Abstract
Older adults (≥ 65 years) are recommended to participate in regular exercise to maintain health in late adulthood. The impact of long-term (20+ years) exercise training that align with the American College of Sports Medicine’s (ACSM) recommended guidelines has not been evaluated for older adults. To address this, a systematic review and meta-analyses were performed regarding the effects of long-term exercise training on older adult aerobic capacity, muscular fitness, and body composition that meet the ACSM’s recommendation for weekly training volume. Ten studies with individuals that performed cardiorespiratory or resistance exercise met the inclusion criteria for the systematic review. Data from five included studies were analyzed in meta-analyses to determine the relationship between the effects of cardiorespiratory training on fitness and body composition measured in the same subjects. Main findings include higher cardiorespiratory fitness (MD: +11.36 mL/kg/min, 95% CI: 5.63 to 17.09 mL/kg/min, p < 0.01) in older adults who performed long-term cardiorespiratory exercise that was found in conjunction with lower percent body fat (MD: −5.41%, 95% CI: −7.65 to −3.17%, p < 0.01). Higher volume of cardiorespiratory exercise beyond the minimum recommendations did not impact benefits. Additionally, resistance-trained older adults showed greater muscular strength and lower percent body fat with comparable cardiorespiratory fitness to sedentary older adults. These findings primarily highlight a preservation of cardiorespiratory fitness and lower risk of mortality and cardiometabolic disease risk for older adults who participate in long-term cardiorespiratory and exercise that meet the ACSM’s recommended weekly training volume.
Keywords: Lifelong exercise, aerobic, elderly, weight training
INTRODUCTION
Aging elicits a general redistribution of bodily constituents, loss of fat-free mass, and a gain in fat mass (35). A sedentary lifestyle in older age further increases the risk of increased fat mass (39) as well as reduced muscle mass (1) and bone mineral density (34) that perpetuate the development of cardiometabolic disorders (37), sarcopenia (12, 31), and osteopenia (12, 31). Chronically active older adults (≥ 65 years) (12, 31) are considered an ideal model of successful aging due to health benefits from long-term exercise training (5). Active older adults are known to have decreased maximal aerobic capacity, a comparable body mass index (BMI) to younger sedentary adults (25, 34), and non-significant changes in fat mass, fat-free mass, resting heart rate, or blood pressure over time (19). Fitness, in particular, is well-known for having a substantial impact on health. For example, maximal aerobic capacity (i.e., VO2max) is a primary measure of cardiorespiratory fitness and is a critical marker of health. VO2max is the best predictor of mortality after age-adjustment (28) and higher cardiorespiratory fitness is inversely associated with all-cause mortality (23). These results imply that a maintenance of cardiorespiratory fitness in older age will certainly be beneficial, but the length of participation in and volume of cardiorespiratory exercise likely determine the degree of potential health benefits.
While the benefits of cardiorespiratory exercise for cardiorespiratory and physical function have been well reported in older adults (4, 17, 26, 30), evaluations of the impact on body composition are less common. Body composition assessment may provide useful information for the effects of exercise with aging on fat distribution as well as bodily constituents of fat mass (e.g., adipose tissue) and fat-free mass (e.g., muscle, protein, and mineral) (42). Additionally, the evidence regarding the effects of long-term resistance training on fitness and body composition is limited. A recent systematic review showed that resistance training interventions in 34 studies were effective in reducing visceral fat, independent of any changes in diet (20). However, these included studies reflect relatively short-term health benefits, when considering the length of the average lifespan, since the duration of resistance training protocols ranged from 2–24 months or until a BMI < 25 kg/m2 was achieved.
The collective benefits of exercise training support recommendations by the American College of Sports Medicine (ACSM) and the American Medical Association (12, 31). Specifically, ACSM recommends the same weekly minimum physical activity guidelines for healthy older adults as for the general adult population to lower the risk of cardiometabolic disease, osteoporosis, and other age-related health consequences of sedentariness. These recommendations are to achieve ≥ 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity cardiorespiratory exercise and resistance training of major muscle groups 2–3 days/week (3). However, only 23 – 47.7% of older adults in 2003–2006 met the current minimum guidelines for weekly training volume (43). Additionally, these results are based on accelerometer data over four days (43) and may not represent the chronic engagement in physical activity or sedentariness. Additionally, the literature is inconsistent with defining older adults and describing the length of training. A previous meta-analysis shows long-term (20+ years) cardiorespiratory and resistance training preserves physical function, muscular strength, and percent body fat (%BF) in adults over 60 years of age (24). However, many of these included subjects did not meet the definition of older adults (≥ 65 years of age) by American-based organizations (ACSM and the American Medical Association) (12, 31). Long-term training has also been defined as short as 1 year (33) to as long as 20+ years (24) of training. Furthermore, since both fitness and body composition are associated with disease risk in older age, questions still remain as to whether the impact of long-term exercise training on fitness are directly reflected to effects on body composition. There has also been a lack of analysis in comparing the amount of training older adults perform each week to determine if there are proportional health benefits with higher volumes of exercise training.
The purpose of this review is to analyze the effects of long-term (20+ years) cardiorespiratory or resistance training, in accordance to the minimum recommendations of weekly training volume by ACSM, on the fitness and body composition of older adults aged ≥ 65 years. A standardized assessment for the effects of long-term exercise training in the current review and meta-analyses based on the minimum exercise recommendations by the American College of Sports Medicine (ACSM) (12) will provide a model of fitness and body composition in active older adults that has not been performed previously. Our hypotheses are that long-term exercise training will result in a preservation of cardiorespiratory fitness or muscular strength as well as an attenuation of age-related changes in body composition.
METHODS
This systematic review and meta-analysis included de-identified data from published sources and, therefore, did not require approval from the University of New Mexico Institutional Review Board.
