Abstract
Objective:
To evaluate the association of number of radical hysterectomies performed per year in each center with disease-free survival and overall survival.
Methods:
We conducted an international, multicenter, retrospective study from patients previously included in the Surveillance in Cervical Cancer collaborative studies. Individuals with FIGO 2009 stage IB1-IIA1 cervical cancer who underwent radical hysterectomy and had negative lymph nodes at final histology were included. Patients were treated in referral centers for gynecologic oncology according to updated national and international guidelines. Optimal cut-offs for surgical volume were identified using an unadjusted Cox proportional hazard model with disease-free survival as the outcome and defined as the value which minimizes the p-value of the split in groups in terms of disease-free survival. Propensity score matching was used to create statistically similar cohorts at baseline.
Results:
2,157 patients were initially included. The two most significant cutoffs for surgical volume were identified in 7 and 17 surgical procedures, dividing the entire cohort in low, middle, and high-volume centers. After propensity score matching, 1,238 patients, distributed as 619 (50.0%) in the high-volume, 523 (42.2%) in the middle-volume and 96 (7.8%) in the low-volume groups, were analyzed. Patients operated in higher volume institutions had a progressively better 5-year disease-free survival than those operated in lower-volume centers (92.3% vs 88.9% vs 83.8%, p=0.029). No 5-year overall survival difference was noted (95.9% vs 97.2% vs 95.2%, p=0.70). Cox multivariable regression analysis showed that FIGO stage >IB1, presence of lymphovascular space invasion, grade >1, tumor diameter >20 mm, minimally invasive surgical approach, non-squamous cell carcinoma histology, and lower-volume centers represented independent risk factors for recurrence.
Conclusion:
Surgical volume of centers represented an independent prognostic factor affecting disease-free survival. Increasing number of radical hysterectomies performed in each center every year was associated with improved disease-free survival.
Précis:
Women with early-stage cervical cancer treated with primary radical hysterectomy had improved disease-free survival when treated in hospitals with a higher surgical volume.
Introduction
Despite the introduction and the implementation of screening and vaccination programs, cervical cancer remains a major burden being the fourth most common cancer diagnosed worldwide [1]. However, the incidence is decreasing in many developed countries, leading to a reduction of the caseload of some centers and of the exposure of trainees [2,3]. The link between hospital case volume and survival improvement has been demonstrated in several cancers, including gynecological malignancies [4–10]. Concerning cervical cancer, few studies have assessed the association between surgical volume and improved survival [11–14].
A recent study aimed to analyze the association between surgical volume and survival of women with early-stage cervical cancer who underwent radical hysterectomy [13]. It concluded that hospital volume for radical hysterectomy may be a prognostic factor for early-stage cervical cancer, as surgery performed at high-volume centers was associated with decreased risk of local recurrence and improved survival. However, this study reported some limitations, such as the use of a single-country national registry database, lack of information on surgical approach and average volume calculated over five calendar years. In addition, improvement of outcomes may not be related only to superior quality of surgery, but also to adherence to guidelines and to the way multidisciplinary care is organized with the availability of imaging and postoperative radiotherapy.
Very recently, the Surveillance in Cervical Cancer (SCCAN) consortium has published two retrospective studies on the annual recurrence risk model for tailored surveillance strategy in patients with cervical cancer [15] and on the post-recurrence survival in patients with cervical cancer [16]. The SCCAN study consortium consisted of 20 tertiary centers of excellence for the treatment of cervical cancer from Europe, Asia, North America, or Latin America. These centers have modern imaging modalities used for clinical staging (magnetic resonance imaging, expert ultrasound, computed tomography, or positron emission tomography/computed tomography). All cases were discussed by a multidisciplinary team, surgery and pathology were performed by surgeon and pathologist with experience in gynecologic oncology, and institutional follow-up was performed by physicians. The present study aimed to assess the prognostic effect (defined in terms of disease-free and overall survival) of surgical volume per center, from patients previously included in the SCCAN collaborative studies.
Methods
The SCCAN is an international, multicenter, retrospective study [15]. Patients were retrospectively included if they met the following inclusion criteria: (i) histologically confirmed cervical cancer treated between 01January 2007 and 31 December 2016; (ii) TNM stage T1a-T2b (based on the preoperative assessment; American Joint Committee on Cancer); (iii) primary surgical management; (iv) and at least one year of follow-up data availability. Patients were treated in national referral centers for gynecologic oncology according to updated national/international guidelines.
For the present study we selected patients with FIGO 2009 stage IB1-IIA1 who underwent type B or C radical hysterectomy [17], who did not undergo neo-adjuvant chemotherapy and with negative lymph nodes at final histology.
The protocol was approved by the institutional review board of the lead institution (General University Hospital in Prague, Czech Republic) in 2016. Institutional review board approval at the participating sites was a prerequisite for participation. The study was performed in accordance with the Declaration of Helsinki.
