Abstract
Background and Aim:
Subclinical mastitis (SCM) caused by erythromycin-resistant Staphylococcus aureus is a significant disease in lactating animals. Therefore, it is crucial to understand the genetic factors contributing to erythromycin resistance in S. aureus. This study aimed to estimate the prevalence of S. aureus in milk from subclinical mastitic cattle and buffaloes and tank milk samples as identified by probe-based real-time polymerase chain reaction (PCR) and the genotypic assessment of macrolide and erythromycin resistance profiles, as well as to analyze the phylogenetic relatedness of our local isolates of S. aureus.
Materials and Methods:
In total, 285 milk samples were analyzed using the California mastitis test to detect SCM. Milk samples were cultured on different specific Staphylococcus media. The presence of S. aureus was confirmed by Gram staining, the catalase and coagulase tests, the detection of hemolytic activity, DNase agar testing, and biofilm activity in Congo red medium. The genotypic identification of S. aureus (nuc) was performed. The determinants of erythromycin (ermA, ermB, ermC, and ermT) and macrolide resistance (msrA) were screened in all isolates. DNA sequencing of our local isolates of S. aureus was used to analyze their phylogenetic relatedness. Moreover, histopathological examination of tissue specimens of mammary gland was performed.
Results:
The S. aureus positivity rates were 36.4%, 48.8%, and 63.6% in cattle, buffalo, and bulk tank milk, respectively. Probe-based real-time PCR molecularly confirmed all 62 S. aureus isolates. Thirty-one isolates were subjected to PCR to create profiles of their genotypic erythromycin resistance. ermA, ermB, ermC, and ermT were present in 5 (8%), 26 (41.9%), 18 (29%), and 15 (24.1%) S. aureus isolates, respectively. Moreover, msrA was found in three (4.8%) strains. Eight PCR products were produced using standard PCR for DNA sequencing. Multiple sequence alignment, phylogenetic tree construction, and analysis of nuc in S. aureus revealed a high degree of homology (100%) with S. aureus strains isolated from milk in cases of bovine mastitis in India and Kenya. Histological analysis of udder tissues revealed extensive aggregation of mononuclear inflammatory cells in the interstitial connective tissue, primarily lymphocytes, and macrophages.
Conclusion:
This study showed a high prevalence of erythromycin resistance in S. aureus isolates. This information is vital for controlling mastitis and the spread of resistance genes between bacterial strains and hosts. Moreover, the probe-based real-time PCR approach is helpful for the rapid screening of S. aureus isolates and the consequent efficient treatment and control of S. aureus mastitis.
Keywords: antimicrobial resistance, bovines, Egypt, erythromycin resistance, genetic diversity, Staphylococcus aureus, subclinical mastitis
Introduction
Bovine mastitis is the most prevalent developing infectious disease globally, affecting the amount and quality of milk [1, 2]. Pathogens, the environment, and animals jointly contribute to the development of this inflammatory illness. Although subclinical mastitis (SCM) lacks overt symptoms, clinical mastitis (CM) can be identified by obvious abnormalities in milk and swelling or discomfort of the udder [3]. Depending on the host and pathogen interactions, mastitis can present as either CM or SCM [4]. Subclinical mastitis is the most prevalent form in all dairy animals [5, 6]. The California mastitis test (CMT), which determines the presence of cellular nuclear protein in milk samples, is an affordable, straightforward, and rapid mastitis screening test. As inflammatory cells are the most prevalent cells observed in mastitic milk, the CMT accurately represents the somatic cell count (SCC), and it is a valid indicator of the extent of infection. The process is sufficiently easy for milking employees and producers to learn, and the test is suitable for the cow-side examination of udder health [7]. For dairy animals, mastitis is an international issue that affects animal health and decreases milk output and quality, resulting in financial losses [8, 9]. Many pathogenic, opportunistic, and spoilage microorganisms best flourish in lactating cow milk, thereby affecting the pathophysiology of mastitis [10, 11]. Such microbes include Staphylococcus aureus, Streptococcus spp., Klebsiella pneumoniae, Escherichia coli, and other less frequent pathogens such as Pseudomonas aeruginosa, Mycoplasma spp., and Mycobacterium spp. [12, 13]. As staphylococci cause contagious infection, unsanitary farming practices contribute to its spread among herds. In multidrug-resistant (MDR) strains in particular, this disease can cause persistent infections that are challenging to treat with conventional therapy, causing enormous financial losses in the dairy business [14, 15]. Another major source of food-borne disease is S. aureus isolates from nursing animals, with bulk and raw milk products playing a key role in bacterial transmission to humans [16]. Moreover, S. aureus is responsible for at least 10% of food-borne illnesses linked to dairy products [17]. Real-time polymerase chain reaction (PCR) is a reliable diagnostic technique for improving bacterial identification and it offers benefits for increasing throughput, permitting completely objective interpretation of results, and quantifying the amount of microbes in milk samples [18, 19].
The rapid detection of S. aureus is a critical issue for treatment and the implementation of infection control measures. In addition, real-time PCR has 100% sensitivity and specificity for these targets compared to culture and conventional techniques [20]. Similarly as all procedures, real-time PCR assays require thorough validation to avoid producing false-positive or false-negative results [21]. The clinical importance of detecting bacterial nucleic acids rather than living cells should be considered. Real-time PCR is limited to the detection of bacterial species for which a test kit has been created [22]. Nonetheless, real-time PCR remains a promising molecular technique for detecting MDR pathogens produced because of the excessive use of antimicrobials in veterinary care and agriculture [15, 23, 24]. Erythromycin is an antibiotic used as an alternative to cephalosporin, penicillin, and other beta-lactams for gram-positive microorganisms, and it has been used for many illnesses for a long time [25, 26]. However, erythromycin use has led to the synthesis of methylase encoded by the erm genes, leading to erythromycin resistance in S. aureus. Erythromycin resistance in bovine mastitis isolates is reportedly caused by ermA, ermB, and ermC [27]. Bovine mastitis and people have both produced strains of erythromycin-resistant S. aureus [2]. Because S. aureus strains are resistant to numerous medicines, many therapeutic approaches for controlling mastitis have been ineffective [28]. Although S. aureus in dairy cattle has long been known cause serious harm, no effective preventive measures or treatments have been suggested. This is attributable to the lack of knowledge regarding the relationship between the bacteria and the host and the rapidly evolving genetic diversity of the pathogens.
This study aimed to estimate the prevalence of S. aureus in bovine subclinical mastitic milk samples, histopathologically examine udder tissues exhibiting mastitis, identify S. aureus isolates by probe-based real-time PCR, and detect the macrolides and erythromycin resistance profile genotypically. Moreover, this study analyzed the phylogenetic relatedness of our local isolates of S. aureus to explain the possible genetic link between other isolates.
Materials and Methods
Ethical approval
This study was approved (no. 231712012023) by Medical Research Ethics Committee at the National Research Centre, Egypt.
