Highlights
-
•
Thuja orientalis folium inhibits influenza A viral infection.
-
•
Thuja orientalis folium significantly inhibits influenza viral binding and entry to the cells by modulating hemagglutinin.
-
•
Thuja orientalis folium represses the neuraminidase activity of the influenza virus.
-
•
Thuja orientalis folium contains a strong virucidal effect at the early phase of viral infection.
Keywords: Thuja orientalis Folium (TOF), Influenza A virus (IAV), Cytopathic effect (CPE), Hemagglutinin (HA), Neuraminidase (NA)
Abstract
Thuja orientalis Folium (TOF) has been prescribed traditionally as an expectorant for inflammatory airway disease. In this study, we evaluated the anti-influenza A virus (IAV) activity of TOF by detecting GFP expressed by influenza A virus (A/PR/8/34-GFP) infection. The fluorescence microscopy and fluorescence-activated cell sorting analysis showed that TOF potently inhibited IAV infection, dose-dependently. Consistently, immunofluorescence and Q-PCR analysis results confirmed TOF significantly represses IAV protein and RNA expression. TOF inhibited IAV infection at the binding and entry step upon viral infection and interferes with HA protein. Further, TOF exhibited a virucidal effect and inhibited the neuraminidase activity of IAV. Additionally, TOF prevented the cytopathic effect caused by H1N1 and H3N2 IAV infection. Amentoflavone among the constituents in TOF exerted the strongest anti-IAV effect. Myricetin, quercetin, and quercitrin also inhibited IAV infection. However, the potent anti-IAV effect of TOF may be related to the synergistic effect of constituents, not by a single specific compound. Our results suggest TOF exhibits a significant inhibitory effect against IAV infection at multi-stages via the blockage of viral attachment and entry, inhibition of neuraminidase, and induction of virucidal effects.
1. Introduction
Influenza A viruses are a major cause of respiratory infection in humans and are responsible for a significant amount of morbidity and mortality worldwide (Cox and Subbarao, 2000). The most common circulating subtypes are the influenza A (H1N1) viruses that cause seasonal epidemics, which are responsible for 290,000–650,000 deaths annually (Liu et al., 2020). Many vaccines and treatments have been developed to prevent the spread of the influenza virus, and these provide the backbone for the current preventive treatment strategy. These vaccines must be modified annually due to the viral tendency to mutate to escape detection in the immune system (Cline et al., 2017). However, the development of new vaccine strains is difficult to predict, which means that it takes time to provide virus pandemic losses (Boltz et al., 2010). The most fundamental countermeasure against the outbreak and pandemic preparation is to develop antiviral drugs to suppress and treat viral infections. Influenza treatments include M2 ion channel blockers (e.g., amantadine, rimantadine), which inhibit virus proliferation by interfering with the process of removing the skin of host cell infection, and neuraminidase inhibitors (e.g., oseltamivir, zanamivir), which inhibit the spread of virus particles by reducing their release from host cells and increasing the formation of virus aggregates (McKimm-Breschkin, 2005; Radosevic et al., 2019). Oseltamivir (Tamiflu), an NA inhibitor, is a successful example of an anti-influenza drug synthesized from natural products, as it was researched and developed using quinic acid and shikimic acid, which were obtained from plants (Gong and Xu, 2008). However, the 2008–2009 seasonal H1N1 influenza virus strain showed almost complete resistance to oseltamivir (Ojcius, 2007). Baloxavir marboxyl was approved for influenza treatment in 2018, but recent studies have shown that virus resistance remains a concern (Lampejo, 2020). It is thus necessary to develop a new agent to treat influenza A with a clear mechanism of action. Natural products have unlimited potential for new drug discoveries because of their chemical diversity. The dried leaf of TOF is a traditional herb, widely used for the treatment of Bronchitis, psoriasis, pancreatitis, and diarrhea (Cho et al., 2000; Naser et al., 2005). TOF contains a lot of chemical components including amentoflavone, quercitrin, myricetin, afzelin, and Kaempferol. Several studies have reported that TOF has a variety of biological functions such as an antioxidant (Ju et al., 2010), anti-inflammatory action (An et al., 2014), anticancer activity (Biswas et al., 2011) as well as reducing fever (Srivastava et al., 2012). The essential oil, in particular, has been used for mycotic infection and the treatment of roundworms (Srivastava et al., 2012). The previous studies showed that TOF contains antiviral activity against influenza virus A/PR8/34 (Won et al., 2013), but the report only demonstrated the inhibitory effect on the cytopathic effect and viral M2 gene expression. In this study, we elucidate the underlying mechanism of antiviral properties of TOF using RAW 264.7 macrophages infected with GFP-expressing H1N1 influenza virus to confirm the potential as an antiviral drug capable of inhibiting the proliferation of influenza viruses.
2. Materials and methods
2.1. Materials, chemicals, and preparation of TOF water extract
The High-Performance Liquid Chromatography (HPLC) system, Thermo Dionex UltiMate 3000, is equipped with a binary pump, an auto-sampler, a column oven, and a diode array UV/VIS detector (Thermo Fisher Scientific, San Jose, CA, USA). HPLC-grade acetonitrile and methanol were purchased from Fisher Scientific (Pittsburgh, PA, USA) and ACS reagent-grade formic acid was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Ultrapure water used for the HPLC analysis was purified by the Puris-Evo UP Water system with Evo-UP Dio VFT and Evo-ROP Dico20 (Mirae ST Co., Ltd., Anyang, Gyeonggi-do, Korea). Reference standard compounds, amentoflavone, and quercetin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Quercitrin was obtained from ChemFaces (Wuhan, China). The purity of all reference standards was above 95%. TOF was purchased from the Yeongcheon Oriental Herbal Market (Yeongcheon, South Korea) and verified by Prof. Kihwan Bae at the College of Pharmacy, Chungnam National University. To prepare TOF water extract, TOF was boiled in the water using a medical heating plate (Gyeongseo Extractor Cosmos-600, Inchon, South Korea). The solution was filtered using standard testing sieves (150 μm; Retsch, Haan, Germany), freeze-dried, and stored at −20 °C. For in vitro antiviral study, the powder of HSF water extract (WHSF) was dissolved in 50% DMSO at the concentration of 100 mg/ml.
2.2. Preparation of standards and sample solution for HPLC-DAD analysis
Five standard compounds, such as amentoflavone, afzelin, myricetin, quercetin, and quercitrin, were prepared by dissolving in methanol at a concentration of 1000 μg/ml. Then, each compound was diluted to a final concentration of 100 μg/ml for preparing the mixed working solution. The water extract of Thuja orientalis Folium (TOF), an analytical sample, was prepared by dissolving in methanol at a concentration of 20 mg/ml. All solutions of standards and samples used for analysis were filtered using 0.22 μm PTFE membrane filters (Whatman International Ltd., Maidstone, UK) and stored at 4 °C until analysis.
