Abstract
Background: Non-transgenic chemical mutagen application, particularly ethyl methanesulfonate (EMS), is an important tool to create mutations and gain a new genetic makeup for plants. It is useful to obtain a sufficient number of mutant plants instead of working with a severe mutation in a few plants. EMS dose and exposure period have been previously studied in several crops; however, EMS used to create point mutations in presoaked rice seeds has not been sufficiently studied and there is no standard protocol for such treatment. The aim of this study is to establish a standard protocol for EMS mutagenesis application in rice.
Methods: Two studies were conducted to evaluate the effect of four durations of rice seed presoaking (0, 6, 12, and 24 hours), four EMS concentration doses (0.0%, 0.5%, 1.0%, and 2.0%), and four EMS exposure periods (6, 12, 24, and 48 hours). Germination rate, plumula and radicle length, seedling survival, shoot length, root length and fresh seedling weight were evaluated.
Results: Results showed that a 12-hour presoaking duration, 0.5% EMS dose, and six hours of EMS exposure were the best practices for the optimum number of mutant plants.
Conclusions: In light of both this study and the literature, a standard application protocol was established. This application protocol, detailed in this article, contains the following guidelines: (1) Presoaking: 12 hours, (2) EMS application: 0.5% dose EMS and six hours, (3) Final washing: six hours, (4) Drying: 72 hours at 38°C. A user-friendly protocol has been presented for utilization by researchers.
Keywords: EMS, dose, mutagenesis, protocol, rice
Introduction
Rice is the staple food for nearly half of the world’s population, most of whom live in developing countries. Rice is currently grown in over a hundred countries, which produce 755 million tons of paddy rice ( FAO, 2019). Asian countries, including China, India, Indonesia, Bangladesh, Vietnam, Myanmar, Thailand, Philippines, Japan, Pakistan, Cambodia, South Korea, Nepal, and Sri Lanka, account for 90% of the world’s total rice production. Europe, however, has several important rice producer countries such as Italy, Spain, Greece, Portugal, France, Bulgaria and Turkey. In the European Union, the rice production area is approximately 418,000 hectares, total production is close to three million tons with average yields of 6.8 tons per hectare ( FAO, 2019).
Rice accounts for a third of the earth's area planted with fields crops and it supplies 35-60% calories of nutrition to the world population. People globally consumed more rice than wheat or maize, the other two staple foods. Both developed and developing nations alike grow and consume rice. Of the major staple foods of rice, wheat, and corn, rice is the most crucial food particularly low- and middle-income nations. Rice is an essential component of complicated cereal product systems that impact issues of worldwide concern, such as food sustainability and security, poverty reduction and protection of social legacy ( Chauhan et al., 2017).
Rice production has some crucial problems such as irrigation scarcity, rice blast disease ( M. oryzae), weeds and red rice. Full yield capability has not been realized due to the damage from insects and diseases, while weeds limit rice through rivalry for daylight, water, and supplements. Weed rivalry can bring about complete yield loss ( Al-Khatib et al., 2018; Brim-Deforest et al., 2017; Espino et al., 2018; Gibson et al., 2002). Intensive research to solve some of these problems is being carried out supported by the European Commission. The problems of weeds and red rice is especially a problem in Europe because of their direct production system of sowing rice. The main rice area, Asia, has a production system of transplanting rice so they have no severe weed problems in their fields. Therefore, chemical companies have not been willing to develop new active ingredients for European countries. Old herbicides do not work effectively over time. The development of herbicide-tolerant rice is a more reasonable approach than developing a new active ingredient. Researchers have developed herbicide resistance systems such as Clearfield, Provisia, and Roxy Rice by mutation application. Most of this research is based on plant EMS mutagenesis application.
Rice plant breeders have used point mutations in their breeding program to overcome these problems. The mutation may exist in nature besides the artificially induced mutation. Physical and chemical mutagens are used to obtain plants by mutation breeding, such as gamma rays, X-rays, fast neutrons and also ethyl methanesulphonate (EMS; CH 3SO 3C 2H 5), diepoxybutane (DEB, C 4H 6O 2) and sodium azide (NaN 3). The chemical mutagen EMS has been widely utilized to induce a large number of functional variations in rice. EMS alkylates guanine bases and leads to mispairing of alkylated G with T instead of C, resulting in primarily G/C- to -A/T transitions ( Bhat et al., 2007).
Chemical mutagen application methods have a draft protocol of presoaking, mutagen application and a final washing process. The implementation phase of these processes differs in many studies and unfortunately, there is no standard protocol for mutagenizing rice seeds. The objective of this study is to develop a standard protocol for EMS mutagenesis application in rice.
Methods
Materials
Osmancik-97 is a Japonica type Turkish rice variety. The variety was released by Trakya Agricultural Research Institute, Edirne, Turkey in 1997. The parents are Rocca and Europe, which originate from Italy. The Osmancik-97 rice variety has a plant length of 105 cm, 85 days of flowering, 135 days of maturity, a semi horizontal 16 cm panicle, 65% milling yield and 8-9 tons per hectare grain yield potential. Material samples have 14% moisture content, 98-100% germination ratio, 24 g milled 1000 grain and 34 g un-milled 1000 grain weight ( Unan et al., 2013).
The molecular formula of EMS (Sigma- Aldrich Inc., USA) is C 3H 8O 3S, molecular weight is 124.2 g, density is 1.206 g ml -1, half-life is 48.5 hours at 25°C. It is a powerful mutagen for plants.
EMS mutagenesis
The experiment was carried out using a randomized parcel design with three replications for the germination experiment and four replications for the seedling experiment, and each replication used 100 seeds under a fume hood in a Fitotron growth chamber. Seeds are sterilized with bleach solution (30% commercial bleach + 0.02% Triton X-100) for 15 min and washed three times with pure water. Seeds were placed in a glass container and pure water was added to a volume of 1 ml seed -1. Seeds were presoaked for 0, 6, 12 or 24 hours at 20°C. Afterwards, the water was decanted and again 1 ml seed -1 of 0.0%, 0.5%, 1%, or 2% concentrations of EMS (v/v) in water was added. Seeds were incubated for six, 12, 24, or 48 hours in different concentrations of EMS solution at 20°C under the fume hood. Subsequently, EMS-treated seeds were washed with pure water five times for five minutes (total 25 minutes) ( Talebi et al., 2012). The seeds were washed again with running tap water for six hours ( Sagel et al., 2017).
