ABSTRACT
Dermatophytes are common causes of skin, hair, and nail infections in humans. The most common species causing infections in humans are Trichophyton rubrum, Trichophyton mentagrophytes, and Trichophyton interdigitale. Outbreaks of recalcitrant dermatophytosis have been reported in parts of South Asia, including those caused by a hypervirulent and resistant species, Trichophyton indotineae. We evaluated the antifungal susceptibility profiles of dermatophytes received by our laboratory from institutions across North America between 2021 and 2022 and performed species identification for isolates deemed to demonstrate in vitro resistance. Susceptibility testing was performed by CLSI broth microdilution methods, and species identification was performed by DNA sequence analysis. During this 2-year period, 271 dermatophyte isolates were included, the majority of which demonstrated low MIC values for terbinafine (geometric mean [GM] and modal MIC, 0.031 μg/mL and 0.008 μg/mL, respectively) and the azoles itraconazole, posaconazole, and voriconazole (0.035 to 0.049 μg/mL and ≤0.03 μg/mL). However, 18.6% of the isolates tested were resistant to terbinafine (MIC ≥ 0.5 μg/mL), including 21 T. rubrum and 21 T. indotineae isolates. These isolates were received from several different states in the United States and two provinces in Canada. In contrast, resistance to itraconazole was relatively rare. We also searched our laboratory database for earlier isolates that were resistant to terbinafine and identified 3 additional T. indotineae isolates, the earliest of which was from 2017. These results demonstrate that terbinafine resistance in dermatophytes was relatively common over this 2-year period and that T. indotineae is present in multiple areas in North America. Continued surveillance is warranted.
KEYWORDS: dermatophytes, Trichophyton species, Trichophyton indotineae, Trichophyton rubrum, terbinafine, antifungal resistance, Trichophyton
INTRODUCTION
Dermatophytosis is the most common fungal infection in the world, affecting approximately 25% of the general population (1, 2). These infections are caused by dermatophytes, which can invade keratinized tissues, such as the skin, hair, and nails. Taxonomically, dermatophytes are grouped into nine separate genera, including Arthroderma, Ctenomyces, Epidermophyton, Guarromyces, Lophophyton, Microsporum, Nannizzia, Paraphyton, and Trichophyton (3). The most common causes of disease in humans include Trichophyton species, with T. rubrum, T. mentagrophytes, and T. interdigitale being the main etiological agents (3). Both topical and orally administered antifungals can be used to treat dermatophytosis. Topical therapies include members of the azole class of antifungal, which target the biosynthesis of ergosterol by inhibiting the enzyme lanosterol 14α-demethylase (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, and sulconazole); the allylamines (naftifine and terbinafine); benzylamines (butenafine), which inhibit the enzyme squalene epoxidase, thus inhibiting the conversion of squalene to 2,3(S)-oxidosqualene; and others (e.g., ciclopirox and tolnaftate). Oral therapies, which are often reserved for more severe infections due to the potential for adverse effects and drug-drug interactions, include the azoles itraconazole and fluconazole, the allylamine terbinafine, and griseofulvin.
Recently, there have been reports of outbreaks of dermatophytosis in parts of South Asia that have reached epidemic levels in some areas (4–6). This includes recalcitrant disease caused by a hypervirulent strain of Trichophyton, T. mentagrophytes genotype VIII, which causes extensive infections in the trunk (tinea corporis), groin (tinea cruris), and face (tinea faciei) (4, 7–9). Trichophyton mentagrophytes genotype VIII has now been formally described as a new fungal species, Trichophyton indotineae (10). Patients with these infections can present with double-edged or multiedged tinea concentric scaly rings. Large lesions with multiple, closely juxtaposed, centrifugally spreading lesions with eczematous centers have also been described (11), as have frequent relapses and treatment failures due to antifungal resistance, including resistance to terbinafine and the azoles itraconazole and voriconazole (4, 6).
Terbinafine-resistant dermatophyte infections are not limited to South Asia, and strains harboring the squalene epoxidase (SQLE) phenylalanine-to-leucine amino acid change at codon 397 (F397L), along with others that cause resistance to this allylamine, have been reported in several other countries, such as Japan, Russia, Iran, and various countries in Europe, including Greece, Switzerland, France, Belgium, Germany, Denmark, Finland, and Poland, and Canada (5, 12, 13). Case reports of terbinafine treatment-resistant infections have also recently been reported in the United States (14, 15). Although the extent of this problem is unknown, infections caused by the hypervirulent species T. indotineae have recently been described in this country (16). The objective of this study was to assess the terbinafine and azole susceptibility profiles of dermatophyte isolates received by our laboratory from institutions in North America over a 2-year period between 2021 and 2022 and to determine if and the extent to which T. indotineae was represented in the resistant strains.
