Abstract
Background
Silver possesses cytotoxic properties against many microorganisms and is regularly used in wound care. Current evidence supporting the use of one type of silver-containing wound dressing (SCWD) is insufficient.
Materials and methods
To examine the ability of selected SCWDs to inhibit the growth of two strains of bacteria (Escherichia coli and Staphylococcus aureus) commonly found in wounds, an in vitro wound model was used. Bacteria were applied to the surface of nutrient agar, and a piece of each SCWD was applied to the bacteria. The plates were incubated at 37°C overnight. The zone of inhibition (ZI) around each SCWD was measured in cm2.
Results
The mean ZI for Acticoat Flex-3 on E. coli was 1.59 ± 0.15 cm2, which was significantly greater than that observed for Aquacel Ag (p<0.001), Mepilex Ag (p<0.0001), Mepitel Ag (p<0.001), Optifoam (p<0.0001), and Tegaderm Alginate Ag (p<0.01), but statistically indistinguishable from Maxorb II Ag. The mean ZI on S. aureus was 1.21 ± 0.16 cm2, which was greater than Aquacel Ag (p<0.05), Mepilex (p<0.0001), Optifoam (p<0.0001), and Tegaderm Alginate Ag (p<0.05), but statistically indistinguishable from Maxorb II Ag or Mepitel Ag.
Conclusion
Of the SCWDs tested, Acticoat Flex-3 demonstrated the most robust antimicrobial effect. Herein, we show that Acticoat Flex-3 may provide the most wound protection against bacterial infection. In conclusion, these data provide clinicians with additional independent evidence to inform their clinical practice on the use of specific wound dressings.
Keywords: escherichia coli, silver ions, wound dressings, antibacterial effects, staphylococcus aureus, silver nanoparticles, wound care management
Introduction
The utilization of silver in wound care has been reported as early as 69 B.C. [1]. Silver’s properties exert cytotoxic effects on fungal, bacterial, and viral microbes [2,3]. The spectrum of bacterial coverage is wide and includes Staphylococcus aureus and Escherichia coli, two of the most commonly implicated bacteria in wound infections [4]. Over the last two decades, studies on wound care products containing silver suggest that silver fulfills a valuable role in wound care [5]. Silver-containing wound dressings (SCWDs) were designed to help decrease wound infection and have transformed the scope of wound care.
Silver must be in a soluble form for it to be biologically active [6]. Silver ions (Ag+) provide cytotoxic activity through the interruption of biofilms, the increased uptake of antibiotics, and the generation of reactive oxygen species [7-10]. Earlier SCWDs provided a quick initial load of Ag+ and would quickly become depleted with prolonged contact with chloride ions in serum. New SCWDs have been designed to release a slow and steady supply of Ag+. For example, dressings under the Acticoat brand contain Ag0 clusters. These silver clusters were designed to slowly release silver upon contact with wound fluid [6].
Previous studies have shown differences in wound treatment outcomes between a variety of wound dressings such as a silver hydrogel dressing, PolyMem Silver, and Acticoat [2]. An additional study found differences in antimicrobial activity among Acticoat, Acticoat Moisture Control, Acticoat Absorbent, SilvercelTM, Aquacel Ag, Urgotul SSD, and Actisorb [11]. The authors reason that the differences were the result of disproportionality in silver concentrations since higher concentrations correlated with more robust antibacterial properties [11,12].
Acticoat Flex-3, an Ag0 silver nanoparticle-based dressing, has been shown to be an effective antimicrobial dressing and has demonstrated effective wound healing properties [13,14]. In a previous study comparing a limited number of other SCWDs, Acticoat Flex-3 had a greater silver release over Actisorb Silver 220, Aquacel Ag, and Mepilex Ag [14]. However, the authors did not investigate their respective antimicrobial properties [15]. Although SCWDs with higher silver release, not to be confused with higher starting concentration, should conceptually exhibit higher antimicrobial effects, yet this has not been previously shown.
