Abstract
Background
Nerve cross-sectional area (CSA) reference values in high-resolution ultrasound for children and adolescents are influenced by demographic and anthropometric factors such as age, height and weight.
Objectives
The influence of hand volume as an additional morphometric factor was evaluated and nerve echogenicity was analyzed in a prospective cross-sectional study.
Methods
CSA were measured in 30 healthy children and adolescents from 2 to 17 years in the median, ulnar, radial, tibial, peroneal and sural nerves. Height, weight, age, handedness and gender were recorded, the volume of the hands was measured using the water displacement method. The intra-nerve CSA variability (INV), left/right ratios and absolute differences were calculated. Age groups were compared by the Kruskal-Wallis test. The influence of demographic factors was analyzed using Spearman correlation and multiple linear regression. Echogenicity and fraction of black were determined for each nerve segment.
Results
Nerve CSA values were consistently lower than those reported for adults and correlated in all measured nerve sites with age, height, weight and hand volume. Weight showed the highest correlation coefficient (R = .95) with the best fitting model predicting CSA. Correlation coefficients were higher in a linear than in a logarithmic model. Ratios were stable, the absolute differences increased with age and were significantly different between age groups. Most nerves showed a mixed or hypoechogenic pattern in echogenicity analysis, hyperechogenicity is less frequently observed.
Conclusions
Nerve CSA in children and adolescents is lower than in adults and increases proportionally during growth with a constant INV and left/right ratio in different age groups. Weight and age are predominant anthropometric factors predicting nerve size. Hand volume is correlated with nerve size, but does not predict CSA independently. Echogenicity can provide additional information on nerve structure.
Keywords: High-resolution nerve ultrasound, pediatric reference values, demographic factors, hand volume, echogenicity
Highlights
Weight and age are predominant factors predicting nerve size in children and adolescents.
Hand volume is correlated with nerve size in children and adolescents.
Peripheral nerves in children and adolescents show a mixed or hypoechogenic pattern, hyperechogenicity is rarely observed.
Introduction
High-resolution ultrasound is of growing importance as a painless method complementary to nerve conduction studies in the workup of disorders of the peripheral nervous system, especially in children and adolescents. However, reference values for this group are not available in most ultrasound laboratories. The correlation of cross-sectional area (CSA) values with demographic and anthropometric data has been analysed in adults, where an influence of gender, age, weight and height has been shown.1-3 As a further morphometric factor, the volume of the ipsilateral hand correlates with median and ulnar nerve CSA in adults. 4
In children and adolescents, the influence of anthropometric data and growth on nerve ultrasound reference values has been assessed in few studies.5-9 A significant correlation of CSA with age and body weight was described by Druzhinin et al, 7 the correlation with age was emphasized by Schubert et al, 8 in a previous study including adolescents and adults height was the most important demographic factor. 6 The correlation of nerve CSA and hand volume as morphometric data has not been studied in children and adolescents. Echogenicity of peripheral nerves has recently been studied for the first time. 8 The present prospective cross-sectional study provides reference values for CSA in upper and lower extremity nerves, reports an estimation of nerve echogenicity and analyses the influence of height, weight, age, gender, and hand volume on CSA values in nerve ultasound in healthy children and adolescents from 2 to 17 years.
Methods
Participants
Participants were children or relatives of hospital employees of the Johannes- Wesling University Hospital Minden, Germany and children admitted to the pediatric department for diseases unrelated to the peripheral nervous system. Participants were recruited and examined between October 2019 and November 2020.
Exclusion criteria
Pre-existing conditions with a potential impact on nerve CSA (eg diabetes, history of entrapment syndromes, peripheral nerve lesions, radiculopathies, peripheral neurosurgical procedures, major trauma to the extremities) led to exclusion from the study. Strength, coordination, sensory symptoms, and reflexes were examined before inclusion. Sensory testing for touch and temperature at the soles of both feet was performed using a 10 g filament (Twin-Tip®), and a Rydel Seiffer 64 Hz tuning fork for testing vibratory sensation at both ankles. Any finding suggestive of neuropathy led to exclusion.
Ultrasound examination
Ultrasound examinations were performed by IY and HM with an Affiniti 50 (Philips, Amsterdam, The Netherlands) device using a 5-18 MHz linear array transducer. Each ultrasound picture and measurement was agreed upon by IY and HM. Measurements were supervised and validated by JP (board certified neurophysiologist with more than 10 years of experience in nerve ultrasound). For inter-rater reliability testing, JP performed blinded CSA measurements of 34 nerve sites on 1 volunteer 2 hours after the first measurement by IY and HM.
The transducer was held at a perpendicular angle to the nerve measured to avoid anisotropic effects caused by nerve structures and to obtain the correct CSA. Zoom was not used to avoid alterations in CSA measurement. For CSA measurements in 1/10 mm2, the nerves were visualized in a transverse plane and measured within the inner border of the hyperechoic epineurium using the free-hand tracer-tool.
Selection of nerve sites
Each nerve was measured bilaterally at predefined sites. The ulnar nerve was measured at Guyon’s canal, in the distal third of the forearm, at the ulnar sulcus and in the upper arm at an equal distance between the epicondylus medialis and the axilla. The median nerve was measured at the carpal tunnel, in the distal third of the forearm and in the upper arm at an equal distance between the epicondylus medialis humeri and the axilla. The ulnar and median nerve were traced from the distal anatomical landmark up to the proximal upper arm to measure the maximum and the minimum CSA. The radial nerve was measured in the upper arm at the level of the spiral groove. The sural nerve was measured between the gastrocnemius heads. The peroneal nerve was localized in the popliteal fossa and at the fibular head. The tibial nerve was measured in the popliteal fossa and at the medial malleolus at the ankle.
