Abstract
Introduction
It remains controversial whether it is better to continue oral low-dose aspirin (LDA) during the perioperative period in spinal surgery. This study aims to evaluate the safety of continued LDA administration in the perioperative periods of microendoscopic laminectomy (MEL) by assessing perioperative complications and clinical outcomes.
Methods
We ultimately included 88 patients (35 males, 53 females) who underwent one level of MEL for lumbar spinal canal stenosis from April 2016 to March 2022. Patients who did not undergo anticoagulation therapy were classified into Group A (65 patients), those who stopped anticoagulation therapy at the perioperative periods were classified into Group B (9 patients), and those who continued oral administration of LDA throughout the perioperative periods were classified into Group C (14 patients). Surgery time, intraoperative estimate blood loss (EBL), differences between hemoglobin (Hb) and platelet (Plt) before and after surgery, perioperative complications, and cross-sectional area of hematoma and dural sac on MRI taken within 1 week and at 6 months or more after surgery were assessed between three groups. The EuroQol-5 dimensions (EQ-5D), Oswestry Disability Index (ODI), and Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ) were also evaluated as the clinical outcomes.
Results
There was no statistically significant difference between the three groups in operation time, intraoperative EBL, differences between Hb and Plt before and after surgery, and cross-sectional area of hematoma and dural sac on MRI. A case of hematoma removal was confirmed in Group A. There was also no statistically significant difference between the three groups in EQ-5D, ODI, and each domain of JOABPEQ.
Conclusions
The continuation of LDA throughout the perioperative periods did not affect perioperative complications and clinical outcomes of one-level MEL. In MEL, it might be possible to continue oral administration of LDA throughout the perioperative periods.
Keywords: Low-dose aspirin, microendoscopic laminectomy, hematoma
Introduction
According to the Japanese nationwide spinal surgery survey, the most frequent age who underwent spinal surgery was 70-79 years, and the patients aged 60 and over account for about 60% of the total1). In addition, the number of patients with comorbidities for the elderly increased in recent years2). Among them, patients with oral anticoagulant and/or antiplatelet drugs due to brain and/or cardiovascular disorders are particularly problematic in spinal surgery. Regarding spinal surgery, it is considered that oral anticoagulant discontinuation before surgery is general because there is a risk of intraoperative hemorrhage and postoperative hematoma. However, problems such as hemorrhagic complications due to preoperative heparin replacement or infarction during the withdrawal period are reported3,4). Among them, low-dose aspirin (LDA) is the most used and studied antiplatelet drug5). While many studies have been conducted, there are various reports that continued oral administration of LDA does not affect perioperative complications6) or reports recommending discontinuation of oral LDA during the perioperative period7). It remains controversial whether it is better to continue oral LDA during the perioperative period in spinal surgery. This study aims to evaluate the safety of continued LDA administration in the perioperative periods of one-level microendoscopic laminectomy (MEL) by assessing perioperative complications and clinical outcomes.
Materials and Methods
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all the individual participants included in this study. This study was approved by the IRB of the authors' affiliated institutions.
This is the retrospective cohort study. All cases were performed by a single surgeon. We decided on the policy, with sufficient informed consent from patients undergoing MEL for lumbar spinal canal stenosis after April 2016, oral LDA administration should be continued during the perioperative period in principle, and other antiplatelet and anticoagulant drugs discontinued perioperatively. The exclusion criteria were as follows: patients who underwent two-level MEL, hemilaminectomy, and concomitant herniotomy. Patients for whom data could not be obtained due to very early discharge were also excluded. The types of anticoagulant and antiplatelet drugs, the causes of oral administration, and comorbidities were also investigated. Data were collected prospectively after April 2016, and patients who met the inclusion criteria were divided into three groups: Group A (no anticoagulant/antiplatelet treatment), Group B (perioperative anticoagulant/antiplatelet treatment discontinuation), and Group C (perioperative LDA oral administration continued).