(This work was presented at the 33rd European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 15 to 18 April 2023).
MATERIALS AND METHODS
Isolates and species identification.
All dermatophyte isolates received by our reference laboratory between 2021 and 2022 were included in this study. In addition, we queried our laboratory database for all dermatophyte isolates that had been received between 2001 and 2020 that demonstrated a high terbinafine MIC (≥0.5 μg/mL). All isolates were initially subcultured onto potato flake agar and incubated at 30°C prior to antifungal susceptibility testing (17). All isolates that demonstrated high terbinafine MICs (≥0.5 μg/mL) were subsequently identified by DNA sequence analysis as previously described (18, 19). This MIC threshold was used as others have used it to identify terbinafine resistance against Trichophyton mentagrophytes complex isolates (6). Briefly, portions of the subcultures were suspended in buffer G2 (Qiagen, Valencia, CA) followed by lysis using a bead beater instrument (Precellys Evolution; Bertin Instruments, Rockville, MD). Proteinase K was then added, and the tubes were incubated at 56°C. DNA was then extracted using an EZ1 DNA tissue kit with a BioRobot EZ1 instrument (Qiagen), and the internal transcribed spacer ribosomal DNA (rDNA) region (ITS) and the D1/D2 domain of the large subunit rDNA (LSU) gene were amplified and sequenced using the primer pairs BMBC-R and NL4R for ITS and D1/D2, respectively (20–22). BLASTn searches were then performed in GenBank, and results were considered significant with an E value of 0.0 at 98 to 100% identity and with at least 90% query coverage. BLASTn searches that showed close matches with type strains for T. indotineae (CBS 146623), T. interdigitale (CBS 428.63), and T. mentagrophytes (IHEM 4268) underwent phylogenetic analysis using maximum likelihood conducted in IQ-TREE (23, 24). The substitution model for maximum-likelihood analysis was determined using the ModelFinder and the Akaike information criterion, and branch support was evaluated using 1,000 resampling of ultrafast bootstrapping (UFBoot), the Bayesian-like modification of the approximate-likelihood ratio test (aLRT; aBayes), and the aLRT with nonparametric Shimodaira-Hasegawa correction (SH-aLRT), all of which were implemented in IQ-TREE (25–27). The substitution model used for maximum likelihood was TN+F+I.
Antifungal susceptibility.
Susceptibility testing was performed by broth microdilution according to the methods published in the CLSI M38 standard (28). Antifungal powders (terbinafine, fluconazole, itraconazole, posaconazole, and voriconazole; Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO) to prepare stock solutions. Further dilutions were prepared in RPMI 1640 buffered with 0.165 M MOPS (morpholinepropanesulfonic acid; pH 7.0) with 0.2% glucose and phenol red, without bicarbonate. Final testing concentrations were 0.004 to 2 μg/mL for terbinafine, 0.125 to 64 μg/mL for fluconazole, and 0.03 to 16 μg/mL for itraconazole, posaconazole, and voriconazole. The final DMSO concentration in the assay was 1% (vol/vol). MICs for terbinafine, itraconazole, posaconazole, and voriconazole were read at 80% inhibition of growth compared to the drug-free growth control after at least 96 h of incubation at 35°C and at 50% inhibition of growth for fluconazole.
Squalene epoxidase sequencing.
The squalene epoxidase (SQLE) gene was also sequenced in a limited number (11) of T. indotineae isolates (4, 29, 30). DNA was extracted as described above, and the entire gene encoding squalene epoxidase was amplified by PCR using primers Tricho SE-F0 (5′-TGTAAAACGACGGCCAGTTGACAGCGACAAGTGCCA-3′) and TINT SE-R0 (5′-CAGGAAACAGCTATGACCAAAGAGCTAGAGATAAGCCTATCTG-3′) and sequenced using internal primers TRI-SE-F3 (5′-TGTAAAACGACGGCCAGTGGAATATCTCCCCATACAACCAG-3′) and TRI SE-R3 (5′-CAGGAAACAGCTATGACCCCTCCCTTCTCCAACGCAG-3′) as previously described (4). Primers were M13 tagged for ease of sequencing. PCR products were enzymatically purified using the ExoSAP-IT PCR cleanup reagent (Thermo Fisher, Waltham, MA) prior to Sanger sequencing. Purified amplicons were sequenced using the BigDye v1.1 Sanger sequencing reagent (Thermo Fisher). Sanger reaction products were run on a 3500xL genetic analyzer (Thermo Fisher). Traces were assembled to contigs using ChromasPro v2.1.10 (Technelysium, South Brisbane, Australia) and aligned to the wild-type SQLE reference sequence for T. interdigitale (GenBank accession number EZF33561) with ClustalW2 (https://www.ebi.ac.uk/Tools/msa/clustalw2/).
