Abstract
Epstein-Barr virus (EBV) is invariably present in undifferentiated nasopharyngeal carcinomas, is found sporadically in other carcinomas, and replicates in the differentiated layer of the tongue epithelium in lesions of oral hairy leukoplakia. However, it is not clear how frequently or by what mechanism EBV infects epithelial cells normally. Here, we report that a human epithelial cell line, 293, can be stably infected by EBV that has been genetically marked with a selectable gene. We show that 293 cells express a relatively low level of CD21, that binding of fluorescein-labeled EBV to 293 cells can be detected, and that both the binding of virus to cells and infection can be blocked with antibodies specific for CD21. Two proteins known to form complexes with CD21 on the surface of lymphoid cells, CD35 and CD19, could not be detected at the surface of 293 cells. All infected clones of 293 cells exhibited tight latency with a pattern of gene expression similar to that of type II latency, but productive EBV replication and release of infectious virus could be induced inefficiently by forced expression of the lytic transactivators, R and Z. Low levels of mRNA specific for the transforming membrane protein of EBV, LMP-1, as well as for LMP-2, were detected; however, LMP-1 protein was either undetectable or near the limit of detection at less than 5% of the level typical of EBV-transformed B cells. A slight increase in expression of the receptor for epidermal growth factor, which can be induced in epithelial cells by LMP-1, was detected at the cell surface with two EBV-infected 293 cell clones. These results show that low levels of surface CD21 can support infection of an epithelial cell line by EBV. The results also raise the possibility that in a normal infection of epithelial cells by EBV, the LMP-1 protein is not expressed at levels that are high enough to be oncogenic and that there might be differences in the cells of EBV-associated epithelial cancers that have arisen to allow for elevated expression of LMP-1.
Accumulating evidence indicates that a typical infection of a person by Epstein-Barr virus (EBV) is primarily an infection of the person’s B cells, both during the acute phase of infection (1, 20, 38) and during life-long latency (34, 35, 42, 49). EBV readily infects human B cells in vitro, by attaching to CD21 at the cell surface, and establishes a latent infection which transforms the B cells into proliferating lymphoblasts (21, 22). It is clear that at some frequency EBV infects nonlymphoid cell types in vivo, since its genomes can be found in a variety of nonlymphoid cancers, primarily epithelial and, most notably, undifferentiated nasopharyngeal carcinoma (NPC) (39). In patient with AIDS, EBV can cause oral hairy leukoplakia, an active EBV infection of the differentiated epithelium of the tongue (15, 56). Very little is known about how frequently EBV infects epithelial cells during normal human infection, about how the virus gains entry into epithelial cells, or whether such an infection typically becomes latent, becomes lytic, or is aborted.
Studies of the infection of epithelial cells by EBV have been limited because EBV does not readily infect epithelial cell lines in culture. The EBV receptor for B cells is CD21, or complement receptor 2 (CR2), which serves as the receptor for complement component C3d,g. EBV binding to CD21 is effected by a viral envelope protein, gp350/220, which shares a region of sequence similarity with C3d,g (8, 10, 36). Expression of CD21 at high levels in epithelial cells from a stably transfected cDNA was shown to be capable of mediating efficient attachment of EBV to epithelial cells, which led to a transient infection (30). Two human epithelial cell lines, RHEK and HeLa, were shown to express very low levels of CD21 and/or its mRNA and were able to bind EBV at the cell surface, but EBV binding was not shown to be dependent on CD21 (3). Since these cell lines were not shown to become infected by EBV, it has not been clear whether such low levels of surface CD21 would be sufficient to support uptake of EBV by these cells. The detection of CD21 by monoclonal antibodies (MAbs) on epithelial surfaces of tissue sections has been called into question (3), and unequivocal evidence has not been obtained to substantiate the presence of CD21 on epithelial cells in vivo. Recent studies in vitro suggest certain epithelial cell lines that do not express CR2 can be infected by EBV if they are cocultivated with virus-releasing cells (19). Cell lines that express the immunoglobulin A (IgA) receptor, SC, are susceptible to uptake of EBV mediated by EBV-specific IgA, suggesting a plausible mechanism for the infection of mucosal epithelial cells in individuals who have acquired immunity to EBV (47).
Here we report using recombinant EBV carrying a G418 resistance gene to test whether epithelial cell lines could be stably infected by EBV to generate drug-resistant cell clones, an approach that allows detection of rare, stably infected cells in a tested population. One cell line among several that were tested, a human embryonic kidney cell line, 293, could be stably infected by EBV at a low efficiency. Our studies indicate that infection of 293 cells by EBV is mediated by CD21. Recently, Takada and coworkers reported using a similar approach to detect stable infection of cell lines derived from human gastric carcinomas by soluble EBV and infection of several other epithelial cell lines with cell-associated virus, but in contrast to our findings, infection of these cell lines did not appear to be mediated by CD21 (19, 55).
Little is known about how EBV gene expression is controlled in epithelial cells. The issue is relevant to the involvement of EBV in NPC, where the EBV transforming membrane protein, LMP-1, is suspected of playing a major role (23, 40). In contrast to the readily detectable expression of LMP-1 in NPC, LMP-1 protein was usually undetectable or near the detection limit in this study and in previous studies of experimentally infected epithelial cell lines (19, 24, 55). This finding suggests that one aspect of NPC may be the abnormal expression of LMP-1. In NPC, the LMP-1 gene is transcribed from two promoters, giving rise to a 3.5-kb mRNA as well as to the more abundant 2.8-kb mRNA, the form which is expressed almost exclusively in B cells (44). In infected 293 cells, only a 3.5-kb LMP-1 mRNA was detected, at low abundance.
MATERIALS AND METHODS
Viruses and cell lines.
293 (13) and RHEK (43) are cell lines that were derived from human embryonic kidney epithelium by transformation with human adenovirus DNA (293) or an adenovirus–simian virus 40 hybrid (RHEK). 293 cells are available from American Type Culture Collection (ATCC), Manassas, Va.; RHEK cells were kindly provided by J. Rhim, Harvard University. P3-531 virus stocks were obtained by transfecting P3HR1 clone 16 cells (16) with p531, a plasmid carrying EBV positions 43935 to 62280 of strain B95-8, with a CMVIE-neo gene (neo gene driven by the cytomegalovirus immediate-early promoter) replacing the BHRF1 open reading frame, as previously described (27). B-652 is a derivative of B95-8 virus that carries the CMVIE-neo gene inserted downstream of the EBNA1 coding exon along with a duplication of a few hundred base pairs in a manner that avoided disrupting any EBV gene or known transcription unit. A clone of B95-8 cells carrying recombinant B-652 was isolated and used to generate stocks of virus containing about equal amounts of B-652 and parental B95-8 viral genomes (27a). Adherent cell lines were cultured in Iscove’s modified Dulbecco’s medium; B-cell lines were cultured in RPMI 1640 medium containing 9% fetal bovine serum and the antibiotics penicillin and ampicillin (all components from Gibco BRL).
Stable infection with recombinant EBV.
The EBV-infected clones of 293 that were characterized in most detail were obtained by cocultivating gamma-irradiated electroporated P3HR1 cells with 293 cells. For all other infections, virus stocks that had been passed through 0.2-μm-pore-size filters were used. Prior to infection, 293 cells were trypsinized from cultures that had just reached confluence and replated at approximately 1:10, usually into six-well culture dishes. The next day, culture medium was removed and fresh medium was added along with dilutions of recombinant virus stock. Two days later, the growth medium was replaced with fresh medium containing G418 (Gibco BRL) at 700 μg/ml (active drug concentration), which was replenished as needed until G418-resistant (G418r) colonies were counted 2 to 3 weeks after infection. To determine titers for Burkitt’s lymphoma line Raji or BL30, cells were infected in 48-well plates containing 25,000 cells and 0.4 ml medium in each well. Different dilutions of virus stock were added to the wells, typically using 24 wells for each dilution. Two days later, approximately half of the medium in each well was removed by gentle aspiration and replaced with fresh medium containing sufficient G418 to achieve active drug concentrations of 840 and 1,120 μg/ml for Raji and BL30, respectively. Medium was replaced periodically as it became acidified, and wells were scored for emergent clones until 3 to 4 weeks after infection. G418r virus titers were determined by using Poisson statistics.
