Graphical Abstract
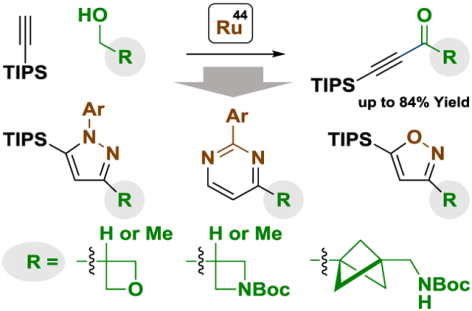
Three-fold scaffold diversification is achieved via ruthenium-catalyzed oxidative alkynylation of commercially available primary alcohols to form α,β-acetylenic ketones (ynones), which provide entry to oxetane-, azetidine- and bicyclopentane-bearing pyrazoles, isoxazoles and pyrimidines.
In connection with the development of ruthenium-catalyzed carbonyl additions via hydrogen transfer,1 our laboratory recently reported the first metal-catalyzed oxidative alkynylations of primary alcohols or aldehydes to form α,β-acetylenic ketones (ynones).2 These processes offer an alternative to multi-step ynone syntheses that rely upon acetylide-mediated aldehyde addition-oxidation or the conversion of carboxylic acids to Weinreb (or morpholine) amides followed by acetylide-mediated acyl substitution.3 To demonstrate how ruthenium-catalyzed oxidative alkynylation can streamline the synthesis of clinical candidates for small molecule drug discovery, this method was applied to the construction of N-heterocycles bearing oxetane,4 azetidine5 and bicyclopentane6 moieties. Such strained rings function as metabolically stable bioisosteres to diverse functional groups and have emerged as important substructures in pharmaceutical and agrochemical ingredients (Figure 1).7,8 Furthermore, as ynones are readily converted to pyrazoles, isoxazoles and pyrimidines via condensation with hydrazines,3,9 hydroxylamine3,10 and amidines,3,11 respectively, the aforementioned strained rings could potentially be appended to three distinct N-heterocycles. In this communication, we report that such three-fold scaffold diversification12 is indeed possible, as illustrated by the rapid assembly of oxetane-, azetidine- and bicyclopentane-bearing pyrazoles, isoxazoles and pyrimidines.
Figure 1.

Azetidine and bicyclopentane bioisosteres in drug discovery.
Optimal conditions identified for the ruthenium-catalyzed oxidative alkynylation, which utilize the catalyst assembled in situ from RuI(CO)3(η3-C3H5)13 and rac-BINAP, were applied to the coupling of triisopropylsilyl (TIPS) acetylene (500 mol%) with commercially available oxetane-, azetidine- and bicyclopentane-containing alcohols 2a-2e (100 mol%) (Scheme 1). Gratifyingly, good to excellent isolated yields of the corresponding ynones 3a-3e were formed. Remarkably, primary alcohols 2b, 2d and 2e each incorporate a quaternary carbon center adjacent to the site of C-C coupling, yet ynone formation remains efficient, although higher catalyst loadings are required. The direct formation of ynones 3a-3e from the corresponding alcohols 2a-2e in the absence of discrete redox reactions or premetalated acetylides is step-economic and minimizes waste generation.
Scheme 1. Ruthenium-catalyzed oxidative alkynylation to form oxetane-, azetidine- and bicyclopentane-bearing ynones 3a-3e.a.
