ABSTRACT
Bacterial vaginosis (BV) is the most common cause of vaginal discharge among reproductive-age women. It is associated with multiple adverse health outcomes, including increased risk of acquisition of HIV and other sexually transmitted infections (STIs), in addition to adverse birth outcomes. While it is known that BV is a vaginal dysbiosis characterized by a shift in the vaginal microbiota from protective Lactobacillus species to an increase in facultative and strict anaerobic bacteria, its exact etiology remains unknown. The purpose of this minireview is to provide an updated overview of the range of tests currently used for the diagnosis of BV in both clinical and research settings. This article is divided into two primary sections: traditional BV diagnostics and molecular diagnostics. Molecular diagnostic assays, particularly 16S rRNA gene sequencing, shotgun metagenomic sequencing, and fluorescence in situ hybridization (FISH), are specifically highlighted, in addition to multiplex nucleic acid amplification tests (NAATs), given their increasing use in clinical practice (NAATs) and research studies (16S rRNA gene sequencing, shotgun metagenomic sequencing, and FISH) regarding the vaginal microbiota and BV pathogenesis. We also provide a discussion of the strengths and weaknesses of current BV diagnostic tests and discuss future challenges in this field of research.
KEYWORDS: bacterial vaginosis, diagnosis, molecular diagnostics, vaginal infection
INTRODUCTION
Bacterial vaginosis (BV) is the most common cause of vaginal discharge among reproductive-age women (1). It is associated with multiple adverse health outcomes, including increased risk of acquisition of HIV (2) and other sexually transmitted infections (STIs) (3), as well as adverse birth outcomes (4). While it is well known that BV is a vaginal dysbiosis characterized by a shift in the vaginal microbiota from protective Lactobacillus species to facultative anaerobic bacteria (Gardnerella species) and strict anaerobic bacteria (BV-associated bacteria [BVAB]; i.e., Prevotella spp., Fannyhessea vaginae [formerly known as Atopobium vaginae], Sneathia spp., Megasphaera spp., etc.), its exact etiology remains unknown (5). This lack of understanding of BV pathogenesis has directly impacted advances in diagnosis, treatment, and prevention.
Since the mid-1950s (6), Gardnerella vaginalis (originally named Haemophilus vaginalis) has been the most frequently studied bacterium in BV pathogenesis, as it is present in 95 to 100% of clinically diagnosed BV cases (7, 8). However, G. vaginalis is found in virgins (9, 10) and in sexually active women with normal vaginal microbiotas (11). In addition, G. vaginalis colonization is not sufficient for BV development (12). Thus, G. vaginalis may be necessary but not sufficient for development of BV (13). Strains of G. vaginalis found in women with normal vaginal microbiotas and those with BV may vary in virulence properties (14). For example, certain genotypes of G. vaginalis produce sialidase, while others do not. Sialidase facilitates the destruction of the protective mucus layer on the vaginal epithelium by hydrolysis of sialic acid on the glycans of mucous membranes and may be involved in BV biofilm development (15). In addition, recent developments in molecular genetics have shed light on the genetic heterogeneity and taxonomic diversity within the genus Gardnerella. Vaneechoutte et al. performed whole-genome sequence analysis (digital DNA-DNA hybridization and average nucleotide identity) for 81 sequenced full genomes of the genus Gardnerella, noting the existence of at least 13 groups distinct enough to be classified as separate species within the taxon formerly known as G. vaginalis (16). This resulted in an emended description of G. vaginalis and the inclusion of more Gardnerella spp., namely, Gardnerella leopoldii, Gardnerella piotii, and Gardnerella swidsinskii.
In addition to Gardnerella spp., given the polymicrobial nature of BV, other BVAB are being investigated in BV pathogenesis research because of their high sensitivity and/or specificity for BV (17) and are correspondingly included in BV diagnostic research. The purpose of this minireview is to provide an updated overview of the range of tests currently used for the diagnosis of BV in both clinical and research settings. This article is divided into two primary sections: traditional BV diagnostics and molecular diagnostics. Molecular diagnostic assays, particularly 16S rRNA gene sequencing, shotgun metagenomic sequencing, and fluorescence in situ hybridization (FISH), are specifically highlighted, in addition to multiplex nucleic acid amplification tests (NAATs), in contrast to prior reviews (18, 19). This is due to their increased use in clinical practice (NAATs) and research studies (16S, shotgun metagenomic sequencing, and FISH) regarding the vaginal microbiota and incident and recurrent BV pathogenesis. We also provide a discussion of the strengths and weaknesses of current BV diagnostic tests and discuss future challenges in the field of BV diagnostic research.
TRADITIONAL BV DIAGNOSTIC METHODS
Laboratory tests: vaginal Gram stain (Nugent score and Ison-Hay criteria).
BV is diagnosed microbiologically using a vaginal Gram stain. There are two traditional scoring systems. The first was initially described by Spiegel et al. in 1983 (20) and later modified by Nugent et al. in 1991 (21). This scoring system quantifies different bacterial morphotypes, rendering the reading subjective and requiring an experienced slide reader. To determine the Nugent score, the vaginal Gram stain is assessed for the presence of large Gram-positive rods (Lactobacillus morphotypes; a decrease in Lactobacillus spp. is scored from 0 to 4), small Gram-variable rods (Gardnerella morphotypes; scored as 0 to 4), and curved Gram-variable rods (Mobiluncus morphotypes; scored as 0 to 2), with scores ranging from 0 to 10. A score of 0 to 3 is consistent with an optimal vaginal microbiota, 4 to 6 is consistent with intermediate vaginal microbiota, and 7 to 10 is consistent with BV. The Nugent score was developed including data from pregnant women (21) and should be used cautiously in the setting of menopause (22). Use of a vaginal Gram stain is more specific for a diagnosis of BV than Amsel’s clinical criteria (see “Amsel criteria” below); however, it is more time-consuming, and results may vary from person to person (18). Results are not available at the point of care (POC) unless clinical staff are appropriately trained to perform this technique and the necessary supplies (i.e., Gram stain solutions) are available. Another disadvantage of the Nugent score is its inability to identify other bacterial morphotypes associated with BV beyond those included in the current scoring system. In addition, it does not define symptom severity of BV.
Given these complexities, a simpler version of the vaginal Gram stain reading system was developed by Ison and Hay in 2002 (23), in which the composition of the vaginal microbiota is classified in one of three categories: normal, intermediate, and BV, depending upon the relative amount of Lactobacillus morphotypes (many, equal amount, or few) compared to Gardnerella morphotypes (few, equal amount, and many) with grades of I (normal), II (intermediate), and III (BV). In contrast to the Nugent score, a quantitative evaluation of bacterial morphotypes is not performed in this scoring system. In a study of 213 nonpregnant Indian women ages 15 to 49 years presenting with or without symptoms of vaginitis, the Ison-Hay criteria were compared to the Nugent score (24). All slides that had a BV Nugent score of ≥7 were found to be grade III (BV) by Ison and Hay’s method. However, among slides with an intermediate Nugent score of 4 to 6, 17 (47.2%) were placed in grade III (BV) and 3 (8.3%) in grade I (normal) using Ison and Hay’s method. Among slides with a normal Nugent score of 0 to 3, 7 (6.5%) were placed in grade II using Ison and Hay’s method. Sensitivity, specificity, positive predictive value, negative predictive value, and kappa value when evaluating Ison and Hay’s criteria using the Nugent score as the gold standard were ≥97.2%, ≥88.1%, ≥80.4%, ≥97.1%, and ≥0.83, respectively. Overall, this study found a strong association of a normal Nugent score with Ison-Hay grade I and a Nugent score of ≥7 with Ison-Hay grade III, with only 6.5% of women with a Nugent score of ≤3 falling in Ison-Hay grade II. The authors concluded that Ison and Hay’s method shows good agreement with the Nugent score and can be used as an alternative method in busy health care settings (24). However, it does not identify any morphotypes of BVAB beyond Gardnerella.