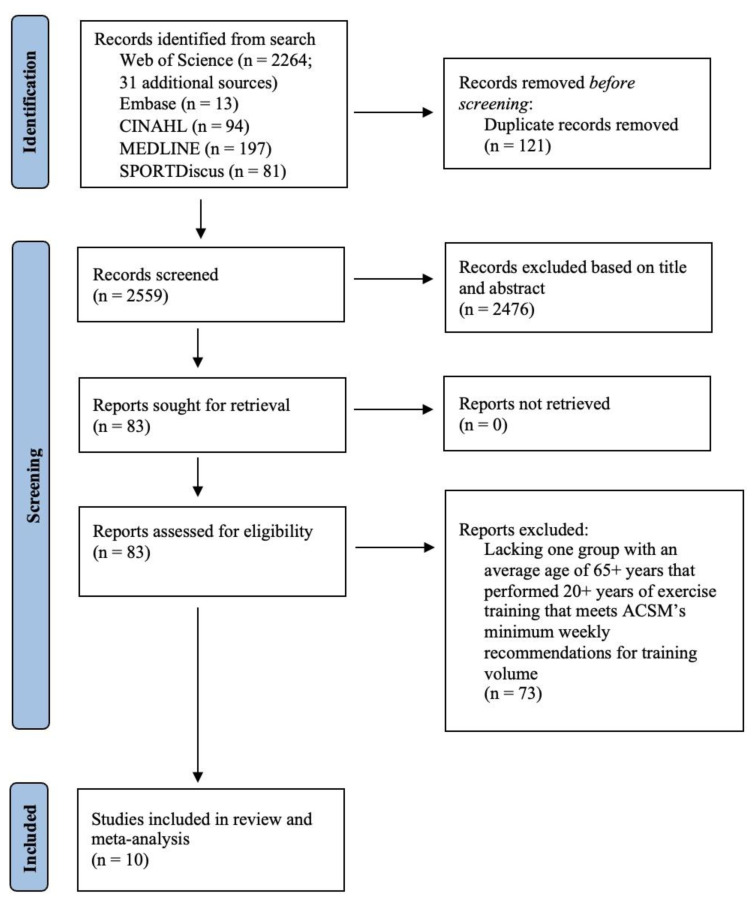
Protocol and Literature Search: This review followed the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) (27). While the protocol was not registered in PROSPERO, duplication of the current review was not found with registered protocols. A literature search in the online databases CINAHL, Embase, Medline, SPORTDiscus, and Web of Science was conducted using key words, free text terms, indexed terms, and Boolean operators and was completed on March 20, 2022. Table 1 provides the complete search that was included into the database and filtered for articles with human subjects published in English with full-text availability. Figure 1 provides the sequenced methodology of the search.
Table 1.
The literature review search with all key words, free text terms, indexed terms, and Boolean operators.
| 1st search field | older adults OR “older adults” OR masters athletes OR “masters athletes” OR older athletes OR “older athletes” |
| AND (2nd search field) | Body composition OR “body composition” OR body parameters OR “body parameters” OR body constitution OR “body constitution” OR body fat OR “body fat” OR BMI OR “BMI” OR body density OR “body density” OR mineral OR “mineral” OR bone OR “bone” |
| AND (third search field) | exercise OR “exercise” OR physical activity OR “physical activity” OR exercise training OR “exercise training” OR endurance exercise OR “endurance exercise” OR endurance training OR “endurance training” OR resistance exercise OR “resistance exercise” OR resistance training OR “resistance training” OR strength training OR “strength training” OR bodyweight exercise OR “body weight exercise” OR concurrent training OR “concurrent training” |
| AND (fourth search field) | long-term OR "long term" OR chronic OR "chronic" OR longitudinal OR "longitudinal" |
Figure 1.
PRISMA flow Diagram.
Inclusion Criteria: The following criteria were used for study inclusion in this literature review. Studies needed an explicit statement of at least one group within the study consisting of adults ≥ 65 years that performed long-term exercise training for 20+ years immediately prior to or throughout the respective study. Older adults are classified as ≥ 65 years by both the American Medical Association (31) and ACSM (12) and the timeframe of long-term exercise training has been used in prior analyses regarding the effects of exercise in adults (24). At least one measurement of body composition collected after 20+ years of exercise training was required. However, preliminary measurements were also recorded if available. Whole body estimations of body composition assessment from any level of body composition (42) were accepted. Only healthy populations free from cardiometabolic, neuromuscular, or neurological disease were be included. Older adults needed to complete the minimum exercise recommendations for weekly training volume for cardiorespiratory or resistance exercise by ACSM (12). Specifically, the participants needed to complete ≥ 2.5 hours/week of cardiorespiratory training or ≥ 2 sessions/week of resistance training. Exercise intensity was recorded if provided but not included for analysis and it was assumed that the older adults completed the minimum exercise intensities recommended by ACSM: at least moderate-intensity cardiorespiratory exercise or ≥ 20% of one-repetition maximum for resistance exercise. Only original studies published in English, with no date restrictions, and full text available were included. Articles that met the inclusion criteria and were cited by relevant literature reviews obtained from the search were also included. For studies with multiple measurements, the longest timespan of measurements separated by ≥ 20 years were used for analysis. Multiple studies from the same longitudinal studies were excluded; only one study with either body composition measures or the longest duration study (if all measured body composition) was used for analysis. Finally, included studies needed to meet the international ethics standards for conducting research (16). By using these criteria, within-study identification of trained and sedentary older adult groups was not used.
Exclusion Criteria: Studies were excluded from the review if: 1) study included another intervention that prevents isolated analysis of the effect of exercise on fitness and body composition; 2) Study did not provide information on weekly training volume that was convertible to hours/week for cardiorespiratory training or sessions/week for resistance training; 3) Sport-specific exercise training that was not categorized as cardiorespiratory or resistance training was excluded.
Study Selection: Titles and abstracts were screened for relevance. Articles included in relevant reviews, systematic reviews, and meta-analyses were also screened. Further evaluation of full-text articles was used to determine if potentially relevant articles met the inclusion criteria. A total of 2,680 studies were obtained from the literature search and an additional 31 articles were obtained by manual search from reference lists from any review articles obtained. After removing duplicate studies (n = 121), 2,559 titles and abstracts were screened, which resulted in 2,476 articles being excluded. The full text of the remaining 83 articles were assessed for eligibility and 10 studies were determined to be eligible for analysis.
Data Extraction: Predetermined variables were extracted from all eligible studies with means and standard deviations reported. Included variables pertained to participant demographics (age, sex, ethnicity, height, weight), self-reported training (years, type, weekly volume or frequency, and intensity) body composition (BMI, percent body fat, fat-free mass, and bone mineral density), cardiorespiratory fitness (VO2max or VO2peak), and muscular strength (limb-specific maximal voluntary contraction and grip strength). The Cochrane formulae were used to combine sample sizes, means, and standard deviations for multiple trained or sedentary groups within the ≥ 65 years age range (18). Any conversions of cardiorespiratory training volumes to hours/week were rounded to the nearest tenth of an hour. For studies reporting a range of weekly exercise sessions performed at or above the recommended duration, the minimum duration was used. For example, if cardiorespiratory exercise was reported as 6–7 sessions per week with a minimum 0.5 hour per session, the group was considered to perform 3 hours/week for analysis.
