Abstract
Objective
The objective of this research is to compare primary and salvage intratympanic (IT) steroid treatments in terms of hearing outcomes in patients with idiopathic sudden sensorineural hearing loss (ISSNHL).
Methods
The patients were randomized into two (primary and salvage) groups. Both groups received systemic steroid treatment for 2 weeks. The primary group also received IT dexamethasone injection three times during the treatment period, whereas the salvage group received IT dexamethasone injection only if no or slight recovery was noted at the 2‐week follow‐up. If needed, salvage steroid injection was administered three times during the following 2 weeks. Hearing recovery was analyzed according to the modified American Academy of Otolaryngology‐Head and Neck Surgery criteria.
Results
The degrees of hearing improvement at the 3‐month follow‐up were similar in the two groups. Compared with baseline, the pure‐tone average values and speech discrimination scores improved by 38.45 ± 21.95 dB HL and 34.32% ± 30.55%, respectively, in the primary group and 36.80 ± 22.33 dB HL and 31.87% ± 27.88%, respectively, in the salvage group (p = .762 and .659, respectively). In addition, the complete or partial hearing recovery rates were also similar in the primary and salvage groups (67.7% vs. 73.3%, respectively; p = .780). In the salvage group, 18 patients required no IT steroid injection because they recovered after systemic steroid treatment.
Conclusion
Primary and salvage IT steroid treatments for ISSNHL led to similar outcomes. In summary, salvage IT steroid injection is recommended for patients with ISSNHL patients to prevent unnecessary IT injection.
Level of evidence
2.
Keywords: intratympanic, salvage treatment, sensorineural hearing loss, steroid injection, sudden hearing loss
Intratympanic steroid treatment for ISSNHL should be administered to only patients with incomplete recovery after conventional systemic steroid treatment.

1. INTRODUCTION
Sudden sensorineural hearing loss is a common condition defined as the sudden onset of sensorineural hearing loss of >30 dB within ≤3 days across at fewest three contiguous frequencies. 1 Approximately 90% of all cases are idiopathic at presentation. 2 The incidence of idiopathic sudden sensorineural hearing loss (ISSNHL) has been reported to be 5–20 per 100,000 persons per year. 3 However, the actual incidence may be considerably higher than the reported value because of underdiagnosed cases, which are resolved spontaneously. The rate of spontaneous recovery ranges from 32% to 65%. 4
Systemic steroid treatment (intravenous or peroral) is the mainstay therapy for ISSNHL. 5 , 6 However, intratympanic (IT) steroid injection has been indicated as an alternative to systemic steroid treatment for ISSNHL. 7 , 8 Some studies have reported higher rates of hearing recovery in patients with ISSNHL receiving both systemic steroid treatment and IT steroid injection than in those receiving systemic steroids alone. 9 , 10 Other studies have indicated high efficacy of IT steroid injection in patients with ISSNHL not responding to systemic steroid treatment. 11 , 12 The clinical practice guideline outlined by the American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS) recommends IT steroid therapy for patients with incomplete recovery after systemic steroid treatment. 5 However, we found no standard protocol for IT steroid treatment in the literature. 13 , 14 Considerable differences among countries in clinical practices for IT steroid treatment for ISSNHL were noted. 15
There is limited data available regarding the efficacy of IT steroid as a salvage therapy for patients who do not respond adequately to systemic steroid treatment. IT steroid injection is administered as either primary or salvage treatment at the discretion of clinicians. Therefore, we conducted the present study to compare primary and salvage IT steroid treatments in terms of hearing outcomes in patients with ISSNHL.
2. METHODS
2.1. Participants and ethics
This prospective randomized controlled trial analyzed patients with ISSNHL who were admitted to a single tertiary hospital in Taiwan between 2021 and 2022. The inclusion criteria were as follows: (1) acute onset of ≥30 dB unilateral sensorineural hearing loss within ≤3 days across at fewest three contiguous frequencies; (2) symptom onset within ≤7 days; (3) initial pure‐tone average (PTA) of >40 dB HL; (4) age of ≥18 years; (5) no identifiable cause of hearing impairment; (6) no recurrent sudden hearing loss; and (7) no neurological signs other than dizziness, vertigo, or tinnitus. In addition, we excluded patients with contraindication for steroid use, a problem in the middle ear, a history of surgery involving the affected ear, or no willingness to participate. Figure 1 depicts the study flowchart. This study was approved by the Institutional Review Board of China Medical University Hospital (approval number: CMUH110‐REC3‐139). All procedures were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all the participants.