2.3. Cells and viruses
RAW 264.7 cells (Mouse Leukemic Monocyte Macrophage cell line; ATCCTIB-71) and MDCK cells (Madin Darby canine kidney; ATCC CCL-34) were acquired originally from ATCC (Manassas, VA, USA) and were passaged in Le Roswell Park Memorial Institute medium (RPMI; Hyclone, Logan, UT) and Eagle's Minimum Essential Medium (EMEM., Lonza, Basel, Switzerland) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and antibiotic/ antimycotic solution (Gibco) at 37 °C and 5% CO2. Green fluorescent protein (GFP)-tagged Influenza A (A/PR8/34(H1N1))-GFP and A/PR/34/(H1N1) viruses were provided by Dr. Jong-Soo Lee (Chungnam National University, Daejeon, South Korea). HBPV-VR-32 (H3N2, A/Korea/33/2005) virus was purchased from the Korea Bank for Pathogenic Viruses). The viruses were propagated in the allantoic fluid of 10-day-old chicken embryos.
2.4. Cytotoxicity test
The cell counting kit-8 (CCK-8) assay was used to evaluate the cytotoxicity of TOF in the cells, according to the manufacturer's recommendation (Dojindo, Rockville, MD, USA). The cells were seeded into 96-well plates at a density of 1 × 105 cells/well for RAW 264.7 cells or 5 × 104 cells/well for MDCK cells. The cells were cultured overnight before the TOF treatment. TOF was added to the wells at various concentrations (0, 10, 100, 200, 500, and 1000 μg/ml). After 24 h of incubation, 10 μl CCK-8 was added to the cells, and they were then incubated for 2 h at 37 °C, after which the absorbance at 450 nm was measured using a spectrophotometer (BioTek, Winooski, VT, USA).
2.5. Anti-viral activity against influenza viruses
The influenza PR8-GFP viruses (10 MOI) were mixed with TOF and incubated for 1 h at 4 °C and the mixtures were then added to RAW 264.7 or MDCK cells for 2 h at 37 °C. After washing with PBS, the cells were further incubated for 24 h until GFP expression. To investigate the effects of TOF, the levels of GFP expression in the cells infected with PR8-GFP IAV were observed under fluorescence microscopy (Nikon ECLIPSE Ti-U, Nikon Co., Japan) and photographed or assessed using fluorescence-activated cell sorting (FACS) analysis. The EC50 of TOF on IAV infection was calculated using online tool: MLA-"Quest Graph™ IC50 Calculator" by AAT Bioquest Inc., https://www.aatbio.com/tools/ic50-calculator.
2.6. Time of addition assay
Time-of-addition assay was performed as previously reported (Cho et al., 2022b). Briefly, for attachment stage, TOF and PR8-GFP IAV (10 MOI) were coinfected to the Raw 264.7 cells for 30 min at 4 °C. After washing with PBS to remove free virus, the cells were further incubated for 24 h at 37 °C. For entry stage, the IAV was infected to RAW 264.7 cells at 4 °C for 30 min. After washing with PBS, TOF was added to the cells for 30 min at 37 °C. The cells were further incubated for 24 h at 37 °C after washing with PBS to remove TOF. For the virucidal stage, IAV and TOF were coincubated at 4 °C for 30 min, and then the mixture was added to the cells for 30 min at 37 °C. The cells were further incubated for 24 h at 37 °C. The cells were analyzed using a fluorescence microscope and FACS.
2.7. Fluorescence-activated cell sorting (FACS) analysis
TOF was incubated with PR8-GFP IAV (10 MOI) for 1 h at 4 °C and then treated with the RAW 264.7 cells for 24 h at 37 °C. The cells were harvested by centrifugation at 5000 rpm for 10 min at 4 °C, washed thrice with cold PBS, and fixed with 4% paraformaldehyde. The cells were resuspended in PBS and analyzed for GFP expression using a CytoPLEX flow cell counter (Beckman Coulter Inc., Pasadena, CA, USA)
2.8. Immunofluorescence staining
Immunofluorescence staining was performed as previously described. RAW 264.7 cells (5 × 104) grown on 12-well tissue culture slides, were incubated at 37 °C for 12 h. The medium was then removed, and the cells were washed with cold PBS three times and infected with PR8-GFP IAV for 2 h. The virus and medium were then removed and the cells were washed with PBS three times. A complete medium was then added to the cells, and the cells were incubated at 37 °C with 5% CO2 for 24 h, cells were washed with cold PBS and fixed with cold absolute methanol for 10 min and 4% paraformaldehyde for 30 min at RT and permeabilized with 1% Triton X-100 in TBS for 15 min at RT. After washing with PBS containing 0.05% Tween 20 (PBST), the cells were subjected to blocking with 1% BSA-PBS for 30 min and incubated with antibodies against influenza viral protein for 1 h at room temperature. After washing with PBS containing 0.05% Tween 20 (PBST), the cells were incubated with Alexa Fluor 594-tagged secondary antibody in PBST for 1 h in the dark, followed by incubation with Hoechst 33,342 for 5 min. The images of the red viral proteins and blue nuclei were visualized under fluorescent microscopy.
2.9. Quantitative reverse transcription polymerase chain reaction
Total RNA from RAW 264.7 cells was precipitated using an Easy spin RNA extraction kit (iNtRON, Seongnam, Korea). After confirmed RNA concentration by Nanodrop DS-11 spectrometer (DeNovix, Wilmington, DE USA), total RNA was reverse-transcribed with iScipt cDNA synthesis kit (Bio-rad, USA) using random primers according to the manufacturer's instructions. Real-time PCR was performed on the Bio-rad real-time PCR system (Bio-rad, USA) with SsoAdvanced™ Universal SYBR® Green Supermix (Bio-rad). Primer sequences used in real-time PCR were indicated in supplemental Table 1. Target mRNA expression in each sample was normalized to the housekeeping gene GAPDH. The 2-ΔΔCt method was used to calculate relative mRNA expression levels.
2.10. Hemagglutination assay
The influenza A (H1N1) viruses were incubated with TOF for 1 h at 4 °C, and the mixture was added to RAW 264.7 cells for 2 h at 37 °C. After washing with PBS, the cells were incubated for another 24 h, then the supernatants were collected for the HA assay. Briefly, the supernatant was subjected to a serial two-fold dilution and added to a round-bottom 96-wellplate. The blank medium was used as a negative control. Each sample was mixed with 1% chicken RBCs (Innovative Research, Inc., Southfield, MI, USA) in PBS buffer. After incubation for 1 h at room temperature, the incubated plates were photographed.
2.11. Neuraminidase inhibition assay
NA-Fluor Influenza Neuraminidase Assay Kit (Life Technologies, Carlsbad, CA, USA) was used to investigate the effect of TOF against neuraminidase activities of H1N1 and H3N2 influenza viruses. TOF or oseltamivir carboxylate was serially diluted in assay buffer and mixed with H1N1 or H3N2 IAV at 96-well black plate. After incubation for 30 min at 37 °C, 200 µM of NA-Fluor Substrate was added to the mixture and incubated for 1 h at 37 °C. Subsequently, the reaction was terminated by NA-Fluor stop solution and monitored using a fluorescence spectrometer (Promega) with an excitation wavelength of 365 nm and an emission wavelength of 445 nm.
