Version Changes
Revised. Amendments from Version 1
The new version of the article has taken into account the suggestions from the peer reviewers, clarifying and correcting the inconsistency across the first version of the manuscript. More citations and consideration had been added for better contextualise the paper to the current literature.
Abstract
It is difficult to model in vitro the intestine when seeking to include crosstalk with the gut microbiota, immune and neuroendocrine systems. Here we present a roadmap of the current models to facilitate the choice in preclinical and translational research with a focus on gut-on-chip. These micro physiological systems (MPS) are microfluidic devices that recapitulate in vitro the physiology of the intestine. We reviewed the gut-on-chips that had been developed in academia and industries as single chip and that have three main purpose: replicate the intestinal physiology, the intestinal pathological features, and for pharmacological tests.
Keywords: Gut-on-a-chip, Intestine-on-a-chip, Microbiota-on-a-chip, Colon-on-a-chip, Organ-on-a-chip, microfluidic, intestinal models
Introduction
The human gastrointestinal (GI) tract primarily processes food and absorbs nutrients, water, and minerals, while also playing key roles in immunity and in different neuroendocrine processes 1 . The physiological environments of different GI lumen sections are distinguished by their pH, redox potential, and transit time and they are deeply influenced by individual condition, diet, circadian clock, and physical activity 2 . A healthy gut is marked by effective digestion and absorption of food, normal and stable intestinal microbiota, effective immune status, and general wellbeing 3 . Poor quality diet, frequent use of antibiotics compromising gut microbiota biodiversity, aging 4 and epigenetic factors have been associated with digestive diseases and linked to non-communicable diseases (NCDs) 5, 6 . Dietary risk factors contribute to 11 million deaths and 255 million cases of morbidity worldwide, according to analysis of the Global Burden of Diseases (GBD) Study 2017 7 . In a more recent GBD report 8 , the annualised rate of change between 2010 and 2019 for the Dietary risk factors assessed a decrease of -0.28, but an increase for the Metabolic risks factors (+1.46%), which can be also associated with the GI diseases 9, 10 .
Considering the important role played by the gut in human physiology and pathology, considerable efforts have been invested to create relevant in vitro models for translational research and personalized medicine. Gut-on-chip (GOC) models provide an advanced and unique approach to combine and preserve the original biological components, the biophysical architecture, and the biophysical phenomena of the gut in vitro. GOCs are organs-on-a-chip (OOC), small in vitro devices based on microfluidic technology that aim to replicate the minimal functional units of the intestine, enabling to culture intestinal cells and bioptic tissues 11 . The GOCs have demonstrated so far capability to replicate: (1) specific physiopathological conditions (e.g. inflammation 12 , intestinal bowel diseases – IBD 13 , colon cancer 14 ); (2) in vitro drug pharmaco-kinetics (e.g. bioavailability assays 15 , drug-to-drug interaction 16 ); (3) host-microbes interactions (HMI) 17– 19 .
Translational potentials and challenges of current gut models
The success rate of drug discovery and development from the preclinical phase to the clinical phases is only about 32% 20 . The same drugs are not necessarily going onto the clinical phase and succeeding. One of the main reasons of the high percentage of failures is due to the difficulty in finding preclinical models, both in vitro and in vivo, that resemble the human physiology, the pathological pathways, and the pharmacological response. Despite the disruptive therapeutic modalities such as gene therapy and immunotherapy, the development of more predictive in vitro model to study the treatment efficacy and toxicity is critical. In the preclinical research, the model roadmap to study the human GI tract pass by in silico, in vitro, and in vivo ( Figure 1). In silico approach is based in computer modelling and aims at producing algorithms or numerical models able to predict the drug effects. They have different level of complexity and include computational fluid dynamics (CFD) 21 , ordinary differential equations (ODEs) 22, 23 , aged-based modelling (ABM) 24, 25 , and genome scale modelling (GSM) 26 . For the development of in silico models, it is critical the reliability of input data that are coming from databases, data banks, data mining, data analysis tools, publications, homology models, and other repositories 27 . Data-based modelling approaches are effective for many ADME (absorption, distribution, metabolism, elimination) properties in relationship with the QSAR (quantitative structure-activity relationship). For example, computational models are used for molecular modelling with enzymes and their docking, drugs solubility and permeability in intestine and brain, prediction of hepatic metabolism and mechanistic models of tissue distribution 28, 29 . The data acquired in silico requires validation to bridge the current gap between theoretical and experimental approaches 30 . In preclinical studies, a range of animal models are used, from small animals (mice and rats) to large animals (pigs, dogs, and non-human primates). This is done to study the effects of a potential treatment in a more complex system than the in vitro systems allow, considering the whole organism. Animal studies require ethical approval and their predictability is challenged by different diets and thus different gut microbiota composition from humans, different genomes, difficulties in handling and maintenance (particularly for large animals), and high costs 30 . The use of animal models is not limited to pharmacological studies as the gut-brain axis research is becoming of critical importance in understanding physiological mechanisms 31, 32 and mental health disorders 33 .
Figure 1. Roadmap of the translation in preclinical studies of gastrointestinal (GI) model in physiology, pharmacology, disease modelling and personalized medicine.
