Abstract
Although herpes simplex virus (HSV) replicates in noncycling as well as cycling cells, including terminally differentiated neurons, it has recently been shown that viral replication requires the activities of cellular cyclin-dependent kinases (cdks) (L. M. Schang, J. Phillips, and P. A. Schaffer, J. Virol. 72:5626–5637, 1998). Since we were unable to isolate HSV mutants resistant to two cdk inhibitors, Olomoucine and Roscovitine (Rosco), we hypothesized that cdks may be required for more than one viral function during HSV replication. In the experiments presented here, we tested this hypothesis by measuring the efficiency of (i) viral replication; (ii) expression of selected immediate-early (IE) (ICP0 and ICP4), early (E) (ICP8 and TK), and late (L) (gC) genes; and (iii) viral DNA synthesis in infected cultures to which Rosco was added after IE or IE and E proteins had already been synthesized. Rosco inhibited HSV replication, transcription of IE and E genes, and viral DNA synthesis when added at 1, 2, or 6 h postinfection or after release from a 6-h cycloheximide block. Transcription of a representative L gene, gC, was also inhibited by Rosco under all conditions examined. We conclude from these studies that cellular cdks are required for transcription of E as well as IE genes. In contrast, steady-state levels of at least one cellular housekeeping gene were not affected by Rosco. The requirement of viral IE and E transcription for cellular cdks may reflect either a requirement for specific cdk-activated cellular and/or viral transcription factors or a more global requirement for cdks in the transcriptional activation of the viral genome.
Herpes simplex virus (HSV) replicates in cycling as well as noncycling cells, including terminally differentiated neurons. HSV replication, however, has long been associated with cellular functions known to be involved in cell cycle progression. Thus, HSV replicates more efficiently in replicating than in growth-arrested cells. Moreover, this difference in replication efficiency is particularly prominent for viral mutants that do not express active forms of certain viral proteins, such as ICP0 and VP16 (4, 7). The phenotypes of these mutants suggest that one of the functions of these viral proteins is to induce or replace cellular activities which are normally activated in a cell-cycle-regulated manner. In addition to the impaired replication efficiency of ICP0 and VP16 mutants in noncycling cells, wild-type HSV cannot replicate at the nonpermissive temperature in several temperature-sensitive (ts) cell lines that are growth arrested in G0/G1, implying that viral replication requires one or more cellular functions activated in a cell-cycle-dependent manner in noninfected cells (62, 67). The cellular protein defective in one of these ts cell lines has been identified as HCF, which is required for binding of a viral transactivator, VP16, to viral immediate-early (IE) promoters. Thus, in addition to its previously recognized role in HSV replication (16, 67), HCF is an important regulator of cell cycle progression (16, 65). The cellular proteins defective in other ts cell lines that arrest in G0/G1 and do not support HSV replication have not yet been characterized but may potentially include any of the cellular proteins involved in cell cycle progression that are also (i) required for efficient HSV replication (16, 20), (ii) activated during HSV infection (25, 30), (iii) localized to the sites of viral replication (11, 64), and/or (iv) interactive physically with HSV DNA replication proteins (34). Consistent with the involvement of certain cell-cycle-related cellular activities in HSV infection, we have recently shown that cyclin-dependent kinases (cdks) are required for HSV replication, at least in cycling cells (54).
Cellular cdks are key regulators of cell cycle progression. Although the precise mechanisms by which cdks accomplish their regulatory roles are largely unclear, cdks are involved in transcriptional regulation, DNA replication, and reorganization of the cellular architecture. Thus, cdk-2, -3, -7, -8, and -9 are involved in transcriptional regulation. Specifically, cdk-2 and -3 are required to activate transcription factors (such as E2F) that are important for cell cycle regulation (9, 10, 26). cdk-7 and -8 are postulated to phosphorylate the carboxy-terminal domain (CTD) of RNA polymerase II (51, 57, 58). Finally, pTEFb (positive transcription elongation factor b), which is required to overcome pausing of the transcriptional complex, is a heterodimer containing and requiring cdk-9 (14, 68, 69, 72). Although the kinase activity of cdk-7, -8, or -9 is disposable for transcription in certain artificial systems, it is likely important in vivo, since phosphorylation of CTD is essential for transcription in vivo (18, 70, 71).
In addition to their physiological roles in cells, cdks are also involved in the replication of DNA-containing viruses. The smaller DNA viruses that replicate only in cells in S phase (parvo-, papova-, and adenoviruses) require cellular cdks for their replication (21, 23, 60). Among larger DNA-containing viruses, cdks have recently been shown to be required for human cytomegalovirus replication (3). The involvement of cdks in viral replication undoubtedly reflects the fact that viral DNA replication of some viruses occurs only in S-phase nuclei, and cdks are required for cellular progression into S phase. In addition, however, cdks are also directly involved in the viral DNA replication process. For instance, the DNA replicative functions of simian virus 40 and polyomavirus large T antigens are activated by cdk-2 phosphorylation in vitro and by phosphorylation at consensus cdk sites in vivo (5, 21, 36). At this writing, however, cdks are not known to be required for transcription of any of the above-mentioned viruses.
Our previous results have demonstrated that cdks are required for HSV replication. Thus, two highly specific cdk inhibitors, Roscovitine (Rosco) and Olomoucine (Olo) (1, 8, 13, 39–42, 55, 63), blocked HSV replication, whereas other purine derivatives and inhibitors of cell cycle progression that do not inhibit cdks did not inhibit HSV replication (54). Rosco and Olo are purine derivatives and display similar inhibitory profiles. They inhibit cdk-1/cyclin B, cdk-2/cyclin A or E, and cdk-5/p25 and extracellular receptor-activated kinases 1 and 2 (which are inhibited at ∼10- to 20-fold-higher concentrations than the cdk targets). These inhibitors do not inhibit 35 other enzymes tested, including protein serine/threonine or tyrosine kinases, phosphatases, topoisomerases, DNA polymerases, and a nucleoside kinase (63). Neither Olo nor Rosco significantly inhibits the kinase activity of cdk-4 or -6, whereas the effects of these drugs on cdk-3, -7, -8, and -9 have not been examined (41, 63). As biological effects, Rosco blocks cell cycle progression both in late G1/early S (when cdk-2 is required prior to the onset of cellular DNA synthesis) and in M (when cdk-1 is required for cell division) in a wide variety of mammalian cells, including Vero and HEL cells (54).
In our previous experiments, we showed that accumulation of the ICP4 IE transcript is inhibited in the presence of Rosco or Olo (54). Accumulation of two HSV early (E) transcripts (those of the ICP8 and TK genes) and viral DNA replication were also shown to be inhibited by these drugs. Neither transcription of E genes nor DNA replication, however, occurs in the absence of IE gene expression during lytic infection of nonneuronal cells. Therefore, the inhibition of E transcript accumulation and viral DNA replication may have been secondary to the block in IE gene expression. Alternatively, inhibition of E transcript accumulation and HSV DNA replication by Rosco or Olo may have been a direct result of inhibition of cellular cdk activities required for these critical processes.
Since wild-type HSV could replicate in the presence of Olo in cells partially resistant to this drug, and because we were unable to isolate viral mutants resistant to Rosco and Olo, we concluded that Rosco most likely does not act on a virally encoded enzyme but rather on cell-encoded cdks (54). Furthermore, we postulated that cellular cdk activities may be required for more than one viral function. More specifically, we hypothesized that cdks may be required for viral functions that occur after IE proteins are expressed. To test this hypothesis, we measured the effects of Rosco on HSV replication, DNA synthesis, and viral transcription under conditions in which IE gene products had already been expressed. If our hypothesis were correct, addition of cdk inhibitors after expression of IE proteins should still block HSV replication. Here we report that Rosco inhibits HSV replication even when added at 6 h postinfection (hpi), after IE and E gene products had already been synthesized. Moreover, Rosco also inhibits HSV replication efficiently after release from a 6-h block in protein synthesis induced by cycloheximide (CHX). In these conditions, inhibition of viral replication occurred at the levels of transcription of both IE and E genes, while translation and stability of viral RNAs were not significantly impaired. Furthermore, transcription of at least one cellular housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was not dependent on the activities of Rosco-sensitive cdks. Hence, transcription of both HSV IE and E genes requires cellular cdk activity, a requirement not shared by at least one cellular gene.
MATERIALS AND METHODS
Cells, virus, plasmids, and drugs.
Methods used for the propagation and maintenance of Vero cells have been described previously (54). A plaque-purified, low-passage (p9) stock of HSV-1, strain KOS, was used throughout these studies and prepared as described previously (54). The construction of plasmids prpTK, prp8, prp4, and prpgC used to synthesize the riboprobes used in this study has been described (31).
Rosco was prepared and diluted as described previously (54). CHX was prepared in phosphate-buffered saline (PBS) as a stock solution at a concentration of 20 mg/ml. Stock CHX solution was diluted to working concentrations in Dulbecco’s minimal essential medium containing 10% fetal bovine serum. Final concentrations of drugs were 100 μM Rosco and 50 μg of CHX/ml in all experiments.