The principal investigator at each institution identified eligible patients, anonymized the data and transferred the data using a web-based system to ensure consistent data collection, which ended in November 2020.
Patients with missing information on key predictor variables, such as tumor and surgery characteristics (tumor type, tumor size), and details about the follow up (date of the last visit, disease status at the last visit and date of recurrence/death) were excluded.
STROBE guidelines were followed in reporting results of this study [18]. Demographics and clinical data were summarized using mean and standard deviation (SD) when considering quantitative variables and absolute counts and percentages if related to categorical items.
Disease-free survival was defined as the time interval between the date of surgery and the evidence of the first disease progression or death from disease. Overall survival was defined as the time interval between the date of surgery and date of death from any cause. Both times were censored at the date of last follow-up if no event was observed.
The Kaplan–Meier method was used to estimate the distribution of time-to event end points of disease-free survival and overall survival and differences among curves were assessed by the log-rank-test [19,20]. Cox regression analysis was performed to estimate Hazard Ratios (HR) and their 95% confidence intervals and to adjust for baseline risk factors [21].
Optimal cut-offs for surgical volume were identified using an unadjusted Cox proportional hazard model with disease-free survival as outcome and defined as the value which minimizes the p-value of the split in groups in terms of disease-free survival. Number of radical hysterectomies was counted as an average over the entire study period per center.
Propensity Score Matching (PSM) was used to adjust the differences between the two groups (high-volume and low-middle volume centers); a ratio 1:1 and the Nearest-Neighbor method was used without replacement and with a caliper of 0.2 SD of the propensity score distribution. Baseline variables used to formulate propensity scores included age, grade, lymphovascular space invasion (LVSI), pathologic stage, type of surgery and maximum tumor diameter. As residual differences in baseline covariates were observed, we performed a multivariable Cox model to better adjust surgical volume effect. IBM SPSS statistical software v. 27.0 and R v. 4.1.2, library MatchIt were used.
Results
