Summary
Background
The epidemiology of human papillomavirus (HPV) in women has been well documented. Less is known about the epidemiology of HPV in men. We aim to provide updated global and regional pooled overall, type-specific, and age-specific prevalence estimates of genital HPV infection in men.
Methods
We conducted a systematic review and meta-analysis to assess the prevalence of genital HPV infection in the general male population. We searched Embase, Ovid MEDLINE, and the Global Index Medicus for studies published between Jan 1, 1995, and June 1, 2022. Inclusion criteria were population-based surveys in men aged 15 years or older or HPV prevalence studies with a sample size of at least 50 men with no HPV-related pathology or known risk factors for HPV infection that collected samples from anogenital sites and used PCR or hybrid capture 2 techniques for HPV DNA detection. Exclusion criteria were studies conducted among populations at increased risk of HPV infection, exclusively conducted among circumcised men, and based on urine or semen samples. We screened identified reports and extracted summary-level data from those that were eligible. Data were extracted by two researchers independently and reviewed by a third, and discrepancies were resolved by consensus. We extracted only data on mucosal α-genus HPVs. Global and regional age-specific prevalences for any HPV, high-risk (HR)-HPV, and individual HPV types were estimated using random-effects models for meta-analysis and grouped by UN Sustainable Development Goals geographical classification.
Findings
We identified 5685 publications from database searches, of which 65 studies (comprising 44 769 men) were included from 35 countries. The global pooled prevalence was 31% (95% CI 27–35) for any HPV and 21% (18–24) for HR-HPV. HPV-16 was the most prevalent HPV genotype (5%, 95% CI 4–7) followed by HPV-6 (4%, 3–5). HPV prevalence was high in young adults, reaching a maximum between the ages of 25 years and 29 years, and stabilised or slightly decreased thereafter. Pooled prevalence estimates were similar for the UN Sustainable Development Goal geographical regions of Europe and Northern America, Sub-Saharan Africa, Latin America and the Caribbean, and Australia and New Zealand (Oceania). The estimates for Eastern and South-Eastern Asia were half that of the other regions.
Interpretation
Almost one in three men worldwide are infected with at least one genital HPV type and around one in five men are infected with one or more HR-HPV types. Our findings show that HPV prevalence is high in men over the age of 15 years and support that sexually active men, regardless of age, are an important reservoir of HPV genital infection. These estimates emphasise the importance of incorporating men in comprehensive HPV prevention strategies to reduce HPV-related morbidity and mortality in men and ultimately achieve elimination of cervical cancer and other HPV-related diseases.
Funding
Instituto de Salud Carlos III, European Regional Development Fund, Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia, and Horizon 2020.
Translations
For the Spanish and French translations of the abstract see Supplementary Materials section.
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted viral infection worldwide, and previous research has shown that most sexually active men and women will acquire at least one genital HPV infection during their lives.1 There are more than 200 HPV types that can be transmitted sexually, and at least 12 types are oncogenic.2 The majority of HPV infections in men and women are asymptomatic, but they can lead to long-term sequelae and mortality. Each year, more than 340 000 women die of cervical cancer.3 In men, HPV infection tends to manifest clinically as anogenital warts, which cause significant morbidity and increase HPV transmission rates.4, 5 HPV infections are also associated with penile, anal, and oropharyngeal cancers, which are commonly linked to HPV type 16.6, 7 The International Agency for Research on Cancer estimated that there were about 69 400 cases of cancer in men caused by HPV in 2018.4
Research in context.
Evidence before this study
The epidemiology of human papillomavirus (HPV) in women is well documented. Prevalence data from the general male population, however, are scarce. We searched Embase, Ovid MEDLINE, and the Global Index Medicus without language restrictions for studies published between Jan 1, 1995, and June 1, 2022. Search terms included “((HPV OR papilloma*) AND (male* OR men OR man) AND (prevalence))”. Most studies in men have been conducted in high-income countries and have focused on subpopulations at increased risk of HPV infection, such as men who have sex with men, men living with HIV, symptomatic men attending sexually transmitted infection clinics, and male partners of women with HPV infection or abnormal cervical cytology. The last comprehensive global review of the prevalence of genital HPV in men was published in 2011 and included studies on men at increased risk of HPV infection. The study reported that HPV prevalence was high among sexually active men in all regions but with considerable variation, from 1% to 84% among men at low risk of HPV infection and from 2% to 93% among men at increased risk of HPV infection. Peak HPV prevalence spanned a wide range of ages, and age-specific prevalence curves were relatively flat or declined only slightly following peak prevalence.
Added value of this study
This study provides global and regional mean benchmark values of genital HPV prevalence in men to inform prevention strategies, on the basis of a systematic review and meta-analysis of studies on HPV infection in general populations of sexually active men who are not reported as being in high-risk subgroups for HPV infection. We were able to generate global and regional prevalence estimates for any HPV, high-risk (HR)-HPV, and specific genotypes and assess age patterns of infection and sources of interstudy heterogeneity. We made a major effort to select only studies that could be considered as representative of the general male population of a country and could be combined by means of meta-analysis with strict selection criteria. We provide support for evidence that the mean global and regional prevalence of high-risk genital HPV in men is very high (globally 21%, 95% CI 18–24) and sustained across the adult lifespan. HR-HPV prevalence estimates were similar across all regions studied except for Eastern and South-Eastern Asia (pooled estimate of 23%, excluding Eastern and South-Eastern Asia), for which estimates were half that of the other regions (10%). We also show that one key factor of interstudy heterogeneity is the anatomical site sampled. HR-HPV prevalence was higher in studies that sampled at least the penile shaft and the glans penis or coronal sulcus (23%) when compared with studies that did not (17%).
Implications of all the available evidence
The results suggest that genital HPV prevalence is high in men and that prevalence remains high throughout heterosexual men's sexual lives. These results are consistent with men being a reservoir of HPV infection and emphasise the importance of incorporating men in efforts to control HPV infection and to reduce the incidence of HPV-related disease. Prevalence data are primarily from high-income countries. Additional epidemiological studies assessing the age-specific prevalence of HPV types in men from additional countries could contribute to impact monitoring efforts both for countries that have HPV vaccination programmes for girls and young women and for the increasing number of countries that are including boys in their vaccination programme.
There are far fewer published reports on the epidemiology of HPV among general populations of men than those among women. Epidemiological studies have primarily focused on women, and data for the prevalence of HPV in men are scarce and predominantly from populations identified as being at increased risk of infection, such as men who have sex with men, men living with HIV, men with symptoms of sexually transmitted infections (STIs) attending STI clinics, and male partners of women with HPV infection or abnormal cervical cytology. The first global review of genital HPV prevalence in men was published in 2006 and identified 40 publications on men at any risk of infection (appendix 3 p 2).8 HPV prevalence in men was 1·3–72·9% in the studies in which more than one anatomical site or specimen were evaluated. HPV prevalence varied on the basis of sampling, testing methods, and the anatomical site or specimen sampled.
A second review in 2011 focused on age-specific prevalence of HPV in men and identified 64 studies, 38 of which included populations at increased risk of HPV infection (appendix 3 p 2).9 HPV prevalence was high among these sexually active men in all regions but varied from 1% to 84% among men with low risk of HPV and from 2% to 93% among men with increased risk of HPV. Peak HPV prevalence spanned a wide range of ages, and age-specific prevalence curves were quite flat or declined only slightly following peak prevalence. A study published in 2018 examined risk factors for HPV in men, including having sex with men, and estimated a global pooled prevalence of any type of genital HPV in men of 49% (95% CI 35–64) and a prevalence of high-risk (HR)-HPV of 35% (26–45) on the basis of data from 16 studies.10 Regional-level reviews from sub-Saharan Africa and Europe have consistently documented a prevalence of any HPV in men exceeding 25%.11, 12
We aimed to update the global and regional estimates of the overall, type-specific, and age-specific prevalence of genital HPV DNA in general populations of men before the onset of widespread HPV gender-neutral vaccination. HPV prevalence data among men are essential to understand disease burden and transmission risk in both men and women and to support the implementation and evaluation of cervical cancer prevention and elimination programmes.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis is reported according to PRISMA13 and GATHER14 guidelines. We searched Embase, Ovid MEDLINE, and the Global Index Medicus using search strategies with exploded MeSH and Emtree terms and broad search criteria and without language restrictions for studies published between Jan 1, 1995, and June 1, 2022. For Embase Classic, Embase, and Ovid MEDLINE, search terms were “((HPV OR papilloma*) AND (male* OR men OR man) AND (prevalence)).mp.”. The search window went back to 1995 to mirror the data included in a meta-analysis of HPV in women.15 The search was supplemented by a bibliographic screening of review papers and papers that met the study entry criteria. The search strategies and full search terms are available in appendix 3 (p 2).
The study eligibility criteria for the systematic review and meta-analysis are summarised in appendix 3 (p 3). Briefly, we included articles that reported HPV prevalence among at least 50 men aged 15 years or older with no HPV-related pathology, used PCR or hybrid capture 2 techniques for HPV DNA detection, and collected samples from penile or anal sites (eg, glans, shaft, scrotum, urethra, anus, or foreskin) mostly after 1995. Studies conducted exclusively in populations that were considered at increased risk for HPV infection were excluded, as were studies conducted exclusively among circumcised men because circumcision is considered a protective factor,16, 17, 18 studies conducted exclusively among men vaccinated against HPV, and studies based on urine or semen samples because the sensitivity of these samples for HPV detection is low.11 We included baseline HPV prevalence data from cohort studies and from the control groups of case-control studies if men were asymptomatic. Titles and abstracts were screened for relevant publications separately by two authors (MT and LB for Embase and Ovid MEDLINE and MT and JR for the Global Index Medicus). Any differences were discussed by the two screeners and, where there was any uncertainty over publication titles and abstracts, the publications were included in the list for full-text screening. Full texts were screened for relevance by GA and LB, and discrepancies were discussed by GA, LB, JR, and MT. In the case of studies where we were uncertain about eligibility, we reached out to the corresponding author (with a minimum of three contact attempts made). We also contacted corresponding authors to access detailed information on HPV prevalence by genotype and age where these data were not reported.