Infections.
Vero cells (2 × 105 cells) were infected with 3 PFU of HSV-1 strain KOS diluted in serum-free medium per cell. After adsorption for 1 h at 37°C, the inoculum was removed, monolayers were washed twice with cold PBS, and standard medium or medium containing drugs was added. When indicated, medium overlaying infected cells was replaced with fresh drug-containing or control (drug-free) medium. Infected cells were scraped into the medium at the indicated times after infection (where time zero is the time of addition of inoculum), and the entire infected cell suspension was transferred to a 5.0-ml tube and frozen at −70°C. After thawing, cells were sonicated for 45 s, and infectious virus was titrated by standard plaque assay. For the experiments in which the time of the addition of Rosco was varied (presented in Fig. 1 to 4), drug-free medium was removed from infected cells at 2 or 6 hpi and replaced with 2 volumes of drug-containing medium. For the drug-replacement and drug-release experiments shown in Fig. 5 to 9, drug-containing medium was removed from infected monolayers at the indicated times postinfection. Infected cells were then washed twice with PBS containing the same concentration of drug to be added to the respective well after the washes. After washing, 2 volumes of drug-free medium or medium containing 50 μg of CHX/ml or 100 μM Rosco was added to each monolayer. Two volumes of medium were added to dilute any residual drug remaining on the monolayers after the washes.
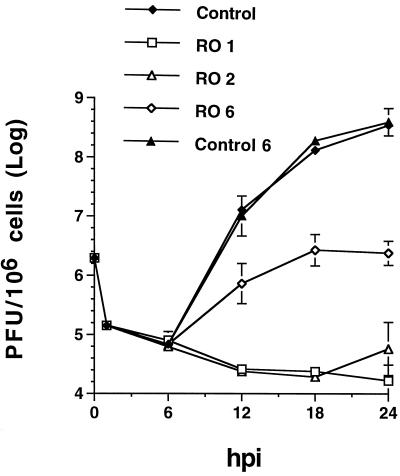
FIG. 1.
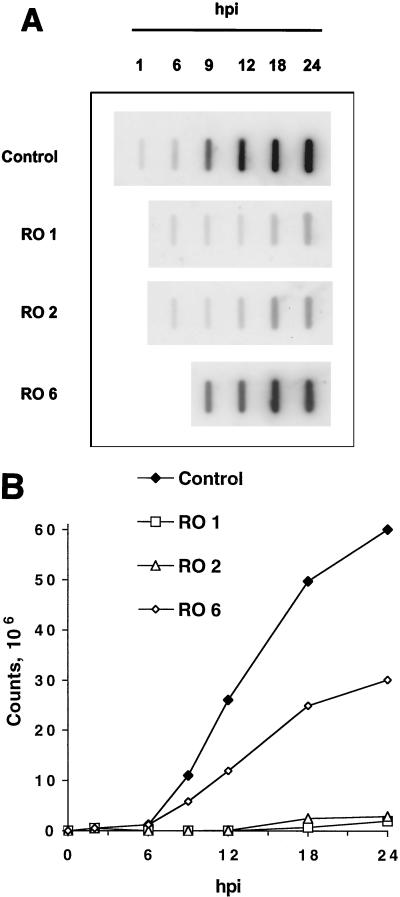
HSV replication in the presence of Rosco added at 1, 2, or 6 hpi. Vero cells were infected with 3 PFU of HSV-1/cell. After 1 h of adsorption, cells were washed and overlaid with drug-free medium or medium containing 100 μM Rosco (RO 1). At 2 (RO 2) and 6 (RO 6) hpi, drug-free medium was removed from replicate series of infected monolayers and replaced with medium containing 100 μM Rosco. An additional series of infected monolayers was incubated continuously in drug-free medium (Control). Medium from yet another series of monolayers was replaced at 6 hpi with fresh drug-free medium (Control 6). At 6, 12, 18, and 24 hpi, cultures were harvested and viral titers were measured by standard plaque assays. Viral titers are plotted against time postinfection (hpi). Each time point indicates the average and range of two independent experiments.
FIG. 4.
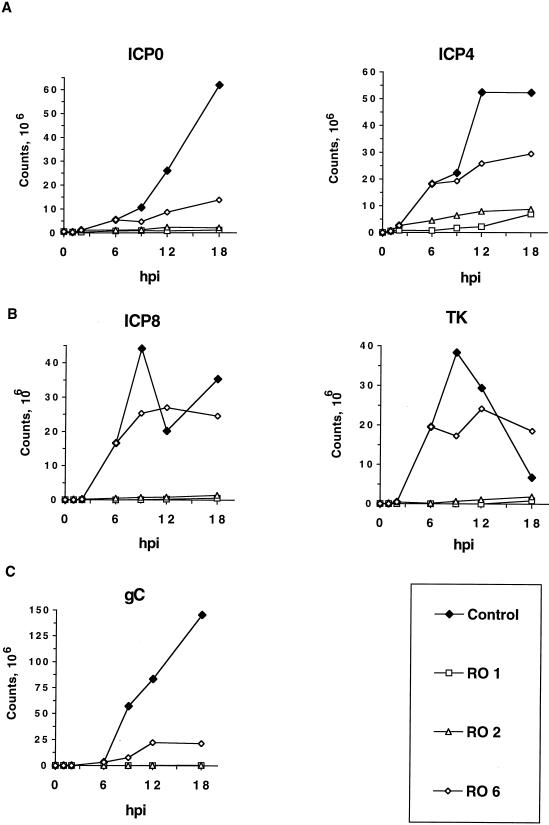
Quantitation of the levels of HSV IE, E, and L transcripts which accumulated in the presence of Rosco added at 1, 2, or 6 hpi. Bands in the gels shown in Fig. 3 were quantitated using the ImageQuant software package (Molecular Dynamics). After the subtraction of background counts, levels of individual viral transcripts were expressed and plotted as counts at the indicated times postinfection (hpi). Although absolute values for levels of individual transcripts in these experiments are comparable, comparisons between the absolute values obtained for different transcripts cannot be made. As noted in the legend to Fig. 3, the apparent drop in the level of ICP8 mRNA at 12 hpi is artifactual and not reproducible.
FIG. 5.
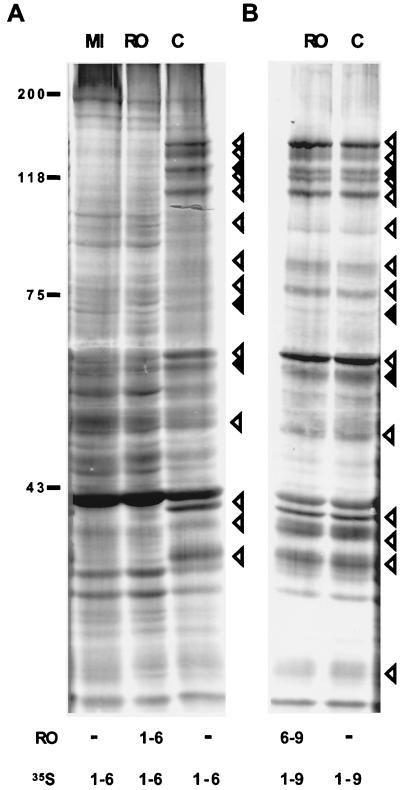
Expression of viral proteins in the presence of Rosco added at 1 or 6 hpi. (A) Vero cells were infected with 6 PFU of HSV-1 KOS/cell and overlaid with medium containing [35S]methionine and 100 μM Rosco (RO) or no drug (C). Six hours later, cells were harvested, and proteins were resolved in a discontinuous 9% polyacrylamide gel. For comparison, mock-infected cells were also labeled in control, drug-free medium (MI). (B) Vero cells were infected and overlaid with drug-free medium containing [35S]methionine. Six hours later, the medium was removed and fresh medium containing [35S]methionine and 100 μM Rosco (RO) or no drug (C) was added. Three hours after the change of medium, cells were harvested, and proteins were resolved in a discontinuous 9% polyacrylamide gel. Thus, infected cells were labeled for 9 h, either for the entire period in drug-free control medium (C) or in control medium for the first 6 h and in Rosco-containing medium for the last 3 h (RO). Molecular weights, estimated from the mobility of markers, are indicated to the left of the gels. On the right, solid arrowheads indicate IE proteins (ICP0, ICP22, and ICP27, from top to bottom [ICP4 and ICP47 are not visible in these gels]). Open arrowheads indicate E and L proteins.
FIG. 9.