Starting from a database of 4,343 patients, we initially included 2,157 (49.7%) patients according to inclusion criteria (baseline characteristics of the entire population are showed in Appendix 1, available online at http://links.lww.com/xxx). Survival associated with continuous cut-offs of average number of radical hysterectomies performed in each center every year is demonstrated in Appendix 2, available online at http://links.lww.com/xxx. The two most significant cut-offs for surgical volume were identified at 7 and 17 surgical procedures per center every year. We stratified the centers in three groups: centers performing less than 7 radical hysterectomies per year were classified as “low volume”, those performing between 7 and 17 surgical procedures per year as “middle volume” and those performing more than 17 radical hysterectomy per year as “high volume”. In view of the difference in baseline characteristics of patients with different surgical volume per year, a PSM analysis was performed, grouping together low and middle versus high volume. After the PSM, 1,238 patients, distributed as 619 (50.0%) in high-volume, 523 (42.2%) in middle-volume and 96 (7.8%) in low-volume groups, were analyzed. Exclusion process is demonstrated in Figure 1.
Figure 1.
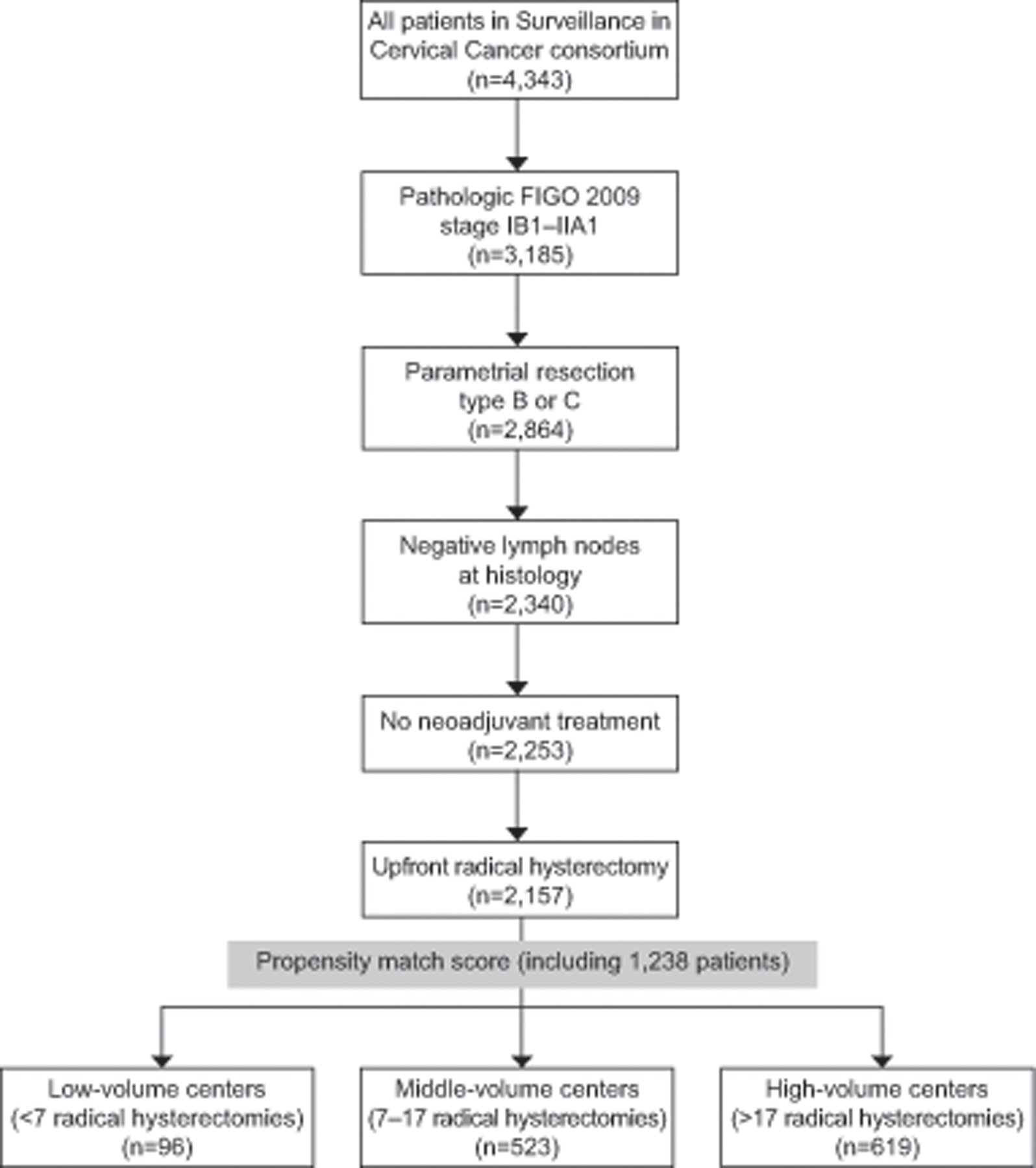
Inclusion and exclusion process. FIGO, International Federation of Gynecology and Obstetrics.
Table 1 shows the clinico-pathological characteristics of the patients analyzed after PSM. Most patients were diagnosed with FIGO stage IB1 (N=1,145, 92.5%), squamous cell carcinoma (N=769, 62.1%), grade 2 (N=920, 74.3%), with negative LVSI (N=593, 47.9%) and underwent open radical hysterectomy (N=885, 71.5%). The majority of patients did not undergo adjuvant treatment after radical surgery (1,124, 90.8%). After PSM the only differences in baseline characteristics were found in grade (higher incidence of grade 3 in low-middle volume centers, p<0.001) and LVSI (higher incidence of negative LVSI in low volume centers, p=0.001). Appendix 3, available online at http://links.lww.com/xxx, shows the temporal matching of the three groups.
Table 1.
Baseline patients’ characteristics after propensity score matching.
| Total (N=1238) | High-volume centers (N=619) | Mid-volume centers (N=523) | Low-volume centers (N=96) | P-value | |
|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 44.7 ±10.4 | 44.7 ± 10.3 | 48.4 ± 11.6 | 46.2 ± 10.1 | 0.89 |
|
| |||||
| Pathological stage | 0.41 | ||||
| IB1 | 1145 (92.5%) | 567 (91.6%) | 485 (92.7%) | 93 (96.9%) | |
| IB2 | 68 (5.5%) | 38 (6.1%) | 27 (5.2%) | 3 (3.1%) | |
| IIA1 | 25 (2.0%) | 14 (2.3%) | 11 (2.1%) | 0 | |