It is also important to note that neither the Nugent score nor Ison and Hay’s method differentiates particular Lactobacillus species. The protective role of Lactobacillus iners in the vaginal microbiota has been questioned as it has been found during vaginal dysbiosis, including BV (25, 26). The genome of L. iners encodes inerolysin, a pore-forming toxin related to vaginolysin in G. vaginalis (26). In addition, L. iners may present as a small Gram-negative coccobacillus, a microscopic feature similar to G. vaginalis, which could increase the Nugent score.
Finally, a convolutional neural network model was recently developed and optimized to evaluate its ability to automatically identify and classify the 3 Nugent score categories from microscopic images (27). This model outperformed health care providers in terms of its accuracy and stability for a diagnosis of BV and may offer a promising future translational approach in automating BV diagnosis with proper supporting hardware.
POC tests.
(i) Amsel criteria. Initially BV diagnosis was entirely based upon nonspecific clinical criteria, resulting in the term “nonspecific vaginitis” (28). The Amsel criteria, first published in 1983, have been the criteria most commonly used to make a clinical diagnosis of BV (29). They include (i) a thin, homogeneous grayish-white vaginal discharge, (ii) a vaginal pH of >4.5, (iii) a positive whiff test (a fishy, amine odor after KOH is mixed with vaginal secretions), and (iv) the presence of ≥20% clue cells (vaginal epithelial cells with a grainy border and speckled appearance due to being coated with coccobacillary microorganisms) per high-power field on wet mount of vaginal secretions. Three or four Amsel criteria are needed to make a clinical diagnosis of BV. The sensitivity and specificity of the Amsel criteria are 37% to 70% and 94% to 99%, respectively, compared with the Nugent score (18). It is noteworthy that the Amsel criteria were developed using data from college-age women (29) and do not account for the effect that hypoestrogenism has on the vaginal pH and the composition of the vaginal microbiota in the setting of menopause. Thus, these criteria should be used cautiously in menopausal women (22). The Amsel criteria also do not define the symptom severity of BV (i.e., mild versus moderate versus severe).
(ii) OSOM BVBlue test. The OSOM BVBlue test (Sekisui Diagnostics, Burlington, MA) is a CLIA-waived chromogenic test that detects increased vaginal sialidase activity (≥7.8 U) produced by G. vaginalis and Prevotella, Bacteroides, and Mobiluncus spp. (30, 31). Results are available within 10 min, allowing quick diagnosis and prompt treatment. Sensitivity is 92.8% and specificity is 98% compared to the Nugent score (31). This test is most useful where microscopic capabilities are not available. One limitation of the OSOM BVBlue Test is that it does not rule out the presence of vaginal yeast infection, Trichomonas vaginalis infection, or other STIs. In addition, similar to the Amsel criteria, it does not define the symptom severity of BV. To prevent adverse performance of the test, it should not be used in women who have douched, engaged in vaginal sexual intercourse, or used spermicides, vaginal lubricants, or feminine deodorant sprays within 72 h prior to testing (31).
(iii) FemExam test card. The FemExam test card (Cooper Surgical, Trumbull, CT) measures vaginal pH and trimethylamine (a metabolic by-product of G. vaginalis) on card 1, while card 2 measures the proline aminopeptidase (PIP) activity of G. vaginalis, eliminating the need for pH paper and KOH for the whiff test (32). For card 1, vaginal fluid is swabbed onto the pH test site, which induces a qualitative colorimetric reaction. If the vaginal fluid pH is ≥4.7, a blue plus sign appears; a minus sign appears if the pH is ≤4.7. The same specimen is then swabbed onto the amine test site, again inducing a colorimetric reaction within 2 min. If trimethylamine is present, a blue plus sign appears, while a minus sign indicates nondetectable levels of trimethylamine. Card 2 contains a test site for G. vaginalis PIP activity that uses an enzymatically activated chromogen. A second vaginal specimen is swabbed onto the test site on card 2 that consists of a chromogen (fast red) and a PIP substrate (l-propyl-β-naphthylamide). In the presence of G. vaginalis PIP activity, a colorimetric reaction takes place, producing a pink color on the swab tip within 5 min (32). Neither test card should be used if blood is present in the vaginal fornix, so women with bloody vaginal discharge should not undergo this test (32). The combined sensitivity and specificity of both cards are 91% and 61.5%, respectively, compared with the Nugent score in a study of 230 women with vaginal discharge syndrome (18). This POC test has the advantage of not requiring a microscope. However, it is not FDA cleared in the United States and is primarily used in resource-poor settings; it is not a preferred diagnostic method for BV due to its lower specificity (33). It, too, does not define symptom severity of BV.
MOLECULAR DIAGNOSTIC METHODS
Molecular diagnostic methods (including direct probe assays, NAATs, 16S rRNA sequencing, shotgun metagenomic sequencing, and fluorescence in situ hybridization) for BV are advantageous over traditional diagnostic methods, as they do not require the use of microscopy or other procedures at the point of care, which reduces the burden on busy clinicians. They are also objective, as they are based on the detection of specific bacterial nucleic acids and are able to detect fastidious BVAB; many can be performed on self-collected vaginal specimens as well as clinician-collected specimens (18, 34). In addition, some BV NAATs are able to detect other microorganisms beyond BVAB (e.g., Candida spp. and T. vaginalis) (35, 36). However, one limitation of these newer methods is their higher cost compared to traditional BV diagnostic methods. In addition, some are either not yet commercially available or not yet a preferred BV diagnostic method in national guideline recommendations (33) (i.e., 16S rRNA gene sequencing, shotgun metagenomics sequencing, fluorescence in situ hybridization) and are currently used primarily in research settings.
Direct probe assays.
Direct probe assays introduce a DNA probe into a vaginal fluid specimen. The probe then binds to specific sequences from a particular bacterium within the specimen and can detect the presence of different bacteria in a single specimen (18). One example of a commonly used direct probe assay used in BV diagnosis is the Affirm VP III assay (Becton Dickinson, Sparks, MD). This is a moderately complex DNA probe test that detects high concentrations of G. vaginalis nucleic acids (>5 × 105 CFU of G. vaginalis/mL) in vaginal fluid, with results being available in 30 min to 1 h. Sensitivity is 90% and specificity is 97% compared with detection of clue cells on vaginal wet mounts, while sensitivity is 94% and specificity is 81% compared to the Nugent score (18). This test is most useful for symptomatic women in conjunction with vaginal pH measurement and presence of amine odor (sensitivity increases to 97%) (18). This test can also be used to detect Candida spp. as well as T. vaginalis; however, it is not FDA cleared for T. vaginalis diagnosis in men. However, it is limited by the fact that it detects only G. vaginalis for BV diagnosis and does not detect other BVAB. This is problematic, as G. vaginalis has been found in sexually active women with normal vaginal microbiotas (11) and G. vaginalis colonization does not always cause BV (12).
NAATs.
NAATs, such as PCR, can detect as little as one microorganism in a vaginal specimen (18); these tests are more sensitive than direct probe assays. Quantitative PCR (qPCR) is used to quantify copy numbers of a given DNA template. Quantification of bacterial species in the vaginal microbiota by qPCR is a popular tool for identifying and measuring specific vaginal microorganisms in research settings (37–39). This method is accurate but time-consuming and requires standard curve generation for each microorganism of interest (40). Research is still in progress to determine specific thresholds for these microorganisms (39) and the concentrations at which key BVAB contribute to BV pathogenesis. qPCR has limitations, including that probes must be designed for each microorganism of interest that target an amplicon within that microorganism but do not cross-react with target DNA sequences in other microorganisms. The design of qPCR primers must also rely on existing sequence databases, which may be incomplete. As such, it is a best-effort process to design a primer that is specific to the microorganism in question. An additional limitation is cost, as this can add up if more than several microorganisms are assayed. Beyond several targeted organisms, other sequencing assays, such as 16S rRNA gene sequencing and shotgun metagenomics sequencing, may be considered, as the cost per sample sequenced does not depend on the number of microorganisms assayed. Nevertheless, qPCR is still a valuable tool to better understand the microenvironment of the vaginal tract and inform development of commercial BV NAAT tests.