Data analysis
Data were extracted by GA and JR and reviewed by LB. Data were extracted using a standardised form. Discrepancies were resolved by consensus. Where the same study population was described in more than one publication, the publication with the highest sample size and most detailed information was used, supplemented by the other publications. Data from multicountry studies were presented by country where possible, and if a study provided data for 2 years or more, we used the starting year or year with the most detailed information (appendix 3 p 4). For studies that reported HPV prevalence data from more than one anatomical site, we prioritised data that included penile shaft, glans, or corona over other sites.19 We also prioritised data from PCR assays over hybrid capture 2 (Qiagen, Gaithersburg, MD, USA) if a study provided data for both. We extracted data only for mucosal α genus HPVs, because the main oncogenic HPV types and types that cause anogenital warts belong to this genus.2, 6 The full list of variables for which data were extracted is shown in appendix 3 (p 4).
The prevalence of high-risk genotypes was based on the high-risk definition used in each publication. If a publication did not provide an HR-HPV estimate, we estimated it as the sum of the prevalence of the 12 HR-HPV genotypes (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) and adjusted for multiple infections using the ratio of the total number of HPV infections to number of individuals who were positive for HPV. Analyses of type-specific HPV prevalence were based on the studies reporting information for that HPV type. Each type (as single or multiple infection) was evaluated independently. Prevalence of HPV types that are included in the two-valent (types 16 and 18), four-valent (types 6, 11, 16, and 18), and nine-valent (types 6, 11, 16, 18, 31, 33, 45, 52, and 58) HPV vaccines were estimated at the global level after adjusting for multiple infections.
To be eligible for inclusion in the age-specific analyses, the range of a reported age category had to be 11 years or less. Age-specific prevalence data from each eligible study were transformed into estimates by 1-year age groups, assuming the number of men and prevalence were equally distributed, and then aggregated into 5-year and 10-year age groups.
The quality of studies was appraised by a risk of bias score (ie, low or high) on the basis of how closely the study population represented the general population of sexually active men, the sampling frame, sample selection, and response rate. The checklist was adapted from publications by Hoy and colleagues20 and Agbor and colleagues21 and is summarised in appendix 3 (p 5).
Regional pooled mean estimates for any HPV, HR-HPV, and HPV type were calculated using the geographical classification of the UN Sustainable Development Goals (SDGs)22 and the World Bank classification of income level.23 Regional estimates were generated only for those regions where the total number of tested men was at least 500.
HPV prevalence estimates were pooled using random-effects models for meta-analyses of binomial data. Meta-analyses were conducted with Stata procedures metaprop, metapreg, and metan.24, 25 Freeman-Tukey arcsine transformation of the prevalence was used to normalise variance; 95% CIs around the study-specific and pooled prevalences were computed on the basis of the score-test statistic. The percentage of total variation due to interstudy heterogeneity was evaluated using the I2 measure. Publication bias was evaluated by visual inspection of funnel plots, the Egger test, and the trim-and-fill method.
Sensitivity analyses were conducted to identify sources of heterogeneity between studies. Subgroup meta-analysis was performed for nine variables and their categories planned a priori (ie, geography [SDG subregions], sample size [>500 or ≤500], risk of bias [low or high], studies conducted in STI clinics or equivalent [no or yes], first year of data collection [before 2006, 2006–13, or 2014 or later], method of sampling [self-collected or clinician collected], anatomical sample site [at least the penile shaft and the glans penis or coronal sulcus sampled or did not collect data from either the shaft or the plans penis or coronal sulcus], number of HPV types tested in the assay [8–15 types, 16–26 types, or 27–50 types], and age [<30 years or ≥30 years]; appendix 3 p 4). The influence of study variables on the variation of the prevalence was assessed by meta-regression, including those variables that caused significant heterogeneity.
All statistical analyses were conducted in Stata (version 16). Although the review was not registered, an internal protocol was followed describing these methods.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
We identified 5685 publications from database searches, of which 65 studies26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 comprising data from 44 769 men were included in the systematic review and meta-analysis (figure 1, table 1). No additional publications were identified from the reference lists of the selected publications identified. Two studies provided data for more than one population. Authors of 49 publications were contacted to provide additional information. 40 of the authors responded and 31 provided unpublished data,27, 28, 32, 35, 38, 39, 41, 42, 43, 50, 51, 53, 55, 56, 57, 59, 60, 62, 63, 64, 66, 71, 73, 75, 80, 81, 83, 86, 87, 90 including 27 authors who shared data for HPV-type distribution or age distribution.
Figure 1.
PRISMA flow diagram
*4938 records were excluded during the screening of publication titles and 352 records were excluded during the screening of abstracts.
Table 1.
Studies included reporting prevalence of genital HPV in men by UN Sustainable Development Goals regional grouping
| Study | Recruitment years | Study population or setting | Study design | Age range, years | Selection of study population | Specimen sample sites | HPV detection method | Risk score | Sample size |
Estimated HPV prevalence |
Inclusion in analyses |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any type | HR-HPV | Overall | Age | Type | |||||||||||
| Australia and New Zealand (Oceania) | |||||||||||||||
| Australia | Machalek et al (2017)55* | 2014–16 | Sexual health clinics, general practice clinics, and outreach using Facebook | Cross-sectional | 16 to 35 | Convenience | Glans, corona, foreskin, and shaft | PCR-PGMY09-PGMY11, Cobas HPV test (Roche Molecular Systems, Alameda, CA, USA) and Linear Array HPV Genotyping Test (Roche Molecular Systems, Alameda, CA, USA) | High | 511 | 28% | 19% | Yes | Yes | Yes |
| Eastern and South-Eastern Asia | |||||||||||||||
| China | Tang et al (2006)76 | 2003–04 | STI clinic | Cross-sectional | 18 to 70 | Convenience | Urethra | PCR-MY09-MY11 and restriction fragment length polymorphisms | High | 305 | 14% | NR | Yes | Yes | Yes |
| China | He et al (2013)41* | 2007–09 | Rural community | Cross-sectional | 25 to 65 | Cluster | Glans, corona, shaft, and scrotum | PCR-SPF1/GP6+ | Low | 2236 | 18% | 6% | Yes | Yes | Yes |
| China | Wei et al (2016)86* | 2014 | Urban community | Cross-sectional | 18 to 55 | Convenience | Glans, corona, penile, perianal, and anal canal | PCR-GP5+/6+ | Low | 1509 | 11% | 10% | Yes | Yes | Yes |
| China | Zhang et al (2018)90* | 2013 | Rural community | Cross-sectional | <30 to >50† | Convenience | Urethra | Human Papillomavirus Genotyping Kit (Gene Chips; Genetel Pharmaceuticals, Shenzhen, China) | High | 104 | 13% | 10% | Yes | No | Yes |
| China | Ma et al (2019)54 | 2016–18 | STI clinic | Cross-sectional | 18 to 67 | Convenience | Glans, corona, shaft, scrotum, and urethral | Real-time PCR genotyping (Liferiver, Shanghai, China) | High | 737 | 26% | 19% | Yes | Yes | Yes |
| China | Wang et al (2021)84 | 2015–20 | Men attending Henan Provincial People's Hospital | Cross-sectional | 20 to 85 | Convenience | Glans, corona, shaft, prepuce, and distal urethra | PCR-HPV genotyping kit (Gene Chips; Genetel Pharmaceuticals, Shenzhen, China) | High | 3690 | 30% | 12% | Yes | Yes | Yes |
| Japan | Takahashi et al (2003)74 | 2002 | University students | Case-control | 18 to 35 | Convenience | Glans, corona, and foreskin | Hybrid Capture 2, for high-risk and low-risk HPV types (Digene, Gaithersburg, MD, USA) | Low | 75 | 1% | 1% | Yes | No | No |
| Japan | Takahashi et al (2005)75* | 2004 | University students | Cross-sectional | 18 to 35 | Convenience | Glans, corona, and foreskin | Hybrid Capture 2, for high-risk and low-risk HPV types (Digene, Gaithersburg, MD, USA) | Low | 150 | 8% | 8% | Yes | No | No |
| Japan | Matsuzawa et al (2020)56* | 2011–15 | Urology clinic | Cross-sectional | 15 to 95 | Convenience | Glans | PCR-GENOSEARCH-HPV31 (Medical and Biological Laboratories, Nagoya, Japan) | High | 759 | 25% | 13% | Yes | Yes | Yes |
| Malaysia | Khoo et al (2021)48 | 2014–16 | Community | Cross-sectional | 18 to 60 | Convenience | Shaft | PCR-BGISEQ-100 (Beijing Genome Institute-assembled Ion Proton Sequencer from Life Technologies, South San Francisco, CA, USA) | Low | 389 | 21% | 19% | Yes | Yes | Yes |
| South Korea | Shin et al (2004)70 | 2002 | University students | Cross-sectional | 18 to 28 | Convenience | Glans, corona, foreskin, penile, and scrotum | PCR line probe assay | Low | 381 | 9% | 4% | Yes | Yes | Yes |
| Philippines, Taiwan, and Australia | Vardas et al (2011)80 | 2004–08 | Community (baseline data of men participating in the Gardasil trial) | Cross-sectional | 16 to 14 | Convenience | Penile, scrotum, and perineal or perianal areas | PCR multiplex real-time fluorescent detection (Atila BioSystems, Mountain View, CA, USA) | Low | 263 | 10% | 9% | Yes | Yes | Yes |
