Abstract
Young adult trans women living with HIV in the US exhibit suboptimal advancement through the HIV Care Continuum relative to national averages. From December 2016 through May 2018, 134 young adult trans women living with HIV enrolled in Text Me, Girl!, a theory-based, trans-specific text-messaging intervention designed to improve HIV Care Continuum outcomes. Participants (N=130) averaged 29.5 years, were predominantly Latinx (43%) or African American/Black (40%). Clustered logistic and ordinal logistic multivariable models (n=105; 366 observations) indicate that through 18-month follow-up, increased exposure to the text-messaging intervention was associated with significant (p<0.05) increased retention to HIV care (adjusted odds ratio [aOR]=1.33) and biomarker-confirmed viral suppression (aOR=1.51); retention in the intervention was associated with significantly increased likelihood of ART uptake (aOR=2.95) and “excellent” ART adherence (aOR=10.44). Text Me, Girl! offers promising evidence that a unidirectional, automated text-messaging intervention can improve HIV care outcomes among young adult trans women living with HIV.
Keywords: HIV/AIDS, transgender women, text messaging, SMS, HIV Care Continuum
Introduction
Trans women are one of the groups most heavily impacted by HIV in the United States (1). Although precise measurements are impossible due to limitations in data collection and reporting standards related to transmission categories (i.e., CDC HIV Surveillance Report still captures trans women either under the MSM risk category or “other”), it is estimated that HIV prevalence among trans women in the United States is approximately 14% (1), which is over 45 times greater than what has been observed in the general adult population (2). However, intervention and epidemiological studies have demonstrated far higher HIV prevalence rates ranging from 18% - 37% (3–5). In addition to exceedingly high prevalence rates, it is also believed that rates of undiagnosed HIV infection are high among trans women (3, 6). Although exact estimates are impossible to calculate, applying the rates listed above to trans population estimates suggests that there could be as many as 1,608 trans women living with HIV in the US who are unaware of their HIV status (7). Further, evidence demonstrates that trans women living with HIV exhibit suboptimal advancement through the HIV Care Continuum relative to other adult groups in the US (8).
Although there is scant research specifically focused on young adult trans women (9), evidence suggests that new HIV infection may be particularly common among trans women aged 20–29 years (10), and studies of young (i.e., 15–24 years) trans women have evidenced HIV positivity rates ranging from 19–22% (11, 12). Additionally, existing evidence reveals even further elevated rates of homelessness, substance use, and engagement in HIV transmission risk behaviors (e.g., unprotected anal intercourse, sex work) (13, 14) among young adult trans women relative to their older trans women counterparts and/or cisgender HIV-positive youth/young adults. Studies also revealed between 27–59% of young trans women report recent engagement in condomless insertive or receptive anal intercourse (3, 15); yet, to date, few HIV prevention and/or treatment interventions have been developed, or shown to be efficacious, for young adult trans women (16).
The same health disparities that place trans women at increased risk of exposure to HIV, including poverty and unstable housing (17), rejection from family and peer networks(18), substance use (19), untreated mental health symptoms (20), engagement in sex work (21), and exposure to transphobic violence and discrimination (22), also stand as obstacles to advancement through the HIV Care Continuum to full viral suppression (8, 23–25). Additionally, evidence demonstrates that many trans women hold fears, suspicions, and mistrust towards institutionalized medicine and traditional healthcare providers (26, 27), likely due to anticipated and/or enacted stigma and discrimination (28, 29), particularly among trans women of color (30). Such obstacles may prompt trans women to fall out of HIV care (31) or avoid healthcare altogether (32), increasing health risks for both themselves and their sexual and needle-sharing partners. Furthermore, once out of care, re-establishing contact and re-linking to care can be a difficult and time-consuming challenge (23), and one that many healthcare providers are unable or unwilling to undertake. As a group, young adult trans women not only demonstrate extremely high and, perhaps age-related, risk of HIV infection (1, 33, 34), they also consistently demonstrate poor rates of advancement through the HIV Care Continuum after infection (25, 35–38).
Technology-facilitated interventions can respond to and work with individuals in real time, and are often low-cost, scalable, and can easily be culturally tailored for specific populations. Several prior studies have evaluated the efficacy of remotely accessed, technology-facilitated interventions (39–45)(39–45), including text-messaging (46, 47) to promote advancement through the HIV Care Continuum among trans women. Specifically, a review of the literature on technology-facilitated interventions addressing the HIV Care Continuum found that about 75% of the reviewed studies showed proven or preliminary efficacy for improving HIV Care Continuum outcomes with 44.4% of all reviewed studies having utilized a mobile texting intervention. However, only six of the 45 reviewed studies included trans participants in their samples, and only five of the studies targeted youth and young adults, illuminating a significant gap in technology-facilitated studies with this population (48).
Due to their mobility, ubiquity, and perceived privacy, technology-facilitated interventions may be particularly capable of overcoming and responding to the unique health disparities facing trans women (45, 48–51). A review of 24 technology-facilitated interventions and platforms designed to improve health outcomes among trans and gender-expansive individuals, primarily adolescents and young adults, found that only nine include HIV care and treatment outcomes, and only five of the interventions that were focused on HIV outcomes included a theoretical framework. Furthermore, only four were conducted in the context of a research study to evaluate feasibility, acceptability, and efficacy. Rather, most focused on other critically important aspects of trans health: psychological well-being, self-affirmation, increased self-esteem and self-efficacy, and physical safety (52).
Given the widespread adoption of cellular phone technology in the United States across every demographic category (53), text-messaging interventions continue to be an optimal platform to provide health interventions to trans women, particularly young adult trans women. Text-messaging interventions do not require an Internet/data connection, apps to download or buy, new interfaces or jargon to learn, or new websites to visit, and fold cleanly into existing communication habits. Furthermore, text messages meet the user wherever they are at, and do not require significant interruptions or new routines in the daily flow of one’s life. Evidence suggests that the uncertain life circumstances facing many trans women, i.e., housing insecurity, un/under employment (17, 19), make it difficult to reliably access computers or other Internet terminals (54, 55), potentially limiting the efficacy of video or graphics-heavy interventions (e.g., smartphone apps and web apps) requiring higher memory requirements or processing speeds. Additionally, the cost of maintaining full Internet connectivity on mobile devices can be prohibitive for many trans women, given their reduced earning potential (56), reducing the potential efficacy of apps that require downloads and updates or HTML-based website interventions designed for smartphone access. As such, media that are both portable and able to be accessed on less expensive devices (i.e., text-based, rather than image-based), may be superior technology-facilitated intervention mediums for young adult trans women. In addition to such promising findings, a systematic review has noted the need for increased application of theory-based content in technology-facilitated interventions for youth at high risk for HIV transmission (48). In the “End the HIV Epidemic” (57) era, the “Treat Pillar” goal calls for significant and lasting improvements in HIV care retention and viral suppression for reducing HIV transmission among trans women and their partners. Thus, given that text messaging is accessible, culturally responsive, private, portable, inexpensive, and already used daily by young trans women (58), theory-based text messaging is a particularly appropriate social media platform for accessing young adult trans women to address the End the HIV Epidemic Treat Pillar and advance through the HIV Care Continuum.