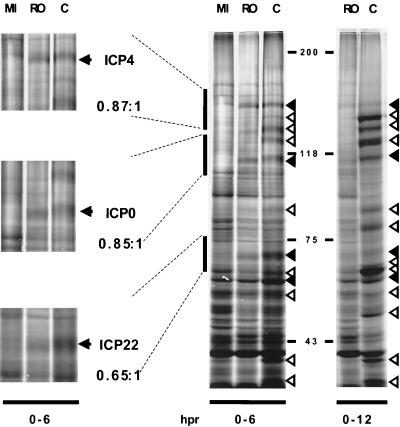
Expression of HSV proteins in the presence of Rosco added after removal of CHX at 6 hpi. Vero cells were infected with 6 PFU of HSV-1/cell for 6 h in the presence of CHX, followed by removal of the drug and incubation in drug-free medium (C) or in medium containing 100 μM Rosco (RO) as described in the legend to Fig. 6. At the time of release, [35S]methionine was added to cultures. For comparison, mock-infected cells were also released and labeled for 6 h (MI). At 6 (0–6 hpr) and 12 (0–12 hpr) h postrelease, cells were harvested, and proteins were resolved in a discontinuous 6% polyacrylamide gel. The regions of the gel labeled 0 to 6 hpr highlighted with vertical lines are shown expanded on the left, where the relevant IE proteins are indicated by arrows. The ratios beneath each protein designation indicate the amount of the indicated protein synthesized in the presence of Rosco relative to the amount synthesized in drug-free medium. Molecular weights, estimated from the mobility of markers, are indicated between the two main gels (0–6 and 0–12 hpr). On the right side of these gels, solid arrowheads indicate IE proteins (from top to bottom: ICP4, ICP0, ICP22, and ICP27, which comigrates with a cellular protein in this concentration of polyacrylamide [ICP47 is not visible in these gels]). Open arrowheads indicate E and L proteins.
Probes.
Plasmids prpgC, prpTK, prp8, prp4 (31), p0Hc-Xh (the generous gift of Robert Jordan, University of Pennsylvania School of Medicine), and pTRI-GAPDH (Ambion, Austin, Tex.) were linearized with BsgI, HindIII, NcoI, XcmI, NruI, and HindIII, respectively. Riboprobes were synthesized using the Riboprobe in vitro Transcription System (Promega, Madison, Wis.), following the manufacturer’s instructions, except that 5 μl of [α-32P]GTP (800 Ci/mmol) was used for labeling, and no cold GTP was included in the transcription mix. Labeled probes were separated from nonincorporated nucleotides using NucTrap probe purification columns according to the manufacturer’s instructions (Stratagene, La Jolla, Calif.).
RNase protection assays.
RNase protection assays were performed using the DirectProtect kit (Ambion) as previously described (54), with minor modifications. Briefly, 4.5 × 106 Vero cells were infected with 3 PFU of HSV-1 per cell in the presence of the indicated drug or in control, drug-free medium. Medium with or without drug was replaced as indicated in the figures for each experiment. At various times after infection, medium was removed, monolayers were washed twice with cold PBS and scraped into 600 μl of RNA extraction buffer (DirectProtect; Ambion), and the resulting cell extracts were transferred to Eppendorf tubes. Aliquots (45 μl) of each sample were annealed with 6 × 105 cpm of each of the virus-specific probes at 55°C. As a control for equal loading, another 45 μl of aliquot of each sample was annealed with 5 × 105 cpm of the GAPDH-specific probe at 37°C. Preliminary experiments had determined that the amount of probe used was saturating at this ratio of cell extract to probe. All annealing reactions were performed overnight in a volume of 50 μl. RNase and proteinase digestions were performed according to manufacturer’s instructions, and the protected fragments were resolved by electrophoresis in 6% denaturing polyacrylamide gels. Dried gels were exposed and analyzed using a Storm PhosphorImager system (Molecular Dynamics, Sunnyvale, Calif.).
Viral DNA replication assays.
A total of 4.0 × 106 Vero cells were infected with 3 PFU of HSV-1 KOS per cell and overlaid with drug-free control medium. Medium was replaced as described for each experiment. At the indicated times after infection, medium was removed, monolayers were washed with cold PBS, and cells were scraped into 360 μl of DNA extraction buffer (Buffer ATL, QIAamp tissue kit; QIAgen, Hilden, Germany). DNA was extracted as recommended by the manufacturer and resuspended to a final concentration of ∼50 ng/ml. Five micrograms of DNA from each sample was diluted to 400 μl with TE (10 mM Tris, 1 mM EDTA [pH 7.6]), blotted, and hybridized as previously described (54), except that a pool of TK, ICP8, and gC riboprobes (3 × 106 cpm of each probe/ml) was used for hybridization.
Metabolic labeling of viral proteins.
A total of 5 × 105 Vero cells were seeded in 60-mm dishes and infected 24 h later with 6 PFU of HSV-1 KOS per cell in the presence of the indicated drugs or in drug-free medium. Medium was replaced for each experiment. At various times after infection, medium was removed, monolayers were washed with warm methionine-free medium (Gibco BRL, Gaithersburg, Md.), and cells were overlaid with 2.0 ml of methionine-free medium supplemented with 100 μCi of [35S]methionine (>1,000 mCi/mmol; DuPont-NEN, Boston, Mass.). At various times after the addition of the label, medium was removed, monolayers were washed once with 1.0 ml of cold PBS, and cells were scraped into 800 μl of cold PBS. Cells in suspension were spun down at 2,000 rpm for 5 min in a Microfuge, and the pellet was resuspended in 45 μl of cell lysis buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40, 0.5% deoxycholate supplemented with 5 mM phenylmethylsulfonyl fluoride, 100 μg of aprotinin/ml, and 10 μM leupeptin). An aliquot of 25 μl of each cell extract was diluted with 25 μl of 2× gel-loading buffer (100 mM Tris [pH 6.8], 200 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol), denatured by boiling for 3 min and loaded onto discontinuous 6% (see Fig. 9) or 9% (see Fig. 5) polyacrylamide gels (29:1; acrylamide:bis-methyl acrylamide). Following electrophoresis, gels were dried, and bands were quantified by PhosphorImager (Molecular Dynamics) analysis.
RESULTS
Rosco inhibits HSV replication when added at 1, 2, or 6 hpi.
To determine whether cdks are required for essential HSV functions that occur after transcription of IE genes, we evaluated the effects on viral replication of adding Rosco at selected times after infection. We have shown previously that Rosco inhibits HSV replication in immortalized African green monkey cells (Vero) as efficiently as in primary human cells (HEL) (54). Since Vero cells are more widely available than HEL cells, we chose the former for all experiments presented herein. Vero cells were infected with 3 PFU of HSV-1/cell. After 1 h of adsorption, inoculum was removed, and monolayers were washed and overlaid with drug-free, control medium or medium containing 100 μM Rosco. At 2 and 6 hpi, medium was removed from a replicate series of monolayers infected in drug-free medium and replaced with medium containing 100 μM Rosco. Two types of control infections were also performed. In one, infected monolayers were left in drug-free medium throughout the experiment. In the other, drug-free medium was replaced with fresh drug-free medium at 6 hpi to control for the effect of medium change on HSV replication.
As shown previously (54), the addition of Rosco immediately after adsorption resulted in an almost total block in HSV replication (Fig. 1). The addition of Rosco at 2 hpi had a nearly identical inhibitory effect on viral replication (Fig. 1). When Rosco was added at 6 hpi, however, 3, 1.5, and 0.4% of wild-type levels of HSV replication were observed at 12, 18, and 24 hpi, respectively. Thus, HSV titers at 24 hpi in the series to which Rosco was added at 6 hpi were more than 2 orders of magnitude below the titers in untreated cultures (Fig. 1). Replacing drug-free medium at 6 hpi with fresh drug-free medium had no measurable effect on the efficiency of HSV replication (Fig. 1). Thus, Rosco inhibits HSV replication even when added after the time of IE protein synthesis (see Fig. 5 below).
Rosco inhibits HSV DNA replication when added at 1, 2, or 6 hpi.
We have shown previously that Rosco inhibits HSV DNA replication when added immediately after adsorption (1 hpi) (54). This block, however, could be a consequence of blocking IE and/or E gene expression. Since Rosco inhibits HSV replication even when added at 6 hpi (Fig. 1), we hypothesized that Rosco may also inhibit a (relatively) late viral function. Since viral DNA synthesis requires previous IE and E gene expression and consequently occurs later in the infection cycle, we investigated whether the efficiency of viral DNA replication was affected by Rosco added at 6 hpi. In these tests, Vero cells were infected with 3 PFU of HSV-1/cell, and Rosco was added to infected cultures at 1, 2, and 6 hpi as described above. No Rosco was added to control cultures. Cells were harvested at the indicated times postinfection, total infected cell DNA was extracted, and levels of viral DNA were determined by slot blot hybridization (Fig. 2).
FIG. 2.
HSV DNA replication in the presence of Rosco added at 1, 2, or 6 hpi. Vero cells were infected with 3 PFU of HSV-1/cell, and drug-free medium was replaced with Rosco-containing medium at 1, 2, and 6 hpi as described in the legend to Fig. 1. A second series of infected monolayers was left in drug-free medium throughout the 18-h experiment (Control). At 1, 2, 6, 9, 12, 18, and 24 hpi, cells were harvested and total cellular DNA was extracted, blotted on a nylon membrane (GeneScreen; New England Nuclear Research Products, Boston, Mass.), and hybridized to a pool of HSV-specific riboprobes. The membrane was then visualized by PhosphorImager analysis (A). The signal that hybridized to each slot was quantitated, and the amount of viral DNA in each sample was plotted as a function of hours postinfection (hpi) after subtraction of background counts (B).