2.12. Cytopathic effect inhibition assay
TOF at 10, 50, 100, 200 μg /ml and H1N1 or H3N2 IAV (20 MOI) were incubated for 1 h at 4 °C. The mixtures were added to RAW 264.7 cells for 2 h at 37 °C. After washing with PBS, the cells were further incubated until cytopathic effect formation. To measure the viability of cells infected by H1N1 and H3N2 IAV with or without TOF, 10 μL CCK-8 was added to cells, and incubated for 2 h at 37 °C. The absorbance at 450 nm was determined by a spectrophotometer (Promega, Madison, WI, USA).
2.13. HPLC analysis conditions
To identify the components from TOF, HPLC analysis was performed via Thermo Dionex UltiMate 3000 HPLC system. The analysis conditions were conducted by referring to and modifying the method of the reported literature (Lu et al., 2006). Chromatographic separation was carried out on a Phenomenex Gemini® C18 column (250 × 4.6 mm, 5 μm), and the mobile phase composition consisted of 0.1% formic acid (v/v) in water (A) and 0.1% formic acid (v/v) acetonitrile (B). To improve chromatographic separation capacity, the gradient elution system was programmed at a flow rate of 1.0 ml/min as follows: 10% B, 0.0 min; 10–60% B, 0.0–40.0 min; 60% B, 40.0–45.0 min; 60–100% B, 45.0–50.0 min; 100% B, 50.0–55.0 min; 100–10% B, 55.0–55.5 min; 10% B, 55.5–60.0 min. The temperature of the column was maintained at 30 °C, and the injection volume of each sample was 10 µL. The detection wavelengths for all analytes, including amentoflavone, afzelin, myricetin, quercetin, and quercitrin were set at 365 nm. Data acquisition and analysis were performed via Dionex Chromelon software (Thermo Fisher Scientific).
3. Results
3.1. The cytotoxicity of TOF
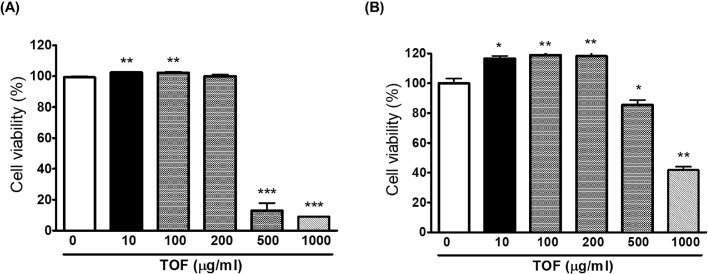
The cytotoxic effects of the TOF on RAW 264.7 and MDCK cells were evaluated using cell viability with the Cell counting Kit-8. The cells were treated with TOF and CCK-8 was added 24 h after the treatment to determine the optimal concentration that would provide anti-viral activity with minimum toxicity. The results showed that ≤ 200 µg/ml of TOF did not affect cell viability. Therefore, the subsequent experiment was performed up to 200 μg/ml TOF (Fig. 1).
Fig. 1.
Determination of the cytotoxicity of TOF in RAW 264.7 (A) and MDCK (B) cells. TOF was added to cells with indicated concentrations for 24 h at 37 °C. Cell viability was examined according to the CCK-8 assay kit. The data represent the mean ± SD based on three replicates in three different experiments. *p< 0.05, ⁎⁎p< 0.01, and ⁎⁎⁎p< 0.001.
3.2. TOF suppressed influenza A virus infection
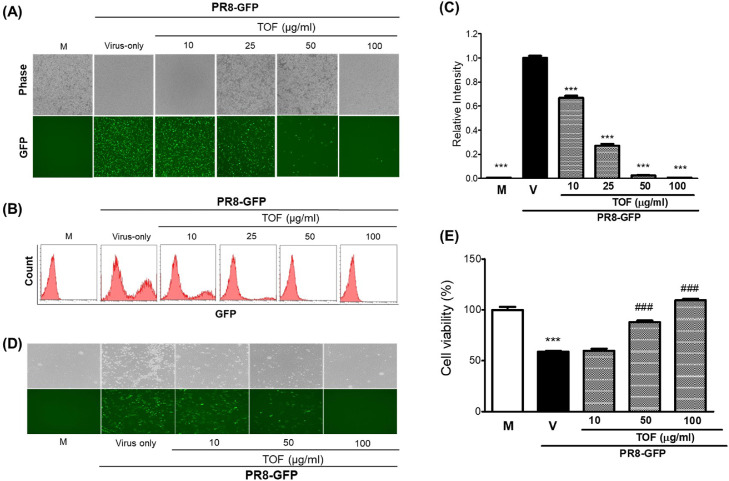
To examine the effect of TOF against influenza A virus infection, we cotreated TOF with various concentrations and influenza A/PR8/34 GFP virus (PR8-GFP IAV) to the RAW 264.7 cells. Since PR8-GFP IAV express high levels of GFP from 24 post-infection in RAW 264.7 cells (Fig. 2, lane V), we could compare the levels of GFP among mock, virus-infected only, virus and TOF cotreated groups. Fig. 2A shows TOF significantly represses the GFP expression in a dose-dependent manner. The inhibitory effect of TOF on PR8-GFP IAV infection was verified by FACS analysis using fixed cells in paraformaldehyde. The levels of GFP expression in each group were compared with the PR8-GFP virus-infected control and graphed as relative intensity. Consistent with Fig. 2A results, TOF significantly reduced the GFP expression by influenza A viral infection (Fig. 2B-C). TOF repressed the GFP expression with a 50% effective concentration (EC50) of 23.6 ± 0.35 μg/ml. Further, TOF completely blocked GFP expression at 100 μg/ml. Next, we cotreated TOF and PR8-GFP IAV to the MDCK cells to confirm the antiviral effect of TOF, as described in Fig 2A. TOF significantly repressed the GFP expression (Fig. 2D) and inhibited the cytopathic effect (Fig. 2E) by PR8-GFP IAV infection in MDCK cells, dose-dependently. These findings indicate that TOF had a potent inhibitory impact on the PR8-GFP influenza virus infection.
Fig. 2.
TOF exhibited a dose-dependent inhibitory effect in influenza A/PR/8/34-GFP virus infection. (A-C) TOF (0, 10, 25, 50, or 100 μg/ml) was mixed with PR8-GFP IAV for 1 h at 4 °C. The mixture was added to RAW 264.7 cells for 2 h at 37 °C. After washing with PBS, the cells were further incubated for 24 h. (A) Brightfield and fluorescence images were captured with the fluorescent microscope at 200x magnification. (B) After fixing with 4% paraformaldehyde and resuspending in PBS, the cells were analyzed for GFP expression by FACS. (C) The levels of GFP expression were depicted as relative intensities compared to the control PR8-GFP IAV. M; Mock group, V; Virus-only group, and TOF; 10, 25, 50, and 100 μg/ml group. Data are expressed as the mean ± SD (n = 3). ⁎⁎⁎p< 0.001 compared with the virus-only group. (D-E) TOF (10, 50, 100 μg/ml) was mixed with PR8-GFP IAV for 1 h at 4 °C before infection to MDCK cells for 2 h at 37 °C. (D-E) After further incubation until GFP expression (D) or CPE formation (E), the images of the cells were captured with a fluorescent microscope, or cell viability was determined using a CCK-8 assay. Data are expressed as the mean ± SD (n = 3). ⁎⁎⁎p< 0.001 compared with the Mock group. ###p< 0.001 compared with the virus-infected group.
