Abstract
Key message
This review summarizes the molecular basis and emerging applications of developmental regulatory genes and nanoparticles in plant transformation and discusses strategies to overcome the obstacles of genotype dependency in plant transformation.
Abstract
Plant transformation is an important tool for plant research and biotechnology-based crop breeding. However, Plant transformation and regeneration are highly dependent on species and genotype. Plant regeneration is a process of generating a complete individual plant from a single somatic cell, which involves somatic embryogenesis, root and shoot organogeneses. Over the past 40 years, significant advances have been made in understanding molecular mechanisms of embryogenesis and organogenesis, revealing many developmental regulatory genes critical for plant regeneration. Recent studies showed that manipulating some developmental regulatory genes promotes the genotype-independent transformation of several plant species. Besides, nanoparticles penetrate plant cell wall without external forces and protect cargoes from degradation, making them promising materials for exogenous biomolecule delivery. In addition, manipulation of developmental regulatory genes or application of nanoparticles could also bypass the tissue culture process, paving the way for efficient plant transformation. Applications of developmental regulatory genes and nanoparticles are emerging in the genetic transformation of different plant species. In this article, we review the molecular basis and applications of developmental regulatory genes and nanoparticles in plant transformation and discuss how to further promote genotype-independent plant transformation.
Keywords: Developmental regulatory genes, Genotype-independent transformation, Nanoparticles, Root organogenesis, Shoot organogenesis, Somatic embryogenesis
Introduction
Plant transformation is a method that delivers foreign DNA into regeneration-competent cells through the Agrobacterium-mediated method, biolistic (also called particle bombardment), pollen tube transformation, electroporation and so on. Among them, Agrobacterium-mediated and biolistic methods are the most common plant transformation methods (An et al. 2019, 2020; Zhang et al. 2018, 2021b; Zhu et al. 2020). Regenerative cells can derive from the proliferation of undifferentiated meristem cells of explants and then develop into intact plants through direct organogenesis. However, in many plants, regenerative cells are derived from the reprogramming of differentiated somatic cells and regain the ability for proliferation competence through dedifferentiation. Regenerative cells derived in this way develop into intact plants by de novo organogenesis and somatic embryogenesis (Feher 2019; Gaillochet and Lohmann 2015; Ikeuchi et al. 2016; Steward et al. 1958; Sugimoto et al. 2011; Xu and Hu 2020). Direct organogenesis regenerates adventitious shoots or roots directly from explants, while indirect organogenesis requires induction of pluripotent non-embryonic callus on the callus-inducing medium (CIM), and the callus then develops into adventitious shoots or roots. The callus is a highly heterogeneous group of cells, and its organized structure resembles lateral root primordia (Atta et al. 2009). Somatic embryo regeneration, namely somatic embryogenesis, depends on the totipotency of plant cells (Feher 2019). Somatic embryogenesis can induce somatic embryos directly without an intermediate embryonic callus or indirectly following an embryonic callus stage. In plant research, both plant genetic engineering and genome editing technologies that promote functional genomic research and accelerate crop trait improvement greatly depend on plant transformation (Altpeter et al. 2016; Fang et al. 2022; Hou et al. 2022a; Jiang et al. 2021; Liu et al. 2022a, b; Wei et al. 2022, 2023). However, plant transformation and regeneration rely highly on species and genotype, which are major limiting factors for developing and applying genetic engineering and genome editing technologies.
Since various factors influence callus formation and regeneration, successful plant transformation has to optimize several external factors such as explant types, pH, and basal media composition. Considering that most regeneration initiates from the cut place, the wound stress may be a trigger of plant regeneration (Ikeuchi et al. 2013). Recent studies showed that hormones and developmental regulatory genes play critical roles in callus induction and plant regeneration. Exogenous hormones induce callus formation in aerial explants with the elimination of leaf identity (He et al. 2012; Lee and Seo 2018). Then, developmental regulatory genes regulate de novo shoot and root regeneration in root and aerial explants (Kareem et al. 2015; Liu et al. 2014). In aerial explant-initiated plant regeneration, the elimination of leaf identity is primarily achieved through epigenetic regulation (He et al. 2012; Lee and Seo 2018). Overall, wounds, hormones, developmental regulatory genes, and epigenetic modifications are essential factors for plant regeneration. Recent studies showed the successful transformation of recalcitrant species through manipulating developmental regulatory genes (Aregawi et al. 2022; Hoerster et al. 2020; Lowe et al. 2018). Alternatively, nanoparticles (NPs) could penetrate the plant cell wall without external force and can be broadly applied to different plant species. In addition, nanomaterials (NMs) can protect cargoes from degradation and reach previously inaccessible plant tissues, cellular and subcellular locations. All these properties make NPs promising materials for exogenous biomolecule delivery and several recent studies showed the successful usage of NPs to deliver genes into plant cells for genetic engineering and genome editing (Demirer et al. 2019a; Kwak et al. 2019; Wang et al. 2020).
