Abstract
Phosphorylation of simian virus 40 large tumor (T) antigen on threonine 124 is essential for viral DNA replication. A mutant T antigen (T124A), in which this threonine was replaced by alanine, has helicase activity, assembles double hexamers on viral-origin DNA, and locally distorts the origin DNA structure, but it cannot catalyze origin DNA unwinding. A class of T-antigen mutants with single-amino-acid substitutions in the DNA binding domain (class 4) has remarkably similar properties, although these proteins are phosphorylated on threonine 124, as we show here. By comparing the DNA binding properties of the T124A and class 4 mutant proteins with those of the wild type, we demonstrate that mutant double hexamers bind to viral origin DNA with reduced cooperativity. We report that T124A T-antigen subunits impair the ability of double hexamers containing the wild-type protein to unwind viral origin DNA, suggesting that interactions between hexamers are also required for unwinding. Moreover, the T124A and class 4 mutant T antigens display dominant-negative inhibition of the viral DNA replication activity of the wild-type protein. We propose that interactions between hexamers, mediated through the DNA binding domain and the N-terminal phosphorylated region of T antigen, play a role in double-hexamer assembly and origin DNA unwinding. We speculate that one surface of the DNA binding domain in each subunit of one hexamer may form a docking site that can interact with each subunit in the other hexamer, either directly with the N-terminal phosphorylated region or with another region that is regulated by phosphorylation.
The initiation of simian virus 40 (SV40) DNA replication by the viral T antigen is a complex series of events that begins when T antigen binds specifically to a palindromic arrangement of four GAGGC pentanucleotide sequences in the minimal origin of viral DNA replication (recently reviewed in references 1, 2, 3, 22, and 48). In the presence of Mg-ATP, T antigen assembles cooperatively on the two halves of the palindrome as a double hexamer (10, 11, 13, 24, 30, 38, 51, 53). The DNA conformation flanking the T-antigen binding sites is locally distorted upon hexamer assembly (reference 7 and references therein). One pair of pentanucleotides is sufficient to direct double-hexamer assembly and local distortion of the origin DNA but not to initiate DNA replication (25). ATP hydrolysis by T-antigen hexamers then catalyzes bidirectional unwinding of the parental DNA (reference 53 and references therein). A mutant origin with a single nucleotide insertion in the center of the palindromic T-antigen binding site prevents cooperative interactions between hexamers and cannot support bidirectional origin unwinding (8, 51), suggesting that both processes require interactions between T-antigen hexamers. After assembly of the two replication forks, bidirectional replication is carried out by 10 cellular proteins and T antigen, which remains at the forks as the only essential helicase (reviewed in references 3, 22, and 48).
The phosphorylation state of SV40 T antigen governs its ability to initiate viral DNA replication (reviewed in references 15 to 17 and 39). T antigen contains two clusters of phosphorylation sites located at the N and C termini (40, 41). Phosphorylation of T antigen on threonine 124 in the N-terminal cluster was shown to be essential for viral DNA replication in monkey cells and in vitro (5, 14, 32–36, 44). Efforts to define what step in viral DNA replication requires modification of threonine 124 revealed that Mg-ATP-induced hexamer formation of T antigen in solution and DNA helicase activity of T antigen did not require phosphorylation at this site (33, 36). Origin DNA binding of T antigen lacking the modification at residue 124 was weaker than that of the modified T antigen (33, 34, 36, 44), but the reduction in binding was modest under the conditions used for SV40 DNA replication in vitro (36). Moreover, a mutant T antigen containing alanine in place of the phosphorylated threonine (T124A) assembled as a double hexamer on the viral origin and altered the conformation of the early palindrome and AT-rich sequences flanking the T-antigen binding sites in the viral origin in the same manner as the wild-type protein, except that higher concentrations were required (36). However, even at an elevated concentration, these mutant double hexamers were unable to unwind closed circular duplex DNA containing the viral origin (33, 36), suggesting that the defect in unwinding was responsible for the inability of T124A T antigen to replicate SV40 DNA. One possible explanation for the unwinding defect of the mutant T antigen, despite its helicase activity, was that some essential interaction between the two hexamers during bidirectional unwinding depended upon phosphorylation of threonine 124. Electron micrographs of SV40 DNA unwinding intermediates, which showed two single-stranded DNA loops protruding between two hexamers of T antigen, provided support for this explanation, implying that a double hexamer pulled the parental duplex DNA into the protein complex and spooled the single-stranded DNA out (53). Furthermore, double-hexamer formation significantly enhanced the helicase activity of T antigen (47, 47a).
Most of the T antigen isolated from mammalian cells is in a hyperphosphorylated form, containing multiple phosphoserines, as well as two phosphothreonines, and supports SV40 DNA replication in vitro poorly but can be stimulated by treatment with alkaline phosphatase or protein phosphatase 2A (19, 28, 37, 42, 49, 50). Hyperphosphorylated T antigen is unable to unwind duplex closed circular duplex DNA harboring the viral origin (4, 6, 51). Dephosphorylation of serines 120 and 123 restores its ability to unwind origin DNA (14, 43, 51). Studies of double-hexamer assembly on the origin indicate that phosphorylation of T antigen on serines 120 and 123 also impairs the cooperativity of double-hexamer assembly (14, 51). These results demonstrate that hyperphosphorylation of T antigen interferes with interactions between hexamers that are required for origin unwinding and raise the question of whether the phosphorylation state of threonine 124 might also affect the cooperativity of double-hexamer assembly on the viral origin.
One class of T antigen mutants with single-amino-acid substitutions in the DNA binding domain (class 4) has been reported to display properties similar to those of the T124A mutant and the hyperphosphorylated form of T antigen (54). Class 4 mutant proteins are defective in viral DNA replication in vivo and in vitro, bind to the viral origin as double hexamers and alter the local DNA conformation, and have helicase activity but do not unwind closed circular duplex viral DNA. The replication and unwinding defects could be due to faulty phosphorylation patterns or to other malfunctions not dependent on phosphorylation status.
The work presented here was undertaken to reevaluate the assembly of wild-type and T124A T antigen on SV40 origin DNA by using more-sensitive quantitative assays and to compare them with the class 4 mutants. We report that cooperativity of T124A T antigen in double-hexamer assembly on the viral origin is impaired. The class 4 mutant T antigens were also found to have defects in cooperativity of double-hexamer assembly. T124A T antigen inhibited the ability of the wild-type protein to unwind closed circular duplex origin DNA. Both T124A and the class 4 mutants displayed dominant-negative phenotypes in viral DNA replication in vitro. Based on these observations, we propose that the N-terminal cluster of phosphorylation sites and the DNA binding domain mediate cooperative hexamer-hexamer interactions during assembly on the viral origin and speculate that these regions of T antigen may interact during origin DNA unwinding.
MATERIALS AND METHODS
Protein purification.
SV40 T antigen and mutant T antigens (classes 4 and 5) (54) were expressed in Sf9 cells infected with recombinant baculoviruses and purified as described earlier (23). T antigen was stored in 20 mM HEPES-KOH (pH 8.5)–50 mM NaCl–0.1 mM EDTA–10% glycerol. Recombinant cyclin A-cdk2 purified by suc1 affinity chromatography (52) was the kind gift of C. Voitenleitner. Escherichia coli single-stranded DNA binding protein (SSB) was generously provided by V. Podust. I. Moarefi kindly provided calf thymus topoisomerase I.
Phosphorylation of T antigen in vitro.