| Europe and Northern America | |||||||||||||||
| Canada | Ogilvie et al (2009)62* | 2006–07 | STI clinic | Cross-sectional | 16 to 69 | Convenience | Glans, foreskin, penile shaft, and scrotum | PCR Linear Array HPV Genotyping Test (Roche Molecular Systems, Alameda, CA, USA) | High | 261 | 61% | 57% | Yes | Yes | Yes |
| Canada | El-Zein et al (2019)37* | 2005–11 | University students | Cohort | 17 to 45 | Convenience | Glans, corona, foreskin, shaft, and scrotum | PCR Linear Array HPV Genotyping Test (Roche Molecular Systems, Alameda, CA, USA) | Low | 535 | 59% | 42% | Yes | Yes | Yes |
| Canada | Nelson et al (2019)59* | 2011–13 | Urban community | Cross-sectional | 16 to 83 | Convenience | Foreskin and shaft | PCR Linear Array HPV Genotyping Test (Roche Molecular Systems, Alameda, CA, USA) | High | 175 | 43% | 24% | Yes | Yes | Yes |
| Croatia | Bosnjak et al (2013)34 | 2009–11 | STI clinic | Cross-sectional | 17 to 60 | Convenience | Genital | PCR-AMPLICOR HPV test (Roche Diagnostics, Mannheim, Germany) | High | 330 | 32% | 32% | Yes | Yes | No |
| Czechia | Jaworek et al (2021)46 | 2013–16 | Men in couples treated for infertility | Cross-sectional | 22 to 57 | Convenience | Glans and corona | PCR-PapilloCheck test (Greiner Bio-One, Frickenhausen, Germany) | High | 195 | 41% | 31% | Yes | No | Yes |
| Denmark | Hebnes et al (2015)42* | 2006–07 | Military conscripts and employees | Cross-sectional | 18 to 65 | Population-based | Glans, corona, foreskin, shaft, scrotum, and perineum | PCR-INNO-LiPA HPV Genotyping Extra II (Innogenetics, Ghent, Belgium) | Low | 2436 | 42% | 30% | Yes | Yes | Yes |
| Denmark | Kjaer et al (2005)49 | 1998 | Miltary conscripts | Cohort | 18 to 29 | Population-based | Glans and corona | PCR-GP5+/6+ | Low | 337 | 34% | NR | Yes | Yes | Yes |
| Finland | Kero et al (2011)47 | No data | Male spouses of pregnant women in their third trimester | Cohort | 19 to 46 | Convenience | Urethra | PCR-GP5+/6+ and MY09/11 | Low | 128 | 23% | 9% | Yes | No | Yes |
| Italy | Bartoletti et al (2014)31 | 2005–06 | STI clinic | Case-control | 18 to 45 | Convenience | Urethra | PCR-INNO-LiPA HPV Genotyping Extra (Innogenetics, Rome, Italy) | High | 1081 | 27% | 11% | Yes | No | No |
| Netherlands | Bleeker et al (2005)33 | 2002 | Outpatient clinic (not an STI clinic) | Cross-sectional | 23 to 73 | Convenience | Glans, corona, and foreskin | PCR-GP5+/6+ | Low | 83 | 25% | 19% | Yes | Yes | Yes |
| Netherlands | Vriend et al (2013)83* | 2009–11 | STI clinic | Cross-sectional | 16 to 24 | Convenience | Glans, corona, and foreskin | PCR-SPF10-DEIA line probe assay (DDL Diagnostic Laboratory, Rijswijk, Netherlands) | High | 414 | 54% | 40% | Yes | Yes | Yes |
| Netherlands | Luttmer et al (2015)53* | 2011–12 | Healthy men | Cross-sectional | 18 to 65 | Convenience | Glans, corona, and foreskin | PCR-SPF10-DNA enzyme-linked immunoassay (DDL Diagnostic Laboratory, Rijswijk, Netherlands) | Low | 170 | 34% | 26% | Yes | Yes | Yes |
| Netherlands | Koene et al (2016)50* | 2011 | STI clinic | Cross-sectional | 18 to 61 | Convenience | Glans, corona, shaft, and urethra | PCR-RHA Kit SPF10-LiPA25 version 1 (Labo Bio-medical Products, Rijswijk, Netherlands) | High | 111 | 65% | 42% | Yes | Yes | Yes |
| Russia | Smelov et al (2013)71* | 2006–09 | STI clinic | Cross-sectional | 16 to 60 | Convenience | Urethra | PCR-Bioplex 200 Luminex system (Bio-Rad, Hercules, CA, USA) | High | 895 | 25% | 17% | Yes | Yes | Yes |
| Slovenia | Golob et al (2014)39* | 2010–13 | Male partners from couples attending a clinic due to inability to conceive within at least one year of unprotected regular sexual intercourse | Cross-sectional | 16 to 55 | Convenience | Penile surface | PCR Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Pleasanton, CA, USA) | High | 299 | 37% | 17% | Yes | Yes | Yes |
| Spain | Álvarez-Argüelles et al (2013)28* | 2002–11 | STI clinic | Cross-sectional | 17 to 87 | Convenience | Balanopreputial or urethral | PCR-MY11/MY09 and GP5+/GP6+ | High | 565 | 12% | 3% | Yes | Yes | Yes |
| Sweden | Wikström et al (2000)89 | No data | STI clinic | Cross-sectional | 18 to 54 | Convenience | Glans, corona, and foreskin | PCR reverse hybridisation DNA sequencing | High | 235 | 13% | 8% | Yes | No | Yes |
| Sweden | Söderlund-Strand et al (2013)79 | 2008 | Sexual health clinic | Cross-sectional | 20 to 29 | Convenience | Urethra | PCR-HPV MassArray (Agena Bioscience, Hamburg, Germany) | High | 597 | 22% | NR | Yes | Yes | No |
| Sweden | Söderlund-Strand et al (2015)72 | No data | Sexual health clinic | Cross-sectional | 21 to 56 | Convenience | Shaft | PCR-HPV MassArray (Agena Bioscience, Hamburg, Germany) | High | 127 | 49% | 34% | Yes | No | Yes |
| UK | Jalal et al (2007)45 | 2003 | STI clinic | Cross-sectional | 15 to 77 | Convenience | Urethra | PCR reverse blot hybridisation | High | 437 | 21% | 13% | Yes | No | Yes |
| UK | Cuschieri et al (2011)35* | 2007–08 | Youth clinic | Cross-sectional | 16 to 25 | Convenience | Shaft | PCR-INNO-LiPA HPV Genotyping Extra II (Fujirebio, Gent, Belgium) | High | 117 | 29% | 20% | Yes | Yes | Yes |
| USA | Baldwin et al (2003)30 | 2000–01 | STI clinic | Cross-sectional | 18 to 70 | Convenience | Glans and corona | PCR-PGMY09/11 and reverse line blot or PCR-L1 consensus | High | 393 | 28% | 12% | Yes | Yes | Yes |
| USA | Weaver et al (2004)85 | 2001–02 | University students | Cross-sectional | 18 to 25 | Population-based | Glans, foreskin, shaft, and scrotum | PCR-MY09/MY11/HMB01 | Low | 317 | 33% | NR | Yes | Yes | No |
| USA | Nielson et al (2007)61 | 2003–06 | STI clinic and the community | Cross-sectional | 18 to 40 | Convenience | Glans, corona, shaft, scrotum, urethra, perianal area, anal canal, and semen | PCR-PGMY09/11 and reverse line blot | High | 463 | 65% | 29% | Yes | No | Yes |
| USA | Partridge et al (2007)65 | 2003–06 | University students | Cohort | 18 to 20 | Population-based | Glans, shaft, and scrotum | PCR-based direct DNA sequencing | Low | 240 | 26% | 20% | Yes | Yes | Yes |
| USA | Hernandez et al (2008)43* | 2004–06 | University population | Cohort | 18 to 79 | Convenience | Glans, corona, shaft, and scrotum | PCR-PGMY09/11 and reverse line blot | Low | 410 | 40% | 24% | Yes | Yes | Yes |
| USA | Hernandez et al (2013)44 | 2010–12 | Community clinic and medical practices | Cross-sectional | 14 to 59 | Convenience | Glans, corona, foreskin, and shaft | PCR Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | High | 450 | 44% | 16% | Yes | Yes | Yes |
| USA | Sudenga et al (2017)73* | 2005–09 | Community (HPV Infection in Men study [HIM]) | Cohort | 18 to 70 | Baseline | Glans, corona, shaft, and scrotum | PCR-L1 consensus and Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 1274 | 47% | 28% | Yes | Yes | Yes |
| USA | Gargano et al (2017)38* | 2013–14 | Community (NHANES) | Cross-sectional | 14 to 59‡ | Population-based | Glans | PCR Linear Array HPV Genotyping Test (Roche Molecular Systems, Alameda, CA, USA) | Low | 1623 | 47% | 26% | Yes | Yes | Yes |
| USA | Widdice et al (2019)88 | 2013–14 | Hospital-based adolescent primary care clinic, STI clinic, and the community | Cross-sectional | 13 to 26 | Sequential sampling | Glans, corona, shaft, scrotum, and perianal or anal areas | PCR Linear Array HPV Genotyping Test (Roche Molecular Systems, Alameda, CA, USA) | High | 310 | 61% | NR | Yes | No | Yes |
| Croatia, Finland, Germany, Netherlands, Norway, Portugal, Spain, and Sweden | Vardas et al (2011)80 | 2004–08 | Community (baseline data of men participating in the Gardasil trial) | Cross-sectional | 16 to 24 | Convenience | Penile, scrotum, and perineal or perianal areas | PCR-Multiplex Real Time Fluorescent Detection (Atila BioSystems, Mountain View, CA, USA) | Low | 354 | 21% | 20% | Yes | Yes | Yes |
| USA and Canada | Vardas et al (2011)80 | 2004–08 | Community (baseline data of men participating in the Gardasil trial) | Cross-sectional | 16 to 24 | Convenience | Penile, scrotum, and perineal or perianal areas | PCR-Multiplex Real Time Fluorescent Detection (Atila BioSystems, Mountain View, CA, USA) | Low | 712 | 27% | 24% | Yes | Yes | Yes |
| Latin America and the Caribbean | |||||||||||||||
| Brazil | Rosenblatt et al (2004)67 | 1999–2001 | Partners of women screened for cervical cancer | Cross-sectional | NR | Convenience | Foreskin, shaft, dorsal and ventral prebalanic area, and urethra | Hybrid Capture 2, for high-risk and low-risk HPV types (Digene, Gaithersburg, MD, USA) | Low | 60 | 15% | 15% | Yes | No | No |
| Brazil | Benzaken et al (2012)32* | 2009 | Community | Cross-sectional | 15 to 64 | Convenience | Urethra | Hybrid Capture 2, for high-risk HPV types (Digene, Gaithersburg, MD, USA) | High | 278 | 14% | 14% | Yes | Yes | No |
| Brazil | Afonso et al (2013)26 | 2000–10 | Partners of women screened for cervical cancer | Cross-sectional | 18 to 51 | Convenience | Penile scrape | PCR-MY09/11 type-specific E6 | Low | 60 | 17% | NR | Yes | No | No |
| Brazil | Menezes et al (2014)58 | 2011–14 | Asymptomatic men from an STI clinic, a dermatology clinic, and a metallurgical factory | Cross-sectional | 18 to 65 | Convenience | Glans, corona, and foreskin | PCR-restriction fragment length polymorphism | High | 550 | 22% | 10% | Yes | No | Yes |
| Brazil | Afonso et al (2016)27* | 2010–11 | Asymptomatic men having laboratory tests done | Cross-sectional | 1867 | Convenience | Glans, corona, and frenulum | PCR-MY09/11 type-specific E6 | High | 110 | 16% | 4% | Yes | No | Yes |