FIGURE 1.
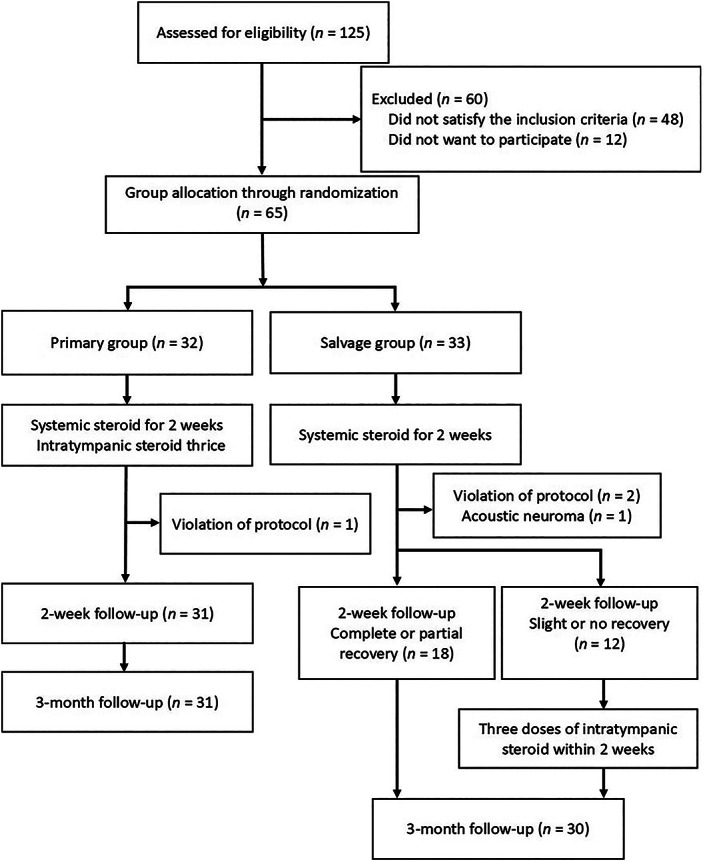
Study flowchart. Hearing recovery was defined according to the modified American Academy of Otolaryngology‐Head and Neck Surgery criteria.
2.2. Randomization and treatment
The participants were randomized into two (primary and salvage) groups. Simple randomization was performed using computer‐generated random numbers. All the patients were hospitalized; they received intravenous low‐molecular‐weight dextran (100 g daily) for 5 consecutive days plus intravenous hydrocortisone (300 mg daily) for the first 3 days followed by oral prednisolone on the fourth (60 mg) and fifth (50 mg) days. After discharge on the sixth day, the patients started receiving 40 mg oral prednisolone for 1 day, which was then tapered (10 mg daily) to a maintenance dose of 10 mg/day. The total duration of systemic steroid treatment was 2 weeks. The primary group simultaneously received IT dexamethasone injection three times during the treatment period, whereas the salvage group received IT dexamethasone injection only if no or slight recovery was noted at the 2‐week follow‐up. For the patients who required salvage IT steroid treatment, injection was administered three times in the following 2 weeks.
IT steroid injection was administered during hospitalization or in the outpatient setting. Patients received IT steroid injection without any analgesia or with 10% lidocaine (pump spray; xylocaine; dose, 10 mg; Mey See Pharm, Kaohsiung, Taiwan) for pain control. Under microscopic guidance, 0.4–0.8 mL of dexamethasone sodium phosphate (5 mg/mL; Taiwan Veterans Pharm, Taoyuan, Taiwan) was injected into the middle ear cavity through the anteroinferior quadrant of the tympanic membrane by using a 25‐gauze needle. After administration, the patients were instructed to remain in the supine position with the affected ear facing upward for 30 min and to avoid speaking, yawning, or swallowing, which could have promoted steroid passage across the round window membrane and avoided steroid leakage into the Eustachian tube.