Rosco added immediately after adsorption (1 hpi) inhibited HSV DNA replication efficiently throughout the 24-h experiment (Fig. 2A and B). Consistent with its inhibitory effect on viral replication, Rosco added at 2 hpi (1 h after adsorption) inhibited HSV DNA replication nearly as efficiently as when added immediately after adsorption (1 hpi) (Fig. 2A and B). When Rosco was added at 6 hpi, however, HSV DNA replication was only partially inhibited (Fig. 2). Thus, as shown in Fig. 2B, the total amount of HSV DNA and the rate of HSV DNA replication were reduced by approximately 50% when Rosco was added at 6 hpi (Fig. 2B). This experiment, however, did not allow us to determine whether inhibition of viral DNA replication is a direct effect of Rosco or whether it results from the reduced levels of E DNA replication proteins as a result of Rosco treatment. The following experiments were designed to directly determine whether E gene expression was impaired by Rosco added at 6 hpi.
Rosco inhibits transcription of IE, E, and L genes when added at 1, 2, or 6 hpi.
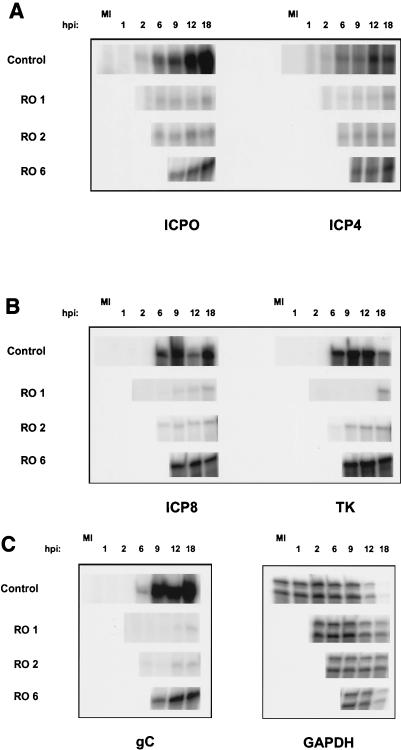
In these tests, we investigated whether the accumulation of viral IE, E, and late (L) transcripts was affected when Rosco was added at 1, 2, or 6 hpi. For this purpose, Vero cells were infected with 3 PFU/cell, incubated for 1 h at 37°C, washed, and overlaid with drug-free (control) or Rosco-containing medium. At 2 and 6 hpi, replicate infected cultures in drug-free medium were transferred into medium containing Rosco. At the indicated times after infection, infected cells were harvested, and total infected cell RNA was extracted. Levels of two IE (ICP0 and ICP4), two E (ICP8 and TK), and one L (gC) viral transcript were measured by RNase protection assays as described previously (54). Equal loading was monitored by measuring the levels of a cellular housekeeping transcript, GAPDH.
As previously observed with HEL cells (54), Rosco inhibited accumulation of HSV IE and E transcripts efficiently when added to Vero cells immediately after adsorption (Fig. 3A and B). Indeed, the inhibition ranged from 25-fold (for ICP4) to more than 1,000-fold (for TK) (Fig. 4A and B). Not surprisingly, levels of an L transcript, whose expression is dependent upon IE and E gene functions, as well as DNA replication, were also very low under these conditions (∼1,000-fold lower than in control infections) (Fig. 3C and 4C). The same or slightly higher levels of all five transcripts examined were observed when Rosco was added at 2 hpi (Fig. 3 and 4). When Rosco was added at 6 hpi, however, the levels of IE transcripts continued to increase between 6 and 18 hpi, although their levels at 18 hpi were still ∼1.8-fold (ICP4) to ∼4.6-fold (ICP0) lower than in the absence of drug (Fig. 3A and 4A). The levels of the E transcripts, ICP8 and TK, increased even less than the levels of the IE transcripts after Rosco was added at 6 hpi, relative to the levels attained immediately before the addition of the drug (Fig. 3B and 4B). The levels of an L transcript continued to increase after addition of Rosco at 6 hpi, although the rate of accumulation in the presence of Rosco was significantly lower than in the absence of drug (Fig. 3C and 4C). Consequently, when Rosco was added at 6 hpi, the levels of gC transcripts at 18 hpi were approximately sevenfold lower than in control infections (Fig. 4). Finally, the levels of the GAPDH transcript demonstrated that loading was equal and that Rosco has no significant effect on the steady-state levels of the transcripts of this representative cellular housekeeping gene (Fig. 3C). The decrease observed in the levels of GAPDH in drug-free (control) infections at late times postinfection is consistent with previous reports of cellular gene expression in HSV-infected cells (31, 54). The relative effects of Rosco on the accumulation of viral transcripts can be seen more clearly in Fig. 4, in which the levels of the viral transcripts shown in Fig. 3 were quantified.
FIG. 3.
Levels of HSV IE, E, and L transcripts which accumulated in the presence of Rosco added at 1, 2, or 6 hpi. Vero cells were infected and medium was changed at selected times postinfection as described in the legend to Fig. 1. At 1, 2, 6, 9, 12, and 18 hpi, cells were harvested, and viral and cellular RNA was extracted. RNA was also extracted from mock-infected cells (MI) as a negative control. Levels of IE transcripts ICP0 and ICP4 (A), E transcripts ICP8 and TK (B), and an L transcript, gC, (C) were evaluated by RNase protection assays. Levels of GAPDH were also measured to ensure equal loading of samples (C). The apparent drop in the level of ICP8 mRNA at 12 hpi in the control (B) is a technical artifact not observed in repeat experiments.
We conclude from these experiments that accumulation of E transcripts is impaired by Rosco even when the drug is added after IE proteins should have been synthesized (see Fig. 5 below), suggesting that Rosco has a direct effect on E gene transcription.
Rosco does not inhibit synthesis of HSV IE proteins when added at 6 hpi.
We next examined whether, as expected, normal levels of IE proteins were synthesized in the presence of Rosco when the drug was added at 1 or 6 hpi. For this purpose, metabolic labeling experiments were performed. Vero cells were infected with 6 PFU/cell, incubated for 1 h at 37°C, and overlaid with control drug-free or Rosco-containing medium supplemented with [35S]methionine (Fig. 5A). For comparison, mock-infected cells were labeled in parallel in the absence of drug. Preliminary experiments had established that Rosco has no detectable effect on the pattern of protein synthesis in mock-infected cells (data not shown). Drug-free medium was removed from two infected monolayers at 6 hpi and replaced with either Rosco-containing medium or fresh drug-free medium supplemented with [35S]methionine (Fig. 5B).
When infected cells were overlaid with Rosco-containing medium at 1 hpi, no viral proteins were synthesized subsequently, whereas the synthesis of cellular proteins was largely unaffected (Fig. 5A). In contrast, and as expected, cells infected in drug-free medium synthesized IE as well as E and L proteins during the same period (Fig. 5A). When Rosco was added to infected cells at 6 hpi, however, the pattern of viral protein synthesis from 1 to 9 hpi was unaffected relative to infections performed in the absence of drug (Fig. 5B). Thus, and as expected, Rosco added at 6 hpi did not impair the synthesis of IE proteins, which are synthesized largely in the first 4 h of infection. Moreover, Rosco added at 6 hpi did not inhibit translation from the E or L viral RNAs synthesized before addition of the drug (Fig. 5). From these experiments, we postulated that Rosco inhibited E transcript accumulation (Fig. 3 and 4) in the presence of normal levels of IE proteins (Fig. 5). To determine unequivocally whether Rosco inhibits any essential viral function that occurs after IE gene expression, the following experiments were performed.
Rosco inhibits HSV replication after removal of a 6-h CHX block.
Although the infections that produced the findings shown in Fig. 1 to 5 were synchronous, it is technically impossible to evaluate the direct effects of Rosco on E gene expression by adding the drug at different times after infection because of the overlap in the times of expression of HSV IE and E proteins. The effects of Rosco on specific stages of the HSV replication cycle can be determined, however, by blocking the progress of infection using drugs with known mechanisms of action and then releasing the block in the presence or absence of Rosco.
Using this approach, we examined the effects of addition of Rosco on viral replication when high levels of IE transcripts had already been expressed. For this purpose, a CHX release experiment was performed (Fig. 6). CHX is a general inhibitor of translation; hence, during infections performed in the presence of this drug, IE transcripts accumulate but are not translated. Consequently, E promoters are not activated, E transcripts and proteins are not expressed, DNA replication does not occur, and infectious virus is not produced. CHX inhibition is, however, reversible such that when the drug is removed, IE proteins are translated from the accumulated transcripts and the replication cycle of the virus resumes. Thus, if cdks are required for HSV replication functions which occur after IE transcript accumulation, Rosco should inhibit viral replication when added after the reversal of a CHX block.
