Abstract
We have analyzed the production of tumor necrosis factor alpha (TNF-α) induced by in vitro infection with African swine fever (ASF) virus (ASFV) and the systemic and local release of this inflammatory cytokine upon in vivo infection. An early increase in TNF-α mRNA expression was detected in ASFV-infected alveolar macrophages, and high levels of TNF-α protein were detected by ELISA in culture supernatants from these cells. When animals were experimentally infected with a virulent isolate (E-75), enhanced TNF-α expression in mainly affected organs correlated with viral protein expression. Finally, elevated levels of TNF-α were detected in serum, corresponding to the onset of clinical signs. TNF-α has been reported to be critically involved in the pathogenesis of major clinical events in ASF, such as intravascular coagulation, tissue injury, apoptosis, and shock. In the present study, TNF-α containing supernatants from ASFV-infected cultures induced apoptosis in uninfected lymphocytes; this effect was partially abrogated by preincubation with an anti-TNF-α specific antibody. These results suggest a relevant role for TNF-α in the pathogenesis of ASF.
African swine fever (ASF) virus (ASFV) is a large, icosahedral DNA virus currently considered the only member of a new family of animal viruses (38). ASFV infects soft ticks of the genus Ornithodoros and different members of the Suidae family. In the natural swine hosts, the warthog and bushpig, ASFV causes inapparent or mild infections with few clinical signs. In contrast, infection of the domestic pig by virulent isolates results in a devastating disease with high mortality (11).
Acute ASF is characterized by disseminated intravascular coagulation with multiple hemorrhages in all tissues, leading to animal death within a few days, as a consequence of shock (37). ASFV replicates mainly in macrophages and monocytes (22, 39), and its ability to infect these cells has been considered to play a critical role in the pathogenicity of the disease (11). It has been demonstrated that, upon in vitro and in vivo infection with ASFV, apoptosis is induced in target cells (31). Moreover, infected animals present marked leukopenia and severe impairment of lymphoid organs, characterized by lymphocyte apoptosis and significant cellular depletion mainly affecting the spleen and lymph nodes (17, 32). Considering the nonsusceptibility of lymphocytes to ASFV infection, the effects observed in this population are most likely due to soluble mediators released by infected cells.
Monocytes-macrophages secrete a large range of soluble mediators, including proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) (35). Among them, TNF-α may particularly contribute to the pathogenesis of ASF (27). TNF-α induces vasodilation, an increase in vascular permeability, and activation of the vascular endothelium, all of which alter the balance between procoagulant and anticoagulant activities and favor the generation of microthrombi (6, 23). Furthermore, TNF-α provides signals involved in the cellular control of programmed cell death (25, 40). Elevated systemic levels of TNF-α result in disseminated intravascular coagulation (with consumption of clotting factors), leading to extensive hemorrages, shock, multiple organ failure, and death (36).
The aim of this study was to analyze the expression pattern of TNF-α following ASFV infection. We evaluated TNF-α production by macrophages induced by in vitro infection and increased levels of TNF-α in the sera and organs of animals experimentally infected with a virulent ASFV isolate (E-75). Altogether, our findings suggest the involvement of TNF-α in the pathogenesis of ASF.
MATERIALS AND METHODS
Virus, cells, and in vitro infections.
The virulent E-75 strain of ASFV was grown in buffy coat cell cultures as previously described (33). Virus was titrated in swine peripheral blood mononuclear cells (PBMC) and expressed as 50% tissue culture-infective doses per milliliter (18). When required, virus inactivation was performed by irradiation with UV light at a distance of 15 cm for 10 min by using a G15T8 UV lamp (15 W; Philips, Eindhoven, The Netherlands). Lack of infectivity of UV-treated virus was confirmed by the absence of a cytophatic effect on macrophage cultures 10 days after inoculation and by the absence of ASFV p73 expression by immunofluorescence.
PBMC were isolated on discontinuous Percoll gradients after blood sedimentation in dextran as previously described (18). Porcine alveolar macrophages were obtained from healthy outbred pigs by alveolar lavage as previously described (9). The cells were washed with Hanks buffer containing 2 mM EDTA and frozen in liquid nitrogen until use.
Alveolar macrophages were thawed, washed immediately with complete medium (RPMI 1640 medium containing 10% fetal calf serum, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 30-μg/ml gentamicin), and seeded onto a six-well plate at 3 × 106 cells/well. After incubation for 6 h at 37°C, nonadherent cells were removed and macrophages were infected with the ASFV isolate E-75 at a multiplicity of infection (MOI) of 5 and incubated in RPMI medium with 20% swine serum. Viral infection of macrophages was monitored by immunoperoxidase staining with polyclonal anti-ASFV antibodies and by PCR detection of p54 and p30 genes.
The absence of endotoxin in virus stocks, cell culture supernatants, and media was assessed with the Limulus amebocyte lysate assay (E-Toxate; Sigma Chemical Co., St. Louis, Mo.). The sensitivity of the assay, with a standard of lipopolysaccharide (LPS) from Escherichia coli O55:B5 (Sigma Chemical Co.), was in the range of 2 to 6 pg/ml.
Animal infection procedures.
Three-month-old Large-White pigs (six for each experiment) were intramuscularly inoculated in the shoulder with 105 50% tissue culture-infective doses of the E-75 isolate. One inoculated pig was killed daily for pathological examination and collection of blood and tissue samples. Clinical symptoms and temperature were recorded. In addition, blood samples were collected daily from one of two additional inoculated animals. Two pigs injected with a saline solution were used as controls. Animals were sedated with azaperone (Stresnil; Esteve) and euthanized with pentobarbital (Doletal; Vetoquine).
Cytokines and antibodies.