Among newer SCWDs, there is limited evidence that one SCWD significantly outperforms others. The different SCWDs are distinguishable by their unique silver compositions and the varied levels of silver embedded within the complex dressing matrices. This can potentially result in different antimicrobial capacities. With continued concerns about multidrug-resistant bacteria as a consequence of prescribing antibiotics to fight infections, a renewed interest in optimizing silver-based wound therapies has emerged [16]. As such, the goal of this study was to compare industry-leading SCWDs in their ability to control bacterial growth.
In the absence of clear experimental evidence, a combination of factors dictates the clinical use of a particular dressing. These include the availability of the dressing, the familiarity of the physician with the dressing, and the type of wound. For patients to achieve better wound care outcomes, independent studies assessing the effectiveness of different SCWDs are needed. An earlier version of this article was previously posted to the bioRxiv preprint server on January 23, 2023.
Materials and methods
Cell density measurements
Colonies of E. coli (ATCC25922 Seattle 1946) and S. aureus (HIP10787 mupA-positive QC strain methicillin-resistant) sourced from Thermo Scientific (LENEXA, KS 66215 USA) were independently inoculated into a 10-mL Luria Broth (LB) medium (Aldon Corporation, Rochester, NY). These were grown overnight to saturation in a 37°C incubator and shaken vigorously at 250 cycles per minute on a rotary shaker. About 10 µL of each saturated broth was then used to independently inoculate another 10 mL of an LB medium of E. coli and S. aureus. Thereafter, the broth cultures were grown until an optical density (OD) setting of 0.595 nm reached 0.6 as measured using a spectrophotometer (CGOLDENWALL 722N Visible Spectrophotometer) in accordance with National Committee for Clinical Laboratory Standards recommendations.
Preparation of in vitro wound model (modified Kirby-Bauer test)
Agar plates containing bacterial lawns were prepared by spreading 100 μL of 1:1000 diluted E. coli or S. aureus. Pieces of each SCWD (Table 1) measuring 1 cm2 were cut using sterile industrial-grade scissors. Subsequently, the pieces of SCWD were placed on the LB agar plates onto which bacteria had been spread. Sterile gauze was used as a negative control. Whatman paper (3M, Saint Paul, MN) cut to 1 cm2 and pre-wetted with 100 μg/mL gentamicin was used as a positive control. The plates were incubated overnight at 37°C and then images were acquired. The area of the zones of inhibition (ZI) was measured for each dressing and plotted as cm2. Ten replicate experiments for each dressing were performed (n=10).
Table 1. Characteristics of silver-containing wound dressings.
| Product | Manufacturer | Composition | Properties | Product number |