Prior to the kinase reaction, samples of T antigen (3 μg) were pretreated with alkaline phosphatase (Merck no. 116072; 0.3 U) in a 20-μl reaction containing 20 mM Tris-HCl (pH 8)–5 mM MgCl2–0.1 mM ZnCl2–10% glycerol for 30 min at 37°C. Pretreatment with acid phosphatase (Merck no. 116071; 3 U) was carried out in a 20-μl reaction containing 50 mM sodium acetate (pH 7.0)–10% glycerol for 30 min at 37°C. Both phosphatases were supplied as ammonium sulfate suspensions, which were collected by centrifugation, resuspended in the appropriate phosphatase buffer, and dialyzed before use. A portion of the dephosphorylated T antigen was then immunoprecipitated in radioimmunoprecipitation assay (RIPA) buffer (20 mM HEPES-KOH, pH 7.8; 150 mM NaCl; 1% sodium dodecyl sulfate [SDS]; 0.2% sodium deoxycholate) with 10 μg of monoclonal antibody Pab 419 (20) and 50 μl of a 50% (vol/vol) protein G-agarose suspension. After incubation at 4°C for 1 h, the agarose beads were washed three times in RIPA buffer, three times in kinase buffer (20 mM HEPES-KOH, pH 7.5; 1 mM dithiothreitol [DTT]; 10 mM MgCl2; 4 mM EGTA; 5 mM NaF; 1 mM EDTA; 0.1 mg of bovine serum albumin [BSA] per ml; 0.1 mM ATP), and resuspended in kinase buffer. Kinase reactions (20 μl) containing kinase buffer, the T antigen bound to the beads, and 1 μCi of [γ-32P]ATP (Amersham) were incubated with an empirically determined amount of purified cyclin A-cdk2 for 15 min at 37°C. The reaction was stopped by adding Laemmli sample buffer (27), and the mixture was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (27) and autoradiography.
DNA binding assays.
An EcoRI-HindIII fragment of pORI DNA (12) containing the minimal SV40 origin was used in band shift assays. The DNA was 5′ end labeled with [γ-32P]ATP and T4 polynucleotide kinase. The concentration of DNA after labeling was determined with the DNA Dipstick Kit from Invitrogen. Concentrations of T antigen were measured by the Bio-Rad protein assay kit with BSA as the standard and are given as molar concentrations of T-antigen monomer.
Filter binding assays.
Next, 2 fmol of labeled origin DNA fragment was incubated in a 10-μl reaction containing 30 mM HEPES-KOH (pH 7.8), 7 mM MgCl2, 1 mM DTT, 40 mM creatine phosphate, 2 μg of creatine kinase, 4 mM AMP-adenosine 5′-β,γ-imino-triphosphate, 100 ng of pBluescript KSII DNA, 1 μg of BSA, and the indicated amount of T antigen for 30 min at 37°C. Protein was cross-linked to DNA by adding glutaraldehyde to a final concentration of 0.2% followed by incubation for another 5 min. Unreacted glutaraldehyde was quenched by adding a 0.1 volume of 10 mM HEPES-KOH (pH 7.8)–100 mM glycine and incubating the mixture for 5 min. The reaction was diluted with 1 ml of filtration buffer (30 mM HEPES-KOH, pH 7.8; 7 mM MgCl2; 0.1 mM DTT) and immediately filtered over alkali-washed nitrocellulose filters (pore size, 45 μm) (31). The filters were washed with 5 ml of filtration buffer, air dried, and evaluated by liquid scintillation counting.
Electrophoretic mobility shift assays.
Assays were performed essentially as described previously (51). Briefly, binding reactions (10 μl) contained 30 mM HEPES-KOH (pH 7.8), 7 mM MgCl2, 1 mM DTT, 40 mM creatine phosphate, 2 μg of creatine kinase, 4 mM AMP-PNP, 100 ng of pBluescript KSII DNA, and 1 μg of BSA and the indicated amounts of labeled origin DNA fragment (specific activity, 10,000 cpm/fmol) and T antigen. The reaction was incubated for 30 min at 37°C unless otherwise stated. T antigen was cross-linked to DNA by the addition of glutaraldehyde to a final concentration of 0.2% and incubation for another 5 min. After addition of a 0.2 volume of loading buffer (10 mM HEPES-KOH, pH 7.8; 2% Ficoll 400; 0.2% bromophenol blue; 0.2% xylene cyanol), protein-DNA complexes were resolved by electrophoresis in a 3.5% native polyacrylamide gel in TBE (89 mM Tris-borate, 89 mM boric acid, 2 mM EDTA) at 200 V. The gel was dried, autoradiographed, and quantitated by densitometry of the autoradiogram.
Determination of origin DNA binding parameters of T antigen.
To measure apparent association rates (konapp), 1.2 × 10−8 M wild-type T antigen or 2 × 10−8 M T124A were incubated with 2 × 10−10 M origin DNA (EcoRI-HindIII fragment of pORI) in a 100-μl mixture. At different times, 10-μl aliquots were removed, and reactions quenched by adding 0.5 μl of unlabeled pPSori64 competitor DNA (2.4 pmol/μl), which contains 64 copies of SV40 origin DNA (51) (104-fold molar excess). After a cross-linking with glutaraldehyde, each sample was immediately loaded on the gel and electrophoresed. After autoradiography and quantitation, 1/([P0] − [D0]) ln {[D0]([P0] − [PD])/[P0]([D0] − [PD])} was plotted as a function of time (t), where [P0] and [D0] are the concentrations of free protein and free DNA at time 0, and [PD] is the concentration of protein-DNA complex at time t. The konapp corresponds to the slope of the line (18).
To measure apparent dissociation rates (koffapp), 1.2 × 10−8 M wild-type T antigen or 2 × 10−8 M T124A were incubated with 2 × 10−10 M origin DNA in a 100-μl reaction for 30 min at 37°C to reach saturation binding of the origin DNA. Then, 5 μl of competitor DNA pPSori64 (2.4 pmol/μl) was added (time 0), giving a final volume of 105 μl. At different times, 10.5-μl portions were withdrawn, cross-linked, loaded directly onto a gel, and electrophoresed. After autoradiography and quantitation, the apparent dissociation rate constant koffapp was determined by plotting ln([PD]/[PD0]) versus time (t), where [PD0] and [PD] are the concentrations of protein-DNA complex at times 0 and t. The slope of the line is equal to −koff (18). Half-lives of the complexes were calculated by the equation: t1/2 = −ln (0.5)/koff.
Origin DNA unwinding assays.
Reactions (total volume, 20 μl) contained 200 ng of supercoiled pUC-HS DNA, 40 mM HEPES-KOH (pH 7.9), 0.5 mM DTT, 8 mM MgCl2, 4 mM ATP, 40 mM creatine phosphate, 0.5 μg of creatine kinase, 2 μg of BSA, 120 ng of topoisomerase I, and 250 ng of E. coli SSB and were started by adding the desired amount of T antigen. After 1 h at 37°C, the mixture was incubated in 0.2% SDS–400 ng of proteinase K at 37°C for 30 min and then ethanol precipitated. The samples were redissolved in 10 mM EDTA–2% Ficoll–2% sucrose–0.01% bromophenol blue–0.1% SDS and electrophoresed in 1.5% agarose gels. The gel was stained with ethidium bromide, and unwound DNA was quantitated by densitometric scanning of the gel by using the Image Store 7500 software (Ultra-Violet Products, Inc.).
SV40 DNA replication assays.
In vitro replication reactions were carried out essentially as described earlier (36) with slight modifications. The reaction (60 μl) contained 30 mM HEPES-KOH, pH 7.8; 7 mM magnesium acetate; 1 mM EGTA; 0.5 mM DTT; 4 mM ATP; 0.2 mM concentrations each of CTP, GTP, and UTP; 0.1 mM concentrations each of dGTP and dATP; 0.05 mM concentrations each of dCTP and dTTP; 5 μCi each of [α-32P]dCTP and [α-32P]dTTP; 40 mM creatine phosphate; 4.8 μg of creatine kinase; 100 ng of pUC-HS DNA; 600 ng of T antigen; and 190 μg of S100 extract prepared from human 293S cells. After 90 min at 37°C, 5-μl portions of the reaction were spotted onto DE81 paper to quantitate incorporated nucleotides. EDTA, SDS, and proteinase K were added to final concentrations of 20 mM, 0.65%, and 1.7 mg/ml, respectively, and incubation was continued for another 30 min. The sample was extracted once with phenol-chloroform, and the DNA was passed over a Sephadex G-50 spin column (Boehringer-Mannheim) equilibrated in TE buffer (10 mM Tris-HCl, pH 8; 1 mM EDTA). DNA was ethanol precipitated and dissolved in 20 μl of TE buffer. Then, 2-μl aliquots were digested with EcoRI or EcoRI/DpnI, and reaction products were separated by 0.8% agarose gel electrophoresis in TBE. The gel was then dried and exposed to X-ray film.
RESULTS
Cooperativity of double-hexamer assembly on viral origin DNA is reduced in the T124A mutant T antigen.