Quality Assessment: A modified STROBE checklist for cohort, case-control, and cross-sectional studies (40) was used to evaluate the quality of the studies included in the systematic review and meta-analyses.
Meta-Analyses: Meta-analyses with random effects were performed using Review Manager ((Revman), Version 5.4.1, The Cochrane Collaboration, 2020), presented as mean difference with confidence interval, from included study data where means and standard deviations were provided for estimations of whole-body body composition along with measures of cardiorespiratory fitness or muscular strength from both trained and sedentary older adult groups in at least two studies. Therefore, meta-analyses were completed with five of the included studies (1, 2, 10, 25, 32) to compare the effects of long-term training in active older adults to sedentary adults related to VO2max, percent body fat, and fat-free mass. Given a systematic review and meta-analysis with the current inclusion and exclusion criteria has not been performed previously, effect sizes were not estimated.
RESULTS
There was a total of 265 participants (229 males, 27 females, 9 of an unknown sex) from the 10 studies included in the analysis (1, 2, 7, 10, 19, 25, 32, 34, 36, 38). Tables 2–5 provide information pertaining to participant characteristics, training experience, body composition assessment, and fitness measures. The quality assessment showed included studies met on average 12.82 ± 1.17 out of 14 criteria with the results for each study provided in Table 6.
Table 2.
Subject characteristics from included studies.
| Study | Groups | Sample (n) | Sex (M/F) | Age (years) | Height (cm or m where indicated) | Weight (kg) |
|---|---|---|---|---|---|---|
| Aagaard et al. (2007) | Resistance trained | 7 | M | 73.9 ± 2.2 | 175.1 ± 4.3 | 78.7 ± 20.1 |
| Sedentary | 8 | M | 70.5 ± 2.8 | 174.8 ± 6.0 | 82.9 ± 6.2 | |
|
| ||||||
| Ari et al. (2004) | Endurance trained | 10 | M | 68 ± 6 | 167 ± 8 | 71 ± 3 |
| Sedentary | 11 | M | 65 ± 5 | 169 ± 5 | 82 ± 11 | |
|
| ||||||
| Buyukyazi et al. (2003) | Endurance Trained | 11 | M | 67.1 ± 6.0 | 167.2 ± 7.7 | 70.9 ± 3.2 |
|
| ||||||
| Carrick- Ranson et al. (2014) | Endurance trained | 25 | 17M/8F | 68 ± 3 | 171 ± 10 | 66 ± 12 |
| Sedentary | 52 | 33M/19F | 70 ± 5.5 | 171.4 ± 10.2 | 75 ± 12.4 | |
|
| ||||||
| Kasch et al. (1999) | Endurance trained (pre) | 11 | M | 43 ± 6 | 177 ± 7.8 | 74.9 ± 5.5 |
| Endurance trained (post) | 11 | M | 76.1 ± 5.7 | 174 ± 7.9 | 72.1 ± 6.1 | |
|
| ||||||
| Mckendry et al. (2020) | Endurance trained | 14 | M | 67.1 ± 6.4 | 1.70 ± 0.1 (m) | 68.7 ± 6.6 |
| Sedentary | 12 | M | 69.8 ± 4.1 | 1.80 ± 0.1 (m) | 77.5 ± 14.2 | |
|
| ||||||
| Pollock et al. (1997) | Endurance trained (pre) | 10 | M | 49.5 ± 10.3 | 179.6 ± 6.2 | 72.3 ± 8.9 |
| Endurance trained (post) | 10 | M | 69.8 ± 10.2 | 177.6 ± 7.0 | 73.1 ± 9.6 | |
| Sedentary (pre) | 2 | M | 52.5 ± 2.1 | 174.3 ± 2.2 | 75.9 ± 1.7 | |
| Sedentary (post) | 2 | M | 73.5 ± 2.1 | 173.1 ± 0.5 | 81.5 ± 0.1 | |
|
| ||||||
| Sanada et al. (2009) | Endurance trained | 24 | M | 65.7 ± 3.0 | - | - |
|
| ||||||
| Stenroth et al. (2016) | Endurance trained | 20 | M | 74.2 ± 2.7 | 175.5 ± 6.8 | 72.1 ± 7.2 |
| Sedentary | 33 | M | 74.8 ± 3.6 | 173 ± 5 | 76.1 ± 7.7 | |
|
| ||||||
| Trappe et al. (2013) | Endurance trained | 9 | - | 81 ± 1 | 172 ± 2 | 68 ±3 |
| Sedentary | 6 | M | 82 ± 2 | 172 ± 4 | 77 ±5 | |
Table 3.
Exercise training descriptions for included participants.
| Study | Groups | Training Type | Length of Training or Sedentariness (years) | Weekly Training Volume | Training Intensity (%HRR) |
|---|---|---|---|---|---|
| Aagaard et al. (2007) | Resistance trained | Resistance | 50+ | 2 – 3 sessions/week | - |
| Sedentary | - | 50+ | - | - | |
|
| |||||
| Ari et al. (2004) | Endurance trained | Endurance | 41 ± 18 | 10 ± 9 hours/week | - |
| Sedentary | - | - | - | - | |
|
| |||||
| Buyukyazi et al. (2003) | Endurance Trained | Endurance: middle- and long-distance running | 38.8 ± 18.5 | 10.0 ± 8.1 hours/week | - |
|
| |||||
| Carrick- Ranson et al. (2014) | Endurance trained | Endurance | 25+ | 3 hours/week | - |
| Sedentary | - | 25+ | - | - | |
|
| |||||
| Kasch et al. (1999) | Endurance trained (pre) | Endurance: running, swimming, cycling | - | 3.3 hours/week | 85 ± 4.7 |
| Endurance trained (post) | Endurance: running, swimming, cycling | 33+ | 5.4 hours/week | 77 ± 6.2 | |
|
| |||||
| Mckendry et al. (2020) | Endurance trained | Endurance: running | 36.5 ± 8.1 | 7.6 ± 4.7 hours/week | - |
| Sedentary | - | - | - | - | |
|
| |||||
| Pollock et al. (1997) | Endurance trained (pre) | Endurance: running and walking | - | 4.5 hours/week | ~60–80 |
| Endurance trained (post) | Endurance: running and walking | 20.4 ± 0.7 | 2.8 hours/week | ~60–80 | |
| Sedentary (pre) | - | - | 2.3 hours/week | ≤70 | |
| Sedentary (post) | - | 20.9 ± 0 | 2 hours/week | ≤70 | |
|
| |||||
| Sanada et al. (2009) | Endurance trained | Endurance: rowing | 46.7 ± 2.8 | 3 hours/week | - |
|
| |||||
| Stenroth et al. (2016) | Endurance trained | Endurance: running | 42.1 ± 20 | 6.5 ± 2.9 hours/week | - |
| Sedentary | - | - | - | - | |
|
| |||||
| Trappe et al. (2013) | Endurance trained | Endurance: cross country skiing, track and field, orienteering | 50+ | 8 hours/week | - |
| Sedentary | - | - | - | - | |
%HRR = percent of heart rate reserve.
