipositor. Many of these events occur on a scale that is too miniscule or fast to see by eye, so high-resolution videography has been used to observe these behaviors instead. However, videography can be labor intensive, require specialized equipment, and often requires restrained animals. We used an efficient, low-cost method to record physical contact between males and females during attempted and successful mating, determined by recording spermathecal filling after dissection. A hydrophobic oil-based fluorescent dye can be applied to the abdominal tip of one animal and can be subsequently transferred to the genitalia of animals of the opposite sex when genital contact occurs. Our data indicate that male mosquitoes make high levels of contact with both receptive and unreceptive females and that males attempt to mate with more females than they successfully inseminate. Female mosquitoes with disrupted remating suppression mate with and produce offspring from multiple males, transferring dye to each. These data suggest that physical copulatory interactions occur independently of the female’s receptivity to mate and that many of these interactions represent unsuccessful mating attempts that do not result in insemination.
Introduction
Aedes aegypti, the yellow fever mosquito, is a global public health concern as a vector of disease-causing pathogens, including yellow fever, dengue fever, chikungunya, and Zika (Rogers et al. 2006; Bhatt et al. 2013; Guerbois et al. 2016; Weaver et al. 2016). Females of this species are obligate blood feeders; to successfully reproduce, they need to mate and consume blood to obtain necessary protein for egg development (Dimond et al. 1956; Attardo et al. 2005).
Although limited remating has been reported across Ae. aegypti populations, this species is generally monandrous—females mate only once (Craig 1967; Gwadz et al. 1971; Jones and Pilitt 1973; Richardson,2015; Helinski et al. 2012). From this single mating event, a female stores sufficient sperm to fertilize all of the eggs produced across multiple clutches for the rest of her life (Craig 1967; Spielman et al. 1967; Carvalho et al. 2018). Specialized organs, called spermathecae, store and maintain sperm after transfer to the female, which can then be released when eggs are ready to be fertilized (Roth 1948). Mating causes dramatic changes in female behavior, including the lifetime suppression of receptivity, inducing her to reject all subsequent mating attempts (Hiss and Fuchs 1972; Clifton et al. 2014). Methods to assess male–female interactions, including attempted mating, successful mating, and rejection, are of great interest to researchers studying Ae. aegypti behavior. A better understanding of the mechanisms by which Ae. aegypti mate will facilitate their exploitation for new population control strategies by preventing mating and reproduction.
Early examinations of Ae. aegypti mating behavior characterized females as highly promiscuous, based on the frequency with which mated females appear to copulate with subsequent males (MacGregor 1915; Roth 1948). However, later studies using genetic markers of offspring paternity showed that Ae. aegypti females become refractory to further mating after successful insemination (Craig 1967; Spielman et al. 1967), and reject future prospective mates by male avoidance, kicking, preventing a male from assuming the correct position, abdominal twisting to prevent copulation, and/or failing to extrude her ovipositor (Roth 1948; Gwadz et al. 1971; Jones and Pilitt 1973; Cator and Harrington 2011). Thus, some forms of female rejection may be indiscernible from successful mating to the naked eye (Eberhard 1991).
This phenomenon, by which male-derived mating signals prevent a female from further mating, is referred to as paternity enforcement. Short-term paternity enforcement within hours of mating is regulated in part by a peptide found in male seminal fluid, Head Peptide (HP-I), which activates a cognate receptor in females, Neuropeptide Y-Like Receptor 1 (NPYLR1) (Naccarati et al. 2012; Duvall et al. 2017). Females mutant for npylr1 are receptive to subsequent mates, bearing mixed-paternity offspring when exposed to successive males within hours. Hp-I mutant males fail to enforce short-term paternity, fathering only a portion of his mate’s offspring if she remates with a subsequently encountered male within hours, although slower-acting paternity-enforcing signals are still present in hp-I mutant males (Duvall et al. 2017). The signals that induce lifetime paternity enforcement and the mechanisms that maintain this behavioral change in females remain unknown (Craig 1967; Fuchs et al. 1968; Hiss and Fuchs 1972).
Assessing the mating status of Ae. aegypti females typically requires dissecting the female reproductive tract to assess the presence of sperm in two of the three spermathecae, although it is also possible to score insemination in a live, immobilized female (Carrasquilla and Lounibos 2015). However, scoring spermathecae for insemination does not capture attempts made, or the identities of animals involved in attempted or successful mating. Because many rejection behaviors occur on a scale too small and fast to see by eye, high-speed, high-resolution videography has been used to observe males mating with tethered females whose scutellum has been glued to a pin to restrict their movement (Aldersley and Cator 2019). While videography can offer high-resolution, detailed observation of mating behavior, it can also be costly, low throughput, and requires restrained animals.
In this study, we utilized an efficient, low-cost method to assay physical contact between males and females during attempted and successful mating. A hydrophobic oil-based fluorescent dye applied to the abdominal tip of one animal is subsequently transferred to the genitalia of animals of the opposite sex during copulation. Females can be scored for insemination to distinguish between attempted and successful mating. Because mating attempts are frequent and do not always result in successful insemination, and failed mating attempts can involve contact between the animals’ abdominal tips that is indistinguishable to the naked eye from successful mating, dye transfer paired with spermathecal dissections can identify females involved in successful and unsuccessful mating attempts as well as males who attempted to mate and were not rejected before making abdominal contact. Our findings reveal males’ propensity to initiate mating regardless of female receptivity, and suggest that males come sufficiently close to mating to make genital contact but are unable to inseminate the female.