T124A and wild-type T antigen unphosphorylated on threonine 124 are able to bind to viral origin DNA, generate the same DNase I protection pattern, and induce the same conformational changes in the origin as the correctly phosphorylated wild-type protein (33, 36). Moreover, T124A and wild-type T antigen contact the same phosphates in the origin DNA backbone (45). Nevertheless, immunoprecipitation assays have indicated that T124A T antigen and wild-type T antigen unphosphorylated at threonine 124 binds to the viral origin with reduced affinity (32–36, 44). However, the cooperativity of T124A T antigen assembly on the viral origin was not assessed.
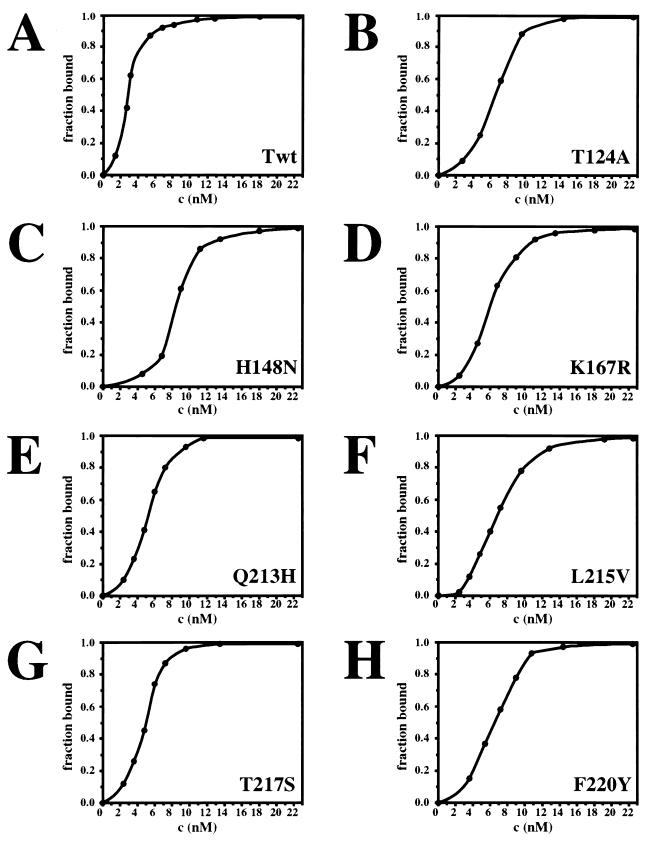
To test whether the reduced origin DNA binding of T124A T antigen might arise through loss of cooperativity, binding was measured with increasing amounts of wild-type and T124A T antigen in a filter-binding assay (Fig. 1A and B). Both proteins gave a sigmoidal binding curve, suggesting that both may bind cooperatively to the viral origin. However, about twofold more T124A T antigen was required to reach half-maximal saturation of the DNA, and the slope of the binding curve was less steep than with the wild-type protein (compare Fig. 1A and B). This could indicate that the affinity of T124A T antigen for origin DNA was reduced in comparison with the wild-type protein and/or that interactions between T124A hexamers were diminished.
FIG. 1.
SV40 origin DNA binding as a function of the concentration of wild-type and mutant T antigens. The indicated amounts of wild-type (A) and mutant (B-H) T antigen were incubated with labeled origin DNA fragment for 30 min and then filtered over nitrocellulose. Protein-DNA complexes retained on the filter were quantitated by liquid scintillation counting. The fraction of bound DNA was plotted as a function of the T-antigen concentration.
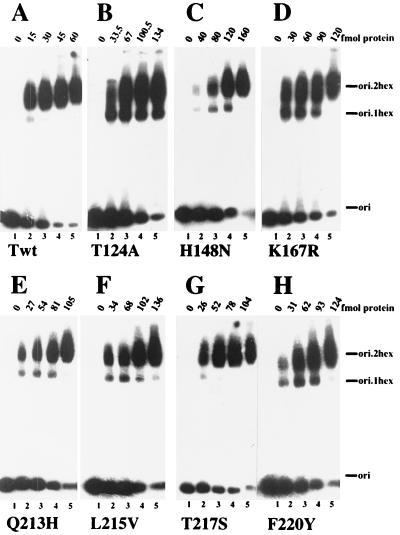
To test for cooperativity of origin DNA binding in more detail, electrophoretic mobility shift assays were used to detect DNA-protein complexes. As in the filter binding assays, about twofold more T124A T antigen than wild type was needed to shift half of the origin DNA into the protein-bound forms (Fig. 2A and B, Table 1). However, single hexamers of T124A T antigen comprised a significant fraction of the DNA-protein complexes at all T-antigen concentrations tested (Fig. 2B, lanes 2 to 5). Quantitation of the fraction of single hexamers in the bound DNA at the point of half-maximal shift, used as a measure of hexamer-hexamer interaction, revealed that only 9% of the wild-type T antigen complexes were single hexamers, whereas about 32% of T124A T-antigen complexes were single hexamers (Table 1). These data suggest that cooperativity between the two hexamers of T124A T antigen in binding to the viral origin DNA is reduced three- to fourfold.
FIG. 2.
Assembly of single and double hexamers of T antigen on viral origin DNA. Increasing amounts of wild type (A) and the indicated mutant T antigens (B to H) were incubated in 10-μl reactions with labeled origin DNA fragment. After cross-linking with glutaraldehyde, free and bound DNA from each reaction were resolved by 3.5% PAGE, and the dried gel was analyzed by autoradiography. The autoradiograms were quantitated densitometrically, and the fraction of the bound DNA in single-hexamer complexes at half-maximal saturation is given in Table 1.
TABLE 1.
SV40 origin DNA binding parameters of wild-type and mutant T antigens
| T antigen (mutant class) | Concn1/2 (nM)a | DNA fraction in single hexamersb |
|---|---|---|
| T (wild type) | 3.0 | 0.09 |
| T124A | 6.7 | 0.32 |
| H148N (4) | 8.0 | 0.20 |
| K167R (4) | 6.0 | 0.24 |
| Q213H (4) | 5.4 | 0.20 |
| L215V (4) | 6.8 | 0.18 |
| T217S (5) | 5.2 | 0.06 |
| F220Y (4) | 6.2 | 0.30 |
The concentrations of protein that led to half-maximal saturation of the origin DNA (concn1/2) were determined in nitrocellulose filter binding assays (Fig. 1).
The amounts of free SV40 origin DNA and origin DNA in hexamer and double-hexamer complexes observed in band shift experiments at half-maximal shift (Fig. 2) were quantitated to give the fraction of DNA in single hexamers, i.e., (hex1)/(hex1 + hex2).
Mutations in the DNA binding domain of T antigen impair cooperative binding to viral origin DNA.
The data above and previous reports (14, 51) implicate the N-terminal cluster of T-antigen phosphorylation sites in regulating cooperative interactions between two T antigen hexamers. However, the surfaces of T antigen that contact each other in these putative hexamer-hexamer interactions are unknown. One might expect T-antigen mutants with defects in hexamer-hexamer interactions to have a phenotype similar to that observed for T124A. One class of T antigen mutants with conservative single-amino-acid substitutions within the origin DNA binding domain (class 4) was reported to be deficient in unwinding superhelical SV40 DNA and in supporting viral DNA replication in vitro and in vivo (54), a phenotype reminiscent of the T124A mutant. These mutants could define a surface of T antigen involved in contacts between hexamers.
To determine whether the class 4 mutations affected hexamer-hexamer interactions, the origin DNA binding properties of these mutants were investigated. A class 5 mutant T antigen T217S that is defective in viral DNA replication in vivo but active in vitro (54) was used as a positive control. Increasing amounts of the class 4 and 5 mutant T antigens were assayed for origin DNA binding in nitrocellulose filter binding assays (Fig. 1) and in electrophoretic mobility shift experiments (Fig. 2). The saturation isotherms measured in filter-binding assays were sigmoidal for the class 4 and 5 mutant proteins (Fig. 1, C to H), just as for the wild-type T antigen and T124A. However, the slopes were less steep with the wild-type protein, and a two- to threefold-greater concentration of the mutant proteins was required to reach half-maximal saturation of the DNA (Table 1). These results suggest that all of the mutants bound cooperatively to the viral origin DNA, although the binding was weaker than that of the wild-type T antigen. In agreement with this, all of the class 4 mutant proteins assembled into double hexamers less efficiently than the wild-type T antigen (Fig. 2, compare A with C to F and H), and single hexamers were nearly as prominent with the class 4 mutants as with T124A (compare B with C to F and H). In contrast, only small amounts of single-hexamer complexes were observed with the class 5 mutant T antigen, a finding comparable to that observed with the wild-type protein (compare A and G).