Data analysis.
Descriptive statistics were used to describe species distributions and culture sites. MIC ranges, MIC50 and MIC90, geometric mean (GM), and modal MIC values were calculated. MIC values greater than the highest concentration tested were assigned a value one dilution higher for the purpose of statistical comparisons. Analysis of variance (ANOVA) with Tukey’s posttest for multiple comparisons was used to assess for significant differences in GM MICs. A P value of ≤0.05 was considered statistically significant.
RESULTS
Species distribution and sources of cultures.
The species distributions of the 271 dermatophytes received by our reference laboratory between 2021 and 2020 are shown in Table 1. The majority of these were members of the T. rubrum series (44.3%; T. rubrum and T. violaceum) followed by those of the T. mentagrophytes series (23.2%; T. mentagrophytes, T. tonsurans, T. indotineae, and T. interdigitale). Members of the T. benhamiae series (T. benhamiae and T. verrucosum) and non-Trichophyton dermatophytes (Microsporum canis and Nannizzia gypsea [formerly Microsporum gypseum]) made up a small proportion (5.2%) of the isolates received during this period. For approximately 27% of isolates received, only a genus-level identification was provided by the submitting laboratory.
TABLE 1.
Species distributions of 217 dermatophyte isolates received between 2021 and 2022a
| Species | % |
|---|---|
| Trichophyton rubrum | 43.9 |
| Trichophyton species not identified | 27.3 |
| Trichophyton tonsurans | 8.1 |
| Trichophyton indotineae | 7.7 |
| Trichophyton mentagrophytes | 5.5 |
| Microsporum canis | 3.3 |
| Trichophyton interdigitale | 1.8 |
| Nannizzia gypsea (formerly Microsporum gypseum) | 0.7 |
| Trichophyton benhamiae | 0.7 |
| Trichophyton verrucosum | 0.4 |
| Trichophyton violaceum | 0.4 |
Species identifications provided by the submitting institutions/laboratories were used except for those with terbinafine MICs of ≥0.25 μg/mL, which were determined by our laboratory by DNA sequence analysis of the ITS rDNA region and the D1/D2 domain of the large subunit rDNA (LSU) gene.
Antifungal susceptibility and species identification.
Antifungal susceptibility profiles against all Trichophyton isolates received between 2021 and 2022 are shown in Table 2. Overall, most isolates demonstrated low MICs for terbinafine (GM MIC, 0.031 μg/mL; mode, 0.008 μg/mL) and the azoles itraconazole, posaconazole, and voriconazole (GM MIC range, 0.035 to 0.049 μg/mL; mode, ≤0.03 μg/mL for each). No significant differences were observed between the GM MICs for the extended-spectrum azoles. Not unexpectedly, fluconazole MICs were higher than the other azoles and terbinafine. During this 2-year period, elevated terbinafine MICs (non-wild type ≥ 0.25 μg/mL [31]) were observed in 52 of 264 (19.7%) isolates tested, and terbinafine resistance, defined as an MIC of ≥0.5 μg/mL (6), was observed in 49 isolates (18.6%), 85.7% of which were confirmed to be T. rubrum or T. indotineae (21 isolates for each species) (Fig. 1). The phylogenetic analysis of the ITS rDNA region using the backbone tree of Kano et al. showed the T. indotineae isolates grouping with the reference strain with a clear delineation from T. interdigitale and T. mentagrophytes (data not shown) (10).
TABLE 2.