Text Me, Girl! was an open-label randomized controlled trial that evaluated the outcomes of a theory-based, trans-specific text-messaging intervention to promote advancement along the HIV Care Continuum among highly impacted young adult trans women, i.e., young adult trans women experiencing multiple syndemic health disparities. This project was guided by one main research question: would the unidirectional, automated delivery of 270 (three daily messages delivered over 90 days) culturally responsive, theory-based text messages be powerful enough to improve HIV health outcomes among such a highly impacted group?
Methods
Participants
From December 2016 through May 2018, 134 trans women were screened, provided informed consent, and were enrolled in Text Me, Girl!. Inclusion criteria for participation were: 1) identified as a trans woman; 2) assigned a biological sex of male at birth; 3) between the ages of 18 and 34 years; 4) confirmed HIV-positive serostatus; 5) tested HIV positive for the first time within the last 12 months, or had not had a HIV care visit in the previous 6 months, or had a viral load of ≥ 200 copies/ml on her last lab test result, or not currently prescribed antiretroviral therapy (ART) medication, or was currently prescribed ART medication but did not rate her ability to take all her medications as “excellent;” and, 6) ability to receive daily text messages on either a personal cell phone or via an email account. Individuals were excluded if they did not meet all eligibility criteria. Potential participants who were unable to provide documentation of their HIV-positive serostatus (e.g., medication prescription, laboratory results) were tested onsite for verification of a positive HIV status. All other eligibility criteria were self-report as biomarkers were not required at screening. Four participants were withdrawn after enrollment due to the discovery that they were outside the 18–34 age range specified in the eligibility criteria (i.e., false screening information was provided), leaving a final sample size of N = 130.
Procedures
Potential participants responded to a community-wide recruitment effort designed to reach a diversity of young adult trans women living with HIV; young adult trans women from different backgrounds and varying life experiences. Six recruitment strategies were employed: 1) online banner ads and digital flyers placed through geo-mapping on websites and social media sites that target trans women; 2) ads placed in print media and via email blasts for trans women or that trans women read; 3) street- and venue-based outreach by two research assistants (RAs) utilizing a modified (i.e., the RAs conducted outreach at the same locations but did not return to each location on the same day and at the same time) semi-structured time-space sampling methodology (59, 60) at locations where young trans women congregate such as boutiques, parks, street corners, bars, clubs, hotels, nail and hair shops, cruising boulevards, hotels; 4) project posters placed at collaborating community-based organizations containing details about how to contact a RA for further information regarding the project; 5) in-services at collaborating community-based organizations and other programs on site; and, 6) long-chain participant referrals. Of the aforementioned recruitment strategies, street- and venue-based outreach was the most successful resulting in 927/1,092 inquiries.
Following screening for eligibility and the informed consent process, potential participants were administered a baseline assessment that took approximately 90 minutes. RAs administered screeners using computer tablets and assessments via Audio Computer-Assisted Self-Interview (ACASI). QDS software was used to implement the ACASI. After completing the baseline assessment, participants were assigned to a study arm through an “urn randomization” procedure (61, 62). The urn randomization procedure provided balance across age (18–24 / 25–34 years old), race/ethnicity (Latinx/Hispanic / all other races/ethnicities), and HIV Care Continuum status (linked / not linked to HIV care). Participants were randomized into one of two arms: Arm A: Immediate Text Message Intervention Delivery (ID: n = 61); or, Arm B: Delayed Text Message Intervention Delivery (DD: n = 69) whereby participants were delivered the text-messaging intervention after a 90-day delay period. Both groups received the same 90-day text-messaging intervention. Potential participants were enrolled in the study following randomization. The randomized two-arm repeated measures design assessed participants at 3-, 6-, 12-, and 18-months post-randomization.
Participants were compensated with a $50 gift card for completion of all baseline procedures, a $50 gift card for completing the 3-month follow-up assessment plus a $20 gift card bonus for completing the 3-month follow-up assessment within +/−5 days of their exact 3-month follow-up date, a $50 gift card each for completing the 6- and 12-month follow-up assessments, and a $100 gift card for completing the 18-month follow-up assessment. Additionally, participants who referred a potential participant to Text Me, Girl! received a small gift (e.g., make-up, earrings; valued at approximately $2) when the potential participant screened, and a $20 gift card if the potential participant was eligible and enrolled, for a maximum of three eligible and enrolled participants per active participant. The total amount a participant could earn for enrolling and participating in the study was $380.
At the completion of the enrollment visit, an RA oriented the participant on how to maintain confidentiality and privacy on mobile devices with respect to the intervention. Participants were shown how to lock their phone, establish and use a pin code to password protect their phone or email account, and were instructed to periodically delete the intervention text messages. Additionally, at the conclusion of the enrollment visit and before each participant left the site, each participant received a welcome message. This initial welcome message was scripted to verify that the participant was properly registered into the text-messaging platform and read, “Welcome to Text Me, Girl!” Finally, participants were asked to notify a RA immediately if they lost their cell phone or changed their cell phone number; approximately 10% of the participants reported that they either lost their cell phone or changed their cell phone number. All study procedures were approved by the Western Institutional Review Board.
The Text Me, Girl! Intervention and Text-message Library
Over the course of 90 days participants received 270 scripted, theory-based, trans-specific text messages that were targeted, tailored, and personalized specifically for young adult trans women living with HIV. Targeted messages are those that are scripted for a specific target population (e.g., young adult trans women living with HIV). Tailored refers to culturally responsive content (e.g., verbiage, language, slang). Personalized refers to the intervention accessibility (e.g., participants’ ability to customize their delivery timeframe and/or their delivery platform). Those randomized to the ID arm began receiving the Text Me, Girl! intervention the day following enrollment. Those randomized to the DD arm began receiving the Text Me, Girl! intervention on the 91st day after enrollment.
Based on optimized outcome findings from previous text-messaging interventions conducted by our research team, each text message was based on one of three theoretical foundations: Social Support Theory, Social Cognitive Theory, and Health Belief Model (63–65). Social Support Theory, which encompasses instrumental, emotional, and informational support, has been shown to mediate the relationship between stressful events and health outcomes (66, 67). Social Cognitive Theory leverages interactive causal relationships among personal determinants, behavior, and environmental influences to increase individuals’ self-efficacy and self-regulation skills (68, 69). The Health Belief Model asserts that individuals’ beliefs regarding threats to their health and their beliefs that specific health behaviors can reduce these threats predict their likelihood of engaging in protective health behaviors (70). Although these three theoretical constructs are unique, when applied in concert they offer a complementary theoretical design and serve as the mechanism of behavioral change.
