ABSTRACT
The regulation of the intracellular level of ATP is a fundamental aspect of bioenergetics. Actin cytoskeletal dynamics have been reported to be an energetic drain in developing neurons and platelets. We addressed the role of actin dynamics in primary embryonic chicken neurons using luciferase assays, and by measurement of the ATP/ADP ratio using the ratiometric reporter PercevalHR and the ATP level using the ratiometric reporter mRuby-iATPSnFR. None of the methods revealed an effect of suppressing actin dynamics on the decline in the neuronal ATP level or the ATP/ADP ratio following shutdown of ATP production. Similarly, we find that treatments that elevate or suppress actin dynamics do not alter the ATP/ADP ratio in growth cones, the subcellular domain with the highest actin dynamics in developing neurons. Collectively, the data indicate that actin cytoskeletal dynamics are not a significant energy drain in developing neurons and that the ATP/ADP ratio is maintained when energy utilization varies. Discrepancies between prior work and the current data are discussed with emphasis on methodology and interpretation of the data.
Keywords: ATP, Actin, Bioenergetics, Cytoskeleton, DRG, Growth cones
Highlighted Article: ATP hydrolysis associated with actin filament dynamics does not contribute significantly to the cytosolic ATP level or ATP/ADP ratio in embryonic sensory neuron growth cones, the sites of highest actin dynamics.
INTRODUCTION
Actin is an ATPase and, in its soluble form, ATP loading promotes polymerization into filaments (Pollard, 2017; Fig. 1A). Following polymerization into a filament, the hydrolytic activity of actin is enhanced, and the bound ATP is hydrolyzed (Fujiwara et al., 1998). Actin filament turnover is thus a source of ATP utilization, and the growth cone contains the highest density of dynamic actin filaments along the developing axon. A prior study presented evidence that actin filament dynamics in embryonic chicken ciliary neurons consume ∼50% of available ATP following blockade of ATP synthesis (Bernstein and Bamburg, 2003). In resting platelets, actin turnover has similarly been suggested to consume approximately a quarter to half of cellular ATP (Daniel et al., 1986). Thus, actin filament turnover has come to be considered a major drain of cellular ATP and has been discussed as such in multiple reviews (for recent reviews addressing this issue see Wolfe et al., 2020; Gallo, 2020; DeWane et al., 2021; Evers et al., 2021; Bülow et al., 2022).
Fig. 1.
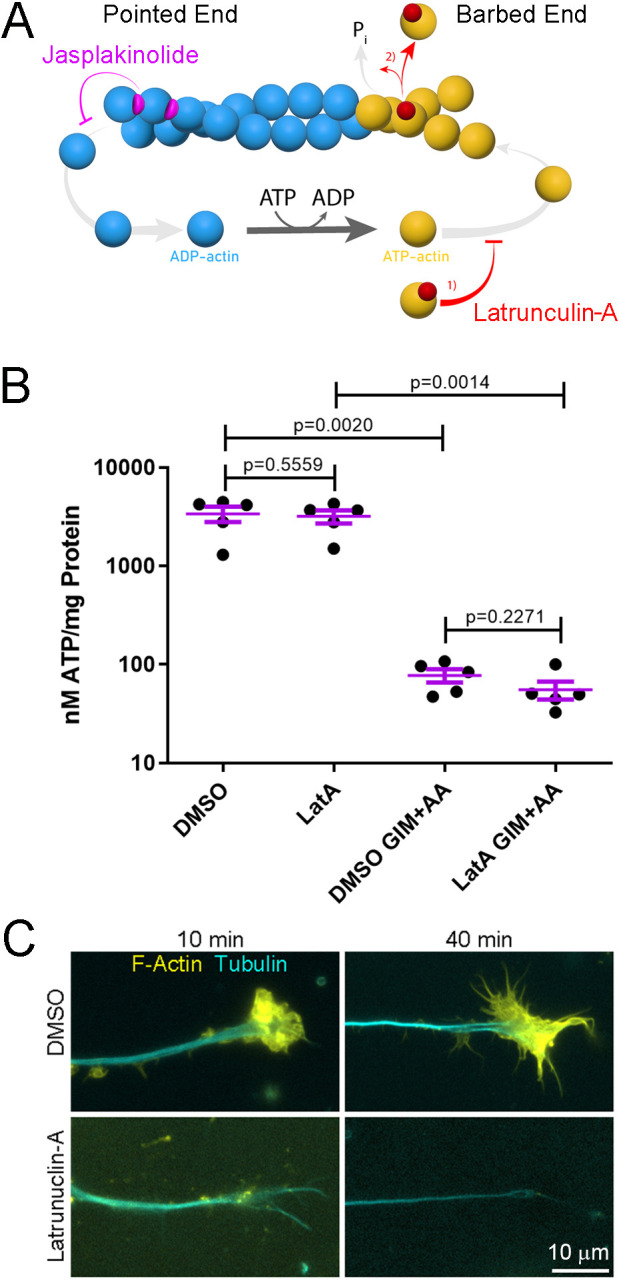
Actin filament depolymerization has no effect on the decrease in the cytosolic ATP level induced by blockade of ATP production in forebrain neurons. (A) Schematic of actin filament polymerization and depolymerization, and the effects of jasplakinolkide and latrunculin-A on actin filament dynamics. Actin monomers (yellow) undergo ATP loading in the cytoplasm priming them for polymerization onto the barbed end of filaments. The ATP is hydrolyzed to ADP+Pi while the actin monomer (blue) is in residence within the filament. ADP actin is then released from the pointed end of the filament and reloaded with ATP. Latrunuclin binds actin monomers and prevents their polymerization (1) and also accelerates the release of actin monomers from filaments (2). Jasplakinolide binds to actin within filaments and inhibits its release, effectively stabilizing the filaments. This leads to the incorporation of the majority of soluble actin into stable filaments when polymerization remains unaltered. (B) A combined suppression of oxidative phosphorylation using antimycin-A (AA) and glycolysis using GIM for 30 min decreases the cellular ATP level as assessed using a luciferase-based assay. A 10 min pretreatment with latrunculin-A does not impact the decrease in the cellular ATP level induced by GIM+AA. Error bars are mean±s.e.m. n=5 for all groups. P-values calculated with an unpaired one-tailed Welch t-test. (C) Representative examples from three cultures per group of the distal axons of forebrain neurons stained to reveal actin filaments (F-actin) and tubulin. The 10 min pre-treatment with latrunculin-A depolymerizes actin filaments and the effect persists until 40 min of treatment reflective of the entire time course of the experiment in B.
Growth cones are dynamic structures localized at the tip of extending axons, and are characterized by the initiation, extension, and retraction of filopodia and lamellipodia (Dent and Gertler, 2003). Filopodia and lamellipodia depend on actin filament nucleation, polymerization and turnover. The plus tips of axonal microtubules penetrate growth cones and undergo bouts of polymerization and depolymerization, collectively referred to as dynamic instability. Microtubule plus tips interact with and regulate the actin cytoskeleton (Pinto-Costa and Sousa, 2021). The bioenergetics of axon extension are not fully understood. Mitochondria and oxidative phosphorylation contribute to developmental and regenerative axon extension (Smith and Gallo, 2018). Although mitochondria are found throughout axons, the density of mitochondria is highest at the growth cone of sensory neurons (Morris and Hollenbeck, 1993; Ketschek and Gallo, 2010). The role of mitochondria, oxidative phosphorylation and glycolysis in regulating growth cone dynamics and extension have begun to be investigated (reviewed in Smith and Gallo, 2018; Ketschek et al., 2021). However, the local regulation of growth cone bioenergetic availability, as reflected by the ATP/ADP ratio and ATP levels, by cytoskeletal dynamics and extrinsic factors that regulate cytoskeletal dynamics is minimally understood.
Neurotrophins are growth factors that control many aspects of neurodevelopment and adult nervous system function (Huang and Reichardt, 2001). Nerve growth factor (NGF) is a major regulator of the biology of dorsal root ganglion (DRG) cutaneous sensory neurons. NGF regulates multiple aspects of mitochondrial function in neurons including mitochondria membrane potential (Verburg and Hollenbeck, 2008), positioning along axons (Chada and Hollenbeck, 2004) and the fission of mitochondria (Armijo-Weingart et al., 2019). Treatment of embryonic sensory neurons with NGF induces rapid elaboration of the growth cone, characterized by increased surface area and filopodia formation driven by actin filament dynamics (Marsick et al., 2010; San Miguel-Ruiz and Letourneau, 2014). However, the effects of NGF on growth cone bioenergetics are not well understood.
The impetus for the current study comes from an earlier report that actin dynamics consume ∼50% of available ATP in developing neurons when ATP production by both oxidative phosphorylation and glycolysis is shut down (Bernstein and Bamburg, 2003), indicating actin filament dynamics and the associated ATP hydrolysis are a major bioenergetic drain for developing neurons. The aim of the current study is to address the role of cytoskeletal dynamics and NGF in the regulation of ATP availability in developing embryonic chicken sensory neurons, specifically at growth cones, as these are the sites of highest actin filament density and turnover in developing neurons. We used modern genetically encoded reporters and imaging approaches for measuring the intracellular ATP/ADP ratio (PercevalHR; Tantama et al., 2013) and ATP level (mRuby-iATPSnFR; Lobas et al., 2019), and luciferase assays to assess the cellular ATP level. We report no detectable role for actin dynamics in either the regulation of growth cone or net cellular ATP. In contrast to Bernstein and Bamburg (2003), we do not find evidence that actin filament dynamics consume detectable amounts of ATP when ATP production is blocked and discuss likely technical reasons for the discrepancy. Based on this evidence we conclude that actin filament dynamics do not impose a detectable energy drain in sensory neuron growth cones, where actin dynamics are highest.
RESULTS
Shutdown of actin filament dynamics does not affect the decrease in cellular ATP level after suppression of oxidative phosphorylation and glycolysis
Bernstein and Bamburg (2003) reported that actin filament dynamics are responsible for ∼50% of ATP hydrolysis in the cell bodies of developing chicken ciliary ganglion neurons using indirect measurements of intracellular ATP levels. The study presented in Bernstein and Bamburg (2003) is briefly summarized below in order to provide a rationale for the current study and approaches. Mg2+ exhibits a 10-fold higher Kd for ATP than ADP or AMP, and thus ATP hydrolysis is expected to result in an increase in cytosolic Mg2+ levels as Mg2+ is released from ATP following hydrolysis. Bernstein and Bamburg (2003) assessed relative changes in ATP as indirectly reflected by measuring changes in intracellular cytosolic Mg2+ levels reported by the Mg2+-binding dye Mg2+ Green, which increases in fluorescence upon binding Mg2+. Bernstein and Bamburg (2003) manipulated actin filament dynamics using the actin-depolymerizing agent latrunculin-A (1 µM) and the actin filament-stabilizing agent jasplakinolide (10 nM) (Fig. 1A). Manipulation of actin dynamics was performed in conjunction with the shutdown of ATP production through combined pharmacological inhibition of oxidative phosphorylation (10 mM sodium azide) and glycolysis (6 mM 2-deoxyglucose). The Bernstein and Bamburg (2003) study reported that impairment of actin filament dynamics suppresses the decline in cytosolic ATP levels after the shutdown of ATP production, as inferred by changes in cytosolic Mg2+ level, by ∼50%.