| Acticoat Flex-3 | Smith & Nephew, London, UK | Silver layer of knitted polyester contains 0.69 mg/cm2 to 1.64 mg/cm2 silver nanocrystalline structure (Ag0, slow release, converts to Ag+) | Will adhere to wound bed and aid in minor debridement with the removal of the dressing | 66800417 |
| Aquacel Ag | ConvaTec, Greensboro, NC | Silver-coated Hydrofiber sodium carboxymethylcellulose and 1.2% Ag+ | Absorbs exudate and forms a tight seal surrounding the wound | 412010 |
| Maxorb II Ag | Manufactured in China for Medline Industries Inc., Northfield, IL. | Highly absorbent silver-coated foam pad contains a maximum of 0.306 mg of ionic silver per square cm, silver sodium hydrogen zirconium phosphate, and 100% calcium Alginate (Ag+) | Manages exudate and bacterial burden | MSC9945EP |
| Mepilex Ag | Mölnlycke Health Care AB, Göteborg, Sweden | Silver-coated foam with silicone interface | Adheres primarily to the skin surrounding the wound and not to the wound bed itself | 287500 |
| Mepitel Ag | Mölnlycke Health Care AB, Göteborg, Sweden | Silver-coated silicone contains 0.13% by weight of silver chloride (Ag+) | Adheres to the skin and not to the wound bed | 391090 |
| Optifoam Ag Non-Adhesive | Manufactured in the UK for Medline Industries Inc., Mundelein, IL. | Non-staining antimicrobial silver-coated pad with silicone adhesive border | Manages bacterial burden and absorbs exudate | MSC9614EP |
| 3M Tegaderm Alginate Ag | 3M Inc., Saint Paul, Minnesota | Silver-coated fiber contains silver sodium hydrogen zirconium phosphate (Ag+) | Absorbs exudate | 90303 |
Unbiased semiquantitative analysis
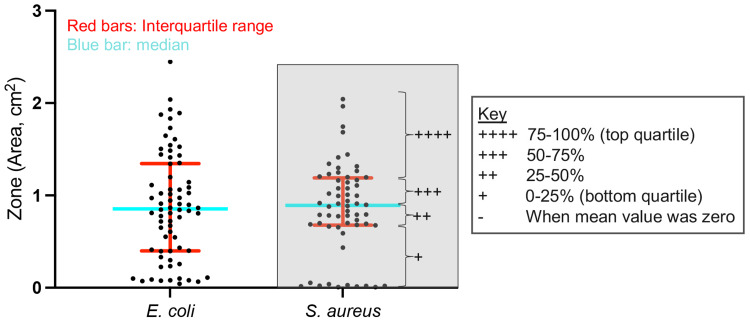
As a summary of the quantitative results obtained using the modified Kirby-Bauer test, we performed a semiquantitative analysis. Each data point corresponding to one of four combinations of bacterial strain and zone (E. coli/ZI, and S. aureus/ZI) was plotted, and the quartiles were determined for each combination. To avoid skewness in the data, zeroes were removed from this analysis. The mean ZI for each SCWD was assigned a number of plus signs (+) based on the quartile range in which it was found. The first/bottom quartile (0%-25%) was given a single plus sign, the second quartile (25%-50%) was given two plus signs, the third quartile (50%-75%) was given three plus signs, and the fourth/top quartile (75%-100%) was given four plus signs. For scoring purposes, the plus sign was defined as one point.
Statistical analysis
The ZI of each SCWD was measured using ImageJ software from the National Institutes of Health. An image of each plate in the same position was captured and measured in ImageJ by tracing the clear zones (inhibition or ZI) of each SCWD. The calculated values for the areas comprising the respective zones were subsequently graphed using GraphPad Prism 9 (GraphPad Software, Inc.). The set of ZI mean values for the SCWDs were statistically analyzed using one-way analysis of variance (ANOVA) and post hoc Tukey test for multiple comparisons (comparing the ZI means to determine which are statistically different from the rest). Statistically significant differences were defined as p<0.05.