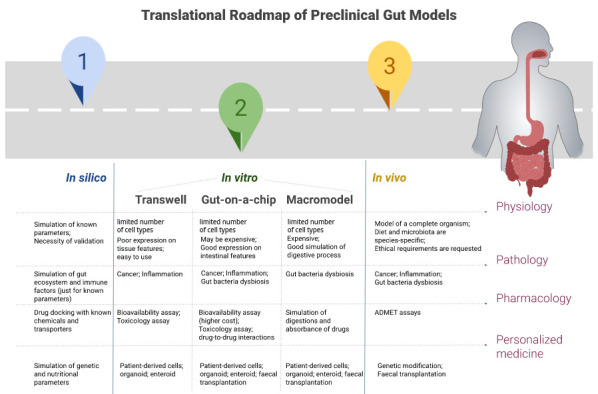
ADMET=Absorption, Distribution, Metabolism, Elimination, and Toxicology.
In vitro models can be distinguished in static and dynamic models; the first are commonly culture epithelial cell lines on Transwell® insert 15 . The most used cell lines are the immortalized human-derived Caco-2, HT29 or HT29-MTX, or the animal-derived IPEC-J2. The advantages of culturing Caco-2 cells in Transwell ®, under static condition, are that: it is the regulatory standard model for drug bioavailability assays 34 , it requires no ethical permissions as cells are commercially available, and it mimics features of both small and large intestine, despite being cells derived by colon cancer. However, there are some limitations to this static Caco-2 in vitro model. For instance, the human intestinal epithelium contains more than one cell type (enterocytes) and it is hard to accurately predict the human response to pathogens and drugs. In fact, the standard bioavailability assay usually does not consider factors like nutrients, microbiota, hormonal factors, plasma carrier proteins, peristalsis speed, or bile acids 35 . Moreover, scientists suggest to consider also the presence of mucus in the bioavailability and in the in vitro digestion, which can be possible by co-culturing Caco-2 and HT29-MTX mucus-producing cells 36, 37 . Recent studies have been working on including bacterial species, representing the gut microbiota, into an in vitro Caco-2/HT29 co-culture. The limitation to this is the restricted nutrient supply, and the time the mammalian and bacterial cells can co-exist in a static environment with build-up of bacterial metabolites and excessive growth rate of bacteria 17, 38 . To overcome these limits, Caco-2 cells have been incorporated into macromodels and GOCs in the dynamic models, which use the fluids flows across the cell cultures.
Macromodels are bioreactors consisting of a series of compartments with different pressures, pH, flow rates, temperatures, and cells aiming to simulate the different GI sections by replicating their biochemical and biophysical parameters 39, 40 . In these models, it is possible to evaluate the bioavailability of drugs and food, and their fermentation by using patient-derived microbiota 41 . However, macromodels require costly lab equipment and space, need stabilization of the microbiota before use, and some of these systems do not mimic peristalsis and lack dialysis for removing microbial acid products 30 .
On the other hand, when Caco-2 are cultivated in alternative GOCs, they express the morphological and functional characteristic of the static in vitro Caco-2 monolayer, both in dynamic fluidic systems with transwells and simpler GOCs 42 . These models have the advantage to control intestinal histogenesis, physiology, mucus production, drugs, and nutrients response. This is possible by modulating several parameters: directional flow rates, mechanical deformation, fluid shear stress, and asymmetric stimulation of the apical and basolateral sides of developing epithelium. Delon et al. used a Hele-Shaw cell to investigate the main features of Caco-2 cells in a microfluidic device by applying several fluid shear stresses 43 . They demonstrated that Caco-2 reach confluency within 5 days (earlier than in the static in vitro models) and that shear stress contributes to morphology, phenotype, and function of the epithelial layer. This turned into better mimicking tight junction expression, mitochondrial activity, mucus production, microvilli density, vacuolization, and cytochrome P450 (CYP450) expression. Gene expression study of Caco-2 on GOCs revealed that expression of MUC17, a transmembrane mucin, was highly enhanced in the 3D villi model compared to a static monolayer culture 43 . In a more recent study, the altered gene expression profile of Caco-2 was compared in static condition versus the flow culturing condition in a GOCs after 21 days. Differences had been spotted in the cellular homeostasis, signal transduction, cell life cycle, and in the immunological responses 44 .
Besides the translational advancement of these GOCs models, there is still a lack of standardization among labs and intrinsic difficulties to scale-up their production. Moreover, like the other aforementioned in vitro models, the currently proposed GOCs are more physiologically relevant model with a reduced number of cell lines, and they generally do not comprise neuroendocrine or immune parameters. Interestingly, some GOCs incorporate organoids, enteroids, and biopsies 11, 45, 46 .
Another commonly used in vitro model of the gut consist of 3D organoids or enteroids, which can be grown from adult intestinal stem cells (ISCs), induced pluripotent stem cells (iPSCs) and primary intestinal epithelial cells (IEC). An advantage of these models is the reproduction of complex structures, including both epithelia and mesenchyme 47 . However, 3D-organoids have lower success in modelling diseases such as IBD because of difficulties maintaining the quality and quantity of cells due to high occurrence of inflammation and pre-apoptosis 48 . Challenges include viability (up to 48h), cost, and difficulties in accessing the lumen of the spheric structure for the application of microbiota and drugs. In pharmacology, there is potential to culture 2D organoids/enteroids in a monolayer to study drug interactions. Also in this case, when biopsies, enteroids or organoids have been integrated in GOCs it was possible to find some advantages in terms of better reproducibility of intestinal cytoarchitecture from a single donor 11, 49– 51 , more reliability in the results for personalized therapy, or longer time in culture in the case of the biopsies 46, 52, 53 .
