Abstract
Objective
To characterize migraine pathophysiology, presentation, and current treatment strategies, specifically in regard to vestibulocochlear manifestations of migraine.
Methods
Narrative review of available literature.
Results
Migraine disorder can be described as a spectrum of otologic manifestations, with vestibular migraine now recognized with fully‐fledged diagnostic criteria. Otologic manifestations are theorized to be due, in part, to trigeminal innervation of the inner ear structures and calcitonin gene‐related peptide (CGRP) expression within the labyrinth. Patients can experience vertigo, aural fullness, enhanced tinnitus, and hearing loss without the characteristic migraine headache, leading to under recognition of these symptoms as migraine‐related. Meniere's disease, mal de débarquement syndrome, persistent postural perceptual dizziness, and recurrent benign paroxysmal positional vertigo have close associations to migraine and may exist on the migraine spectrum. Migraine treatment consists of two goals: halting acute attacks (abortive therapy) and preventing attacks (prophylactic therapy). Abortive medications include triptans, corticosteroids, anti‐histamines, and anti‐emetics. Pharmacologic prophylaxis in conjunction with lifestyle modifications can decrease frequency and severity of symptoms and include tricyclic antidepressants, calcium channel blockers, anti‐epileptic medications, selective serotonin reuptake inhibitors, serotonin‐norepinephrine reuptake inhibitors, beta‐blockers, gepants, and monoclonal antibodies to CGRP. Promising evidence is emerging regarding the ability of migraine medications to positively treat the various otologic symptoms of migraine.
Conclusion
Migraine disorder manifesting with primarily cochleovestibular symptoms can be challenging to diagnose and manage for practicing clinicians. Patients with various vestibulopathies that are closely related to migraine may benefit from migraine treatment. Lifestyle choices and prophylactic medications are key to satisfactorily preventing acute migrainous attacks and improve function.
Keywords: lifestyle modifications, migraine diagnosis and evaluation, migraine spectrum, prophylactic medications, tinnitus, vestibular migraine, vestibulocochlear migraine
Migraine is a spectrum of diseases and can manifest with otologic symptoms, including vertigo, disequilibrium, hearing loss, aural fullness, and enhancement of tinnitus. Pharmacologic prophylaxis combined with lifestyle modifications have been shown to decrease the frequency and severity of vestibular migraine attacks. Meniere's disease, recurrent benign paroxysmal positional vertigo, and persistent postural perceptual dizziness are vestibulopathies that may benefit from migraine treatment.
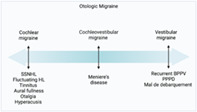
1. INTRODUCTION
Migraine disorder, estimated to affect 15%–18% of the population, 1 is a leading cause of disability worldwide in young and middle‐aged adults. 2 , 3 Originally considered a vascular headache disorder, the understanding of migraine and its variable presentations have evolved tremendously. 4 A spectrum of disease, migraine disorder, and its associated neural dysfunction can cause numerous cochleovestibular complaints, bringing this neurological disease into the purview of otolaryngologists (Figure 1 ). An estimated 5%–7% of patients seen in neurotology outpatient clinics are vestibular migraneurs, 5 which is under diagnosed 6 , 7 , 8 due to the underrecognized intersection of migraine and other vertigo‐causing pathologies. It is imperative that otolaryngologists feel confident diagnosing and managing this disabling disease.
FIGURE 1.
Spectrum of migraine inner ear disease. Created with BioRender.com.
2. EPIDEMIOLOGY AND EFFECT ON PATIENTS
Vestibular migraine impacts an estimated 1%–2.7% of the population 9 , 10 , 11 and accounts for 25% of patients with vertigo. 8 Median age of vestibular migraine onset is quoted between 30 and 40 years, and women are affected three times more than men. 12 , 13 , 14 Patients who suffer from vestibular migraine are more disabled with lower health‐related quality of life than migraineurs without vestibular symptoms. 10 , 12 , 15 , 16
3. PATHOPHYSIOLOGY
Migraine attacks are thought to occur in multiple phases: premonitory phase, trigeminal activation, cortical spreading depression, and sustained inflammation. 17 Activation of the trigeminal neurovascular complex is considered a forefront mechanism underlying migraine 1 and is mediated by endogenous signaling neuropeptides inducing neurogenic and meningeal inflammation. 8 , 17 Cortical spreading depression is a slowly propagating wave of neuronal depolarization accompanied by ion and electrical charge fluctuation followed by a period of depressed activity 17 ; this phenomenon is thought to be responsible for migrainous auras and could play a role in hypersensitivity to surrounding stimuli. 8
3.1. Pathophysiology specific to vestibular migraine
The exact mechanism for vestibular migraine remains debated, but it is likely a peripheral manifestation of a central process in which the vestibular system is the end‐organ affected. Leading theories include neurovascular inflammation secondary to an initial trigeminal neurovascular complex activation propagated to the inner ear, 18 , 19 , 20 genetic abnormalities in neuronal membrane ion channels, 21 and hyperactivation of cortical sensory processing sites due to cortical spreading depression. 18 , 21 Calcitonin gene‐related peptide (CGRP) is expressed in trigeminal neurons and efferent synapses of hair cells in the cochlea and semicircular canals. This neuropeptide is currently thought to play a role in nociception and cause vasodilation. 22 Functional magnetic resonance imaging has identified that chronic migraineurs have abnormal activity at the amygdala, thalamus, and temporal cortex, even when not experiencing an attack, 23 suggesting pathology regarding nociceptive processing.
Family studies focusing on vestibular migraine report autosomal dominant inheritance with incomplete penetrance but with high penetrance reported in women. 19 , 20 Familial prevalence rates of vestibular migraine have been shown to be 4–10 times higher than that of the general population. 24 Vestibular migraine is suspected to be a complex multigenic disease with significant variability in presentation. 8 , 25
4. DIAGNOSIS AND SYMPTOMATOLOGY
4.1. Vestibular migraine diagnostic criteria
Vestibular migraine is defined as recurrent, episodic vertigo caused by migraine and excludes dizziness due to other organic vestibular and non‐vestibular causes. 8 , 26 This disorder is characterized by episodic vertigo with associated migraine features in patients with a history of migraine. The Bárány Society diagnostic criteria are listed in Table 1. 26 Vestibular symptoms can include spontaneous vertigo (internal or external), positional vertigo, visually‐induced vertigo, and head motion‐induced vertigo with and without nausea. 26 Patients can experience vestibular symptoms with and without headache, and may even experience aura without the characteristic headache. 27 Vestibular migraine is juxtaposed against common migraine due to misleading patient reports of attenuated headache, scalp tingling, head pressure, sinus pressure, or absence of headache altogether. Moderate vertiginous symptoms interfere with daily activities but do not prohibit function. Vestibular symptoms are classified as severe if daily activities cannot be executed. 26 The current criteria may be too stringent and possibly misclassifies patients with vestibular migraine, but do not meet the criteria for migraine headaches. 28
TABLE 1.
Bárány Society and International Headache Society consensus criteria for vestibular migraine. 26
| Bárány Society Vestibular Migraine criteria | ||
|---|---|---|
| Vestibular migraine | ||
| A | At least five episodes with vestibular symptoms of moderate or severe intensity, lasting 5–72 h | |
| B | Current or previous history of migraine with or without aura according to ICHD‐3 | |
| C | One or more migraine features with at least 50% of the vestibular episodes: | |
| 1 | Headache with at least 2 of the following: unilateral, pulsating quality, moderate or severe pain intensity, aggravation by routine physical activity | |
| 2 | Photophobia and phonophobia | |
| 3 | Visual aura | |
| D | Not better accounted for by another vestibular or ICHD diagnosis | |
| Probable vestibular migraine | ||
| A | At least five episodes with vestibular symptoms of moderate or severe intensity, lasting 5–72 h | |
| B | Only one of B or C criteria from VM diagnosis is fulfilled | |
| C | Not better accounted for by another vestibular or ICHD diagnosis | |
Abbreviation: ICHD, International Classification of Headache Disorders.
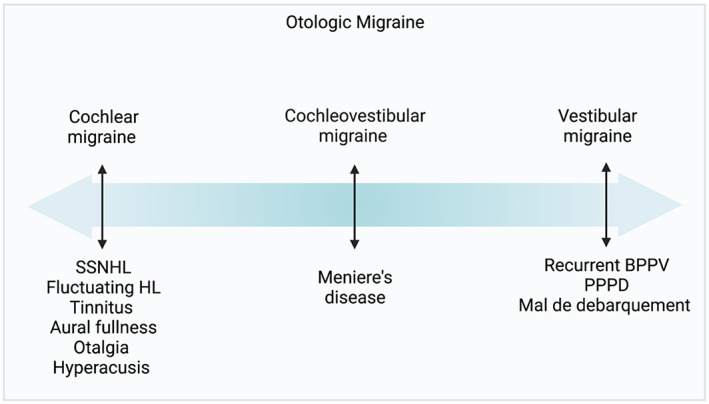
For patients who experience migraine episodes for at least 15 days a month with interictal symptoms, the term chronic migraine is used. Patients with interictal vestibular symptoms can be considered akin to chronic migraineurs, but with vestibular symptoms rather than headaches. Vestibular symptoms are more common in patients with chronic migraine symptoms. 15 Figure 2 shows the type and frequency of symptoms experienced by patients with vestibular migraine. 5 , 29 , 30 Episodic vertigo episodes can be spontaneous, positional, visual, or a mixed picture. 5 , 8 Over half of vestibular migraine patients experience episodic spontaneous and positional vertigo. 5 Patients can also experience interictal head motion and visually‐stimulated dizziness. 6
FIGURE 2.