| Brazil | Sudenga et al (2017)73* | 2005–09 | Community (HPV in Men study [HIM]) | Cross-sectional | 18 to 70 | Baseline | Glans, corona, shaft, and scrotum | PCR-L1 consensus and Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 1398 | 60% | 34% | Yes | Yes | Yes |
| Brazil | Wendland et al (2020)87* | 2016–17 | Young people who use public health system | Cross-sectional | 15 to 25 | Convenience | Glans, corona, shaft, and scrotum | PCR-L1 consensus and Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 1120 | 53% | 29% | Yes | Yes | Yes |
| Chile | Guzmán et al (2008)40 | No data | University students | Cross-sectional | 20 to 51 | Convenience | Corona and shaft | PCR-GP5+/6+ and reverse line blot | Low | 61 | 84% | 75% | Yes | No | Yes |
| Mexico | Lazcano-Ponce et al (2001)52 | 1998 | University students and industry workers | Cross-sectional | 14 to 55 | Convenience | Corona and urethra | PCR-GP5+/6+ | Low | 96 | 43% | 20% | Yes | No | No |
| Mexico | Sánchez-Alemán et al (2002)69 | 2000–01 | University students | Cross-sectional | ≥16 | Convenience | Glans, corona, and foreskin | Hybrid Capture 2, for high-risk HPV types (Digene, Gaithersburg, MD, USA) | Low | 71 | 9% | 9% | Yes | Yes | No |
| Mexico | Lajous et al (2005)51* | 2000–03 | Military | Cross-sectional | 16 to 40 | Convenience | Corona, shaft, scrotum, and urethra | PCR strip assay using the reverse line blot | Low | 1045 | 43% | 32% | Yes | Yes | Yes |
| Mexico | Vaccarella et al (2006)78 | 2003–04 | Men seeking vasectomy | Cross-sectional | 15 to 65 | Convenience | Glans, corona, foreskin, shaft, scrotum, and urethra | PCR-L1 consensus | Low | 779 | 9% | 6% | Yes | Yes | Yes |
| Mexico | Parada et al (2010)64* | 2002–03 | Partners of women attending a primary health centre | Cross-sectional | 18 to 75 | Convenience | Foreskin, shaft, scrotum, and urethra | PCR-L1 consensus and reverse line blot | Low | 504 | 20% | 8% | Yes | Yes | Yes |
| Mexico | Vera-Uehara et al (2014)82 | 2002–03 | University students | Cross-sectional | ≥18 | Convenience | Penis, glans, and corona | PCR-MY09/MY11 and Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 253 | 19% | 17% | Yes | Yes | Yes |
| Mexico | Sudenga et al (2017)73* | 2005–09 | Community (HPV in Men study [HIM]) | Cross-sectional | 18 to 70 | Baseline | Glans, corona, shaft, and scrotum | PCR-L1 consensus and Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 1342 | 50% | 27% | Yes | Yes | Yes |
| Brazil, Costa Rica, Mexico, Peru | Vardas et al (2011)80 | 2004–08 | Community (baseline data of men participating in the Gardasil trial) | Cross-sectional | 16 to 24 | Convenience | Penile, scrotum, and perineal or perianal areas | PCR-Multiplex Real Time Fluorescent Detection (Atila BioSystems, Mountain View, CA, USA) | Low | 1301 | 29% | 26% | Yes | Yes | Yes |
| Sub-Saharan Africa | |||||||||||||||
| Botswana | Ramogola-Masire et al (2022)66* | 2019–21 | Men without HIV infection from the University of Botswana | Cross-sectional | 18 to 22 | Convenience | Penile, shaft, glans, and foreskin | PCR real-time Anyplex II HPV28 assay (Seegene, Seoul, South Korea) | Low | 493 | 31% | 24% | Yes | Yes | Yes |
| Kenya | Ng'ayo et al (2008)60* | 2005 | Fishermen from the community | Cross-sectional | 18 to 68 | Population-based | Glans, corona, shaft, scrotum, and perianal region | PCR-MY09/MY11/HMB01 and Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 186 | 53% | 32% | Yes | Yes | Yes |
| Kenya | Rositch et al (2012)68 | 2002–05 | Community (circumcision trial) | Cross-sectional | 17 to 28 | Population-based | Glans, corona, foreskin, and shaft | PCR-GP5+/6+ and reverse line blot hybridisation | Low | 2702 | 51% | 35% | Yes | No | Yes |
| Mozambique | Edna Omar et al (2017)36 | 2009–11 | Young people attending a youth clinic | Cross-sectional | 18 to 24 | Convenience | Urethra | PCR-Clart HPV 2 (Genomica, Madrid, Spain) | High | 176 | 10% | 6% | Yes | Yes | Yes |
| Rwanda | Veldhuijzen et al (2012)81* | 2007–09 | Control group in an infertility study | Case-control | 21 to 57 | Convenience | Glans, corona, foreskin, shaft, and scrotum | PCR Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 166 | 43% | 27% | Yes | Yes | Yes |
| South Africa | Mbulawa et al (2010)57* | 2006–09 | Couples studies | Cross-sectional | 19 to 67 | Convenience | Glans, foreskin, and shaft | PCR Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | High | 313 | 51% | 25% | Yes | Yes | Yes |
| South Africa | Auvert et al (2010)29 | 2005–06 | Community (circumcision trial) | Cross-sectional | 18 to 24 | Population-based | Urethra | PCR Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 1683 | 19% | 17% | Yes | Yes | Yes |
| South Africa | Vardas et al (2011)80 | 2004–08 | Community (baseline data of men participating in the Gardasil trial) | Cross-sectional | 16 to 24 | Convenience | Penile, scrotal, and perineal or perianal areas | PCR-Multiplex Real Time Fluorescent Detection (Atila BioSystems, Mountain View, CA, USA) | Low | 518 | 42% | 36% | Yes | Yes | Yes |
| Tanzania | Olesen et al (2013)63* | 2009 | Urban and rural community | Cross-sectional | 16 to 65 | Convenience | Glans, corona, and shaft | Hybrid Capture 2 HPV DNA Test, for high-risk and low-risk HPV types (Qiagen Gaithersburg, MD, USA) and INNO-LiPA HPV Genotyping Extra II (Fujirebio, Gent, Belgium) | Low | 1343 | 20% | 15% | Yes | Yes | Yes |
| Uganda | Tobian et al (2013)77 | 2003–06 | Community (circumcision trial) | Cross-sectional | 15 to 49 | Population-based | Glans and corona | PCR Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA) | Low | 978 | 61% | 39% | Yes | Yes | Yes |
HPV=human papillomavirus. HR-HPV=high-risk HPV. NR=not reported. STI=sexually transmitted infection.
Authors provided additional information.
Data were reported from men younger than 30 years, men aged 30–49 years, and men aged 50 years or older; the full range of ages included was not reported.
To exclude HPV-vaccinated cohorts, only data for people aged 20–59 years were considered.
The 65 studies26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 provided data from 35 countries, with 16 countries having more than one study. One SDG region (Europe and Northern America) accounted for 31 (48%) of the studies. No prevalence data were found from three SDG regions (Northern Africa and Western Asia, Central and Southern Asia, and Oceania [excluding Australia and New Zealand]). 36 (55%) studies were from high-income countries and only six (9%) studies were from low or lower-middle-income countries (table 2).
Table 2.
Meta-analyses of studies reporting prevalence of any HPV, HR-HPV, HPV-16, and HPV-6 by region and income classification
|
Any type |
HR-HPV |
HPV-16 |
HPV-6 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Number of men | HPV prevalence, % (95% CI) | Number of studies | Number of men | HPV prevalence, % (95% CI) | Number of studies | Number of men | HPV prevalence, % (95% CI) | Number of studies | Number of men | HPV prevalence, % (95% CI) | ||
| Region | |||||||||||||
| Global* | 65 | 44 769 | 31% (27–35) | 56 | 41 617 | 21% (18–24) | 54 | 41 482 | 5% (4–7) | 53 | 41 045 | 4% (3–5) | |
| Australia and New Zealand (Oceania) | 1 | 511 | 28% (24–32) | 1 | 511 | 19% (16–23) | 1 | 511 | 3% (2–5) | 1 | 511 | 3% (2–5) | |
| Eastern and South-Eastern Asia† | 11 | 10 335 | 15% (11–21) | 10 | 10 030 | 10% (7–13) | 9 | 10 110 | 2% (1–5) | 9 | 10 110 | 3% (0–10) | |
| Eastern Asia | 10 | 9946 | 15% (10–20) | 9 | 9641 | 9% (6–12) | 8 | 9721 | 2% (1–5) | 8 | 9721 | 3% (0–11) | |
| Europe and Northern America‡ | 31 | 16 074 | 36% (32–41) | 26 | 13 947 | 24% (20–28) | 27 | 13 577 | 7% (5–9) | 26 | 13 142 | 4% (3–5) | |
| Europe | 19 | 8911 | 31% (25–37) | 16 | 7412 | 22% (17–28) | 16 | 6732 | 6% (4–7) | 15 | 6296 | 3% (2–5) | |
| Northern America | 13 | 7163 | 45% (38–51) | 11 | 6535 | 27% (21–32) | 12 | 6845 | 9% (6–12) | 12 | 6846 | 5% (3–7) | |
| Latin America and the Caribbean | 15 | 9028 | 30% (21–40) | 12 | 8308 | 22% (16–29) | 10 | 8463 | 7% (4–10) | 10 | 8461 | 4% (3–6) | |
| Central America | 7 | 4090 | 26% (13–41) | 7 | 4090 | 16% (8–26) | 5 | 3923 | 5% (2–9) | 5 | 3923 | 2% (0–4) | |
| South America | 8 | 3637 | 34% (19–50) | 5 | 2917 | 31% (21–43) | 5 | 3239 | 11% (5–20) | 5 | 3239 | 7% (6–9) | |
| Sub-Saharan Africa | 10 | 8558 | 37% (26–49) | 10 | 8558 | 25% (18–32) | 10 | 8558 | 4% (3–7) | 10 | 8558 | 4% (3–6) | |
| Eastern Africa | 6 | 5551 | 38% (23–55) | 6 | 5551 | 24% (15–35) | 6 | 5551 | 5% (2–9) | 6 | 5551 | 4% (2–5) | |
| Southern Africa | 4 | 3007 | 35% (21–51) | 4 | 3007 | 25% (17–34) | 4 | 3007 | 4% (3–5) | 4 | 3007 | 6% (3–10) | |
| Income level | |||||||||||||
| High income | 36 | 17 116 | 34% (29–39) | 31 | 14 989 | 23% (19–27) | 30 | 14 394 | 7% (5–9) | 29 | 13 959 | 4% (3–5) | |
| Low and middle income | 31 | 27 390 | 28% (22–34) | 27 | 26 365 | 19% (15–24) | 26 | 26 825 | 4% (3–6) | 26 | 26 823 | 4% (2–6) | |
| Upper-middle income | 25 | 21 839 | 26% (20–32) | 21 | 20 814 | 18% (14–22) | 20 | 21 274 | 4% (3–6) | 20 | 21 272 | 4% (2–7) | |
| Lower-middle income | 3 | 4231 | 40% (18–65) | 3 | 4231 | 26% (12–44) | 3 | 4231 | 6% (1–13) | 3 | 4231 | 4% (2–5) | |
| Low income | 3 | 1320 | 36% (9–70) | 3 | 1320 | 22% (5–46) | 3 | 1320 | 4% (0–9) | 3 | 1320 | 3% (1–6) | |
UN Sustainable Development Goals regional or subregional groupings were used for regional classification and World Bank income classification was used for income level. HR-HPV=high-risk HPV. HPV=human papillomavirus.