Results
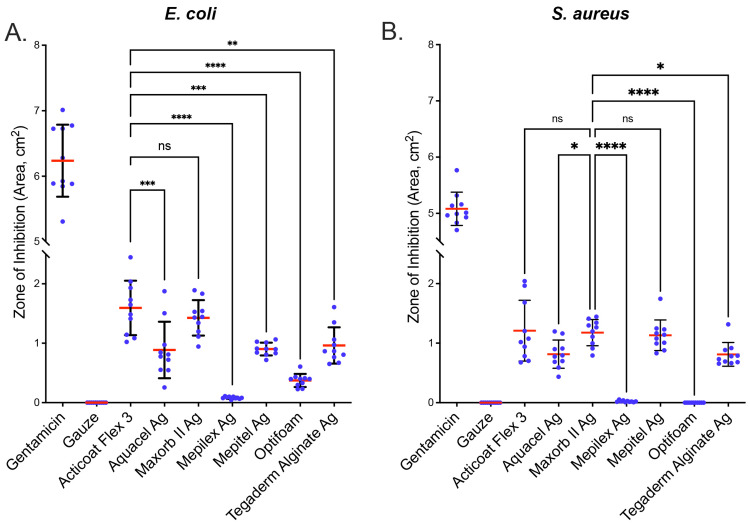
To examine the inhibitory effect of each SCWD on E. coli and S. aureus, we performed a modified Kirby-Bauer test. We obtained representative images for each of the SCWDs for the two strains of bacteria tested (Figure 1). Images were analyzed for zones of inhibition on E. coli and S. aureus lawns, and the data were graphed (Figure 2). With respect to the effect of the SCWDs on E. coli growth, Acticoat Flex-3 showed the most robust inhibitory effect with a mean ZI of 1.59 ± 0.15 cm2 (Figure 2A). The mean ZI of Aquacel Ag (0.89 ± 0.15 cm2) was significantly lower than that of Acticoat Flex-3 (p<0.01). The mean ZI of Maxorb II Ag (1.43 ± 0.09 cm2) was not statistically significantly lower (p=0.96) compared with Acticoat Flex-3. Mepilex Ag (0.08 ± 0.01 cm2) had a significantly lower mean ZI than that of Acticoat Flex-3 (p<0.01). Similarly, Mepitel Ag (0.90 ± 0.03 cm2, p<0.001), Optifoam (0.37 ± 0.03 cm2, p<0.0001), and Tegaderm Alginate Ag (0.96 ± 0.10 cm2, p<0.01) each had a mean ZI significantly lower than that of Acticoat Flex-3.
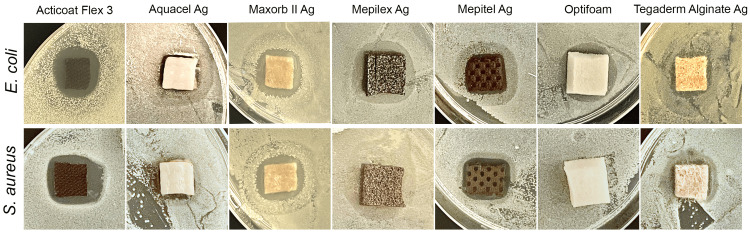
Figure 1. SCWDs demonstrate variable antimicrobial effects against E. coli and S. aureus.
Representative images of each SCWD for E. coli and S. aureus. The zone of inhibition is defined by clear zones adjacent to the SCWD. SCWD: silver-containing wound dressing, E. coli: Escherichia coli, S. aureus: Staphylococcus aureus.
Figure 2. SCWDs differ in their antibacterial effect.
Antibacterial effect of SCWDs against (A) E. coli and (B) S. aureus. Gentamicin was used as a positive control, and gauze only was used as a negative control. Scatter dot plot with zone of inhibition given in cm2. Ten replicates (n=10) were performed for each SCWD. Statistical analysis was performed using ordinary one-way ANOVA with Tukey’s multiple comparisons test. Error bars = mean ± SD. Mean is represented as a red line. **p<0.01, ***p<0.001, and ****p<0.0001. ns: not significant, SCWD: silver-containing wound dressing, E. coli: Escherichia coli, S. aureus: Staphylococcus aureus.
The effect of the SCWDs on the growth of S. aureus was also examined (Figure 2B). We obtained the highest mean ZI for Maxorb II Ag; therefore, the results and statistical analyses described here were obtained using Maxorb II Ag as the reference. The mean ZI for Acticoat Flex-3 (1.21 ± 0.16 cm2) was statistically indistinguishable from that obtained with Maxorb II Ag (1.18 ± 0.07 cm2, p=0.99). The mean ZI for Aquacel Ag (0.82 ± .08 cm2, p<0.05), Mepilex Ag (0.02 ± 0.01 cm2, p<0.0001), Optifoam (0.00 ± 0.00 cm2, p<0.0001), and Tegaderm Alginate Ag (0.81 ± 0.06 cm2 p<0.05) were significantly lower than Maxorb II Ag (1.18 ± 0.07 cm2). The mean ZI for Mepitel Ag (1.13 ± 0.08 cm2) was statistically indistinguishable from the mean ZI for Maxorb II Ag (1.18 ± 0.07 cm2, p=0.99).