Symptomatology among vestibular migraine patients. Data from Young et al., 5 Preysner et al., 29 Lepcha et al. 30 Created with BioRender.com.
4.2. Cochlear migraine
Aural symptoms, such as increased tinnitus, aural pressure, and subjective hearing loss, 5 , 6 , 12 can accompany vestibular migraine in a bilateral or unilateral configuration, raising the question whether vestibular migraine should be termed vestibulocochlear migraine. Lai and Liu 31 described a subset of patients with long‐term, unilateral fluctuating hearing loss associated with aural fullness and tinnitus that did not meet the criteria for vestibular migraine. 31 This group of patients also reported migraine features and family histories pertinent for migraine. Shi et al. 12 demonstrated that tinnitus, otalgia, and hearing loss were connected among vestibular migraine patients. 12 Similarly, Ma et al. 32 detailed a close relationship between migraine, sudden sensorineural hearing loss, deafness, and tinnitus. 32 Goshtasbi et al. 33 found that 35.6% of patients with migraine reported tinnitus. 33 Twenty‐one percent of patients with vestibular symptoms exhibited unilateral or bilateral objective hearing loss; these patients experienced a higher rate of aural‐related symptoms and shorter periods of vertigo. 12 Cochlear migraine was introduced in 2018 to explain the relationship between migraine and auditory symptoms. Although vestibular migraine is fully recognized with diagnostic criteria, cochlear migraine requires further study. Studies dedicated to explaining and characterizing the combined vestibulocochlear manifestations of migraine are continuing to emerge.
5. EVALUATION
The history of present illness should include prominent vestibular symptoms, timing, personal and family history of migraine, and headache symptoms. A comprehensive neuro‐otologic assessment should be performed for any patient presenting with vertigo. For patients who suffer from vestibular migraine, otoscopy is typically normal, though some patients with cochlear migraine, on our clinical observation, will have dilation of the capillaries over the malleus on the involved side. Patients actively experiencing an acute vertiginous attack will likely have low‐amplitude spontaneous, positional, or mixed‐pattern nystagmus upon examination. 5 , 34 Neurological exams between attacks are typically normal, 18 , 34 although nystagmus has been reported. 35 Audiologic assessment is warranted for patients who report aural symptoms or subjective hearing changes. 36 As previously mentioned, hearing loss on pure tone audiometry has been reported in up to 21% of patients with vestibular symptoms. 12 , 35 , 37 Hearing loss pattern is typically mild, sensorineural, and centered in the low‐frequency range; it can present both unilaterally, which is more common, and bilaterally. 12 Most patients who suffer from VM will have normal audiometric testing results and normal results on caloric and vestibular evoked myogenic potential testing. 5
Psychiatric co‐morbidities, notably generalized anxiety, major depressive, and panic disorders, have been shown to be related to vestibular symptoms. 6 , 38 Patients with vestibular migraine experience higher levels of anxiety, 38 , 39 which can lead to a positive feedback loop of continued heightened awareness of vestibular symptoms and anxiety. 8 Screening for anxiety in patients with vestibular migraine has been proposed to identify patients who would benefit from prophylactic migraine treatment that has been repurposed from psychiatric treatment.
6. MIGRAINE AND OVERLAP WITH OTHER VESTIBULOPATHIES
6.1. Mal de débarquement syndrome
Mal de débarquement syndrome (MDDS) is a distinct type of disequilibrium characterized by the sensation of rocking or swaying, as if on a boat, typically starting after prolonged exposure to passive motion following travel by air, boat, or car. 40 Symptoms need to be present for at least 1 month. 41 The sensation improves with passive motion. MDDS is closely associated with migraine and may even be part of the migraine disorder spectrum 40 , 42 ; the pathognomonic symptom, rocking and swaying sensation, worsens during acute vestibular migraine attacks. 43 Patients who experience both MDDS and vestibular migraine exhibit increased severity of attacks and disability. 43 Patients with MDDS have been shown to respond well to migraine therapy. 40
6.2. Benign paroxysmal positional vertigo
Benign paroxysmal positional vertigo (BPPV) is characterized by fleeting, seconds‐to‐minutes‐long vertigo occurring with head position changes. The general consensus of causative mechanism is semicircular canal otolith mispositioning 44 and is usually idiopathic or secondary to other vestibular pathology. Co‐incidence of migraine disorder in patients with BPPV of unknown etiology is three times higher than in the general population, and onset of BPPV at a younger age is strongly associated with a migraine history. 45 , 46 It has been suggested that the two diseases may have similar pathophysiology and that BPPV may be on the disease presentation spectrum for migraine with vestibulocochlear symptoms. 44 , 47 , 48
6.3. Meniere's disease
Meniere's disease (MD), or endolymphatic hydrops, is an inner ear disorder caused by increased intralabyrinthine pressure. 49 , 50 , 51 Symptoms of MD include aural pressure, fluctuating hearing loss, and low‐pitched tinnitus accompanied by episodic vertigo lasting 20 min–12 h, 50 which falls within the accepted time frame for VM attacks. Audiometric testing reveals a unilateral or asymmetric low‐frequency sensorineural hearing loss, and hearing loss on audiometry has been suggested as a method for distinguishing MD as a diagnosis over vestibular migraine. 52 Asymmetric hearing loss, tinnitus, and aural fullness have been found to be more prevalent in MD than in VM. 52 It has been demonstrated that patients with MD respond well to migraine therapy. 53
6.4. Persistent postural perceptual dizziness
Persistent postural perceptual dizziness (PPPD) is a chronic vestibular dysfunction hallmarked by visually‐induced vertigo, space motion discomfort, and subjective chronic dizziness. 54 A majority of patients with PPPD suffer from migraine. 55 Co‐morbid anxiety and depression are considered to play key roles in the pathogenesis of PPPD and often require treatment for optimal treatment outcomes. Vestibular migraine may interact with PPPD as a trigger or perpetuating co‐morbidity 56 ; treatment for PPPD and co‐morbid vestibular migraine requires a holistic biopsychosocial approach with medical therapy targeted at migraine and co‐morbid psychiatric diagnoses. 57 Vestibular rehabilitation (VR) with habituation exercises has been shown to be beneficial. 58 , 59
7. VESTIBULAR MIGRAINE TREATMENT
Vestibular migraine is challenging to treat with no widespread consensus because of the lack of evidence‐based treatment and randomized controlled clinical trials. 9 , 18 , 60 As a result, the current treatment options for vestibular migraine rely on those used for migraine headaches. 18 , 61 , 62 Abortive medications are used to alleviate symptoms during an acute attack, whereas prophylactic medications are considered in patients with frequent and/or severe attacks that impact their quality of life.
7.1. Abortive therapy
The goal of abortive therapy is to halt or lessen the severity of an acute vestibular attack. There are few studies investigating abortive medications and their efficacy in vestibular migraine attacks. Triptans (selective serotonin receptor 5‐hydroxytyramine receptor one agonists) have been shown to be effective in managing migraine with vestibular symptoms (Table 2 ). 37 , 63 , 64 In our clinical practice, we have not found triptans to be as effective against vertigo, especially for those with chronic symptoms.
TABLE 2.
