Abstract
Per- and polyfluoroalkyl substances (PFAS) are a group of persistent environmental pollutants that are ubiquitously found in the environment and virtually in all living organisms, including humans. PFAS cross the blood–brain barrier and accumulate in the brain. Thus, PFAS are a likely risk for neurotoxicity. Studies that measured PFAS levels in the brains of humans, polar bears, and rats have demonstrated that some areas of the brain accumulate greater amounts of PFAS. Moreover, in humans, there is evidence that PFAS exposure is associated with attention-deficit/hyperactivity disorder (ADHD) in children and an increased cause of death from Parkinson’s disease and Alzheimer’s disease in elderly populations. Given possible links to neurological disease, critical analyses of possible mechanisms of neurotoxic action are necessary to advance the field. This paper critically reviews studies that investigated potential mechanistic causes for neurotoxicity including (1) a change in neurotransmitter levels, (2) dysfunction of synaptic calcium homeostasis, and (3) alteration of synaptic and neuronal protein expression and function. We found growing evidence that PFAS exposure causes neurotoxicity through the disruption of neurotransmission, particularly the dopamine and glutamate systems, which are implicated in age-related psychiatric illnesses and neurodegenerative diseases. Evaluated research has shown there are highly reproduced increased glutamate levels in the hippocampus and catecholamine levels in the hypothalamus and decreased dopamine in the whole brain after PFAS exposure. There are significant gaps in the literature relative to the assessment of the nigrostriatal system (striatum and ventral midbrain) among other regions associated with PFAS-associated neurologic dysfunction observed in humans. In conclusion, evidence suggests that PFAS may be neurotoxic and associated with chronic and age-related psychiatric illnesses and neurodegenerative diseases. Thus, it is imperative that future mechanistic studies assess the impact of PFAS and PFAS mixtures on the mechanism of neurotransmission and the consequential functional effects.
Graphical Abstract
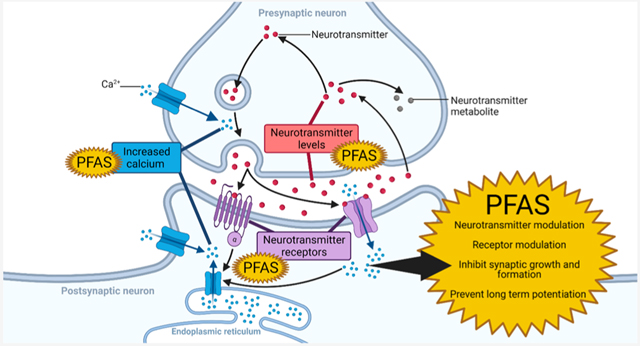
1. INTRODUCTION
1.1. PFAS Overview.
Per- and polyfluoroalkyl substances (PFAS) [previously called perfluorochemicals (PFCs)] are a group of thousands of man-made chemicals that are now ubiquitously found in the environment and in living organisms.1–3 PFAS have been manufactured for over 70 years due to their useful characteristics that include the ability to repel dirt and water, act as a surfactant, and resist high temperatures.3 Products containing PFAS include carpets, furniture, corrosion resistant pipes, nonstick cookware, food packaging, firefighting foam, and microwaveable food containers.2,4 PFAS have been identified as persistent organic pollutants (POPs) or “forever chemicals” and continue to be found in the environment and animals across the globe, even in the Arctic and Antarctic regions, due to the decades of large-scale manufacturing and uncontrolled pollution in the environment.5–13
1.2. PFAS Exposure in Humans: Overview of Widespread Public Health Concern.
1.2.1. Legacy and Emerging PFAS Exposure.
In 1938, polytetrafluoroethylene (PTFE) (also known Teflon, which is used for nonstick coatings) first manufactured by DuPont was produced using perfluorooctanoic acid (PFOA, C-8), one of the common legacy PFAS.14 A decade later, the 3M Company began producing perfluorooctane sulfonyl fluoride (POSF), a PFAS precursor that degraded into perfluorooctanesulfonate (PFOS), the most prevalent legacy PFAS.14 Both PFOS and PFOA contain eight carbons with seven carbons saturated with highly stable carbon–fluorine bonds, and they differ by a single functional group.7,14 By the late 1970s, PFAS were found in the blood of PFAS industrial workers and in drinking water near PFAS factories.14 In 1998, PFAS were found in nearly all human blood bank samples in the United States.14
Due to concerns of bioaccumulation and toxicity, the U.S. Environmental Protection Agency (U.S.EPA) convinced industry to voluntarily stop or restrict production of PFOA, PFOS, their precursors, and other related compounds between 2000 and 2007.6,7,14 Although the production of these PFAS was reduced by 95% in 2010, PFOS and PFOA remain prevalent in the environment in the United States due to historical pollution, and they continue to be produced in some countries worldwide.7,10,13,14 A 2020 study in Japan found that 100% of over 12 000 sampled mothers had PFOS measured in their serum.15 A recent report on European PFAS exposure reported PFAS with the highest levels in human serum are PFOS (~65% of total PFAS in adults, ~35% of total PFAS in children) and PFOA (~15% of total PFAS in adults, ~35% of total PFAS in children).16 In a 2021 survey of environmental PFAS contamination, the greatest contributing chemicals were PFOS (29% of total PFAS) and perfluorobutyric acid (PFBA; a four-carbon PFAS) (25% of total PFAS).13
Highly industrialized regions, such as south China, have recently reported increasing environmental levels of PFAS including both legacy and emerging PFAS.13 Furthermore, the legacy PFAS have been replaced by PFAS containing fewer carbons with less known toxic effects. For example, 2,3,3,3- tetrafluoro-2-(heptafluoropropoxy)-propanoate (HFPO–DA, GenX) has been used to replace PFOA in the manufacturing of PTFE by DuPont.1 These emerging PFAS have been presumed to have less propensity to bioaccumulate because of having fewer carbons.1 Recent studies demonstrate the replacement PFAS are being detected in the environment and in animals including polar bears, ringed seals, and orca whales.17 In 2021, Wang et al. reported the PFAS perfluorohexanesulfonic acid (PFHxS) reached detectable levels in the environment since 2016 in south China,13 and PFHxS is currently detectable in the serum of adults and children in Japan, Europe, and the United States,15,16,18 suggesting the PFAS with less carbons are likely able to accumulate in the environment and in humans. Furthermore, recent studies of HFPO–DA (GenX) have demonstrated systemic and developmental toxicity, supporting the urgent need to assess the toxicity of other emerging PFAS.19–21
1.2.2. PFAS Contamination of Food Sources and Health Concerns.
PFAS are currently found in human food and water sources worldwide.10,13,16,22 PFOS continues to be the dominant PFAS polluting the environment in the southern Chinese rivers, streams, and ocean coastal regions.10,13 An environmental exposure assessment determined that PFAS accumulation is present in seafood consumed by humans.13 Crustaceans and fishes predominantly accumulated PFOS, and noncrustacean shellfish accumulated more PFBA.13 The same study found the largest PFAS quantities in the water to be PFOS and PFBA with one region also having a higher percentage of perfluorononanoic acid (PFNA) in the samples.13 In 2021, Huang et al. found additional PFAS that are gaining concern as potential health hazards in the south Chinese rivers and streams, which include PFNA, perfluorohexanesulfonate (PFHxS), PFBA, and perfluorobutanesulfonate (PFBS).10 A European report of PFAS food contamination found PFOS to be the highest PFAS contaminant in food, and it was found in the highest quantities in the nonmeat tissues of nonfish animals (game and farmed) and fishes, followed by game animal meat and eggs/egg products.16 Another recent study comparing PFAS plasma levels in humans with different diets demonstrated that people with omnivorous diets had significantly higher levels of PFOS and PFNA compared to those with vegan diets.22 Therefore, PFAS exposure in humans through the environment and diet as well as the consequences of PFAS exposure to human health are a major concern.
The most studied PFAS, PFOS and PFOA, have been demonstrated to produce toxic effects during development and on the nervous, endocrine (including thyroid, sex, and stress hormone pathways), immune, reproductive, cardiovascular, and hepatic systems.1–3,18 However, neurotoxic adverse effects and their mechanisms of action for PFOS and PFOA are not well understood. Associations between PFOS and PFOA neurotoxicity at chronic, low-dose exposures (most relevant to what is observed in humans) have been demonstrated in epidemiological studies and in animal models. With PFOS and PFOA having the best understood and potentially more robust neurotoxic effects, they will be the major focus of many studies in this Review, although other PFAS will be included where applicable (most frequently in PFAS complex mixtures).
Ongoing research has identified that the PFAS’s neurotoxic targets for neurotransmission alter neurotransmitter levels,6,23–36 synaptic protein expression and function,25,26,30,31,34,37–49 and calcium signaling 38–43,45,49–54 This Review aims to critically analyze the state of PFAS neurotoxicity with focus on neurotransmission-related end points and to reveal PFAS’s potential targets of neurological disease that require epidemiological evaluation in the future. Recent reviews have broadly focused on brain accumulation and neurotoxicity.3,7,55–58 This critical analysis of the field focuses specifically on neurotransmission targets, which may underlie adverse neurological outcomes. Furthermore, this Review identifies the current state of PFAS neurotoxicity research and gaps that are critical to address in future studies.
2. PFAS IN THE BRAIN
2.1. Overall Weight of Evidence of PFAS Deposition in the Brain.
There is a limited understanding about how PFAS are distributed to and eliminated from the brain. PFAS including PFOS, PFOA, and PFHxS found in human blood have been declining since 200059 with blood half-life levels estimated in humans to be 3–6, 2–3, and 4–10 years, respectively.60 However, the half-life in the brain is unknown. Males on average have higher PFAS blood and serum levels compared to females, especially during female reproductive ages.18,59,60 The distribution of PFOS and PFOA to liver tissue in rats was higher in males compared to females (no difference in the kidney, heart, lung, and spleen), but the brain was not evaluated.61 The analysis of brain half-life and potential sex differences in brain distribution will require investigation in the future to better understand the mechanism of PFAS neurotoxicity.
It has been hypothesized that the incorporation of PFAS into the cell membrane due to their surfactant nature may disrupt membrane integrity to allow entry into the brain.50,62 Additionally, damage to the endothelial cells and astrocytes of the blood–brain barrier has been demonstrated in PFOS-treated mice, which would also increase permeability of the blood–brain barrier, making the brain susceptible not only to PFAS but also to other neurotoxins/toxicants.62 Furthermore, PFAS have been detected in the cerebrospinal fluid (CSF) of humans63,64 Most convincingly, PFAS have been demonstrated to be present in the brains of exposed laboratory animals,23,40,62 environmentally exposed animals,8,9,44 and humans.65–68 A study of rats with in utero exposure to PFOS demonstrated that the rat pups had a greater elevation of PFOS in the brain compared to the mother’s brain PFOS levels.40 A recent study on five post-mortem individuals from a heavily PFAS contaminated region of Italy has confirmed PFAS, including PFOA, PFHxS, and perfluorohexanoate (PFHxA), continue to be found systemically in the body and deposits in the brain, even after PFAS levels in drinking water were reduced to undetectable levels.12,65 In 2022, Xie et al. quantified multiple PFAS from Chinese glioma brain cancer tissue and found PFAS were present in 96% of the samples.68 Furthermore, the highest median PFAS concentrations (0.27 to 0.77 ng/g) in the glioma tissue were the short-chain PFAS: PFBS and PFHxA; emerging PFAS: GenX and perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid) (6:2Cl-PFESA); the PFAS precursor: perfluorooctanesulfonamide (PFOSA); legacy PFAS: PFOS, PFOA, and perfluoroundecanoic acid (PFUA).68 PFAS with the highest detection rate in the gliomas were PFOSA (88%) and PFOS (85%), followed by 6:2Cl-PFESA (73%), PFOA (65%), PFNA (58%), PFHxS (50%), and PFUA (50%).68 The studies of PFAS deposition in the brain demonstrate that many types of PFAS are capable of accumulating in the brain and are present even after measures to eliminate exposure are taken.
Mounting data suggest PFAS are likely neurotoxic and may modulate neurological function (exemplified by an outstanding recent review by Cao and Ng in 2021,55 broadly addressing neurotoxicity). PFAS accumulate in the greatest amounts in the blood, liver, lung, and kidney.69 Reports of neurotoxicity are present at relatively low amounts accumulating in the brain.23,35,66,67 However, recent experiments quantifying PFAS in cerebrospinal fluid (CSF) and post-mortem human brains suggest these earlier studies may be underestimating the concentrations that PFAS accumulate in the brain.63 When a single dose of PFOS was administered, it rapidly accumulated in the brain of rats within hours of administration of a single dose.35 PFAS have been demonstrated to accumulate in the brains of many species including adult post-mortem humans, 65–67 human fetuses,70 polar bears,8,9,44 mice,36 rats,23,35,61,63 and northern leopard frogs.6 Considering the heterogeneity of the brain structure, studies have additionally investigated the region-specific accumulation of PFAS, which could potentially target which brain functions are at greatest risk of dysfunction and disease.
2.2. PFAS Distribution in Brain vs Blood.
Thus far, the brain region-specific accumulation of PFAS in the brain has been performed on humans,65 polar bears,9,44 and rats.23 Table 1 summarizes the studies that have quantified PFAS accumulation (PFOS, PFOA, PFHxS, and PFHxA) in different regions of the brain and blood or serum. Importantly, systemic PFAS levels are what is most often used as a biomarker of organismal PFAS exposure and have been reported to be magnitudes higher than the levels that accumulate in the brain.23,35,69 A study of Chinese people found that PFAS in CSF were only 2–3-fold lower in the CSF compared to plasma levels of PFAS,63 which demonstrated the plasma levels may be an underestimation of how much PFAS get distributed to and accumulate in the brain.An earlier 2006 human study of PFAS in brain and blood sample levels (sampled from northern Italy) demonstrated a 5-fold lower difference between blood and brain PFOA or PFOS levels.67
Table 1.
