The authors conclude that patient-oncologist cost communication and financial distress were associated with medication nonadherence, suggesting that cost discussions are important for patients forced to make cost-related behavior alterations.
Abstract
Background:
Little is known about the association between patient-oncologist discussion of cancer treatment out-of-pocket (OOP) cost and medication adherence, a critical component of quality cancer care.
Methods:
We surveyed insured adults receiving anticancer therapy. Patients were asked if they had discussed OOP cost with their oncologist. Medication nonadherence was defined as skipping doses or taking less medication than prescribed to make prescriptions last longer, or not filling prescriptions because of cost. Multivariable analysis assessed the association between nonadherence and cost discussions.
Results:
Among 300 respondents (86% response), 16% (n = 49) reported high or overwhelming financial distress. Nineteen percent (n = 56) reported talking to their oncologist about cost. Twenty-seven percent (n = 77) reported medication nonadherence. To make a prescription last longer, 14% (n = 42) skipped medication doses, and 11% (n = 33) took less medication than prescribed; 22% (n = 66) did not fill a prescription because of cost. Five percent (n = 14) reported chemotherapy nonadherence. To make a prescription last longer, 1% (n = 3) skipped chemotherapy doses, and 2% (n = 5) took less chemotherapy; 3% (n = 10) did not fill a chemotherapy prescription because of cost. In adjusted analyses, cost discussion (odds ratio [OR] = 2.58; 95% CI, 1.14 to 5.85; P = .02), financial distress (OR = 1.64, 95% CI, 1.38 to 1.96; P < .001) and higher financial burden than expected (OR = 2.89; 95% CI, 1.41 to 5.89; P < .01) were associated with increased odds of nonadherence.
Conclusion:
Patient-oncologist cost communication and financial distress were associated with medication nonadherence, suggesting that cost discussions are important for patients forced to make cost-related behavior alterations. Future research should examine the timing, content, and quality of cost-discussions.
Introduction
The financial burden of cancer care has increased considerably with the advent of new technology for diagnosis and treatment.1 Growing financial burden has not only resulted in higher national expenditure, but has also placed a higher out-of-pocket expense burden on patients.1,2 These increased costs are caused by rising deductibles, higher copayments, and coinsurance.3
The higher out-of-pocket expenses resulting from cancer care can have a considerable impact on patients' lives.3 Patients have reported coping with costs by reducing spending on leisure activities, decreasing spending on food and clothing, and using savings to defray treatment-related expenses.3 Cost is also known to affect medication adherence, with known financial barriers such as higher out-of-pocket deductibles.4 For example, Neugut et al5 found that higher prescription copayments are associated with nonadherence to adjuvant hormonal therapy in female patients with breast cancer. There are significant downstream effects of medication nonadherence, including worse disease-related outcomes and higher long-term systemic health care costs.6 However, identifying those patients most susceptible to nonadherence has proven difficult.6
In an era of increased cost sharing and growing out-of-pocket costs, what is known about cost discussions between patients and oncologist? Studies have shown that patients and physicians express desire to discuss costs, but rarely do those discussions actually take place.7,8 Furthermore, little evidence exists to explain what prompts patients to discuss costs with their doctors. Recently ASCO identified patient-physician discussions of the cost of cancer care as a critical component of high-quality care,9 and the Institute of Medicine has listed cost discussions as a critical component of patient-oriented, high-quality care.10 Because nonadherence to medication has been previously associated with financial burden, our objective was to explore the relationship between nonadherence, financial distress, and patient-physician discussions of cancer treatment-related cost.
Methods
Participants
A convenience sample of adult patients was recruited from Duke Cancer Institute, a quaternary referral cancer center, and three affiliated rural oncology clinics. Enrollment occurred between November 2012 and June 2013. Potential participants were identified through a review of clinic schedules, with electronic record assessment for eligibility criteria. In order to be eligible, patients had to be diagnosed with a solid-tumor malignancy, have insurance coverage, and have received at least 1 month of anticancer therapy at the time of enrollment. Patients who met eligibility criteria were approached while receiving chemotherapy or while in the clinic waiting room. After providing informed consent, participants were surveyed in person by trained interviewers, at the time of enrollment and 3 months later. Participants received $10 for completing each survey.