2.3. Data collection and analysis
The following data were collected: age, sex, affected ear, period from hearing loss onset to therapy initiation, associated symptoms (tinnitus, dizziness, or vertigo), comorbidities, and initial audiogram patterns. Audiogram patterns were categorized according to Sheehy classification: low‐tone loss, high‐tone loss, flat loss, and total loss. 16 Audiometric (pure‐tone and speech) data were obtained upon admission (before the initiation of treatment); on the day of discharge; at the 2‐week follow‐up; and at the 1‐, 2‐, and 3‐month follow‐ups. Air conduction thresholds were recorded at 0.25, 0.5, 1, 2, 4, and 8 kHz. The PTA was the average value of the 0.5‐, 1‐, 2‐, and 4‐kHz thresholds of air conduction. The speech discrimination score (SDS) was calculated at each follow‐up.
We compared the patients' hearing test results before the treatment with those obtained after 2 weeks and 3 months of follow‐up. The groups were compared in terms of the improvements in their PTA values and SDSs. Furthermore, pure‐tone threshold improvements for each tone (0.25, 0.5, 1, 2, 4, and 8 kHz) were recorded and compared between the groups. Hearing recovery was classified according to the modified AAO‐HNS criteria: complete recovery, PTA within 10 dB and SDS within 5%–10% of the corresponding values of the unaffected ear; partial recovery, improvement of >10 dB in PTA or >10% in SDS, PTA of ≤50 dB, and SDS of >50%; slight recovery, improvement of >10 dB in PTA or >10% in SDS, PTA of >50 dB, and SDS of ≤50%; and no recovery, improvement of <10 dB in PTA and <10% in SDS. 5 , 17 The initial hearing level of the unaffected ear was used as the baseline value for evaluating hearing recovery. The rate of hearing recovery was compared between the groups. Subgroup analysis was performed by comparing the hearing results of patients with severe to profound hearing loss (PTA > 70 dB HL). Early recovery was defined as partial or complete recovery at the 2‐week follow‐up.
2.4. Statistical analysis
Statistical analyses were performed using SPSS (version 25.0; IBM Corporation, Armonk, NY, USA). The Mann–Whitney U test was used to compare numerical variables between the groups. Fisher's exact test was used for categorical variables. A p value of <.05 indicated statistical significance.
3. RESULTS
3.1. Patient demographics
In total, 125 patients were assessed for eligibility; from these patients, 60 were excluded (Figure 1). Thus, 65 patients (age range, 18–83 years) were included in the analysis. After randomization, 32 and 33 were in the primary and salvage groups, respectively. A total of 3 patients (primary group, 1; salvage group, 2) were excluded because of treatment protocol violations. A salvage group patient was excluded because of a diagnosis of acoustic neuroma. Thus, 31 primary group patients (mean age, 48.4 ± 12.7 years) and 30 salvage group patients (mean age, 49.9 ± 12.8 years) were included in the final analysis. Table 1 presents the patient demographics and baseline auditory data. No significant between‐group difference was noted before the treatment.
TABLE 1.
Patient demographics and baseline auditory data a .
| Variable | Primary group | Salvage group | p value |
|---|---|---|---|
| (n = 31) | (n = 30) | ||
| Age (years) | 48.4 ± 12.7 | 49.9 ± 12.8 | .762 |
| Sex (male/female) | 14:17 (45.2:54.8) | 15:15 (50.0:50.0) | .800 |
| Affected ear (left/right) | 16:15 (51.6:48.4) | 18:12 (60.0:40.0) | .609 |
| Onset (days) | 3.7 ± 2.0 | 4.2 ± 2.1 | .286 |
| Dizziness or vertigo | 17 (54.8) | 17 (56.7) | 1.000 |
| Tinnitus | 29 (93.5) | 25 (83.3) | .255 |
| Diabetes mellitus | 3 (9.7) | 2 (6.7) | 1.000 |
| Hypertension | 6 (19.4) | 7 (23.3) | .762 |
| Hyperlipidemia | 3 (9.7) | 2 (6.7) | 1.000 |
| Initial audiogram patterns b | |||
| Low‐tone loss | 3 (9.7) | 5 (16.7) | .901 |
| High‐tone loss | 6 (19.4) | 6 (20.0) | |
| Flat loss | 15 (48.4) | 13 (43.3) | |
| Total loss | 7 (22.6) | 6 (20.0) | |
| Initial PTA (dB) of the affected ear | 80.4 ± 17.1 | 76.4 ± 18.9 | .359 |
| Initial SDS (%) of the affected ear | 48.9 ± 30.7 | 53.9 ± 31.6 | .432 |
| PTA (dB) of the unaffected ear | 18.1 ± 8.4 | 18.6 ± 7.8 | .851 |
| SDS (%) of the unaffected ear | 100 ± 0 | 100 ± 0 | 1.000 |
Abbreviations: PTA, pure‐tone average; SDS, speech discrimination score.