Blocking infection with antibodies.
293 cells were seeded into six-well plates from just-confluent cultures, using a 1:4 split for use the next day. Anti-CD21 or control antibodies were added to each well at their working concentrations, determined as described below, along with 0.4 ml of medium, and the plates were sealed with Parafilm and rocked for 30 min at 24°C; 30 μl of virus stock was added, and the plates were sealed and rocked at 24°C for 1 h. The medium with virus was then removed, and the cells were rinsed twice with medium and returned to culture. Alternatively, after incubation with the first antibody, the medium with antibody was removed, the cells were rinsed once, and cells were incubated with a secondary (cross-linking) antibody for 30 min at 24°C before virus was added. The following day, cells in each well were removed by using trypsin, washed free of trypsin, and replated into three wells of a six-well plate. Beginning the second day after infection, the cells were cultured in medium containing 740 μg of G418 (active) per ml, and viable clones were counted 12 days later. The EBV strain used for this experiment, P3-ΔDS-42, will be described elsewhere. It is a P3HR1 derivative that has the same selective marker insertion as P3-531, and was produced in B95-8 cells at a high titer.
EBV labeling and binding to cells.
Purification of EBV, coupling to fluorescein isothiocyanate (FITC), and EBV binding and binding inhibition assays were essentially as described elsewhere (8), except that in some experiments EBV was coupled to FITC (isomer I; Sigma, St. Louis, Mo.) that had been dissolved in dimethyl sulfoxide at a concentration of 5 mg/ml and incubated 1:100 with purified virus in carbonate-bicarbonate buffer.
Flow cytometry.
For cytometric analysis, we used 1 × 105 to 5 × 105 cells per sample. Adherent cells were suspended by incubation in 20 mM EDTA min phosphate-buffered saline (without calcium and magnesium) at 37°C for 10 min. Adherent cells or cells grown in suspension culture were then washed twice with fresh culture medium containing 2% heat-inactivated fetal bovine serum. Cells were incubated with antibody for 3 min on ice, washed with phosphate-buffered saline, incubated with a secondary antibody for another 30 min on ice, and then washed two additional times. Propidium iodide was added to give a final concentration of 5 μg/ml. Live cells were analyzed by flow cytometry on a FACScan (Becton Dickinson). The histograms presented in Fig. 1A, 2, 3 and 8 are representative of independent analyses that were performed two to seven times.
FIG. 1.
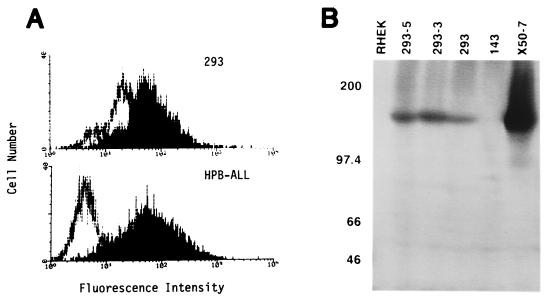
Detection of CD21 on 293 cells with a monospecific rabbit antiserum. (A) Detection of CD21 on the cell surface of 293 compared with HPB-ALL by flow cytometry. Cells were stained either with rabbit anti-CD21 (block) or with normal rabbit serum (line). (B) Detection of CD21 protein in extracts of 293 cells by Western analysis. Total soluble protein from Nonidet P-40 lysis of 500,000 cells was analyzed for the cell lines indicated. Two clones of 293 that are stably infected with the EBV recombinant P3-531 are indicated as 293-5 and 293-3. At the left, positions are indicated (in kilodaltons) for standards.
FIG. 2.
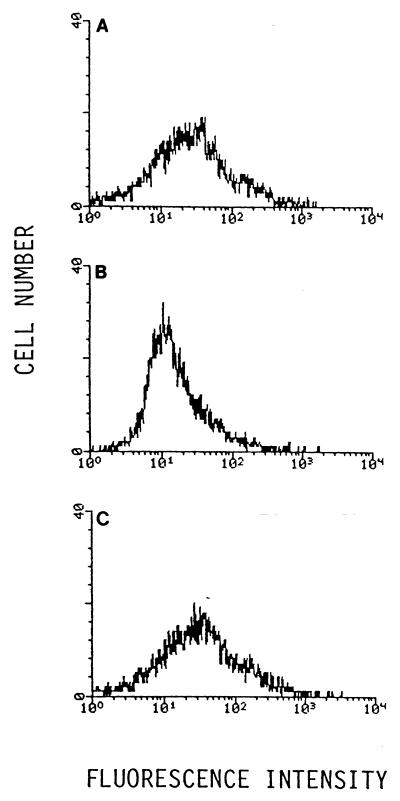
CD21-dependent binding of FITC-labeled EBV to 293 cells, as determined by flow cytometry. (A) Binding of FITC-labeled EBV to 293 cells without pretreatment. (B) Before incubating 293 cells with FITC-labeled EBV, surface CD21 was cross-linked with anti-CD21 mouse MAb HB-5 followed by goat F(ab′)2 against mouse IgG. (C) 293 cells were treated identically except that the irrelevant control MAb UPC10 was used for cross-linking.
FIG. 3.
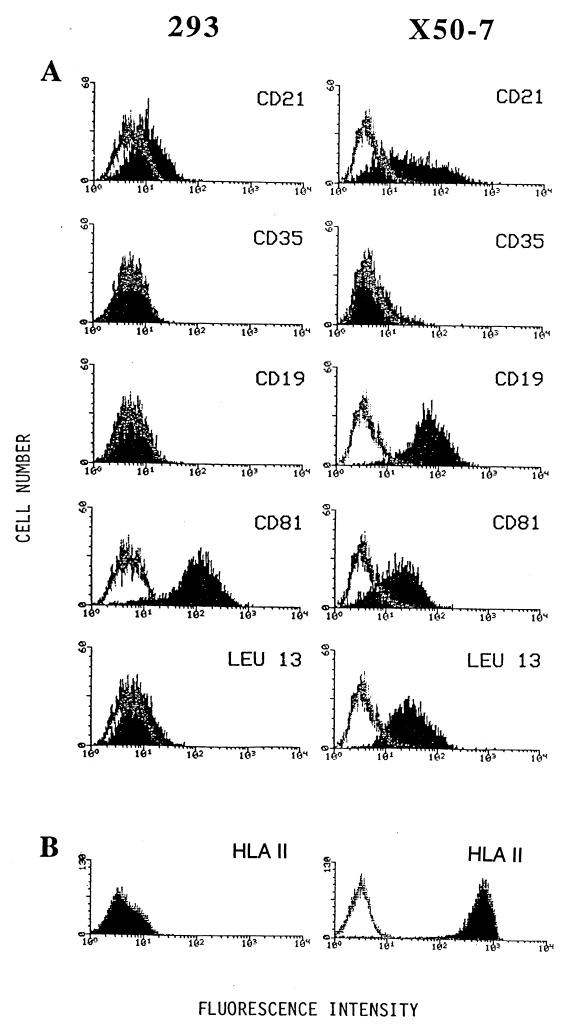
Analysis of surface expression of CD21 and proteins reported to form membrane complexes with CD21 for 293 cells and X50-7 cells. Binding is indicated (block) for mouse MAbs specific to CD21 (HB-5), to CD35/CR1 (543), to CD19 (B-C3), to CD81/TAPA-1 (5A6), and to Leu-13 (anti-Leu-13). Each is shown in comparison to binding by an irrelevant antibody, UPC10 (line).