Brain Region-Specific Accumulation of PFAS in Humans, Polar Bears, and Ratsa
| study description | human 202265 | polar bear 20069 | polar bear 2011–201244 | rat 1 mg/kg/d 200323 | rat 10 mg/kg/d 200323 | rat dams 1 mg/kg/d 201740 | rat dams 2 mg/kg/d 201740 | rat pups 1 mg/kg/d 201740 | rat pups 2 mg/kg/d 201740 |
|---|---|---|---|---|---|---|---|---|---|
| blood or serum (ng/mL)b | |||||||||
| total PFAS | 291.9 | 158.4 | – | 10 480.0 | 45 446.0 | 20 400.0 | 38 200.0 | 19 300.0 | 37 200.0 |
| PFOS | 0.8 | 135.6 | – | 10 480.0 | 45 446.0 | 20 400.0 | 38 200.0 | 19 300.0 | 37 200.0 |
| PFOA | 217.4 | 3.7 | – | – | – | – | – | – | – |
| PFHxS | 71.0 | 19.1 | – | – | – | – | – | – | – |
| PFHxA | 2.6 | n.d. | – | – | – | – | – | – | – |
| Brain Subregions (ng/g) | |||||||||
| cerebral cortexc | |||||||||
| total PFAS | 29.6 | 32.2–34.2 | 22.2–31.3 | 294.0 | 4487.0 | 1500.0 | 3100.0 | 6100.0 | 11 200.0 |
| PFOS | n.d. | 30.5–32.8 | 20.5–29.7 | 294.0 | 4487.0 | 1500.0 | 3100.0 | 6100.0 | 11 200.0 |
| PFOA | 22.2 | 0.2–0.3 | 0.6 | – | – | – | – | – | – |
| PFHxS | 4.6 | 1.4–1.5 | 1.0–1.2 | – | – | – | – | – | – |
| PFHxA | 2.8 | n.d. | 0.0–0.1 | – | – | – | – | – | – |
| hypothalamus | |||||||||
| total PFAS | 206.9 | 60.1 | 23.2 | <50 | 15 706.0 | – | – | – | – |
| PFOS | n.d. | 58.8 | 20.5 | <50 | 15 706.0 | – | – | – | – |
| PFOA | 158.9 | n.d | 1.5 | – | – | – | – | – | – |
| PFHxS | 31.6 | 1.3 | 1.0 | – | – | – | – | – | – |
| PFHxA | 16.4 | n.d. | 0.2 | – | – | – | – | – | – |
| hippocampus | |||||||||
| total PFAS | – | – | 28.2 | 115.0 | 8966.0 | 1200.0 | 2300.0 | 5000.0 | 9800.0 |
| PFOS | – | – | 23.7 | 115.0 | 8966.0 | 1200.0 | 2300.0 | 5000.0 | 9800.0 |
| PFOA | – | – | 2.6 | – | – | – | – | – | – |
| PFHxS | – | – | 1.6 | – | – | – | – | – | – |
| PFHxA | – | – | 0.4 | – | – | – | – | – | – |
| brain stem (midbrain, pons/medulla)d | |||||||||
| total PFAS | 23.0 | 48.6 | 35.7 | 363.0 | 5346.0 | – | – | – | – |
| PFOS | n.d. | 46.9 | 33.4 | 363.0 | 5346.0 | – | – | – | – |
| PFOA | 13.3 | 0.2 | 0.9 | – | – | – | – | – | – |
| PFHxS | 8.6 | 1.3 | 1.2 | – | – | – | – | – | – |
| PFHxA | 1.1 | n.d. | 0.1 | – | – | – | – | – | – |
| cerebellum | |||||||||
| total PFAS | 54.4 | 38.6 | 26.3 | 289.0 | 5540.0 | 1700.0 | 3100.0 | 7600.0 | 14 600.0 |
| PFOS | n.d. | 36.5 | 24.5 | 289.0 | 5540.0 | 1700.0 | 3100.0 | 7600.0 | 14 600.0 |
| PFOA | 32.3 | 0.3 | 0.6 | – | – | – | – | – | – |
| PFHxS | 8.7 | 1.8 | 1.2 | – | – | – | – | – | – |
| PFHxA | 13.4 | n.d. | 0.0 | – | – | – | – | – | – |
| striatume | |||||||||
| total PFAS | 45.3–109.2 | 33.7 | 21.6 | 396.0 | 4256.0 | – | – | – | – |
| PFOS | n.d. | 31.9 | 19.2 | 396.0 | 4256.0 | – | – | – | – |
| PFOA | 31.1–93.3 | 0.2 | 1.4 | – | – | – | – | – | – |
| PFHxS | 8.4–12.1 | 0.9 | 0.8 | – | – | – | – | – | – |
| PFHxA | 2.1–7.4 | n.d. | 0.2 | – | – | – | – | – | – |
| caudate nucleus | |||||||||
| total PFAS | 109.2 | – | – | – | – | – | – | – | – |
| PFOS | n.d. | – | – | – | – | – | – | – | – |
| PFOA | 93.3 | – | – | – | – | – | – | – | – |
| PFHxS | 8.4 | – | – | – | – | – | – | – | – |
| PFHxA | 7.4 | – | – | – | – | – | – | – | – |
| lenticular nucleus | |||||||||
| total PFAS | 45.3 | – | – | – | – | – | – | – | – |
| PFOS | n.d. | – | – | – | – | – | – | – | – |
| PFOA | 31.1 | – | – | – | – | – | – | – | – |
| PFHxS | 12.1 | – | – | – | – | – | – | – | – |
| PFHxA | 2.1 | – | – | – | – | – | – | – | – |
| thalamuse | |||||||||
| total PFAS | 19.7 | 41.2 | 23.7 | 396.0 | 4256.0 | – | – | – | – |
| PFOS | n.d. | 38.8 | 21.5 | 396.0 | 4256.0 | – | – | – | – |
| PFOA | 14.4 | 0.9 | 1.1 | – | – | – | – | – | – |
| PFHxS | 2.7 | 1.3 | 1.0 | – | – | – | – | – | – |
| PFHxA | 2.7 | n.d. | 0.2 | – | – | – | – | – | – |
| brain average or whole brainf | |||||||||
| total PFAS | 97.5 | 37.5 | 25.2 | 247.0 | 12821.0 | 1900.0 | 3310.0 | 8400.0 | 15 100.0 |
| PFOS | n.d. | 35.2 | 22.9 | 247.0 | 12821.0 | 1900.0 | 3310.0 | 8400.0 | 15 100.0 |
| PFOA | 73.0 | 0.3 | 1.1 | – | – | – | – | – | – |
| PFHxS | 18.5 | 1.4 | 1.1 | – | – | – | – | – | – |
| PFHxA | 6.1 | n.d. | 0.1 | – | – | – | – | – | – |
The PFAS included PFOS, PFOA, PFHxS, and PFHxA because these are the only PFAS included in brain region-specific quantification in the human study. The human study by Di Nisio et al. in 2022 analyzed samples taken from five Caucasian males that were non-occupationally exposed (exposure characterized by Pitter et al.) to elevated levels of PFASs (especially PFOA) in Veneto Region, Italy.12,65 The polar bear studies collected samples from East Greenland in 20069 and 2011–2012.44 The PFAS totals in the table are only for the PFAS PFOS, PFOA, PFHxS, and PFHxA to compare with the human levels, although the polar bear studies included many additional PFAS. The polar bear and human studies only received environmental exposure to PFAS mixtures.9,44 The female adult rat study conducted by Austin et al. in 2003 and Ishida et al. in 2017 (gestational rat study) were the only controlled exposure laboratory studies that analyzed brain region-specific PFAS accumulation. These studies only assessed PFOS exposure; therefore, PFOS levels are equal to the total PFAS levels.23,40 The adult rat study dosed female rats with 1 or 10 mg/kg/d for 14 days, and tissue samples were immediately collected at the end of the exposure.23 The gestational rat study exposed pregnant female rats (dams) to 1 or 2 mg/kg/d from gestation day (GD) 11–20 (10 days total).40 PFOS was quantified on postnatal day 4 in the dams and pups.40 For both rat studies, control rat PFOS serum brain levels were below the limit of detection and not represented in the table.23,40 Quantities are given as average PFAS levels in the table, and average ranges are given if multiple subregions were samples in one study. Blood or serum levels are presented as ng/ mL, and all brain and brain levels are presented as ng/g. “n.d.” = below the detection limit; “–” = not quantified in the study.
Human and polar bear studies used whole blood, whereas the rat study used serum. Polar bear PFAS blood levels were converted from ng/g to ng/mL using 1 g wet blood = 0.9434 mL blood volume.72
The polar bear study quantified many subregions of the cerebral cortex including frontal, occipital, and temporal regions separately; the human study only quantified the frontal cortex, and the rat study quantified the cortex in its entirety.
The adult rat study was the only study to quantify the brain stem altogether. The human study only quantified the midbrain portion of the brainstem, and the polar bear studies only quantified the pons/medulla together.
The adult rat study quantified the rest of the brain together, which included the striatum and thalamus. The striatum and thalamus were individually quantified in the human and polar bear studies. The human study only quantified subregions of the dorsal striatum, whereas the polar bear studies quantified the entire striatum. Both the polar bear and human studies quantified the thalamus separately.
The 2017 gestational rat study quantified PFOS in the whole brain instead of averaging the measured regions.
The recently published human study by Di Nisio et al. quantified PFAS in the post-mortem brains of five males that did not have a known occupational exposure to PFAS and had lived in the heavily PFAS-contaminated area of Veneto region, Italy.65 Veneto, Italy is the location of extremely elevated levels of PFAS (primarily PFOA) in drinking water.12 In 2013, the median PFOA levels in the drinking water were 319 ng/L and the max PFOA levels were 1475 ng/L.12 Between 2013 and 2018, measures were taken to reduce the PFAS levels in drinking water to below the limits of detection.12 The collection date for the human subjects was not reported, but interestingly, the mean total PFAS blood level of 291.9 ng/mL is <3 times that found in the average whole brain level of 97.5 ng/g.65 The Maestri et al.67 and Di Nisio et al.65 human brain studies both measured post-mortem human brain PFAS in individuals from northern Italy. However, the composition of PFOA and PFOS greatly differed between the Italian studies, whereas the 2006 Italian study67 and the Xie et al.68 Chinese glioma study in 2022 demonstrate similar mean PFOS and PFOA levels.65,67,68 Taken together, the human data suggest a high variability of PFAS accumulation and composition, which may be due to the composition and abundance of PFAS pollution in the environment.
Interestingly, a lack of major differences between blood and brain PFAS levels for assumed chronic environmental exposure was also observed in the Greaves et al. study in 2013 that evaluated PFAS levels in polar bears.9 However, in the 14 day 1 or 10 mg/kg/d exposure to PFOS, female rats produced serum PFAS levels that were orders of magnitude higher in the blood than in the brain,23,35,69 suggesting that there may be significant interspecies differences in brain accumulations and brain accumulation kinetics may be far different than that in blood (i.e., chronic dosing is required to achieve high brain levels).71 There was a very large magnitude of difference between PFAS levels in the plasma and brain of human fetal tissue,70 which closely resembles what was observed in the acute exposures in animal studies.23,35 Therefore, we can hypothesize that the duration of the PFAS exposure may be key for the prediction of the amount of PFAS in the brain. Considering the PFAS levels in the human blood have been dropping since 200059 and brain vs blood PFAS in humans65 and polar bears9 are <10-fold different as reported in laboratory animal experimental exposures,23,35 it is possible that the half-lives of PFAS in the brain are much higher than that of the standard PFAS elimination half-lives calculated from blood in humans, rodents, and other animals.60,71 Interestingly, the polar bear studies (which collected samples in 2006 and 2011–2012) demonstrate the total PFAS levels are declining in polar bear brains and brain regions, but the rate of decrease appears to be dependent on the brain region and PFAS.9,44 Changes in polar bear blood and brain PFAS levels over time could not be compared because the second study did not include blood levels.44 Furthermore, a recent metanalysis by Starnes et al. assessed brain/serum ratios for PFOS, PFOA, and PFNA levels from multiple studies and compared the brain/serum PFAS levels of environmentally and experimentally exposed animals.56 The key finding was that all three PFAS had statistically significant elevated brain/serum PFAS levels in environmental exposures compared to experimental exposures. It is important that future studies evaluate the elimination rate of PFAS in the brain so estimations for the brain half-life of PFAS in humans can be calculated in future studies. Subsequent research that measured blood vs brain PFAS in humans and in polar bears gives more direct evidence of PFAS distribution and accumulation in the brain.
2.3. Distribution of PFAS Brain Regions.
The human brain regions with the highest total PFAS levels were the hypothalamus with 206.9 ng/g and the caudate nucleus with 109.2 ng/g, followed by the cerebellum (54.4 ng/g) and lenticular nucleus (45.3 ng/g).65 Interestingly, the polar bear samples collected in 2006 also had the largest total PFAS in the hypothalamus (60.1 ng/g), followed by the pons/medulla (48.6 ng/g) and thalamus (41.2 ng/g), whereas the 2011–2012 polar bear samples had the highest PFAS levels in the brain stem (35.7 ng/g) and occipital lobe (31.3 ng/g).9,44 In the rats that were only exposed to high levels of PFOS, the 1 mg/kg/d exposure had the highest accumulation in the brain stem (363 ng/g) and the rest of the brain (striatum and thalamus with 396 ng/g) and the 10 mg/kg/d dose had the highest levels in the hypothalamus (15 706 ng/g) and hippocampus (8966 ng/g).23
The cross-species variability, variability in composition and levels of the exposed PFAS mixtures, and differences in distribution and elimination of PFAS from these brain regions may explain the variability in the brain region PFAS levels. Importantly, the regions with the highest long-term accumulation of PFAS may not necessarily be at the highest risk of neurotoxicity due to the many region-specific neuron and glia populations and unique connectivity to other regions of the brain.45 Therefore, to understand the mechanism of how PFAS are inducing neurotoxicity and the impact on human disease, we must look at the functional targets within cells that are most susceptible to adverse effects from PFAS exposure, which includes neurotransmission.
3. PFAS EFFECTS ON NEUROTRANSMITTERS
3.1. PFAS Change Neurotransmitter Levels in the Brain.
There is growing evidence that PFOS, PFOA, and PFAS mixtures lead to changes in neurotransmitter levels,6,23–36 alter neurotransmitter receptor and transporter expression and function,25,26,30,31,34,37–49 and change neurotransmitter metabolism.6,24–27,30,31,33,34,36,46,48,65,73,74 Although the major focus for the evaluation of PFAS neurotoxicity has been on PFAS developmental neurotoxicity,6,20,21,24–26,30–32,32,36,40,42,48,65,73,75–82 PFAS exposure during both development and adulthood may change neurotransmitter levels in a brain region-specific manner (Table 2). Lifelong exposure of humans to PFAS may result in dynamic changes in neurological function resulting from PFAS accumulation over time.
Table 2.