Table 4.
Body composition assessment.
| Study | Groups | Assessment | BMI | %BF | FFM (kg) | LBM (kg) | FM (kg) | BMD (g/cm2) | SMI (%) | ALM/ht (kg/m2) | C (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aagaard et al. (2007) | Resistance trained | Skinfold | 50+ | 22.0 ± 7.8 | - | - | - | - | - | - | - |
| Sedentary | 50+ | 28.6 ± 2.1 | - | - | - | - | - | - | - | ||
|
| |||||||||||
| Ari et al. (2004) | Endurance trained | - | 41 ± 18 | 14 ± 2 | - | - | - | - | - | - | - |
| Sedentary | - | 18 ±2 | - | - | - | - | - | - | |||
|
| |||||||||||
| Buyukyazi et al. (2003) | Endurance Trained | Skinfold | 38.8 ± 18.5 | 14.2 ± 1.8 | - | - | - | - | - | - | - |
|
| |||||||||||
| Carrick- Ranson et al. (2014) | Endurance trained | - | 25+ | 22 ± 7 | 51 ± 8 | - | - | - | - | - | - |
| Sedentary | 25+ | 31 ± 7.5 | 52 ± 9.5 | - | - | - | - | - | - | ||
|
| |||||||||||
| Kasch et al. (1999) | Endurance trained (pre) | Skinfold | - | 12.9 ± 2.2 | - | 65.2 ± 5.5 | - | - | - | - | - |
| Endurance trained (post) | 33+ | 12.1 ± 1.8 | - | 63.4 ± 5.3 | - | - | - | - | - | ||
|
| |||||||||||
| Mckendry et al. (2020) | Endurance trained | DXA (Hologic) | 36.5 ± 8.1 | 19.2 ± 4.1 | 52.2 ± 3.5 | - | 13.3 ± 3.9 | - | 77 ± 4 | 7.71 ± 0.54 | - |
| Sedentary | - | 26.8± 5.4 | 52.9 ± 7.8 | - | 20.9± 7.1 | - | 69.8± 5.1 | 7.39 ± 0.95 | - | ||
|
| |||||||||||
| Pollock et al. (1997) | Endurance trained (pre) | Skinfold | - | 13.2 ± 5.3 | 62.7 ± 7.5 | - | - | - | - | - | Waist: 83.1 ± 6.5 Hip: 94.6 ± 3.7 |
| Endurance trained (post) | 20.4 ± 0.7 | 17.7 ± 4.8 | 60 ± 7.6 | - | - | - | - | - | Waist: 87.4 ± 7.1 Hip: 93.6 ± 3.8 | ||
| Sedentary (pre) | - | 15.7 ± 1.1 | 63.9 ± 0.6 | - | - | - | - | Waist: 82.7 ± 0.7 Hip: 97.8 ± 2.2 | |||
| Sedentary (post) | 20.9 ± 0 | 21.8 ± 0.6 | 63.7 ± 0.6 | - | - | - | - | - | Waist: 94.6 ± 3.3 Hip: 98.6 ± 2.8 | ||
|
| |||||||||||
| Sanada et al. (2009) | Endurance trained | DXA (Hologic) | 46.7 ± 2.8 | 18.8 ± 5.0 | - | - | - | 1.16 ± 0.09 | - | - | - |
|
| |||||||||||
| Stenroth et al. (2016) | Endurance trained | - | 42.1 ± 20 | - | - | - | - | - | - | - | - |
| Sedentary | - | - | - | - | - | - | - | - | - | ||
|
| |||||||||||
| Trappe et al. (2013) | Endurance trained | DXA (Lunar) | 50+ | - | - | 50 ± 2 | - | - | - | - | - |
| Sedentary | - | - | - | 50 ± 2 | - | - | - | - | - | ||
BMI = body mass index, %BF = percent body fat, FFM = fat-free mass, LBM = lean body mass, FM = fat mass, BMD = bone mineral density, SMI = skeletal muscle index, ALM/ht = appendicular lean mass / height, C = circumference.
Table 5.
Measures of cardiorespiratory fitness and muscular strength.