In this review, we performed a bibliometric analysis of 2414 publications selected by the related searches of plant transformation methods to gain a brief overview of the research history and status. We summarize and discuss the molecular basis of wounds, hormones, developmental regulatory genes, and epigenetic modifications in plant regeneration and the application of developmental regulatory genes in plant transformation. In addition, we also summarize the uptake and translocation of recently emerged NPs in plant cells and their application in plant transformation. Finally, we discuss the potential for genotype-independent plant transformation based on these advances.
A brief overview of plant transformation methods
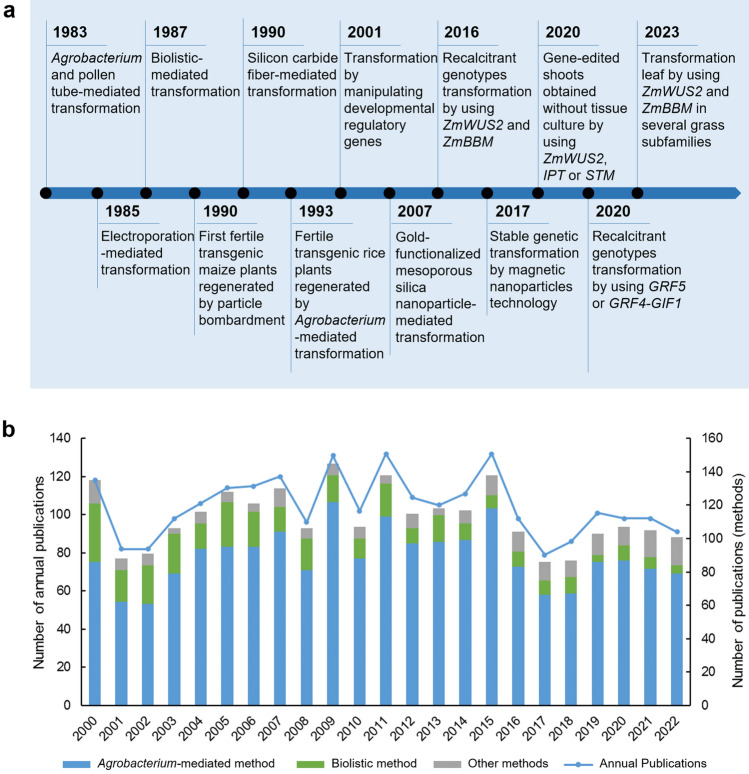
The mechanism of Agrobacterium-mediated plant transformation is the transfer of foreign genes carried between the Ti plasmid T-DNA boundaries to the plant cell nucleus and subsequent transient transgene expression or integration into the plant genome. In 1983, with the successful regeneration of the transgenic Nicotiana tabacum transformed using the Agrobacterium-mediated method, the “starting line” was drawn for plant transformation (Fraley et al. 1983; Herrera-Estrella et al. 1983). Meanwhile, a study reported that a pollen tube-mediated method could successfully transform Gossypium hirsutum (Zhou et al. 1983). From then, leaf discs of a wide range of dicotyledon plants were successfully infected by Agrobacterium (Horsch et al. 1985). However, monocots, particularly the graminaceous crops, cannot be infected for a long time via this method. Other methods, such as biolistic, electroporation and silicon carbide fiber-mediated methods, are developed and applied to monocot transformation (Fromm et al. 1985; Kaeppler et al. 1990; Klein et al. 1987). In 1990, the first fertile transgenic Zea mays plants transformed by the biolistic method were regenerated (Gordon-Kamm et al. 1990). This method can deliver biomolecules to a broader range of plant species, but plant tissue is often damaged under high bombardment pressures. In addition, biolistic technology requires specialized equipment, which limits its wide application. It is necessary to develop Agrobacterium infecting method for monocots because the Agrobacterium-mediated method is easy to perform, low cost, and shows higher transformation efficiency than other methods. Several studies found that monocots can not produce enough inducers, such as phenolic compounds, at injury sites (Stachel et al. 1985). This could be the reason for the recalcitrance to Agrobacterium infection as these compounds are required for the activation of virulence (Vir) genes on the Ti plasmid, which is needed for inducing plant tumor production (Stachel et al. 1985). Indeed, transgenic Oryza sativa is obtained through Agrobacterium-mediated infection of immature embryos with the addition of phenolic compounds (Chan et al. 1993). These landmark events in plant transformation are illustrated in Fig. 1A. These methods were widely used in plant transformation from 2000 to 2022, among which the Agrobacterium-mediated method is the most used, followed by the biolistic method (Fig. 1B). However, the bottleneck of plant transformation and regeneration is species and genotype dependence.