MIC ranges, MIC50 and MIC90 values, modal MICs, and GM MIC values for terbinafine, fluconazole, itraconazole, posaconazole, and voriconazole against dermatophyte clinical isolates between 2021 and 2022a
| Species | Antifungal (no. of isolates tested) | MIC/MEC range | MIC50 | MIC90 | Modal MIC | GM MIC |
|---|---|---|---|---|---|---|
| All dermatophyte isolates | Terbinafine (264) | ≤0.004 to >2 | 0.015 | >2 | 0.008 | 0.031 |
| Fluconazole (190) | 0.25 to >64 | 2 | 16 | 1 | 2.84 | |
| Itraconazole (213) | ≤0.03 to 2 | ≤0.03 | 0.125 | ≤0.03 | 0.040 | |
| Posaconazole (40) | ≤0.03 to 0.125 | ≤0.03 | 0.06 | ≤0.03 | 0.035 | |
| Voriconazole (126) | ≤0.03 to 4 | ≤0.03 | 0.125 | ≤0.03 | 0.049 | |
| Trichophyton rubrum | Terbinafine (117) | ≤0.004 to >2 | 0.008 | >2 | 0.008 | 0.029 |
| Fluconazole (68) | 0.25 to >64 | 1 | 4 | 1 | 1.28 | |
| Itraconazole (88) | ≤0.03 to 0.25 | ≤0.03 | ≤0.03 | ≤0.03 | 0.033 | |
| Posaconazole (16) | ≤0.03 to 0.06 | ≤0.03 | ≤0.03 | ≤0.03 | 0.031 | |
| Voriconazole (47) | ≤0.03 to 4 | ≤0.03 | 0.06 | ≤0.03 | 0.038 | |
| Trichophyton indotineae | Terbinafine (21) | 0.5 to >2 | >2 | >2 | >2 | >2 |
| Fluconazole (20) | 8 to >64 | 16 | >64 | 16 | 19.0 | |
| Itraconazole (21) | ≤0.03 to 0.125 | ≤0.03 | 0.125 | ≤0.03 | 0.046 | |
| Posaconazole (5) | ≤0.03 to 0.125 | |||||
| Voriconazole (4) | ≤0.03 to 1 |
MICs for terbinafine, itraconazole, posaconazole, and voriconazole were measured after 96 h of incubation at 35°C as the lowest concentration that resulted in at least 80% inhibition of growth compared to the drug-free growth control, while that of fluconazole was measured at 50% growth inhibition. All values are in micrograms per milliliter. MIC50, MIC90, modal MIC values, and GM MICs were not calculated for posaconazole or voriconazole against T. indotineae as the number of isolates tested was less than 10.
FIG 1.
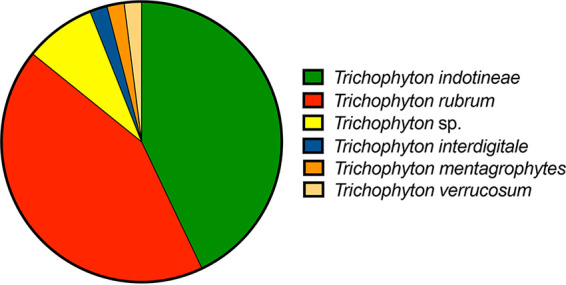
Species distribution of 49 terbinafine-resistant Trichophyton isolates. Resistance was defined as a terbinafine MIC of ≥0.5 μg/mL. Four isolates that were no longer available to be identified to the species level by DNA sequence analysis are included as Trichophyton spp.
All T. indotineae isolates identified in this study had terbinafine MICs of ≥0.5 μg/mL, with 18 of 21 having values of ≥2 μg/mL. Itraconazole susceptibility results were available against 56 isolates with elevated terbinafine MICs (≥0.5 μg/mL), and MICs for this azole ranged from ≤0.03 to 0.125 μg/mL with a mode of ≤0.03 μg/mL. Only a single Trichophyton species isolate had an elevated itraconazole MIC (2 μg/mL), and the corresponding terbinafine MIC was low (≤0.004 μg/mL). Unfortunately, this isolate was not able to be sequenced for species identification. Fluconazole MICs against the terbinafine-resistant isolates were more variable, ranging from 0.25 to >64 μg/mL, with a mode of 16 μg/mL. Both T. indotineae isolates for which the fluconazole MIC was ≥64 μg/mL also had elevated voriconazole MICs (1 μg/mL for each), while the posaconazole MICs were lower (0.06 and 0.125 μg/mL). None of the non-Trichophyton isolates (9 Microsporum canis and 2 Nannizzia gypsea) isolates were resistant to terbinafine or itraconazole, although one Nannizzia gypsea isolate did have a fluconazole MIC of 16 μg/mL.