Triptan dosing and efficacy in acute VM attack.
| Abortive therapy | ||||
|---|---|---|---|---|
| Medication | Dose | Study | Study type | Effect found |
| Almotriptan | 12.5 mg PO within 1 hour of attack | Cassano et al. | Retrospective, multicenter open‐label (n = 18) | 55% complete vertigo resolution, 28% of patients reported over 50% reduction of vertigo |
| Sumatriptan | Unspecified | Bikhazi et al. | Retrospective survey (n = 111) | Excellent efficacy at improving headaches (4) and vertigo (3) on a numerical scale from 1 to 4 |
| Zolmitriptan | 2.5 mg PO during attack | Neuhauser et al. | RCT (n = 10) | 38% of patients had improvement in vertigo at 2 hours following treatment versus 22% for placebo, results inconclusive due to limited power |
Other medications used for acute migrainous vertigo include those routinely used for acute vertigo from other causes. Despite inconclusive evidence detailed in McCoul et al., 65 systemic corticosteroids have been broadly used in acute vestibular neuritis and sudden sensorineural hearing loss. 65 Based on a 65‐year systematic review, corticosteroids are as effective as migraine abortive medications for acute attacks. 66 Prakash and Shah detail improvement of migrainous vertigo after intravenous methylprednisolone administration in acute and chronic vertigo recalcitrant to other medications. Antiemetics (ondansetron) and antihistamines (meclizine, diphenhydramine) are commonly employed despite sparse well‐established evidence of their efficacy in treating vestibular migraine. 22 , 67 Similarly, antidopaminergic medications (metoclopramide) are effective in treating migrainous nausea. 68
7.2. Pharmacological prophylaxis
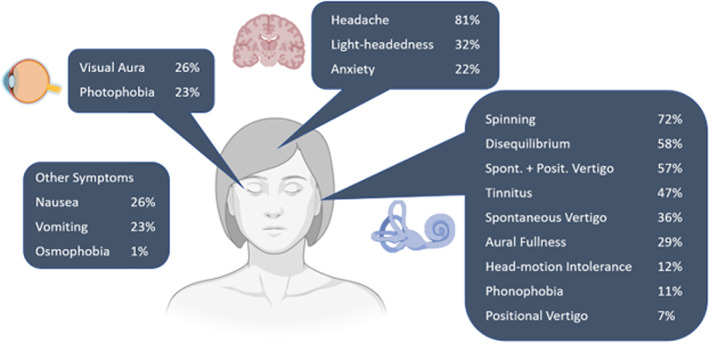
The goal of prophylactic medication for vestibular migraine is to reduce attack frequency and severity. Most of the medications used are extrapolated from the prophylactic medications for treating migraine headaches with no evidence of superiority of any medication over others. 67 These treatments include beta‐blockers (propranolol, metoprolol), 69 , 70 , 71 antiepileptics (topiramate, lamotrigine, valproic acid), 72 calcium channel blockers (verapamil, flunarizine, lomerizine, cinnarizine), 30 , 71 , 73 , 74 tricyclic antidepressants (amitriptyline, nortriptyline), 71 , 72 serotonin‐norepinephrine reuptake inhibitors (SNRI) (venlafaxine), 69 and selective serotonin reuptake inhibitors (SSRI) (paroxetine). 75 Flunarizine and cinnarizine are not currently available in the United States. If dizziness is not controlled with one class of medication, another class should be considered to achieve symptomatic control for a few months before tapering. Some have suggested maintenance therapy for a year prior to tapering off medication. 76 The algorithmic approach to vestibular migraine prophylactic management is detailed in Figure 3. Outcomes from prophylactic management of VM have been encouraging, with many cohort studies reporting a majority of patients with symptom resolution, reduction in frequency of attacks, and satisfactory symptom control. 30 , 35 , 69 , 77 , 78 VM patients anecdotally are very sensitive to medication changes and side effects; common side effects are detailed in Table 3. The American College of Obstetricians and Gynecologists recommends avoiding prophylactic migraine treatment in pregnancy. 79
FIGURE 3.
Sample algorithmic approach to otologic migraine prophylactic management. †CGRP: calcitonin‐gene related peptide; HR, heart rate; SBP, systolic blood pressure.
TABLE 3.
Side effects and contraindications of migraine prophylactic medications.
| Medication | Side‐effects | Contraindications |
|---|---|---|
| Antiepileptics | ||
| Topiramate | Cognitive slowing, weight loss, depression, paresthesia | Pregnancy |
| Lamotrigine | Rash, nausea, dizziness | Cardiac arrhythmias |
| Valproic acid | Nausea, vomiting, hair loss, easy bruising, tremor, weight gain, hepatotoxicity | Pregnancy |
| Gabapentin | Somnolence, cognitive slowing | Pregnancy |
| Calcium channel blockers | ||
| Flunarizine, cinnarizine (not FDA approved) | Headache, lightheadedness, flushing, parkinsonism (rare) | Pregnancy |
| Verapamil | ECG abnormalities at doses above 480 mg/day, edema, gastrointestinal discomfort, dull headache, gingival hyperplasia | Underlying heart disease (relative) |
| Tricyclic anti‐depressants | ||
| Amitriptyline | Hypersomnolence (higher than nortriptyline), orthostasis, mild QTC prolongation, dry mouth, constipation, tachycardia, palpitations, weight gain, blurred vision, urinary retention, lower seizure threshold at high doses, sexual dysfunction | Underlying heart disease (relative) |
| Nortriptyline | Hypersomnolence (less than amitriptyline), orthostasis, dry mouth, mild QTC prolongation, constipation, tachycardia, palpitations, weight gain, blurred vision, urinary retention, lower seizure threshold at high doses | Underlying heart disease (relative) |
| SNRIs and SSRIs | ||
| Venlafaxine | Nausea, hypertension, dizziness, dry mouth, sexual dysfunction | ‐ |
| Paroxetine | Orthostasis, sexual dysfunction, weight gain, drowsiness | Pregnancy |
| Beta‐blockers | ||
| Propranolol | Fatigue, depression, sexual dysfunction, hypoglycemia, hyperkalemia | Severe bronchospastic disease |
| Metoprolol | Fatigue, depression, sexual dysfunction | Severe bronchospastic disease (less than propranolol) |
| Angiotensin II receptor antagonists | ||
| Candesartan | Back pain, dizziness, and cold‐ or flu‐like symptoms | None |
Note: Sources include Ko et al., 80 Beumer and Hardonk, 81 Singh et al., 82 Skinner et al., 83 Reid et al., 84 Castellino et al., 85 Deacon and Barnett, 86 Shorr et al., 87 Capella et al., 88 Chouza et al., 89 Anderson et al., 90 Stahl et al., 91 Wenzel‐Seifert et al., 92 Hirsch et al., 93 Arif et al., 94 Abou‐Khalil, 95 Biton et al., 96 Nasreddine and Beydoun, 97 Cohen et al. 98 and Tomson et al. 99
7.3. New and emerging therapies
Gepants (CGRP receptor antagonists) are a new class of medication with the potential to absolve and possibly prevent acute attacks. Rimegepant, ubrogepant, and zavegepant have been shown in clinical trials to be efficacious for acute attacks; rimegepant and atogepant both have promising preventative applications. 100 Trials to show non‐inferiority of gepants when compared to triptans have not occurred yet, and studies assessing the efficacy of gepants in patients with vestibular migraine are yet to be conducted despite promising animal studies. 101 , 102
Russo et al. 103 were the first to report the efficacy of anti‐CGRP monoclonal antibodies (erenumab, fremanezumab, or galcanezumab) on vestibular migraine, particularly when started early in the disease course. They found that nearly 80% of the patients experienced at least a concurrent 50% reduction in migraine days, vertigo days, and migraine disability assessment scores. 103 Furthermore, Lasmiditan, a highly selective 5‐hydroxytryptamine receptor 1F agonist, was approved by the US Food and Drug Administration for the treatment of acute migraine and might be a promising therapy for vestibular migraine by blocking trigeminally‐mediated responses, such as CGRP release. 104
7.4. Non‐pharmacological prophylaxis
Many patients with vestibular migraine seek non‐pharmacological treatment due to medication side effects. Non‐pharmacological approaches focus on managing migraine triggers, which can be categorized into five main categories: stress (psychological or physical), hormonal changes (menstrual cycle, menopause, hormone replacement therapy, etc.), changes in sleep (too much or too little sleep, interrupted sleep, obstructive sleep apnea), diet (skipping meals; eating certain foods such as caffeine, monosodium glutamate, glutamate, tyramine, and histamine; and dehydration), and intense stimulations (bright lights, intense sound, visual motion, intense smells, weather changes, heat, and atmospheric pressure changes) (Table 4 ). 5 , 6 , 8 , 105 , 106 , 107 A personalized treatment plan to control or avoid patients' identified triggers may reduce the frequency and severity of their vestibular migraine symptoms. Additionally, dietary supplementation such as magnesium (400 mg PO twice daily) and riboflavin (vitamin B2) (200 mg PO once daily) has been proven helpful in preventing migraine. 108 , 109 , 110 , 111 , 112 Roberts et al. 113 found that lifestyle modifications in patients with definite vestibular migraine, specifically restful sleep, are associated with improvement in dizziness handicap inventory (DHI) and headache. It should be noted, however, that 32% of patients were excluded due to their request for pharmacological treatment or failure to follow up. 113
TABLE 4.