Seedling survival rate is the ratio of survive seedlings 21 days after sowing of seeds ( Evangelina et al., 2010). In the seedling experiment, seedling survival of rice seeds with each of the four presoaking durations, four EMS doses and four exposure periods was determined as the percentage of seedlings that survived 21 days after seeding in the fitotron chamber.
Seedling survival (%) = (survived rice seedlings / sowed rice seeds) × 100
Imbibition rate was calculated as the percentage of water intake of seeds hourly. 100g of seeds which had 14% water content were incubated in pure water at 20°C and the weight noted each hour for 48 hours with three replications. The seeds were removed from the water, drained for one minute and dried with blotting paper for 30 seconds and then measured with an analytical balance (AS 3Y, Radwag Wagi Elektroniczne, Poland). Imbibition rate was calculated using the following formula:
Imbibition rate (%) = (last weight - first weight) × 100 / first weight
Experiment 1: Germination experiment
The experiment was carried out using a randomized parcel design with three replications for germination. Experiment factors were four presoaking durations, four EMS doses, and four EMS exposure periods. 100 EMS-treated seeds were used for each treatment besides 100 untreated control seeds on filter paper soaked in 30ml of pure water in petri dishes. Untreated control seeds were managed under the same conditions except EMS exposure. The seeds were then put in the Fitotron at 25°C and 30°C for 12-hour cycles of light and dark conditions for seven days. After seven days, the number of seeds that germinated, with 5 mm plumula being accepted as germinated ( Cruz & Milach, 2004), under these conditions was recorded. Seedling length of the plants were measured using a digital caliper (Insize standart calipper, Germany). The roots were scanned using an Epson 11000XL scanner at a resolution of 600 dpi. Root traits were obtained using WinRHIZO 2009 Pro software (Regent Instruments). The equation to calculate germination percentage was (seeds germinated / total seeds) × 100 ( IRRI, 2002).
Experiment 2: Seedling experiment
The experiment was carried out using a randomized parcel design with four replications. Experiment factors are four presoaking durations, four EMS doses, four EMS exposure periods, and their controls. Twenty seeds for each presoaking duration, EMS-treatment and EMS exposure duration seeds and their controls were sown in a plastic plant viol. Control seeds included no-presoaked seeds and no-EMS exposure seeds. Sterilized soil was used in the experiment. The 28-cell plant viol had a diameter of 7 cm and a depth of 7.4 cm. The plant viols were then put in the Fitotron at 25°C and 30°C for 12-hour dark and 12-hour light cycles for 21 days, respectively. After 21 days the surviving seedlings’ length, root length and fresh plant weight were measured ( IRRI, 2002). The fresh plant weight measurement equipment used for analytical weighing was manufactured by Radwag Wagi Elektroniczne, Poland (Radwag, AS 3Y analytical balances). The length of the plants was measured using a digital caliper (Insize standart calipper, Germany). The roots were scanned using an Epson 11000XL scanner at a resolution of 600 dpi. Root traits were obtained using WinRHIZO 2009 Pro software (Regent Instruments).
Factsheet and flowchart of protocol for EMS mutagenesis application in rice
A one-page user protocol might be useful in laboratory studies. Hence, a single page user protocol has been created. The materials used in the protocol are simply defined in the factsheet. Protocol application stages and durations are given for presoaking, EMS application, final washing, and drying. In addition, a flowchart is supplied for users. This flowchart shows a schematic for how to utilize the protocol. The factsheet and flowchart of the protocol for EMS mutagenesis application in rice are supplied as Extended data ( Unan, 2021b).
Statistical analysis
Three-way analysis of variance was used in order to detect any statistically significant differences between presoaking duration, EMS dose, and EMS exposure period. Significant differences between the averages were evaluated using the Tukey least significant difference (LSD) test at p-value <0.01. LSD tested the differences in observed averages of all tested parameters between treatment and non-treatment seeds. Statistical analysis was conducted using JMP 7.0 software.
Results
Imbibition rate
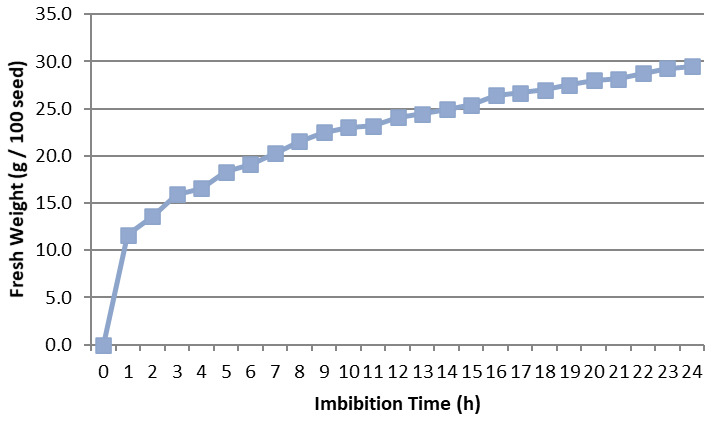
The imbibition rate was calculated for Osmancik-97 rice at the start of the experiment. The increase in seed weight happening over the imbibition time period hourly and every six hours at 20°C in the Fitotron was determined ( Figure 1 and Figure 2). Initial moisture content was 14%. The seeds with 14% moisture were considered to have 0% water intake; water intake was calculated as a percentage increase in moisture content. Rapid increases of water uptake were calculated in first hour as more than 10%. Subsequently, the rapid rising proceeded up to 25% in the first 12 hours. Finally, the increase reached 30% in the first 24 hours. No significant increase was seen after 24 hours. The seeds weight reached equilibrium as around 30% in the pure water. During the 0, 6, 12 and 24 hours presoaking (imbibition) stage, the seeds had 0%, 19.1%, 24.1%, and 29.5% water intake, respectively ( Unan, 2021a).