| Study | Groups | VO2max or VO2peak where indicated (mL/kg/min) | Knee Extension MVC | Elbow Flexion MVC | Plantar Flexion MVC (Nm) | Grip Strength (kg) |
|---|---|---|---|---|---|---|
| Aagaard et al. (2007) | Resistance trained | 26.6 ± 3.6 | 2.88 ± 0.63 Nm/kg | - | - | - |
| Sedentary | 25.9 ± 3.2 | 2.21 ± 0.52 Nm/kg | - | - | - | |
|
| ||||||
| Ari et al. (2004) | Endurance trained | 31.2 ± 6.2 | - | - | - | - |
| Sedentary | 18.8 ± 5.1 | - | - | - | - | |
|
| ||||||
| Buyukyazi et al. (2003) | Endurance Trained | 31.4 ± 5.9 | - | - | - | - |
|
| ||||||
| Carrick-Ranson et al. (2014) | Endurance trained | 40 ± 5 | - | - | - | - |
| Sedentary | 25 ± 5.1 | - | - | - | - | |
|
| ||||||
| Kasch et al. (1999) | Endurance trained (pre) | 45.9 ± 5.5 | - | - | - | - |
| Endurance trained (post) | 37.0 ± 5.2 | - | - | - | - | |
|
| ||||||
| Mckendry et al. (2020) | Endurance trained | 49.3 ± 3.6 (estimated) | 520 ± 105 Nm; 62 ± 13 Nm/kg leg FFM | 223 ± 43 Nm; 68 ± 12 Nm/kg leg FFM | - | 47 ± 5.9 |
| Sedentary | 36.7 ± 6.5 (estimated) | 471 ± 118 Nm; 57 ± 13 Nm/kg leg FFM | 257 ± 49 Nm; 77 ± 10 Nm/kg leg FFM | - | 46.3 ± 8.4 | |
|
| ||||||
| Pollock et al. (1997) | Endurance trained (pre) | 54.2 ± 7.7 | - | - | - | - |
| Endurance trained (post) | 40.8 ± 9.5 | - | - | - | - | |
| Sedentary (pre) | 50 ± 8.7 | - | - | - | - | |
| Sedentary (post) | 27 ± 0.1 | - | - | - | - | |
|
| ||||||
| Sanada et al. (2009) | Endurance trained | 35.0 ± 6.5 (VO2peak) | - | - | - | 48.8 ± 6.9 |
|
| ||||||
| Stenroth et al. (2016) | Endurance trained | - | - | - | 134.5 ± 37.1 | - |
| Sedentary | - | - | - | 132 ± 21 | - | |
|
| ||||||
| Trappe et al. (2013) | Endurance trained | 38 ± 1 | - | - | - | - |
| Sedentary | 21 ± 1 | - | - | - | - | |
FFM = fat-free mass, MVC = maximal voluntary contraction, VO2max = maximal oxygen consumption, VO2peak = peak oxygen consumption
Table 6.
Quality Assessment for each included study using a modified STROBE checklist.
| # | Strobe reference | Aagaard et al. (2007) | Anselme et al. (1994) | Ari et al. (200 4) | Buyukyazi et al. (2003) | Sanada et al. (2009 ) | Trappe et al. (2012 ) | Carric k- Ranson et al. (2014) | Kasch et al. (199 9) | McKendry et al. (2020) | Pollock et al. (1997 ) | Stenroth et al. (2015) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 12a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | 12c | N/A | 1 | N/ | 1 | N/A | 1 | 1 | N/ | N/A | 1 | 1 |
| 7 | 9 | 1 | 1 | A 1 | 0 | 1 | 1 | 0 | A 1 | 1 | 1 | 1 |
| 8 | 10 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| 9 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 7 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 0 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 14a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 2 | 14a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 3 | 19 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
|
| ||||||||||||
| - 4 | Sum | 12 | 14 | 10 | 13 | 13 | 14 | 13 | 12 | 13 | 13 | 14 |
Quality assessment criteria: 1) indicate the study’s design with a commonly used term in the title or the abstract; 2) state specific objectives, including any prespecified hypotheses; 3) describe length of follow-up; 4) describe body composition equipment for non-BMI-related variables; 5) describe all statistical methods, including those used to control for confounding; 6) explain how missing data were addressed; 7) describe any effort to address potential sources of bias; 8) explain how the study size was arrived at; 9) clearly define weekly training volume or the means to calculate this from the available data; 10) sufficiently describe types of training; 11) provide fitness and body composition for older adult group; 12) outline the number of males and females included in the study; 13) outline anthropometric features of participants (height, age, weight); 14) discuss limitations of the study, taking into account sources of potential bias or imprecision, as well as direction and magnitude of any potential bias
Training Experience: All 10 studies included at least one trained older adult group that performed long-term cardiorespiratory or resistance exercise for ≥ 20 years. Two studies obtained pre- and post-measurements from a training period of ≥ 20 years (20–33 years) (19, 32) while the other eight studies collected measurements only after a training period of ≥ 20 years. There were seven older adults from the same study that performed long-term resistance training, 134 older adults from nine studies that performed long-term cardiorespiratory training, and 124 sedentary older adults from seven studies for comparison.
The endurance-trained older adults performed various types of cardiorespiratory exercise (e.g., running, swimming, cycling, walking, cross-country skiing, track and field, and orienteering) for an average volume of 2.8–10.0 hours/week, which is near or significantly above ACSM’s minimum recommended volume of 2.5 hours/week (12). Two studies reported a weekly training intensity ((19): 77–85% heart rate reserve (HRR); (32): 60–80% HRR), collectively showing these older adults regularly participated in moderate-to-vigorous cardiorespiratory exercise (12) over 20–33 years. The resistance-trained older adults performed an average of 2–3 resistance training sessions per week (1), which is consistent with ACSM’s minimum weekly guidelines for frequency of resistance training (12).
Body Composition: The most common measure of body composition reported, percent body fat, was reported by eight studies (1, 2, 7, 10, 19, 25, 32, 34), wherein percent body fat was obtained with skinfolds in four studies (1, 7, 19, 32) and with DXA in two studies (25, 34). The two remaining studies did not report the technique used to measure percent body fat (2, 10). Body mass index and fat-free mass were the next most commonly reported values in five (7, 19, 34, 36, 38) and three (10, 25, 32) studies, respectively, although BMI was reported from sedentary adults in one study. Lean body mass was reported in two studies (19, 38) and all other assessments of body composition (fat mass, bone mineral density, skeletal muscle index, appendicular lean mass, and circumferences) were reported in one of the included studies. Descriptive analysis of the data shows trained older adults have generally lower percent body fat (12.1–22% vs. 18–31%) and BMI (23–25.5 vs. 26) after 20+ years of exercise training when compared to sedentary older adults. However, trained older adults also showed a slightly lower range of fat-free mass (51–60 kg vs. 52–63.7 kg) compared to their sedentary counterparts. Another notable finding is from (32) in which circumference measures were reported. Endurance-trained older adults had smaller values and changes in waist-to-hip ratios (0.88 to 0.93) when compared to sedentary adults (0.85 to 0.96), showing a lower risk of myocardial infarction using waist-to-hip ratio as a predictor (9). However, the n = 2 for the sedentary group from Pollock et al. (32) needs to be considered when interpreting these findings.