For a comprehensive comparison of the results described above, we performed a semiquantitative analysis (Figure 3 and Table 2). Acticoat Flex-3 had a score of 8 points (1 point for each “+”, with a maximum of 4 points for each SCWD/bacterial strain combination), which was the most robust effect against both E. coli and S. aureus. Maxorb II Ag had a score of 7 each and also showed a fairly strong antibacterial effect against both strains. Mepitel Ag (6 points), Aquacel Ag (5 points), and Tegaderm Alginate Ag (5 points) had overall moderate antibacterial effects. Strikingly, Mepilex Ag (2 points) and Optifoam (1 point) showed the weakest antibacterial effects, with the former showing 1 point for each bacterial strain and the latter showing 1 and zero points for E. coli and S. aureus, respectively.
Table 2. Results of unbiased semiquantitative analysis of bacterial inhibition.
Notes: Each plus sign contributes 1 point to the score. Hyphen (-), no effect on bacterial growth. SCWD: silver-containing wound dressing, E. coli: Escherichia coli, S. aureus: Staphylococcus aureus.
| SCWD | E. coli | S. aureus | Score |
| Acticoat Flex-3 | ++++ | ++++ | 8 |
| Aquacel Ag | +++ | ++ | 5 |
| Maxorb II Ag | ++++ | +++ | 7 |
| Mepilex Ag | + | + | 2 |
| Mepitel Ag | +++ | +++ | 6 |
| Optifoam | + | - | 1 |
| Tegaderm Alginate Ag | +++ | ++ | 5 |
Figure 3. Defining the quartile ranges for semiquantitative analysis.
To perform the unbiased semiquantitative analysis, we first defined quartile ranges for all data grouped by bacterial strain. Scatter dot plot with all data from E. coli or S. aureus. To remove skewness in the data, zero values were removed from this analysis. Red bar represents the interquartile range comprising the center 50% of data. Blue bar represents the median, which separates the top 50% from the bottom 50% of data. The key shows the number of plus signs (“+”) assigned to each quartile. This is given as an example in the gray-filled box for S. aureus. E. coli: Escherichia coli, S. aureus: Staphylococcus aureus.
Discussion
Wound infection is the most common postoperative complication, often causing debilitating pain that leads to significant suffering [17]. In addition, this complication has been associated with negative economic impact, increased morbidity, extended postoperative hospital stay, readmission, sepsis, and death [17,18]. In the United States and Europe, the world’s largest wound-dressing markets, there has been an increasingly high demand for wound healing products, where in 2014, the global annual cost for wound care averaged $2.8 billion [19]. Additionally, Medicare cost estimates for chronic and acute wound treatments in 2018 were between $28.1 billion and $96.8 billion [19]. Silver-containing wound dressings (SCWDs) are an attractive and practical choice for wound treatment as silver has been shown to possess antimicrobial properties. Silver is detrimental to bacteria in part through its ability to damage the bacterial cell wall (resulting in increased membrane permeability), block enzyme and solute transport systems, prevent DNA and RNA replication, and block cellular respiration [20,21]. During wound healing, microorganisms such as S. aureus and E. coli can predominate [22]. Our comparative study demonstrated the variability in the antimicrobial effectiveness of selected SCWDs against these bacteria. Nevertheless, Acticoat Flex-3 demonstrated the most consistent antimicrobial properties. Other studies have shown the effectiveness of different Acticoat-branded dressings; however, the relative effectiveness of Acticoat Flex-3 in an in vitro wound model, in particular, has not been investigated [11].