Study period and location
This study was conducted from January to June 2022 at the Veterinary Research Institute, National Research Center, Dokki, Egypt.
Animals and milk samples processing
Seventy-five bovine herds raised in private dairy farms located in Cairo, Giza, Kalyobia, Fayoum, and Kafr El-Sheikh, Egypt, were included in the study. Each herd consisted of 30–50 milking animals. Some farms contained more than one bulk tank. Samples were taken from each bulk tank in sterile vials from the top surface of the milk under agitation according to the recommendations of the National Mastitis Council, USA. [29]. Agitation was permitted to last for at least 10 min. The CMT was used to identify SCM and milk samples were obtained aseptically as suggested by Andrews et al. [30]. Positive CMT milk samples were tightly closed, refrigerated at 4°C, and transported to the laboratory, where they were cultivated on agar medium for 24 or 48 h.
Furthermore, 285 milk samples were collected from 150 dairy cattle and 135 dairy buffalo raised by smallholders in different governments in Egypt (Cairo, Giza, Kalyobia, Fayoum, and Kafr El-Sheikh). The CMT was applied to detect SCM and milk samples were aseptically collected as described by Andrews et al. [30]. Milk samples were tightly closed, refrigerated at 4°C, and transported to the Laboratory of Bacteriology, Department of Microbiology and Immunology, Veterinary Research Division, National Research Center for bacteriological examination as rapidly as possible.
Bacteriological isolation and identification
To isolate Staphylococcus spp., milk samples of cattle and buffalo with SCM were centrifuged, and the sediment was then cultured on Baird-Parker agar, Mannitol salt agar, and blood agar 10% (Oxoid, UK) and then incubated for 1–2 days at 37°C [31]. Confirmation for S. aureus was achieved through gram staining, catalase and coagulase tests [32], hemolytic activity testing, DNase agar testing [33], and the biofilm activity onto Congo red medium [34].
DNA extraction
DNA extraction from bacterial cultures was performed using a QIAamp DNA Mini kit (Qiagen, Germany, GmbH) following the manufacturer’s recommendations. Briefly, bacterial pellets were re-suspended in 200 μL of PBS and incubated with 20 μL of proteinase K and 200 μL of lysis buffer at 56°C for 10 min. After incubation, 200 μL of 100% ethanol was added to the lysate. The sample was then washed and centrifuged. Nucleic acid was eluted with 50 μL of elution buffer.
Molecular identification using real-time PCR
The PCR TaqMan assay targeting S. aureus was performed using qTOWER 3G (Analytik Jena, Germany), which was used for thermocycling and fluorescence detection. Real-time PCR amplification was performed in a total volume of 20 µL containing 10 µL of 2× TOPreal TaqMan Probe quantitative PCR mixture (Cat RT600, Enzynomics) according to the manufacturer’s instructions. In addition, 0.2 µL (10 µm) of each primer, 0.4 µL (10 µm) the TaqMan probe mixture, and 2 µL of template DNA were mixed, and then distilled water was added for a final volume of 20 µL. The specific primers (nuc2) and probes used to identify nuc in S. aureus are listed in Table-1 [35–39]. The cycling conditions are listed in Table-2.
Table-1.
Polymerase chain reaction primers and probes used in the study.
| Gene | Sequence (5´-3´) | Amplicon size (bp) | References |
|---|---|---|---|
| NUC1 | CTG GCA TAT GTA TGG CAA TTG TT TAT TGA CCT GAA TCA GCG TTG TCT | 664 bp | [35] |
| NUC2 | AAAGCGATTGATGGTGATACGGTT TGCTTTGTTTCAGGTGTATCAACCA FAM-Probe ATGTACAAAGGTCAACCAATGACATTYAGA | ------- | [36] |
| ermA | TATCTTATCGTTGAGAAGGGATT CTACACTTGGCTTAGGATGAAA | 139 bp | [37] |
| ermB | CTATCTGATTGTTGAAGAAGGATT GTTTACTCTTGGTTTAGGATGAAA | 142 bp | [37] |
| ermC | CTTGTTGATCACGATAATTTCC ATCTTTTAGCAAACCCGTATTC | 190 bp | [37] |
| ermT | ATTGGTTCAGGGAAAGGTCA GCTTGATAAAATTGGTTTTTGGA | 536 bp | [38] |
| msrA | TCCAATCATTGCACAAAATC AATTCCCTCTATTTGGTGGT | 163 bp | [39] |
Table-2.
Cycling conditions for the detection of genes in this study.
| Gene | Init. Denat. | Denat. | Anneal. | Extention | Final ext. | Cycles |
|---|---|---|---|---|---|---|
| NUC1 | 95°C 2 min | 95°C 20 s | 60°C 30 s | 72°C 45 s | 72°C 10 min | 35 |
| NUC2 (Q-PCR) | 95°C 10 min | 95°C 10 s | 56°C 20 s | 60°C 40 s | ----- | 40 |
| ermA | 95°C 3 min | 95°C 20 s | 59°C 30 s | 72°C 45 s | 72°C 10 min | 40 |
| ermB | 94°C 2 min | 94°C 30 s | 55°C 30 s | 72°C 30 s | 72°C 7 min | 35 |
| ermC | 95°C 3 min | 95°C 20 s | 55°C 30 s | 72°C 45 s | 72°C 7 min | 40 |
| ermT | 95°C 2 min | 95°C 30 s | 57°C 30 s | 72°C 30 s | 72°C 7 min | 35 |
| msrA | 94°C 2 min | 94°C 20 s | 54°C 30 s | 72°C 45 s | 72°C 7 min | 35 |
PCR=Polymerase chain reaction
Conventional PCR to detect S. aureus
To identify S. aureus (nuc) and detect erythromycin resistance determinants (ermA, ermB, ermC, and ermT) and macrolide-resistant determinants (msrA), PCR was performed. A GS-96 gradient thermocycler (Hercuvan, Malaysia) was used for PCR in a final volume of 25 μL containing 12.5 μL of 2× COSMO PCR RED Master Mix (Cat. W1020300X, Willofort Co., UK), 0.5 μL (10 μM) of each primer (Vivantis, Malaysia), and 1 μL of target DNA. The PCR products were separated by electrophoresis on 1.5% agarose gels and then photographed and analyzed using the InGenius 3 gel documentation system (Syngene, UK). The used primers and cycling conditions are listed in Tables-1 and 2.
DNA sequencing
The positive PCR products targeting nuc1 were cleaned using a GeneJET™ Gel Extraction Kit (K0691, Thermo Fisher Scientific, USA) and then sequenced by MACROGEN Company (Korea) on 3730XL sequencers (Applied Biosystems, USA).
Eight PCR sequences identified in this study have been deposited in the GenBank database under the accession numbers OP821405–OP821408 and OP821409–OP821412 for S. aureus isolates from cattle and buffalo with SCM, respectively.