Data are presented as mean ± standard deviation values or numbers with percentage values in parentheses.
Audiogram patterns were categorized according to the Sheehy classification.
3.2. Treatment outcomes
In the salvage group, 18 patients (60%) had complete or partial recovery at the 2‐week follow‐up and thus required no IT steroid injection. However, 12 patients (40%) in the salvage group showed slight or no recovery at the 2‐week follow‐up; therefore, they received salvage IT steroid injection 3 times in the following 2 weeks.
At the 2‐week follow‐up, significant improvements were noted in the average PTA values of the primary and salvage groups (29.9 ± 24.1 and 28.3 ± 21.4 dB, respectively); however, the between‐group difference was nonsignificant (p = .734). The improvements in the SDSs of the primary and salvage groups were 25.9% ± 32.5% and 24.0% ± 27.1%, respectively; however, the between‐group difference was nonsignificant (p = .761). At the 2‐week follow‐up, the rates of complete, partial, slight, and no recovery were 29.0%, 29.0%, 12.9%, and 29.0% in the primary group and 33.3%, 26.7%, 13.3%, and 26.7% in the salvage group, respectively. The early recovery (complete and partial) rates were 58.1% and 60.0% in the primary and salvage groups, respectively; no significant between‐group difference was observed (p = 1.000). Table 2 shows the hearing gain and recovery results of the primary and salvage groups at the 2‐week follow‐up.
TABLE 2.
Hearing recovery after 2 weeks and 3 months of treatment a .
| Variable | 2‐week follow‐up | 3‐month follow‐up | ||||
|---|---|---|---|---|---|---|
| Primary group (n = 31) | Salvage group (n = 30) | p value | Primary group (n = 31) | Salvage group (n = 30) | p value | |
| PTA improvement (dB) | 29.9 ± 24.1 | 28.3 ± 21.4 | .734 | 38.5 ± 22.0 | 36.8 ± 22.3 | .762 |
| SDS improvement (%) | 25.9 ± 32.5 | 24.0 ± 27.1 | .761 | 34.3 ± 30.6 | 31.9 ± 27.9 | .659 |
| Complete recovery b | 9 (29.0) | 10 (33.3) | .786 | 14 (45.2) | 17 (56.7) | .446 |
| Partial recovery b | 9 (29.0) | 8 (26.7) | 1.000 | 7 (22.6) | 5 (16.7) | .749 |
| Slight recovery b | 4 (12.9) | 4 (13.3) | 1.000 | 6 (19.4) | 4 (13.3) | .731 |
| No recovery b | 9 (29.0) | 8 (26.7) | 1.000 | 4 (12.9) | 4 (13.3) | 1.000 |
| Complete and partial recovery | 18 (58.1) | 18 (60.0) | 1.000 | 21 (67.7) | 22 (73.3) | .780 |
| Complete, partial, and slight recovery | 22 (71.0) | 22 (73.3) | 1.000 | 27 (87.1) | 26 (86.7) | 1.000 |
Abbreviations: PTA, pure‐tone average; SDS, speech discrimination score.
Data are presented as mean ± standard deviation values or numbers with percentage values in parentheses.
Treatment outcomes were analyzed according to the modified American Academy of Otolaryngology‐Head and Neck Surgery criteria.