Figure 1. Water uptake measurement compared to imbibition time interval at six hours in Osmancik-97 rice variety.
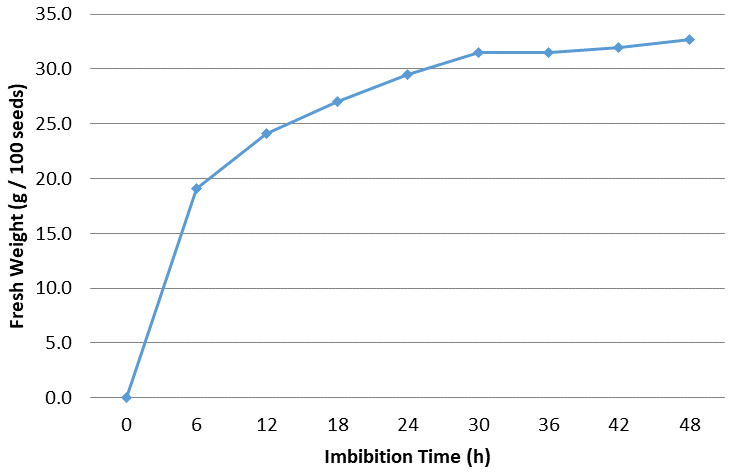
Figure 2. Water uptake measurement compared to imbibition time hourly in Osmancik-97 rice variety.
Germination experiment
Germination is a crucial factor for EMS mutagenesis experiments. The analysis of variance revealed significant (P < 0.01) differences in germination percentage between each EMS dose, exposure period and presoaking and their combinations. Germination was ranked from 0% to 100% in this study. When evaluated in terms of the EMS dose, the lowest average germination observed was 33.4% for the 2% EMS dose. The highest mean germination observed was 98.8% for the control plot ( Table 1). Statistical analysis on germination showed an attendant decrease in germination with applied increases in the concentration of EMS. As per Table 1, the outcomes acquired show that a decrease in seed germination occurred with a corresponding increase in EMS dose (P < 0.01). Considering EMS exposure period, mean germination percentage was 91.9%, 81.3%, 55.6%, and 24.7% for six, 12, 24, and 48-hour exposure periods, respectively. When evaluated in terms of presoaking, the lowest mean germination was observed 49.1% for zero hours (dry seed) presoaking. The highest mean germination observed was 71.8% at 12 hours presoaking. EMS application without presoaking and 48 hours of EMS application and their combinations almost prevented germination. It should be emphasized that the chemical reduces the germination ability of dry seeds and also EMS application for more than 24 hours prevents germination to a high extent. Most combinations resulted in 100% germination. However, six hours application, 0.5% EMS dose, and 12 hours presoaking interaction might be preferred for maximum germination of mutant seeds.
Table 1. Effect of EMS application dose, EMS exposure period and presoaking duration on germination in rice (%).
| EMS application
dose (%) |
EMS
exposure period (h) |
Presoaking duration (hours) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 100a | 100a | 95b | 95b | 97.5 c |
| 12 | 100a | 100a | 100a | 100a | 100.0 a | |
| 24 | 100a | 95b | 100a | 100a | 98.8 b | |
| 48 | 100a | 100 | 95b | 100a | 98.8 b | |
| Mean | 100a | 98.8b | 97.5c | 98.8b | 98.8 A | |
| 0.5% | 6 | 100a | 100a | 100a | 100a | 100.0 a |
| 12 | 75e | 95b | 100a | 100a | 92.5 d | |
| 24 | 80d | 100a | 100a | 55g | 83.8 f | |
| 48 | 0l | 0l | 0l | 0l | 0.0 k | |
| Mean | 63.g | 73.8e | 75.0d | 63.8g | 69.1 B | |
| 1% | 6 | 60f | 100a | 100a | 100a | 90.0 e |
| 12 | 35i | 100a | 100a | 100a | 83.8 f | |
| 24 | 0l | 75e | 50h | 15j | 35.0 i | |
| 48 | 0l | 0l | 0l | 0l | 0.0 k | |
| Mean | 23.8m | 68.8f | 62.5h | 53.8i | 52.2 C | |
| 2% | 6 | 35i | 100a | 100a | 85c | 80.0 g |
| 12 | 0l | 15j | 100a | 80d | 48.8 h | |
| 24 | 0l | 10k | 10k | 0l | 5.0 j | |
| 48 | 0l | 0l | 0l | 0l | 0.0 k | |
| Mean | 8.8n | 31.3l | 52.5j | 41.3k | 33.4 D | |
| General Average | 49.1D | 68.1B | 71.8A | 64.4C | 63 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 0,09; LSD Duration= 0.09; LSD Presoaking = 0.11; LSD Dose×Duration=0.22; LSD Dose×Presoaking=0.22; LSD Duration×Presoaking=0.22; LSD Dose×EMSduration×Presoaking = 0,45; CV (%) = 4.44. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
Plumula length is another indicator factor used in EMS mutagenesis experiments. There are significant (P < 0.01) differences in plumula length with each EMS dose, exposure period, presoaking, and their combinations according to the analysis of variance. Plumula length ranged from 0 mm to 62.0 mm in the germination experiment. In terms of the EMS dose, the lowest mean plumula length measured was 20.0 mm for the 2% EMS dose plot. The highest mean plumula length measured was 52.6 mm for the control plot ( Table 2). Statistical analysis on plumula length showed an attendant decrease in plumula length with applied increases in the concentration of EMS. As per Table 2, the outcomes acquired show that a decrease in plumula length was observed with a corresponding increase in EMS dose (P < 0.01). When evaluated in terms of the exposure period, the mean plumula length was 50.1, 41.8, 29.6, and 13.0 mm for the six, 12, 24, and 48 hours exposure periods, respectively. Regarding presoaking, the lowest mean plumula length observed was 11.2 mm for the zero hours (dry seed) presoaking plot. The highest mean plumula length observed was 33.3 mm for the 12 hours presoaking plot. EMS application without presoaking and 48 hours of EMS application and their combinations nearly forestalled plumula length. It should be underlined that the EMS harms the plumula length capacity of dry seeds and furthermore EMS application for over 24 hours forestalls plumula length. Many of the combinations had a 5 mm plumula length. However, six hours application, 0.5% EMS dose, and 24 hours presoaking showed the best results except for the 0% (control) EMS dose application.