FIG. 8.
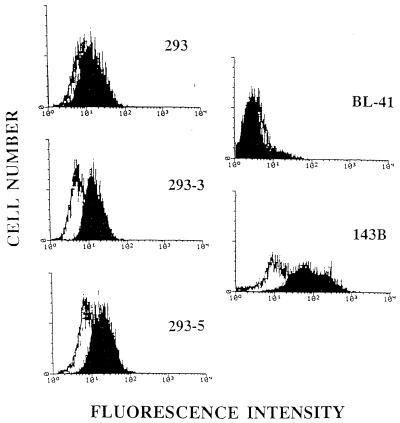
Detection by fluorescence cytometry of EGFR on 293 and on EBV-infected 293 cells. Cells stained with a mouse IgG2a Mab to the human EGFR (block) were compared with an isotype-matched irrelevant control MAb, UPC10 (black line), and analyzed by flow cytometry. BL-41, an EBV-negative Burkitt’s lymphoma line, was used as a negative control; 143B, an osteosarcoma cell line that expresses moderate amounts of EGFR, was used as a positive control.
Antibodies. (i) For cell surface antigens:
Mouse MAbs were as follows: HB-5 (anti-CD21) and 543 (anti-CD35), obtained from the ATCC; OKB7 (anti-CD21), purchased from Ortho Diagnostics, Raritan, N.J.; B-C3 (anti-CD19), from Biosource, Camarillo, Calif.; 5A6 (anti-CD81, TAPA-1) a gift of Shoshana Levy, Stanford University School of Medicine, Stanford, Calif.; anti-Leu-13, a gift from Ron Evans and Sharon Evans, Roswell Park Cancer Institute, Buffalo, N.Y.; and LB3.1 (anti-HLA class II) and W6/32 (anti-HLA class I), gifts from Jack Strominger, Harvard University, Cambridge, Mass. Each antibody was titered and used for cell staining or for blocking experiments at a concentration that was 10-fold in excess of saturation based on cytometric analysis using a standard lymphoblastoid cell line such as X50-7 (or, in the case of CD35, THP-1). Anti-epidermal growth factor receptor (EGFR) (Ab-1, clone 528; Oncogene Sciences, Cambridge, Mass.) was used at 10 μg/ml.
(ii) Control MAb.
UPC10, an IgG2a mouse MAb with hapten specificity for β-2-6-linked fructosan, was purchased from Cappel-Organon Teknika, Durham, N.C. MOPC21, an IgG2b mouse MAb, was purchased from Cappel; P3, an IgG1 MAb was from the ATCC. UPC10, MOPC21, and P3 have no known specificity for human cells. They were used as isotype controls at a concentration of 10 μg/ml unless stated otherwise.
(iii) Rabbit antisera.
Rabbit anti-human CD21 was prepared as described elsewhere (7). Preimmune or normal rabbit serum was used as a control. Rabbit sera were used at a dilution of 1:200. FITC-labeled goat F(ab′)2 anti-rabbit IgG was obtained from Biosource and was used at 10 μg/ml.
(iv) EBV-specific antibodies.
Anti-BZLF1 MAb BZ.1 (56) was from Dako (Denmark); anti-EA-D antibody R3 (41) was from ABI (Columbia, Md.); anti-gp350/220 antibody 2L10 was obtained from G. Pearson; anti-LMP-1 MAb S12 was produced from the hybridoma (31). MAbs were used at 40 μg/ml or according to recommendations of the manufacturers and detected with FITC-labeled goat F(ab′)2 anti-mouse IgG (Biosource) at 10 μg/ml. Affinity-purified rabbit antibody specific for the carboxyl-terminal cytoplasmic domain of LMP-1 (2) was generously provided by B. Sugden. A human anti-viral capsid antigen (VCA) serum that was shown to recognize primarily late EBV antigens by immunofluorescence assay was used at a 1:20 dilution.
Hybridization probes.
Random-prime-labeled probes were prepared and RNA blot hybridization was performed as described previously (8). To detect LMP-1 mRNA, the 495-bp NcoI-NcoI fragment of exon C, positions 168263 to 168758 of EBV genomic DNA, was used. An LMP-2A cDNA was excised from a pBluescript KS+ clone by using EcoRI (45). For glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a cDNA clone, pHcGAP (50), was obtained from the ATCC. An EagI fragment of EBV DNA (66823 to 67633) was the U-exon probe. pRA386 (54) was used to probe for EBER1.
RESULTS
Stable infection of 293 cells.
To explore whether EBV could stably infect cell lines of epithelial origin, we tested whether EBV that had been genetically altered to carry a G418 resistance gene could stably infect a given cell line and yield G418r colonies. For most experiments, we used a recombinant EBV designated P3-531, which was generated prior to each infection by homologous recombination between P3HR1 virus and plasmid p531, resulting in replacement of the viral BHRF1 gene with a CMVIE-neo gene (27). Stocks of P3-531 recombinants, typically containing 40 to 200 infectious recombinants per ml, were added to dishes containing subconfluent cultures of the cell lines of epithelial origin, using up to 1 ml of P3-531 stock per 106 adherent cells. After 2 or 3 days, the culture medium was replaced with medium containing G418, and the plates were inspected periodically for emerging G418r clones. A small number of G418r colonies appeared reproducibly when the cell line 293 was tested. We failed to obtain any G418r colonies after attempting to infect another adenovirus-transformed epithelial cell line, RHEK, and three different cell lines derived from human carcinomas, HeLa, D98, and HEp2.
The number of stably infected 293 clones that could be obtained from a given virus stock was consistently lower than would be expected from the titer of recombinant virus that could be measured by infecting the Burkitt’s lymphoma-derived cell lines Raji and BL30. As shown in Table 1, a stock of P3-531 virus with a titer of 55 G418-resistance-conferring units per ml, as measured by infecting BL30 cells, gave an effective titer of only 1.3 for stable infection of 293 cells. The difference was more dramatic for a G418-resistance-conferring virus derived from the B95-8 strain of EBV. This recombinant virus, B-652, has been recovered in the producer cell line, B95-8, from which it and the parental B95-8 virus are released in comparable amounts (27a). Two different stocks of B-652 virus that were tested on the cell lines at different times could stably infect the Burkitt’s lymphoma cell lines several hundred to a few thousand times more efficiently than they could stably infect 293 cells (Table 1).
TABLE 1.
Abilities of 293 and two Burkitt’s lymphoma cell lines to become stably infected with two different strains of EBV
| Virus | Titer of G418 EBV (CFU/ml)a after infection with:
|
||
|---|---|---|---|
| BL30 | Raji | 293 | |
| P3-531 | 55 | ND | 1.3 |
| B95-652 | 120,000 | 63,000 | 20 |
| B95-652 | 65,000 | 33,000 | 80 |
Measured as described in Materials and Methods. Two different stocks of B95-652 were tested at different times. Repeated determinations were within twofold of the average shown in each case. ND, not determined.
EBV binds to CD21 on the surface of 293 cells.
293 cells were examined for the presence of CD21 molecules at their surface by using flow cytometry to measure the binding to intact cells by rabbit antibodies specific for human CD21. As shown in Fig. 1A, the CD21-specific serum produced a histogram that was clearly shifted to a higher fluorescent intensity than that obtained with normal rabbit serum. This result indicated that most 293 cells contained CD21 molecules at their surface. A similar result was obtained with the CD21-specific MAb HB-5 compared to an irrelevant, isotype-matched control antibody (see Fig. 3). Proteins extracted from 293 cells were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting using the rabbit anti-CD21 serum (Fig. 1B). A protein with an apparent size of 145 kDa was detected at the same positions as the authentic CD21 glycoprotein extracted from the B-cell line X50-7, although at a much lower quantity. CD21 was not detected in extracts of an osteosarcoma-derived cell line, 143, or an adenovirus-transformed human kidney epithelial cell line, RHEK. We conclude that 293 cells contain authentic CD21 at their surface.