The operation time, intraoperative estimate blood loss (EBL), treated level, pre- and postoperative hemoglobin level changes (1st and 7th postoperative days), pre- and postoperative platelet level changes (1st and 7th postoperative days), and perioperative complications (presence or absence of hematoma removal, myocardial infarction, cerebral infarction, and blood transfusion) were evaluated. In patients who could be followed up 6 months or more, clinical results (Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ), Oswestry Disability Index (ODI), EuroQol-5 dimensions (EQ-5D)) were evaluated. Furthermore, images could be taken within 1 week after surgery, and the cross-sectional area of the dural canal and hematoma on MRI was also evaluated (Fig. 1). The cross-sectional area was measured three times and the average value was adopted. Regarding the MRI imaging criteria, from April 2016 to March 2018, MRI imaging was performed in all cases within 1 week after surgery regardless of symptoms. Due to the influence of the hospital system that was transferred in April 2018, MRI imaging was temporarily stopped, and from October 2019 to March 2022, MRI imaging was performed within 1 week after surgery for all patients taking perioperative LDA regardless of symptoms. More than 6 months after surgery, the MRI was taken to measure the cross-sectional area of the dural sac in the same way (Fig. 2).
Figure 1.
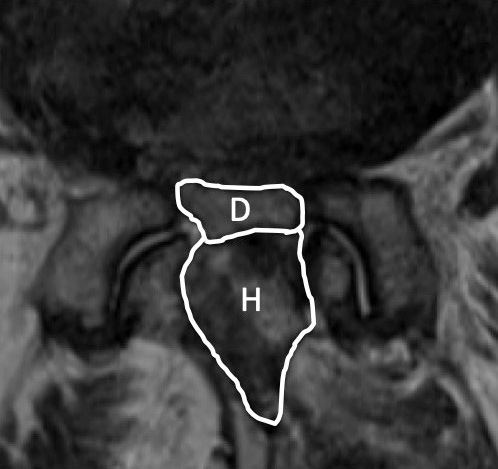
MRI taken within 1 week after surgery demonstrates the dural sac and hematoma. Area D means a cross-sectional area of the dural sac. Area H means a cross-sectional area of the hematoma.
Figure 2.
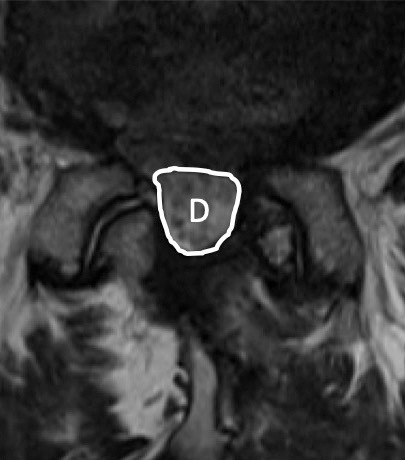
MRI taken more than 6 months after surgery demonstrates the dural sac without hematoma. Area D means a cross-sectional area of the dural sac.
The Kruskal-Wallis test was used for group comparisons, and p<0.05 was considered significant.
Results
Between April 2016 and March 2022, 106 patients underwent MEL. Eighteen patients were excluded (1 patient underwent two-level MEL, 6 underwent hemilaminectomy, 10 underwent concomitant herniotomy, 1 patient was discharged very early), and 88 patients (male: 35, female: 53) were ultimately included.