The historical data within our antifungal susceptibility database were also queried for terbinafine MICs of ≥0.5 μg/mL between 2001 and 2020 to identify earlier dermatophyte isolates that were terbinafine resistant. A total of 32 isolates were found, all of which were Trichophyton isolates and had terbinafine MICs of ≥2 μg/mL. Of these additional terbinafine-resistant isolates, 3 were identified as T. indotineae, the earliest of which was from 2017. The others were T. rubrum, although species identification was not confirmed as part of this study. Interestingly, none of the T. indotineae isolates were cultured from nails but instead came from skin and tissue samples collected from different parts of the body (e.g., chest, axilla, thighs, abdomen, buttocks). In contrast, the terbinafine-resistant T. rubrum isolates were cultured from skin, tissue, and nails. The terbinafine-resistant type isolates came from several different geographic locations in North America, including institutions in Arkansas, Arizona, California, Kansas, Michigan, Montana, North Carolina, Nebraska, Texas, Washington, Wisconsin, and two provinces in Canada (Quebec and Ontario). However, the geographic spread could be even wider, as 31 of the 62 non-wild-type isolates were received from large commercial laboratories, so their origin could not be determined.
Variants in squalene epoxidase.
Sequence analysis of the SQLE gene was performed in 11 of the 21 T. indotineae isolates (Table 3), and variants leading to the phenylalanine to F397L amino acid substitution within the squalene epoxidase enzyme were found in 8 strains. Interestingly, this amino acid substitution was not found in the earliest T. indotineae isolate received by our laboratory in 2017. Instead, a leucine-to-serine amino acid change at codon 393 (L393S), which is also known to lead to high-level terbinafine resistance, was identified. This variant was not found in the other 10 isolates for which SQLE analysis was performed. Of the remaining 2 isolates without an F397L or L393S variant in SQLE, both Q408L and A448T were found. While the A448T variant was also present in an isolate with L393S, the Q408L variant was unique to terbinafine-resistant isolates without other known resistance-driving variations.
TABLE 3.
Amino acid changes detected at specific codons within the squalene epoxidase enzyme in 11 Trichophyton indotineae isolatesa
| Isolate no. | Terbinafine MIC | V24A | T52A | E174D | D352N | A392S | L393S | F397L | Q408L | A448T | I483V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DI23-52b | 2 | + | + | + | + | + | + | − | − | − | + |
| DI23-53 | >2 | + | + | + | + | + | − | − | + | + | + |
| DI23-54 | >2 | + | + | + | + | + | − | − | + | + | + |
| DI23-55 | >2 | + | + | + | + | + | − | + | − | + | + |
| DI23-56 | >2 | + | + | + | + | + | − | + | − | − | + |
| DI23-57 | >2 | + | + | + | + | + | − | + | − | − | + |
| DI23-58 | >2 | + | + | + | + | + | − | + | − | − | + |
| DI23-59 | >2 | + | + | + | + | + | − | + | − | − | + |
| DI23-60 | >2 | + | + | + | + | + | − | + | − | − | + |
| DI23-61 | >2 | + | + | + | + | + | − | + | − | − | + |
| DI23-62 | >2 | + | + | + | + | + | − | + | − | − | + |
Terbinafine MICs for each isolate are also provided (all values in micrograms per milliliter). +, presence of amino acid change; −, absence of change.
First T. indotineae isolate received by the Fungus Testing Laboratory in 2017.
DISCUSSION
Dermatophytes are common causes of infections in humans, capable of infecting skin, hair, and nails throughout the body (3, 32). These fungi are also the most common causes of fungus infections in humans, with some estimates that a quarter of the world’s population is affected by dermatophytes. In addition to the high prevalence of dermatophytosis, certain species within this group are also some of the few fungi capable of being transmitted from person to person (3). Treatment of dermatophytosis can range from short-term topical treatment of skin infections with over-the-counter antifungal preparations to longer durations of oral therapy with prescription medications against onychomycosis, which can have low success rates due to poor penetration of the antifungals into the nails (32). Different classes of antifungals are available for treatment, including azoles, allylamines, and benzylamines, which target steps within the ergosterol biosynthesis pathway, and agents with mechanisms of action independent of lanosterol 14α-demethylase and squalene epoxidase. As with many microbes, dermatophytes can develop resistance to different antifungals following exposure, and this has been documented to occur in Trichophyton, the genus most commonly associated with human dermatophytosis, against terbinafine and the azoles, which are commonly used agents for the treatment of these infections. Variants within the SQLE gene leading to amino acid changes in the squalene epoxidase protein are known to lead to terbinafine resistance, while single nucleotide variants within the genes encoding lanosterol 14α-demethylase and overexpression of efflux pump genes, including MDR1, MDR2, MDR3, and MDR5 of the ATP-binding cassette super family and MFS1 and MFS2 in the major facilitator superfamily (MFS), are known to lead to azole resistance (33, 34).