3.3. TOF reduced influenza viral RNA and protein expression
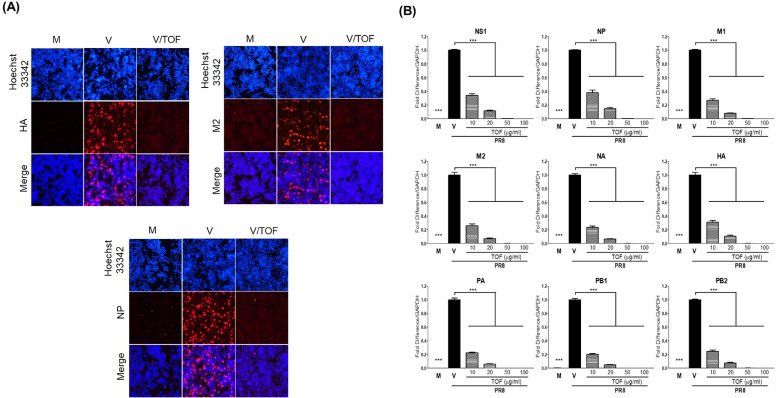
The effect of TOF on viral RNA and protein expression was examined by immunofluorescence and q-PCR (Fig. 3). TOF and PR8-GFP IAV were co-incubated at 4 °C for 1 h before infection to RAW 264.7 cells. The cells treated with mixtures were incubated at 37 °C for 18 h (for RNA) or 24 h (for protein). As shown in Fig. 3A, TOF decreased HA, M2, and NP IAV proteins. Consistently, Q-PCR performed using viral RNA-specific primers showed that the expression of all viral genes was significantly decreased by TOF in a dose-dependent manner (Fig. 3B).
Fig. 3.
The repressive effect of TOF on influenza A/PR/8/34-GFP viral RNA and protein expression. (A) HA, M2, and NP protein expression. TOF (100 μg/ml) or mock was co-treated to RAW 264.7 cells. At 24 h post-infection, the cells were fixed with paraformaldehyde, stained with antibodies against (HA, M2, and NP) (Red), and Hoechst 33,342 for nuclei (Blue). The viral protein and nuclei were observed and captured under a fluorescent microscope. (B) Viral RNA level in RAW 264.7 cells with different dosages of TOF. At 18 h post-infection, total RNA was isolated from cells. After synthesizing cDNA, q-PCR was performed using viral-specific primers. M; Mock group, V; Virus-only group, V/TOF; Virus and TOF cotreated group. The data represent the mean ± SD based on three independent experiments. Statistical significance was assessed via an unpaired Student T-test. ***P<0.001 compared with the virus-infected group.
3.4. TOF inhibited viral infection at attachment, entry, and virucidal stages
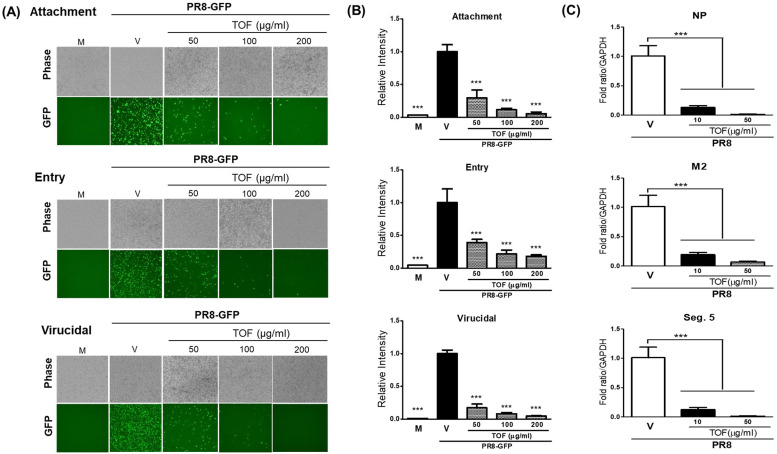
Since TOF significantly exhibited anti-influenza viral activity, we investigate whether the antiviral effect of TOF is related to the inhibitory effect on viral attachment and entry or direct killing at the early stages upon viral infection. We performed the time-of-addition experiments using cotreatment of PR8-GFP IAV and TOF at 50, 100, or 200 μg/ml in RAW 264.7 cells, described in materials and methods. Fig. 4A and B represent TOF dose-dependently inhibits viral attachment and entry and further exerts virucidal effect, implying killing the virus before entering into the cells. To confirm the effect of TOF on virus entry and virucidal impact, the level of entered viral genome RNA in cells after 1 h infection was checked using q-PCR. The levels of two translated regions (NP and M2) and one non-translated region, which is located at segment 5 of the viral genome (Seg.5), were significantly decreased by 10 or 50 μg/ml of TOF. These results mean that TOF potently inhibits influenza viral infection at an early stage (Fig. 4C).
Fig. 4.
The inhibitory effect of TOF against viral infection at early stages. (A, B) TOF at concentrations of 50, 100, and 200 μg/ml or medium were mixed with H1N1 IAV at 4 °C for 1 h to treat RAW 264.7 cells. The detailed methods for evaluating to effect on attachment, entry, and virucidal stages were shown in the materials and methods. (A) The brightfield and fluorescent images were captured under fluorescence microscopy at a magnification of 200x. (B) The cells were fixed and analyzed by FACS. (C) TOF at 10, 50 μg/ml, or medium were mixed with H1N1 IAV at 4 °C for 1 h to treat RAW 264.7 cells for 2 h at 37 °C. The cells were washed with PBS and subjected to RNA isolation. The relative level of three regions of viral RNA (segment 5, NP, and M2) entered in RAW 264.7 cells. Data are expressed as the mean ± SD (n = 3). ⁎⁎⁎p< 0.001 compared with the virus-only group. M; Mock group, V; Virus-only group, and TOF; 50, 100, and 200 μg/ml group.
3.5. Effect of TOF on HA activity
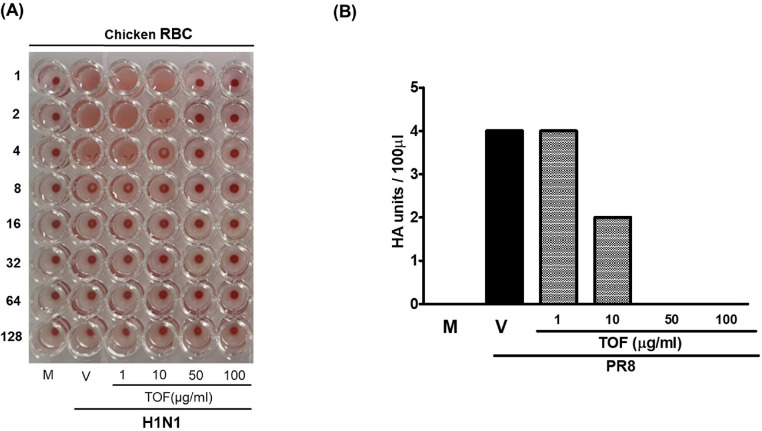
Because the time-of-addition assay showed that TOF directly blocks the IAV infection at attachment and entry, we investigated whether TOF affects the HA activity of IAV. HA of IAV is a crucial role in viral binding into the cell at an early stage and cause hemagglutination of red blood cell (RBC). When we co-incubated the chicken RBC and supernatants of cells infected by IAV and TOF mixtures, we found that TOF significantly represses the hemagglutination by IAV, as shown in Fig. 5. In the absence of TOF, HA units of IAV were 4 units, but 10 μg/ml TOF lowered HA units by 2-fold compared to virus only group. In the presence of 50 or 100 μg/ml TOF hemagglutination was completely blocked (Fig. 5). These findings confirm that TOF limits IAV infection by blocking viral attachment to the cells via inhibiting the HA of influenza virus.
