Abstract
The genus Taxus (yews) is the largest genus of the family Taxaceae. It comprises about 24 species with 55 varieties distributed mainly in Asia, Europe, North Africa, and North America. In addition to the taxane diterpenoids and the cancer drug taxol, its species contain many essential oils with actual or potential biological activity. This review covers the chemical constituents as well as biological activities of these oils that have been studied in fourteen countries over 46 years (1975–2021). It also discusses the biotic and abiotic factors that limit the regeneration of these economically and medicinally important plants.
Keywords: Medicinal plants, Taxus species, taxol and its precursors, endangered yews, essential oils, fatty acids, biological activities, regeneration factors
1. Introduction
The genus Taxus is the most important member of the family Taxaceae from a phytochemical perspective. Its species are in high demand for the extraction of taxol or related taxanes, a drug for the treatment of various cancers. Essential oils extracted from the studied Taxus plant parts were found to be composed mainly of alcohols. 1-Octen-3-ol, cis-3-hexen-1-ol, caryophyllene oxide, myrtenol, elemicin, trans-2-hexenal, α-pinene, and laminitol were the most frequent components with high concentrations of these essential oils [1–8]. Palmitic, oleic, linoleic, taxoleic, and α-linolenic acids were the most predominant and frequently reported fatty acid constituents of the oils (lipids) of Taxus plants from different regions [9–15]. The oils (essential oils and/or lipids) of the investigated plants of the genus Taxus have demonstrated powerful antifungal, antibacterial, antioxidant, and antihypertensive activities. However, the species of the genus Taxus are the most threatened and endangered plants in their geographical ranges [16,17]. Various factors are affecting the survival of these precious species and due to these, their regeneration was very poor. Therefore, to protect these plants, urgent conservation actions must be taken for all of the plants in their geographical sites. At the present time, the chemical constituents of the oils of only eight and the biological activities of the oils of only four Taxus species have been reported, which have been discussed in the later parts of this review.
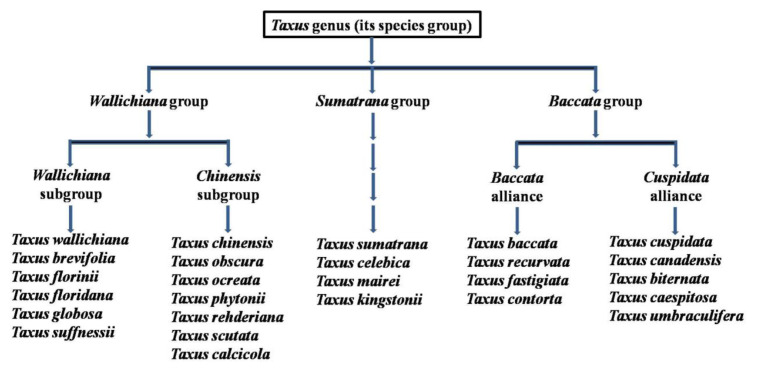
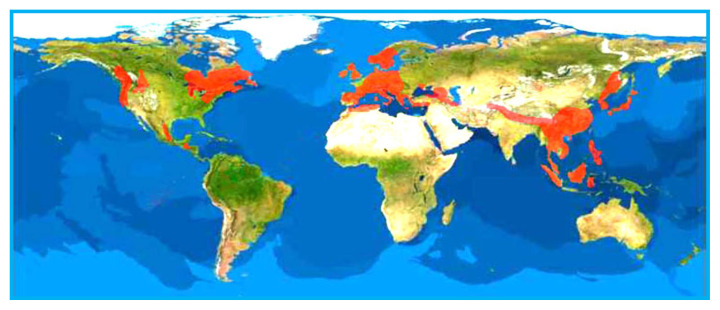
Taxus (yews) is the largest genus of slow-growing long lived evergreen coniferous trees in the family Taxaceae. It comprises about 24 species with 55 varieties [18], distributed mainly in Asia (Pakistan, North India, Japan and China), Europe, North Africa and North America (see Figures 1 and 2) [19,20]. These plants are classified into three groups that are Wallichiana, Baccata, and Sumatrana (Figure 1) based on morphology and geographic distribution, such as European yews (Europe), Canadian yews (North America), and Himalayan yews (Asia) [21]. In Asia, Himalayan yews have a wide distribution in Hindu-Kush Himalaya (HKH) and neighboring regions, ranging from Afghanistan to Philippines [21]. Almost ten plants of the genus Taxus are distributed in this HKH region. These are T. contorta Griff., T. contorta Griff. var. contorta, T. wallichiana Zucc., T. yunnanensis, T. mairei (Lemée&H. Léveillé) S.Y. Hu ex T.S. Liu, T. contorta Griff. var. mucronata Spjut, T. sumatrana (Miq.) de Laubenfels, T. phytonii Spjut, T. celebica (Warb.) H.L. Li and T. baccata L. [22–24]. In North America, four Taxus species namely, T. canadensis, T. floridana (T. globosa var. floridana sensu Spjutis), T. brevifolia, and T. globosa Schltdl. are widely recognized [25]. In China, there are four species of the genus Taxus and one subspecies commonly found in the south-western and north-eastern regions of the country [26,27]. These are T. yunnanensis Cheng et L.K.Fu, T. wallichiana Zucc., T. chinensis (Pilg) Rehd., T. chinensis var. mairei (Lemee et Levl.) Cheng et L.K.Fu, and T. cuspidata Sieb.et Zucc. [27]. However, ten Taxus species such as T. wallichiana Zucc., T. chinensis (Pilg.) Rehder, T. celebica (Warb.) H.L. Li, T. biternata Spjut, T. contorta Griff., T. mairei (Lemée&Lév) S.Y.Huex T.S. Liu, T. umbraculifera (Sieb. ex Endl.) C. Lawson, T. kingstonii Spjut, T. sumatrana (Miq.) de Laub., T. yunnanensis W.C. Cheng & L.K. Fu, all of which are referred to as Chinese yews are reported to be native species [28]. Only one species, Taxus baccata L. (European yew) is found growing in Turkey [29].
Figure 1.
Figure 2.
Worldwide distributions of the Taxus species.
Among all the identified Taxus species and subspecies or varieties, T. contorta Griff. (syn. T. fuana), T. yunnanensis, T. baccata subsp. wallichiana, T. globosa Schltdl., T. cuspidata Sieb.et Zucc., T. chinensis var. mairei, T. wallichiana var. maireii, T. calcicola L.M. Gao & Mich. Möller, T. floridana Nutt. ex Chapm., T. florinii Spjut, T. chinensis (Pilg.) Rehd. and T. wallichiana Zucc. are endangered/critically endangered species due to their low growth, regeneration, and overharvesting for several applications and medicinal uses [24,25,27,32–37]. These endangered species are also listed in https://threatenedconifers.rbge.org.uk/taxonomy/taxaceae/taxus.
The leaves, roots, twigs, and dried bark of plants of the genus Taxus are used to relieve edema and remove toxicity from the body in traditional Chinese medicine (TCM) for a long time [26]. The leaves of Taxus plants have various types of medicinal uses to treat diseases like lung disorders, epilepsy, nervousness, hysteria, malaria, nephropathy, and diabetic nephropathy [19,38]. Various species of this genus have also been reported to exhibit a number of biological activities including antileukemic, analgesic, cytotoxic, antiinflammatory, sedative, anticancer, anticonvulsant, antipyretic, antibacterial, antimitotic, tranquilising, antifungal, and antiseptic [19,39]. Yews have also several applications in making of local beverages using their leaves extract, high-priced furniture, oil extraction, timber, fuel, traditional tea, and for woodcarving [34,36]. However, they gained global notoriety for their FDA (US) approved anticancer/cardiovascular drug paclitaxel (taxol) (Figure 3) which was recognized as one of the most effective and powerful antitumor agents [40]. Nowadays, as an option, this drug is largely produced from its precursors like 10-deacetyl baccatin III (10 DAB III), cephalomannine and baccatin III which are also more readily available in different parts of plants of the genus Taxus (see Table 1 and Figure 3) [41].
Figure 3.
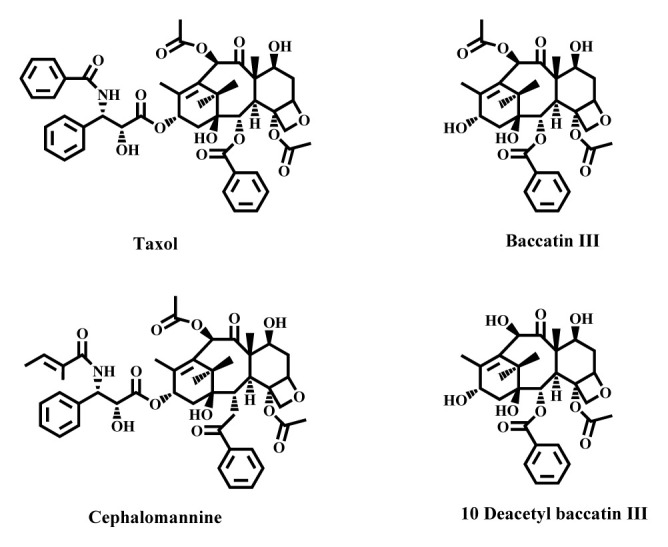
Chemical structure of taxol and its precursors encountered in Taxus species.
Table 1.
Content of taxol and other taxoids (precursors to taxol) in different parts of Taxus species.