Along these lines, Fredricks et al. developed a panel of taxon-directed 16S rRNA gene PCR assays for the detection of 17 vaginal bacterial species (17). Using vaginal specimens from 81 women with and 183 women without BV, they assessed the prevalence of each of these vaginal bacterial species. Women with BV had an average of 11.1 species (range, 5 to 16). In contrast, women without BV had an average of 3.6 species (range, 0 to 14). The detection of either BVAB2 or Megasphaera type 1 had a sensitivity of 95.9% and specificity of 93.7% compared to the Nugent score (17). Since this study and given the polymicrobial nature of BV, quantitative multiplex PCR assays have become a focus of development for a commercial diagnosis of BV (18). Multiplex PCR uses unique primer and probe sets that bind to regions of the 16S rRNA gene to provide quick and simple results for the molecular diagnosis of BV using proprietary algorithms for each assay (18). Various BVAB have different positive predictive values for BV diagnosis if used alone. However, the combined detection of several BVAB might improve test characteristics (18).
As of January 2023, there are six multiplex NAATs commercially available for BV diagnosis in cis-gender women in the United States. These tests include the BD MAX vaginal panel (Becton Dickinson, Sparks, MD) (36), Aptima BV (Hologic, Marlborough, MA) (41), GeneXpert Xpress multiplex vaginal panel (MVP) (Cepheid, Sunnyvale, CA) (35), NuSwab VG (LabCorp, Burlington, NC) (42), OneSwab BV panel PCR with Lactobacillus profiling by qPCR (Medical Diagnostic Laboratory, Hamilton, NJ) (43), and SureSwab BV (Quest Diagnostics, Secaucus, NJ) (Table 1). Three are FDA cleared for use in symptomatic women (BD MAX vaginal panel, Aptima BV, and GeneXpert Xpress MVP), while the others are laboratory-developed tests that have to be internally validated prior to their use. The assays that have received FDA clearance for BV diagnosis all have excellent sensitivity as a requirement for clearance. They have not been shown to be substantively different in accuracy; however, there are few studies that show head-to-head comparisons of these tests (35). The specific vaginal bacterial targets included in each of the tests, relative cost, specimen types, time to result, and additional comments pertinent to each test (i.e., sensitivity and specificity) are listed in Table 1. It is important to note that the vaginal bacterial targets included in the assays vary, due to the etiology of BV remaining incompletely understood. These tests can be performed on clinician-collected and self-collected vaginal specimens, with results being available within 60 min to 24+ h, depending upon the molecular diagnostic platform used. Use of these assays removes the need for use of microscopy, reader expertise, and maintenance of equipment, which are requirements when Amsel or vaginal Gram stain criteria (Nugent score or Ison-Hay criteria) are used for a diagnosis of BV. In addition, use of a BV NAAT test was shown to have higher sensitivity and specificity for BV (≥96.2% and ≥92.4%, respectively) than clinician diagnoses (83.4% and 85.5%, respectively) and in-clinic assessments (75.9% and 94.4% for the Amsel criteria, respectively) in one study (41). However, these tests are more costly than traditional BV diagnostic methods (19), have not been studied in transgender populations, are recommended only for use in symptomatic cis-gender women (33), do not define the severity of BV symptoms, and are unable to differentiate persistence, relapse, or reinfection among women with recurrent BV. In addition, the gold standard used to compare the performance of the new BV NAATs is difficult to define, although Nugent score criteria are often used. This is a limitation for truly defining the clinical sensitivity and specificity for commercially available NAATs and should be kept in mind when these new assays are evaluated for use by laboratories.
TABLE 1.
BV nucleic acid amplification tests currently available in the United Statesa
| Assay | Time to result | Equipment requirement | Specimen type(s) | Bacterial targets | Relative costb | Comments |
|---|---|---|---|---|---|---|
| BD MAX vaginal panel | <8 h | BD Max automated system | Clinician-collected, self-collected vaginal swabs | Lactobacillus spp. (L. crispatus and L. jensenii), G. vaginalis, F. vaginae, BVAB2, and Megasphaera type 1 | $$$ | CLIA high complexity; DNA amplification; results reported as BV positive or negative; FDA cleared; has 90.5% sensitivity and 85.8% specificity for BV diagnosis compared to Amsel criteria and Nugent score |
| Aptima BV assay | <8 h | Tigris or Panther automated system | Clinician-collected vaginal swabs, endocervical swabs, or endocervical samples in PreservCyt medium | Lactobacillus spp. group (L. gasseri, L. crispatus, L. jensenii), G. vaginalis, F. vaginae | $$$ | CLIA high complexity; RNA amplification; results reported as BV positive or negative; FDA cleared; sensitivity and specificity are 95.0%–97.3% and 85.8%–89.6%, respectively, compared to the Nugent score (plus the Amsel criteria for intermediate Nugent scores), depending on the use of clinician- or patient-collected vaginal swabs |
| GeneXpert Xpress multiplex vaginal panel | 60 min | GeneXpert Instrument (variable module no. available) | Clinician-collected, self-collected vaginal swabs | F. vaginae, BVAB2, Megasphaera type 1 | $$$$ | CLIA moderate complexity; DNA amplification; results reported as BV positive or negative; FDA cleared; high positive percent agreement, 93.6–99.0% for both clinician-collected and self-collected vaginal swabs, and negative percent agreement of 92.1%–99.8% for both types of specimens (comparator, BD MAX vaginal panel) |
| NuSwab VG | 3–4 days (per LabCorp website) | PCR machine | Vaginal specimen collected in Aptima transport tube | F. vaginae, BVAB2, Megasphaera type 1 | $$$ | CLIA high complexity; laboratory-developed test; has to be internally validated; DNA amplification; results reported as species with interpretation for BV: score: negative for BV (0–1), intermediate (2), positive for BV (3–6); sensitivity of 96.7% and specificity of 92.% compared to Amsel and Nugent |
| OneSwab BV panel PCR with Lactobacillus profiling by qPCR | >24 h | Real-time PCR system | Vaginal specimens (OneSwab transport tube), ThinPrep Pap specimens | Lactobacillus spp. (L. crispatus, L. gasseri, L. jensenii, L. iners), G. vaginalis, F. vaginae, BVAB2, Megasphaera types 1 and 2 | $$$ | CLIA high complexity; laboratory-developed test; has to be internally validated; results reported as species with interpretation for BV: normal microbiota, transitional microbiota, abnormal microbiota; sensitivity of 92%, specificity 95% compared to Amsel and Nugent |
| SureSwab BV | >24 h | PCR machine | Vaginal specimens collected in Aptima transport tube | Lactobacillus spp., G. vaginalis, F. vaginae, BVAB2, Megasphaera type 1 | $$$ | CLIA high complexity; laboratory-developed test; has to be internally validated; results reported as species with interpretation for BV: not supportive, equivocal, supportive |
BD, Becton Dickinson; BV, bacterial vaginosis; CLIA, Clinical Laboratory Improvement Amendments; FDA, Food and Drug Administration.
Costs vary by location and laboratory testing volume. Relative costs are shown as higher ($$$$) or lower ($$$) than other tests, assuming all factors are equal.
16S rRNA gene sequencing.