Dye applied to either males or females is transferred to the abdominal tip of animals of the opposite sex during successful mating that results in insemination, and also during unsuccessful mating attempts in which the abdominal tips come into contact. Since successful insemination requires contact between the mating pair, we did not expect to see any instance of insemination without dye transfer and did not observe any such occurrences. Previous work suggests that many mating attempts are unsuccessful (Aldersley and Cator 2019) and that males attempt to mate with unreceptive females. Because dye transfer reports both successful and unsuccessful attempts in which genital contact is achieved, we expected rates of dye transfer to exceed rates of insemination.
Our experimental methods for dye application and scoring are provided in the “Methods” section. In the “Results” section, we present our experiments showing that dye applied to either males or females is transferred to the abdominal tip of animals of the opposite sex during successful mating that results in insemination, and also during unsuccessful mating attempts in which the abdominal tips come into contact. In the “Discussion” section, we discuss our results and avenues for future research.
Methods
Rearing
Aedes aegypti wild-type laboratory strain (Orlando) and mutant strain (npylr1-/-, Liesch et al. 2013) were reared in environmental rooms at 70–80% relative humidity, 25–28°C, with a photoperiod of 14 h light and 10 h dark, as described in DeGennaro et al. (2013). Eggs were hatched in hatch broth (one crushed TetraMin fish food tablet in 1L deionized, deoxygenated water). Larvae were fed crushed TetraMin fish food tablets. The npylr1 mutant strain was selected for experiments in which females encounter successive males because these mosquitoes lack functional NPYLR1, and the females are receptive to remating, but males of this strain do not show any mating deficits (Duvall et al. 2017).
For assays involving unmated females, animals were separated by sex as pupae to ensure the unmated status of the females. Unmated males and females were housed separately until behavioral assays were performed. For assays involving mated females, males and females were allowed to cohabit as adults for at least 5 days to ensure that females were mated. Adult mosquitoes were housed in custom cages (216 mm diameter and 181 mm height) and provided access to 10% sucrose ad libitum. For all assays, males and unmated females were 5–14 days post-eclosion, and mated females were 7–21 days post-eclosion at the beginning of the assay.
Dye application and scoring
Mosquitoes were cold anesthetized at 4°C for 10 min prior to dye application, then placed in plastic cups on ice. Using forceps, mosquitoes were transferred from the plastic cups to a chilled glass petri dish, where fluorescent oil-based dye (ACDelco 1,148,963 GM Original Equipment 10–5045 Multi-Purpose Fluorescent Leak Detection Dye) was applied to the terminal two segments of the abdomen. Dye application was performed under a Leica MZ10 F fluorescence stereomicroscope in the Cy3 channel (Fig. 1A) using a modified fine-tip paintbrush (Amazon Catalog #B073YDKWWP). Prior to dye application, all but 20 bristles were removed from a fine-tip paintbrush to minimize the amount of dye applied. After dye application, animals were placed on ice in a plastic cup lined with a Kim-wipe to absorb any excess dye and allowed to recover for ∼5 min. To confirm that dye transfer reported direct physical interactions between animals, we performed control assays in which 10 painted females were housed in a custom cage (216 mm diameter and 181 mm height) for 10 h, then removed. A new set of 10 unpainted female mosquitoes was then housed in the same cage for 12 h, then scored for the presence of dye to confirm that no dye was transferred incidentally via cage surfaces (Supplementary Table S1). To confirm that dye was not transferred between female mosquitoes during non-mating interactions, dye was applied to 10 unmated female mosquitoes, which were cohoused with 10 unmated, unpainted female mosquitoes for 20 h, after which all animals were cold-anesthetized and scored for dye presence. No instances of dye transfer between painted and unpainted females were recorded (Supplementary Table S3).
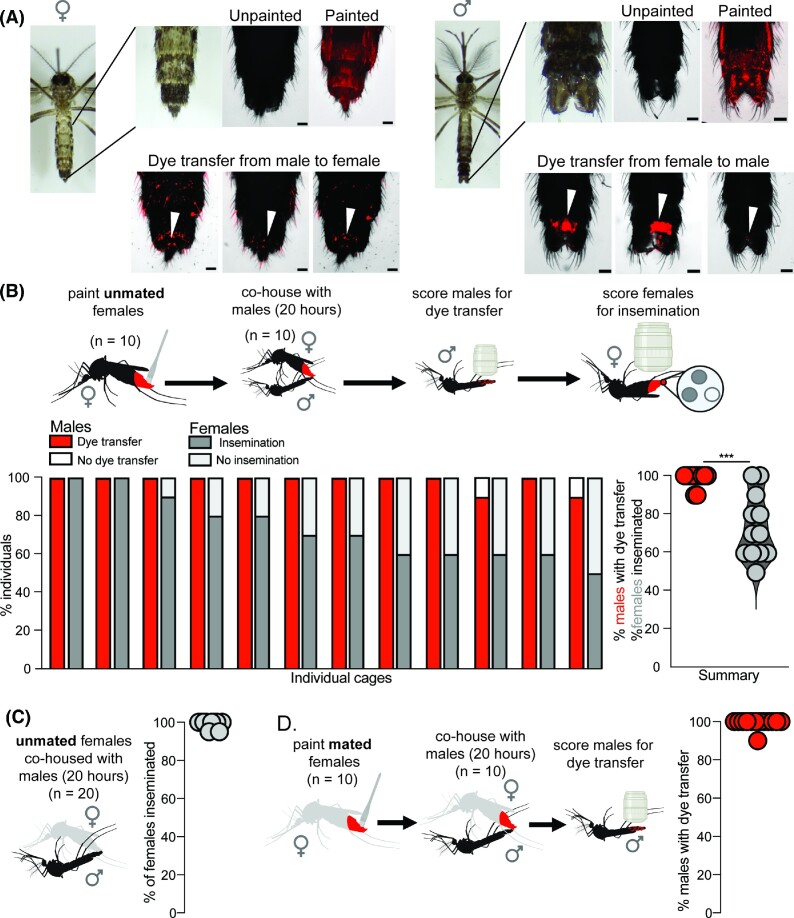
Fig. 1.