Measurement of hand volume
Hand volume was measured separately for each hand by submerging the hand into an overflow vessel filled with water up to the second skin fold at the wrist. The forearm was held to avoid excessive movements of the young participants. The displaced water corresponding to the hand volume was collected in a measuring cylinder and measured in cubic centimeters (cm3).
Determination of echogenicity
The fraction of black 10 of each nerve site was determined using ImageJ (imagej.nih.gov) to assess nerve echogenicity: First, CSA was circled manually inside the already marked nerve limits to avoid additional white pixels of the epineurium. Second, images were converted to 8-bit, assigning a grey value ranging from 0 (black) to 255 (white) to each pixel. In this image, the automated thresholding function of ImageJ was used to segment greyscale image into black and white. The hypoechoic fraction was measured as fraction of black in percent of the nerve CSA at a specific nerve site. Nerves were assigned a category of echogenicity: Hypoechogenic, mixed hypo-/hyperechogenic, and hyperechogenic. Hypoechogenic is defined as a fraction of black above 67%. Mixed hypo-/hyperechogenic is defined as a proportion of black between 33 and 67% and hyperechogenic as a proportion of black below 33%. 11
Calculations
The intra-nerve CSA variability, defined as the ratio of the largest to the smallest CSA of 1 nerve, was calculated for the median, ulnar and tibial nerves. The absolute difference (delta) between right and left side CSA of each nerve and the ratio of the larger and the smaller CSA value of each nerve site was calculated. As a further nerve-specific side-to-side comparison value, we calculated the side-to-side difference ratio of the intra-nerve CSA variability (SSDIVA) for the median, ulnar and tibial nerves, defined as the ratio of the intra-nerve CSA variability on the side with the larger intranerve CSA variability to that on the side with the smaller intra-nerve CSA variability. 12 The body mass index (BMI) was calculated as weight (kg)/height (m)2.
Statistical analyses
Normal distribution of the data was tested using the Shapiro-Wilk test. Means, SDs and 95% confidence intervals of nerve CSA were calculated for the right and left side separately in order to avoid artificial reduction of variance. Sample size calculations were based on previously published data 7 and yielded a mininum of 5 individual sets of measurements per age group to calculate single means (alpha error .01 and beta error .1), thus 4 age groups (2-5; 6-9; 10-13; 14-17 years) with 5-9 individuals were generated to calculate means, SDs, and 95% confidence intervals of nerve CSA. Additionally, different weight groups 7 were created to compare mean CSA values. Intra-class correlation coefficients for repeated single measurements with fixed observers and absolute agreement were calculated to assess inter-rater reliability. The Mann-Whitney-U test (non-normally distributed data) and paired t test (normally distributed data) were appplied to compare paired data. The Kruskal-Wallis test was applied to compare mean CSA values of corresponding nerve sites between age groups, and absolute differences and ratios between right and left body side in each age group, as some age groups’ CSA data was non-normally distributed. Spearman’s rho and Pearson’s correlation coefficient was calculated for CSA, intra-nerve CSA variability, SSDIVA and age, weight, gender and hand volume. Multiple linear regression analysis with backward exclusion was used to calculate the best model predicting CSA with age, weight, height, and volume of the hands as independent variables. Model selection was based on the Akaike information criterion. IBM SPSS for Windows version 27 (IBM, Armonk, NY, USA) was used for statistical analysis.
Results
Study population
Thirty participants were included, data were obtained in 30 participants for the upper extremities and 29 participants in ultrasound of the lower extremities, as 1 participant declined further examinations after ultrasound of the upper extremities. Missing data were not replaced. Examples of ultrasound images are shown in Figure 1. The demographic characteristics of the participants in 4 age groups are shown in Table 1. The Shapiro-Wilk test showed a normal distribution of demographic data. Six participants were left handed or preferred the left hand according to their parents. A paired t test of hand volume measurements revealed no significant differences between left and right hands (P > .05).
Figure 1.
Examples of nerve ultrasound images with anatomical landmarks.
Table 1.
Demographic and Anthropometric Characteristics in Different Age Groups.
| Sex | Height (cm) mean (SD) | Weight (kg) mean (SD) | BMI (kg/m2) mean (SD) | Volume right hand (cm3) mean (SD) | Volume left hand (cm3) mean (SD) | |
|---|---|---|---|---|---|---|
| Group 1 (2-5 years) | 5 f, 4 m | 105.5 (7.6) | 16.2 (2.5) | 14.5 (.9) | 95.5 (11.3) | 96.7 (9.9) |
| Group 2 (6-9 years) | 6 f, 3 m | 129.1 (8.6) | 24.7 (3.6) | 14.8 (1.9) | 134.7 (18.6) | 140.7 (19.1) |
| Group 3 (10-13 years) | 1 f, 4 m | 153.0 (15.6) | 41.0 (11.1) | 17.6 (2.0) | 197.2 (46.2) | 229.3 (91.2) |
| Group 4 (14-17 years) | 4 f, 3 m | 173.3 (8.8) | 63.2 (15.3) | 20.8 (3.3) | 258.7 (43.3) | 253.6 (58.6) |
Inter-rater reliability
The inter-rater reliability was .93 (.87-.96, P < .01).
CSA reference values
Mean CSA values of the 4 age groups for the median, ulnar, radial, peroneal, tibial and sural nerves at different sites, the intra-nerve CSA variability and SSDIVA are shown in Table 2.
Table 2.