At the 3‐month follow‐up, significant improvements were noted in the average PTA values of both the primary and salvage groups (38.5 ± 22.0 and 36.8 ± 22.3 dB, respectively); however, no significant between‐group difference was noted (p = .762; Figure 2). The improvements in the SDSs of the primary and salvage groups were 34.3% ± 30.6% and 31.9% ± 27.9%, respectively; however, no significant between‐group difference was noted (p = .659). For 0.25, 0.5, 1, 2, 4, and 8 kHz, the average hearing gains in terms of PTA were 37.8 ± 29.8, 40.8 ± 27.1, 40.5 ± 24.5, 39.0 ± 25.5, 33.4 ± 23.4, and 19.4 ± 17.8 dB in the primary group and 38.5 ± 26.1, 45.7 ± 24.7, 41.7 ± 26.1, 34.3 ± 24.3, 25.0 ± 20.7, and 21.7 ± 17.7 dB in the salvage group, respectively. No significant between‐group difference was noted for hearing improvement (Figure 3) or recovery (Table 2). The rates of complete, partial, slight, and no recovery were 45.2%, 22.6%, 19.4%, and 12.9% in the primary group and 56.7%, 16.7%, 13.3%, and 13.3% in the salvage group, respectively. At the 3‐month follow‐up, the overall recovery (complete and partial) 17 rates were 67.7% and 73.3% in the primary and salvage groups, respectively; no significant between‐group difference was observed (p = .780). Moreover, no significant between‐group difference was noted in the rate of complete, partial, or slight recovery (p = 1.000).
FIGURE 2.

PTA values of the primary and salvage groups before treatment and after 2 weeks and 1, 2, and 3 months of treatment. In both groups, significant improvement was noted in the average PTA value at the 3‐month follow‐up (***p < .001). However, no significant between‐group difference was noted (p > .05). PTA, pure‐tone average.
FIGURE 3.
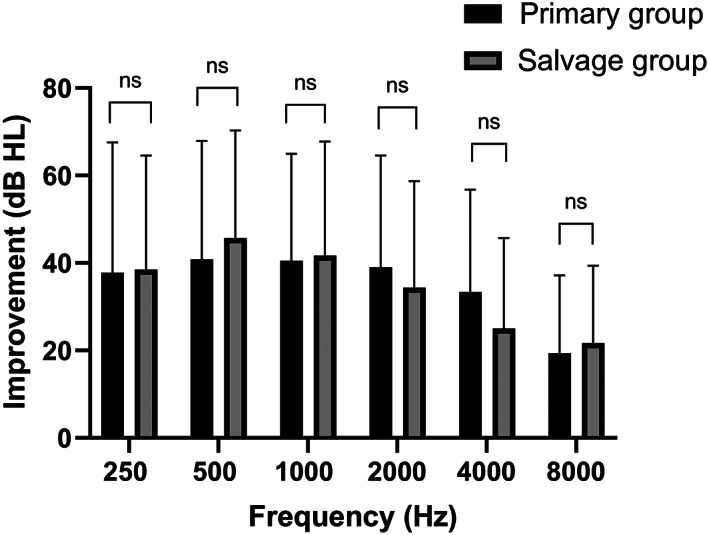
Improvement in hearing threshold (in terms of frequency) after 3 months of treatment. No significant between‐group difference was noted (p > .05).
A total of 43 patients (primary group, 31; salvage group, 12) received IT steroid injection. Postinjection transient dizziness was noted in 7 of these patients (16.3%), and postinjection vertigo was observed in 1 (2.3%); this patient had complete recovery within 3 h after receiving the injection. Twenty‐five (58.1%) patients had mild, tolerable pain during the procedure. Tongue numbness was reported by 1 patient (2.3%); this patient had complete recovery 1 week posttreatment.
3.3. Subgroup analysis results
Table 3 presents the results of subgroup analysis for patients with severe to profound hearing loss (PTA > 70 dB HL). At the 2‐week and 3‐month follow‐ups, no significant between‐group difference was noted for PTA or SDS improvement. The early recovery rates were 50% and 38.9% in the primary and salvage groups, respectively (p = .532). At the 3‐month follow‐up, the overall recovery rates were 42.9% and 45.8% in the primary and salvage groups, respectively (p = 1.000). No significant between‐group difference was noted for the rate of hearing recovery.
TABLE 3.