Dye transfer from females to males. (A) Images of a female (left) and male (right) mosquito. Top rows from left to right: a photograph of an unpainted mosquito, a photograph of the most posterior abdominal segments, and images of unpainted and painted mosquitoes. Bottom row: examples of dye transfer to unpainted females (left) and males (right) from painted partners (scale bar = 100 m). White arrowheads indicate specific areas of transfer to the genital region. (B) Dye transfer and insemination between painted, unmated females and unpainted males. Dye was applied to unmated females who were co-housed with unpainted males for 20 h. Males were then scored to determine the rate of dye transfer (98.3 ± 3.89%) and female spermathecae were scored to determine the rate of insemination (73.3 ± 16.7%) (n = 12 replicate cages; 10 males and 10 females/replicate). ***P < 0.01 in the Mann–Whitney test (U = 15, P = 0.0002). (C) Insemination rates during 20 h of co-housing (99.0 ± 2.1%, n = 20 females). Unmated females were co-housed with males for 20 h. Female spermathecae were dissected and scored for insemination (10 replicate cages; 20 females/replicate). (D) Dye transfer rates between painted, mated females and unpainted males (99.2 ± 2.8%). Dye was applied to mated females who were co-housed with unpainted males for 20 h. Males were then scored for dye transfer (n = 11 replicate cages; 10 males/replicate). Females are shown in gray to indicate that they were co-housed with males prior to the assay. Cartoons were created with BioRender.com.
To score for dye transfer in all assays, unpainted animals were cold anesthetized at 4°C, and dye transfer was scored using a Leica MZ10 F fluorescence stereomicroscope. Animals were scored as positive if a fluorescent signal was detected on the genitals or abdomen. Representative images for dye transfer were taken on a Nikon Ti2-E Inverted Fluorescent Stereomicroscope in the Cy3 channel (Fig. 1A). The excitation and emission spectrum of this fluorescent dye is wide, so the Cy3 channel was selected for scoring dye transfer because autofluorescence of the cuticle is minimal while signal from the dye is strong in that channel; however, fluorescence from the dye can also be visualized in the CFP and GFP channels.
Insemination status of females was determined by spermathecal dissection. A pair of fine-tipped forceps (Dumont #5 Biology/Inox Forceps, Fine Science Tools 11,252-20) were used to separate the last two abdominal segments from the rest of the abdomen, exposing the spermathecae. The spermathecae were then examined for the presence of sperm, and females were scored as inseminated if sperm was detected in the spermathecae. In all inseminated females, two of three spermathecae contained sperm, always the larger medial spermatheca and one smaller lateral spermatheca, in line with previously established patterns of sperm distribution in the spermathecae of mated Ae. aegypti (Roth 1948).
Dye-transfer behavioral assays
All behavioral assays were performed in environmental rooms at 70–80% relative humidity, 25–28°C, with a photoperiod of 14 h light and 10 h dark. Dye-transfer assays began in mid-to-late afternoon for a duration of 20 h, ending the following morning, except where otherwise noted in remating encounter experiments. Animals were housed in custom cages (216 mm diameter and 181 mm height) and provided with 10% sucrose ad libitum during the assay to reduce mortality. Replicates with <70% survival in either sex were excluded.
Single encounter—transfer from females to males
To score dye transfer from females to males, dye was applied to 10 females as described above. After recovery, 10 painted females were co-housed with 10 unpainted males for 20 h, after which males were scored for dye transfer and females were dissected and scored for insemination (Fig. 1B and D). We performed 13 replicate cages with painted, unmated females, one of which was excluded due to mortality >70% among the painted females. We performed 13 replicate cages with painted, mated females, none of which were excluded.
Single encounter—transfer from males to females
To score dye transfer from males to females, dye was applied to one male as described above. After recovery, the single painted male was co-housed with 10 unpainted females for 20 h, after which females were scored for dye transfer and then dissected and scored for insemination (Fig. 2). We performed 14 replicate cages with unpainted, unmated females, four of which were excluded due to the death of the single painted male. We performed 14 replicate cages with unpainted, mated females, four of which were excluded due to the death of the single painted male. Female mortality was below our threshold for exclusion in all painted male replicates.