Quantitative evaluation of the data yielded the T-antigen concentrations that led to half-maximal saturation of the DNA, as well as the fraction of bound DNA in single hexameric complexes under the conditions of half-maximal saturation. All of the mutant T antigens, including the class 5 mutant, bound about two- to threefold less origin DNA than did the wild-type protein (Fig. 1C to H, Table 1). The class 5 mutant formed double hexamers as well as did the wild-type protein (Fig. 2, compare A and G), whereas the class 4 mutants were clearly defective in their ability to assemble double hexamers on the viral origin. Comparison of the fraction of bound DNA in single hexamers of class 4 T antigen with that of class 5 and wild-type T antigen suggests that interactions between class 4 hexamers were reduced from two- to fivefold (Table 1). Although the defects observed with some of the class 4 T antigens were less marked than with T124A, the results indicate that all of these class 4 mutations impair interactions between two hexamers of T antigen.
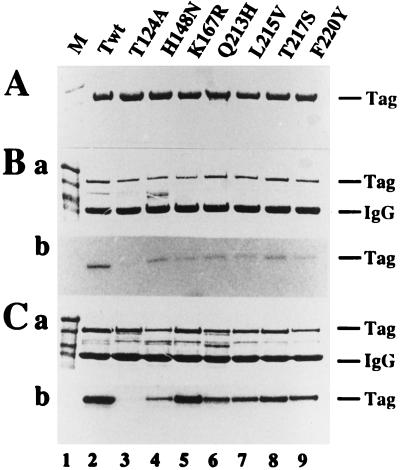
A trivial explanation for the similar phenotypes of the class 4 mutants and T124A would be that class 4 mutant T antigens were not properly phosphorylated. The class 4 and 5 T antigens were purified from insect cells infected with the corresponding recombinant baculovirus (Fig. 3A). Like the wild-type T antigen (23), they were hypophosphorylated (data not shown). However, to verify that threonine 124 was properly phosphorylated, the purified T antigens were phosphorylated with purified cyclin A-cdk2 (52), which specifically modifies this site in T antigen (23, 32). Since baculovirus-expressed wild-type T antigen is almost quantitatively phosphorylated on threonine 124, it was poorly modified by cyclin-dependent kinases (23). Similarly, only low levels of phosphorylation were detected with the class 4 and 5 proteins (data not shown). To ensure that threonine 124 in the mutant proteins was accessible for the kinase, T antigens were pretreated with alkaline or acid phosphatase prior to the phosphorylation reaction. Alkaline phosphatase removes phosphate only from phosphoserines on T antigen (19, 26, 46), whereas acid phosphatase targets phosphothreonine 124 and the phosphoserines, but not phosphothreonine 701 (19, 26). Thus, by comparing cyclin A-cdk2 phosphorylation of class 4 T antigens pretreated with alkaline phosphatase or acid phosphatase with phosphorylation of the pretreated wild-type protein, the phosphorylation state of threonine 124 in the mutant proteins can be compared with that in the wild type. Alkaline phosphatase-treated class 4 T antigens were phosphorylated at a low level by cyclin A-cdk2, one comparable to the level observed with wild-type T antigen (Fig. 3Bb). In contrast, acid phosphatase-pretreated T antigens were phosphorylated more efficiently than the alkaline phosphatase-pretreated proteins (compare Fig. 3Cb and Bb). No phosphorylation was observed with T124A T antigen (Fig. 3B and C, lanes 3), confirming the specificity of cyclin A-cdk2 for threonine 124 in this experiment. The results demonstrate that the baculovirus-expressed class 4 and 5 mutant proteins, like the wild-type T antigen, were phosphorylated on threonine 124. Hence, the impaired assembly of double hexamers with class 4 mutants is probably not caused by defects in phosphorylation.
FIG. 3.
In vitro phosphorylation of threonine 124 in class 4 and 5 mutant T antigens is strongly enhanced by pretreatment with acid phosphatase. (A) Each of the indicated T antigens was purified and analyzed by electrophoresis in 10% denaturing gels and then Coomassie blue stained. (B and C) Each of the purified T antigens was treated with alkaline (B) or acid (C) phosphatase, immunoprecipitated, and then phosphorylated with purified cyclin A-cdk2 and [γ-32P]ATP. Proteins were separated by electrophoresis on 10% denaturing gels, Coomassie stained (a), and visualized by autoradiography (b). The positions of molecular weight marker proteins (lanes 1), T antigen (Tag) (lanes 2 to 9), and the immunoglobulin heavy chain (IgG) are indicated.
Kinetics of wild-type and T124A T antigen interaction with SV40 origin DNA.
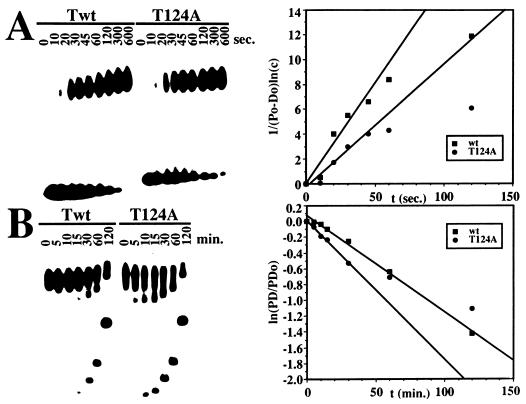
The equilibrium DNA binding data presented above suggest that interactions between hexamers of T124A and class 4 mutants in assembly on the origin are reduced. If interactions between T-antigen hexamers are indeed impaired in the T124A and class 4 mutants in assembly on the origin are reduced. If interactions between T-antigen hexamers are indeed impaired in the T124A mutant, one might expect the assembly of double hexamers to be slower than with the wild-type protein and the dissociation of double hexamers to be faster. This prediction was tested by measuring on- and off-rates for wild-type and T124A T antigen on origin DNA in band shift experiments (Fig. 4). The overall on-rate of T124A was about 1.5-fold lower and the off-rate about 1.5-fold higher than that of wild-type T antigen (Table 2). Using the off-rates, the half-life of T antigen-DNA complexes was calculated to be approximately 60 min for wild type and 40 min for mutant T antigen (Table 2). The on-rates for both proteins were clearly slower than expected for a diffusion-controlled rate of 108 to 109 M−1 s−1 (9). The slow on-rate probably reflects the assembly of T-antigen monomers into hexamers on the origin DNA (11, 24), which could be the rate-limiting step in origin binding. Dissociation constants calculated from the observed on- and off-rates indicate that the affinity of T124A for the origin DNA was two- to threefold lower than that of wild-type T antigen (Table 2). These values agree well with the results of the filter-binding assays (Table 1).
FIG. 4.
Time course of wild type (Twt) and T124A T antigen binding to origin DNA and dissociation. (A) On-rates were determined by incubating 1.2 pmol of Twt (left panel, first nine lanes) or 2 pmol of T124A (second nine lanes) with 200 fmol of labeled origin DNA fragment in a volume of 100 μl. At the indicated times, samples were withdrawn and the binding reaction was quenched by the addition of excess unlabeled competitor DNA. After cross-linking, each sample was immediately electrophoresed in a 3.5% native gel. Note that the samples from short binding reactions were electrophoresed for longer times and thus migrated farther into the gel than the samples from longer binding reactions. (Right) Bound and free DNA were quantitated, and 1/(P0-D0) ln(c) (107 liters/mol) was plotted as a function of incubation time as described in Materials and Methods. The slope of the line equals konapp. (B) Off-rates were determined by first incubating 1.2 pmol of Twt (first seven lanes) or 2 pmol T124A (second seven lanes) to equilibrium with 200 fmol of labeled origin DNA fragment in a volume of 100 μl. Then, excess unlabeled competitor DNA was added. At the indicated times after the addition of competitor, samples were withdrawn, treated with glutaraldehyde, and immediately electrophoresed in a 3.5% polyacrylamide gel. Note that samples electrophoresed for longer times migrated farther into the gel than those electrophoresed for shorter times. (Right) Bound and free DNA were quantitated, and ln(PD/PD0) was plotted as a function of reaction time as described in Materials and Methods. The negative of the slope is equal to koffapp.