Brain Neurotransmitter and Metabolite Levels Following PFAS Exposure in Animal Modelsa
| PFAS (PFAS/PFAS mixture) and dose | model/age of exposure/exposure duration | brain region | neurotransmitter or metabolite | dose-related change | source |
|---|---|---|---|---|---|
| PFOS: 0, 3.0, 6.0 mg/kg/d | male SD rats/adult/28 days | anterior hypothalamus | DA | increase | 33 |
| DOPAC/DA | decrease | ||||
| HVA/DA | decrease | ||||
| GABA | increase | ||||
| mediobasal hypothalamus | DA | no change | |||
| DOPAC/DA | no change | ||||
| HVA/DA | no change | ||||
| GABA | no change | ||||
| PFOS: 0, 0.5, 1, 3, 6 mg/kg/d | male SD rats/adult/28 days | anterior hypothalamus | NE | increase | 28 |
| mediobasal hypothalamus | NE | no change | |||
| median eminence | NE | increase | |||
| PFOS: 0, 0.5, 1, 3, 6 mg/kg/d | male SD rats/adult/28 adults | anterior hypothalamus, mediobasal hypothalamus, and median eminence | 5-HT | increase | 29 |
| 5-HIAA/5-HT | decrease | ||||
| PFOS: 0, 1.0, 10.0 mg/kg/d | female SD rats/adult/14 days | hypothalamus paraventricular nucleus | DA | no change | 23 |
| NE | no change | ||||
| hypothalamus medial preoptic area | DA | no change | |||
| NE | increase | ||||
| PFOS: 0, 0.5, 1.0, 3.0, 6.0 mg/kg/d | male SD rats/adult/28 days | amygdala | DA | no change | 34 |
| DOPAC | no change | ||||
| DOPAC/DA | no change | ||||
| HVA | decrease | ||||
| HVA/DA | no change | ||||
| PFOS: 0, 0.5, 1.0, 3.0, 6.0 mg/kg/d | male SD rats/adult/28 days | prefrontal cortexb | DA | no changeb | 34 |
| DOPAC | no changeb | ||||
| DOPAC/DA | no changeb | ||||
| HVA | no changeb | ||||
| HVA/DA | no changeb | ||||
| PFOS: 0, 250 mg/kg | male SD rats/adult/single dose | cerebrum | DA | no change | 35 |
| 5-HT | no change | ||||
| NE | no change | ||||
| GLU | no change | ||||
| GABA | no change | ||||
| GLY | no change | ||||
| PFOS: 0, 1 mg/kg/d | SD rats (sex not specified)/development: gestational and postnatal/dosed GD1-PND21 | cortex | ASP | increase | 32 |
| SER | increase | ||||
| GLY | increase | ||||
| taurine | increase | ||||
| GABA | increase | ||||
| PFOS: 0, 0.5, 1.0, 3.0, 6.0 mg/kg/d | male SD rats/adult/28 days | hippocampus | DA | increase | 34 |
| DOPAC | no change | ||||
| DOPAC/DA | decrease | ||||
| HVA | no change | ||||
| HVA/DA | no change | ||||
| PFOS: 0.00, 0.43, 2.15, 10.75 mg/kg/d | C57BL/6 mice (half male, half female)/adult/subchronic (3 months) | hippocampus | GLU | increase | 27 |
| GABA | no change | ||||
| PFOS: 0, 1 mg/kg/d | male C57BL/6 mice/development: postnatal/PND0-PND14/sampled at adulthood | dorsal hippocampus | GLU | increase | 30 |
| GLN | no change | ||||
| GLY | no change | ||||
| GABA | increase | ||||
| PFOS: 0.00, 0.43, 2.15, 10.75 mg/kg/d | C57BL/6 mice (half male, half female)/adult/subchronic (3 months) | dorsal striatum (caudate putamen) | DA | decrease | 27 |
| DOPAC | decrease | ||||
| HVA | no change | ||||
| (DOPAC + HVA)/DA | no change | ||||
| PFOS: 0, 250 mg/kg | male SD rats/adult/single dose | cerebellum, pons, and medulla | DA | no change | 35 |
| 5-HT | no change | ||||
| NE | no change | ||||
| GLU | no change | ||||
| GABA | no change | ||||
| GLY | no change | ||||
| PFOS: 0, 1 mg/kg/d; 14 days from birth | male C57BL/6 mice/development: postnatal/PND0-PND14/sampled at adulthood | cerebellum | GLU | no change | 31 |
| GLN | no change | ||||
| GLY | no change | ||||
| GABA | no change | ||||
| PFOA: 0, 0.5, 2.5 mg/kg/d | male BALB mice/adolescent/28 days; 6 weeks-old at start | whole brain | DA | increase | 36 |
| L-DOPA | increase | ||||
| 5-HT | increase | ||||
| NE | decrease | ||||
| GLU | decrease | ||||
| PFNA, PFDA, PFUA, PFDoDA, PFTrDA, PFTeDA, PFOA, PFOS, PFAS feed mixC | male and female A/J mice/adolescent/10 weeks; PND21 at start | whole brain: females | DA | no change | 25 |
| whole brain: males | DA | decrease | |||
| PFOS, PFTrDA, PFNA, PFOA | female juvenile Atlantic cod (G. morhua)/adolescent/once per week for 2 weeks | whole brain | DA | decrease | 26 |
| DOPAC | no change | ||||
| PFAS mix:d low dose (1×) and high dose (20×) | DOPAC/DA | no change | |||
| HVA | no change | ||||
| (DOPAC + HVA)/DA | no change | ||||
| PFOA or PFOS: 0, 0.01, 0.1, 1.0 ppm (mg/L) | northern leopard frogs (Rana pipens)/development: larval/30 days | whole brain: PFOA | DA | decrease | 24 |
| whole brain: PFOS | DOPAC | no change | |||
| HVA | no change | ||||
| (DOPAC + HVA)/DA | increase | ||||
| NE | no change | ||||
| 5-HT | no change | ||||
| 5-HIAA | no change | ||||
| 5-HIAA/5-HT | no change | ||||
| GLU | no change | ||||
| GABA | no change | ||||
| ACh | no change | ||||
| PFOS alone or PFAS mix (PFOS, PFOA, PFHxS, PFPeA): 0, 10 ppb (0.01 mg/L); PFOS or PFAS mix (4.0 ppb PFOS, 1.25 ppb PFOA, 3.0 ppb PFHxS, 1.25 ppb PFHxA, 0.5 ppb PFPeA) | northern leopard frogs (Rana pipens)/development: larval or larval through metamorphosis/30 days during larval development or until metamorphosis was complete (juvenile frogs) | whole brain: PFOS, 30 days, larva | DA | no change | 6 |
| DOPAC | no changeg | ||||
| HVA | no change | ||||
| (DOPAC + HVA)/DA | no change | ||||
| NE | no change | ||||
| 5-HT | decrease | ||||
| 5-HIAA | no change | ||||
| 5-HIAA/5-HT | no change | ||||
| GLU | decrease | ||||
| GABA | no change | ||||
| GLU/GABA | no change | ||||
| ACh | no change | ||||
| whole brain: PFAS mix, 30 days, larva | DA | no change | |||
| DOPAC | no change | ||||
| HVA | no change | ||||
| (DOPAC + HVA)/DA | no change | ||||
| NE | no change | ||||
| 5-HT | decrease | ||||
| 5-HIAA | no change | ||||
| 5-HIAA/5-HT | no change | ||||
| GLU | decrease | ||||
| GABA | no change | ||||
| GLU/GABA | no change | ||||
| ACh | no change | ||||
| whole brain: PFOS, juvenile frogs; PFAS, mix juvenile frogs | DA | no change | |||
| DOPAC | no change | ||||
| HVA | no change | ||||
| (DOPAC + HVA)/DA | no change | ||||
| NE | no change | ||||
| 5-HT | no change | ||||
| 5-HIAA | no change | ||||
| 5-HIAA/5-HT | no change | ||||
| GLU | no change | ||||
| GABA | no change | ||||
| GLU/GABA | no change | ||||
| ACh | increase |
Animal studies were included that assessed the effect of PFAS exposure on neurotransmitter levels and metabolite levels in the brain compared to vehicle controls. When multiple doses were included, a dose-related change was only included when significant changes were detecting with ascending doses. Studies that had no control group were excluded. Abbreviations: acetylcholine (ACh), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), gamma-aminobutyric acid (GABA), homovanillic acid (HVA), glutamate (GLU), glutamine (GLN), glycine (GLY), l-3,4-dihydroxyphenylalanine (L-DOPA), norepinephrine (NE), per- and polyfluoroalkyl substance (PFAS), perfluorodecanoic acid (PFDA), perfluorododecanoic acid (PFDoDA), perfluorohexanoate (PFHxA), perfluorohexanesulfonate (PFHxS), perfluorononanoic acid (PFNA), perfluorooctanoic acid (PFOA), perfluorooctanesulfonate (PFOS), perfluoro-n-pentanoate (PFPeA), perfluorotetradecanoic acid (PFTeDA), perfluorotridecanoic acid (PFTrDA), perfluoroundecanoic acid (PFUA), postnatal day (PND), serotonin (5-HT), Sprague–Dawley (SD), and 5-hydroxyindoleacetic acid (5-HIAA).
Tissue degradation may have influenced results.
PFAS feed mix containing 2 ng/g PFNA, 3 ng/g PFDA, 3 ng/g PFUA, 8 ng/g PFDoDA, 16 ng/g PFTrDA, and 20 ng/g PFTeDA (6 d/wk) and gel diet containing 17.5 ng/g PFOA and 9.1 ng/g PFOS (1 d/wk).
PFAS injection mix low dose (1×) or high dose (20×) containing 25 or 500 mg/g PFOS, 16.95 or 339 mg/g PFTrDA, 5.925 or 118.5 mg/g PFNA, and 3.825 or 76.5 mg/g PFOA, respectively.
An overview of PFAS effects on neurotransmitter levels in controlled laboratory studies is presented in Table 2. Studies that have analyzed neurotransmitters have exposure lengths ranging from a single dose to 3 month subchronic exposures. Interestingly, it is challenging to discern a clear neurotoxic pattern, as the effects vary widely between brain region, dose, and sex. A study performed in rodents has determined that the biodistribution of PFAS is variable between sexes, which may explain the variation between sexes,61 but very few studies have been appropriately powered to confirm sex effects in neurotransmission. Furthermore, species differences in the absorption, distribution, and excretion of PFAS have previously been described by Pizzurro et al. in 2019 and may also contribute to variation in the effects of PFAS on neurotransmitter levels in the brain.71
The developmental PFAS exposure studies have largely relied on analyzing whole brain changes in neurotransmitters and not specific regions/subregions.6,24–26,73 Developmental or adolescent exposures to PFAS mixtures, PFOS only, or PFOA only in mice, female juvenile Atlantic cod, and northern leopard frog tadpoles resulted in decreased whole brain dopamine.24–26 Two mice studies that exposed mice to different PFAS mixtures during adolescence demonstrated that a PFAS mixture decreased whole brain dopamine in male mice, whereas male mice exposed to PFOA alone demonstrated increased whole brain dopamine.25,36 Multiple species also demonstrated decreased whole brain glutamate after developmental or adolescent exposure to PFOS or a PFAS mixture in northern leopard frog tadpoles and PFOA in male mice.6,36
Multiple studies that evaluated PFOS exposure in adult rats reported highly reproducible increases in norepinephrine and increases in other monoamines such as dopamine and serotonin in the hypothalamus.23,28,29,33 The consistency of these results is likely because the studies were conducted in the same species and were dosed at the same life stage, the same brain region was sampled, and the same PFAS was used in all studies. The final notable alteration in the neurotransmitters was increased glutamate in the hippocampus of mice exposed to PFOS during adulthood or development.27,30 Similar to the changes in neurotransmitters in the hypothalamus, the same species and PFAS were used in both studies. However, these studies are evidence that exposure to PFOS at any age may increase hippocampal glutamate. Existing data suggest that some brain subregions are specifically resistant to the effects on neurotransmission, whereas PFAS exposure in adult animals has little to no significant effect on the neurotransmitter or neurotransmitter metabolite levels in the cortex, cerebellum, pons, and medulla (Table 2).
3.1.1. Acetylcholine.
Hallgren et al. showed male mice that were lactationally exposed to PFOS had changes in locomotor behavior; treated mice had less activity at the beginning and elevated activity at the end of the trial.82 These behavioral changes indicate changes in acclimation and exploratory behavior. These mice also displayed decreased transcription of the acetylcholinesterase (AChE) gene and one subunit of the nicotinic acetylcholine receptor (nAChR) gene in the cortex and increased transcription of a subunit of the muscarinic acetylcholine receptor (mAChR) gene in the hippocampus.82 Interestingly, male mice given a single neonatal oral dose of PFOS or PFOA demonstrated hypoactive locomotor behavior in a nicotine stimulant challenge performed four months after exposure.83 In northern leopard frogs, developmental PFOS exposure demonstrated increased acetylcholine in young frogs.6 A study by Lau et al. in 2003 determined that choline acetyltransferase (ChAT) activity (the enzyme responsible for generating ACh from choline and acetyl-CoA) was reduced in the prefrontal cortex, but not the hippocampus, of rat pups developmentally exposed to PFOS.76 Decreases in AChE protein expression and decreased ChAT activity could explain the increased acetylcholine observed in young frogs, but this needs to be studied further in both rodent and frog models. A study by Eggers Pedersen et al. found decreased AChE activity correlated with an increase in PFAS levels in the thalamus, cerebellum, and frontal cortex of polar bear brains.44 Additionally, PFAS levels correlated with elevated mAChR activity in the frontal cortex.44 Taken together, there are likely region-specific effects of PFAS on cholinergic neurotransmission, and the frontal cortex is likely a key region involved. Further exploration should quantify ACh in brain regions to better understand how cholinergic neurotransmission is affected by PFAS exposure.
3.1.2. Dopamine and Glutamate.
In the studies utilizing sentinel species exposed to PFAS in feed during late or early brain development (Atlantic cod and northern leopard frogs) as well as mice fed a PFAS mix starting at weaning, the whole brain dopamine levels decreased.24–26 An exception was a vole study that found PFAS levels to be positively associated with dopamine levels (negatively associated with the metabolite/parent neurotransmitter ratio). Importantly, this was a sentinel study with uncontrolled exposures, where a ski area with voles containing elevated PFOS, PFHxS, PFDA, PFUA, perfluorododecanoic acid (PFDoDA), perfluorotridecanoic acid (PFTrDA), and perfluorotetradecanoic acid (PFTeDA) was compared to a reference area with voles that had elevated PFHxA;37,84 thus, the study compared two different PFAS mixes without a control. With respect to the sentinel vole study, the same group conducted a controlled exposure in mice on the basis of PFAS earthworm levels in a contaminated ski area and found the PFAS exposure to be associated with decreased whole brain dopamine levels similar to other sentinel studies.24–26,37,84 Another laboratory study that dosed male mice with PFOA during adolescence found elevation in whole brain dopamine.36 Taken together, these studies suggest the effects of PFAS on dopamine levels during development may depend on the exposure of one or more PFAS.
Rodent models exposed to PFAS before weaning and near and after sexual maturity had increases in dopamine and serotonin in the whole brain,36 hippocampus,30,34 and hypothalamus.28,33 Interestingly, male mice developmentally exposed to PFAS mixtures, frogs developmentally exposed to PFOS or PFOA, and female juvenile Atlantic cod exposed to a PFAS mixture resulted in decreases in whole brain dopamine levels.24–26 Long et al. detected decreases in dopamine levels in the dorsal striatum and increases in hippocampal glutamate levels of male and female mice subchronically exposed to PFAS for 3 months during adulthood.27 These studies suggest potential deficits in dopaminergic neurotransmission and glutamatergic excitotoxity from longer PFAS exposures.27
Mshaty et al. found that the adult male mice developmentally exposed to PFOS had deficits in learning and memory, but there were no changes in gene expression of γ-aminobutyric acid (GABA) receptors and most of the N-methyl-d-aspartate (NMDA) glutamate receptors.30 Although decreases in α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) glutamate receptor subunit A3 (Gria3) mRNA expression were detected, there were no changes in protein expression.30 These results suggest that PFOS and PFOA may change levels of glutamate and GABA but not their receptor protein levels. Additionally, other neurotransmitters and amino acids important in neurotransmission and development, such as glycine and taurine, should be evaluated in the brain for PFAS-related changes.
3.1.3. GABA.
There is some evidence in animal and cell models that the PFAS, PFOS and PFOA, may alter GABAergic neurotransmission. Adult male mice lactationally exposed to PFOS were determined to have elevated levels of extracellular glutamate and GABA and no changes in extracellular glycine in the dorsal hippocampus.30 Another developmental PFOS study in rats conducted metabolomic analysis of the cortex and found elevated levels of multiple inhibitory neurotransmitters including taurine, GABA, and glycine.32 Furthermore, in human induced pluripotent stem cell (hiPSC)-derived neurons, Tukker et al. demonstrated PFOS and PFOA acted as noncompetitive GABAA receptor antagonists.85 Taken together, it can be hypothesized that PFAS inhibit GABA synaptic neurotransmission by competitively inhibiting the GABAA receptor and increased GABA excretion (and other inhibitory neurotransmitters) may be a brain region-dependent compensatory mechanism.