Recombinant bovine (rBo) TNF-α was kindly supplied by Rolf Steiger of Ciba-Geigy Ltd. (Basel, Switzerland) and had a specific activity of 1.5 × 106 U/mg (lot no. 4447-95, code CGA 214 666). Bovine and porcine TNF-α proteins show 84% homology (5). Recombinant porcine TNF-α (specific activity, 108 U/mg) and antiporcine TNF-α monoclonal antibody (MAb) 4F4 were purchased from Endogen.
New Zealand rabbits were inoculated subcutaneously with 20 μg of rBo TNF-α emulsified in complete Freund’s adjuvant. At monthly intervals, they received subcutaneous booster injections (20 μg per dose) of rBo TNF-α in incomplete Freund’s adjuvant, and blood was periodically collected. Serum immunoglobulin G was purified by affinity chromatography with protein A-Sepharose CL4B (Pharmacia, Uppsala, Sweden) and biotin labeled as described elsewhere (12).
RT-PCR analysis of TNF-α mRNA expression.
Total RNA was isolated by using the Tripure Isolation reagent (Boehringer Mannheim, Indianapolis, Ind.) by a method based on that described by Chomczynski and Sacchi (10). First-strand cDNA was obtained from 5 μg of total RNA (previously denatured by heating for 2 min at 65°C and immediately placed on ice) with 5 μl of a reverse transcriptase (RT) reaction mixture containing 10× Moloney murine leukemia virus RT buffer (Epicentre), 10 mM dithiothreitol, 0.5 mM oligo(dT), 50 μM each deoxynucleoside triphosphate, 12.5 U of Moloney murine leukemia virus RT (Epicentre), and 20 U of RNasin (Promega). The reaction mixtures, at a final volume of 50 μl with RNase-free water, were incubated for 1 h at 37°C. For PCR, a variable amount of the cDNA (typically, 2.5 μl) was used in a total volume of 25 μl of a PCR mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.005% Tween 20, 0.005% Nonidet P-40, 0.5 to 1 mM MgCl2 (depending on the oligonucleotide pair), 50 μM each deoxynucleoside triphosphate, 10 pmol each of specific oligonucleotides (forward and reverse primers), and 1 U of Dnazyme II DNA Polymerase (Finnzymes Oy). PCR amplification was carried out with 30 cycles for TNF-α and 40 cycles for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) (denaturation at 94°C for 1 min, annealing at 60°C for 90 s, and extension at 72°C for 2 min). PCR products were electrophoresed on 2% agarose gels containing ethidium bromide and analyzed by an image densitometer coupled to a computer program (TDI, Gelstation). Under these conditions, the intensity of amplified bands was proportional to the amount of cDNA templates (data not shown). The primer sequences and expected amplified-fragment sizes are shown in Table 1. G3PDH expression was used as a control for RNA content and integrity.
TABLE 1.
Primer sequences and product sizes
| Primer | Sequence | Product size (bp) |
|---|---|---|
| TNF-α 5′ | CCCAGAAGGAAGAGTTTCCAG | |
| TNF-α 3′ | CAGCAAAGTCCAGATAGTCGG | 489 |
| p30 5′ | GCGCGGATCCATGGATTTTATTTTAAATAT | |
| p30 3′ | GCGCTCTAGACAATCATATAAGAATAACTA | 615 |
| p54 5′ | GCGCGGATCCTGGCCCGCCAGGTTTAC | |
| p54 3′ | GCGCGCGAATTCATGGATTCTGAATTTTTTCAACC | 561 |
| G3PDH 5′ | CCATCACTATCTTCCAGGAGCGTGAC | |
| G3PDH 3′ | AAGTTGTCATGGATGACCTTGGCCA | 285 |
Quantitation of TNF-α in sera and culture supernatants.
TNF-α protein levels in sera and cell culture supernatants were measured with an enzyme-linked immunosorbent assay (ELISA) obtained from Endogen by following the manufacturer’s recommendations. Recombinant porcine TNF-α was used as the standard, and the assay sensitivity was 38 pg/ml.
Immunohistochemical analyses.
Tissues were snap frozen in isopentane-liquid nitrogen and stored at −70°C. Frozen sections of 6 μm were fixed in cold acetone for 10 min and then washed with phosphate-buffered saline (PBS). Sections were stained with MAbs recognizing different macrophage subsets or with an anti-CD62E MAb by an indirect immunoperoxidase technique as previously described (24). Briefly, frozen sections were incubated for 90 min at room temperature with hybridoma supernatants. After washing in PBS, sections were incubated with a 1/40 dilution of polyclonal rabbit anti-mouse antibodies conjugated with peroxidase (Dako) for 60 min. After extensive washing with PBS, sections were incubated for 5 min in a solution of 0.6-mg/ml diaminobenzidine (DAB; Sigma) and 0.02% hydrogen peroxide in PBS. Finally, they were washed with water, counterstained with methylene blue, dehydrated, and mounted with DePex (Serva, Heidelberg, Germany). The MAbs used in this study were MAb 1.2B6 (anti-CD62E), which was obtained from Serotec, and MAb 2A10 (anti-porcine macrophages), which was generated in our laboratory and whose specificity has been previously reported (8).
For detection of ASFV-infected cells, sections were incubated with a 1/100 dilution of biotinylated swine anti-ASFV immunoglobulins for 90 min. After washing of the sections with PBS, a 1/40 dilution of streptavidin-peroxidase (Dako) in PBS was applied, and the sections were incubated for 60 min. Visualization of positively stained cells was carried out as described above.