|
| |||||
| Histology | 0.18 | ||||
| Squamous | 769 (62.1%) | 383 (61.9%) | 333 (63.7%) | 53 (55.2%) | |
| Adenocarcinoma | 395 (31.9%) | 193 (31.2%) | 168 (32.1%) | 34 (35.4%) | |
| Adenosquamous | 50 (4.0%) | 27 (4.4%) | 16 (3.1%) | 7 (7.3%) | |
| Others | 22 (1.8%) | 16 (2.5%) | 6 (1.2%) | 0 | |
| Unknown | 2 (0.2%) | 0 | 0 | 2 (2.1%) | |
|
| |||||
| Grade | <0.001* | ||||
| 1 | 147 (11.9%) | 27 (4.4%) | 97 (18.5%) | 23 (24.0%) | |
| 2 | 920 (74.3%) | 540 (87.2%) | 334 (63.9%) | 46 (47.9%) | |
| 3 | 171 (13.8%) | 52 (8.4%) | 92 (17.6%) | 27 (28.1%) | |
|
| |||||
| LVSI | 0.001* | ||||
| No | 593 (47.9%) | 277 (44.7%) | 255 (48.8%) | 61 (63.5%) | |
| Yes | 345 (27.9%) | 169 (27.3%) | 159 (30.4%) | 17 (17.7%) | |
| Unknown | 300 (24.2%) | 173 (27.9%) | 109 (20.8%) | 18 (18.8%) | |
|
| |||||
| Diameter (mm) | 0.77 | ||||
| (mean ± SD) | 20.4±11.9 | 20.2 ± 12.1 | 20.6 ± 11.7 | 19.8 ± 11.1 | |
|
| |||||
| Diameter | 0.68 | ||||
| ≤ 20 mm | 761 (61.5%) | 379 (61.2%) | 319 (61.0%) | 63 (65.6%) | |
| > 20 mm | 477 (38.5%) | 240 (38.8%) | 204 (39.0%) | 33 (34.4%) | |
|
| |||||
| Surgical Approach | 0.12 | ||||
| Open | 885 (71.5%) | 429 (69.3%) | 390 (74.6%) | 66 (68.8%) | |
| Others | 353 (28.5%) | 190 (30.7%) | 133 (25.4%) | 30 (31.2%) | |
|
| |||||
| Adjuvant therapy | 0.62 | ||||
| No | 1124 (90.8%) | 557 (90.0%) | 479 (91.6%) | 88 (91.7%) | |
| Yes | 114 (9.2%) | 62 (10.0%) | 44 (8.4%) | 8 (8.3%) | |
p<0.05
The median follow-up time of the included patients was 5.2 years (IQR: 3.5–7.4). 5-year disease-free survival in the entire cohort was 90.6% (95%CI, 88.8%−92.4%) and 5-year overall survival was 96.4% (95%CI, 95.2%−97.6%). 112 (9.0%) patients had recurrence and 48 (3.9%) patients died in the entire cohort.
A multivariable analysis performed on the 2,157 included patients before performing PSM is demonstrated in Appendix 4, available online at http://links.lww.com/xxx.
Patients operated in higher volume institutions had a progressively better 5-year disease-free survival compared to those operated in lower volume centers (92.3% vs 88.9% vs 83.8%, p=0.029) (Figure 2). However, no 5-year overall survival difference was noted between high, middle, and low volume centers (95.9% vs 97.2% vs 95.2%, p=0.70) (Figure 3).
Figure 2.
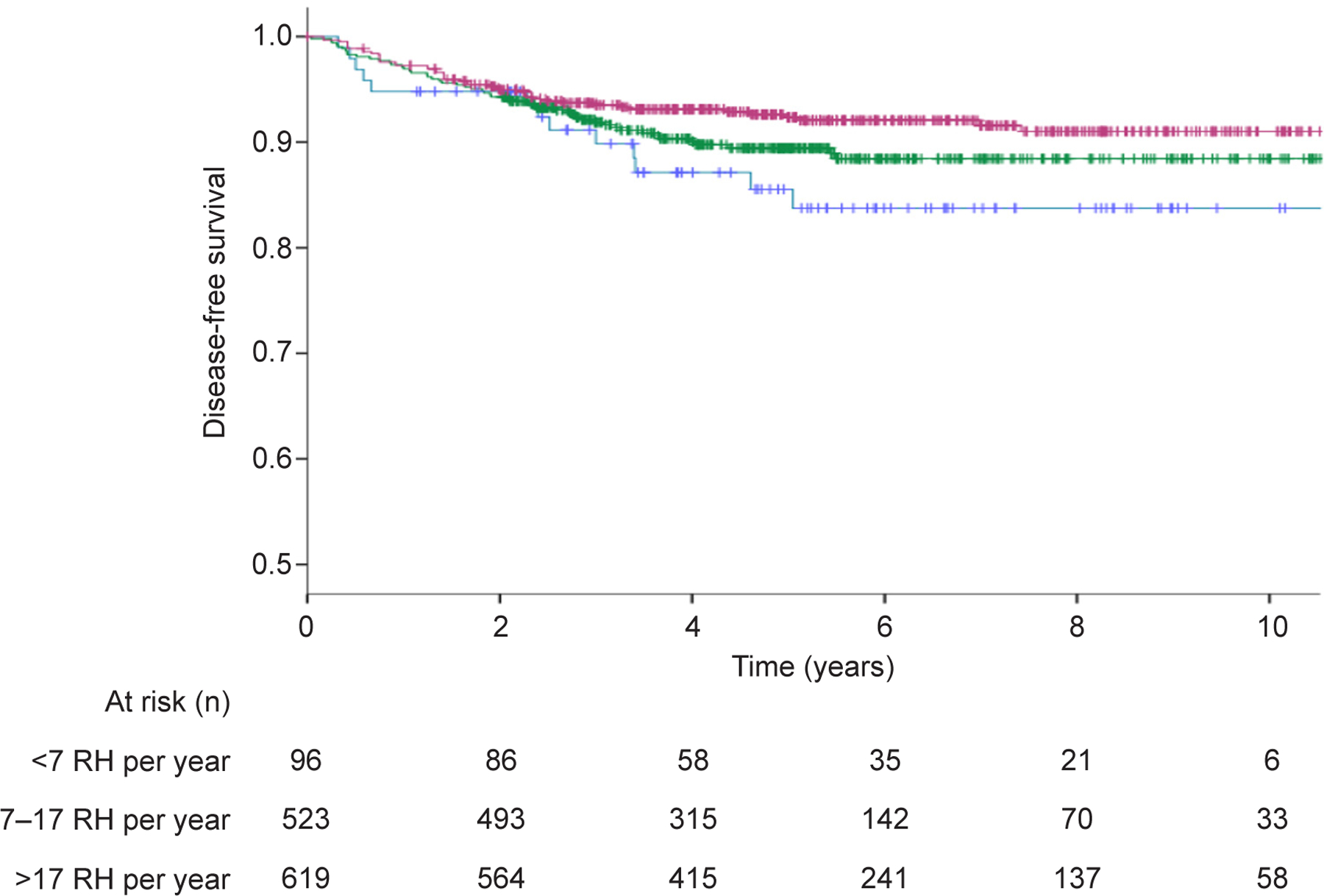
Disease-free survival comparison in patients undergoing radical hysterectomy (RH) in high-volume, middle-volume, and low-volume centers (P=.029). Blue line indicates <7 RH per year; green line indicates 7‒17 RH per year; purple line indicates >17 RH per year.