Dietary and physiologic migraine triggers.
| Migraine triggers | Recommendations |
|---|---|
| Stress (conflict at home/work, death of relative, physical pain, infection, other illness, etc.) |
|
| Sleep (too much sleep, too little sleep, interrupted sleep, shifting sleep schedule, different sleep schedule on weekends, etc.) |
|
| Diet (skipping meals, eating certain foods, and dehydration) |
|
7.5. Vestibular rehabilitation
VR is a non‐pharmacologic approach that involves gaze stability exercises, habituation exercises, gait and balance training, and walking to improve endurance, with the aim of alleviating dizziness and balance dysfunction in patients with vestibular disorders including vestibular migraine. 59 , 114 VR can improve functional outcomes in various vestibulopathies, 115 , 116 including in patients who suffer from vestibular symptoms due to migraine. 58 , 117 Patients with migraine undergoing VR may report persistent or worsening symptoms, particularly after a session of therapy. Although we recommend stopping VR in patients who experience worsening symptoms with therapy, one study showed improvement in patient‐reported outcome measures and objective performance measures for vestibular migraineurs who completed a 6‐month program. 58 Additional investigation is crucial to determine the impact of vestibular suppressant medications and the timing of their administration on the outcomes of VR.
7.6. Treatment of MD as a migraine variant
Current strategies for treatment of MD as shown in Figure 4 include lifestyle changes (low‐sodium diet, good hydration, caffeine restriction), medication trial (betahistine and diuretics), 118 , 119 and intratympanic steroid injection. Surgical or ablative therapies include endolymphatic sac surgery, intratympanic gentamicin, labyrinthectomy, and vestibular neurectomy, as detailed in Figure 4. 118 , 119 , 120 , 121
FIGURE 4.
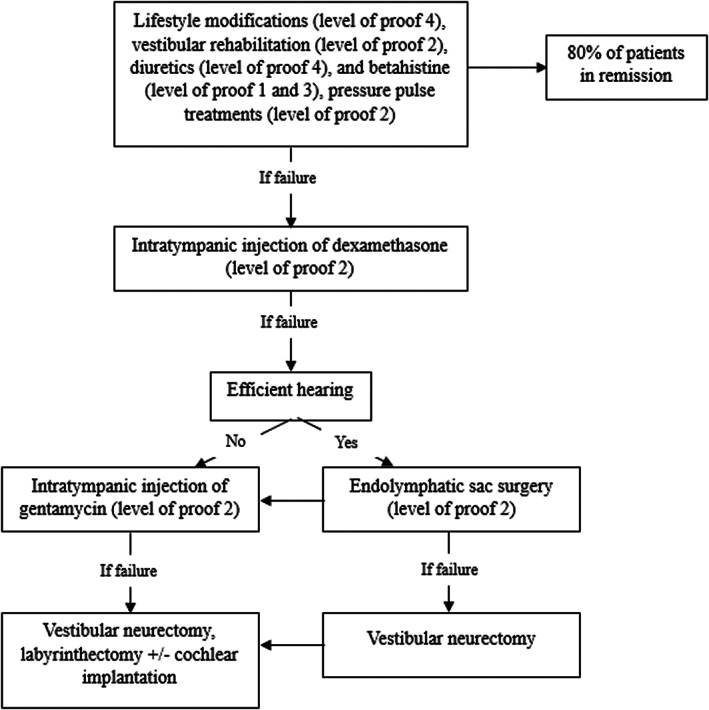
The international consensus algorithm to treat Meniere's disease. 118 , 119
Migraine diet and lifestyle modifications and medications as treatment for MD patients have sparked a debate among otolaryngologists. Proponents assert that MD, also referred to as “cochleovestibular migraine,” encompasses vestibular and cochlear migraine symptoms. As a result, some argue that patients with MD may benefit from migraine treatments. 53 , 106 , 122 , 123 Migraine treatment options include the migraine diet and lifestyle modifications that focus on avoiding migraine triggers that potentially provoke MD attacks (Table 4 ). Importantly, in contrast to the belief that a salt‐restricted diet is beneficial, the key may lie in maintaining adequate hydration. Prophylactic medications previously discussed can be started at a low dose (10 mg of nortriptyline or 25 mg of topiramate) and increased weekly or bi‐weekly, following the steps outlined in Figure 3. 122 Oral or intravenous steroids could be considered in managing acute MD attacks by preventing the spreading cortical depression secondary to migrainous central phenomena. 66 Finally, patients who have identified barometric changes as a migraine trigger may benefit from tympanostomy tubes. 124 , 125
7.7. Treatment of other migraine‐related otologic symptoms
7.7.1. Tinnitus
A significant percentage of migraine patients experience enhanced tinnitus, 5 , 33 and clinicians should educate patients with persistent, bothersome or fluctuating tinnitus about management strategies. Clinicians should also recommend cognitive behavioral therapy (CBT) with a potential consideration of sound therapy (ST). 36 Abouzari et al. 126 showed that an 8‐week course of CBT, which included emotional awareness, stress management, and sleeping techniques among others, and personalized pitch‐matched ST, led to a notable improvement in tinnitus handicap inventory scores. 126 Other studies have shown that customized ST and CBT are helpful in tinnitus management. 127 , 128 Migraine diet, lifestyle modifications, and medications may also have potential in treating tinnitus. Sullivan et al. 129 demonstrated the effectiveness of nortriptyline in reducing the severity of tinnitus. 129 In a recent network meta‐analysis including 36 randomized controlled trials with 2761 participants, Chen et al. 130 reported that pharmacological interventions that act on the brain (amitriptyline, acamprosate, and gabapentin) and those with anti‐inflammation effects (steroids and melatonin) exhibit a significant reduction in tinnitus severity and response rate when compared to the placebo or waiting‐list control groups. 130 In our clinical practice, we have found improvement in the fluctuation of tinnitus and reduction of loud tinnitus in patients treated with a migraine regimen and control of migraine triggers. Other keys to tinnitus management include dietary modifications such as having regular eating habits and avoiding migraine food triggers, maintaining a regular sleep schedule, gaining control over stress, and use of supplements such as magnesium and vitamin B2 (Figure 3). 131 , 132
7.7.2. Persistent postural perceptual dizziness
Currently, there is no definitive explanation for how pharmacological and non‐pharmacological approaches work in treating PPPD. 133 , 134 Main therapeutic approaches include CBT, SSRIs, SNRIs, and VR with the aim of breaking the maladaptive cycle. 135 Staab et al. 136 reported that sertraline (25 –200 mg) significantly reduced DHI scores and psychological distress in patients with PPPD. Yu et al. 137 compared the use of sertraline alone to sertraline plus CBT and found that adding CBT alongside sertraline significantly enhanced the effectiveness of sertraline in reducing scores from the DHI, Hamilton Anxiety Rating Scale, and Hamilton Depression Rating Scale. In addition, CBT reduced the dosage of sertraline used in the experiment group. 137 Finally, since several studies show an association between PPPD and migraine headaches, it may be prudent to expand PPPD treatment to include migraine treatment. 55 , 138 , 139
7.7.3. Treatment of sudden sensorineural hearing loss (SSNHL)
According to clinical guidelines, when patients present with SSNHL, clinicians should administer corticosteroids as soon as possible and consider intratympanic steroid injection between two to 6 weeks from onset if recovery is incomplete. 140 Concurrent oral and intratympanic steroids may have a stronger positive effect than either alone 141 ; however, Plontke et al. 142 demonstrated little to no effect difference for combination oral and intratympanic steroids versus unimodal administration. An association between SSNHL and migraine has been described; Abouzari et al. 111 found that migraine prophylactic treatment may be efficacious in treating SSNHL. They found that patients with SSNHL who received oral steroids, intratympanic dexamethasone injections, migraine lifestyle modifications, as well as a combination of nortriptyline and topiramate, demonstrated greater improvements in thresholds at the low frequencies and pure tone average and required fewer intratympanic injections when compared to those who received oral steroids and intratympanic dexamethasone only. 111 Moreover, Goshtasbi et al. showed that patients with long‐term SSNHL had statistically significant improvement in hearing thresholds, low‐frequency pure tone averages, speech‐frequency pure tone averages, word recognition scores, and speech recognition thresholds after receiving migraine prophylactic medication including nortriptyline, topiramate, and/or verapamil, lifestyle changes, and intratympanic steroid injections. These findings serve as a positive indicator for the use of migraine treatment for long‐term hearing loss. 143
8. CONCLUSION
There is increasing evidence regarding the association between migraine and various otologic symptoms, which carries significant implications for the development and investigation of therapies to address these challenging cochleovestibular presentations. An ideal treatment would be integrative neurosensory rehabilitation including migraine diet and lifestyle modifications, supplements (magnesium and vitamin B2), and migraine prophylactic medications. Further randomized placebo‐controlled clinical trials are required to provide higher evidence regarding migraine treatment's effectiveness on various symptoms of otologic migraine.
CONFLICT OF INTEREST STATEMENT
Dr. Brooks, Dr. Tawk, and Dr. Hobson do not have any relevant financial disclosures. Dr. Djalilian holds equity in Neuromed Care LLC, MindSet Technologies, Elinava Technologies, and Cactus Medical LLC. He is a consultant to NXT Biomedical.