TABLE 2.
SV40 origin DNA binding parameters of wild-type T antigen and mutant T124A
| DNA binding parametera | T antigen (wild type) | T124A |
|---|---|---|
| konapp (M−1 s−1) | 1.6 × 106 | 1.0 × 106 |
| koffapp (s−1) | 2.0 × 10−4 | 3.0 × 10−4 |
| t1/2 (min) | 57 | 39 |
| koffapp/konapp (M) | 1.2 × 10−10 | 3.0 × 10−10 |
Apparent association rates konapp (M−1 s−1) and dissociation rates koffapp (s−1) were determined in band shift experiments (Fig. 4) by quantitating the amounts of shifted and unshifted DNA. Apparent dissociation constants Kdapp were calculated by the formula Kdapp = koffapp/konapp. The stabilities t1/2 (min) of the protein-DNA complexes were calculated from the equation t1/2 = −ln (0.5)/koffapp.
T124A shows reduced stability as a DNA-bound double hexamer.
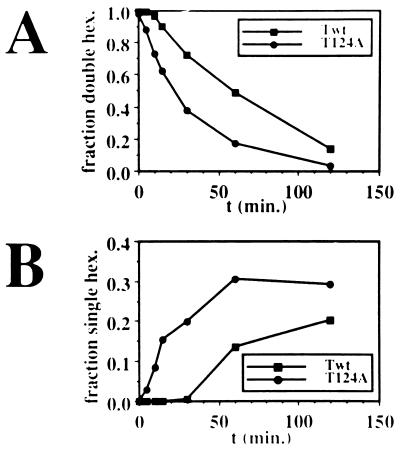
To further assess the possible contributions of hexamer-hexamer interactions to the assembly of T124A T antigen on the origin, we compared the rates of dissociation of the double-hexamer DNA complexes of T124A and wild-type T antigen into the single-hexamer complexes in the off-rate experiments (Fig. 4B). The amounts of DNA in each form were quantitated and plotted as a function of time. The double-hexamer form of T124A T antigen decreased significantly faster than did the wild-type double-hexamer complex (Fig. 5A). The half-life of the wild-type double hexamer was about 60 min, whereas that of the T124A double hexamer was only 20 min. Conversely, the single-hexamer form of T124A increased much more rapidly than that of wild-type T antigen (Fig. 5B). After 30 min, when about 20% of the DNA was already present in single hexamers with T124A T antigen, only traces of single hexamer were observed with the wild-type protein. These results indicate that the stability of the mutant T antigen as a double hexamer on the origin is impaired to a greater extent than binding as a single hexamer.
FIG. 5.
The stability of T antigen double hexamers bound to origin DNA. From the data in Fig. 4B, the fractions of DNA in (A) double-hexamer complexes (A) and single-hexamer complexes (B) were plotted as a function of time.
Dominant-negative phenotypes of T124A and the class 4 mutants.
Defects in cooperativity of double-hexamer assembly of T124A and the class 4 mutant T antigens appeared to correlate with their inability to unwind closed circular duplex origin DNA, an essential step in the initiation of viral DNA replication. This correlation could be interpreted to indicate that interactions between hexamers are necessary for bidirectional unwinding of the origin. Yet the observed reduction in cooperativity was not more than about fourfold, raising the question of how this relatively minor defect could inhibit the unwinding and replication activities of these proteins so dramatically. One possible solution to this conundrum that is consistent with the importance of the double hexamer in origin unwinding (47, 47a, 53) would be that multiple cycles of hexamer association and dissociation occur during the unwinding reaction, whereas only one stable association between hexamers is required for the initial double-hexamer assembly on the origin. If each hexamer had six possible binding sites in the other hexamer and each of these interactions were less stable with the mutant than with the wild-type protein, the necessity for repeated interactions during unwinding would quickly amplify the effect of a small reduction in each individual interaction. If repeated interactions between hexamers are indeed required for unwinding, one would predict that the mutant T antigens should impair the unwinding activity of the wild-type protein.
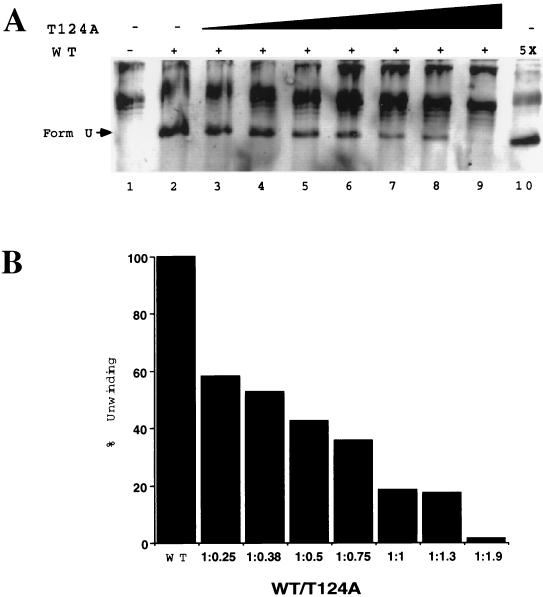
This prediction was tested with mixtures of T124A and wild-type T antigen (Fig. 6). Supercoiled SV40 origin DNA incubated without T antigen, but with topoisomerase I and SSB, became relaxed (lane 1). When wild-type T antigen was present in the reaction, an underwound form-U DNA was generated (lane 2). Addition of increasing amounts of T124A T antigen suppressed the appearance of the unwinding products in a dose-dependent manner (lanes 3 to 9). A mixture containing 800 ng each of T124A and wild-type T antigen generated only about 20% of the underwound DNA generated by 800 ng of the wild-type protein alone. Even the smallest amount of T124A T antigen tested reduced the unwinding activity of the wild type by 40%. These results indicate that the presence of T124A T antigen subunits in double hexamers inhibited the unwinding activity of the wild-type subunits.
FIG. 6.
Unwinding of closed circular duplex SV40 origin DNA by wild-type (WT) T antigen is suppressed by T124A. (A) Supercoiled SV40 origin DNA was incubated with topoisomerase I and E. coli SSB without T antigen (lane 1) or with 800 ng of wild type (lane 2) or with 800 ng of wild type mixed with 200 ng (lane 3), 300 ng (lane 4), 400 ng (lane 5), 600 ng (lane 6), 800 ng (lane 7), 1 μg (lane 8), or 1.5 μg (lane 9) of T124A T antigen. Only background unwinding activity was observed with T124A (data not shown and reference 36). Lane 10 shows a control reaction containing 4 μg of wild type to test whether excess T antigen would inhibit unwinding. Reaction products were analyzed by agarose gel electrophoresis and ethidium bromide staining. (B) Unwound forms of DNA in each lane were quantitated by densitometry. After subtraction of background (panel A, lane 1), unwinding activity at different ratios of wild type to T124A T antigen was expressed as a percentage of the unwinding activity of wild type (panel A, lane 2), which was set to 100%.
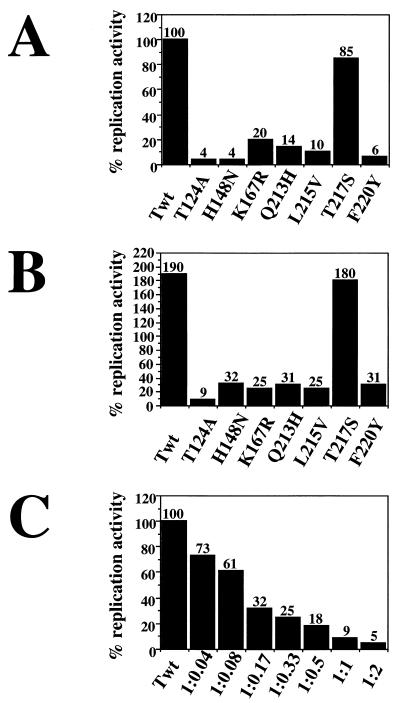
The dominant-negative inhibition by T124A of origin unwinding by the wild-type protein suggested that the T124A mutant should also inhibit replication of viral DNA by wild-type T antigen. Similarly, one might expect the class 4 T antigens to have a dominant-negative phenotype in replication assays. SV40 replication reactions were performed with each of the purified mutant T antigens and the wild type to compare their activities (Fig. 7A). As reported previously (35, 36, 54), little or no SV40 DNA replication was detected with T124A or the class 4 mutants, while the class 5 mutant protein was nearly as active as wild-type T antigen. Mixtures containing equal amounts of a mutant protein and the wild-type T antigen were then tested for replication activity. Doubling the amount of T antigen nearly doubled the amount of replication products observed with the wild-type protein and the class 5 mutant (Fig. 7B). In contrast, the replication activity of the wild-type protein was potently inhibited by T124A and, slightly less strongly, by the class 4 mutant proteins (Fig. 7B). To facilitate a comparison of the effects of T124A on unwinding and replication, the replication activity of wild-type T antigen was tested in the presence of increasing amounts of T124A T antigen. The replication activity of the wild-type protein was almost abolished in the presence of an equal amount of T124A T antigen (Fig. 7C). Even the smallest amount of T124A clearly inhibited the replication activity of the wild-type protein (Fig. 7C). Comparison of the ability of T124A T antigen to inhibit origin unwinding and replication by the wild-type protein indicates that at a given ratio of mutant to wild-type protein, the inhibition of replication was stronger (compare Fig. 6B and 7C), probably because a complete round of replication requires more extensive unwinding than production of form-U DNA.