The number of studies in each region does not add to the global number of studies because Vardas et al80 and Sudenga et al73 included more than one country or region.
The number of studies in Eastern Asia is different to the number of studies in Eastern and South-Eastern Asia because subregional estimates were generated only for studies in the subregions for which the total number of men was at least 500, which excluded one study in South-Eastern Asia.48
Vardas et al80 was included in both subregions of Europe and Northern America but is counted only once for the region of Europe and Northern America.
The data included in the analysis came from prevalence surveys, case-control studies, and cohort studies conducted in a range of populations, including university students, the military, different occupational groups, participants of circumcision trials, outpatient clinics, and asymptomatic men attending STI or sexual health services (table 1). Of the 62 studies with information on date of specimen collection, 30 (48%) had samples collected before 2006, 25 (40%) between 2006 and 2013, and only seven (11%) from 2014 or later. 32 (49%) of 65 studies sampled at least the penile shaft and the glans of the penis or coronal sulcus. The most sampled genital anatomical sites were glans of the penis and corona sulcus (44 [68%] studies) and the penile shaft (32 [49%] studies); prepuce, foreskin, or frenulum (22 [34%] studies); the urethra or meatus (21 [32%] studies); and the scrotum (19 [29%] studies).
The estimated overall HPV prevalence (of any type) among men was 31% (95% CI 27–35) with study-specific prevalences ranging from 1% to 84% (Table 1, Table 2; appendix 3 p 12). The pooled prevalence estimates for any HPV, HR-HPV, HPV-16, and HPV-6 are shown in table 2; forest plots for the 56 studies with data for HR-HPV, any HPV, HPV-16, and HPV-6 are shown in appendix 3 (pp 11–14).
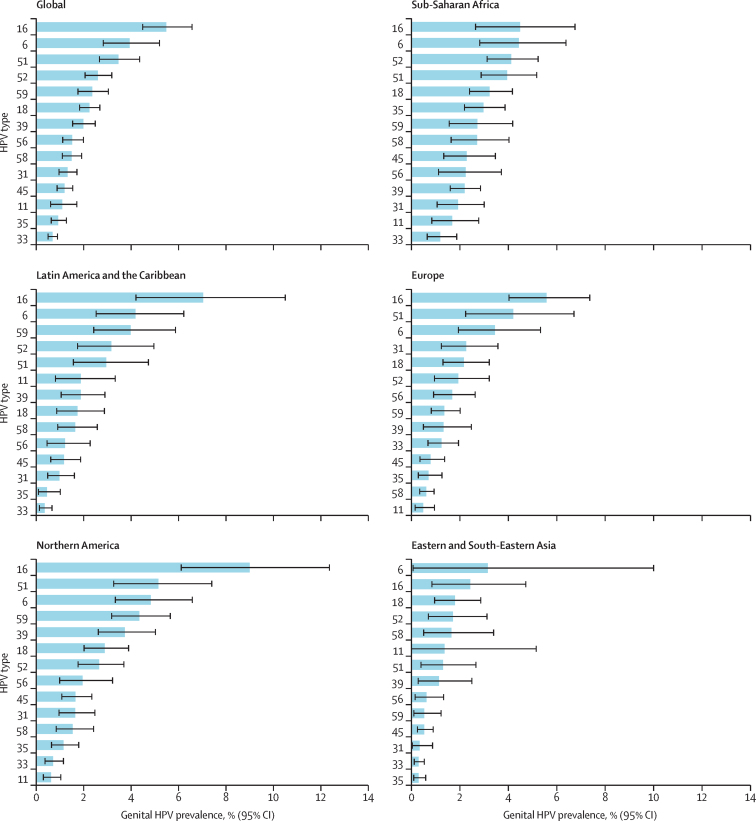
The overall prevalence of HR-HPV was 21% (95% CI 18–24) with study-specific prevalences ranging from 1% to 75%. Globally, HPV-16 was the most frequent HR-HPV type at 5% (95% CI 4–7) followed by types 51 (3%, 3–4), 52 (3%, 2–3), 59 (2%, 2–3), and 18 (2%, 2–3; figure 2; appendix 3 p 6). HPV-6, a non-HR-HPV type, was the second most prevalent HPV type globally (4%, 95% CI 3–5; table 2, figure 2; appendix 3 p 6). The pooled prevalence was 7% (95% CI 6–8) for the HPV types found in the two-valent vaccine, 11% (95% CI 9–13%) for the four-valent vaccine, and 16% (95% CI 14–18%) for the nine-valent vaccine (appendix 3 p 7).
Figure 2.
Pooled prevalence of HR-HPV types, HPV-6, and HPV-11 in men by region
Regions are defined by the UN Sustainable Development Goals regional or subregional grouping. Europe and Northern America are shown separately, and Central America and South America were the only subregions for Latin America and the Caribbean. HR-HPV types were defined as types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. HPV types are ranked by prevalence. Error bars represent the 95% CIs. Numbers of studies with available data for each HPV type and the number of men for whom data were contributed are shown in appendix 3 (p 6). HR-HPV=high-risk HPV. HPV=human papillomavirus.
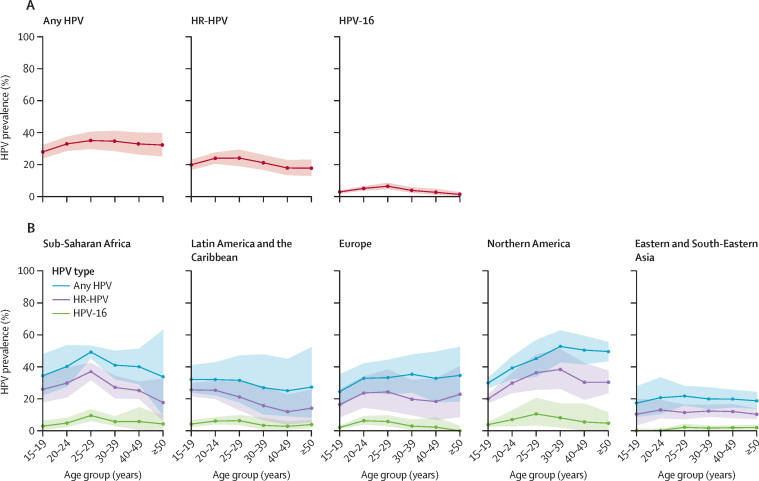
47 studies (39 183 individuals) had sufficient age-specific information to estimate prevalence by age (figure 3). The age-specific prevalence curves show a high prevalence of HPV in young adult men, which remains high throughout adulthood. Prevalence was highest in people aged 25–29 years for any HPV (35%, 95% CI 30–41), HR-HPV (24%, 19–29), and HPV-16 (6%, 5–9). Prevalence in people aged 15–19 years was also high (28% [95% CI 24–32] for any HPV; 20% [17–23] for HR-HPV; and 3% [2–4] for HPV-16). HPV-16 age-specific prevalence curves for HR-HPV in Eastern and South-Eastern Asia and Europe and Northern America reflect upward prevalence from ages 15 years to 20 years followed by stable prevalence in older age groups, whereas in Latin America and the Caribbean and Sub-Saharan Africa, the curves appear to trend downwards after they peak (figure 3).
Figure 3.
Age-specific prevalence of genital HPV infection in men
Global (A) and regional (B) prevalence of any HPV, HR-HPV, and HPV-16 by age group. Areas represent 95% CIs. Regions are defined by the UN Sustainable Development Goals regional or subregional grouping. Europe and Northern America are shown separately, and Central America and South America were the only subregions for Latin America and the Caribbean. The number of studies for which age-specific data were available, the number of men for whom data were contributed, and the countries included in each region are shown in appendix 3 (p 6). HR-HPV=high-risk HPV. HPV=human papillomavirus.
There were sufficient data to generate regional estimates for five SDG regions. The pooled prevalence for any HPV was highest in Sub-Saharan Africa (37%, 95% CI 26–49), followed by Europe and Northern America (36%, 32–41; table 2). The lowest prevalence was in Eastern and South-Eastern Asia (15%, 95% CI 11–21) and was the only regional prevalence estimate that was significantly different from the others. The same pattern is true for HR-HPV; for all ten of the studies from Eastern and South-Eastern Asia, the prevalence of HR-HPV was 10% (95% CI 7–13), below the global pooled HR-HPV prevalence (21%, 18–24; table 2; appendix 3 p 11). Although there were no significant differences, the pooled prevalence for any HPV, HR-HPV, and HPV-16 was slightly higher in high-income countries than in low-income and middle-income countries; for example, the prevalence of any HPV was 34% (95% CI 29–39) in high-income countries and 28% (22–34) in low-income and middle-income countries (table 2).