Furthermore, each text message corresponded to a placement along the HIV Care Continuum: HIV Positivity/Physical and Emotional Health, Linkage/Retention in HIV Care, and ART Adherence/Viral Load Suppression. To maintain interest and enthusiasm in the project, each text message was unique; participants did not receive the same scripted text message twice. As illustrated in Table 1, the Text Me, Girl! text message library was evenly distributed across the three theoretical foundations and the HIV Care Continuum; text messages were uniformly dispersed through unidirectional automation administration. Thus, each day a participant received one HIV Positivity/Physical and Emotional Health message, one Linkage/Retention in HIV Care message, and one ART Adherence/Viral Load Suppression message; and, each of these three daily HIV Care Continuum messages was based on a theoretical foundation, either Social Support Theory, or Social Cognitive Theory, or Health Belief Model. Table 2 provides examples of theory-based, trans-specific text messages along the HIV Care Continuum from the Text Me, Girl! library and illustrates how each HIV Care Continuum message has a theoretical foundation. The intervention dosing (i.e., three messages per day) and the timeframe (i.e., 10 hours) were determined as optimal based on prior text-messaging interventions designed and implemented by our research team (64, 65). Participants received a text message every five hours (e.g., at 12:00 PM, at 5:00 PM, and at 10:00 PM) and could choose to have the text-messaging intervention delivered through their cell phone or email inbox. The Text Me, Girl! library can be accessed by contacting the first author or via the website (www.friendscommunitycenter.org).
Table 1:
Text Me, Girl! Text Message Intervention by HIV Care Continuum by Theoretical Foundation Content
| HIV Care Continuum | |||||
|---|---|---|---|---|---|
| HIV Positivity/Physical and Emotional Health | Linkage/Retention in HIV Care | ART Medication Adherence/Viral Load Suppression | Total: | ||
| Theoretical Foundation | Social Support Theory | 30 | 30 | 30 | 90 |
| Social Cognitive Theory | 30 | 30 | 30 | 90 | |
| Health Belief Model | 30 | 30 | 30 | 90 | |
| Total: | 90 | 90 | 90 | 270 | |
Table 2:
Sample Text Messages by HIV Care Continuum and Theoretical Foundation
| HIV Positivity/Physical and Emotional Health | Linkage/Retention in HIV Care | ART Medication Adherence/Viral Load Suppression | ||
|---|---|---|---|---|
| Theoretical Foundation | Social Support Theory | Trans women, living positive, loving life. | When you stay in HIV care you can expose your heart, not your partner. | HIV meds work, your trans beautiful body is worth protecting. |
| Social Cognitive Theory | Make no compromise. You can protect yourself, girl. | Stay on top of your numbers with your doctor’s help, now that’s Trans Pride. | You can take care of yourself and your trans community, take your meds. | |
| Health Belief Model | Be smart and sexy. | Don’t be a statistic, see your doctor. We need every trans woman we have. | HIV meds can keep your trans body strong and healthy. | |
Post-intervention Opt-in/Opt-out Retention and Engagement Text Messages
Following the 90-day theory-based, trans-specific Text Me, Girl! intervention, participants in both ID and DD arms were offered an opportunity to opt-in to receive additional weekly text messages at a reduced schedule through their final distal follow-up assessment. Post-intervention retention/engagement messages consisted of two topic areas: 1) linkage/retention support; and, 2) ART adherence reminders. Participant could opt-in to either one or both of the topic areas. Each of the two topic areas were transmitted once a week for a maximum of two weekly messages. Post-intervention retention/engagement messages were derived directly from the text messages already available through the HRSA-funded UCARE4LIFE library. At each follow-up visit, participants were asked if they wanted to opt-in/opt-out to receive these additional retention/engagement text messages and were informed that they could stop the additional messages at any time by texting back “stop” to the system.
Text-messaging Platform
QualtricsXM LLC (www.qualtrics.com) programmed the text-messaging software system and hosted the system on their HIPAA-secure server. The research team provided Qualtrics staff with 334 scripted text messages (1 welcome message, 270 Text Me, Girl! library messages, and 63 scripted messages from the HRSA-funded UCARE4LIFE library) and the delivery schedule for the Text Me, Girl! library. Enrolled participants were added to the Qualtrics panel database with the delivery method (SMS or email), phone number or email address, and the delivery start time (ID or DD). Cost for programming a text-messaging library varies by the development company.
Measures
Social Media Initiative Multisite Assessment. The Social Media Initiative Multisite Assessment gathered information on participant sociodemographics and risks (e.g., age, racial/ethnic identity, educational attainment, income, current homeless status, and self-reported engagement in sex work in the past six months), at baseline and 6-, 12-, and 18-month follow-ups (except age, racial/ethnic identity, and educational attainment, which were only assessed at baseline). Ten, four, two, and four participants declined to report their current income at baseline and at 6-, 12-, and 18-month follow-ups, respectively; nine, fourteen, and six participants declined to report on their sex work behaviors at baseline and at 6-, and 18-month follow-ups, respectively; data was not imputed and these participants were excluded from those specific time points in both the tables and multivariable analyses.
The Social Media Initiative Multisite Assessment also assessed each participants’ HIV Care Continuum placement at each time point including attendance to at least one HIV care appointment in the past six months (self-reported by all participants; yes/no), being currently prescribed ART (self-reported by all participants; yes/no), ART adherence in the past month (self-reported by all participants currently on ART; six category ordinal from “very poor” to “excellent”), and being currently virally suppressed (self-reported by all participants; yes/no). One participant declined to answer any HIV Care Continuum questions at both 6- and 18-month follow-ups, and was thus excluded from these specific time points on both longitudinal tables and in multivariable analyses. Participants were also asked how many of the theory-based text messages they read during the intervention period, and were provided the following response categories: “None/Did Not Receive;” “Less than Half;” “Half;” “A Lot;” “All.” Twenty-five participants did not respond to the intervention exposure question due to missing the follow-up time point that assessed it. Missing intervention exposure data were not imputed, and thus these twenty-five participants were not included in the multivariable analyses, decreasing the final analytical sample to n = 105 (366 observations over time).
Electronic Health Records. Release of information forms requesting access to Electronic Health Records were requested for all participants; 18 participants refused access to their Electronic Health Records (14%), 47 participants (36%) signed release of information forms granting access but records were never successfully obtained from the healthcare provider, and 65 participants (50%) signed release of information forms granting access and medical records were obtained. Electronic Health Record data was queried in six-month waves from March 2017 through August 2019, and included information on ambulatory care visits, as well as viral load and CD4 test results. For the lone biomarker-confirmed outcome variable in the current study (i.e., most recent viral load), participants were marked “1” at a given time point if 1) a viral load test occurred during the data collection wave associated with that time point; 2) lab results were successfully acquired from the clinic/health care provider; and, 3) lab results indicated viral suppression; participants were coded “0” otherwise.
Method of Message Delivery and Optional Post-Intervention Retention and Engagement Messages. Two additional variables were created from study records. First, a variable was created to denote how each participant chose to receive their theory-based messages (0 = SMS; 1 = emails). Second, a variable was created to denote whether a participant opted-in to receive the post-intervention retention and engagement messages (0 = did not opt-in; 1 = opted-in).
Statistical Analysis
With the exception of Electronic Health Records and unless otherwise indicated, all data were self-reported. Descriptive statistics are provided for all variables; counts and their corresponding percentages are reported for categorical variables, and means/standard deviations are reported for numerical variables. Bivariate associations between participant outcomes and study time point were derived using Pearson’s Chi-square tests (denoted as χ2 in all tables, with subscripted text to denote the degrees of freedom), while contrasts across study arms used both Chi-square tests and one-way ANOVAs (denoted as F in all tables, with subscripted text to denote degrees of freedom). Multivariable associations were derived using clustered variations of the generalized linear model to account for the intraclass correlation introduced through repeated measurements of the same individual over time (i.e., 366 observations nested in 105 participants). Specifically, models reported here were robustly estimated logistic and ordinal logistic regressions of participants’ HIV Care Continuum outcomes (i.e., retention in HIV care, ART uptake, ART adherence, viral suppression) on self-reported intervention exposure, study time point, random study arm assignment, and opting-in to receive the post-intervention retention and engagement messages. Sociodemographics, risk variables, and method of message delivery were applied as controls in all multivariable models. Tests of significance were two-tailed wherever possible, and are flagged for discussion beginning at α ≤ 0.05.
