Abstract
Context/Objective
Prevention of urinary tract infection (UTI) after spinal cord injury is an important goal. Intravesical hyaluronic acid with chondroitin sulphate (HA+CS) has been effective in preventing UTI in other settings. We aimed to demonstrate safety and feasibility of a standard treatment course of 7 intravesical HA+CS instillations over 12 weeks, in patients with acute (Arm A) and chronic (Arm B) spinal cord injury (SCI).
Design
Follow-up of adverse events, quality of life bladder management difficulty (BMD) and bladder complication (BC) T-scores at baseline (Arm B only), 12 and 24 weeks, and symptomatic urinary tract infection (UTI).
Results
Of 33 and 14 individuals screened, 2 and 8 participants were recruited to the study for Arm A and Arm B respectively. Of the 10 participants, 8 completed all 7 instillations. HA+CS commonly caused cloudy urine with urinary sediment which was mild and short-lived. In Arm B, a mean reduction in BMD and BC T-scores was observed from baseline (57.3 and 54.4 respectively), of 6.8 and 4.3 at 12 weeks and 1.6 and 2.8 at 24 weeks, respectively. Four participants with a history of frequent UTI in the prior 12 months did not have UTI in the 24 weeks of the study.
Conclusions
HA+CS was well tolerated. Recruitment was more difficult in early acute SCI; participants with chronic SCI were highly motivated to reduce UTI and manage self-administration without difficulty. Larger case–control or randomized controlled trials in patients with neurogenic bladder from SCI are warranted.
Trial registration
ClinicalTrials.gov identifier: NCT03945110.
Keywords: Urinary tract infection, UTI, Spinal cord injury, Hyaluronic acid, Chondroitin sulphate
Introduction
Traumatic spinal cord injury (SCI) presents significant impacts and considerable costs to affected individuals and society. In addition to paralysis and sensation loss, the effects of SCI on the urinary bladder are immediate, with altered voiding dynamics and pathogenic mechanisms including bladder ischemia, hypoperfusion, abnormal microbiological architecture, damage to the urothelial barrier and dysregulated inflammatory responses, rendering patients susceptible to urinary tract infection (UTI).1 Acute bladder management requires urethral indwelling catheterization (IDC), which further increases UTI susceptibility. Following SCI, genitourinary diseases including UTI result in significant long-term morbidity are the leading cause of re-hospitalization,2–5 and the second leading cause of death.6 Individuals with chronic SCI and neurogenic bladder require ongoing IDC or intermittent catheterization (IC), which further increases the risks of mucosal irritation, catheter cystitis and recurrent UTI. People with chronic SCI experience a mean 2.14 UTIs per year,7–9 though up to 19% have >6 infections per year.8 There are few prophylactic measures with strong evidence-based efficacy for people with neurogenic bladder,10 and antibiotic prophylaxis is not generally advisable, since this may promote antibiotic resistance.11
Our recent audit of acute SCI bladder management and UTI in Western Australia (WA), 2015–2017, found that for patients with ≥1 UTIs (n = 43/70), a more prolonged duration of initial IDC was associated with shorter time to first UTI, which in turn was associated with an increased frequency of subsequent UTI.12 These findings indicate that prevention of UTI during early acute SCI may reduce subsequent rates of infection.
Restoring the integrity of the urothelium has been an effective strategy to reduce recurrent UTI in neurologically intact populations.13 The healthy urothelium is coated with a film consisting of glycosaminoglycans (GAGs), highly hydrophilic molecules that create a protective barrier. Animal models have demonstrated disruption of this layer following SCI, exposing the urothelium to toxins in the urine, leading to increased UTIs. Infection further damages the GAG layer, promoting progressive damage and recurrent infection.14 Studies over the last 20 years have explored ways to restore the GAG layer, including introducing hyaluronic acid (HA) and chondroitin sulphate (CS) intravesically via a catheter.15 HA and/or CS bladder instillations are safe and produced promising results for recurrent UTI in the general population.13 For individuals hospitalized with metastatic spinal cord compression requiring IDC, intravesical HA instillation was effective in preventing UTI.16 There is very limited research into the effectiveness of intravesical GAGs at preventing UTI in people with neurogenic bladder, including those with SCI,17,18 and none have examined these instillations in the early acute phase post-injury. The non-antibiotic prevention approach has minimal administrative burden for individuals who routinely require catheterization. We, therefore, conducted an exploratory study primarily on the feasibility and tolerability of intravesical HA + CS in people with SCI. Measures to prevent UTI need to be tested in both acute and chronic SCI as there may be differences between the two groups in the efficacy and implementation of an intervention.