FIG. 7.
T124A and class 4 T antigens are dominant-negative inhibitors of SV40 DNA replication. (A) The in vitro SV40 DNA replication activities of the indicated mutant T antigens were assayed and are expressed as a percentage of the deoxyribonucleoside monophosphate incorporation (35 pmol) by an equal amount of wild-type T antigen (Twt), which was set to 100%. (B) Replication reactions contained either 1200 ng of Twt (column 1) or a mixture of 600 ng of Twt and 600 ng of the indicated mutant T antigen. The replication activities of each mixture were expressed as a percentage of the dNMP incorporation that was obtained with 600 ng of Twt alone. (C) Replication reactions were carried out with Twt alone (column 1) or with mixtures of T124A and Twt in the indicated ratios (wild type/T124A). Incorporation of dNMP by Twt alone was set to 100%, and the replication activities of the mixtures were expressed as a percentage of the activity of Twt alone.
DISCUSSION
Two regions of T antigen contribute to cooperative interactions between hexamers on the SV40 origin of DNA replication.
Quantitative DNA binding experiments have shown that T124A T antigen is compromised in its ability to form double hexamers on the SV40 origin (Fig. 1, 2, 4, and 5; Table 1 and 2). The overall difference between wild type T antigen and T124A in apparent origin binding affinity was only about twofold. However, double hexamers of T124A bound to the origin dissociated to single hexamers about threefold faster than wild-type double hexamers (Fig. 5A) and accumulated as single hexamers (Fig. 5B). These observations suggest that the impaired interactions between the mutant hexamers may be sufficient to account for the observed reduction in origin binding affinity. Taken together, the DNA binding data implicate the phosphorylation of threonine 124 in cooperative hexamer-hexamer interactions.
The cooperativity of hexamer-hexamer interactions on SV40 origin DNA has also been shown to depend on the absence of phosphorylation on serines 120 and 123 (14, 51). Phosphorylation of these serines reduced cooperativity even more strongly than the lack of phosphorylation on threonine 124. The dissociation of double hexamers phosphorylated on serines 120 and 123 was ninefold faster than that of double hexamers unphosphorylated on the serines (51), compared with a threefold difference between T124A and wild-type T antigens (Fig. 5B). These findings indicate that the modification pattern of the N-terminal cluster of phosphorylation sites plays a critical role in the interactions between hexamers of T antigen.
The class 4 T-antigen mutants (54) were also reduced in origin DNA binding and cooperativity of double-hexamer assembly on the viral origin (Fig. 1 and 2, Table 1). In contrast, a class 5 mutant T antigen with reduced origin DNA binding displayed no defect in cooperativity of double-hexamer assembly. Since the phosphorylation pattern of the class 4 mutant proteins resembled that of the wild type (Fig. 3; data not shown), the reduced cooperativity of double-hexamer formation observed with the class 4 mutants appears to be due directly to structural alterations in the DNA binding domain itself rather than to secondary changes in the phosphorylation pattern of the mutant proteins.
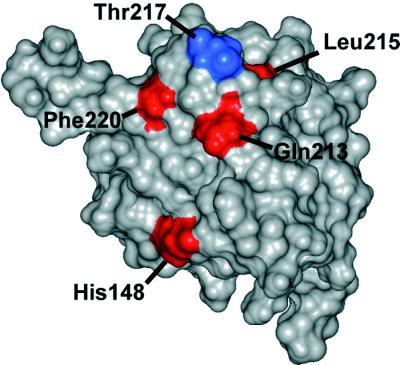
The observation that changes in the modification pattern in the N-terminal phosphorylation region, as well as mutations in the DNA binding domain of T antigen, impair the cooperativity of hexamer assembly on origin DNA suggests that these two regions of T antigen contribute to the cooperativity between hexamers. The simplest explanation for this cooperativity would be that these two regions participate directly in hexamer-hexamer contacts. Inspection of the structure of the DNA binding domain (residues 131 to 260) determined by nuclear magnetic resonance spectroscopy (29) reveals that three of the class 4 mutations and the class 5 mutation target a cluster of residues on the surface of the protein. These residues form a cone, with Gln-213, Leu-215, and Phe-220 at the base and Thr-217 at the apex. Thus, it is possible that this surface forms a docking site in each subunit of one hexamer for a second region in each subunit of the other hexamer (Fig. 8; class 4 mutation sites are depicted in red). Interestingly, substitution of Thr-217 by Ser (class 5 mutant depicted in blue in Fig. 8) did not lead to a loss of cooperativity in double-hexamer assembly, probably because the two amino acids are similar enough to substitute for each other in viral DNA replication, at least in vitro (Fig. 7 [54]). One class 4 mutation targets His-148, which resides on the surface near the cone-shaped structure (29) and may constitute part of the proposed docking site (Fig. 8, red residue). The remaining class 4 mutation targets a residue (Lys-167) that is buried in the isolated DNA binding domain (29), but it seems possible that phosphorylation of Thr-124 in the full-length T antigen could alter the conformation of this domain, making Lys-167 accessible on the surface and thereby strengthening interactions between hexamers. Interestingly, two previously described T-antigen mutants, C8A and SVR9D, which were defective in DNA replication but retained some origin DNA binding and DNA helicase activity target residues (i.e., residues 224 and 214), reside on this same surface of the DNA binding domain (36a, 48a).
FIG. 8.
Proposed hexamer-hexamer docking site in the DNA binding domain of T antigen. A docking site in the DNA binding domain of one subunit of a hexamer is postulated to contact the phosphorylated region of one subunit in the other hexamer or another region regulated by phosphorylation. A space-filling representation of the DNA binding domain of T antigen (29) is shown with the residues comprising the proposed docking site in color: His-148, Gln-213, Leu-215, and Phe-220 (in red) and Thr-217 (in blue). See the text for details.
The isolated DNA binding domain is monomeric (data not shown [29]), suggesting that it may not be sufficient by itself to mediate contacts between hexamers and that a second region of T antigen could be involved. The structure of the N-terminal cluster of phosphorylation sites is not yet known, but based on the properties of T124A and T antigen phosphorylated on serines 120 and 123, we speculate that this region may constitute part of a surface with which the putative docking site interacts. Consistent with this proposal, there is some evidence that the N-terminal region of T antigen is sufficient to mediate oligomerization that is distinct from hexamer formation because it depends on the phosphorylation state. Band shift origin DNA binding experiments performed with T-antigen residues 1 to 259 lacking any phosphorylation showed no evidence of higher-order protein-protein complexes, whereas after phosphorylation of the peptide with cyclin-dependent kinase, higher-order complexes became detectable (34). However, it is also possible that the N-terminal cluster of phosphorylation sites regulates formation of protein-protein contacts rather than comprising part of the contact site, which could be made up of other sequences in the peptide 1 to 259.
Cooperativity in T antigen double-hexamer assembly correlates with the ability to unwind closed circular duplex origin DNA and support viral DNA replication.