Brooks KA, Tawk K, Djalilian HR, Hobson CE. Migraine management for the otolaryngologist. Laryngoscope Investigative Otolaryngology. 2023;8(4):1080‐1093. doi: 10.1002/lio2.1109
Kaitlyn A. Brooks and Karen Tawk contributed equally to this study.
Presented as a panel at the 2023 Triological Society Combined Sections Meeting titled “Migraine Management for the Otolaryngologist” Otology/Neurotology Session B, Thursday, January 26, 2023, in Coronado, California, USA.
Contributor Information
Hamid R. Djalilian, Email: hdjalili@hs.uci.edu.
Candace E. Hobson, Email: candace.hobson@emory.edu.
REFERENCES
- 1. Khan J, Asoom LIA, Sunni AA, et al. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother. 2021;139:111557. doi: 10.1016/j.biopha.2021.111557 [DOI] [PubMed] [Google Scholar]
- 2. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the global burden of Disease study 2015. Lancet. 2016;388(10053):1545‐1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163‐2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174‐182. doi: 10.1016/S1474-4422(17)30435-0 [DOI] [PubMed] [Google Scholar]
- 5. Young AS, Nham B, Bradshaw AP, et al. Clinical, oculographic, and vestibular test characteristics of vestibular migraine. Cephalalgia. 2021;41(10):1039‐1052. doi: 10.1177/03331024211006042 [DOI] [PubMed] [Google Scholar]
- 6. Beh SC. Vestibular migraine: how to Sort it out and what to Do about it. J Neuroophthalmol. 2019;39(2):208‐219. doi: 10.1097/WNO.0000000000000791 [DOI] [PubMed] [Google Scholar]
- 7. Lauritsen CG, Marmura MJ. Current treatment options: vestibular migraine. Curr Treat Options Neurol. 2017;19(11):38. doi: 10.1007/s11940-017-0476-z [DOI] [PubMed] [Google Scholar]
- 8. Krishnan PS, Carey JP. Vestibular migraine: clinical aspects and pathophysiology. Otolaryngol Clin North Am. 2022;55(3):531‐547. doi: 10.1016/j.otc.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 9. Webster K, Dor A, Galbraith K, et al. Pharmacological interventions for prophylaxis of vestibular migraine. Cochrane Database Syst Rev. 2023;2023(4):7‐53. doi: 10.1002/14651858.CD015187.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028‐1033. doi: 10.1212/01.wnl.0000237539.09942.06 [DOI] [PubMed] [Google Scholar]
- 11. Formeister EJ, Rizk HG, Kohn MA, Sharon JD. The epidemiology of vestibular migraine: a population‐based survey study. Otol Neurotol. 2018;39(8):1037‐1044. doi: 10.1097/MAO.0000000000001900 [DOI] [PubMed] [Google Scholar]
- 12. Shi S, Wang D, Ren T, Wang W. Auditory manifestations of vestibular migraine. Front Neurol. 2022;13:944001. doi: 10.3389/fneur.2022.944001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246(10):883‐892. doi: 10.1007/s004150050478 [DOI] [PubMed] [Google Scholar]
- 14. Power L, Shute W, McOwan B, Murray K, Szmulewicz D. Clinical characteristics and treatment choice in vestibular migraine. J Clin Neurosci. 2018;52:50‐53. doi: 10.1016/j.jocn.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 15. Carvalho GF, Vianna‐Bell FH, Florencio LL, et al. Presence of vestibular symptoms and related disability in migraine with and without aura and chronic migraine. Cephalalgia. 2019;39(1):29‐37. doi: 10.1177/0333102418769948 [DOI] [PubMed] [Google Scholar]
- 16. Ozdemir S, Ozdemir D, Terzi O, Mehel DM, Ozgur A. The economic burden of vertigo: results from the hospitalized and outpatients. Ear Nose Throat J. 2021;100(5_suppl):707S‐711S. doi: 10.1177/0145561320906330 [DOI] [PubMed] [Google Scholar]
- 17. Sutherland HG, Albury CL, Griffiths LR. Advances in genetics of migraine. J Headache Pain. 2019;20(1):72. doi: 10.1186/s10194-019-1017-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12(7):706‐715. doi: 10.1016/S1474-4422(13)70107-8 [DOI] [PubMed] [Google Scholar]
- 19. Huang TC, Wang SJ, Kheradmand A. Vestibular migraine: an update on current understanding and future directions. Cephalalgia. 2020;40(1):107‐121. doi: 10.1177/0333102419869317 [DOI] [PubMed] [Google Scholar]
- 20. von Brevern M, Lempert T. Vestibular migraine. Handb Clin Neurol. 2016;137:301‐316. doi: 10.1016/B978-0-444-63437-5.00022-4 [DOI] [PubMed] [Google Scholar]
- 21. Lempert T, Neuhauser H. Migrainous vertigo. Neurol Clin. 2005;23(3):715‐730. doi: 10.1016/j.ncl.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 22. Raymond MJ, Vivas EX. Current and emerging medical therapies for dizziness. Otolaryngol Clin North Am. 2021;54(5):1037‐1056. doi: 10.1016/j.otc.2021.05.019 [DOI] [PubMed] [Google Scholar]
- 23. Schwedt TJ, Schlaggar BL, Mar S, et al. Atypical resting‐state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53(5):737‐751. doi: 10.1111/head.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paz‐Tamayo A, Perez‐Carpena P, Lopez‐Escamez JA. Systematic review of prevalence studies and familial aggregation in vestibular migraine. Front Genet. 2020;11:954. doi: 10.3389/fgene.2020.00954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albury CL, Stuart S, Haupt LM, Griffiths LR. Ion channelopathies and migraine pathogenesis. Mol Genet Genomics. 2017;292(4):729‐739. doi: 10.1007/s00438-017-1317-1 [DOI] [PubMed] [Google Scholar]
- 26. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167‐172. doi: 10.3233/VES-2012-0453 [DOI] [PubMed] [Google Scholar]
- 27. Lempert T, von Brevern M. Vestibular migraine. Neurol Clin. 2019;37(4):695‐706. doi: 10.1016/j.ncl.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 28. Risbud A, Muhonen EG, Tsutsumi K, Martin EC, Abouzari M, Djalilian HR. Migraine features in patients with isolated aural fullness and proposal for a new diagnosis. Otol Neurotol. 2021;42(10):1580‐1584. doi: 10.1097/MAO.0000000000003324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Preysner TA, Gardi AZ, Ahmad S, Sharon JD. Vestibular migraine: cognitive dysfunction, mobility, falls. Otol Neurotol. 2022;43(10):1216‐1221. doi: 10.1097/MAO.0000000000003700 [DOI] [PubMed] [Google Scholar]
- 30. Lepcha A, Amalanathan S, Augustine AM, Tyagi AK, Balraj A. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol. 2014;271(11):2931‐2936. doi: 10.1007/s00405-013-2786-4 [DOI] [PubMed] [Google Scholar]
- 31. Lai JT, Liu TC. Proposal for a new diagnosis for Cochlear migraine. JAMA Otolaryngol Head Neck Surg. 2018;144(3):185‐186. doi: 10.1001/jamaoto.2017.2427 [DOI] [PubMed] [Google Scholar]
- 32. Ma X, Ke YJ, Jing YY, Diao TX, Yu LS. Migraine and Cochlear symptoms. Curr Med Sci. 2021;41(4):649‐653. doi: 10.1007/s11596-021-2410-6 [DOI] [PubMed] [Google Scholar]
- 33. Goshtasbi K, Abouzari M, Risbud A, et al. Tinnitus and subjective hearing loss are more common in migraine: a Cross‐sectional NHANES analysis. Otol Neurotol. 2021;42(9):1329‐1333. doi: 10.1097/MAO.0000000000003247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polensek SH, Tusa RJ. Nystagmus during attacks of vestibular migraine: an aid in diagnosis. Audiol Neurootol. 2010;15(4):241‐246. doi: 10.1159/000255440 [DOI] [PubMed] [Google Scholar]
- 35. Maione A. Migraine‐related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope. 2006;116(10):1782‐1786. doi: 10.1097/01.mlg.0000231302.77922.c5 [DOI] [PubMed] [Google Scholar]
- 36. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(2 Suppl):S1‐S40. doi: 10.1177/0194599814545325 [DOI] [PubMed] [Google Scholar]
- 37. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine‐associated dizziness. Am J Otol. 1997;18(3):350‐354. [PubMed] [Google Scholar]
- 38. Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. 2016;87(7):741‐749. doi: 10.1136/jnnp-2015-312233 [DOI] [PubMed] [Google Scholar]
- 39. Kutay O, Akdal G, Keskinoglu P, Balci BD, Alkin T. Vestibular migraine patients are more anxious than migraine patients without vestibular symptoms. J Neurol. 2017;264(Suppl 1):37‐41. doi: 10.1007/s00415-017-8439-6 [DOI] [PubMed] [Google Scholar]