We used a cell lysate luciferase-based assay to assess net cellular ATP levels. For these studies, we used cultured chicken embryonic forebrain neurons (Kollins et al., 2009) in order to obtain sufficient sample for the biochemical analysis. As in our prior work (Ketschek et al., 2021), to inhibit oxidative phosphorylation we used antimycin-A (AA; 20 µM) and glycolysis was inhibited by transferring the neurons to medium containing no glucose and 10 mM 2-deoxygluose, a glycolysis inhibitor, a treatment condition we refer to as glycolysis inhibition medium (GIM). Consistent with Bernstein and Bamburg (2003), joint suppression of oxidative phosphorylation and glycolysis (GIM+AA) for 30 min decreased cellular ATP levels (Fig. 1B). Treatment with latrunculin-A (4 µM) alone for 30 min did not affect cellular ATP levels (Fig. 1B). Pretreatment (10 min) with latrunculin-A did not impact the decrease in cellular ATP levels induced by treatment with GIM+AA (Fig. 1B). As a positive control, the effectiveness of latrunculin-A treatment on depolymerization of actin filament levels was assessed through phalloidin staining (Fig. 1C). These data are not consistent with the hypothesis that actin filament dynamics are a significant drain for net cellular ATP.
Inhibition of actin filament dynamics does not impact the decline in the ATP/ADP ratio or ATP levels induced by joint suppression of oxidative phosphorylation and glycolysis in either growth cones or cell bodies
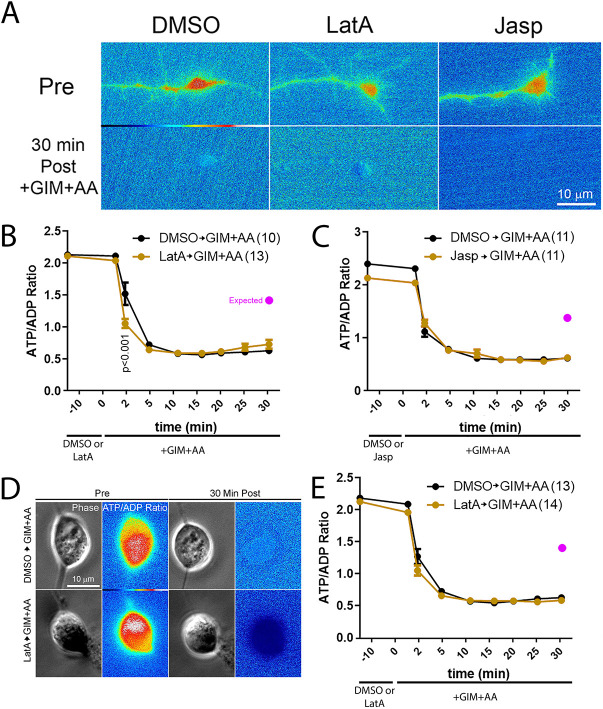
Bernstein and Bamburg (2003) assessed the contributions of actin filament dynamics to the decrease in intracellular ATP after ATP shutdown in the cell bodies of embryonic chicken ciliary neurons. Given the absence of an effect of manipulating actin filament dynamics on the decline in net cellular ATP after blockade of ATP production (Fig. 1), we addressed whether actin dynamics might have a specific role in the cell bodies and growth cones of embryonic chicken sensory neurons raised in continuous NGF. Growth cones contain the highest density of dynamic actin filaments and we thus expected that an effect may be greatest in this subcellular domain. We monitored the ATP/ADP ratio in living neurons using the genetically encoded single molecule sensor PercevalHR (Tantama et al., 2013). PercevalHR binds to either ATP or ADP but exhibits fluorescence emission at differing wavelengths based on the nucleotide binding it, thereby allowing for derivation of the ratio of ATP/ADP. Consistent with our prior assessment (Ketschek et al., 2021), treatment with GIM+AA decreased the ATP/ADP ratio in the growth cones of neurons cultured in continuous NGF (Fig. 2A–C).
Fig. 2.
Manipulation of actin filament dynamics does not impact the decrease in the ATP/ADP ratio or ATP level induced by blockade of ATP production in either growth cones or cell bodies. (A) Representative ratiometric images of the ATP/ADP ratio assessed in growth cones using PercevalHR in growth cones pretreated for 10 min with DMSO, latrunculin-A or jasplakinolide prior to a 30 min treatment with GIM+AA. In all conditions, the ATP/ADP ratio is similarly suppressed. (B) Graph of the ATP/ADP ratio in growth cones as a function of time after a 10 min pretreatment with DMSO or latrunculin-A prior to a 30 min treatment with GIM+AA. Bonferroni post-hoc time point-matched tests yield P>0.05 at all the time points after GIM+AA treatment with the exception of 2 min of GIM+AA treatment in latrunculin-A treatment conditions. (C) Graph of the ATP/ADP ratio in growth cones as a function of time after a 10 min pretreatment with DMSO or jasplakinolide prior to a 30 min treatment with GIM+AA. Bonferroni post-hoc time point-matched tests yield P>0.05 at all the time points after GIM+AA treatment. (B,C) Power analysis, as described in the Materials and Methods section, indicates that to detect a 50% difference in the extent of the decrease of the ATP/ADP ratio following GIM+AA treatment at the latest time point imaged (30 min) a sample size of 2 and 2 in the experimental groups for latrunculin-A or jasplakinolide, respectively, would be required, and the samples sizes were between 10–13 in all groups. (D) Representative ratiometric images of the ATP/ADP ratio assessed in the cell bodies of sensory neurons using PercevalHR that were pretreated for 10 min with DMSO or latrunculin-A prior to a 30 min treatment with GIM+AA. (E) Graph of the ATP/ADP ratio in cell bodies as a function of time after a 10 min pretreatment with DMSO or latrunculin-A prior to a 30 min treatment with GIM+AA. Bonferroni post-hoc time point-matched tests yield P>0.05 at all the time points after GIM+AA treatment. (D,E) As above, power analysis indicates that to detect a 50% difference in the extent of the decrease of ATP/ADP ratio following GIM+AA treatment at the latest time point a sample size of 2 in the experimental group for latrunculin-A would be required, and the data set consisted of 13 cell bodies. In panels B, C and E the purple circle denotes the expected mean for a 50% decrease in the decline of the ATP/ADP ratio. Results are shown as mean±s.e.m.
Similar to Bernstein and Bamburg (2003), who used 1 µM latrunculin-A to block actin filament polymerization, we treated neurons with 4 µM latrunculin-A. Latrunculin-A treatment results in rapid depolymerization of actin filaments in chicken sensory neuron growth cones and loss of filopodia and lamellipodia dynamics at concentrations of 2 µM and higher (Gallo et al., 2002). Bernstein and Bamburg (2003) used 10 nM jasplakinolide to inhibit actin filament turnover. We have previously shown that 40 nM jasplakinolide exhibits maximal effects on stabilizing actin filaments in chicken sensory neuron growth cones (Gallo et al., 2002) and thus used this concentration. Neither pretreatment with jasplakinolide (40 nM) nor latrunculin-A (4 µM) prior to treatment with GIM+AA impacted the time course of the decline in the ATP/ADP ratio induced by GIM+AA treatment in growth cones (Fig. 2B,C). The only difference detected at P<0.05 was a greater decrease in the ATP/ADP ratio in the latrunculin-A treatment conditions at the 2 min of GIM+AA treatment time point relative to GIM+AA treatment alone, counter to the expectation. Measurements of the ATP/ADP ratio were performed over a 30 min period, twice as long as in the study by Bernstein and Bamburg (2003). Analysis of the ATP/ADP ratio in neuronal cell bodies did not reveal any effect of latrunculin-A on the decline in the ATP/ADP ratio induced by GIM+AA (Fig. 2D,E).
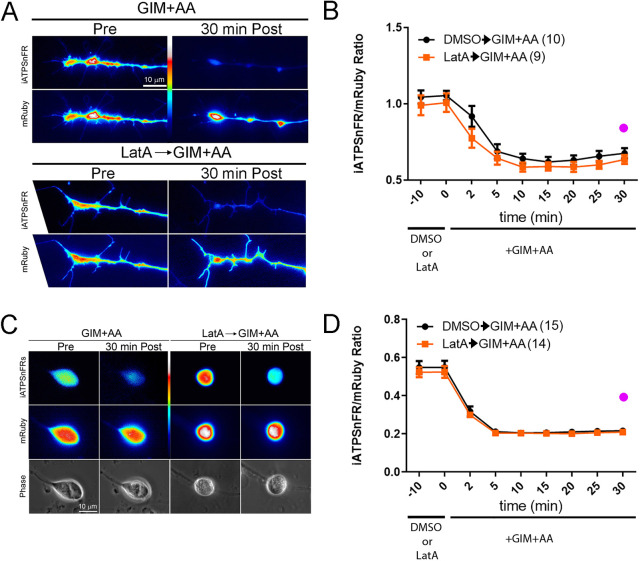
To address the ATP level, as an alternative approach to measuring the ATP/ADP ratio, we used the ratiometric intracellular ATP reporter mRuby-iATPSnFR (Lobas et al., 2019). In this reporter, the ATP-insensitive mRuby is fused to the ATP-sensitive iATPSnFR, and the intensity of mRuby serves as the denominator while the intensity of iATPSnFR as the numerator for the ratio. ATP levels were measured in both the cell bodies and the growth cones. Treatment with GIM+AA decreased the ATP level in growth cones (Fig. 3A,B) and cell bodies (Fig. 3C,D). Pretreatment with latrunculin-A had no impact on the decrease in the level of ATP in either growth cones (Fig. 3A,B) or cell bodies (Fig. 3C,D). Collectively, the data from PercevalHR and mRuby-iATPSnFR measurements fail to demonstrate a role for actin filament dynamics in the depletion of intracellular ATP levels after a shutdown of ATP synthesis at both the cell body and growth cone.
Fig. 3.