Figure 2 shows 14 HPV types (ie, the 12 main HR-HPV types, HPV-6, and HPV-11) by region. HPV-16 was the most common HPV type in four of five regions. The exception was Eastern and South-Eastern Asia, where HPV-6 was the most prevalent, although the 95% CIs were wide. The second most common HPV types were HPV-51 in Europe and Northern America, HPV-6 in Sub-Saharan Africa and Latin America and the Caribbean, and HPV-16 in Eastern and South-Eastern Asia.
Studies had a median HR-HPV prevalence of 20% (IQR 12–29; appendix 3 p 8) with significant heterogeneity across the studies (I2=98%, p<0·0001; appendix 3 p 11). No study size effects were observed (appendix 3 p 9), and an influence analysis did not show influential studies in the overall estimate. The Egger test had a p value of 0·92, and the adjusted overall HR-HPV prevalence using both the observed and imputed potentially non-published studies was 24% (95% CI 21–27) versus 21% (18–24) using observed data only (appendix 3 p 16). Similar results were observed for any HPV (I2=99%, p<0·0001), HPV-16 (I2=95%, p<0·0001), and HPV-6 (I2=97%, p=0·027; appendix 3 pp 12–14).
We performed subgroup meta-analyses for nine variables looking at the prevalence of any HPV, HR-HPV, HPV-16, and HPV-6 (appendix 3 p 9). No differences were observed by sample size, risk of bias score, inclusion of men recruited from STI or sexual health clinics, start year of data collection, method of sampling, or age. For any HPV, significant between-group heterogeneity was observed for three variables: Eastern and South-Eastern Asia compared with the other regions (p<0·0001), anatomic sites sampled (p=0·001), and number of HPV types tested in the HPV detection test (p<0·0001). Overall, any HPV prevalence was lower in studies from Eastern and South-Eastern Asia (15%, 95% CI 11–21%) than in studies from other regions (34%, 30–38). Prevalence was significantly greater in studies that sampled at least the penile shaft and the glans penis or coronal sulcus (37%, 95% CI 31–42) than in studies that did not (24%, 20–30). As expected, and as a proxy of the HPV detection method (table 1), the prevalence of any HPV increased with the number of HPV types tested for, from 20% (95% CI 15–25) in studies that tested for eight to 15 HPV types, 25% (18–33) in those that tested for 16 to 26 HPV types, and to 38% (33–43) in those that tested for more than 26 HPV types. After adjusting for geography, anatomical sites sampled, and number of HPV types tested for, any HPV prevalence was 29% (95% CI 26–33). Similar results were observed for HR-HPV and HPV-16. For HR-HPV, after adjusting for geography and anatomical sites sampled prevalence was 19% (95% CI 17–22). For HPV-16, after adjusting for geography, anatomical sites sampled, and number of HPV types included in the assay, prevalence was 5% (95% CI 4–6).
Discussion
We estimated the global pooled prevalence for genital HPV infection among men to be 31% (95% CI 27–35) for any HPV and 21% (18–24) for HR-HPV on the basis of data from 65 studies (including 44 769 men) conducted between Jan 1, 1995, and June 1, 2022.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 HPV-16 was the dominant HPV type (5%, 95% CI 4–7), followed by HPV-6 (4%, 3–5). The age-specific analysis showed a high prevalence of HPV in young adult men, which remained high throughout adulthood. These estimates are consistent with the hypothesis that sexually active men, regardless of age, are at risk of HPV-related morbidity and are a reservoir of sexually transmissible HPV infection.
Globally in men, as in women, the most common oncogenic and preventable HPV type is HPV-16.6 The roll-out of HPV vaccination in young women, and increasingly in young men, is beginning to have a beneficial effect on reducing the prevalence of the specific genotypes targeted by the different HPV vaccines and on HPV-related disease in men and women.91 Three HPV vaccine formulations are available on the market: two-valent (HPV types 16 and 18), four-valent (HPV types 6, 11, 16, and 18), and nine-valent (HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58). All three vaccine formulations target HPV-16 and HPV-18, the two HR-HPV types that are most often associated with HPV-related cancers.6 By 2022, 45 countries were providing HPV vaccination for boys.92, 93 And, as a result of the synergy of the protective effects of vaccinating boys and girls, mathematical modelling studies suggest that the HPV types targeted by the different HPV vaccines could be eliminated from circulation with gender-neutral vaccination.94
HPV prevalence in men peaked in the group aged 25–29 years and remained high until at least age 50 years. Prevalence in the group aged 15–19 years was also high, suggesting that young men are being infected rapidly following first sexual activity. These estimates are consistent with data from studies on the natural history of HPV infection in men, which show stable rates of detection of incident genital HPV across age groups and low seroconversion rates following HPV infection, suggesting that men remain susceptible to HPV infection across the lifespan.95, 96 This age profile of infection in men is different from the profile in women, for whom genital HPV prevalence peaks soon after first sexual activity and declines with age, with a slight rebound after age 50–55 years (ie, often after or around the time of menopause) in some populations.97, 98
Regional pooled prevalence estimates for the five SDG regional groupings with data were similar apart from for Eastern and South-Eastern Asia. Eastern and South-Eastern Asia had the lowest pooled prevalence for both any HPV (15%, 95% CI 11–21) and HR-HPV (10%, 7–13). These results mirror the lower HPV prevalence reported in women in Asia15, 98 and among Asian men compared with other world regions and the global population.99 A prominent presence of HPV-35 in Sub-Saharan Africa was also confirmed (figure 2). HPV-35 has been consistently identified to be more prevalent among African women or women of African descent, with a greater contribution to cancers and precancerous lesions than in women from other regions.15, 100, 101
Our estimates are based on a systematic review of published prevalence surveys and unpublished data shared by study authors. Few studies provide data for heterosexual men (65 studies from 35 countries), whereas the amount of data available for women is much higher, and over half of the studies (55%) included were from high-income countries. To be eligible for inclusion, studies were required to have collected samples in 1995 or later, and only seven (11%) studies included specimens collected in 2014 or later. Therefore, our estimates do not reflect the effect of the roll-out of HPV vaccination in women on the prevalence of HPV in men,38, 55, 91 but the prevalence in general populations of men before HPV-vaccinated cohorts reached adult ages, either with girls-only or gender-neutral strategies.
One of the major efforts made when selecting studies was to select studies that could be viewed as representative of the general male population of the country. There was high heterogeneity observed in HR-HPV prevalence in the studies, with values ranging from 1% to 75% (appendix 3 p 9). High heterogeneity has also been noted in studies in women.15 We excluded studies done exclusively, or primarily, in men who have sex with men, men living with HIV, and men with signs or symptoms of HPV-related disease and studies conducted solely in men who were circumcised. These men, however, are included in many of the studies as part of the general population. Regarding including studies conducted in STI clinics, as long as the study population met the selection criteria (appendix 3 p 2), our sensitivity analyses (appendix 3 p 9) identified no significant differences in HPV prevalence between study settings (STI clinic vs others) or differences by risk of bias. Most studies combined samples from more than one anatomical location; 49% of studies included at least the penile shaft and the glans penis or coronal sulcus as recommended19 (table 1, appendix 3 p 9). We were limited in our ability to compare data across studies by differences in the numbers of HPV types tested for and specific anatomical sites sampled (appendix 3 p 9) and were not able to evaluate HPV prevalence by circumcision status, as only 26 of 65 studies provided stratified data by this variable. We could not evaluate the sensitivity of HPV sampling of abraded skin, the yield from specific genital locations, or sampling at more than one timepoint to discern contamination versus sustained infection. Despite these limitations, the pooled estimates serve to establish mean benchmark prevalences that can inform prevention strategies.
Global and regional pooled estimates provide us with mean baseline values for HPV prevalence in general populations of men. Our study draws attention to the high prevalence, ranging from 20% to 30% for HR-HPV in men across most regions, and the need for strengthening HPV prevention within overall STI control efforts. It also emphasises the scarcity of HPV data among men from some parts of the world and the importance of expanding HPV prevalence surveys in these areas to inform and measure the effects of prevention efforts. Incorporating HPV vaccination for adolescent males into national immunisation schedules can be further considered as vaccine supplies allow and single-dose strategies are assessed.102 Future epidemiological studies are needed to monitor trends in prevalence in men, especially considering the roll-out of HPV vaccination in girls and young women and that many countries are beginning to vaccinate boys.
Data sharing
All datasets generated and analysed, including the search strategy, list of the included and excluded studies, data extracted, and quality assessment, are available in the Article and on request from the corresponding author. On request, the statistical source code is available in the IDIBELL repository.
For the IDIBELL repository see https://repository.idibell.cat
Declaration of interests
The Cancer Epidemiology Research Program (with which LB, LA, and GA are affiliated) has received sponsorship for grants from Merck and HPV test kits at no cost from Roche for research purposes. ARG reports payments from Merck to her institution, and as consulting fees to herself, and payments from Merck for participation on an advisory board. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was partially supported by a grant from the Instituto de Salud Carlos III through the project PI18/01137 and PI21/00982. The Agency for Management of Universities and Research Grants, Secreteriat for Universities, and Research of the Department of Business and Knowledge of the Government of Catalonia supported the activities of our research group through the Support for Research Groups grants in 2017–19 and 2021–23 (grant numbers 2017SGR1718, 2017SGR1085, 2021SGR01029, and 2021SGR01354). LB and MA received support from the EU's Horizon 2020 research and innovation programme under grant agreement number 847845, via the Risk-based Strategy for Cervical Cancer Screening Network. We thank the Centres de Recerca de Catalunya Program of the Generalitat de Catalunya for institutional support. None of these entities played a role in data collection or analysis, or in the interpretation of the results. This work was also supported by both the Department of Sexual and Reproductive Health and Research (WHO) and the US Centers for Disease Control and Prevention. We thank all authors of the publications included in the meta-analysis and the authors of publications listed in table 1, who extracted additional information from the original data files. We also thank Xin Wang, who helped with extracting data from publications in Chinese, and Victoria Nyaga, who developed the statistical packages metaprop and metapreg. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published tables, text, and figures.