A body of evidence has accumulated to suggest that the phosphorylation state of the N-terminal cluster of phosphorylation sites regulates the ability of T antigen to unwind closed circular duplex origin DNA and hence to replicate viral DNA (reviewed in references 3 and 16). The mutation T124A potently inhibited bidirectional unwinding of closed circular duplex origin DNA and viral DNA replication, but it has little effect on many other properties of the protein (33, 36). Phosphorylation of serines 120 and 123 strongly inhibited unwinding of closed circular duplex origin DNA and viral DNA replication (4–6, 14, 51). Both the absence of phosphorylation at threonine 124 and the presence of phosphorylation on serines 120 and 123 also led to a loss of cooperativity in interactions between hexamers on the origin (the present study and reference 51). Consistent with the involvement of the N-terminal cluster of phosphorylation sites in origin unwinding, a truncated T antigen lacking the first 123 residues was able to assemble double hexamers on the origin, but it could not distort the AT-rich element or unwind closed circular duplex origin DNA (7), whereas T antigen lacking the first 109 residues was reported to form double hexamers on the origin, alter the local DNA conformation normally, and replicate viral DNA (7). These results support the interpretation that the N-terminal cluster of phosphorylation sites located between residues 110 and 123 is essential for functional interactions between the two hexamers in unwinding and replication.
The class 4 mutant T antigens were characterized by their inability to unwind closed circular duplex origin DNA and to support viral DNA replication, while the other properties of T antigen were not comparably defective (54). We show here that the class 4 mutants assembled double hexamers with reduced cooperativity. Further evidence suggesting a critical role for cooperative interactions between hexamers in origin unwinding comes from the ability of TATA binding protein (TBP) complexes bound to T antigen to block origin unwinding (21). The TBP binding site of T antigen has been mapped in the DNA binding domain directly adjacent to the proposed docking site defined by the class 4 mutations, implying that TBP binding may interfere sterically with hexamer-hexamer contacts at this site that are required for unwinding. Based on this series of correlations, we suggest that contacts between hexamers, mediated through the DNA binding domain and the N-terminal cluster of phosphorylated sites, participate in a critical way in the unwinding process during viral DNA replication.
The modest reduction in cooperativity of hexamer interactions on the origin makes it difficult to understand how these interactions could give rise to the inability of T124A and the class 4 mutants to unwind the origin and replicate viral DNA. However, the simplest interpretation of the dominant-negative phenotypes of T124A and the class 4 mutants in unwinding and DNA replication (Fig. 6 and 7) is that mixed hexamers of wild-type and T124A T antigen were formed and that even a few mutant subunits were sufficient to significantly reduce the interactions between the hexamers. Dominant-negative replication phenotypes have also been observed with other mutants bearing lesions in the DNA binding domain (C6-2, T22, and C8A) (reviewed in reference 3).
It is unclear what might happen during unwinding and replication when a weak interaction between mutant and wild-type hybrid hexamers is encountered. One possibility would be that one hexamer might dissociate from the DNA, as was observed with mutant double hexamers on the viral origin (Fig. 4B and Fig. 5), thereby disrupting bidirectional unwinding. Another possibility would be that the unwinding process would become uncoordinated at the two replication forks. Based on the correlations between the loss of cooperative interactions between hexamers of T124A and class 4 mutants on the origin, their inability to unwind viral origin DNA, and their trans-dominant inhibition of wild-type T antigen in unwinding and replication, we propose that repeated interactions between hexamers are required to unwind, and hence to replicate, the viral DNA. However, elucidation of the contact sites between hexamers and how these hexamer interactions are related to T-antigen translocation on the DNA and ATP hydrolysis during unwinding awaits further work.
ACKNOWLEDGMENTS
We thank C. Voitenleitner, T. Kelly, I. Moarefi, and V. Podust for reagents. We are very grateful to S.-G. Huang and T. Kelly for stimulating discussions, V. Podust for constructive criticism of the manuscript, and A. Krezel and A. Altman for help with illustrations.
The financial support of the NIH (GM52948 to E.F.), Vanderbilt University, and the NSF (Shared Instrumentation grant BIR-9419667) is gratefully acknowledged. These studies were begun with a grant from the German Science Foundation to E.F.
REFERENCES
- 1.Brinton B, Hassell J A. SV40 and polyoma DNA replication. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 639–677. [Google Scholar]
- 2.Brush G S, Kelly T J. Mechanisms for replicating DNA. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 1–43. [Google Scholar]
- 3.Bullock P A. The initiation of simian virus 40 DNA replication in vitro. Crit Rev Biochem Mol Biol. 1997;32:503–568. doi: 10.3109/10409239709082001. [DOI] [PubMed] [Google Scholar]
- 4.Cegielska A, Moarefi I, Fanning E, Virshup D M. T-antigen kinase inhibits simian virus 40 DNA replication by phosphorylation of intact T antigen on serines 120 and 123. J Virol. 1994;68:269–275. doi: 10.1128/jvi.68.1.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cegielska A, Shaffer S, Derua R, Goris J, Virshup D M. Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol Cell Biol. 1994;14:4616–4623. doi: 10.1128/mcb.14.7.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cegielska A, Virshup D M. Control of simian virus 40 replication by the HeLa nuclear kinase casein kinase I. Mol Cell Biol. 1993;13:1202–1211. doi: 10.1128/mcb.13.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Joo W S, Bullock P A, Simmons D T. The N-terminal side of the origin-binding domain of simian virus 40 large T antigen is involved in A/T untwisting. J Virol. 1997;71:8743–8749. doi: 10.1128/jvi.71.11.8743-8749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen G L, Wright P J, DeLucia A L, Lewton B A, Anderson M E, Tegtmeyer P. Critical spatial requirement within the origin of simian virus 40 DNA replication. J Virol. 1984;51:91–96. doi: 10.1128/jvi.51.1.91-96.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creighton T E. Proteins: structures and molecular properties. W. H. New York, N.Y: Freeman and Co.; 1984. [Google Scholar]
- 10.Dean F B, Dodson M, Echols H, Hurwitz J. ATP-dependent formation of a specialized nucleoprotein structure by simian virus 40 (SV40) large tumor antigen at the SV40 replication origin. Proc Natl Acad Sci USA. 1987;84:8981–8985. doi: 10.1073/pnas.84.24.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean F B, Borowiec J A, Eki T, Hurwitz J. The simian virus 40 T antigen double hexamer assembles around the DNA at the replication origin. J Biol Chem. 1992;267:14129–14137. [PubMed] [Google Scholar]
- 12.DeLucia A L, Deb S, Partin K, Tegtmeyer P. Functional interactions of the simian virus 40 core origin of replication with flanking regulatory sequences. J Virol. 1986;57:138–144. doi: 10.1128/jvi.57.1.138-144.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodson M, Dean F B, Bullock P, Echols H, Hurwitz J. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science. 1987;238:964–968. doi: 10.1126/science.2823389. [DOI] [PubMed] [Google Scholar]
- 14.Erdile L F, Collins K L, Russo A, Simancek P, Small D, Umbricht C, Virshup D, Cheng L, Randall S, Weinberg D, Moarefi I, Fanning E, Kelly T J. Initiation of SV40 DNA replication: mechanism and control. Cold Spring Harbor Symp Quant Biol. 1991;56:303–313. doi: 10.1101/sqb.1991.056.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992;66:1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanning E. Control of SV40 DNA replication by protein phosphorylation: a model for cellular DNA replication? Trends Cell Biol. 1994;4:250–255. doi: 10.1016/0962-8924(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 17.Fanning E, Knippers R. Structure and function of simian virus 40 large T antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- 18.Fried M G, Crothers D M. Kinetics and mechanism in the reaction of gene regulatory proteins with DNA. J Mol Biol. 1984;172:263–282. doi: 10.1016/s0022-2836(84)80026-1. [DOI] [PubMed] [Google Scholar]