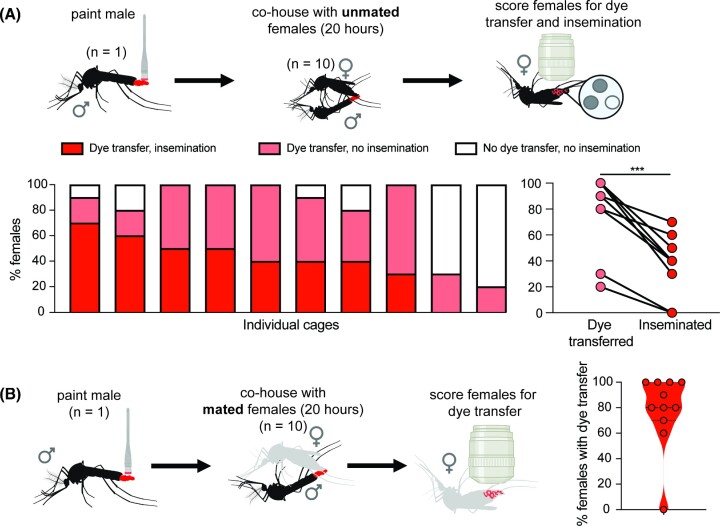
Fig. 2.
Dye transfer from males to females. (A) Dye transfer and insemination between a single painted male and unpainted, unmated females. An individual painted male was co-housed with unpainted, unmated females for 20 h. Females were then scored to determine the rate of dye transfer (79 ± 29.6%) and insemination (38.0 ± 21.8%) (10 replicate cages; 10 females/replicate). ***P < 0.01, Mann–Whitney test (U = 15.5, P = 0.0075). (B) Dye transfer between a single painted male and unpainted, mated females. An individual painted male was co-housed with unpainted, mated females for 20 h. Females were then scored to determine rates of dye transfer (86.0 ± 12.3%) (n = 11 replicate cages; 10 females/replicate). Cartoons were created with BioRender.com.
Remating encounter
To investigate how attempted mating interactions may differ between initial mating encounters and subsequent encounters, unmated females were given the opportunity to mate with successive males of different genotypes. To evaluate dye transfer during instances of remating, we utilized females mutant for npylr1, which have been previously shown to mate with multiple males if they are encountered within hours (Duvall et al. 2017). In these assays, painted unmated females were sequentially exposed to two groups of unpainted males whose offspring can be genetically distinguished. Group 1 males were co-housed with females for 90 min; this duration was chosen because it falls within the window of HP-I/NPYLR1 paternity enforcement. Group 1 males were removed from the cage and replaced with group 2 males, which were co-housed with females for 20 h. Males were scored for dye transfer after each encounter, and offspring paternity was assigned for each female by individually genotyping offspring (Fig. 3A).
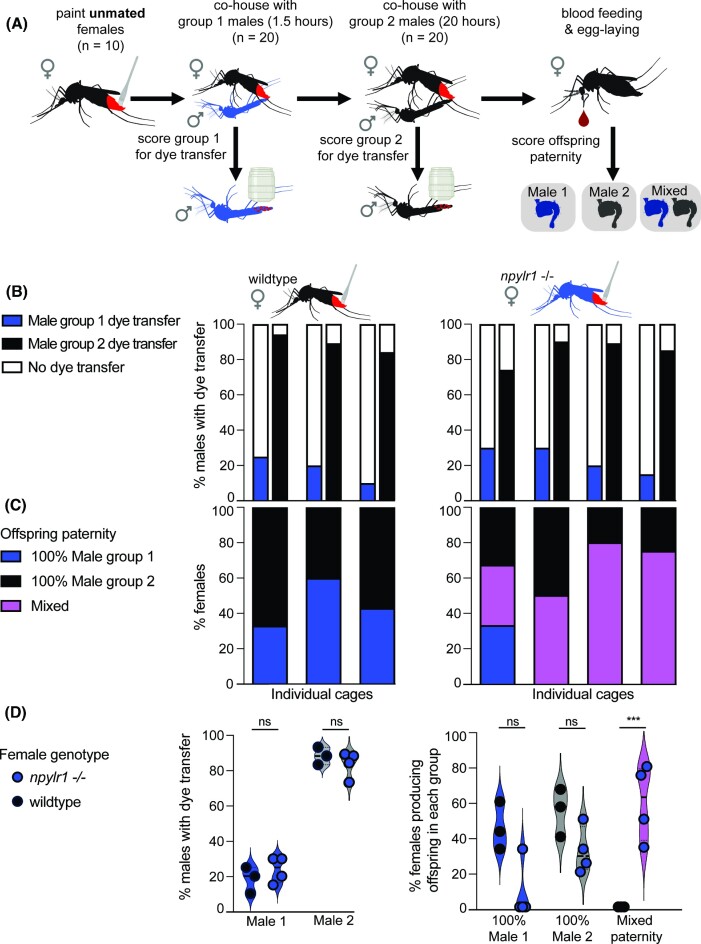
Fig. 3.
