Abstract
Background
Cardiopulmonary bypass (CPB) is frequently employed for cardiac surgery, and selecting a suitable priming fluid is a prerequisite for CPB. Currently, the commonly used priming fluids in clinics are classified as crystalloids and colloids, including balanced crystalloids, albumin, dextran, gelatin and hydroxyethyl starch (HES). This network meta-analysis compared the effects of eight fluids used during CPB in adults to determine optimal priming fluid during CPB surgery.
Methods
Randomised controlled trials assessing priming fluids for CPB in adult cardiac surgery published before 13 April 2023 were searched across Ovid MEDLINE(R) ALL, OVID EMbase, and Cochrane Central Register of Controlled Trials. Various priming fluids were classified into eight categories, including balanced crystalloids, 0.9% NaCl, iso-oncotic human albumin, hyperoncotic human albumin, HES with molecular weight 130k, HES with molecular weight 200k, gelatin and dextran.
Results
The NMA of platelet counts revealed no significant differences in any result. In direct comparison results, only the comparison of HES with molecular weight 130k vs. gelatin (standard mean difference = −0.40, 95% confidence interval [95%CI: −0.63, −0.16) revealed a significant difference. According to the SUCRA, balanced crystalloids had the highest platelet count, followed by gelatin, and HES with a molecular weight of 130k had the lowest platelet, followed by HES with a molecular weight of 200k.
Conclusion
Patients using dextran have a low mortality rate and a short mean CPB time, the use of balanced crystalloids is beneficial in terms of platelet count, and HES with molecular weight 130k is beneficial for postoperative urine volume at 24h. However, all priming fluids have pros and cons quite, and the optimal choice of priming fluids remains unsupported by current evidences. When performing CPB surgery, the type of priming fluid should be selected according to the actual situation in CPB for adult cardiac surgery.
Registration: PROSPERO CRD42023416194
Keywords: Cardiopulmonary bypass, priming fluid, colloid, crystalloid, hydroxyethyl starch
KEY MESSAGES
When dextran was used as the CPB priming fluid, patients had the lowest mortality and shortest mean CPB time.
With iso-oncotic HA, patients had the shortest length of ICU stay, the least blood loss 24h after surgery, and the lowest chest tube output 24h after surgery.
The use of balanced crystalloids was beneficial for platelet count, the use of L-HES was beneficial for urine output 24h after surgery, and the use of H-HES resulted in the shortest hospital stay.
In summary, each of these fluids has pros and cons quite, and an optimal choice of priming fluids during CPB surgery remains unsupported by current evidence.
When performing CPB surgery, the type of priming fluids should be selected according to the actual condition of the patient’s body.
1. Introduction
Cardiopulmonary bypass (CPB) is a technique that temporarily takes over cardiopulmonary function and maintains blood circulation and oxygen levels in the body during surgery and is commonly used in cardiac surgery. During cardiopulmonary bypass, an adequate priming solution allows the pretreatment of the line, oxygenator, and blood pump, draining the air in the arterial duct, and performing proper blood dilution [1]. However, the priming solution may affect the physicochemical and homeostasis balance in the blood and alter the metabolic response when CPB is used in cardiac surgery [2]. Therefore, finding a suitable priming fluid is very important for patients who need CPB. Despite extensive research and discussion on CPB priming solutions in the past decades, there is still no consensus and agreement on the best optimal CPB priming solution [3].
Owing to surgical requirements, most cardiothoracic surgeries still rely on the use of CPB, and the selection of appropriate priming fluid is a crucial prerequisite for the initiation of CPB [4]. Two types of priming fluids are commonly used in clinics: crystalloids and colloids. Crystalloid priming solutions usually consist of full electrolyte solutions, rarely of glucose, and often have additional additives such as mannitol. Colloid priming solutions include human albumin (HA), dextran, gelatin, and hydroxyethyl starch (HES) [5]. The effectiveness of these fluids during CPB varies, with both advantages and disadvantages [6]. For example, the use of HES in CPB may significantly affect the clotting system, and HA does not cause clotting dysfunction compared to HES [6]. However, the high cost of albumin must be considered [7]. Hemodilution occurs when the crystalloids are used as priming fluids, reducing colloid osmotic pressure and causing interstitial oedema. Although the colloid can maintain osmotic pressure, it increases the formation of oedema when they migrate into the interstitium. In addition, the use of colloids can also cause non-surgical bleeding [8]. In addition, the marketing authorisation for HES infusion solutions was suspended by the European Commission in 2022 due to the risk of renal injury and death in patients with critically ill sepsis. However, there have always been different opinions about whether to using of HES. Studies have demonstrated conflicting evidence regarding the best choice for CPB with colloids, crystalloids, and different types of fluids in these categories [9]. Thus, although studies on various types of crystalloids and colloids have been conducted for at least 30 years, direct guidelines are lacking on the type of priming fluid to choose for CPB in adults [5].
Meta-analyses of the priming fluids used in CPB are scarce. Previous studies [10–13] on the effect of priming fluid are traditional meta-analyses. No study has comprehensively compared the effects of using various types of starting fluid in adults. This network meta-analysis (NMA) will evaluate the effects of eight fluids used during CPB in adults undergoing cardiac surgery based on platelet count, mortality, mean CPB time, urine output 24h after surgery, length of intensive care unit (ICU) and hospital stay, blood loss 24h after surgery, and chest tube output within 24h after surgery, and further provide more comprehensive advice for the choice of priming fluid in CPB surgery.
2. Materials and methods
The NMA was developed using the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Network Meta-Analyses (PRISMA-NMA) guidelines [14], and this NMA was registered at PROSPERO (CRD42023416194).
2.1. Literature search and selection criteria
Relevant clinical studies were obtained by searching for studies published by Ovid MEDLINE(R) ALL, OVID EMbase, and Cochrane Central Register of Controlled Trials before 13 April 2023. The search terms included priming fluid, colloid, crystalloid, albumin, gelatin, hetastarch, plasma and dextran. Supplementary Method 1 showed detailed electronic search strategies.
Two independent reviewers (Chen-Yang Xian-Yu and Yu-Tong Ma) investigated the qualified title, abstract, and full-text content, and addressed differences in opinion through discussion. If there were any disagreements, a third reviewer (Chao Zhang) was consulted.
2.2. Inclusion and exclusion criteria
Included clinical researches met the following criteria: (1) Population: All adults who underwent cardiac surgery; (2) Interventions: Various priming fluids were classified into eight categories, including balanced crystalloids (including lactated ringer’s, ringer acetate or plasmalytes), 0.9% NaCl, iso-oncotic HA (including 4% iso-oncotic HA or 5% iso-oncotic HA), hyperoncotic HA (25%), HES with molecular weight 130k (L-HES), HES with molecular weight 200k (H-HES), gelatin (including 3.5% gelatin, 4% gelatin or gelofusine), and dextran; (3) Outcomes: Platelet count, mortality, mean CPB time, urine output 24h after surgery, length of ICU and hospital stay, blood loss 24h after surgery, and chest tube output within 24h after surgery; and (4) study design: All included clinical researches were randomised controlled trials (RCTs). The exclusion criteria were as follows: (1) missing and incomplete study data; (2) repetitive studies; and (3) drug combinations.
2.3. Data extraction and quality assessment
Two authors (Chen-Yang Xian-Yu and Yu-Tong Ma) independently extracted relevant information and data, including details of the year, study design, participants, interventions, and all outcomes. For the missing data of outcomes, the original study authors for clarification were contacted by email address. Finally, all arguments were resolved by an investigator (Chao Zhang).