| Taxus species | Country | Plant part | Content | References |
|---|---|---|---|---|
| T. baccata | Poland | Needles | Taxol: 0.011% dw | [54] |
| France | Taxol: 0.0005%–0.0184% dw | [55] | ||
| 10 DAB III: 0%–0.099% dw | ||||
| Britain | Taxol: 0.0055%–0.0065% dw | |||
| 10 DAB III: 0.062%–0.073% dw | ||||
| Switzerland | Taxol: 0.0072% dw | |||
| 10 DAB III: 0.054% dw | ||||
| Germany | Taxol: 0.0008%–0.0186% dw | |||
| 10 DAB III: 0.0003%–0.075% dw | ||||
| Ireland | Taxol: 0.00064%–0.0115% dw | |||
| 10 DAB III: 0.00292%–0.08828% dw | ||||
| Georgia | Twigs and leaves | Taxol: 0.0057%–0.0122% dw | [50] | |
| Cephalomannine: 0.0032%–0.0067% dw | ||||
| Baccatin III: 0.0044%–0.0088% dw | ||||
| Russia | Taxol: 0.0033%–0.0125% dw | |||
| Cephalomannine: 0.0018%–0.0079% dw | ||||
| Baccatin III: 0.0022%–0.0097% dw | ||||
| Ukraine | Taxol: 0.0018%–0.0151% dw | |||
| Cephalomannine: 0.0007%–0.0104% dw | ||||
| Baccatin III: 0.0022%–0.0134% dw | ||||
| Bark | Taxol: 0.0148% dw | |||
| USA | Stems | Taxol: 0.001% dw | [51] | |
| Twigs | Taxol: 0.0006% dw | |||
| Leaves | Taxol: 0.003% dw | |||
| T. baccata (female) | Hungary | Foliage | Taxol: 0.0029% | [48] |
| T. baccata (male) | Hungary | Bark | Taxol: 0.0061% | |
| T. baccata “a” sign male clone | Hungary | Foliage | Taxol: 0.0027% | |
| T. baccata “b” sign clone | Hungary | Bark | Taxol: 0.0040% | |
| T. baccata “c” sign clone | Hungary | Bark | Taxol: 0.0029% | |
| T. baccata “d” sign clone | Hungary | Bark | Taxol: 0.0068% | |
| T. baccata “e” sign clone | Hungary | Bark | Taxol: 0.0093% | |
| T. baccata L. | Poland | Needles | Taxol: 0.0105% dw | [54] |
| Taxol: 0.00251% dw** | [56] | |||
| Cephalomannine: 0.00139% dw** | ||||
| Taxol: 0.00194% dw*** | ||||
| Cephalomannine: 0.00102% dw*** | ||||
| Twigs | Taxol: 0.0016% dw ** | |||
| Cephalomannine: 0.0004% dw** | ||||
| Taxol: 0.00187% dw *** | ||||
| Cephalomannine: 0.00055% dw*** | ||||
| Surface of twigs | Taxol: 0.00000084% fw | [57] | ||
| Baccatin III: 0.00000258% fw | ||||
| 10 DAB III: 0.00000148% fw | ||||
| Cephalomannine: n.d. | ||||
| Needles | Taxol: 0.00117%–0.00337% dw | |||
| Baccatin III: 0.00193%–0.00453% dw | ||||
| 10 DAB III: 0.00502%–0.01459% dw | ||||
| Cephalomannine: 0.00346%–0.02048% dw | ||||
| Stems | Taxol: 0.00023%–0.00189% dw | |||
| Baccatin III: 0.00091%–0.00471% dw | ||||
| 10 DAB III: 0.0068%–0.03022% dw | ||||
| Cephalomannine: 0.00062%–0.00528% dw | ||||
| Leaves | Taxol: 0.01167% | [52] | ||
| On the surface of the needles | Taxol: 0.00001% | |||
| Netherlands and UK | Needles | Taxol: 0.0041% dw | [53] | |
| Cephalomannine: 0.0022% dw | ||||
| Baccatin III: 0.0014% dw | ||||
| 10 DAB III: 0.0762% dw | ||||
| Poland | Red arils | Taxol: 0.000002% dwZ | [9] | |
| Cephalomannine: 0.000005% dwZ | ||||
| Baccatin III: 0.00063% dwZ | ||||
| 10 DAB III: 0.00198% dw Z | ||||
| Taxol: 0.00001% dwW | ||||
| Cephalomannine: 0.000018% dwW | ||||
| Baccatin III: 0.0002% dwW | ||||
| 10 DAB III: 0.00039% dwW | ||||
| Taxol: 0.000005% dwK | ||||
| Cephalomannine: 0.000012% dwK | ||||
| Baccatin III: 0.00023% dwK | ||||
| 10 DAB III: 0.00074% dwK | ||||
| Taxol: 0.000005% dwC | ||||
| Cephalomannine: 0.000012% dwC | ||||
| Baccatin III: 0.00024% dwC | ||||
| 10 DAB III: 0.00041% dwC | ||||
| T. baccata basic species | Hungary | Foliage | Taxol: 0.0146% | [48] |
| T. baccata “Adpressa” | Hungary | Bark | Taxol: 0.0047% | |
| Ireland | Needles | Taxol: 0.00762% dw | [55] | |
| 10 DAB III: 0.01674% dw | ||||
| France | Taxol: 0.0012%–0.0023% dw | |||
| 10 DAB III: 0.01368%–0.0663% dw | ||||
| T. baccata adpressa aurea | France | Needles | Taxol: 0.0005%–0.0005% dw**** | |
| 10 DAB III: 0%–0.002759% dw | ||||
| T. baccata “’Aurea” | Hungary | Foliage | Taxol: 0.0025%–0.0053% | [48] |
| Bark | Taxol: 0.0056% | |||
| Ireland | Needles | Taxol: 0.0101% dw | [55] | |
| 10 DAB III: 0.02382% dw | ||||
| France | Taxol: 0.0018%–0.004% dw | |||
| 10 DAB III: 0.00458%–0.02004% dw | ||||
| T. baccata Barronii | France | Needles | Taxol: 0.0035%–0.0051% dw | |
| 10 DAB III: 0.02101%–0.02162% dw | ||||
| T. baccata “Dovastoniana” | Hungary | Foliage | Taxol: 0.0071% | [48] |
| Ireland | Needles | Taxol: 0.00736% dw | [55] | |
| 10 DAB III: 0.01011% dw | ||||
| T. baccata “Elegantissima” | Hungary | Foliage | Taxol: 0.0029% | [48] |
| Poland | Needles | Taxol: 0.017% dw | [54] | |
| Taxol: 0.00299% dw** | [56] | |||
| Cephalomannine: 0.00271% dw** | ||||
| Taxol: 0.00244% dw*** | ||||
| Cephalomannine: 0.002% dw*** | ||||
| Twigs | Taxol: 0.00086% dw ** | |||
| Cephalomannine: 0.00039% dw** | ||||
| Taxol: 0.00063% dw *** | ||||
| Cephalomannine: 0.00035% dw*** | ||||
| Ireland | Needles | Taxol: 0.00316% dw | [55] | |
| 10 DAB III: 0.009% dw | ||||
| Poland | Leaves | Taxol: 0.002591% | [52] | |
| On the surface of needles | Taxol: 0.000015% | |||
| T. baccata erecta | Ireland | Needles | Taxol: 0.00848% dw | [55] |
| 10 DAB III: 0.009% dw | ||||
| T. baccata “Fastigiata” | Hungary | Foliage | Taxol: 0.0027%–0.01% | [48] |
| France | Needles | Taxol: 0.0041%–0.0142% dw | [55] | |
| 10 DAB III: 0.00462%–0.04179% dw | ||||
| Ireland | Taxol: 0.00475%–0.019% dw | |||
| 10 DAB III: 0.01253%–0.04542% dw | ||||
| T. baccata “Fastigiata” “Aurea “ | Hungary | Bark | Taxol: 0.0023%–0.0037% | [48] |
| France | Needles | Taxol: 0.0021%–0.0099% dw | [55] | |
| 10 DAB III: 0.01298%–0.04439% dw | ||||
| T. baccata fastigiata aurea marginata | France | Needles | Taxol: 0.0015%–0.0028% dw | |
| 10 DAB III: 0.01005%–0.01179% dw | ||||
| T. baccata fructolutea | Ireland | Needles | Taxol: 0.00929% dw | |
| 10 DAB III: 0.0233% dw | ||||
| T. baccata glauca | Ireland | Needles | Taxol: 0.00489% dw | |
| 10 DAB III: 0.017% dw | ||||
| T. baccata “Lutea” (female) | Hungary | Bark | Taxol: 0.0179% | [48] |
| T. baccata marginata aurea | France | Needles | Taxol: 0.0024%–0.0043% dw | [55] |
| 10 DAB III: 0.01722%–0.02674% dw | ||||
| T. baccata “Overeyndenri” | Hungary | Bark | Taxol: 0.0024%–0.0079% | [48] |
| T. baccata “Repanda” | Hungary | Bark | Taxol: 0.0048% | |
| T. baccata ‘Repandens’ | France | Needles | Taxol: 0.0012%–0.0034% dw | [55] |
| 10 DAB III: 0.02302%–0.04125% dw | ||||
| USA | Taxol: 0.003% dw | [58] | ||
| 10 DAB III: 0.02% dw | ||||
| Stems | Taxol: 0.001% dw | |||
| 10 DAB III: n.q. | ||||
| T. baccata “Semperaurea” | Hungary | Bark | Taxol: 0.0049% | [48] |
| France | Needles | Taxol: 0.0054%–0.0067% dw | [55] | |
| 10 DAB III: 0.01869%–0.0272% dw | ||||
| T. baccata variegata | France | Needles | Taxol: 0.0007%–0.0038% dw | |
| 10 DAB III: 0.00823%–0.013% dw | ||||
| T. brevifolia | Netherlands and UK | Needles | Taxol: 0.013% dw | [53] |
| Cephalomannine: 0 | ||||
| Baccatin III: 0.0296% dw | ||||
| 10 DAB III: 0.0041% dw | ||||
| Hungary | Bark | Taxol: 0.0048% | [48] | |
| USA | Taxol: 0.02%–0.06% dw | [59] | ||
| 10 DAB III: 0.03%–0.03% dw***** | ||||
| Ireland | Needles | Taxol: 0.00116% dw | [55] | |
| 10 DAB III: 0.013%–0.014% dw | ||||
| France | Taxol: 0.0008%–0.0015% dw | |||
| 10 DAB III: 0.00774%–0.02976% dw | ||||
| USA and Canada | Bark | Taxol: 0.015% dw | [51] | |
| Roots | Taxol: 0.004% dw | |||
| Wood | Taxol: 0.0006% dw | |||
| Wood with Bark | Taxol: 0.0003% dw | |||
| Branches | Taxol: 0.0017% dw | |||
| Leaves/needles | Taxol: 0.0015% dw | |||
| Twigs | Taxol: 0.0012% dw | |||
| Seedlings | Taxol: 0.0058% dw | |||
| USA | Shoots | Taxol: 0.001%–0.033% dw | [60] | |
| Bark | Taxol: 0.001%–0.013% dw | |||
| Cephalomannine: 0.002%–0.027% dw | ||||
| Baccatin III: 0.001%–0.050% dw | ||||
| Needles | Taxol: 0.001%–0.003% dw | |||
| Cephalomannine: 0.002%–0.008% dw | ||||
| Baccatin III: 0.013%–0.030% dw | ||||
| Taxol: 0.006% dw | [58] | |||
| 10 DAB III: 0.01% dw | ||||
| T. canadensis | Netherlands and UK | Needles | Taxol: 0.0285% dw | [53] |
| Cephalomannine: 0.0289% dw | ||||
| Baccatin III: 0.0224% dw | ||||
| 10 DAB III: 0.2665% dw | ||||
| Ireland | Taxol: 0.00158% dw | [55] | ||
| 10 DAB III: 0.016% dw | ||||
| France | Taxol: 0.0036%–0.0046% dw | |||
| 10 DAB III: 0.02919%–0.04753% dw | ||||
| Canada | Taxol: 0.00975%–0.01561% dw | |||
| 10 DAB III: 0.02818%–0.04279% dw | ||||
| USA | Taxol: 0.009% dw | [58] | ||
| 10 DAB III: 0.002% dw | ||||
| Stems | Taxol: 0.002% dw | |||
| 10 DAB III: 0.005% dw | ||||
| Hungary | Foliage | Taxol: 0.0095% | [48] | |
| T. celebica | Netherlands and UK | Needles | Taxol: 0.0026% dw | [53] |
| Cephalomannine: 0 | ||||
| Baccatin III: 0 | ||||
| 10 DAB III: 0.007% dw | ||||
| T. chinensis | China | Needles | Taxol: 0.0039% | [61] |
| Cephalomannine: 0.0112% | ||||
| 10 DAB III: 0.0168% | ||||
| Leaves | Taxol: 0.0088% dw | [49] | ||
| Cephalomannine: 0.0058% dw | ||||
| Ireland | Needles | Taxol: 0.00286% dw | [55] | |
| 10 DAB III: 0.006% dw | ||||
| China | Taxol: 0.01135% | [62] | ||
| Cephalomannine: 0.00899% | ||||
| 10 DAB III: 0.00559% | ||||
| Baccatin III: 0.00338% | ||||
| T. cuspidata | China | Needles | Taxol: 0.005% | [61] |
| 10 DAB III: 0.0046% | ||||
| Cephalomannine: 0.0093% | ||||
| Netherlands and UK | Taxol: 0.0105% dw | [53] | ||
| Cephalomannine: 0.004% dw | ||||
| Baccatin III: 0.0015% dw | ||||
| 10 DAB III: 0.012% dw | ||||
| Stem bark | Taxol: 0.013%–0.017% dw | [49] | ||
| Cephalomannine: 0.0080%–0.032% dw | ||||
| Hungary | Foliage | Taxol: 0.0037% | [48] | |
| Ireland | Needles | Taxol: 0.00728% dw | [55] | |
| 10 DAB III: 0.002% dw | ||||
| France | Taxol: 0.0008%–0.0169% dw | |||
| 10 DAB III: 0%–0.05319% dw | ||||
| Roumania | Taxol: 0%–0.00186% dw | |||
| 10 DAB III: 0%–0.02493% dw | ||||
| USA | Twigs | Taxol: 0.0006% dw | [51] | |
| China | Needles | Taxol: 0.00996% | [62] | |