Fitness: Cardiorespiratory fitness measures were reported in 10 studies (1, 2, 7, 10, 19, 25, 32, 34, 38). Endurance-trained older adults from nine studies had higher cardiorespiratory fitness (31.2–49.3 mL/kg/min), determined with VO2peak and measured or estimated VO2max, when compared to resistance trained (26.6 mL/kg/min) and sedentary older adults (18.8–36.7 mL/kg/min) from one and seven studies, respectively. This shows a distinct difference between cardiorespiratory and resistance exercise on cardiorespiratory fitness in older age. From studies that included pre- and post-measures of cardiorespiratory fitness, there was a smaller decline in VO2max in endurance-trained older adults (−8.9 to −13.4 mL/kg/min) after 20+ years of cardiorespiratory training when compared to sedentary older adults (−23.0 mL/kg/min), although sedentary adults were evaluated in only one of these studies. Limited evidence was available regarding muscular strength measures; these were only found in four studies (1, 25, 34, 36). Both endurance- and resistance-trained older adults demonstrated greater leg muscular strength based on maximal voluntary contraction in knee extension. However, the results are equivocal for the effects of endurance training on arm strength given higher absolute and relative strength in sedentary adults in elbow flexion and similar results for endurance-trained and sedentary older adults based on grip strength.
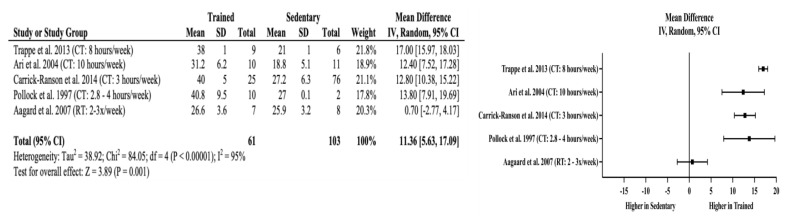
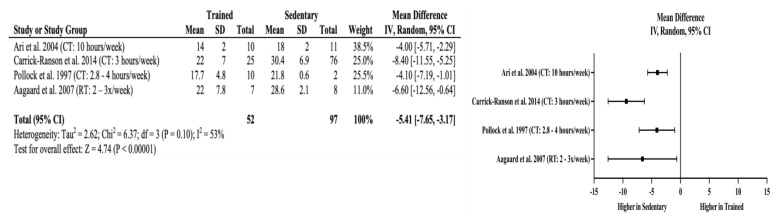
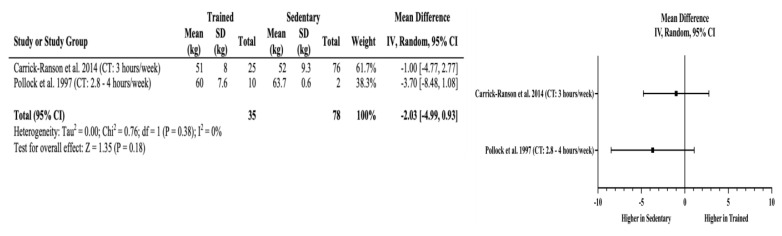
Meta-Analyses: Trained older adults had significantly higher VO2max (mean difference (MD) = +11.36 mL/kg/min, 95% CI = 5.63 to 17.09 mL/kg/min, p < 0.01) than sedentary adults. The effects of long-term training on cardiorespiratory fitness appear independent of the amount of cardiorespiratory training older adults performed when the minimum recommendation for weekly cardiorespiratory training volume was achieved. This is evident in four studies that showed higher VO2max (MD = +12.4 to +17.0 ml/kg/min) in the endurance-trained older adults where the weekly training volume ranged from 2.8–10 hours per week. The lack of effects of training above minimum recommendations for improved cardiorespiratory fitness are also reflected in body composition assessments. Trained older adults also had significantly lower percent body fat (MD = −5.41%, 95% CI = −7.65 to −3.17%, p < 0.01) without an observable trend with increasing amount of cardiorespiratory exercise. Non-significant differences were found in fat-free mass in the studies by Carrick-Ranson et al. (10) and Pollock et al. (32) (MD = −2.03 kg, 95% CI = −4.99 to 0.93 kg, p = 0.18), which indicates that significant improvements in cardiorespiratory fitness are not matched with changes in fat-free mass. Figures 2–4 include the meta-analyses and forest plots pertaining to VO2max, percent body fat, and fat-free mass.
Figure 2.
VO2max random effects meta-analysis and forest plot. Squares represent the mean difference and the horizontal line through each square represents the confidence interval. CI = confidence interval, CT = cardiorespiratory training, RT = resistance training.
Figure 3.
Percent body fat random effects meta-analysis and forest plot. Squares represent the mean difference and the horizontal line through each square represents the confidence interval. CI = confidence interval, CT = cardiorespiratory training, RT = resistance training.
Figure 4.
Fat-free mass random effects meta-analysis and forest plot. Squares represent the mean difference and the horizontal line through each square represents the confidence interval. CI = confidence interval, CT = cardiorespiratory training, RT = resistance training.
DISCUSSION
To our knowledge, this is the first review to analyze the effects long-term exercise training equaling or exceeding the minimum recommendations of weekly training volume (12) has on the fitness and body composition levels of older adults. A combination of both aging and inactivity place all individuals at higher risk of age-related diseases and comorbidities such as age-related sarcopenia that is associated with a loss of fat-free mass (mostly muscle mass and bone mineral density) as well as cardiometabolic disease from reduced cardiorespiratory fitness (decreased or greater decline in VO2max) and increased percent body fat. Long-term training from the included studies showed a favorable effect on fitness in older adults as shown through higher or better maintenance of cardiorespiratory fitness and generally higher leg muscular strength. Furthermore, long-term training confers positive impacts on body composition through lower or better maintenance of percent body fat for included subjects. Overall, these collective benefits highlight lower risks in many of the clinical complications associated with aging and physical inactivity, particularly those related to cardiometabolic health (8, 9, 23, 28, 37).