Detection of TNF-α-producing cells was performed by incubating tissue sections with biotin-labeled rabbit anti-TNF-α immunoglobulins diluted 1:100 in TBS (Tris-buffered saline, pH 7.6) for 90 min. After washing with TBS, sections were further incubated with streptavidin-alkaline phosphatase (Dako) diluted 1/50 in TBS for 60 min. After extensive washing with TBS, a solution containing 1-mg/ml Fast Red TR, 0.4-mg/ml Naphthol AS-MX, and 0.15-mg/ml levamisole in Tris buffer (0.1 M pH 7.6) was applied to the sections, and they were incubated for 25 min. Finally, the sections were washed with water, counterstained with hematoxylin, and mounted with Clearmount (Zymed). All steps were carried out in a dark, humidified chamber at room temperature.
In vitro effects of supernatants containing TNF-α.
PBMC were plated in a 96-well plate (Costar, Cambridge, Mass.) at 15 × 104 cells per well in 150 μl of RPMI medium supplemented with 1% fetal calf serum. Alveolar macrophage cultures were infected with ASFV isolate E-75 at an MOI of 5. At 8 and 24 h postinfection, 50-μl volumes of infected culture supernatants were collected and added to the PBMC. Supernatants from noninfected macrophages and from macrophages stimulated for 24 h with 1-μg/ml LPS (Sigma) were also added to the PBMC. Recombinant porcine TNF-α (Endogen) (as a potent inducer of apoptosis in lymphocytes) was used at a final concentration of 6 ng/ml, which had been previously determined in titration assays (data not shown) to be the highest nonsaturating concentration to induce significant apoptosis. In order to block TNF-α activity contained in supernatants, they were preincubated for 2 h with either medium or various amounts of anti-TNF-α MAb (Endogen) (concentrations of 0 to 100 μg/ml, as indicated). After 6, 12, and 24 h of treatment, PBMC were collected to analyze DNA fragmentation.
DNA fragmentation assays.
An ELISA kit (Boehringer GmbH, Mannheim, Germany) was used to quantitate low-molecular-weight DNA linked to histones by following the manufacturer’s instructions. Briefly, a 96-well plate (Costar) was coated with antihistone antibody and incubated overnight at 4°C. Approximately 4 × 104 cells were treated with lysis buffer, and the cytoplasmic extract was obtained by centrifugation. A dilution of the cytoplasmic extract was added to the wells and incubated for 90 min (together with the controls and blanks to measure the background of the assay). After washing, an anti-DNA antibody conjugated with peroxidase was added, and the mixture was incubated for 90 min at room temperature. Finally, 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate was added, and color was evaluated by spectrophotometric analysis at 405 nm.
RESULTS
In vitro induction of TNF-α mRNA and protein expression by ASFV.
To investigate whether ASFV was able to induce the production of TNF-α, comparative PCR analyses were performed on RNA isolated from alveolar macrophages that had been cultured in the presence of ASFV (MOI of 5) or UV-inactivated ASFV or in medium alone (Fig. 1). Total RNA was extracted at 2, 4, 6, 8, and 24 h postinfection (hpi), reversed transcribed into cDNA, and subsequently amplified with TNF-α-specific primers. Equivalent PCR products for G3PDH indicated similar levels of reverse transcription efficiency and similar amounts of input cDNA between samples.
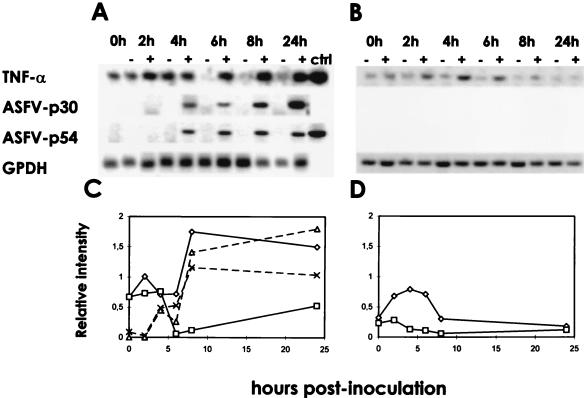
FIG. 1.
Comparison of TNF-α mRNA levels in ASFV-infected and uninfected alveolar macrophages. Alveolar macrophages were infected with ASFV strain E-75 (MOI of 5) (A, + lanes), incubated with an equivalent amount of UV-inactivated ASFV (B, + lanes), or left uninfected (A and B, − lanes). At the times indicated, RNA was purified, reverse transcribed, and amplified by PCR using specific primers. The amplified products were visualized under UV light in 2% agarose gels. Bands were analyzed with an image densitometer coupled to a computer program (C and D). The data correspond to the ratio of the densitometry units of TNF-α (◊, □), p30 (▵), or p54 (×) to those of G3PDH bands; the latter was used as a control (ctrl) for RNA content and integrity.
In ASFV-infected macrophages, expression of TNF-α mRNA rapidly increased at 4 hpi (time at which p30 mRNA became detectable), peaked at 8 hpi, and persisted at least until 24 hpi. In contrast, in cells cultured with medium alone, TNF-α expression was only detectable during the first 4 h. In cells incubated with UV-inactivated virus, an increase in TNF-α expression was also induced but it was clearly shorter and lower than that obtained in infected cultures.
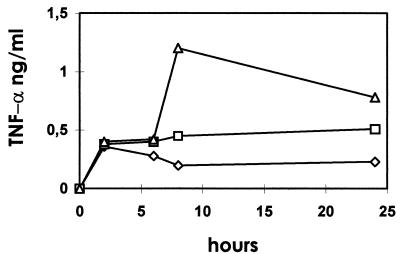
TNF-α protein in culture supernatants harvested at 2, 6, 8, and 24 hpi was determined by ELISA (Fig. 2). As had been observed by RT-PCR, live virus was more effective for induction of TNF-α than the equivalent dose of a UV-inactivated preparation. At 8 hpi, the amount of TNF-α in supernatants of infected cultures was sixfold higher than that in mock-infected cultures, whereas TNF-α in supernatants of cells incubated with UV-inactivated virus was only twofold higher. Production of TNF-α in ASFV-infected cells was also clearly demonstrated by immunocytochemistry by using a cross-reactive polyclonal rabbit anti-bovine TNF-α serum (data not shown).