During the last decade, outbreaks of antifungal-resistant dermatophytosis have been documented throughout India, with some reaching epidemic levels (6, 35). These have included recalcitrant disease in which patients can present with large multiedged lesions of bizarre shapes that can cover multiple parts of the body (e.g., trunk and groin areas and upper and lower extremities) (9, 11). These infections are often caused by the hypervirulent species T. indotineae, which is a member of the T. mentagrophytes series and is also referred to as T. mentagrophytes genotype VIII (6, 7, 10). Many patients who suffer from these extensive infections have reported self-medication with over-the-counter products that contain steroids, antifungals such as terbinafine or miconazole, and antibiotics prior to seeing a primary care provider (6, 11, 35). Some products are known to contain steroids, such as clobetasol, which is a U.S. group 1 super-high-potency drug with anabolic properties (11). While these products may be associated with temporary relief of symptoms, they may also lead to proliferation of infection due to suppression of the immune response, leading to further opportunities for antifungal resistance to develop when antifungals are used concurrently.
In the current study, we document a high rate of terbinafine resistance in Trichophyton isolates sent to our reference laboratory for testing during years between 2021 and 2022. The prevalence of resistance to this antifungal was 18.6% during this 2-year period. However, resistance to the azole itraconazole was low, with only a single Trichophyton isolate that demonstrated a high MIC of 2 μg/mL. Of the 49 isolates that were terbinafine resistant (MIC ≥ 0.5 μg/mL) during this period, there was an equal distribution between T. rubrum and T. indotineae species. This also represents the first documentation of T. indotineae in several different geographic areas of North America, as isolates were received from different states across the United States as well as different provinces in Canada. Recently, cases of T. indotineae infections have been documented in New York (16). In a review of our historical database, we identified additional isolates between 2001 and 2020 that had elevated terbinafine MICs, and 3 additional T. indotineae isolates were identified, the earliest of which was from 2017. We also documented the presence of variants within SQLE that lead to amino acid changes within the squalene epoxidase gene, several of which are associated with terbinafine resistance, including those in the F397 and S393 codons (4, 9, 29, 36). Further work is needed to identify and characterize variants associated with terbinafine resistance that may be used in the future to design molecular assays to detected resistance mechanisms in direct specimens.
The majority of the T. indotineae isolates that we have identified thus far have had terbinafine MICs at or above the highest concentration that our laboratory tests (2 μg/mL), and all were cultured from skin or tissues and not from nails. The source of T. indotineae isolates in this study is consistent with what has been reported by others, and to date, T. indotineae has only been cultured from the nail of one patient in France (37). However, we did not identify to the species level all isolates, including in this study, but only those that were resistant to terbinafine (MIC ≥ 0.5 μg/mL). Thus, T. indotineae isolates with lower terbinafine MICs may have not been identified. In other studies, the rates of terbinafine resistance in T. indotineae isolates have ranged from approximately 32% to 68% (4, 6, 30). Thus, although many strains of this species are resistant to terbinafine, this species does not meet the threshold of having intrinsic resistance to this allylamine (≥97% per the cutoff used by CLSI) (38).
Interestingly, the T. indotineae isolates in our study did not have elevated itraconazole MICs, which ranged from ≤0.03 to 0.125 μg/mL. These results are somewhat consistent with a previous report from a large study in India which included 308 dermatophyte isolates from multiple centers in different geographic locations (6). In that study, terbinafine-resistant isolates were more common in the azole-susceptible group (77%) than in the azole-resistant group (51%), and conversely, azole-resistant isolates were more common in the terbinafine-susceptible group (42%) than in the terbinafine-resistant group (18%). Similarly, in an in vitro study that included 64 T. indotineae isolates, 53% of which had terbinafine MICs of >16 μg/mL, itraconazole MICs ranged from 0.016 to 2 μg/mL, with an MIC90 of 0.25 μg/mL and GM MIC of 0.15 μg/mL (30). Low itraconazole MICs were also observed in a limited number of T. indotineae isolates in Denmark (39).
There are some limitations to this study that need to be considered. Foremost, we do not have clinical information associated with these dermatophyte isolates. Thus, we are unable to determine if the T. indotineae isolates cultured from patients in North America were associated with recalcitrant infections similar to those reported in South Asia. In addition, we were not able to determine if there may be an association between resistance development and use of over-the-counter products that contain terbinafine, nor were we able to determine what types of infections occurred in patients from which resistant T. rubrum isolates were cultured. In terms of clinical application, many clinical microbiology laboratories do not identify dermatophytes to the species level by DNA sequence analysis, and it is currently unknown if T. indotineae can be distinguished from T. mentagrophytes or T. interdigitale by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). One possible means to potentially help identify T. indotineae isolates from T. mentagrophytes/Trichophyton interdigitale may be the use of the urease test, which is reported to be negative for T. indotineae but positive for these other two species (7, 10). Further studies are needed to confirm if this can reliably help identify potential T. indotineae isolates as part of routine clinical workflows.