Mean Cross-Sectional Area of the Left Side, Intranerve Cross-Sectional Area Variability and Side-to-Side Difference Ratio of the Intranerve Cross-Sectional Area Variability in Different Age Groups.
| Group 1 (2-5 years) CSA mean (SD; 95% CI) (mm2) | Group 2 (6-9 years) CSA mean (SD; 95% CI) (mm2) | Group 3 (10-13 years) CSA mean (SD; 95% CI) (mm2) | Group 4 (14-17 years) CSA mean (SD; 95% CI) (mm2) | |
|---|---|---|---|---|
| Median nerve | ||||
| CT | 4.3 (.8; 3.7-4.9) | 5.3 (.4; 5.0-5.7) | 5.5 (1.2; 4.1-7.0) | 7.6 (1.0; 6.7-8.5) |
| FA | 4.0 (.6; 3.5-4.5) | 4.7 (1.1; 3.9-5.5) | 5.0 (1.2; 3.5-6.6) | 6.8 (1.0; 5.9-7.7) |
| UA | 5.3 (1.1; 4.5-6.1) | 6.7 (.8; 6.0-7.3) | 7.3 (2.0; 4.8-9.7) | 8.9 (1.5; 7.5-10.2) |
| INV | 1.5 (.3; 1.2-1.7) | 1.5 (.4; 1.2-1.8) | 1.7 (.5; 1.1-2.3) | 1.5 (.2; 1.3-1.7) |
| SSDIVA | 1.2 (.2; 1.0-1.3) | 1.3 (.2; 1.1-1.5) | 1.3 (.2; 1.1-1.5) | 1.4 (.2; 1.2-1.6) |
| Ulnar nerve | ||||
| LdG | 2.8 (.6; 2.3-3.3) | 3.0 (.4; 2.7-3.4) | 3,6 (1.2; 2.1-5.0) | 4.9 (1.1; 3.9-6.0) |
| FA | 2.7 (.5; 2.4-3.1) | 3.1 (.6; 2.6-3.6) | 3.9 (1.2; 2.4-5.4) | 5.3 (.9; 4.5-6.2) |
| EB | 2.7 (.5; 2.3-3.1) | 3.6 (1.2; 2.6-4.5) | 5.6 (2.2; 2.9-8.3) | 7.4 (1.9; 5.7-9.2) |
| UA | 3.3 (.8; 2.7-3.9) | 3.9 (1.1; 3.0-4.8) | 5.0 (1.2; 3.5-6.5) | 6.5 (1.5; 5.2-7.9) |
| INV | 1.5 (.2; 1.3-1.7) | 1.6 (.6; 1.2-2.0) | 1.9 (.2; 1.7-2.2) | 1.8 (.5; 1.4-2.3) |
| SSDIVA | 12 (.1; 1.1-1.3) | 1.4 (.3; 1.2-1.7) | 1.3 (.1; 1.2-1.5) | 1.3 (.3; 1.1-1.6) |
| Radial nerve | 3.2 (.5; 2.7-3.6) | 3.5 (1.0; 2.8-4.3) | 4.0 (.7; 3.1-5.0) | 6.1 (1.4; 4.7-7.4) |
| Peroneal nerve | ||||
| FP | 5.6 (1.3; 4.6-6.7) | 5.8 (2.0; 4.3-7.3) | 7.8 (2.0; 5.4-10.3) | 10.7 (6.2; 5.0-16.4) |
| FH | 4.8 (1.2; 3.8-5.8) | 6.0 (2.4; 4.2-7.8) | 7.0 (.6; 6.3-7.8) | 11.2 (2.7; 7.8-14.7) |
| Tibial nerve | ||||
| FP | 10.4 (2.6; 8.2-12.6) | 12.5 (2.7; 10.4-14.5) | 15.3 (3.0; 11.6-19.0) | 18.8 (6.6; 12.7-25.0) |
| T | 5.8 (1.0; 5.0-6.6) | 6.8 (1.3; 5.8-7.8) | 9.1 (1.5; 7.3-10.9) | 10.4 (4.4; 6.3-14.5) |
| INV | 1.8 (.4; 15-2.1) | 1.9 (.5; 1.5-2.3) | 1.7 (.4; 1.2-2.2) | 2.0 (.8; 1.2-2.7) |
| SSDIVA | 1.2 (.3; 1.0-1.4) | 1.4 (.2; 1.2-1.5) | 1.6 (.6; .9-2.3) | 15 (.6; 1.9-2.1) |
| Sural nerve | 1.0 (.2; .8-1.2) | .8 (.2; .7-.9) | 2.1 (1.5; .2-4.0) | 2.2 (1.0; 1.4-3.1) |
CT, carpal tunnel; FA, forearm; LdG, loge de Guyon; EB, elbow; FH, fibular head; FP, popliteal fossa; T, tarsal. r, right; l, left; CSA, cross-sectional area; INV, Intranerve CSA variability; SSDIVA, side-to-side difference ratio of the intra-nerve CSA variability. INV and SSDIVA are ratios without unit. n = 30 for upper extremity nerves, n = 29 for lower extremity nerves.