The density of CD21 molecules at the surface of most 293 cells appears to be much lower than it is with two B-cell lines that EBV readily infects, Raji and BL30 (data not shown). The T-lymphoma cell line HPB-ALL, which expresses levels of surface CD21 similar to those of the B-lymphoma lines Raji and BL30, appeared to bind about the same amount of HB5 antibody as did 293 cells in the experiment of Fig. 1A. But 293 cells are larger than HPB-ALL cells and also bind more antibody nonspecifically (Fig. 1A), and so the surface density of CD21 molecules must be significantly lower for 293 cells than for HPB-ALL cells. The number of CD21 molecules per cell is clearly much lower for 293 cells than it is for the EBV-immortalized cell line X50-7 (Fig. 1B and 3), where a high level of CD21 is induced by expression of EBV type III latency gene products.
If the uptake of EBV by a cell is to some extent dependent on the surface density of CD21, then it is possible that EBV infects 293 cells less efficiently than B cells because 293 cells have a lower density of CD21 receptor molecules on their surface. In this case, the stably infected clones of 293 cells might have arisen from variants that expressed higher levels of CD21. Two EBV-infected clones of 293 were examined for CD21 content by immunoblot analysis and were found to have perhaps twice as much CD21 as the uninfected 293 cell population (Fig. 1B). This could mean that there is some heterogeneity with respect to the level of CD21 in the uninfected population and that cells having more CD21 than the population average might have been selectively infected. It is also conceivable that this slight increase in CD21 is a consequence of low levels of EBV-encoded LMP-1 expressed in these cells (see below), since LMP-1 has been shown to induce CD21 gene expression in B-cell lines (51).
To test whether binding of EBV to 293 cells could be detected, purified EBV of strain B95-8 was labeled with FITC and allowed to bind to 293 cells, which were then washed free of unbound virus and examined by flow cytometry. A histogram with a broad peak, perhaps with a shoulder at higher fluorescent signal, was obtained (Fig. 2A). The histogram shown is representative of results obtained from three independent preparations of labeled virus, each analyzed at least twice. The brighter fluorescence exhibited by about half of the cells in the population was not observed if, before the addition of fluoresceinated EBV, the 293 cells were treated with HB-5 followed by goat F(ab′)2 specific to mouse IgG in order to cross-link the CD21 molecules on the cell surface (Fig. 2B). The cross-linking step had no effect on the binding of fluoresceinated EBV when the irrelevant MAb UPC10 was used instead of HB-5 (Fig. 2C). This is evidence that EBV can bind to a large fraction of a population of 293 cells and that this binding is mediated by CD21.
CD21-specific antibodies block infection of 293 cells by EBV.
To examine whether the virus binding that is mediated by CD21 is responsible for the cells becoming infected, we tested whether antibodies specific to CD21 would block infection of 293 cells. To do this, 293 cells in six-well dishes were incubated with the CD21-specific MAb OKB7, with an isotype-matched control antibody, MOPC21, or with an anti-HLA class I monoclonal antibody, W6/32, for 30 min at room temperature (24°C). The cells were then washed to remove antibody and incubated with virus for 1 h before being washed and returned to culture to monitor outgrowth of G418r clones. Alternatively, after washing away the primary antibody, a secondary antibody was added for 30 min in order to cross-link the receptor before infection with EBV. As shown in Table 2, 10 to 23 infected clones were generated from each infection when cells were either not treated with antibody or treated with control antibody MOPC21, which does not bind to 293 cells, or W6/32, which recognizes abundant class I HLA molecules at the surface of 293 cells. However, when cells were treated with CD21-specific OKB7, no infected clones emerged. OKB7 blocked infection with or without the cross-linking step. Previously, OKB7 had been shown to block EBV attachment to B cells directly, without a need to cross-link with a secondary antibody (36). A rabbit antiserum specific to CD21 also blocked infection with or without treatment with a cross-linking antibody, while the preimmune serum from the same rabbit had little or no effect. These results provide strong evidence that most or all infections of 293 cells by EBV are mediated by CD21.
TABLE 2.
Blocking infection of 293 cells with antibodies specific to CD21a
| Antibody
|
No. of G418r clones
|
||
|---|---|---|---|
| 1st | 2nd | Obtained | Expectedb |
| Not applicable (No virus) | 0 | ||
| None | 16 | ||
| MOPC21 | 23 | ||
| W6/32 | 15 | ||
| OKB7 | 0 | 19 | |
| MOPC21 | α-cMouse IgG | 10 | |
| W6/32 | α-Mouse IgG | 18 | |
| OKB7 | α-Mouse IgG | 0 | 14 |
| Rabbit preimmune | 14 | ||
| Rabbit α-CD21 | 0 | 14 | |
| Rabbit preimmune | α-Rabbit IgG | 12 | |
| Rabbit α-CD21 | α-Rabbit IgG | 1 | 12 |
| Total with α-CD21 | 1 | 59 | |
See Materials and Methods for details.
Number or average number observed with the matched-antibody controls.
α, anti.
Examining 293 cells for the presence of proteins that form complexes with CD21.
On B-cell membranes, CD21 is reported to reside in two distinct complexes, one with CD35 (CR1, the receptor for complement component C3b) and one with CD19, CD81 (TAPA-1), and Leu-13 (6, 48). It is possible that EBV infects 293 cells less efficiently than B cells because CD21 is present independently on 293 cell surfaces or in complexes with different proteins at the surfaces of the different types of cells. As shown in Fig. 3, we could not detect either CD35 or CD19 on 293 cells. The 293 cells expressed a higher level of CD81 (TAPA-1) than did X50-7 cells. Anti-Leu-13 gave a slight shift in the histogram relative to the isotype-matched control antibody, which might indicate the presence of small amounts of Leu-13 on 293 cells.
In the case of the infection of B cells by EBV, while surface CD21 is sufficient for B cells to bind EBV, efficient infection requires the presence of HLA class II molecules, which associate with a minor EBV glycoprotein, gp42 (28). As shown in Fig. 3, 293 cells do not express class II HLA at detectable levels.
Stably infected 293 clones exhibit type II latency with minimal expression of LMP-1.
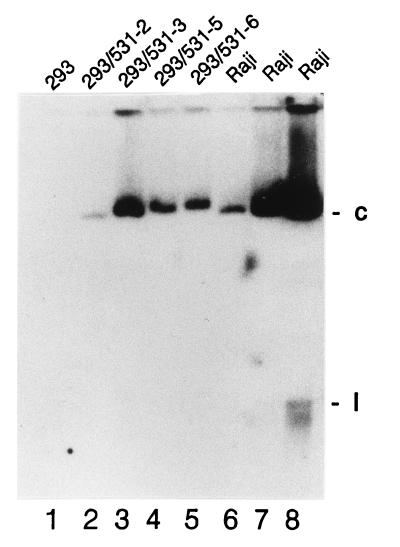
The EBV genomes of several stably infected 293 cells were analyzed. Four clones that had been infected with P3-531 recombinant EBV were examined for the presence of covalently closed, circular EBV genomes by electrophoresis through an in situ lysis gel, as shown in Fig. 4. The average number of circular EBV chromosomes per cell varied among the clones, from close to 1 to about 10. The recombinant EBV genomes carried by each clone were found to have the expected structure in the vicinity of the inserted G418 resistant gene (data not shown). Clone 3 was found also to carry nonrecombinant P3HR1 genomes. The number of viral genomes per cell did not diminish appreciably after several weeks of culture in the absence of G418 (data not shown).