A total of 65 patients corresponded to Group A, 9 corresponded to Group B, and 14 corresponded to Group C (Fig. 3) A total of 23 patients were taking some anticoagulant or antiplatelet drug. The types of oral medications were Warfarin Potassium in 1 case, Apixaban in 3, Rivaroxaban in 1, Clopidogrel Sulfate in 3, and LDA in 15. The reasons for taking anticoagulant or antiplatelet drug were eight cases of cerebrovascular accident prophylaxis, ten cases of cardiovascular accident prophylaxis, and five cases of unknown details. Hypertension was the most common comorbidity in 58 cases and diabetes in 14 cases. One of the patients who took LDA was classified as Group B due to self-interruption before surgery. Basic characteristics such as preoperative hemoglobin level and preoperative platelet level except age did not show any significant difference among the three groups (Table 1). Regarding the evaluation items related to surgery, there was no significant difference among the three groups in each item, such as the operation time, intraoperative EBL, pre- and postoperative hemoglobin level changes (1st and 7th postoperative days), and pre- and postoperative platelet level changes (1st and 7th postoperative days). A case of hematoma removal was confirmed in Group A (Table 2). Regarding the evaluation items related to the clinical results, the ODI and EQ-5D did not show any significant difference among the three groups at any time before surgery, 6 months after surgery, and 1 year after surgery (Fig. 4). There was no significant difference in preoperative JOABPEQ among the three groups in each domain, such as pain-related disorders, lumbar spine function, walking ability, social life function, and mental health (Table 3). Moreover, there was no significant difference between the three groups in the amount acquired JOABPEQ in each domain 6 months after surgery and 1 year after surgery (Table 4). Regarding the MRI imaging evaluation, there was no significant difference between the three groups in the cross-sectional areas of the dural sac and hematoma on MRI taken within 1 week after surgery. There was also no significant difference between the three groups in the cross-sectional area of the dural sac on MRI at least 6 months after surgery (Table 5).
Figure 3.
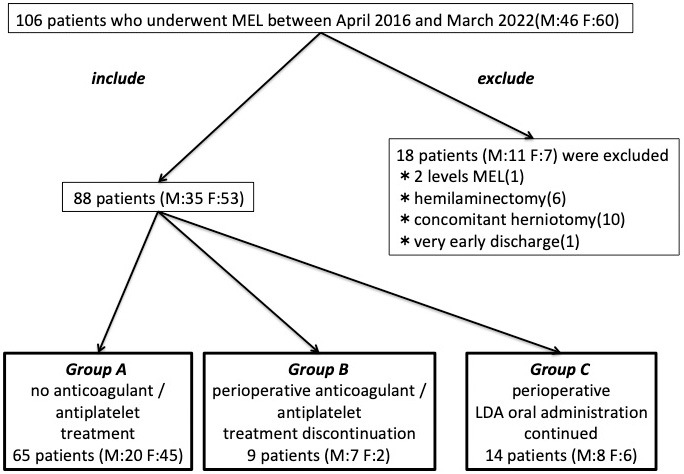
Summary of inclusion/exclusion and grouping of the patient selection flow.
Table 1.
Basic Characteristics of the Patients in Each Group.
| Characteristics | Group A (N=65) | Group B (N=9) | Group C (N=14) | P value |
|---|---|---|---|---|
| Age | 70.4±1.2 | 77.7±1.1 | 78.1±1.4 | P=0.001 |
| Gender (male/female) | 20/45 | 7/2 | 8/6 | |
| Treated level | L2/3: 4, L3/4: 17, L4/5: 43, L5/6: 1 | L3/4: 2, L4/5: 7 | L2/3: 1, L3/4: 3, L4/5: 10 | |
| Preoperative Hb (g/dl) | 13.5±0.2 | 14.0±0.6 | 13.7±0.3 | P=0.67 |
| Preoperative Plt (×103/μl) | 22.6±0.6 | 22.2±1.2 | 20.0±1.2 | P=0.15 |
| Reason for internal use | N.A | Brain: 3, Heart: 5, unknown: 1 | Brain: 5, Heart: 5, unknown: 4 | |
| Types of medicines | N.A | Warfarin: 1, Apixaban: 3,
Rivaroxaban: 1, Clopidogrel: 3, LDA: 1 |
LDA: 14 | |
| Comorbidity | HT: 37, DM: 10 | HT: 7, DM: 1 | HT: 14, DM: 3 |
*Plus-minus values are means±SE.
Table 2.