3.2. PFAS Alters Monoamine Metabolism.
Recent studies have been evaluating metabolism-related mechanisms to explain PFAS-induced changes in neurotransmitter levels. The monoamine neurotransmitters include norepinephrine, dopamine, and serotonin and share metabolic enzymes and precursors.86,87 There is evidence in the animal data that PFAS exposure changes monoamine metabolism.6,23–29,33,34,36,37,44,48,73 Multiple studies demonstrated PFOS-treated male rats had decreased dopamine or serotonin turnover in the hypothalamus29,33 and hippocampus.34 Studies that developmentally exposed northern leopard frog tadpoles to PFOS or PFOA found no changes in whole-brain serotonin levels or serotonin turnover.6,24 This suggests that the effects of PFAS on monoamine metabolism may depend on the species and brain region. As an example, whole brain studies do not always capture changes in the expression to metabolic enzymes.37
In humans, elevated serum PFAS correlated with changes in systemic metabolic pathways that provide precursors for monoamine synthesis.88 The changes in the metabolic pathway for tryptophan, an amino acid precursor for serotonin synthesis, were found to correlate with PFHxS and PFNA exposure. Moreover, changes in the metabolic pathway for glutamate, the most prevalent amino acid and neurotransmitter in the brain, were correlated with PFOS, PFHxS, and PFNA exposure.88 Rat kidney cells exposed to PFOS or PFOA demonstrated decreases in phenylalanine (a tyrosine precursor), tyrosine (a dopamine and norepinephrine precursor), and tryptophan (a serotonin precursor) levels compared to the controls.74 Interestingly, mice exposed to PFOA had elevated liver tyrosine, dopamine, and tryptophan and decreased liver serotonin, but their brain levels of both dopamine and serotonin were elevated with decreases in norepinephrine and glutamate.36 Metabolomic brain analysis from rats developmentally exposed to PFOS demonstrated elevated metabolite levels for phenylalanine, tyrosine, tryptophan, glutamate, glycine, and taurine.32 Therefore, circulating levels of metabolic precursors for serotonin, norepinephrine, and dopamine synthesis may have downstream effects resulting in altered brain neurotransmitter levels.
Many studies have determined that PFAS can alter brain tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine production. Developmental exposure of zebrafish larva to PFOA increased TH mRNA expression.73 A study in male mice given a single developmental dose of PFOS had elevated TH levels in the hippocampus 24 h after the exposure and decreased TH levels in the hippocampus a month and a half after the exposure.48 Interestingly, there were no changes in TH in the cortex at either time point. This suggests that even a single high dose exposure to PFAS can have lasting effects on developmental and adult brain function and there is more evidence for specific regions with higher susceptibility to PFAS neurotoxicity. Furthermore, a study by Grønnestad et al. demonstrated male mice exposed to a PFAS mixture during late brain development and adulthood had decreases in whole brain TH expression, whereas no changes were detected in female mice.25 Female Atlantic cod exposed to a PFAS mixture also had no change in TH RNA expression.26 Therefore, sex-dependent differences in response to PFAS may greatly affect the downstream neurotoxic effects and must be considered when evaluating the neurotoxic mechanisms of PFAS on neurotransmission. Many studies that have exposed animals to PFOA, PFOS, or PFAS mixtures have reported that PFAS decrease or increase TH RNA expression,25,26,48,73 but there is a clear need to evaluate TH protein expression and activity to understand if these changes in TH mRNA correlate with functional changes in metabolism.
Additional evidence found by mice and ecologically sampled bank voles exposed to similar PFAS mixtures did not find changes in mRNA expression of monoamine oxidase (MAO), an enzyme used to breakdown dopamine into DOPAC and HVA25,37,86,87 and serotonin into 5-HIAA.86,87 However, decreased MAO-A mRNA in the bank vole brains was associated with both PFTrDA and PFOS exposure when individual PFAS were evaluated.37 The inconsistent changes in RNA expression largely conflict and do not aid in the interpretation of mechanistic changes resulting from PFAS exposure. Finally, the effects of PFAS on other important monoamine metabolic enzymes (besides TH) have not yet been assessed for expression or function, including aromatic amino acid decarboxylase (AADC), catechol-O-methyltransferase (COMT), tryptophan hydroxylase (TrpH), and dopamine β-hydroxylase (DBH). These gaps in knowledge for functional enzymatic changes in catecholamine metabolism resulting from PFAS exposure should be addressed in future experiments.
4. SYNAPTIC CALCIUM SIGNALING TARGETS FOR PFAS NEUROTOXICITY
PFAS including PFOA, PFOS, PFHxS, PFDA, and PFTA have been found to increase the frequency of synaptic calcium currents, and PFOS and PFTA increase calcium amplitude.52 Furthermore, PFOA, PFOS, PFHxS, PFDA, PFBS, and PFOC increase intracellular calcium.52 There is accumulating evidence that PFOS and PFOA disrupt calcium signaling in the neurons of the hippocampus41,42,51,52,89 and cerebellar Purkinje cells.50 Research discussed in this section discusses PFAS neurotoxic effects on calcium homeostasis in neurons. Major mechanistic targets include the cell membrane calcium channels L-type high-voltage calcium channels, NMDA glutamate receptor, and AMPA glutamate receptor and the intracellular calcium channels RyR and IP3R.
4.1. PFAS Alters Intracellular Calcium Storage.
Calcium signaling plays an important role in neuronal function, including regulating action potentials and cell death pathways, and is primarily stored in the endoplasmic reticulum and mitochondria of cells.89 PFOS has been demonstrated to produce a larger magnitude of deleterious effects on calcium signaling compared to PFOA, and it is hypothesized that this is due to the different properties of their functional groups.89
Liu et al. demonstrated that hippocampal primary rat neurons exposed to PFOS or PFOA disrupt calcium homeostasis by increasing the extracellular calcium release as well as the release of calcium from intracellular storage in the endoplasmic reticulum.89 The release of calcium from the endoplasmic reticulum was through the RyR and IP3R calcium channels. The authors have hypothesized this is driven by low-voltage gated “T-type” calcium channels.89 However, there are no PFAS studies that specifically target the T-type calcium channels in neurons. Future studies should evaluate how PFAS affect calcium stored in the mitochondria in addition to the endoplasmic reticulum because mitochondria may be another synaptic target of the PFAS and contribute to neuron survival.
4.2. PFAS Affects Glutamate Receptor Calcium Signaling.
PFAS effects on the NMDA and AMPA glutamate receptors have been extensively studied. The mechanism of calcium influx into neurons and through the glutamate receptor has also been described in rodent cortex,40 cerebellum,38,39,43,50 and hippocampus.36,41,45,51,54,57,89
Liao et al. demonstrated in rat hippocampal primary neurons that PFOS increases glutamate-activated currents through the NMDA and AMPA receptors.41 A study using primary rat cerebellar granular neurons and PC12 cells (with or without NMDA receptor expression) demonstrated that PFOS, but not PFOA, is dependent on an NMDA receptor-mediated influx of calcium ions.38 Berntsen et al. in rat cerebellar granular neurons demonstrated 20–40 μM PFOS induced NMDA receptor-mediated cytotoxicity.39 Furthermore, PFOS (60 μM) and PFOA (300 μM) demonstrated elevated levels of calcium in the extracellular space.39 A study in chicken embryo primary cerebellar granular neurons demonstrated that PFOS induced cytotoxicity through the NMDA receptor in both the presence and absence of glutamate. Additionally, this study found that PFOS-induced elevation of intracellular calcium was dependent on the NMDA receptor and glutamate.43
Increased AMPA and NMDA calcium currents with exposure to PFOS exposure may be through changes in protein expression. Studies have demonstrated that PFOS decreases protein expression of the AMPA receptor subunit 2 (GluA2).40,49 The presence of GluA2 in the AMPA receptor causes sodium but not calcium to enter the activated receptor, and the absence of GluA2 induces calcium but not sodium to enter the cell through the activated AMPA receptor.90 Therefore, decreased GluA2 protein expression could further increase the PFOS-induced increase in intracellular calcium.90 Additionally, the increased influx of calcium could be mediated through PFOS-induced upregulation of the NMDA receptor. Studies have indicated that PFOS increases protein expression of NMDA receptor subunits NR1, NR2A, and NR2B.45 Taken together, PFOS is likely the only known PFAS that selectively targets glutamate-receptor mediated calcium influx. More research needs to evaluate if PFAS mixtures and emerging PFAS target glutamate receptors.
4.3. L-Type Calcium Channel Currents Are Altered by PFAS.
Both PFOS and PFOA have been extensively linked to the interaction with L-type high-voltage calcium channels (L-VGCCs).45,50,52,89 In primary rat Purkinje cells, PFOS prevented the function of calcium ion gated channels, causing a positive shift in the action potential, hyperpolarization of the resting membrane potential, and a decrease in the firing rate.50 Harada et al. hypothesized that PFOS’s inhibitory effect of neurotransmission and disruption through voltage-gated calcium channels in Purkinje cells may be caused by the incorporation of PFOS into the outer cell membranes.50 Results from Liao et al.41,51 demonstrated that PFOS increased calcium influx in primary hippocampal neurons and inducted excitotoxicity, which contrasts the results from Harada et al.50 Furthermore, inhibition of synaptogenesis is a downstream effect that resulted from PFOS’s enhancement of inward calcium currents via the L-type calcium channels in hippocampal neurons.51 These results largely support that PFOS stimulates the influx of intracellular calcium, leading to downstream inhibition of synaptogenesis.48,91–93
Harada et al. and Liu et al. performed their experiments in media lacking calcium ions, and the presence of PFOS or PFOA would increase the levels of extracellular calcium,50,89 whereas Liao et al. used calcium containing media, which may result in influx into the cell.51 One may hypothesize that the effect PFOS or PFOA on intracellular and extracellular calcium is dependent on the basal extracellular conditions and they contribute to the movement of calcium across the concentration gradient. Additionally, these effects are likely dependent on the levels of extracellular calcium. Interestingly, Liu et al. found that the intracellular concentrations of PFOS and PFOA were significantly increased in the presence of calcium-containing media compared to calcium-free media.89 Therefore, the investigation of calcium channels as possible symporters for PFAS may be required.
The voltage-gated and glutamate receptor activated calcium channels expressed in the brain, including if they have been assessed for PFAS toxicity, are summarized in Table 3. Although many different types of voltage-gated calcium channels are expressed in the brain, only the L-type calcium channels have been demonstrated to have currents affected by PFAS in the studies that were discussed above.42,50 As outlined in Table 3, many of the neurological functions associated with L-type channels including learning and memory (subtype 1.2), striatal dopaminergic motor function (subtype 1.3), and psychiatric mental health (subtypes 1.2 and 1.3) have been demonstrated to be negatively affected by PFAS exposure.31,94 Currently, the NMDA and AMPA glutamate receptors are the best characterized calcium transporters to be affected by PFAS, followed by the L-type voltage-gated calcium channels.
Table 3.
Calcium Channels and Transporters Important to Neurotransmission and Possible Roles in PFAS Neurotoxicitya
| type | neurological functions | mechanisms related to neurotransmission | PFAS neurotoxicity | source |
|---|---|---|---|---|
| Calcium Channel Group: High Voltage-Gated | ||||
| L-type “long-lasting” | 42, 50–52 | |||
| all subtypes (Cav1.2, Cav1.3, and Cav.1.,4) | tonic and slow release of neurotransmitters | subtypes have not been evaluated individually | ||
| CaM/CaMK/CREB transcription-signaling and CaM/Fas/MAPK-signaling inhibits nicotinic signaling to CREB | PFOS and PFOA induce effects on calcium currents and enhance neurotransmission | |||
| studies did not demonstrate complete block of all other calcium channels in the neuronal cells (effect may not be specific to L-type calcium channels) | ||||
| studies have demonstrated that PFOS has downstream effects on the CaM/CaMKII/CREB signaling pathway including deficits in neuronal process formation | ||||
| Cav1.2 | regulates working memory, social behavior, and adaptation to novel situations | in cortex and cerebellum: Cavl.2 C-terminus (CCAT) translocates and acts as a transcription factor for NMDA subunits, K+ channel subunits, and gap junction proteins; promotes neurite outgrowth | ||
| in hippocampus: essential for spatial learning and memory. | expression in increased by cocaine exposure | |||
| loss of function associated with psychiatric disorders including depression, bipol | induces calcium influx that drives the release of catecholamines | |||
| required for neuronal circuit formation and amyglala functional disorder, and schizophrenia | ||||
| Cav1.3 | in VTA and nucleus accumbens: involved with cocaine-induced chronic behavioral changes | expression is decreased with cocaine exposure | ||
| increased expression in early stage Parkinson’s disease | in hippocampus: better coupling to CREB compared to Cav1.2 | |||
| channel blocker is a potential target for Parkinson’s disease treatment | in striatal spiny neurons: the only channel that couples to CREB | |||
| induces calcium influx that drives the release of catecholamines in substantia nigra dopaminergic neurons: expression and regulation of pacemaking increases with age | ||||
| Calcium Channel Group: High Voltage-Gated | ||||
| N-type “neural” | needs evaluation | |||
| Cav2.2 | regulates working memory, social behavior, and adaptation to novel situations | rapid synaptic neurotransmitter release | ||
| loss of function reduces ethanol consumption and increases activity | involved in GABA release in some cells in the hippocampus | |||
| primary channel for synaptic glutamate release in many brain regions in hippocampus: partial functional compensation when Cav2.1 has lost function | ||||
| Calcium Channel Group: High Voltage-Gated | ||||
| P-type/Q-type | needs evaluation | |||
| “Purkinje”/“Q after P” Cav2.1 | hippocampus: loss of function induces seizures | rapid synaptic neurotransmitter release | ||
| cerebellum: loss of function induces ataxia and seizures | primary channel for synaptic glutamate release in many brain regions | |||
| gain-of-function mutation induces migraines and cortical depression | involved in GABA release of interneurons in the cortex, pyramidal neurons, and many other neurons | |||
| Calcium Channel Group: High Voltage-Gated | ||||
| R-type “residual” | needs evaluation | |||
| Cav2.3 | loss of function decreases pain sensitivity | rapid synaptic neurotransmitter release | ||
| in hippocampus: partial functional compensation when the Cav2.1 function is lost Calcium Channel Group: Low Voltage-Gated | ||||
| Calcium Channel Group: Low Voltage-Gated | ||||
| T-type “transient” Cav3.1, Cav3.2, and Cav3.3 | involved in regulating sleep patterns and arousal | drives low-threshold exocytosis of neurotransmitters | needs evaluation | |
| mutations associated with epilepsy and autism spectrum disorders | key role in regulating the thalmocortical circuit | |||
| induces calcium influx that drives the release of catecholamines | ||||
| Calcium Channel Group: Ionotropic Glutamate Receptor | ||||
| AMPA receptor | synaptic plasticity, long-term potentiation, and action potentials | glutamate-dependent activation of the calcium channel | PFOS decreases subunit expression, thus allowing the AMPA-R type that is permeable to calcium | 40–42, 49, 90 |
| calcium or sodium permeability is determined by the GluA2 subunit; AMPA-R allows calcium to penetrate without the GluA2 subunit or if the GluA2 subunit is the unedited GluA2(Q) isomer; the edited GluA2(R) isomer is normally expressed in neurons | PFOS increases intracellular calcium through the AMPA-R | |||
| expression of edited and nonedited GluA2 receptors after PFAS exposure needs evaluation | ||||
| NMDA receptor | synaptic plasticity, long-term potentiation, and action potentials | produces excitoxicity-induced cell death by C/EBPβ transcriptional activity | PFOS increases intracellular calcium through glutamateactivated NMDA-R, inducing excitotoxicity | 38, 39, 41, 43, 54 |
| glutamate-dependent activation of the calcium channel | PFOS can increase excitoxicity through the NMDA-R using glutamate-independent toxicity, through an unknown mechanism | |||
| inhibited by L-type induced CREB signaling NMDA-induced increases in calcium activate CaMKIIa | ||||
Neurological functions of voltage-gated calcium channels are summarized from reviews by Dolphin and Lee (2020),95 Saravanaraman et al. (2014),91 Simms and Zamponi (2014),96 and Wang and Jin (2012).53 The calcium channels that are primarily linked to PFAS neurotoxicity are the L-type high-voltage gated channels and the NMDA and AMPA glutamate receptors. Nearly all studies on the toxic effects of PFAS exposure on these calcium channels have only tested PFOS and PFOA.38–43,50–52,54 There is much to be learned regarding the roles of calcium channels as primary neurotoxic targets. The primary goal of this table is to prompt mechanistic research.