Higher levels of VO2max are inversely related to all-cause mortality. This has strong clinical significance since every 1 mL/kg/min increase in VO2max reduces mortality risk by 9% (22). The higher VO2max values found in endurance-trained older adults was expected and highlights the importance of cardiorespiratory exercise for older adult health by suppressing the age-related decline in VO2max. Additionally, trained older adults had similar magnitudes of improved cardiorespiratory fitness compared to the sedentary adults within their respective studies. This may indicate that older adults who consistently perform near the recommended minimum weekly volume of cardiorespiratory exercise (10, 32) may achieve similar health benefits, particularly related to mortality, obtained by older adults training at volumes far in excess of the minimum recommended volume (2, 38). However, the results from the current analysis related to cardiorespiratory fitness do not reflect those from a recent study reporting that individuals doubling the recommended weekly training volume had the lowest risk for onset of hypertension during adult life (from 18 to 60 years of age) (29). This may indicate the VO2max-related benefits, a key marker for mortality, may plateau beyond 2.5 hours/week of cardiorespiratory exercise while optimal health benefits that lower hypertension risk still require higher volumes of exercise training. This is important information for professionals when developing exercise programs for client or patient goals primarily related to physical fitness, health, or both. In addition to weekly training volume, consistent future reporting of training intensity will help to discern potential reasons for these opposing findings regarding the amount of weekly exercise that is required for optimal benefits related to both mortality and cardiovascular health.
From the included studies, appendicular lean mass / height was higher in endurance-trained older adults and has a positive association with survival rates (6). The findings related to percent body fat in the current study are similar to results in another meta-analysis that investigated the impact of long-term exercise training despite slight differences for systematic review inclusion criteria (24). In the current review, weekly cardiorespiratory or resistance exercise needed to be quantified and the minimum older adult group age was 65 years, which resulted in only four studies that directly compared the percent body fats of older trained and sedentary adults. Of course, the purposes of each review vary with the current analyses, specifically, showing strong positive effects of long-term cardiorespiratory training on VO2max and body composition related to body fat. The results related to percent body fat need to be interpreted while considering the subject population in the current review: older adults (≥ 65 years). Meeting the ACSM minimum guidelines of 150 minutes of moderate to vigorous physical activity can prevent ≥ 3% weight gain in adults, which is significant to reduce cardiovascular disease (11). However, the clinical significance of health measures related to weight, specifically, for older adults is questionable. For example, sarcopenia may be caused by obesity (i.e., sarcopenic obesity) and unintentional weight loss, common conditions associated with advanced aging (13). Obesity also does not predict mortality risk for older adults as it does for young adults (21). Therefore, lower weights and percent body fat, alone, in endurance-trained older adults do not necessarily represent improved health.
Possible reduction in overall fat-free mass (kg) between endurance-trained and sedentary adults, particularly from Pollock et al. (32), was an interesting finding. A preservation of fat-free mass reduces the risk of age-related sarcopenia, which has previously been observed to develop in as little as three years (15). It is unlikely that the results from Pollock et al. (32) indicate cardiorespiratory exercise perpetuates loss of fat-free mass since fat-free mass is an absolute measurement and the trained older adults, across all included studies, generally had lower body weights. Therefore, absolute measures of fat-free mass may not accurately represent the health of these participants and relative measures are preferred to account for inter-individual variability in body size.
As demonstrated in the included study by Aagaard et al. (1), resistance training may improve the quality of life of older adults by increasing muscular strength (14). Despite these resistance-trained older adults having similar levels of cardiorespiratory fitness to sedentary older adults, they had a lower percent body fat. This implies the results of long-term resistance training can favorably impact body composition despite a lack of crossover benefit to cardiorespiratory fitness. However, endurance-trained older adults also had higher leg strength measured using knee extension. This shows cardiorespiratory exercise that targets the lower body preserves or increases knee extension maximal voluntary contraction in older age while also improving cardiorespiratory fitness. Given the small number of studies in this review and meta-analyses, further research is required the verify the above findings from long-term exercise participation are generalizable to the general older adult population.
Limitations: There are notable limitations to this study that need to be considered when interpreting the results. None of the included studies reported ethnicity and only 10.2% of the participants are known to be female. Therefore, it is difficult to determine potential variation regarding the benefits of long-term exercise on body composition for older females and diverse ethnic populations. Exercise intensity was also underreported, impairing the ability to differentiate long-term exercise adaptations from activities that may be categorized differently based on metabolic equivalents (e.g., walking and running). Finally, large heterogeneity was found in the meta-analysis for the differences in VO2max (I2 = 95%), indicating variability in the extracted data from included studies. This is a common limitation for small meta-analyses, such as in the current review, and may not reflect the true heterogeneity of the data set (41).
Conclusions: Long-term exercise training variably suppresses age-related changes in cardiorespiratory fitness and body composition. Both endurance- and resistance-trained older adults had greater knee extensor strength and lower percent body fat while only endurance-trained older adults showed greater cardiorespiratory fitness. Therefore, meeting ACSM’s guidelines for minimum weekly training volume, with a combination of cardiorespiratory and resistance exercise, is recommended to achieve the full health benefits of long-term exercise participation in older age.