Table 2. Effect of EMS application dose, EMS exposure period and presoaking duration on plumula length in rice (mm).
| EMS
application dose (%) |
EMS
exposure period (h) |
Presoaking duration (hours) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 62.2ab | 60.1ac | 49.7hl | 52.9ej | 56.2 a |
| 12 | 50.6fl | 50.5fl | 51.1fl | 51.8fk | 51.0 b | |
| 24 | 47.5ln | 52.9ej | 49.5hl | 54.6df | 51.1 b | |
| 48 | 58.1bd | 63.0a | 54.0dg | 32.9t | 52.0 b | |
| Mean | 54.6a | 56.6a | 51.1b | 48.1c | 52.6 A | |
| 0.5% | 6 | 42.4oq | 53.4ei | 49.4hl | 57.1ce | 50.6 b |
| 12 | 31.3t | 51.2fl | 50.6fl | 52.2fk | 46.3 c | |
| 24 | 38.1rs | 47.0ln | 49.8gl | 38.6qs | 43.4 d | |
| 48 | 0.0w | 0.0w | 0.0w | 0.0w | 0.0 h | |
| Mean | 27.9f | 37.9d | 37.5d | 36.9d | 35.1 B | |
| 1% | 6 | 18.0u | 49.1jm | 54.1df | 53.6eh | 43.7 d |
| 12 | 40.3pr | 44.9mo | 47.3ln | 43.4np | 43.9 d | |
| 24 | 0.0w | 42.6oq | 34.8st | 7.6v | 21.2 f | |
| 48 | 0.0w | 0.0w | 0.0w | 0.0w | 0.0 h | |
| Mean | 14.6h | 34.1e | 34.0e | 26.2f | 27.2 C | |
| 2% | 6 | 44.9mo | 59.8ac | 53.3ei | 48.1km | 51.5 b |
| 12 | 0.0w | 15.3u | 49.3il | 39.5pr | 26.0 e | |
| 24 | 0.0w | 4.3v | 6.8v | 0.0w | 2.8 g | |
| 48 | 0.0w | 0.0w | 0.0w | 0.0w | 0.0 h | |
| Mean | 11.2i | 19.9g | 27.3f | 21.9g | 20.0 D | |
| General Average | 27.1C | 37.1A | 37.5A | 33.3B | 33.7 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 1.0; LSD Duration= 1.0; LSD Presoaking = 1.0; LSD Dose×Duration=2.1; LSD Dose×Presoaking=2.1; LSD Duration×Presoaking=2.1; LSD Dose×EMSduration×Presoaking = 4.2; CV (%) = 7.7. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
Radicle length ranged from 0.0 to 47.8 mm in this study. The analysis of variance showed significant (P < 0.01) differences in radicle length with each presoaking duration, EMS dose, exposure period, and their combinations. When evaluated in terms of the EMS dose, the lowest and highest mean radicle length observed was 10.7 mm and 33.7 mm for the 2% EMS dose plot and control plot, respectively ( Table 3). Increasing EMS doses caused shortening of the radicle length. Considering each exposure period, the mean radicle length was 33.7, 19.8, 14.9, and 10.7 mm for the six, 12, 24, and 48 hours exposure periods, respectively. When evaluated in terms of presoaking, the lowest and highest mean radicle length observed was 14.9 mm and 23.8 mm for the zero hours (dry seed) and 12 hours presoaking plot, respectively. Many of the combinations resulted in 20 mm radicle length, which is the optimum radicle length. However, 12 hours application, 0.5% EMS dose, and 24 hours presoaking combinations showed the best results except for the 0% (control) EMS dose application.
Table 3. Effect of EMS application dose, EMS exposure period and presoaking duration on radicle length in rice (mm).
| EMS application dose (%) | EMS exposure period(hour) | Presoaking duration (hours) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 25.7jk | 31.7fg | 43c | 46.2ab | 36.7 b |
| 12 | 42.8c | 43.9bc | 47.8a | 33.3eg | 41.9 a | |
| 24 | 30.8gh | 32.1eh | 38.4d | 29.6hi | 32.7 c | |
| 48 | 32.5eh | 29.5hi | 21.4lm | 11.1o | 23.6 f | |
| Mean | 32.9b | 34.3b | 37.7a | 30.1c | 33.7 A | |
| 0.5% | 6 | 13.6no | 27.5ij | 34.ef | 35.1e | 27.5 e |
| 12 | 15.2n | 29.6hi | 42.0c | 46.ab3 | 33.3 c | |
| 24 | 19.0m | 19.6m | 15.1n | 12.9no | 16.7 h | |
| 48 | 0.0r | 0.0r | 0.0r | 0.0r | 0.0 k | |
| Mean | 11.9ij | 19.2f | 22.7d | 23.5d | 19.8 B | |
| 1% | 6 | 10.7o | 15.4n | 32.0fg | 29.7hi | 21.9 g |
| 12 | 23.kl | 34.2ef | 32.3eh | 19.6m | 27.3 e | |
| 24 | 0.0r | 20.0m | 18.8m | 3.7pq | 10.6 j | |
| 48 | 0.0r | 0.0r | 0.0r | 0.0r | 0.0 k | |
| Mean | 8.4k | 17.4g | 20.8e | 13.2hi | 14.9 C | |
| 2% | 6 | 25.1jk | 34.7ef | 34.1ef | 23.1kl | 29.3 d |
| 12 | 0.0r | 6.7p | 18.6m | 24.3kl | 12.4 i | |
| 24 | 0.0r | 1.7qr | 3.5q | 0.0r | 1.3 k | |
| 48 | 0.0r | 0.0r | 0.0r | 0.0r | 0.0 k | |
| Mean | 6.3l | 10.7j | 14.1h | 11.9ij | 10.7 D | |
| General average | 14.9C | 20.4B | 23.8A | 19.9B | 19.7 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 0.7; LSD Duration= 0.7; LSD Presoaking = 0.7; LSD Dose×Duration=1.5; LSD Dose×Presoaking=1.5; LSD Duration×Presoaking=1.5; LSD Dose×EMSduration×Presoaking = 3.0; CV (%) = 9.4. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
Seedling experiment
Germinated seeds might lose their vitality over time at the seedling stage. Hence, seedling survival is a crucial factor for mutation experiments. In this study, the germination rate was 98.8% in the germination experiment and survival seedling rate was determined as 90.2% in the seedling experiment in the control plots. Although all conditions and applications are the same, a loss of 8.6% was experienced. This illustrates the importance of seedling trials in addition to germination trials in mutation experiments.