Focusing on GOCs: from academia to industries and their proof-of-concept
GOCs are microfluidic devices hosting cell or tissue cultures in a single chip. In Table 1, we list each chip, its main features, and the level of industry involvement. GOCs may be used for bioavailability assays, intestinal absorption of nutrients 12 and drugs, and real time evaluation of uptake and transports of drugs. The US Food and Drug Administration ( FDA), the European Medicine Agency ( EMA), and the World Health Organization ( WHO) recommend Caco-2 intestinal permeability assays as the standard model to determine the intestinal permeability rate and ratio of active pharmaceutical ingredients (API). These studies permit to compare the drug permeability from the apical to the basolateral side by considering the involvement of efflux transporter and active uptake transporters ( EMA Guideline on the investigation of drug interaction). Multiple transporters of the adenosine triphosphate (ATP) binding cassette (ABC) active transporter family such as P-glycoprotein (P-gp) or multidrug resistance protein- (MDRP1 or ABCB1) and multidrug resistance protein-2 (MRP-2 or ABCC2) efflux pumps are expressed by Caco-2 54 . A pharmaceutical compound needs to exhibit an apparent permeability (Papp) coefficient of > 90% compared with metoprolol, the gold standard for positive control in Caco-2 cells to be considered for exemption from bioequivalence studies; according to the Biopharmaceutical Classification System (BCS) 55 . A systematic approach for the comparison of the BCS in static and in dynamic conditions on a GOC was done by Kulthong et al. 15 , but no significant improvements were found in drug bioavailability, probably due to the very low shear stress applied in the GOC. In fact, in another GOC model based on 12-wells transwell insert connected to a bioreactor (Quasi-Vivo Kirkstall Ltd), applied fluid mechanical forces enhanced the absorbance of the fluorescein in a time-dependent manner 56 . Comparing a thiol-ene GOC with static in vitro culture 42 , the permeabilities of mannitol, insulin, and fluorescein isothiocyanate were not significantly higher. However, the Caco-2 grew and differentiated faster in the thiol-ene GOC, expressing P-glycoprotein 1 (P-gp), aminopeptidase activity and mucous proteins, which play important roles in the oral bioavailability. A GOC with integrated optical fibers developed by Kimura enabled to observe the transport of rhodamine 123 in real time 57 . Two organoid-derived intestine-on-chip used the Emulate commercially available chip, also containing a polydimethylsiloxane (PDMS) membrane, for a small intestine-on-chip 16 and colon-on-chip 11 models. The advantage of using organoids derived from healthy donors compared to the Caco-2 model is that they better reproduce the intestinal cytoarchitecture, cell-cell interactions, transporters, and the expression of the CYP3A4. This is particularly relevant in studies on pharmacokinetics and pharmacodynamics. Duodenal epithelial cells are cultivated on top of the membrane, while human intestinal microvascular endothelial cells (HIMECs) grown at the bottom. Sontheimer-Phelps et al. have isolated human donor crypts, growing organoids, dissociating the spheres, and seeding the cell mixture onto the chip 11 . This method replicated the mucus bilayer of the colon to a full diameter of 0.6mm. Unfortunately, they did not report how this affected the fluid velocity of the apical channel (height: 1.0 mm), nor did they take this into consideration when reporting the effect of shear on villi bending.
Table 1. List of main of gut-on-chip (GOC)s models and their characteristics, including those developed in academia, in industries or in collaboration.
AOI=Anoxic-oxic interfase; COC=Cyclic Olefin Copolymer; GOC=gut-on-chip; HMI=Host Microbes Interaction; IBD=Intestinal Bowel Disease; IOC=Intestine-on-chip; PC=Polycarbonate; PDMS=Polydimethylsiloxane; PE=Polyester; PET=Polyethylene terephthalate; PMI-CHIP=physiodynamic mucosal interfase-on-a-chip; PS=Polystyrene. Caco-2, CCD-18Co, CRC, and HCT-116 are colon cancer cell lines; HCoMEC=Human Colonic Microvascular Endothelial Cells; HIMECs=Human Intestinal Microvascular Endothelial Cells; HUVEC=Human umbilical vein endothelial cells; iPSC=Induced pluripotent stem cells; PBMC=Peripheral blood mononuclear cell; U937=human lung lymphoblast.