FIG. 4.
Detection of circular EBV genomes in stably infected 293 cell clones. Cells were lysed in the wells of a 0.8% agarose gel, allowing nonintegrated viral genomes to be electrophoresed into the gel (12). After electrophoresis and transfer of DNA to a nylon membrane, EBV genomes were detected by hybridization to random-prime-labeled BamHI W repeat. “c” and “l” indicate positions of supercoiled and linear (broken) EBV genomes, respectively. Integrated and nicked circular EBV genomes do not migrate through the gel appreciably. Lanes 1 through 5 contained 200,000 293 cells or 293 cell clones infected with P3-531, as indicated. Lanes 6 to 8 contained 10,000, 40,000, and 200,000, Raji cells, with EBV-negative DG75 cells also added to lanes 6 and 7 so that a total of 200,000 cells were loaded into every well.
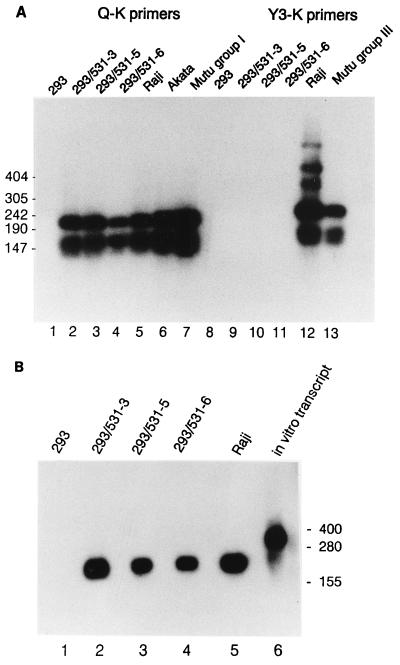
The pattern of EBV gene expression was found to resemble type I or type II latency in that EBNA1 mRNA contained the Q exon, indicative of transcription initiated from the Qp promoter, as revealed by reverse transcription (RT)-PCR analysis (Fig. 5A). The absence of detectable Cp or Wp promoter usage for synthesis of EBNA1 mRNA implied that the remaining EBNAs were not expressed. EBNA1 was detected in all clones examined by Western analysis, and EBNA2 was not detectable (data not shown). The EBV-encoded small RNA, EBER1, which has been detected in all types of latent infection, was abundant in three clones examined (Fig. 5B).
FIG. 5.
Detection of EBNA1 mRNA and EBER1 RNA in infected 293 cells. (A) Spliced EBNA1 transcripts were detected in 2 μg of total RNA from the indicated cell lines by RT-PCR using primers specific for the 3′K (coding) exon and for one of the upstream exons, either Q or Y3 as indicated, to reveal type I or type III latency promoter usage (46), as previously described (26). Amplification products were detected by Southern analysis using labeled internal exon U as a probe. Positions of DNA size markers are indicated (in base pairs) at the left. (B) EBER1 RNA was detected by Northern analysis of 20 μg of total RNA extracted from each cell line. An in vitro transcript (lane 6) includes the EBER1 sequence and additional RNA from the vector, p386 (54). Positions of RNA size markers are shown (in bases) at the right.
An LMP-1 specific RNA was detected in two clones that were examined by Northern analysis (Fig. 6A). By analyzing the hybridization signals using a PhosporImager (Molecular Dynamics), we determined that RNA from these two EBV-infected 293 clones contained only 1/12 as much LMP-1 transcript as a similar amount of RNA from the EBV-immortalized B-cell line, X50-7. For a comparison, the blot was stripped and reprobed to detect GAPDH mRNA, but it was found that 293 cells contain significantly lower levels of GAPDH mRNA as a fraction of total RNA than is the case for the B-cell lines (Fig. 6C). The ethidium bromide-stained gel showed that similar amounts of cellular RNA had been loaded with all samples (Fig. 6D).
FIG. 6.
Northern analysis of RNA transcripts specific to LMP-1 and LMP-2A/2B genes in infected 293 cells. Fifteen micrograms of total RNA from each cell line was analyzed. The blot was first probed with exon 3 of LMP-1 (A), then stripped and reprobed to detect cellular GAPDH mRNA (C), and finally stripped and reprobed to detect LMP-2A/2B mRNA (B). Probes are described in Materials and Methods. Because 293 cells contain significantly less GADPH mRNA than the B-cell line X50-7 and the pre-B-cell line NALM-6, a photograph of the ethidium bromide-stained gel is shown (D) to demonstrate that similar amounts of rRNA were loaded on the gel. RNA was analyzed from three different sources of 293 cells, designated 293 C, 293 J, and 293 Y. (E) Detection of LMP-1 mRNA by another Northern blot performed in the same manner.
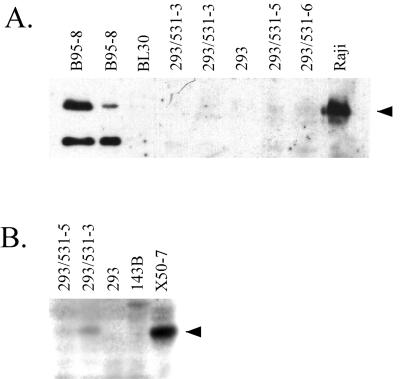
The LMP-1-specific RNA present in the infected 293 clones appears to be a few hundred nucleotides longer than the 2.8-kb LMP-1 mRNA detected in X50-7 cells. The LMP-1 mRNA that is expressed in infected 293 cells may be identical to a 3.5-kb mRNA that was detected in an NPC cell line, C15, and was shown to initiate from a site within the nearest terminal repeat sequence and several hundred bases pairs upstream of the major promoter that is used in B cells (44). In contrast to C15 NPC cells, which were found to express both 2.8- and 3.5-kb LMP-1 mRNA at about equal levels, EBV-infected 293 cells express only the 3.5-kb mRNA detectably by Northern analysis. The amount of LMP-1 protein in the infected 293 cells was determined by Western analysis to be less than 5% of the level present in the EBV-immortalized B-cell line B95-8 (Fig. 7A, left blot) and usually below our limit of detection, although LMP-1 protein appeared to have been detected on more than one occasion (e.g., Fig. 7A, right blot).
FIG. 7.
LMP-1 protein, if present in infected 293 cells, is present at less than 5% of the level typical of EBVC-immortalized B cells. (A) Western analysis using affinity-purified rabbit antibodies directed against LMP-1, detected by chemiluminescence using a horseradish peroxidase-conjugated secondary antibody. From left to right, extracts from 40,000 B95-8 cells, 10,000 B95-8 cells, and 200,000 cells of each of the remaining cell lines were analyzed. (B) Western analysis using LMP-1-specific MAb S12, detected by 125I-labeled secondary antibody. Extracts from 500,000 cells were analyzed for each cell line.
LMP-2-specific RNAs were also detected at low levels in the EBV-infected 293 cell clones (Fig. 6B). LMP-2A and LMP-2B mRNAs, which are about 2.3 and 2.0 kb in size, respectively, differ in that their first exons are initiated from different promoters (25, 45). The predominant LMP-2 mRNA in infected 293 cells appears to be LMP-2B, based on its size.
Increased EGFR on EBV-infected 293 cells.
Previously, forced expression of LMP-1 in an epithelial cell line was shown to result in a large increase in expression of EGFR (33). When two EBV-infected 293 clones were tested for surface EGFR by flow cytometry, a small but reproducible increase was detected in comparison to uninfected 293 cells (Fig. 8). The amount of EGFR present on the EBV-infected 293 cells was lower than the amount detected on 143B cells, a human osteosarcoma-derived cell line which expresses moderate levels of EGFR.
Induction of lytic infection in 293 cells.