Depolymerization of actin filaments has no effect on the decline in the level of ATP induced by blockade of ATP production in either growth cones or cell bodies. (A) Examples of the effects of GIM+AA and pretreatment with latrunculin-A or jasplakinolide on the intensity of iATPSnFR and mRuby in growth cones. For presentation, the iATPSnFR and mRuby channels are shown separately as the ensuing average ratio at baseline is approximately one (growth cones, B) or lower (cell bodies, D). The iATPSnFR intensity in growth cones decreases to a similar extent from the initial baseline with or without latrunculin-A pretreatment (10 min) after a 30 min treatment with GIM+AA. (B) Graph of the iATPSnFR/mRuby ratio in growth cones as a function of time after a 10 min pretreatment with latrunculin-A or DMSO followed by treatment with GIM+AA. Bonferroni post-hoc time point-matched tests after GIM+AA treatment between DMSO and LatA treatment yield P>0.05 at all time points. (C) Examples of the iATPSnFR and mRuby channels in the cell bodies of sensory neurons pretreated with DMSO or latrunculin-A followed by a 30 min treatment with GIM+AA. Phase-contrast images of the cell bodies are also shown. (D) Graph of the iATPSnFR/mRuby ratio in cell bodies as a function of time after a 10 min pretreatment with latrunculin-A or DMSO followed by treatment with GIM+AA. Bonferroni post-hoc time point-matched tests after GIM+AA treatment between DMSO and LatA treatment yield P>0.05 at all time points. (A–D) Power analyses indicate that to detect a 50% difference in the extent of the decrease of the mRuby-iATPSnFR ATP ratio following GIM+AA treatment at the latest time point sample sizes of 2 and 5 in the experimental groups for latrunculin-A would be required for cell bodies and growth cones, respectively, and 14 and 9 cell bodies and growth cones were sampled, respectively. In panels B and D, the purple circle denotes the expected mean for a 50% decrease in the decline of the iATPSnFR/mRuby ratio. Results are shown as mean±s.e.m.
Inhibition of actin filament dynamics does not alter the ATP/ADP ratio in growth cones in continuous NGF treatment conditions
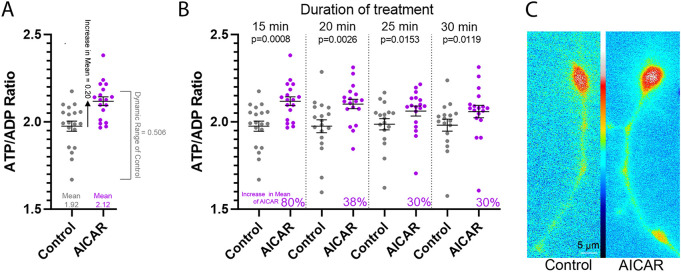
Growth cones contain the highest density of dynamic actin filaments in neurons and are thus expected to be a subcellular domain wherein actin filaments dynamics could be contributing significantly to the consumption of ATP. In order to further test the hypothesis that actin filament dynamics are a drain on cytosolic ATP, we sought to determine whether suppression of actin filament dynamics would impact the endogenous ATP/ADP ratio in growth cones in the absence of the shutdown of ATP production in neurons cultured in continuous NGF. Based on the report by Bernstein and Bamburg (2003), we predicted that we would detect an increase in the ATP/ADP ratio in growth cones due to decreased actin filament dynamics driven consumption of ATP. We first assessed whether PercevalHR could report on increases in the ATP/ADP ratio in growth cones. To increase the ATP level we treated cells with the AMP-activated protein kinase activator AICAR (1 mM). AICAR has been previously shown to increase the cellular level of ATP in multiple cell types including neurons (Rosenkranz et al., 1986; Galiñanes et al., 1992; Hardie and Carling, 1997; Dagher et al., 1999; Menze et al., 2010; Anilkumar et al., 2013; Chen et al., 2015). Treatment with AICAR increased the ATP/ADP ratio in a time-dependent manner; by 80% at 15 min to 30% at 30 min of treatment (Fig. 4). These data indicate that PercevalHR is competent to reveal a 50% increase in the ATP/ADP ratio, as expected based on the estimated 50% consumption of ATP by actin dynamics reported by Bernstein and Bamburg (2003).
Fig. 4.
AICAR treatment increases the ATP/ADP ratio in growth cones. (A) Example of the calculation of the percent increase in the mean after treatment with AICAR. The 15 min time point from B is used for demonstration. Mean±s.e.m. shown throughout the figure. The dynamic range of the PercevalHR ATP/ADP ratio measurements in the control is determined by subtracting the lowest (min) measurement from the highest (max). The increase in the absolute value of the mean after AICAR treatment is determined by subtracting the AICAR mean from the control mean. The percentage increase in the mean within the dynamic range of the control is then determined by dividing the increase in the absolute value of the mean after AICAR treatment by half of the dynamic range. A 50% increase in the control mean would be represented by an increase of 25% of the dynamic range. (B) Treatment with 1 mM AICAR for 15–30 min increases the ATP/ADP ratio in growth cones. The percentage increase in the mean after AICAR treatment is shown within each time point and derived as shown in A. Pretreatment mean ratios for the control and AICAR treatment groups did not differ (P=0.22) and sampling was performed at the denoted times after treatment. Control group, n=17–19; AICAR treatment group, n=18. P-values calculated with an unpaired one-tailed Welch t-test. (C) Representative ratiometric image of the ATP/ADP ratio before and after AICAR treatment (20 min). Although the ATP/ADP ratio is increased this results in a minimally visible change in the coloring of the ratio image because the value of the pixels representing the ratio is mapped using the associated look up table (between panels) that covers the whole range of pixel values, as in other ATP/ADP ratiometric images throughout.
We used power analysis to address whether the sample sizes of the data sets in the presented experiments have sufficient statistical power to resolve increases in the means of the ATP/ADP ratio. Power analysis was performed for 80% power and an alpha value of P=0.05. In pre–after treatment comparison experiments (e.g. before and after inhibition of actin filament dynamics), the expected mean after an experimental treatment was estimated as a percentage change from the mean of the pretreatment time point. For example, considering a pre-treatment data set assessing the ATP/ADP ratio exhibiting a mean of 1.9066±0.0987 (s.d.) and maximum and minimum ATP/ADP ratio measurements of 2.123 and 1.669, respectively, this yields a dynamic range of measurement of 0.454 (2.123−1.669). For power analysis to predict the sample size needed to detect a 50% difference in the control pre-treatment mean (1.9066) within the pretreatment dynamic range of measurement, the expected mean was predicted to be 1.9066+[(2.123−1.669)/4]=2.0201. These calculations were selected to be most stringent in the calculation of the power analysis and rule out the likelihood of increases being below the range of measurement.
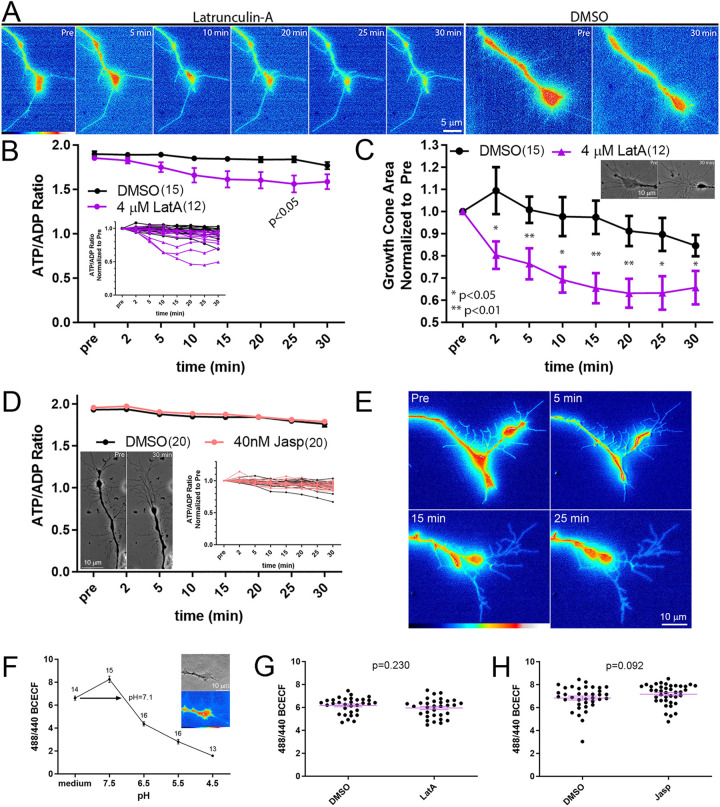
Timelapse imaging of the ATP/ADP ratio failed to reveal a change in the ATP/ADP ratio of growth cones in response to a 30 min treatment with 4 µM latrunculin-A (Fig. 5A,B), while the area of the growth cones decreased as an expected consequence of actin filament depolymerization by 2 min of treatment (Fig. 5C). Although there was a trend toward a decrease in the ATP/ADP ratio after latrunculin-A treatment, counter to the expectation, the only time point at which a difference was detected at P<0.05 was at 25 min of treatment (Fig. 5B). The difference reflected a decrease in the ATP/ADP ratio in latrunculin-A-treated growth cones, which is counter to the expected increase due to suppression of actin filament dynamics-mediated consumption of ATP if the former were a drain of ATP. Considering that the ATP/ADP ratio after latrunculin-A treatment exhibited a minor trend toward a decrease, the treatment with latrunculin-A is assessed as having no effect on increasing the ATP/ADP ratio in growth cones as would be expected if actin dynamics reflected a considerable source of ATP hydrolysis.
Fig. 5.
Neither depolymerization nor hyper-stabilization of actin filaments impacts the ATP/ADP ratio of growth cones in continuous NGF. (A) Representative ratiometric images of the ATP/ADP ratio assessed in growth cones using PercevalHR. The ratio is shown before and at multiple time points (min) after treatment with 4 µM latrunculin-A and before and 30 min after treatment with DMSO. (B) Graph of ATP/ADP ratio before and 2–30 min after treatment with 4 µM latrunculin-A (LatA) or DMSO (mean±s.e.m.). Throughout this and other figures, when the error bars are not evident, it is because they overlap with the symbol representing the mean. The inset shows the same data set, but measurements from individual growth cones are graphed as separate lines normalized to the ratio of the individual growth cone at the pre-treatment time point. Power analysis indicates that a sample size of 12 growth cones is required to detect a 50% difference in the mean of the ATP/ADP ratio from pre-treatment, and the data set contains 15 and 12 growth cones for the control and latrunculin-A groups, respectively. (C) Graph of the measurement of growth cone area as a function of time after treatment with LatA or DMSO. Inset shows an example of a growth cone collapse before and at 30 min of LatA treatment. Mean±s.e.m. P-values in B and C calculated using Bonferroni post-hoc multiple comparison tests between groups within each time point. (D) Graph of ATP/ADP ratio before and 2–30 min after treatment with 40 nM jasplakinolide (Jasp) or DMSO (mean±s.e.m.). The s.e.m. bars at some time points overlap with the symbol and are not visible. The graph inset shows the same data set but measurements from individual growth cones are graphed as separate lines normalized to the ratio for the individual growth cone at the pre-treatment time point. The image inset shows the characteristic response of growth cones to Jasp (Gallo et al., 2002). Power analysis showed that a sample size of 12 growth cones would be required to detect a 50% difference in the pre-treatment mean of the ATP/ADP ratio, and the data set consisted of 20 growth cones in each group. (E) The ATP/ADP ratio is shown before and at multiple time points (min) after treatment with 40 nM jasplakinolide. (F) Example of calibration curve for BCECF measurements of pH. The estimated growth cone intracellular pH reported by BCECF (25 nM, 5 min loading) in cultures is 7.1, within the expected physiological range. Mean±s.e.m. is shown throughout the figure. At 4.5 pH, the error bars are too small to be resolved. The inset shows a growth cone, imaged using phase-contrast in the top image, and the resultant BCECF ratiometric image providing an estimate of pH 7.4. (G) A 30 min treatment with 4 µM latrunculin-A does not impact BCECF ratiometric measurements. DMSO, n=31; LatA, n=32. (H) A 30 min treatment with 40 nM jasplakinolide does not impact BCECF ratiometric measurements. DMSO, n=37; Jasp, n=38. Mean±s.e.m. is shown in G and H. P-values calculated with an unpaired two-tailed Welch t-test.