Study Design
This was a cross-sectional survey study with follow-up assessing medication adherence, patient out-of pocket costs related to cancer care, and financial distress. Demographic data were abstracted from the medical record and included cancer diagnosis, stage of disease (eg, metastatic v recurrent cancer), type of treatment (eg, oral v intravenous chemotherapy), and duration of treatment at the time of enrollment. The survey was pilot tested in a group of 20 patients receiving care at Duke Cancer Institute. The Duke University Medical Center Institutional Review Board approved this study.
The survey contained questions regarding the following: self-reported demographic data, cost-related decision making, objective and subjective financial burden, and medication nonadherence. Participants were asked whether they had previously discussed out-of-pocket costs of cancer care with their oncologist. Subjective financial distress was measured using the InCharge Financial Distress/Financial Well-Being Scale, an instrument designed to assess financial state on a continuum ranging from “overwhelming financial distress/lowest level of financial well-being” to “no financial distress/highest level of financial well-being.11 This validated measure asked respondents to grade, on a scale from 1 to 10, questions such as: “What do you feel is the level of your financial stress today?” and “How often do you worry about not being able to meet normal monthly living expenses?”11
The outcome variable was self-reported medication nonadherence. A binary measure was used, with nonadherence defined as answering yes to any of the following: (1) skipping medication doses to make the prescription last longer, (2) taking less medication than prescribed to make it last longer, or (3) not filling a prescription because of cost. Participants who answered yes were questioned further to determine whether their nonadherence applied specifically to a chemotherapy prescription.
Statistical Analyses
Descriptive statistics were used to assess cohort demographics. Univariable analyses, specifically χ2 or Fisher's exact test depending on the context, were used to compare baseline demographics of patients who reported complete adherence with patients who reported medication nonadherence. Multivariable logistic regression was performed, with the outcome variable defined as medication nonadherence. The multivariable model controlled for financial distress (modeled as a continuous, inverted variable), age (units = 5 years), sex, race (white v nonwhite), postsecondary education, marriage, curative versus palliative treatment, days receiving treatment (unit = 180 days), private insurance, higher than expected subjective financial burden, and patient-reported discussion of costs with an oncologist. All variables were entered simultaneously. The logistic regression analysis used the 300 patients with complete data. Statistical significance was defined as P < .05. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patients
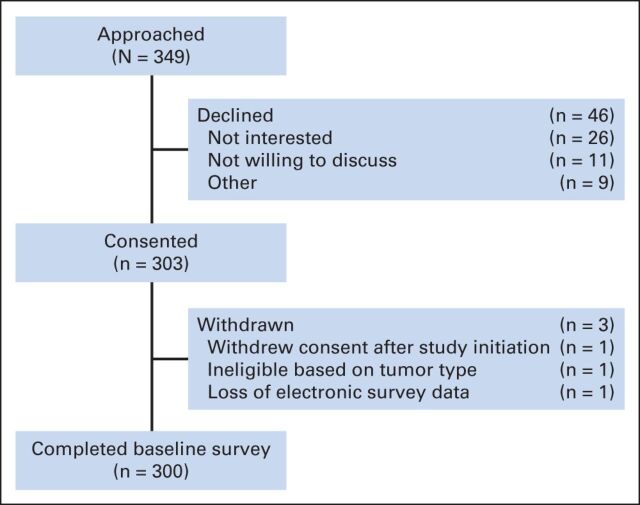
Overall, 349 patients met eligibility criteria and were approached for participation. Of those, 300 participants completed the survey for a response of 86% (Appendix Figure A1, online only). Of the 300 who completed the baseline survey, 246 (82%) completed the 3-month follow-up survey. Patient characteristics are described in Table 1. All patients were insured, with the majority having private insurance. Ninety-seven percent of patients (n = 290) had prescription drug coverage. All patients were receiving anticancer therapy at the time of study enrollment. Sixteen percent of patients (n = 49) reported high or overwhelming subjective financial distress. Twenty-seven percent of patients (n = 81) reported taking any oral chemotherapy, 5% (n = 16) reported taking only oral chemotherapy, and 22% (n = 65) received both intravenous (IV) and oral chemotherapy. Seventy two percent (n = 217) reported IV chemotherapy alone.
Table 1.