FIG. 2.
TNF-α production by ASFV-infected macrophages. Alveolar macrophages (3 × 106) were incubated with UV-inactivated (□) or live (▵) ASFV isolate E-75 at an MOI of 5 or mock infected (◊), and their supernatants were harvested at the times postinfection indicated. TNF-α protein levels were quantified by ELISA. The data are means of triplicates of a representative experiment.
Levels of TNF-α in serum of ASFV-infected animals.
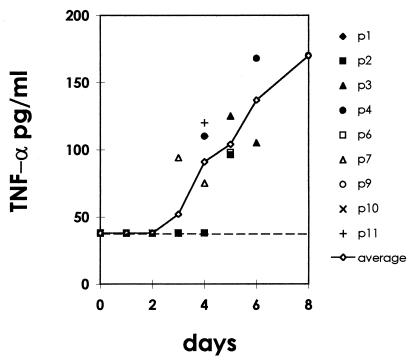
We next analyzed levels of TNF-α in the sera of animals experimentally infected with virulent strain E-75 of ASFV. Before virus inoculation, TNF-α levels in serum were below the detection level of the ELISA (38 pg/ml). In ASFV-infected pigs, at 4 or 5 days postinfection, serum TNF-α increased to values higher than 100 pg/ml, coincident with the appearance of leukopenia and other clinical signs and remained elevated thereafter until the animal died (Fig. 3). No increase in TNF-α levels was observed in the sera of animals inoculated with a saline solution.
FIG. 3.
Concentration of TNF-α in the serum of ASFV-infected animals as determined by ELISA. Data from individual animals are plotted together with the average (solid line). The broken line represents the limit of detection of the ELISA (38 pg/ml).
TNF-α expression in tissues from ASFV-infected animals.
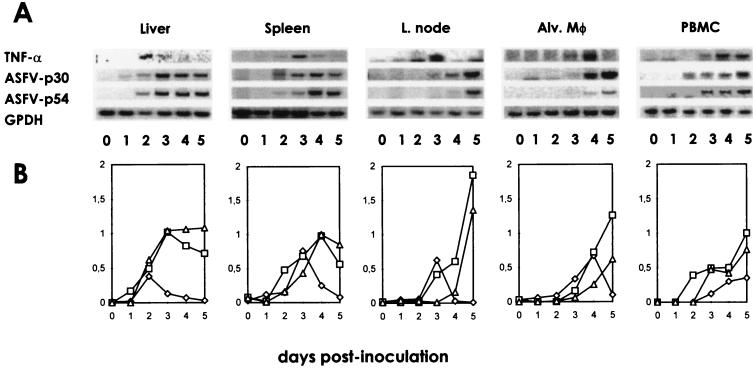
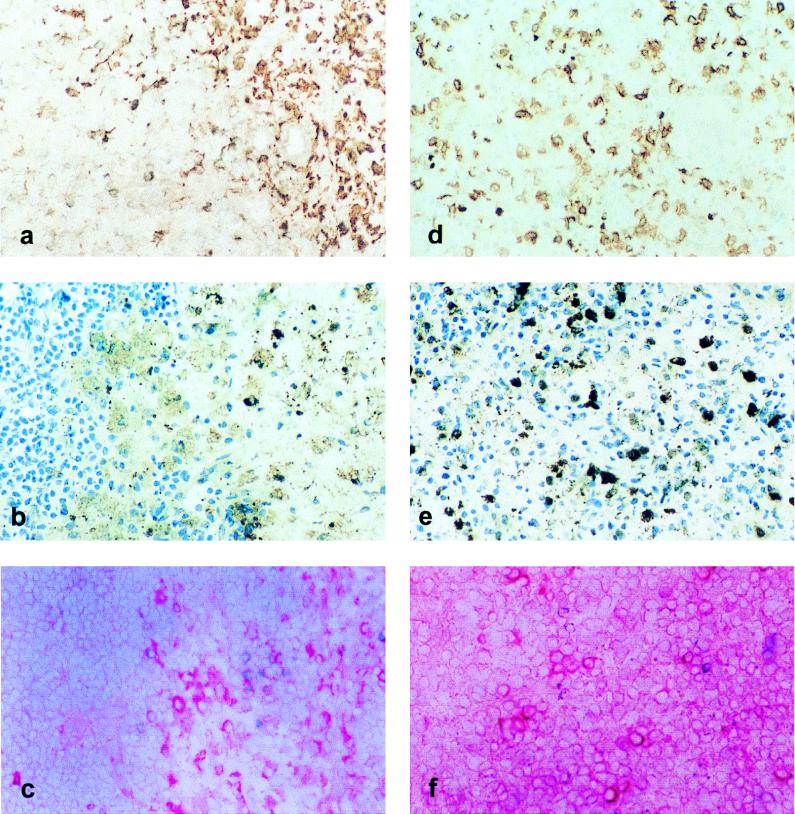
The expression of TNF-α mRNA was also analyzed in spleen, liver, lymph node, alveolar macrophage, and PBMC samples at different days postinfection (dpi) by using the RT-PCR assay (Fig. 4). In ASFV-infected animals, TNF-α transcripts were clearly detectable in the liver at 2 to 3 dpi and in the spleen and mesenteric, submandibular, and mediastinal lymph nodes at 3 dpi, usually corresponding to the time at which mRNA for viral proteins p30 and p54 began to be detectable in these organs. However, whereas p30 and p54 expression increased progressively with time or remained high, TNF-α expression rapidly declined. In alveolar macrophages and PBMC, TNF-α mRNA was detectable slightly later, at 4 dpi. TNF-α-producing cells, showing intense cytoplasmic staining, were detected by immunohistochemistry in the spleens and lymph nodes of ASFV-infected animals but not in those of controls inoculated with a saline solution (Fig. 5). They were detectable in the spleen at 2 dpi and in lymph nodes at 3 dpi, their number increasing on the following day (3 dpi in the spleen and 4 dpi in lymph nodes) and rapidly decreasing thereafter. Most TNF-α-producing cells were located in the red pulp of the spleen and in the sinuses and medulla of the lymph nodes; they exhibited morphological features of macrophages. The majority of cells in these areas expressed the 2A10 antigen, a specific marker for porcine macrophages. These areas also contained most of the ASFV-infected cells. However, cells expressing viral antigens were more numerous than TNF-α-producing cells and could be detected for longer periods.