The five aspects, including the randomisation process, deviations from intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result, based on a revised tool for assessing the risk of bias in randomised trials from Cochrane handbook (RoB-2) [15] were employed.
2.4. Statistical analysis
The binary classification data were represented by the relative risk (RR) with a 95% confidence interval (CI). Continuous data were represented by the standard mean difference (SMD) or mean difference (MD) with 95% CI. If a study only reported the sample median and range or the first and third quartiles in continuous data, we estimated the sample mean and standard deviation using calculation [16–18]. Heterogeneity between studies was evaluated by I2 statistic. I2 ≥40% denoted significant heterogeneity, and the random-effects model was involved in all outcomes.
In the statistical model of NMA, a design-by-treatment interaction model was employed [19]. The ‘loop inconsistency’ is applied to evaluate network inconsistencies [20]. The surface under the cumulative ranking curve (SUCRA) was employed for providing summary statistics of the cumulative ranking of all priming fluids [21]. The STATA 15.0 was performed for all statistical analyses of NMA.
3. Results
3.1. Literature identification
We conducted an extensive search for studies published on Ovid MEDLINE(R) ALL, OVID EMbase, and Cochrane Central Register of Controlled Trials before 13 April 2023 and identified 27,983 studies. After preliminary screening, 24,557 studies met the requirements and were included, while 24,441 studies did not unqualify the requirements. Finally, 21 studies were selected after careful reading of the full articles. Figure 1 shows the work of selected studies.
Figure 1.
Study selection.
3.2. Study characteristics
Twenty-one studies were included [22–42]. The basic characteristics of included studies, including age, sex, type of surgery, type of priming fluid, and dose of priming fluid, are shown in Table 1. Of these, 1457 participants were older than 18 years, and all studies were mixed-sex population trials. Eight interventions were used: balanced crystalloids, 0.9% NaCl, iso-oncotic HA, hyperoncotic HA, L-HES, H-HES, gelatin, and dextran. All studies evaluated one or more of the following phenomena: platelet count, mortality, mean CPB time, urine output 24h after surgery, length of ICU and hospital stay, blood loss 24h after surgery, and chest tube output within 24h after surgery. These phenomena were summarised and studied to determine the best priming fluid. The results of RoB-2 are shown in Supplementary Table 1.
Table 1.
Summary basic characteristics of included studies.
| Study | Year of publication | Sample size | Mean age (SD) | Gender (M/F) | Type of cardiac surgery | Type 1 of priming fluid | Type 2 of priming fluid | Type 3 of priming fluid | Volume of priming fluid |
|---|---|---|---|---|---|---|---|---|---|
| Barbu | 2020 | 39/41 | 65.8(6.1)/67.0(7.6) | 28/11;29/12 | Elective cardiac surgery | Dextran-based solution | Ringer acetate | NR | 1300 ml |
| Choi | 2010 | 18/18 | 54(12)/55(14) | 5/13;6/12 | Elective mitral valvular heart surgery | 6% HES 130/0.4 | 5% HA | NR | 500 ml |
| Gurbuz | 2013 | 100/100 | 61.52(9.29)/61.81(10.12) | 74/26;77/23 | CABG surgery | 6% HES 130/0.4 | Balanced multielectrolyte solution | NR | NR |
| Kamra | 2013 | 10/10 | 55(18.8)/59(7.1) | 9/1;9/1 | CABG surgery | Plasmalytes | 5% HA | NR | NR |
| Kolsrud | 2022 | 39/41 | 65.8(6.1)/67.7(7.6) | 28/11;29/12 | Elective cardiac surgery | Dextran 40 | Ringer-acetate plus mannitol | NR | 1300 ml |
| Kuitunen | 2004 | 15/15/15 | 57/60/58 | 13/2;14/1;15/0 | Elective CABG surgery | 4% HA | 6% HES 120 | 6% HES 400 | 20 ml/kg |
| Kuitunen | 1993 | 15/15/15 | 57(2)/57(2)/57(2) | 12/3;12/3;12/3 | Elective primary CABG | Low molecular weight-HES | High molecular weight-HES | Ringer’s solution | 2000 ml |
| Linden | 2004 | 55/55 | 63(8)/63(11) | 35/20;37/18 | Elective coronary artery or single valve surgery | 6% HES 200/0.5 | 3.5% UREA-linked gelatin | NR | 30 ± 3 ml/kg/day |
| Liou | 2012 | 10/10/10 | 69.8(2.7)/62.4(2.9)/64.1(4.3) | 7/3;8/2;9/1 | Elective CABG surgery | 10% HES 200/0.5 | Ringer’s solution | 25% HA | 950 ml |
| Lou | 2012 | 35/35 | 49.9(13.2)/51.5(13.7) | 23/12;19/16 | Elective first-time cardiac surgery | 6% HES 130/0.4 | 4% Gelatin | NR | NR |
| Maleki | 2016 | 30/30 | 61.85(9.10)/66.07(8.82) | 17/13;21/9 | Cardiopul monary CABG surgery | 6% HES 130/0.4 | 5% HA | NR | NR |
| Min | 2013 | 45/45 | 56.4(10.7)/59.7(9.0) | 39/6;36/9 | Elective CABG surgery | 6% HES 130/0.4 | 4% succinylated gelatin | NR | HES: 1,942.3 ± 1,046.1 ml, Gelatin: 1973.3 ± 728.8 ml |
| Moerman | 2016 | 20/20 | 65(9)/68(10) | 15/5;18/2 | Elective CABG surgery | 6% HES 130/0.4 | Modified fluid gelatin | NR | NR |
| Schramko | 2015 | 19/16 | 73.6(7.8)/73.6(6.4) | 13/6;8/7 | CABG or Valve procedure | 6% HES 130/0.42 | Ringer’s acetate solution | NR | 2000 ml |
| Schweizer | 2019 | 15/15 | >18 | / | Elective conventional adult cardiac surgery | 6% HES 130/0.4 | Sodium chloride (NaCl) 0.9% | NR | 1000 mL |
| Shahbazi | 2010 | 35/35 | 57.4(9.3)/57.6(9.0) | 26/9;21/14 | Elective CABG surgery | HES 130/0.4 | Ringer’s lactate | NR | NR |
| Svendsen | 2018 | 20/18 | 67(10)/62(10) | 17/3;18/0 | Elective CABG surgery | 6% HES 130/0.42 | Ringer’s solution | NR | 1700 ml |
| Tamayo | 2008 | 22/22 | 67.8(8.1)/66.50(6.9) | 19/3;17/5 | Elective CABG surgery | Gelatin-containing solution | Ringer’s lactate | NR | NR |
| Tiryakioğlu | 2008 | 70/70 | 56(8)/58(7) | 60/10;60/10 | CABG surgery | HES 130/0.4 | Ringer solution | NR | 1500 ml |
| Vanhoonacker | 2009 | 82/72 | 67.31(9.30)/66.34(9.51) | 66/16;56/16 | CABG surgery | 6% HES 130/0.4 | Modified fluid gelatin | NR | 1500 ml |
| Yap | 2007 | 20/20 | 59/63 | 17/3;17/3 | CABG surgery | HES 130/0.4 | Gelofusine | NR | NR |
HA: Human albumin; HES: Hydroxyethyl starch; CABG: Coronary artery bypass graft; NR: Not reported.