Contributors
GA, LB, JR, and MT designed the study and did the literature searches. GA, LB, and JR designed the data-extraction form. JR extracted the data. GA, LB, and MT cross-checked the data extraction. GA did the statistical analyses. LB supervised the statistical analyses. MA conceptualised the statistical packages metaprop and metapreg. GA, LB, JR, and MT wrote the manuscript. All authors critically revised subsequent drafts and read and approved the submitted version. GA and JR accessed and verified the data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660–664. doi: 10.1097/OLQ.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AR, Anic G, Nyitray AG. Epidemiology and pathology of HPV disease in males. Gynecol Oncol. 2010;117(suppl):S15–S19. doi: 10.1016/j.ygyno.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sanjosé S, Serrano B, Tous S, et al. Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2019;2 doi: 10.1093/jncics/pky045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga S, Wang X, Luttropp K, et al. Global burden of HPV-related cancers in men: a systematic literature review. J Clin Oncol. 2019;37 [Google Scholar]
- 8.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 9.Smith JS, Gilbert PA, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of human papillomavirus infection in males: a global review. J Adolesc Health. 2011;48:540–552. doi: 10.1016/j.jadohealth.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Álvarez MI, Gómez-Urquiza JL, Husein-El Ahmed H, Albendín-García L, Gómez-Salgado J, Cañadas-De la Fuente GA. Prevalence and risk factors of human papillomavirus in male patients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebnes JB, Olesen TB, Duun-Henriksen AK, Munk C, Norrild B, Kjaer SK. Prevalence of genital human papillomavirus among men in Europe: systematic review and meta-analysis. J Sex Med. 2014;11:2630–2644. doi: 10.1111/jsm.12652. [DOI] [PubMed] [Google Scholar]
- 12.Olesen TB, Munk C, Christensen J, Andersen KK, Kjaer SK. Human papillomavirus prevalence among men in sub-Saharan Africa: a systematic review and meta-analysis. Sex Transm Infect. 2014;90:455–462. doi: 10.1136/sextrans-2013-051456. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 15.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro SB, Laurie C, El-Zein M, Franco EL. Association between male circumcision and human papillomavirus infection in males and females: a systematic review, meta-analysis, and meta-regression. Clin Microbiol Infect. 2023;29:968–978. doi: 10.1016/j.cmi.2023.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Albero G, Castellsagué X, Giuliano AR, Bosch FX. Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sex Transm Dis. 2012;39:104–113. doi: 10.1097/OLQ.0b013e3182387abd. [DOI] [PubMed] [Google Scholar]
- 18.Castellsagué X, Bosch FX, Muñoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AR, Nielson CM, Flores R, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007;196:1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Agbor VN, Takah NF, Aminde LN. Prevalence and factors associated with medication adherence among patients with hypertension in sub-Saharan Africa: protocol for a systematic review and meta-analysis. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UN Department of Economic and Social Affairs. Statistics Division SDG indicators. Regional groupings used in report and statistical annex. 2016. https://unstats.un.org/sdgs/indicators/regional-groups
- 23.World Bank World Bank country and lending groups. 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 24.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyaga VN. METAPREG: Stata module to compute fixed and random effects meta-analysis and meta-regression of proportions. 2019. https://ideas.repec.org/c/boc/bocode/s458693.html
- 26.Afonso LA, Rocha WM, Carestiato FN, et al. Human papillomavirus infection among sexual partners attending a sexually transmitted disease clinic in Rio de Janeiro, Brazil. Braz J Med Biol Res. 2013;46:533–538. doi: 10.1590/1414-431X20132519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afonso LA, Cordeiro TI, Carestiato FN, Ornellas AA, Alves G, Cavalcanti SMB. High risk human papillomavirus infection of the foreskin in asymptomatic men and patients with phimosis. J Urol. 2016;195:1784–1789. doi: 10.1016/j.juro.2015.12.096. [DOI] [PubMed] [Google Scholar]
- 28.Álvarez-Argüelles ME, Melón S, Junquera ML, et al. Human papillomavirus infection in a male population attending a sexually transmitted infection service. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auvert B, Lissouba P, Cutler E, Zarca K, Puren A, Taljaard D. Association of oncogenic and nononcogenic human papillomavirus with HIV incidence. J Acquir Immune Defic Syndr. 2010;53:111–116. doi: 10.1097/QAI.0b013e3181b327e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin SB, Wallace DR, Papenfuss MR, et al. Human papillomavirus infection in men attending a sexually transmitted disease clinic. J Infect Dis. 2003;187:1064–1070. doi: 10.1086/368220. [DOI] [PubMed] [Google Scholar]
- 31.Bartoletti R, Cai T, Meliani E, et al. Human papillomavirus infection is not related with prostatitis-related symptoms: results from a case-control study. Int Braz J Urol. 2014;40:247–256. doi: 10.1590/S1677-5538.IBJU.2014.02.16. [DOI] [PubMed] [Google Scholar]
- 32.Benzaken A, Sabidó M, Galban E, Rodrigues Dutra DL, Leturiondo AL, Mayaud P. HIV and sexually transmitted infections at the borderlands: situational analysis of sexual health in the Brazilian Amazon. Sex Transm Infect. 2012;88:294–300. doi: 10.1136/sextrans-2011-050309. [DOI] [PubMed] [Google Scholar]
- 33.Bleeker MCG, Hogewoning CJA, Voorhorst FJ, et al. HPV-associated flat penile lesions in men of a non-STD hospital population: less frequent and smaller in size than in male sexual partners of women with CIN. Int J Cancer. 2005;113:36–41. doi: 10.1002/ijc.20502. [DOI] [PubMed] [Google Scholar]
- 34.Bosnjak Z, Perić M, Krizan IR, et al. Prevalence and genotype distribution of high-risk human papillomavirus (HR HPV) in male genital samples of Osijek-Baranja County. Coll Antropol. 2013;37:1203–1208. [PubMed] [Google Scholar]
- 35.Cuschieri K, Nandwani R, McGough P, et al. Urine testing as a surveillance tool to monitor the impact of HPV immunization programs. J Med Virol. 2011;83:1983–1987. doi: 10.1002/jmv.22183. [DOI] [PubMed] [Google Scholar]
- 36.Edna Omar V, Orvalho A, Nália I, et al. Human papillomavirus prevalence and genotype distribution among young women and men in Maputo city, Mozambique. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Zein M, Coutlée F, Tellier P-P, Roger M, Franco EL, Burchell AN. Human papillomavirus infection and transmission among couples through heterosexual activity (HITCH) cohort study: protocol describing design, methods, and research goals. JMIR Res Protoc. 2019;8 doi: 10.2196/11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013–2014. J Infect Dis. 2017;215:1070–1079. doi: 10.1093/infdis/jix057. [DOI] [PubMed] [Google Scholar]
- 39.Golob B, Poljak M, Verdenik I, Kolbezen Simoniti M, Vrtačnik Bokal E, Zorn B. High HPV infection prevalence in men from infertile couples and lack of relationship between seminal HPV infection and sperm quality. BioMed Res Int. 2014;2014 doi: 10.1155/2014/956901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guzmán P, Ili C, Rifo P, et al. Prevalence of human papillomavirus genital infection among male university students. Rev Med Chil. 2008;136:1381–1389. doi: 10.4067/s0034-98872008001100003. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 41.He Z, Liu Y, Sun Y, et al. Human papillomavirus genital infections among men, China, 2007–2009. Emerg Infect Dis. 2013;19:992–995. doi: 10.3201/eid1906.111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hebnes JB, Munk C, Nøhr B, et al. Human papillomavirus infection among 2460 men in Denmark: prevalence in relation to age using 2 human papillomavirus DNA testing methods. Sex Transm Dis. 2015;42:463–467. doi: 10.1097/OLQ.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–794. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez BY, Wilkens LR, Unger ER, et al. Evaluation of genital self-sampling methods for HPV detection in males. J Clin Virol. 2013;58:168–175. doi: 10.1016/j.jcv.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 45.Jalal H, Stephen H, Bibby DF, Sonnex C, Carne CA. Molecular epidemiology of genital human papillomavirus and Chlamydia trachomatis among patients attending a genitourinary medicine clinic—will vaccines protect? Int J STD AIDS. 2007;18:617–621. doi: 10.1258/095646207781568501. [DOI] [PubMed] [Google Scholar]
- 46.Jaworek H, Koudelakova V, Oborna I, et al. Prevalence and genotype distribution of human papillomavirus in Czech non-vaccinated heterosexual couples. Virol J. 2021;18:80. doi: 10.1186/s12985-021-01551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kero K, Rautava J, Syrjänen K, Grenman S, Syrjänen S. Human papillomavirus genotypes in male genitalia and their concordance among pregnant spouses participating in the Finnish Family HPV study. J Sex Med. 2011;8:2522–2531. doi: 10.1111/j.1743-6109.2011.02378.x. [DOI] [PubMed] [Google Scholar]
- 48.Khoo SP, Shafii MKA, Bhoo-Pathy N, et al. Prevalence and sociodemographic correlates of anogenital human papillomavirus (HPV) carriage in a cross-sectional, multi-ethnic, community-based Asian male population. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kjaer SK, Munk C, Winther JF, Jørgensen HO, Meijer CJLM, van den Brule AJC. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14:1528–1533. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- 50.Koene F, Wolffs P, Brink A, et al. Comparison of urine samples and penile swabs for detection of human papillomavirus in HIV-negative Dutch men. Sex Transm Infect. 2016;92:467–469. doi: 10.1136/sextrans-2015-052054. [DOI] [PubMed] [Google Scholar]
- 51.Lajous M, Mueller N, Cruz-Valdéz A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–1716. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 52.Lazcano-Ponce E, Herrero R, Muñoz N, et al. High prevalence of human papillomavirus infection in Mexican males: comparative study of penile-urethral swabs and urine samples. Sex Transm Dis. 2001;28:277–280. doi: 10.1097/00007435-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Luttmer R, Dijkstra MG, Snijders PJF, et al. Presence of human papillomavirus in semen of healthy men is firmly associated with HPV infections of the penile epithelium. Fertil Steril. 2015;104:838–844. doi: 10.1016/j.fertnstert.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Ma D-M, Sun M-X, Li X-Y, et al. Distribution of high-risk human papillomavirus genotypes in male attendees at a clinic for sexually transmitted infections in Northern China. Eur Rev Med Pharmacol Sci. 2019;23:9714–9720. doi: 10.26355/eurrev_201911_19533. [DOI] [PubMed] [Google Scholar]