Fig. 1.
Landmark events and related publications of plant transformation. a Timeline of landmark events in plant transformation. b Publications related to plant transformation from 2000 to 2022. Publications on seven plant transformation methods, including the Agrobacterium-mediated method, biolistic, pollen tube-mediated method, electroporation, silicon carbide fiber-mediated method, polyethylene glycol-mediated method, and nanoparticles delivery
Several studies have recently attempted to transform recalcitrant plant species and genotypes. For example, a method called in planta particle bombardment (iPB) is developed for Triticum aestivum transformation (Hamada et al. 2018). The iPB method is used to deliver Cas9/gRNA plasmids to shoot apical meristem (SAM) of imbibed seeds and regenerated genome-edited plants (Hamada et al. 2018). This method makes it possible to transform other recalcitrant plant species. Agrobacterium-mediated Vigna unguiculata embryonic axis transformation achieved transformation frequencies between 4 and 37% in many genotypes (Che et al. 2021). In addition, SAM cells were used as explants to transform recalcitrant G. hirsutum genotypes and successfully obtained transgenic plants (Ge et al. 2023). However, few studies showed the use of SAM or embryonic axis as explants to promote genotype-independent transformation. Another strategy for transforming recalcitrant species and genotypes is manipulating developmental regulatory genes and this strategy is widely used in genotype-independent plant transformation (Aregawi et al. 2022; Hoerster et al. 2020; Lowe et al. 2018). Recently, several plant species have achieved stable genetic transformation via magnetic nanoparticles (MNPs) technology (Wang et al. 2022b; Zhao et al. 2017). In the following sections, we will discuss in detail the mechanism and application of developmental regulatory genes and NPs to promote genotype-independent plant transformation.
Molecular basis of somatic embryogenesis, root and shoot organogeneses
Intact plant regeneration from a single somatic cell has to experience somatic embryogenesis, root and shoot organogeneses, and these processes require proper in vitro conditions and involve complicated in vivo signaling and transcriptional networks triggered or regulated by wounds, hormones, developmental regulatory genes and epigenetic reprogramming. Factors affecting somatic embryogenesis, root and shoot organogeneses and their molecular basis are summarized in the succeeding texts.
Molecular basis of somatic embryogenesis
Somatic embryogenesis occurs in many plant species when they are incubated on an auxin-containing medium and then transferred to an auxin-free medium (Ikeda-Iwai et al. 2002; Lu et al. 1983; Wernicke and Brettell 1980). During indirect somatic embryogenesis, embryonic callus formation is first activated on an auxin-rich medium (Ikeda-Iwai et al. 2002). The subsequent absence of auxin in the medium leads to the de novo establishment of auxin gradients in the embryonic callus (Fig. 2A). The gradient auxin distribution initiates a developmental program similar to zygotic embryogenesis, possibly activating the auxin transporter PIN-PORMED1 (PIN1) polar localization (Liu et al. 1993; Su et al. 2009). WUSCHEL (WUS), which determines stem cell fate in SAM, is induced by the established auxin gradient and polar auxin transport, and promotes somatic embryogenesis (Su et al. 2009).