3.3. Result of NMA
3.3.1. Platelet count
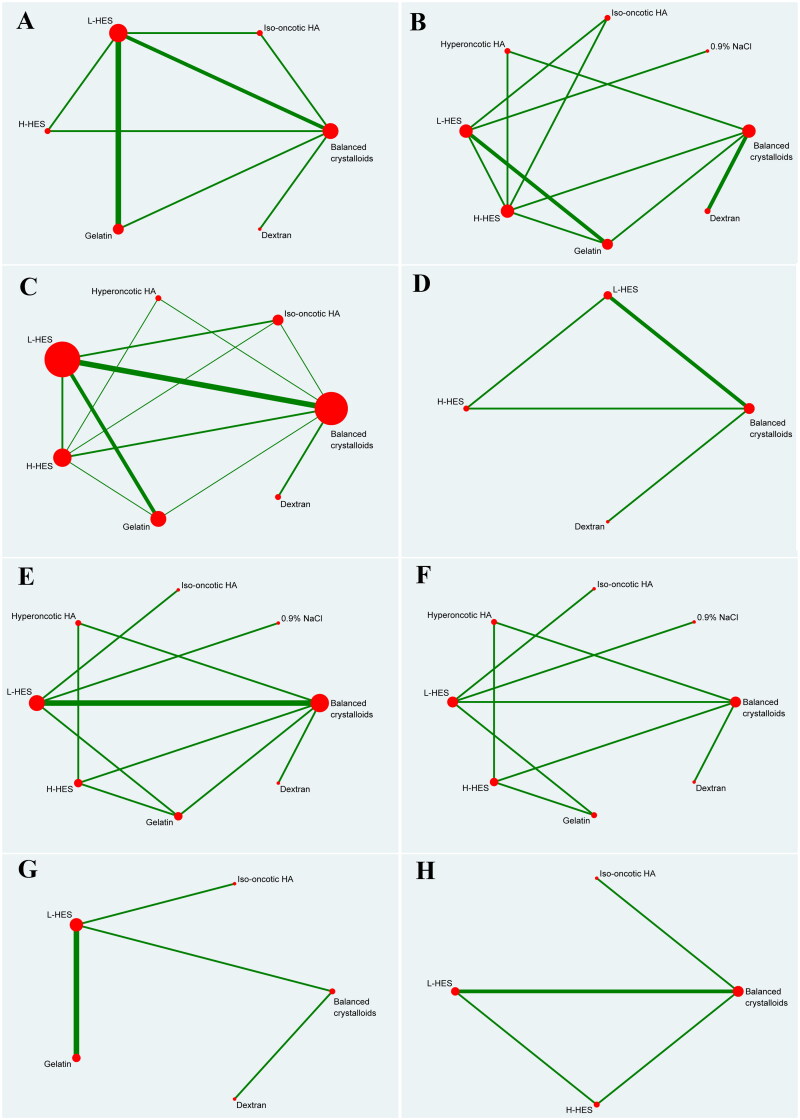
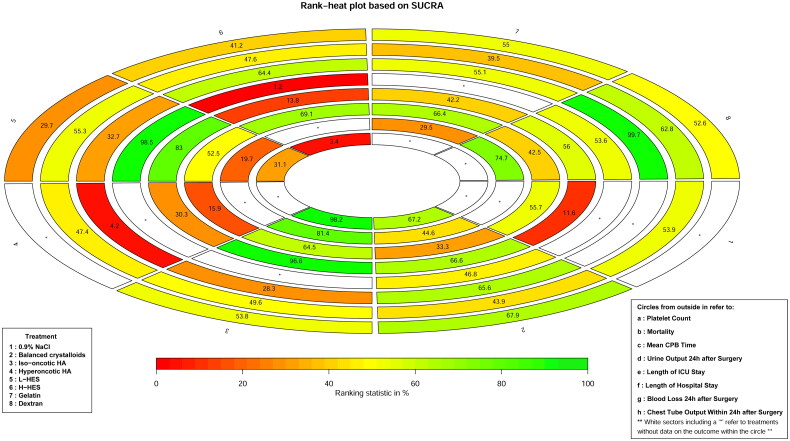
Nine studies [23,26–29,31,34,35,38] included data on platelet count. Based on the six priming fluids, Figure 2(A) shows the qualified network diagram of the platelet count for balanced crystalloids, iso-oncotic HA, L-HES, H-HES, gelatin, and dextran. No inconsistencies are found, except between balanced crystalloids, L-HES, and H-HES, as is shown in Supplementary Figure 1(A). Table 2 presents all results regarding platelet counts, including the results of network comparison and direct comparison. All network results did not show statistical significance. In the direct results, only L-HES as bridge intervention vs. gelatin (SMD = −0.40, 95%CI: −0.63, −0.16) is statistically significant, and the other results show no significant statistical difference. All interventions are ranked according to SUCRA (Figure 3), with the highest platelet counts being observed in balanced crystalloids, followed by gelatin, and the lowest being observed in L-HES, followed by H-HES. In addition, no publication bias with respect to platelet count is observed, as is shown in Supplementary Figure 2(A).
Figure 2.
Network plot of priming fluids for all outcomes.
Table 2.
Results of network and traditional paired meta-analysis for platelet count.
| Balanced crystalloids | 0 (0, 0) | 1.32 (−2.76, 5.40) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| 0.27 (−1.63, 2.18) | Iso-oncotic HA | 0 (0, 0) | – | – | – |
| 0.73 (−0.65, 2.11) | 0.46 (−1.44, 2.35) | L-HES | 0 (0, 0) | −0.40 (−0.63, −0.16) | – |
| 0.66 (−1.60, 2.93) | 0.39 (−2.40, 3.18) | −0.06 (−2.32, 2.19) | H-HES | – | – |
| 0.23 (−1.37, 1.83) | −0.04 (−2.21, 2.13) | −0.50 (−1.76, 0.77) | −0.43 (−2.93, 2.06) | Gelatin | – |
| 0.36 (−2.06, 2.78) | 0.09 (−2.99, 3.17) | −0.37 (−3.16, 2.42) | −0.30 (−3.62, 3.01) | 0.13 (−2.77, 3.03) | Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin; L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
Figure 3.
Ranking of priming fluids for all outcomes.
3.3.2. Mortality
Nine studies [22,25,26,30,35,36,38,40,41] included data on mortality. Based on the eight priming fluids, Figure 2(B) demonstrates a qualified network diagram of mortality for balanced crystalloids, 0.9% NaCl, iso-oncotic HA, hyperoncotic HA, L-HES, H-HES, gelatin, and dextran. Supplementary Figure 1 (B)shows that there are no inconsistencies in the number of patients who died. All results for mortality are shown in Table 3, and those including network comparisons and direct comparisons are not statistically significant. All interventions are ranked according to SUCRA (Figure 3), with the lowest mortality rate being observed in dextran, followed by L-HES, and the highest in gelatin, followed by balanced crystalloids. In addition, no publication bias with respect to mortality is observed, as is shown in Supplementary Figure 2 (B).
Table 3.