Histopathological examination
Twelve udder tissue samples were fixed in formalin solution (10%), dehydrated in different concentrations of alcohol, embedded in paraffin, sectioned at a thickness of 4–5 μm, and stained with hematoxylin and eosin. The tissue sections were examined and photographed using a light microscope (Olympus BX 51, Tokyo, Japan) [40].
Results
Using bulk milk samples from apparently normal animals, 18 (38.2%) and 15 (53.6%) cattle and buffaloes, respectively, exhibited SCM and reacted positively in the CMT, giving a total prevalence of SCM of 44% (Table-3).
Table-3.
Prevalence of subclinical mastitis from cattle and buffaloes bulk tank milk using CMT.
| Number | Governorate | Cattle (%) | Buffalo (%) | Total (%) |
|---|---|---|---|---|
| 1 | Cairo | 1/3 (33.3) | - | 1/3 (33.3) |
| 2 | Giza | 2/8 (25) | 3/6 (50) | 5/14 (35.7) |
| 3 | Kalyobia | 1/4 (25) | 0/2 (0) | 1/6 (16.7) |
| 4 | Fayoum | 5/12 (41.7) | 5/7 (71.4) | 10/19 (52.6) |
| 5 | Kafr El-Sheikh | 9/20 (45) | 7/13 (53.8) | 16/33 (48.5) |
| Total | 18/47 (38.2) | 15/28 (53.6) | 33/75 (44) |
CMT=California mastitis test
Using 33 bulk tank milk samples from cattle and buffalo with SCM, 12 (66.7%) and 9 (60%) S. aureus isolates were identified in 18 and 15 cattle and buffalo milk samples, respectively, giving a prevalence of 63.6% (Table-4 and Figure-1).
Table-4.
Prevalence of Staphylococcus aureus isolates in bulk tank milk samples from cattle and buffaloes with subclinical mastitis.
| Number | Governorate | Cattle (%) | Buffalo (%) | Total (%) |
|---|---|---|---|---|
| 1 | Cairo | 1/1 (100) | - | 1/1 (100) |
| 2 | Giza | 2/2 (100) | 1/3 (33.3) | 3/5 (60) |
| 3 | Kalyobia | 1/1 (100) | 0 | 1/1 (100) |
| 4 | Fayoum | 4/5 (80) | 2/5 (40) | 6/10 (60) |
| 5 | Kafr El-Sheikh | 4/9 (44.4) | 6/7 (85.7) | 10/16 (62.5) |
| Total | 12/18 (66.7) | 9/15 (60) | 21/33 (63.6) |
Figure-1.

Agarose gel electrophoresis of polymerase chain reaction product amplified from Staphylococcus aureus nuc gene (664 bp). Lane 1–100 bp DNA Ladder; Lanes 2–15, representative positive samples.
In apparently normal cattle and buffaloes, 55 (36.7%) and 43 (31.9%) examined milk samples from cattle and buffaloes, respectively, exhibited SCM and reacted positively in the CMT, giving a prevalence of SCM of 34.4% (Table-5).
Table-5.
Prevalence of SCM in dairy cattle and buffaloes at different governorates of Egypt (Smallholders).
| Number | Governorate | Cattle (%) | Buffalo (%) | Total (%) |
|---|---|---|---|---|
| 1 | Cairo | 4/12 (33.3) | 5/15 (33.3) | 9/27 (33.3) |
| 2 | Giza | 11/27 (40.7) | 6/25 (24) | 17/52 (32.7) |
| 3 | Kalyobia | 10/23 (43.5) | 13/37 (35.1) | 23/60 (38.3) |
| 4 | Fayoum | 14/45 (31.1) | 9/41 (22) | 23/86 (26.7) |
| 5 | Kafr El-Sheikh | 16/43 (37.2) | 10/17 (58.8) | 26/60 (43.3) |
| Total | 55/150 (36.7) | 43/135 (31.9) | 98/285 (34.4) |
SCM = Subclinical mastitis
Using milk from subclinically mastitic cattle and buffaloes raised by smallholders, 20 (36.4%) and 21 (48.8%) S. aureus isolates were obtained from 55 and 43 cattle and buffaloes, respectively, giving a total prevalence of 41.8% (Table-6 and Figure-1).
Table-6.
Prevalence of Staphylococcus aureus isolates in milk samples from cattle and buffaloes with subclinical mastitis (Smallholders).
| Number | Governorate | Cattle (%) | Buffalo (%) | Total (%) |
|---|---|---|---|---|
| 1 | Cairo | 1/4 (25) | 4/5 (80) | 5/9 (55.6) |
| 2 | Giza | 3/11 (27.3) | 6/6 (100) | 9/17 (52.9) |
| 3 | Kalyobia | 5/10 (50) | 4/13 (30.8) | 9/23 (39.1) |
| 4 | Fayoum | 4/14 (28.6) | 4/9 (44.4) | 8/23 (34.8) |
| 5 | Kafr El-Sheikh | 7/16 (43.8) | 3/10 (30) | 10/26 (38.5) |
| Total | 20/55 (36.4) | 21/43 (48.8) |
Molecular identification using real-time PCR
In total, 62 S. aureus isolates that were bacteriologically isolated and identified from the milk of cattle and buffaloes with SCM were subjected to molecular confirmation using probe-based real-time PCR. The probe-based real-time PCR confirmed all 62 (100%) bacteriologically identified isolates.
Molecular identification using conventional PCR
Conventional PCR was performed using the positive probe-based reverse-transcription-PCR samples for DNA sequencing. All samples were positive using conventional PCR, as shown in Figure-1.
Prevalence of antimicrobial resistance genes
Erythromycin and macrolide genes were detected in the genomic DNA of 31 S. aureus strains. Concerning erythromycin resistance, ermA-, ermB-, ermC-, and ermT-specific amplicons were detected in 5 (8%), 26 (41.9%), 18 (29%), and 15 (24.1%) strains, respectively, as shown in Figures-2 and Table-7. msrA was harbored in three (4.8%) S. aureus strains exhibiting macrolide resistance, as shown in Figure-3 and Table-7.
Figure-2.

Polymerase chain reaction analysis of erythromycin-resistant determinants: Lane 1, 100 bp DNA ladder; Lanes 2 and 3, ermT (536 bp); Lanes 4 and 5, ermC (190 bp); Lanes 6 and 7, ermB (142 bp); Lanes 8 and 9; ermA (139 bp).
Table-7.
Prevalence of Staphylococcus aureus antibiotic resistance genes (erythromycin and macrolides).
| Number | Gene | Total (%) |
|---|---|---|
| 1 | nuc | 62 (100) |
| 2 | ermA | 5 (8) |
| 3 | ermB | 26 (41.9) |
| 4 | ermC | 18 (29) |
| 5 | ermT | 15 (24.1) |
| 6 | msrA | 3 (4.8) |
Figure-3.