Conversely, we blocked actin filament turnover by treating with jasplakinolide, as also performed by Bernstein and Bamburg (2003). A 30 min treatment with jasplakinolide treatment failed to impact the ATP/ADP ratio in growth cones (Fig. 5D). However, jasplakinolide treatment led to retraction of the axon tip (Fig. 5D,E), as expected by its previously described effects due to myosin-driven contraction of the stabilized filaments (Gallo et al., 2002). Similar to what was seen with latrunculin-A, jasplakinolide was not found to appreciably alter the ATP/ADP ratio in growth cones.
Neither latrunculin A nor jasplakinolide changed intracellular pH, which might impact PercevalHR measurements (Tantama et al., 2013), as determined by 2′,7′-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF; James-Kracke, 1992) ratiometric measurements of pH (Fig. 5F–H). Collectively, these data indicate that inhibition of actin filament dynamics does not impact the ATP/ADP ratio in growth cones.
Inhibition of actin dynamics during acute treatment with NGF does not alter the ATP/ADP ratio in growth cones as the growth cones undergo NGF-induced morphologic elaboration
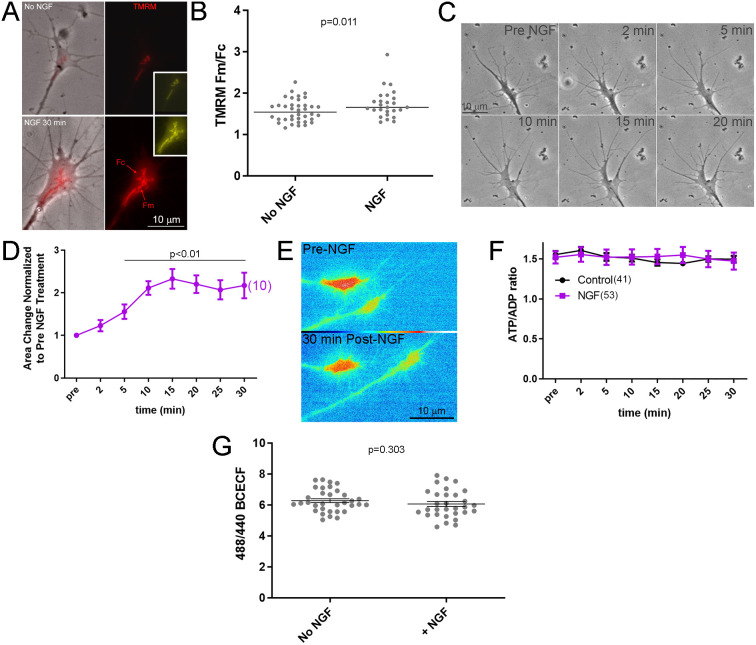
Treatment with NGF hyperpolarizes axonal mitochondria (Verburg and Hollenbeck, 2008), suggesting elevated ATP production. Using the same approach as Verburg and Hollenbeck (2008), imaging of the dye TMRM, which accumulates in mitochondria as a function of hyperpolarization, we also detected that treatment with NGF results in a hyperpolarization of mitochondria in sensory growth cones as reflected by an increase in TMRM fluorescence (Fig. 6A,B). We used live imaging of the ATP/ADP ratio to address whether NGF altered the ATP/ADP ratio during the period when NGF signaling is at its maximum amplitude (Kaplan et al., 1991; Vetter et al., 1991; Soltoff et al., 1992 ; Sainath and Gallo, 2021) and growth cones undergo an increase in surface area and morphologic complexity due to increased actin filament nucleation and polymerization (Marsick et al., 2010; San Miguel-Ruiz and Letourneau, 2014). The surface area of growth cones was expectedly increased by 5 min of treatment with NGF (Fig. 6C,D). However, the ATP/ADP ratio in growth cones did not change during the same period (Fig. 6E,F). Acute NGF treatment did not affect pH in growth cones (Fig. 6G).
Fig. 6.
Acute NGF treatment does not change the ATP/ADP ratio in growth cones but hyperpolarizes mitochondria. (A) Examples of TMRM staining intensity in growth cones not treated with NGF (No NGF; 30 min treatment with NGF vehicle) and at 30 min after treatment with NGF (NGF). The TMRM signal is also shown in yellow for colorblind individuals. (B) NGF treatment (30 min) increases TMRM staining intensity in growth cone mitochondria assessed as in Verburg and Hollenbeck (2008) and described in the Materials and Methods. The data reflect the ratio of mean TMRM staining intensity within regions of interest defined by the presence of mitochondria (Fm) and the cytoplasm devoid of mitochondria (Fc) (see A, NGF-treated growth cone). The median is highlighted. No NGF, n=38; NGF, n=26. P-value calculated with an unpaired one-tailed Welch t-test. (C) Phase-contrast imaging of the elaboration of growth cone area in response to treatment with NGF (2–20 min after NGF treatment is shown). (D) The area of growth cones increases by 5 min of treatment with NGF. Mean±s.e.m. P-value calculated with Dunnett multiple comparisons to pre-treatment group. (E) Ratiometric images of the ATP/ADP ratio in two growth cones before and at 30 min after NGF treatment. (F) Graph of the ATP/ADP ratio in growth cones before treatment (Pre, no NGF) with NGF and as a function of time after treatment with NGF. Control reflects treatment with NGF vehicle in no NGF conditions. Mean±s.e.m. Dunnett post-hoc tests within the NGF treatment group relative to the pretreatment ratio did not yield P<0.05 for any time point. Similarly, Bonferroni post-hoc analysis between no NGF and NGF treatment groups yielded P>0.05 at all time points. (G) Graph of intracellular pH measurements assessed using BCECF in no NGF and at 30 min treatment with NGF. Mean±s.e.m. No NGF, n=33; +NGF, n=30. P-value calculated with an unpaired two-tailed Welch t-test.
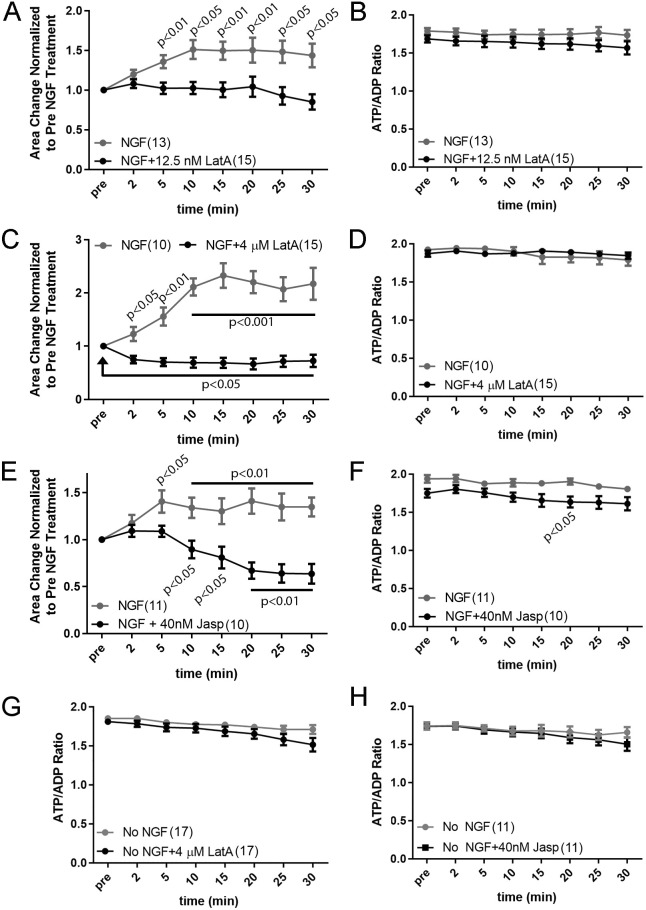
In response to acute NGF treatment, the actin filament level in growth cones is elevated by 100% and 180% at 10 and 15 min of treatment, respectively (Roche et al., 2009; Marsick et al., 2010). As actin filament content and dynamics are increased by NGF, and as proposed by Bernstein and Bamburg (2003), actin dynamics might be a pronounced sink of neuronal ATP utilization and the NGF-induced increase in actin filaments in growth cones could be masking an NGF-induced increase in ATP production. We thus sought to address the impact of increased actin filament dynamics and content on the ATP/ADP ratio in growth cones following acute NGF treatment. First, through a concentration–response curve (data not shown), we determined that treatment with 12.5 nM latrunculin-A along with NGF blocked the NGF-induced increase in growth cone surface area and maintained the area at pre-NGF treatment conditions (Fig. 7A). However, treatment with 12.5 nM latrunculin-A did not impact the ATP/ADP ratio in growth cones following NGF treatment relative to NGF treatment alone (Fig. 7B), as predicted if an increase in ATP utilization by the ensuing NGF-induced increase in actin filament dynamics were driving growth cone elaboration and masking an increase in ATP production. Furthermore, pretreatment with 4 µM latrunculin-A, which depolymerizes filaments and decreases growth cone surface area following acute NGF treatment (Fig. 7C), also did not impact the ATP/ADP ratio after NGF treatment relative to NGF treatment alone (Fig. 7D). Similarly, treatment with 40 nM jasplakinolide prior to NGF blocked the NGF-induced increase in growth cone surface area (Fig. 7E) but did not alter the ATP/ADP ratio in growth cones following NGF treatment with the exception of the 20 min post-treatment time point at which the jasplakinolide treatment group exhibited a decrease (Fig. 7F). Neither latrunculin-A nor jasplakinolide had an effect on the ATP/ADP ratio in the absence of NGF treatment (Fig. 7G,H). These data indicate that increased actin filament dynamics and filament levels in response to acute NGF treatment are not impacting the ATP/ADP ratio in growth cones.