Results
Figure 1 illustrates the progress and retention from initial screening through 18-month follow-up assessments. A total of 229 potential participants screened, 146 screened eligible, and 134 participants enrolled in Text Me, Girl!, of which, 61 were randomized into the ID arm and 69 were randomized into the DD arm (N = 130; final sample size). Total participant follow-up rates were 74% at the 3-month follow-up assessment, 92% at 6-months, 92% at 12-months, and 93% at 18-months.
Figure 1:
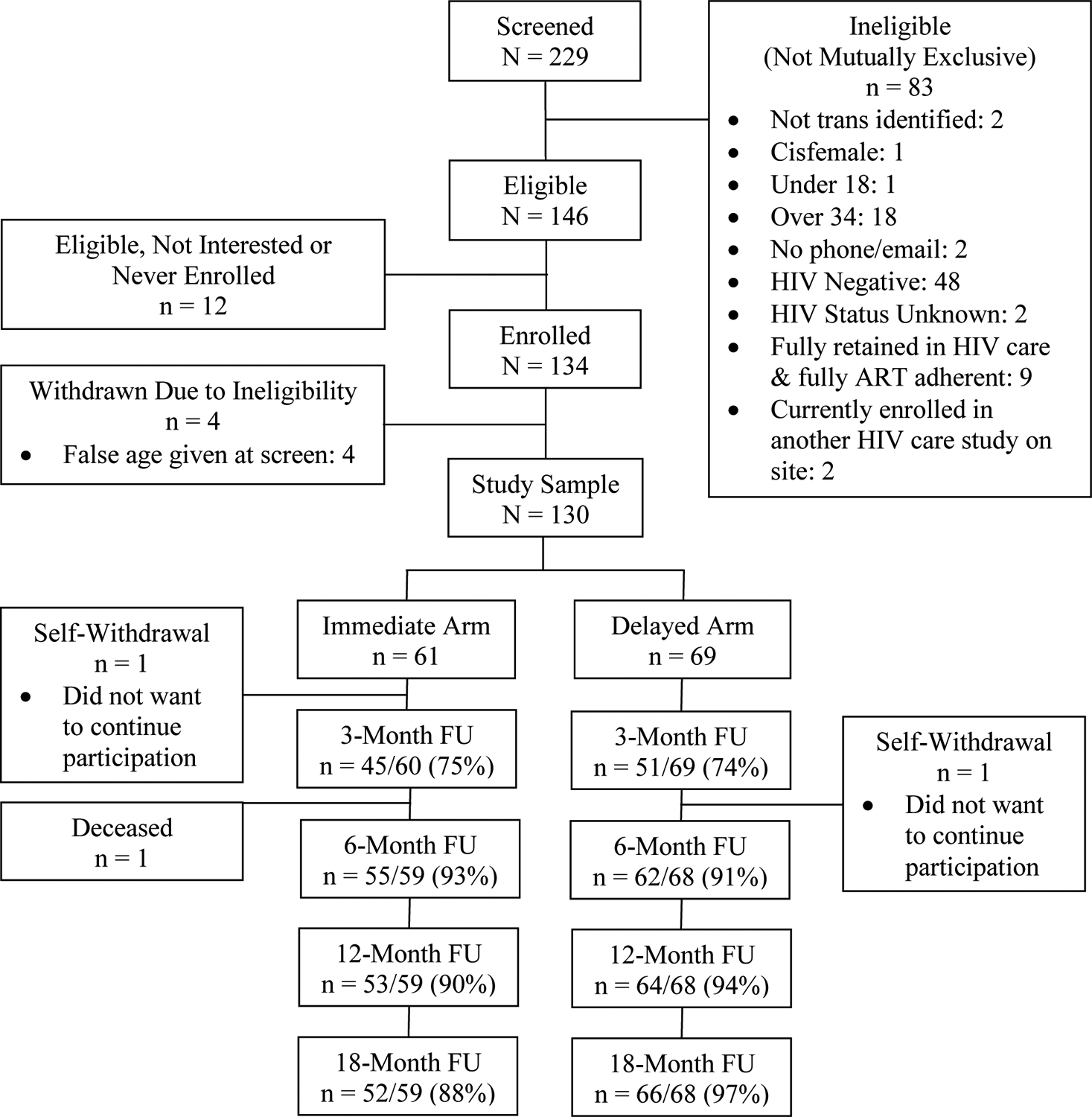
CONSORT Diagram of Study Progression and Retention
Table 3 provides participants’ sociodemographic characteristics by study arm. Participants averaged 29 and-a-half years of age (range = 19 thru 34), most self-identified as Latinx (43%) or African American/Black (40%). At baseline, 41% had educational attainment of less than a high school diploma, the average monthly income for all participants was $783, and 44% reported current housing instability. In the six months prior to baseline, 22% reported engagement in sex work. Participants randomized into the Delayed Delivery arm reported significantly higher average incomes than participants in the Immediate Delivery arm ($783 vs. $522; p < 0.05), otherwise no significant differences were observed across arms.
Table 3:
Participant Sociodemographics and Health Disparities by Study Arm
| Immediate Delivery (n = 61) | Delayed Delivery (n = 69) | Total (N = 130) | Test for Differences Across Arms | |||||
|---|---|---|---|---|---|---|---|---|
| Age | Mean or N | SD or % | Mean or N | SD or % | Mean or N | SD or % | ||
| [range = 19 thru 34 years; Md = 31] | 29.5 | 4.0 | 29.5 | 3.7 | 29.5 | 3.8 | F(1,128) = 0.00; ns | |
| 18–24 years | 9 | 14.8% | 7 | 10.1% | 16 | 12.3% | χ2(2) = 2.40; ns | |
| 25–29 years | 14 | 23.0% | 24 | 34.8% | 38 | 29.2% | ||
| 30–35 years | 38 | 62.3% | 38 | 55.1% | 76 | 58.5% | ||
| Racial/Ethnic Identity | ||||||||
| Latinx | 23 | 37.7% | 33 | 47.8% | 56 | 43.1% | χ2(2) = 1.48; ns | |
| African American/Black | 26 | 42.6% | 26 | 37.7% | 52 | 40.0% | ||
| Non-Black/Non-Latinx | 12 | 19.7% | 10 | 14.5% | 22 | 16.9% | ||
| Educational Attainment | ||||||||
| Less than HS | 27 | 44.3% | 26 | 37.7% | 53 | 40.8% | χ2(2) = 1.33; ns | |
| HS Grad/GED | 18 | 29.5% | 27 | 39.1% | 45 | 34.6% | ||
| More than HS | 16 | 26.2% | 16 | 23.2% | 32 | 24.6% | ||
| Monthly Income (n = 57; n = 63; n = 120) | ||||||||
| [range = $0 thru $9,000; Md = $495] | $519.68 | $521.73 | $1,020.67 | $1,688.17 | $782.70 | $1,294.62 | F(1,128) = 4.62; p = 0.034 | |
| Housing Status | ||||||||
| Stably Housed | 33 | 54.1% | 40 | 58.0% | 73 | 56.1% | χ2(1) = 0.20; ns | |
| Unstably Housed | 28 | 45.9% | 29 | 42.0% | 57 | 43.9% | ||
| Sex Work (past 6 months; n = 121) | ||||||||
| No | 50 | 82.0% | 52 | 75.4% | 102 | 78.5% | χ2(1) = 0.84; ns | |
| Yes | 11 | 18.0% | 17 | 24.6% | 28 | 21.5% | ||
N & n: Sample size; SD: Standard Deviation; Md: Median; ns: not significant at p < 0.05; p: probability
Table 4 provides participants’ observed HIV Care Continuum outcomes (i.e., linkage to care, retention in care, ART uptake, viral suppression) at each study time point for each arm. Participants in both arms significantly increased ART uptake and “excellent” ART adherence over time, but no other longitudinal improvements were observed. Two-sample z-tests of proportions revealed no differences between study arms at any time point, indicating study arms did not significantly diverge over time.