In conclusion, terbinafine resistance was relatively common in Trichophyton isolates received by our reference laboratory between 2021 and 2022 from institutions throughout North America, and T. indotineae, a new and hypervirulent species responsible for outbreaks of dermatophytosis in parts of South Asia, was found. During this period, resistance to expanded-spectrum azoles, including itraconazole, was relatively rare. However, continued surveillance is needed, as are studies to determine if this resistance is associated with recalcitrant infections and what risk factors may predispose patients to them.
ACKNOWLEDGMENTS
N.P.W. has received grant support from Astellas, bioMérieux, F2G, Maxwell Biosciences, Mycovia, and Sfunga. All other authors report no conflicts.
Footnotes
Supplemental material is available online only.
Contributor Information
Nathan P. Wiederhold, Email: wiederholdn@uthscsa.edu.
Kimberly E. Hanson, University of Utah
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Havlickova B, Czaika VA, Friedrich M. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 3.de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Goker M, Rezaei-Matehkolaei A, Mirhendi H, Graser Y. 2017. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, Meis JF, Chowdhary A. 2018. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 61 61:477–484. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 5.Shen JJ, Arendrup MC, Verma S, Saunte DML. 2022. The emerging terbinafine-resistant trichophyton epidemic: what is the role of antifungal susceptibility testing? Dermatology 238:60–79. doi: 10.1159/000515290. [DOI] [PubMed] [Google Scholar]
- 6.Ebert A, Monod M, Salamin K, Burmester A, Uhrlaß S, Wiegand C, Hipler U-C, Krüger C, Koch D, Wittig F, Verma SB, Singal A, Gupta S, Vasani R, Saraswat A, Madhu R, Panda S, Das A, Kura MM, Kumar A, Poojary S, Schirm S, Gräser Y, Paasch U, Nenoff P. 2020. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: a multicentre study. Mycoses 63:717–728. doi: 10.1111/myc.13091. [DOI] [PubMed] [Google Scholar]
- 7.Tang C, Kong X, Ahmed SA, Thakur R, Chowdhary A, Nenoff P, Uhrlass S, Verma SB, Meis JF, Kandemir H, Kang Y, de Hoog GS. 2021. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex harboring the highly virulent, multiresistant genotype T. indotineae. Mycopathologia 186:315–326. doi: 10.1007/s11046-021-00544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Chandra U, Anchan VN, Verma P, Tilak R. 2020. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: results of a randomized pragmatic trial. Br J Dermatol 183:840–846. doi: 10.1111/bjd.19146. [DOI] [PubMed] [Google Scholar]
- 9.Sacheli R, Hayette MP. 2021. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel) 7:983. doi: 10.3390/jof7110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kano R, Kimura U, Kakurai M, Hiruma J, Kamata H, Suga Y, Harada K. 2020. Trichophyton indotineae sp. nov.: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 185:947–958. doi: 10.1007/s11046-020-00455-8. [DOI] [PubMed] [Google Scholar]
- 11.Verma SB, Zouboulis C. 2018. Indian irrational skin creams and steroid-modified dermatophytosis–an unholy nexus and alarming situation. J Eur Acad Dermatol Venereol 32:e426–e427. doi: 10.1111/jdv.15025. [DOI] [PubMed] [Google Scholar]
- 12.Siopi M, Efstathiou I, Theodoropoulos K, Pournaras S, Meletiadis J. 2021. Molecular Epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: emergence of terbinafine-resistant Trichophyton mentagrophytes type VIII locally and globally. J Fungi (Basel) 7:419. doi: 10.3390/jof7060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posso-De Los Rios CJ, Tadros E, Summerbell RC, Scott JA. 2022. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J Cutan Med Surg 26:371–376. doi: 10.1177/12034754221077891. [DOI] [PubMed] [Google Scholar]
- 14.Gu D, Hatch M, Ghannoum M, Elewski BE. 2020. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep 6:1153–1155. doi: 10.1016/j.jdcr.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edriss MT, Parker JJ, Pritchett EN. 2022. Response to Gu et al’s “Treatment resistant dermatophytosis: a representative case highlighting an emerging public health threat.” JAAD Case Rep 32:88–89. doi: 10.1016/j.jdcr.2021.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan AS, Chaturvedi S, Zhu Y, Todd GC, Yin L, Lopez A, Travis L, Smith DJ, Chiller T, Lockhart SR, Alroy KA, Greendyke WG, Gold JAW. 2023. Notes from the field: first reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morb Mortal Wkly Rep 72:536–537. doi: 10.15585/mmwr.mm7219a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinaldi MG. 1982. Use of potato flakes agar in clinical mycology. J Clin Microbiol 15:1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, Danziger L. 2019. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis 6:ofz262. doi: 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babiker A, Gupta N, Gibas CFC, Wiederhold NP, Sanders C, Mele J, Fan H, Iovleva A, Haidar G, Fernandes C. 2019. Rasamsonia sp: an emerging infection amongst chronic granulomatous disease patients. A case of disseminated infection by a putatively novel Rasamsonia argillacea species complex involving the heart. Med Mycol Case Rep 24:54–57. doi: 10.1016/j.mmcr.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 21.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J Clin Microbiol 48:741–752. doi: 10.1128/JCM.01948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5' end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- 25.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akaike H. 1974. A new look at the statistical model identification, p 215–222. In Parzen E, Tanabe K, Kitagawa G (ed), Selected papers of Hirotugu Akaike. Springer New York, New York, NY. [Google Scholar]
- 27.Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O. 2011. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60:685–699. doi: 10.1093/sysbio/syr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed. CLSI standard M38. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, Salamin K, Fratti M, Monod M. 2017. Terbinafine resistance of trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother 61:e00115-17. doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong X, Tang C, Singh A, Ahmed SA, Al-Hatmi AMS, Chowdhary A, Nenoff P, Graser Y, Hainsworth S, Zhan P, Meis JF, Verweij PE, Liu W, de Hoog GS. 2021. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the Trichophyton mentagrophytes species complex. Antimicrob Agents Chemother 65:e00056-21. doi: 10.1128/AAC.00056-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arendrup MC, Jorgensen KM, Guinea J, Lagrou K, Chryssanthou E, Hayette MP, Barchiesi F, Lass-Florl C, Hamal P, Dannaoui E, Chowdhary A, Meletiadis J. 2020. Multicentre validation of a EUCAST method for the antifungal susceptibility testing of microconidia-forming dermatophytes. J Antimicrob Chemother 75:1807–1819. doi: 10.1093/jac/dkaa111. [DOI] [PubMed] [Google Scholar]
- 32.Thomas J, Jacobson GA, Narkowicz CK, Peterson GM, Burnet H, Sharpe C. 2010. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther 35:497–519. doi: 10.1111/j.1365-2710.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- 33.Fachin AL, Ferreira-Nozawa MS, Maccheroni W, Martinez-Rossi NM. 2006. Role of the ABC transporter TruMDR2 in terbinafine, 4-nitroquinoline N-oxide and ethidium bromide susceptibility in Trichophyton rubrum. J Med Microbiol 55:1093–1099. doi: 10.1099/jmm.0.46522-0. [DOI] [PubMed] [Google Scholar]
- 34.Monod M, Feuermann M, Salamin K, Fratti M, Makino M, Alshahni MM, Makimura K, Yamada T. 2019. Trichophyton rubrum azole resistance mediated by a new ABC transporter, TruMDR3. Antimicrob Agents Chemother 63:e00863-19. doi: 10.1128/AAC.00863-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhary A, Singh A, Kaur A, Khurana A. 2022. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: a new challenge in the management of dermatophytosis. PLoS Pathog 18:e1010795. doi: 10.1371/journal.ppat.1010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, Gupta A, Gautam RK, Sharma PK, Jain D. 2018. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother 62:e01038-18. doi: 10.1128/AAC.01038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno-Sabater A, Normand AC, Bidaud AL, Cremer G, Foulet F, Brun S, Bonnal C, Ait-Ammar N, Jabet A, Ayachi A, Piarroux R, Botterel F, Houze S, Desoubeaux G, Hennequin C, Dannaoui E. 2022. Terbinafine resistance in dermatophytes: a French multicenter prospective study. J Fungi (Basel) 8:220. doi: 10.3390/jof8030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2022. Performance standards for antifungal susceptibility testing of filamentous fungi, 3rd ed. CLSI supplement M38M51S. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Astvad KMT, Hare RK, Jorgensen KM, Saunte DML, Thomsen PK, Arendrup MC. 2022. Increasing terbinafine resistance in Danish Trichophyton isolates 2019–2020. J Fungi (Basel) 8:150. doi: 10.3390/jof8020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jcm.00562-23-s0001.docx, DOCX file, 0.01 MB (14.8KB, docx)