Baseline Patient Characteristics (N = 300)
| Characteristic | No. | % |
|---|---|---|
| Median age, years | 60 | |
| Sex | ||
| F | 143 | 47.67 |
| M | 157 | 52.33 |
| Race | ||
| White | 229 | 76.33 |
| Nonwhite | 71 | 23.67 |
| Education | ||
| Advanced degree | 60 | 20 |
| Bachelor's degree | 71 | 23.67 |
| Associate's degree | 86 | 28.67 |
| High school/GED | 64 | 21.33 |
| Some high school or less | 19 | 6.33 |
| Insurance | ||
| Not private | 132 | 44 |
| Private | 168 | 56 |
| Employment | ||
| Full-time | 80 | 26.67 |
| Part-time | 18 | 6 |
| Retired | 110 | 36.67 |
| Unemployed | 80 | 26.66 |
| Other | 12 | 4 |
| Income, $ | ||
| At least 60,000 | 239 | 79.67 |
| 40,000 to 59,999 | 10 | 3.33 |
| 20,000 to 39,999 | 11 | 3.67 |
| < 20,000 | 18 | 6 |
| Prefer not to answer | 22 | 7.33 |
| Prescription coverage | ||
| No | 7 | 2.33 |
| Yes | 290 | 96.67 |
| Relationship | ||
| Married, married-like | 205 | 68.33 |
| Not married | 94 | 31.33 |
| Financial distress | ||
| High/overwhelming | 49 | 16.33 |
| Low/average | 251 | 83.67 |
| Financial burden | ||
| Higher than expected | 118 | 39.33 |
| Lower than expected | 170 | 56.67 |
| Diagnosis | ||
| Breast | 53 | 17.67 |
| Colorectal | 81 | 27 |
| Esophageal, gastric | 10 | 3.33 |
| Kidney | 4 | 1.33 |
| Lung | 52 | 17.33 |
| Pancreas | 39 | 13 |
| Prostate | 22 | 7.33 |
| Sarcoma | 9 | 3 |
| Testicular | 4 | 1.33 |
| Uterine | 4 | 1.33 |
| Other | 22 | 7.33 |
| Stage | ||
| Localized | 4 | 1.33 |
| Metastatic recurrence | 73 | 24.33 |
| Regionally advanced | 3 | 1 |
| Stage I | 8 | 2.67 |
| Stage II | 18 | 6 |
| Stage III | 29 | 9.67 |
| Stage IV | 162 | 54 |
| Unresectable | 3 | 1 |
Abbreviation: GED, general educational development.
Cost Discussions and Medication Nonadherence
Nineteen percent of patients (n = 56) reported discussing treatment-related out-of-pocket costs with their oncologist. Fifty-two percent of patients (n = 155) reported some desire to talk to their oncologist about the out-of-pocket cost of their cancer care.
Overall, 73% (n = 220) of patients reported complete adherence to medications, whereas 27% (n = 77) of patients reported at least one form of medication nonadherence. Table 2 lists the proportion of patients with each type of self-reported medication nonadherence, and by whether patients were nonadherent to chemotherapy. When comparing baseline responses to 3-month follow-up responses, a similar proportion of patients (1) skipped medication doses to make medication last longer (14% v 15%), (2) took less medication than prescribed to make medication last longer (11% v 15%), and (3) did not fill a prescription because of cost (22% v 22%).
Table 2.
Patterns of Medication Nonadherence
| Pattern | No. | % |
|---|---|---|
| Medication nonadherence | ||
| Patients who reported any nonadherence | 80 | 27 |
| Patients who reported only one type of nonadherence | 41 | 14 |
| Patients who reported > one type of nonadherence | 39 | 13 |
| Types of medication nonadherence | ||
| Skipped medication doses to make the prescription last longer | 42 | 14 |
| Took less medication than prescribed to make the prescription last longer | 33 | 11 |
| Did not fill a prescription because of cost | 66 | 22 |
| Chemotherapy nonadherence | ||
| Patients who reported any nonadherence with chemotherapy | 14 | 4.67 |
| Patients who reported only one type of chemotherapy nonadherence | 12 | 4 |
| Patients who reported > 1 type of chemotherapy nonadherence | 2 | 0.67 |
| Types of chemotherapy nonadherence | ||
| Skipped chemotherapy doses to make the prescription last longer | 3 | 1 |
| Took less chemotherapy to make the prescription last longer | 5 | 1.67 |
| Did not fill a chemotherapy prescription because of cost | 10 | 3.33 |
NOTE. Percentages do not equal 100% because more than one response was allowed.