Fig. 7.
Suppression of actin filament dynamics in response to acute NGF treatment blocks NGF-induced growth cone elaboration but does not affect the ATP/ADP ratio. (A) 12.5 nM latrunculin-A (LatA) prevents the NGF-induced increase in growth cone area. Bonferroni post-hoc time point-matched tests between NGF and NGF+LatA are shown. Throughout the figure, NGF treatment groups included treatment with equivalent volumes of DMSO, the vehicle for both LatA and jasplakinolide. Dunnett multiple comparisons test P-values for NGF compared to the pre-treatment values are as indicated; for each time point after LatA treatment Dunnett multiple comparisons test to the pre-treatment values all yield P>0.05. (B) 12.5 nM latrunculin-A treatment does not alter the ATP/ADP ratio following treatment with NGF. P>0.05 for all Bonferroni post-hoc time point-matched tests. (C) 4 µM latrunculin-A decreases growth cone area following treatment with NGF. Bonferroni post-hoc time point-matched tests between NGF+DMSO and NGF+LatA are shown associated with the NGF treatment data. Dunnett multiple comparisons for each time point after LatA treatment to the pre-treatment values all yield P<0.05. (D) 4 µM latrunculin-A treatment does not alter the ATP/ADP ratio following treatment with NGF. P>0.05 for all Bonferroni post-hoc time point-matched tests. (E) 40 nM jasplakinolide (Jasp) decreases growth cone area following treatment with NGF. Bonferroni post-hoc time point-matched tests between NGF+DMSO and NGF+Jasp are shown associated with the NGF treatment data. Dunnett multiple comparisons for each time point after jasplakinolide treatment relative to pre-treatment are shown associated with the jasplakinolide treatment data. (F) 40 nM jasplakinolide treatment does not alter the ATP/ADP ratio following treatment with NGF. Bonferroni post-hoc time point-matched tests between NGF and NGF+Jasp only reveal a difference at 20 min of treatment. (G) 4 µM latrunculin-A does not alter the ATP/ADP ratio in the no NGF condition. P>0.05 for all Bonferroni post-hoc time point-matched tests. (H) 40 nM jasplakinolide does not alter the ATP/ADP ratio in the no NGF condition. P>0.05 for all Bonferroni post-hoc time point-matched tests. For B, D, F–H, power analysis to assess required sample sizes to detect a 50% difference in the means of the ATP/ADP ratios after treatment with latrunculin-A or jasplakinolide based on the NGF treatment control, or control treatment in the absence of NGF treatment control, provide required samples sizes of 10 axons per group and sample sizes were equal to or greater than 10 in all groups. Results are shown as mean±s.e.m.
Suppression of microtubule plus tip dynamics during the initial stages of NGF signaling does not impact the ATP/ADP ratio in growth cones but prevents growth cone elaboration
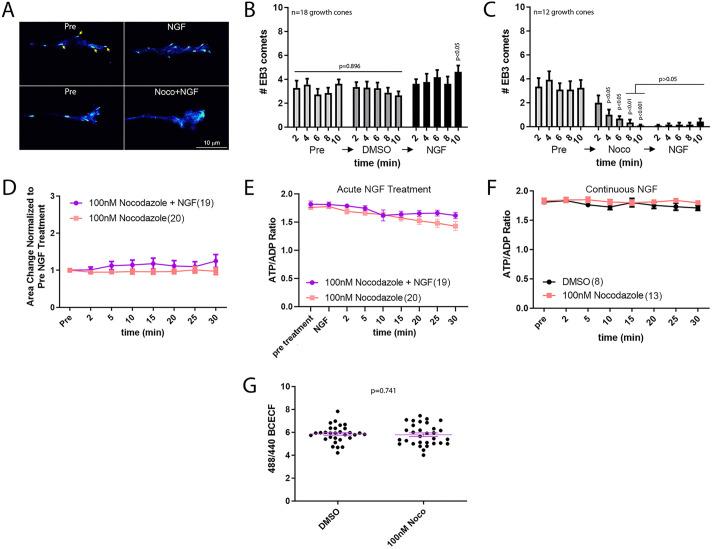
Dynamic instability and polymerization of the plus tips of microtubules regulate axon extension, growth cone dynamics and guidance, actin filament dynamics and a variety of other processes in cells (Dent and Gertler, 2003). We have previously reported that a 30 min treatment with NGF results in a doubling of the microtubule mass in distal sensory axons and increases the number of actively polymerizing microtubule plus tips along axons (Spillane et al., 2012). Microtubule plus tip polymerization and dynamics regulate actin filament polymerization in growth cones and lamellipodial and filopodial dynamics (Tanaka et al., 1995; Gallo, 1998; Rochlin et al., 1999). Thus, we sought to determine whether suppression of microtubule plus tip dynamics during the initial stages of NGF treatment might contribute to the effects of NGF on growth cone elaboration. To suppress dynamic instability, we used a previously detailed pharmacological approach using low concentrations of the microtubule assembly inhibitor nocodazole (Rochlin et al., 1996; Gallo and Letourneau, 1999). As a positive control for the efficacy of nocodazole in suppressing microtubule plus tip dynamics, we imaged plus tip dynamics using GFP-tagged end-binding protein 3 (GFP–EB3; EB3 is also known as MAPRE3), which targets polymerizing plus tips resulting in ‘comets’ reflective of polymerizing plus tips in timelapse videos (Fig. 8A). A dose–response curve (data not shown) revealed that treatment with 100 nM nocodazole effectively suppressed plus tip dynamics in growth cones as reflected by the decreased number of EB3 comets forming under conditions of no NGF treatment and in response to NGF treatment (Fig. 8B,C).
Fig. 8.
Suppression of microtubule plus tip dynamic instability blocks actin filament dependent growth cone elaboration in response to acute NGF treatment but does not alter the ATP/ADP ratio. (A) Examples of EB3 comets (yellow arrows) in growth cones before (pre) and after treatment for 10 min with DMSO followed by 10 min with NGF (top row) or before and after treatment for 10 min with 100 nM nocodazole (Noco) followed by 10 min with NGF (bottom row). Graphs in B and C show the quantification of the number of comets. Note that in the nocodazole+NGF treatment condition there are no visible comets. (B) Graph of the effects of NGF treatment on the number of EB3 comets in growth cones. The pre group represents the untreated no NGF condition, the DMSO group (control for nocodazole treatment) is not different at any time points (2–10 min of treatment) from any time points in the pre-treatment group (one-way ANOVA). For analysis of the effects of NGF the pre and DMSO treatment groups were thus compiled and used for comparison to each time point after NGF treatment using Dunn's multiple comparison tests. NGF increased the number of comets in growth cones at 10 min of treatment. (C) Graph of the effects of treatment with nocodazole on the number of EB3 comets in growth cones. By 4 min of treatment the number of comets in growth cones decreases as assessed using Dunn's time point-matched multiple comparison tests between pre and nocodazole treatment. The time points after NGF treatment were compared to the compiled data from 8 and 10 min of nocodazole treatment, when the effect of nocodazole is greatest prior to treatment with NGF, using Dunn's multiple comparison tests. Subsequent treatment with NGF did not elevate the number of comets relative to 8-10 min of nocodazole treatment at any time point. (D) Treatment with nocodazole blocks the NGF-induced increase in growth cone area. Bonferroni post-hoc time point-matched tests yield P>0.05 at all time points. (E) Treatment with nocodazole does not affect the ATP/ADP ratio after NGF treatment. Bonferroni post-hoc time point-matched tests yield P>0.05 at all time points. (F) Treatment with nocodazole does not affect the ATP/ADP ratio in no NGF treatment condition. Bonferroni post-hoc time point-matched tests yield P>0.05 at all time points. (G) Treatment with nocodazole for 30 min does not alter intracellular pH in growth cones cultured in continuous NGF. Results are shown as mean±s.e.m. DMSO, n=8; 100 nM Noco, n=31. P-value calculated with an unpaired two-tailed Welch t-test.
Nocodazole blocked the NGF-induced increase in growth cone area (Fig. 8D), supporting the notion that NGF-induced increases in microtubule dynamics are required for NGF-induced actin filament-dependent growth cone elaboration. However, nocodazole did not alter the ATP/ADP ratio in growth cones during the same period (Fig. 8E). Similarly, nocodazole treatment had no effect on the ATP/ADP ratio of growth cones in the continuous presence of NGF during a 30 min period (Fig. 8F). Nocodazole did not alter intracellular pH as determined by BCECF ratiometric measurements in the presence of NGF (Fig. 8G). Collectively the data indicate that microtubule plus tip dynamics do not impact the ATP/ADP ratio in growth cones under conditions of acute NGF treatment but are required for the NGF-induced actin filament-based elaboration of the growth cone.