Fig. 5.
The inhibitory effect of TOF on hemagglutination against IAV infection. TOF at concentrations of 1, 10, 50, and 100 μg/ml or medium was mixed with H1N1 IAV at 4 °C for 1 h and added to RAW 264.7 cells for 24 h at 37 °C. The supernatant of the cells was 2-fold serially diluted and mixed with PBC cells for 1 h at room temperature. M; Mock, V; Virus-only, and TOF; 1, 10, 50, and 100 μg/ml.
3.6. Effect of TOF on NA activity of IAV
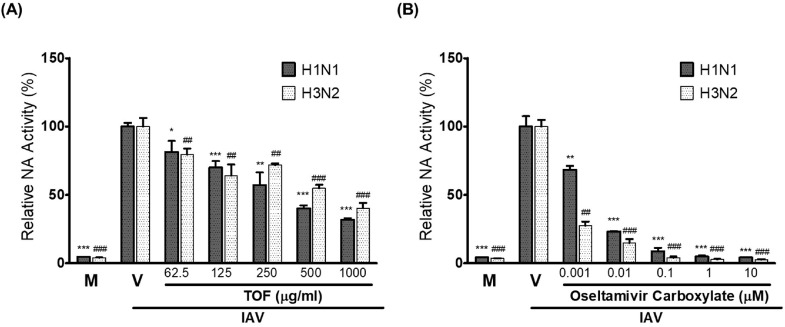
Neuraminidase of IAV plays a critical role in the viral progeny release from the infected cells. Oseltamivir and zanamivir are the anti-influenza viral drugs targeting NA activity of IAV currently in use. We investigated whether TOF affects the NA activity of H1N1 and H3N2 IAV. As shown in Fig. 6, TOF inhibited NA activities of both H1N1 and H3N2 viruses, dose-dependently. Oseltamivir carboxylate used as a positive control exerted a strong inhibitory effect on H1N1 and H3N2 viruses.
Fig. 6.
The inhibitory effect of TOF on neuraminidase activity of H1N1 and H3N2 IAV. (A) TOF at concentrations from 1000 to 62.5 μg/ml or medium was mixed with H1N1 or H3N2 IAV in a 96-well black plate. (B) Oseltamivir carboxylate with the indicated concentration was used as a positive control. The NA activities were determined using the NA-Fluor Influenza Neuraminidase assay kit according to the manufacturer's instructions. Data are expressed as the mean ± SD (n = 3). *p< 0.05, ⁎⁎p< 0.01 and ⁎⁎⁎p< 0.001 compared with H1N1 virus-infected group. ##p< 0.01 and ###p< 0.001 compared with H3N2 virus-infected group. M; Mock, V; Virus-only group.
3.7. TOF inhibited cytopathic effect by H1N1 and H3N2 IAV infection
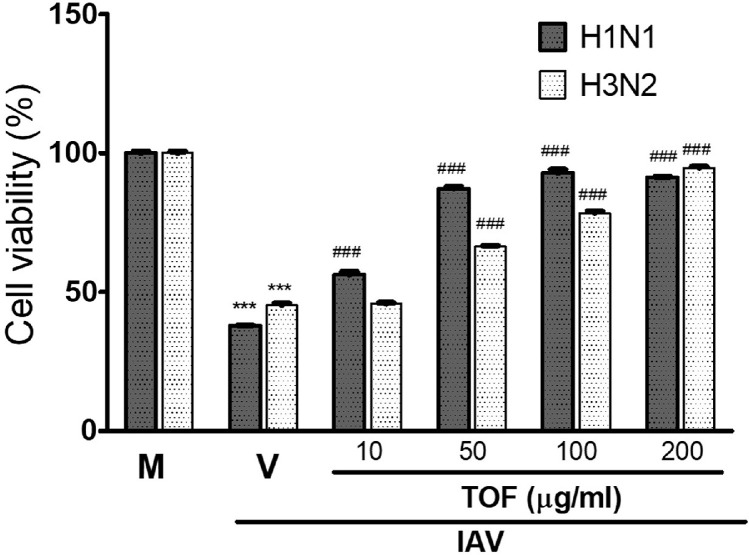
We examined the effect of TOF on H1N1 wild type and H3N2 IAV infection. H1N1 and H3N2 influenza viruses decreased the cell viability by inducing cytopathic effects (CPE) in RAW 264.7 cells. However, TOF inhibited the cytopathic effect caused by H1N1 and H3N2 IAV and increased the cell viability reduced by viruses, dose-dependently (Fig. 7). TOF at 200 μg/ml suppressed the CPE effect by H1N1 and H3N2 viral infection up to more than 90% compared to virus-infected control. These results indicate TOF has a potent antiviral efficacy against influenza A viruses including PR8, H1N1, and H3N2.
Fig. 7.
TOF suppresses the CPE by H1N1 or H3N2 influenza viral infection. TOF (10, 50, 100, and 200 μg/ml) or medium (mock) was incubated with H1N1 or H3N2 IAV at 4 °C for 1 h. The mixtures were added to RAW 264.7 cells at 37 °C for 24 h. The cell viability was detected using a CCK-8 kit. Data are expressed as the mean ± SD (n = 3). ⁎⁎⁎p< 0.001 compared with the Mock group, ###p< 0.001 compared with virus-infected group. M; Mock, V; Virus-only group.