Figure 3.
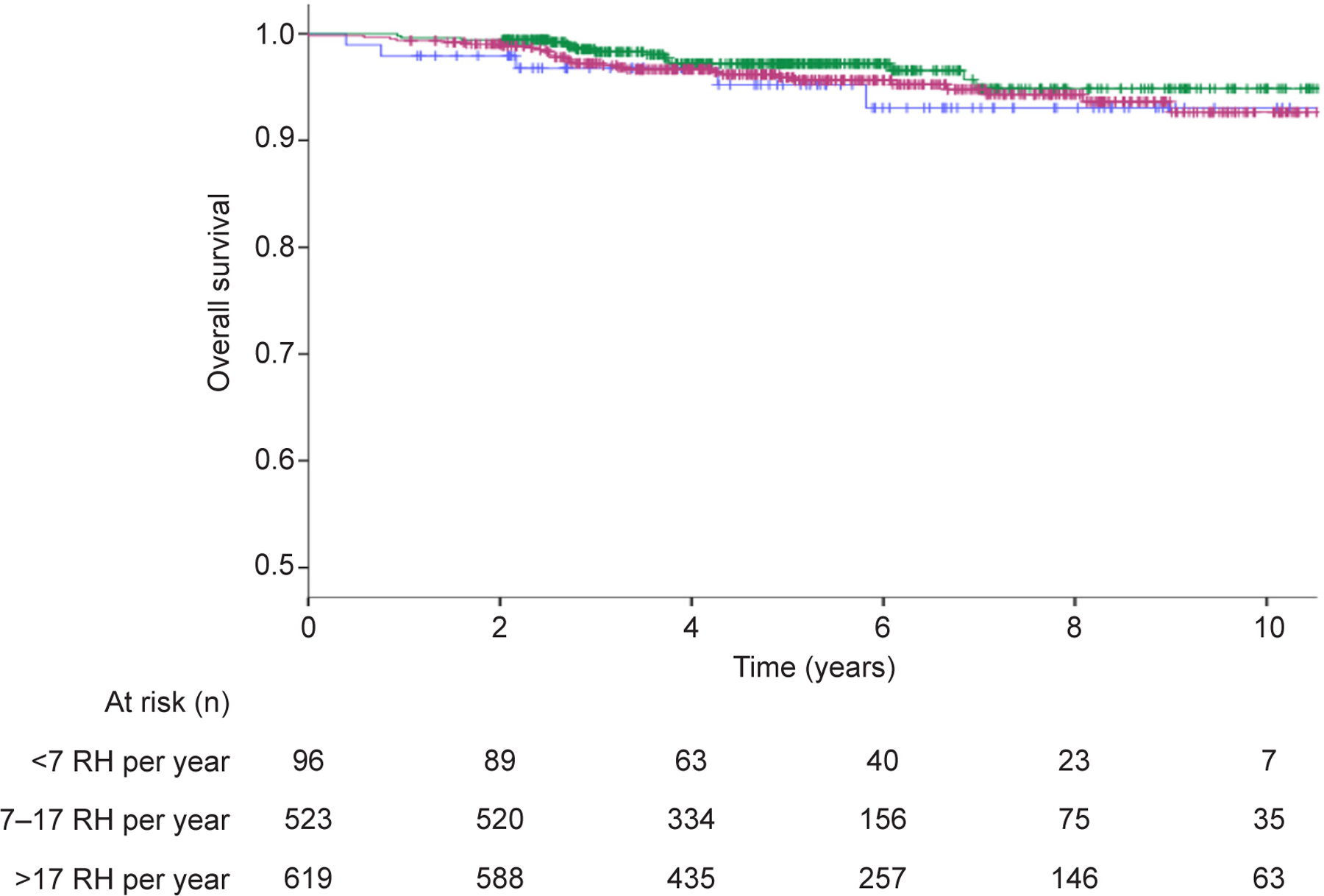
Overall survival comparison in patients undergoing radical hysterectomy in high-volume, middle-volume, and low-volume centers (P=.070). Blue line indicates <7 RH per year; green line indicates 7‒17 RH per year; purple line indicates >17 RH per year.