| Cephalomannine: 0.02486% | ||||
| 10 DAB III: 0.00277% | ||||
| Baccatin III: 0.00254% | ||||
| T. cuspidata ‘Capitata’ | USA | Needles | Taxol: 0.008% dw | [58] |
| 10 DAB III: 0.002% dw | ||||
| Stems | Taxol: 0.004% dw | |||
| 10 DAB III: 0.002% dw | ||||
| T. cuspidata Sieb. et Zucc. | China | Stem bark* | Taxol: 0.031% dw | [49] |
| Cephalomannine: 0.023% dw | ||||
| Root bark* | Taxol: 0.018% dw | |||
| Cephalomannine: 0.018% dw | ||||
| Fibrous roots* | Taxol: 0.014% dw | |||
| Cephalomannine: 0.010% dw | ||||
| Twigs and leaves* | Taxol: 0.0059% dw | |||
| Cephalomannine: 0.0055% dw | ||||
| Poland | Needles | Taxol: 0.0105% dw | [54] | |
| Taxol: 0.0181% dw** | [56] | |||
| Cephalomannine: 0.00309% dw** | ||||
| Taxol: 0.01284% dw*** | ||||
| Cephalomannine: 0.00286% dw*** | ||||
| Twigs | Taxol: 0.00036% dw ** | |||
| Cephalomannine: 0.00019% dw** | ||||
| Taxol: 0.00027% dw *** | ||||
| Cephalomannine: 0.00024% dw*** | ||||
| Leaves | Taxol: 0.04643% | [52] | ||
| On the surface of needles | Taxol: 0.000118% | |||
| T. floridana | Ireland | Needles | Taxol: 0.0076% dw | [55] |
| 10 DAB III: 0.003% dw | ||||
| Netherlands and UK | Taxol: 0.0516% dw | [53] | ||
| Cephalomannine: 0 | ||||
| Baccatin III: 0 | ||||
| 10 DAB III: 0.1689% dw | ||||
| T. globosa | Netherlands and UK | Stems | Taxol: 0.0064% | [63] |
| Cortex | Taxol: 0.0085% | |||
| Needles | Taxol: 0.0130% | |||
| Taxol: 0.0433% dw | [53] | |||
| Cephalomannine: 0.048% dw | ||||
| Baccatin III: 0.0168% dw | ||||
| 10 DAB III: 0.1395% dw | ||||
| T. hunevelliata | Hungary | Foliage | Taxol: 0.0032% | [48] |
| T. x hunnewelliana | France | Needles | Taxol: 0.0083%–0.0104% dw | [55] |
| 10 DAB III: 0%–0.00867% dw | ||||
| Netherlands and UK | Taxol: 0.0041% dw | [53] | ||
| Cephalomannine: 0 | ||||
| Baccatin III: 0 | ||||
| 10 DAB III: 0.0063% dw | ||||
| T. mairei | China | Leaves | Taxol: 0.0069%–0.0127% dw | [64] |
| T. x media | Hungary | Foliage | Taxol: 0.0036% | [48] |
| Poland | Needles | Taxol: 0.036% dw | [54] | |
| USA | Stems | Taxol: 0.002% dw | [51] | |
| Twigs | Taxol: 0.009% dw | |||
| Leaves | Taxol: 0.002% dw | |||
| China | Needles | Taxol: 0.01301% | [62] | |
| Cephalomannine: 0.00715% | ||||
| 10 DAB III: 0.00875% | ||||
| Baccatin III: 0.00405% | ||||
| Taxol: 0.0051% | [61] | |||
| 10 DAB III: 0.0132% | ||||
| Cephalomannine: 0.0122% | ||||
| T. x media Brownii | France | Needles | Taxol: 0.0041%–0.0064% dw | [55] |
| 10 DAB III: 0.007%–0.03316% dw | ||||
| T. x media ‘Densiformis’ | France | Needles | Taxol: 0.004%–0.007% dw | |
| 10 DAB III: 0.0078%–0.03202% dw | ||||
| USA | Taxol: 0.002% dw | [58] | ||
| 10 DAB III: 0.007% dw | ||||
| Stems | Taxol: 0.003% dw | |||
| 10 DAB III: 0.002% dw | ||||
| T. x media var. Hatfieldii | Poland | Needles | Taxol: 0.02% dw | [54] |
| Taxol: 0.00128% dw** | [56] | |||
| Cephalomannine: 0.00043% dw** | ||||
| Taxol: 0.0013% dw*** | ||||
| Cephalomannine: 0.00048% dw*** | ||||
| Twigs | Taxol: 0.00201% dw ** | |||
| Cephalomannine: 0.00045% dw** | ||||
| Taxol: 0.00211% dw *** | ||||
| Cephalomannine: 0.00056% dw*** | ||||
| France | Needles | Taxol: 0.0087%–0.0115% dw | [55] | |
| 10 DAB III: 0.00393%–0.01008% dw | ||||
| Poland | Leaves | Taxol: 0.04852% | [52] | |
| On the surface of needles | Taxol: 0.00008% | |||
| T. x media “Hicksii” | Hungary | Foliage | Taxol: 0.0056% | [48] |
| Bark | Taxol: 0.0031% | |||
| Poland | Needles | Taxol: 0.015%–0.02% dw | [54] | |
| Taxol: 0.00658% dw** | [56] | |||
| Cephalomannine: 0.0047% dw** | ||||
| Taxol: 0.0054% dw*** | ||||
| Cephalomannine: 0.00403% dw*** | ||||
| Twigs | Taxol: 0.00236% dw ** | |||
| Cephalomannine: 0.0022% dw** | ||||
| Taxol: 0.00183% dw *** | ||||
| Cephalomannine: 0.00162% dw*** | ||||
| Britain | Needles | Taxol: 0.00507%–0.0069% dw | [55] | |
| 10 DAB III: 0.0487%–0.08754% dw | ||||
| France | Taxol: 0.0109%–0.0112% dw | |||
| 10 DAB III: 0.00418%–0.03025% dw | ||||
| USA | Taxol: 0.01% dw | [58] | ||
| 10 DAB III: 0.009% dw | ||||
| Stems | Taxol: 0.005% dw | |||
| 10 DAB III: 0.002% dw | ||||
| Poland | Leaves | Taxol: 0.08859% | [52] | |
| On the surface of the needles | Taxol: 0.000129% | |||
| T. x media stricta viridis | France | Needles | Taxol: 0.0049%–0.0088% dw | [55] |
| 10 DAB III: 0.01045%–0.0134% dw | ||||
| T. wallichiana | Pakistan | Leaves | Taxol: 0.018%–0.022 wt % | [40] |
| Stem | Taxol: 0.005%–0.006 wt % | |||
| Bark | Taxol: 0.049%–0.066 wt % | |||
| Root | Taxol: 0.023%–0.087 wt % | |||
| India | Stem bark | Taxol: 0.011%–0.043% dw | [65] | |
| Baccatin III: 0.38%–3.44% dw | ||||
| 10 DAB III: 0.081%–0.704% dw | ||||
| Needle leaves | Taxol: 0.016%–0.031% dw | |||
| Baccatin III: 0.065%–1.442% dw | ||||
| 10 DAB III: 0.015%–0.621% dw | ||||
| Stems | Taxol: 0.001%–0.012% dw | |||
| Baccatin III: 0.011%–0.382% dw | ||||
| 10 DAB III: 0.035%–0.454% dw | ||||
| Bark**** | Taxol: 0.064%–8.032 g/plant dw | [42] | ||
| Bark of male trees | Taxol: 0.0376–0.1167% | |||
| Bark of female trees | Taxol: 0.0129–0.0810% | |||
| India | Needles | Taxol: 0.00183%–0.00406% dw | [55] | |
| 10 DAB III: 0.02476%–0.05949% dw | ||||
| Netherlands and/or UK | Taxol: 0.0272% dw | [53] | ||
| Cephalomannine: 0 | ||||
| Baccatin III: 0 | ||||
| 10 DAB III: 0.1092% dw | ||||
| T. yunnanensis | China | Stem bark | Taxol: 0.024%–0.030% dw | [49] |
| Cephalomannine: 0.0088%–0.018% dw |
10 DAB III: 10 deacetyl baccatin III; fw: fresh weight; dw: dry weight; n.d.: nondetectable; n.q.: not quantifiable;
plant age = 15 years;
obtained by using SPE-HPLC;
obtained by using TLC-HPLC;
plant age from 27 to 136 years and the concentration was expressed by gram per each plant;
obtained from a variety of sources/multiple times;
samples collected from Zielona Gora, Warsaw, Koszalin, and Cracow sites, Poland, respectively.
According to the literature survey, over 550 taxanes including taxol and a number of other different classes of compounds (e.g., phenolic compounds, abietanes, lignans, phytosterols, glycosides, fatty alcohol, steroids, flavonoids, sesquiterpene, and ecdysteroids) were isolated and reported from organic solvent extracts of different parts such as bark, needles, stems, leaves, seeds, twigs, heartwood, roots, and branches of various Taxus species (yews). Several reviews have also compiled these Taxus phytoconstituents [19,43–47]. However, only few Taxus plants have been studied concerning the chemical compositions and biological activities of their oils. To the best of our knowledge, there is no review paper published on these oils and their biological activities. Therefore, this review paper compiles a brief overview on the chemical compositions of the oils of Taxus plants and their biological activities reported in the published literature, using Google Scholar, Google, PubMed, and ScienceDirect databases which might be important in the pharmaceutical industries and drug formulation principles. Moreover, the review presents biotic and abiotic factors that limit the regeneration of these economically and medicinally important plants because many of them are listed as highly endangered species. Thus, the review is very useful for the researchers who have interest in performing further studies on Taxus plants.
1.1. Chemical constituents of oils of Taxus plants
The oils obtained from plants and their constituents are extensively used in cosmetics, detergents, perfumes, agriculture, soaps, foods, and pharmaceutical and other industries [66–71]. They are reported to have analgesic, antitumorigenic, repellent, insecticidal, AChE inhibitory, antifungal, antihypertensive, anticarcinogenic, antiviral, antiinflammatory, antibacterial, antioxidant, and antiparasitic properties [66,67,69]. Nowadays, the investigation on these oils and their constituents has been an interesting, attractive, and hot research area. Therefore, the analysis of the oils and their components including fatty acids is very important for complement of new information on plant applications, for the description of fresh perspective on the potential uses of these organic natural ingredients, and to help meet the requirements of the steadily increasing global edible oil markets.
The main target of this review is also to give an overview on the chemical constituents of the oils from different members of the genus Taxus worldwide. Table 2 shows the collection of the available literature data regarding the oil composition of these plants. According to the literature, among the identified Taxus plants, only eight of them, namely T. chinensis, T. media, T. baccata, T. canadensis, T. chinensis var. mairei, T. cuspidata, T. wallichiana, and T. wallichiana var mairei were investigated concerning the chemical constituent of their oils. Of these, T. baccata was the most studied plant. As presented in the table, the plant part, the most abundant components, country of study, and extraction and analysis methods of oils relating to different plants of this genus have been pointed out. Generally, the dominant chemical class of compounds of the essential oils (EOs) obtained by different methods from the species in the genus Taxus is alcohols (Table 2 and Figure 4) [1–5,72]. Alkanes, alkenes, aldehydes, ketones, flavonoids, fatty alcohols, aromatic compounds, fatty acids, fatty acid esters, ethers, phthalates, phenols, pyridines, steroids, alkaloids, monoterpenes, sesquiterpenes, diterpenes, tetraterpenes, and their derived compounds were also identified and reported from the EOs profiles of these plants from different areas/countries [5–7,10,11,72–78].
Table 2.
Constituents of oils of different Taxus species worldwide.