Table 4:
Participant HIV Care Continuum Outcomes by Study Arm and Time Point
| Baseline (N = 130) | 6-Month Follow-Up (n = 116) | 12-Month Follow-Up (n = 117) | 18-Month Follow-Up (n = 117) | Test for Change Over Time w/in Arms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immediate Delivery (n = 61) | Delayed Delivery (n = 69) | Immediate Delivery (n = 55) | Delayed Delivery (n = 61) | Immediate Delivery (n = 53) | Delayed Delivery (n = 64) | Immediate Delivery (n = 53) | Delayed Delivery (n = 64) | Immediate Delivery | Delayed Delivery | |
| HIV Care Visit in Past 6 Months | 37 (60.7%) | 44 (63.8%) | 39 (70.9%) | 40 (65.6%) | 32 (60.4%) | 40 (62.5%) | 30 (56.6%) | 40 (62.5%) | ns | ns |
| Currently on ART | 34 (55.7%) | 29 (42.0%) | 38 (69.1%) | 40 (65.6%) | 39 (73.6)% | 47 (73.4%) | 42 (79.3%) | 49 (76.6%) | χ2(3) = 8.13; p = 0.043 | χ2(3) = 21.37; p < 0.001 |
| ART Adherence (Participants on ART only) | (n = 34) | (n = 29) | (n = 38) | (n = 40) | (n = 39) | (n = 47) | (n = 42) | (n = 49) | ||
| Excellent | 1 (2.9%) | 2 (6.9%) | 14 (36.8%) | 12 (30.0%) | 11 (28.2%) | 11 (23.4%) | 18 (42.9%) | 22 (44.9%) | χ2(3) = 16.35; p < 0.001 | χ2(3) = 13.76; p = 0.003 |
| Viral Suppression | ||||||||||
| Self-Reported | 23 (37.7%) | 22 (31.9%) | 32 (58.2%) | 26 (42.6%) | 30 (56.6%) | 27 (42.2%) | 28 (52.8%) | 32 (49.2%) | χ2(3) = 6.19; ns | χ2(3) = 4.27; ns |
| Biomarker-Confirmed | 24 (39.3%) | 27 (39.1%) | 21 (38.2%) | 26 (42.6%) | 23 (43.4%) | 25 (39.1%) | 23 (43.4%) | 26 (40.0%) | χ2(3) = 0.50; ns | χ2(3) = 0.22; ns |
HIV: Human Immunodeficiency Virus: ART: Antiretroviral Therapy; N & n: Sample Size; ns: not significant at p < 0.05; p: probability; χ2: Chi-Square
Table 5 regresses these same HIV Care Continuum outcomes onto participants’ self-reported level of intervention exposure, study time point, random study arm assignment, receipt of the post-intervention retention and engagement messages, the sociodemographic/health disparity variables from Table 3, and method of message delivery (i.e., SMS or email). Multivariable results indicate that random arm assignment (i.e., delayed intervention administration) was unassociated with all outcomes, including attendance to HIV care visits, ART uptake and adherence, and both self-reported and biomarker-confirmed viral suppression.
Table 5:
Clustered Robust Multivariable Regressions of Participant HIV Care Continuum Outcomes on Intervention Exposure, Time Point, Random Study Arm Assignment, and Receipt of Post-Intervention Messages (366 observations nested in 105 participants unless otherwise noted)
| Outcome | Past 6m: Attend | Currently | ART Adherence | Viral Supp. | Viral Supp. |
|---|---|---|---|---|---|
| HIV Care Visit | On ART | (257 obs.; n = 96) | (self-reported) | (biomarker) | |
| Predictor | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) |
| Intervention Exposure (Scale; 0–4) | 1.33** (1.10; 1.61) | 1.24 (0.99; 1.54) | 1.01 (0.84; 1.20) | 1.33*** (1.12; 1.57) | 1.51** (1.11; 2.07) |
| Time Point | |||||
| Baseline | Reference Category | Reference Category | Reference Category | Reference Category | Reference Category |
| 6-Month Follow-Up | 1.17 (0.61; 2.24) | 2.06* (1.10; 3.85) | 5.93*** (2.63; 13.38) | 2.30* (1.20; 4.43) | 1.02 (0.79; 1.31) |
| 12-Month Follow-Up | 0.82 (0.42; 1.62) | 2.24** (1.22; 4.12) | 6.19*** (3.13; 12.25) | 2.44** (1.25; 4.79) | 1.15 (0.89; 1.47) |
| 18-Month Follow-Up | 0.90 (0.45; 1.81) | 2.95** (1.41; 6.16) | 10.44*** (5.48; 19.89) | 2.38* (1.07; 5.27) | 1.21 (0.92; 1.59) |
| Study Arm | |||||
| Immediate Delivery | Reference Category | Reference Category | Reference Category | Reference Category | Reference Category |
| Delayed Delivery | 1.78 (0.98; 3.21) | 0.97 (0.44; 2.13) | 0.78 (0.46; 1.34) | 0.77 (0.43; 1.38) | 1.45 (0.50; 4.23) |
| Post-Intervention Retention and Engagement Messages | |||||
| Opted Out | Reference Category | Reference Category | Reference Category | Reference Category | Reference Category |
| Opted In | 3.56** (1.51; 8.39) | 2.05 (0.79; 5.29) | 1.43 (0.72; 2.85) | 2.47** (1.35; 4.53) | 3.04 (0.93; 9.96) |
| N & n: Sample Size; HIV: Human Immunodeficiency Virus: ART: Antiretroviral Therapy; obs: observations; aOR: adjusted odds ratio; CI = confidence interval | Logistic Regression | Logistic Regression | Ordinal Logistic Regression | Logistic Regression | Logistic Regression |
p < 0.05;
p < 0.01;
p ≤ 0.001
Statistical Controls: Age, Race/Ethnicity, Educational Attainment, Monthly Income, Housing Status, Sex Work (past 6m), Method of Message Delivery (SMS/email)
In contrast, Table 5 also demonstrates that for participants in both arms each level of increased self-reported exposure to the Text Me, Girl! intervention was associated with an estimated 33% increase in the odds of self-reported retention in HIV care (adjusted odds ratio [aOR] = 1.33; 95% Confidence Interval [CI] = 1.10–1.61), an estimated 12%−57% increase in the odds of self-reported viral suppression (aOR = 1.33), and a corresponding 51% estimated increase in the odds of biomarker-confirmed viral suppression (95% CI = 1.11–2.07) at a given time point. Furthermore, retention in Text Me, Girl! through the 18-month follow-up assessment was associated with an estimated 195% increase in the odds of self-reporting being currently on ART (95% CI = 1.41 – 6.16), with an estimated 944% increase in the proportional odds of self-reported “excellent” ART adherence (95% CI = 5.48 – 19.89), and with an estimated 138% increase in the odds of self-reported viral suppression (95% CI = 1.07 – 5.27). Opting in to receive the UCARE4LIFE post-intervention retention and engagement messages was associated with a 256% increase in the odds of attending an HIV care visit in the past 6 months (95% CI = 1.51; 8.39) as well as a 147% increase in the odds of self-reporting viral suppression at a given time point (95% CI = 1.35; 4.53). Finally, participants who chose to receive their theory-based messages by email, rather than by SMS, were significantly less likely to self-report viral suppression (aOR = 0.47; 95% CI = 0.27–0.80), although method of message delivery was unassociated with any other outcome (coefficients not tabulated).