DISCUSSION
The rationale for the experiments performed herein was based on the report by Bernstein and Bamburg (2003) estimating that actin filament dynamics account for ∼50% of ATP hydrolysis in developing neurons. Owing to the absence of live cell reporters for ATP at the time of their study, Bernstein and Bamburg (2003) indirectly monitored ATP levels in the cell bodies of ciliary neurons as reflected by changes in intracellular Mg2+ levels. In support of this interpretation, Bernstein and Bamburg (2003) provided evidence that in the context of blockade of ATP production similar curves for ATP depletion were obtained whether through measuring intracellular Mg2+ level or ATP directly through luciferase using cell extracts. However, direct ATP measurements were not performed in the context of manipulations of actin cytoskeletal dynamics, and the cytosolic Mg2+ level was used as the indirect metric for the ATP level. In the current study, we used genetically encoded reporters to monitor the ATP/ADP ratio and ATP level. Bernstein and Bamburg (2003) found that treatment with 1 µM latrunculin-A or 10 nM jasplakinolide attenuated the increase in Mg2+ Green fluorescence following blockade of ATP production in neuronal cell bodies, interpreted as indicative of a decrease in the levels of ATP by ∼50%. In our study, we did not observe effects of either 4 µM latrunculin-A or 40 nM jasplakinolide on the decline in the ATP/ADP ratio or ATP level after blockade of ATP production. Furthermore, the luciferase-based assessment of cytosolic ATP performed using forebrain neurons generated results consistent with those obtained through live imaging of reporters in sensory neurons. As both the current and Bernstein and Bamburg (2003) studies were performed using embryonic chicken peripheral nervous system neurons, differences in species or cell type are unlikely to account for the discrepancy in the results. The current study used higher concentrations of latrunculin-A and jasplakinolide but with shorter pretreatment times. Both latrunculin-A and jasplakinolide have maximal effects on actin filaments in growth cones within minutes of treatment and the pretreatment times were selected to ensure the drugs had maximal effects prior to other manipulations or readouts. Given that the current study primarily focuses on growth cones rather than cell bodies, longer pretreatment periods were avoided. This decision was made to prevent extensive retraction of growth cones and axons, which complicates sampling. At this juncture, it is worth considering that the interpretation of Mg2+ levels as a metric for ATP levels might have been confounded. Actin binds Mg2+ at a high-affinity site, associated with ATP, and low-affinity sites where the binding is considered to regulate the actin monomer polymerization competency (Zimmerle et al., 1987; Carlier, 1991). Whether Mg2+ binding to actin might be impacted by experimental manipulations that alter actin dynamics is not known but could underly aspects of the results tracking cytosolic Mg2+ in the study by Bernstein and Bamburg (2003). Alternatively, and not mutually exclusively, actin filaments contribute to a multitude of cellular processes and manipulations of actin dynamics might impact aspects of Mg2+ handling by neurons, for example through its storage in endomembrane compartments, such as mitochondria and the endoplasmic reticulum, or through changes in binding availability to the multitude of additional Mg2+-binding proteins in cells (Yamanaka et al., 2019), although this has not been determined. In conclusion, we suggest that the differences in the interpretation of the role of actin filament dynamics in ATP hydrolysis under conditions of ATP synthesis blockade between the current study and that of Bernstein and Bamburg (2003) are due to the methodologies used to monitor ATP. Using direct ATP monitoring sensors in live neurons and luciferase assays for cytosolic ATP levels, the results of the current study falsify the hypothesis that actin filament dynamics are a major source of ATP hydrolysis in the growth cones and cell bodies of sensory neurons. We cannot exclude that there are changes in ATP below our detection sensibility but overall the data did not show any trends in the expected directions in multiple experimental contexts. The current data are consistent with the theoretical calculations of Engl and Attwell (2015) predicting that actin dynamics would account for less than 1% of ATP utilization in nervous tissue. However, using rat postnatal day 10 hippocampal slices Engl et al. (2017) report that actin dynamics account for 25% of oxygen consumption. This could be attributable to the contribution of actin turnover in synaptic vesicle dynamics, which has been estimated to account for 44% of energy utilization in presynaptic terminals of cultured hippocampal neurons that have established synaptic networks (Pulido and Ryan, 2021). Possible differences in actin filament dynamics driven ATP consumption between embryonic and adult or synaptically mature systems will require further consideration. Finally, suppression of ATP production blocks actin filament dynamics in cells including neurons (Atkinson et al., 2004; Ketschek and Gallo, 2010; Calabrese et al., 2022). Thus, it is counterintuitive that actin filament dynamics would be involved in ATP consumption after shutdown of ATP production as actin dynamics are strongly suppressed under these conditions.
The other report indicating that actin filament dynamics constitute a 29–51% drain of cellular ATP was performed in resting platelets (Daniel et al., 1986). Although our study did not address platelets, we provide a few considerations regarding the likely sources of the discrepancy between the conclusions of the current study and Daniel et al. (1986). In Daniel et al. (1986) the level of ADP-bound actin, but not ATP-bound, was assessed biochemically and the results were interpreted in the context of the understanding of actin filament dynamics and actin-driven ATP hydrolysis available in 1986. The Daniel et al. (1986) study did not manipulate actin dynamics or ATP levels, and the conclusions are derived from theoretical considerations based on the at-the-time current understanding of filament dynamics. The conclusion of a 29–51% drain of cellular ATP due to filament dynamics was based on multiple assumptions that are not tenable in the context of the current understanding of ADP-ATP exchange along filaments wherein ADP actin is found along the length of the filament and would thus persist for as long as the filament until the ADP-actin is released through filament depolymerization (Pollard, 2017). Additionally, the activity of proteins that sequester monomeric actin or alter ADP–ATP exchange was not considered (Pollard, 2017). Furthermore, the study was performed in resting platelets and activation of platelets results in an ∼75% increase in actin filament levels and increase in dynamics by 5 min (Falet et al., 2005). Thus, if actin dynamics in resting platelets were consuming 29–51% of ATP, then in activated platelets, the estimate might range to 50–89%, the latter estimation leaving just above 10% of ATP to support all other cellular processes. The role of actin filament dynamics in ATP utilization in platelets would benefit from additional scrutiny.
The current data indicate that although actin dynamics require ATP hydrolysis by actin, the ATP/ADP ratio in growth cones is not impacted by suppression of actin filament dynamics or microtubule dynamics that contribute to the promotion of actin dynamics. The mechanism by which cells maintain a steady level of ATP and ATP/ADP ratio is not clear and will require further analysis. Treatment with AICAR increased the ATP/ADP ratio. This observation suggests that AMP-activated protein kinase might be a component of the mechanism that sets the ATP/ADP ratio in developing axons and prior evidence implicates this kinase in energetic homeostasis (Sharma et al., 2023). It will be of interest to further consider the role of AMP-activated protein kinase in the regulation of ATP homeostasis and ATP/ADP level in the context of manipulations of actin dynamics and other cellular functions. Analysis of the maintenance of the ATP/ADP ratio in neuronal cell bodies in the face of varying degrees of electrical activity, an energy consuming process, showed that the Na+ pump is a major mediator of maintenance (Baeza-Lehnert et al., 2019). The possible role of the Na+ pump in developing axons remains to be addressed. It is also possible that mitochondrial superoxide flashes might be regulated to maintain bioenergetic homeostasis as observed in other electrically active cells (Wang et al., 2017).
In conclusion, the data presented herein fail to provide evidence in support of the hypothesis that actin dynamics are a major determinant of the intracellular ATP/ADP ratio or ATP level in either the cell bodies or growth cones of embryonic sensory neurons. Similarly, in forebrain neurons lysates, luciferase-based quantification of cellular ATP failed to reveal any effect of filament depolymerization at steady state or in response to the shutdown of ATP synthesis. It remains possible that, in additional subcellular domains, actin filament dynamics act as a drain for ATP hydrolysis (e.g. in small and confined domains such as synapses wherein oxidative phosphorylation and glycolysis might contribute differently to the net local ATP level; Rangaraju et al., 2014). The original study by Bernstein and Bamburg (2003) has been a driving force in understanding how cytoskeletal dynamics might impact ATP utilization in neurons. Although the current study, using multiple approaches, fails to replicate the basic conclusion of the original study, collectively they emphasize the need for further investigation into the local mechanisms of intracellular ATP utilization and how these could change with developmental stage. Finally, the mechanism that maintains a steady ATP/ADP ratio and cytosolic ATP level in axons remains to be elucidated.
MATERIALS AND METHODS
Sensory neuron cultures
Embryonic day 7 chicken (Gallus gallus) embryos were dissected and cultured according to Lelkes et al. (2005) on coverslips coated with polylysine (100 µg/ml; Cat # P9011, Sigma, St Louis, MO, USA) and laminin (25 µg/ml (Cat # 23017-015, Life Technologies, Carlsbad, CA 92008, USA) subsequentially. Briefly, F12HS10 dissection consisted of Hams F12 1× with L-glutamine (#MT10080CV, Thermo Fisher Scientific) containing 10% fetal bovine serum (#MT35011CV, Thermo Fisher Scientific) and 1% HEPES 1 M (#BP299-100, Thermo Fisher Scientific). F12H culturing medium consisted of F12 nutrient mix (#21700075, Thermo Fisher Scientific) dissolved in 1 l of distilled water and supplemented with the following ingredients: 1% HEPES 1 M (#BP299-100, Thermo Fisher Scientific), 1% PSF (#BW17745E, Thermo Fisher Scientific), 1% L-glutamine 200 mM (#MT25005CI, Thermo Fisher Scientific), 4% sodium pyruvate 100 mM (#11360070, Gibco, Grand Island, NY, USA), 5 µM phosphocreatine (#P-7936, Sigma), 100 mg/l apo-transferrin (#T-2252, Sigma), 0.1 µg/ml sodium selenate (#S-8295, Sigma), 20 nM progesterone (#P-8783, Sigma) and 0.87 µM insulin (#I-5500, Sigma). For glucose deprivation experiments neurons were cultured in DMEM lacking glucose (#11966025, Gibco), supplemented with 10 mM D-glucose (#G7021, Sigma), 10 mM Hepes (#BP299-100, Thermo Fisher Scientific), 1% PSF (penicillin-streptomycin mixture; Thermo Fisher Scientific, #BW17745E) and 1 mM sodium pyruvate (#11360-070, Gibco). Glycolysis inhibition medium (GIM) was made using the same formulation but replacing D-glucose with 10 mM 2-deoxyglucose (#D8375, Sigma). Unless otherwise noted, all media were supplemented with 20 ng/ml NGF (R&D Systems, #256-GF-100, Minneapolis, MN, USA). All animal experiments were performed according to approved guidelines.
Forebrain cultures
Embryonic day 8 chicken (Gallus gallus) forebrains were dissected, dissociated and neurons cultured as described in Kollins et al. (2009). Briefly, forebrains were dissociated using 0.1% trypsin (#25-050-Cl, Corning, Manasses, VA, USA) diluted with DPBS (#14190, Gibco), then, cultured in F12 nutrient mix (#21700075, #21700075, Thermo Fisher Scientific) supplemented with 1% Hepes 1 M (#BP299-100, Thermo Fisher Scientific), 1% PSF (#BW17745E, Thermo Fisher Scientific), 1% L-glutamine 200 mM (#MT25005CI, Thermo Fisher Scientific), 4% sodium pyruvate 100 mM (#11360070, Gibco), 5 µM phosphocreatine (#P-7936, Sigma,), 100 mg/l apo-transferrin (#T-2252, Sigma), 0.1 µg/ml sodium selenate (#S-8295, Sigma), 20 nM progesterone (#P-8783, Sigma), 0.87 µM insulin (#I-5500, Sigma) and 10% fetal bovine serum (#MT35011CV, Thermo Fisher Scientific) on polylysine (100 µg/ml; #P9011, Sigma)-coated culture dishes for 3 days.
Transfection and plasmids
Transfection was performed as previously described (Armijo-Weingart et al., 2019). For transfection of plasmids into neurons, 40 chicken DRGs were dissociated and suspended in 100 µl nucleofector solution (#VPG-1002, Lonza, Köln, Germany) through gentle trituration. The neuron suspension was transferred to a nucleofector cuvette containing 15 µg of plasmid DNA, and electroporated using an Amaxa Nucleofector (program G-13; Lonza). The electroporated solution was then immediately transferred to a tube containing culturing media as described above before plating. Plasmids used in this study were: cyto-Ruby3-iATPSnFR1.0 (Addgene plasmid #102551), FUGW-PercevalHR (Addgene plasmid #49083) and GFP–EB3 (Spillane et al., 2012).