Seedling survival decreased substantially with increasing EMS dose ( Table 4). To investigate the reasons behind this dramatic decrease in seedling survival with increasing EMS dose, the level of seedling damage by EMS exposure period in presoaked and dry seeds before sowing was examined. The presoaking of seeds before sowing has a strong effect on seedling survival rate. This may suggest that presoaked seeds could tolerate EMS exposure periods up to 24 hours, as they tolerate high EMS doses during the seedling stage.
Table 4. Effect of EMS application dose, EMS exposure period and presoaking duration on surviving seedling in rice seedling experiment (%).
| EMS
application dose (%) |
EMS
exposure period (hours) |
Presoaking duration (hours) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 68.8bc | 93.8a | 56.3ce | 31.3fh | 62.5 c |
| 12 | 100.0a | 93.8a | 100.0a | 100.0a | 98.4 a | |
| 24 | 100.0a | 100.0a | 100.0a | 100.0a | 100.0 a | |
| 48 | 100.0a | 100.0a | 100.0a | 100.0a | 100.0 a | |
| Mean | 92.1ab | 96.9a | 89.1ab | 82.8b | 90.2 A | |
| 0.5% | 6 | 18.8hj | 31.3fh | 37.5eh | 25.0gi | 28.1 f |
| 12 | 6.3ij | 18.8hj | 56.3ce | 56.3ce | 34.4 ef | |
| 24 | 62.5bd | 93.8a | 100.0a | 62.5bd | 79.7 b | |
| 48 | 0.0j | 0.0j | 0.0j | 0.0j | 0.0 i | |
| Mean | 21.9fg | 35.9d | 48.4c | 35.9d | 35.5 B | |
| 1% | 6 | 0.0j | 43.8dg | 81.3ab | 62.5bd | 46.9 d |
| 12 | 0.0j | 68.8bc | 50.0cf | 56.3ce | 43.8 de | |
| 24 | 0.0j | 25.0gi | 68.8bc | 0.0j | 23.4 fg | |
| 48 | 0.0j | 0.0j | 0.0j | 0.0j | 0.0 i | |
| Mean | 0.0i | 34.4de | 50.0c | 29.7df | 28.5 C | |
| 2% | 6 | 25.0gi | 43.8dg | 50.0cf | 43.8dg | 40.6 de |
| 12 | 0.0j | 0.0j | 25.0gi | 25.0gi | 12.5 gh | |
| 24 | 0.0j | 6.3ij | 18.8hj | 0.0j | 6.25hi | |
| 48 | 0.0j | 0.0j | 0.0j | 0.0j | 0.0 I | |
| Mean | 6.25hi | 12.5gh | 23.4eg | 17.2gh | 14.8 D | |
| General average | 30.1C | 44.9B | 52.7A | 41.4B | 42.3 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 5.7; LSD Duration= 5.7; LSD Presoaking = 5.7; LSD Dose×Duration=11.42; LSD Dose×Presoaking=11.42; LSD Duration×Presoaking=11.42; LSD Dose×EMSduration×Presoaking = 23.0; CV (%) = 3.9. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
A significant interaction was also observed between the EMS exposure period and EMS dose. This is might be a result of EMS concentration in seeds increasing with increasing exposure time, particularly when the seeds are incubated in EMS solution for longer.
The analysis of variance revealed significant (P < 0.01) differences in surviving seedlings with each EMS dose, exposure period, presoaking period, and their interactions. The surviving rate was ranked from 0% to 100% in this study. When evaluated in terms of the EMS dose, the lowest survival rate observed was 14.8% for the 2% EMS dose plot. The highest surviving rate observed was 90.2% for the control plot ( Table 4). Statistical analysis on survival rate showed an attendant decrease in germination with applied increases in the concentration of EMS. As per Table 4, the outcomes acquired show that a decrease in seed germination occurred with a corresponding increase in EMS dose (P < 0.01). Considering each exposure period, the mean germination percentage was 44.5%, 47.3%, 52.3%, and 25.0% for 6-, 12-, 24-, and 48-hour exposure periods, respectively. When evaluated in terms of presoaking, the lowest mean survival rate observed was 30.1% for the zero hours (dry seed) presoaking plot. The highest mean survival rate observed was 52.7% for the 12 hours presoaking plot. EMS application without presoaking and 48 hours of EMS application and their combinations almost prevented seedling survival. It should be emphasized that the chemical reduces the germination ability of dry seeds and also EMS application for more than 24 hours inhibit germination to a high extent. Correspondingly, the survival rate also decreased. In addition, there was a difference between germination rate and survival rate up to 8.6%. It could be reasoned that seedlings that germinated weakly after the mutation application were unable to survive.
There were significant effects of presoaking duration, EMS exposure period, EMS dose, and some of the combinations provided a 100% survival rate. Survival rates were similar for 24-hour exposure period, 0.5% EMS dose and 12 hours presoaking plots compared with control plots.