FIG. 6.
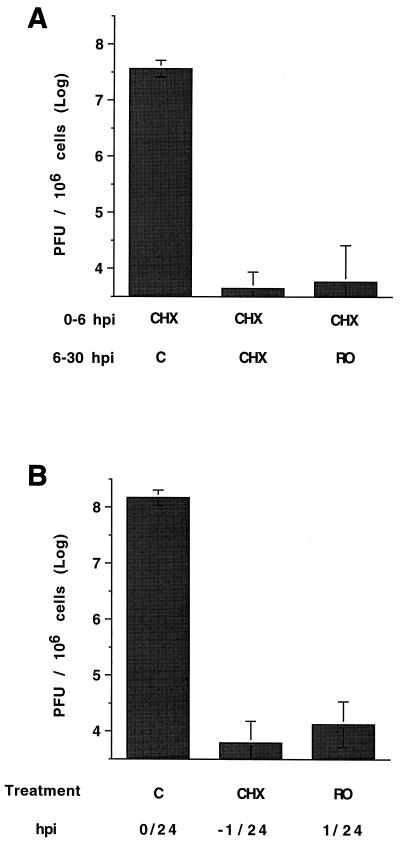
HSV replication in the presence of Rosco added after removal of CHX at 6 hpi. (A) Vero cells were pretreated with CHX for 1 h, infected with 3 PFU of HSV-1/cell, washed, and overlaid with medium containing 50 μg of CHX/ml. At 6 hpi, CHX-containing medium was removed, cells were washed twice with PBS, and fresh medium containing no drug (C), 50 μg of CHX/ml, or 100 μM Rosco (RO) was added. The PBS used for the washes contained the same drugs at the same concentrations as the medium added to respective cultures after washing. Twenty-four hours after the change of medium, cells were harvested, and viral titers were measured by standard plaque assay. Each bar represents the average and range of two experiments. (B) Vero cells were infected with 3 PFU of HSV-1/cell, washed, and overlaid with medium containing no drug (C), 50 μg of CHX/ml, or 100 μM Rosco (RO). Cells infected in the presence of CHX had been pretreated with the same drug for 1 h before infection. Twenty-four hours after infection, cells were harvested, and viral titers were measured by standard plaque assay. Each bar represents the average and range of two experiments.
To test this possibility, Vero cells were pretreated with CHX for 1 h, infected with 3 PFU of HSV-1/cell, and overlaid with medium containing 50 μg of CHX/ml. The concentration of CHX used in these and subsequent reversal experiments was minimized to permit efficient reversal. Six hours later, medium was removed, and monolayers were washed twice with PBS and overlaid with fresh medium containing no drug (control), 50 μg of CHX/ml, or 100 μM Rosco. The PBS used for the washes contained the same drugs as the media added after the washes. Twenty-four hours after the change of medium, cells were harvested, and infectious virus was measured by standard plaque assay.
The results presented in Fig. 6A demonstrate that Rosco inhibits HSV replication efficiently when added after removal of CHX at 6 hpi. Indeed, inhibition of HSV replication by Rosco under these conditions was almost as efficient as when CHX itself was added back after removal of CHX at 6 hpi. For comparison, cells in three dishes were infected for 24 h without change of medium. Infected cells in one dish were incubated for 24 h in drug-free medium, infected cells in a second dish were incubated in the presence of CHX from 1 h before infection through 24 hpi, and cells in a third dish were incubated in the presence of Rosco from 1 to 24 hpi (Fig. 6B). A comparison of Fig. 6A and B demonstrates that Rosco added after removal of CHX at 6 hpi inhibited HSV replication nearly as efficiently as when it was added immediately after infection.
The inhibitory effect of Rosco on HSV replication after reversal of a CHX block could be the result of inhibition of translation of IE proteins, of E or L gene transcription, DNA replication, encapsidation, viral egress, or other viral replication processes. Therefore, we next investigated whether transcription of E genes in the presence of IE proteins required cdk activities.
Rosco inhibits HSV transcription when added after removal of CHX at 6 hpi.
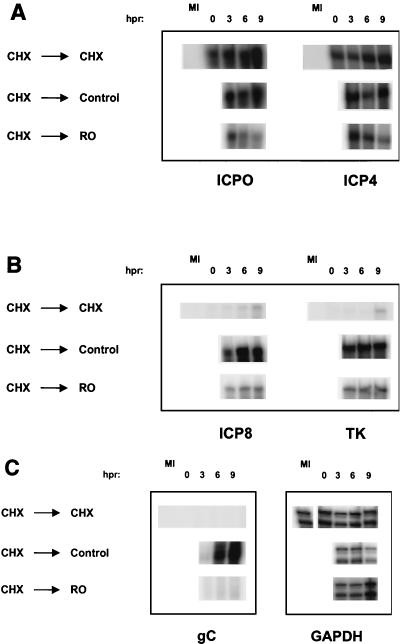
To determine if Rosco inhibits transcription of viral genes after removal of a 6-h CHX block, we measured the levels of representative viral IE, E, and L transcripts at selected times after the release of the CHX block in the presence or absence of Rosco. For this purpose, Vero cells were pretreated with CHX for 1 h, HSV infected, overlaid with CHX-containing medium, and released as described above. At the time of the change of medium (time zero) and 3, 6, and 9 h after the change of medium cells were harvested, and total RNA was extracted and measured as previously described (54).
As expected, when infected cells were released from the CHX block into drug-free medium, levels of both IE transcripts remained stable for 9 h (Fig. 7A and 8A). Moreover, when fresh CHX-containing medium was added immediately after release of the 6-h CHX block, IE transcripts continued to accumulate such that their levels increased 1.7-fold (ICP4) and 2.3-fold (ICP0) in 9 h (Fig. 7A and 8A). In contrast, when infected cells were released into Rosco-containing medium, levels of ICP4 and ICP0 decreased approximately 2.5- to fourfold, respectively, through the 9-h test period (Fig. 7A and 8A).
FIG. 7.
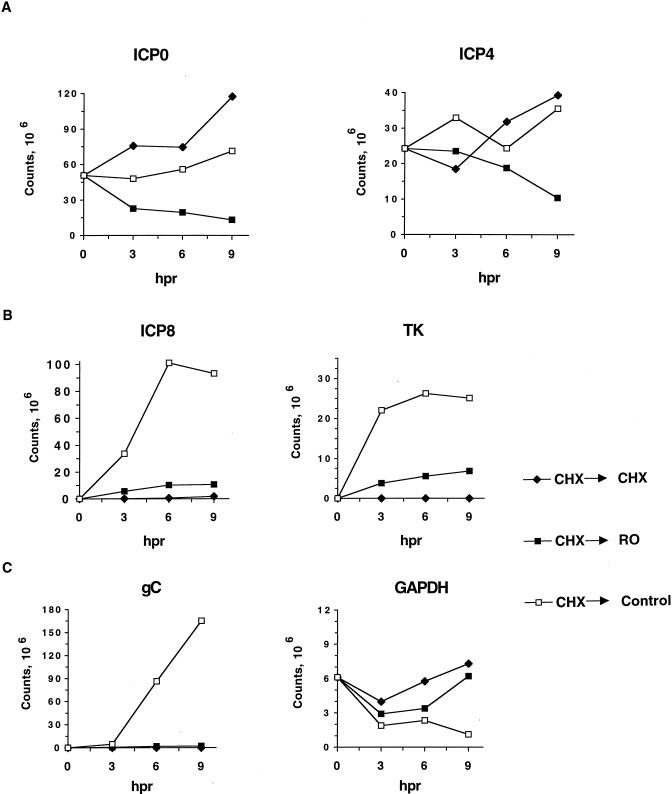
Levels of HSV IE, E, and L transcripts in the presence of Rosco added after removal of CHX at 6 hpi. Vero cells were infected with 3 PFU of HSV-1/cell, washed, and overlaid with medium containing 50 μg of CHX/ml. At 6 hpi, medium was removed, cells were washed twice with drug-containing PBS, and fresh medium containing 50 mg of CHX/ml, no drug (Control), or 100 μM Rosco (RO) was added. Immediately before (0), and at 3, 6, and 9 h postrelease (hpr) of the CHX block and addition of the secondary drug, cells were harvested and RNA extracted. RNA was also extracted from mock-infected cells as a negative control (MI). Levels of ICP0 and ICP4 (IE), ICP8 and TK (E), and gC (L) transcripts were evaluated by RNase protection. Levels of GAPDH were also measured to ensure equal loading of individual samples (C).
FIG. 8.
Quantitation of HSV IE, E, and L transcripts in the presence of Rosco added after removal of CHX at 6 hpi. The transcripts shown in Fig. 7 were quantitated with the ImageQuant software package (Molecular Dynamics). After subtraction of background, levels of individual transcripts were plotted as counts as a function of hours postrelease (hpr). Comparisons should be made not between the absolute values obtained for different transcripts but rather between levels of a given transcript after different drug treatments.