Results of network and traditional paired meta-analysis for mortality.
| Balanced crystalloids | – | – | 1 (1, 1) | – | 1 (1, 1) | 1 (1, 1) | 1.90 (0.36, 10.09) |
| 1.53 (0.01, 273.39) | 0.9% NaCl | – | – | 1 (1, 1) | – | – | – |
| 1.31 (0.02, 111.33) | 0.85 (0.00, 168.79) | Iso-oncotic HA | – | 1 (1, 1) | 1 (1, 1) | – | – |
| 1.05 (0.03, 40.28) | 0.69 (0.00, 224.05) | 0.81 (0.01, 121.83) | Hyperoncotic HA | – | 1 (1, 1) | – | – |
| 1.53 (0.05, 49.00) | 1.00 (0.02, 47.35) | 1.17 (0.03, 43.75) | 1.45 (0.02, 108.40) | L-HES | 1 (1, 1) | 0.33 (0.01, 7.72) | – |
| 1.11 (0.05, 22.62) | 0.73 (0.01, 84.24) | 0.85 (0.02, 31.76) | 1.05 (0.03, 40.28) | 0.73 (0.05, 11.69) | H-HES | 1 (1, 1) | – |
| 0.90 (0.04, 18.47) | 0.58 (0.01, 49.51) | 0.69 (0.01, 33.16) | 0.85 (0.01, 50.60) | 0.58 (0.06, 5.26) | 0.81 (0.06, 10.91) | Gelatin | – |
| 1.90 (0.36, 10.09) | 1.24 (0.01, 287.90) | 1.46 (0.01, 168.19) | 1.80 (0.03, 99.14) | 1.24 (0.03, 58.10) | 1.71 (0.05, 53.52) | 2.12 (0.07, 67.27) | Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin, L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
3.3.3. Mean CPB time
A total of 18 studies [22,23,25–27,29–39,41,42] included data on the mean CPB time. Based on the seven priming fluids, Figure 2(C) shows the qualified network diagram of the mean CPB time for balanced crystalloids, iso-oncotic HA, hyperoncotic HA, L-HES, H-HES, gelatin, and dextran. Supplementary Figure 1 (C) shows that there are no inconsistencies in the mean CPB time. All results for the mean CPB time are shown in Table 4. In the network results, these comparisons, including L-HES vs. dextran as a bridge intervention (MD = 19.77, 95%CI: 8.22, 31.33) exhibit a significant difference. Moreover, balanced crystalloids vs. hyperoncotic HA (MD = −15.15, 95%CI: −28.06, −2.24), balanced crystalloids vs. dextran (MD = 16.00, 95%CI: 5.33, 26.67), iso-oncotic HA vs. dextran (MD = 23.47, 95%CI [5.93, 41.01), hyperoncotic HA vs. H-HES (MD = 15.14, 95%CI: 2.75, 27.52), hyperoncotic HA vs. gelatin (MD = 14.10, 95%CI: 0.36, 27.84), hyperoncotic HA vs. dextran (MD = 31.15, 95%CI: 14.41, 47.90), H-HES vs. dextran (MD = 16.01, 95%CI: 3.81, 28.21), and gelatin vs. dextran (MD = 17.05, 95%CI: 4.51, 29.59), exhibited significant difference. In the direct results, only the comparison of balanced crystalloids vs. dextran (MD = 16.00, 95%CI: 5.90, 26.10) reveals a significant difference. All interventions are ranked according to SUCRA (Figure 3), with the shortest mean CPB time being observed in dextran, followed by balanced crystalloids, and the longest mean CPB time being observed in hyperoncotic HA, followed by iso-oncotic HA. Furthermore, no publication bias regarding the mean CPB time is observed, as is shown in Supplementary Figure 2 (C).
Table 4.
Results of network and traditional paired meta-analysis for mean CPB time.
| Balanced crystalloids | 0(0, 0) | 0(0, 0) | −4.83 (−11.41, 1.76) | 2.07 (−3.16, 7.29) | 0(0, 0) | 16.00 (5.90, 26.10) |
| −7.47 (−21.40, 6.46) | Iso-oncotic HA | – | 1.18 (−15.54, 17.89) | 0(0, 0) | – | – |
| −15.15 (−28.06, −2.24) | −7.68 (−26.38, 11.01) | Hyperoncotic HA | – | 0(0, 0) | – | – |
| −3.77 (−8.22, 0.67) | 3.69 (−10.08, 17.47) | 11.38 (−1.68, 24.43) | L-HES | 1.89 (−3.80, 7.57) | 3.23 (−2.84, 9.30) | – |
| −0.01 (−5.93, 5.91) | 7.46 (−7.15, 22.07) | 15.14 (2.75, 27.52) | 3.76 (−1.96, 9.48) | H-HES | 0(0, 0) | – |
| −1.05 (−7.65, 5.55) | 6.42 (−8.34, 21.18) | 14.10 (0.36, 27.84) | 2.73 (−2.88, 8.33) | −1.04 (−8.00, 5.93) | Gelatin | – |
| 16.00 (5.33, 26.67) | 23.47 (5.93, 41.01) | 31.15 (14.41, 47.90) | 19.77 (8.22, 31.33) | 16.01 (3.81, 28.21) | 17.05 (4.51, 29.59) | Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin; L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
3.3.4. Urine output 24h after surgery
Three studies [25,34,37] included data on urine output 24h after surgery. Based on the four priming fluids, Figure 2(D) provides a qualified network diagram for urine output 24h after surgery for balanced crystalloids, L-HES, H-HES, and dextran. No inconsistencies are found, except between balanced crystalloids, L-HES, and H-HES, as is shown in Supplementary Figure 1 (D). All results regarding urine output 24h after surgery are shown in Table 5. In the network results, the comparisons, including balanced crystalloids vs. L-HES as a bridge intervention (MD = −290.94, 95%CI: −492.44, −89.43), and L-HES vs. H-HES (MD = 592.81, 95%CI: 390.12, 795.50), reveal significant difference. In addition, balanced crystalloids vs. H-HES (MD = 301.87, 95%CI: 57.37, 546.37) is statistically significant. The direct results reveal no significant statistical differences in all results. All interventions are ranked according to SUCRA (Figure 3), with the lowest urine output 24h after surgery using the H-HES, followed by balanced crystalloids, and the highest from the L-HES group, followed by dextran. Furthermore, no publication bias regarding urine output 24h after surgery is observed, as is shown in Supplementary Figure 2 (D).
Table 5.
Results of network and traditional paired meta-analysis for urine output 24h after surgery.
| Balanced crystalloids | −72.44 (−904.46, 759.58) | 0(0, 0) | 0(0, 0) |
| −290.94 (−492.44, −89.43) | L-HES | 0(0, 0) | – |
| 301.87 (57.37, 546.37) | 592.81 (390.12, 795.50) | H-HES | – |
| −26.00 (−257.94, 205.94) | 264.94 (−42.31, 572.18) | −327.87 (−664.88, 9.14) | Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin, L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
3.3.5. Length of ICU stay
Ten studies [22,23,25,31,32,35,38,40–42] included data on length of ICU stay. Based on the eight priming fluids, Figure 2(E) exhibits the qualified network diagram of the length of ICU stay for balanced crystalloids, 0.9% NaCl, iso-oncotic HA, hyperoncotic HA, L-HES, H-HES, gelatin, and dextran. Supplementary Figure 1 (E) shows an inconsistency between balanced crystalloids, H-HES and gelatin and no inconsistency between balanced crystalloids, L-HES and gelatin. All results regarding the length of ICU stay are shown in Table 6. In the network results, the comparisons, including balanced crystalloids vs. L-HES as a bridge intervention (MD = 2.15, 95%CI: 0.06, 4.24), 0.9% NaCl vs. L-HES (MD = 27.64, 95%CI: 1.94, 53.34), hyperoncotic HA vs. L-HES (MD = 14.78, 95%CI: 1.87, 27.69), and L-HES vs. H-HES (MD = −22.50, 95%CI: −38.81, −6.19), reveal significant differences. In addition, the comparisons, including balanced crystalloids vs. H-HES (MD = −20.35, 95%CI: −36.71, −3.99), 0.9% NaCl vs. iso-oncotic HA (MD = 34.81, 95%CI: 6.83, 62.79), iso-oncotic HA vs. hyperoncotic HA (MD = −21.95, 95%CI: −38.95, −4.95), iso-oncotic HA vs. H-HES (MD = −29.67, 95%CI: −49.38, −9.96), and iso-oncotic HA vs. gelatin (MD = −17.45, 95%CI: −33.50, −1.40), reveal significant differences. In the direct results, only the interaction of balanced crystalloids vs. L-HES (MD = 2.31, 95%CI: 0.21, 4.41) reveals significant differences. All interventions are ranked according to SUCRA (Figure 3), with the shortest ICU stay being observed in iso-oncotic HA, followed by L-HES, and the longest being observed in 0.9% NaCl, followed by H-HES. Furthermore, no publication bias regarding the length of ICU stay is observed, as is shown in Supplementary Figure 2 (E).