Table 2 demonstrates the Cox multivariable regression analysis for risk of recurrence in the PSM population. FIGO stage >IB1, presence of LVSI, grade >1, tumor diameter >20 mm, minimally invasive surgical approach, non-squamous cell carcinoma histology, and lower volume centers represented independent risk factors for recurrence. Table 3 shows the Cox multivariable regression analysis for risk of death in the PSM population. FIGO stage >IB1, presence of LVSI, tumor diameter >20 mm and non-squamous cell carcinoma histology represented independent risk factors for death.
Table 2.
Proportional hazard model after propensity score matching for disease-free survival.
| MULTIVARIABLE HR (95% CI) |
||
|---|---|---|
| Age in years | 1.01 (0.99–1.03) | p=0.35 |
|
| ||
| Stage | p=0.03* | |
| 1b1 | 1.00 | |
| 1b2 | 2.18 (1.16–4.08) | |
| 2a1 | 2.00 (0.73–5.52) | |
|
| ||
| LVSI | p=0.005* | |
| No | 1.00 | |
| Yes | 1.79 (1.15–2.78) | |
| unknown | 0.82 (0.48–1.43) | |
|
| ||
| Grade | p=0.002* | |
| 1 | 1.00 | |
| 2 | 4.14 (1.63–10.54) | |
| 3 | 2.08 (0.71–6.12) | |
|
| ||
| Adjuvant therapy | p=0.99 | |
| No | 1.00 | |
| Yes | 1.00 (0.59–1.70) | |
|
| ||
| Diameter | p<0.001* | |
| ≤ 20 mm | 1.00 | |
| > 20 mm | 2.32 (1.53–3.51) | |
|
| ||
| Surgical Approach | p<0.001* | |
| Open | 1.00 | |
| Others | 2.65 (1.78–3.95) | |
|
| ||
| Histotype | p=0.003* | |
| Squamous | 1.00 | |
| Adenocarcinoma | 1.27 (0.81–1.99) | |
| Other | 2.77 (1.53–5.02) | |
|
| ||
| Number of radical hysterectomies per year | p=0.001* | |
| <7 | 1.00 | |
| 7–17 | 0.58 (0.31–1.09) | |
| >17 | 0.32 (0.17–0.61) | |
p<0.05
Table 3.
Proportional hazard model after propensity score matching for overall survival.
| MULTIVARIABLE HR (95% CI) |
||
|---|---|---|
| Age in years | 1.02 (0.99–1.05) | p=0.11 |
|
| ||
| Stage | p=0.004* | |
| 1b1 | 1.00 | |
| 1b2 | 3.90 (1.76–8.64) | |
| 2a1 | 1.31 (0.18–9.75) | |
|
| ||
| LVSI | p=0.045* | |
| No | 1.00 | |
| Yes | 1.98 (1.01–3.91) | |
| unknown | 0.82 (0.35–1.92) | |
|
| ||
| Grade | p=0.30 | |
| 1 | 1.00 | |
| 2 | 3.03 (0.68–13.42) | |
| 3 | 2.22 (0.42–11.62) | |
|
| ||
| Adjuvant therapy | p=0.67 | |
| No | 1.00 | |
| Yes | 1.18 (0.56–2.46) | |
|
| ||
| Diameter | p<0.001* | |
| ≤ 20 mm | 1.00 | |
| > 20 mm | 2.08 (1.07–4.03) | |
|
| ||
| Surgical Approach | p=0.054 | |
| Open | 1.00 | |
| Others | 1.88 (0.99–3.57) | |
|
| ||
| Histotype | p=0.008* | |
| Squamous | 1.00 | |
| Adenocarcinoma | 1.42 (0.69–2.92) | |
| Other | 3.19 (1.35–7.55) | |
|
| ||
| Number of radical hysterectomies per year | p=0.27 | |
| <7 | 1.00 | |
| 7–17 | 0.43 (0.15–1.21) | |
| >17 | 0.55 (0.20–1.48) | |
p<0.05
Discussion
The present study aimed to assess the prognostic effect of radical hysterectomy volume in the SCCAN database consisting of patients from 20 tertiary international centers of excellence for the treatment of cervical cancer. We identified surgical volume of centers as an independent prognostic factor affecting disease-free survival. Higher numbers of radical hysterectomies performed in each center every year was associated with improved disease-free survival.