Spontaneous expression of lytic antigens was not detected in any of the infected clones, nor could lytic antigens by induced by treating cells with phorbol myristate acetate or with sodium butyrate. Lytic infection could be induced by transfecting cells with a plasmid, pCMV-RZ (17), which forces expression of the EBV lytic activators R and Z. From the data shown in Table 3 and Fig. 9, it can be seen that the amount of Z expressed in transiently transfected 293(P3-531) cells was comparable to the amount expressed spontaneously in the semipermissive marmoset B-cell line, B95-8. Late antigens, VCA and gp350/220, were detected in transfected 293(P3-531) cells but at levels lower than in B95-8 cells (Table 3). Small amounts of G418-resistance-conferring virus were present in the culture fluids two to 4 days following transfection, as detected by infecting BL30 cells (Table 3).
TABLE 3.
Induction of EBV replication and virus production in infected 293 cells
| Cell line | Transfection | % Positivea
|
G418r virus released (CFU/ml)b | ||
|---|---|---|---|---|---|
| Z | VCA | gp350/220 | |||
| 293 | pMCV-RZ | 5.6 | 0.0 | 0.0 | 0 |
| 293(P3-531)-3c | Mock | 0.0 | 0.0 | 0.0 | 0 |
| pCMV-RZ | 5.9 | 1.6 | 0.3 | 22 ± 5 | |
| 293(P3-531)-5 | Mock | 0.0 | 0.0 | 0.0 | 0 |
| pCMV-RZ | 9.3 | 0.8 | 0.0 | 3.7 ± 1.0 | |
| B95-8 | NAd | 6.6 | 6.5 | 4.0 | NAd |
Percentage of cells positive for the antigen by immunofluorescence.
Determined by infection of BL30; average of two measurements ± standard error of the mean.
Carries both parental P3HR1 and recombinant P3-531 EBV genomes.
NA, not applicable.
FIG. 9.
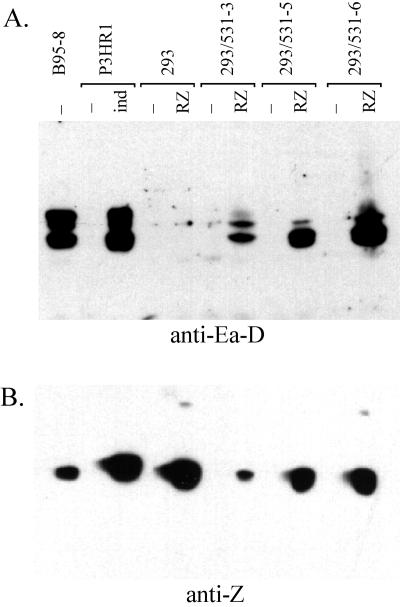
Induction of lytic antigens in infected 293 cells. (A) Detection of EA-D by Western analysis using MAb R3 and a horseradish peroxidase-conjugated secondary antibody. Infected clones of 293 cells were either transfected with pCMV-RZ (17) by the calcium phosphate method (14) with a 20% glycerol shock 5 h later or mock transfected, as indicated. P3HR1 c1 16 cells were induced by diluting a nearly saturated culture into fresh medium containing 3 mM sodium butyrate. Lysates from 200,000 cells of each cell line were analyzed. (B) Detection of EBV Z transactivator by Western analysis using MAb BZ1 as for panel A.
Lytic EBV DNA replication was also very weak in the 293(P3-531) cells transfected with pCMV-RZ in comparison to B95-8 cells (data not shown). We examined these cells for expression of EA-D, several closely related proteins encoded by the BMRF1 open reading frame. BMRF1 encodes a polymerase accessory protein that is essential for EBV lytic DNA replication (9). Expression of EA-D proteins was induced by R and Z in the infected 293 cells (Fig. 8A). A form of EA-D that migrates most slowly by SDS-PAGE was present at very low levels in 293 cells, whereas this form was a major component of EA-D in lytically infected B cells. We do not know whether the limited abundance of the slowest-migrating form of EA-D is related to the low efficiency of induction of lytic replication in these cells.
In other experiments, we found that virus released from the infected 293 cells immortalized B cells with close to the same efficiency that it could stably infect BL30 or Raji cells to provide G418 resistance. These experiments suggest that the viruses isolated in the infected clones of 293 cells are biologically normal, although an ultimate proof of this could be difficult to achieve.
DISCUSSION
We have presented evidence that EBV binds to the surface of 293 cells, a human epithelial cell line, and infects them in a CD21-dependent manner (Fig. 2 and Table 2). At a low efficiency, EBV establishes a latent infection of 293 cells that can be scored by clonal outgrowth if the infecting viral genome carries a selective marker. Previously, it was shown that EBV could infect two epithelial cell lines that had been altered to express high levels of CD21 from a stably transfected cDNA (30). This study is the first to indicate that EBV can infect an epithelial cell line in a CD21-dependent manner when CD21 is expressed endogenously and at a level that is lower than is typical of B cells. Recent studies that are discussed below have suggested that infection of several epithelial cell lines by EBV does not require CD21. While CD21-specific antibodies blocked infection of 293 cells almost completely in our study (Table 2), it remains possible that some infection of these or other epithelial cells could occur independently of CD21 but with a lower efficiency.
In a study similar to ours, Yoshiyama et al. found that recombinant EBV of the Akata strain carrying a G418 resistance gene could be used to stably infect three different gastric carcinoma cell lines to yield G418r colonies (55). Gastric carcinoma is one of several types of carcinoma that sometimes carry EBV genomes (39). The gastric carcinoma lines did not express CD21 mRNA that could be readily detected by RT-PCR, although one of the cell lines gave a weak positive signal. Furthermore, MAb OKB7, which competes with EBV gp350/220 for binding to CD21, was ineffective at blocking infection of the AGS gastric carcinoma cells at antibody concentrations that interfered with the infection of a B-cell line, leading Yoshiyama et al. to conclude that EBV gains entry into the gastric carcinoma cell lines through a receptor other than CD21. More recently the same group reported that many other human epithelial cell lines, most of which expressed little or no detectable CD21 mRNA, were susceptible to infection by EBV if the virus-releasing Akata cells were cocultured with the recipient epithelial cells (19). Perhaps it is worth noting that among several EBV strains examined by Li et al., Akata was the most efficient at infecting epithelial cells expressing an introduced CD21 cDNA, although in this case the infection was clearly CD21 dependent (30).
These results suggest that EBV may be able to infect epithelial cells by more than one mechanism; however, such a conclusion should be approached with caution. First, in the survey of epithelial cell lines made by Imai et al. (19), the six cell lines that were infected most efficiently either transiently or stably were all found to express CD21 or its mRNA at detectable levels either by the authors of the study (cell lines Nu-GC-3, MKN74, DLD-1, and LoVo) or by other labs (293 [this report] and HepG2 [32]). For the remaining cell lines, it is possible that a low level of CD21 expression in a very small fraction of cells in the populations escaped detection but was sufficient to support the amount of EBV infection observed. CD21 expression has been examined very carefully in only a few human epithelial cell lines. Birkenbach et al. detected trace amounts of mRNA in HeLa and RHEK cell lines and, in the case of RHEK, detected CD21 at the cell surface by a very sensitive method of iodination and immunoprecipitation and further showed that it could bind to the EBV membrane protein pg350/220 (3). We did not detect CD21 in extracts of RHEK cells by Western analysis, indicating that they contain much less CD21 than 293 cells. HeLa cells were later reported to express readily detectable amounts of CD21 mRNA when cultured without serum (32), which raises the possibility that for some epithelial cell lines, culture conditions affect the level of CD21 and consequently the susceptibility to infection by EBV.