REFERENCES
- 1.Aagaard P, Magnusson PS, Larsson B, Kjær M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc. 2007;39(11):1989–1996. doi: 10.1249/mss.0b013e31814fb402. [DOI] [PubMed] [Google Scholar]
- 2.Ari Z, Kutlu N, Uyanik BS, Taneli F, Buyukyazi G, Tavli T. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int J Neurosci. 2004;114(5):623–637. doi: 10.1080/00207450490430499. [DOI] [PubMed] [Google Scholar]
- 3.Ashe MC, Miller WC, Eng JJ, Noreau L. Older adults, chronic disease and leisure-time physical activity. Gerontology. 2009;55(1):64–72. doi: 10.1159/000141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black MA, Cable NT, Thijssen DHJ, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:1109–1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortz WM, 4th, Bortz WM., 2nd How fast do we age? Exercise performance over time as a biomarker. J Gerontol A Biol Sci Med Sci. 1996;5(5):223–225. doi: 10.1093/gerona/51a.5.m223. [DOI] [PubMed] [Google Scholar]
- 6.Bunout D, de la Maza MP, Barrera G, Leiva L, Hirsch S. Association between sarcopenia and mortality in healthy older people. Australas J Ageing. 2011;30(2):89–92. doi: 10.1111/j.1741-6612.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 7.Buyukyazi G. Differences in the cellular and humoral immune system between sedentary and endurance-trained elderly males. Science and Sports. 2004;19(3):130–135. [Google Scholar]
- 8.Cameron AJ, Romaniuk H, Orellana L, Dallongeville J, Dobson AJ, Drygas W, Ferrario M, Ferrieres J, Giampaoli S, Gianfagna F, Iacoviello L, Jousilahti P, Kee F, Moitry M, Niiranen TJ, Pająk A, Palmieri L, Palosaari T, Satu M, Tamosiunas A, Thorand B, Toft U, Vanuzzo D, Veikko S, Veronesi G, Wilsgaard T, Kuulasmaa K, Söderberg S. Combined influence of waist and hip circumference on risk of death in a large cohort of European and Australian adults. J Am Heart Assoc. 2020;9(13):e015189. doi: 10.1161/JAHA.119.015189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Q, Yu S, Xiong W, Li Y, Li H, Li J, Li F. Waist-hip ratio as a predictor of myocardial infarction risk: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97(30):e11639. doi: 10.1097/MD.0000000000011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol. 2014;116:736–745. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 12.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 13.Gill LE, Bartels SJ, Batsis JA. Weight management in older adults. Curr Obes Rep. 2015;4(3):379–388. doi: 10.1007/s13679-015-0161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliano C, Karahalios A, Neil C, Allen J, Levinger I. The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure — a meta-analysis. Int J Cardiol. 2017;227:413–423. doi: 10.1016/j.ijcard.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 16.Harriss DJ, Macsween A, Atkinson G. Standards for ethics in sport and exercise science research 2018 update. Int J Sports Med. 2017;38(14):1126–1131. doi: 10.1055/s-0043-124001. [DOI] [PubMed] [Google Scholar]
- 17.Hayes LD, Grace FM, Sculthorpe N, Herbert P, Kilduff LP, Baker JS. Does chronic exercise attenuate age-related physiological decline in males? Res Sports Med. 2013;21(4):343–354. doi: 10.1080/15438627.2013.825799. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Li T, Deeks J, editors. Chapter 6: choosing effect measures and computing estimates of effect. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. (updated July 2019 Cochrane, 2019) [Google Scholar]
- 19.Kasch FW, Boyer JL, Schmidt PK, Wells RH, Wallace JP, Verity LS, Guy H, Schneider D. Ageing of the cardiovascular system during 33 years of aerobic exercise. Age Ageing. 1999;28:531–536. doi: 10.1093/ageing/28.6.531. [DOI] [PubMed] [Google Scholar]
- 20.Khalafi M, Malandish A, Rosenkranz SK, Ravasi AA. Effect of resistance training with and without caloric restriction on visceral fat: a systemic review and meta-analysis. Obes Rev. 2021;22(9):e13275. doi: 10.1111/obr.13275. [DOI] [PubMed] [Google Scholar]
- 21.Kuk JL, Ardern CI. Influence of age on the association between various measures of obesity and all-cause mortality. J Am Geriatr Soc. 2009;57(11):2077–2084. doi: 10.1111/j.1532-5415.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 22.Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R. Long-term change in cardiorespiratory fitness and all-cause mortality: a population-based follow-up study. Mayo Clin Proc. 2016;91(9):1183–1188. doi: 10.1016/j.mayocp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6):1–12. doi: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mckendry J, Breen L, Shad BJ, Greig CA. Muscle morphology and performance in master athletes: a systematic review and meta-analyses. Ageing Res Rev. 2018;45:62–82. doi: 10.1016/j.arr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Mckendry J, Joanisse S, Baig S, Liu B, Parise G, Greig CA, Breen L. Superior aerobic capacity and indices of skeletal muscle morphology in chronically trained master endurance athletes compared with untrained older adults. J Gerontol A Biol Sci Med Sci. 2020;75(6):1079–1088. doi: 10.1093/gerona/glz142. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen UR, Couppé C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, Klausen HH, Magnusson SP, Kjaer M. Life-long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev. 2013;134:531–540. doi: 10.1016/j.mad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood E. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 29.Nagata JM, Vittinghoff E, Gabriel KP, Garber AK, Moran AE, Sidney S, Rana JS, Reis JP, Bibbins-Domingo K. Physical activity and hypertension from young adulthood to middle age. Am J Prev Med. 2021;60(6):757–765. doi: 10.1016/j.amepre.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce GL, Donato A, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10(6):1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollock ML, Mengelkoch LJ, Graves JE, Lowenthal DT, Limacher MC, Foster C, Wilmore JH. Twenty-year follow-up of aerobic power and body composition of older track athletes. J Appl Physiol. 1997;82(5):1508–1516. doi: 10.1152/jappl.1997.82.5.1508. [DOI] [PubMed] [Google Scholar]
- 33.Pyka G, Lindenberger E, Charette S, Marcus R. Muscle strength and fiber adaptations to a year-long resistance training program in elderly men and women. J Gerontol. 1994;49(1):22–27. doi: 10.1093/geronj/49.1.m22. [DOI] [PubMed] [Google Scholar]
- 34.Sanada K, Miyachi M, Tabata I, Suzuki K, Yamamoto K, Kawano H, Usui C, Higuchi M. Differences in body composition and risk of lifestyle-related diseases between young and older male rowers and sedentary controls. J Sports Sci. 2009;27(10):1027–1034. doi: 10.1080/02640410903081852. [DOI] [PubMed] [Google Scholar]
- 35.Santanasto AJ, Goodpaster BH, Kritchevsky SB, Miljkovic I, Satterfield S, Schwartz AV, Cummings SR, Boudreau RM, Harris TB, Newman AB. Body composition remodeling and mortality: the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2017;72(4):513–519. doi: 10.1093/gerona/glw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenroth L, Cronin N, Peltonen J, Korhonen M, Sipilä S, Finni T. Triceps surae muscle-tendon properties in older endurance-and sprint-trained athletes. J Appl Physiol. 2016;120(1):63–69. doi: 10.1152/japplphysiol.00511.2015. [DOI] [PubMed] [Google Scholar]
- 37.Stevens J, Cai J, Juhaeri, Thun MJ, Wood JL. Evaluation of WHO and NHANES ll standards for overweight using mortality rates. J Am Diet Assoc. 2000;100(7):825–827. doi: 10.1016/s0002-8223(00)00238-8. [DOI] [PubMed] [Google Scholar]
- 38.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol. 2013;114(1):3–10. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol. 1996;80(1):285–290. doi: 10.1152/jappl.1996.80.1.285. [DOI] [PubMed] [Google Scholar]
- 40.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. [Google Scholar]
- 41.von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang ZM, Pierson RN, Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr. 1992;56(1):19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Zenko Z, White WE. Proportion of adults meeting the 2018 physical activity guidelines for Americans according to accelerometers. Front Public Health. 2018;7:135. doi: 10.3389/fpubh.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]