When the levels of two E transcripts were measured, transcript accumulation in the presence of CHX was minimal (Fig. 7B and 8B) but both transcripts accumulated more than 1,000-fold above prerelease levels within the first 6 h following release into drug-free medium (Fig. 7B and 8B), as expected. Recall that the concentration of CHX was minimized to allow for efficient reversal and hence CHX did not block transcription of E genes completely, as seen in Fig. 7B. After release of the CHX block into Rosco-containing medium, E transcripts accumulated only to low levels, and levels of the two transcripts increased only modestly at later times postrelease (Fig. 7B and 8B). Thus, the levels of E transcripts in infections released into Rosco were approximately sevenfold lower than in infections released into drug-free medium (Fig. 8B).
Consistent with the inhibition of accumulation of E or IE and E transcripts, the levels of a representative L transcript remained very low during the 9-h period following release into CHX- or Rosco-containing medium but reached high levels when released into drug-free medium (Fig. 7C and 8C; gC). Thus, the levels of gC transcripts were ∼75-fold lower when infected cells were released into Rosco-containing medium than when they were released into drug-free medium.
In contrast to viral transcripts, the levels of a cellular transcript, that of the GAPDH gene, were not reduced in infected cultures released into CHX or Rosco relative to those released into drug-free medium. In fact, and as observed in previous experiments (reference 54 and Fig. 3 and 4), levels of GAPDH transcripts decreased approximately threefold as infection progressed in cultures released into drug-free medium (Fig. 7C and 8C). In contrast, the levels of the GAPDH transcripts were not significantly affected following release of the CHX block into CHX- or Rosco-containing media (Fig. 7C and 8C), likely as a result of the block in viral replication.
The inhibition of accumulation of E (and L) transcripts when Rosco was added after a 6-h CHX block could be mediated either by direct inhibition of E gene transcription or by the inhibition of IE protein synthesis from the accumulated IE transcripts. We tested the latter possibility.
Rosco inhibits accumulation of E but not IE proteins when added after removal of CHX at 6 hpi.
Although the cdks known to be inhibited by Rosco have not been reported to be required for translation, this remained a theoretical possibility. Thus, we determined if the IE transcripts detected in the experiments shown in Fig. 7 and 8 were indeed translated into proteins in the presence of Rosco. For this purpose, we performed a metabolic labeling experiment. Vero cells were treated with CHX for 1 h, infected with 6 PFU of HSV/cell, and maintained in the presence of CHX for 6 h. At 6 hpi, CHX-containing medium was removed, and cells were washed with PBS containing either no drug or 100 μM Rosco as required. After washing, medium containing [35S]methionine and 100 μM Rosco or no drug was added to the monolayers. Six and 12 h after release from the CHX block in the presence of label and drug, cells were harvested and labeled proteins were resolved by SDS-polyacrylamide gel electrophoresis. For comparison, mock-infected cells were blocked with CHX for 6 h and released in the presence of label-containing, drug-free medium. In preliminary experiments, we had determined that Rosco has no visible effect on cellular protein synthesis in uninfected cells after release from a 6-h CHX block (data not shown).
Six hours after release into Rosco-containing medium, the majority of the labeled proteins comigrated with cellular proteins in the SDS-polyacrylamide gel (Fig. 9). At least four labeled bands derived from infected cells released into Rosco-containing medium, however, comigrated with labeled bands derived from infected cells released into drug-free medium (Fig. 9). Based on the molecular weights and migration patterns of these bands, the four proteins were identified as IE proteins ICP0, ICP4, ICP22, and ICP27. Notably, the levels of these proteins in infected cells released into Rosco-containing and drug-free medium were similar (Fig. 9). Thus, the levels of ICP0 and ICP4 synthesized in the presence of Rosco were ∼85% of the levels synthesized in drug-free medium, as measured by PhosphorImager analysis. Only the levels of ICP22 were slightly reduced in the presence of Rosco (∼65% of levels in drug-free medium in the experiment presented in Fig. 9). Although the half-lives of the IE proteins in the presence or absence of Rosco were not measured, any change in half-life would be physiologically irrelevant, as it would not affect the total amount of protein that accumulated in the first 6 h after release from the CHX block.
In infected cells released into drug-free medium, several E and L, as well as IE, proteins were detected 6 h after release (Fig. 9), and the levels of most of these proteins increased during the first 12 h after release. In contrast, in the infections released in the presence of Rosco, the levels of (IE) viral proteins did not increase after 6 h postrelease (Fig. 9). In both control and Rosco-treated cultures, the levels of IE proteins were lower when cells were labeled for 12 h after release than when they were labeled for 6 h. Therefore, as expected, synthesis of IE proteins was not maintained indefinitely after release. Moreover, the enhanced decrease in levels of IE protein synthesis at later times after release of the CHX block in the presence of Rosco (Fig. 9) correlated with the previously observed decrease in the steady-state levels of IE transcripts (Fig. 7A and 8A). No E or L proteins were detected in cells released from the 6-h CHX block into Rosco-containing medium (Fig. 9), consistent with the low levels of E and L transcripts that accumulated under these conditions (Fig. 7B and C and Fig. 8).
In sum, although the levels of IE proteins synthesized in the presence of Rosco after a 6-h CHX block were not significantly lower than in infected cells released into control medium (Fig. 9), E transcript accumulation was significantly impaired by Rosco under these conditions (Fig. 7 and 8). Based on a comparison of the results presented in Fig. 7, 8, and 9, we conclude that Rosco inhibits transcription of E genes in the presence of normal levels of IE proteins.
DISCUSSION
cdks are required for HSV transcription.
We have shown previously that cellular cdks are required for HSV replication (54). In the experiments reported in this manuscript, we have established that at least two viral functions require cellular cdks during HSV replication in cycling cells. Thus, accumulation of both IE and E transcripts was dependent on cdk activity. The effects of Rosco on L gene transcription, on the other hand, may well reflect secondary effects of the inhibition of E gene expression, and consequently viral DNA replication, given that expression of gC is directly dependent on these viral functions. As an alternative explanation, Rosco may have inhibited some yet-unknown viral or cellular kinase whose function is required for HSV IE, E, and L transcription. Notably, however, none of the three acknowledged or putative viral protein kinases is required for expression of all five viral genes tested in the experiments presented in this article (15, 38, 47, 48).
Inhibition of HSV transcription by Rosco added 6 h after infection (Fig. 3 and 4) or after release of a CHX block (Fig. 7 and 8) proves that the effects of Rosco on transcription, and hence on HSV replication, are not due to a block in the transport of capsids to the nucleus or to a defect in uncoating. Moreover, Rosco does not appear to inhibit transcript accumulation by stimulating RNA degradation, in that the levels of two E transcripts (ICP8 and TK) and one L transcript (gC) remained stable or increased slightly after addition of Rosco (Fig. 3, 4, 7, and 8). On the other hand, the levels of ICP0 (and to a lesser extent ICP4) transcripts decreased after the addition of Rosco following release of a 6-h CHX block (Fig. 7 and 8). In these experiments, the estimated half-life of ICP0 mRNAs was ∼4.5 h and that of the ICP4 mRNAs was slightly longer. Although our experimental conditions preclude precise determination of the half-lives of ICP0 or ICP4 transcripts, the stability of both transcripts in the presence of Rosco is consistent with their previously documented half-lives, which are estimated to be between 1.5 and 5 h (24, 44). More importantly, the estimated half-lives are inconsistent with the hypothesis that the low levels of these mRNAs can be explained by a decrease in their half-lives. The apparently long half-lives of E and L transcripts in the presence of Rosco added after release of a 6-h CHX block may be due either to indirect stabilization resulting from Rosco treatment or to low levels of transcription of these genes upon addition of Rosco. These (and other) viral transcripts may have been stabilized indirectly by Rosco inhibition of vhs expression, as vhs itself is an L gene product. Consistent with this possibility, the half-lives of ICP0, ICP4, TK, ICP8, and other HSV mRNAs increased from ∼1.5-fold to ∼4.5-fold in 8 h during infection with a vhs− HSV mutant (44).
We hypothesized previously that inhibition of IE transcription by Rosco or Olo might be mediated by inhibition of cdk-mediated phosphorylation of one or more of the proteins required for transcription of IE genes (54). These proteins include members of the basal cellular transcriptional complex as well as promoter-specific viral and cellular transactivators. Notably, the proteins that activate HSV IE promoters do not activate E promoters during infection. Consequently, if Rosco inhibited transcription of IE genes exclusively by inhibiting phosphorylation of promoter-specific transactivators, the requirement of E gene transcription for cdks would indicate that cdks activate at least two independent transcriptional activities required for HSV replication. For instance, HSV IE proteins, which activate E promoters and are themselves phosphorylated, may be directly or indirectly regulated by cdk phosphorylation. Interestingly, the relative mobilities of the IE proteins after release from a CHX block in the presence of Rosco were distinct from the mobility of the same viral proteins after release in drug-free medium (Fig. 9). Experiments to determine whether this change in migration pattern reflects differences in the phosphorylation states of these proteins are in progress.