FIG. 4.
Kinetics of TNF-α mRNA expression in organs of ASFV-infected animals. Total RNA was isolated from different tissues, reverse transcribed, and amplified by PCR using specific primers. The amplified products were visualized under UV light in 2% agarose gels (A). Bands were analyzed with an image densitometer coupled to a computer program (B). The data represent the ratio of the densitometry units of TNF-α (◊), p30 (□), or p54 (▵) to those of G3PDH bands, which was used as a control for RNA content and integrity. L., lymph; Alv. Mφ, alveolar macrophages.
FIG. 5.
TNF-α producing cells in lymphoid organs from ASFV-infected pigs. Panels: a to c, renal lymph nodes; d to f, spleen; a and d, distribution of cells stained with specific macrophage marker 2A10 by using indirect immunoperoxidase staining revealed with DAB and counterstained with methylene blue; b and e, positive staining of infected cells equally labeled with a polyclonal anti-ASFV antibody in a medullar sinus of a renal lymph node (b) and in the splenic red pulp (e); c and f, TNF-α-producing cells labeled with an anti-rBo TNF-α antibody using an indirect alkaline phosphatase technique revealed with fast red. Original magnification, ×250.
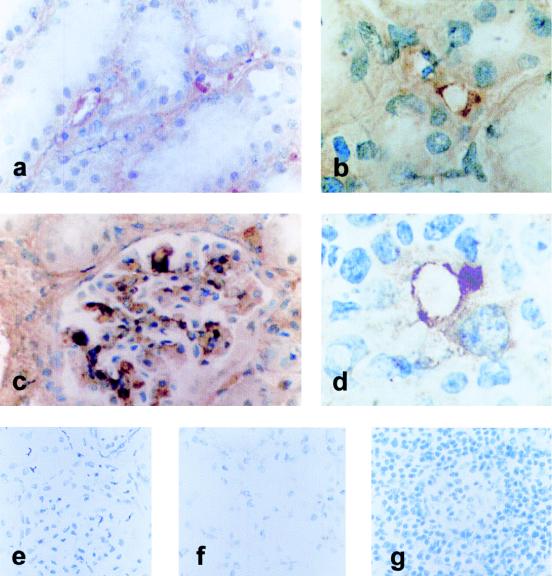
The expression of CD62E (ELAM-1), an activation molecule observed in certain endothelia and induced by TNF-α, was also analyzed. CD62E expression was detected at 3 to 4 dpi in the small vessels of the kidney interstitium and the lymph nodes exclusively in ASFV-infected animals but not in control animals (Fig. 6).
FIG. 6.
CD62E expression in blood vessels from ASFV-infected tissues detected with immunoperoxidase and revealed with DAB. Panels: a and b, staining of small interstitial vessels in intertubular spaces in the kidney; c, strong positive staining in the glomerular capillary tuft in the kidney; d, small vessel in the medulla of a renal lymph node. Magnifications: a and c, ×400; b and d, ×1,000. Control kidney (e and f) and lymph node (g) tissues were treated identically. Original magnification, ×250.
TNF-α production by ASFV-infected macrophages: apoptotic induction in uninfected lymphocytes.
Since ASFV-infected macrophages were shown to produce TNF-α, we tested the potential apoptotic effect of supernatants from infected cells on uninfected swine PBMC. Therefore, PBMC from uninfected pigs were incubated for 0, 6, 12, and 24 h with supernatants of ASFV-infected alveolar macrophages collected at various times (0, 8, and 24 hpi). Recombinant porcine TNF-α and supernatants from alveolar macrophages stimulated for 24 h with LPS were used as positive controls; supernatants of uninfected alveolar macrophages were used as negative controls. Apoptosis was determined by analysis of DNA fragmentation with an ELISA to quantify the histone-associated DNA fraction.