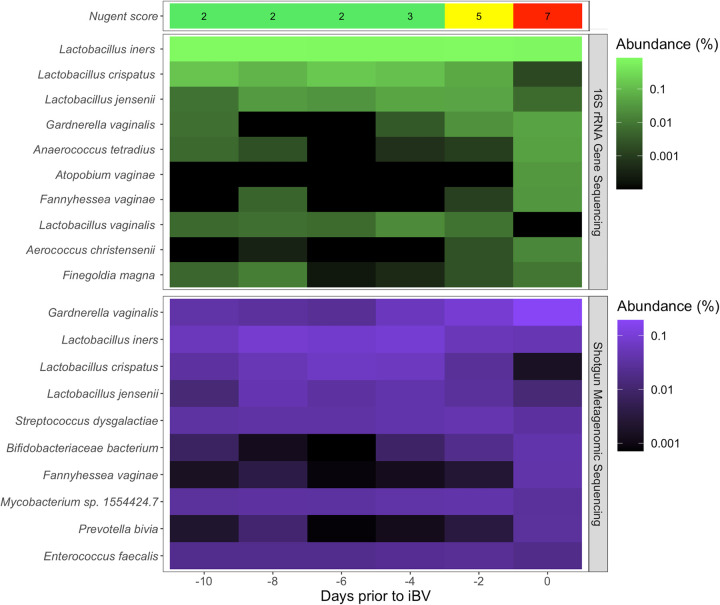
To address the limitations of qPCR, researchers sought out universal genes that exist in all microorganisms of interest. The 16S rRNA gene was selected to assay bacterial communities (44). Universal primers were designed to allow the amplification of a given portion of this gene, an amplicon, to apply PCR to the entire pool of DNA isolated from a bacterial community (45). In practice, these primers achieve in excess of 95% amplification, missing only a small proportion of microorganisms in the community depending upon the primer selected (46). In order to achieve a high level of universality, the primers are often degenerate, including a mixture of closely related sequences (47). After amplification of the amplicon in question, which also ligates to sequencing primers, the resulting DNA can be subjected to a sequencing assay (48). Originally, only Sanger sequencing was available, allowing researchers to look at the breadth of microorganisms existing in a community, which could not be done with individual qPCR assays. With the advent of less expensive high-throughput sequencing technologies in the early 2000s, this technique was applied to more research studies at a much lower cost. The sequencing reads produced are matched against a 16S rRNA database to classify each sequencing read and determine the composition and relative abundance of all microorganisms in the community (34, 49) (Fig. 1).
FIG 1.
Comparison of 16S rRNA gene sequencing and shotgun metagenomics sequencing results for bacterial microorganisms in woman who developed incident BV (34). The Nugent score is presented at the top and indicates normal (0 to 3), intermediate (4 to 6), or high (7 to 10), and the number of days prior to the day of incident BV (iBV) (day 0) is given at the bottom. Days sequenced using 16S rRNA gene sequencing are in green, while the same days sequenced using shotgun metagenomics are colored in purple. Each bacterial microorganism’s abundance is displayed as a log-normalized relative abundance.
Despite these advances, 16S rRNA gene sequencing has its limitations (50). The PCR that is performed to amplify DNA from the entire community subjects the assay to bias, as some microorganisms are amplified at a higher efficiency than others. Further, if the universal primer selected does not amplify a given microorganism’s 16S rRNA gene, that microorganism will be missing from the sequencing data. The 16S rRNA gene databases also present a limitation, as only microorganisms that have been deposited in these databases will be matched when the sequencing reads are mapped. Finally, the specific amplicon chosen that covers a given region of the 16S rRNA gene can influence the ability to detect certain microorganisms (51). Hence, several blind spots can exist whereby certain microorganisms in the community may not be seen or may be misclassified due to limitations of the universal primers or 16S rRNA gene databases. Nonetheless, this technique provides a cost-effective method to assay an entire community of vaginal bacteria, achieving a breadth of community analysis which is not possible with individual qPCR assays. This presents a powerful tool for researchers investigating the vaginal microbiota and BV pathogenesis (34, 52–54) but is not typically performed in clinical practice, as several hundred specimens must be batch tested in order to make the method cost-effective and the amount of time needed to perform these assays would introduce too large a delay in patient care to be clinically useful.
SMS.
As sequencing costs have rapidly declined, a more comprehensive approach to sequencing bacterial communities has come into more common use. The use of shotgun metagenomic sequencing (SMS) can sequence all DNA from the vaginal microbiota (55). The DNA is fragmented into small pieces and sequenced using a high-throughput sequencing technology. This method bypasses the initial PCR using universal primers, eliminating this bias. It also ensures that microorganisms which are not well matched by universal primers are not missed, because all of the DNA is sequenced. While 16S rRNA gene sequencing performs a simple bacterial census, SMS sequences all of the DNA and allows assessment of not only the identity of all of the bacteria present in a community but also the entire DNA sequences of these bacteria (Fig. 2). However, this requires much more sequencing effort than 16S rRNA gene sequencing, and hence, the cost of SMS is often more that 10-fold higher per sample (56). The amount of additional cost is dictated by how much sequencing per sample is desired. More sequencing per sample will provide a more comprehensive picture of the microorganisms present in the community, along with their functional potential.
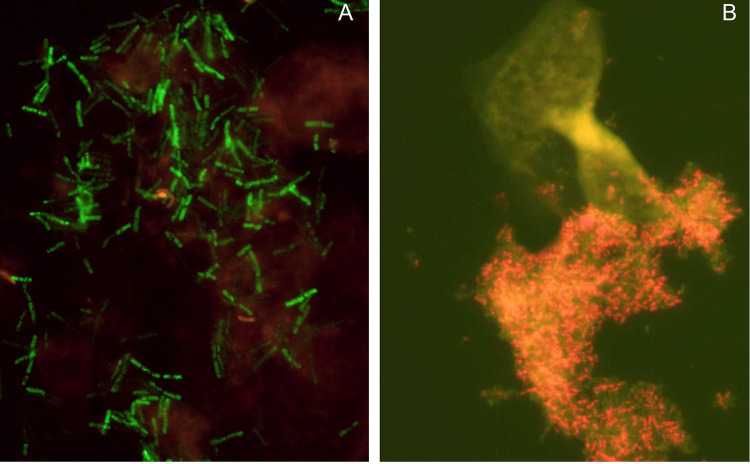
FIG 2.
Example of BV diagnosis using fluorescence in situ microscopy and PNA FISH probes. Lactobacillus-specific (Lac633-Alexa Fluor 488) and Gardnerella-specific (Gard162-Alexa Fluor 594) PNA FISH probes allowed a quick (<3-h) and accurate (85% sensitivity and 98% specificity) BV diagnosis following Ison and Hay’s criteria. The Lac633 probe marked the Lactobacillus spp. with a strong green signal, while Gard162 marked the Gardnerella spp. with a red signal. Sample A was obtained from a woman with a Nugent score of <3, while sample B was obtained from a woman with a Nugent score of >7. Some nonspecific fluorescence can be observed in both images, but the difference between the woman without BV and the woman with BV is evident. These images were obtained from a previous study (82).
In addition to the higher cost, SMS has other limitations. Since a specific gene is not PCR amplified out of the entire DNA, as is done in 16S rRNA gene sequencing, much of the sequencing effort in SMS may be expended on sequencing host DNA (56). For this reason, SMS was initially applied to bacterium-rich environments, such as microbial mats (57) and the gut microbiome (58). The ratio of bacterial DNA to other DNA is a paramount factor that drives the cost of SMS per sample (59). For example, if a given sample, such as a stool sample, includes roughly 50% bacterial DNA, 600 Gb of sequencing performed on a high-throughput sequencer will yield about 300 Gb of bacterial sequencing reads. On the other hand, if an ocular swab contains about 1% bacterial DNA compared to 99% other DNA (host, etc.), then the same expenditure for 600 Gb of high-throughput sequencing will yield only about 6 Gb of bacterial DNA. Hence, to achieve the coverage of 300 Gb of bacterial sequencing reads on ocular swabs, one would have to pay for 50 times as much sequencing and buy 30,000 Gb of sequencing. This can be cost prohibitive and a major driving factor in the adoption of SMS to assay high-bacterial-burden communities (59). However, as the cost of sequencing decreases, it may become more feasible to use SMS to assay lower-bacterial-burden samples. Providing a clinically relevant interpretation of SMS data is currently a challenge given the ongoing uncertainties in BV etiology and pathogenesis, as is widespread availability of the equipment and expertise needed to obtain and analyze these data. Interpretable software for SMS would be beneficial to provide clinicians with actionable results.