| MODEL OF GOC | APPLICATION | CELLS/TISSUES | MEMBRANE
(Y/N) |
BULK MATERIALS | FLOW RATE
(µL/MIN) |
ACADEMIA
(Y/N) |
INDUSTRY
(Y/N) |
|---|---|---|---|---|---|---|---|
| HUMIX 18, 59 | - HMI
- Disease modelling (colorectal cancer) - Pharmacology (pre- and probiotics) |
Caco-2+ CCD-
18Co; primary CRC cells (T6) |
Yes
PC 1 µm pores |
PC and silicone
gaskets |
25 | Yes | No |
| GOC 17, 48 | - Physiological characterization
- HMI |
Caco-2 + HIMECs | Yes
PDMS |
PDMS | 0.5 | Yes | Modified from
Emulate |
| AOI 19 | - HMI | Caco-2 + HIMECs | Yes
PDMS |
PDMS | 0.833-3.333 | Yes | Modified from
Emulate |
| IOC 11, 16 | - Physiological characterization
- HMI - Drug-to-drug interaction |
Primary, human
derived organoids + HIMECs |
Yes
PDMS |
PDMS | 1 | Yes | Yes
Emulate |
| PMI-CHIP 60 | - HMI
- Disease modelling (IBD) |
Caco-2 or patients’
organoids |
Yes
PDMS |
PDMS | 0.833-1667 | Yes | No |
|
INTESTINAL
MICROFLUIDIC MODEL 57 |
- Pharmaceutical testing | Caco-2 | Yes
PE |
PDMS and PE | N/A | Yes | No |
| TUMOR-ON-A-CHIP 14 | - Disease modelling (Colorectal
Cancer) - Pharmaceutical testing |
HCT-116 +
HCoMECs |
No | PDMS | 0.133 | Yes | No |
| GOFLOWCHIP 45 | - Physiological characterization | iPSC derived
organoids |
No | matrigel, clear
cast acrylic plastic, silicone gasket, borosilicate glass |
0.083 | Yes | Yes |
| ORGANOTYPIC-ON-A-CHIP 53 | - Physiological characterization
- HMI |
Biopsy (mouse
intestinal section) |
No | PDMS, collagen
gel matrix |
16.67 | Yes | No |
| DUAL FLOW BIOREACTOR 56 | - Physiological characterization
- Pharmaceutical testing |
Caco-2 | Yes
PC |
PDMS | 100-400 | Yes | Yes
Kirkstall |
|
USSING CHAMBER ON A
CHIP 46 |
- Disease modelling (IBD) | Human Intestinal
Biopsy |
Yes
PDMS |
Glass, petroleum
jelly |
4 | Yes | No |
| MOTIF 61 | - Physiological characterization
- HMI |
Caco-2, HUVECs,
PBMCs, primary macrophages |
Yes
PET |
COC | 25-50 | Yes | Yes
ChipShop GmbH |
|
THIOL-ENE BASED
MICROFLUIDIC CHIP FOR INTESTINAL TRANSPORT STUDIES 42 |
- Physiological characterization
- Pharmaceutical testing |
Caco-2 | Yes
Thiol-ene coated Teflon |
PMMA, PDMS,
tetra-thiol moieties |
0.5-3 | Yes | No |
| GOC 15 | - Physiological characterization
- Pharmaceutical testing |
Caco-2 | Yes
PET |
Glass, PET | 0.4167 | Yes | Yes
Micronit |
| NUTRICHIP 12 | - Physiological characterization
- Disease modelling (inflammation) |
Caco-2 + U937 | Yes
PET |
PMMA, PS, PDMS | 0.6-2 | Yes | No |
| ORGANOPLATE 13, 58 | - Physiological characterization
- Disease modelling (IBD) - Pharmaceutical testing |
Caco-2 | No | PS, glass,
proprietary polymers |
N/A | No | Yes
Mimetas |
Several GOCs aim to target a specific disease, as in the case of the tumor-on-a-chip for nanoparticles developed by Carvalho and colleagues 14 . Shear stress on HCT-116 cells (a human colon cancer cell line) and human colonic microvascular endothelial cells (HCoMECs) recreated the angiogenesis sprouting typical of colon cancer. To replicate the intestinal tubules, Beaurivage C et al. integrated extracellular matrix (ECM)-supported intestinal tubules grown from Caco-2 cells into their perfused microfluidic devices, OrganoPlate® 13 . In this device, the cells exhibit cellular polarization, tight junction formation, and express key receptors. This GOC is easy to handle and allows different experimental settings for physiological, pathological, and pharmacological studies. However, limitations of this model are 1) the use of a rocker that, by switching inclination of +/- 7 degree every 8 minutes, results in non-uniform bidirectional shear stress; 2) the Caco-2 tubular structure of the chip remain stable only for 6 - 8h of perfusion 58 .
Dawson and colleagues developed their dual-flow biopsy-holding chamber as an improved Ussing chamber 46 . Biopsy culture was maintained for 68h at which point 80% of the tissue was alive as shown with lactate dehydrogenase (LDH) activity upon cell lysis. The longest culture time of intestinal explant tissue in a microfluidic device was reported by Baydoun and colleagues 52 . In their study on a PDMS GOC, they demonstrated 3 of 9 biopsies to be intact upon histological observation after 8 days. Yissachar and colleagues implemented a gut organ culture, accommodating a mice gut tissue fragment in a bath of nutrients 53 . The researchers cocultured ex vivo intestinal tissue with intestinal microbiota and investigated crosstalk with the immune system and expression of neuronal-specific genes. Limits of this model include the short length of experiments (structure degradation after 30–40 hours) and the microbiota overgrowth (24 hours). Scientists from Paul Wilmes group have developed and patented HuMiX, the “Human Microbial Cross-talk” model 59 . This GOC co-cultures Caco-2 and bacteria, either Lactobacillus rhamnosus GG (LGG) or Bacteroides caccae 18 . HuMiX is made from polycarbonate (PC) and therefore has the potential of large-scale production. However, the Caco-2 and the microbiota are separated by a PC membrane which may be a limitation, because only indirect interactions can be assessed. Furthermore, the rigid membrane does not allow the chip to simulate peristalsis. On the contrary, the peristalsis is part of the GOC described by Jalili-Firoozinezhad and colleagues 17, 62 . This GOC is a Polydimethylsiloxane (PDMS) microfluidic two-channel device containing a porous membrane coated with ECM. The Caco-2 cells are cultured on top of the membrane, while below the human intestinal microvascular endothelial cells (HIMECs) lies. The peristaltic movement is controlled by two lateral vacuum chambers that stretch the membrane and regulate the suction force 48 , like in the Emulate chips. The gut microbiota in the chip lived for up to 5 days, more than doubling the 48h of static Caco-2 monoculture. A modified chip, called anoxic-oxic interface-on-a-chip (AOI Chip) 19 , was made by co-cultivating the Caco-2 cells with two obligate anaerobic bacteria, Bifidobacterium adolescentis and Eubacterium hallii. The authors demonstrated that AOI does not compromise the viability, mucin production, barrier function, and the expression of proteins in the intestinal epithelial layer. Moreover, to produce the anoxic environment in the chip while oxic culture media was flowed in the oxic chamber, it was sufficient to precondition culture media in an anoxic chamber. The same research group have more recently developed their own GOC called 3D physiodynamic mucosal interface-on-a-chip (PMI Chip) 60 . The novelty introduced with the PMI Chip is the multiaxial stretching motion that provides the tortuosity of hydrodynamic flow with approximately 5% in cell strain at 0.15 Hz frequency. MOTiF biochips, designed by microfluidic ChipShop GmbH, is a microfluidic chip in polystyrol (PS) initially used to seed endothelial cells, human umbilical vein endothelial cells (HUVEC) 61 . A limitation of the study is the oxygen gradient, which is difficult to measure or control, because bacteria and fungi are sensitive to the gas composition, temperature, and humidity 63 . Following the differentiation of Caco-2 cells (which was faster compared to the transwell model), the authors demonstrated the possibility of colonization with bacteria ( L. rhamnosus) and the fungal pathogen Candida albicans showing the competitive mechanism in vitro.