Compared with those who had not discussed cost, patients who had discussed out-of-pocket expenses with their oncologist were more likely to also have reported medication nonadherence (OR = 2.58; 95% CI, 1.14 to 5.85; P = .02, Table 3). Patients who reported high or overwhelming financial distress were more likely to report nonadherence (OR = 1.64; 95% CI, 1.38 to 1.96; P < .01), as were those who experienced higher financial burden than they expected at the start of treatment (OR = 2.89; 95% CI, 1.42 to 5.89; P < .01). When compared with those with nonprivate insurance, patients with private insurance had decreased odds of medication nonadherence (OR = 0.31; 95% CI, 0.14 to 0.72; P < .01).
Table 3.
Characteristics Associated With Nonadherence in an Adjusted Multivariable Analysis (N = 300)
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Financial distress (continuous, inverted) | 1.64 | 1.38 to 1.96 | < .001 |
| Age (unit = 5 yr) | 1.06 | 0.89 to 1.26 | .541 |
| Sex F v M | 2.00 | 0.98 to 4.08 | .058 |
| Race white v nonwhite | 1.21 | 0.55 to 2.69 | .639 |
| Some college education v no college education | 1.43 | 0.65 to 3.14 | .379 |
| Married v not married | 0.81 | 0.39 to 1.68 | .565 |
| Curative v palliative | 0.90 | 0.41 to 1.96 | .786 |
| Days on treatment (unit = 180 d) | 0.95 | 0.87 to 1.04 | .286 |
| Private insurance v not private | 0.31 | 0.14 to 0.72 | .006 |
| Higher financial burden than expected v lower or as expected | 2.90 | 1.42 to 5.89 | .004 |
| Reported prior cost discussion v did not report prior cost discussion | 2.58 | 1.14 to 5.85 | .023 |
Discussion
In this study of insured patients receiving anticancer therapy, having discussed out-of-pocket costs with an oncologist was associated with medication nonadherence. A growing body of evidence suggests that treatment-related out-of-pocket expenses affect patient well-being.12 Until now, little work had been done to understand which factors, if any, are associated with having cost conversations. The directionality or reasons for the relationship between discussion of cost with a physician and nonadherence is not clear. Increased rates of cost-related communication may be a marker for people stressed by the personal cost implications of their health care and at risk for nonadherence, or the relationship between cost discussions and nonadherence might have been influenced by an unmeasured factor. Nonetheless, these data suggest that discussing cost of care is important for patients who must alter their behaviors as a result of financial distress.
The clinical implications of these data relate to the importance of patient-physician communication in preserving the quality of cancer care. Instead of waiting for behavioral change—in this case, medication nonadherence—caused by financial distress, broaching the topic of costs earlier in the course of treatment might prevent cost-related behavior alterations that are detrimental to cancer care quality. In support of this hypothesis, our analysis also found that patients who experienced higher financial burden than they expected were more likely to be nonadherent with medications. In other words, if expectations of out-of-pocket costs were set by cost discussions early in the treatment course, nonadherence might have been avoided. For example, patients might be better able to prepare emotionally and financially for significant expenses, or be offered a less costly treatment alternative. Future research should investigate the timing of cost discussions along the treatment continuum, and assess the role of early patient and physician cost communication education in reducing financial distress and nonadherence due to cost. Further research is also needed to explore the decisions physicians make when they are mindful of cost implications early in the treatment planning, and how this relates to the patient's personal experience of financial distress.
Financial distress can negatively affect patients' subjective well-being, affect patients' families, and influence patients' treatment choices.3,13,14 Our study suggests that high out-of-pocket costs can cause significant problems for patients, specifically related to not taking medications as prescribed. Cost-related nonadherence occurs frequently. In a study of patients receiving copayment assistance, nearly half of patients experienced cost-related nonadherence.15 Through the Quality Oncology Practice Initiative, ASCO has included careful monitoring of medication compliance as an essential component of safe chemotherapy administration.16 Patients in our study reported nonadherence in the form of skipping medications, taking less medication than prescribed, and not filling prescriptions in an attempt to reduce costs. Not properly taking cancer-related medication and chemotherapy as prescribed may have serious health consequences and has been associated with increased mortality.17 Chemotherapy nonadherence in our study was lower than suggested in similar studies. In a recent study by Dusetzina et al,18 nonadherence to tyrosine kinase inhibitors for chronic myelogenous leukemia was 21% to 30%.18 Among women with breast cancer, Neugut et al19 found a lower rate of nonadherence with aromatase inhibitors (10.6%). However, our study estimated overall chemotherapy nonadherence at 5%. The difference might be accounted for by varying definitions of nonadherence, patient sample composition, or our smaller sample size compared with the other two studies. Indeed, our smaller sample size prevented additional comparison of oral versus IV chemotherapy nonadherence beyond descriptive statistics.