- 55.Machalek DA, Chow EPF, Garland SM, et al. Human papillomavirus prevalence in unvaccinated heterosexual men after a national female vaccination program. J Infect Dis. 2017;215:202–208. doi: 10.1093/infdis/jiw530. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzawa Y, Kitamura T, Suzuki M, Koyama Y, Shigehara K. Prevalence, genotype distribution, and predictors against HPV infections targeted by 2-, 4-, 9-valent HPV vaccines among Japanese males. Vaccines (Basel) 2020;8:221. doi: 10.3390/vaccines8020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mbulawa ZZA, Marais DJ, Johnson LF, Boulle A, Coetzee D, Williamson A-L. Influence of human immunodeficiency virus and CD4 count on the prevalence of human papillomavirus in heterosexual couples. J Gen Virol. 2010;91:3023–3031. doi: 10.1099/vir.0.020669-0. [DOI] [PubMed] [Google Scholar]
- 58.Menezes W, Ceperuelo DL, Cordeiro TI, et al. Human papillomavirus infection in healthy men from Rio de Janeiro, Brazil. J Bras Doenças Sex Transm. 2014;26:21–24. [Google Scholar]
- 59.Nelson LE, Tharao W, Husbands W, et al. The epidemiology of HIV and other sexually transmitted infections in African, Caribbean and Black men in Toronto, Canada. BMC Infect Dis. 2019;19:294. doi: 10.1186/s12879-019-3925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng'ayo MO, Bukusi E, Rowhani-Rahbar A, et al. Epidemiology of human papillomavirus infection among fishermen along Lake Victoria Shore in the Kisumu District, Kenya. Sex Transm Infect. 2008;84:62–66. doi: 10.1136/sti.2007.027508. [DOI] [PubMed] [Google Scholar]
- 61.Nielson CM, Flores R, Harris RB, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev. 2007;16:1107–1114. doi: 10.1158/1055-9965.EPI-06-0997. [DOI] [PubMed] [Google Scholar]
- 62.Ogilvie GS, Taylor DL, Achen M, Cook D, Krajden M. Self-collection of genital human papillomavirus specimens in heterosexual men. Sex Transm Infect. 2009;85:221–225. doi: 10.1136/sti.2008.033068. [DOI] [PubMed] [Google Scholar]
- 63.Olesen TB, Iftner T, Mwaiselage J, et al. Prevalence and type distribution of human papillomavirus among 1813 men in Tanzania and the relationship to HIV status. Sex Transm Dis. 2013;40:592–598. doi: 10.1097/OLQ.0b013e31828fcf57. [DOI] [PubMed] [Google Scholar]
- 64.Parada R, Morales R, Giuliano AR, Cruz A, Castellsagué X, Lazcano-Ponce E. Prevalence, concordance and determinants of human papillomavirus infection among heterosexual partners in a rural region in central Mexico. BMC Infect Dis. 2010;10:223. doi: 10.1186/1471-2334-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 66.Ramogola-Masire D, McClung N, Mathoma A, et al. Human papillomavirus prevalence in male and female university students in Gaborone, Botswana. Epidemiol Infect. 2022;150:1–25. doi: 10.1017/S0950268822000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenblatt C, Lucon AM, Pereyra EA, Pinotti JA, Arap S, Ruiz CA. HPV prevalence among partners of women with cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2004;84:156–161. doi: 10.1016/j.ijgo.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Rositch AF, Poole C, Hudgens MG, et al. Multiple human papillomavirus infections and type competition in men. J Infect Dis. 2012;205:72–81. doi: 10.1093/infdis/jir709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Alemán MA, Uribe-Salas F, Conde-González CJ. Human papillomavirus infection, a possible biological marker of sexual behavior among university students. Salud Publica Mex. 2002;44:442–447. (in Spanish). [PubMed] [Google Scholar]
- 70.Shin H-R, Franceschi S, Vaccarella S, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–476. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 71.Smelov V, Eklund C, Bzhalava D, Novikov A, Dillner J. Expressed prostate secretions in the study of human papillomavirus epidemiology in the male. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Söderlund-Strand A, Wikström A, Dillner J. Evaluation of human papillomavirus DNA detection in samples obtained for routine Chlamydia trachomatis screening. J Clin Virol. 2015;64:88–91. doi: 10.1016/j.jcv.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Sudenga SL, Torres BN, Silva R, et al. Comparison of the natural history of genital HPV infection among men by country: Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:1043–1052. doi: 10.1158/1055-9965.EPI-17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takahashi S, Shimizu T, Takeyama K, et al. Detection of human papillomavirus DNA on the external genitalia of healthy men and male patients with urethritis. Sex Transm Dis. 2003;30:629–633. doi: 10.1097/01.OLQ.0000085184.68124.B3. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S, Takeyama K, Miyamoto S, et al. Incidence of sexually transmitted infections in asymptomatic healthy young Japanese men. J Infect Chemother. 2005;11:270–273. doi: 10.1007/s10156-005-0411-1. [DOI] [PubMed] [Google Scholar]
- 76.Tang X, Xu A-E, Dong X-P, Sun X-K, Shen H, Liu J-F. Epidemiological investigation of human papillomavirus infection in men attending a sexually transmitted disease clinic in Hangzhou area. Biomed Environ Sci. 2006;19:153–157. [PubMed] [Google Scholar]
- 77.Tobian AAR, Grabowski MK, Kigozi G, et al. High-risk human papillomavirus prevalence is associated with HIV infection among heterosexual men in Rakai, Uganda. Sex Transm Infect. 2013;89:122–127. doi: 10.1136/sextrans-2012-050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaccarella S, Lazcano-Ponce E, Castro-Garduño JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- 79.Söderlund-Strand A, Dillner J. High-throughput monitoring of human papillomavirus type distribution. Cancer Epidemiol Biomarkers Prev. 2013;22:242–250. doi: 10.1158/1055-9965.EPI-12-1003. [DOI] [PubMed] [Google Scholar]
- 80.Vardas E, Giuliano AR, Goldstone S, et al. External genital human papillomavirus prevalence and associated factors among heterosexual men on 5 continents. J Infect Dis. 2011;203:58–65. doi: 10.1093/infdis/jiq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veldhuijzen NJ, Dhont N, Vyankandondera J, et al. Prevalence and concordance of HPV, HIV, and HSV-2 in heterosexual couples in Kigali, Rwanda. Sex Transm Dis. 2012;39:128–135. doi: 10.1097/OLQ.0b013e3182367c4c. [DOI] [PubMed] [Google Scholar]
- 82.Vera-Uehara C, Sánchez-Alemán MA, Uribe-Salas FJ, Ramos-Castañeda J, Olamendi-Portugal ML, Conde-Glez CJ. HPV infection, risk factors and viral load among Mexican male college students. Braz J Infect Dis. 2014;18:71–76. doi: 10.1016/j.bjid.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vriend HJ, Bogaards JA, van der Klis FRM, et al. Patterns of human papillomavirus DNA and antibody positivity in young males and females, suggesting a site-specific natural course of infection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H, Zhao J, Liu X, Yan W, Li G, Yuan Y. The prevalence and genotype distribution of human papillomaviruses among men in Henan province of China. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.676401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–685. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 86.Wei F, Yin K, Wu X, et al. Human papillomavirus prevalence and associated factors in women and men in south China: a population-based study. Emerg Microbes Infect. 2016;5:e119. doi: 10.1038/emi.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wendland EM, Villa LL, Unger ER, et al. Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: the POP-Brazil study. Sci Rep. 2020;10 doi: 10.1038/s41598-020-61582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Widdice LE, Bernstein DI, Franco EL, et al. Decline in vaccine-type human papillomavirus prevalence in young men from a Midwest metropolitan area of the United States over the six years after vaccine introduction. Vaccine. 2019;37:6832–6841. doi: 10.1016/j.vaccine.2019.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wikström A, Popescu C, Forslund O. Asymptomatic penile HPV infection: a prospective study. Int J STD AIDS. 2000;11:80–84. doi: 10.1177/095646240001100203. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R-J, Jiang Z-X, Lin N, et al. Prevalence of human papilloma virus infection and its risk factors in some rural areas of Jiangsu, China. Zhonghua Nan Ke Xue. 2018;24:795–801. (in Chinese). [PubMed] [Google Scholar]
- 91.Drolet M, Bénard É, Pérez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi: 10.1016/S0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruni L, Saura-Lázaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 93.WHO Human papillomavirus (HPV) vaccination coverage dashboard. 2022. https://immunizationdata.who.int/pages/coverage/hpv.html
- 94.Vänskä S, Luostarinen T, Baussano I, et al. Vaccination with moderate coverage eradicates oncogenic human papillomaviruses if a gender-neutral strategy is applied. J Infect Dis. 2020;222:948–956. doi: 10.1093/infdis/jiaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ingles DJ, Lin H-Y, Fulp WJ, et al. An analysis of HPV infection incidence and clearance by genotype and age in men: the HPV Infection in Men (HIM) study. Papillomavirus Res. 2015;1:126–135. doi: 10.1016/j.pvr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giuliano AR, Viscidi R, Torres BN, et al. Seroconversion following anal and genital HPV infection in men: the HIM study. Papillomavirus Res. 2015;1:109–115. doi: 10.1016/j.pvr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 98.Campos NG, Demarco M, Bruni L, et al. A proposed new generation of evidence-based microsimulation models to inform global control of cervical cancer. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2021.106438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akogbe GO, Ajidahun A, Sirak B, et al. Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States. Int J Cancer. 2012;131:E282–E291. doi: 10.1002/ijc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 101.Pinheiro M, Gage JC, Clifford GM, et al. Association of HPV35 with cervical carcinogenesis among women of African ancestry: evidence of viral-host interaction with implications for disease intervention. Int J Cancer. 2020;147:2677–2686. doi: 10.1002/ijc.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.WHO Human papillomavirus vaccines: WHO position paper (2022 update) Dec 16, 2022. https://www.who.int/publications/i/item/who-wer9750-645-672
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated and analysed, including the search strategy, list of the included and excluded studies, data extracted, and quality assessment, are available in the Article and on request from the corresponding author. On request, the statistical source code is available in the IDIBELL repository.
For the IDIBELL repository see https://repository.idibell.cat