In 2007, Castellano et al. demonstrated that several Acticoat-branded wound dressings (also containing Ag), in addition to Aquacel Ag, were effective at inhibiting the growth of several strains of bacteria (Staphylococcus aureus, Streptococcus faecalis, Escherichia coli, and Pseudomonas aeruginosa) [11]. Similar to our study, Aquacel Ag performed at a moderate level compared with the other dressings tested. In our study, the most robust inhibitory effect against S. aureus was observed with Acticoat Flex-3, Maxorb II Ag, and Mepitel Ag, which were statistically indistinguishable from each other. Overall, the inhibitory effects of the selected SCWDs show that most silver-containing wound dressings can negatively impact bacterial growth. Taken together, we show through our comparative study that the antimicrobial effectiveness of SCWDs commonly used in current clinical settings varies significantly. Studies show that Acticoat-branded SCWDs (e.g., Acticoat Flex-3) provide a slower release and prolonged exposure to bioactive silver cations as a result of their proprietary nanocrystalline composition [23]. Because of this variability, we performed a semi-quantitative analysis that identified Acticoat Flex-3 as the most consistent antimicrobial SCWD in our study. The semi-quantitative analysis showed that Acticoat Flex-3 demonstrated the most robust antimicrobial effectiveness across conditions, but Maxorb II Ag was also effective across conditions to a marginally lesser extent. Aquacel Ag, Mepitel Ag, and Tegaderm Alginate Ag performed moderately well, while Mepilex Ag and Optifoam performed poorly with a weak or no antimicrobial response against E. coli and S. aureus.
Many factors may explain the variability we observed with the selected SCWDs, but the factor that is likely to drive the biggest effect is the bioavailability of the Ag+ ions. In 2012, Rigo et al. reported the amounts and rate of silver released from a number of SCWDs that we also tested in our study (Acticoat Flex-3, Aquacel Ag, and Mepilex Ag) [14]. Initially, the authors independently verified the silver concentrations in the dressings and found that they were consistent with those provided by the vendors (1.379 ± 0.091 mg/cm2 for Acticoat Flex-3, 0.993 ± 0.078 mg/cm2 for Mepilex Ag, and 0.111 ± 0.004 mg/cm2 for Aquacel Ag). Interestingly, these concentrations do not correlate with the antimicrobial effects we observed. This suggests that other factors influence the effectiveness of a particular SCWD. The authors also measured the rate and amount of silver released by placing the dressings in several solutions, including a bioengineered serum substitute [14]. They showed Acticoat Flex-3 and Aquacel Ag continued to release silver up to at least three days in the serum substitute. This was in contrast with Mepilex Ag, which has a lower concentration of Ag than Acticoat Flex-3, but nonetheless showed a very high initial rate of release and reached maximum concentration (greater than that achieved by Acticoat Flex-3 by day 3) within an hour. In our study, if we assume slow release from Acticoat Flex-3 and Aquacel Ag, then inhibition of E. coli and S. aureus inversely correlates with the rate of release but not with the amount of Ag in solution (or agar matrix in our case). Taken together, these data suggest that the degree to which Ag is bioavailable, which defines the antimicrobial profile of any SCWD, is subject to a combination of technology- and wound-specific variables.
In addition to in vitro studies, in vivo models have also been used to study the effectiveness of SCWDs. The advantage of these models is that they establish a more physiologically relevant wound environment. Recently, a study showed that SCWDs containing nanocrystalline silver (e.g., Acticoat Flex3) were superior to silver-plated dressings and non-SCWDs in an in vivo wound model [24]. However, these studies were limited in the number of products they compared. A review of the literature spanning the last 10 years reveals a paucity of in vitro studies investigating the antibacterial efficacy of SCWDs [15,22,25,26]. Additionally, earlier in vitro studies showed conflicting results [26-28]. As such, our study represents the most comprehensive list of SCWDs investigated for their effectiveness in inhibiting bacterial growth.