Bulk and membrane materials
Materials employed in fabrication represent a crucial step and choosing a right material based on the application of the chip is not straightforward 64 . One of the main bottlenecks to scale up the GOC are the materials used to manufacture them 65 . PDMS is easy to prototype, elastic and optically transparent, but the costs are higher for mass production, it absorbs low molecular weight hydrophobic molecules, such as drug compounds, it is permeable to carbon dioxide (CO 2) and it has rather unstable surface properties 66 . However, limited gas permeability of PDMS has been turned into an advantage in HMI studies, controlling for oxygen and anoxic flows to grow different species of gut bacteria 19 . Thermoplastic materials, such as polycarbonate (PC), Poly(methyl methacrylate) PMMA, or Cycloolefins such as cyclic olefin polymers (COP) and copolymers (COC) are easier to produce in larger scale, through injection molding strategies 67 . However, they need to be accurately selected to facilitate sterilization and the needed optical properties for a given assay. PC is easier to produce in larger scale, through injection molding strategies, and can be sterilized in autoclave, but it is more rigid, limiting its use to induce peristaltic deformations, and it has a poor resistance to organic solvents as well as some autofluorescence and sensitivity to ultraviolet (UV) radiation which could be minor inconveniences. COP and COC show low molecules absorption, minimum autofluorescence and excellent optical properties. However, thermoplastics are generally rigid materials and a flexible membrane, or a suitable biological structure should also be provided to induce realistic peristaltic deformations when needed in some GOC models 68 . In most of the GOCs reviewed, membranes serve as support for cell culture (Caco-2 or primary cells) and to simulate peristalsis in combination with flow. They vary not only in manufacturing process and material, but also with regards to pore size, cell-to-cell distance, and overall porosity. Membrane permeability, a function of porosity, pore sizes and specific material properties like charge, is highly relevant for pharmacodynamic testing, such as bioavailability tests conducted in GOCs and other in vitro models. All of these GOCs have been trialed with synthetic membranes such as nylon, PDMS, PC, or polyester such as polyethylene terepthalate (PET). Some, for example Esch et al. 69 and Kim et al. 62 precondition or coat these membranes with collagen 1 to promote cell adhesion. Several papers lacked detail on the exact characteristics of the materials, simply stating that PC or PE from commercial transwells were used.
PC is one of the more commonly used synthetic membrane material due to low cost and rigid nature, as well as its resistance to autoclave pressure and temperature. Aspects such as thickness and porosity can be precisely controlled. However, it is not naturally biocompatible, leading some researchers to precondition the surface with collagen or mucin 18, 70 . Other popular membrane materials are polyesters, including PET. Along with PC, they are widely established in transwell inserts and do not optically interfere in a critical way with microscopy.
Other bioengineering approaches for mimicking the villi structure had been explored and included in larger scaffolds (like the macromodels above described), but not in the GOC models. Other membranes that may be tested in GOC are a combination of synthetic and natural components 71– 73 . Examples include 3D bioprinted membranes made of Poly(ethylene glycol) dimethacrylate (PEGDMA); gelatin methacrylate (GelMA); Lutrol; gelatin also mixed with chitosan; combination of fibrinogen, alginate, gelatin, and polyacrylamide; collagen; or silk proteins with spiral pattern 30 .
Conclusions and future perspective of GOC
GOCs are microfluidic devices that respond to the need of GI models that consider the ethical dilemmas involved in direct studies on humans (Declaration of Helsinki 74 ) and animal testing. In fact, The final aim of these devices is to refine, reduce, and ultimately replace animal testing based on the 3Rs’ 75 and utilise a closer model to the human physiology. Considering that the gut microbiota is also specie-specific and is influenced by nutrition 76 , animal models are often less reliable models for GI compared to other organs 77 . Efforts have been made to produce new GOCs or modify existing ones for new applications. There is however a lack of reported effort on stabilizing protocols to be applied on larger scales and ensuring the product is “fit for a purpose” 78 . In fact, modifications to the geometry design and the protocols seems to be the major concerns of researchers in this field, making OOCs a niche not ready for a larger market and even less ready for the development and testing of therapeutic compounds. In the future, the GOCs described may have a higher output in in vitro studies on HMI, disease modelling, personalized medicine, and pharmacological studies. Fluid mechanical forces in GOCs enable to achieve intestinal physiological features more realistically when compared to other in vitro methods not incorporating biophysical stimulus 79 . Therefore, GOCs can reduce the time for drug development and translational approaches with fewer ethical concerns than animal testing. The GOC approach is very promising but translation into industrial and commercial products aimed to cover the drug industry and healthcare markets require a larger effort to achieve robustness, to guarantee repeatability and to prove reliability.