293 was among the cell lines found by Imai et al. to be susceptible to EBV infection by cell-to-cell contact but, in contrast to our study, not with cell-free virus; in their study, 293 cells did not express CD21 detectably (19). We detected CD21 reproducibly in 293 cells obtained from three different sources, and the cells could be infected either with cell-free EBV or by cocultivation at similar efficiencies, using EBV of P3HR1 strain derivation. It is possible that the 293 cells used by the Takada group are genuinely different from the 293 cell lines used in our study.
For the infection of B cells by EBV, the association of the EBV membrane protein gp350/220 with CD21 is sufficient for the virus to bind to cells, but efficient penetration of the cell membrane has been found to depend on an interaction between a minor EBV membrane glycoprotein, gp42, and HLA class II molecules (28). 293 cells do not have surface HLA class II molecules (Fig. 3). A gp42-specific MAb that can block infection of B cells was found to be incapable of blocking infection of an epithelial cell line that artificially expressed CD21 (29), and an EBV mutant lacking gp42 was able to infect this epithelial cell line at normal efficiency (52). The infection of epithelial cells by EBV must differ mechanistically from the infection of B cells and perhaps involves a coreceptor other than HLA class II, as discussed previously (52).
On the surface of B cells, CD21 is believed to exist in distinct complexes, one with CD35 (CR1) and another with CD19 and TAPA-1 (CD81) and possibly Leu-13 (6, 48). Among these proteins, only TAPA-1 would be available to associate with CD21 on the surface of 293 cells (Fig. 4). It thus appears that none of these proteins, with the possible exception of TAPA-1, is needed for CD21-dependent infection of epithelial cells infection by EBV, although CD21-associated proteins might contribute to some of the differences between epithelial cells and B cells with regard to EBV infection.
These differences between the two cell types are worth considering in light of the low efficiency with which EBV was found to stably infect 293 epithelial cells compared with their infectivity toward B-cell lines (Table 1). In the case of strain P3-531, a derivative of strain P3HR1, much of the 40-fold reduction in efficiency of infecting 293 cells compared to B-cell lines might be explained by the lower surface density of CD21 on 293 cells than on the B cell lines. However, low CD21 levels on 293 cells cannot by itself explain why strain B-652, a derivative of B95-8, infects 293 cells several-thousand-fold less efficiently than it infects B cells (Table 1). Previously, different strains of EBV were found to differ significantly in the ability to infect epithelial cells that were made to express CD21 artificially, relative to their titers in a B-cell transformation assay (30). Strain B95-8 showed the lowest relative efficiency for infecting the epithelial cell line among several EBV strains tested, but a quantitative comparison to P3HR1 was not made since this virus does not transform B cells. It has not been determined whether these strain differences arise from the viruses themselves or instead from the different cell lines used to produce them. Virus stocks of B95-8 and its derivative B-652 were produced in a marmoset B-cell line (also called B95-8), while stocks of the other viruses were released from human B-cell lines. Whatever the explanation is, the strain difference indicates that even though EBV infects these epithelial cells in a CD21-dependent manner, the mechanism of infection must be somewhat different than it is with B cells.
In the case of 293 cells, we know that most cells in a population are capable of binding to EBV in a CD21-dependent manner (Fig. 2) but also that at least one subsequent step that leads to stable, latent infection must be much less efficient (Table 1). Knox et al. selected stably infected clones of SVK-CR2 cells by brute-force screening and found that latent infection became stabilized in few percent of infected cells, which then maintained the infection for over a year without any selection (24). This finding suggests that latent infection of 293 cells may also become stable with a relatively low efficiency.
In the EBV-infected 293 cell clones that were characterized, the infection was tightly latent. Lytic phase gene expression and viral replication could be induced by forcing expression of R and Z transactivators by DNA tansfection (Table 3; Fig. 9) but not by agents that are often effective with B-cell lines (phorbol ester, sodium butyrate, iododeoxyuridine, and nutrient depletion) (data not shown). Even when late gene expression was induced in some cells by forcing expression of R and Z, the amount of virus released per VCA-positive cell was orders of magnitude lower than with the more permissive B-cell lines such as B95-8 (Table 3), indicating that late events in virus replication or maturation are hindered, too, in 293 cells that reach this stage of lytic development. In contrast to our results, Delecluse et al. recently reported that the stable introduction of the EBV genome carrying a selective marker into 293 cells by transfection produced stably infected clones that were partially permissive for EBV lytic development (5), suggesting a significant difference between the two lineages of 293 cells used by separate laboratories. Stably infected clones of gastric carcinoma cell lines were also somewhat permissive for EBV lytic replication (55). SV-CR2 cells could be induced to express differentiation markers and to support EBV lytic replication soon after infection, but both properties were diminished in several stably infected clones that were isolated (24, 30). Permissiveness for EBV replication in epithelial cells has been linked to the extent of epithelial differentiation (56), but the basis for the differences among epithelial cell lines in supporting EBV lytic development remains to be explored.
The pattern of gene expression observed in latently infected 293 cells, which we might call weakly type II because of the low-level expression of LMP-1 and LMP-2, is similar to what has been observed with previous experimental infections of epithelial cell lines. It is notable that in previous studies while LMP-1 mRNA could be detected at low levels by RT-PCR, LMP-1 protein has been either undetectable (24, 30) or only sporadically detected at low levels in rare clones (19, 55). In the present study, LMP-1 was detected in extracts of EBV-infected 293 cells on occasion, but the level was clearly less than 5% of the amount present in an EBV-immortalized B-cell clone, B95-8. In EBV-infected 293 cell clones, we detected at low abundance an LMP-1-specific mRNA that appeared to be several hundred nucleotides longer than the major 2.8-kb mRNA that is expressed in EBV-transformed B-cell lines. This LMP-1 mRNA appears to be the 3.5-kb mRNA that initiates in the nearest terminal repeat sequence about 700 bp upstream of the major LMP-1 gene promoter (data not shown) that was identified previously (44). The 3.5-kb mRNA was detected previously as a minor LMP-1 mRNA in certain EBV-infected B-cell lines but was nearly as abundant as the 2.8-kb LMP-1 mRNA in NPCs and in an NPC cell line (44). A function for this mRNA is not known. Until now, the 3.5-kb LMP-1 mRNA has been observed only in the presence of an equal or greater amount of the 2.8-kb mRNA. Because EBV released from the infected 293 cells appears to immortalize B cells with normal efficiency, it does not seem likely that the virus would have acquired mutations that alter the expression of the LMP-1 gene. The previous studies of experimental infection of epithelial cell lines did not include Northern analysis of LMP-1 mRNA, and so it remains to be determined whether exclusive expression of the 3.5-kb mRNA will be general to infection of experimentally infected epithelial cell lines.
LMP-1 protein is clearly detectable in developing NPC and is likely to be the critical link that EBV contributes to this disease (40). Expression of LMP-1 in the skin of transgenic mice leads to epidermal hypertrophy (53). When expressed from a constitutive, heterologous promoter in human epithelial cell lines, LMP-1 can alter growth characteristics and increase tumorigenicity (4, 18, 37), increase expression of EGFR (33), and interfere wit p53-mediated apoptosis by elevating the level of A20 gene expression (11). It is questionable whether similar effects would result from the very low levels of LMP-1 expression that have been observed in experimentally infected epithelial cell lines. EBV-infected 293 cells appeared to express slightly more EGFR than uninfected 293 cells (Fig. 8), perhaps due to low-level LMP-1 expression, but in a preliminary experiment we were unable to detect any EGF-dependent increase in survival or growth of the EBV-infected lines in medium lacking serum or with reduced serum (data not shown). Since LMP-1 protein is usually either undetectable or present at very low levels in experimentally infected epithelial cell lines, it is reasonable to speculate that EBV infection of undifferentiated epithelial cells might normally result in expression of LMP-1 at levels that would be too low to be oncogenic. In this case, the inappropriate expression of LMP-1 would be a rate-limiting step in the development of NPC, an idea that has been stated before (30).