There is a gap in knowledge about the impact PFAS has on the RNA editing of GluA2 mRNA. The GluA2 subunit of the AMPA receptor is edited in 99% of expressed proteins to contain the GluA2(R) isomer, which selectively allows sodium to enter the cell when glutamate is bound.90 The minority GluA2 isomer is the unedited GluA2(Q), which has selective permeability to calcium over sodium.90 Therefore, in addition to decreases in total GluA2 protein expression, GluA2 mRNA editing is another potential target for PFAS toxicity that would produce elevated calcium influxes. There is a gap in knowledge about the impact that PFAS have on other types of high-voltage calcium channels expressed in the brain, including the N-, P-, Q-, and R-types and the low-voltage T-types. More work needs to be done to determine how PFAS may be altering these different calcium channels and transporters in different brain regions, especially the hippocampus, to understand PFAS-induced calcium dyshomeostasis and the mechanism of synaptic toxicity.
5. FUNCTIONAL CONSEQUENCES OF PFAS EXPOSURE
5.1. PFAS Alter Synaptic Formation and Growth.
Changes in other proteins involved in neuronal function and disease have been reported to change with PFOS and PFOA exposure. Johansson et al. demonstrated that acute postnatal oral exposure to PFOS or PFOA changes the expression of proteins important for growth and synaptogenesis in neurons.97 Rats gestationally and lactationally exposed to PFOS demonstrated transient changes in gene expression (mRNA levels) for proteins important to calcium signaling in the hippocampus.42 Interestingly, increased expressions of calmodulin-dependent kinase IIa (CaMKIIa) and cAMP-response element-binding (CREB) were normalized by postnatal day (PND)30, while calmodulin (CaM, a subunit of high voltage gated calcium channels) expressions were decreased by PND30.42 Another study that injected a single oral dose of PFOS or PFOA on PND10 increased protein levels of CaMKII (CaM-dependent kinase II, a kinase that becomes activated when it dimerizes with CaM bound to calcium) in the hippocampus but not the cerebral cortex of young mice.97 PFOS or PFOA increased CREB, CaMKII, and GAP-43, and the alteration of these calcium-related pathways by PFAS may lead to downstream inhibition of synaptogenesis.42,48,91–93,97
Recently, it was found that PFOA exposure has an adverse effect on the maturation of dopaminergic neurons, resulting in the decreased expression of TH and dopamine transporter (DAT) with the greatest effects on dopaminergic neuroprogenitors that were further along in differentiation.65 It was demonstrated in hIPSC-derived neuroprogenitors that PFOA decreased the expression of markers of dopaminergic neuron maturation [TH and neurofilament heavy (NFH)] when hIPSCs were exposed during the neuronal precursor phase (DP2) of dopaminergic differentiation. PFOA exposure at the mature dopaminergic differentiation phase (DP3) induced a major reduction of TH, NFH, and dopamine transporter (DAT) expression, which is involved in the reuptake of DA in the presynaptic neuron.65 The aforementioned reduction in TH and DAT could relate to a mechanism that was first identified by Slotkin et al., in which PFAS (including PFOS and PFOA) decreased neuroprogenitor differentiation into dopaminergic neurons in a cell model.80 However, the alteration in DAT RNA and protein expression was only observed after PFOA exposure; PFOS and PFAS mixtures failed to change DAT RNA expression. Furthermore, since PFAS upregulate CAMKIIa, a protein that regulates TH activity by phosphorylation, changes in dopamine levels in some parts of the brain (e.g., increases) may be driven by the changes in CaMKIIa levels, which may drive increased TH activity.98
Studies have demonstrated PFAS may alter protein expression of dopamine receptor 1 (DR1) and dopamine receptor 2 (DR2). The alteration of these dopamine receptors could explain changes in multiple signaling cascades. The activation of the DR1/DR2 complex induces release of intracellular calcium storage from the endoplasmic reticulum, which increases CaMKIIa activity. DR1 activation increases phosphokinase A (PKA), leading to activation of the L-VGCC channels, CREB activity, and GABAA receptor activity. Alternatively, DR2 activation inhibits PKA and the downstream targets. Eggers Pedersen et al.44 demonstrated that polar bears exposed to PFAS have decreased DR2 activity, which may contribute to elevated calcium levels observed with PFOS, PFOA, or PFHxS exposure,38,40,43,45,49,52,54,89 the inhibitory effects of PFOS and PFOA on the GABAA receptor activity,85 and subsequent inhibition of neurite growth by PFAS. Interestingly, the cotreatment of cerebellar neurons exposed to PFHxS with memantine or MK-801 (NMDA, nACh, DA, and 5-HT receptor inhibitors) but not cotreatment with CPP (only the NMDA receptor inhibitor) or NBQX (only the AMPA receptor inhibitor) rescued PFHxS-induced cytotoxicity. These inhibitors suggest that PFHxS-induced neurotoxicity is through a glutamate receptor-independent mechanism, most likely through the interaction with nACh, dopamine, or serotonin receptors38 (Figure 1).
Figure 1.
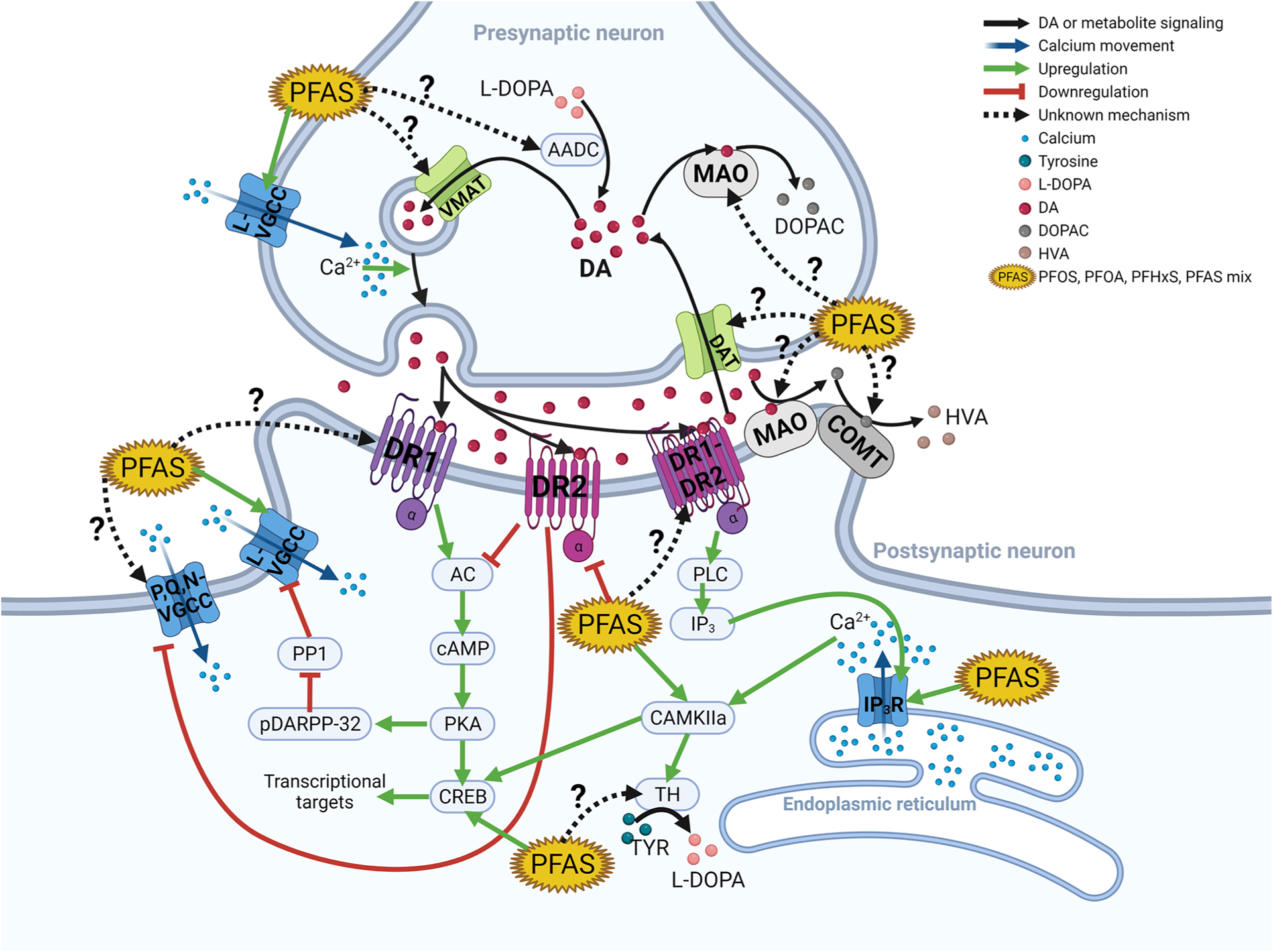
PFAS neurotoxicity through dopamine and calcium signaling. This figure illustrates the synaptic pathways connecting PFAS, dopamine, and calcium. The key concept is that dopamine, like glutamate, is a target for PFAS-induced modulation of calcium currents. Importantly, PFAS interacts with many known proteins that are a part of dopaminergic postsynaptic signal transduction, but future studies need to investigate the interaction of PFAS and dopamine receptors (DRs) 1 and 2 and the DR1–2 dimer as well as DR3, −4, and −5 (not depicted in the figure). Some evidence suggests DR2 activity, protein levels, and RNA expression may decrease with PFAS exposure. This would result in PFAS inhibiting DR2’s inhibition of AC, resulting in the upregulation of the L-VGCC’s activity. PFAS has been extensively demonstrated to induce increased cytosolic calcium through the L-VGCC and to a lesser extent in the IP3R. Future studies also need to investigate PFAS’s effect on the monoamine metabolic enzymes and transporters. AADC, aromatic amino acid decarboxylase; AC, adenylyl cyclase; CAM, calmodulin; CAMKIIa, CAM kinase IIa; cAMP, cyclic adenosine monophosphate; COMT, catechol-O-methyltransferase; CREB, cAMP-response element-binding; DA, dopamine; DAT, dopamine transporter; DOPAC, 3,4-dihydroxyphenylacetic acid; DR1, dopamine receptor 1; DR1-DR2, DR1–DR2 dimer; DR2, dopamine receptor 2; IP3, inositol triphosphate; IP3R, inositol triphosphate receptor; L-DOPA, l-3,4-dihydroxyphenylalanine; HVA, homovanillic acid; L-VGCC, L-type voltage-gated calcium channel; MAO, monoamine oxidase; pDARPP-32, dopamine and cAMP-regulated phosphor-protein 32 kDa; PKA, protein kinase A; PLC, protein lipase C; P,Q,N-VGCC, P,Q,N-type voltage-gated calcium channel; PP1, protein phosphatase 1; VMAT, vesicular monoamine transporter.
The PFAS decrease the long-term potentiation in neurons, resulting in a neurotoxic effect with lasting functional consequences.49,51,54 Long-term potentiation is important for the formation and strengthening of synaptic connections, which are imperative for learning and memory.49 In rats dosed with PFOS, PFBS, and PFHxS, Zhang et al. demonstrated there was decreased long-term potentiation in the hippocampus.54 The authors suggest this could be due to a postsynaptic mechanism. Long-term potentiation is driven by patterns of synaptic activity, including neurotransmitter release and synaptic calcium signaling (discussed in Sections 3 and 4), which provide further mechanisms that lead to the alteration of long-term potentiation. Therefore, PFAS may induce lasting changes in neuronal connectivity and increase the risk of neurological dysfunction.
5.2. PFAS Alter Behavioral Function.
The most reproduced functional deficits resulting from PFAS exposure are the effects that PFOS and PFOA have on motor function31,82,83,99–101 and learning and memory.27,30,102 Additionally, there is some mechanistic and functional laboratory evidence that may explain the controversial association between attention-deficit/hyperactivity disorder (ADHD) and PFAS exposure.3,94,103–105 This section aims to relate the alteration of neurotransmission to functional deficits and human disease with mechanistic evidence.
A study that exposed male mouse pups to a single dose of PFOS found that, 24 h after PFOS exposure, there was decreased transcription of dopamine receptor 5 (DR5) in the cortex and increased TH in the hippocampus.48 After 2 months, there was a significant decrease in TH and dopamine receptor 2 (DR2) transcription in the hippocampus and no changes in the cortex.48 Another study with the same exposure found there was decreased gene translation for AChE and nAChR-β2 in the cortex and increased mAChR-5 in the hippocampus of mice with a single developmental exposure to PFOS.82 No changes were present 2 months after PFOS exposure. Taken together, PFAS exposure (most evidence from PFOS exposure) produces persistent transcriptional changes of genes expressed in dopaminergic neurons, whereas PFAS-transcriptional effects on the cholinergic system have evidence of transient changes that recover after the exposure is removed.82
The evidence of PFAS exposure’s persistent and transient changes in the dopaminergic and cholinergic systems, respectively, can potentially inform the neurotransmitter systems that are likely responsible for PFAS-induced motor behavioral changes.31,75,82,83,99 Hallgren et al. demonstrated that mice with a single developmental exposure to PFOS had persistent deficits to acclimation in the open-field locomotor experiment. The deficit to acclimation was apparent by the PFOS-treated mice having hypoactivity in the first 20 min and hyperactivity in the last 20 min of 1 h in the activity chamber, when compared to the control animals.82 The behavioral effects from Hallgren et al. are most likely dopaminergic and not cholinergic. However, the Hallgren et al.’s82 transcriptional data suggest that PFAS may affect addiction, as the decreased expression of nAChR-β2 and increased expression of nAChR-α5 may be associated with decreased nicotine addiction and may have implications on other psychiatric conditions.106
PFAS exposure has been associated with ADHD in some epidemiological studies.3,94,103–105 This association remains controversial, and other studies did not find consistent patterns of association between PFAS and ADHD.107–109 The alteration of dopaminergic and serotonergic neurotransmission by PFAS exposure may also explain the mechanism for functional deficits attributed to ADHD. Hyperactivity, one of the key symptoms of ADHD, has been demonstrated in animal models exposed to PFAS as discussed in the previous paragraph. Studies have demonstrated that PFOS-exposed zebrafish and PFOA-exposed mice demonstrated significantly decreased locomotor response when given dexamphetamine or methamphetamine, respectively.100,110 These results may suggest the pathway involved in the treatment of ADHD symptoms (dopamine) may be impacted by PFOS and PFOA.111 The effects of amphetamines should be investigated with exposure to PFAS-induced behavioral changes to investigate if a similar therapeutic effect is present as seen in ADHD patients. While the direct relationship between PFAS-induced neurotransmitter alterations and functional deficits is unclear, detected alterations in neurotransmission are likely to have functional consequences as observed in many established neurotoxicity models and in human neurodegenerative diseases.