Potential roles of cellular cdks in HSV transcription.
Since cdks are involved in regulating the activities of specific cellular transcription factors (reviewed in reference 9), phosphorylation of these cellular factors could explain the requirement of HSV transcription for cdks. For instance, the activity of oct-1, which is required for HSV IE gene expression, is regulated by cdk phosphorylation (19, 53). Other cellular transcription factors, including E2F, are further regulated by cdk-2 through more complex mechanisms (2, 10, 12, 32, 43, 45, 52, 66). It has been reported that the HSV TK promoter is activated by E2F in transient transfection assays through a cell cycle-independent process (59). Interestingly, free E2F and E2F in complexes resembling those present in cycling cells at the G1/S transition are induced during HSV infection (25). Although the precise mechanism leading to E2F induction during HSV infection has not been characterized (25), free E2F and E2F in G1/S-specific complexes are physiologically induced by cdks (2, 10, 43).
Complexes of transcription factors, which generally contain E2F and cdk-2, bind to cellular promoters in a constitutive manner, but activate transcription only under certain circumstances (37). Cyclin A and p107 bind to some of these transcription factor complexes in an inducible manner (37). In theory, binding of cyclin A should activate cdk-2 already bound to the promoter. Complexes containing cdk-2 bound to cyclin A and p107/p130 have unique substrate specificity (22). The altered substrate specificity of cdk-2 in such complexes has been postulated to indicate that cdk-2 bound to promoters through its interaction with p107/p130 may specifically activate transcription factors constitutively bound to the same promoters, thus explaining the inducibility of these promoters (22, 37). It is possible, therefore, that a Rosco-sensitive cdk binds indirectly to HSV promoters during infection and activates other viral or cellular transcription factors already bound to these promoters.
Considering the results of the experiments reported herein, we must now consider an alternative but not mutually exclusive explanation for the requirement of both IE and E transcription for cellular cdk. cdks may be required for global transcriptional activation of the viral genome. Interestingly, a uniquely phosphorylated form of the CTD of RNA Pol II appears to be required for the switch from cellular to viral transcription during infection (49, 50). Physiologically, the CTD is phosphorylated by cdk-7, -8, and -9 (33, 51, 57, 58, 61, 68). The kinase that catalyzes the unique phosphorylation of the CTD during HSV infection has not been identified. Given that the vast majority of the phosphorylation sites in the CTD are cdk sites (6), however, the infection-specific phosphorylation of the CTD is likely also catalyzed by a cdk. We previously performed an analysis of the published structure and sequence data of cdk-7 and -8 to evaluate whether they may be inhibited by Rosco (54). Considering the results presented herein, it is now imperative to determine experimentally whether Rosco inhibits cdk-7 or -8. Experiments to address this issue are currently in progress.
In addition to RNA Pol II itself, most, if not all, components of the basal transcriptional complex are differentially phosphorylated (17). Furthermore, the activities of many of these components are regulated by phosphorylation, and in some cases, cdks have been identified as the relevant kinases (17, 35, 56). In addition to the basal transcription complex, transcriptional coactivators, such as CBP and p300, are also regulated by cdk phosphorylation (46). Therefore, differential phosphorylation of one or more of the components of the basal or inducible transcriptional complexes by cdks may be required for transcription of the viral genome. Notably, cellular protein synthesis is not grossly affected by Rosco (Fig. 5 and 9), suggesting that transcription of most cellular genes does not require a Rosco-sensitive cdk (Fig. 3 and 7).
Involvement of cdks in viral functions.
Cellular cdks have long been known to be involved in the replication of DNA-containing viruses (21, 23, 60). For example, the replication of simian virus 40 and polyomavirus DNAs is activated by cdk-2 (5, 21, 36), and human cytomegalovirus DNA replication is inhibited by both Rosco and Olo (3). HSV appears to be unique, however, in that it requires cellular cdks for transcription of both IE and E genes. Since IE and E proteins are required for HSV DNA replication, we have not yet determined whether cdks are also required for this latter viral function. Experiments designed to address this issue are in progress. All experiments presented here and in our previous article (54) have been performed in cycling cells. Of the cdks known to be sensitive or which may be sensitive to Rosco, cdk-1, -2, and -7 are active in cycling cells. Moreover, cdk-3 and -5 are expressed at high levels in these cells and may also be active, although currently available techniques do not allow detection of cdk-3- or -5-dependent kinase activity in cycling cells. We must emphasize that we do not yet know which of the Rosco-sensitive cdks is required for each specific HSV replication function; thus, we cannot yet determine whether these cdks are specifically induced during infection. If the required cdks are in fact those that are activated in a cell-cycle-specific manner, one would predict that HSV infection of cells in stages of the cell cycle in which these cdks are inactive should induce them efficiently and rapidly to facilitate full viral gene expression and successful lytic infection. This hypothesis further suggests that HSV infection of neurons, in which several of the Rosco-sensitive cdks are inactive or not expressed, may well lead to a latent state in which most viral transcription is repressed.
Since at least two distinct HSV functions require the activities of cellular cdks (transcription of IE and of E genes), resistance of HSV replication to cdk inhibitors would require multiple mutations. The results presented herein are therefore consistent with our previously reported inability to isolate HSV variants resistant to Rosco or Olo (54). Both our inability to isolate drug-resistant mutants and the ability of these drugs to prevent the inflammatory and immune responses required for controlling and clearing viral infections suggest that cdk inhibitors may be useful as anti-(herpes)viral drugs. Knowledge of the processes that require cdk activity should provide an interesting new series of molecular targets for the development of novel antiviral compounds.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants (R37CA20260 from the National Cancer Institute and PO1NS35138 from the National Institute of Neurological Disorders and Stroke).
We thank Robert Jordan and William Halford for helpful discussions and ideas and Amy Francis, Jennifer Isler, and David Davido for critically reading the manuscript.
REFERENCES
- 1.Abraham R T, Acquarone M, Andersen A, Asensi A, Belle R, Berger F, Bergounioux C, Brunn G, Buquet-Fagot C, Fagot D, et al. Cellular effects of olomoucine, an inhibitor of cyclin-dependent kinases. Biol Cell. 1995;83:105–120. doi: 10.1016/0248-4900(96)81298-6. [DOI] [PubMed] [Google Scholar]
- 2.Adams P D, Kaelin W G., Jr Transcriptional control by E2F. Semin Cancer Biol. 1995;6:99–108. doi: 10.1006/scbi.1995.0013. [DOI] [PubMed] [Google Scholar]
- 3.Bresnahan W A, Boldogh I, Chi P, Thompson E A, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, Schaffer P A. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee A, Bockus B J, Gjørup O V, Schaffhausen B S. Phosphorylation sites in polyomavirus large T antigen that regulate its function in viral, but not cellular, DNA synthesis. J Virol. 1997;71:6472–6478. doi: 10.1128/jvi.71.9.6472-6478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahmus M E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 7.Daksis J I, Preston C M. Herpes simplex virus immediate early gene expression in the absence of transinduction by Vmw65 varies during the cell cycle. Virology. 1992;189:196–202. doi: 10.1016/0042-6822(92)90695-l. [DOI] [PubMed] [Google Scholar]
- 8.De Azevedo W F, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim S H. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 9.Dynlacht B. Regulation of transcription by proteins that control the cell cycle. Nature. 1997;389:149–152. doi: 10.1038/38225. [DOI] [PubMed] [Google Scholar]
- 10.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 11.Ebert S, Dubramanian D, Shtrom S, Chung I, Parris D, Muller M. Association between the p170 form of human topoisomerase II and progeny viral DNA in cells infected with herpes simplex virus type 1. J Virol. 1994;68:1010–1020. doi: 10.1128/jvi.68.2.1010-1020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewen M E. The cell cycle and the retinoblastoma protein family. Cancer Metastasis Rev. 1994;13:45–66. doi: 10.1007/BF00690418. [DOI] [PubMed] [Google Scholar]
- 13.Glab N, Labidi B, Qin L X, Trehin C, Bergounioux C, Meijer L. Olomoucine, an inhibitor of the cdc2/cdk2 kinases activity, blocks plant cells at the G1 to S and G2 to M cell cycle transitions. FEBS Lett. 1994;353:207–211. doi: 10.1016/0014-5793(94)01035-8. [DOI] [PubMed] [Google Scholar]