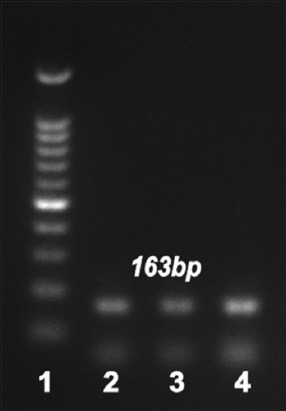
Polymerase chain reaction analysis of macrolide-resistant determinants: msrA (163 bp). Lane 1, 100 bp DNA ladder; Lanes, 2–4, positive samples.
Phylogenetic analysis
All eight PCR products had the same nucleotide sequence and they were named OP821405–Op821412. Multiple sequence alignment, phylogenetic analysis, and tree construction of S. aureus nuc confirmed its high homology (100%) with that of S. aureus strains isolated from milk in bovines with mastitis in India and Kenya (JX240349, JN247783, GU129659, and MW826579), as shown in Figure-4.
Figure-4.

Phylogenetic relationship of selected strains of Staphylococcus aureus from different sources, based on the nuc gene.
Histopathological examination
Microscopic examination revealed lymphocytic mastitis in the mammary glands of the examined animals characterized by massive aggregation of mononuclear inflammatory cells, mainly lymphocytes and macrophages, in the interstitial connective tissue. The secretory acini showed vacuolar degeneration of the epithelial lining in some cases (Figure-5).
Figure-5.

Mammary gland showing lymphocytic mastitis as diffuse infiltration of lymphocytes in the intralobular interstitium associated with homogenous esinophilic masses corpora amylacea (H and E, ×100).
Discussion
In Egypt and other countries, S. aureus is the most frequent cause of CM and SCM [1, 2, 41]. Bovine mastitis is the most common and economically significant disease in dairy animals. Among 33 bulk tank milk samples from cattle and buffaloes with SCM, S. aureus was isolated from 12/18 (66.7%) and 9/15 (60%) samples, respectively. In the present study, the incidence of SCM in apparently normal bulk tank milk from cattle and buffaloes from different governments in Egypt was 44% (33/75). The prevalence of SCM in milk samples collected from individual quarters using the CMT was 44% (88/200) in buffaloes and 52.1% (146/280) in cattle. In contrast, the incidence of SCM in milk samples from animals with no obvious abnormalities was 36.7% (55/150) in cattle and 31.9% (43/135) in buffaloes. Between cattle and buffaloes, there was no appreciable variation in the occurrence of SCM.
According to Hoque et al. [14], the proportion of riverine buffaloes with SCM was 37.6% (188/500). However, using the CMT, Abed et al. [31] reported that the prevalence of SCM was 46%. Our investigation detected SCM in 98/285 animals (34.4%). Many earlier investigations in Egypt and other countries supported these conclusions [42–45]. Poor housing and bedding materials, unsanitary conditions, a history of mastitis, improper milking techniques, and contaminated milking equipment can all contribute to high rates of SCM in dairy herds [46–48]. To reduce the adverse effects of SCM, dairy farms’ entire farming and housing systems and udder health management procedures should be addressed.
Bovine mastitis, a condition that significantly affects the dairy supply chain, is caused by S. aureus, which has been the focus of extensive research in the veterinary area [3]. According to Table-6, the prevalence of S. aureus in milk from animals with SCM raised by smallholders was 36.4% (20/55) in cattle and 48.8% (21/43) in buffaloes, whereas the overall prevalence of S. aureus in SCM milk samples was 41.8% (41/98). Hoque et al. [14] reported that 291 isolates were recovered from 188 SCM samples, and the prevalence of S. aureus was 37.4% (109/291), almost identical to our findings. Moreover, the prevalence of S. aureus in the milk samples obtained from individual quarters utilizing the CMT was reported as 35.9% (84/234), including rates of 36.3% (53/146) in cattle and 31% (31/88) in buffaloes. This result was much higher than the prevalence (6.5%) reported by Haltia et al. [49] but similar to findings in earlier research [50, 51]. Nevertheless, Rasool et al. [2] indicated that the prevalence of S. aureus in mastitis-affected buffaloes was 75%, whereas De Los Santos et al. [52] revealed that subclinical staphylococcal mastitis was present in most farms (82%). This prevalence resembles that revealed in a recent study by Akkou et al. [53]. The incidence reported by Ashfaq and Muhammad [54] was lower than that reported by Akkou et al. [53], which was higher than our findings in both cattle and buffaloes. S. aureus is spread among animals through the use of contaminated milk utensils and the hands of the milker [55]. Moreover, the keratin coating in the teat canals of healthy cows allows S. aureus to persist and resist phagocytosis [56, 57]. This emphasizes the importance of good hygiene and management procedures in dairy farms. Furthermore, this poses a major risk to public health because mastitic milk is typically added to bulk milk tanks, particularly in areas in which some people might consume raw milk or non-heated dairy products such as yogurt or cheese [58].
Molecular techniques such as PCR are employed to rapidly identify S. aureus, which is crucial for both treatment and the adoption of infection control strategies to stop the spread of sickness and outbreaks [20, 59]. Real-time PCR offers high sensitivity and specificity for the detection of mastitis-causing pathogens in bulk tank milk [21]. In this study, 62 S. aureus isolates were molecularly confirmed using probe-based real-time PCR. Our findings are consistent with those of Katholm et al. [60], who used real-time PCR to test bulk tank milk from 4258 Danish dairy herds. In their study, Staphylococcus spp. were found in all bulk tank milk samples, whereas Koskinen et al. [22] obtained false-positive results using real-time PCR to test quarter milk samples from clinically healthy cows with a low milk SCC. As with all techniques, real-time PCR requires comprehensive validation to prevent false-positive or false-negative results caused by its ability to identify low copy numbers of bacteria originating from the environment or by components in the sample that inhibit the reaction [21]. According to prior research, real-time PCR is more sensitive and specific than bacterial cultures for detecting mastitis pathogens [61, 62]. In herds with a high prevalence of contagious mastitis, dairy farmers frequently use antibiotic therapy as a crucial tool to prevent intramammary infection, particularly before calving, or to treat persistent and chronic udder infections, as well as for dry cow therapy at the end of the lactation season [63]. Because of the risk of resistance spreading to people and its impact on the effectiveness of current antimicrobial therapy procedures, excessive antimicrobial use in dairy farms has led to the emergence of resistance among several bacteria [64]. The macrolide erythromycin exhibits strong antibacterial activity against Gram-positive and Gram-negative bacteria, including Staphylococcus, Streptococcus, and E. coli [65, 66]. Staphylococcus aureus is a primary cause of mastitis, and it is often resistant to antibiotics. Rasool et al. [2] identified the genetic factors of erythromycin resistance in S. aureus, such as ermA, ermB, and ermC. This investigation used PCR to detect the erythromycin resistance profiles of the genomic DNA of 31 S. aureus isolates. As indicated in Figures-2 and Table-7, most S. aureus isolates tested positive for ermA, ermB, ermC, or ermT. Moreover, the existence of msrA was examined in S. aureus isolates resistant to macrolides, and this gene was found in three (4.8%) strains, as shown in Figure-3 and Table-7. Our findings are consistent with those of a study conducted in Pakistan that assessed erythromycin resistance profiles in milk samples and found that 8 (52.1%), 3 (21.4%), and 5 (35.7%) S. aureus isolates were positive for ermA, ermB, and ermC, respectively [2]. Our resistance rate was similar to that Rusenova et al. [67] reported in another investigation in Bulgaria, in which erythromycin resistance was identified in 7/12 (58%) samples. Additionally, our research is supported by a Chinese study that detected macrolide resistance in 34.08% of patients [68]. By contrast, Algammal et al. [42] conducted a study in Egypt to estimate the antimicrobial resistance profiles and determine the prevalence of S. aureus on farms in Ismailia Province. Their study revealed that 63.1% of the S. aureus strains were sensitive to erythromycin, and the different findings could be attributable to the different locations in which the studies were conducted. De Los Santos et al. [52] examined subclinical bovine mastitis associated with Staphylococcus in Uruguayan dairy farms, and their results disagreed with our findings. None of their Staphylococcus isolates showed resistance to erythromycin. In addition, isolates identified by Hoque et al. [14] were extremely susceptible to azithromycin (80.7%) and erythromycin (60.5%). Their earlier investigations and several other studies reported the highest vulnerability of mastitis-causing bacterial infections to macrolides, such as azithromycin and erythromycin [4, 69, 70].