Comparison of groups
The Kruskal-Wallis test showed significant differences (P < .05) in all nerve sites between age group 1 and group 4 except for the right peroneal nerve at the fibular head, comparison between groups 2 and 4 and 1 and 3 yielded significant differences in the median, ulnar and radial nerve and in the tibial, peroneal and sural nerves. Mean CSA of the median nerves in age group 1-4 are shown in Figure 2. Mean total CSA (sum of CSA of all nerve sites 5 ) for different weight groups are displayed in Figure 3. The Kruskal-Wallis test showed significant differences (P < .01) between the weight groups. Comparison of nerve CSA according to gender using the Mann-Whitney-U test showed no differences in almost all nerve sites except for the peroneal nerve in group 1 and the sural nerve in group 2 (P < .05). A paired t test of all left and right side CSA measurement revealed no significant differences in all nerve sites except in the ulnar nerve in the forearm. The absolute difference (delta) between right and left side CSA of each nerve increased with age (mean 1.2 mm2, SD 1.44 mm2), with significant differences between the age groups in the Kruskal-Wallis test (P < .01). The ratio of the larger and the smaller CSA value of each nerve site remained stable (Kruskal-Wallis test P = .18) with a mean of 1.2 and SD of .26 (see Figure 4).
Figure 2.
Mean cross-sectional area of the median nerve in age group 1-4. r, right; l, left; CT, carpal tunnel; FA, forearm; UA, upper arm. n = 30.
Figure 3.
Mean total cross-sectional area in different weight groups. Total cross-sectional area represents the sum of all measured nerve sites’ cross-sectional area in an individual participant (n = 30). Means and 95% confidence intervals are displayed.
Figure 4.
Absolute differences (delta) and ratio of right and left nerve cross-sectional area in different age groups. The means and 95% confidence intervals of the cross-sectional area ratios (blue line) and the absolute differences (green line) between all corresponding right and left side nerve sites are displayed for different age groups.
Regression analysis
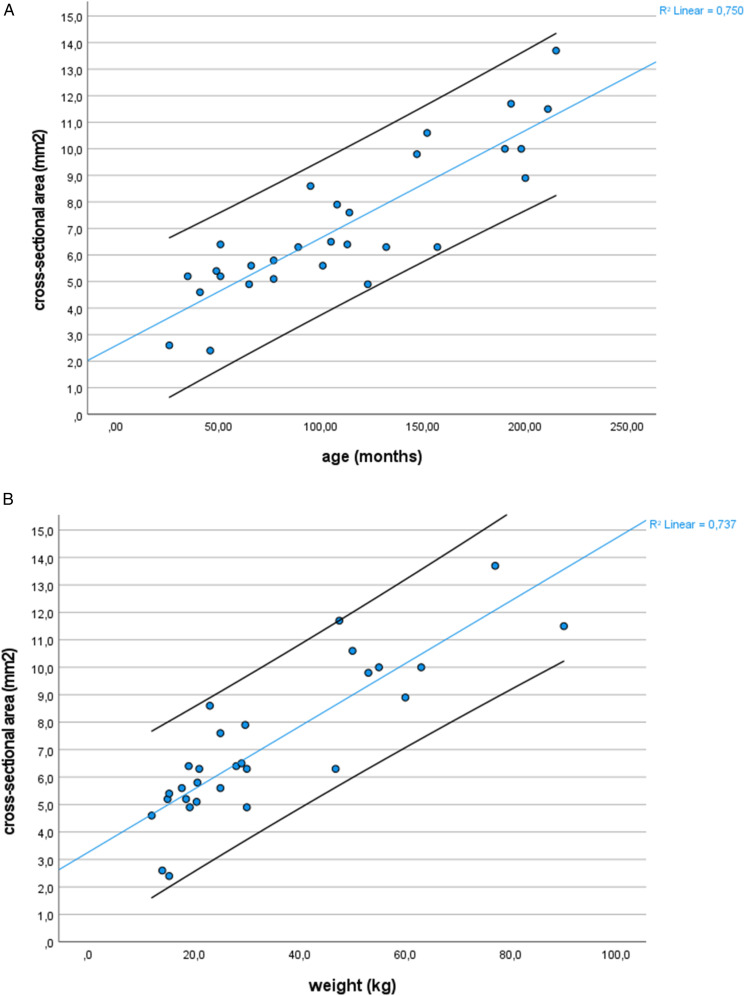
Spearman correlation showed significant correlations between CSA and age, weight, height and volume of the hands in all measured nerve sites (P < .05), correlation coefficients ranging from .38 to .85. Correlation coefficients of individual nerve sites (some examplary nerve sites are shown in Table 3) and age or weight were always higher in a linear correlation model than in a logarithmic correlation model. The correlation coefficient for a linear correlation between weight and total CSA was .95.
Table 3.
Spearman Correlation Coefficients of Median Nerve (in the Forearm), Radial and Sural Nerve Cross-Sectional Area and Anthropometric Factors.
| Radial nerve right | Radial nerve left | Sural nerve right | Sural nerve left | Median nerve right | Median nerve left | |
|---|---|---|---|---|---|---|
| Age (mo) | .62 (P < .01) | .72 (P < .01) | .63 (P < .01) | .63 (P < .01) | .73 (P < .01) | .72 (P < .01) |
| Height (cm) | .69 (P < .01) | .68 (P < .01) | .59 (P < .01) | .60 (P < .01) | .75 (P < .01) | .73 (P < .01) |
| Weight (kg) | .70 (P < .01) | .72 (P < .01) | .67 (P < .01) | .69 (P < .01) | .75 (P < .01) | .75 (P < .01) |
| Hand volume (cm3) | .72 (P < .01) | .67 (P < .01) | .61 (P < .01) | .58 (P < .01) | .74 (P < .01) | .77 (P < .01) |
Linear regression curves with upper and lower 95% CI of CSA and body weight and age for the left sural nerve and the right median nerve in the upper arm are shown in Figure 5.
Figure 5.