| Taxus species | Plant part | The most dominant components (%) | Extraction Method | Country | Analysis method | References |
|---|---|---|---|---|---|---|
| T. baccata | Fresh leaves | A1-Octen-3-ol (32.4%); trans-2-hexen-1-ol (8.2%); caryophyllene oxide (7.2%) and hexahydrofarnesyl acetone (6.8%) | Hydrodistillation | Turkey | GC and GC-MS | [4] |
| M1-Octen-3-ol (20.7%); 1-hexanol (10.9%) and trans-2-hexen-1-ol (7.3%) | ||||||
| Fresh needles and twigs | a1-Octen-3-ol (15.56%); myrtenol (13.30%) and cis-3-hexen-1-ol (6.84%) | Hydrodistllation in a Clevenger-type apparatus | Serbia | GC-FID and GC-MS | [5] | |
| b1-Octen-3-ol (27.55%); myrtenol (12.88%) and cis-3-hexen-1-ol (4.77%) | ||||||
| c1-Octen-3-ol (22.18%); cis-3-hexen-1-ol (19.78%) and myrtenol (9.22%) | ||||||
| d1-Octen-3-ol (23.48%); cis-3-hexen-1-ol (11.46%) and myrtenol (11.38%) | ||||||
| Fresh needles and branches | Hexahydrofarnesyl acetone (18.3%); myrtenol (18.3%); cis-3-hexen-1-ol (6.0%); senecioic acid (5.9%) and tricosane (5.5%) | Hydrodistllation in a Clevenger-type apparatus | Serbia | GC and GC-MS | [80] | |
| - | 1-Octen-3-ol (>50%), eugenol (0.5–5%) and cis-3-hexen-1-ol (<0.5%) | Hydrodistillation followed by enzymatic hydrolysis with -glucosidase | Netherlands | GC and GC-MS | [1] | |
| T. baccata L. | Leaves | Oleic acid (20.87 %); 9,12-octadecadien-1-ol (17.77 %); 4-hydroxyphenylacetic acid (9.67 %); 2-methyl-1-thia-cyclopentane (8.87%); 3,5-dimethoxyphenol (7.65%) and pluchidiol (5.05%) | Water:methanol extract | Iran | GC-MS | [11] |
| Male Cones | 3-O-methyl-D-glucose (64.00%); oleic acid (13.32%); 9,12-octadecadien-1-ol (7.70%) and 2-ethylidene-6-methyl-3,5-heptadienal (2.66%) | |||||
| Fresh needles | Palmitic acid (19.6%); capric acid (19.5%); lauric acid (8.1%); decanol (5.4%) and ethyl linolenate (4.2%) | Enzymatic Hydrolysis followed by hydrodistllation in a Clevenger-type apparatus | Turkey | GC-MS | [10] | |
| Dried needles | Palmitic acid (22.5%); capric acid (12.6%); myristic acid (8.0%); lauric acid (5.9%) and hexahydrofarnesyl acetone (4.7%) | |||||
| Red arils | ZLinoleic acid (30.92%); palmitic acid (20.43%); α-linolenic acid (18.53%); myristic acid (9.84%) and oleic acid (9.52%) | Folch’s method with chloroform-methanol mixture (2:1, v/v) | Poland | GC-FID | [9] | |
| Wα-Linolenic acid (25.18%); palmitic acid (22.66%); linoleic acid (20.99%); myristic acid (10.76%) and oleic acid (6.65%) | ||||||
| Kα-Linolenic acid (23.43%); palmitic acid (22.37%); linoleic acid (21.33%); oleic acid (12.35%) and myristic acid (6.76%) | ||||||
| Cα-Linolenic acid (26.50%); palmitic acid (24.37%); linoleic acid (19.40%); myristic acid (10.39%) and oleic acid (6.59%) | ||||||
| Seeds | Oleic acid (54.78%); linoleic acid (23.08%) and taxoleic acid (9.50%) | Folch’s method with chloroform-methanol mixture (2:1, v/v) | Britain or France | GLC | [15] | |
| Seeds | Oleic acid (59.3%); linoleic acid (16.8%) and taxoleic acid (12.2%) | Petroleum ether extract | USA | GLC | [12] | |
| Seeds | Oleic acid (56.00%); linoleic acid (22.81%) and taxoleic acid (9.57%) | Folch’s method with chloroform-methanol mixture (2:1, v/v) | France | GLC | [14] | |
| T. canadensis | Fresh twigs and needles | 1-Octen-3-ol (44.64%) and trans-2-hexenal (24.13%) | Steam distillation | Canada | GC-MS | [2] |
| 3,5-Dimethoxyphenol (48.65%); 1-octen-3-ol (23.05%) and cis-3-hexen-1-ol (3.68%) | Enzymatic hydrolysis with -glucosidase | |||||
| 1-Octen-3-ol (39.11%); 3,5-dimethoxyphenol (26.29%) and cis-3-hexen-1-ol (4.09%) | Enzymatic hydrolysis with cellulose | |||||
| Seeds | Oleic acid (46.77%); linoleic acid (27.93%) and taxoleic acid (13.65%) | Bligh and Dyer method using chloroform and methanol | Canada | GLC-FID | [13] | |
| Leaves | 1-Propanone (36.38%); morpholine (10.95%); methylamine (9.10%); methanone (8.14%) and caryophylleneoxide (4.05%) | HS-SPME | Canada | GC-MS | [75] | |
| T. chinensis | Stems | α-Pinene (34.8%); caryophyllene oxide (17.1%); trans-verbenol (5.0%) and verbenone (4.6%) | Hydrodistllation in a Clevenger-type apparatus | Vietnam | GC-FID and GC-MS | [7] |
| Leaves | α-Pinene (24.2%); sabinene (19.5%); α-terpinyl acetate (12.8%); 1,8-cineole (11.7%); β-pinene (6.1%) and manoyl oxide (4.3%) | Hydrodistllation in a Clevenger-type apparatus | Vietnam | GC and GC-MS | [8] | |
| Woods | α-Pinene (20.0%); photosantalol (10.2%); caryophyllene oxide (8.9%); spathulenol (7.6%); guaiol (6.8%); β-pinene (5.6%) and bornyl acetate (5.4%) | |||||
| Seeds | Oleic acid (34.31%); linoleic acid (34.22%) and taxoleic acid (16.08%) | Folch’s method with chloroform-methanol mixture (2:1, v/v) | Britain or France | GLC | [15] | |
| Bark | Elemicin (47.50%); 4,6-diamino-3-[4-methoxyben zyl]-1H-pyrazolo[3,4-d]pyrimidine (3.21%) and butyl isodecyl phthalate (0.63%) | Ethanol extract | China | GC-MS | [6] | |
| Elemicin (29.89%) and asarone (0.53%) | Ethanol/methanol mixture extract | |||||
| Elemicin (46.23%); diisobutyl phthalate (3.11%); 4,6-diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine (3.11%) and dibutyl phthalate (2.32%) | Ethanol/benzene mixture extract | |||||
| Sapwood | Elemicin (30.61%) and γ-sitosterol (2.29%) | Ethanol extract | ||||
| Elemicin (18.24%); 2,3,5,6-tetrahydro-3,3,4,5,5,8-hexamethyl-s-indacene-1,7-dione (14.46%); macckiain (5.12%) and 4,6-diamino-3-[4-methoxybenzyl]-1H-pyrazolo[3,4-d]pyrimidine (2.57%) | Ethanol/methanol mixture extract | |||||
| Elemicin (29.69%); laminitol (5.16%); γ-sitosterol (2.52%) and diisobutyl phthalate (2.13%) | Ethanol/benzene mixture extract | |||||
| Heartwood | Formononetin (17.71%); laminitol (8.19%); pseudobaptigenin (5.40%); 2,3,5,6-tetrahydro-3,3,4,5,5,8-hexamethyl-s-indacene-1,7-dione and macckiain (2.32%) | Ethanol extract | ||||
| Elemicin (4.69%); laminitol (3.79%) and nerolidol (1.27%) | Ethanol/methanol mixture extract | |||||
| Laminitol (14.48%); nerolidol (7.04%); γ-sitosterol (4.99%); diisobutyl phthalate (3.82%); 3-O-methyl-D-glucose (3.33%) and dibutyl phthalate (2.76%) | Ethanol/benzene mixture extract | |||||
| T. chinensis var. mairei | Leaves | cis-Vaccenic acid (36.96%); trans-palmitoleic acid (24.05%); palmitic acid (6.19%); hexadecanoic acid methyl ester (4.82%) and ethyl oleate (3.37%) | Hydrodistllation in a Clevenger-type apparatus | China | GC-MS | [77] |
| cis-Vaccenic acid (36.73%); trans-palmitoleic acid (23.66%); palmitic acid (6.19%); hexadecanoic acid methyl ester (4.84%) and ethyl oleate (3.44%) | Microwave-assisted simultaneous distillation extraction | |||||
| Aerial stems | HPhthalic acid mono-2-ethylhexyl ester (21.36%); palmitic acid (16.60%); butylated hydroxytoluene (7.75%); stearic acid (7.27%) and ethylbenzene (5.04%) | SFE-CO2 extraction | China | GC-MS | [78] | |
| QPhthalic acid mono-2-ethylhexyl ester (25.21%); palmitic acid (19.37%); 7,9-di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione (9.69%); ethylbenzene (6.36%); stearic acid (6.29%) and butylated hydroxytoluene (5.71%) | ||||||
| SPhthalic acid mono-2-ethylhexyl ester (26.38%); palmitic acid (12.31%); butylated hydroxytoluene (7.51%) and stearic acid (5.06%) | ||||||
| XHeptacosane (24.93%); palmitic acid (5.97%) and 7,9-di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione (5.82%) | ||||||
| Leaves | Benzene propanenitrile (49.39%); 1-hydroxy-2-butanone (12.72%); acetic acid (5.39%); 1-octen-3-ol (4.28%) and trans-2-hydroxycinnamic acid (3.53%) | Steam distillation | China | GC-MS | [73] | |
| T. cuspidata | Fresh stems | Ethyl linoleolate (9.0%); longiborneol (7.9%); 13-diepoxy-14,15-bisnorlabdane (7.0%) and ambrettolide (4.5%) | Microwave-assisted hydrodistillation | Korea | GC-MS | [76] |
| Seeds | Oleic acid (39.21%); linoleic acid (29.35%) and taxoleic acid (16.16%) | Folch’s method with chloroform-methanol mixture (2:1, v/v) | Britain or France | GLC | [15] | |
| Seeds | Oleic acid (36.50%); linoleic acid (32.88%) and taxoleic acid (16.02%) | Bligh and Dyer method using chloroform and methanol | Japan | GLC-FID | [13] | |
| Leaves | Ethyl phthalate (28.15%); E-procainamide (4.59%); 3-methyl-4,4-diphenyl-2-cyclohexen-1-one (4.20%) and n-hexyl vinyl alcohol (3.54%) | Microwave-assisted hydrodistillation | Korea | GC-MS | [74] | |
| T. media | Leaves | Benzene propanenitrile (21.30%); 1,4-dioxane-2,3-diol (20.13%); 3-bromo-3-methyl-butyric acid (17.92%) and 1-hydroxy-2-butanone (9.85%) | Steam distillation | China | GC-MS | [73] |
| T. wallichiana | Fresh leaves | trans-2-Octen-1-ol (14.5%); pentacosane (8.1%); caryophyllene oxide (7.1%); 1-octanol (6.5%); caproic acid (5.5%) and cis-3-hexen-1-ol (4.1%) | Hydrodistllation in a Clevenger-type apparatus | India | GC-MS | [72] |
| T. wallichiana var mairei | Leaves | cis-3-Hexen-1-ol (12.14%); 1-octen-3-ol (9.56%); 2-hexenal (7.45%); hexyl formate (4.24%); 2-penten-1-ol (3.71%); 3-octanone (3. 65%) and 1-penten-3-ol (3.51%) | Simultaneous distillation and diethyl ether extraction | China | GC-MS | [3] |
| 2-Hexenal (7.03%); cis-3-hexen-1-ol (4.99%); palmitic acid (4.77%); hexanol (4.44%) and 3-octanone (4.06%) | Simultaneous distillation and dichloromethane extraction |
Population I/Tara,
Population II/Kopaonik,
Population III/Malinik and
Population I – III, Serbia.
Samples from Zielona Gora, Warsaw, Koszalin and Cracow sites, Poland, respectively.
Plant samples collected respectively from Huangshan city, Qingyang county, Shucheng county and Xuancheng city, China. GLC: Gas–liquid chromatography. GC-MS: Gas chromatography–mass spectrometry. SFE-CO2: Supercritical fluid extraction using carbon dioxide.
Samples collected from western (Aegean region) and southern (Mediterranean region), Turkey, respectively. HS-SPME: Head space solid phase micro-extraction. − : Missing data.
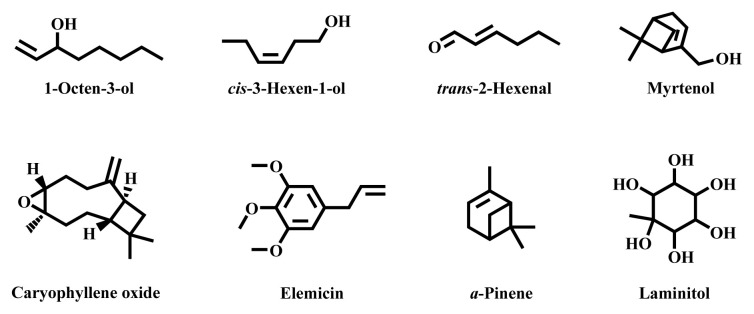
Figure 4.
Chemical structures of most frequently reported constituents of essential oils from Taxus species.