FISH.
A prominent feature of BV is the presence of clue cells, one of the Amsel criteria (29). Despite being a known feature of BV for decades, it was not until 2005 that the seminal work of Swidsinski et al. confirmed that the clue cell was a vaginal epithelial cell coated with an adherent bacterial biofilm (60). It is now well established that BV is a biofilm infection. The biofilm is composed mainly of Gardnerella spp., although it is polymicrobial (61). Being able to accurately detect the presence of this polymicrobial biofilm is a highly specific marker for BV diagnosis (62). To achieve this goal, FISH is a promising probe-based technique, as it combines visual information from microscopy with histochemical techniques and specificity provided by molecular probes (63).
Traditionally, the use of FISH for bacterial identification is based on the hybridization of a synthetic DNA oligomer coupled to a fluorophore that is complementary to a target 16S RNA sequence (64). FISH requires the fixation of a sample that will improve bacterial cell wall permeabilization, prior to the hybridization step, which normally occurs at temperatures ranging from 35 to 60°C, depending upon the sequence of the probe (65). Sample observation is then performed using a fluorescence light microscope. Although this technique can be very sensitive and specific, it is more time-consuming and less sensitive that qPCR (66). However, unlike NAATs, FISH does not require extraction and amplification of target biological material or controls for absolute quantification of bacteria in biological samples (67). Importantly, FISH can be combined with flow cytometry for high-resolution automated analysis of mixed microbial populations (68). A possible strategy for high-throughput diagnosis would first analyze the samples by flow cytometry. Once large numbers of the target species are found, the sample would then be used for direct visualization by fluorescence microscopy.
The use of FISH for BV diagnosis has been shown in multiple studies. The first was performed by Swidsinski et al., using 38 DNA-based genus- and species-specific probes, including a novel probe targeting Gardnerella spp. (60). While this was a relatively small study with 3 groups of 20 women each, the results confirmed that Gardnerella spp. was the predominant bacterial species in specimens obtained from women with BV, while Lactobacillus spp. were the main constituents in specimens from healthy premenopausal women. Furthermore, Gardnerella spp. dominated the BV biofilm and were detected only in BV cases, while only small numbers of dispersed Gardnerella spp. were found in a few healthy controls. Importantly, BV biofilms included other species besides Gardnerella spp., but only at very low concentrations (60). The central role of Gardnerella spp. in the BV biofilm has been confirmed by multiple subsequent studies. In a follow-up study by Swidsinski et al. in women scheduled to undergo curettage or laparoscopic salpingectomy, Gardnerella spp. biofilms were only found in women with BV (69). More recently, in a study involving 196 women and using 2 multiplex DNA probes assays, Jung et al. confirmed that Gardnerella spp. dominated biofilms were present in women with BV while Lactobacillus spp. dominated the vaginal microbiota of healthy controls (70). A more recent study of 60 pregnant women demonstrated that, besides Gardnerella spp., F. vaginae, and Sneathia spp. were major constituents of the BV biofilm (71). In an additional study of 500 vaginal samples, Gardnerella spp. and F. vaginae were found to be the most commonly represented species in the BV biofilm (72).
DNA-based Gardnerella spp.-specific probes have also been used to assess the presence and relevance of Gardnerella in other medical conditions, such as inflammatory bowel disease (73). Other target species have also been considered. Srinivasan et al. compared the abundances of two curved Gram-negative rods, Mobiluncus spp. and BVAB1 (recently renamed “Candidatus Lachnocurva vaginae” [74]), with DNA-based FISH probes, in women with BV or normal vaginal microbiota, comparing these results with qPCR and metagenomic analyses to determine if Mobiluncus spp. observed in vaginal Gram stains are actually BVAB1 (“Candidatus Lachnocurva vaginae”) (75).
Over the years, several improvements in FISH technology have been achieved (76). A major breakthrough was the substitution of DNA probes by peptide-nucleic acid (PNA) probes, which allowed significantly better permeabilization steps and enhanced sensitivity and specificity of the FISH technique (77). PNA probes are analogues of DNA probes but have an uncharged polyamide backbone instead of a sugar-phosphate backbone (78). This results in stronger hybridization due to the absence of electrostatic repulsion between the PNA probe and the negatively charged sugar-phosphate backbone of the target (79). The first PNA probes designed to study BVAB were highly specific for Gardnerella spp. (80) and Lactobacillus spp. (81). Machado et al. then demonstrated the high specificity and accuracy of a duplex PNA approach for the diagnosis of BV in clinical samples (82), following the Ison-Hay criteria (23). Hardy et al. used a different approach, by developing a PNA probe specific for F. vaginae (83). While the dual detection of Gardnerella spp. and F. vaginae serves as a highly specific marker for BV (84), the F. vaginae probe alone had a lower sensitivity (~67%) (83). In an attempt to improve this sensitivity, a more robust F. vaginae probe was recently developed that can be used in a multiplex assay together with a Gardnerella spp. probe (83). While data reveal in vitro sensitivity and specificity of 100% and 99.9%, respectively, the real-life efficiency of this probe in vaginal samples of women with BV has yet to be determined.
While there is yet no commercial FISH-based assay for BV diagnosis, the success of FISH as a diagnostic tool to detect bacterial infections has been well established through decades of research, and FISH has been approved by the U.S. FDA as well as the European Medicines Agency (EMA) for application in microbiological clinical analysis (85). Prominent existing clinical applications of FISH include diagnosis of bloodstream infections (86–88), infective endocarditis (89), and gastrointestinal infections (90), among others. The limitations of FISH are that it can be expensive and specialized laboratory equipment and expertise are needed to perform this technique. However, results are robust and can be obtained in as little as 6 h, and bacterial microorganisms of any kind can be detected in clinical specimens.
FUTURE CHALLENGES IN BV DIAGNOSIS
There has been considerable progress for clinicians in establishing a functional diagnosis of BV, facilitating therapeutic decisions, and allowing comparative scientific studies worldwide dealing with pathogenesis, epidemiology, natural history, complications, and treatment efficacy. Without consensus on diagnostic definitions, progress in any of these fields is not possible. The development of the Amsel criteria (29) represented a critical advance, particularly for clinicians, and it is still widely used in clinical practice. The limitations of this diagnostic criteria are now widely recognized and were followed by vaginal Gram stain diagnostic criteria (Nugent and Ison-Hay criteria) (21, 23) and a new era followed, allowing limited progress to be made in all the above-mentioned scientific fields. Moving forward, the use of artificial intelligence (AI) for BV microscopy interpretation may address the need for manual and time-consuming interpretation of vaginal Gram stains (27). However, the absence of vaginal microbiota culture data prevented further progress, which was rescued by the arrival of molecular diagnostic techniques described in the above-mentioned text.
Despite the plethora of molecular data now available describing the vaginal microbiota in women with and without BV, additional questions emerge. Molecular-based diagnostic tests are now widely available commercially, utilizing a variety of algorithms to establish diagnostic validity; hence the need for awareness for potential areas of conflict, especially in comparative therapeutic and other longitudinal studies. These new tests also have other limitations, as mentioned above, and have not been studied in transgender populations. A positive BV molecular diagnostic test, as with traditional methods of BV diagnosis (i.e., Amsel, Nugent, etc.), does not yet define the symptom severity of BV (i.e., mild versus moderate versus severe). In addition, BV molecular diagnostic tests today are also unable to differentiate persistence, relapse, or reinfection as the causal mechanism in women with recurrent BV. The ability to make the latter distinction could influence management of patients, as treatment may differ based on diagnosis (33, 91). Such diagnostic tests were also not designed as a test of cure following drug administration and should not be used as such. Most importantly, diagnostic molecular microbiology is only starting to be used as a prognostic marker with regard to risk of early or late recurrence of BV.