5.3. PFAS and Neurodegeneration.
Rats developmentally exposed to PFOS increased Tau phosphorylation and protein expression as well as amyloid precursor protein (APP) and amyloid-β 1–42 (Aβ1–42) in adults,79,81 suggesting that the developmental exposure to PFOS may be involved in the development of neurodegenerative diseases, particularly Alzheimer’s disease (AD) later in life. Another study determined that rats exposed to PFOS increased hippocampal protein levels of total Tau, phospho-Tau-S199, and amyloid-β (Aβ) as well as increased the amount of amyloid precursor protein (APP) mRNA.79 Thus, it can be hypothesized that PFAS may increase the risk of developing Alzheimer’s disease due to the changes in key proteins involved in this disease. In C. elegans, PFOS exposure induced motor dysfunction and decreases in dopaminergic, GABAergic, serotonergic, and cholinergic neurons, suggesting PFOS may also have mechanisms related to Parkinson’s disease (PD) and other neurodegenerative diseases.75 Furthermore, Sammi et al. demonstrated that not only dopaminergic neurons but also serotonergic, GABAergic, and cholinergic neurons have elevated neurodegeneration when C. elegans were developmentally exposed to PFOS.75 Although Salgado et al. evaluated dopamine in many brain regions of rats exposed to PFOS,34 future studies should study additional brain regions that rely heavily on dopamine, including the ventral striatum and substantia nigra, especially since these regions are susceptible to PFAS accumulation. These studies indicate PFAS exposure causing mitochondrial dysfunction, increases in ROS, and increased cell death may also give evidence of PFOS and PFOA potentially increasing risk factors for the development of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease, which warrant further investigation.
A study of human mortality in people from the highly PFAS-contaminated Veneto Region in Italy determined that female individuals living in the contaminated sight were more likely to die from complications related to Parkinson’s disease compared to females living in the uncontaminated site.11 Furthermore, people of both sexes were more likely to die from Alzheimer’s disease-related causes when living in the PFAS-contaminated area.11 The epidemiological study further highlights the importance of studying how PFAS are affecting lifelong neurological health and the need for increased understanding of the effects of not only developmental but also lifelong PFAS exposure.
6. CONCLUSION
The bioaccumulating and persistent nature of PFAS like PFOS and PFOA will cause these chemicals to be present in the environment and humans for the foreseeable future. The major focus of the neurotoxic effects of the PFASs has been on the resulting effects of developmental exposures that are halted before adulthood, adult only exposures, or studies that have focused on neuroendocrine effects. Therefore, there are significant gaps in the field that need to be addressed, which have been discussed in the body of the paper. Because these compounds were first created in the 1930s, populations with lifelong exposure are beginning to reach ages where epidemiological research can test associations between lifelong PFAS exposure and neurodegeneration in the aging population. Despite changes in neurotransmission being a risk factor for age-related neurodegeneration, there are no studies that evaluate the PFAS exposure’s impact on neurotransmitter changes in chronically exposed animals. Furthermore, the PFAS exposure’s effects on end points relating to neurodegeneration such as protein aggregation, oxidative stress, and region-specific neuron-type-specific neurodegeneration require investigation. While there have been some attempts cited above to identify mechanisms of neurotransmitter modulation, these have been far from definitive. Moreover, the direct impact of PFAS-induced neurotransmitter modulation on neurobehavior outcomes has yet to be identified.
The emerging PFAS, such as GenX, need to undergo evaluation for neurotoxicity. Furthermore, toxicity testing of these compounds needs to include male and female animals. Recent epidemiological studies have shown changes in thyroid hormone levels correlated with PFAS exposure, and other studies have determined females may be at greater risk for ADHD when exposed to PFAS.3,94,103,104 Finally, considering there is evidence more people are dying from age-related neurodegenerative disease in an area with high PFAS contamination, it is imperative that mechanistic studies are conducted to better understand the long-term consequences of PFAS on aging and neurological function.
ACKNOWLEDGMENTS
J.R.C. was supported by AG068787 from the National Institutes of Health. The authors would like to thank Drs. Tauqeer Syeda and Shreesh Sammi for helpful discussions during manuscript preparation. Figure 1 and the graphical abstract were created with BioRender.com.
ABBREVIATIONS
- AADC
aromatic amino acid decarboxylase
- Aβ
amyloid-β
- AC
adenylyl cyclase
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ADHD
attention deficit hyperactivity disorder
- AD
Alzheimer’s disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- APP
amyloid precursor protein
- CAM
calmodulin
- CAMKIIa
CAM kinase IIa
- cAMP
cyclic adenosine monophosphate
- CCAT
Cav1.2 C-terminus
- ChAT
choline acetyltransferase
- COMT
catechol-O-methyltransferase
- CREB
cAMP-response element binding
- CSF
cerebrospinal fluid
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- DP2
neuronal precursor phase
- DP3
mature dopaminergic differentiation phase
- DR1
dopamine receptor 1
- DR1-DR2
DR1-DR2 dimer
- DR2
dopamine receptor 2
- GABA
gamma-aminobutyric acid
- GenX or HFPO–DA
2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate
- GLU
glutamate
- GLN
glutamine
- GLY
glycine
- Gria3
AMPA glutamate receptor subunit A3
- hiPSC
human induced pluripotent stem cells
- HPA
hypothalamus–pituitary–adrenal
- HVA
homovanillic acid
- IP3
inositol triphosphate
- IP3R
inositol triphosphate receptor
- L-DOPA
l-3,4-dihydroxyphenylalanine
- L-VGCC
L-type voltage-gated calcium channel
- MAO
monoamine oxidase
- nAChR
nicotinic ACh receptor
- NE
norepinephrine
- NFH
neurofilament heavy
- NMDA
N-methyl-d-aspartate
- NR2B
NMDA receptor subtype-2B
- PD
Parkinson’s disease
- pDARPP-32
dopamine and cAMP-regulated phosphor-protein 32 kDa
- PFAS
per- and polyfluoroalkyl substances
- PFBA
perfluorobutyric acid
- PFBS
perfluorobutanesulfonate
- PFCs
perfluorochemicals
- PFDA
perfluorodecanoic acid
- PFDoDA
perfluorododecanoic acid
- PFHxA
perfluorohexanoate
- PFHxS
perfluorohexanesulfonate
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctanesulfonic acid
- PFOSA
perfluorooctanesulfonamide
- PFPeA
perfluoro-n-pentanoate
- PFTeDA
perfluorotetradecanoic acid
- PFTrDA
perfluorotridecanoic acid
- PFUA
perfluoroundecanoic acid
- PKA
protein kinase A
- PND
postnatal day
- PP1
protein phosphatase 1
- POPs
persistent organic pollutants
- PTFE
Teflon or polytetrafluoroethylene
- PLC
protein lipase C
- POSF
perfluorooctane sulfonyl fluoride
- P,Q,N-VGCC
P,Q,N-type voltage-gated calcium channel
- VMAT
vesicular monoamine transporter
- 5-HT
serotonin
- SD
Sprague–Dawley
- TH
tyrosine hydroxylase
- 6:2Cl-PFESA
perfluoro-(2-((6-chlorohexyl)oxy)ethanesulfonic acid)
- 5-HIAA
5-hydroxyindoleacetic acid
Biographies
Josephine M. Brown-Leung is a toxicology Ph.D. graduate student in the School of Health Science, Purdue University. She received her M.S. in toxicology from the University of Cincinnati College of Medicine in 2019. Her Ph.D. dissertation research evaluates the mechanisms of PFAS neurotoxicity with particular interest in Parkinson’s and Alzheimer’s disease etiology. She uses rodent models to characterize neurobehavioral, histological, and neurochemical effects of toxicants.
Jason R. Cannon is a Professor of Toxicology in the School of Health Sciences, Purdue University. Dr. Cannon received a B.S. in physiology from Michigan State University, a Ph.D. in toxicology from the University of Michigan, and postdoctoral training at the Pittsburgh Institute for Neurodegenerative Diseases, University of Pittsburgh. Dr. Cannon studies mechanisms of environmentally induced neurodegeneration. He recently gave a keynote speech at EUROTOX on this topic.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrestox.2c00072
REFERENCES
- (1).ATSDR Toxicological Profile for Perfluoroalkyls, Peer Review Agenda; https://www.atsdr.cdc.gov/sites/peer_review/tox_profile_perfluoroalkyls.html (accessed 2021-03-15).
- (2).Coperchini F; Croce L; Ricci G; Magri F; Rotondi M; Imbriani M; Chiovato L Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol 2021, 11, 612320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Piekarski D; Diaz K; McNerney M Perfluoroalkyl Chemicals in Neurological Health and Disease: Human Concerns and Animal Models. NeuroToxicology 2020, 77, 155–168. [DOI] [PubMed] [Google Scholar]
- (4).Teymourian T; Teymoorian T; Kowsari E; Ramakrishna S A Review of Emerging PFAS Contaminants: Sources, Fate, Health Risks, and a Comprehensive Assortment of Recent Sorbents for PFAS Treatment by Evaluating Their Mechanism. Res. Chem. Intermed 2021, 47 (12), 4879–4914. [Google Scholar]
- (5).Ramírez Carnero A; Lestido-Cardama A; Vazquez Loureiro P; Barbosa-Pereira L; Rodríguez Bernaldo de Quirós A; Sendón R Presence of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Food Contact Materials (FCM) and Its Migration to Food. Foods 2021, 10 (7), 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Foguth RM; Hoskins TD; Clark GC; Nelson M; Flynn RW; de Perre C; Hoverman JT; Lee LS; Sepulveda MS; Cannon JR Single and Mixture Per- and Polyfluoroalkyl Substances Accumulate in Developing Northern Leopard Frog Brains and Produce Complex Neurotransmission Alterations. Neurotoxicol. Teratol 2020, 81, No. 106907. [DOI] [PubMed] [Google Scholar]
- (7).Foguth R; Sepuĺveda MS; Cannon J Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics 2020, 8 (2), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Greaves AK; Letcher RJ Linear and Branched Perfluorooctane Sulfonate (PFOS) Isomer Patterns Differ among Several Tissues and Blood of Polar Bears. Chemosphere 2013, 93 (3), 574–580. [DOI] [PubMed] [Google Scholar]
- (9).Greaves AK; Letcher RJ; Sonne C; Dietz R Brain Region Distribution and Patterns of Bioaccumulative Perfluoroalkyl Carboxylates and Sulfonates in East Greenland Polar Bears (Ursus Maritimus). Environ. Toxicol. Chem 2013, 32 (3), 713–722. [DOI] [PubMed] [Google Scholar]
- (10).Huang C; Zhang J; Hu G; Zhang L; Chen H; Wei D; Cai D; Yu Y; Li X; Ding P; Li J Characterization of the Distribution, Source, and Potential Ecological Risk of Perfluorinated Alkyl Substances (PFAS) in the Inland River Basin of Longgang District, South China. Environ. Pollut 2021, 287, 117642. [DOI] [PubMed] [Google Scholar]
- (11).Mastrantonio M; Bai E; Uccelli R; Cordiano V; Screpanti A; Crosignani P Drinking Water Contamination from Perfluoroalkyl Substances (PFAS): An Ecological Mortality Study in the Veneto Region, Italy. Eur. J. Public Health 2018, 28 (1), 180–185. [DOI] [PubMed] [Google Scholar]
- (12).Pitter G; Da Re F; Canova C; Barbieri G; Zare Jeddi M; Dapr F; Manea F; Zolin R; Bettega AM; Stopazzolo G; Vittorii S; Zambelli L; Martuzzi M; Mantoan D; Russo F Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect 2020, 128 (2), No. 027007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang X-F; Mo M-S; Chen H-G; Wang Q; Yang J-L; Zhao D-H Distribution and Exposure Risk Assessment of Perfluorinated Alkyl Substances in Aquatic Products along the Coastal Region of the South China Sea. Expo. Health 2021, 13 (3), 505–515. [Google Scholar]
- (14).Lindstrom AB; Strynar MJ; Libelo EL Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol 2011, 45 (19), 7954–7961. [DOI] [PubMed] [Google Scholar]
- (15).Kashino I; Sasaki S; Okada E; Matsuura H; Goudarzi H; Miyashita C; Okada E; Ito YM; Araki A; Kishi R Prenatal Exposure to 11 Perfluoroalkyl Substances and Fetal Growth: A Large-Scale, Prospective Birth Cohort Study. Environ. Int 2020, 136, No. 105355. [DOI] [PubMed] [Google Scholar]
- (16).EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk D; Bignami M; Bodin L; Chipman JK; del Mazo J; Grasl-Kraupp B; Hogstrand C; Hoogenboom LR; Leblanc J; Nebbia CS; Nielsen E; Ntzani E; Petersen A; Sand S; Vleminckx C; Wallace H; Barregård L; Ceccatelli S; Cravedi J; Halldorsson TI; Haug LS; Johansson N; Knutsen HK; Rose M; Roudot A; Van Loveren H; Vollmer G; Mackay K; Riolo F; Schwerdtle T Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18 (9), e06223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Gebbink WA; Bossi R; Rigét FF; Rosing-Asvid A; Sonne C; Dietz R Observation of Emerging Per- and Polyfluoroalkyl Substances (PFAS) in Greenland Marine Mammals. Chemosphere 2016, 144, 2384–2391. [DOI] [PubMed] [Google Scholar]
- (18).Yu CH; Riker CD; Lu S.-e.; Fan ZT Biomonitoring of Emerging Contaminants, Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS), in New Jersey Adults in 2016–2018. Int. J. Hyg. Environ. Health 2020, 223, 34–44. [DOI] [PubMed] [Google Scholar]