Objectives
Acute Arm (Arm A): To examine the safety and feasibility of 7 bladder instillations of HA and CS (HA + CS) over 12 weeks, commencing within 10 days of traumatic SCI (before the onset of UTI)
Chronic Arm (Arm B): To examine the safety and feasibility of 7 bladder instillations of HA + CS over 12 weeks, performed by patients with chronic SCI (rehabilitation phase or community) and a history of recurrent UTI.
Materials and methods
Trial design and setting
This is an exploratory study involving patients with acute and chronic SCI. All Participants followed Western Australian (WA) standard care pathways in relation to bladder management following SCI.
The trial was conducted across the two main hospital sites managing SCI in WA: Royal Perth Hospital (RPH, “Surgical Hospital”) and the State Rehabilitation Service at Fiona Stanley Hospital (FSH, “Rehabilitation Hospital”), and a private urology clinic (“Clinic”), over a 12-month period.
Eligibility and recruitment
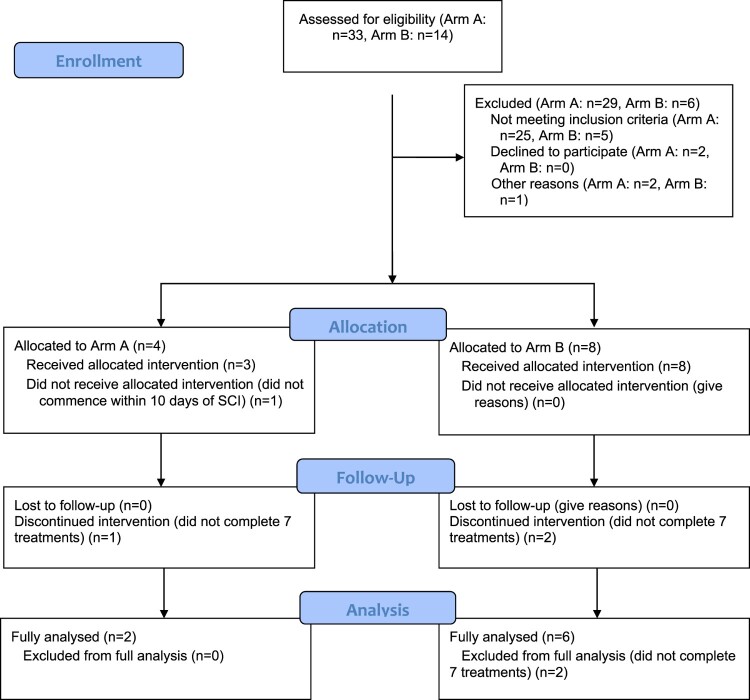
Inclusion and exclusion criteria for Arm A and B are displayed in Table 1. Figure 1 summarizes screening and enrolment in Arm A and Arm B. For Arm A, of 33 potential patients at screening, only four ultimately met eligibility criteria and two completed the seven HA + CS instillations. The main exclusions were >70 years of age (n = 9) and spontaneous voiding of urine prior to day 10 (n = 6).
Table 1.
Inclusion and exclusion criteria for Arms A and B of the study.
| Inclusion criteria | Arm A | Arm B |
|---|---|---|
| Age 18–70 years | Yes | Yes |
| Traumatic SCI | Yes | Yes |
| Atraumatic sudden onset SCI | No | Yes |
| Within 10 days post-SCI | Yes | No |
| History of recurrent UTI | No | Yes |
| Exclusion criteria | ||
| Admission to hospital outside Western Australia | Yes | No |
| Inability to commence HA + CS Within 10 days of SCI | Yes | No |
| Spontaneous voiding | Yes | Yes |
| Bladder/urethral trauma on Admission | Yes | Yes |
| Bladder cancer/other bladder Pathology | Yes | Yes |
| Known hypersensitivity to HA or CS | Yes | Yes |
| UTI before commencing Study intervention | Yes | No |
| Current pregnancy | Yes | Yes |
| History of neurological disorder | Yes | Yes |
| Inability to provide consent | Yes | Yes |
| History of autonomic Dysreflexia with urological procedures | No | Yes |
CS = chrondroitin sulphate, HA = hyaluronic acid, SCI = spinal cord injury, UTI = urinary tract infection.