Eight PCR products were obtained in DNA sequencing in this study using standard PCR, and multiple sequence alignment, phylogenetic analysis, and tree construction of the S. aureus nuc gene revealed a high degree of homology (100%) with that in S. aureus strains isolated from milk in bovines with mastitis in India and Kenya.
The production of different toxins, virulence factors, and cell wall adhesion proteins is most likely attributable to the ability of S. aureus to cause mastitis [71, 72]. The bacterium can withstand phagocytosis in the udder, and it frequently leads to persistent inflammation [71, 73]. Because changes in udder tissue occur far sooner than they become obvious, early identification of SCM is crucial [14].
Our histological analysis of udder tissues revealed significant accumulation of mononuclear inflammatory cells, primarily lymphocytes and macrophages, in the interstitial connective tissue. In some instances, the epithelial lining of the vacuoles in the secretory acini was degenerated. This finding aligns with that of Fasulkov et al. [74], who recorded histological findings revealing epithelial cell vacuolar degeneration, interstitial alterations, edema, and mononuclear inflammatory cell proliferation (lymphocytes and histiocytes).
Conclusion
Our findings showed that S. aureus had a high prevalence in raw milk. Moreover, many erythromycin and macrolide resistance genes were present in the identified S. aureus isolates. Therefore, developing treatment strategies based on the various regional and seasonal characteristics linked to the occurrence and resistance of S. aureus strains is extremely important. Our findings will assist in safeguarding public and animal health by preventing S. aureus infections in cattle on dairy farms. The S. aureus strains isolated from submastitic milk samples could be recognized within 2 h based on the high sensitivity and specificity of probe-based real-time PCR. This approach is probably useful for the rapid screening of S. aureus isolates and the efficient treatment and control of S. aureus mastitis.
Authors’ Contributions
KAA, EAF, and AAA: Study design, conducted the PCR, genetic markers of virulence, and antibiotic resistance. AHS: Pathological examination. ME: Diagnosis of SCM using CMT and statistical analysis. AMA: Bacterial isolation and identification and nucleic acid extraction. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
The authors acknowledge financial support from the Science and Technology Development Fund (STDF), Egyptian Ministry of Higher Education and Scientific Research (Project No. 34805).
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Abdalhamed A.M, Zeedan G.S.G, Arafa A.A, Ibrahim E.S, Sedky D, Hafez A.A.N. Detection of methicillin-resistant Staphylococcus aureus in clinical and subclinical mastitis in ruminants and studying the effect of novel green synthesized nanoparticles as one of the alternative treatments. Vet. Med. Int. 2022;2022:6309984. doi: 10.1155/2022/6309984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasool Z, Noreen H, Anjum A, Rizvi A, Rabaan A.A, Halwani M.A, Sabour A.A, Aljeldah M, Al Shammari B.R, Alhajri S.M, Alshubaith I.H, Garout M, Firyal S, Ahmed N. Genotypic and phenotypic characterization of erythromycin-resistant Staphylococcus aureus isolated from bovine mastitis and humans in close contact. Trop. Med. Infect. Dis. 2023;8(1):26. doi: 10.3390/tropicalmed8010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos B, Pickering A.C, Rocha L.S, Aguilar A.P, Fabres-Klein M.H, de Oliveira Mendes T.A, Fitzgerald J.R, de Oliveira Barros Ribon A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis:Current understanding and future perspectives. BMC Vet. Res. 2022;18:115. doi: 10.1186/s12917-022-03197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoque M.N, Istiaq A, Clement R.A, Gibson K.M, Saha O, Islam O.K, Abir R.A, Sultana M, Siddiki A, Crandall K.A. Insights into the resistome of bovine clinical mastitis microbiome, a key factor in disease complication. Front. Microbiol. 2020;11:860. doi: 10.3389/fmicb.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvador R.T, Beltran J.M, Abes N.S, Gutierrez C.A, Mingala C.N. Short communication:Prevalence and risk factors of subclinical mastitis as determined by the California Mastitis Test in water buffaloes (Bubalis bubalis) in Nueva Ecija, Philippines. J. Dairy Sci. 2012;95(3):1363–1366. doi: 10.3168/jds.2011-4503. [DOI] [PubMed] [Google Scholar]
- 6.Hoque M.N, Das Z.C, Talukder A.K, Alam M.S, Rahman A.N.M. Different screening tests and milk somatic cell count for the prevalence of subclinical bovine mastitis in Bangladesh. Trop. Anim. Health Prod. 2015;47(1):79–86. doi: 10.1007/s11250-014-0688-0. [DOI] [PubMed] [Google Scholar]
- 7.Kandeel S.A, Morin D.E, Calloway C.D, Constable P.D. Association of California mastitis test scores with intramammary infection status in lactating dairy cows admitted to a veterinary teaching hospital. J. Vet. Intern. Med. 2018;32(1):497–505. doi: 10.1111/jvim.14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F, Zhang S, Shang X, Li H, Zhang H, Cui D, Wang X, Wang L, Yan Z, Sun Y. Short communication:Detection and molecular characterization of methicillin-resistant Staphylococcus aureus isolated from subclinical bovine mastitis cases in China. J. Dairy Sci. 2020;103(1):840–845. doi: 10.3168/jds.2019-16317. [DOI] [PubMed] [Google Scholar]