Next-generation sequencing, by allowing specific taxon and strain definition of newly recognized potential pathogens, will allow new progress to be made in understanding the pathogenesis of both incident and recurrent BV. The next challenge of diagnostic microbiology is the identification of antimicrobial resistance genes, including among uncultivatable BVAB (92), as prior studies have demonstrated metronidazole resistance among some common BVAB such as F. vaginae (93–95). This would potentially fulfill a massive clinical need expressed by frustrated clinicians dealing with the frequent challenge of women with refractory and recurrent BV. The future of vaginal microbiota studies is not just of critical value to advance BV diagnosis but will also play a vital role in answering multiple scientific questions regarding the pathogenesis and treatment of this common vaginal infection.
CONCLUSION
In conclusion, there are a growing number of BV diagnostic tests, particularly molecular diagnostic assays, which are available in clinical and research settings. The application of recently developed molecular diagnostic assays has dramatically transformed our approach to a poorly understood but extremely common clinical entity. Many of the traditional BV diagnostic tests have been simplistic, if not primitive, for far too long. Traditional diagnostic methods have remained stationary for multiple decades and facilitated, if not enabled, empiricism in therapeutic decisions. Recently, scientific leadership and hence progress have transferred to the diagnostic laboratory employing a variety of state-of-the-art molecular methodologies. Methods are emerging to rapidly provide clinicians, laboratorians, and researchers alike with diagnostic information to optimize therapeutic drug choice and more. New sophisticated microbiologic information provided to clinicians treating women with lower-genital-tract infections such as BV must be accompanied by simultaneous mandatory education, recognizing that not all clinicians are necessarily up to date on or proficient in nucleic acid amplification methods or gene biology. In addition, laboratorians should understand the limitations and gold standards used to determine the clinical sensitivity/specificity of the new BV molecular diagnostic assays to better aid in interpretation of results. It would be unfortunate if advances in scientific technology are not accompanied by progress not only in diagnostic accuracy but also in the understanding of the pathogenesis of BV.
ACKNOWLEDGMENTS
Christina A. Muzny, Nuno Cerca, Jacob H. Elnaggar, and Christopher M. Taylor are currently supported by R01AI146065-01A1 from the National Institute of Allergy and Infectious Diseases (NIAID). Christina A. Muzny, Jacob H. Elnaggar, and Christopher M. Taylor are also supported by R21AI167754-01 from NIAID.
Christina A. Muzny reports receiving grants to her institution from NIAID, Lupin, Abbott Molecular, and Gilead. She also reports honorarium and/or consulting fees from Scynexis, Cepheid, BioNTech, Visby Medical, Elsevier, UpToDate, Abbott Molecular, and Roche. Barbara Van Der Pol reports receiving grants to her institution, honoraria, and/or consulting fees from Abbott Molecular, Becton, Dickinson and Company, Binx Health, BioFire, Cepheid, Hologic, Rheonix, Roche, and SpeeDx. The other authors have no conflicts of interest to declare.
Contributor Information
Christina A. Muzny, Email: cmuzny@uabmc.edu.
Romney M. Humphries, Vanderbilt University Medical Center
REFERENCES
- 1.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. 2019. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 46:304–311. doi: 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 2.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, Donnell D, Celum C, Kapiga S, Delany S, Bukusi EA. 2012. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, Schwebke JR. 2010. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 202:1907–1915. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, II, Rao AV. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 5.Muzny CA, Schwebke JR. 2016. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis 214(Suppl 1):S1–S5. doi: 10.1093/infdis/jiw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner HL, Dukes CD. 1955. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol 69:962–976. doi: 10.1016/0002-9378(55)90095-8. [DOI] [PubMed] [Google Scholar]
- 7.Catlin BW. 1992. Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin Microbiol Rev 5:213–237. doi: 10.1128/CMR.5.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill GB. 1993. The microbiology of bacterial vaginosis. Am J Obstet Gynecol 169:450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 9.Bump RC, Buesching WJ, III.. 1988. Bacterial vaginosis in virginal and sexually active adolescent females: evidence against exclusive sexual transmission. Am J Obstet Gynecol 158:935–939. doi: 10.1016/0002-9378(88)90097-x. [DOI] [PubMed] [Google Scholar]
- 10.Bump RC, Sachs LA, Buesching WJ, III.. 1986. Sexually transmissible infectious agents in sexually active and virginal asymptomatic adolescent girls. Pediatrics 77:488–494. doi: 10.1542/peds.77.4.488. [DOI] [PubMed] [Google Scholar]
- 11.Aroutcheva AA, Simoes JA, Behbakht K, Faro S. 2001. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin Infect Dis 33:1022–1027. doi: 10.1086/323030. [DOI] [PubMed] [Google Scholar]
- 12.Hickey RJ, Forney LJ. 2014. Gardnerella vaginalis does not always cause bacterial vaginosis. J Infect Dis 210:1682–1683. doi: 10.1093/infdis/jiu303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwebke JR, Flynn MS, Rivers CA. 2014. Prevalence of Gardnerella vaginalis among women with lactobacillus-predominant vaginal flora. Sex Transm Infect 90:61–63. doi: 10.1136/sextrans-2013-051232. [DOI] [PubMed] [Google Scholar]
- 14.Castro J, Alves P, Sousa C, Cereija T, Franca A, Jefferson KK, Cerca N. 2015. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci Rep 5:11640. doi: 10.1038/srep11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. 2017. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One 12:e0172522. doi: 10.1371/journal.pone.0172522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaneechoutte M, Guschin A, Van Simaey L, Gansemans Y, Van Nieuwerburgh F, Cools P. 2019. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol 69:679–687. doi: 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 17.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. 2007. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 45:3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman JS, Gaydos CA. 2018. Molecular diagnosis of bacterial vaginosis: an update. J Clin Microbiol 56:e00342-18. doi: 10.1128/JCM.00342-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzny CA, Balkus J, Mitchell C, Sobel JD, Workowski K, Marrazzo J, Schwebke JR. 2022. Diagnosis and management of bacterial vaginosis: summary of evidence reviewed for the 2021 Centers for Disease Control and Prevention sexually transmitted infections treatment guidelines. Clin Infect Dis 74:S144–S151. doi: 10.1093/cid/ciac021. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel CA, Amsel R, Holmes KK. 1983. Diagnosis of bacterial vaginosis by direct Gram stain of vaginal fluid. J Clin Microbiol 18:170–177. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Gerwen OT, Smith SE, Muzny CA. 2023. Bacterial vaginosis in post-menopausal women. Curr Infect Dis Rep 25:7–15. doi: 10.1007/s11908-022-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ison CA, Hay PE. 2002. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect 78:413–415. doi: 10.1136/sti.78.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawla R, Bhalla P, Chadha S, Grover S, Garg S. 2013. Comparison of Hay's criteria with Nugent's scoring system for diagnosis of bacterial vaginosis. Biomed Res Int 2013:365194. doi: 10.1155/2013/365194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak J, Ravel J, Ma B, Ferreira CST, Tristao ADR, Silva MG, Marconi C. 2022. Characteristics associated with Lactobacillus iners-dominated vaginal microbiota. Sex Transm Infect 98:353–359. doi: 10.1136/sextrans-2020-054824. [DOI] [PubMed] [Google Scholar]