Figure 1.
Consort study diagram.
Fourteen individuals from the community were screened and eight participants were recruited to Arm B. Reasons for exclusion were; not sudden onset SCI (n = 3), remote location so unable to attend clinic (n = 1), no history of recurrent UTI (n = 2). Six participants completed all installations to be fully analyzed. One participant (participant 9) completed six instillations. The final instillation for participant 9 was not given due to an inability to attend the clinic. One participant (participant 10) received a single instillation in hospital but due to COVID-19 restrictions he couldn’t attend clinic for further instillations, and follow-up was limited to 46 days (see Fig. 1).
Intervention
The 12-week protocol consisted of seven bladder instillations of HA + CS (iAluRil®) which were administered according to the product insert at weeks 1, 2, 3, 4, 6, 8 and 12 through a urinary catheter using 50 mL pre-filled syringes containing a sterile solution of sodium hyaluronate (1.6% 800 mg/50 mL) and sodium chondroitin sulphate (2%–1 g/50 mL). iAluRil® syringes are registered on the Australian Register of Therapeutics Goods as a class III medical device.
Arm A procedure
Instillations were administered by attending nurses who were trained by study author GKK, who has extensive SCI nursing experience. Following complete bladder emptying, HA + CS was instilled at room temperature slowly (over 5 min) via the catheter which was then clamped for a minimum 30 min. Standard observations were recorded before and after instillation. Participants were monitored for adverse events, including signs of AD, a potentially life-threatening condition occurring in some patients with injuries at or above neurological level T7 and is characterized by a sudden increase in blood pressure and may be triggered in susceptible individuals by instilling a fluid into the bladder and/or catheter clamping. We administered intravesical instillations slowly at room-temperature to minimizes this risk.
Arm B procedure
Instillations were administered by attending nurses per Arm A for inpatients who were not performing self-intermittent catheterization (self-IC). All other participants administered HA + CS themselves at an outpatient clinic supervised by spinal urology nurses, who provided instruction and assistance, and ensured good technique with full emptying of the bladder via self-IC. Participants remained in clinic for 30 min after HA + CS instillation for the monitoring of adverse events. Pre- and post- instillation observations were recorded.
Data collection
All participants were followed for 24 weeks, to allow surveillance for outcomes up to 12 weeks after the final HA + CS instillation. The following data were collected from medical records for inpatients and from a Log Book* provided to all Arm B participants and to Arm A participants discharged before 24 weeks:
Demographics, medical history, injury details and neurological status
Bladder management methods and durations
Concurrent medications, illnesses and urological complications
Episodes of symptomatic UTI, defined according to guidelines of the Infectious Diseases Society of America as bacteriuria (≥104 CFU/mL of ≥1 bacterial species in catheter or midstream urine samples), plus symptomatology with no other identifiable cause.19
Renal ultrasound and urodynamic assessment results (conducted ∼12 weeks post-SCI as part of standard care for acute patients).
Two validated bladder-related quality of life surveys (SCI-QoL ‘Bladder Management Difficulties SF8a’ and ‘Bladder Complications’),20,21 were conducted with each participant at baseline (Arm B only), 12 and 24 weeks post-enrolment. The “Bladder Management Difficulties” (BMD) survey involves eight questions related to how bladder management interferes with a person’s life. Each question is graded 1 (not at all) to 5 (very much), with the raw score converted to a T-score ranging from 40.9 to 81.6. The “Bladder Complications” (BC) survey involves 5 questions related to the interference of UTI on quality of life and bladder issues on sex life, rated 1 (not at all) to 5 (very much), converted to a T-score ranging from 39.7 to 76.7. T-score of 50 reflects the mean score from a calibration study in the SCI population, with each difference of 10 points representing one standard deviation. Higher scores indicate increased difficulties or complications. A short survey on satisfaction and ease of instillation was also conducted at 24 weeks. Arm B participants were questioned about UTIs (requiring antibiotics) during the 12 months before enrolment.
Statement of ethics
The study was conducted with informed patient consent, received ethical approval from The South Metropolitan Health Service Human Research Ethics Committee (RGS-2994), and is registered on ClinicalTrials.gov (NCT03945110) and the Australian and New Zealand Clinical Trials Registry.