Linear regression curves of cross-sectional area and body weight or age. Linear regression curves with upper and lower 95% confidence intervals of cross-sectional area and age (A) or body weight (B) for the right median nerve in the upper arm (n = 29, one outlier excluded).
Regression models in multiple linear regression analysis with automated backward exclusion were significant (P < .05) in all 26 nerve sites: Weight was a predictive factor for CSA in 17 nerve sites, height in 7, age (in months) in 11 and hand volume in 6 nerve sites. Corrected R2 for significant regression models ranged from .57 to .74 in the upper extremity and from .36 to .72 in lower extremity nerve sites. If the BMI (replacing weight and height) was used in multiple linear regression analysis, age (in months) was the most important predictive factor. Sex and handedness were not predictive for CSA. Regression model analysis with automated backward exclusion for the sum of CSA of all nerve sites (total CSA) 5 yielded weight as the most important predictive factor in the model with the lowest Akaike information criterion.
Echogenicity
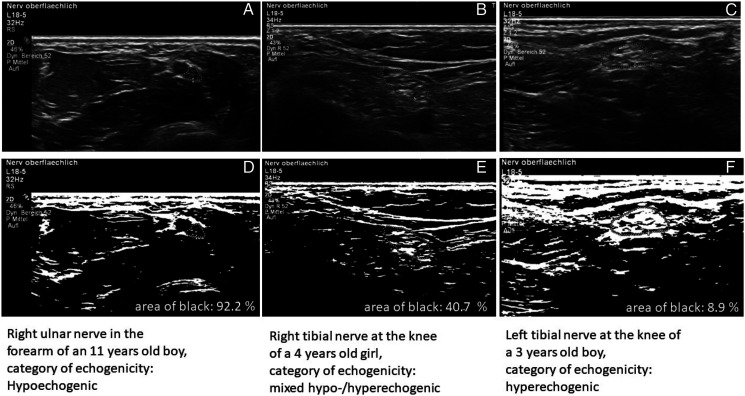
The distribution of the assigned categories of echogenicity according to their fraction of black (hypoechogenic, mixed hyper-/hypoechogenic and hyperechogenic, see Figure 6) for all nerve sites of the right side is shown in Table 4. Echogenicity measured as proportion of area of black at different nerve sites of the left side is displayed in Figure 7. Overall, nerves tended to show a hypoechogenic or mixed internal pattern with the exception of a higher hyperechogenicity in the tibial and peroneal nerve at the knee.
Figure 6.
Examples of echogenicity measurements using ImageJ. Echogenicity analysis in ImageJ. (A-C): Original ultrasound images; (D-F): Images after conversion to 8 bit in black and white, determination of the area of black in the manually selected area.
Table 4.
Distribution of the Categories of Echogenicity According to Their Fraction of Black.
| Nerve site | Hyperechogenic % | Mixed hypo-/hyperechogenic % | Hypoechogenic % |
|---|---|---|---|
| Median nerve CT | 10.3 | 41.4 | 48.3 |
| Median nerve FA | 6.9 | 65.5 | 27.6 |
| Median nerve UA | 3.4 | 24.1 | 72.4 |
| Ulnar nerve LdG | 3.4 | 44.8 | 51.7 |
| Ulnar nerve FA | 0 | 44.8 | 55.2 |
| Ulnar nerve EB | 3 | 27.6 | 69.0 |
| Ulnar nerve UA | 0 | 33.3 | 66.7 |
| Radial nerve UA | 3.4 | 41.4 | 55.2 |
| Peroneal nerve FP | 7.1 | 50.0 | 42.9 |
| Peroneal nerve FH | 32.1 | 50.0 | 17.9 |
| Tibial nerve FP | 10.7 | 57.1 | 32.1 |
| Tibial nerve T | 11.1 | 7.4 | 81.5 |
| Sural nerve | 7.1 | 35.7 | 57.1 |
CT, carpal tunnel; FA, forearm; UA, upper arm; LdG, loge de Guyon; EB, elbow; FH, fibular head; FP, popliteal fossa; T, tarsal. Results are reported for the right side. n = 30 for upper extremity nerves, n = 29 for lower extremity nerves.
Figure 7.
Echogenicity measured as area of black at different nerve sites. CT, carpal tunnel; FA, forearm; UA, upper arm; LdG, loge de Guyon; EB, elbow; FH, fibular head; FP, popliteal fossa; T, tarsal. Results are displayed for the left side. (Upper extremity, n = 30; lower extremity, n = 29).
Discussion
The present study provides normative values of peripheral nerves in the upper and lower extremities for children and adolescents from 2 to 18 years and their correlation with demographic and anthropometric data including volume of the hands. Nerve CSA significantly correlates with weight, height, age (in months), and volume of the hands, with weight being the most important predicting factor.