ACKNOWLEDGMENTS
We thank Bill Sugden for generously providing affinity-purified antibody against LMP-1.
This work was supported by a grant in aid from the American Heart Association to J.D.F. and grants from the NIH, R01 CA4312212 to J.L.Y. and R01 DE12186 to J.D.F.
REFERENCES
- 1.Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. [PubMed] [Google Scholar]
- 2.Baichwal V R, Sugden B. Posttranslational processing of an Epstein-Barr virus-encoded membrane protein expressed in cells transformed by Epstein-Barr virus. J Virol. 1987;61:866–875. doi: 10.1128/jvi.61.3.866-875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkenbach M, Tong X, Bradbury L E, Tedder T F, Kieff E. Characterization of an Epstein-Barr virus receptor on human epithelial cells. J Exp Med. 1992;176:1405–1414. doi: 10.1084/jem.176.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson C W, Rickinson A B, Young L S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature. 1990;344:777–780. doi: 10.1038/344777a0. [DOI] [PubMed] [Google Scholar]
- 5.Delecluse H J, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci USA. 1998;95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon D T, Carter R H. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 7.Fingeroth J D, Benedict M A, Levy D N, Strominger J L. Identification of murine complement receptor type 2. Proc Natl Acad Sci USA. 1989;86:242–246. doi: 10.1073/pnas.86.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingeroth J D, Weis J J, Tedder T F, Strominger J L, Biro P A, Fearon D T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci USA. 1984;81:4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fixman E D, Hayward G S, Hayward S D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992;66:5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frade R, Barel M, Ehlin-Henriksson B, Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc Natl Acad Sci USA. 1985;82:1490–1493. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fries K L, Miller W E, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardella T, Medveczky P, Sairenji T, Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984;50:248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 14.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan J S, Greenspan D, Lennette E T, Abrams D I, Conant M A, Petersen V, Freese U K. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313:1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 16.Heston L, Rabson M, Brown N, Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982;295:160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- 17.Holley-Guthrie E A, Quinlivan E B, Mar E C, Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L F, Chen F, Zheng X, Ernberg I, Cao S L, Christensson B, Klein G, Winberg G. Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene. 1993;8:1575–1583. [PubMed] [Google Scholar]
- 19.Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karajannis M A, Hummel M, Anagnostopoulos I, Stein H. Strict lymphotropism of Epstein-Barr virus during acute infectious mononucleosis in nonimmunocompromised individuals. Blood. 1997;89:2856–2862. [PubMed] [Google Scholar]
- 21.Kieff E, Rickinson A B. Epstein-Barr virus. In: Knipe D M, Fields B N, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 22.Kieff E. Epstein-Barr virus and its replication. In: Knipe D M, Fields B N, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2324–2396. [Google Scholar]
- 23.Kieff E. Epstein-Barr virus—increasing evidence of a link to carcinoma. N Engl J Med. 1995;333:724–726. doi: 10.1056/NEJM199509143331110. . (Editorial; comment.) [DOI] [PubMed] [Google Scholar]
- 24.Knox P G, Li Q X, Rickinson A B, Young L S. In vitro production of stable Epstein-Barr virus-positive epithelial cell clones which resemble the virus: cell interaction observed in nasopharyngeal carcinoma. Virology. 1996;215:40–50. doi: 10.1006/viro.1996.0005. [DOI] [PubMed] [Google Scholar]
- 25.Laux G, Perricaudet M, Farrell P J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 1988;7:769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lear A L, Rowe M, Kurilla M G, Lee S, Henderson S, Kieff E, Rickinson A B. The Epstein-Barr virus (EBV) nuclear antigen 1 BamHI F promoter is activated on entry of EBV-transformed B cells into the lytic cycle. J Virol. 1992;66:7461–7468. doi: 10.1128/jvi.66.12.7461-7468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M A, Yates J L. BHRF1 of Epstein-Barr virus, which is homologous to human proto-oncogene bcl2, is not essential for transformation of B cells or for virus replication in vitro. J Virol. 1992;66:1899–1906. doi: 10.1128/jvi.66.4.1899-1906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Lee, M. A., et al. Unpublished data.
- 28.Li Q, Spriggs M K, Kovats S, Turk S M, Comeau M R, Nepom B, Hutt-Fletcher L M. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Turk S M, Hutt-Fletcher L M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q X, Young L S, Niedobitek G, Dawson C W, Birkenbach M, Wang F, Rickinson A B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 31.Mann K P, Staunton D, Thorley-Lawson D A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985;55:710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael E J, McClellan M, Huebner H, Hostetter M K. Expression of CD21 and synthesis of its ligands by HeLa cells after growth in serum-free medium. J Lab Clin Med. 1995;125:102–112. [PubMed] [Google Scholar]
- 33.Miller W E, Earp H S, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyashita E M, Yang B, Babcock G J, Thorley-Lawson D A. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyashita E M, Yang B, Lam K M, Crawford D H, Thorley-Lawson D A. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 36.Nemerow G R, Wolfert R, McNaughton M E, Cooper N R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2) J Virol. 1985;55:347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson L J, Hopwood P, Johannessen I, Salisbury J R, Codd J, Thorley-Lawson D, Crawford D H. Epstein-Barr virus latent membrane protein does not inhibit differentiation and induces tumorigenicity of human epithelial cells. Oncogene. 1997;15:275–283. doi: 10.1038/sj.onc.1201187. [DOI] [PubMed] [Google Scholar]
- 38.Niedobitek G, Agathanggelou A, Herbst H, Whitehead L, Wright D H, Young L S. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol. 1997;182:151–159. doi: 10.1002/(SICI)1096-9896(199706)182:2<151::AID-PATH824>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Niedobitek G, Young L S. Epstein-Barr virus persistence and virus-associated tumours. Lancet. 1994;343:333–335. doi: 10.1016/s0140-6736(94)91167-3. [DOI] [PubMed] [Google Scholar]
- 40.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 41.Pearson G R, Vroman B, Chase B, Sculley T, Hummel M, Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983;47:193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu L, Rowe D T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhim J S, Jay G, Arnstein P, Price F M, Sanford K K, Aaronson S A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985;227:1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- 44.Sadler R H, Raab-Traub N. The Epstein-Barr virus 3.5-kilobase latent membrane protein 1 mRNA initiates from a TATA-less promoter within the first terminal repeat. J Virol. 1995;69:4577–4581. doi: 10.1128/jvi.69.7.4577-4581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sample J, Liebowitz D, Kieff E. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J Virol. 1989;63:933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaefer B C, Strominger J L, Speck S H. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sixbey J W, Yao Q Y. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science. 1992;255:1578–1580. doi: 10.1126/science.1312750. [DOI] [PubMed] [Google Scholar]
- 48.Tedder T F, Zhou L J, Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol Today. 1994;15:437–442. doi: 10.1016/0167-5699(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 49.Tierney R J, Stevens N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tso J Y, Sun X H, Kao T H, Reece K S, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Kenyon W J, Li Q, Mullberg J, Hutt-Fletcher L M. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson J B, Weinberg W, Johnson R, Yuspa S, Levine A J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990;61:1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]
- 54.Wu T C, Mann R B, Epstein J I, MacMahon E, Lee W A, Charache P, Hayward S D, Kurman R J, Hayward G S, Ambinder R F. Abundant expression of EBER1 small nuclear RNA in nasopharyngeal carcinoma. A morphologically distinctive target for detection of Epstein-Barr virus in formalin-fixed paraffin-embedded carcinoma specimens. Am J Pathol. 1991;138:1461–1469. [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997;71:5688–5691. doi: 10.1128/jvi.71.7.5688-5691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young L S, Lau R, Rowe M, Niedobitek G, Packham G, Shanahan F, Rowe D T, Greenspan D, Greenspan J S, Rickinson A B, et al. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991;65:2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]