Results
Arm A
Participant 1 was a 32-year-old male with injury at neurological level C4 who had an IDC for 65 days, followed by staff-IC for 66 days, and at study completion bladder was managed with IDC awaiting final management via suprapubic catheter (SPC). His Surgical Hospital stay was 37 days and Rehabilitation Hospital stay 94 days. He had two UTIs. BMD T-scores were 53.2 at 12 weeks and 49.8 at 24 weeks. BC T-scores were 55.5 at 12 weeks and 45.8 at 24 weeks. Participant 2 was an 18-year-old male with injury at neurological level T5 who had IDC for 21 days followed by self-IC. His Surgical Hospital stay was 17 days and Rehabilitation Hospital stay 56 days. He had no UTIs. BMD T-scores were 53.2 at 12 weeks and 54.8 at 24 weeks. BC T-scores were 45.8 at 12 weeks and 45.8 at 24 weeks (see Table 2). Neither participant found instillations bothersome and both were glad to have participated in the study.
Table 2.
Demographics and outcomes of participants in Arm A (acute spinal cord injury [SCI]), Arm B (chronic SCI), indicating ISNSCI spinal cord level (Le), Complete SCI (Co), Years since injury (Ye), Bladder management during the treatment phase (BM; IDC = indwelling catheter, IC = intermittent catheter), urinary tract infection (UTI) in the 12 months before study, UTI during the study, SCI-QoL Bladder Management Difficulty (BMD) and Bladder Complication (BC) T-scores at baseline (Ba), 12 and 24 weeks, and median/geometric mean Arm A, Arm B, Arm A + B for Age, Ye, UTI 12M, UTI study, BMD, BC.
| Sex | Age | Le | Co | Ye | BM | UTI 12M | UTI study | BMD Ba | BMD 12W | BMD 24W | BC Ba | BC 12W | BC 24W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm A | ||||||||||||||
| 1 | M | 32 | C4 | Y | 0 | IDC | N/A | 2 | N/A | 53.2 | 49.8 | N/A | 55.5 | 45.8 |
| 2 | M | 19 | T5 | Y | 0 | IDC/IC | N/A | 0 | N/A | 53.2 | 54.8 | N/A | 45.8 | 45.8 |
| Median/Mean | 25.5/24.6 | 0/0 | 1/1 | 53.2/53.2 | 52.3/52.3 | 50.6/50.6 | 45.8/45.8 | |||||||
| Arm B | ||||||||||||||
| 3 | F | 42 | T5 | Y | 22 | IC | 1–3 | 1 | 58.9 | 51.8 | 53.2 | 45.8 | 58.6 | 45.8 |
| 4 | F | 31 | T5 | Y | 3 | IC | 4–6 | 0 | 62.2 | 56 | 66.7 | 45.8 | 45.8 | 66.9 |
| 5 | M | 37 | C7 | N | 11 | IC | 6+ | 0 | 40.9 | 40.9 | 40.9 | 45.8 | 45.8 | 45.8 |
| 6 | M | 39 | L1 | Y | 16 | IC | 1–3 | 4 | 62.9 | 59.8 | 65.9 | 64.4 | 61.3 | 64.4 |
| 7 | F | 33 | T9 | N | 3 | IC | 6+ | 2 | 57 | 57 | 57 | 63.1 | 45.8 | 45.8 |
| 8 | M | 42 | T10 | Y | 25 | IC | 6+ | 0 | 65.9 | 40.9 | 54.8 | 66.2 | 45.8 | 45.8 |
| 9 | M | 32 | T10 | Y | 5 | IC | 6+ | 0 | – | – | – | – | – | – |
| 10 | M | 18 | C5 | N | 0 | IDC | 6 | – | – | – | – | – | – | – |
| Median/Mean | 35/33.3 | 8/8.9 | 6/4.5 | 0/1 | 60.6/57.3 | 53.9/50.5 | 55.9/55.7 | 54.4/54.4 | 45.8/50.1 | 45.8/51.6 | ||||
| Arm A + B | ||||||||||||||
| Median/Mean | 33/31.3 | 4/8.9 | 6/4.5 | 0/1 | 60.6/57.3 | 53.2/51.1 | 54.8/54.8 | 54.4/54.4 | 45.8/50.2 | 45.8/50.1 |
Arm B
Demographics and outcomes are recorded in Table 2. Participants were young and many years post SCI, all were managing their neurogenic bladder with self-IC, and had median 6 UTIs in the last 12 months. Four participants remained free of UTI in the 24 weeks of the trial. Bladder Management Difficulties and Bladder Complication T-scores had a mean reduction from baseline 57.3 and 54.4, of 6.8 and 4.3 at 12 weeks and 1.6 and 2.8 at 24 weeks, respectively. At the end of study survey, 5/6 said installations were not bothersome at all, and all were glad they participated in the study. Since instillations finished, comments were “my bladder health was generally better until I got a UTI”, “I haven’t had a UTI for a couple of months”, and “it seems that I’m more at risk of UTI since HA + CS stopped.” Two participants reported an intention to remain on HA + CS supervised by their general practitioner. Other comments were that the HA + CS packaging was difficult to open and that a three-way tap would help by allowing the HA + CS syringe to be attached to the catheter before insertion into the bladder.