Seedling shoot length is an important feature showing the development of seedlings after mutation application. Seedling shoot length was significantly (P < 0.01) affected by presoaking duration, EMS dose, EMS exposure period, and their combinations. Seedling shoot length varied between 0.0-36.3 mm and the experiment average was 16.0 mm. The highest mean shoot length was measured 30.6 mm on the control plot ( Table 5). The consequences of the seedling experiment indicated that increasing EMS doses caused a significant decrease in seedling shoot development ( Table 5). A significant decrease was observed of over 50% when EMS dose was 0.5% and higher. As EMS exposure period increased, a significant decrease in seedling shoot length occurred, especially for the 24-hour EMS exposure period. At a dose of 0.5% EMS, the lowest EMS dose, an exposure period of 48 hours resulted in a significant decrease (no growth of shoots) in seedling shoot length compared with the control. The results indicated that no presoaking caused a significant decrease in seedling shoot development. A significant decrease was observed of approximately 50% in the non-presoaked plot. In terms of the interaction between presoaking duration and EMS exposure period, a significant decrease in seedling shoot length occurred, especially for no presoaking and 48-hour EMS exposure period. The longest seedling shoots were observed for the 12 hours presoaking duration, 0.5% EMS dose and 12-hour exposure period conditions when compared to the other combinations except for 0% EMS dose.
Table 5. Effect of EMS application dose, EMS exposure period and presoaking duration on shoot in rice seedling experiment (mm).
| EMS
application dose (%) |
EMS
exposure period (h) |
Presoaking duration (h) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 31.9ae | 30.5af | 26.5bj | 19.3im | 27.0 b |
| 12 | 33.1ac | 31.5af | 33.1ac | 31.8af | 32.4 a | |
| 24 | 32.6ae | 31.7af | 33.9ab | 30.2ag | 32.1 a | |
| 48 | 21.4hl | 36.3a | 33.7ab | 33.1ad | 31.1 ab | |
| Mean | 29.7a | 32.5a | 31.8a | 28.6a | 30.6 A | |
| 0.5% | 6 | 18.8jm | 17.4ko | 24.9ck | 18.9jm | 19.9 c |
| 12 | 4.3pq | 12.4mp | 30.0ag | 26.2bj | 18.2 cd | |
| 24 | 20.7hm | 19.6im | 24.6ek | 18.2jn | 20.8 c | |
| 48 | 0.0q | 0.0q | 0.0q | 0.0q | 0.0 f | |
| Mean | 10.9de | 12.3ce | 19.9b | 15.8bc | 14.7 B | |
| 1% | 6 | 0.0q | 22.0gl | 27.6bi | 26.3bj | 18.9 cd |
| 12 | 0.0q | 23.5fl | 18.2jn | 20.3hm | 15.5 d | |
| 24 | 0.0q | 9.9np | 17.6kn | 0.0q | 6.9 e | |
| 48 | 0.0q | 0.0q | 0.0q | 0.0q | 0.0 f | |
| Mean | 0.0f | 13.8cd | 15.8bc | 11.6ce | 10.3 C | |
| 2% | 6 | 10.3np | 28.3ah | 16.0lo | 24.6dk | 19.8 c |
| 12 | 0.0q | 0.0q | 9.1op | 25.5bk | 8.6 e | |
| 24 | 0.0q | 4.3pq | 16.0lo | 0.0q | 5.1 e | |
| 48 | 0.0q | 0.0q | 0.0q | 0.0q | 0.0 f | |
| Mean | 2.6f | 8.1e | 10.3de | 12.5cd | 8.4 C | |
| General average | 10.8C | 16.7B | 19.4A | 17.1B | 16.2 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 2.1; LSD Duration= 2.1; LSD Presoaking = 2.1; LSD Dose×Duration=4.1; LSD Dose×Presoaking=4.1; LSD Duration×Presoaking=4.1; LSD Dose×EMSduration×Presoaking = 8.2; CV (%) = 37.5. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
Seedling root length is another important character of seedling stage development in rice. Seedling root length was significantly (P < 0.01) affected by EMS dose, EMS exposure period, presoaking duration, and their combinations. Seedling root length varied between 0.0–5.1 cm and the experiment average was 2.2 cm. The highest mean root length was measured at 3.5 cm for the 0% EMS dose control plot ( Table 6). The result of the seedling experiment indicated that increasing EMS doses caused a significant decrease in seedling root development. A significant decrease was observed of over 50% when EMS dose was 1% and higher. As EMS exposure period increased, a significant decrease in seedling root occurred, especially for the 24-hour EMS exposure period. At doses of 0.5% EMS and higher, an exposure period of 48 hours resulted in a significant decrease (no growth of roots) in seedling root length compared with the control. The results indicated that no presoaking caused a significant decrease in seedling root development. A significant decrease was observed of approximately 50% when seeds were not presoaked. In terms of presoaking duration, a significant decrease in seedling root length occurred especially for the 48-hour EMS exposure period. The longest seedling roots were obtained for the 12 hours presoaking duration, 2% EMS dose and six-hour exposure period conditions when compared to the other combinations.
Table 6. Effect of EMS application dose, EMS exposure period and presoaking duration on root in rice seedling experiment (cm).