Dye transfer and paternity outcomes during remating. (A) Dye was applied to unmated female mosquitoes, which were co-housed with group 1 males (npylr1-/-, blue) for 90 min. Group 1 males were removed and replaced with group 2 males (Orlando, black) and co-housed for 20 h. Males were scored for dye transfer immediately after removal. Offspring were collected individually, and each female is categorized as bearing group 1 male’s offspring, group 2 male’s offspring, or mixed paternity (some offspring from each) based on genotypic differentiation of the npylr1 locus. (B) Rates of dye transfer from wild-type (Orlando) females (18.3 ± 7.6%, three replicate cages; 20 of each male/replicate) and npylr1-/- females (23.8 ± 7.5%, n = 4 replicate cages; 20 males/replicate) to group 1 male (npylr1-/-) (84.5 ± 7.3%), and group 2 male (89.0 ± 5.0%) (Orlando). (C) Percent of wild-type (Orlando) females (n = 3 replicate cages; 5–7 females/replicate; n = 10–55 offspring/female) bearing offspring exclusively from group 1 male (45.3 ± 13.7%, n = 3) and group 2 male (54.7 ± 13.7%, n = 3) and percent of npylr1-/- females (n = 4 replicate cages; 5–8 females/replicate; n = 11–71 offspring/female) bearing offspring from group 1 male (8.3 ± 16.5%, n = 4), group 2 male (32.0 ± 13.1%, n = 4), or mixed paternity offspring (59.8 ± 21.6%, n = 4). No wild-type (Orlando) females bore offspring of mixed paternity. Bars in (B) and (C) are stacked vertically, representing dye transfer to males and paternity outcomes for the same cage of animals. (D) shows summary data. ***P < 0.001, two-way ANOVAs analyzing the impact of genotype on both rates of dye transfer to group 1 males and group 2 males [F (1,10) = 0.01451, P = 0.9065], and rate of mixed paternity offspring [F (2,15) = 20.16, P < 0.0001]. Cartoons were created with BioRender.com.
To establish a baseline mating rate of painted, unmated females exposed to males for 90 min, dye was applied to 10 unmated wild-type (Orlando) or npylr1-/- females (Liesch et al. 2013) as described above. After recovery, 20 males were introduced to the females’ cage. After 90 min, these males were removed and scored for dye transfer. The females were cold-anesthetized at 4°C for 10 min, then placed on ice and scored for insemination.
To score dye transfer from females to successive males, dye was applied to 10 unmated females as described above. After recovery, 20 unpainted males (group 1) were introduced to the females’ cage for 90 min. At the end of 90 min, all males were removed, and 20 new unpainted males (group 2) were introduced into the cage for 20 h. In these assays, group 1 males were npylr1-/-, which do not show mating deficits and were used because their offspring can be unambiguously differentiated from wild-type (Orlando), which were used as group 2 males. A sex ratio of two males to one female was chosen to account for the reduced time that the females had with group 1 males (90 min) compared with other assays (20 h).
We performed four replicates with painted, unmated npylr1-/- females, none of which were excluded, and three replicates with painted, unmated wild type (Orlando) females, none of which were excluded.
Offspring paternity assessment
For paternity assessment, females were fed a blood meal of defibrinated sheep blood (Hardy Diagnostics DSB100) supplemented with adenosine 5’-triphosphate (ATP, 200 mM in aqueous NaHCO3), which serves as a phagostimulant. The meal was delivered using a Hemotek artificial membrane feeder (Hemotek Ltd. SP6W1-3), and females were allowed to feed to repletion. After feeding, each replete female was individually housed in a wide fly vial with 5 mL of deionized water and a 55 mm-diameter Whatman filter paper cone egg-laying substrate, as in Duvall et al. (2017). Females were allowed 7 days after feeding to oviposit. After ovipositing, each female was removed, and the egg paper was pulled out of the water to prevent premature hatching. After 7 days, each egg paper was placed in a separate plastic cup and hatched in 10 mL of hatch broth (see Rearing above). Offspring were collected for genotyping at the pupal stage, or L4 in the case of a small number of animals that were developing slowly, to genotype them alongside their siblings.
Females that did not feed to repletion or that produced fewer than 10 viable offspring were excluded from analysis. In the first replicate with wild-type females, one unfed female and three females that laid fewer than 10 eggs were excluded. In the second replicate with wild-type females, one unfed female and two females that laid fewer than 10 eggs were excluded. In the third replicate with wild-type females, two unfed females and three females that laid fewer than 10 eggs were excluded. In the first replicate with npylr1-/- females, one unfed female and three females that laid fewer than 10 eggs were excluded. In the second replicate with npylr1-/- females, one female that laid fewer than 10 eggs was excluded. In the third replicate with npylr1-/- females, one unfed female and one female that laid fewer than 10 eggs were excluded. In the fourth replicate with npylr1-/- females, four females that laid fewer than 10 eggs were excluded.
The offspring of each female were then individually genotyped using PCR and gel electrophoresis to determine their paternity. DNA extraction was performed from whole L4 larvae or pupae using Phire Tissue Direct (ThermoFisher Scientific F170), and paternity was determined by PCR-amplification of the npylr1 locus that differentiates group 1 males (npylr1-/-) from group 2 males. Presence of the wild-type npylr1 allele was detected with npylr1 forward and reverse primers (npylr1 forward primer: 5’-TAATCGTGTGGACTAGAAGAGGG-3’, npylr1 reverse primer: 5’-AGCTCTTCGCAGTAGAATGTACG-3’). Presence of the mutant npylr1-/- allele was detected with the npylr1 reverse primer and a forward primer embedded in the large polyubiquitin insert in the mutant (polyubiquitin forward primer: 5’- CGACTAACAGACACAAGCAC-3’; reverse primer as above). PCR products of the expected size in the agarose gel were Sanger sequenced (Genewiz) to confirm the presence of the wild-type or mutant allele.