Adverse events
Thirteen adverse events were recorded, 8 within 24 h of instillation. Twelve were of mild severity. The most common AE was cloudy urine +/- urinary sediment, usually noted with the IC following intravesical HA + CS, and resolving within 24 h. (see Table 3).
Table 3.
Adverse events following intravesical hyaluronic acid + chondroitin sulphate (HA + CS).
| Timing of AE | Description | Severity | Relationship to HA + CS | Outcome |
|---|---|---|---|---|
| <24 h | Nausea/chills/headache | Mild | Unlikely | Resolved |
| <24 h | Headache/fatigue | Mild | Unlikely | Resolved |
| <24 h | Bladder spasms | Mild | Suspected | Resolved |
| <24 h | Cloudy urine/ bladder pain | Mild | Suspected | Resolved |
| <24 h | Cloudy urine/sediment | Mild | Suspected | Resolved |
| <24 h | Cloudy urine | Mild | Suspected | Resolved |
| <24 h | Sediment in urine | Mild | Suspected | Resolved |
| <24 h | Sediment in urine | Mild | Suspected | Resolved |
| <7 days | UTI | Mild | Suspected | Antibiotics |
| <7 days | Bladder pain/cloudy urine | Mild | Unlikely | Resolved |
| <7 days | Bladder pain | Mild | Unlikely | Resolved |
| >7 days | Chest infection | Moderate | Unlikely | Antibiotics |
| >7 days | Infected SPC site | Mild | Unlikely | Resolved |
AE = adverse event, SPC = suprapubic catheter, UTI = urinary tract infection.
Discussion
The current study shows intravesical HA + CS in people with SCI is feasible. It did however reveal significant barriers to recruiting acute patients within 10 days of SCI, with only 2/33 screened individuals completing the 24-week study. This is partly a reflection of our exclusion criteria of age >70 years and the requirement for informed patient consent. Given that specialist nursing staff could administer HA + CS for the hospitalized period, an upper age limit for exclusion may not be necessary. For patients unable to give informed consent, this could be obtained from relatives.
In contrast, recruitment of people with chronic SCI was more successful; there was a larger population to draw from, bladder management was established, and they were clinically stable. They had also recognized the reduced quality of life associated with recurrent UTI,22 in contrast to people with acute SCI who were adjusting to the various implications of an SCI diagnosis. Although we recruited individuals managed by self-IC, HA + CS could also be utilized and studied in people with SPC or IDC. Most participants reported that installation was easy or very easy and the adverse risk profile was predominated by short-term mild cloudy urine, which leads us to suggest HA + CS could be administered by individuals at home after initial education and supervision. This would also make HA + CS more accessible to individuals residing in remote locations-one such person was excluded from Arm B of this study. The study was not powered to examine efficacy, but four out of eight chronic SCI participants were free of UTI in the 24 weeks of the study in contrast to frequent UTIs (median 6, mean 4.5) in the 12 months before the study. These findings support two small studies of 10 and 11 people with chronic neurogenic bladder that suggested effectiveness of HA + CS at reducing UTI incidence.17,18 Indeed, systemic review and meta-analysis demonstrated efficacy in non-injured women with recurrent UTI.23 Quality of life scores in the chronic SCI group were also improved during the treatment period. Numbers were too small in the acute SCI group to comment on efficacy.