Comparison with previous studies
Our study confirms that nerve CSA in children and adolescents is lower than in adults5,6,8,9 and grows gradually up to adult CSA values. Compared to infants younger than 2 years, where a logarithmic model describes correlation to age better than a linear model, 13 a linear correlation seems to be the best model to describe the relation between demographic factors and nerve CSA in children and adolescents older than 2 years. A direct statistical comparison of nerve sizes and age groups between different studies is affected by differences in age groups, exact ultrasound examination sites, resolution of ultrasound transducers, tracing methods and precision of measurements. In the present study nerve CSA is measured in 1/10 mm2 using the free-hand tracing tool. Given the small nerve CSA observed especially in younger children, using a higher precision of measurement 14 is recommended both in establishing normative values and in daily routine. Schubert et al 8 and Druzhinin et al 7 assumed that nerve growth reaches a maximum during late adolescence or early adulthood. This is in line with the findings of the present study, nerve CSA values in the upper age and weight groups are comparable to those observed for European ultrasound laboratories in a meta-analysis,15,16 with an exception of slightly lower CSA values for the tibial nerve in the popliteal fossa. The INV and the SSDIVA 12 (as a nerve-specific side-to-side comparison value) of the median, ulnar and tibial nerve remains constant in the different age groups. As reported previously,8,9 the INV is comparable to values reported by Padua et al, 17 Zaidman et al 6 and Kerasnoudis et al 3 in adults. An elevated INV is of diagnostic relevance independently of an absolute increase in nerve CSA especially in demyelinating polyneuropathies. An elevated SSDIVA indicates a relevant CSA difference in nerve segments between left and right side in a specific nerve that can be observed in multifocal neuropathies. Recently, Voltan et al 18 analyzed the absolute difference (delta) and ratios between right and left side nerve CSA values in 66 healthy volunteers including 17 children aged 7-14, finding stable absolute and relative differences in all age groups. In the present study, the ratios were stable with results comparable to Voltan et al, the delta increased with age and was significantly different in the age groups (Figure 4). The INV and the SSDIVA were stable as well, so the present data suggests the use of comparison tools based on ratios rather than on absolute differences for children.
Influence of demographic and anthropometric factors
The evident growth of nerve CSA in childhood and adolescence can be correlated with demographic and anthropometric factors. In addition to the correlation to height and age,5,6,8 Druzhinin et al 7 layed emphasis on the association with body weight and age, rather than height. For adults, correlations with age and weight have been described by different authors,2,4,6,19,20 the influence of age being controversial. Kerasnoudis et al 3 and Tahmaz et al 4 described a significant decrease in CSA of the radial nerve in the spiral groove with increasing age, whereas Cartwright et al 5 reported a significant increase in CSA with increasing age in all nerves.
In the present study, hand volume is measured for the first time as an additional anthropometric marker in children and adolescents. In adults, there is a significant correlation between median and ulnar nerve CSA and hand volume. 4 In children and adolescents, hand volume is correlated to nerve CSA in all nerve sites including the lower extremities. Thus, hand volume is rather an additional marker of growth in children than a predictor of the size of nerves innervating the hand. We found correlations of CSA with all anthropometric factors, weight is significantly correlated to nerve CSA and can thus represent growth in a similar manner as age, even if the latter is measured in months to improve measurement precision in younger age. Given that hand volume, weight, height and age interact statistically and as biological markers of an indidvidual’s development, further analyses have been performed to find out each factor’s individual contribution. Multiple linear regression models with automated backward exclusion are significant and show that weight is the predominant anthropometric factor predicting nerve CSA. Schubert et al 8 decided not to correlate nerve CSA with weight and height as those parameters were considered to be too heterogeneous in children of same age (measured in years). As a demographic factor, BMI was used instead. In the present study as well as in Schubert et al, 8 the use of BMI instead of weight and height in multiple linear regression yields age as predominant predicting factor of nerve CSA. This phenomenon can be explained as the BMI is not a marker of growth, but rather of body proportion (malnutrition or obesity); height in the denominator can thus not be detected as a predicting factor for nerve size and levels off the effect of weight. Weight can serve as a marker of nutritional and growth status in children and shows a considerable world-wide variation. 21 Weight represents muscle mass unless there is severe obesity or malnutrition. Muscle mass is a marker of motor unit size and number, 4 nerve CSA usually represents motor unit number in the absence of demyelinisation and is correlated to muscle mass.22,23 Height is strongly influenced by genetic factors even in early childhood and represents bone growth rather than muscular development. Age does not reflect nutritional and growth status unless the developmental quantile of an individual child is known. Stratifying CSA values according to 5%-95% quantiles 9 necessitates a very high sample size 24 and makes the use of age groups to predict nerve CSA in an individual child more complicated than using the child’s actual weight.
Predictive nomograms
As the existing normative values found in the literature cannot be transferred to all ultrasound laboratories and patients from different ethnic, general nutritional and socio-economic background, the recommendation that every laboratory should establish its own reference values for adult nerve CSA values 3 should be kept in mind even more for children and adolescents. A regression diagram (with upper and lower 95% CI line) as shown in Figure 5 can serve as a predictive nomogram using age or weight as independent variables. Predictive nomograms based on limited local sample sizes are frequently used for the evaluation of somatosensory evoked potentials with respect to height in children 25 and to adjust F- wave latencies for limb length in adults. 26 Although they do not replace authoritative large scale studies or meta-analyses on normative values, they are useful in daily routine and have the advantage of identical operators and equipment in establishing and using the predictive nomogram. Some studies have been performed with limited numbers of participants in somatosensory evoked potentials (n children = 32 25 ) as well as in nerve ultrasound (n children = 40 6 , n children = 32 5 , n children = 50 7 ). The upper limits of nerve CSA found using such nomogramms for individual nerve sites in the present study correspond well with those published by Schubert 8 for school children and Grimm 9 for preschool children. This is plausible as the German population studied by both authors is likely to be similar to the participants of the present study.
However, distributions and variance may vary in different age and weight clusters. This can be observed in the 2 weight groups above 30 kg and age groups over 10 years, probably because of more rapid and highly individual changes of weight and growth velocity during puberty. As both age and weight can be misleading as a marker of individual growth even more in children affected by acute or chronic diseases, nerve CSA could be evaluated using both reference methods. A nerve enlargement found in both nomograms (age- and weight-based) is likely to be relevant.