Analysis
GraphPad Prism was used to perform statistical analysis to assess the significance of observed results and generate associated graphs. The alpha level for all statistical analyses was set at 0.05. A Mann–Whitney test was utilized to compare overall rates of dye transfer and insemination in single encounter assays involving painted males, as well as rates of insemination in wild-type (Orlando) and npylr1-/- females in 90-min cohousing control assays. Two-way ANOVA analyses were conducted to examine the effects of female genotype on both the rates of dye transfer from different groups of males in remating experiments, and the proportion of females with mixed paternity offspring.
Results
Dye transfer from females to males
Males were able to mate successfully with receptive females when dye was applied to the females, and nearly all males attempted to mate with females regardless of receptivity. In single encounter assays involving painted, unmated females and unpainted males (n = 12 replicate cages), the rate of insemination was 73.3 ± 16.7%, while 98.3 ± 3.9% of males had dye transfer from females (Fig. 1B), indicating that males will attempt to mate with, and can successfully inseminate females regardless of dye application. Mann–Whitney analysis showed that the proportion of males with dye transfer was significantly higher than the proportion of females that were inseminated (U = 15, P = 0.0002). In control experiments, we confirmed that neither incidental transfer from painted mosquitoes to the walls of the cage and then to unpainted mosquitoes, nor female-to-female transfer was observed (Supplementary Tables S1 and S3).
Female Ae. aegypti are non-receptive to males after they have successfully mated (Gwadz et al. 1971). We find that rates of insemination in control assays are maximal after 20 h of cohousing with males; 99.0 ± 2.0% of females are inseminated at this timepoint in control experiments (Fig. 1C), confirming that 5 days of cohousing provides ample time to ensure that females are mated before the assay. In single encounter assays with painted, previously mated females and unpainted males (n = 12 replicate cages), mated females transfer dye to 99.2 ± 2.8% of males, despite mated females being unreceptive and refractory to subsequent mating (Gwadz et al. 1971) (Fig. 1D).
Dye transfer from males to females
Painted males were able to mate with and inseminate receptive females, and males attempted to mate with females regardless of receptivity. In single encounter assays involving painted males and unmated, unpainted females (n = 10 replicate cages), 79.0 ± 29.6% of females had dye transfer, and 38.0 ± 21.8% of females were inseminated (Fig. 2A). In each cage, all inseminated females had dye transfer, and in each cage, dye transfer exceeded insemination. A Mann–Whitney test revealed that the overall level of dye transfer to females was significantly higher than insemination (38.0 ± 21.8%) (U = 15.5, P = 0.0075) (Fig. 2A), indicating that a single male will make contact with more females than he will successfully inseminate.
Single-encounter assays with a single painted male housed with unpainted, previously mated females (n = 11 replicate cages) resulted in dye transfer to 86.0 ± 12.3% of females. In one case, the male did not transfer dye to any females (Fig. 2B), indicating either that the male did not attempt to mate or that the females rejected this male before he was able to make contact.
These data show that males frequently make dye-transferring contact with both unmated and previously mated, unreceptive females. This indicates a male will attempt to mate, regardless of the female’s receptivity, consistent with previous studies (Gwadz et al. 1971; Jones and Pilitt 1973).
Dye transfer during remating
In order to determine whether rates of dye transfer differ between wild-type (Orlando) females and those with disrupted mating pathways, we scored interactions between wild-type (Orlando) and npylr1 mutant females who sequentially encountered two groups of males. Rates of dye transfer to group 1 males during the 90-min exposure window were 18.3 ± 7.6% (n = 3 replicate cages) in wild-type (Orlando) and 23.8 ± 7.5% (n = 4 replicate cages) in npylr1-/- females. Rates of dye transfer to group 2 males were 89.0 ± 5.0% (n = 3 replicate cages) from wild-type females and 84.5 ± 7.3% (n = 4 replicate cages) from npylr1-/- females (Fig. 3B). A two-way ANOVA showed that in both wild-type (Orlando) and npylr1-/- females, the rates of dye transfer to group 1 males were significantly lower than rates to group 2 males [F (1,10) = 298.2, P < 0.0001], but that female genotype did not impact rates of transfer [F (1,10) = 0.01451, P = 0.9065] (Fig. 3D). Mann–Whitney analysis showed that within a 90-min window, insemination rates of painted npylr1 mutants (21.3 ± 20.8%, n = 3 replicate cages) and wild-type (Orlando) females (32.5 ± 19.1%) were not significantly different (U = 3.0, P = 0.7) (Supplementary Table S2). These data indicate that wild-type (Orlando) and npylr1-/- females make comparable dye-transferring contacts with both groups of males.
To score offspring genotype, females were blood fed and housed in individual vials for oviposition so that offspring could be attributed to a single female. Wild-type (Orlando) females produced offspring fathered exclusively by group 1 (45.3 ± 13.7%) or group 2 (54.7 ± 13.7%) but never produced any mixed-paternity offspring, despite dye-transferring interactions with both males (n = 3 replicate cages) (Fig. 3C and D). Although some npylr1 mutant females produced offspring fathered exclusively by group 1 (8.3 ± 16.5%) or group 2 (32.0 ± 13.1%), this group also included individual females who bore offspring of mixed paternity within a single clutch (59.8 ± 21.6%) (n = 4 replicate cages), a phenomenon that was never observed in the wild-type females (Fig. 3C and D). Two-way ANOVA analysis showed that npylr1 mutants have significantly more mixed paternity offspring than wild-type (Orlando) females [F (2,15) = 20.16, P < 0.0001]. These data indicate that although both wild-type (Orlando) and npylr1 mutant females make similar copulatory contacts with both groups of males, only npylr1 mutant females are ever successfully inseminated by and produce offspring fathered by both group 1 and group 2 males.