Surgery-related Evaluation Items of the Patients in Each Group.
| Group A (N=65) | Group B (N=9) | Group C (N=14) | P value | |
|---|---|---|---|---|
| Operation time (min) | 123.2±3.4 | 119.8±7.7 | 127.9±5.9 | 0.56 |
| Intraoperative estimate blood loss (g) | 49.7±8.0 | 28.8±12.7 | 38.7±10.9 | 0.87 |
| Hemoglobin level change at 1st postoperative day (g/dl) | 1.4±0.1 | 1.2±0.3 | 1.5±0.2 | 0.89 |
| Hemoglobin level change at 7th postoperative day (g/dl) | 1.4±0.1 | 1.1±0.3 | 1.7±0.2 | 0.19 |
| Platelet level change at 1st postoperative day (×103/μl) | 2.4±0.3 | 2.3±0.8 | 1.9±0.5 | 0.78 |
| Platelet level change at 7th postoperative day (×103/μl) | 2.9±0.4 | 2.4±0.8 | 3.3±0.6 | 0.81 |
| Perioperative complications | Hematoma removal: 1 | None | None |
*Plus-minus values are means±SE.
Figure 4.
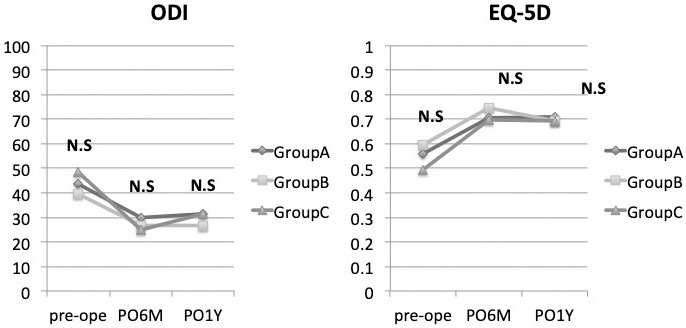
Clinical outcomes of EQ-5D and ODI show that there were no significant differences in the evaluation items at any time point during the follow-up period from before surgery to 1 year after surgery.
Table 3.
Each Domain of Preoperative JOABPEQ in Each Group.
| Preoperative JOABPEQ | Group A (N=65) | Group B (N=9) | Group C (N=14) | P value |
|---|---|---|---|---|
| Pain-related disorders | 48.8±4.5 | 64.9±6.3 | 47.9±8.7 | 0.33 |
| Lumbar function | 50.5±3.5 | 48.0±9.6 | 54.1±8.1 | 0.88 |
| Walking ability | 26.1±2.8 | 24.6±4.7 | 22.4±6.9 | 0.67 |
| Social life function | 35.3±2.4 | 39.0±6.2 | 36.4±5.6 | 0.82 |
| Mental health | 43.3±1.8 | 44.1±3.0 | 42.8±4.7 | 0.72 |
*Plus-minus values are means±SE.
Table 4.
Each Domain of Postoperative JOABPEQ in Each Group at 6 Months and 1 Year after Surgery.
| PO6M | Group A (N=55) | Group B (N=9) | Group C (N=12) | P value |
|---|---|---|---|---|
| Pain-related disorders | 18.4±5.2 | 11.2±7.4 | 39.3±9.0 | 0.11 |
| Lumbar function | 17.7±3.2 | 19.3±9.0 | 17.4±13.0 | 0.92 |
| Walking ability | 29.7±4.1 | 27.9±9.6 | 36.3±10.8 | 0.87 |
| Social life function | 21.8±3.7 | 18.2±7.7 | 16.4±9.0 | 0.70 |
| Mental health | 7.0±2.1 | 6.6±5.3 | 9.1±4.8 | 0.72 |
| *Plus-minus values are means±SE. | ||||
| PO1Y | Group A (N=45) | Group B (N=7) | Group C (N=11) | P value |
|---|---|---|---|---|
| Pain-related disorders | 14.5±5.8 | 16.3±15.1 | −2.5±15.6 | 0.62 |
| Lumbar function | 11.6±4.2 | 19.1±12.4 | −1.5±15.7 | 0.61 |
| Walking ability | 24.8±4.5 | 28.7±9.4 | 23.5±11.4 | 0.97 |
| Social life function | 17.9±4.1 | 17.3±7.2 | 11.3±9.8 | 0.94 |
| Mental health | 5.3±3.0 | 7.3±4.9 | −4.4±8.2 | 0.38 |
| *Plus-minus values are means±SE. | ||||
Table 5.