- 14.Gold M O, Yang X, Herrmann C H, Rice A P. PITALRE, the catalytic subunit of TAK, is required for human immunodeficiency virus Tat transactivation in vivo. J Virol. 1998;72:4448–4453. doi: 10.1128/jvi.72.5.4448-4453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein D J, Weller S K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 16.Goto H, Motomura S, Wilson A C, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 17.Gottesfeld J M, Forbes D J. Mitotic repression of the transcriptional machinery. Trends Biochem Sci. 1997;22:197–202. doi: 10.1016/s0968-0004(97)01045-1. [DOI] [PubMed] [Google Scholar]
- 18.Greenblatt J. RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 19.Grenfell S J, Latchman D S, Thomas N S. Oct-1 and Oct-2 DNA-binding site specificity is regulated in vitro by different kinases. Biochem J. 1996;315:889–893. doi: 10.1042/bj3150889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammersten O, Xiaodan Y, Elias P. Inhibition of topoisomerase II by ICRF-193 prevents efficient replication of herpes simplex virus type 1. J Virol. 1996;70:4523–4529. doi: 10.1128/jvi.70.7.4523-4529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassell J A, Brinton B. SV40 and polyomavirus DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 639–677. [Google Scholar]
- 22.Hauser P J, Agrawi D, Chu B, Pledger W J. p107 and p130 associated cyclin A has altered substrate specificity. J Biol Chem. 1997;272:22954–22959. doi: 10.1074/jbc.272.36.22954. [DOI] [PubMed] [Google Scholar]
- 23.Hay R. Adenovirus DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 699–719. [Google Scholar]
- 24.Henley D C, Weir J P. The relative stability of selected herpes simplex virus type 1 mRNAs. Virus Res. 1991;20:121–132. doi: 10.1016/0168-1702(91)90104-4. [DOI] [PubMed] [Google Scholar]
- 25.Hilton M J, Mounghane D, McLean T, Contractor N V, O’Neil J, Carpenter K, Bachenheimer S L. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology. 1995;213:624–638. doi: 10.1006/viro.1995.0034. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann F, Livingston D M. Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes Dev. 1996;10:851–861. doi: 10.1101/gad.10.7.851. [DOI] [PubMed] [Google Scholar]
- 27.Honess R W, Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973;12:1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hossain A, Holt T, Ciacci-Zanella J, Jones C. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J Gen Virol. 1997;78:3341–3348. doi: 10.1099/0022-1317-78-12-3341. [DOI] [PubMed] [Google Scholar]
- 31.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa M, Higashi H, Suzuki-Takahashi I, Segawa K, Hanks S K, Taya Y, Nishimura S, Okuyama A. Phosphorylation of E2F-1 by cyclin A-cdk2. Oncogene. 1995;10:229–236. [PubMed] [Google Scholar]
- 33.Leclerc V, Tassan J P, O’Farrell P H, Nigg E A, Leopold P. Drosophila Cdk8, a kinase partner of cyclin C that interacts with the large subunit of RNA polymerase II. Mol Biol Cell. 1996;7:505–513. doi: 10.1091/mbc.7.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S-K, Dong Q, Lehman I. Interaction of herpes simplex virus 1 origin-binding protein with DNA polymerase α. Proc Natl Acad Sci USA. 1995;92:7882–7886. doi: 10.1073/pnas.92.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leresche A, Wolf V J, Gottesfeld J M. Repression of RNA polymerase II and III transcription during M phase of the cell cycle. Exp Cell Res. 1996;229:282–288. doi: 10.1006/excr.1996.0373. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Bhattacharye S, Prives C. Cyclin-dependent kinase regulation of the replication functions of polyomavirus large T antigen. J Virol. 1997;71:6479–6485. doi: 10.1128/jvi.71.9.6479-6485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L-J, Naeve G S, Lee A S. Temporal regulation of cyclin A-p107 and p33cdk2 complexes binding to a human thymidine kinase promoter element important for G1-S phase transcriptional regulation. Proc Natl Acad Sci USA. 1993;90:3554–3558. doi: 10.1073/pnas.90.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeoch D, Barnett B, McLean C. Emerging functions of alphaherpesvirus genes. Semin Virol. 1993;4:125–134. [Google Scholar]
- 39.Meijer L. Chemical inhibitors of cyclin-dependent kinases. Prog Cell Cycle Res. 1995;1:351–363. doi: 10.1007/978-1-4615-1809-9_29. [DOI] [PubMed] [Google Scholar]
- 40.Meijer L. Chemical inhibitors of cyclin-dependent kinases. Trends Cell Biol. 1996;6:393–397. doi: 10.1016/0962-8924(96)10034-9. [DOI] [PubMed] [Google Scholar]
- 41.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 42.Meijer L, Kim S H. Chemical inhibitors of cyclin-dependent kinases. Methods Enzymol. 1997;283:113–128. doi: 10.1016/s0076-6879(97)83011-x. [DOI] [PubMed] [Google Scholar]
- 43.Nevins J R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 44.Oroskar A A, Read S G. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989;63:1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeper D S, Keblusek P, Helin K, Toebes M, van der Eb A J, Zantema A. Phosphorylation of a specific cdk site in E2F-1 affects its electrophoretic mobility and promotes pRB-binding in vitro. Oncogene. 1995;10:39–48. [PubMed] [Google Scholar]
- 46.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-kappaB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 47.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice S, Long M, Lam V, Spencer C. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice S A, Long M C, Lam V, Schaffer P A, Spencer C A. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rickert P, Seghezzi W, Shanahan F, Cho H, Lees E. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene. 1996;12:2631–2640. [PubMed] [Google Scholar]
- 52.Rieber M, Rieber M S. Cyclin-dependent kinase 2 and cyclin A interaction with E2F are targets for tyrosine induction of B16 melanoma terminal differentiation. Cell Growth Differ. 1994;5:1339–1346. [PubMed] [Google Scholar]
- 53.Roberts S B, Segil N, Heintz N. Differential phosphorylation of the transcription factor Oct1 during the cell cycle. Science. 1991;253:1022–1026. doi: 10.1126/science.1887216. [DOI] [PubMed] [Google Scholar]
- 54.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulze-Gahmen U, Brandsen J, Jones H D, Morgan D O, Meijer L, Vesely J, Kim S H. Multiple modes of ligand recognition: crystal structures of cyclin-dependent protein kinase 2 in complex with ATP and two inhibitors, olomoucine and isopentenyladenine. Proteins. 1995;22:378–391. doi: 10.1002/prot.340220408. [DOI] [PubMed] [Google Scholar]
- 56.Segil N, Guermah M, Hoffmann A, Roeder R G, Heintz N. Mitotic regulation of TFIID: inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev. 1996;10:2389–2400. doi: 10.1101/gad.10.19.2389. [DOI] [PubMed] [Google Scholar]
- 57.Serizawa H, Makela T P, Conaway J W, Conaway R C, Weinberg R A, Young R A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995;374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- 58.Shiekhattar R, Mermelstein F, Fisher R P, Drapkin R, Dynlacht B, Wessling H C, Morgan D O, Reinberg D. Cdk-activating kinase complex is a component of human transcription factor. Nature. 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- 59.Shin E K, Tevosian S G, Yee A S. The N-terminal region of E2F-1 is required for transcriptional activation of a new class of target promoter. J Biol Chem. 1996;271:12261–12268. doi: 10.1074/jbc.271.21.12261. [DOI] [PubMed] [Google Scholar]
- 60.Stenlund A. Papillomavirus DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 679–697. [Google Scholar]
- 61.Tassan J P, Jaquenoud M, Leopold P, Schultz S J, Nigg E A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc Natl Acad Sci USA. 1995;92:8871–8875. doi: 10.1073/pnas.92.19.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Umene K, Nishimoto T. Inhibition of generation of authentic genomic termini of herpes simplex virus type 1 DNA in temperature-sensitive mutant BHK-21 cells with a mutated CCG1/TAF(II)250 gene. J Virol. 1996;70:9008–9012. doi: 10.1128/jvi.70.12.9008-9012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vesely J, Havlicek L, Strnad M, Blow J J, Donella-Deana A, Pinna L, Letham D S, Kato J, Detivaud L, Leclerc S, Meijer L. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 64.Wilcock D, Lane D P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991;349:429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- 65.Wilson A C, Freiman R N, Goto H, Nishimoto T, Herr W. VP16 targets an amino-terminal domain of HCF involved in cell cycle progression. Mol Cell Biol. 1997;17:6139–6146. doi: 10.1128/mcb.17.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu M, Sheppard K A, Peng C Y, Yee A S, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanagi K, Talavera A, Nishimoto T, Rush M G. Inhibition of herpes simplex virus type 1 replication in temperature-sensitive cell cycle mutants. J Virol. 1978;25:42–50. doi: 10.1128/jvi.25.1.42-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang X, Gold M O, Tang D N, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Herrmann C H, Rice A P. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J Virol. 1996;70:4576–4584. doi: 10.1128/jvi.70.7.4576-4584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yankulov K, Yamashita K, Roy R, Egly J M, Bentley D L. The transcriptional elongation inhibitor 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole inhibits transcription factor IIH-associated protein kinase. J Biol Chem. 1995;270:23922–23925. doi: 10.1074/jbc.270.41.23922. [DOI] [PubMed] [Google Scholar]
- 71.Yankulov K Y, Pandes M, McCracken S, Bouchard D, Bentley D L. TFIIH functions in regulating transcriptional elongation by RNA polymerase II in Xenopus oocytes. Mol Cell Biol. 1996;16:3291–3299. doi: 10.1128/mcb.16.7.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M, Price D. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1996;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]