| EMS
application dose (%) |
EMS
exposure period (h) |
Presoaking duration (h) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 4.8ab | 3.9ae | 3.7af | 1.4il | 3.5 ac |
| 12 | 3.5ag | 3.1ci | 3.1ci | 4.1ac | 3.4 ac | |
| 24 | 4.0ad | 4.1ac | 3.3bh | 3.2bh | 3.6 ab | |
| 48 | 2.3ej | 3.9ae | 4.2ac | 3.3bh | 3.4 ac | |
| Mean | 3.7ab | 3.7a | 3.6ab | 3.0ac | 3.5 A | |
| 0.5% | 6 | 3.3bh | 2.4dj | 3.8af | 3.2bh | 3.1 bd |
| 12 | 0.8jl | 1.5il | 3.3bh | 3.8af | 2.3 de | |
| 24 | 3.5ag | 2.9ci | 4.4ac | 2.7ci | 3.4 ac | |
| 48 | 0.0l | 0.0l | 0.0l | 0.0l | 0.0 i | |
| Mean | 1.9eg | 1.7eg | 2.9bd | 2.4ce | 2.2 B | |
| 1% | 6 | 6.66E-16 | 3.3bh | 3.9ae | 3.5ag | 2.7 ce |
| 12 | 1.80E-16 | 3.4bh | 2.9ci | 2.8ci | 2.3 ef | |
| 24 | 0.0l | 2.0gk | 2.3ej | 0.0l | 1.1 gh | |
| 48 | 0.0l | 0.0l | 0.0l | 0.0l | 0.0 i | |
| Mean | 0.0h | 2.2dg | 2.3cf | 1.6fg | 1.5 C | |
| 2% | 6 | 2.1fk | 5.1a | 5.1a | 4.1ac | 4.1 a |
| 12 | 0.0l | 0.0l | 2.0gk | 3.8af | 1.4 fg | |
| 24 | 0.0l | 0.5kl | 1.8hk | 0.0l | 0.6 hi | |
| 48 | 0.0l | 0.0l | 0.0l | 0.0l | 0.0 i | |
| Mean | 0.5h | 1.4g | 2.2cg | 1.9eg | 1.5 C | |
| General average | 1.5C | 2.3B | 2.7A | 2.2B | 2.2 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 0.4; LSD Duration= 0.4; LSD Presoaking = 0.4; LSD Dose×Duration=0.82; LSD Dose×Presoaking=0.82; LSD Duration×Presoaking=0.82; LSD Dose×EMSduration×Presoaking = 1.65; CV (%) = 54.5. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
Fresh seedling weight is another notable parameter that indicates seedling development after mutation. Fresh seedling weight was significantly (P < 0.01) affected by presoaking duration, EMS dose, EMS exposure period, and their combinations. Fresh seedling weight varied between 0.0-195.9 mg and the experiment average was 99.5 mg. The conclusion of the seedling experiment indicated that increasing EMS doses caused a significant decrease in fresh seedling weight. In terms of EMS dose, the highest fresh seedling weight measured was 170.2 mg for the control plot ( Table 7). A significant decrease was observed of approximately 50% when the EMS dose was 0.5% and higher. There was a significant decrease in fresh seedling weight with the increase in EMS application time, especially for the 48-hour EMS application time. It was determined that the seedlings did not develop and fresh weight was not obtained for plots with 48 hours of EMS exposure combined with EMS doses of 0.5%, 1%, and 2%. In addition, it was observed that the fresh seedling weight was dramatically decreased in the plots without presoaking. A significant decrease was observed of more than 30% when non-presoaked. In terms of presoaking duration, a significant decrease in fresh seedling weight occurred especially for the 48-hour EMS exposure period. The highest fresh seedling weight was calculated for the 12 hours presoaking duration, 0.5% EMS dose and six-hour exposure period conditions when compared to the other combinations.
Table 7. Effect of EMS application dose, EMS exposure period and presoaking duration on fresh seedling weight in rice seedling experiment (mg).
| EMS
application dose (%) |
EMS
exposure period (h) |
Presoaking duration (hours) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | |||
| 0.0% | 6 | 192.5ab | 168.4ag | 143.6ai | 121.5el | 156.5 bd |
| 12 | 195.9a | 178.0ad | 187.1ab | 176.0ae | 184.3 a | |
| 24 | 188.5ab | 168.9ag | 186.1ab | 163.2ai | 176.7 ab | |
| 48 | 150.2ai | 183.4ac | 165.8ah | 153.2ai | 163.1 ac | |
| Mean | 181.8a | 174.7ab | 170.7ab | 153.5b | 170.2 A | |
| 0.5% | 6 | 123.3dk | 110.9hm | 177.5ad | 118.1gm | 132.4 df |
| 12 | 33.8no | 81.5jn | 170.7ag | 175.2af | 115.3 eg | |
| 24 | 148.0ai | 142.9ai | 142.5ai | 108.3im | 135.4 ce | |
| 48 | 0.0o | 0.0o | 0.0o | 0.0o | 0.0 i | |
| Mean | 76.3df | 83.8de | 122.7c | 100.4cd | 95.8 B | |
| 1% | 6 | 0.0o | 128.4cj | 147.4ai | 145.7ai | 105.4 fg |
| 12 | 0.0o | 150.4ai | 120.6fl | 138.2bi | 102.3 g | |
| 24 | 0.0o | 68.5kn | 116.4gm | 0.0o | 46.2 h | |
| 48 | 0.0o | 0.0o | 0.0o | 0.0o | 0.0 i | |
| Mean | 0.0g | 86.8de | 96.1ce | 70.9ef | 63.5 C | |
| 2% | 6 | 63.0mn | 169.7ag | 108.3im | 139.3bi | 120.1 eg |
| 12 | 0.0o | 0.0o | 66.6ln | 156.0ai | 55.6 h | |
| 24 | 0.0o | 30.0no | 109.5im | 0.0o | 34.9 h | |
| 48 | 0.0o | 0.0o | 0.0o | 0.0o | 0.0 i | |
| Mean | 15.8g | 49.9f | 71.1ef | 73.8df | 52.6 C | |
| General average | 68.4C | 98.8B | 115.1A | 99.7B | 95.5 | |
| ** | ** | ** | ||||
**: significant at the 1% level; NS: no significant differences. Values followed by the same letter are not statistically significantly different. LSD dose = 13.8; LSD Duration= 13.8; LSD Presoaking = 13.8; LSD Dose×Duration=27.7; LSD Dose×Presoaking=27.7; LSD Duration×Presoaking=27.7; LSD Dose×EMSduration×Presoaking = 55.3; CV (%) = 40.8. CV, coefficient of variation; EMS, ethyl methanesulfonate; LSD, least significant difference.
Discussion
The experimental results of both the germination experiment and the seedling experiment revealed that the presoaking duration, EMS dose, EMS exposure period, and their interactions were significant. The result of the experiment was similar to study results of Talebi et al. (2012), and Ramchander et al. (2014). Slight variations appeared in terms the most suitable combination of factors. However, results were obtained that could be used to make a standard protocol. Presoaking is an important stage for the EMS solution to diffuse into the seed and optimum presoaking duration is expressed as the presoak duration when the seed reaches full saturation. Although from previous experiments it was recommended that maximum water intake of the seeds is reached for EMS mutation application, water intake level at around 25%, reached in 12 hours, was determined to be useful in this research. The results illustrated that the rice seed reached full saturation after 24 hours presoaking. However, when the presoaking duration was evaluated on its own and with other conditions, it was determined that the 12-hour period was the most suitable time for EMS application. In addition, EMS exposure periods of more than six hours might be damaging to the seed. The seeds might tolerate a long exposure period of 12 hours or 24 hours. However, 48 hours of application caused the seed to irreversibly lose its germination ability. EMS application doses of 0.5%, 1%, and 2% reduced surviving seeds by roughly 50%, 60%, and 80%, compared to the 0% EMS dose. Furthermore, the mutated seeds can be stored for three to four weeks after drying and retain more than 85% of their germination ability; the result of Tonthong et al. (2018) also supported this process. In this study, 12 hours presoaking duration, a six-hours EMS exposure period, and 0.5% EMS dose were determined to be the most appropriate combination. The EMS application protocol might be successfully utilized in rice mutation research.