Table 6.
Results of network and traditional paired meta-analysis for length of ICU stay.
| Balanced crystalloids | – | – | 0 (0, 0) |
2.31
(0.21, 4.41) |
0 (0, 0) |
0 (0, 0) |
0 (0, 0) |
| −25.49 (−51.27, 0.29) |
0.9% NaCl | – | – | 0 (0, 0) |
– | – | – |
| 9.32 (−1.95, 20.58) |
34.81
(6.83, 62.79) |
Iso-oncotic HA | – | 0 (0, 0) |
– | – | – |
| −12.63 (−25.37, 0.11) |
12.86 (−15.90, 41.61) |
−21.95
(−38.95, −4.95) |
Hyperoncotic HA | – | 0 (0, 0) |
– | – |
|
2.15
(0.06, 4.24) |
27.64
(1.94, 53.34) |
−7.17 (−18.24, 3.90) |
14.78
(1.87, 27.69) |
L-HES | – | 0 (0, 0) |
– |
|
−20.35
(−36.71, −3.99) |
5.14 (−25.29, 35.57) |
−29.67
(−49.38, −9.96) |
−7.72 (−28.08, 12.64) |
−22.50
(−38.81, −6.19) |
H-HES | 0 (0, 0) |
– |
| −8.13 (−19.85, 3.59) |
17.36 (−10.84, 45.56) |
−17.45
(−33.50, −1.40) |
4.50 (−12.60, 21.61) |
−10.28 (−21.90, 1.35) |
12.22 (−0.16, 24.60) |
Gelatin | – |
| −4.00 (−17.27, 9.27) |
21.49 (−7.51, 50.49) |
−13.32 (−30.73, 4.09) |
8.63 (−9.77, 27.03) |
−6.15 (−19.59, 7.29) |
16.35 (−4.72, 37.42) |
4.13 (−13.58, 21.84) |
Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin; L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
3.3.6. Length of hospital stay
Seven studies [22,23,25,35,40–42] included data on the length of hospital stay. Based on the eight priming fluids, Figure 2(F) shows the qualified network diagram of the length of hospital stay for balanced crystalloids, 0.9% NaCl, iso-oncotic HA, hyperoncotic HA, L-HES, H-HES, gelatin, and dextran. Supplementary Figure 1 (F) shows that there are no inconsistencies in the length of stay. All results regarding the length of hospital stay are shown in Table 7, and those including network comparisons and direct comparisons are not significant. All interventions are ranked according to the SUCRA (Figure 3), with the shortest hospital stay being observed in H-HES, followed by gelatin, and the longest being observed in hyperoncotic HA, followed by balanced crystalloids. Furthermore, no publication bias regarding patient hospital stay is observed, as is shown in Supplementary Figure 2 (F).
Table 7.
Results of network and traditional paired meta-analysis for length of hospital stay.
| Balanced crystalloids | – | – | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | – | 0 (0, 0) |
| 0.59 (−4.07, 5.26) | 0.9% NaCl | – | – | 0 (0, 0) | – | – | – |
| 0.72 (−1.57, 3.02) | 0.13 (−4.99, 5.25) | Iso-oncotic HA | – | 0 (0, 0) | – | – | – |
| −1.05 (−3.38, 1.29) | −1.64 (−6.84, 3.56) | −1.77 (−5.02, 1.48) | Hyperoncotic HA | – | 0 (0, 0) | – | – |
| 0.32 (−0.32, 0.97) | −0.27 (−4.89, 4.35) | −0.40 (−2.60, 1.80) | 1.37 (−1.02, 3.77) | L-HES | – | 0 (0, 0) | – |
| 0.73 (−0.42, 1.87) | 0.13 (−4.65, 4.91) | 0.00 (−2.52, 2.52) | 1.77 (−0.44, 3.98) | 0.40 (−0.83, 1.63) | H-HES | 0 (0, 0) | – |
| 0.60 (−0.47, 1.67) | 0.01 (−4.71, 4.72) | −0.12 (−2.51, 2.26) | 1.65 (−0.83, 4.12) | 0.28 (−0.65, 1.20) | −0.13 (−1.46, 1.21) | Gelatin | – |
| 0.10 (−1.07, 1.27) | −0.49 (−5.30, 4.31) | −0.62 (−3.20, 1.95) | 1.15 (−1.47, 3.76) | −0.22 (−1.56, 1.11) | −0.63 (−2.26, 1.01) | −0.50 (−2.08, 1.08) | Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin; L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
3.3.7. Blood loss 24h after surgery
Six studies [24,26–28,33,35] included data on blood loss 24h after surgery. Based on the five priming fluids, Figure 2(G) displays a qualified network diagram of blood loss 24h after surgery for balanced crystalloids, iso-oncotic HA, L-HES, gelatin, and dextran. All results regarding blood loss 24h after surgery are shown in Table 8, and those including network comparisons and direct comparisons are not statistically significant. All interventions are ranked according to the SUCRA (Figure 3), with the lowest postoperative transfusion at 24h being observed in iso-oncotic HA, followed by dextran, and the highest being observed in L-HES, followed by gelatin. Furthermore, no publication bias regarding blood loss 24h after surgery is observed, as is shown in Supplementary Figure 2 (G).
Table 8.
Results of network and traditional paired meta-analysis for blood loss 24h after surgery.
| Balanced crystalloids | – | 0 (0, 0) | – | 0 (0, 0) |
| 107.24 (−66.26, 280.74) | Iso-oncotic HA | 0 (0.0) | – | – |
| −44.38 (−147.76, 58.99) | −151.62 (−332.74, 29.49) | L-HES | 11.49 (−96.25, 119.23) | – |
| −31.25 (−161.31, 98.82) | −138.49 (−336.84, 59.86) | 13.14 (−78.05, 104.32) | Gelatin | – |
| 110.00 (−121.91, 341.91) | 2.76 (−286.87, 292.38) | 154.38 (−99.52, 408.29) | 141.25 (−124.64, 407.14) | Dextran |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin; L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
3.3.8. Chest tube output within 24h after surgery
Three studies [29,34,37] included data on chest tube output within 24h after surgery. Based on the four priming fluids, Figure 2(H) offers a qualified network diagram of chest tube output within 24h after surgery for balanced crystalloids, iso-oncotic HA, L-HES, and H-HES. Supplementary Figure 1 (G) shows that there is no inconsistency in the chest tube output within 24h after surgery. All results regarding chest tube output within 24h after surgery are shown in Table 9. In the network results, the comparisons, including balanced crystalloids vs. L-HES as a bridge intervention (MD = −73.26, 95%CI: −143.40, −3.13) and iso-oncotic HA vs. L-HES (MD = −228.26, 95%CI: −428.56, −27.97), reveal significant differences. In addition, the comparisons, including balanced crystalloids vs. H-HES (MD = −144.28, 95%CI: −251.47, −37.10) and iso-oncotic HA vs. H-HES (MD = −299.28, 95%CI: −515.35, −83.21), reveal significant differences. In the direct results, the comparison of balanced crystalloids vs L-HES (MD = −73.26, 95%CI: −143.40, −3.13) reveals a significant difference. All interventions are ranked according to SUCRA (Figure 3), with the lowest 24h postoperative chest tube output being observed in iso-oncotic HA, followed by balanced crystalloids, and the highest being observed in H-HES, followed by L-HES. Furthermore, no publication bias regarding patient chest tube output within 24h after surgery is observed, as is shown in Supplementary Figure 2 (H).