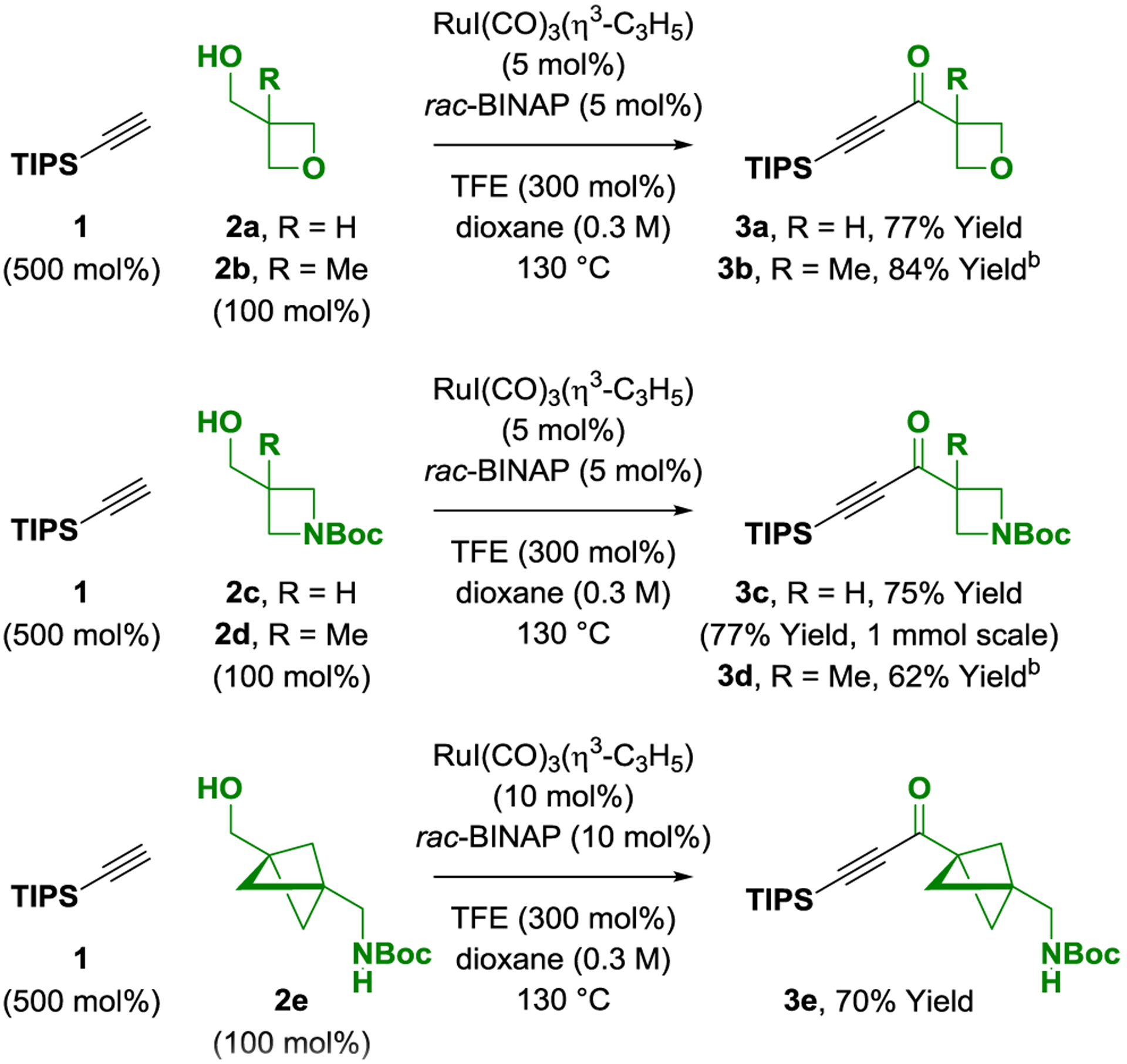
a Yields of material isolated by silica gel chromatography. bCatalyst (10 mol%). See Supporting Information for further details.
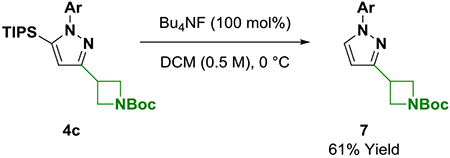
With ynones 3a-3e in hand, condensation to form the corresponding pyrazoles, isoxazoles and pyrimidines was explored (Scheme 2). Exposure of ynones 3a-3e to p-bromophenyl hydrazine in the presence of cuprous acetate (5 mol%) enabled efficient formation of the respective pyrazoles 4a-4e.9 The conversion of ynones 3a-3e to isoxazoles 5a-5e required a two-step protocol involving isolation of the (Z)-oximes followed by gold-catalyzed cycloisomerization.10,14,15 Finally, access to pyrimidines 6a-6e was achieved by condensation with p-bromophenyl amidine.11 The quaternary carbon center adjacent to the ketone functional group of ynones 3b, 3d and 3e did not impede condensation to form the pyrazoles (4b, 4d and 4e), isoxazoles (5b, 5d and 5e) or pyrimidines (6b, 6d and 6e), which is notable as α-quaternary heterobenzylic centers are challenging to prepare via metal-catalyzed cross-coupling. Whereas condensation to form pyrazoles 4a-4e and isoxazoles 5a-5e occurs with retention of the TIPS moiety, formation of pyrimidines 6a-6e is accompanied by desilylation. The carbon-silicon bond of pyrazoles (4b, 4d and 4e) and isoxazoles (5b, 5d and 5e) were resistant to functionalization under a variety of conditions; however, protodesilylation mediated by TBAF occurs efficiently, as illustrated by formation of the des-silyl compound 7 (eq. 1). Exposure of pyrazole 7 to N,N-dibromo-5,5-dimethylhydantoin delivered 4-bromopyrazole 8 in excellent yield as a single regioisomer (eq. 2).