Funding Statement
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No [845036],(Human gut microbiota on gut-on-a-chip [Goc-MM]) and grant agreement No [814168], (Research and Training in Early Life Nutrition to Prevent Disease [GROWTH]).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
Data availability
No data are associated with this article.
References
- 1. Vanner SJ, Greenwood-Van Meerveld B, Mawe GM, et al. : Fundamentals of neurogastroenterology: Basic science. Gastroenterology. 2016;150(6):1280–1291. 10.1053/j.gastro.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoyama S, Shibata S: Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front Nutr. 2020;7:18. 10.3389/fnut.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bischoff SC: “Gut health”: A new objective in medicine? BMC Med. 2011;9:24. 10.1186/1741-7015-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ianiro G, Tilg H, Gasbarrini A: Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut. 2016;65(11):1906–1915. 10.1136/gutjnl-2016-312297 [DOI] [PubMed] [Google Scholar]
- 5. Malaguarnera G, Leggio F, Vacante M, et al. : Probiotics in the gastrointestinal diseases of the elderly. J Nutr Heal Aging. 2012;16(4):402–10. 10.1007/s12603-011-0357-1 [DOI] [PubMed] [Google Scholar]
- 6. GBD 2017 Causes of Death Collaborators: Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GBD 2017 Diet Collaborators: Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. GBD 2019 Risk Factors Collaborators: Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe S, Hojo M, Nagahara A: Metabolic syndrome and gastrointestinal diseases. J Gastroenterol. 2007;42(4):267–274. 10.1007/s00535-007-2033-0 [DOI] [PubMed] [Google Scholar]
- 10. De Filippis A, Ullah H, Baldi A, et al. : Gastrointestinal disorders and metabolic syndrome: Dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int J Mol Sci. 2020;21(14):4929. 10.3390/ijms21144929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sontheimer-phelps A, Chou DB, Tovaglieri A, et al. : Colon In Vivo Inner & Outer Mucus Layer Colon Chip Mucus Layer Swelling Mucus Layer Colon Organoids. Cell Mol Gastroenterol Hepatol. 2019. [Google Scholar]
- 12. Ramadan Q, Jafarpoorchekab H, Huang C, et al. : NutriChip: Nutrition analysis meets microfluidics. Lab Chip. 2013;13(2):196–203. 10.1039/c2lc40845g [DOI] [PubMed] [Google Scholar]
- 13. Beaurivage C, Naumovska E, Chang YX, et al. : Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int J Mol Sci. 2019;20(22):5661. 10.3390/ijms20225661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carvalho MR, Barata D, Teixeira LM, et al. : Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Sci Adv. 2019;5(5):eaaw1317. 10.1126/sciadv.aaw1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulthong K, Duivenvoorde L, Sun H, et al. : Microfluidic chip for culturing intestinal epithelial cell layers: Characterization and comparison of drug transport between dynamic and static models. Toxicol In Vitro. 2020;65:104815. 10.1016/j.tiv.2020.104815 [DOI] [PubMed] [Google Scholar]
- 16. Kasendra M, Tovaglieri A, Sontheimer-Phelps A, et al. : Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci Rep. 2018;8(1):2871. 10.1038/s41598-018-21201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, et al. : A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019;3(7):520–531. 10.1038/s41551-019-0397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah P, Fritz JV, Glaab E, et al. : A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat Commun. 2016;7:11535. 10.1038/ncomms11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin W, Wu A, Massidda MW, et al. : A robust longitudinal co-culture of obligate anaerobic gut microbiome with human intestinal epithelium in an anoxic-oxic interface-on-a-chip. Front Bioeng Biotechnol. 2019;7:13. 10.3389/fbioe.2019.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takebe T, Imai R, Ono S: The Current Status of Drug Discovery and Development as Originated in United States Academia: The Influence of Industrial and Academic Collaboration on Drug Discovery and Development. Clin Transl Sci. 2018;11(6):597–606. 10.1111/cts.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrua MJ, Singh RP: Computational Modeling of Gastrointestinal Fluid Dynamics. In: Cheng LK, Pullan AJ, Farrugia G, eds. New Advances in Gastrointestinal Motility Research. Dordrecht: Springer Netherlands;2013;243–266. 10.1007/978-94-007-6561-0_13 [DOI] [Google Scholar]
- 22. Wilkinson MHF: Model intestinal microflora in computer simulation: A simulation and modeling package for host-microflora interactions. IEEE Trans Biomed Eng. 2002;49(10):1077–1085. 10.1109/TBME.2002.803548 [DOI] [PubMed] [Google Scholar]
- 23. Taghipoor M, Lescoat P, Licois JR, et al. : Mathematical modeling of transport and degradation of feedstuffs in the small intestine. J Theor Biol. 2012;294:114–121. 10.1016/j.jtbi.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 24. Hoertel N, Blachier M, Blanco C, et al. : A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat Med. 2020;26(9):1417–1421. 10.1038/s41591-020-1001-6 [DOI] [PubMed] [Google Scholar]
- 25. Shashkova T, Popenko A, Tyakht A, et al. : Agent based modeling of human gut microbiome interactions and perturbations. PLoS One. 2016;11(12):e0148386. 10.1371/journal.pone.0148386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Feunteun S, Mackie AR, Dupont D: In silico trials of food digestion and absorption: how far are we? Curr Opin Food Sci. 2020;31:121–125. 10.1016/j.cofs.2020.04.006 [DOI] [Google Scholar]