Microscopy and imaging
Neurons were imaged using a Zeiss Axio Observer Z1 microscope (Carl Zeiss Microscopy, Göttingen, Germany) equipped with an Orca-R2 camera (Hamamatsu) and Zeiss Pan Neofluar 100× and 63× objectives. Zeiss Axiovision software was used for Image acquisition and analysis. Temperature was kept constant at 39°C using a heated microscope stage (Zeiss heating insert P with objective heater). PercevalHR (Tantama et al., 2013) was imaged with Zeiss filter set 48 (#1196-684) and 52 HE (#424920). cyto-Ruby3-iATPSnFR1.0 (Lobas et al., 2019) was imaged using Zeiss filter set 20 (#488020-9901-000) and 38 HE (#489038-9901-000). For glucose deprivation experiments using DMEM medium, CO2 was kept constant at 5% using a cover for the heating insert P (#0441-341, Carl Zeiss Microscopy) in conjunction with a 5% balance air mixture.
2′,7′-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF; B-1150, Invitrogen, Eugene, OR, USA) was used to determine intracellular pH. 25 nM was loaded on cells for 5 min. Following a wash, neurons were treated as indicated for 20 min and imaged with Zeiss filter set 48 (#1196-684) and 52 HE (#424920) for not longer than 10 min (maximum BCECF-AM exposure of 35 min). Cells with compartmentalized signal were excluded from analysis. Calibration was done using the Intracellular pH Calibration Buffer Kit (Invitrogen P35379, Invitrogen) following the manufacturer's direction.
The final concentration used for ImageiT tetramethylrhodamine methyl ester (TMRM) reagent (#I34361, Invitrogen) and MitoTracker Green FM (#M7514, Invitrogen) was 5 nM. They were imaged using Zeiss filter set 20 (#488020-9901-000) and 38 HE (#489038-9901-000). The following inhibitors were used at concentrations indicated in figure legends or the text: antimycin A (#A8674, Sigma), latrunculin-A (#BML-T119-0100, Enzo, Farmingdale, NY, USA), jasplakinolide (#J7473, Invitrogen), Nocodazole (#M1404, Sigma) and AICAR (#2840, Tocris, Bristol, UK,).
Quantification of fluorescence and ratiometric analysis
The growth cone was defined as the distal 10 µm of the axon shaft. If a growth cone exhibited lamellipodia, then the distal most extent of the lamellipodium was taken as the distal most point on the growth cone; if not, the distal most extent of the axon shaft was used as the defining distal point. Quantitative analysis of images was performed using Zeiss AxioVision software. The region of interest encompassing the growth cone was outlined and measurements of mean pixel fluorescence intensity in all relevant channels was determined for generation of ratios. For each channel, the mean intensity value obtained from the background of the image not containing any cells was subtracted from the mean intensity in the growth cone region of interest prior to determination of the ratio. Acquisition settings were chosen to generate maximum pixel values between 20% and 30% of the dynamic range to allow for detection of both decreases and increases in intensity in response to experimental treatments. For presentation, ratiometric images were generated using ImageJ (NIH) RatioPlus plugin and false-colored using the Royal look-up-table.
ATP luciferase bioluminescence assay
ATP was detected in lysates of forebrain neurons by luciferase driven bioluminescence using the ATP Bioluminescence Assay Kit CLS II (#11 699 695 001, Roche, Mannheim, Germany) and boiling hot cell suspension buffer (100 mM Tris-HCl, 4 mM EDTA, pH 7.75). Luminescence was measured using a SpectraMax i3x spectrophotometer using SoftMax Pro 7 software (Molecular Devices, San Jose, CA, USA). The blank value (no ATP) was subtracted from raw data and ATP concentrations were interpolated from a log-log plot of the standard curve data. Values were then normalized to total protein concentration in the lysate determined using Quick Start Bradford Dye Reagent (#500-0205, Bio-Rad, Hercules, CA, USA).
Immunocytochemistry
Actin filaments and microtubules were stained using Rhodamine–phalloidin (#R415, Invitrogen) and anti-α-tubulin DM1A-FITC (1:400; #F2168, Sigma), respectively. Therefore, cultures were simultaneously fixed (0.25% glutaraldehyde, #16300, Electron Microscopy Sciences, Hatfield, PA, USA) and extracted (0.1% Triton X-100, #T-9284, Sigma, St Louis, MO, USA) to reveal polymeric cytoskeletal components as described in Gallo and Letourneau (1999).
Experimental design and statistical analysis
All data were analyzed using GraphPad software (Boston, MA 02110, USA). Determination of the normalcy of data sets was performed using the Kolmogorov and Smirnov tests. Normal data sets were analyzed using the Welch t-test for independent groups or the paired t-test for before-after treatment experimental designs. If non-normal data sets were present, then non-parametric analysis was used (Mann–Whitney test). For multiple comparison tests within experimental designs, parametric Bonferroni or non-parametric Dunn's post-hoc tests were used according to the normalcy of the data sets. One- or two-tailed P-values are reported based on whether the hypothesis predicted the directionality of the expected difference in mean or median, respectively. Sample sizes and qualitative statistical presentation are denoted in figure legends or figures or in the text. For experiments involving live imaging and pre-post treatment experimental design from a single subject or growth cone tracked over time, imaging was sampled from at least three different independent replicate experiments sampled equally across the multiple groups in the experimental design. For each replicate experiment neurons were pooled from two to four embryos.
For power analysis addressing experiments involving ATP production blockade (GIM+antimycin-A treatment; Figs 2 and 3), the dynamic range of the measurements was determined by subtracting the mean at 30 min of treatment from the mean of the pre-treatment time point, and the expected mean if the treatment (e.g. latrunculin-A or jasplakinolide) decreased ATP utilization by 50% was predicted by dividing the dynamic range by 2 and adding it to the mean at 30 min of treatment. For example, for a pretreatment mean of 1.9431 and a 30 min time point mean of 0.6732, the expected mean for a 50% decrease in ATP utilization was estimated to be 0.6732+[(1.9431−0.6732)/2]. Power analysis was performed at https://clincalc.com/stats/samplesize.aspx.
For presentation, data sets are shown as individual data points when possible. In the case of time course data, if the data sets were normally distributed, which ratiometeric data sets invariably were, then the mean±s.e.m. of measurement are shown. The latter presentation style was opted for as otherwise the data sets overlapped significantly and obscured one another.
Supplementary Material
Acknowledgements
The authors thank Dr S. Fossati (Lewis Katz School of Medicine at Temple University) and her laboratory for assistance with the luciferase assay.
Footnotes
Author contributions
Conceptualization: S.M.H., G.G.; Methodology: S.M.H., G.G.; Software: G.G.; Validation: G.G.; Formal analysis: S.M.H., G.G.; Investigation: S.M.H., G.G.; Resources: G.G.; Data curation: S.M.H., G.G.; Writing - original draft: G.G.; Writing - review & editing: S.M.H.; Visualization: S.M.H., G.G.; Supervision: G.G.; Project administration: G.G.; Funding acquisition: G.G.
Funding
This work was supported by National Institutes of Health awards NS118000 and NS128049 (to G.G.). Deposited in PMC for release after 12 months.
Data availability
Quantitative data files are available at Figshare, https://doi.org/10.6084/m9.figshare.23696910.v1.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/lookup/doi/10.1242/jcs.261356.reviewer-comments.pdf.