- 9.Sharun K, Dhama K, Tiwari R, Gugjoo M.B, Yatoo M.I, Patel S.K, Pathak M, Karthik K, Khurana S.K, Singh R, Puvvala B, Amarpal Singh R, Singh K.P, Chaicumpa W. Advances in therapeutic and managemental approaches of bovine mastitis:A comprehensive review. Vet. Q. 2021;41(1):107–136. doi: 10.1080/01652176.2021.1882713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoque M.N, Istiaq A, Clement R.A, Sultana M, Crandall K.A, Siddiki A.Z, Hossain M.A. Metagenomic deep sequencing reveals association of microbiome signature with functional biases in bovine mastitis. Sci. Rep. 2019;9:13536. doi: 10.1038/s41598-019-49468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zayda M.G, Masuda Y, Hammad A.M, Honjoh K.I, Elbagory A.M, Miyamoto T. Molecular characterization of methicillin-resistant (MRSA) and methicillin-susceptible (MSSA) Staphylococcus aureus isolated from bovine subclinical mastitis and Egyptian raw milk cheese. Int. Dairy J. 2020;104:104646. [Google Scholar]
- 12.Ardicli O, Demirbilek S.K, Carli K.T. Pathogens isolated from bovine clinical mastitis and their antimicrobial resistance. Med. Weter. 2022;78(1):6606–2022. [Google Scholar]
- 13.Shoaib M, Aqib A.I, Naseer M.A, Bhutta Z.A, Wanxia P.U, Tanveer Q, Hammad M. Mastitis in Dairy Cattle, Sheep and Goats. London: IntechOpen; 2022. Etiology of Bovine Mastitis. [Google Scholar]
- 14.Hoque M.N, Talukder A.K, Saha O, Hasan M.M, Sultana M, Rahman A.A, Das Z.C. Antibiogram and virulence profiling reveals multidrug resistant Staphylococcus aureus as the predominant aetiology of subclinical mastitis in riverine buffaloes. Vet. Med. Sci. 2022;8(6):2631–2645. doi: 10.1002/vms3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Chen Y, Li X, Wang X, Li H. Detection of antibiotic resistance, virulence gene, and drug resistance gene of Staphylococcus aureus isolates from bovine mastitis. Microbiol. Spectr. 2022;10(4):e0047122. doi: 10.1128/spectrum.00471-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagundes H, Barchesi L, Filho A.N, Ferreira L.M, Oliveira C.A. Occurrence of Staphylococcus aureus in raw milk produced in dairy farms in São Paulo state, Brazil. Braz. J. Microbiol. 2010;41(2):376–380. doi: 10.1590/S1517-838220100002000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigo F, Vasil M, Ondrašovičová S, Výrostková J, Bujok J, Pecka-Kielb E. Maintaining optimal mammary gland health and prevention of mastitis. Front. Vet. Sci. 2021;8:607311. doi: 10.3389/fvets.2021.607311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koskinen M.T, Holopainen J, Pyörälä S, Bredbacka P, Pitkälä A, Barkema H.W, Bexiga R, Roberson J, Sølverød L, Piccinini R, Kelton D, Lehmusto H, Niskala S, Salmikivi L. Analytical specificity and sensitivity of a real-time polymerase chain reaction assay for identification of bovine mastitis pathogens. J. Dairy Sci. 2009;92(3):952–959. doi: 10.3168/jds.2008-1549. [DOI] [PubMed] [Google Scholar]
- 19.Viguier C, Arora S, Gilmartin N, Welbeck K, O'Kennedy R. Mastitis detection:Current trends and future perspectives. Trends Biotechnol. 2009;27(8):486–493. doi: 10.1016/j.tibtech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Galia L, Ligozzi M, Bertoncelli A, Mazzariol A. Real-time PCR assay for detection of Staphylococcus aureus, Panton-Valentine Leucocidin and Methicillin Resistance directly from clinical samples. AIMS Microbiol. 2019;5(2):138–146. doi: 10.3934/microbiol.2019.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi Y, Wang Y.J, Qin Y, Vallverdú R.G, García J.M, Sun W, Li S, Cao Z. Prevalence of bovine mastitis pathogens in bulk tank milk in China. PLoS One. 2016;11(5):e0155621. doi: 10.1371/journal.pone.0155621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koskinen M.T, Wellenberg G.J, Sampimon O.C, Holopainen J, Rothkamp A, Salmikivi L, van Haeringen W.A, Lam T.J.M, Pyörälä S. Field comparison of real-time polymerase chain reaction and bacterial culture for identification of bovine mastitis bacteria. J. Dairy Sci. 2010;93(12):5707–5715. doi: 10.3168/jds.2010-3167. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Wang Z, Yan Z, Wu J, Ali T, Li J, Lv Y, Han B. Bovine mastitis Staphylococcus aureus:Antibiotic susceptibility profile, resistance genes and molecular typing of methicillin-resistant and methicillin-sensitive strains in China. Infect. Genet. Evol. 2015;31:9–16. doi: 10.1016/j.meegid.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Brahma U, Suresh A, Murthy S, Bhandari V, Sharma P. Antibiotic resistance and molecular profiling of the clinical isolates of Staphylococcus aureus causing bovine mastitis from India. Microorganisms. 2022;10(4):833. doi: 10.3390/microorganisms10040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leclercq R. Mechanisms of resistance to macrolides and lincosamides:Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002;34(4):482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 26.Rabello R.F, Souza C.R.V.M, Duarte R.S, Lopes R.M.M, Teixeira L.M, Castro A.C.D. Characterization of Staphylococcus aureus isolates recovered from bovine mastitis in Rio de Janeiro, Brazil. J. Dairy Sci. 2005;88(9):3211–3219. doi: 10.3168/jds.S0022-0302(05)73004-6. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Ferreri M, Liu X.Q, Chen L.B, Su J.L, Han B. Development of multiplex polymerase chain reaction assay for rapid detection of Staphylococcus aureus and selected antibiotic resistance genes in bovine mastitic milk samples. J. Vet. Diagn. Invest. 2011;23(5):894–901. doi: 10.1177/1040638711416964. [DOI] [PubMed] [Google Scholar]
- 28.Rabbani R.A, Ahmad I, Lodhi L, Ahmad N, Muhammad G. Prevalence of various reproductive disorders and economic losses caused by genital prolapse in buffaloes. Pak. Vet. J. 2010;30:44–48. [Google Scholar]
- 29.National Mastitis Council. Laboratory Handbook on Bovine Mastitis. Verona, WI: National Mastitis Council; 1999. [Google Scholar]
- 30.Andrews A.H, Blowey R.W, Boyd W, Eddy R.G. Bovine Medicine Diseases and Husbandry of Cattle. United Kingdom: Blackwell Scientific Publications; 1992. [Google Scholar]