- 27. Havenaar R, Bellmann S: Results from a Validated in vitro Gastrointestinal Model (TIM) used as input Data for in silico Modeling Give Highly Predictive Information for the Human Situation. Med Rec Arch. [S.l.],2022;10(9). Date accessed: 29 oct. 2022. 10.18103/mra.v10i9.3136 [DOI] [Google Scholar]
- 28. Yamashita F, Hashida M: In silico approaches for predicting ADME properties of drugs. Drug Metab Pharmacokinet. 2004;19(5):327–38. 10.2133/dmpk.19.327 [DOI] [PubMed] [Google Scholar]
- 29. Parenti R, Vicario N: Peer Review Report For: The translational roadmap of the gut models, focusing on gut-on-chip [version 1; peer review: 1 approved, 1 approved with reservations]. Open Res Europe. 2021;1:62. Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fois CAM, Le TYL, Schindeler A, et al. : Models of the Gut for Analyzing the Impact of Food and Drugs. Adv Healthc Mater. 2019;8(21):e1900968. 10.1002/adhm.201900968 [DOI] [PubMed] [Google Scholar]
- 31. Tan HE, Sisti AC, Jin H, et al. : The gut-brain axis mediates sugar preference. Nature. 2020;580(7804):511–516. 10.1038/s41586-020-2199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zimmerman CA, Huey EL, Ahn JS, et al. : A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature. 2019;568(7750):98–102. 10.1038/s41586-019-1066-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nithianantharajah J, Balasuriya GK, Franks AE, et al. : Using Animal Models to Study the Role of the Gut-Brain Axis in Autism. Curr Dev Disord Rep. 2017;4(2):28–36. 10.1007/s40474-017-0111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hubatsch I, Ragnarsson EGE, Artursson P: Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2(9):2111–2119. 10.1038/nprot.2007.303 [DOI] [PubMed] [Google Scholar]
- 35. Costa J, Ahluwalia A: Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest. Front Bioeng Biotechnol. 2019;7:144. 10.3389/fbioe.2019.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahler GJ, Shuler ML, Glahn RP: Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J Nutr Biochem. 2009;20(7):494–502. 10.1016/j.jnutbio.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 37. Malik M, Yang Y, Fathi P, et al. : Critical Considerations for the Design of Multi-Organ Microphysiological Systems (MPS). Front Cell Dev Biol. 2021;9:721338. 10.3389/fcell.2021.721338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hickman JJ, Malik M: Peer Review Report For: The translational roadmap of the gut models, focusing on gut-on-chip [version 1; peer review: 1 approved, 1 approved with reservations]. Open Res Europe. 2021;1:62. 10.21956/openreseurope.14783.r28371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Wiele T, den Abbeele P, Ossieur W, et al. : The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). In: Verhoeckx K, Cotter P, López-Expósito I, et al,.eds. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models. Cham: Springer International Publishing;2015;305–317. 10.1007/978-3-319-16104-4_27 [DOI] [Google Scholar]
- 40. Wiese M, Khakimov B, Nielsen S, et al. : CoMiniGut-A small volume in vitro colon model for the screening of gut microbial fermentation processes. PeerJ. 2018;6(1):e4268. 10.7717/peerj.4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guzman-Rodriguez M, McDonald JAK, Hyde R, et al. : Using bioreactors to study the effects of drugs on the human microbiota. Methods. 2018;149:31–41. 10.1016/j.ymeth.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 42. Tan HY, Trier S, Rahbek UL, et al. : A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS One. 2018;13(5):e0197101. 10.1371/journal.pone.0197101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Delon LC, Guo Z, Oszmiana A, et al. : A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials. 2019;225:119521. 10.1016/j.biomaterials.2019.119521 [DOI] [PubMed] [Google Scholar]
- 44. Kulthong K, Hooiveld GJEJ, Duivenvoorde L, et al. : Transcriptome comparisons of in vitro intestinal epithelia grown under static and microfluidic gut-on-chip conditions with in vivo human epithelia. Sci Rep. 2021;11(1):3234. 10.1038/s41598-021-82853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sidar B, Jenkins BR, Huang S, et al. : Long-term flow through human intestinal organoids with the gut organoid flow chip (GOFlowChip). Lab Chip. 2019;19(20):3552–3562. 10.1039/c9lc00653b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dawson A, Dyer C, Macfie J, et al. : A microfluidic chip based model for the study of full thickness human intestinal tissue using dual flow. Biomicrofluidics. 2016;10(6):064101. 10.1063/1.4964813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoo JH, Donowitz M: Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases. World J Gastroenterol. 2019;25(30):4125–4147. 10.3748/wjg.v25.i30.4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim HJ, Ingber DE: Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb). 2013;5(9):1130–40. 10.1039/c3ib40126j [DOI] [PubMed] [Google Scholar]
- 49. Sontheimer-Phelps A, Chou DB, Tovaglieri A, et al. : Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cell Mol Gastroenterol Hepatol. 2020;9(3):507–526. 10.1016/j.jcmgh.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tovaglieri A, Sontheimer-Phelps A, Geirnaert A, et al. : Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome. 2019;7(1):43. 10.1186/s40168-019-0650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sances S, Ho R, Vatine G, et al. : Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Stem Cell Reports. 2018;10(4):1222–1236. 10.1016/j.stemcr.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baydoun M, Treizeibré A, Follet J, et al. : An interphase microfluidic culture system for the study of ex vivo intestinal tissue. Micromachines (Basel). 2020;11(2):150. 10.3390/mi11020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yissachar N, Zhou Y, Ung L, et al. : An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell. 2017;168(6):1135–1148.e12. 10.1016/j.cell.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pham-The H, Garrigues T, Bermejo M, et al. : Provisional classification and in silico study of biopharmaceutical system based on Caco-2 cell permeability and dose number. Mol Pharm. 2013;10(6):2445–2461. 10.1021/mp4000585 [DOI] [PubMed] [Google Scholar]