Echogenicity
Echogenicity seems to be highly variable in healthy individuals 27 and overlap in most polyneuropathies with a tendency towards hyperechogenicity in unstable CIDP 28 as an indicator of tissue repair. Hypoechogenic nerves are frequently seen in acute demyelinating polyneuropathies. 29 For children, Schubert et al 8 reported absolute grey scale values for the first time without finding differences according to age groups and gender. They report lower echogenicity in the distal tibial nerve than in the upper limb nerves. In the present study, the relative fraction of black method 10 as an evaluated method to measure echogenicity in nerve ultrasound is applied to children. As shown in Table 4, most nerve sites display mixed or hypoechogenic echogenicity, whereas hyperechogenic nerves with an area of black below 40% (Figure 7) are rarely observed. As an exemption, the tibial nerve in the popliteal fossa and the peroneal nerve at the fibular head show higher rates of hyperechogenicity. The reason of an alteration in echogenicity might be a higher probability of mechanical irritation 29 in hypermobile nerve segments. Given the generally lower frequency of hyperechogenicity, the finding of hyperechogenic nervs in an individual patient should be noted and interpreted cautiously in the context of the clinical and elektrophysiological findings. The significance of echogenicity in peripheral nerves should be discussed in further studies.
Limitations
A limitation is the low number of participants, non-parametric tests were used to compare age- and weight groups. Another limitation is the lack of nerve conduction studies; exclusion of peripheral neurological disorders was based on history and clinical examination. With regard to echogenicity, the selection of the region of interest inside the already marked nerve limits might have biased the calculations of the area of black in small nerves.
Conclusions
Nerve CSA in children and adolescents is lower than in adults and increases proportionally during growth with a constant INV and left/right ratio in different age groups. Weight and age are predominant anthropometric factors predicting nerve size. Hand volume is correlated with nerve size, but does not predict CSA independently. In order to establish local normal values of nerve size in children and adolescents, predictive nomograms can be derived from regression curves. Most nerves show a mixed or hypoechogenic pattern, echogenicity can provide additional information on nerve structure.
Footnotes
Author contributions: Ifirae Yusuf: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Hannah Mork: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Bernhard Erdlenbruch: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing. Jörg Philipps: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Peter Dieter Schellinger: Conceptualization, Methodology, Project administration, Supervision, Writing - original draft, Writing - review & editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Research ethics: The local ethics committee of the Ruhr University of Bochum, Germany, approved the study protocol compliant with the declaration of Helsinki (Approval number: 2019-477).
Informed consent: All participants’ legal representatives signed informed consent, participants were additionally informed using a graphic information sheet appropriate for children.
Consent for Publication: The above mentioned written informed consent included participant/legal representatives consent for publication of anonymized data and images.
Data availability: Data is available at osf.io/zhj25 identified as DOI 10.17605/OSF.IO/JCDEK or upon request to the corresponding author.
ORCID iD
Jörg Philipps https://orcid.org/0000-0003-3327-3550
References
- 1.Cartwright MS, Passmore LV, Yoon JS, Brown ME, Caress JB, Walker FO. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve. 2008;37:566-571. DOI: 10.1002/mus.21009 [DOI] [PubMed] [Google Scholar]
- 2.Won SJ, Kim BJ, Park KS, Yoon JS, Choi H. Reference values for nerve ultrasonography in the upper extremity. Muscle Nerve. 2013;47:864-871. DOI: 10.1002/mus.23691 [DOI] [PubMed] [Google Scholar]
- 3.Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Cross sectional area reference values for sonography of peripheral nerves and brachial plexus. Clin Neurophysiol. 2013;124:1881-1888. DOI: 10.1016/j.clinph.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Tahmaz M, Yoon MS, Schellinger PD, Philipps J. Cross-sectional area in median and ulnar nerve ultrasound correlates with hand volume. Muscle Nerve. 2020;62:83-88. DOI: 10.1002/mus.26881 [DOI] [PubMed] [Google Scholar]
- 5.Cartwright MS, Mayans DR, Gillson NA, Griffin LP, Walker FO. Nerve cross-sectional area in extremes of age. Muscle Nerve. 2013;47:890-893. DOI: 10.1002/mus.23718 [DOI] [PubMed] [Google Scholar]
- 6.Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: An ultrasound study. Muscle Nerve. 2009;40:960-966. DOI: 10.1002/mus.21431 [DOI] [PubMed] [Google Scholar]
- 7.Druzhinin D, Naumova Е, Nikitin S. Nerve ultrasound normal values in children and young adults. Muscle Nerve 2019; 60: 757-761. DOI: 10.1002/mus.26715 [DOI] [PubMed] [Google Scholar]
- 8.Schubert C, Grimm AS, Stahl JH, et al. Nerve ultrasound reference data in children from two to seven years. Clin Neurophysiol 2020; 131: 859-865. DOI: 10.1016/j.clinph.2019.12.404 [DOI] [PubMed] [Google Scholar]