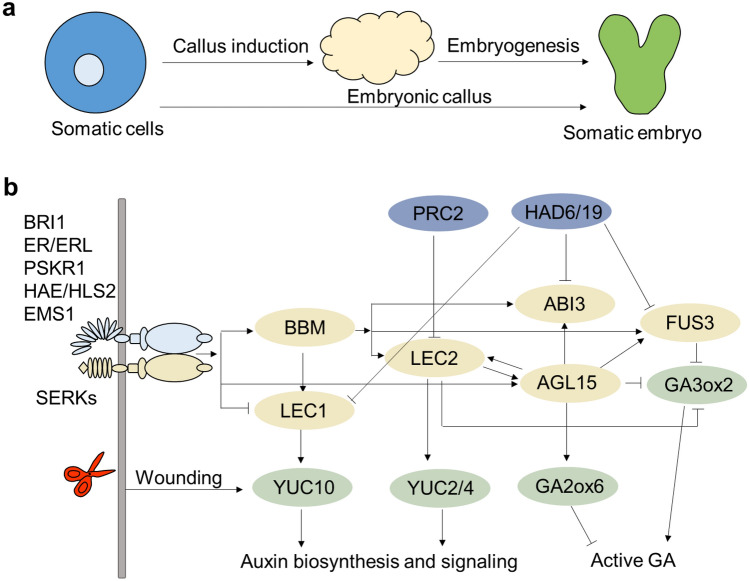
Fig. 2.
Schematic representation and molecular regulatory network of somatic embryogenesis. a Schematic representation of somatic embryogenesis. Somatic embryos can be induced through somatic embryogenesis directly or indirectly. In indirect induction, embryonic callus is induced from plant somatic cells on the callus-inducing medium (CIM), and then somatic embryo formation. b Molecular regulatory network of somatic embryogenesis. Developmental regulatory genes are in yellow; hormone biosynthesis- and signaling-related genes are in green and epigenetic modification-related genes are in blue. Arrows and bar-head arrows represent activation and repression, respectively (color figure online)
Since most regeneration occurs at wounded loci, wound stress has long been considered a trigger for plant regeneration (Ikeuchi et al. 2013). Wound stress is perceived via damage-associated molecular modules, including cell wall-derived oligogalacturonic acid (Bishop et al. 1981) and extracellular adenosine triphosphate (ATP) (Choi et al. 2014; Tanaka et al. 2014). The ATP is released as a danger signal during plant damage, inducing cytoplasmic calcium signaling and a burst of reactive oxygen species (Choi et al. 2014; Tanaka et al. 2014). The local wound signals are further translated into electrical signals, such as cation channel GLUTAMATE RECEPTOR-LIKEs, which are transmitted to other parts of the plant to induce epigenetic modifications, transcriptional changes and phytohormone synthesis (Ikeuchi et al. 2017; Mousavi et al. 2013).
Somatic embryogenesis receptor-like kinase1 (SERK1) is a Leu-rich repeat (LRR) transmembrane receptor-like kinase (PLK) that might co-regulate the plant differentiation process with other specific receptor-like kinases. Ectopic expression of SERK1 has been taken as a strategy for improving the somatic embryogenesis efficiency of Coffea canephora (Perez-Pascual et al. 2018), Arabidopsis thaliana (Hecht et al. 2001), and O. sativa (Hu et al. 2005). SERK1 regulates somatic embryogenesis by activation of auxin biosynthesis, auxin transport, and probably also auxin perception, leading to the expression of early-stage homeotic genes, including WUS, the AP2/ERF transcription factor Baby boom (BBM) and the MADS-box transcription factor Agamous-like15 (AGL15), and the repression of late-stage homeotic genes such as Leafy cotyledon1 (LEC1) (Perez-Pascual et al. 2018). The BBM, LEC1, LEC2, and AGL15 transcription factors play essential roles in early embryogenesis. LEC1 and LEC2, as well as two other transcription factors, Abscisic acid insensitive3 (ABI3) and FUSCA3 (FUS3) are up-regulated by BBM in somatic embryogenesis (Horstman et al. 2017). In addition, LEC2 rapidly activates the expression of AGL15 (Braybrook et al. 2006). Interestingly, LEC2, FUS3, and ABI3 were identified as direct target genes of AGL15 (Zheng et al. 2009). These data suggest that feedback regulation exists in gene regulatory networks during embryogenesis. In addition, LEC2 is a mediator of auxin biosynthesis and signaling. LEC2 induces YUCCA2 (YUC2) and YUC4 (Stone et al. 2008), which encode auxin biosynthesis enzymes, while LEC1 activates the YUC10 (Junker et al. 2012). AGL15 directly upregulates GA2ox6, a GA catabolic enzyme, and represses the GA biosynthesis gene GA3ox2 leading to a reduction of biologically active GA in Arabidopsis (Wang et al. 2004; Zheng et al. 2009). Ga3ox2 is also repressed by LEC2 and FUS3 and is ectopically activated in the loss-of-function mutants of lec2 and fus3 (Curaba et al. 2004).