Table 9.
Results of network and traditional paired meta-analysis for chest tube output within 24h after surgery.
| Balanced crystalloids | 0(0, 0) | −73.26 (−143.40, −3.13) | 0(0, 0) |
| 155.00 (−32.61, 342.61) | Iso-oncotic HA | – | – |
| −73.26 (−143.40, −3.13) | −228.26 (−428.56, −27.97) | L-HES | 0(0, 0) |
| −144.28 (−251.47, −37.10) | −299.28 (−515.35, −83.21) | −71.02 (−175.79, 33.76) | H-HES |
Comparison results between priming fluids should be interpreted from column to row, the priming fluids on the column is the intervention group and the priming fluids on the row is the control group. Result of bold and underline is statistically significant. HA: human albumin; L-HES: hydroxyethyl starch with molecular weight 130k; H-HES: hydroxyethyl starch with molecular weight 200k; –: not available.
4. Discussion
This NMA evaluated the clinical benefit of different priming fluids during CPB in adults undergoing cardiac surgery. When dextran as the priming fluid was associated with the lowest mortality, and the shortest mean CPB time. When iso-oncotic HA was used as a priming fluid, the ICU stay was the shortest, the amount of blood loss 24h after surgery was the lowest, and the chest tube output within 24h after surgery was the lowest. In addition, patients with balanced crystalloids had the highest platelet counts, those using L-HES had the highest postoperative urine volume, and those using H-HES had the shortest hospital stay.
4.1. Crystalloids: balanced crystalloids and saline
The NMA demonstrated that the balanced crystalloids had a favourable effect on platelet counts when used as CPB priming fluid, with shorter mean CPB time and less chest tube output in the 24h after surgery. While 0.9% NaCl was performed generally, patients using it had the longest ICU stay. A study found that crystal priming induces an increase in platelet activation, whereas albumin priming results in a rapid decrease in platelet count [43]. In addition, HES alters platelet function by reducing plasma von willebrand factor levels and coating platelet membranes [34]. The HES solution as a priming fluid may increase the tendency of patients to bleed due to fibrinolysis after CPB [44]. These results are consistent with those of our study, in which crystalloids significantly outperformed other colloids in terms of platelet count. This also marked a significant decrease in the incidence of nonsurgical bleeding due to platelet dysfunction in patients using balanced crystalloids.
4.2. Human albumin: iso-oncotic and hyperoncotic Human albumin
Compared with other fluids, patients who used iso-oncotic HA had the shortest ICU stay, the least blood loss at 24h after surgery, and the lowest chest tube output within 24h after surgery. In contrast to the good results of iso-oncotic HA, hyperoncotic HA had the longest hospital stay and mean CPB time, which clearly indicated the poor effect of hyperoncotic HA. Albumin is an ideal humanized colloid with minimal side effects while having less effect on platelet count [45]. Compared to synthetic colloids, it can better reduce the risk of bleeding after CPB [46]. Total chest tube drainage would decrease the platelet count and decrease aggregation in patients undergoing CPB surgery [44]. This is identical to our finding that iso-oncotic HA during CPB is associated with the least output from the thoracic duct within 24h after surgery, resulting in less postoperative nonoperative bleeding due to platelet depletion and dysfunction. Furthermore, the reduction in urine volume due to the use of albumin is associated with osmotic pressure. The osmolality of iso-oncotic HA was analogous to that of plasma osmolality, and hyperoncotic HA was higher than plasma osmolality [47]. Compared with iso-oncotic HA, hyperoncotic HA increases osmotic pressure higher, leading to intraglomerular oncotic force changes or severe renal injury, such as osmotic nephropathy [48]. However, no results of either iso-oncotic HA or hyperoncotic HA regarding urine output were collected in this study.
4.3. HES: L-HES and H-HES
L-HES and H-HES exhibited high chest output and significantly lower platelet counts 24h after surgery than those of the other assessed agents. This indicates that patients using HES are more prone to nonsurgical bleeding due to platelet dysfunction than patients using other fluids. A study on the effect of HES on postoperative haemostatic function noted that HES (120 and 400) were associated with prolonged thrombosis, reduced clot stiffness, and large blood loss from chest drainage in patients undergoing cardiac surgery [30]. This was also evidenced by the maximum 24h blood loss of L-HES in all fluids in this study. In one study, HES was associated with an increased use of allogeneic blood products [49]. This may suggest that patients who use H-HES have a higher risk of allogeneic blood exposure. Furthermore, there is substantial evidence that the use of HES is associated with renal dysfunction in various types of critically ill patients [50]. This study also showed that patients primed with L-HES had more urine volume at 24h after surgery, while those primed with H-HES had significantly less urine. Overall, H-HES is not recommended as a priming fluid. In daily surgical practice, the HES with high molecular weight has been withdrawn from the market, while the HES of low molecular weight is still used [50].
4.4. Gelatin and dextran
No significant advantage was observed with the use of gelatin as a priming fluid. Notably, it had the highest rate of death among all fluids. A meta-analysis demonstrated that gelatin increased the risk of allergic reactions, mortality, kidney failure and bleeding [51]. In addition, the use of gelatin as a priming fluid is more expensive than other liquids. In contrast, dextran is a more effective and safer priming fluid. This study found that patients using dextran had lower mortality, shorter mean CPB, and less blood loss 24h after surgery. In addition, no other obvious disadvantages are observed. Clinical research found that compared with albumin, the extra did not compromise organ function, and no significant difference in blood loss or transfusion volume [26]. Normally, high doses of artificial colloids impair haemostatic function, but dextran does not [26]. However, the use of dextran may also lead to allergies. Studies have shown that the incidence of allergic reactions to dextran was 21.9 per 100,000 injections (0.0219%) [52].
Inconsistencies were found in the three outcomes of platelet count, urine output 24h after surgery, and length of ICU stay. Among them, the time of 24h postoperative urine output was not all 24h, and different units were used in different studies of platelet count. In addition, different doses of priming fluid were used and different types of surgery were performed by patients in each of the three outcome studies. These factors might account for the inconsistency in outcomes.
Of concern, a meta-analysis [53] provided that HES was associated with a significantly increased risk of acute kidney injury (AKI) and death, a significant change from the original results after the German anesthesiologist Joachim Boldt’s trial was excluded. Since then, Boldt has had nearly 90 fraudulent studies withdrawn [54]. A consensus recommendation from the European Society of Intensive Care Medicine states that HES with high molecular weight should not be recommended to patients with severe sepsis or patients with a risk of AKI [55]. On 24 May 2022, the European Commission confirmed the suspension of marketing authorisation for HES solutions of infusion. These events had an impact on the recommendation of priming fluid in clinical evidence, HES will no longer be recommended, and literature involving fraud was excluded from this study.