- 31.Abed A.H, Menshawy A.M.S, Zeinhom M.M.A, Hossain D, Khalifa E, Wareth G, Awad M.F. Subclinical mastitis in selected bovine dairy herds in North upper Egypt:Assessment of prevalence, causative bacterial pathogens, antimicrobial resistance and virulence-associated genes. Microorganisms. 2021;9(6):1175. doi: 10.3390/microorganisms9061175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schalm O.W, Noorlander D.O. Experiments and observations leading to development of the California mastitis test. J. Am. Vet. Med. Assoc. 1957;130(5):199–204. [PubMed] [Google Scholar]
- 33.American Public Health Association “APHA”. Standard Methods for the Examination of Dairy Products. 15th ed. Washington, USA: American Public Health Association; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arciola C.R, Campoccia D, Ravaioli S. Polysaccharide intercellular adhesin in biofilm:Structural and regulatory aspects. Front. Cell. Infect. Microbial. 2015;5:7. doi: 10.3389/fcimb.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graber H.U, Casey M.G, Naskova J, Steiner A, Schaeren W. Development of a highly sensitive and specific assay to detect Staphylococcus aureus in bovine mastitic milk. J. Dairy Sci. 2007;90(10):4661–4669. doi: 10.3168/jds.2006-902. [DOI] [PubMed] [Google Scholar]
- 36.Wang H. Y, Kim S, Kim J, Park S. D, Uh Y, Lee H. Multiplex real-time PCR assay for rapid detection of methicillin-resistant staphylococci directly from positive blood cultures. J. Clin. Microbiol. 2014;52(6):1911–1920. doi: 10.1128/JCM.00389-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martineau F, Picard F. J, Grenier L, Roy P. H, Ouellette M, Bergeron M. G. Multiplex PCR assays for the detection of clinically relevant antibiotic resistance genes in staphylococci isolated from patients infected after cardiac surgery. The ESPRIT Trial. J. Antimicrob. Chemother. 2000;46(4):527–534. doi: 10.1093/jac/46.4.527. [DOI] [PubMed] [Google Scholar]
- 38.Fessler A, Scott C, Kadlec K, Ehricht R, Monecke S, Schwarz S. Characterization of methicillin-resistant Staphylococcus aureus ST398 from cases of bovine mastitis. J. Antimicrob. Chemother. 2010;65(4):619–625. doi: 10.1093/jac/dkq021. [DOI] [PubMed] [Google Scholar]
- 39.Aktas Z, Aridogan A, Kayacan C. B, Aydin D. Resistance to macrolide, lincosamide and streptogramin antibiotics in staphylococci isolated in Istanbul, Turkey. J. Microbiol. 2007;45(4):286–290. [PubMed] [Google Scholar]
- 40.Suvarna K.S, Layton C, Bancroft J.D. British Library Cataloguing in Publication Data. 7th ed. China: Elsevier Health Sciences; 2018. Bancroft's Theory and Practice of Histological Techniques. [Google Scholar]
- 41.Cote-Gravel J, Malouin F. Symposium review:Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies. J. Dairy Sci. 2019;102(5):4727–4740. doi: 10.3168/jds.2018-15272. [DOI] [PubMed] [Google Scholar]
- 42.Algammal A.M, Enany M.E, El-Tarabili R.M, Ghobashy M.O.I, Helmy Y.A. Prevalence, antimicrobial resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens. 2020;9(5):362. doi: 10.3390/pathogens9050362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azooz M.F, El-Wakeel S.A, Yousef H.M. Financial and economic analyses of the impact of cattle mastitis on the profitability of Egyptian dairy farms. Vet. World. 2020;13(9):1750–1759. doi: 10.14202/vetworld.2020.1750-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He W, Ma S, Lei L, He J, Li X, Tao J, Wang X, Song S, Wang Y, Wang Y, Shen J, Cai C, Wu C. Prevalence, etiology, and economic impact of clinical mastitis on large dairy farms in China. Vet. Microbiol. 2020;242:108570. doi: 10.1016/j.vetmic.2019.108570. [DOI] [PubMed] [Google Scholar]
- 45.Samuel T.M, De Castro C.A, Dubascoux S, Affolter M, Giuffrida F, Billeaud C, Picaud J.C, Agosti M, Al-Jashi I, Pereira A.B, Costeira M.J, Silva M.G, Marchini G, Rakza T, Haaland K, Stiris T, Stoicescu S.M, Martínez-Costa C, Vanpee M, Domellöf M, Silva-Zolezzi I. Subclinical mastitis in a European multicenter cohort:Prevalence, impact on human milk (HM) composition, and association with infant HM intake and growth. Nutrients. 2020;12(1):105. doi: 10.3390/nu12010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis:Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016;12(1):270. doi: 10.1186/s12917-016-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed H.F, Straubinger R.K, Hegazy Y.M, Ibrahim S. Subclinical mastitis in dairy cattle and buffaloes among smallholders in Egypt:Prevalence and evidence of virulence of Escherichia coli causative agent. Trop. Biomed. 2018;35(2):321–329. [PubMed] [Google Scholar]
- 48.Rahularaj R, Deshapriya R.M.C, Ranasinghe R.M.S.B.K. Influence of bovine sub-clinical mastitis and associated risk factors on calving interval in a population of crossbred lactating cows in Sri Lanka. Trop. Anim. Health Prod. 2019;51(8):2413–2419. doi: 10.1007/s11250-019-01957-4. [DOI] [PubMed] [Google Scholar]
- 49.Haltia L, Honkanen-Buzalski T, Spiridonova I, Olkonen A, Myllys V. A study of bovine mastitis, milking procedures and management practices on 25 Estonian dairy herds. Acta Vet. Scand. 2006;48(1):22. doi: 10.1186/1751-0147-48-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dego O.K, Tareke F. Bovine mastitis in selected areas of southern Ethiopia. Trop. Anim. Health Prod. 2003;35(3):197–205. doi: 10.1023/a:1023352811751. [DOI] [PubMed] [Google Scholar]
- 51.Whist A.C, Østerås O, Sølverød L. Staphylococcus aureus and Streptococcus dysgalactiae in Norwegian herds after introduction of selective dry cow therapy and teat dipping. J. Dairy Res. 2006;74(1):1–8. doi: 10.1017/S0022029906002135. [DOI] [PubMed] [Google Scholar]
- 52.De Los Santos R, González-Revello Á, Majul L, Umpiérrez A, Aldrovandi A, Gil A, Hirigoyen D, Zunino P. Subclinical bovine mastitis associated with Staphylococcus spp. in eleven Uruguayan dairy farms. J. Infect. Dev. Ctries. 2022;16(4):630–637. doi: 10.3855/jidc.12960. [DOI] [PubMed] [Google Scholar]
- 53.Akkou M, Antri K, Bachtarzi M.A, Bes M, Tristan A, Dauwalder O, Kaidi R, Meugnier H, Tazir M, Etienne J. Phenotypic and genotypic characterization of Staphylococcus aureus strains associated with bovine mastitis and nasal carriage of workers in contact to animals in Algeria. Pak. Vet. J. 2016;36(2):184–188. [Google Scholar]