Discussion
Results demonstrated that increased self-reported exposure to the Text Me, Girl! theory-based text messages was associated with significantly increased likelihood of retention in HIV care and self-reported achievement of full viral suppression, and that delayed administration of the Text Me, Girl! intervention was not associated with any measurable decline in efficacy. Both those randomized to receive the intervention content immediately upon enrollment (the ID arm) and those randomized to receive the intervention content after a 90-day delay (the DD arm) exhibited significant improvements in HIV Care Continuum outcomes, implying observed intervention results were likely not the result of spurious short-term improvements prompted merely through the process of self-selected enrollment into a HIV care intervention. However, given that there was no standard of care control arm it is impossible to determine if these significant outcomes were solely in response to the unidirectional delivery of 270 culturally responsive, theory-based text messages over the course of 90 days.
Although effective methods of linking and retaining trans women in HIV care have been developed (23), trans women remain one of the more challenging groups to maintain consistently in care due to syndemic health disparities (e.g., poverty, housing instability) that serve as barriers to linkage and retention. Furthermore, trans women report pervasive medical mistrust of medicine, doctors, and standardized care models (26, 27). In-person interventions, i.e., interventions that are delivered in brick-and-mortar facilities, are limited by the fact that these interventions can only reach those who are able and willing to physically enter the facility. As a result of the often chaotic life circumstances experienced by highly impacted trans women, many do not attend to in-person interventions as is evidenced by the low baseline HIV linkage and retention rates. Given the intersectionality of multiple health disparities and individual and structural barriers, interventions to improve HIV health outcomes must be realistically scalable, also noting the high probability that the resultant intervention will need to be applied in resource-limited settings.
Low cost and scalability are critical characteristics for interventions designed for trans women at elevated risk of HIV acquisition or transmission as many are disproportionately likely to live in poverty and experience housing instability (71, 72). As such, interventions that can employ free-to-use services are critical for improving health outcomes. Evidence suggests (65) that automated text messaging is both an efficacious and a cost-effective means of intervening in the lives of extremely impacted and/or stigmatized populations. In this sample of highly impacted young adult trans women living with HIV, increased exposure to and retention within the Text Me, Girl! intervention was associated with increased likelihood of attending ongoing HIV care visits, increased ART uptake and adherence, and increased likelihood of viral suppression.
Conclusions
These results must be interpreted in the context of the study limitations. This study utilized a convenience sample of young adult trans women living with HIV who self-selected by volunteering to receive a HIV care-related text-messaging intervention. Although the application of a delayed intervention arm reduces concerns of results being due to short-term effects and self-selection bias, this possibility cannot be ruled out. All behavioral data was self-reported, which has inherent liabilities such as recall errors and/or deliberate falsification. Although social desirability bias may also influence responses, this limitation was mitigated by the use of an ACASI. The text-messaging platform lacked the ability to directly count the number of text messages read by each participant and, therefore, intervention exposure was based on self-report. There were a significant number of missing data from Electronic Health Records that were never received either due to participants opting-out of their data abstraction or due to the limitations of data abstraction from collaborating health clinics. Although potentially concerning, it is also important to note: 1) all participants for whom Electronic Health Record data could not be obtained (n = 65) were coded as “Detectable” in the biomarker-confirmed viral load variable to provide a maximally conservative estimate of intervention efficacy even although actual viral suppression numbers were likely higher; and, 2) multivariable analyses of biomarker-confirmed data still revealed significant intervention effects on viral suppression even under the maximally conservative conditions applied. Nevertheless, the introduction of so much missing biologic data and the lack of true random selection of participants increased risk for significant residual confounding. As there was no standard of care control arm it is impossible to firmly attribute the significant advancement along the HIV Care Continuum solely to the intervention. A further limitation is that the study was not sufficiently powered to explore all possible interactions between the intervention and included covariates (e.g., age, racial/ethnic identity). Finally, the highly specific nature of the sample (i.e., young adult trans women living with HIV from a large metropolitan city, most of whom were racial/ethnic minority trans women) may limit the generalizability of these findings to similar samples and may not apply among, for example, rural or predominantly white young adult trans women or older trans women living with HIV.
In spite of these limitations, results presented here provide promising evidence that the delivery of a unidirectional text-messaging intervention that was targeted, tailored, and personalized improved HIV care outcomes among young adult trans women living with HIV. Despite experiencing several health disparities including low educational attainment, low income, and housing instability, participants demonstrated significant increases in ART uptake, significant improvements in ART adherence, and significant increases in achievement of an undetectable viral load, and these improvements were durable through 18-month follow-up.
Text Me, Girl! is a fully automated and unidirectional text messaging intervention that is highly replicable and versatile, thus offering several advantages to both participants and providers. The scripted text-message library can be easily parsed to the needs of a specific agency (e.g., delivered in its entirety, or parceled to only the content/theory subsets that meet agency goals/needs). For example, a social service provider working with those out of care may choose to only adopt the 90 messages specific to Linkage/Retention in HIV Care. Whereas, an HIV clinic interested in maintaining their patients’ medication adherence may choose to only adopt the 90 messages specific to ART Medication Adherence/Viral Load Suppression. Or, a provider may be interested in only the 90 Social Support theoretical messages. Findings reported here, coupled with the inherent flexibility of the Text Me, Girl! text-message library, provides much promise for improving HIV health outcomes among young adult trans women living with HIV.
Acknowledgments
This project was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number H97HA28889 in the last annual award amount of $300,000 awarded to Friends Research Institute (PI: C. Reback). Dr. Reback acknowledges additional support from the National Institute of Mental Health (P30 MH58107). The authors would like to thank Raymond P. Mata for his outstanding work as the Research Coordinator on this study.