- 26.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. 2017. Lactobacillus iners: friend or foe? Trends Microbiol 25:182–191. doi: 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Zhang L, Zhao M, Wang Y, Bai H, Wang Y, Rui C, Fan C, Li J, Li N, Liu X, Wang Z, Si Y, Feng A, Li M, Zhang Q, Yang Z, Wang M, Wu W, Cao Y, Qi L, Zeng X, Geng L, An R, Li P, Liu Z, Qiao Q, Zhu W, Mo W, Liao Q, Xu W. 2021. Deep neural networks offer morphologic classification and diagnosis of bacterial vaginosis. J Clin Microbiol 59:e02236-20. doi: 10.1128/JCM.02236-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pheifer TA, Forsyth PS, Durfee MA, Pollock HM, Holmes KK. 1978. Nonspecific vaginitis: role of Haemophilus vaginalis and treatment with metronidazole. N Engl J Med 298:1429–1434. doi: 10.1056/NEJM197806292982601. [DOI] [PubMed] [Google Scholar]
- 29.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 30.Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. 2005. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol 43:1304–1308. doi: 10.1128/JCM.43.3.1304-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myziuk L, Romanowski B, Johnson SC. 2003. BVBlue test for diagnosis of bacterial vaginosis. J Clin Microbiol 41:1925–1928. doi: 10.1128/JCM.41.5.1925-1928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West B, Morison L, Schim van der Loeff M, Gooding E, Awasana AA, Demba E, Mayaud P. 2003. Evaluation of a new rapid diagnostic kit (FemExam) for bacterial vaginosis in patients with vaginal discharge syndrome in The Gambia. Sex Transm Dis 30:483–489. doi: 10.1097/00007435-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. 2021. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muzny CA, Blanchard E, Taylor CM, Aaron KJ, Talluri R, Griswold ME, Redden DT, Luo M, Welsh DA, Van Der Pol WJ, Lefkowitz EJ, Martin DH, Schwebke JR. 2018. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect Dis 218:966–978. doi: 10.1093/infdis/jiy243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillis RA, Parker RL, Ackerman R, Ackerman J, Young S, Weissfeld A, Trevino E, Nachamkin I, Crane L, Brown J, Huang C, Liu X, Van Der Pol B. 2023. Clinical evaluation of a new molecular test for the detection of organisms causing vaginitis and vaginosis. J Clin Microbiol 61:e01748-22. doi: 10.1128/jcm.01748-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaydos CA, Beqaj S, Schwebke JR, Lebed J, Smith B, Davis TE, Fife KH, Nyirjesy P, Spurrell T, Furgerson D, Coleman J, Paradis S, Cooper CK. 2017. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol 130:181–189. doi: 10.1097/AOG.0000000000002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jespers V, Menten J, Smet H, Poradosu S, Abdellati S, Verhelst R, Hardy L, Buve A, Crucitti T. 2012. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol 12:83. doi: 10.1186/1471-2180-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. 2009. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol 47:721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. 2010. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol 48:1812–1819. doi: 10.1128/JCM.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tettamanti Boshier FA, Srinivasan S, Lopez A, Hoffman NG, Proll S, Fredricks DN, Schiffer JT. 2020. Complementing 16S rRNA gene amplicon sequencing with estimates of total bacterial load to infer absolute species concentrations in the vaginal microbiome. mSystems 5:e00777-19. doi: 10.1128/mSystems.00777-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwebke JR, Taylor SN, Ackerman R, Schlaberg R, Quigley NB, Gaydos CA, Chavoustie SE, Nyirjesy P, Remillard CV, Estes P, McKinney B, Getman DK, Clark C. 2020. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol 58:e01643-19. doi: 10.1128/JCM.01643-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cartwright CP, Lembke BD, Ramachandran K, Body BA, Nye MB, Rivers CA, Schwebke JR. 2012. Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J Clin Microbiol 50:2321–2329. doi: 10.1128/JCM.00506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilbert DW, Smith WL, Chadwick SG, Toner G, Mordechai E, Adelson ME, Aguin TJ, Sobel JD, Gygax SE. 2016. Development and validation of a highly accurate quantitative real-time PCR assay for diagnosis of bacterial vaginosis. J Clin Microbiol 54:1017–1024. doi: 10.1128/JCM.03104-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woese CR, Fox GE. 1977. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA 74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker GC, Smith JJ, Cowan DA. 2003. Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555. doi: 10.1016/j.mimet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Thijs S, Op De Beeck M, Beckers B, Truyens S, Stevens V, Van Hamme JD, Weyens N, Vangronsveld J. 2017. Comparative evaluation of four bacteria-specific primer pairs for 16S rRNA gene surveys. Front Microbiol 8:494. doi: 10.3389/fmicb.2017.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambo F, Finotello F, Lavezzo E, Baruzzo G, Masi G, Peta E, Falda M, Toppo S, Barzon L, Di Camillo B. 2018. Optimizing PCR primers targeting the bacterial 16S ribosomal RNA gene. BMC Bioinformatics 19:343. doi: 10.1186/s12859-018-2360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janda JM, Abbott SL. 2007. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45:2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abellan-Schneyder I, Matchado MS, Reitmeier S, Sommer A, Sewald Z, Baumbach J, List M, Neuhaus K. 2021. Primer, pipelines, parameters: issues in 16S rRNA gene sequencing. mSphere 6:e01202-20. doi: 10.1128/mSphere.01202-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Der Pol WJ, Kumar R, Morrow CD, Blanchard EE, Taylor CM, Martin DH, Lefkowitz EJ, Muzny CA. 2019. In silico and experimental evaluation of primer sets for species-level resolution of the vaginal microbiota using 16S ribosomal RNA gene sequencing. J Infect Dis 219:305–314. doi: 10.1093/infdis/jiy508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, Sakamoto J, Koenig SS, Fu L, Zhou X, Hickey RJ, Schwebke JR, Forney LJ. 2013. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 1:29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feehily C, Crosby D, Walsh CJ, Lawton EM, Higgins S, McAuliffe FM, Cotter PD. 2020. Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. NPJ Biofilms Microbiomes 6:50. doi: 10.1038/s41522-020-00162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wensel CR, Pluznick JL, Salzberg SL, Sears CL. 2022. Next-generation sequencing: insights to advance clinical investigations of the microbiome. J Clin Invest 132:e154944. doi: 10.1172/JCI154944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klatt CG, Wood JM, Rusch DB, Bateson MM, Hamamura N, Heidelberg JF, Grossman AR, Bhaya D, Cohan FM, Kuhl M, Bryant DA, Ward DM. 2011. Community ecology of hot spring cyanobacterial mats: predominant populations and their functional potential. ISME J 5:1262–1278. doi: 10.1038/ismej.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, Huttenhower C, Ley RE. 2013. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput Biol 9:e1002863. doi: 10.1371/journal.pcbi.1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, Wang G, Lau HC, Yu J. 2022. Metagenomic sequencing for microbial DNA in human samples: emerging technological advances. Int J Mol Sci 23:2181. doi: 10.3390/ijms23042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP, Lochs H. 2005. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106:1013–1023. doi: 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 61.Rosca AS, Castro J, Sousa LGV, Cerca N. 2020. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol Rev 44:73–105. doi: 10.1093/femsre/fuz027. [DOI] [PubMed] [Google Scholar]