- 55. Dahan A, Miller JM, Amidon GL: Prediction of solubility and permeability class membership: provisional BCS classification of the world's top oral drugs. AAPS J. 2009;11(4):740–746. 10.1208/s12248-009-9144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giusti S, Sbrana T, La Marca M, et al. : A novel dual-flow bioreactor simulates increased fluorescein permeability in epithelial tissue barriers. Biotechnol J. 2014;9(9):1175–1184. 10.1002/biot.201400004 [DOI] [PubMed] [Google Scholar]
- 57. Kimura H, Yamamoto T, Sakai H, et al. : An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip. 2008;8(5):741–746. 10.1039/b717091b [DOI] [PubMed] [Google Scholar]
- 58. Trietsch SJ, Naumovska E, Kurek D, et al. : Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat Commun. 2017;8(1):262. 10.1038/s41467-017-00259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Greenhalgh K, Ramiro-Garcia J, Heinken A, et al. : Integrated In Vitro and In Silico Modeling Delineates the Molecular Effects of a Synbiotic Regimen on Colorectal-Cancer-Derived Cells. Cell Rep. 2019;27(5):1621–1632.e9. 10.1016/j.celrep.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 60. Shin YC, Shin W, Koh D, et al. : Three-Dimensional Regeneration of Patient-Derived Intestinal Organoid Epithelium in a Physiodynamic Mucosal Interface-on-a-Chip. Micromachines (Basel). 2020;11(7):663. 10.3390/mi11070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maurer M, Gresnigt MS, Last A, et al. : A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials. 2019;220:119396. 10.1016/j.biomaterials.2019.119396 [DOI] [PubMed] [Google Scholar]
- 62. Kim HJ, Huh D, Hamilton G, et al. : Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–2174. 10.1039/c2lc40074j [DOI] [PubMed] [Google Scholar]
- 63. Andresen LC, Dungait JAJ, Bol R, et al. : Bacteria and fungi respond differently to multifactorial climate change in a temperate heathland, traced with 13C-glycine and FACE CO 2. inc. PLoS One. 2014;9(1):e85070. 10.1371/journal.pone.0085070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ren K, Zhou J, Wu H: Materials for microfluidic chip fabrication. Acc Chem Res. 2013;46(11):2396–2406. 10.1021/ar300314s [DOI] [PubMed] [Google Scholar]
- 65. Zhang B, Radisic M: Organ-on-A-chip devices advance to market. Lab Chip. 2017;17(14):2395–2420. 10.1039/c6lc01554a [DOI] [PubMed] [Google Scholar]
- 66. Whitesides GM: The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- 67. Qin D, Xia Y, Whitesides GM: Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5(3):491–502. 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 68. Chae SK, Ryoo JH, Lee SH: Thin and large free-standing PDMS membrane by using polystyrene Petri dish. Biochip J. 2012;6(2):184–190. 10.1007/s13206-012-6211-7 [DOI] [Google Scholar]
- 69. Esch MB, Sung JH, Yang J, et al. : On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic 'body-on-a-chip' devices. Biomed Microdevices. 2012;14(5):895–906. 10.1007/s10544-012-9669-0 [DOI] [PubMed] [Google Scholar]
- 70. Chen HJ, Miller P, Shuler ML: A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip. 2018;18(14):2036–2046. 10.1039/c8lc00111a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bakarich SE, Panhuis MIH, Beirne S, et al. : Extrusion printing of ionic-covalent entanglement hydrogels with high toughness. J Mater Chem B. 2013;1(38):4939–4946. 10.1039/c3tb21159b [DOI] [PubMed] [Google Scholar]
- 72. Hong S, Sycks D, Chan HF, et al. : 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv Mater. 2015;27(27):4035–4040. 10.1002/adma.201501099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Skardal A, Zhang J, McCoard L, et al. : Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A. 2010;16(8):2675–2685. 10.1089/ten.TEA.2009.0798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. World Medical Association: World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 75. Herrmann K, Pistollato F, Stephens ML: Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. ALTEX. 2019;36(3):343–352. 10.14573/altex.1907031 [DOI] [PubMed] [Google Scholar]
- 76. Gevers D, Knight R, Petrosino JF, et al. : The Human Microbiome Project: A Community Resource for the Healthy Human Microbiome. PLoS Biol. 2012;10(8):e1001377. 10.1371/journal.pbio.1001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kennedy EA, King KY, Baldridge MT: Mouse microbiota models: Comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. 10.3389/fphys.2018.01534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Taute F, Homs-Corbera A, Gaudriault P: The challenges and considerations for emerging or future entrepreneurial researchers in microphysiological systems [version 1; peer review: 2 approved with reservations]. Open Res Eur. 2021;1:38. 10.12688/openreseurope.13335.1 [DOI] [Google Scholar]
- 79. Polacheck WJ, Li R, Uzel SGM, et al. : Microfluidic platforms for mechanobiology. Lab Chip. 2013;13(12):2252–2267. 10.1039/c3lc41393d [DOI] [PMC free article] [PubMed] [Google Scholar]