Scheme 2. Conversion of ynones 3a-3e to oxetane-, azetidine- and bicyclopentane-bearing pyrazoles 4a-4e, isoxazoles 5a-5e and pyrimidines 6a-6e.a .
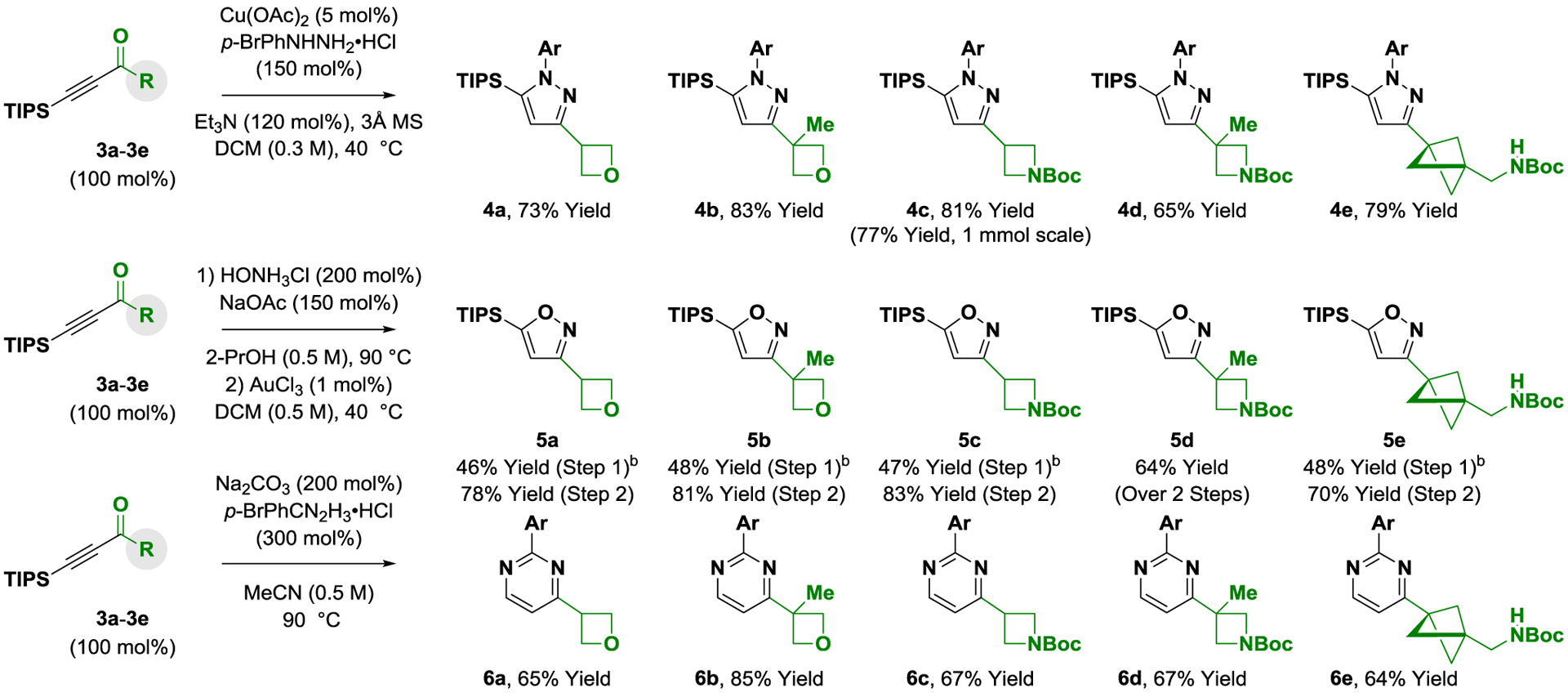
aYields of material isolated by silica gel chromatography. Ar = p-bromophenyl. bYield of chromatographically purified (Z)-oxime. See Supporting Information for further details.