There are variations and slight similarity in the contents and chemical constituents or classes of compounds of the EOs obtained from the same plant organs or among the species of the genus Taxus. The most frequent components with high concentrations of this genus are 1-octen-3-ol, cis-3-hexen-1-ol (aliphatic unsaturated alcohols), caryophyllene oxide (oxygenated sesquiterpene), myrtenol (oxygenated monoterpene), elemicin (phenylpropanoid), trans-2-hexenal (aldehyde), α-pinene (monoterpene hydrocarbon), and laminitol (cyclic polyhydroxy alcohol) (Table 2). The structures of these chemical compounds are appeared in Figure 4. Of these compounds, 1-octen-3-ol was detected to be a predominant compound of the EO isolated from T. canadensis of Canada [2], T. baccata growing in Turkey [4], Serbia [5], and Netherlands [1]. cis-3-Hexen-1-ol was also predominant in the EO obtained by simultaneous distillation extraction using diethyl ether as a solvent from T. wallichiana var mairei from China [3]. Elemicin was the most abundant volatile component in the oil obtained from the sapwood and bark of T. chinensis and from heartwood extracted using ethanol and methanol as solvent [6]. The highest content of α-pinene was also found in the EOs isolated from T. chinensis stems [7], leaves, and woods [8]. However, the oil isolated from the heart wood of the same plant by using ethanol and benzene as solvent was characterized by high amount of laminitol [6].
On the other hand, the fatty acid (FA) compositions of oils extracted from Taxus species with different methods showed that they constitute many saturated and unsaturated (both monounsaturated and polyunsaturated) fatty acid compounds. Palmitic acid and oleic acid were identified as the most predominant components of saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA), respectively, whereas linoleic acid was reported as a principal compound of polyunsaturated fatty acid (PUFA) followed by taxoleic and α-linolenic acids. All these saturated and unsaturated FAs were frequently reported as major fatty acid compositions from the oils of plants of the genus Taxus from different regions. However, the concentrations of the FAs and the overall fatty acids profiles of these oils showed variations. Oleic acid was identified as the most abundant component (20.87%) of the oil extracted from T. baccata leaves of Iran [11]. The oils isolated from the fresh and dried needles of the same plant of Turkey were dominated mostly (19.6%–22.5%) by palmitic acid [10].
The major fatty acid in the oil obtained from the red arils of this plant collected from Zielona Gora, Poland was linoleic acid (30.92%), followed by palmitic (20.43%), α-linolenic (18.53%), myristic (9.84%), and oleic (9.52%) acids. However, α-linolenic acid was the most abundant (23.43%–26.50%) fatty acid component of the same part of this plant collected from Warsaw, Koszalin and Cracow sites, Poland, followed by palmitic (22.37%–24.37%) and linoleic (19.40%–21.33%) acids [9]. The seeds oil of T. chinensis, T. canadensis, T. cuspidata, and T. baccata mainly composed of oleic (34.31%–59.3%), linoleic (16.8%–34.22%) and taxoleic (9.5%–16.16%) acids [12–15]. Other MUFAs such as cis-vaccenic (36.73 – 36.96%) and trans-palmitoleic (23.66%–24.05%) acids together with palmitic acid (6.19%) were also reported as the most predominant compositions of the oils isolated from T. chinensis var. mairei leaves [77]. All the variations in the contents and compositions of the oils of Taxus species may be due to different factors including extraction and analysis methods [66,69–71].
1.2. Biological activities of oils of Taxus species
There is a shortage of literature on the biological activities of oils of Taxus species. However, the previously reported results on the investigated antimicrobial activities of the oils of these plants evaluated using MIC, MBC, ZI, and IC50 approaches against pathogenic yeast, Candida albicans and various gram (+) and gram (−) bacteria are presented in Table 3. Table 4 also represents the compilation of the results on their antioxidant activities. Among all Taxus plants from different regions, only the oils of T. chinensis, T. cuspidata, T. chinensis var. mairei, and T. media were evaluated for their biological activities.
Table 3.
Antimicrobial activities of the oils of the species of the genus Taxus worldwide.
| Taxus species | Plant part | Sample | ZI (mm) | MIC value | MBC value | IC50 | Bacterial strain | References |
|---|---|---|---|---|---|---|---|---|
| T. chinensis | Leaves | Essential oil extracted by hydrodistllation in a Clevenger-type apparatus | - | 16.0 μg/mL | - | 3.98 μg/mL | E. faecalis ATCC 299212 | [8] |
| - | 256.0 μg/mL | - | 100.56 μg/mL | S. aureus ATCC 25923 | ||||
| - | 64.0 μg/mL | - | 19.78 μg/mL | B. cereus ATCC 14579 | ||||
| - | NA | - | NA | E. coli ATCC 25922 | ||||
| - | NA | - | NA | P. aeruginosa ATCC 27853 | ||||
| - | NA | - | NA | S. enterica ATCC 13076 | ||||
| - | 128.0 μg/mL | - | 55.67 μg/mL | C. albicans ATCC 10231 | ||||
| Woods | - | 64.0 μg/mL | - | 20.33 μg/mL | E. faecalis ATCC 299212 | |||
| - | 128.0 μg/mL | - | 56.78 μg/mL | S. aureus ATCC 25923 | ||||
| - | NA | - | NA | B. cereus ATCC 14579 | ||||
| - | 256.0 μg/mL | - | 87.78 μg/mL | E. coli ATCC 25922 | ||||
| - | NA | - | NA | P. aeruginosa ATCC 27853 | ||||
| - | NA | - | NA | S. enterica ATCC 13076 | ||||
| - | 256.0 μg/mL | - | 89.67 μg/mL | C. albicans ATCC 10231 | ||||
| T. chinensis var. mairei | Leaves | Essential oil obtained by steam distillation | - | 95% | - | - | E. coli | [73] |
| - | 98% | - | - | S. aureus | ||||
| T. cuspidata | Leaves | Essential oil isolated by microwave-assisted hydrodistillation | 34.0 | 250 μg/mL | 500 μg/mL | - | B. cereus ATCC 13061 | [74] |
| 27.0 | 500 μg/mL | 1000 μg/mL | - | L. monocytogenes ATCC 7644 | ||||
| 34.0 | 250 μg/mL | 500 μg/mL | - | S. aureus ATCC 12600 | ||||
| 22.0 | 500 μg/mL | 1000 μg/mL | - | S. typhimurium ATCC 43174 | ||||
| 24.0 | 500 μg/mL | 1000 μg/mL | - | E. coli ATCC 43889 | ||||
| T. media | Leaves | Essential oil extracted by steam distillation | - | 5% | - | - | E. coli | [73] |
| - | 5% | - | - | S. aureus |
ZI: Zone of inhibition. MIC: Minimum inhibitory concentration. MBC: Minimum bactericidal concentration.
Table 4.
Antioxidant activities of the oils of Taxus species.
| Taxus species | Plant part | Sample | Assay | Inhibitory effect (%) | References |
|---|---|---|---|---|---|
| T. cuspidata | Fresh stems | Essential oil extracted by microwave-assisted hydrodistillation | DPPH | 92.8%a | [76] |
| Nitric oxide radical | 80.0%b | ||||
| Superoxide radical | 71.7%c | ||||
| Hydroxyl radical | 73.7%d | ||||
| Lipid peroxidation | 80.2%e | ||||
| Reducing power activity | 1.1f |
At the concentration of 500 μg/mL.
At the concentration of 300 μg/mL.
At the concentration of 250 μg/mL.
At the concentration of 500 μg/mL.
At the concentration of 250 μg/mL.
Absorbance value at 25 μg/mL concentration.
Almost all the investigated oils displayed strong antimicrobial activity toward the tested strains of bacteria and a fungus. In general, these oils are more susceptible toward gram (+) bacteria than gram (−) ones. The oil from T. chinensis leaves with high amount of α-pinene showed the highest antibacterial activity with MIC value of 16.0 μg/mL as well as IC50 value of 3.98 μg/mL against E. faecalis (gram-positive bacterium). This same oil also demonstrated potent activity towards a fungus, C. albicans (MIC = 128.0 μg/mL, IC50 = 55.67 μg/mL) and other gram-positive bacteria such as B. cereus (MIC = 64.0 μg/mL, IC50 = 19.78 μg/mL) and S. aureus (MIC = 256.0 μg/mL, IC50 = 100.56 μg/mL), but no activity towards the gram (−) pathogens like S. enterica, E. coli, and P. aeruginosa. The oil obtained from the woods of the same plant with high content of α-pinene also displayed strong antimicrobial activities against E. faecalis (MIC = 64.0 μg/mL, IC50 = 20.33 μg/mL), S. aureus (MIC = 128.0 μg/mL, IC50 = 56.78 μg/mL), E. coli (MIC = 256.0 μg/mL, IC50 = 87.78 μg/mL), and C. albicans (MIC = 256.0 μg/mL, IC50 = 89.67 μg/mL). However, this oil showed no activity towards P. aeruginosa and S. enterica [8]. The powerful antimicrobial activities of these oils are probably related to the high content of α-pinene. This compound was reported to have antimicrobial activities [66,79].
Bajpai et al. [74] also reported the good bactericidal potential of the leaves oil of T. cuspidata. This oil was very active against B. cereus (ZI = 34.0 mm, MIC = 250 μg/mL, MBC = 500 μg/mL), S. aureus (ZI = 34.0 mm, MIC = 250 μg/mL, MBC = 500 μg/mL), L. monocytogenes (ZI = 27.0 mm, MIC = 500 μg/mL, MBC = 1000 μg/mL), E. coli (ZI = 24.0 mm, MIC = 500 μg/mL, MBC = 1000 μg/mL) and S. typhimurium (ZI = 22.0 mm, MIC = 500 μg/mL, MBC = 1000 μg/mL). The antibacterial activity of the leaves oil of T. media was stronger than that of T. chinensis var. mairei leaves oil [73]. The MIC values for the oil of T. chinensis var. mairei for S. aureus and E. coli were 98% and 95%, respectively. However, the values for the oil of T. media on these bacteria were both 5%. These oils demonstrated high activity to E. coli in comparison to S. aureus. The different chemical compositions and their percentages of the oils are most likely responsible for the different properties found towards the microbes.
According to the literature survey, the antioxidant activities of only the oil of T. cuspidata fresh stems have been investigated and reported. To determine the activities of the oil, antioxidant assays such as DPPH, reducing power activity, lipid peroxidation, nitric oxide, superoxide, and hydroxyl radicals were employed. In Table 4, results of these activities of the oil of this Taxus plant are shown. The results demonstrated that the oil exhibited powerful antioxidant activity in DPPH assay with an inhibitory effect of 92.8% at 500 μg/mL concentration. At 100 μg/mL, the inhibitory effects of α-tocopherol and ascorbic acid standards were 73.4% and 72.9%, respectively. The oil also had strong inhibitory effects (71.7%, 73.7%, and 80.0%) which were comparable to the standards on superoxide, hydroxyl, and nitric oxide radicals, respectively (Table 4). The inhibitory effects of α-tocopherol and ascorbic acid were 74.4% and 73.0% on superoxide radicals, whereas BHA and ascorbic acid were 70% and 73.3% on hydroxyl radicals, respectively. Moreover, the oil showed better lipid peroxidation inhibition (80.2%) than the standards, α-tocopherol (80.1%) and BHA (76.5%) all at 250 μg/mL concentration. The same oil also exhibited significant reducing power activity (absorbance value, 1.1) in comparison to the reference compounds, α-tocopherol (absorbance value, 1.1) and ascorbic acid (absorbance value, 1.2) at 25 μg/mL concentration. The strong antioxidant property of the oil was due to the existence of phenolic compounds such as umbelliferon and eugenol and fatty acids in the oil [76]. The volatile chemical constituents of the leaves of T. chinensis var. mairei were also proved to be used as natural and supplementary reagents to treat hypertension [38].
2. Factors in the regeneration of the endangered Taxus plants
As discussed in detail in Section 1, Taxus species have a variety of medicinal and economic values; their oils also have several biological activities and bioactive chemical constituents. However, they are highly endangered plants principally due to their high demand for the extraction of taxol drug [81] and regeneration of these plants have been of large concern worldwide [20]. The seeds of Taxus species are highly dormant and due to this, they are extremely difficult to germinate. These and other factors such as low seed production, slow growth, overexploitation, lack of awareness, narrow range, slow propagation, destructive harvesting, habitat specificity, high value, climate change, habitat loss or destruction, over-grazing and changes in forest management were the reasons identified by several researchers why Taxus plants face extinction and need urgent conservation [17,20,36,37,81]. All these diverse factors have negative impact on the anatomy, physiology, and behavioral peculiarities of yews that ultimately impact their regeneration. In this review paper, in this section, the major biotic and abiotic factors that limit the regeneration and growth of these important and useful but endangered plants are explained in detail based on the data collected from several research papers and the literature.