Larger case–control or randomized controlled trials in SCI should be undertaken to properly examine efficacy for preventing UTI. Ideally trials would be placebo-controlled because this cohort of patients are often desperate for an intervention to be effective, and this may lead to a significant placebo effect. Also the diagnosis of UTI needs to be robust according to recognized criteria as symptoms may occur due to pathologies other than UTI, and bacteriuria alone is not sufficient to diagnose UTI. While establishing the protocol appeared to be easier in a chronic SCI population, overcoming barriers to commencing HA + CS prophylaxis in acute SCI may provide long-term benefits, since lengthening the time to first UTI may reduce subsequent UTI rates 12. Antibiotic prophylaxis was effective in reducing UTI in several studies of people with neurogenic bladder after SCI, although antibiotic resistance was demonstrated,11,24,25 and other studies did not show efficacy.24 Options for antimicrobial prophylaxis following SCI include fosfomycin which has a very high urinary and a broad activity against uropathogens, although it was not effective at preventing UTI after kidney transplantation.26 Sublingual vaccines containing inactivated uropathogens have been shown to greatly reduce UTI in women with recurrent UTI,27–29 and may warrant investigation in those with SCI.
In summary, we have demonstrated that recruitment to studying HA + CS in neurogenic bladder due to SCI is easier in chronic SCI than acute SCI. In addition, individuals can self-administer without serious adverse events, with a suggestion it may be effective. Larger trials of HA + CS in this group are warranted to evaluate efficacy.
Conflicts of interest
No potential conflict of interest was reported by the author(s).
Funding
This study was funded via the NRP/ICWA (Neurotrauma Research Program [Perron Institute for Neurological and Translational Science, Western Australia] and the Insurance Commission of Western Australia) Grant Program.
Author contributions
GK King: Project development, data collection, data analysis, manuscript writing; LM Goodes: project development, data collection, data analysis, manuscript writing; C Hartshorn: project development, data collection, manuscript writing; J Thavaseelan: project development, manuscript writing; S Jonescu: data collection; A Watts: project development, data collection; M Rawlins: project development, data analysis, manuscript writing; P Woodland: project development, manuscript writing; E-L Synnott: project development, data analysis, manuscript writing; T Barrett: project development, manuscript writing; D Hayne: project development, manuscript writing; P Boan: project development, data collection, data analysis, manuscript writing; SA Dunlop: project development, data collection, data analysis, manuscript writing.
Data deposition
Data is completely supplied in this manuscript. There is no supplemental data.
References
- 1.Vigil HR, Hickling DR.. Urinary tract infection in the neurogenic bladder. Transl Androl Urol 2016;5(1):72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardenas DD, Hoffman JM, Kirshblum S, McKinley W.. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004;85(11):1757–1763. [DOI] [PubMed] [Google Scholar]
- 3.DeJong G, Tian W, Hsieh CH, Junn C, Karam C, Ballard PH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil 2013;94(4 Suppl):S87–S97. [DOI] [PubMed] [Google Scholar]
- 4.Gabbe BJ, Nunn A.. Profile and costs of secondary conditions resulting in emergency department presentations and readmission to hospital following traumatic spinal cord injury. Injury 2016;47(8):1847–1855. [DOI] [PubMed] [Google Scholar]
- 5.Watts A. (2012). An audit of readmissions of people with spinal cord injury to a major metropolitan teaching hospital in Perth, Western Australia.
- 6.Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 2002;113(Suppl 1A):67S–79S. [DOI] [PubMed] [Google Scholar]
- 7.Esclarin De Ruz A, Garcia Leoni E, Herruzo Cabrera R.. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol 2000;164(4):1285–1289. [PubMed] [Google Scholar]
- 8.Roth JD, Pariser JJ, Stoffel JT, Lenherr SM, Myers JB, Welk B, et al. Patient subjective assessment of urinary tract infection frequency and severity is associated with bladder management method in spinal cord injury. Spinal Cord 2019;57(8):700–707. [DOI] [PubMed] [Google Scholar]
- 9.Stillman MD, Barber J, Burns S, Williams S, Hoffman JM.. Complications of spinal cord injury over the first year after discharge from inpatient rehabilitation. Arch Phys Med Rehabil 2017;98(9):1800–1805. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Arguello LY, O'Horo JC, Farrell A, Blakney R, Sohail MR, Evans CT, et al. Infections in the spinal cord-injured population: a systematic review. Spinal Cord 2017;55(6):526–534. [DOI] [PubMed] [Google Scholar]