In considering the conclusions of our study, we recognize a few study limitations. One such limitation is that our in vitro model does not fully recapitulate wounds in humans. However, this affords us the ability to study the effectiveness of the selected SCWDs in a straightforward and highly controlled environment. In addition, our study is limited by having examined only two bacterial strains, although wounds can contain a wide spectrum of bacteria, such as Pseudomonas aeruginosa, Proteus mirabilis, and Streptococcus pyogenes [22]. Finally, one must also be aware of the potential cytotoxic effects that silver may have on keratinocytes and fibroblasts in and near the wound [29]. Nevertheless, our study provides additional evidence for consideration of the clinical application of these SCWDs.
Conclusions
Silver-containing wound dressings show differential antibacterial effects on bacteria commonly infecting wounds in an in vitro wound model. In particular, Acticoat Flex-3 possessed the highest antibacterial properties compared with other contemporary SCWDs we tested. However, Maxorb II Ag also showed robust antibacterial effects. As such, our study provides valuable insight into the effectiveness of commonly employed SCWDs that can be used as one factor to inform their clinical application. In conclusion, the nanocrystalline silver layer of knitted polyester outperforms other silver-containing wound dressings in an in vitro wound model.
Acknowledgments
Data are available on reasonable request.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Advances in wound healing: a review of current wound healing products. Murphy PS, Evans GR. Plast Surg Int. 2012;2012:190436. doi: 10.1155/2012/190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat™ and PolyMem Silver(®) Boonkaew B, Kempf M, Kimble R, Supaphol P, Cuttle L. Burns. 2014;40:89–96. doi: 10.1016/j.burns.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 3.An in vitro analysis of the antimicrobial properties of 10 silver-containing dressings. Thomas S, McCubbin P. J Wound Care. 2003;12:305–308. doi: 10.12968/jowc.2003.12.8.26526. [DOI] [PubMed] [Google Scholar]
- 4.Comparison of the efficacy of silver-based antimicrobial burn dressings in a porcine model of burn wounds. Ross JA, Allan N, Olson M, et al. Burns. 2020;46:1632–1640. doi: 10.1016/j.burns.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 5.An historical overview of the use of silver in wound management. White RJ. Br J Nurs. 2001;10:3–8. [Google Scholar]
- 6.The role of Acticoat with nanocrystalline silver in the management of burns. Dunn K, Edwards-Jones V. Burns. 2004;30:1–9. doi: 10.1016/s0305-4179(04)90000-9. [DOI] [PubMed] [Google Scholar]
- 7.Evidence of oxidative stress in chronic venous ulcers. James TJ, Hughes MA, Cherry GW, Taylor RP. Wound Repair Regen. 2003;11:172–176. doi: 10.1046/j.1524-475x.2003.11304.x. [DOI] [PubMed] [Google Scholar]
- 8.Impact of silver-containing wound dressings on bacterial biofilm viability and susceptibility to antibiotics during prolonged treatment. Kostenko V, Lyczak J, Turner K, Martinuzzi RJ. Antimicrob Agents Chemother. 2010;54:5120–5131. doi: 10.1128/AAC.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Park HJ, Kim JY, Kim J, Lee JH, Hahn JS, Gu MB, Yoon J. Water Res. 2009;43:1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Mechanism of prophylaxis by silver compounds against infection of burns. Ricketts CR, Lowbury EJ, Lawrence JC, Hall M, Wilkins MD. Br Med J. 1970;2:444–446. doi: 10.1136/bmj.2.5707.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comparative evaluation of silver-containing antimicrobial dressings and drugs. Castellano JJ, Shafii SM, Ko F, et al. Int Wound J. 2007;4:114–122. doi: 10.1111/j.1742-481X.2007.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver I: its antibacterial properties and mechanism of action. Lansdown AB. J Wound Care. 2002;11:125–130. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 13.Active silver nanoparticles for wound healing. Rigo C, Ferroni L, Tocco I, et al. Int J Mol Sci. 2013;14:4817–4840. doi: 10.3390/ijms14034817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Characterization and evaluation of silver release from four different dressings used in burns care. Rigo C, Roman M, Munivrana I, Vindigni V, Azzena B, Barbante C, Cairns WR. Burns. 2012;38:1131–1142. doi: 10.1016/j.burns.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Use of internally validated in vitro biofilm models to assess antibiofilm performance of silver-containing gelling fibre dressings. Suleman L, Purcell L, Thomas H, Westgate S. J Wound Care. 2020;29:154–161. doi: 10.12968/jowc.2020.29.3.154. [DOI] [PubMed] [Google Scholar]