Hearing recovery after 2 weeks and 3 months of treatment for severe to profound idiopathic sudden sensorineural hearing loss a .
| Variable | 2‐week follow‐up | 3‐month follow‐up | ||||
|---|---|---|---|---|---|---|
| Primary group (n = 20) | Salvage group (n = 18) | p value | Primary group (n = 20) | Salvage group (n = 18) | p value | |
| PTA improvement | 34.76 ± 26.07 | 29.65 ± 24.16 | .432 | 44.05 ± 22.07 | 43.35 ± 23.71 | .954 |
| SDS improvement | 36.38 ± 34.13 | 34.35 ± 32.41 | .601 | 47.43 ± 27.72 | 47.53 ± 28.00 | .977 |
| Complete and partial recovery b | 10 (50.0) | 7 (38.9) | .532 | 6 (42.9) | 11 (45.8) | 1.000 |
Abbreviations: PTA, pure‐tone average; SDS, speech discrimination score.
Data are presented as mean ± standard deviation values or numbers with percentage values in parentheses.
Treatment outcomes were analyzed according to the modified American Academy of Otolaryngology‐Head and Neck Surgery criteria.
4. DISCUSSION
We compared primary and salvage IT steroid treatments for ISSNHL and found that primary IT steroid injection was not superior to salvage IT steroid injection. In addition, no considerable improvement in early or overall hearing recovery, evaluated in terms of PTA and SDS and assessed according to the modified AAO‐HNS criteria, was observed in the patients receiving primary IT steroid injection compared with those receiving salvage IT injection. In the patients with severe to profound ISSNHL, the two treatments led to similar hearing outcomes.
The incorporation of IT steroid injection into systemic steroid treatment increases the likelihood of hearing recovery in patients with ISSNHL. 9 , 10 Battaglia et al. found that patients receiving a combination therapy (IT steroid and high‐dose prednisolone taper) exhibited, on average, 44% improvement in SDS and 40 dB improvement in PTA after 4 weeks of treatment. 10 The combination therapy significantly improved the patients' SDSs; a higher proportion of patients receiving the combination therapy exhibited substantial improvements in PTA (>15 dB) compared with those receiving prednisolone alone. 10 In their prospective study, Arslan et al. reported that the average PTA gains after 2 weeks of treatment were higher in patients receiving a combination therapy than in those receiving systemic steroid alone (21.8 and 13.0 dB, respectively). 9 On the basis of the improvements in hearing outcomes (>10 dB gain for PTA), the rate of hearing recovery was higher in the combination therapy group than in the systemic steroid alone group. 9 However, Ahn et al. found that the total rate of hearing recovery (more than slight recovery according to Siegel's criteria) after 3 months of treatment was 73.3% in a combination therapy group and 70% in a systemic steroid alone group; thus, they concluded that the incorporation of IT steroid treatment into systemic steroid treatment did not enhance hearing outcomes in patients with ISSNHL. 18
The use of IT steroid injection as a salvage treatment after systemic steroid treatment for ISSNHL has been proposed in several studies. 4 , 11 , 19 , 20 The AAO‐HNS clinical practice guideline for sudden hearing loss recommends IT steroid treatment for patients with ISSNHL who have incomplete recovery after conventional systemic steroid treatment. However, a key problem is that the definition of hearing recovery varies across trials. 5 A study exploring the real‐world clinical practice of IT steroid injection for ISSNHL revealed wide differences across countries: 41% of UK clinicians reported prescribing IT steroids with oral steroids as the first‐line therapy, 86% of US clinicians reported prescribing IT steroids with systemic steroids as the first‐line therapy, and 74% of Austrian or German clinicians reported not prescribing IT steroids as the first‐line therapy. 14 These findings indicate the need for a standard protocol for IT steroid treatment.