3.8. HPLC analysis of the constituents in TOF
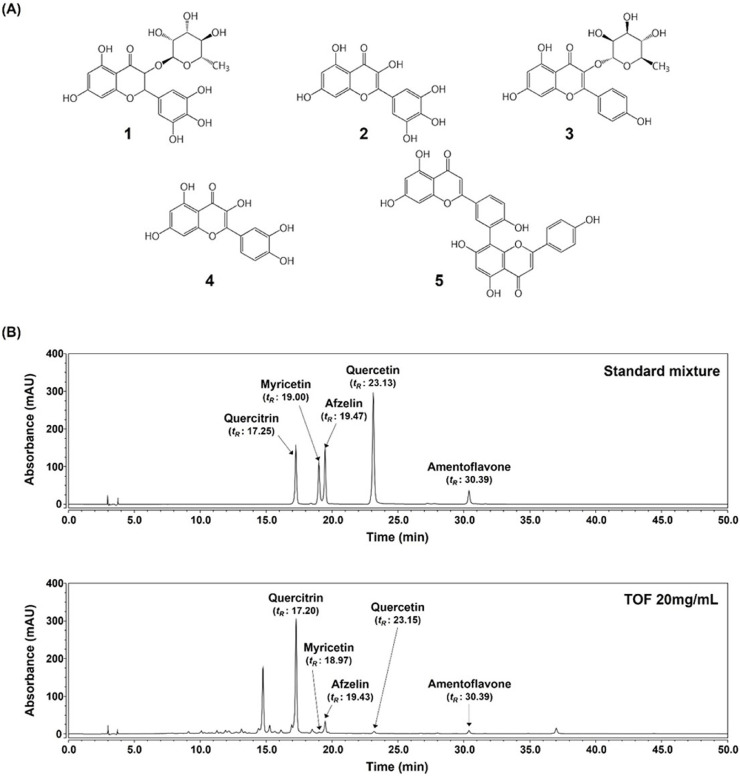
The five components having flavonoid backbone, including amentoflavone, afzelin, myricetin, quercetin, and quercitrin (Fig. 8A), were identified by comparing the maximum UV absorbance and the retention times (tR) with those of reference compounds. As shown in Fig. 8B, quercitrin (17.25 min), myricetin (19.00 min), afzelin (19.47 min), quercetin (23.13 min), and amentoflavone (30.39 min) had a good selectivity at 365 nm in standard mixture with each compound at 100μg/ml final concentrations. Under the same analytical condition, quercitrin (17.20 min), myricetin (18.97 min), afzelin (19.43 min), quercetin (23.15 min), and amentoflavone (30.39 min) contained in TOF were respectively detected with the comparable retention time and UV spectrum.
Fig. 8.
HPLC-DAD analysis of the components in a standard mixture and TOF at 365 nm. (A) Chemical structures of the components in standard mixtures. 1, Quercitrin; 2, Myricetin; 3, Afzelin; 4, Quercetin; 5, Amentoflavone. (B) Detection of the five components in TOF. Each standard concentration was 100 μg/ml.
3.9. Anti-IAV activity of constituents in TOF
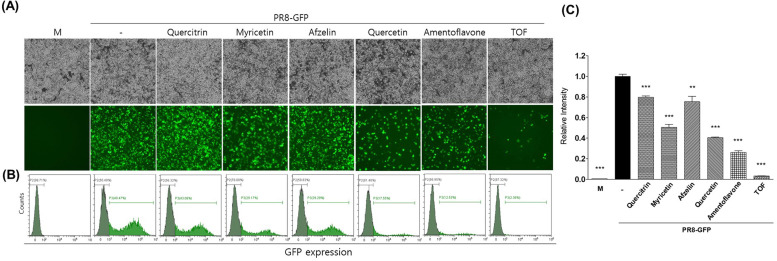
To determine which constituents in TOF are related to the antiviral effect, we compared the anti-viral effect of each constituent in TOF with the method described in Fig. 2. Fig. 9 represents that amentoflavone among five constituents exerted the strongest anti-IAV effect. Quercetin and myricetin also showed a strong inhibitory effect against IAV infection compared to the virus-infected group. Amentoflavone and quercetin present in the small peak among the five components in TOF showed strong antiviral efficacy, whereas Quercitrin and afzelin present in large amounts showed less efficacy against IAV infection. These results indicate that the potent anti-IAV efficacy of TOF is related to the synergistic effect of components present in TOF, including amentoflavone and quercetin.
Fig. 9.
Anti-influenza viral effect of five constituents in TOF. Each compound (50 μM) or TOF (100 μg/ml) was mixed with PR8-GFP IAV (10 MOI). The cytotoxicity of each compound was determined using a CCK-8 assay. Each compound was not toxic at 50 μM (Sup. Fig. 1). The mixtures were added to RAW 264.7 cells and incubated for GFP expression. Fluorescence and brightfield images were captured (A) and GFP expression was analyzed by FACS (B, C). ⁎⁎p< 0.01 and ⁎⁎⁎p< 0.001 compared with the virus-infected group. M; Mock group.
4. Discussion
TOF has been widely used as a botanical medicine herb to treat regular seasonal influenza (Srivastava et al., 2012). It has been demonstrated to have antibacterial activity against methicillin-resistant Staphylococcus aureus (Jain and Sharma, 2017), antiviral action on Fig leaf mottle-associated virus 1(FLMaV-1) (Aldhebiani et al., 2017), watermelon mosaic virus (WMV) (Elbeshehy et al., 2015), especially fungi toxic activity in essential oils (Guleria et al., 2008), as well as anti-inflammation (Shin et al., 2015; Silva et al., 2017), anti-cancer (R et al., 2018) and neurotoxicity effects (Park et al., 2014). A previous report (Won et al., 2013) showed the anti-viral potential of TOF by reducing the cytopathic effect by the influenza A virus in MDCK cells.
In this study, we demonstrated that the antiviral efficacy of TOF is closely related to the inhibition of viral binding and release via the modulation of HA and NA of IAV during viral infection. We confirmed the antiviral effect of TOF using the PR8-GFP influenza virus. Fluorescence microscopy and FACS analysis showed that TOF potently inhibits IAV infection, dose-dependently. The immunofluorescence staining for the IAV protein confirmed that TOF substantially reduces IAV protein expression. Based on the strong inhibitory effects of TOF on IAV infection, we investigated whether TOF could prevent viral attachment and entry into cells at early stages during viral infection. The time-of-addition assay revealed that TOF significantly reduces viral binding and penetration into the cells, in a dose-dependent manner. HA inhibition assay confirmed the inhibitory effect of TOF on the hemagglutination (HA) of IAV. HA of IAV is responsible for viral binding on the cells and main target for antiviral therapies. There are several reports on natural herbal extracts such as broccoli leaves (Cho et al., 2022a), Eupatorium perfoliatum (Derksen et al., 2016), cranberry (Luganini et al., 2018), and jatropha curcas Linn. leaf (Patil et al., 2013), which inhibited HA activity and viral attachment into the cells. Especially, we have reported that Epimedium Koreanum Nakai (EKN) water extract directly inhibits influenza virus binding to cells and has virucidal effects before entering the cell (Cho and Ma, 2022). The virucidal effects of EKN are due to specific components of the extract which can directly interact with the virus, and this inhibits viral particles from adhering to the cells. Viral invasion inhibitors have attracted considerable attention from synthetic chemists and biochemists as a new type of antiviral drug (Cho et al., 2015). Zhou et al. found that plant-derived pentacyclic triterpenes act as highly potent antiviral agents by efficiently preventing viral attachment to the host cells (Xiao et al., 2018). Additionally, TOF exhibited an inhibitory effect on neuraminidase (NA), which is responsible for viral progeny release. Our results suggest both HA and NA inhibition by TOF are closely related to the potent anti-IAV effect. A previous report showed that Lotus (Nelumbo nucifera Gaertn.) leaf inhibits IAV infection via modulating HA and NA activities (Cho et al., 2022c). We also confirmed that TOF protects the cells from cytopathic effects caused by H1N1 and H3N2 IAV infection. HPLC analysis detected quercitrin, myricetin, afzelin, quercetin, and amentoflavone in TOF. Several researches showed that quercitrin blocks IAV infection by controlling TLR signaling and inhibiting NA and NS1 proteins in the docking studies (Ahmad et al., 2015; Ling et al., 2020; Sun et al., 2020; Tang et al., 2023). Our previous study showed that quercetin inhibits IAV attachment and entry at an early infection (Cho and Ma, 2022). Myricetin was also reported to have an anti-IAV effect through the inhibition of PB2 and TLR3 signaling (Sang et al., 2021). Amentoflavone reportedly has anti-viral effects on HSV-1 (Li et al., 2019), Coxsackie virus B3 (Wilsky et al., 2012), and respiratory syncytial virus (RSV) (Ma et al., 2003). In this study, we first found that amentoflavone contains an anti-influenza viral effect. We identified several anti-IAV compounds in TOF, but the strong anti-viral efficacy of TOF may be related to the synergistic effect of them. It remains to be identified which components in TOF play a critical role in the virucidal effect or HA, NA inhibition. Our results showed that TOF has strong antiviral efficacy against IAV by inhibiting viral binding, penetration, and progeny release, and inducing the virucidal of IAV. Although further study is needed to confirm the antiviral effects of TOF through in vivo experiments, our results suggest the possibility that TOF could be developed as a natural antiviral drug preventing influenza A viral infection.