- 16.Skin and soft-tissue infections: impact of resistant gram-positive bacteria. Wilson MA. Am J Surg. 2003;186:35–41. doi: 10.1016/j.amjsurg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Risk of surgical site infections after colorectal surgery and the most frequent pathogens isolated: a prospective single-centre observational study. Panos G, Mulita F, Akinosoglou K, et al. Med Glas (Zenica) 2021;18:438–443. doi: 10.17392/1348-21. [DOI] [PubMed] [Google Scholar]
- 18.Postoperative sepsis after colorectal surgery: a prospective single-center observational study and review of the literature. Mulita F, Liolis E, Akinosoglou K, et al. Prz Gastroenterol. 2022;17:47–51. doi: 10.5114/pg.2021.106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human wounds and its burden: an updated compendium of estimates. Sen CK. Adv Wound Care (New Rochelle) 2019;8:39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanocrystalline silver dressings in wound management: a review. Fong J, Wood F. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2676636/#:~:text=In%20summary%2C%20the%20literature%20review,decreases%20the%20matrix%20metalloproteinase%20activity%2C. Int J Nanomedicine. 2006;1:441–449. doi: 10.2147/nano.2006.1.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Should one size fit all? An overview and critique of the VULCAN study on silver dressings. Leaper D, Drake R. Int Wound J. 2011;8:1–4. doi: 10.1111/j.1742-481X.2010.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Bessa LJ, Fazii P, Di Giulio M, Cellini L. Int Wound J. 2015;12:47–52. doi: 10.1111/iwj.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A matched-pair, randomized study evaluating the efficacy and safety of Acticoat silver-coated dressing for the treatment of burn wounds. Tredget EE, Shankowsky HA, Groeneveld A, Burrell R. https://pubmed.ncbi.nlm.nih.gov/9848045/ J Burn Care Rehabil. 1998;19:531–537. doi: 10.1097/00004630-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Therapeutic efficacy of three silver dressings in an infected animal model. Heggers J, Goodheart RE, Washington J, et al. https://pubmed.ncbi.nlm.nih.gov/15640735/ J Burn Care Rehabil. 2005;26:53–56. doi: 10.1097/01.bcr.0000150298.57472.26. [DOI] [PubMed] [Google Scholar]
- 25.Silver-containing dressing for surgical site infection in clean and clean-contaminated operations: a systematic review and meta-analysis of randomized controlled trials. Li HZ, Zhang L, Chen JX, Zheng Y, Zhu XN. J Surg Res. 2017;215:98–107. doi: 10.1016/j.jss.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Burns: dressings. Wasiak J, Cleland H. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4501337/ BMJ Clin Evid. 2015;2015:1903. [PMC free article] [PubMed] [Google Scholar]
- 27.In vitro efficacy of various topical antimicrobial agents in different time periods from contamination to application against 6 multidrug-resistant bacterial strains isolated from burn patients. Hajská M, Slobodníková L, Hupková H, Koller J. Burns. 2014;40:713–718. doi: 10.1016/j.burns.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Comparative study of silver sulfadiazine with other materials for healing and infection prevention in burns: a systematic review and meta-analysis. Nímia HH, Carvalho VF, Isaac C, Souza FÁ, Gemperli R, Paggiaro AO. Burns. 2019;45:282–292. doi: 10.1016/j.burns.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Silver in wound care-friend or foe?: A comprehensive review. Khansa I, Schoenbrunner AR, Kraft CT, Janis JE. Plast Reconstr Surg Glob Open. 2019;7:0. doi: 10.1097/GOX.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]