- (19).Conley JM; Lambright CS; Evans N; McCord J; Strynar MJ; Hill D; Medlock-Kakaley E; Wilson VS; Gray LE Hexafluoropropylene Oxide-Dimer Acid (HFPO-DA or GenX) Alters Maternal and Fetal Glucose and Lipid Metabolism and Produces Neonatal Mortality, Low Birthweight, and Hepatomegaly in the Sprague-Dawley Rat. Environ. Int 2021, 146, No. 106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Blake BE; Cope HA; Hall SM; Keys RD; Mahler BW; McCord J; Scott B; Stapleton HM; Strynar MJ; Elmore SA; Fenton SE Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice Following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ. Health Perspect 2020, 128 (2), No. 027006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cope HA; Blake BE; Love C; McCord J; Elmore SA; Harvey JB; Chappell VA; Fenton SE Latent, Sex-Specific Metabolic Health Effects in CD-1 Mouse Offspring Exposed to PFOA or HFPO-DA (GenX) during Gestation. Emerg. Contam 2021, 7, 219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Menzel J; Abraham K; Dietrich S; Fromme H; Völkel W; Schwerdtle T; Weikert C Internal Exposure to Perfluoroalkyl Substances (PFAS) in Vegans and Omnivores. Int. J. Hyg. Environ. Health 2021, 237, No. 113808. [DOI] [PubMed] [Google Scholar]
- (23).Austin ME; Kasturi BS; Barber M; Kannan K; MohanKumar PS; MohanKumar SMJ Neuroendocrine Effects of Perfluorooctane Sulfonate in Rats. Environ. Health Perspect 2003, 111 (12), 1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Foguth RM; Flynn RW; de Perre C; Iacchetta M; Lee LS; Sepulveda MS; Cannon JR Developmental Exposure to Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Selectively Decreases Brain Dopamine Levels in Northern Leopard Frogs. Toxicol. Appl. Pharmacol 2019, 377, No. 114623. [DOI] [PubMed] [Google Scholar]
- (25).Grønnestad R; Johanson SM; Müller MHB; Schlenk D; Tanabe P; Krøkje Å; Jaspers VLB; Jenssen BM; Ræder EM; Lyche JL; Shi Q; Arukwe A Effects of an Environmentally Relevant PFAS Mixture on Dopamine and Steroid Hormone Levels in Exposed Mice. Toxicol. Appl. Pharmacol 2021, 428, No. 115670. [DOI] [PubMed] [Google Scholar]
- (26).Khan EA; Bertotto LB; Dale K; Lille-Langøy R; Yadetie F; Karlsen OA; Goksøyr A; Schlenk D; Arukwe A Modulation of Neuro-Dopamine Homeostasis in Juvenile Female Atlantic Cod (Gadus Morhua) Exposed to Polycyclic Aromatic Hydrocarbons and Perfluoroalkyl Substances. Environ. Sci. Technol 2019, 53 (12), 7036–7044. [DOI] [PubMed] [Google Scholar]
- (27).Long Y; Wang Y; Ji G; Yan L; Hu F; Gu A Neurotoxicity of Perfluorooctane Sulfonate to Hippocampal Cells in Adult Mice. PLoS One 2013, 8 (1), No. e54176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).López-Doval S; Salgado R; Pereiro N; Moyano R; Lafuente A Perfluorooctane Sulfonate Effects on the Reproductive Axis in Adult Male Rats. Environ. Res 2014, 134, 158–168. [DOI] [PubMed] [Google Scholar]
- (29).López-Doval S; Salgado R; Fernández-Pérez B; Lafuente A Possible Role of Serotonin and Neuropeptide Y on the Disruption of the Reproductive Axis Activity by Perfluorooctane Sulfonate. Toxicol. Lett 2015, 233 (2), 138–147. [DOI] [PubMed] [Google Scholar]
- (30).Mshaty A; Haijima A; Takatsuru Y; Ninomiya A; Yajima H; Kokubo M; Khairinisa MA; Miyazaki W; Amano I; Koibuchi N Neurotoxic Effects of Lactational Exposure to Perfluorooctane Sulfonate on Learning and Memory in Adult Male Mouse. Food Chem. Toxicol 2020, 145, No. 111710. [DOI] [PubMed] [Google Scholar]
- (31).Ninomiya A; Mshaty A; Haijima A; Yajima H; Kokubo M; Khairinisa MA; Ariyani W; Fujiwara Y; Ishii S; Hosoi N; Hirai H; Amano I; Koibuchi N The Neurotoxic Effect of Lactational PFOS Exposure on Cerebellar Functional Development in Male Mice. Food Chem. Toxicol 2022, 159, No. 112751. [DOI] [PubMed] [Google Scholar]
- (32).Reardon AJF; Karathra J; Ribbenstedt A; Benskin JP; MacDonald AM; Kinniburgh DW; Hamilton TJ; Fouad K; Martin JW Neurodevelopmental and Metabolomic Responses from Prenatal Coexposure to Perfluorooctanesulfonate (PFOS) and Methylmercury (MeHg) in Sprague–Dawley Rats. Chem. Res. Toxicol 2019, 32 (8), 1656–1669. [DOI] [PubMed] [Google Scholar]
- (33).Salgado R; Pereiro N; López-Doval S; Lafuente A Initial Study on the Possible Mechanisms Involved in the Effects of High Doses of Perfluorooctane Sulfonate (PFOS) on Prolactin Secretion. Food Chem. Toxicol 2015, 83, 10–16. [DOI] [PubMed] [Google Scholar]
- (34).Salgado R; López-Doval S; Pereiro N; Lafuente A Perfluorooctane Sulfonate (PFOS) Exposure Could Modify the Dopaminergic System in Several Limbic Brain Regions. Toxicol. Lett 2016, 240 (1), 226–235. [DOI] [PubMed] [Google Scholar]
- (35).Sato I; Kawamoto K; Nishikawa Y; Tsuda S; Yoshida M; Yaegashi K; Saito N; Liu W; Jin Y Neurotoxicity of Perfluorooctane Sulfonate (PFOS) in Rats and Mice after Single Oral Exposure. J. Toxicol. Sci 2009, 34 (5), 569–574. [DOI] [PubMed] [Google Scholar]
- (36).Yu N; Wei S; Li M; Yang J; Li K; Jin L; Xie Y; Giesy JP; Zhang X; Yu H Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-Throughput Targeted Metabolomics Approach. Sci. Rep 2016, 6 (1), 23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Grønnestad R; Schlenk D; Krøkje Å; Jaspers VLB; Jenssen BM; Coffin S; Bertotto LB; Giroux M; Lyche JL; Arukwe A Alteration of Neuro-Dopamine and Steroid Hormone Homeostasis in Wild Bank Voles in Relation to Tissue Concentrations of PFAS at a Nordic Skiing Area. Sci. Total Environ 2021, 756, 143745. [DOI] [PubMed] [Google Scholar]
- (38).Berntsen HF; Bjørklund CG; Strandabø R; Haug TM; Moldes-Anaya A; Fuentes-Lazaro J; Verhaegen S; Paulsen RE; Tasker RA; Ropstad E PFOS-Induced Excitotoxicity Is Dependent on Ca2+ Influx via NMDA Receptors in Rat Cerebellar Granule Neurons. Toxicol. Appl. Pharmacol 2018, 357, 19–32. [DOI] [PubMed] [Google Scholar]
- (39).Berntsen HF; Moldes-Anaya A; Bjørklund CG; Ragazzi L; Haug TM; Strandabø RAU; Verhaegen S; Paulsen RE; Ropstad E; Tasker RA Perfluoroalkyl Acids Potentiate Glutamate Excitotoxicity in Rat Cerebellar Granule Neurons. Toxicology 2020, 445, No. 152610. [DOI] [PubMed] [Google Scholar]
- (40).Ishida K; Tsuyama Y; Sanoh S; Ohta S; Kotake Y Perfluorooctane Sulfonate Induces Neuronal Vulnerability by Decreasing GluR2 Expression. Arch. Toxicol 2017, 91 (2), 885–895. [DOI] [PubMed] [Google Scholar]
- (41).Liao C; Cui L; Zhou Q; Duan S; Jiang G Effects of Perfluorooctane Sulfonate on Ion Channels and Glutamate-Activated Current in Cultured Rat Hippocampal Neurons. Environ. Toxicol. Pharmacol 2009, 27 (3), 338–344. [DOI] [PubMed] [Google Scholar]
- (42).Liu X; Liu W; Jin Y; Yu W; Wang F; Liu L Effect of Gestational and Lactational Exposure to Perfluorooctanesulfonate on Calcium-Dependent Signaling Molecules Gene Expression in Rats’ Hippocampus. Arch. Toxicol 2010, 84 (1), 71–79. [DOI] [PubMed] [Google Scholar]
- (43).Yadav A; Verhaegen S; Verbruggen E; Kerhoas M; Willemijn Huiberts EH; Hadera MG; Berntsen HF; Zimmer KE; Ropstad E; Paulsen RE A Human Relevant Mixture of Persistent Organic Pollutants (POPs) and Perfluorooctane Sulfonic Acid (PFOS) Differentially Affect Glutamate Induced Excitotoxic Responses in Chicken Cerebellum Granule Neurons (CGNs) in Vitro. Reprod. Toxicol 2021, 100, 109–119. [DOI] [PubMed] [Google Scholar]
- (44).Eggers Pedersen K; Basu N; Letcher R; Greaves AK; Sonne C; Dietz R; Styrishave B Brain Region-Specific Perfluor-oalkylated Sulfonate (PFSA) and Carboxylic Acid (PFCA) Accumulation and Neurochemical Biomarker Responses in East Greenland Polar Bears (Ursus Maritimus). Environ. Res 2015, 138, 22–31. [DOI] [PubMed] [Google Scholar]
- (45).Wang R; Wang R; Niu X; Cheng Y; Shang X; Li Y; Li S; Liu X; Shao J Role of Astrocytes-Derived d-Serine in PFOS-Induced Neurotoxicity through NMDARs in the Rat Primary Hippocampal Neurons. Toxicology 2019, 422, 14–24. [DOI] [PubMed] [Google Scholar]
- (46).Li Z; Liu Q; Liu C; Li C; Li Y; Li S; Liu X; Shao J Evaluation of PFOS-Mediated Neurotoxicity in Rat Primary Neurons and Astrocytes Cultured Separately or in Co-Culture. Toxicol. In Vitro 2017, 38, 77–90. [DOI] [PubMed] [Google Scholar]
- (47).Patel R; Bradner JM; Stout KA; Caudle WM Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo. Med. Sci 2016, 4 (3), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hallgren S; Viberg H Postnatal Exposure to PFOS, but Not PBDE 99, Disturb Dopaminergic Gene Transcription in the Mouse CNS. Environ. Toxicol. Pharmacol 2016, 41, 121–126. [DOI] [PubMed] [Google Scholar]
- (49).Zhang Q; Liu W; Zhao H; Zhang Z; Qin H; Luo F; Niu Q Developmental Perfluorooctane Sulfonate Exposure Inhibits Long-Term Potentiation by Affecting AMPA Receptor Trafficking. Toxicology 2019, 412, 55–62. [DOI] [PubMed] [Google Scholar]
- (50).Harada KH; Ishii TM; Takatsuka K; Koizumi A; Ohmori H Effects of Perfluorooctane Sulfonate on Action Potentials and Currents in Cultured Rat Cerebellar Purkinje Cells. Biochem. Biophys. Res. Commun 2006, 351 (1), 240–245. [DOI] [PubMed] [Google Scholar]
- (51).Liao C; Li X; Wu B; Duan S; Jiang G Acute Enhancement of Synaptic Transmission and Chronic Inhibition of Synaptogenesis Induced by Perfluorooctane Sulfonate through Mediation of Voltage-Dependent Calcium Channel. Environ. Sci. Technol 2008, 42 (14), 5335–5341. [DOI] [PubMed] [Google Scholar]
- (52).Liao C; Wang T; Cui L; Zhou Q; Duan S; Jiang G Changes in Synaptic Transmission, Calcium Current, and Neurite Growth by Perfluorinated Compounds Are Dependent on the Chain Length and Functional Group. Environ. Sci. Technol 2009, 43 (6), 2099–2104. [DOI] [PubMed] [Google Scholar]
- (53).Wang Y; Jin Y Perfluorooctane Sulfonate (PFOS) and Calcium Channel Downstream Signaling Molecules. Toxicol. Res 2012, 1 (2), 103. [Google Scholar]
- (54).Zhang Q; Liu W; Niu Q; Wang Y; Zhao H; Zhang H; Song J; Tsuda S; Saito N Effects of Perfluorooctane Sulfonate and Its Alternatives on Long-Term Potentiation in the Hippocampus CA1 Region of Adult Rats in Vivo. Toxicol. Res 2016, 5 (2), 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Cao Y; Ng C Absorption, Distribution, and Toxicity of per- and Polyfluoroalkyl Substances (PFAS) in the Brain: A Review. Environ. Sci. Process. Impacts 2021, 23 (11), 1623–1640. [DOI] [PubMed] [Google Scholar]
- (56).Starnes HM; Rock KD; Jackson TW; Belcher SM A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior. Front. Toxicol 2022, 4, 881584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Wang Y; Wang L; Chang W; Zhang Y; Zhang Y; Liu W Neurotoxic Effects of Perfluoroalkyl Acids: Neurobehavioral Deficit and Its Molecular Mechanism. Toxicol. Lett 2019, 305, 65–72. [DOI] [PubMed] [Google Scholar]
- (58).Zeng Z; Song B; Xiao R; Zeng G; Gong J; Chen M; Xu P; Zhang P; Shen M; Yi H Assessing the Human Health Risks of Perfluorooctane Sulfonate by in Vivo and in Vitro Studies. Environ. Int 2019, 126, 598–610. [DOI] [PubMed] [Google Scholar]
- (59).Olsen GW; Mair DC; Lange CC; Harrington LM; Church TR; Goldberg CL; Herron RM; Hanna H; Nobiletti JB; Rios JA; Reagen WK; Ley CA Per- and Polyfluoroalkyl Substances (PFAS) in American Red Cross Adult Blood Donors, 2000–2015. Environ. Res 2017, 157, 87–95. [DOI] [PubMed] [Google Scholar]
- (60).Li Y; Fletcher T; Mucs D; Scott K; Lindh CH; Tallving P; Jakobsson K Half-Lives of PFOS, PFHxS and PFOA after End of Exposure to Contaminated Drinking Water. Occup. Environ. Med 2018, 75 (1), 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Kim S-J; Heo S-H; Lee D-S; Hwang IG; Lee Y-B; Cho H-Y Gender Differences in Pharmacokinetics and Tissue Distribution of 3 Perfluoroalkyl and Polyfluoroalkyl Substances in Rats. Food Chem. Toxicol 2016, 97, 243–255. [DOI] [PubMed] [Google Scholar]
- (62).Yu Y; Wang C; Zhang X; Zhu J; Wang L; Ji M; Zhang Z; Ji X-M; Wang S-L Perfluorooctane Sulfonate Disrupts the Blood Brain Barrier through the Crosstalk between Endothelial Cells and Astrocytes in Mice. Environ. Pollut 2020, 256, No. 113429. [DOI] [PubMed] [Google Scholar]
- (63).Wang J; Pan Y; Cui Q; Yao B; Wang J; Dai J Penetration of PFAS Across the Blood Cerebrospinal Fluid Barrier and Its Determinants in Humans. Environ. Sci. Technol 2018, 52 (22), 13553–13561. [DOI] [PubMed] [Google Scholar]
- (64).Liu Y; Zhou X; Wu Y; Yang X; Wang Y; Li S; Bai X; Schlenk D; Liu W Exposure and Blood–Cerebrospinal Fluid Barrier Permeability of PFAS in Neonates. Environ. Sci. Technol. Lett 2022, 9 (1), 64–70. [Google Scholar]
- (65).Di Nisio A; Pannella M; Vogiatzis S; Sut S; Dall’Acqua S; Rocca MS; Antonini A; Porzionato A; De Caro R; Bortolozzi M; Toni LD; Foresta C Impairment of Human Dopaminergic Neurons at Different Developmental Stages by Perfluoro-Octanoic Acid (PFOA) and Differential Human Brain Areas Accumulation of Perfluoroalkyl Chemicals. Environ. Int 2022, 158, No. 106982. [DOI] [PubMed] [Google Scholar]
- (66).Pérez F; Nadal M; Navarro-Ortega A; Fàbrega F; Domingo JL; Barceló D; Farré M Accumulation of Perfluoroalkyl Substances in Human Tissues. Environ. Int 2013, 59, 354–362. [DOI] [PubMed] [Google Scholar]
- (67).Maestri L; Negri S; Ferrari M; Ghittori S; Fabris F; Danesino P; Imbriani M Determination of Perfluorooctanoic Acid and Perfluorooctanesulfonate in Human Tissues by Liquid Chromatography/Single Quadrupole Mass Spectrometry. Rapid Commun. Mass Spectrom 2006, 20 (18), 2728–2734. [DOI] [PubMed] [Google Scholar]
- (68).Xie M-Y; Lin Z-Y; Liu L-Y; Wu C-C; Liu Y-W; Huang G-L; Zeng EY Use of Glioma to Assess the Distribution Patterns of Perfluoroalkyl and Polyfluoroalkyl Substances in Human Brain. Environ. Res 2022, 204, No. 112011. [DOI] [PubMed] [Google Scholar]
- (69).Li X; Li T; Wang Z; Wei J; Liu J; Zhang Y; Zhao Z Distribution of Perfluorooctane Sulfonate in Mice and Its Effect on Liver Lipidomic. Talanta 2021, 226, No. 122150. [DOI] [PubMed] [Google Scholar]
- (70).Björvang RD; Vinnars M-T; Papadogiannakis N; Gidlöf S; Mamsen LS; Mucs D; Kiviranta H; Rantakokko P; Ruokojärvi P; Lindh CH; Andersen CY; Damdimopoulou P Mixtures of Persistent Organic Pollutants Are Found in Vital Organs of Late Gestation Human Fetuses. Chemosphere 2021, 283, No. 131125. [DOI] [PubMed] [Google Scholar]
- (71).Pizzurro DM; Seeley M; Kerper LE; Beck BD Interspecies Differences in Perfluoroalkyl Substances (PFAS) Toxicokinetics and Application to Health-Based Criteria. Regul. Toxicol. Pharmacol 2019, 106, 239–250. [DOI] [PubMed] [Google Scholar]
- (72).National Institute of Standards and Technology; https://www.nist.gov/ (accessed 2022-02-27).