The favorable survival effect of treating oncologic patients in referral centers has already been demonstrated for multiple cancers [4,5], including gynecological malignancies [6–10]. Regarding cervical cancer, previous studies suggested a possible survival benefit for patients treated in large volume centers [11,13,14]. Lee et al. [12] reported results from a meta-analysis showing comparable survival outcomes in low and high-volume hospitals, but with higher number of patients with poorer prognosis in the latter, and concluded that the benefit of hospital volume should be investigated in well-designed studies. Matsuo et al. [13] conducted a large national registry database retrospective study demonstrating that hospital volume for radical hysterectomy may be a prognostic factor for early-stage cervical cancer and that surgery at high volume centers was associated with decreased local recurrence risk and improved survival. Few differences between our study and the one from Matsuo et al. [13] need to be highlighted. The proposed cut-offs were calculated based on the number of surgeries per center in a five-year period. Moreover, the characteristics of the included patients were different - only 50% of patients had stage IB1 disease, 20% had parametrial involvement, 26% had metastatic lymph nodes and almost 60% of patients received adjuvant treatment. These might represent a limitation when analyzing the association of radical surgery with survival.
We tried to overcome the potential limitations of previous studies, such as the use of national registry databases, lack of information on surgical approach or analysis of a population treated with laparoscopic radical hysterectomy only (now defined as a well-known risk factor, after LACC trial results have been published [22]) and lack of cut-off based on surgeries per year [12–14]. Particularly, with regards to the surgical approach, we have to highlight that in the present study, minimally invasive approaches were associated with a significant risk of recurrence, but not death, at multivariable analysis (Table 2 and 3).
With the use of PSM analysis we tried to adjust the potential differences between baseline groups. However, patients in the high-volume centers still had a higher incidence of LVSI whereas those in the low volume group had higher incidence of grade 3 tumors. These discrepancies could have affected our findings. Our PSM survival analysis showed that patients operated in centers performing more than 17 radical hysterectomies per year had better disease-free survival. This finding was confirmed in multivariable analysis. The lack of overall survival difference may be explained by the relatively low number of events in the included patients (48 deaths, 3.9%). With our results we aim to define a minimum number of radical hysterectomies per year used to describe a center as “high volume”. The need for the identification of a “safe” minimum number of procedures per year was one of the topics discussed in the European Society of Gynaecological Oncology (ESGO) quality indicators for surgical treatment of cervical cancer [23].
There is a clear link between the volume of centers and the surgeons’ learning curve and proficiency. A recent study demonstrated that surgeon’s experience was an independent prognostic factor in the outcome of minimally invasive radical hysterectomy, with a minimum of 18 radical hysterectomies per surgeon as threshold for an improved survival [24]. This hypothesis was confirmed also in case of open radical hysterectomy [25].
In order to quantify the surgical activity across Europe, it should be reported that in a previously published ESGO survey on clinical practice in cervical cancer surgery, only 8% of institutions reported less than 5, 26% of centers performed 10–20, and about 50% reported more than 20 radical hysterectomies annually [26]. Furthermore, it should be highlighted that the inclusion criteria in the present study might have led to an underestimation of the number of radical surgeries per center per year. In our cohort we excluded those patients who had a radical hysterectomy but had unexpected lymph node metastasis on final pathology and patients who had a hysterectomy after neo-adjuvant chemotherapy. As a result, the actual threshold for the number of radical hysterectomies per year that is associated with better outcome may be slightly higher.
We have to recognize few limitations of the present study. First of all, the retrospective nature of the analysis. Secondly, the baseline patients’ characteristics had minor differences even after PSM Thirdly, data on LVSI was missing in 24.2% of cases. Moreover, only 3/20 (15%) centers performed more than 17 radical hysterectomies per year and were considered high volume. Lack of the information on individual surgeon volume and number of surgeons per center. Lastly, we did not report information about peri-operative morbidity. On the other hand, we acknowledge the fact that the present study overcomes limitations such as single country/national registry database, lack of information on surgical approach and the fact we defined a calculated cut-off of number of cases per year to define centers volume. Moreover, this study recorded data from pre-selected academic referral centers adhering to national and international guidelines.
Conclusion
Surgical volume of centers represented an independent prognostic factor affecting DFS in the present retrospective analysis. Increasing number of radical hysterectomies performed in each center every year was associated with improved disease-free survival (but not overall survival).
Supplementary Material
Acknowledgments
The authors thank Martina Borčinová for her contribution in the assistance to the SCCAN collaborative group.