Discussion
In this study, we utilize a hydrophobic oil-based fluorescent dye to score physical interactions between males and females to assess attempted and successful mating in Ae. aegypti. We show that both painted males and females transfer dye to the posterior abdominal segments and areas immediately surrounding the genitals of unpainted animals of the opposite sex, whether the female is receptive or not. The absence of insemination without dye transfer validates the method as faithfully reporting attempted mating. Previous observations have shown that a single male mosquito can inseminate five to seven females before exhausting his sperm, which is in line with our observations (Gwadz et al. 1971; Jones 1973). Our observation that a single painted male always transfers dye to more receptive females than he will successfully inseminate also suggests that male mosquitoes continue to attempt to mate when given the opportunity, even if they have depleted their sperm stores.
Although female rejection has been characterized as flying away from the male, abdominal twisting, flicking of wings, kicking males, and/or failure to open vaginal plates and extrude the ovipositor, we lack a mechanistic understanding of how and when these behaviors are deployed (Roth 1948; Gwadz et al. 1971; Jones and Pilitt 1973; Cator and Harrington 2011). The presence of dye transfer to the genitalia of the opposite sex, especially in the case of unreceptive females, supports the observation of “pseudocopulation” reported in Gwadz (1971), where there was genital contact, but no transfer of semen. These observed high rates of attempted mating culminating in contact of the external genitalia suggest the importance of mechanisms of rejection once animals reach the point of genital contact and imply additional methods of mate rejection on the part of unreceptive females. Such methods include external barriers such as closing of the vaginal sclerite to prevent insemination, or sperm rejection after copulation before the sperm reach the spermathecae.
There are limitations to this method, which must be considered when designing assays. Our assays were not designed to identify pair-wise mating interactions, although individual housing or combinatorial use of multiple dyes could be used to achieve this. Although dye transfer reports mating attempts in which genital contact was achieved, it does not report all mating attempts; previous work indicates that many female rejection behaviors preclude physical contact and would not result in dye transfer (Aldersley and Cator 2019).
Although painted males can inseminate females and painted females can be inseminated, rates of mating in cages with painted animals were lower than in assays in which both partners were unpainted, regardless of the genotype (compare Fig. 1B and C). It is possible that painted animals require longer to reach maximal mating rates. This may be because dye application or cold stress leads to reluctance to mate, reduces painted females’ attractiveness to potential mates, or reduces animals’ ability to maneuver.
There was no discernible difference in spatial patterns of dye transfer between wild-type and mutant animals or receptive and non-receptive females. Dye transfer indicates attempts at mating and must be considered along with female mating status at the beginning of the assay and insemination status after the assay to determine whether mating attempts were successful.
Although we developed this assay for use in Ae. aegypti, it is likely to succeed across many insect species. It may offer further insight and greater resolution to researchers interested in assessing males’ attempted interspecific mating, as in the case of Ae. aegypti and Ae. albopictus. Ae. albopictus males are known to attempt to mate with females of closely related species, including Ae. aegypti (Leahy and Craig 1967; Tripet et al. 2011; Bargielowski et al. 2015; Lounibos et al. 2016). Disrupting mating systems to exogenously suppress receptivity and effectively sterilize female mosquitoes is also of potential interest to mosquito control programs. A well-established method of controlling invasive and pest insect populations in the wild involves the mass introduction of sterilized or genetically modified males. The success of this approach relies on the fitness of the released males and their ability to successfully compete with wild males to mate with wild females (Alphey et al. 2010; Benelli 2015; Lees et al. 2015). The effectiveness and sustainability of these control strategies in the long run will be improved by releasing males with a high probability of mating success, which will facilitate the success of the program. Researchers could test lab strains’ ability to compete with wild populations to interact with females. It is our hope that the accessibility of our method will be of use to researchers working in a variety of insect mating systems, from cryptic mating behaviors to biological control.
Supplementary Material
Acknowledgement
We thank the reviewers and members of the Duvall Lab for their critical comments on the manuscript. We thank Lauren Subramaniam and Candace Cochran for their assistance in completing assays and data collection.
Notes
From the symposium “Neuroethology in the age of gene editing: New tools and novel insights into the molecular and neural basis of behavior’’ presented at the annual meeting of the Society for Integrative and Comparative Biology virtual annual meeting, January 16–March 31, 2023.
Contributor Information
Monica M Cramer, Department of Biological Sciences, Columbia University, New York, NY 10027, USA.
Thomas M Gabel, Department of Biological Sciences, Columbia University, New York, NY 10027, USA.
Laura B Duvall, Department of Biological Sciences, Columbia University, New York, NY 10027, USA.
Funding
This work was supported by a Beckman Young Investigator Award, a Pew Scholar in Biomedical Sciences Award, a Klingenstein-Simons Fellowship Award in Neuroscience, and R35 GM137888 from NIGMS.
Conflict of interest
The authors declare no conflict of interest. All data is available in Supplemental Data File.