5. Conclusion
TOF inhibits IAV infection by preventing early viral binding and entry by interfering with HA protein and directly killing the virus. TOF also affects viral progeny release by repression of NA activity. Our results show the potential of TOF to be used as a natural anti-influenza viral agent through the synergistic effects of various compounds in TOF.
CRediT authorship contribution statement
Myong-Min Lee: Investigation, Validation, Writing – original draft. Won-Kyung Cho: Conceptualization, Methodology, Investigation, Validation, Writing – original draft, Writing – review & editing. Min Ho Cha: Investigation, Validation, Writing – original draft. Nam-Hui Yim: Investigation, Validation, Writing – original draft. Hye Jin Yang: Investigation, Validation, Writing – original draft. Jin Yeul Ma: Conceptualization, Supervision, Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Funding
This work was supported by Grant numbers KSN1812101 and KSN1823233 from KIOM (Korea Institute of Oriental Medicine), provided by the Ministry of Science and ICT, Republic of Korea.
Acknowledgments
We thank Dr. Jong-Soo Lee (Chungnam National University, Republic of Korea) for providing GFP-tagged Influenza A/PR/8/34 and H1N1 influenza viruses.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199199.
Contributor Information
Won-Kyung Cho, Email: wkcho@kiom.re.kr.
Jin Yeul Ma, Email: jyma@kiom.re.kr.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Ahmad A., Ahad A., Rao A.Q., Husnain T. Molecular docking based screening of neem-derived compounds with the NS1 protein of Influenza virus. Bioinformation. 2015;11(7):359–365. doi: 10.6026/97320630011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldhebiani A.Y., Elbeshehy E.K., Baeshen A.A., Elbeaino T. Inhibitory activity of different medicinal extracts from Thuja leaves, ginger roots, Harmal seeds and turmeric rhizomes against Fig leaf mottle-associated virus 1 (FLMaV-1) infecting figs in Mecca region. Saudi J. Biol. Sci. 2017;24(4):936–944. doi: 10.1016/j.sjbs.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S.M., Kim H.G., Choi E.J., Hwang H.H., Lee E., Baek J.H., Boo Y.C., Koh J.S. Screening for anti-inflammatory activities in extracts from Korean herb medicines. J. Soc. Cosmetic Sci. Korea. 2014;40(1):95–108. [Google Scholar]
- Biswas R., Mandal S.K., Dutta S., Bhattacharyya S.S., Boujedaini N., Khuda-Bukhsh A.R. Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: evidences from in vitro studies on A375 cells. Evid.-Based Complement. Alternat. Med. 2011. 2011 doi: 10.1093/ecam/neq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz D.A., Aldridge J.R., Webster R.G., Govorkova E.A. Drugs in development for influenza. Drugs. 2010;70(11):1349–1362. doi: 10.2165/11537960-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.Y., Kim P.S., Park J., Yoo E.S., Baik K.U., Kim Y.-K., Park M.H. Inhibitor of tumor necrosis factor-α production in lipopolysaccharide-stimulated RAW264. 7 cells from Amorpha fruticosa. J. Ethnopharmacol. 2000;70(2):127–133. doi: 10.1016/s0378-8741(99)00154-3. [DOI] [PubMed] [Google Scholar]
- Cho W.-K., Weeratunga P., Lee B.-H., Park J.-S., Kim C.-J., Ma J.Y., Lee J.-S. Epimedium koreanum Nakai displays broad spectrum of antiviral activity in vitro and in vivo by inducing cellular antiviral state. Viruses. 2015;7(1):352–377. doi: 10.3390/v7010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.-K., Yim N.-H., Lee M.-M., Han C.-H., Ma J.Y. Broccoli Leaves Attenuate Influenza A Virus Infection by Interfering With Hemagglutinin and Inhibiting Viral Attachment. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.899181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.K., Lee M.M., Ma J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.K., Ma J.Y. Antiviral activity of Epimedium koreanum Nakai water extract against influenza viruses. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112581. [DOI] [PubMed] [Google Scholar]
- Cho W.K., Yang H.J., Ma J.Y. Lotus (Nelumbo nucifera Gaertn.) leaf water extracts suppress influenza a viral infection via inhibition of neuraminidase and hemagglutinin. J. Funct. Foods. 2022;91 2022. 93. [Google Scholar]
- Cline T.D., Beck D., Bianchini E. Influenza virus replication in macrophages: balancing protection and pathogenesis. J. Gen. Virol. 2017;98(10):2401. doi: 10.1099/jgv.0.000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N.J., Subbarao K. Global epidemiology of influenza: past and present. Annu. Rev. Med. 2000;51(1):407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- Derksen A., Kühn J., Hafezi W., Sendker J., Ehrhardt C., Ludwig S., Hensel A. Antiviral activity of hydroalcoholic extract from Eupatorium perfoliatum L. against the attachment of influenza A virus. J. Ethnopharmacol. 2016;188:144–152. doi: 10.1016/j.jep.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Elbeshehy E.K., Metwali E.M., Almaghrabi O.A. Antiviral activity of Thuja orientalis extracts against watermelon mosaic virus (WMV) on Citrullus lanatus. Saudi J. Biol. Sci. 2015;22(2):211–219. doi: 10.1016/j.sjbs.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Xu W. Different synthetic strategies of oseltamivir phosphate: a potent influenza neuraminidase inhibitor. Curr. Med. Chem. 2008;15(30):3145–3159. doi: 10.2174/092986708786848497. [DOI] [PubMed] [Google Scholar]
- Guleria S., Kumar A., Tiku A.K. Chemical composition and fungitoxic activity of essential oil of Thuja orientalis L. grown in the north-western Himalaya. Zeitschrift für Naturforschung C. 2008;63(3-4):211–214. doi: 10.1515/znc-2008-3-409. [DOI] [PubMed] [Google Scholar]
- Jain N., Sharma M. Ethanobotany, phytochemical and pharmacological aspects of Thuja orientalis. A review. Int. J. Pure Appl. Biosci. 2017;5:73–83. [Google Scholar]