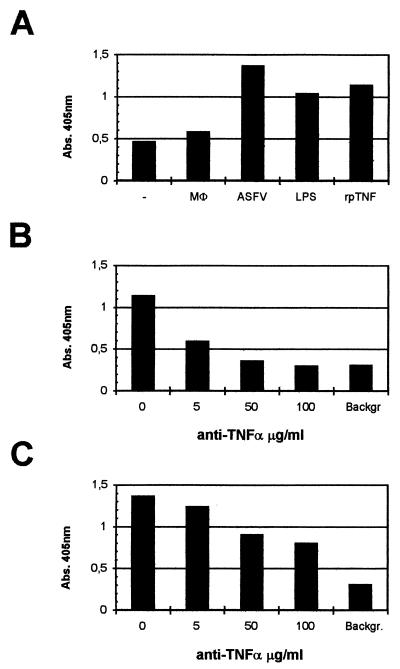
As shown in Fig. 7 (A and C), incubation with supernatants from ASFV-infected macrophages resulted in apoptosis of PBMC, as was observed with supernatants of LPS-stimulated macrophages or recombinant porcine TNF-α. Cell death was observed after 6 (Fig. 7), 12, or 24 h of incubation with supernatants (data not shown) collected at 8 (Fig. 7A and C) or 24 (data not shown) hpi. To confirm the contribution of TNF-α to the apoptotic process, the supernatants were preincubated for 2 h with different amounts of a neutralizing anti-TNF-α MAb previous to the addition to PBMC. In these circumstances, the fraction of dead cells was reduced more than 50%; however, when recombinant porcine TNF-α was preincubated with the neutralizing antibody, cell death was completely abrogated. These results reflect the involvement of TNF-α in lymphocyte apoptosis induced by ASFV-infected macrophage supernatants. However, the incomplete inhibition of cell death observed after anti-TNF-α MAb treatment of these supernatants suggests the presence of additional apoptotic factors.
FIG. 7.
ASFV-induced TNF-α production triggers lymphocyte apoptosis. (A) Apoptotic cell death induced in PBMC after 6 h of incubation with culture supernatants from 8-h ASFV-infected (ASFV), LPS-stimulated (LPS), or uninfected (Mφ) macrophages; recombinant porcine TNF-α (rpTNF); or RPMI medium (−) was measured by quantifying low-molecular-weight DNA linked to histones and represented as absorbance (Abs.) at 405 nm in a specific ELISA. Apoptosis was reduced when recombinant porcine TNF-α (B) or ASFV (C) supernatants were preincubated with a neutralizing anti-TNF-α MAb at the indicated concentrations. The background (Backgr.) is the value obtained when PBMC were incubated with medium and 100-μg/ml MAb.
DISCUSSION
The findings reported here indicate that ASFV infection is associated with the production of TNF-α and suggest a possible contribution of this cytokine to the major clinical manifestations of the disease. First, in vitro ASFV infection of porcine alveolar macrophages resulted in increased expression of TNF-α at both the mRNA and protein levels. Second, high levels of TNF-α protein were detected in the sera of ASFV-infected animals. Third, TNF-α-producing cells were detected in tissues of ASFV-infected animals but not in those from controls.
Tissue macrophages appear to be the main source of TNF-α. Abundant TNF-α-producing cells with the typical morphology of macrophages were detected by immunohistochemistry in the spleens and lymph nodes of ASFV-infected animals. In the different organs analyzed, the increase in TNF-α-specific mRNA correlated with the expression of viral genes encoding p30 or p54. However, TNF-α mRNA decreased to basal levels after 2 days, whereas viral mRNA persisted or increased until death. Similarly, the number of TNF-α-producing cells was always lower than that of ASFV-infected cells. This may reflect the tightly regulated expression of this cytokine or be a result of the cellular shutoff produced by virus infection. This down-regulation may be also due to products released by neighboring cells (IL-10, transforming growth factor β, prostaglandins, etc.) or viral products (i.e., IκB homologue) capable of interfering with TNF-α production. On the other hand, the persistence of high TNF-α levels in serum may be explained by successive waves of viral replication as the virus spreads to different compartments, reflected by the asynchronous TNF-α gene expression in the different organs. In this context, a substantial contribution to the TNF-α levels in serum could be provided by circulating monocytes and neutrophils (13), which have been reported to become infected at a low percentage (30) during the later periods of infection.
Our results are in contrast to those of Powell et al. (29), who found that ASFV inhibited the transcription of TNF-α and other proinflammatory cytokines induced by phorbol myristate acetate (PMA). However, in their experiments, UV-inactivated virus alone was able to stimulate IFN-α and TNF-α secretion. The inhibitory effect observed in infected macrophages was attributed to inactivation by viral protein A238L of host transcription factor NF-κB, which is homologous to porcine IκBα. The discrepancy with our results could be due to the different ASFV isolates tested or the experimental conditions used, particularly to PMA prestimulation of macrophages. Whereas TNF-α induction by PMA seems to be coupled to NF-κB activation (3), ASFV might trigger TNF-α production through an NF-κB-independent pathway. In this regard, the NF-κB binding sites on the human TNF-α promoter do not appear to be required for LPS or virus induction of TNF-α gene expression in vitro (16).
The mechanisms by which ASFV triggers TNF-α production are unknown. The fact that the UV-inactivated virus is able to induce TNF-α production suggests that the virion or viral proteins, either by binding to cell surface receptors or after cell entry, might trigger signals for TNF-α synthesis. However, since cells incubated for 8 h with live virus produce fourfold more TNF-α than cells incubated with the same amount of UV-inactivated virus, additional mechanisms requiring intracellular viral replication, and/or triggered by viral gene products, seem to be required for enhanced TNF-α synthesis.
The IκB homologous protein encoded by the virus has been considered as a strategy to evade the host inflammatory response. However, TNF-α does not show any inhibitory effect on ASFV production in either monocytes or alveolar macrophages (14). On the other hand, a viral mutant with the IκB homologue deleted is the same as the wild type in the ability to replicate in macrophage cell cultures and in infection virulence in domestic pigs (26).