Few studies have compared simultaneous systemic plus IT steroid treatment and salvage IT steroid treatment alone in terms of their efficacy. In our study, the primary and salvage groups did not differ in terms of demographics, auditory data, or initial audiogram patterns. Compared with other patterns, a low‐tone loss pattern indicated improved prognosis. 16 We found no significant between‐group difference in the distribution of the initial audiometric patterns, which suggests high reliability in our findings. In addition, our study revealed that primary IT steroid injection may not be superior to salvage IT steroid injection. Overall recovery rates were similar between the primary and salvage groups (67.7% vs 73.3%, respectively; p = .780). This finding corroborates that of a prospective randomized controlled trial indicating that simultaneous (primary) use of IT dexamethasone did not result in more hearing gain, earlier recovery, or higher overall recovery rate compared with the outcomes of subsequent (salvage) use of IT dexamethasone. 21 The aforementioned study also reported that at the 3‐month follow‐up, the overall recovery rates were 63.6% and 56.8% in the primary and salvage groups, respectively; no significant between‐group difference was noted. 21
The present study found no significant between‐group difference in hearing gain, early recovery, or overall recovery for patients with severe to profound hearing loss. The early and overall recovery rates were 50% and 42.9% in the primary group and 38.9% and 45.8% in the salvage group (p = .532 and 1.000), respectively. Our findings are consistent with those of Yoo et al., who compared primary and salvage IT steroid treatments in terms of their efficacy against severe to profound ISSNHL. 17 They reported that the patients had complete or partial recovery at the 2‐month follow‐up according to modified AAO‐HNS criteria. The recovery rates were 33% and 35% in the primary and salvage groups, respectively; the between‐group difference was nonsignificant. 17
In our study, some complications were noted during IT dexamethasone injection; these problems included transient dizziness (16.3%), postinjection vertigo (2.3%), mild painful sensation (58.1%), and tongue numbness (2.3%). Similar complications were reported by Liu et al., namely tinnitus (5.4%), transient dizziness (16.9%), postinjection vertigo (1.8%), mild (tolerable) pain (75.9%), tongue numbness (0.7%), and persisting eardrum perforation (1.7%). 22 Most of our patients had no or very few complications during IT steroid treatment, and such problems can be avoided. Eighteen of the patients (60%) in the salvage group had complete or partial recovery at the 2‐week follow‐up; therefore, these patients did not receive any IT steroid injection. Clinicians should weigh the benefits and harms of IT steroid injection before prescribing it to treat ISSNHL.
Our study had some limitations. First, the sample size was small; hence, we could not identify any significant relationships. Nevertheless, we used nonparametric analysis methods in this study to reduce the risk of inaccurate conclusions. Second, the combined use of low‐molecular‐weight dextran might have influenced the outcomes. Nevertheless, we used dextran in both groups to reduce any likely biases. Finally, because of the lack of a standard definition for hearing recovery, comparing studies is challenging 5 , 23 ; in addition, this deficiency leads to wide variation in the protocol for IT steroid treatment. 24 Thus, in the future, a standard definition of hearing recovery must be proposed; furthermore, large, multicenter randomized controlled trials should use this definition to comprehensively compare primary and salvage IT steroid treatments in terms of their efficacy.
5. CONCLUSION
In this study, primary and salvage IT dexamethasone treatments led to similar hearing outcomes in patients with ISSNHL. To avoid unnecessary postinjection complications, IT steroid treatment should be administered to only patients with incomplete recovery after conventional systemic steroid treatment.
FUNDING INFORMATION
The authors have no funding or financial relationship to disclose.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank our colleagues at the Department of Otolaryngology Head and Neck Surgery, China Medical University Hospital, for providing insightful suggestions and valuable assistance. This manuscript was edited by Wallace Academic Editing.
Lan W‐C, Lin C‐D, Tsou Y‐A, et al. Primary versus salvage intratympanic steroid treatment for idiopathic sudden sensorineural hearing loss. Laryngoscope Investigative Otolaryngology. 2023;8(4):1029‐1035. doi: 10.1002/lio2.1088
REFERENCES
- 1. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1‐S35. [DOI] [PubMed] [Google Scholar]