 |
(eq. 1) |
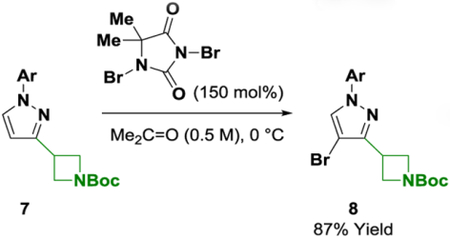 |
(eq. 2) |
A one-pot oxidative alkynylation-condensation would enable direct access to pyrazoles and pyrimidines bearing oxetane, azetidine and bicyclopentane moieties from alcohols 2a-2e (Scheme 3). Toward this end, azetidinol 2c was exposed to conditions for ruthenium-catalyzed oxidative alkynylation and the crude reaction mixture was treated p-bromophenyl hydrazine in the presence of substoichiometric cuprous acetate. The azetidine-containing pyrazole 4c was isolated in 53% yield over the two-step one-pot operation. Similarly, addition of p-bromophenyl amidine to the crude reaction mixture from the ruthenium-catalyzed oxidative alkynylation of azetidinol 2c leads to direct formation of pyrimidine 6c in 45% yield.
Scheme 3. One-pot oxidative alkynylation-condensation to form pyrazole 4c and pyrimidine 6c.a.
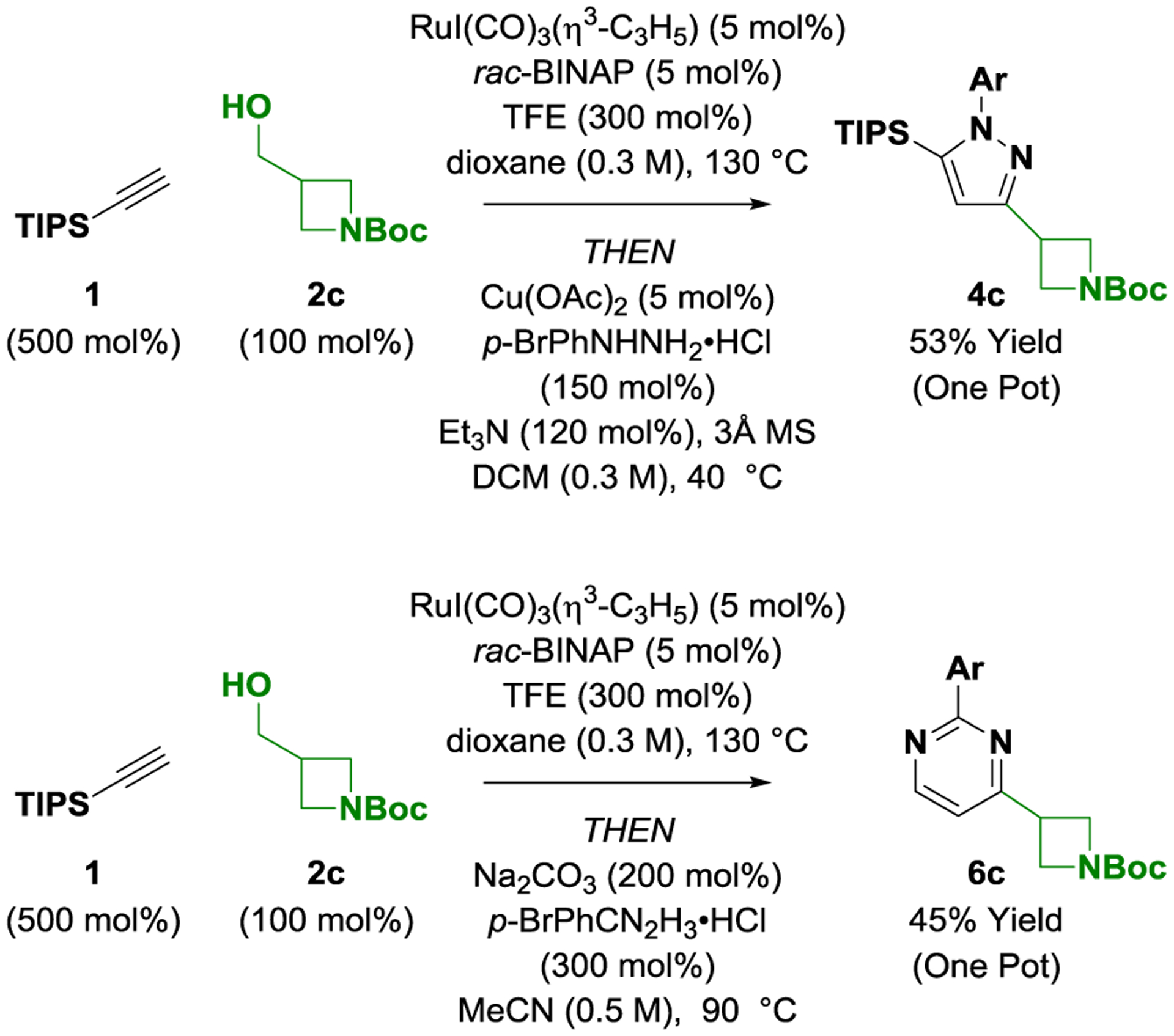
a Yields of material isolated by silica gel chromatography. Ar = p-bromophenyl. See Supporting Information for further details.
In summary, we report a concise routes to oxetane-, azetidine- and bicyclopentane-bearing pyrazoles, isoxazoles and pyrimidines via oxidative alkynylation of alcohols 2a-2e to form ynones 3a-3e. Whereas classical ynone formation typically requires multiple steps,3 oxidative alkynylation merges redox and C-C bond construction events, enabling ynone formation in a single manipulation. Furthermore, by telescoping oxidative alkynylation and condensation events, the targeted pyrazoles and pyrimidines are directly accessible from alcohols 2a-2e.
Supplementary Material
Acknowledgments.
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445) are acknowledged for partial support of this research.
Footnotes
Supporting Information Available.
Experimental procedures, spectroscopic and chromatographic data for all new compounds (1H NMR, 13C NMR, IR, HRMS). This material is available free of charge via the internet at http://pubs.acs.org.
Notes.
The authors declare no competing financial interest.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
■ REFERENCES
- (1).(a) Moran J; Krische MJ Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation. Pure Appl. Chem 2012, 84, 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Perez F; Oda S; Geary LM; Krische MJ Ruthenium Catalyzed Transfer Hydrogenation for C-C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs. Top. Curr. Chem 2016, 374, 365–387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Santana CG; Krische MJ From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present and Future. ACS Catal. 2021, 11, 5572–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ortiz E; Shen W; Shezaf JZ; Krische MJ Ruthenium-Catalyzed Dehydrogenation: Historical Perspective and Survey of Enantioselective for Conversion of Lower Alcohols to Higher Alcohols. Chem. Sci 2022, 13, 12625–12633. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Carbonyl Allylation and Crotylation: Historical Perspective, Relevance to Polyketide Synthesis and Evolution of Enantioselective Ruthenium-Catalyzed Hydrogen Auto-Transfer Processes,” Ortiz E; Saludares C; Wu J; Cho Y; Santana CG; Krische MJ Synthesis 2023, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ortiz E; Evarts MM; Strong ZH; Shezaf JZ; Krische MJ Ruthenium-Catalyzed C-C Coupling of Terminal Alkynes with Primary Alcohols or Aldehydes: α,β-Acetylenic Ketones (Ynones) via Oxidative Alkynylation. Angew. Chem. Int. Ed 2023, 62, e2023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For selected reviews on the synthesis and reactions of α,β-acetylenic ketones (ynones), see:; (a) Katkevich RI; Vereshchagin LI Advances in the Synthesis of α-Ethynylcarbonyl Compounds. Russ. Chem. Rev 1969, 38, 900-912. [Google Scholar]; (b) Fraile A; Parra A; Tortosa M; Aleman J Organocatalytic Transformations of Alkynals, Alkynones, Propiolates, and Related Electron-Deficient Alkynes. Tetrahedron 2014, 70, 9145-9173 [Google Scholar]; (c) Najera C; Sydnes LK; Yus M Conjugated Ynones in Organic Synthesis. Chem. Rev 2019, 119, 11110-11244. [DOI] [PubMed] [Google Scholar]; (d) Wang L; Zhu H; Peng T; Yang D Conjugated Ynones in Catalytic Enantioselective Reactions. Org. Biomol. Chem 2021, 19, 2110-2145. [DOI] [PubMed] [Google Scholar]; (e) Niedballa J; Mueller TJJ Heterocycles by Consecutive Multicomponent Syntheses via Catalytically Generated Alkynoyl Intermediates. Catalysts 2022, 12, 90. [Google Scholar]; (f) Worch JC; Stubbs CJ; Price MJ; Dove AP Click Nucleophilic Conjugate Additions to Activated Alkynes: Exploring Thiol-yne, Amino-yne, and Hydroxyl-yne Reactions from (Bio)Organic to Polymer Chemistry. Chem. Rev 2021, 121, 6744-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For selected reviews on the synthesis of oxetanes and their use in chemical synthesis, see:; (a) Mack DJ; Njardarson JT Recent Advances in The Metal-Catalyzed Ring Expansions of Three- and Four-Membered Rings. ACS Catal. 2013, 3, 272–286. [Google Scholar]; (b) Njardarson JT Catalytic Ring Expansion Adventures. Synlett 2013, 787–803. [Google Scholar]; (c) D’Auria M; Racioppi R Oxetane Synthesis through the Paternò-Büchi Reaction. Molecules 2013, 18, 11384–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang Z; Chen Z; Sun J Catalytic Asymmetric Nucleophilic Openings of 3-Substituted Oxetanes. Org. Biomol. Chem 2014, 12, 6028–6032. [DOI] [PubMed] [Google Scholar]; (e) Carreira EM; Fessard TC Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev 2014, 114, 8257–8322. [DOI] [PubMed] [Google Scholar]; (f) Malapit CA; Howell AR Recent Applications of Oxetanes in