- 11.Jahromi MS, Mure A, Gomez CS.. UTIs in patients with neurogenic bladder. Curr Urol Rep 2014;15(9):433. [DOI] [PubMed] [Google Scholar]
- 12.Goodes LM, King GK, Rea A, Murray K, Boan P, Watts A, et al. Early urinary tract infection after spinal cord injury: a retrospective inpatient cohort study. Spinal Cord 2020;58(1):25–34. [DOI] [PubMed] [Google Scholar]
- 13.Damiano R, Cicione A.. The role of sodium hyaluronate and sodium chondroitin sulphate in the management of bladder disease. Ther Adv Urol 2011;3(5):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L.. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol 2003;284(5):F966–F976. [DOI] [PubMed] [Google Scholar]
- 15.Madersbacher H, van Ophoven A, van Kerrebroeck PE.. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans–a review. Neurourol Urodyn 2013;32(1):9–18. [DOI] [PubMed] [Google Scholar]
- 16.Manas A, Glaria L, Pena C, Sotoca A, Lanzos E, Fernandez C, et al. Prevention of urinary tract infections in palliative radiation for vertebral metastasis and spinal compression: a pilot study in 71 patients. Int J Radiat Oncol Biol Phys 2006;64(3):935–940. [DOI] [PubMed] [Google Scholar]
- 17.Cicek N, Yildiz N, Alpay H.. Intravesical hyaluronic acid treatment in recurrent urinary tract infections in children with spina bifida and neurogenic bladder. J Pediatr Urol 2020;16(3):366.e1–366.e5. [DOI] [PubMed] [Google Scholar]
- 18.Havloka K, Rejchrt M, Kriz J.. Prevention of recurrent urinary tract infections with the use of intravesical instillation of hyaluronic acid and chondroitin sulfate in patients after spinal cord injury. Eur Urol Suppl 2016;15(11):e1441. [Google Scholar]
- 19.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50(5):625–663. [DOI] [PubMed] [Google Scholar]
- 20.Tulsky DS, Kisala PA, Tate DG, Spungen AM, Kirshblum SC.. Development and psychometric characteristics of the SCI-QOL Bladder Management Difficulties and bowel Management Difficulties item banks and short forms and the SCI-QOL Bladder Complications scale. J Spinal Cord Med 2015;38(3):288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tulsky DS, Kisala PA, Victorson D, Tate DG, Heinemann AW, Charlifue S, et al. Overview of the spinal cord injury–quality of life (SCI-QOL) measurement system. J Spinal Cord Med 2015;38(3):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke JA, Welk B, Macnab A, Rivers CS, Kurban D, Nigro M, et al. Exploring the relationship between self-reported urinary tract infections to quality of life and associated conditions: insights from the spinal cord injury community survey. Spinal Cord 2019;57(12):1040–1047. [DOI] [PubMed] [Google Scholar]
- 23.Goddard JC, Janssen DAW.. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: systematic review and meta-analysis. Int Urogynecol J 2018;29(7):933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morton SC, Shekelle PG, Adams JL, Bennett C, Dobkin BH, Montgomerie J, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83(1):129–138. [DOI] [PubMed] [Google Scholar]
- 25.Salomon J, Denys P, Merle C, Chartier-Kastler E, Perronne C, Gaillard JL, et al. Prevention of urinary tract infection in spinal cord-injured patients: safety and efficacy of a weekly oral cyclic antibiotic (WOCA) programme with a 2 year follow-up–an observational prospective study. J Antimicrob Chemother 2006;57(4):784–788. [DOI] [PubMed] [Google Scholar]
- 26.Arreola-Guerra JM, Rosado-Canto R, Alberu J, Maravilla E, Torres-Gonzalez P, Criollo E, et al. Fosfomycin trometamol in the prophylaxis of post-kidney transplant urinary tract infection: A controlled, randomized clinical trial. Transpl Infect Dis 2018;20(5):e12980. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzo-Gomez MF, Padilla-Fernandez B, Garcia-Cenador MB, Virseda-Rodriguez AJ, Martin-Garcia I, Sanchez-Escudero A, et al. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front Cell Infect Microbiol 2015;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzo-Gomez MF, Padilla-Fernandez B, Garcia-Criado FJ, Miron-Canelo JA, Gil-Vicente A, Nieto-Huertos A, et al. Evaluation of a therapeutic vaccine for the prevention of recurrent urinary tract infections versus prophylactic treatment with antibiotics. Int Urogynecol J 2013;24(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang B, Foley S.. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine uromune((R)). BJU Int 2018;121(2):289–292. [DOI] [PubMed] [Google Scholar]