4.5. Clinical implications
This NMA examined previous RCTs of priming fluids and compared the effects of eight fluids commonly used in CPB. The effects and safety of these eight priming fluids in CPB were different and had both disadvantages and advantages. Among them, iso-oncotic HA and dextran showed relatively better efficacy and safety than those of the remaining fluids, whereas six other liquids were found to have significant drawbacks. However, they have certain disadvantages. Other study47 has found that iso-oncotic HA leads to higher mortality rates and dextran may cause allergies. In summary, the choice of the CPB priming fluid varies from case to case. This study compared and ranked the effects of the priming fluid used in CPB using a NMA, which provides a theoretical basis for clinical staff to select the priming fluid. Moreover, the findings of this study further define the effect of the commonly used priming fluid in the adult CPB process, providing a basis for subsequent studies.
4.6. Limitations
In order to make a systematic comparison of various liquids, there are some limitations. Firstly, the number of studies in many trials was limited, and some drugs might have lacked some aspects of the outcome, which might have led to biases in the results. Secondly, many studies on the priming fluid consider colloid osmotic pressure a major indicator; however, the data in this study were insufficient to support this outcome, so colloid osmotic pressure was not included as an outcome. In addition, because each of the eight priming fluids had advantages and disadvantages, we could not determine an optimal choice. Future studies should provide more precise evidence to help accurately select a priming fluid for CPB.
5. Conclusion
The study found that the use of dextran as the CPB priming fluid may be associated with lower mortality and a shorter mean CPB time. The use of iso-oncotic HA was probably associated with shorter length of ICU stay and both the blood loss and the chest tube output were the lowest 24h after surgery. In addition, the use of balanced crystalloids was beneficial for platelet counts, the use of L-HES was beneficial for urine output 24h after surgery, and the use of H-HES resulted in the shortest hospital stay. In summary, each of these fluids has pros and cons quite, and an optimal choice of priming fluids during CPB surgery remains unsupported by current evidence. When performing CPB surgery, the type of priming fluids should be selected according to the actual condition of the patient’s body.
Supplementary Material
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Author contribution
CZ and JYY designed the research. CYXY, JBX, TYG and HYL collected the data and verified the accuracy of the data. YTM, JBX, NJD and CYXY verified the accuracy of the data. YTT, JBX, HJL and YTM contributed to data interpretation. CZ, CYXY and YTT performed the statistical analysis and visualization. CYXY, CZ and JYY wrote the manuscript. All authors read, critically reviewed, and approved the final manuscript.
Disclosure statement
No conflict of interest was reported by the author(s).
Data availability statement
The data are available from the corresponding author upon reasonable request.
References
- 1.Patel J, Prajapati M, Solanki A, et al. Comparison of albumin, hydroxyethyl starch and ringer lactate solution as priming fluid for cardiopulmonary bypass in paediatric cardiac surgery. J Clin Diagn Res. 2016;10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himpe D, Van Cauwelaert P, Neels H, et al. Priming solutions for cardiopulmonary bypass: comparison of three colloids. J Cardiothorac Vasc Anesth. 1991;5(5):457–466. doi: 10.1016/1053-0770(91)90120-i. [DOI] [PubMed] [Google Scholar]
- 3.Lilley A. The selection of priming fluids for cardiopulmonary bypass in the UK and Ireland. Perfusion. 2002;17(5):315–319. doi: 10.1191/0267659102pf538oa. [DOI] [PubMed] [Google Scholar]
- 4.Baehner T, Boehm O, Probst C, et al. Cardiopulmonary bypass in cardiac surgery. Anaesthesist. 2012;61(10):846–856. doi: 10.1007/s00101-012-2050-0. [DOI] [PubMed] [Google Scholar]
- 5.Gu YJ, Boonstra PW.. Selection of priming solutions for cardiopulmonary bypass in adults. Multimed Man Cardiothorac Surg. 2006;2006:001198. [DOI] [PubMed] [Google Scholar]
- 6.Wilkes MM, Navickis RJ, Sibbald WJ.. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001;72(2):527–533; discussion 534. doi: 10.1016/s0003-4975(01)02745-x. [DOI] [PubMed] [Google Scholar]
- 7.Rozga J, Piątek T, Małkowski P.. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205–217. doi: 10.12659/AOT.889188. [DOI] [PubMed] [Google Scholar]
- 8.Hirleman E, Larson DF.. Cardiopulmonary bypass and edema: physiology and pathophysiology. Perfusion. 2008;23(6):311–322. doi: 10.1177/0267659109105079. [DOI] [PubMed] [Google Scholar]
- 9.Miles LF, Coulson TG, Galhardo C, et al. Pump priming practices and anticoagulation in cardiac surgery: results from the global cardiopulmonary bypass survey. Anesth Analg. 2017;125(6):1871–1877. doi: 10.1213/ANE.0000000000002052. [DOI] [PubMed] [Google Scholar]
- 10.Russell JA, Navickis RJ, Wilkes MM.. Albumin versus crystalloid for pump priming in cardiac surgery: meta-analysis of controlled trials. J Cardiothorac Vasc Anesth. 2004;18(4):429–437. doi: 10.1053/j.jvca.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Beukers AM, de Ruijter JAC, Loer SA, et al. Effects of crystalloid and colloid priming strategies for cardiopulmonary bypass on colloid oncotic pressure and haemostasis: a meta-analysis. Interact Cardiovasc Thorac Surg. 2022;35:ivac127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiedermann CJ. Human albumin and 6% hydroxyethyl starches (130/0.4) in cardiac surgery: a meta-analysis revisited. BMC Surg. 2022;22(1):140. doi: 10.1186/s12893-022-01588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himpe D. Colloids versus crystalloids as priming solutions for cardiopulmonary bypass: a meta-analysis of prospective, randomised clinical trials. Acta Anaesthesiol Belg. 2003;54(3):207–215. [PubMed] [Google Scholar]
- 14.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 17.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–654. [DOI] [PubMed] [Google Scholar]
- 19.Jackson D, Barrett JK, Rice S, et al. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Statist Med. 2014;33(21):3639–3654. doi: 10.1002/sim.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song F, Xiong T, Parekh-Bhurke S, et al. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ. 2011;343:d4909. doi: 10.1136/bmj.d4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rücker G, Schwarzer G.. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Linden PJ, De Hert SG, Daper A, et al. 3.5% Urea-linked gelatin is as effective as 6% HES 200/0.5 for volume management in cardiac surgery patients. Can J Anaesth. 2004;51(3):236–241. doi: 10.1007/BF03019102. [DOI] [PubMed] [Google Scholar]
- 23.Choi YS, Shim JK, Hong SW, et al. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 on coagulation and inflammatory response when used as priming solutions for cardiopulmonary bypass. Minerva Anestesiol. 2010;76:584–591. [PubMed] [Google Scholar]
- 24.Hosseinzadeh Maleki M, Derakhshan P, Rahmanian Sharifabad A, et al. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 (voluven) on renal function as priming solutions for cardiopulmonary bypass: a randomized double blind clinical trial. Anesth Pain Med. 2016;6(1):e30326. doi: 10.5812/aapm.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolsrud O, Barbu M, Dellgren G, et al. Dextran-based priming solution during cardiopulmonary bypass attenuates renal tubular injury – a secondary analysis of randomized controlled trial in adult cardiac surgery patients. Acta Anaesthesiol Scand. 2022;66(1):40–47. doi: 10.1111/aas.13975. [DOI] [PubMed] [Google Scholar]