The low rate and lateness of infection of endothelial cells (17) suggest that the coagulation and vascular disorders characteristic of ASF do not depend on the direct effect of the virus on these cells, but more probably they are triggered by factors released by ASF-infected macrophages. In this regard, TNF-α seems a likely candidate. This cytokine exerts important effects on endothelial cells, leading to loss of the anticoagulant activity of the endothelium and increased permeability and inducing the expression of adhesion molecules for leukocytes and platelets (15, 36). In addition, TNF-α can stimulate the synthesis of other cytokines, such as IL-1 or IL-6, which have overlapping effects on the endothelium, and has been implicated as an important mediator of shock and tissue injury resulting from microbial infection. TNF-α also induces a disseminated form of endothelial cell apoptosis involving ceramide generation, and this effect was shown to mediate the endotoxic shock syndrome (19). In ASFV-infected animals, concentrations of TNF-α in serum increased at 3 to 4 dpi and remained elevated until the time of death. These concentrations of TNF-α in serum are similar to those found in other severe viral hemorrhagic diseases, such as those caused by dengue virus, Pichinde virus or Junin virus, where TNF-α levels have been correlated with the severity of disease (1, 20, 21).
High expression of E-selectin was detected in the small vessels of the kidneys and lymph nodes of ASFV-infected animals, which is indicative of an activated state of the endothelium. TNF-α concentrations equivalent to those found in culture supernatants of ASFV-infected macrophages were sufficient to induce expression of E-selectin in in vitro cultures of porcine endothelial cells (4, 34). Other products released by infected cells (i.e., IL-1, oxygen radicals, prostaglandins, etc.) might also contribute to activation of the endothelium, reducing the amount of TNF-α required (7, 15). Local differences in TNF-α synthesis and/or in the response of endothelial cells to this cytokine may explain the absence of E-selectin expression in other organs.
TNF-α has been shown to induce apoptosis in different cell populations (25). Therefore, overproduction of TNF-α may also account for the extensive apoptosis seen in the lymphoid organs in ASF. For instance, spleen sections of acutely ASFV-infected animals, presenting high TNF-α expression in the red pulp, exhibited extensive apoptotic destruction of lymphocytes in the periarterial lymphoid sheath. Recombinant TNF-α added to in vitro lymphocyte cultures at concentrations comparable to those found in culture supernatants of virus-infected macrophages induced apoptosis. Likewise, supernatants from ASFV-infected alveolar macrophages were able to induce apoptosis in lymphocytes. Moreover, preincubation of these supernatants with neutralizing antibodies to TNF-α significantly reduced lymphocyte apoptosis, suggesting that another factor(s) contributes to lymphocyte apoptosis, as has been described in other systems (2, 28).
In conclusion, the results of this study demonstrate overproduction of TNF-α following ASFV infection and suggest the contribution of this cytokine to some of the major clinical manifestations of acute ASF, such as intravascular coagulation, apoptosis, and shock. In vivo treatment with anti-TNF-α neutralizing antibodies or soluble TNF-α receptors will help to determine the relevance of this cytokine in ASF pathogenesis.
ACKNOWLEDGMENTS
This work was supported by CICYT grant BIO97-0402-CO2-01 and INIA grants SC93-155, SC93-160, and SC97-066. M.G.M. was a recipient of a graduate fellowship from the Spanish Ministry of Education.
REFERENCES
- 1.Aronson J F, Herzog N K, Jerrells T R. Tumor necrosis factor and the pathogenesis of Pichinde virus infection in guinea pigs. Am J Trop Med Hyg. 1995;52:262–269. doi: 10.4269/ajtmh.52-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Henkel T. Function and activation of NFκB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Batten P, Yacoub M H, Rose M L. Effect of human cytokines (IFNγ, TNF-α, IL1β, IL-4) on porcine endothelial cells: induction of MHC and adhesion molecules and functional significance of these changes. Immunology. 1996;87:127–133. [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoni G, Kuhnert P, Peterhans E, Pauli U. Improved bioassay for the detection of porcine tumor necrosis factor using a homologous cell line: PK(15) J Immunol Methods. 1993;160:267–271. doi: 10.1016/0022-1759(93)90187-c. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, Cerami A. The biology of cathectin/TNF. A primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 7.Bevilacqua M P, Pober J S, Majeau G R, Fiers W, Cotran R S, Gimbone M A. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci USA. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullido R, Gómez del Moral M, Alonso F, Ezquerra A, Zapata A, Sánchez C, Ortuño E, Alvarez B, Domínguez J. Monoclonal antibodies specific for porcine monocytes/macrophages: macrophage heterogeneity in the pig evidenced by the expression of surface antigens. Tissue Antigens. 1997;49:403–413. doi: 10.1111/j.1399-0039.1997.tb02769.x. [DOI] [PubMed] [Google Scholar]
- 9.Carrascosa A L, Santarén J, Viñuela E. Production and titration of African swine fever virus in porcine alveolar macrophages. J Virol Methods. 1982;3:303–310. doi: 10.1016/0166-0934(82)90034-9. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Colgrove G S, Haelterman E O, Coggins L. Pathogenesis of African swine fever in young pigs. Am J Vet Res. 1969;30:1343–1359. [PubMed] [Google Scholar]
- 12.Domínguez J, Hedrick R P, Sánchez-Vizcaíno J M. Use of monoclonal antibodies for detection of infectious pancreatic necrosis virus by the enzyme-linked immunosorbent assay. Dis Aquat Org. 1990;8:157–163. [Google Scholar]