- 2. Khater A, El‐Anwar MW, Nofal AA, Elbahrawy AT. Sudden sensorineural hearing loss: comparative study of different treatment modalities. Int Arch Otorhinolaryngol. 2018;22:245‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie W, Dai Q, Liu J, Liu Y, Hellström S, Duan M. Analysis of clinical and laboratory findings of idiopathic sudden sensorineural hearing loss. Sci Rep. 2020;10:6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salvador P, Moreira da Silva F, Fonseca R. Idiopathic sudden sensorineural hearing loss: effectiveness of salvage treatment with low‐dose intratympanic dexamethasone. J Otol. 2021;16:6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161:S1‐S45. [DOI] [PubMed] [Google Scholar]
- 6. Leung MA, Flaherty A, Zhang JA, Hara J, Barber W, Burgess L. Sudden sensorineural hearing loss: primary care update. Hawaii J Med Public Health. 2016;75:172‐174. [PMC free article] [PubMed] [Google Scholar]
- 7. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071‐2079. [DOI] [PubMed] [Google Scholar]
- 8. Plontke SK, Meisner C, Agrawal S, et al. Intratympanic corticosteroids for sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2022;7:Cd008080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arslan N, Oğuz H, Demirci M, et al. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2011;32:393‐397. [DOI] [PubMed] [Google Scholar]
- 10. Battaglia A, Burchette R, Cueva R. Combination therapy (intratympanic dexamethasone + high‐dose prednisone taper) for the treatment of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2008;29:453‐460. [DOI] [PubMed] [Google Scholar]
- 11. Plaza G, Herraiz C. Intratympanic steroids for treatment of sudden hearing loss after failure of intravenous therapy. Otolaryngol Head Neck Surg. 2007;137:74‐78. [DOI] [PubMed] [Google Scholar]
- 12. Lee JB, Choi SJ, Park K, Park HY, Choo OS, Choung YH. The efficiency of intratympanic dexamethasone injection as a sequential treatment after initial systemic steroid therapy for sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2011;268:833‐839. [DOI] [PubMed] [Google Scholar]
- 13. Bear ZW, Mikulec AA. Intratympanic steroid therapy for treatment of idiopathic sudden sensorineural hearing loss. Mo Med. 2014;111:352‐356. [PMC free article] [PubMed] [Google Scholar]
- 14. Lechner M, Sutton L, Ferguson M, Abbas Y, Sandhu J, Shaida A. Intratympanic steroid use for sudden sensorineural hearing loss: current otolaryngology practice. Ann Otol Rhinol Laryngol. 2019;128:490‐502. [DOI] [PubMed] [Google Scholar]
- 15. Wu Y, Song Z, Wang Y, et al. Optimal timing of salvage intratympanic steroids in idiopathic sudden sensorineural hearing loss. Laryngoscope Investig Otolaryngol. 2022;7:1559‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang NC, Ho KY, Kuo WR. Audiometric patterns and prognosis in sudden sensorineural hearing loss in southern Taiwan. Otolaryngol Head Neck Surg. 2005;133:916‐922. [DOI] [PubMed] [Google Scholar]
- 17. Yoo MH, Lim WS, Park JH, et al. Simultaneous versus sequential intratympanic steroid treatment for severe‐to‐profound sudden sensorineural hearing loss. Audiol Neurootol. 2016;21:399‐405. [DOI] [PubMed] [Google Scholar]
- 18. Ahn JH, Yoo MH, Yoon TH, Chung JW. Can intratympanic dexamethasone added to systemic steroids improve hearing outcome in patients with sudden deafness? Laryngoscope. 2008;118:279‐282. [DOI] [PubMed] [Google Scholar]
- 19. Ng JH, Ho RC, Cheong CS, Ng A, Yuen HW, Ngo RY. Intratympanic steroids as a salvage treatment for sudden sensorineural hearing loss? A meta‐analysis. Eur Arch Otorhinolaryngol. 2015;272:2777‐2782. [DOI] [PubMed] [Google Scholar]
- 20. Dispenza F, De Stefano A, Costantino C, Marchese D, Riggio F. Sudden sensorineural hearing loss: results of intratympanic steroids as salvage treatment. Am J Otolaryngol. 2013;34:296‐300. [DOI] [PubMed] [Google Scholar]
- 21. Park MK, Lee CK, Park KH, Lee JD, Lee CG, Lee BD. Simultaneous versus subsequent intratympanic dexamethasone for idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2011;145:1016‐1021. [DOI] [PubMed] [Google Scholar]
- 22. Liu YC, Chi FH, Yang TH, Liu TC. Assessment of complications due to intratympanic injections. World J Otorhinolaryngol Head Neck Surg. 2016;2:13‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue DP, Bogaz EA, Barros F, Penido NO. Comparison of hearing recovery criteria in sudden sensorineural hearing loss. Braz J Otorhinolaryngol. 2012;78:42‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferri E, Frisina A, Fasson AC, Armato E, Spinato G, Amadori M. Intratympanic steroid treatment for idiopathic sudden sensorineural hearing loss after failure of intravenous therapy. ISRN Otolaryngol. 2012;2012:647271. [DOI] [PMC free article] [PubMed] [Google Scholar]