the Synthesis of Heterocyclic Compounds. J. Org. Chem 2015, 80, 8489–8495. [DOI] [PubMed] [Google Scholar]; (g) Davis OA; Bull JA Recent Advances in the Synthesis of 2-Substituted Oxetanes. Synlett 2015, 1283–1288. [Google Scholar]; (h) Also see, Rojas JJ; Croft RA; Sterling AJ; Briggs EL; Antermite D; Schmitt DC; Blagojevic L; Haycock P; White AJP; Duarte F; Choi C; Mousseau JJ; Bull JA Amino-Oxetanes as Amide Isosteres by an Alternative Defluorosulfonylative Coupling of Sulfonyl Fluorides. Nat. Chem 2022, 14, 160–169. [DOI] [PubMed] [Google Scholar]
- (5).For selected reviews on the synthesis of azetidines and their use in chemical synthesis, see:; (a) Couty F; Evano G Azetidines: new tools for the synthesis of nitrogen heterocycles. Synlett 2009, 3053–3064. [Google Scholar]; (b) Bott TM; West FG Preparation and Synthetic Applications of Azetidines. Heterocycles 2012, 84, 223–264. [Google Scholar]; (c) Mehra V; Lumb I; Anand A; Kumar V Recent Advances in Synthetic Facets of Immensely Reactive Azetidines. RSC Advances 2017, 7, 45763–45783. [Google Scholar]; (d) Richardson AD; Becker MR; Schindler CS Synthesis of Azetidines by aza Paterno-Buchi Reactions. Chem. Sci 2020, 11, 7553–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Milton JP; Fossey JS Azetidines and Their Applications in Asymmetric Catalysis. Tetrahedron 2021, 77, 131767. [Google Scholar]; (f) Mughal H; Szostak M Recent Advances in The Synthesis and Reactivity of Azetidines: Strain-Driven Character of The Four-Membered Heterocycle. Org. Biomol. Chem 2021, 19, 3274–3286. [DOI] [PubMed] [Google Scholar]
- (6).For selected reviews on the synthesis of bicyclopentanes and their use in chemical synthesis, see:; (a) Levin MD; Kaszynski P; Michl J Bicyclo[1.1.1]pentanes, [n]Staffanes, [1.1.1]Propellanes, and Tricyclo[2.1.0.02,5]pentanes. Chem. Rev 2000, 100, 169–234. [DOI] [PubMed] [Google Scholar]; (b) Kanazawa J; Masanobu U Recent Advances in the Synthetic Chemistry of Bicyclo[1.1.1]pentane. Synlett 2019, 30, 1–11. [Google Scholar]; (c) Anderson JM; Measom ND; Murphy JA; Poole DL Bridge Functionalisation of Bicyclo[1.1.1]pentane Derivatives. Angew. Chem. Int. Ed 2021, 60, 24754–24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).For selected reviews on oxetanes, azetidines and bicyclopentanes in medicinal chemistry and crop protection, see:; (a) Wuitschik G; Carreira EM; Wagner B; Fischer H; Parrilla I; Schuler F; Rogers-Evans M; Mμller K Oxetanes in Drug Discovery: Structural and Synthetic Insights. J. Med. Chem 2010, 53, 3227-3246. [DOI] [PubMed] [Google Scholar]; (b) Burkhard JA; Wuitschik G; Rogers-Evans M; Müller K; Carreira EM Oxetanes as Versatile Elements in Drug Discovery and Synthesis. Angew. Chem. Int. Ed 2010, 49, 9052–9067. [DOI] [PubMed] [Google Scholar]; (c) St. Jean DJ Jr; Fotsch C Mitigating Heterocycle Metabolism in Drug Discovery. J. Med. Chem 2012, 55, 6002–6020. [DOI] [PubMed] [Google Scholar]; (d) Bull JA; Croft RA; Davis OA; Doran R; Morgan KF Oxetanes: Recent Advances in Synthesis, Reactivity, and Medicinal Chemistry. Chem. Rev 2016, 116, 12150–12233. [DOI] [PubMed] [Google Scholar]; (e) Delost Michael D.; Smith David T.; Anderson Benton J.; Njardarson Jon T. Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem 2018, 61, 10996–11020. [DOI] [PubMed] [Google Scholar]; (f) Lamberth C Small Ring Chemistry in Crop Protection. Tetrahedron 2019, 75, 4365–4383. [Google Scholar]; (g) Bauer MR; Di Fruscia P; Lucas SCC; Michaelides IN; Nelson JE; Storer RI; Whitehurst BC Put a Ring on It: Application of Small Aliphatic Rings in Medicinal Chemistry. RSC Med. Chem 2021, 12, 448–471. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Subbaiah MAM; Meanwell NA Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem 2021, 64, 14046–14128. [DOI] [PubMed] [Google Scholar]
- (8).(a) Butler CR; Beck EM; Harris A; Huang Z; McAllister LA; Ende CWA; Fennell K; Foley TL; Fonseca K; Hawrylik SJ; Johnson SD; Knafels JD; Mente S; Noell GS; Pandit J; Phillips TB; Piro JR; Rogers BN; Samad TA; Wang J; Wan S; Brodney MA Azetidine and Piperidine Carbamates as Efficient, Covalent Inhibitors of Monoacylglycerol Lipase. J. Med. Chem 2018, 60, 9860–9873. [DOI] [PubMed] [Google Scholar]; (b) Stepan AF; Subramanyam C; Efremov IV; Dutra JK; O’Sullivan TJ; DiRico KJ; McDonald SW; Won A; Dorff PD; Nolan CE; Beck SL; Pustilnik LR; Riddell RD; Kauffman GW; Kormos BL; Zhang L; Lu Y; Capetta SH; Green ME; Karki K; Sibley E; Atchison KP; Hallgren AJ; Obroski CE; Robshaw AE; Sneed B; O’Donnell CJ Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem 2012, 55, 3414–3424. [DOI] [PubMed] [Google Scholar]
- (9).Specific conditions used for pyrazole formation were derived from the following reports:; (a) Hsieh M-T; Kuo S-C; Lin H-C Solvent- and Transition Metal Catalyst-Dependent Regioselectivity in the [3+2] Cyclocondensation of Trifluoromethyl-α,β-ynones with Hydrazines: Switchable Access to 3- and 5-Trifluoromethylpyrazoles. Adv. Synth. Catal 2015, 357, 683–689. [Google Scholar]; (b) Chalyk BA; Khutorianskyi A; Lysenko A; Fil Y; Kuchkovska YO; Gavrilenko KS; Bakanovych I; Moroz YS; Gorlova AO; Grygorenko OO Regioselective Synthesis of Functionalized 3- or 5-Fluoroalkyl Isisoxazoles and Pyrazoles from Fluoroalkyl Ynones and Binucleophiles. J. Org. Chem 2019, 84, 15212–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Specific conditions used for isoxazole formation were derived from the following reports:; (a) Sobenina LN; Tomilin DN; Gotsko MD; Ushakov IA; Mikhaleva AI; Tofimov BA From 4,5,6,7-Tetrahydroindoles to 3- or 5-(4,5,6,7-Tetrahydroindol-2-yl)isisoxazoles in Two Steps: A Regioselective Switch between 3- and 5-Isomers. Tetrahedron, 2014. ¸ 70, 5168–5174. [Google Scholar]; (b) Jeyaveeran JC; Praveen C; Arun Y; Prince AAM; Perumal PT Cycloisomerization of Acetylenic Oximes and Hydrazones under Gold Catalysis: Synthesis and Cytotoxic Evaluation of Isisoxazoles and Pyrazoles. J. Chem. Sci 2016, 128, 73–83. [Google Scholar]