- 54.Ashfaq K, Muhammad G. Pathogens associated with bovine and bubaline mastitis in peri-urban areas of Faisalabad, Pakistan. Pak. J. Life Soc. Sci. 2008;6:86–88. [Google Scholar]
- 55.Scherrer D, Corti S, Muehlherr J.E, Zweifel C, Stephan R. Phenotypic and genotypic characteristics of Staphylococcus aureus isolates from raw bulk-tank milk samples of goats and sheep. Vet. Microbiol. 2004;101(2):101–107. doi: 10.1016/j.vetmic.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Alekish M.O, Al-Qudah K.M, Al-Saleh A. Prevalence of antimicrobial resistance among bacterial pathogens isolated from bovine mastitis in northern Jordan. Rev. Med. Vet. 2013;164(6):319–326. [Google Scholar]
- 57.Constable P.D, Hinchcliff K.W, Done S.H, Grünberg W. Veterinary Medicine-e-Book:A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 11th ed. Amsterdam, The Netherlands: Elsevier; 2017. pp. 220–465. [Google Scholar]
- 58.Awad A, Ramadan H, Nasr S, Ateya A, Atwa S. Genetic characterization, antimicrobial resistance patterns and virulence determinants of Staphylococcus aureus isolated from bovine mastitis. Pak. J. Biol. Sci. 2017;20(6):298–305. doi: 10.3923/pjbs.2017.298.305. [DOI] [PubMed] [Google Scholar]
- 59.Al-Talib H, Yean C.Y, Al-Khateeb A, Hasan H, Ravichandran M. Rapid detection of methicillin-resistant Staphylococcus aureus by a newly developed dry reagent-based polymerase chain reaction assay. J. Microbiol. Immunol. Infect. 2014;47(6):484–490. doi: 10.1016/j.jmii.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Katholm J, Bennedsgaard T.W, Koskinen M.T, Rattenborg E. Quality of bulk tank milk samples from Danish dairy herds based on real-time polymerase chain reaction identification of mastitis pathogens. J. Dairy Sci. 2012;95(10):5702–5708. doi: 10.3168/jds.2011-5307. [DOI] [PubMed] [Google Scholar]
- 61.Mweu M.M, Toft N, Katholm J, Nielsen S.S. Evaluation of two herd-level diagnostic tests for Streptococcus agalactiae using a latent class approach. Vet. Microbiol. 2012;159(1–2):181–186. doi: 10.1016/j.vetmic.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 62.Mahmmod Y.S, Toft N, Katholm J, Grønbæk C, Klaas I.C. Estimation of test characteristics of real-time PCR and bacterial culture for diagnosis of subclinical intramammary infections with Streptococcus agalactiae in Danish dairy cattle in 2012 using latent class analysis. Prev. Vet. Med. 2013;109(3–4):264–270. doi: 10.1016/j.prevetmed.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Rowe S.M, Nydam D.V, Godden S.M, Gorden P.J, Lago A, Vasquez A.K, Royster E, Timmerman J, Thomas M.J, Lynch R.A. Partial budget analysis of culture- and algorithm-guided selective dry cow therapy. J. Dairy Sci. 2021;104(5):5652–5664. doi: 10.3168/jds.2020-19366. [DOI] [PubMed] [Google Scholar]
- 64.Abed A.H, Al Sayed R.A, Atia A.A. Genotyping of b-lactams resistant staphylococci isolated from bovine subclinical mastitis. Beni Suef Univ. J. Basic Appl. Sci. 2018;7(4):499–504. [Google Scholar]
- 65.Feucht C, Patel D.R. Principles of pharmacology. Pediatr. Clin. North Am. 2011;58(1):11–19. doi: 10.1016/j.pcl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Enany M.E, Algammal A.M, Nasef S.A, Abo-Eillil S.A.M, Bin-Jumah M.N, Taha A.E, Allam A.A. The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express. 2019;9(1):192. doi: 10.1186/s13568-019-0920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rusenova N, Vasilev N, Rusenov A, Milanova A, Sirakov I. Comparison between some phenotypic and genotypic methods for assessment of antimicrobial resistance trend of bovine mastitis Staphylococcus aureus isolates from Bulgaria. Vet. Sci. 2022;9(8):401. doi: 10.3390/vetsci9080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang K, Cha J, Liu K, Deng J, Yang B, Xu H, Wang J, Zhang L, Gu X, Huang C, Qu W. The prevalence of bovine mastitis-associated Staphylococcus aureus in China and its antimicrobial resistance rate:A meta-analysis. Front. Vet. Sci. 2022;9:1006676. doi: 10.3389/fvets.2022.1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bantawa K, Sah S.N, Limbu D.S, Subba P, Ghimire A. Antibiotic resistance patterns of Staphylococcus aureus, Escherichia coli, Salmonella, Shigella and Vibrio isolated from chicken, pork, buffalo and goat meat in eastern Nepal. BMC Res. Notes. 2019;12(1):766. doi: 10.1186/s13104-019-4798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shrestha A, Bhattarai R.K, Luitel H, Karki S, Basnet H.B. Prevalence of methicillin-resistant Staphylococcus aureus and pattern of antimicrobial resistance in mastitis milk of cattle in Chitwan, Nepal. BMC Vet. Res. 2021;17(1):239. doi: 10.1186/s12917-021-02942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El-Ashker M, Gwida M, Tomaso H, Monecke S, Ehricht R, El-Gohary F, Hotzel H. Staphylococci in cattle and buffaloes with mastitis in Dakahlia Governorate, Egypt. J. Dairy Sci. 2015;98(11):7450–7459. doi: 10.3168/jds.2015-9432. [DOI] [PubMed] [Google Scholar]
- 72.Haran K.P, Godden S.M, Boxrud D, Jawahir S, Bender J.B, Sreevatsan S. Prevalence and characterization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J. Clin Microbiol. 2012;50(3):688–695. doi: 10.1128/JCM.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoque M.N, Das Z.C, Rahman A.N.M.A, Haider M.G, Islam M.A. Molecular characterization of Staphylococcus aureus strains in bovine mastitis milk in Bangladesh. Int. J. Vet. Sci. Med. 2018;6(1):53–60. doi: 10.1016/j.ijvsm.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fasulkov I, Karadaev M, Vasilev N, Simeonov R, Urumova V, Mladenova E. Ultrasound and histopathological investigations of experimentally induced Staphylococcus aureus mastitis in goats. Small Rumin. Res. 2015;129:114–120. [Google Scholar]