- 40. Ghavami Y, Haidar YM, Ziai KN, et al. Management of mal de debarquement syndrome as vestibular migraines. Laryngoscope. 2017;127(7):1670‐1675. doi: 10.1002/lary.26299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Ombergen A, van Rompaey V, Maes LK, van de Heyning PH, Wuyts FL. Mal de debarquement syndrome: a systematic review. J Neurol. 2016;263(5):843‐854. doi: 10.1007/s00415-015-7962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cha YH, Cui Y. Rocking dizziness and headache: a two‐way street. Cephalalgia. 2013;33(14):1160‐1169. doi: 10.1177/0333102413487999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beh SC, Chiang HS, Sanderson C. The interconnections of mal de debarquement syndrome and vestibular migraine. Laryngoscope. 2021;131(5):E1653‐E1661. doi: 10.1002/lary.29214 [DOI] [PubMed] [Google Scholar]
- 44. Bruss D, Abouzari M, Sarna B, et al. Migraine features in patients with recurrent benign paroxysmal positional vertigo. Otol Neurotol. 2021;42(3):461‐465. doi: 10.1097/MAO.0000000000002976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishiyama A, Jacobson KM, Baloh RW. Migraine and benign positional vertigo. Ann Otol Rhinol Laryngol. 2000;109(4):377‐380. doi: 10.1177/000348940010900407 [DOI] [PubMed] [Google Scholar]
- 46. Kim EK, Pasquesi L, Sharon JD. Examining migraine as a predictor of benign paroxysmal positional vertigo onset, severity, recurrence, and associated falls. Cureus. 2022;14(8):e28278. doi: 10.7759/cureus.28278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lempert T, Leopold M, von Brevern M, Neuhauser H. Migraine and benign positional vertigo. Ann Otol Rhinol Laryngol. 2000;109(12 Pt 1):1176. [PubMed] [Google Scholar]
- 48. Batuecas‐Caletrio A, Martin‐Sanchez V, Cordero‐Civantos C, et al. Is benign paroxysmal vertigo of childhood a migraine precursor? Eur J Paediatr Neurol. 2013;17(4):397‐400. doi: 10.1016/j.ejpn.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 49. Teggi R, Fabiano B, Recanati P, Limardo P, Bussi M. Case reports on two patients with episodic vertigo, fluctuating hearing loss and migraine responding to prophylactic drugs for migraine. Meniere's disease or migraine‐associated vertigo? Acta Otorhinolaryngol Ital. 2010;30(4):217. [PMC free article] [PubMed] [Google Scholar]
- 50. Espinosa‐Sanchez JM, Lopez‐Escamez JA. Meniere's disease. Handb Clin Neurol. 2016;137:257‐277. doi: 10.1016/B978-0-444-63437-5.00019-4 [DOI] [PubMed] [Google Scholar]
- 51. Lopez‐Escamez JA, Carey J, Chung WH, et al. M. Meniere: Diagnostische Kriterien des Internationalen Klassifikationskomitees der Barany‐Gesellschaft [Diagnostic criteria for Meniere's disease according to the classification Committee of the Barany Society]. HNO. 2017;65(11):887‐893. doi: 10.1007/s00106-017-0387-z [DOI] [PubMed] [Google Scholar]
- 52. van Esch BF, van Wensen E, van der Zaag‐Loonen HJ, Benthem P, van Leeuwen RB. Clinical characteristics of benign recurrent vestibulopathy: clearly distinctive from vestibular migraine and Meniere's Disease? Otol Neurotol. 2017;38(9):e357‐e363. doi: 10.1097/MAO.0000000000001553 [DOI] [PubMed] [Google Scholar]
- 53. Ghavami Y, Haidar YM, Moshtaghi O, Lin HW, Djalilian HR. Evaluating quality of life in patients with Meniere's disease treated as migraine. Ann Otol Rhinol Laryngol. 2018;127(12):877‐887. doi: 10.1177/0003489418799107 [DOI] [PubMed] [Google Scholar]
- 54. Gambacorta V, D'Orazio A, Pugliese V, di Giovanni A, Ricci G, Faralli M. Persistent postural perceptual dizziness in episodic vestibular disorders. Audiol Res. 2022;12(6):589‐595. doi: 10.3390/audiolres12060058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarna B, Risbud A, Lee A, Muhonen E, Abouzari M, Djalilian HR. Migraine features in patients with persistent postural‐perceptual dizziness. Ann Otol Rhinol Laryngol. 2021;130(12):1326‐1331. doi: 10.1177/00034894211007233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ma Y, Zhang D, Wang W, Zhang Z, Zhang H. The comorbid mechanism of vestibular migraine and persistent postural‐perceptual dizziness. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke za Zhi. 2022;36(4):321‐324. doi: 10.13201/j.issn.2096-7993.2022.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Popkirov S, Staab JP, Stone J. Persistent postural‐perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness. Pract Neurol. 2018;18(1):5‐13. doi: 10.1136/practneurol-2017-001809 [DOI] [PubMed] [Google Scholar]
- 58. Vitkovic J, Winoto A, Rance G, Dowell R, Paine M. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol. 2013;260(12):3039‐3048. doi: 10.1007/s00415-013-7116-7 [DOI] [PubMed] [Google Scholar]
- 59. Enticott JC, Vitkovic JJ, Reid B, O'Neill P, Paine M. Vestibular rehabilitation in individuals with inner‐ear dysfunction: a pilot study. Audiol Neurootol. 2008;13(1):19‐28. doi: 10.1159/000107434 [DOI] [PubMed] [Google Scholar]
- 60. Webster KE, Dor A, Galbraith K, et al. Non‐pharmacological interventions for prophylaxis of vestibular migraine. Cochrane Database Syst Rev. 2023;4(4):CD015321. doi: 10.1002/14651858.CD015321.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mallampalli MP, Rizk HG, Kheradmand A, et al. Care gaps and recommendations in vestibular migraine: an expert panel summit. Front Neurol. 2021;12:812678. doi: 10.3389/fneur.2021.812678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Byun YJ, Levy DA, Nguyen SA, Brennan E, Rizk HG. Treatment of vestibular migraine: a systematic review and meta‐analysis. Laryngoscope. 2021;131(1):186‐194. doi: 10.1002/lary.28546 [DOI] [PubMed] [Google Scholar]
- 63. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo‐controlled trial. Neurology. 2003;60(5):882‐883. doi: 10.1212/01.wnl.0000049476.40047.a3 [DOI] [PubMed] [Google Scholar]
- 64. Cassano D, Pizza V, Busillo V. P074. Almotriptan in the acute treatment of vestibular migraine: a retrospective study. J Headache Pain. 2015;16(Suppl 1):A114. doi: 10.1186/1129-2377-16-S1-A114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCoul ED, Megwalu UC, Joe S, et al. Systemic steroids for otolaryngology‐head and neck surgery disorders: an evidence‐based primer for clinicians. Otolaryngol Head Neck Surg. 2023;168(4):643‐657. doi: 10.1177/01945998221087664 [DOI] [PubMed] [Google Scholar]
- 66. Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65‐year systematic review with pooled analysis and critical appraisal. Cephalalgia. 2015;35(11):996‐1024. doi: 10.1177/0333102414566200 [DOI] [PubMed] [Google Scholar]
- 67. von Brevern M, Lempert T. Vestibular migraine: treatment and prognosis. Semin Neurol. 2020;40(1):83‐86. doi: 10.1055/s-0039-3402067 [DOI] [PubMed] [Google Scholar]
- 68. Kirthi V, Derry S, Moore RA, McQuay HJ. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;2010(4):CD008041. doi: 10.1002/14651858.CD008041.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salviz M, Yuce T, Acar H, Karatas A, Acikalin RM. Propranolol and venlafaxine for vestibular migraine prophylaxis: a randomized controlled trial. Laryngoscope. 2016;126(1):169‐174. doi: 10.1002/lary.25445 [DOI] [PubMed] [Google Scholar]
- 70. Baier B, Winkenwerder E, Dieterich M. "vestibular migraine": effects of prophylactic therapy with various drugs. A retrospective study. J Neurol. 2009;256(3):436‐442. doi: 10.1007/s00415-009-0111-3 [DOI] [PubMed] [Google Scholar]
- 71. Gorur K, Gur H, Ismi O, Ozcan C, Vayisoglu Y. The effectiveness of propranolol, flunarizine, amitriptyline and botulinum toxin in vestibular migraine complaints and prophylaxis: a non‐randomized controlled study. Braz J Otorhinolaryngol. 2022;88(6):975‐981. doi: 10.1016/j.bjorl.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol. 2012;33(1):121‐127. doi: 10.1016/j.amjoto.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 73. Taghdiri F, Togha M, Razeghi Jahromi S, Refaeian F. Cinnarizine for the prophylaxis of migraine associated vertigo: a retrospective study. Springerplus. 2014;3:231. doi: 10.1186/2193-1801-3-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Teggi R, Colombo B, Gatti O, Comi G, Bussi M. Fixed combination of cinnarizine and dimenhydrinate in the prophylactic therapy of vestibular migraine: an observational study. Neurol Sci. 2015;36(10):1869‐1873. doi: 10.1007/s10072-015-2270-6 [DOI] [PubMed] [Google Scholar]