- 19.Grässer F A, Mann K, Walter G. Removal of serine phosphates from simian virus 40 T antigen increases its ability to stimulate DNA replication in vitro but has no effect on ATPase and DNA binding. J Virol. 1987;61:3373–3380. doi: 10.1128/jvi.61.11.3373-3380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigen. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbig U, Weisshart K, Taneja P, Fanning E. Interaction of the transcription factor TFIID with simian virus 40 (SV40) large T antigen interferes with replication of SV40 DNA in vitro. J Virol. 1999;73:1099–1107. doi: 10.1128/jvi.73.2.1099-1107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herendeen D, Kelly T J. SV40 DNA replication. In: Blow J J, editor. Eukaryotic DNA replication. New York, N.Y: Oxford University Press; 1996. pp. 29–65. [Google Scholar]
- 23.Höss A, Moarefi I, Scheidtmann K-H, Cisek L J, Corden J L, Dornreiter I, Arthur A, Fanning E. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J Virol. 1990;64:4799–4807. doi: 10.1128/jvi.64.10.4799-4807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S-G, Weisshart K, Gilbert I, Fanning E. Stoichiometry and mechanism of assembly of simian virus 40 T antigen complexes with the viral origin of DNA replication and DNA polymerase α-primase. Biochemistry. 1998;37:15345–15352. doi: 10.1021/bi9810959. [DOI] [PubMed] [Google Scholar]
- 25.Joo W S, Kim H Y, Purviance J D, Sreekumar K R, Bullock P A. Assembly of T-antigen double hexamers on the simian virus 40 core origin requires only a subset of the available binding sites. Mol Cell Biol. 1998;18:2677–2687. doi: 10.1128/mcb.18.5.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klausing K, Scheidtmann K-H, Baumann E A, Knippers R. Effects of in vitro dephosphorylation of DNA-binding and DNA helicase activities of simian virus 40 large T antigen. J Virol. 1988;62:1258–1265. doi: 10.1128/jvi.62.4.1258-1265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lawson R, Cohen P, Lane D P. SV40 large T antigen-dependent DNA replication is activated by protein phosphatase 2A in vitro. J Virol. 1990;64:2380–2383. doi: 10.1128/jvi.64.5.2380-2383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo X, Sanford D G, Bullock P A, Bachovchin W W. Solution structure of the origin specific DNA binding domain from simian virus 40 T-antigen. Nat Struct Biol. 1996;3:1034–1039. doi: 10.1038/nsb1296-1034. [DOI] [PubMed] [Google Scholar]
- 30.Mastrangelo I A, Hough P V C, Wall J S, Dodson M, Dean F B, Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of replication. Nature (London) 1989;338:658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- 31.McEntee K, Winstock G M, Lehman I R. RecA protein catalyzed strand assimilation: stimulation by Escherichia coli single-stranded DNA-binding protein. Proc Natl Acad Sci USA. 1980;77:857–861. doi: 10.1073/pnas.77.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McVey D, Brizuela L, Mohr I, Marshak D R, Gluzman Y, Beach D. Phosphorylation of large tumor antigen by cdc2 stimulates SV40 DNA replication. Nature (London) 1989;341:503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- 33.McVey D, Ray S, Gluzman Y, Berger L, Wildeman A G, Marshak D R, Tegtmeyer P. Cdc2 phosphorylation of threonine 124 activates the origin-unwinding functions of simian virus 40 T antigen. J Virol. 1993;67:5206–5215. doi: 10.1128/jvi.67.9.5206-5215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McVey D, Woelker B, Tegtmeyer P. Mechanisms of simian virus 40 T-antigen activation by phosphorylation of threonine 124. J Virol. 1996;70:3887–3893. doi: 10.1128/jvi.70.6.3887-3893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moarefi I, Schneider C, van Zee K, Höss A, Arthur A K, Fanning E. Control of SV40 DNA replication by protein phosphorylation. In: Fanning E, Knippers R, Winnacker E-L, editors. DNA replication and the cell cycle. Berlin, Germany: Springer-Verlag; 1993. pp. 157–169. [Google Scholar]
- 36.Moarefi I, Small D, Gilbert I, Höpfner M, Randall S K, Schneider C, Russo A A R, Ramsperger U, Arthur A K, Stahl H, Kelly T J, Fanning E. Mutation of cyclin-dependent kinase phosphorylation site in simian virus 40 (SV40) large T antigen specifically blocks SV40 origin DNA unwinding. J Virol. 1993;67:4992–5002. doi: 10.1128/jvi.67.8.4992-5002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Mohr I J, Fairman M P, Stillman B, Gluzman Y. Large T antigen mutants define multiple steps in the initiation of simian virus 40 DNA replication. J Virol. 1989;63:4181–4188. doi: 10.1128/jvi.63.10.4181-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohr I J, Stillman B, Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987;6:153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons R, Stenger J E, Ray S, Welker R, Anderson M E, Tegtmeyer P. Cooperative assembly of simian virus 40 T-antigen hexamers on functional halves of the replication origin. J Virol. 1991;65:2798–2806. doi: 10.1128/jvi.65.6.2798-2806.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prives C. The replication functions of SV40 T antigen are regulated by phosphorylation. Cell. 1990;61:735–738. doi: 10.1016/0092-8674(90)90179-i. [DOI] [PubMed] [Google Scholar]
- 40.Scheidtmann K-H, Buck M, Schneider J, Kalderon D, Fanning E, Smith A E. Biochemical characterization of phosphorylation site mutants of simian virus 40 large T antigen: evidence for interaction between amino- and carboxy-terminal domains. J Virol. 1991;65:1479–1490. doi: 10.1128/jvi.65.3.1479-1490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheidtmann K-H, Echle B, Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982;44:116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheidtmann K-H, Hardung M, Echle B, Walter G. DNA binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984;50:1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheidtmann K-H, Virshup D M, Kelly T J. Protein phosphatase 2A dephosphorylates simian virus 40 large T antigen specifically at residues involved in regulation of the DNA-binding activity. J Virol. 1991b;65:2098–2101. doi: 10.1128/jvi.65.4.2098-2101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider J, Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alters its origin DNA-binding specificity for site I or II and affect SV40 DNA replication activity. J Virol. 1988;62:1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SenGupta, D. J., and J. A. Borowiec. Personal communication.
- 46.Shaw S B, Tegtmeyer P. Binding of dephosphorylated A protein to SV40 DNA. Virology. 1981;115:88–96. doi: 10.1016/0042-6822(81)90091-x. [DOI] [PubMed] [Google Scholar]
- 47.Smelkova N V, Borowiec J A. Dimerization of simian virus 40 T-antigen hexamers activates T-antigen DNA helicase activity. J Virol. 1997;71:8766–8773. doi: 10.1128/jvi.71.11.8766-8773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Smelkova N V, Borowiec J A. Synthetic DNA replication bubbles bound and unwound with twofold symmetry by a simian virus 40 T-antigen double hexamer. J Virol. 1998;72:8676–8681. doi: 10.1128/jvi.72.11.8676-8681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stillman B. Comparison of DNA replication in cells from prokarya and eukarya. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 435–460. [Google Scholar]
- 48a.Stringer J R. Mutant of simian virus 40 large T-antigen is defective for viral DNA synthesis, but competent for transformation of cultured rat cells. J Virol. 1982;42:854–864. doi: 10.1128/jvi.42.3.854-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virshup D M, Kauffman M G, Kelly T J. Activation of SV40 DNA replication in vitro by cellular protein phosphatase 2A. EMBO J. 1989;8:3891–3898. doi: 10.1002/j.1460-2075.1989.tb08568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virshup D M, Kelly T J. Purification of replication protein C, a cellular protein involved in the initial stages of simian virus 40 DNA replication in vitro. Proc Natl Acad Sci USA. 1989;86:3584–3588. doi: 10.1073/pnas.86.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virshup D M, Russo A, Kelly T J. Mechanism of activation of simian virus 40 DNA replication by protein phosphatase 2A. Mol Cell Biol. 1992;12:4883–4895. doi: 10.1128/mcb.12.11.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voitenleitner C, Fanning E, Nasheuer H-P. Phosphorylation of DNA polymerase α-primase by cyclin A-dependent kinases regulates initiation of DNA replication in vitro. Oncogene. 1997;14:1611–1615. doi: 10.1038/sj.onc.1200975. [DOI] [PubMed] [Google Scholar]
- 53.Wessel R, Schweizer J, Stahl H. Simian virus 40 T-antigen DNA helicase is a hexamer which forms a binary complex during bidirectional unwinding from the viral origin of DNA replication. J Virol. 1992;66:804–815. doi: 10.1128/jvi.66.2.804-815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wun-Kim K, Upson R, Young W, Melendy T, Stillman B, Simmons D T. The DNA-binding domain of simian virus tumor antigen has multiple functions. J Virol. 1993;67:7608–7611. doi: 10.1128/jvi.67.12.7608-7611.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]