- 13.Dubravec D B, Spriggs D R, Mannick J A, Rodrick M L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor α. Proc Natl Acad Sci USA. 1990;87:6758–6761. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esparza Y, Gonzalez J C, Viñuela E. Effect of interferon-α, interferon-γ and tumor necrosis factor on African swine fever virus replication in porcine monocytes and macrophages. J Gen Virol. 1988;69:2973–2980. doi: 10.1099/0022-1317-69-12-2973. [DOI] [PubMed] [Google Scholar]
- 15.Feldmann H, Bugany H, Mahner F, Klenk H D, Drenckhahn D, Schnittler H J. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol. 1996;70:2208–2214. doi: 10.1128/jvi.70.4.2208-2214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldfeld A E, Doyle C, Maniatis T. Human tumor necrosis factor α gene regulation by virus and lipopolysaccharide. Proc Natl Acad Sci USA. 1990;87:9769–9773. doi: 10.1073/pnas.87.24.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Villamandos J C, Hervás J, Méndez A, Carrasco L, Martín de las Mulas J, Villeda C J, Wilkinson P J, Sierra M A. Experimental African swine fever: apoptosis of lymphocytes and virus replication in other cells. J Gen Virol. 1995;76:2399–2405. doi: 10.1099/0022-1317-76-9-2399. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez S, Mendoza C, Sánchez-Vizcaíno J M, Alonso F. Inhibitory effect of African swine fever virus on lectin-dependent swine lymphocyte proliferation. Vet Immunol Immunopathol. 1990;26:71–80. doi: 10.1016/0165-2427(90)90133-d. [DOI] [PubMed] [Google Scholar]
- 19.Haimowitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards C K, Schuchman E H, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heller M V, Saavedra M C, Falcoff R, Maiztegui J I, Molinas F C. Increased tumor necrosis factor-α levels in Argentine hemorrhagic fever. J Infect Dis. 1992;166:1203–1204. doi: 10.1093/infdis/166.5.1203. [DOI] [PubMed] [Google Scholar]
- 21.Hober D, Poli L, Roblin B, Gestas P, Chunge E, Granic G, Imbert P, Pecarere J L, Vergez-Pascal R, Wattre P, Maniez-Montreuil M. Serum levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 22.Malmquist W A, Hay D. Hemadsorption and cytopathic effect produced by African swine fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. 1960;21:104–108. [PubMed] [Google Scholar]
- 23.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today. 1997;18:231–240. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 24.Minguez I, Rueda A, Domínguez J, Sánchez-Vizcaíno J M. Double labeling immunohistological study of African swine fever virus-infected spleen and lymph nodes. Vet Pathol. 1988;25:193–198. doi: 10.1177/030098588802500302. [DOI] [PubMed] [Google Scholar]
- 25.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 26.Neilan J G, Lu Z, Kutish G F, Zsak L, Lewis T L, Rock D L. A conserved African swine fever virus IkB homolog, 5EL, is nonessential for growth in vitro and virulence in domestic swine. Virology. 1997;235:377–385. doi: 10.1006/viro.1997.8693. [DOI] [PubMed] [Google Scholar]
- 27.Peterhans E, Jungi T W, Stocker R. Autotoxicity and reactive oxygen in viral disease. In: Cerutti P A, Fridovich I, McCord J M, editors. Oxy-radicals in molecular biology and pathology. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 543–562. [Google Scholar]
- 28.Pitti R M, Marsters S A, Ruppert S, Donahue C J, Moore A, Ashkenazi A. Induction of apoptosis by APO-2 ligand. A new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 29.Powell P P, Dixon L K, Parkhouse R M E. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J Virol. 1996;70:8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramiro-Ibañez F, Escribano J M, Alonso C. Application of monoclonal antibody recognizing protein p30 to detect African swine fever virus-infected cells in peripheral blood. J Virol Methods. 1995;55:339–345. doi: 10.1016/0166-0934(95)00071-1. [DOI] [PubMed] [Google Scholar]
- 31.Ramiro-Ibañez F, Ortega A, Brun A, Escribano J M, Alonso C. Apoptosis: a mechanism of cell killing and lymphoid organ impairment during acute African swine fever virus infection. J Gen Virol. 1996;77:2209–2219. doi: 10.1099/0022-1317-77-9-2209. [DOI] [PubMed] [Google Scholar]
- 32.Ramiro-Ibañez F, Ortega A, Ruiz-Gonzalvo F, Escribano J M, Alonso C. Modulation of immune cell populations and activation markers in the pathogenesis of African swine fever virus infection. Virus Res. 1997;47:31–40. doi: 10.1016/s0168-1702(96)01403-7. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Gonzalvo F, Carnero M E, Caballero C, Martínez J. Inhibition of African swine fever infection in presence of immune sera in vivo and in vitro. Am J Vet Res. 1986;47:1249–1252. [PubMed] [Google Scholar]
- 34.Tsang Y T M, Stephens P E, Licence S T, Haskard D O, Binns R M, Robinson M K. Porcine E-selectin: cloning and functional characterization. Immunology. 1995;85:140–145. [PMC free article] [PubMed] [Google Scholar]
- 35.Unanue E R. Macrophages, antigen-presenting cells, and the phenomena of antigen handling and presentation. In: Paul W, editor. Fundamental immunology, third edition. New York, N.Y: Raven Press; 1993. pp. 111–144. [Google Scholar]
- 36.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 37.Villeda C J, Williams S M, Wilkinson P J, Viñuela E. Consumption coagulopathy associated with shock in acute African swine fever. Arch Virol. 1993;133:467–475. doi: 10.1007/BF01313784. [DOI] [PubMed] [Google Scholar]
- 38.Viñuela E. Molecular biology of African swine fever virus. In: Becker Y, editor. African swine fever virus. Boston, Mass: Martinus Nijhoff Publishers; 1987. pp. 31–50. [Google Scholar]
- 39.Wardley R C, Hamilton F, Wilkinson P J. The replication of virulent and attenuated strains of African swine fever virus in porcine macrophages. Arch Virol. 1979;61:217–225. doi: 10.1007/BF01318056. [DOI] [PubMed] [Google Scholar]
- 40.Zheng L, Fisher G, Miller R, Peschon J, Lynch D, Lenardo M. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]