The Cross-sectional Area of the Dural Sac and Hematoma on MRI in Each Group within 1 Week and at 6 Months or More.
| Group A (N=22) | Group B (N=5) | Group C (N=9) | P value | |
|---|---|---|---|---|
| Dural canal within 1 week | 121.9±9.5 | 96.7±9.5 | 99.0±9.3 | 0.07 |
| Hematoma within 1 week | 199.4±16.4 | 286.9±31.0 | 210.8±32.8 | 0.07 |
| *Plus-minus values are means±SE. | ||||
| Group A (N=21) | Group B (N=5) | Group C (N=9) | P value | |
|---|---|---|---|---|
| Dural canal at 6 months or more | 160.1±11.9 | 146.5±38.3 | 118.8±19.0 | 0.15 |
| *Plus-minus values are means±SE. | ||||
Discussion
The safety of continued LDA administration in the perioperative periods of one-level MEL was evaluated in this study. Continued oral administration of LDA perioperative period did not affect the operation time, intraoperative EBL, changes in hemoglobin/platelets before and after surgery, and perioperative complications. Furthermore, continued oral administration of LDA perioperative period did not affect the clinical result up to 1 year after surgery, the cross-sectional area of the dural sac and hematoma on MRI taken within 1 week after surgery, and the cross-sectional area of the dural sac on MRI more than 6 months after surgery.
Patients receiving anticoagulant therapy (Groups B and C) were significantly elderly. It has been reported that patients undergoing spinal surgery are aging1), and it is expected that the treatment of patients undergoing anticoagulant therapy will become even more important in the future.
In the past, many reports have recommended the discontinuation of LDA concerned about perioperative bleeding complications during the perioperative period7-10). Many of these reports recommended oral LDA for at least 7 days prior to surgery. Although many of these reports focus on spinal fusion surgery, based on these reports, it seems that spine surgeons stopped taking LDA 7-10 days before surgery under the advice of a cardiologist or a cerebrovascular physician in each institution. On the other hand, there are some reports that thrombotic complications increase due to the discontinuation of LDA11,12). In such a historical background, there are increasing reports that oral administration of LDA does not affect perioperative complications such as postoperative epidural hematoma and massive bleeding even in spinal surgery13-16). Therefore, we considered that it would be preferable to continue oral LDA in spinal surgery, especially in minimally invasive decompression surgery, and considered continuing oral LDA in the perioperative period in one-level MEL. However, there was a problem with the report on whether or not the LDA could be continued in the past. Previous reports have focused only on perioperative thrombosis or hemorrhagic complications and have not evaluated clinical outcomes. Ikuta et al.17) reported that asymptomatic postoperative hematomas were actually more abundant than previously reported. They also reported that those with hematomas had poorer dural canal enlargement and clinical results were inferior to those without hematomas. In other words, not only the evaluation of perioperative thrombosis or hemorrhagic complications but also the postoperative asymptomatic hematoma and clinical results should be evaluated. Otherwise, it cannot be determined whether it is really possible to continue taking LDA perioperatively safely and without affecting clinical results. In our study, in addition to perioperative complications, the cross-sectional area of the dural sac and epidural hematoma on MRI and clinical results were evaluated, although there was no significant difference in all items.
Our study had some limitations. First, the number of cases in each group varies. Further accumulation of the number of cases should be necessary. Second, this research is limited to MEL among various surgical procedures. Therefore, whether or not LDA can be discontinued requires a thorough discussion with relevant departments such as cardiologist, neurologist, and anesthesiologist for each case.