Epigenetic reprogramming occurs in many plant developmental processes and regeneration (Hou and Wan 2021; Hou et al. 2022b). Studies have shown that epigenetic modifications, including histone modifications and DNA methylation, suppress regenerative potential and maintain the differentiated status of plant cells (Chen and Dent 2014; Ikeuchi et al. 2015a; Lee and Seo 2018). A chromatin regulator POLYCOMB REPRESSIVE COMPLEX 2 (PRC2) promotes trimethylation on lysine 27 of histone H3 (H3K27me3) to represses gene expression (Holec and Berger 2012). In Arabidopsis, loss-of-function mutants in PRC2 complex develop normal root hairs but fail to maintain the differentiated state and generate callus and somatic embryos (Ikeuchi et al. 2015b). The Wound-induced dedifferentiation 3 (WIND3) and LEC2 are target genes of PRC2, and ectopic overexpression of WIND3 and LEC2 partly phenocopies the prc2 mutants (Ikeuchi et al. 2015b). These findings suggested that PRC2-mediated gene repression is essential for maintaining the differentiated cell state. Histone acetylation is a permissive histone mark and plays an essential role in somatic embryogenesis (Kadosh and Struhl 1998; Rundlett et al. 1998). Arabidopsis plants treated with trichostatin A (TSA), an inhibitor of histone deacetylases (HDAC), resulted in growth arrest and enhanced transcription of LEC1, FUS3, and ABI3 during germination (Tanaka et al. 2008). In addition, an HAD6/HAD19 double-repression line generated embryo-like structures on the true leaves. These phenotypes of the repression line can be rescued by lec1 (Tanaka et al. 2008). Thus, HDA6 and HDA19 redundantly regulate the inhibition of embryonic properties by repressing embryo-specific genes during germination in Arabidopsis. In Brassica napus, repressing histone deacetylase activity with TSA resulted in a significant increase in cell transition from pollen to embryogenic growth in male gametophytes (Li et al. 2014). Interestingly, TSA with heat treatment greatly increased the formation of somatic embryos (Li et al. 2014). Thus, heat stress and histone deacetylation may synergistically regulate somatic embryogenesis (Fig. 2B).
Molecular basis of de novo root organogenesis
The pericycle cells between the endodermis and stele have the potential to generate new lateral roots (Beeckman and De Smet 2014). Arabidopsis explants incubation on CIM and the root-inducing medium (RIM) strongly promote root regeneration from pericycle cells. Culturing hypocotyl explants on RIM after pretreatment on CIM induces a large number of roots, whereas only a few roots form when they are inoculated on RIM without the pretreatment. In contrast to hypocotyl explants, root explants with lateral root meristem primordia efficiently promote root formation when directly cultured on RIM. These results suggest that CIM induces the pluripotent non-embryonic callus generation and these cells then further develop into adventitious roots on RIM (Fig. 3A).
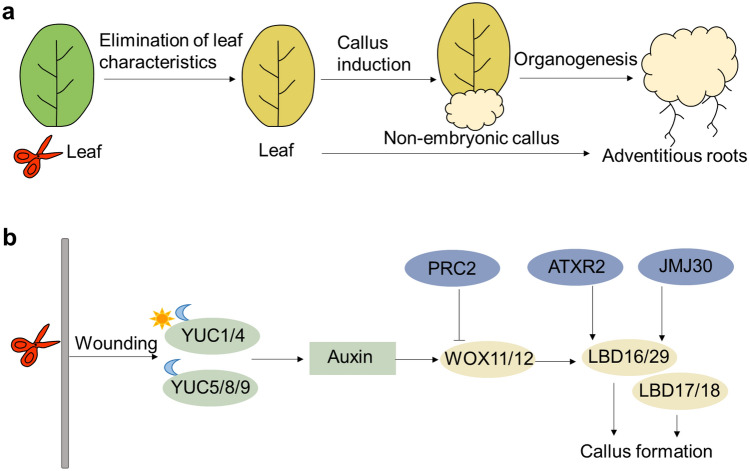
Fig. 3.