- 75. Iwasaki S, Ushio M, Chihara Y, Ito K, Sugasawa K, Murofushi T. Migraine‐associated vertigo: clinical characteristics of Japanese patients and effect of lomerizine, a calcium channel antagonist. Acta Otolaryngol Suppl. 2007;559:45‐49. doi: 10.1080/03655230701596491 [DOI] [PubMed] [Google Scholar]
- 76. Lapira A. Vestibular migraine treatment and prevention. HNO. 2019;67(6):425‐428. Behandlung und Pravention von vestibularer Migrane. doi: 10.1007/s00106-019-0661-3 [DOI] [PubMed] [Google Scholar]
- 77. Gode S, Celebisoy N, Kirazli T, et al. Clinical assessment of topiramate therapy in patients with migrainous vertigo. Headache. 2010;50(1):77‐84. doi: 10.1111/j.1526-4610.2009.01496.x [DOI] [PubMed] [Google Scholar]
- 78. Reploeg MD, Goebel JA. Migraine‐associated dizziness: patient characteristics and management options. Otol Neurotol. 2002;23(3):364‐371. doi: 10.1097/00129492-200205000-00024 [DOI] [PubMed] [Google Scholar]
- 79. ACOG clinical practice guideline No. 3: headaches in pregnancy and postpartum: correction. Obstet Gynecol. 2022;140(2):344. doi: 10.1097/AOG.0000000000004878 [DOI] [PubMed] [Google Scholar]
- 80. Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta‐blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. Jama. 2002;288(3):351‐357. doi: 10.1001/jama.288.3.351 [DOI] [PubMed] [Google Scholar]
- 81. Beumer HM. Adverse effects of beta‐adrenergic receptor blocking drugs on respiratory function. Drugs. 1974;7(1):130‐138. doi: 10.2165/00003495-197407010-00009 [DOI] [PubMed] [Google Scholar]
- 82. Singh BN, Whitlock RM, Comber RH, Williams FH, Harris EA. Effects of cardioselective beta adrenoceptor blockade on specific airways resistance in normal subjects and in patients with bronchial asthma. Clin Pharmacol Ther. 1976;19(5 Pt 1):493‐501. doi: 10.1002/cpt1976195part1493 [DOI] [PubMed] [Google Scholar]
- 83. Skinner C, Gaddie J, Palmer KN. Comparison of effects of metoprolol and propranolol on asthmatic airway obstruction. Br Med J. 1976;1(6008):504. doi: 10.1136/bmj.1.6008.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reid JL, Whyte KF, Struthers AD. Epinephrine‐induced hypokalemia: the role of beta adrenoceptors. Am J Cardiol. 1986;57(12):23F‐27F. doi: 10.1016/0002-9149(86)90884-2 [DOI] [PubMed] [Google Scholar]
- 85. Castellino P, Bia MJ, DeFronzo RA. Adrenergic modulation of potassium metabolism in uremia. Kidney Int. 1990;37(2):793‐798. doi: 10.1038/ki.1990.47 [DOI] [PubMed] [Google Scholar]
- 86. Deacon SP, Barnett D. Comparison of atenolol and propranolol during insulin‐induced hypoglycaemia. Br Med J. 1976;2(6030):272‐273. doi: 10.1136/bmj.2.6030.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Antihypertensives and the risk of serious hypoglycemia in older persons using insulin or sulfonylureas. Jama. 1997;278(1):40‐43. [PubMed] [Google Scholar]
- 88. Capella D, Laporte JR, Castel JM, Tristan C, Cos A, Morales‐Olivas FJ. Parkinsonism, tremor, and depression induced by cinnarizine and flunarizine. BMJ. 1988;297(6650):722‐723. doi: 10.1136/bmj.297.6650.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chouza C, Scaramelli A, Caamano JL, de Medina O, Aljanati R, Romero S. Parkinsonism, tardive dyskinesia, akathisia, and depression induced by flunarizine. Lancet. 1986;1(8493):1303‐1304. doi: 10.1016/s0140-6736(86)91223-7 [DOI] [PubMed] [Google Scholar]
- 90. Anderson IM, Ferrier IN, Baldwin RC, et al. Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008;22(4):343‐396. doi: 10.1177/0269881107088441 [DOI] [PubMed] [Google Scholar]
- 91. Stahl SM, Entsuah R, Rudolph RL. Comparative efficacy between venlafaxine and SSRIs: a pooled analysis of patients with depression. Biol Psychiatry. 2002;52(12):1166‐1174. doi: 10.1016/s0006-3223(02)01425-7 [DOI] [PubMed] [Google Scholar]
- 92. Wenzel‐Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687‐693. doi: 10.3238/arztebl.2011.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hirsch LJ, Arif H, Nahm EA, Buchsbaum R, Resor SR Jr, Bazil CW. Cross‐sensitivity of skin rashes with antiepileptic drug use. Neurology. 2008;71(19):1527‐1534. doi: 10.1212/01.wnl.0000334295.50403.4c [DOI] [PubMed] [Google Scholar]
- 94. Arif H, Buchsbaum R, Weintraub D, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68(20):1701‐1709. doi: 10.1212/01.wnl.0000261917.83337.db [DOI] [PubMed] [Google Scholar]
- 95. Abou‐Khalil BW. Antiepileptic drugs. Continuum. 2016;22(1):132‐156. doi: 10.1212/CON.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 96. Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology. 2001;56(2):172‐177. doi: 10.1212/wnl.56.2.172 [DOI] [PubMed] [Google Scholar]
- 97. Nasreddine W, Beydoun A. Valproate‐induced thrombocytopenia: a prospective monotherapy study. Epilepsia. 2008;49(3):438‐445. doi: 10.1111/j.1528-1167.2007.01429.x [DOI] [PubMed] [Google Scholar]
- 98. Cohen AS, Matharu MS, Goadsby PJ. Electrocardiographic abnormalities in patients with cluster headache on verapamil therapy. Neurology. 2007;69(7):668‐675. doi: 10.1212/01.wnl.0000267319.18123.d3 [DOI] [PubMed] [Google Scholar]
- 99. Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530‐538. doi: 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 100. Moreno‐Ajona D, Villar‐Martinez MD, Goadsby PJ. New generation Gepants: migraine acute and preventive medications. J Clin Med. 2022;11(6):1‐11. doi: 10.3390/jcm11061656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tian R, Zhang Y, Pan Q, et al. Calcitonin gene‐related peptide receptor antagonist BIBN4096BS regulates synaptic transmission in the vestibular nucleus and improves vestibular function via PKC/ERK/CREB pathway in an experimental chronic migraine rat model. J Headache Pain. 2022;23(1):35. doi: 10.1186/s10194-022-01403-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Negro A, Martelletti P. Gepants for the treatment of migraine. Expert Opin Investig Drugs. 2019;28(6):555‐567. doi: 10.1080/13543784.2019.1618830 [DOI] [PubMed] [Google Scholar]
- 103. Russo CV, Sacca F, Braca S, et al. Anti‐calcitonin gene‐related peptide monoclonal antibodies for the treatment of vestibular migraine: a prospective observational cohort study. Cephalalgia. 2023;43(4):3331024231161809. doi: 10.1177/03331024231161809 [DOI] [PubMed] [Google Scholar]
- 104. Mecklenburg J, Raffaelli B, Neeb L, Sanchez Del Rio M, Reuter U. The potential of lasmiditan in migraine. Ther Adv Neurol Disord. 2020;13:1756286420967847. doi: 10.1177/1756286420967847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27(5):394‐402. doi: 10.1111/j.1468-2982.2007.01303.x [DOI] [PubMed] [Google Scholar]
- 106. Benjamin T, Gillard D, Abouzari M, Djalilian HR, Sharon JD. Vestibular and auditory manifestations of migraine. Curr Opin Neurol. 2022;35(1):84‐89. doi: 10.1097/WCO.0000000000001024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hindiyeh NA, Zhang N, Farrar M, Banerjee P, Lombard L, Aurora SK. The role of diet and nutrition in migraine triggers and treatment: a systematic literature review. Headache. 2020;60(7):1300‐1316. doi: 10.1111/head.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Koseoglu E, Talaslioglu A, Gonul AS, Kula M. The effects of magnesium prophylaxis in migraine without aura. Magnes Res. 2008;21(2):101‐108. [PubMed] [Google Scholar]
- 109. Tarighat Esfanjani A, Mahdavi R, Ebrahimi Mameghani M, Talebi M, Nikniaz Z, Safaiyan A. The effects of magnesium, L‐carnitine, and concurrent magnesium‐L‐carnitine supplementation in migraine prophylaxis. Biol Trace Elem Res. 2012;150(1–3):42‐48. doi: 10.1007/s12011-012-9487-5 [DOI] [PubMed] [Google Scholar]
- 110. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high‐dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50(2):466‐470. doi: 10.1212/wnl.50.2.466 [DOI] [PubMed] [Google Scholar]