- 62.Redelinghuys MJ, Geldenhuys J, Jung H, Kock MM. 2020. Bacterial vaginosis: current diagnostic avenues and future opportunities. Front Cell Infect Microbiol 10:354. doi: 10.3389/fcimb.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moter A, Gobel UB. 2000. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods 41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 64.Amann RI. 1995. Fluorescently labelled, rRNA-targeted oligonucleotide probes in the study of microbial ecology. Mol Ecol 4:543–554. doi: 10.1111/j.1365-294X.1995.tb00255.x. [DOI] [Google Scholar]
- 65.Fontenete S, Guimaraes N, Wengel J, Azevedo NF. 2016. Prediction of melting temperatures in fluorescence in situ hybridization (FISH) procedures using thermodynamic models. Crit Rev Biotechnol 36:566–577. doi: 10.3109/07388551.2014.993589. [DOI] [PubMed] [Google Scholar]
- 66.Frickmann H, Zautner AE, Moter A, Kikhney J, Hagen RM, Stender H, Poppert S. 2017. Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: a review. Crit Rev Microbiol 43:263–293. doi: 10.3109/1040841X.2016.1169990. [DOI] [PubMed] [Google Scholar]
- 67.Lima A, Franca A, Muzny CA, Taylor CM, Cerca N. 2022. DNA extraction leads to bias in bacterial quantification by qPCR. Appl Microbiol Biotechnol 106:7993–8006. doi: 10.1007/s00253-022-12276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freen-van Heeren JJ. 2021. Flow-FISH as a tool for studying bacteria, fungi and viruses. BioTech (Basel) 10:21. doi: 10.3390/biotech10040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swidsinski A, Verstraelen H, Loening-Baucke V, Swidsinski S, Mendling W, Halwani Z. 2013. Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS One 8:e53997. doi: 10.1371/journal.pone.0053997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung H, Ehlers MM, Peters RPH, Lombaard H, Redelinghuys MJ, Bezuidenhoudt JE, Kock MM. 2020. Growth forms of Gardnerella spp. and Lactobacillus spp. on vaginal cells. Front Cell Infect Microbiol 10:71. doi: 10.3389/fcimb.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shcherbina IM, Plakhotna IY. 2021. Features of violations of the state of the vaginal ecosystem in pregnant women with bacterial vaginosis. Wiad Lek 74:460–464. doi: 10.36740/WLek202103114. [DOI] [PubMed] [Google Scholar]
- 72.Swidsinski A, Loening-Baucke V, Swidsinski S, Sobel JD, Dorffel Y, Guschin A. 2022. Clue cells and pseudo clue cells in different morphotypes of bacterial vaginosis. Front Cell Infect Microbiol 12:905739. doi: 10.3389/fcimb.2022.905739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schilling J, Loening-Baucke V, Dorffel Y. 2014. Increased Gardnerella vaginalis urogenital biofilm in inflammatory bowel disease. J Crohns Colitis 8:543–549. doi: 10.1016/j.crohns.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 74.Holm JB, France MT, Ma B, McComb E, Robinson CK, Mehta A, Tallon LJ, Brotman RM, Ravel J. 2020. Comparative metagenome-assembled genome analysis of “Candidatus Lachnocurva vaginae”, formerly known as bacterial vaginosis-associated bacterium-1 (BVAB1). Front Cell Infect Microbiol 10:117. doi: 10.3389/fcimb.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srinivasan S, Morgan MT, Liu C, Matsen FA, Hoffman NG, Fiedler TL, Agnew KJ, Marrazzo JM, Fredricks DN. 2013. More than meets the eye: associations of vaginal bacteria with Gram stain morphotypes using molecular phylogenetic analysis. PLoS One 8:e78633. doi: 10.1371/journal.pone.0078633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vance GH, Khan WA. 2022. Utility of fluorescence in situ hybridization in clinical and research applications. Clin Lab Med 42:573–586. doi: 10.1016/j.cll.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 77.Nacher-Vazquez M, Santos B, Azevedo NF, Cerqueira L. 2022. The role of nucleic acid mimics (NAMs) on FISH-based techniques and applications for microbial detection. Microbiol Res 262:127086. doi: 10.1016/j.micres.2022.127086. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen PE, Egholm M, Berg RH, Buchardt O. 1991. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 79.Nielsen PE. 2004. PNA technology. Mol Biotechnol 26:233–248. doi: 10.1385/MB:26:3:233. [DOI] [PubMed] [Google Scholar]
- 80.Machado A, Almeida C, Salgueiro D, Henriques A, Vaneechoutte M, Haesebrouck F, Vieira MJ, Rodrigues L, Azevedo NF, Cerca N. 2013. Fluorescence in situ hybridization method using peptide nucleic acid probes for rapid detection of Lactobacillus and Gardnerella spp. BMC Microbiol 13:82. doi: 10.1186/1471-2180-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machado A, Almeida C, Carvalho A, Boyen F, Haesebrouck F, Rodrigues L, Cerca N, Azevedo NF. 2013. Fluorescence in situ hybridization method using a peptide nucleic acid probe for identification of Lactobacillus spp. in milk samples. Int J Food Microbiol 162:64–70. doi: 10.1016/j.ijfoodmicro.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 82.Machado A, Castro J, Cereija T, Almeida C, Cerca N. 2015. Diagnosis of bacterial vaginosis by a new multiplex peptide nucleic acid fluorescence in situ hybridization method. PeerJ 3:e780. doi: 10.7717/peerj.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardy L, Jespers V, Dahchour N, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. 2015. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS One 10:e0136658. doi: 10.1371/journal.pone.0136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Menard JP, Fenollar F, Henry M, Bretelle F, Raoult D. 2008. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis 47:33–43. doi: 10.1086/588661. [DOI] [PubMed] [Google Scholar]
- 85.Alexander BD, Ashley ED, Reller LB, Reed SD. 2006. Cost savings with implementation of PNA FISH testing for identification of Candida albicans in blood cultures. Diagn Microbiol Infect Dis 54:277–282. doi: 10.1016/j.diagmicrobio.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Zhang B, Maimaiti Y, Liu C, Li J, Wang H, Lin H, Deng Z, Lu X, Zhang X. 2019. Direct detection of Staphylococcus aureus in positive blood cultures through molecular beacon-based fluorescence in situ hybridization. J Microbiol Methods 159:34–41. doi: 10.1016/j.mimet.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Klingspor L, Lindback E, Ullberg M, Ozenci V. 2018. Seven years of clinical experience with the yeast Traffic Light PNA FISH: assay performance and possible implications on antifungal therapy. Mycoses 61:179–185. doi: 10.1111/myc.12722. [DOI] [PubMed] [Google Scholar]
- 88.Da Silva RM, Da Silva Neto JR, Santos CS, Frickmann H, Poppert S, Cruz KS, Koshikene D, De Souza JVB. 2015. Evaluation of fluorescence in situ hybridisation (FISH) for the detection of fungi directly from blood cultures and cerebrospinal fluid from patients with suspected invasive mycoses. Ann Clin Microbiol Antimicrob 14:6. doi: 10.1186/s12941-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumpf O, Dohmen P, Ertmer M, Knebel F, Wiessner A, Kikhney J, Moter A, Treskatsch S. 2016. Rapid molecular diagnosis of infective aortic valve endocarditis caused by Coxiella burnetii. Infection 44:813–817. doi: 10.1007/s15010-016-0916-9. [DOI] [PubMed] [Google Scholar]
- 90.Prudent E, Raoult D. 2019. Fluorescence in situ hybridization, a complementary molecular tool for the clinical diagnosis of infectious diseases by intracellular and fastidious bacteria. FEMS Microbiol Rev 43:88–107. doi: 10.1093/femsre/fuy040. [DOI] [PubMed] [Google Scholar]
- 91.Muzny CA, Sobel JD. 2022. The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment. Antibiotics (Basel) 11:500. doi: 10.3390/antibiotics11040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 93.Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH. 2007. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol 45:1016–1018. doi: 10.1128/JCM.02085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, Fidel PL, Jr, Martin DH. 2004. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis 4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geissdorfer W, Bohmer C, Pelz K, Schoerner C, Frobenius W, Bogdan C. 2003. Tuboovarian abscess caused by Atopobium vaginae following transvaginal oocyte recovery. J Clin Microbiol 41:2788–2790. doi: 10.1128/JCM.41.6.2788-2790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]