- 9.Grimm AS, Schubert C, Grimm A, et al. Normative observational nerve ultrasound values in school-age children and adolescents and their application to hereditary neuropathies. Front Neurol. 2020;11:303. DOI: 10.3389/fneur.2020.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisse AL, Pitarokoili K, Motte J, et al. Nerve echogenicity and intranerve CSA variability in high-resolution nerve ultrasound (HRUS) in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol. 2019;266:468-475. DOI: 10.1007/s00415-018-9158-3 [DOI] [PubMed] [Google Scholar]
- 11.Padua L, Granata G, Sabatelli M, et al. Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin Neurophysiol. 2014;125:160-165. DOI: 10.1016/j.clinph.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 12.Kerasnoudis A, Klasing A, Behrendt V, Gold R, Yoon MS. Intra- and internerve cross-sectional area variability: New ultrasound measures. Muscle Nerve. 2013;47:146-147. DOI: 10.1002/mus.23520 [DOI] [PubMed] [Google Scholar]
- 13.Jenny C, Lutschg J, Broser PJ. Change in cross-sectional area of the median nerve with age in neonates, infants and children analyzed by high-resolution ultrasound imaging. Eur J Paediatr Neurol. 2020;29:137-143. DOI: 10.1016/j.ejpn.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 14.Walter U, Tsiberidou P. Differential age-gender-and side-dependency of vagus, spinal accessory, and phrenic nerve calibers detected with precise ultrasonography measures. Muscle Nerve. 2019;59:486-491. DOI: 10.1002/mus.26412 [DOI] [PubMed] [Google Scholar]
- 15.Fisse AL, Katsanos AH, Gold R, Pitarokoili K, Krogias C. Cross-sectional area reference values for peripheral nerve ultrasound in adults: A systematic review and meta-analysis-Part I: Upper extremity nerves. Eur J Neurol 2021; 28: 1684-1691. DOI: 10.1111/ene.14759 [DOI] [PubMed] [Google Scholar]
- 16.Fisse AL, Katsanos AH, Gold R, Krogias C, Pitarokoili K. Cross-sectional area reference values for peripheral nerve ultrasound in adults: A systematic review and meta-analysis-Part II: Lower extremity nerves. Eur J Neurol. 2021;28:2313-2318. DOI: 10.1111/ene.14850 [DOI] [PubMed] [Google Scholar]
- 17.Padua L, Martinoli C, Pazzaglia C, et al. Intra- and internerve cross-sectional area variability: New ultrasound measures. Muscle Nerve. 2012;45:730-733. DOI: 10.1002/mus.23252 [DOI] [PubMed] [Google Scholar]
- 18.Voltan G, Bernardes Filho F, Lugao HB, Nogueira-Barbosa MH, Frade MAC. Ultrasound reference values for peripheral nerve cross-sectional areas and indices in a sample of healthy individuals in Brazil. Radiol Bras. 2022;55:337-345. DOI: 10.1590/0100-3984.2022.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehm J, Scheidl E, Bereczki D, Schelle T, Arányi Z. High-resolution ultrasonography of peripheral nerves: Measurements on 14 nerve segments in 56 healthy subjects and reliability assessments. Ultraschall Med. 2014;35:459-467. DOI: 10.1055/s-0033-1356385 [DOI] [PubMed] [Google Scholar]
- 20.Grimm A, Axer H, Heiling B, Winter N. Nerve ultrasound normal values - readjustment of the ultrasound pattern sum score UPSS. Clin Neurophysiol. 2018;129:1403-1409. DOI: 10.1016/j.clinph.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 21.Natale V, Rajagopalan A. Worldwide variation in human growth and the World Health Organization growth standards: A systematic review. BMJ Open 2014; 4: e003735. DOI: 10.1136/bmjopen-2013-003735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz R, Westenberg RF, Langhammer CG, Knaus WJ, Chen NC, Eberlin KR. Nerve diameter in the hand: A cadaveric study. Plast Reconstr Surg Glob Open 2019; 7: e2155. DOI: 10.1097/GOX.0000000000002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciniak C, Caldera F, Welty L, et al. High-resolution median nerve sonographic measurements: Correlations with median nerve conduction studies in healthy adults. J Ultrasound Med. 2013;32:2091-2098. doi: 10.7863/ultra.32.12.2091 [DOI] [PubMed] [Google Scholar]
- 24.Rasenack M, Decard BF, Schadelin S, Grimm A, Fischer D, Hafner P. Ultrasonographic reference values for peripheral nerves and nerve roots in the normal population of children and adolescents: Study protocol for an observational-prospective trial. BMJ Open 2016; 6: e014662. DOI: 10.1136/bmjopen-2016-014662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore RL, Bass NH, Wright EA, Greathouse D, Stanback K, Norvell E. Developmental assessment of spinal cord and cortical evoked potentials after tibial nerve stimulation: Effects of age and stature on normative data during childhood. Electroencephalogr Clin Neurophysiol. 1985;62:241-251. DOI: 10.1016/0168-5597(85)90002-4 [DOI] [PubMed] [Google Scholar]
- 26.Nobrega JA, Pinheiro DS, Manzano GM, Kimura J. Various aspects of F-wave values in a healthy population. Clin Neurophysiol. 2004;115:2336-2342. DOI: 10.1016/j.clinph.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 27.Gamber D, Motte J, Kerasnoudis A, et al. High-resolution nerve ultrasound to assess nerve echogenicity, fascicular count, and cross-sectional area using semiautomated analysis. J Neuroimaging. 2020;30:493-502. DOI: 10.1111/jon.12717 [DOI] [PubMed] [Google Scholar]
- 28.Erdmann A, Motte J, Brunger J, et al. Nerve echogenicity in polyneuropathies of various etiologies-results of a retrospective semi-automatic analysis of high-resolution ultrasound images. Diagnostics (Basel). 2022;12:1341. DOI: 10.3390/diagnostics12061341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartig F, Ross M, Dammeier NM, et al. Nerve ultrasound predicts treatment response in chronic inflammatory demyelinating polyradiculoneuropathy-a prospective follow-up. Neurotherapeutics. 2018;15:439-451. DOI: 10.1007/s13311-018-0609-4 [DOI] [PMC free article] [PubMed] [Google Scholar]