- 26.Barbu M, Kolsrud O, Ricksten SE, et al. Dextran- versus crystalloid-based prime in cardiac surgery: a prospective randomized pilot study. Ann Thorac Surg. 2020;110(5):1541–1547. doi: 10.1016/j.athoracsur.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Vanhoonacker J, Ongenae M, Vanoverschelde H, et al. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for cardiopulmonary bypass priming: the effects on postoperative bleeding and volume expansion needs after elective CABG. Acta Anaesthesiol Belg. 2009;60(2):91–97. [PubMed] [Google Scholar]
- 28.Yap WW, Young D, Pathi V.. Effects of gelatine and medium molecular weight starch as priming fluid in cardiopulmonary bypass – a randomised controlled trial. Perfusion. 2007;22(1):57–61. doi: 10.1177/0267659107077903. [DOI] [PubMed] [Google Scholar]
- 29.Kamra C, Beney A.. Human albumin in extracorporeal prime: effect on platelet function and bleeding. Perfusion. 2013;28(6):536–540. doi: 10.1177/0267659113492836. [DOI] [PubMed] [Google Scholar]
- 30.Kuitunen AH, Hynynen MJ, Vahtera E, et al. Hydroxyethyl starch as a priming solution for cardiopulmonary bypass impairs hemostasis after cardiac surgery. Anesth Analg. 2004;98(2):291–297. doi: 10.1213/01.ANE.0000096006.60716.F6. [DOI] [PubMed] [Google Scholar]
- 31.Tiryakioğlu O, Yildiz G, Vural H, et al. Hydroxyethyl starch versus ringer solution in cardiopulmonary bypass prime solutions (a randomized controlled trial). J Cardiothorac Surg. 2008;3:45. doi: 10.1186/1749-8090-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurbuz HA, Durukan AB, Salman N, et al. Hydroxyethyl starch 6%, 130/0.4 vs. a balanced crystalloid solution in cardiopulmonary bypass priming: a randomized, prospective study. J Cardiothorac Surg. 2013;8:71. doi: 10.1186/1749-8090-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svendsen ØS, Farstad M, Mongstad A, et al. Is the use of hydroxyethyl starch as priming solution during cardiac surgery advisable? A randomized, single-center trial. Perfusion. 2018;33(6):483–489. doi: 10.1177/0267659117746235. [DOI] [PubMed] [Google Scholar]
- 34.Kuitunen A, Hynynen M, Salmenperä M, et al. Hydroxyethyl starch as a prime for cardiopulmonary bypass: effects of two different solutions on haemostasis. Acta Anaesthesiol Scand. 1993;37(7):652–658. doi: 10.1111/j.1399-6576.1993.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 35.Ooi JS, Ramzisham AR, Zamrin MD.. Is 6% hydroxyethyl starch 130/0.4 safe in coronary artery bypass graft surgery? Asian Cardiovasc Thorac Ann. 2009;17:368–372. [DOI] [PubMed] [Google Scholar]
- 36.Moerman A, Van Eeckhout C, Vanderstraeten K, et al. The effect of hydroxyethyl starch 6% 130/0.4 compared with gelatin on microvascular reactivity. Anaesthesia. 2016;71(7):798–805. doi: 10.1111/anae.13388. [DOI] [PubMed] [Google Scholar]
- 37.Schramko A, Suojaranta-Ylinen R, Niemi T, et al. The use of balanced HES 130/0.42 during complex cardiac surgery; effect on blood coagulation and fluid balance: a randomized controlled trial. Perfusion. 2015;30(3):224–232. doi: 10.1177/0267659114540022. [DOI] [PubMed] [Google Scholar]
- 38.Tamayo E, Alvarez FJ, Alonso O, et al. The inflammatory response to colloids and crystalloids used for pump priming during cardiopulmonary bypass. Acta Anaesthesiol Scand. 2008;52(9):1204–1212. doi: 10.1111/j.1399-6576.2008.01758.x. [DOI] [PubMed] [Google Scholar]
- 39.Lou S, Bian L, Long C, et al. Does 6% hydroxyethyl starch 130/0.4 impact differently on blood glucose than 4% gelatin in patients receiving open heart surgery? Perfusion. 2012;27(2):113–118. doi: 10.1177/0267659111426920. [DOI] [PubMed] [Google Scholar]
- 40.Schweizer R, Lameche M, Coelembier C, et al. Cardiopulmonary bypass priming with hydroxyethyl starch 6% 130/0.4 or sodium chloride 0.9%: a preliminary Double-Blind randomized controlled study in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(12):3534–3535. doi: 10.1053/j.jvca.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Liou HL, Shih CC, Chao YF, et al. Inflammatory response to colloids compared to crystalloid priming in cardiac surgery patients with cardiopulmonary bypass. Chin J Physiol. 2012;55(3):210–218. doi: 10.4077/CJP.2012.BAA028. [DOI] [PubMed] [Google Scholar]
- 42.Shahbazi S, Zeighami D, Allahyary E, et al. Effect of colloid versus crystalloid administration of cardiopulmonary bypass. Prime Solution Tissue Organ Perfusion. 2010;5:e14235. [Google Scholar]
- 43.Adrian K, Mellgren K, Skogby M, et al. The effect of albumin priming solution on platelet activation during experimental long-term perfusion. Perfusion. 1998;13(3):187–191. doi: 10.1177/026765919801300306. [DOI] [PubMed] [Google Scholar]
- 44.Holloway DS, Summaria L, Sandesara J, et al. Decreased platelet number and function and increased fibrinolysis contribute to postoperative bleeding in cardiopulmonary bypass patients. Thromb Haemost. 1988;59(01):062–067. doi: 10.1055/s-0038-1646770. [DOI] [PubMed] [Google Scholar]
- 45.Finfer S. Reappraising the role of albumin for resuscitation. Curr Opin Crit Care. 2013;19(4):315–320. doi: 10.1097/MCC.0b013e3283632e42. [DOI] [PubMed] [Google Scholar]
- 46.Schramko A, Suojaranta-Ylinen R, Kuitunen A, et al. Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Br J Anaesth. 2010;104(6):691–697. doi: 10.1093/bja/aeq084. [DOI] [PubMed] [Google Scholar]
- 47.Tseng CH, Chen TT, Wu MY, et al. Resuscitation fluid types in sepsis, surgical, and trauma patients: a systematic review and sequential network meta-analyses. Crit Care. 2020;24(1):693. doi: 10.1186/s13054-020-03419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finfer S, Myburgh J, Bellomo R.. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14(9):541–557. doi: 10.1038/s41581-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 49.Hecht-Dolnik M, Barkan H, Taharka A, et al. Hetastarch increases the risk of bleeding complications in patients after off-pump coronary bypass surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2009;138(3):703–711. doi: 10.1016/j.jtcvs.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Moret E, Jacob MW, Ranucci M, et al. Albumin-Beyond fluid replacement in cardiopulmonary bypass surgery: why, how, and when? Semin Cardiothorac Vasc Anesth. 2014;18(3):252–259. doi: 10.1177/1089253214535667. [DOI] [PubMed] [Google Scholar]
- 51.Moeller C, Fleischmann C, Thomas-Rueddel D, et al. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J Crit Care. 2016;35:75–83. doi: 10.1016/j.jcrc.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Barron ME, Wilkes MM, Navickis RJ.. A systematic review of the comparative safety of colloids. Arch Surg. 2004;139(5):552–563. doi: 10.1001/archsurg.139.5.552. [DOI] [PubMed] [Google Scholar]
- 53.Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309(7):678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 54.Wise J. Boldt: the great pretender. BMJ. 2013;346:f1738. doi: 10.1136/bmj.f1738. [DOI] [PubMed] [Google Scholar]
- 55.Zacharowski K, Van Aken H, Marx G, et al. Comments on Reinhart et al.: consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. 2012;38(9):1556–1557. doi: 10.1007/s00134-012-2641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author upon reasonable request.