Funding
This work was supported by Charles University in Prague (UNCE 204065 and PROGRES Q28/LF1) and the NIH/NCI Cancer Center Support Grant (P30 CA008748). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Financial Disclosure
Henrik Falconer reports receiving payment from Intuitive Surgical and Surgical Science. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.He WQ, Li C. Recent global burden of cervical cancer incidence and mortality, predictors, and temporal trends. Gynecol Oncol 2021;163(3):583–592. doi: 10.1016/j.ygyno.2021.10.075 [DOI] [PubMed] [Google Scholar]
- 3.Bizzarri N, Pletnev A, Razumova Z, et al. Quality of training in cervical cancer radical surgery: a survey from the European Network of Young Gynaecologic Oncologists (ENYGO) [published online ahead of print, 2022 Jan 6]. Int J Gynecol Cancer 2022;ijgc-2021–002812. doi: 10.1136/ijgc-2021-002812 [DOI] [PubMed]
- 4.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol 2000;18:2327–40. [DOI] [PubMed] [Google Scholar]
- 5.Killeen SD, O’Sullivan MJ, Coffey JC, et al. Provider volume and outcomes for oncological procedures. Br J Surg 2005;92:389–402. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Palis BE, Chi DS, et al. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol 2010;118:262–7. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Chang J, Ziogas A, et al. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol 2014;132:403–10. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Chen L, Hou JY, et al. Association of Hospital Volume and Quality of Care With Survival for Ovarian Cancer. Obstet Gynecol 2017;130(3):545–553. doi: 10.1097/AOG.0000000000002164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JD, Hershman DL, Burke WM, et al. Influence of surgical volume on outcome for laparoscopic hysterectomy for endometrial cancer. Ann Surg Oncol 2012;19(3):948–958. doi: 10.1245/s10434-011-2090-8 [DOI] [PubMed] [Google Scholar]
- 10.Matsuo K, Nishio S, Matsuzaki S, Machida H, Mikami M. Hospital volume-outcome relationship in vulvar cancer treatment: a Japanese Gynecologic Oncology Group study. J Gynecol Oncol 2021;32(2):e24. doi: 10.3802/jgo.2021.32.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JD, Huang Y, Ananth CV, et al. Influence of treatment center and hospital volume on survival for locally advanced cervical cancer. Gynecol Oncol 2015;139(3):506–512. doi: 10.1016/j.ygyno.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee B, Kim K, Park Y, Lim MC, Bristow RE. Impact of hospital care volume on clinical outcomes of laparoscopic radical hysterectomy for cervical cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97(49):e13445. doi: 10.1097/MD.0000000000013445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Shimada M, Yamaguchi S, et al. Association of Radical Hysterectomy Surgical Volume and Survival for Early-Stage Cervical Cancer. Obstet Gynecol 2019;133(6):1086–1098. doi: 10.1097/AOG.0000000000003280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennari P, Gerken M, Mészáros J, et al. Minimal-invasive or open approach for surgery of early cervical cancer: the treatment center matters. Arch Gynecol Obstet 2021;304(2):503–510. doi: 10.1007/s00404-020-05947-y [DOI] [PubMed] [Google Scholar]
- 15.Cibula D, Dostálek L, Jarkovsky J, et al. The annual recurrence risk model for tailored surveillance strategy in patients with cervical cancer [published online ahead of print, 2021 Oct 16]. Eur J Cancer 2021;158:111–122. doi: 10.1016/j.ejca.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cibula D, Dostálek L, Jarkovsky J, et al. Post-recurrence survival in patients with cervical cancer. Gynecol Oncol 2022;164(2):362–369. doi: 10.1016/j.ygyno.2021.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Querleu D, Cibula D, Abu-Rustum NR. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann Surg Oncol 2017;24(11):3406–3412. doi: 10.1245/s10434-017-6031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 20.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50(3):163–70. [PubMed] [Google Scholar]
- 21.Cox DR. Models and Life-Tables Regression. J R Stat Soc Ser B (Methodological) 1972;34(2):187–220. [Google Scholar]
- 22.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med 2018;379(20):1895–1904. doi: 10.1056/NEJMoa1806395 [DOI] [PubMed] [Google Scholar]
- 23.Cibula D, Planchamp F, Fischerova D, et al. European Society of Gynaecological Oncology quality indicators for surgical treatment of cervical cancer. Int J Gynecol Cancer 2020;30(1):3–14. doi: 10.1136/ijgc-2019-000878 [DOI] [PubMed] [Google Scholar]
- 24.Pedone Anchora L, Bizzarri N, Gallotta V, et al. Impact of surgeon learning curve in minimally invasive radical hysterectomy on early stage cervical cancer patient survival. Facts Views Vis Obgyn 2021;13(3):231–239. doi: 10.52054/FVVO.13.3.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li LY, Wen LY, Park SH, et al. Impact of the Learning Curve on the Survival of Abdominal or Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. Cancer Res Treat 2021;53(1):243–251. doi: 10.4143/crt.2020.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dostalek L, Åvall-Lundqvist E, Creutzberg CL, et al. ESGO Survey on Current Practice in the Management of Cervical Cancer. Int J Gynecol Cancer 2018;28(6):1226–1231. doi: 10.1097/IGC.0000000000001314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