Although nonadherence may lead to cost savings at the individual level (eg, fewer payments for prescription refills or copayments), medication nonadherence has important financial and policy implications for the overall health care system. Approximately 33% to 69% of all hospital admissions are due to nonadherence, resulting in an estimated added cost of approximately $100 billion per year.6
Our data suggest a need to engage patients regarding costs before financial burden forces nonadherence. Unfortunately, identifying those patients most susceptible to nonadherence has proven difficult. Previously identified risk factors for nonadherence include race, sex, and socioeconomic status.6 This is an important area for research in patient-level interventions. In addition, the study by Dusetzina et al,18 which found that higher copayments for imatinib were associated with increased odds of chemotherapy discontinuation or nonadherence, further underscores the importance of addressing cost in the clinical encounter given its relationship to nonadherence, a quality measure.
Our study has several limitations. First, these data suggest correlation but not evidence of direction. The temporal relationship of cost discussion and medication nonadherence is unknown, as is the frequency of conversations. Secondly, this was a convenience sample. Given that this study this was conducted at a tertiary referral center, the stage and oncologic diagnoses in the study sample may not be representative of the typical oncologic patient population. Patients were surveyed at a clinic visit; the fact that there were present for a visit may potentially skew the population toward higher compliance. The results of this study included only a single point in time; it is not clear how patients treatment trajectory, and thus their financial toxicity and medication adherence, may have changed over time. This study included only insured patients. Although these results may not be generalizable to uninsured patients, it would be expected that insured patients would be less likely to experience cost-related medication nonadherence.20 Furthermore, details of the cost discussions between patients and physicians were not recorded. Specifically, it is unclear what event triggered the discussion or whether patient or provider initiated the conversation. Recollection of the cost conversation itself is subject to recall bias as well. For example, those having financial difficulties may be more likely to recall the cost conversation. Surveying patients at multiple visits, surveying their oncologists about the occurrence of the conversation, or recording the patient-physician discussion may improve accuracy of reporting these conversations. Adherence was self-reported, leading to the possibility of recall bias and socially desirable response bias. Patient copayment information was not collected. Prior studies have suggested that higher copayments are associated with greater risk of nonadherence. Future research should correlate copayment information with incidence of patient-oncologist cost discussions as well.21,22 Adherence is a continuum of behaviors, but this study used a binary measure of medication nonadherence. Future studies might use a continuum measure of nonadherence to validate the results from this binary assessment study.
In conclusion, we found that discussion of cancer treatment-related out-of-pocket costs between patient and oncologist was associated with medication nonadherence. Patients might be waiting to have a cost discussion with their oncologist until financial burden has already altered their behavior and quality of treatment. Further research is needed to evaluate the directionality and temporality of this relationship, as well as to determine the opportunity for intervention through cost-related discussions on nonadherence.
Acknowledgment
Previously presented at the ASCO Quality Care Symposium, San Diego, CA, November 1-2, 2013.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Amy P. Abernethy, Athena Health (C), Orange Leaf Associates (C), Advoset (C), AAHPM (C); Jeffrey Peppercorn, GlaxoSmithKline (C) Consultant or Advisory Role: Deborah Schrag, New Century Health (C), Ohio State University (C); Amy P. Abernethy, Novartis (C), Pfizer (C), BMS (C) Stock Ownership: Jeffrey Peppercorn, GlaxoSmithKline Honoraria: None Research Funding: Amy P. Abernethy, Dara, Celgene, Helsinn, Dendreon, Pfizer Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
Author Contributions
Conception and design: Fumiko Chino, Ivy Altomare, Deborah Schrag, Jonathan Nicolla, Amy P. Abernethy, Jeffrey Peppercorn, S. Yousuf Zafar
Financial support: S. Yousuf Zafar
Collection and assembly of data: Christel Rushing, Fumiko Chino, Deborah Schrag, Jonathan Nicolla, S. Yousuf Zafar
Data analysis and interpretation: Christine M. Bestvina, Leah L. Zullig, Christel Rushing, Gregory P. Samsa, James Tulsky, Peter Ubel, Deborah Schrag, S. Yousuf Zafar
Manuscript writing: All authors
Final approval of manuscript: All authors
Appendix
Figure A1.