One-level MEL with continued oral administration of perioperative LDA can be performed safely without any perioperative complications or significant impact on clinical results. LDA might be used safely to reduce the risk of brain and/or cardiovascular disorders during the perioperative period in minimally invasive spinal surgery.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Sources of Funding: None
Author Contributions: KT contributed to the conception and design of the study, acquisition of the data, analysis and interpretation of the data, and drafting the manuscript. All authors read and approved the final manuscript.
Ethical Approval: This study was approved by the ethics committee of Kyushu University Hospital (Approval code: No. 2022-60).
Informed Consent: Informed consent was obtained from all the individual participants included in this study.
References
- 1.Imajo Y, Taguchi T, Yone K, et al. Japanese 2011 nationwide survey on complications from spine surgery. J Orthop Sci. 2015;20(1):38-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsutake S, Ishizaki T, Teramoto C, et al. Patterns of co-occurrence of chronic disease among older adults in Tokyo, Japan. Prev Chronic Dis. 2019;16:E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015; 373(8):823-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari E, Benhamou M, Cerboni P, et al. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol. 2005;45(3):456-9. [DOI] [PubMed] [Google Scholar]
- 5.Cohen AT, Imfeld S, Markham J, et al. The use of aspirin for primary and secondary prevention in venous thrombo-embolism and other cardiovascular disorders. Thromb Res. 2015;135(2):217-25. [DOI] [PubMed] [Google Scholar]
- 6.Cuellar JM, Petrizzo A, Vaswani R, et al. Does aspirin administration increase perioperative morbidity in patients with cardiac stents undergoing spinal surgery? Spine. 2015;40(9):629-35. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Ahn Y, Choi BS, et al. Antithrombotic effects of aspirin on 1- or 2-level lumbar spinal fusion surgery: a comparison between 2 groups discontinuing aspirin use before and after 7 days prior to surgery. Spine. 2013;38(18):1561-5. [DOI] [PubMed] [Google Scholar]
- 8.Park HJ, Kwon KY, Woo JH. Comparison of blood loss according to use of aspirin in lumbar fusion patients. Eur Spine J. 2014;23(8):1777-82. [DOI] [PubMed] [Google Scholar]
- 9.Kang SB, Cho KJ, Moon KH, et al. Does low-dose aspirin increase blood loss after spinal fusion surgery? Spine J. 2011;11(4):303-7. [DOI] [PubMed] [Google Scholar]
- 10.Epstein NE. When and if to stop low-dose aspirin before spine surgery? Surg Neurol Int. 2018;9(3):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari E, Benhamou M, Cerboni P, et al. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol. 2005;45:456-9. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein NS, Schulman PM, Gerstein WH, et al. Should more patients continue aspirin therapy perioperatively?: clinical impact of aspirin withdrawal syndrome. Ann Surg. 2012;255(5):811-9. [DOI] [PubMed] [Google Scholar]
- 13.Soleman J, Baumgarten P, Perrig WN, et al. Non-instrumented extradural lumbar spine surgery under low-dose acetylsalicylic acid: a comparative risk analysis study. Eur Spine J. 2016;25(3):732-9. [DOI] [PubMed] [Google Scholar]
- 14.Goes R, Muskens IS, Smith TR, et al. Risk of aspirin continuation in spinal surgery: a systematic review and meta-analysis. Spine J. 2017;17(12):1939-46. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Wang G, Liu X, et al. Safety of continuing aspirin therapy during spinal surgery: a systematic review and meta-analysis. Medicine. 2017;96(46):e8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue T, Mizutamari M, Hatake K. Safety of continuous low-dose aspirin therapy for cervical laminoplasty. Spine Surg Relat Res. 2022;6(3):240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikuta K, Tono O, Tanaka T, et al. Evaluation of postoperative spinal epidural hematoma after microendoscopic posterior decompression for lumbar spinal stenosis: a clinical and magnetic resonance imaging study. J Neurosurg Spine. 2006;5(5):404-9. [DOI] [PubMed] [Google Scholar]