2.1. Climate change and temperature effects
Various climatic and environmental factors can affect the distribution and regeneration of Taxus species in the forest [36]. In addition to fungi, insects, viruses, bacteria, rodents, and pests; climate change and disturbances from fires have a significant impact on the establishment, growth, and spread of these plants [36,81,82]. High temperatures and their variations have a direct influence on the conditions for the growth and development of these plants [83]. Forest fires are one of the major causes for the increased temperatures in the forests worldwide [84]. The high temperatures negatively affect the plant regeneration, mostly in the southern aspect resulting in excessive loss of moisture due to an increase in evapotranspiration [83]. Losses of several plant species worldwide have been attributed to temperature fluctuations [85]. Climate change is one of the major problems of the 21st century [86]. Climatic changes due to temperature variation have also been reported to result in decreased pollination and seed production [87]. Thus, climate change and the drought occurrences because of these changes also have negative effects on the regeneration of Taxus plants [36,81] because the rate of growth and survival of the seedlings of yews could be determined on the basis of their resistance to these and the aforesaid destructive components of the environment as well as climate shocks and events [36,88].
2.2. Canopy closure
Local environmental conditions can also affect the germination of the seeds of the endangered Taxus species [89]. The seeds of these plants germinate in the shady areas under the canopy of the trees than in canopy gaps [90,91]. Most seedlings which are found under the mother trees of their geographical sites clearly indicate the requirement of minimal light for the germination of the seeds of Taxus species and their regeneration potential on deep shady, moist, and sheltered sites [90]. However, the availability of light is necessary for regeneration [88]. The stand structure and canopy cover have played a major role in the establishment of the seedlings of Taxus plants [91]. Due to these effects, the rates of the establishment of the seedlings were very low and hence influence the regeneration and vitality of the plants [92]. Although Taxus plants are known to thrive under dense forest canopy for a long time in the seedling stage, at maturity, they need canopy gaps without which they may lose the competition for essential resources [91]. It has been reported that a higher percentage of the living crown of associated species can harm Taxus species formation [93]. Sometimes, herbivores also play a key role in the development of canopy gaps [82,93,94]. Hence, good regeneration or survival of Taxus plants is dependent on the suitability of the local environments.
2.3. Herbivores
Herbivores (insects, deer, rabbit, moose, rodents, goats, horses, cattle, sheep, and others) adversely affect the regeneration of plants particularly concerning the overall growth of seedlings and saplings, their proliferation and attainment of luxuriance [82,93,95]. As compared to healthy plants, in the plants damaged by the herbivores, besides the overall plant height pollination, seed production and stand structural dynamism are significantly different. One of the reasons for the poor regeneration of plants of the genus Taxus has been attributed to the damage caused by the abovementioned grazing animals [93,95,96]. The immense browsing pressure of the plants by these grazing animals sometimes even proves lethal to their establishment, growth, and development because the animals readily eat the seedlings as well as the needles/leaves, buds, shoots, and bark of Taxus trees [82,94–99]. In some areas, the seeds of these plants along with their red arils were also eaten by monkeys, rats, birds (especially Turdus species), and children [36,90,97]. Thus, herbivory can also be the main factor influencing the growth, development, and regeneration of yews.
2.4. Availability of water and species competition
The availability of water in the forest in the areas where Taxus plants are found can also play a great role in their regeneration. As reported by the researchers, there is a scarcity of water in the temperate regions of the southern aspect harboring natural habitats such as forests, which is a major constraint in the regeneration of these plants while northern aspects are impacted more by shade [83,88]. In general, landscapes with more availability of water, humidity and rainfall have a higher density of regeneration in comparison to drier places at both regional as well as continental scales [88,100]. Thus, regeneration of Taxus plants is closely associated to an abiotic factor, water availability. Moreover, Taxus plants strongly face competition for light, nutrients, and water availability with other plants or the same species that decrease the numbers of their populations by affecting the seedlings’ survival rates [82,91]. Hence, the availability of sufficient water resources and protecting the plants from other competing species are obligatory requirements for saving Taxus species from getting into a more endangered status and also preventing fragmentation into small as well as marginal populations.
2.5. Dispersal of seeds
The dispersals of seeds of plants can also play an important role in their regeneration. The dispersal of seeds in Taxus species is a pivotal phenomenon due to unsuitable microsites and the role of predators in seed dispersion phenomena [101]. The seeds of these plants are dispersed to unfavorable sites mainly by birds and monkeys [36,82,89,90,98]. They are also not able to survive if dispersed in the places that are cleared for the purposes of agricultural activities [36]. This is because the dispersed seeds of Taxus plants have been reported to be highly dormant and hard to germinate [98]. During the postdispersal stage, the seeds can also be destroyed by rodents [90,98,99]. The rodent populations in the forest are quite high and they eat seeds of Taxus species, which significantly reduces the chances for regeneration and contributes to low numbers of seedlings [97]. Not only rodents, birds, and monkeys, but also humans are equally responsible in this regard [92,99]. Moreover, in their natural conditions and inside their geographical ranges, the ripe seeds of Taxus plants dispersed in autumn and in the late summer do not germinate before the second spring, and germinate in the next spring or maybe later [89,93]. Furthermore, the geostatistical investigation has demonstrated that seedlings that grow in patches in the forest areas avoid their direct competition with mature trees for resource mobilization [102]. Thus, the dispersal of seeds is also a factor that strongly affects the regeneration of the endangered yews.
2.6. Anthropogenic disturbances
Nowadays, anthropogenic activities are playing a significant role in the decline of Taxus species populations [103]. These activities are closely related to agricultural practices, destruction of habitats, deforestation, fuel, lopping, regular removal of bark, overexploitation, and unsustainable extraction and burning [36,88,90,92,103]. They are major reasons that highly affect the growth and regeneration of these endangered plants. Of all these human disturbances, overexploitation of the bark and leaves of Taxus species for pharmaceutical uses are listed as primary reasons for their unsustainable regeneration [19]. Overharvesting of plant parts for domestic purposes has also brought the plants under severe threat [22]. Additionally, browsing and bark peeling by domestic cattle adversely affect the growth of seedlings, saplings, and their vitality [99]. Therefore, not only climatic changes and all the abovementioned factors, but also anthropogenic disturbances play a detrimental role in the proliferation of the population of yews in their region [83,104].
In summary, all the abovementioned major biotic and abiotic factors are bringing plants of the genus Taxus to severe endangerment. Therefore, to protect these natural wild resources, urgent conservation actions must be taken for all of the plants in their region. Some of these conservation actions include building fences for the protection of Taxus plant’s natural regeneration, protecting them by guards, raising awareness in local people, and limiting the big game hunting of the ungulates to reduce their population. In addition, the forest managements can also save the older or matured Taxus trees because they are sources of seeds that can ensure the regeneration of other Taxus trees and also maintain the ecological integrity of these plants stands. Taxus plants are very sensitive at their seedling stage and protecting the seedlings from grazing damage and browsing is also needed for the growth, establishment, and regeneration of these plants. Artificial regenerations of Taxus plants from their seeds are extremely poor because of the hard-coated seeds, and the growth and development of the seedlings are very slow [20]. Therefore, tissue, hairy root, cell, and other organ cultures technology by specialists, reported as very fast, effective, and successful tools for the regeneration and propagation of plants [18,81] are required as an alternative technique to save Taxus plants from extinction and endangerment. This technique is very helpful for the production of a high concentration of taxol and its precursors and other important secondary metabolites from Taxus trees without destroying them [18,105].
3. Conclusions
Taxus is the largest genus of the family Taxaceae and comprises about 24 species with 55 varieties, distributed mainly in Asia, Europe, North Africa, and North America. Its species gained global recognition for their anticancer drug taxol. Taxus species are also used to relieve edema, to remove toxicity from the body, and to treat diseases like lung disorders, epilepsy, nervousness, hysteria, malaria, nephropathy, and diabetic nephropathy. They are reported to exhibit antileukemic, analgesic, cytotoxic, antiinflammatory, sedative, anticancer, anticonvulsant, antipyretic, antibacterial, antimitotic, tranquilizing, antifungal, and antiseptic properties. According to the literature, among the identified plants of the genus Taxus, only eight of them, namely T. baccata, T. chinensis, T. canadensis, T. media, T. cuspidata, T. wallichiana, T. wallichiana var mairei, and T. chinensis var. mairei, were studied concerning the chemical constituent and only four, such as T. chinensis, T. cuspidata, T. chinensis var. mairei, and T. media, have been studied in terms of biological activities (only antifungal, antibacterial, antioxidant, and antihypertensive activities) of their oils. Generally, essential oils of the investigated Taxus species were dominated mostly by alcohols. The most frequent components with high concentrations of these essential oils are cis-3-hexen-1-ol, 1-octen-3-ol, caryophyllene oxide, myrtenol, elemicin, trans-2-hexenal, α-pinene, and laminitol. Palmitic, oleic, linoleic, taxoleic, and α-linolenic acids were the most predominant and frequently reported fatty acid constituents of the oils (lipids) of Taxus plants from different regions. The oils of the investigated plants of the genus Taxus have demonstrated powerful antifungal, antibacterial, antioxidant and antihypertensive activities. However, the species of this genus are the most threatened and endangered plants in their geographical ranges. Various biotic and abiotic factors are affecting the survival of these precious species and due to these, their regeneration is very poor. Of these, climatic and environmental factors and anthropogenic disturbances are the main reasons for the poor regeneration. Therefore, to protect plants of the genus Taxus, urgent conservation actions must be taken by forest managers, local communities, governments and other stakeholders for all of the plants in their region. In the future, studies are also needed in the researches of pharmacists, chemists, biologists, and phytochemists to investigate the chemical constituents and biological activities of oils of the unstudied and less studied Taxus plants and foresters, ecologists, and environmentalists regarding their most effective regeneration.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1. Merkx YM, Svendsen AB. Glycosidically bound volatile aliphatic and aromatic alcohols—a common feature in the vegetable kingdom? Journal of Essential Oil Research. 1990;2(4):207–208. [Google Scholar]