- Ju M.S., Lee P., Kim H.G., Lee K.Y., Hur J., Cho S.H., Sung S.H., Oh M.S. Protective effects of standardized Thuja orientalis leaves against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Toxicol. in Vitro. 2010;24(3):759–765. doi: 10.1016/j.tiv.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Lampejo T. Influenza and antiviral resistance: an overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(7):1201–1208. doi: 10.1007/s10096-020-03840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Song X., Su G., Wang Y., Wang Z., Jia J., Qing S., Huang L., Wang Y., Zheng K. Amentoflavone inhibits HSV-1 and ACV-resistant strain infection by suppressing viral early infection. Viruses. 2019;11(5):466. doi: 10.3390/v11050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L.J., Lu Y., Zhang Y.Y., Zhu H.Y., Tu P., Li H., Chen D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153150. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang B., Wang Y., Haymour H.S., Zhang F., Xu L.-c., Srinivasarao M., Low P.S. A universal dual mechanism immunotherapy for the treatment of influenza virus infections. Nat. Commun. 2020;11(1):1–14. doi: 10.1038/s41467-020-19386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.-h., Liu Z.-y., Wang Z.-t., Wei D.-z. Quality evaluation of Platycladus orientalis (L.) Franco through simultaneous determination of four bioactive flavonoids by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2006;41(4):1186–1190. doi: 10.1016/j.jpba.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Luganini A., Terlizzi M.E., Catucci G., Gilardi G., Maffei M.E., Gribaudo G. The cranberry extract oximacro® exerts in vitro virucidal activity against influenza virus by interfering with hemagglutinin. Front. Microbiol. 2018;9:1826. doi: 10.3389/fmicb.2018.01826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.-Y., Ma S.-C., Wei F., Lin R.-C., But P.P.-H., Lee S.H.-S., Lee S.F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003;51(11):1264–1267. doi: 10.1248/cpb.51.1264. [DOI] [PubMed] [Google Scholar]
- McKimm-Breschkin J.L. Management of influenza virus infections with neuraminidase inhibitors. Treat. Respir. Med. 2005;4(2):107–116. doi: 10.2165/00151829-200504020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser B., Bodinet C., Tegtmeier M., Lindequist U. Thuja occidentalis (Arbor vitae): a review of its pharmaceutical, pharmacological and clinical properties. Evid.-Based Complement. Altern. Med. 2005;2(1):69–78. doi: 10.1093/ecam/neh065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojcius D. Tamiflu side effects in the spotlight. Nat. Rev. Microbiol. 2007;5(5):335–336. [Google Scholar]
- Park G., Kim H.G., Ju M.S., Kim A.-J., Oh M.S. Thuja orientalis leaves extract protects dopaminergic neurons against MPTP-induced neurotoxicity via inhibiting inflammatory action. Korea J. Herbol. 2014;29(3):27–33. [Google Scholar]
- Patil D., Roy S., Dahake R., Rajopadhye S., Kothari S., Deshmukh R., Chowdhary A. Evaluation of Jatropha curcas Linn. leaf extracts for its cytotoxicity and potential to inhibit hemagglutinin protein of influenza virus. Indian J. Virol. 2013;24(2):220–226. doi: 10.1007/s13337-013-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R E.B., Jesubatham P.D., V M.B., S V., S S. Non-toxic and non teratogenic extract of Thuja orientalis L. inhibited angiogenesis in zebra fish and suppressed the growth of human lung cancer cell line. Biomed. Pharmacother. 2018;106:699–706. doi: 10.1016/j.biopha.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Radosevic D., Sencanski M., Perovic V., Veljkovic N., Prljic J., Veljkovic V., Mantlo E., Bukreyeva N., Paessler S., Glisic S. Virtual screen for repurposing of drugs for candidate influenza a M2 ion-channel inhibitors. Front. Cell. Infect. Microbiol. 2019;9:67. doi: 10.3389/fcimb.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang H., Huang Y., Tian Y., Liu M., Chen L., Li L., Liu S., Yang J. Multiple modes of action of myricetin in influenza A virus infection. Phytother. Res. 2021 doi: 10.1002/ptr.7025. [DOI] [PubMed] [Google Scholar]
- Shin I.S., Shin N.R., Jeon C.M., Kwon O.K., Hong J.M., Kim H.S., Oh S.R., Ahn K.S. Thuja orientalis reduces airway inflammation in ovalbumin-induced allergic asthma. Mol Med Rep. 2015;12(3):4640–4646. doi: 10.3892/mmr.2015.3910. [DOI] [PubMed] [Google Scholar]
- Silva I.S., Nicolau L.A.D., Sousa F.B.M., Araujo S., Oliveira A.P., Araujo T.S.L., Souza L.K.M., Martins C.S., Aquino P.E.A., Carvalho L.L., Silva R.O., Rolim-Neto P.J., Medeiros J.V.R. Evaluation of anti-inflammatory potential of aqueous extract and polysaccharide fraction of Thuja occidentalis Linn. in mice. Int. J. Biol. Macromol. 2017;105(Pt 1):1105–1116. doi: 10.1016/j.ijbiomac.2017.07.142. [DOI] [PubMed] [Google Scholar]
- Srivastava P., Kumar P., Singh D., Singh V. Biological properties of Thuja orientalis Linn. Adv Life Sci. 2012;2(2):17–20. [Google Scholar]
- Sun X., Zhang L., Cao Y., Li J., Atanasov A.G., Huang L. Anti-neuraminidase activity of chemical constituents of Balanophora involucrata. Biomed. Chromatogr. 2020;34(12):e4949. doi: 10.1002/bmc.4949. [DOI] [PubMed] [Google Scholar]
- Tang J., Zhou L., Yuan G., Liu Y., Shi X., Lu Y., Chen D. Therapeutic effects on H1N1-induced pneumonia in mice and intestinal bacteria biotransformation of four main flavonoids from Houttuynia cordata Thunb. J. Pharm. Biomed. Anal. 2023;233 doi: 10.1016/j.jpba.2023.115469. [DOI] [PubMed] [Google Scholar]
- Wilsky S., Sobotta K., Wiesener N., Pilas J., Althof N., Munder T., Wutzler P., Henke A. Inhibition of fatty acid synthase by amentoflavone reduces coxsackievirus B3 replication. Arch. Virol. 2012;157(2):259–269. doi: 10.1007/s00705-011-1164-z. [DOI] [PubMed] [Google Scholar]
- Won J.N., Lee S.Y., Song D.S., Poo H. Antiviral activity of the plant extracts from Thuja orientalis, Aster spathulifolius, and Pinus thunbergii against influenza virus A/PR/8/34. J. Microbiol. Biotechnol. 2013;23(1):125–130. doi: 10.4014/jmb.1210.10074. [DOI] [PubMed] [Google Scholar]
- Xiao S., Tian Z., Wang Y., Si L., Zhang L., Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018;38(3):951–976. doi: 10.1002/med.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.