Footnotes
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Centers for Disease Control and Prevention (CDC). HIV and Transgender People. Available at: https://www.cdc.gov/hiv/pdf/group/gender/transgender/cdc-hiv-transgender-factsheet.pdf. Accessed February 15, 2020.
- 2.Centers for Disease Control and Prevention (CDC). US Statistics. Available at: https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics. Accessed February 15, 2020.
- 3.Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: A systematic review. AIDS Behav. 2008;12(1):1–17. [DOI] [PubMed] [Google Scholar]
- 4.Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. [DOI] [PubMed] [Google Scholar]
- 5.Kussin-Shoptaw AL, Fletcher JB, Reback CJ. Physical and/or sexual abuse is associated with increased psychological and emotional distress among transgender women. LGBT Health. 2017;4(4):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitasi MA, Oraka E, Clark H, Town M, DiNenno EA. HIV testing among transgender women and men—27 states and Guam, 2014–2015. MMWR Morb Mortal Wkly Rep. 2017;66(33):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. HIV Surveillance Report, 2018 (Updated); Vol. 31. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed January 7, 2021. [Google Scholar]
- 8.Reisner SL, Jadwin-Cakmak L, Hughto JMW, Martinez M, Salomon L, Harper GW. Characterizing the HIV prevention and care continua in a sample of transgender youth in the US. AIDS Behav. 2017;21(12):3312–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garofalo R, Johnson AK, Kuhns LM, Cotten C, Joseph H, Margolis A. Life skills: Evaluation of a theory-driven behavioral HIV prevention intervention for young transgender women. J Urban Health. 2012;89(3):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulden JD, Song B, Barros A, et al. Rapid HIV testing in transgender communities by community-based organizations in three cities. Public Health Rep. 2008;123(3_suppl):101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garofalo R, Deleon J, Osmer E, Doll M, Harper GW. Overlooked, misunderstood and at-risk: Exploring the lives and HIV risk of ethnic minority male-to-female transgender youth. J Adolesc Health. 2006;38(3):230–236. [DOI] [PubMed] [Google Scholar]
- 12.Wilson EC, Garofalo R, Harris RD, et al. Transgender female youth and sex work: HIV risk and a comparison of life factors related to engagement in sex work. AIDS Behav. 2009;13(5):902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan J, Kuhns LM, Johnson AK, et al. Syndemic theory and HIV-related risk among young transgender women: The role of multiple, co-occurring health problems and social marginalization. Am J Public Health. 2012;102(9):1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowshen N, Matone M, Luan X, et al. Behavioral and health outcomes for HIV+ young transgender women linked to and engaged in medical care. LGBT Health. 2016;3(2):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garofalo R, Osmer E, Sullivan C, Doll M, Harper G. Environmental, psychosocial, and individual correlates of HIV risk in ethnic minority male-to-female transgender youth. J HIV AIDS Prev Child Youth. 2007;7(2):89–104. [Google Scholar]
- 16.Johnson A, Garofalo R, Kuhns L. Life Skills: Evaluation of a novel behavioral HIV-prevention intervention for male-to-female transgender youth. J Adolesc Health. 2010;46(2):S75–S76.20172462 [Google Scholar]
- 17.Fletcher JB, Kisler KA, Reback CJ. Housing status and HIV risk behaviors among transgender women in Los Angeles. Arch Sex Behav. 2014;43(8):1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevelius JM, Keatley J, Gutierrez-Mock L. HIV/AIDS programming in the United States: Considerations affecting transgender women and girls. Womens Health Issues. 2011;21(6):S278–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr (1999). 2016;72(3):S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman RS, Benotsch EG, Shoemaker S, et al. Mediational models linking psychosocial context, mental health problems, substance use, and HIV risk behaviors in transgender women. Health Psychol Behav Med. 2015;3(1):379–390. [Google Scholar]
- 21.Nuttbrock LA, Hwahng SJ. Ethnicity, sex work, and incident HIV/STI among transgender women in New York City: A three year prospective study. AIDS Behav. 2017;21(12):3328–3335. [DOI] [PubMed] [Google Scholar]
- 22.Arayasirikul S, Wilson EC, Raymond HF. Examining the effects of transphobic discrimination and race on HIV risk among transwomen in San Francisco. AIDS Behav. 2017;21(9):2628–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reback CJ, Ferlito D, Kisler KA, Fletcher JB. Recruiting, linking, and retaining high-risk transgender women into HIV prevention and care services: An overview of barriers, strategies, and lessons learned. Int J Transgend. 2015;16(4):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno Y, Beer L, Huang P, Frazier EL. Factors associated with antiretroviral therapy adherence among transgender women receiving HIV medical care in the United States. LGBT Health. 2017;4(3):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bukowski LA, Chandler CJ, Creasy SL, Matthews DD, Friedman MR, Stall RD. Characterizing the HIV care continuum and identifying barriers and facilitators to HIV diagnosis and viral suppression among black transgender women in the United States. J Acquir Immune Defic Syndr (1999). 2018;79(4):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seelman KL, Colón-Diaz MJ, LeCroix RH, Xavier-Brier M, Kattari L. Transgender noninclusive healthcare and delaying care because of fear: Connections to general health and mental health among transgender adults. Transgend Health. 2017;2(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler KG, Strang JF, Call D, et al. Healthcare experiences of transgender and gender diverse youth: A qualitative analysis. J Pediatr Adolesc Gynecol. 2018;31(2):182. [Google Scholar]
- 28.Aylagas-Crespillo M, García-Barbero Ó, Rodríguez-Martín B. Barriers in the social and healthcare assistance for transgender persons: A systematic review of qualitative studies. Enfermería Clínica (English Edition). 2018;28(4):247–259. [DOI] [PubMed] [Google Scholar]
- 29.Heng A, Heal C, Banks J, Preston R. Transgender peoples’ experiences and perspectives about general healthcare: A systematic review. Int J Transgend. 2018;19(4):359–378. [Google Scholar]
- 30.Howard SD, Lee KL, Nathan AG, Wenger HC, Chin MH, Cook SC. Healthcare experiences of transgender people of color. J Gen Intern Med. 2019;34(10):2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. 2014;47(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White Hughto JM, Murchison GR, Clark K, Pachankis JE, Reisner SL. Geographic and individual differences in healthcare access for US transgender adults: A multilevel analysis. LGBT Health. 2016;3(6):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin H, Restar A, Biello K, et al. Burden of HIV among young transgender women: Factors associated with HIV infection and HIV treatment engagement. AIDS Care. 2019;31(1):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark H, Babu AS, Wiewel EW, Opoku J, Crepaz N. Diagnosed HIV infection in transgender adults and adolescents: Results from the National HIV Surveillance System, 2009–2014. AIDS Behav. 2017;21(9):2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowshen N, Lee S, Franklin J, Castillo M, Barg F. Access to medical and mental health services across the HIV care continuum among young transgender women: A qualitative study. Transgend Health. 2017;2(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper GW, Jadwin-Cakmak LA, Popoff E, et al. Transgender and other gender-diverse youth’s progression through the HIV continuum of care: Socioecological system barriers. AIDS Patient Care STDS. 2019;33(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reback CJ, Kisler KA, Fletcher JB. A novel adaptation of peer health navigation and contingency management for advancement along the HIV care continuum among transgender women of color. AIDS Behav. 