- 2. Jean FI, Garneau FX, Collin GJ, Bouhajib M, Zamir LO. The essential oil and glycosidically bound volatile compounds of Taxus canadensis Marsh. Journal of Essential Oil Research. 1993;5(1):7–11. [Google Scholar]
- 3. Xie JC, Lu LH, Zeng H, Han HL, Sun BG. Volatile Constituents of Taxus wallichiana var mairei Leaves. Journal of Essential Oil Bearing Plants. 2012;15(5):724–730. [Google Scholar]
- 4. Yasar S. Volatile constituents of Taxus baccata L. leaves from western and southern Turkey. Asian Journal of Chemistry. 2013;25(16):9123–9125. [Google Scholar]
- 5. Stefanović M, Ristić M, Popović Z, Matić R, Nikolić B, et al. Chemical composition and interpopulation variability of essential oils of Taxus baccata L. from Serbia. Chemistry and Biodiversity. 2016;13(7):943–953. doi: 10.1002/cbdv.201500326. [DOI] [PubMed] [Google Scholar]
- 6. Hu Z, Chen JT, Jiang SC, Liu Z, Ge SB, et al. Chemical components and functions of Taxus chinensis extract. Journal of King Saud University-Science. 2020;32(2):1562–1568. [Google Scholar]
- 7. Huong LT, Thuong NT, Chac LD, Dai DN, Giwa-Ajeniya AO, et al. The stem essential oil of Taxus chinensis (Rehder & EH Wilson) Rehder (Taxaceae) from Vietnam. American Journal of Essential Oils and Natural Products. 2020;8(3):9–12. [Google Scholar]
- 8. Huong LT, Thuong NT, Chac LD, Dai DN, Ogunwande IA. Antimicrobial activity and chemical constituents of essential oils from the leaf and wood of Taxus chinensis (Rehder & EH Wilson) Rehder (Taxaceae) from Vietnam. Journal of Biologically Active Products from Nature. 2020;10(1):8–17. [Google Scholar]
- 9. Tabaszewska M, Rutkowska J, Skoczylas Ł, Słupski J, Antoniewska A, et al. Red Arils of Taxus baccata L.—a new source of valuable fatty acids and nutrients. Molecules. 2021;26(3):723. doi: 10.3390/molecules26030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erdemoglu N, Sener B, Demirci B, Baser KHC. The glycosidically bound volatile compounds of Taxus baccata. Chemistry of Natural Compounds. 2003;39(2):195–198. [Google Scholar]
- 11. Shirmohammadli Y, Hosseinihashemi SK, Jalaligoldeh A, Efhamisisi D, Mousavinezhad SH, et al. Chemical composition of Taxus baccata L. leaves and male cones water: methanol extracts. Celal Bayar University Journal of Science. 2020;16(3):251–255. [Google Scholar]
- 12. Madrigal RV, Smith CR. Taxus baccata seed oil: a new source of cis-5, cis-9-octadecadienoic acid. Lipids. 1975;10(8):502–504. doi: 10.1007/BF02532438. [DOI] [PubMed] [Google Scholar]
- 13. Takagi T, Itabashi Y. cis-5-Olefinic unusual fatty acids in seed lipids of gymnospermae and their distribution in triacylglycerols. Lipids. 1982;17(10):716–723. [Google Scholar]
- 14. Wolff RL, Deluc LG, Marpeau AM. Conifer seeds: oil content and fatty acid composition. Journal of the American Oil Chemists’ Society. 1996;73(6):765–771. [Google Scholar]
- 15. Wolff RL, Pédrono F, Marpeau AM, Christie WW, Gunstone FD. The seed fatty acid composition and the distribution of Δ5-olefinic acids in the triacylglycerols of some Taxaceae (Taxus and Torreya) Journal of the American Oil Chemists’ Society. 1998;75(11):1637–1641. [Google Scholar]
- 16. Poudel RC, Möller M, Gao LM, Ahrends A, Baral SR, et al. Using morphological, molecular and climatic data to delimitate yews along the Hindu Kush-Himalaya and adjacent regions. PloS One. 2012;7(10):e46873. doi: 10.1371/journal.pone.0046873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karami-Kordalivand P, Esmailzadeh O, Willner W, Noroozi J, Alavi SJ. Classification of forest communities (co-) dominated by Taxus baccata in the Hyrcanian forests (northern Iran) and their comparison with southern Europe. European Journal of Forest Research. 2021;140(2):463–476. [Google Scholar]
- 18.Sykłowska-Baranek K, Sygitowicz G, Pietrosiuk A. Development of Taxus spp Hairy Root Cultures for Enhanced Taxane Production Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications. Switzerland: Springer Nature; 2021. pp. 541–559. [Google Scholar]
- 19. Juyal D, Thawani V, Thaledi S, Joshi M. Ethnomedical properties of Taxus wallichiana zucc. (Himalayan yew) Journal of traditional and complementary medicine. 2014;4(3):159–161. doi: 10.4103/2225-4110.136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishtiyak P, Puni L. Standardization of in-situ propagation technique for Taxus baccata Linn. an endangered medicinal plant of Western Himalayas. Studies on Ethno-Medicine. 2017;11(4):332–340. [Google Scholar]
- 21. Sharma H, Garg M. A review of traditional use, phytoconstituents and biological activities of Himalayan yew, Taxus wallichiana. Journal of integrative medicine. 2015;13(2):80–90. doi: 10.1016/S2095-4964(15)60161-3. [DOI] [PubMed] [Google Scholar]
- 22. Poudel RC, Gao LM, Möller M, Baral SR, Uprety Y, et al. Yews (Taxus) along the Hindu Kush-Himalayan region: exploring the ethnopharmacological relevance among communities of Mongol and Caucasian origins. Journal of ethnopharmacology. 2013;147(1):190–203. doi: 10.1016/j.jep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 23. Poudel RC, Möller M, Li DZ, Shah A, Gao LM. Genetic diversity, demographical history and conservation aspects of the endangered yew tree Taxus contorta (syn. Taxus fuana) in Pakistan. Tree Genetics & Genomes. 2014;10(3):653–665. [Google Scholar]
- 24. Yu C, Luo X, Zhan X, Hao J, Zhang L, et al. Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biology. 2018;18(1):1–12. doi: 10.1186/s12870-018-1412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-Upton J, Garcia-Martí X. Taxus globosa Schltdl. (Taxaceae). Distribution and diagnosis of an endangered yew. Earth Sciences. 2015;4(3):80–88. [Google Scholar]
- 26. Xiao L, Lao WG, Tan Y, Qu X. In vitro investigation of anti-diabetic effect of Taxus cuspidata extracts by ultrasound assisted method. The American Journal of Chinese Medicine. 2012;40(06):1205–1215. doi: 10.1142/S0192415X12500899. [DOI] [PubMed] [Google Scholar]
- 27. Wang S, Xie Y. China species red list. Beijing: Higher Education Press. 2004;1:300–309. [Google Scholar]
- 28. Hao DC, Xiao PG, Ge GB, Liu M. Biological, chemical, and omics research of Taxus medicinal resources. Drug Development Research. 2012;73(8):477–486. [Google Scholar]
- 29. Küpeli E, Erdemoğlu N, Yeşilada E, Şener B. Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. Journal of Ethnopharmacology. 2003;89(2–3):265–270. doi: 10.1016/j.jep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30. Spjut RW. Taxonomy and nomenclature of Taxus (Taxaceae) Journal of the Botanical Research Institute of Texas. 2007;1(1):203–289. [Google Scholar]
- 31. Spjut RW. A phytogeographical analysis of Taxus (Taxaceae) based on leaf anatomical characters. Journal of the Botanical Research Institute of Texas. 2007;1(1):291–332. [Google Scholar]
- 32. Shi GL, Bai B, Lu CH. Seed rain and seed bank of Chinese yew (Taxus chinensis var. mairei) population in Tianmu Mountain. Acta Ecologica Sinica. 2010;30(5):276–279. [Google Scholar]
- 33. Zhang JT, Ru W. Population characteristics of endangered species Taxus chinensis var. mairei and its conservation strategy in Shanxi, China. Population Ecology. 2010;52(3):407–416. [Google Scholar]
- 34. Zhang DQ, Zhou N. Genetic diversity and population structure of the endangered conifer Taxus wallichiana var. mairei (Taxaceae) revealed by simple sequence repeat (SSR) markers. Biochemical Systematics and Ecology. 2013;49:107–114. [Google Scholar]
- 35. Majeed A, Singh A, Choudhary S, Bhardwaj P. Transcriptome characterization and development of functional polymorphic SSR marker resource for Himalayan endangered species, Taxus contorta (Griff) Industrial Crops and Products. 2019;140:111600. [Google Scholar]
- 36. Iqbal J, Meilan R, Khan B. Assessment of risk, extinction, and threats to Himalayan yew in Pakistan. Saudi Journal of Biological Sciences. 2020;27(2):762–767. doi: 10.1016/j.sjbs.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu C, Zhang C, Xu X, Huang J, Chen Y, et al. Omic analysis of the endangered Taxaceae species Pseudotaxus chienii revealed the differences in taxol biosynthesis pathway between Pseudotaxus and Taxus yunnanensis trees. BMC Plant Biology. 2021;21(1):1–13. doi: 10.1186/s12870-021-02883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang WX, Zhao ZG, Wang LH, Yu SJ, Liang ZS. Control of hypertension in rats using volatile components of leaves of Taxus chinensis var. mairei. Journal of Ethnopharmacology. 2012;141(1):309–313. doi: 10.1016/j.jep.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 39. Olsen CE, Singh R, Gupta S, Bisht KS, Malhotra S, et al. Chemical constituents of Taxus canadensis. Indian Journal of Chemistry. 1998;37B:828–831. [Google Scholar]
- 40. Ghaffar N, Lee LS, Choi YJ, Perre CD, Khan B. Efficient heated ultrasound assisted extraction and clean-up method for quantifying paclitaxel concentrations in Taxus Wallichiana. International Journal of Environmental Analytical Chemistry. 2019;101(4):549–560. [Google Scholar]
- 41. Hao J, Guo H, Shi X, Wang Y, Wan Q, et al. Comparative proteomic analyses of two Taxus species (Taxus × media and Taxus mairei) reveals variations in the metabolisms associated with paclitaxel and other metabolites. Plant and Cell Physiology. 2017;58(11):1878–1890. doi: 10.1093/pcp/pcx128. [DOI] [PubMed] [Google Scholar]
- 42. Nadeem M, Rikhari HC, Kumar A, Palni LMS, Nandi SK. Taxol content in the bark of Himalayan Yew in relation to tree age and sex. Phytochemistry. 2002;60(6):627–631. doi: 10.1016/s0031-9422(02)00115-2. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Yu S, Dong M, Zhang M, Huo C, Shi Q. Chemical studies on Taxus cuspidata. Chemistry and Biodiversity. 2010;7(7):1698–1716. doi: 10.1002/cbdv.200800295. [DOI] [PubMed] [Google Scholar]
- 44. Miele M, Mumot AM, Zappa A, Romano P, Ottaggio L. Hazel and other sources of paclitaxel and related compounds. Phytochemistry Reviews. 2012;11(2–3):211–225. [Google Scholar]
- 45. Li Y, Qin F, Wang SM, Guo RX, Zhang YF, et al. Chemical studies on Taxus canadensis. Chemistry and Biodiversity. 2013;10(10):1729–1753. doi: 10.1002/cbdv.201200032. [DOI] [PubMed] [Google Scholar]
- 46. Wahab A, Khera RA, Rehman R, Mushtaq A, Blama A, et al. A review on phytochemistry and medicinal uses of Taxus wallichiana L. (Himalayan Yew) International Journal of Chemical and Biochemical Sciences. 2016;9:116–120. [Google Scholar]
- 47. Sinha A. A review on taxanes: an important group of anticancer compound obtained from Taxus sp. International Journal of Pharmaceutical Sciences and Research. 2020;11(5):1969–1985. [Google Scholar]
- 48. Németh-Kiss V, Forgács E, Cserháti T, Schmidt G. Taxol content of various Taxus species in Hungary. Journal of Pharmaceutical and Biomedical Analysis. 1996;14(8–10):997–1001. doi: 10.1016/0731-7085(95)01682-1. [DOI] [PubMed] [Google Scholar]
- 49. Fang W, Wu Y, Zhou J, Chen W, Fang Q. Qualitative and quantitative determination of taxol and related compounds in Taxus cuspidata Sieb et Zucc. Phytochemical Analysis. 1993;4(3):115–119. [Google Scholar]
- 50. Elias TS, Korzhenevsky VV. The presence of taxol and related compounds in Taxus baccata native to the Ukraine (Crimea), Georgia, and southern Russia. Aliso: A Journal of Systematic and Evolutionary Botany. 1992;13(3):463–470. [Google Scholar]
- 51. Vidensek N, Lim P, Campbell A, Carlson C. Taxol content in bark, wood, root, leaf, twig, and seedling from several Taxus species. Journal of Natural Products. 1990;53(6):1609–1610. doi: 10.1021/np50072a039. [DOI] [PubMed] [Google Scholar]
- 52. Zobel AM, Furmanowa M, Glowniak K, Cragg C. Taxol on the surface of leaves and inside the needles of three species and two varieties of Taxus. Phytomedicine. 1996;3(3):287–291. doi: 10.1016/S0944-7113(96)80068-7. [DOI] [PubMed] [Google Scholar]