- 111. Abouzari M, Goshtasbi K, Chua JT, et al. Adjuvant migraine medications in the treatment of sudden sensorineural hearing loss. Laryngoscope. 2021;131(1):E283‐E288. doi: 10.1002/lary.28618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dolati S, Rikhtegar R, Mehdizadeh A, Yousefi M. The role of magnesium in pathophysiology and migraine treatment. Biol Trace Elem Res. 2020;196(2):375‐383. doi: 10.1007/s12011-019-01931-z [DOI] [PubMed] [Google Scholar]
- 113. Roberts RA, Watford KE, Picou EM, Hatton K, Trone TH, Brignola EY. Effects of lifestyle modification on vestibular migraine. Otol Neurotol. 2021;42(10):e1537‐e1543. doi: 10.1097/MAO.0000000000003297 [DOI] [PubMed] [Google Scholar]
- 114. Alghadir AH, Anwer S. Effects of vestibular rehabilitation in the management of a vestibular migraine: a review. Front Neurol. 2018;9:440. doi: 10.3389/fneur.2018.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brown KE, Whitney SL, Marchetti GF, Wrisley DM, Furman JM. Physical therapy for central vestibular dysfunction. Arch Phys Med Rehabil. 2006;87(1):76‐81. doi: 10.1016/j.apmr.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 116. Brown KE, Whitney SL, Wrisley DM, Furman JM. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111(10):1812‐1817. doi: 10.1097/00005537-200110000-00027 [DOI] [PubMed] [Google Scholar]
- 117. Whitney SL, Wrisley DM, Brown KE, Furman JM. Physical therapy for migraine‐related vestibulopathy and vestibular dysfunction with history of migraine. Laryngoscope. 2000;110(9):1528‐1534. doi: 10.1097/00005537-200009000-00022 [DOI] [PubMed] [Google Scholar]
- 118. Magnan J, Ozgirgin ON, Trabalzini F, et al. European position statement on diagnosis, and treatment of Meniere's Disease. J Int Adv Otol. 2018;14(2):317‐321. doi: 10.5152/iao.2018.140818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nevoux J, Barbara M, Dornhoffer J, Gibson W, Kitahara T, Darrouzet V. International consensus (ICON) on treatment of Meniere's disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1S):S29‐S32. doi: 10.1016/j.anorl.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 120. Ozturk K, Ata N. Intratympanic mixture gentamicin and dexamethasone versus dexamethasone for unilateral Meniere's disease. Am J Otolaryngol. 2019;40(5):711‐714. doi: 10.1016/j.amjoto.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 121. Geng Y, Cao W, Xu H, Wu F, Feng T. Effects of an intratympanic injection of dexamethasone combined with gentamicin on the expression level of serum P0 protein antibodies in patients with Meniere's disease. Clinics. 2020;75:e1622. doi: 10.6061/clinics/2020/e1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sarna B, Abouzari M, Lin HW, Djalilian HR. A hypothetical proposal for association between migraine and Meniere's disease. Med Hypotheses. 2020;134:109430. doi: 10.1016/j.mehy.2019.109430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Abouzari M, Abiri A, Djalilian HR. Successful treatment of a child with definite Meniere's disease with the migraine regimen. Am J Otolaryngol. 2019;40(3):440‐442. doi: 10.1016/j.amjoto.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ogawa Y, Otsuka K, Hagiwara A, et al. Clinical study of tympanostomy tube placement for patients with intractable Meniere's disease. J Laryngol Otol. 2015;129(2):120‐125. doi: 10.1017/S0022215115000079 [DOI] [PubMed] [Google Scholar]
- 125. Sugawara K, Kitamura K, Ishida T, Sejima T. Insertion of tympanic ventilation tubes as a treating modality for patients with Meniere's disease: a short‐ and long‐term follow‐up study in seven cases. Auris Nasus Larynx. 2003;30(1):25‐28. doi: 10.1016/s0385-8146(02)00105-0 [DOI] [PubMed] [Google Scholar]
- 126. Abouzari M, Goshtasbi K, Sarna B, et al. Adapting personal therapies using a mobile application for tinnitus rehabilitation: a preliminary study. Ann Otol Rhinol Laryngol. 2021;130(6):571‐577. doi: 10.1177/0003489420962818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mahboubi H, Haidar YM, Kiumehr S, Ziai K, Djalilian HR. Customized versus noncustomized sound therapy for treatment of tinnitus: a randomized crossover clinical trial. Ann Otol Rhinol Laryngol. 2017;126(10):681‐687. doi: 10.1177/0003489417725093 [DOI] [PubMed] [Google Scholar]
- 128. Hesser H, Weise C, Westin VZ, Andersson G. A systematic review and meta‐analysis of randomized controlled trials of cognitive‐behavioral therapy for tinnitus distress. Clin Psychol Rev. 2011;31(4):545‐553. doi: 10.1016/j.cpr.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 129. Sullivan MD, Dobie RA, Sakai CS, Katon WJ. Treatment of depressed tinnitus patients with nortriptyline. Ann Otol Rhinol Laryngol. 1989;98(11):867‐872. doi: 10.1177/000348948909801107 [DOI] [PubMed] [Google Scholar]
- 130. Chen JJ, Chen YW, Zeng BY, et al. Efficacy of pharmacologic treatment in tinnitus patients without specific or treatable origin: a network meta‐analysis of randomised controlled trials. EClinicalMedicine. 2021;39:101080. doi: 10.1016/j.eclinm.2021.101080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Weinreich HM, Carey JP. Prevalence of pulsatile tinnitus among patients with migraine. Otol Neurotol. 2016;37(3):244‐247. doi: 10.1097/MAO.0000000000000968 [DOI] [PubMed] [Google Scholar]
- 132. Abouzari M, Tawk K, Lee D, Djalilian HR. Migrainous vertigo, tinnitus, and ear symptoms and alternatives. Otolaryngol Clin North Am. 2022;55(5):1017‐1033. doi: 10.1016/j.otc.2022.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Webster KE, Harrington‐Benton NA, Judd O, et al. Pharmacological interventions for persistent postural‐perceptual dizziness (PPPD). Cochrane Database Syst Rev. 2023;3(3):CD015188. doi: 10.1002/14651858.CD015188.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Webster KE, Kamo T, Smith L, et al. Non‐pharmacological interventions for persistent postural‐perceptual dizziness (PPPD). Cochrane Database Syst Rev. 2023;3(3):CD015333. doi: 10.1002/14651858.CD015333.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Axer H, Finn S, Wassermann A, Guntinas‐Lichius O, Klingner CM, Witte OW. Multimodal treatment of persistent postural‐perceptual dizziness. Brain Behav. 2020;10(12):e01864. doi: 10.1002/brb3.1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Staab JP, Ruckenstein MJ, Amsterdam JD. A prospective trial of sertraline for chronic subjective dizziness. Laryngoscope. 2004;114(9):1637‐1641. doi: 10.1097/00005537-200409000-00025 [DOI] [PubMed] [Google Scholar]
- 137. Yu YC, Xue H, Zhang YX, Zhou J. Cognitive behavior therapy as augmentation for sertraline in treating patients with persistent postural‐perceptual dizziness. Biomed Res Int. 2018;2018:8518631. doi: 10.1155/2018/8518631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133(2):170‐176. doi: 10.1001/archotol.133.2.170 [DOI] [PubMed] [Google Scholar]
- 139. Bittar RS, Lins EM. Clinical characteristics of patients with persistent postural‐perceptual dizziness. Braz J Otorhinolaryngol. 2015;81(3):276‐282. doi: 10.1016/j.bjorl.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161(1_suppl):S1‐S45. doi: 10.1177/0194599819859885 [DOI] [PubMed] [Google Scholar]
- 141. Battaglia A, Lualhati A, Lin H, Burchette R, Cueva R. A prospective, multi‐centered study of the treatment of idiopathic sudden sensorineural hearing loss with combination therapy versus high‐dose prednisone alone: a 139 patient follow‐up. Otol Neurotol. 2014;35(6):1091‐1098. doi: 10.1097/MAO.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 142. Plontke SK, Meisner C, Agrawal S, et al. Intratympanic corticosteroids for sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2022;7(7):CD008080. doi: 10.1002/14651858.CD008080.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Goshtasbi K, Chua JT, Risbud A, et al. Treatment of long‐term sudden sensorineural hearing loss as an otologic migraine phenomenon. Otol Neurotol. 2021;42(7):1001‐1007. doi: 10.1097/MAO.0000000000003111 [DOI] [PMC free article] [PubMed] [Google Scholar]