Derivation of the study cohort.
References
- 1. Meropol NJ, Schulman KA: Cost of cancer care: Issues and implications J Clin Oncol 25: 180–186,2007. [DOI] [PubMed] [Google Scholar]
- 2. Bernard DS, Farr SL, Fang Z: National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008 J Clin Oncol 29: 2821–2826,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zafar SY, Peppercorn J, Schrag D , etal: The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience Oncologist 18: 381–390,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cutler DM, Everett W: Thinking outside the pillbox–medication adherence as a priority for health care reform N Engl J Med 362: 1553–1555,2010. [DOI] [PubMed] [Google Scholar]
- 5. Neugut AI, Subar M, Wilde ET , etal: Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer J Clin Oncol 29: 2534–2542,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osterberg L, Blaschke T: Adherence to medication N Engl J Med 353: 487–497,2005. [DOI] [PubMed] [Google Scholar]
- 7. Alexander GC, Casalino LP, Meltzer DO: Patient-physician communication about out-of-pocket costs JAMA 290: 953–958,2003. [DOI] [PubMed] [Google Scholar]
- 8. Schrag D, Hanger M: Medical oncologists' views on communicating with patients about chemotherapy costs: A pilot survey J Clin Oncol 25: 233–237,2007. [DOI] [PubMed] [Google Scholar]
- 9. Meropol NJ, Schrag D, Smith TJ , etal: American Society of Clinical Oncology guidance statement: The cost of cancer care J Clin Oncol 27: 3868–3874,2009. [DOI] [PubMed] [Google Scholar]
- 10. Ganz PA, Levit LA: Charting a new course for the delivery of high-quality cancer care J Clin Oncol 31: 4485–4487,2013. [DOI] [PubMed] [Google Scholar]
- 11. Prawitz AD, Garman ET, Sorhaindo B , etal: Incharge financial distress/financial well-being scale: Development, administration, and score interpretation Fin Couns Plan 17: 34–50,2006. [Google Scholar]
- 12. Ubel PA, Abernethy AP, Zafar SY: Full disclosure–out-of-pocket costs as side effects N Engl J Med 369: 1484–1486,2013. [DOI] [PubMed] [Google Scholar]
- 13. Zafar SY, Abernethy AP: Financial toxicity, part I: A new name for a growing problem Oncology (Williston Park) 27: 80–81,2013. 149 [PMC free article] [PubMed] [Google Scholar]
- 14. Mosher CE, Champion VL, Azzoli CG , etal: Economic and social changes among distressed family caregivers of lung cancer patients Supportive Care Cancer 21: 819–826,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zullig LL, Peppercorn J, Schrag D , etal: Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer J Oncol Pract 9: 60s–63s,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobson JO, Polovich M, McNiff KK , etal: American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards J Clin Oncol 27: 5469–5475,2009. [DOI] [PubMed] [Google Scholar]
- 17. Hershman DL, Shao T, Kushi LH , etal: Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer Breast Cancer Res Treatment 126: 529–537,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dusetzina SB, Winn AN, Abel GA , etal: Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia J Clin Oncol 32: 306–311,2013. [DOI] [PubMed] [Google Scholar]
- 19. Neugut AI, Subar M, Wilde ET , etal: Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer J Clin Oncol 29: 2534–2542,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Briesacher BA, Gurwitz JH, Soumerai SB: Patients at-risk for cost-related medication nonadherence: A review of the literature J Gen Int Med 22: 864–871,2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dusetzina SB, Winn AN, Abel GA , etal: Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia J Clin Oncol 32: 306–311,2014. [DOI] [PubMed] [Google Scholar]
- 22. Gellad WF: Targeted cancer therapy: From bench to bedside to patient J Clin Oncol 32: 268–270,2014. [DOI] [PubMed] [Google Scholar]