References
- Anilkumar, U., Weisová, P., Düssmann, H., Concannon, C. G., König, H. G. and Prehn, J. H. (2013). AMP-activated protein kinase (AMPK)-induced preconditioning in primary cortical neurons involves activation of MCL-1. J. Neurochem. 124, 721-734. 10.1111/jnc.12108 [DOI] [PubMed] [Google Scholar]
- Armijo-Weingart, L., Ketschek, A., Sainath, R., Pacheco, A., Smith, G. M. and Gallo, G. (2019). Neurotrophins induce fission of mitochondria along embryonic sensory axons. Elife 8, e49494. 10.7554/eLife.49494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, S. J., Hosford, M. A. and Molitoris, B. A. (2004). Mechanism of actin polymerization in cellular ATP depletion. J. Biol. Chem. 279, 5194-5199. 10.1074/jbc.M306973200 [DOI] [PubMed] [Google Scholar]
- Baeza-Lehnert, F., Saab, A. S., Gutiérrez, R., Larenas, V., Díaz, E., Horn, M., Vargas, M., Hösli, L., Stobart, J., Hirrlinger, J.et al. (2019). Non-canonical control of neuronal energy status by the Na+ pump. Cell Metab. 29, 668-680.e4. 10.1016/j.cmet.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Bernstein, B. W. and Bamburg, J. R. (2003). Actin-ATP hydrolysis is a major energy drain for neurons. J. Neurosci. 23, 1-6. 10.1523/JNEUROSCI.23-01-00002.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow, P., Patgiri, A. and Faundez, V. (2022). Mitochondrial protein synthesis and the bioenergetic cost of neurodevelopment. iScience 25, 104920. 10.1016/j.isci.2022.104920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese, B., Jones, S. L., Shiraishi-Yamaguchi, Y., Lingelbach, M., Manor, U., Svitkina, T. M., Higgs, H. N., Shih, A. Y. and Halpain, S. (2022). INF2-mediated actin filament reorganization confers intrinsic resilience to neuronal ischemic injury. Nat. Commun. 13, 6037. 10.1038/s41467-022-33268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier, M. F. (1991). Actin: protein structure and filament dynamics. J. Biol. Chem. 266, 1-4. 10.1016/S0021-9258(18)52391-7 [DOI] [PubMed] [Google Scholar]
- Chada, S. R. and Hollenbeck, P. J. (2004). Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 14, 1272-1276. 10.1016/j.cub.2004.07.027 [DOI] [PubMed] [Google Scholar]
- Chen, X., Zhao, X., Zhang, M. and Wei, S. (2015). Nuclear respiratory factor-2α and adenosine triphosphate synapses in rat primary cortical neuron cultures: The key role of adenosine monophosphate-activated protein kinase. Mol. Med. Rep. 12, 6323-6329. 10.3892/mmr.2015.4140 [DOI] [PubMed] [Google Scholar]
- Daniel, J. L., Molish, I. R., Robkin, L. and Holmsen, H. (1986). Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur. J. Biochem. 156, 677-684. 10.1111/j.1432-1033.1986.tb09631.x [DOI] [PubMed] [Google Scholar]
- Dent, E. W. and Gertler, F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209-227. 10.1016/S0896-6273(03)00633-0 [DOI] [PubMed] [Google Scholar]
- Dagher, Z., Ruderman, N., Tornheim, K. and Ido, Y. (1999). The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 265, 112-115. 10.1006/bbrc.1999.1635 [DOI] [PubMed] [Google Scholar]
- Dewane, G., Salvi, A. M. and Demali, K. A. (2021). Fueling the cytoskeleton - links between cell metabolism and actin remodeling. J. Cell Sci. 134, jcs248385. 10.1242/jcs.248385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engl, E. and Attwell, D. (2015). Non-signalling energy use in the brain. J. Physiol. 593, 3417-3429. 10.1113/jphysiol.2014.282517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engl, E., Jolivet, R., Hall, C. N. and Attwell, D. (2017). Non-signalling energy use in the developing rat brain. J. Cereb. Blood Flow Metab 37, 951-966. 10.1177/0271678X16648710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers, T. M. J., Holt, L. J., Alberti, S. and Mashaghi, A. (2021). Reciprocal regulation of cellular mechanics and metabolism. Nat. Metab 3, 456-468. 10.1038/s42255-021-00384-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falet, H., Chang, G., Brohard-Bohn, B., Rendu, F. and Hartwig, J. H. (2005). Integrin alpha(IIb)beta3 signals lead cofilin to accelerate platelet actin dynamics. Am. J. Physiol. Cell Physiol. 289, C819-C825. 10.1152/ajpcell.00587.2004 [DOI] [PubMed] [Google Scholar]
- Fujiwara, I., Takeda, S., Oda, T., Honda, H., Narita, A. and Maéda, Y. (1998). Polymerization and depolymerization of actin with nucleotide states at filament ends. Biophys. Rev. 10, 1513-1519. 10.1007/s12551-018-0483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiñanes, M., Bullough, D., Mullane, K. M. and Hearse, D. J. (1992). Sustained protection by acadesine against ischemia- and reperfusion-induced injury. Studies in the transplanted rat heart. Circulation 86, 589-597. 10.1161/01.CIR.86.2.589 [DOI] [PubMed] [Google Scholar]
- Gallo, G. (1998). Involvement of microtubules in the regulation of neuronal growth cone morphologic remodeling. J. Neurobiol. 35, 121-140. [DOI] [PubMed] [Google Scholar]
- Gallo, G. (2020). The bioenergetics of neuronal morphogenesis and regeneration: Frontiers beyond the mitochondrion. Dev. Neurobiol. 80, 263-276. 10.1002/dneu.22776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, G. and Letourneau, P. C. (1999). Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J. Neurosci. 19, 3860-3873. 10.1523/JNEUROSCI.19-10-03860.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, G., Yee, H. F., Jr and Letourneau, P. C. (2002). Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J. Cell Biol. 158, 1219-1228. 10.1083/jcb.200204140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie, D. G. and Carling, D. (1997). The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259-273. 10.1111/j.1432-1033.1997.00259.x [DOI] [PubMed] [Google Scholar]
- Huang, E. J. and Reichardt, L. F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677-736. 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Kracke, M. R. (1992). Quick and accurate method to convert BCECF fluorescence to pHi: calibration in three different types of cell preparations. J. Cell. Physiol. 151, 596-603. 10.1002/jcp.1041510320 [DOI] [PubMed] [Google Scholar]
- Kaplan, D. R., Hempstead, B. L., Martin-Zanca, D., Chao, M. V. and Parada, L. F. (1991). The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science 252, 554-558. 10.1126/science.1850549 [DOI] [PubMed] [Google Scholar]
- Ketschek, A. and Gallo, G. (2010). Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J. Neurosci. 30, 12185-12197. 10.1523/JNEUROSCI.1740-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek, A., Sainath, R., Holland, S. and Gallo, G. (2021). The axonal glycolytic pathway contributes to sensory axon extension and growth cone dynamics. J. Neurosci. 41, 6637-6651. 10.1523/JNEUROSCI.0321-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins, K. M., Hu, J., Bridgman, P. C., Huang, Y. Q. and Gallo, G. (2009). Myosin-II negatively regulates minor process extension and the temporal development of neuronal polarity. Dev. Neurobiol. 69, 279-298. 10.1002/dneu.20704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelkes, P. I., Unsworth, B. R., Saporta, S., Cameron, D. F. and Gallo, G. (2005). Culture of Neuroendocrine and Neuronal Cells for Tissue Engineering. In Culture of Cells for Tissue Engineering (Culture of Specialized Cells) (ed. Vunjak-Novakovic G., Freshney R. I.), p. 375. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Lobas, M. A., Tao, R., Nagai, J., Kronschläger, M. T., Borden, P. M., Marvin, J. S., Looger, L. L. and Khakh, B. S. (2019). A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun. 10, 711. 10.1038/s41467-019-08441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsick, B. M., Flynn, K. C., Santiago-Medina, M., Bamburg, J. R. and Letourneau, P. C. (2010). Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev. Neurobiol. 70, 565-588. 10.1002/dneu.20800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menze, M. A., Chakraborty, N., Clavenna, M., Banerjee, M., Liu, X. H., Toner, M. and Hand, S. C. (2010). Metabolic preconditioning of cells with AICAR-riboside: improved cryopreservation and cell-type specific impacts on energetics and proliferation. Cryobiology 61, 79-88. 10.1016/j.cryobiol.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, R. L. and Hollenbeck, P. J. (1993). The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 104, 917-927. 10.1242/jcs.104.3.917 [DOI] [PubMed] [Google Scholar]
- Pinto-Costa, R. and Sousa, M. M. (2021). Microtubules, actin and cytolinkers: how to connect cytoskeletons in the neuronal growth cone. Neurosci. Lett. 747, 135693-135703. 10.1016/j.neulet.2021.135693 [DOI] [PubMed] [Google Scholar]
- Pollard, T. D. (2017). What we know and do not know about actin. In The Actin Cytoskeleton. Handbook of Experimental Pharmacology (ed. Jockusch B.), vol. 235, pp. 331-347, Cham: Springer. [DOI] [PubMed] [Google Scholar]
- Pulido, C. and Ryan, T. A. (2021). Synaptic vesicle pools are a major hidden resting metabolic burden of nerve terminals. Sci. Adv. 7, eabi9027. 10.1126/sciadv.abi9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju, V., Calloway, N. and Ryan, T. A. (2014). Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825-835. 10.1016/j.cell.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche, F. K., Marsick, B. M. and Letourneau, P. C. (2009). Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J. Neurosci. 29, 638-652. 10.1523/JNEUROSCI.3845-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin, M. W., Wickline, K. M. and Bridgman, P. C. (1996). Microtubule stability decreases axon elongation but not axoplasm production. J. Neurosci. 16, 3236-3246. 10.1523/JNEUROSCI.16-10-03236.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin, M. W., Dailey, M. E. and Bridgman, P. C. (1999). Polymerizing microtubules activate site-directed F-actin assembly in nerve growth cones. Mol. Biol. Cell 10, 2309-2327. 10.1091/mbc.10.7.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz, E. R., Okamoto, F., Buckberg, G. D., Vinten-Johansen, J., Allen, B. S., Leaf, J., Bugyi, H., Young, H. and Barnard, R. J. (1986). Biochemical studies: failure of tissue adenosine triphosphate levels to predict recovery of contractile function after controlled reperfusion. J. Thorac. Cardiovasc. .Surg. 92, 488-501. 10.1016/S0022-5223(19)36500-6 [DOI] [PubMed] [Google Scholar]
- Sainath, R. and Gallo, G. (2021). Bioenergetic requirements and spatiotemporal profile of nerve growth factor induced PI3K-Akt signaling along sensory axons. Front. Mol. Neurosci. 14, 726331. 10.3389/fnmol.2021.726331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel-Ruiz, J. E. and Letourneau, P. C. (2014). The role of Arp2/3 in growth cone actin dynamics and guidance is substrate dependent. J. Neurosci. 34, 5895-5908. 10.1523/JNEUROSCI.0672-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A., Anand, S. K., Singh, N., Dwivedi, U. N. and Kakkar, P. (2023). AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell Res. 428, 113614. 10.1016/j.yexcr.2023.113614 [DOI] [PubMed] [Google Scholar]
- Smith, G. M. and Gallo, G. (2018). The role of mitochondria in axon development and regeneration. Dev. Neurobiol. 78, 221-237. 10.1002/dneu.22546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff, S. P., Rabin, S. L., Cantley, L. C. and Kaplan, D. R. (1992). Nerve growth factor promotes the activation of phosphatidylinositol 3-kinase and its association with the trk tyrosine kinase. J. Biol. Chem. 267, 17472-17477. 10.1016/S0021-9258(18)41950-3 [DOI] [PubMed] [Google Scholar]
- Spillane, M., Ketschek, A., Donnelly, C. J., Pacheco, A., Twiss, J. L. and Gallo, G. (2012). Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J. Neurosci. 32, 17671-17689. 10.1523/JNEUROSCI.1079-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, E., Ho, T. and Kirschner, M. W. (1995). The role of microtubule dynamics in growth cone motility and axonal growth. J. Cell Biol. 128, 139-155. 10.1083/jcb.128.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama, M., Martínez-François, J. R., Mongeon, R. and Yellen, G. (2013). Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat. Commun. 4, 2550. 10.1038/ncomms3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg, J. and Hollenbeck, P. J. (2008). Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J. Neurosci. 28, 8306-8315. 10.1523/JNEUROSCI.2614-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, M. L., Martin-Zanca, D., Parada, L. F., Bishop, J. M. and Kaplan, D. R. (1991). Nerve growth factor rapidly stimulates tyrosine phosphorylation of phospholipase C-gamma 1 by a kinase activity associated with the product of the trk protooncogene. Proc. Natl. Acad. Sci. U S A 88, 5650-5654. 10.1073/pnas.88.13.5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Zhang, X., Wu, D., Huang, Z., Hou, T., Jian, C., Yu, P., Lu, F., Zhang, R., Sun, T.et al. (2017). Mitochondrial flashes regulate ATP homeostasis in the heart. Elife 6, e23908. 10.7554/eLife.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, K., Kamata, R., Coutinho, K., Inoue, T. and Sasaki, A. T. (2020). Metabolic compartmentalization at the leading edge of metastatic cancer cells. Front. Oncol. 10, 554272. 10.3389/fonc.2020.554272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, R., Shindo, Y. and Oka, K. (2019). Magnesium is a key player in neuronal maturation and neuropathology. Int. J. Mol. Sci. 20, 3439-3465. 10.3390/ijms20143439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerle, C. T., Patane, K. and Frieden, C. (1987). Divalent cation binding to the high- and low-affinity sites on G-actin. Biochemistry 26, 6545-6552. 10.1021/bi00394a039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.