- 53. van Rozendaal EL, Lelyveld GP, van Beek TA. Screening of the needles of different yew species and cultivars for paclitaxel and related taxoids. Phytochemistry. 2000;53(3):383–389. doi: 10.1016/s0031-9422(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 54. Matysik G, Głowniak K, Józefczyk A, Furmanowa M. Stepwise gradient thin-layer chromatography and densitometric determination of taxol in extracts from various species of Taxus. Chromatographia. 1995;41(7):485–487. [Google Scholar]
- 55. Poupat C, Hook I, Guéritte F, Ahond A, Guénard D, et al. Neutral and basic taxoid contents in the needles of Taxus species. Planta Medica. 2000;66(06):580–584. doi: 10.1055/s-2000-8651. [DOI] [PubMed] [Google Scholar]
- 56. Glowniak K, Zgórka G, Józefczyk A, Furmanowa M. Sample preparation for taxol and cephalomannine determination in various organs of Taxus sp. Journal of Pharmaceutical and Biomedical Analysis. 1996;14(8–10):1215–1220. doi: 10.1016/s0731-7085(96)01728-1. [DOI] [PubMed] [Google Scholar]
- 57. Glowniak K, Mroczek T, Zobel AM. Seasonal changes in the concentrations of four taxoids in Taxus baccata L. during the autumn-spring period. Phytomedicine. 1999;6(2):135–140. doi: 10.1016/S0944-7113(99)80049-X. [DOI] [PubMed] [Google Scholar]
- 58. Witherup KM, Look SA, Stasko MW, Ghiorzi TJ, Muschik GM, et al. Taxus spp. needles contain amounts of taxol comparable to the bark of Taxus brevifolia: analysis and isolation. Journal of Natural Products. 1990;53(5):1249–1255. doi: 10.1021/np50071a017. [DOI] [PubMed] [Google Scholar]
- 59. Rao KV. Taxol and related taxanes. I. Taxanes of Taxus brevifolia bark. Pharmaceutical Research. 1993;10(4):521–524. doi: 10.1023/a:1018937700459. [DOI] [PubMed] [Google Scholar]
- 60. Wheeler NC, Jech K, Masters S, Brobst SW, Alvarado AB, et al. Effects of genetic, epigenetic, and environmental factors on taxol content in Taxus brevifolia and related species. Journal of Natural Products. 1992;55(4):432–440. doi: 10.1021/np50082a005. [DOI] [PubMed] [Google Scholar]
- 61. Fu YJ, Sun R, Zu YG, Li SM, Liu W, et al. Simultaneous determination of main taxoids in Taxus needles extracts by solid-phase extraction-high-performance liquid chromatography with pentafluorophenyl column. Biomedical Chromatography. 2009;23(1):63–70. doi: 10.1002/bmc.1085. [DOI] [PubMed] [Google Scholar]
- 62. Zu Y, Fu Y, Li S, Sun R, Li Q, et al. Rapid separation of four main taxoids in Taxus species by a combined LLP-SPE-HPLC (PAD) procedure. Journal of Separation Science. 2006;29(9):1237–1244. doi: 10.1002/jssc.200500483. [DOI] [PubMed] [Google Scholar]
- 63. Soto M, Sanjurjo M, González MT, Cruz D, Giral F. El tejo mexicano (Taxus globosa Sch.). Potencial de su aprovechamiento en taxol. CIENCIA ergo-sum, Revista Científica Multidisciplinaria de Prospectiva. 2000;7(3):277–279. [Google Scholar]
- 64. Xi XJ, Guo J, Zhu YG, Yang XL, Yu Y, et al. Genetic diversity and taxol content variation in the Chinese yew Taxus mairei. Plant Systematics and Evolution. 2014;300(10):2191–2198. [Google Scholar]
- 65. Mukherjee S, Ghosh B, Jha TB, Jha S. Variation in content of taxol and related taxanes in Eastern Himalayan populations of Taxus wallichiana. Planta Medica. 2002;68(08):757–759. doi: 10.1055/s-2002-33808. [DOI] [PubMed] [Google Scholar]
- 66. Getahun T, Sharma V, Gupta N. The genus Laggera (Asteraceae)–ethnobotanical and ethnopharmacological information, chemical composition as well as biological activities of its essential oils and extracts: a review. Chemistry and Biodiversity. 2019;16(8):e1900131. doi: 10.1002/cbdv.201900131. [DOI] [PubMed] [Google Scholar]
- 67.Thormar H, editor. Lipids and Essential Oils as Antimicrobial Agents. Chichester, England: John Wiley & Sons; 2011. [Google Scholar]
- 68. Cansu TB, Yaylı B, Özdemir T, Batan N, Karaoğlu ŞA, et al. Antimicrobial activity and chemical composition of the essential oils of mosses (Hylocomium splendens (Hedw.) Schimp. and Leucodon sciuroides (Hedw.) Schwägr.) growing in Turkey. Turkish Journal of Chemistry. 2013;37(2):213–219. [Google Scholar]
- 69. Getahun T, Sharma V, Gupta N. Chemical composition, antibacterial and antioxidant activities of oils obtained by different extraction methods from Lepidium sativum L. seeds. Industrial Crops and Products. 2020;156:112876. [Google Scholar]
- 70. Getahun T, Sharma V, Gupta N. Chemical composition and biological activity of essential oils from Aloe debrana roots. Journal of Essential Oil Bearing Plants. 2020;23(3):493–502. [Google Scholar]
- 71. Getahun T, Sharma V, Kumar D, Gupta N. Chemical composition, and antibacterial and antioxidant activities of essential oils from Laggera tomentosa Sch. Bip. ex Oliv. et Hiern (Asteraceae) Turkish Journal of Chemistry. 2020;44(6):1539–1548. doi: 10.3906/kim-2004-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Khan M, Verma SC, Srivastava SK, Shawl AS, Syamsundar KV, et al. Essential oil composition of Taxus wallichiana Zucc. from the Northern Himalayan region of India. Flavour and Fragrance Journal. 2006;21(5):772–775. [Google Scholar]
- 73.Zhang J, Yuan K, Jin YC. Advanced Materials Research. Vol. 343. Trans Tech Publications Ltd; 2012. Comparison of chemical composition and antimicrobial activities of the essential oil of Taxus media and Taxus chinensis var. mairei leaves; pp. 1092–1097. [Google Scholar]
- 74. Bajpai VK, Sharma A, Moon B, Baek KH. Chemical composition analysis and antibacterial mode of action of Taxus cuspidata leaf essential oil against foodborne pathogens. Journal of Food Safety. 2014;34(1):9–20. [Google Scholar]
- 75. Kılıç Ö, Koçak A. Volatile constituents of Juniperus communis L., Taxus canadensis Marshall. and Tsuga canadensis (L.) Carr. from Canada. Journal of Agricultural Science and Technology. 2014;B 4:135–140. [Google Scholar]
- 76. Bajpai VK, Baek KH. Characterization of microwave extracted essential oil from Taxus cuspidata stem and determination of its phenolic content, antioxidant and free radical scavenging activities. Journal of Essential Oil Bearing Plants. 2016;19(5):1051–1065. [Google Scholar]
- 77. Zhao C, He X, Li C, Yang L, Fu Y, et al. A microwave-assisted simultaneous distillation and extraction method for the separation of polysaccharides and essential oil from the leaves of Taxus chinensis var. mairei. Applied Sciences. 2016;6(2):19. [Google Scholar]
- 78. Wei Q, Yin CW. Chemical composition of essential oils from the stems of Taxus chinensis var. mairei. Journal of Essential Oil Bearing Plants. 2019;22(4):1144–1149. [Google Scholar]
- 79. Pirbalouti AG, Izadi A, Poor FM, Hamedi B. Chemical composition, antioxidant and antibacterial activities of essential oils from Ferulago angulata. Pharmaceutical Biology. 2016;54(11):2515–2520. doi: 10.3109/13880209.2016.1162816. [DOI] [PubMed] [Google Scholar]
- 80. Radulović N, Blagojević P, Palić R, Zlatković B. Chemical composition of the essential oil hydrodistilled from Serbian Taxus baccata L. Journal of Essential Oil Research. 2010;22(5):458–461. [Google Scholar]
- 81. Lanker U, Malik AR, Gupta NK, Butola JS. Natural regeneration status of the endangered medicinal plant, Taxus baccata Hook. F. syn. T. wallichiana, in northwest Himalaya. International Journal of Biodiversity Science, Ecosystem Services and Management. 2010;6(1–2):20–27. [Google Scholar]
- 82. Svenning JC, Magård E. Population ecology and conservation status of the last natural population of English yew Taxus baccata in Denmark. Biological Conservation. 1999;88(2):173–182. [Google Scholar]
- 83. Thomas PA, Garcia-Martí X. Response of European yews to climate change: a review. Forest Systems. 2015;24(3):eR01-1–11. [Google Scholar]
- 84. Prasanna KR, Mathana JM, Ramya TA, Nirmala R. LoRa network based high performance forest fire detection system. Materials Today: Proceedings. 2021 [Google Scholar]
- 85. Hanewinkel M, Cullmann DA, Schelhaas MJ, Nabuurs GJ, Zimmermann NE. Climate change may cause severe loss in the economic value of European forest land. Nature Climate Change. 2013;3(3):203–207. [Google Scholar]
- 86.Dhakal S, Mohanty A, Rijal KP. Assessment of Carbon Sequestration and Tree Diversity in Gokarna Forest. Kathmandu, Nepal: Sustainable Climate Action and Water Management; 2021. pp. 167–180. [Google Scholar]
- 87. Singh RP, Prasad PV, Reddy KR. Impacts of changing climate and climate variability on seed production and seed industry. Advances in Agronomy. 2013;118:49–110. [Google Scholar]
- 88. Sanz R, Pulido F, Nogués-Bravo D. Predicting mechanisms across scales: amplified effects of abiotic constraints on the recruitment of yew Taxus baccata. Ecography. 2009;32(6):993–1000. [Google Scholar]
- 89. Suszka B. Conditions for after-ripening and germination of seeds and for seedling emergence of English yew (Taxus baccata L.) Arboretum Kórnickie. 1985;30:285–338. [Google Scholar]
- 90. Rikhari HC, Palni LMS, Sharma S, Nandi SK. Himalayan yew: stand structure, canopy damage, regeneration and conservation strategy. Environmental Conservation. 1998;25(4):334–341. [Google Scholar]
- 91. Dobrowolska D, Niemczyk M, Olszowska G. The influence of stand structure on European yew Taxus baccata populations in its natural habitats in central Poland. Polish Journal of Ecology. 2017;65(3):369–384. [Google Scholar]
- 92. Martínez D, García D. Role of avian seed dispersers in tree recruitment in woodland pastures. Ecosystems. 2017;20(3):616–629. [Google Scholar]
- 93. Sedmáková D, Kýpet’ová M, Saniga M, Pittner J, Vencurik J, et al. Deer game, a key factor affecting population of European yew in beech forests of the Veľká Fatra Mts, Slovakia. Folia Oecologica. 2018;45(1):1–7. [Google Scholar]
- 94. Mysterud A, Østbye E. Roe deer Capreolus capreolus feeding on yew Taxus baccata in relation to bilberry Vaccinium myrtillus density and snow depth. Wildlife Biology. 1995;1(1):249–253. [Google Scholar]
- 95. Mysterud A, Østbye E. Roe deer (Capreolus capreolus) browsing pressure affects yew (Taxus baccata) recruitment within nature reserves in Norway. Biological Conservation. 2004;120(4):545–548. [Google Scholar]
- 96. Perrin PM, Kelly DL, Mitchell FJ. Long-term deer exclusion in yew-wood and oakwood habitats in southwest Ireland: natural regeneration and stand dynamics. Forest Ecology and Management. 2006;236(2–3):356–367. [Google Scholar]
- 97. Hulme PE. Natural regeneration of yew (Taxus baccata L.): microsite, seed or herbivore limitation? Journal of Ecology. 1996;84(6):853–861. [Google Scholar]
- 98. García D, Zamora R, Hódar JA, Gómez JM, Castro J. Yew (Taxus baccata L.) regeneration is facilitated by fleshy-fruited shrubs in Mediterranean environments. Biological Conservation. 2000;95(1):31–38. [Google Scholar]
- 99. Farris E, Filigheddu R. Effects of browsing in relation to vegetation cover on common yew (Taxus baccata L.) recruitment in Mediterranean environments. Plant Ecology. 2008;199(2):309–318. [Google Scholar]
- 100. Pers-Kamczyc E, Iszkuło G, Rabska M, Wrońska-Pilarek D, Kamczyc J. More isn’t always better–the effect of environmental nutritional richness on male reproduction of Taxus baccata L. Environmental and Experimental Botany. 2019;162:468–478. [Google Scholar]
- 101. Hulme PE. Post-dispersal seed predation and the establishment of vertebrate dispersed plants in Mediterranean scrublands. Oecologia. 1997;111(1):91–98. doi: 10.1007/s004420050212. [DOI] [PubMed] [Google Scholar]
- 102. Vessella F, Salis A, Scirè M, Piovesan G, Schirone B. Natural regeneration and gender-specific spatial pattern of Taxus baccata in an old-growth population in Foresta Umbra (Italy) Dendrobiology. 2015;73:75–90. [Google Scholar]
- 103. Paul A, Bharali S, Khan ML, Tripathi OP. Anthropogenic disturbances led to risk of extinction of Taxus wallichiana Zuccarini, an endangered medicinal tree in Arunachal Himalaya. Natural Areas Journal. 2013;33(4):447–454. [Google Scholar]
- 104. Ahmadi K, Alavi SJ, Amiri GZ, Hosseini SM, Serra-Diaz JM, et al. The potential impact of future climate on the distribution of European yew (Taxus baccata L.) in the Hyrcanian Forest region (Iran) International Journal of Biometeorology. 2020;64:1451–1462. doi: 10.1007/s00484-020-01922-z. [DOI] [PubMed] [Google Scholar]
- 105. Zhang CH, Wu JY. Ethylene inhibitors enhance elicitor-induced paclitaxel production in suspension cultures of Taxus spp. cells. Enzyme and Microbial Technology. 2003;32(1):71–77. [Google Scholar]