Conclusion
The most suitable EMS application practice was determined to be 12 hours presoaking, 0.5% EMS dose, and six hours EMS exposure for rice. The protocol includes the following: (1) Presoaking: 12 hours, (2) EMS application: 0.5% dose EMS and six hours, (3). Final washing: six hours, (4) Drying: 72 hours at 38°C. In addition, the protocol sheets are presented as a user-friendly protocol as Extended data ( Unan, 2021b).
Data availability
Underlying data
Zenodo: Dataset related paper "protocol for ems mutagenesis application in rice". https://doi.org/10.5281/zenodo.4549457 ( Unan, 2021a).
This project contains the following underlying data:
-
-
Protocol_for_EMS_application_in_rice_data.xlsx
Extended data
Zenodo: Factsheet related paper "protocol for ems mutagenesis application in rice".
https://doi.org/10.5281/zenodo.4587383 ( Unan, 2021b).
This project contains the following extended data:
-
-
Factsheet of Protocol for EMS mutagenesis application in rice.pdf
-
-
Flow Chart of Protocol for EMS Mutagenesis Application in Rice.pdf
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No [897192], (project HerbaRice).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved with reservations]
References
- Al-Khatib K, Godar AS, Brim-DeForest WB: Community efforts to detect and manage herbicide resistant weeds in California. Proceeding of 37th Rice Technical Working Group. 2018;37:110–111. [Google Scholar]
- Bhat R, Upadhyaya N, Chaudhury A, et al. : Chemical-and Irradiation-Induced Mutants and Tilling, In: N. M. Upadhyaya, Ed., Rice Functional Genomics: Challenges, Progress and Prospects. Springer, New York,2007;148–180. 10.1007/0-387-48914-2_8 [DOI] [Google Scholar]
- Brim-DeForest WB, Al-Khatib K, Fischer AJ: Predicting Yield Losses in Rice Mixed-Weed Species Infestations in California. Weed Sci. 2017;65(1):61–72. 10.1614/WS-D-16-00079.1 [DOI] [Google Scholar]
- Chauhan BS, Jabran K, Mahajan K: Rice production worldwide.2017;117–135. 10.1007/978-3-319-47516-5_5 [DOI] [Google Scholar]
- Cruz RP, Milach SCK: Cold tolerance at the germination stage of rice: Methods of evaluation and characterization of genotypes. Sci Agric. 2004;61(1):1–8. 10.1590/S0103-90162004000100001 [DOI] [Google Scholar]
- Espino L, Brim-DeForest W, Al-Khatib K, et al. : Weedy rice in California: Addressing an emerging pest through outreach and research. Proceeding of 37th Rice Technical Working Group. 2018;37:108–109. [Google Scholar]
- Evangelina SE, Maribel LD, Abdelbagi MI: Proper Management Improves Seedling Survival and Growth during Early Flooding in Contrasting Rice Genotypes. Crop Sci. 2010;50(5):1997–2008. 10.2135/cropsci2009.09.0492 [DOI] [Google Scholar]
- FAO: Faostat.2019. Access date is 24.01.2021. Reference Source [Google Scholar]
- Gibson KD, Fischer AJ, Foin TJ, et al. : Implication of delayed Echinocla spp. Germination and duration of competition for integrated weed management in water-seeded rice. Weed Res. 2002;42(5):351–358. 10.1046/j.1365-3180.2002.00295.x [DOI] [Google Scholar]
- IRRI: Standard evaluation system for rice. International Rice Research Institute, Philippines.2002. Reference Source [Google Scholar]
- Ramchander S, Pillai MA, Ushakumar R: Determination of Lethal Dose and Effect of Ethyl Methane Sulphonate in Rice Varieties. Trends Biosci. 2014;7(11):1151–1156. Reference Source [Google Scholar]
- Sagel Z, Tutluer Mİ, Peskircioglu H, et al. : Determination of Effect of Chemical Mutagen EMS on TAEK A-3 and TAEK C-10 Mutant Soybean Varieties in M 1 Generation. Ekin Journal of Crop Breeding and Genetics. 2017;3(1):19–24. Reference Source [Google Scholar]
- Talebi AB, Talebi AB, Shahrokhifar B: Ethyl Methane Sulphonate (EMS) Induced Mutagenesis in Malaysian Rice (cv. MR219) for Lethal Dose Determination. Am J Plant Sci. 2012;3(12):1661–1665. 10.4236/ajps.2012.312202 [DOI] [Google Scholar]
- Tonthong Y, Chanprasert W, Romkaew J, et al. : Open Access Germinability and storability of pre-germinated rice ( Oryza sativa L.) seeds. Seed Sci Technol. 2018;46(1):119–129. 10.15258/sst.2018.46.1.12 [DOI] [Google Scholar]
- Unan R, Sezer I, Sahin M, et al. : Control of lodging and reduction in plant length in rice ( Oryza sativa L.) with the treatment of Trinexapac-Ethyl and sowing density. Turk J Agric For. 2013;37:253–264. 10.3906/tar-1207-72 [DOI] [Google Scholar]
- Unan R: Dataset related paper "protocol for ems mutagenesis application in rice" [Data set]. Zenodo. 2021a. 10.5281/zenodo.4549457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unan R: Factsheet related paper "protocol for ems mutagenesis application in rice" [Data set]. Zenodo. 2021b. 10.5281/zenodo.4587636 [DOI] [PMC free article] [PubMed] [Google Scholar]