- (73).Yu T; Zhou G; Cai Z; Liang W; Du Y; Wang W Behavioral Effects of Early-Life Exposure to Perfluorooctanoic Acid Might Synthetically Link to Multiple Aspects of Dopaminergic Neuron Development and Dopamine Functions in Zebrafish Larvae. Aquat. Toxicol 2021, 238, No. 105926. [DOI] [PubMed] [Google Scholar]
- (74).Gong X; Yang C; Hong Y; Chung ACK; Cai Z PFOA and PFOS Promote Diabetic Renal Injury in Vitro by Impairing the Metabolisms of Amino Acids and Purines. Sci. Total Environ 2019, 676, 72–86. [DOI] [PubMed] [Google Scholar]
- (75).Sammi SR; Foguth RM; Nieves CS; De Perre C; Wipf P; McMurray CT; Lee LS; Cannon JR Perfluorooctane Sulfonate (PFOS) Produces Dopaminergic Neuropathology in Caenorhabditis Elegans. Toxicol. Sci 2019, 172 (2), 417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Lau C; Thibodeaux JR; Hanson RG; Rogers JM; Grey BE; Stanton ME; Butenhoff JL; Stevenson LA Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. II: Postnatal Evaluation. Toxicol. Sci 2003, 74 (2), 382–392. [DOI] [PubMed] [Google Scholar]
- (77).Lau C; Butenhoff JL; Rogers JM The Developmental Toxicity of Perfluoroalkyl Acids and Their Derivatives. Toxicol. Appl. Pharmacol 2004, 198 (2), 231–241. [DOI] [PubMed] [Google Scholar]
- (78).Lau C; Thibodeaux JR; Hanson RG; Narotsky MG; Rogers JM; Lindstrom AB; Strynar MJ Effects of Perfluorooctanoic Acid Exposure during Pregnancy in the Mouse. Toxicol. Sci 2006, 90 (2), 510–518. [DOI] [PubMed] [Google Scholar]
- (79).Zhang Q; Zhao H; Liu W; Zhang Z; Qin H; Luo F; Leng S Developmental Perfluorooctane Sulfonate Exposure Results in Tau Hyperphosphorylation and β-Amyloid Aggregation in Adults Rats: Incidence for Link to Alzheimer’s Disease. Toxicology 2016, 347–349, 40–46. [DOI] [PubMed] [Google Scholar]
- (80).Slotkin TA; MacKillop EA; Melnick RL; Thayer KA; Seidler FJ Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro. Environ. Health Perspect 2008, 116 (6), 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Basaly V; Hill J; Bihaqi SW; Marques E; Slitt AL; Zawia NH Developmental Perfluorooctanesulfonic Acid (PFOS) Exposure as a Potential Risk Factor for Late-Onset Alzheimer’s Disease in CD-1 Mice and SH-SY5Y Cells. NeuroToxicology 2021, 86, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Hallgren S; Fredriksson A; Viberg H More Signs of Neurotoxicity of Surfactants and Flame Retardants – Neonatal PFOS and PBDE 99 Cause Transcriptional Alterations in Cholinergic Genes in the Mouse CNS. Environ. Toxicol. Pharmacol 2015, 40 (2), 409–416. [DOI] [PubMed] [Google Scholar]
- (83).Johansson N; Fredriksson A; Eriksson P Neonatal Exposure to Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Causes Neurobehavioural Defects in Adult Mice. Neuro-Toxicology 2008, 29 (1), 160–169. [DOI] [PubMed] [Google Scholar]
- (84).Grønnestad R; Vázquez BP; Arukwe A; Jaspers VLB; Jenssen BM; Karimi M; Lyche JL; Krøkje Å Levels, Patterns, and Biomagnification Potential of Perfluoroalkyl Substances in a Terrestrial Food Chain in a Nordic Skiing Area. Environ. Sci. Technol 2019, 53 (22), 13390–13397. [DOI] [PubMed] [Google Scholar]
- (85).Tukker AM; Bouwman LMS; van Kleef RGDM; Hendriks HS; Legler J; Westerink RHS Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) Acutely Affect Human α 1 β 2 γ 2L GABA A Receptor and Spontaneous Neuronal Network Function in Vitro. Sci. Rep 2020, 10 (1), 5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Lo A; Guibal P; Doummar D; Rodriguez D; Hautem J-Y; Couderc R; Billette De Villemeur T; Roze E; Chaminade P; Moussa F Single-Step Rapid Diagnosis of Dopamine and Serotonin Metabolism Disorders. ACS Omega 2017, 2 (9), 5962–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Cho H-U; Kim S; Sim J; Yang S; An H; Nam M-H; Jang D-P; Lee CJ Redefining Differential Roles of MAO-A in Dopamine Degradation and MAO-B in Tonic GABA Synthesis. Exp. Mol. Med 2021, 53 (7), 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Chang C-J; Barr DB; Ryan PB; Panuwet P; Smarr MM; Liu K; Kannan K; Yakimavets V; Tan Y; Ly V; Marsit CJ; Jones DP; Corwin EJ; Dunlop AL; Liang D Per- and Polyfluoroalkyl Substance (PFAS) Exposure, Maternal Metabolomic Perturbation, and Fetal Growth in African American Women: A Meet-in-the-Middle Approach. Environ. Int 2022, 158, No. 106964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Liu X; Jin Y; Liu W; Wang F; Hao S Possible Mechanism of Perfluorooctane Sulfonate and Perfluorooctanoate on the Release of Calcium Ion from Calcium Stores in Primary Cultures of Rat Hippocampal Neurons. Toxicol. In Vitro 2011, 25 (7), 1294–1301. [DOI] [PubMed] [Google Scholar]
- (90).Wright A; Vissel B The Essential Role of AMPA Receptor GluR2 Subunit RNA Editing in the Normal and Diseased Brain. Front. Mol. Neurosci 2012, 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Saravanaraman P; Chinnadurai RK; Boopathy R Why Calcium Channel Blockers Could Be an Elite Choice in the Treatment of Alzheimer’s Disease: A Comprehensive Review of Evidences. Rev. Neurosci 2014, 25 (2), 231–246. [DOI] [PubMed] [Google Scholar]
- (92).Tarazi FI; Baldessarini RJ Regional Localization of Dopamine and Ionotropic Glutamate Receptor Subtypes in Striatolimbic Brain Regions. J. Neurosci. Res 1999, 55 (4), 401–410. [DOI] [PubMed] [Google Scholar]
- (93).Tarazi FI; Campbell A; Yeghiayan SK; Baldessarini RJ Localization of Ionotropic Glutamate Receptors in Caudate-Putamen and Nucleus Accumbens Septi of Rat Brain: Comparison of NMDA, AMPA, and Kainate Receptors. Synapse 1998, 30 (2), 227–235. [DOI] [PubMed] [Google Scholar]
- (94).Lenters V; Iszatt N; Forns J;Čechová E; Kočan A; Legler J; Leonards P; Stigum H; Eggesbø M Early-Life Exposure to Persistent Organic Pollutants (OCPs, PBDEs, PCBs, PFAS) and Attention-Deficit/Hyperactivity Disorder: A Multi-Pollutant Analysis of a Norwegian Birth Cohort. Environ. Int 2019, 125, 33–42. [DOI] [PubMed] [Google Scholar]
- (95).Dolphin AC; Lee A Presynaptic Calcium Channels: Specialized Control of Synaptic Neurotransmitter Release. Nat. Rev. Neurosci 2020, 21 (4), 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Simms BA; Zamponi GW Neuronal Voltage-Gated Calcium Channels: Structure, Function, and Dysfunction. Neuron 2014, 82 (1), 24–45. [DOI] [PubMed] [Google Scholar]
- (97).Johansson N; Eriksson P; Viberg H Neonatal Exposure to PFOS and PFOA in Mice Results in Changes in Proteins Which Are Important for Neuronal Growth and Synaptogenesis in the Developing Brain. Toxicol. Sci 2009, 108 (2), 412–418. [DOI] [PubMed] [Google Scholar]
- (98).Kawaai K; Mizutani A; Shoji H; Ogawa N; Ebisui E; Kuroda Y; Wakana S; Miyakawa T; Hisatsune C; Mikoshiba K IRBIT Regulates CaMKIIα Activity and Contributes to Catecholamine Homeostasis through Tyrosine Hydroxylase Phosphorylation. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (17), 5515–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Onishchenko N; Fischer C; Wan Ibrahim WN; Negri S; Spulber S; Cottica D; Ceccatelli S Prenatal Exposure to PFOS or PFOA Alters Motor Function in Mice in a Sex-Related Manner. Neurotox. Res 2011, 19 (3), 452–461. [DOI] [PubMed] [Google Scholar]
- (100).Goulding DR; White SS; McBride SJ; Fenton SE; Harry GJ Gestational Exposure to Perfluorooctanoic Acid (PFOA): Alterations in Motor Related Behaviors. NeuroToxicology 2017, 58, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Butenhoff JL; Ehresman DJ; Chang S-C; Parker GA; Stump DG Gestational and Lactational Exposure to Potassium Perfluorooctanesulfonate (K+PFOS) in Rats: Developmental Neurotoxicity. Reprod. Toxicol 2009, 27 (3–4), 319–330. [DOI] [PubMed] [Google Scholar]
- (102).Wang Y; Liu W; Zhang Q; Zhao H; Quan X Effects of Developmental Perfluorooctane Sulfonate Exposure on Spatial Learning and Memory Ability of Rats and Mechanism Associated with Synaptic Plasticity. Food Chem. Toxicol 2015, 76, 70–76. [DOI] [PubMed] [Google Scholar]
- (103).Tsuda S Differential Toxicity between Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). J. Toxicol. Sci 2016, 41 (Special), SP27–SP36. [DOI] [PubMed] [Google Scholar]
- (104).Forns J; Verner M-A; Iszatt N; Nowack N; Bach CC; Vrijheid M; Costa O; Andiarena A; Sovcikova E; Høyer BB; Wittsiepe J; Lopez-Espinosa M-J; Ibarluzea J; Hertz-Picciotto I; Toft G; Stigum H; Guxens M; Liew Z; Eggesbø M Early Life Exposure to Perfluoroalkyl Substances (PFAS) and ADHD: A Meta-Analysis of Nine European Population-Based Studies. Environ. Health Perspect 2020, 128 (5), No. 057002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Lien G-W; Huang C-C; Shiu J-S; Chen M-H; Hsieh W-S; Guo Y-L; Chen P-C Perfluoroalkyl Substances in Cord Blood and Attention Deficit/Hyperactivity Disorder Symptoms in Seven-Year-Old Children. Chemosphere 2016, 156, 118–127. [DOI] [PubMed] [Google Scholar]
- (106).D’Souza MS; Markou A Neuronal Mechanisms Underlying Development of Nicotine Dependence: Implications for Novel Smoking-Cessation Treatments. Addict. Sci. Clin. Pract 2011, 6 (1), 4–16. [PMC free article] [PubMed] [Google Scholar]
- (107).Liew Z; Ritz B; von Ehrenstein OS; Bech BH; Nohr EA; Fei C; Bossi R; Henriksen TB; Bonefeld-Jørgensen EC; Olsen J Attention Deficit/Hyperactivity Disorder and Childhood Autism in Association with Prenatal Exposure to Perfluoroalkyl Substances: A Nested Case–Control Study in the Danish National Birth Cohort. Environ. Health Perspect 2015, 123 (4), 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Skogheim TS; Weyde KVF; Aase H; Engel SM; Surén P; Øie MG; Biele G; Reichborn-Kjennerud T; Brantsæter AL; Haug LS; Sabaredzovic A; Auyeung B; Villanger GD Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Associations with Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Children. Environ. Res 2021, 202, No. 111692. [DOI] [PubMed] [Google Scholar]
- (109).Dalsager L; Jensen TK; Nielsen F; Grandjean P; Bilenberg N; Andersen HR No Association between Maternal and Child PFAS Concentrations and Repeated Measures of ADHD Symptoms at Age 21/2 and 5 Years in Children from the Odense Child Cohort. Neurotoxicol. Teratol 2021, 88, No. 107031. [DOI] [PubMed] [Google Scholar]
- (110).Spulber S; Kilian P; Wan Ibrahim WN; Onishchenko N; Ulhaq M; Norrgren L; Negri S; Di Tuccio M; Ceccatelli S PFOS Induces Behavioral Alterations, Including Spontaneous Hyperactivity That Is Corrected by Dexamfetamine in Zebrafish Larvae. PLoS One 2014, 9 (4), No. e94227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Punja S; Shamseer L; Hartling L; Urichuk L; Vandermeer B; Nikles J; Vohra S Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents. Cochrane Database Syst. Rev 2016, No. 2, CD009996. [DOI] [PMC free article] [PubMed] [Google Scholar]