2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reback CJ, Rünger D, Fletcher JB. Drug use is associated with delayed advancement along the HIV care continuum among transgender women of color. AIDS Behav. 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalichman SC, Kalichman MO, Cherry C, et al. Brief behavioral self-regulation counseling for HIV treatment adherence delivered by cell phone: An initial test of concept trial. AIDS Patient Care STDS. 2011;25(5):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hersch RK, Cook RF, Billings DW, Kaplan S, Murray D, Safren S, et al. Test of a web-based program to improve adherence to HIV medications. AIDS Behav. 2013;17(9):2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingersoll K, Dillingham R, Reynolds G, et al. Development of a personalized bidirectional text messaging tool for HIV adherence assessment and intervention among substance abusers. J Subst Abuse Treat. 2014;46(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurth AE, Chhun N, Cleland CM, et al. Linguistic and cultural adaptation of a computer-based counseling program (CARE+ Spanish) to support HIV treatment adherence and risk reduction for people living with HIV/AIDS: A randomized controlled trial. J Med Internet Res. 2016;18(7):e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephenson R, Metheny N, Sharma A, Sullivan S, Riley E. Providing home-based HIV testing and counseling for transgender youth (Project Moxie): Protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2017;6(11):e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeGrand S, Muessig KE, Platt A, et al. Epic allies, a gamified mobile phone app to improve engagement in care, antiretroviral uptake, and adherence among young men who have sex with men and young transgender women who have sex with men: Protocol for a randomized controlled trial. JMIR Res Protoc. 2018;7(4):e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanner AE, Song EY, Mann-Jackson L, et al. Preliminary impact of the weCare social media intervention to support health for young men who have sex with men and transgender women with HIV. AIDS Patient Care STDS. 2018;32(11):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garofalo R, Kuhns LM, Hotton A, Johnson A, Muldoon A, Rice D. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 2016;20(5):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalichman SC, Kalichman MO, Cherry C, Eaton LA, Cruess D, Schinazi RF. Randomized factorial trial of phone-delivered support counseling and daily text message reminders for HIV treatment adherence. J Acquir Immune Defic Syndr (1999). 2016;73(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henny KD, Wilkes AL, McDonald CM, Denson DJ, Neumann MS. A rapid review of eHealth interventions addressing the continuum of HIV care (2007–2017). AIDS Behav. 2018;22(1):43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowen D, Jabson J, Kamen C. mHealth: An avenue for promoting health among sexual and gender minority populations? mHealth. 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun CJ, Anderson KM, Mayer L, Kuhn T, Klein CH. Findings from formative research to develop a strength-based HIV prevention and sexual health promotion mHealth intervention for transgender women. Transgend Health. 2019;4(1):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirtz AL, Cooney EE, Chaudhry A, Reisner SL. Computer-mediated communication to facilitate synchronous online focus group discussions: Feasibility study for qualitative HIV research among transgender women across the United States. J Med Internet Res. 2019;21(3):e12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skeen SJ, Cain D, Gamarel KE, Hightow-Weidman L, Reback CJ. mHealth for transgender and gender-expansive youth: Harnessing gender-affirmative cross-disciplinary innovations to advance HIV prevention and care interventions. mHealth. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pew Research Center. Mobile Fact Sheet. Available at: https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed February 15, 2020.
- 54.Gelaude DJ, Sovine ML, Swayzer III R, Herbst JH. HIV prevention programs delivered by community-based organizations to young transgender persons of color: Lessons learned to improve future program implementation. Int J Transgend. 2013;14(3):127–139. [Google Scholar]
- 55.Singh AA. Transgender youth of color and resilience: Negotiating oppression and finding support. Sex Roles. 2013;68(11):690–702. [Google Scholar]
- 56.Human Rights Campaign, National Center for Transgender Equality and Center for American Progress, Movement Advancement Project. A Broken Bargain for Transgender Workers. Available at: http://www.lgbtmap.org/file/a-broken-bargain-for-transgender-workers.pdf. Accessed March 26, 2015.
- 57.Office of Infectious Disease and HIV/AIDS Policy, HHS. What is ending the HIV epidemic: A plan for America? Available at: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview. Accessed January 22, 2021.
- 58.Holloway IW, Jordan SP, Dunlap SL, Ritterbusch A, Reback CJ. Leveraging social networks and technology for HIV prevention and treatment with transgender women. AIDS Educ Prev. 2020;32(2):83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semaan S. Time-space sampling and respondent-driven sampling with hard-to-reach populations. Methodological Innovations. 2010;5(2):60–75. [Google Scholar]
- 60.MacKellar DA, Gallagher KM, Finlayson T, Sanchez T, Lansky A, Sullivan PS. Surveillance of HIV risk and prevention behaviors of men who have sex with men—A national application of venue-based, time-space sampling. Public Health Rep. 2007;122(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9(4):345–364. [DOI] [PubMed] [Google Scholar]
- 62.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994(s12):70–75. [DOI] [PubMed] [Google Scholar]
- 63.Reback CJ, Grant DL, Fletcher JB, et al. Text messaging reduces HIV risk behaviors among methamphetamine-using men who have sex with men. AIDS Behav. 2012;16(7):1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reback CJ, Fletcher JB, Shoptaw S, Mansergh G. Exposure to theory-driven text messages is associated with HIV risk reduction among methamphetamine-using men who have sex with men. AIDS Behav. 2015;19(2):130–141. [DOI] [PubMed] [Google Scholar]
- 65.Reback CJ, Fletcher JB, Leibowitz AA. Cost effectiveness of text messages to reduce methamphetamine use and HIV sexual risk behaviors among men who have sex with men. J Subst Abuse Treat. 2019;100:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derlega VJ, Winstead BA, Oldfield EC, Barbee AP. Close relationships and social support in coping with HIV: A test of sensitive interaction systems theory. AIDS Behav. 2003;7(2):119–129. [DOI] [PubMed] [Google Scholar]
- 67.Turner RJ, Turner JB. Social Integration and Support. In: Aneshensel CS, Phelan JC, eds. Handbook of the Sociology of Mental Health. New York, NY: Kluwer Academic/Plenum; 1999. p. 301–319. [Google Scholar]
- 68.Bandura A. Social Cognitive Theory and Exercise of Control over HIV Infection. In: DiClemente RJ, Peterson JL, eds. Preventing AIDS: Theories and Methods of Behavioral Interventions. New York, NY: Plenum Press; 1994. p. 25–59. [Google Scholar]
- 69.Bandura A. Social cognitive theory of mass communication. Media Psychol. 2001;3(3):265–299. [Google Scholar]
- 70.Janz NK, Becker MH. The health belief model: A decade later. Health Educ Q. 1984;11(1):1–47. [DOI] [PubMed] [Google Scholar]
- 71.Conron KJ, Scott G, Stowell GS, Landers SJ. Transgender health in Massachusetts: Results from a household probability sample of adults. Am J Public Health. 2012;102(1):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frazer S, Howe E. Transgender Health and Economic Insecurity: A Report from the 2015 LGBT Health and Human Services Needs Assessment Survey. Available at: http://strengthinnumbersconsulting.com/wp-content/uploads/2017/06/TG-health-and-economic-insecurity-report-FINAL.pdf. Accessed May 29, 2020.