- (11).Specific conditions used for pyrimidine formation were derived from the following report:; Karpov AS; Müller TJJ Straightforward Noel One-Pot Enaminone and Pyrimidine Syntheses by Coupling-Addition-Cyclocondensation Sequences. Synthesis 2003, 18, 2815–2826. [Google Scholar]
- (12).For selected reviews on scaffold diversification in small molecule drug discovery, see:; (a) Böhm H-J; Flohr A; Stahl M Scaffold Hopping. Drug Discovery Today: Technol. 2004, 1, 217–224. [DOI] [PubMed] [Google Scholar]; (b) Cramer RD; Jilek JR; Guessregen S; Clark J; Wendt B; Clark RD “Lead Hopping”. Validation of Topomer Similarity as a Superior Predictor of Similar Biological Activities. J. Med. Chem 2004, 47, 6777–6779. [DOI] [PubMed] [Google Scholar]; (c) Schneider G; Schneider P; Renner S Scaffold-Hopping: How Far Can You Jump? QSAR Comb Sci. 2006, 25, 1162–1171. [Google Scholar]; (d) Brown N; Jacoby E On Scaffolds and Hopping in Medicinal Chemistry. Mini-Rev. Med. Chem 2006, 6, 1217–1229. [DOI] [PubMed] [Google Scholar]; (e) Zhao H Scaffold Selection and Scaffold Hopping in Lead Generation: A Medicinal Chemistry Perspective. Drug Discovery Today, 2007, 12, 149–155. [DOI] [PubMed] [Google Scholar]; (f) Langdon RS; Ertl P; Brown N Bioisosteric Replacement and Scaffold Hopping in Lead Generation and Optimization. Mol. Inf 2010, 29, 366–385. [DOI] [PubMed] [Google Scholar]; (g) Classification of Scaffold-Hopping Approaches. Drug Discov. Today 2012, 17, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Trabocchi A; Lenci E Smart Design of Small-Molecule Libraries: When Organic Synthesis Meets Cheminformatics. ChemBioChem. 2019, 20, 1115–1123. [DOI] [PubMed] [Google Scholar]; (i) Lazzara RP; Moore WT Scaffold-Hopping as a Strategy to Address Metabolic Liabilities of Aromatic Compounds. RSC Med. Chem 2020, 11, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Sbrana G; Braca G; Piacenti F; Pino P Synthesis of π-Allylruthenium Tricarbonyl Halides. J. Organomet. Chem 1968, 13, 240–242. [Google Scholar]
- (14).Unlike hydrazones, oxime (E/Z)-isomers are configurationally stable:; Bohle SD; Chua Z; Perepichka I; Rosadiuk K E/Z Oxime Isomerism in PhC(NOH)CN. Chem. Eur. J 2013, 19, 4223–4229. [DOI] [PubMed] [Google Scholar]
- (15).For the seminal review on metal-catalyzed cycloisomerization, see:; Trost B; Krische MJ Transition Metal Catalyzed Cycloisomerizations. Synlett 1998, 1–16.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