References
- Aldersley A, Cator LJ. 2019. Female resistance and harmonic convergence influence male mating success in Aedes aegypti. Sci Rep. 9: 2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Service MW, Dobson SL. 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 10: 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. 2005. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol. 35: 661–75. [DOI] [PubMed] [Google Scholar]
- Bargielowski IE, Lounibos LP, Shin D, Smartt CT, Carrasquilla MC, Henry A, Navarro JC, Paupy C, Dennett JA. 2015. Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: culicidae) in nature. Infect Genet Evol. 36:456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G. 2015. Research in mosquito control: current challenges for a brighter future. Parasitol Res. 114: 2801–5. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh Oet al. 2013. The global distribution and burden of dengue. Nature. 496: 504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquilla MC, Lounibos LP. 2015. Satyrization without evidence of successful insemination from interspecific mating between invasive mosquitoes. Biol Lett. 11: 20150527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DO, Chuffi S, Ioshino RS, Marques ICS, Fini R, Costa MK, Araújo HRC, Costa-da-Silva AL, Kojin BB, Capurro ML. 2018. Mosquito pornoscopy: observation and interruption of Aedes aegypti copulation to determine female polyandric event and mixed progeny. PLoS One. 13: e0193164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Harrington LC. 2011. The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim Behav. 82: 627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Correa S, Rivera-Perez C, Nouzova M, Noriega FG. 2014. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J Insect Physiol. 64: 40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig GB. 1967. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 156: 1499–501. [DOI] [PubMed] [Google Scholar]
- Dimond JB, Lea AO, Hahnert WF, DeLong DM. 1956. The amino acids required for egg production in Aedes aegypti. Can Entomol. 88: 57–62. [Google Scholar]
- Duvall LB, Basrur NS, Molina H, McMeniman CJ, Vosshall LB. 2017. A peptide signaling system that rapidly enforces paternity in the Aedes aegypti mosquito. Curr Biol. 27: 3734–42.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard WG. 1991. Copulatory courtship and cryptic female choice in insects. Biol Rev. 66: 1–31. [Google Scholar]
- Fuchs MS, Craig GB, Hiss EA. 1968. The biochemical basis of female monogamy in mosquitoes I. Extraction of the active principle from Aedes aegypti. Life Sci. 7: 835–9. [DOI] [PubMed] [Google Scholar]
- Guerbois M, Fernandez-Salas I, Azar SR, Danis-Lozano R, Alpuche-Aranda CM, Leal G, Garcia-Malo IR, Diaz-Gonzalez EE, Casas-Martinez M, Rossi SLet al. 2016. Outbreak of Zika virus infection, Chiapas State, Mexico, 2015, and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. J Infect Dis. 214: 1349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz RW, Craig GB, Hickey WA. 1971. Female sexual behavior as the mechanism rendering Aedes aegypti refractory to insemination. Biol Bull. 140: 201–14. [DOI] [PubMed] [Google Scholar]
- Helinski MEH, Valerio L, Facchinelli L, Scott TW, Ramsey J, Harrington LC. 2012. Evidence of polyandry for Aedes aegypti in semifield enclosures. Am J Trop Med Hyg. 86: 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiss EA, Fuchs MS. 1972. The effect of matrone on oviposition in the mosquito, Aedes aegypti. J Insect Physiol. 18: 2217–27. [DOI] [PubMed] [Google Scholar]
- Jones JC, Pilitt DR. 1973. Observations on the sexual behavior of free flying mosquitoes. Biol Bull. 144: 480–8. [Google Scholar]
- Leahy MG, Craig GB. 1967. Barriers to hybridization between Aedes aegypti and Aedes albopictus (Diptera: culicidae). Evolution. 21: 41–58. [DOI] [PubMed] [Google Scholar]
- Lees RS, Gilles JR, Hendrichs J, Vreysen MJ, Bourtzis K. 2015. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Ins Sci. 10: 156–62. [DOI] [PubMed] [Google Scholar]
- Liesch J, Bellani LL, Vosshall LB. 2013. Functional and genetic characterization of neuropeptide Y-like receptors in Aedes aegypti. PLoS Negl Trop Dis. 7: e2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Bargielowski I, Carrasquilla MC, Nishimura N. 2016. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: culicidae) in peninsular florida two decades after competitive displacements. J Med Entomol. 53: 1385–90. [DOI] [PubMed] [Google Scholar]
- MacGregor M.E. 1915. Notes on the rearing of Stegomyia fasdata in London. J Trop Med Hyg. 18: 193–6. [Google Scholar]
- Naccarati C, Audsley N, Keen JN, Kim JH, Howell GJ, Kim YJ, Isaac RE. 2012. The host-seeking inhibitory peptide, Aea-HP-1, is made in the male accessory gland and transferred to the female during copulation. Peptides. 34: 150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JB, Jameson SB, Gloria-Soria A, Wesson DM, Powell J. 2015. Evidence of limited polyandry in a natural population of Aedes aegypti. Am J Trop Med Hyg. 93: 189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ, Wilson AJ, Hay SI, Graham AJ. 2006. The global distribution of yellow fever and dengue. Adv Parasitol. 62: 181–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth LM. 1948. A study of mosquito behavior. An experimental laboratory study of the sexual behavior of Aedes aegypti (Linnaeus). Am Midland Naturalist. 40: 265. [Google Scholar]
- Spielman A, Leahy MG, Skaff V. 1967. Seminal loss in repeatedly mated female Aedes aegypti. Biol Bull. 132: 404–12. [Google Scholar]
- Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. 2011. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg. 85: 265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. 2016. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 130: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. 2013. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 498:487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.