Schematic representation and molecular regulatory network of de novo root organogenesis. a Schematic representation of de novo root organogenesis. First, leaf explants have to eliminate leaf characteristics. Then, adventitious roots are induced directly or indirectly through de novo root organogenesis. In indirect induction, the non-embryonic callus is induced on the callus-inducing medium (CIM), and these cells then develop into adventitious roots on the root-inducing medium (RIM). b Molecular regulatory network of de novo root organogenesis. Developmental regulatory genes are in yellow; hormone biosynthesis- and signaling-related genes are in green and epigenetic modification-related genes are in blue. Symbols of the sun and the moon represent light and dark conditions, respectively. Arrows and bar-head arrows represent activation and repression, respectively (color figure online)
Several studies have shown that wounds, hormones, developmental regulatory genes, and epigenetic modifications affect de novo root organogenesis. Under either light or dark conditions, YUC1 and YUC4 are rapidly activated in response to wounding, promoting auxin biogenesis in mesophyll and competent cells, while YUC5, YUC8, and YUC9 mainly respond to dark conditions. Overall, YUC genes enhanced the auxin level in leaf explants during de novo root organogenesis (Chen et al. 2016). Wuschel related homeobox11 (WOX11), a homeobox gene, responds to wounding-induced auxin signaling together with its homolog WOX12 to upregulate Lateral organ boundaries domain 16 (LBD16) and LBD29, resulting in the fate transition from leaf procambium or parenchyma cells to root founder cells (Liu et al. 2014). Notably, the auxin response elements (AuxREs) in the promotor of WOX11 are essential for its induction in leaf explants, indicating that the auxin signaling pathway directly activates WOX11 expression during root regeneration (Liu et al. 2014). Thus, this novel regulatory mechanism links wounding and hormonal signaling to organ formation during regeneration. The other two LBD genes, LBD17 and LBD18 are also rapidly and significantly induced by CIM. In Arabidopsis, ectopic expression of each of the four LBD genes is sufficient for spontaneous callus formation in the absence of exogenous phytohormones, and inhibition of LBD function suppresses CIM-induced callus formation (Fan et al. 2012). These results support that LBD transcription factors play essential roles during the callus induction process. Collectively, these regulatory pathways together promote the auxin-mediated establishment of root meristems.
Epigenetic regulation, such as PRC2-mediated repression, regulates WOX11 to influence plant cell fate transition (Ikeuchi et al. 2013; Liu et al. 2014). In addition, JUMONJI C domain-containing protein 30 (JMJ30) binds to the promoters of LBD16 and LBD29 with Auxin response factor 7/19 (ARF7/ARF19) which are transcriptional activators of early auxin response, removes the methyl groups from H3K9me3, and promotes LBD expression (Lee et al. 2018). Arabidopsis trithorax-related 2 (ATXR2) is recruited to LBD16 and LBD29 promoters through ARF-JMJ30 complex and promotes trimethylation on lysine 36 of histone H3 (H3K36me3) to further promotes LBD expression during callus formation (Lee et al. 2017). A schematic gene regulatory network during root organogenesis is illustrated in Fig. 3B.
Molecular basis of de novo shoot organogenesis
Like many other plants, Arabidopsis explants do not readily regenerate shoots. However, culturing Arabidopsis explants on CIM and the shoot-inducing medium (SIM) rich in cytokinin strongly promotes shoot regeneration from pericycle cells (Atta et al. 2009; Che et al. 2007; Valvekens et al. 1988). The CIM-induced callus possesses root meristem characteristics. Thus, it is easy to regenerate roots when the CIM-induced callus is transferred to RIM. Establishing the identity of root meristem and further root development can be regulated through auxin-induced transcriptional cascade (Ozawa et al. 1998). In contrast, shoot regeneration may be more complex because it requires the transition from root meristem fate to shoot meristem fate. CIM induces pluripotent non-embryonic callus, which develops into adventitious shoots through two developmental processes: shoot progenitor cell regeneration and shoot formation after transferring to SIM (Kareem et al. 2015) (Fig. 4A).
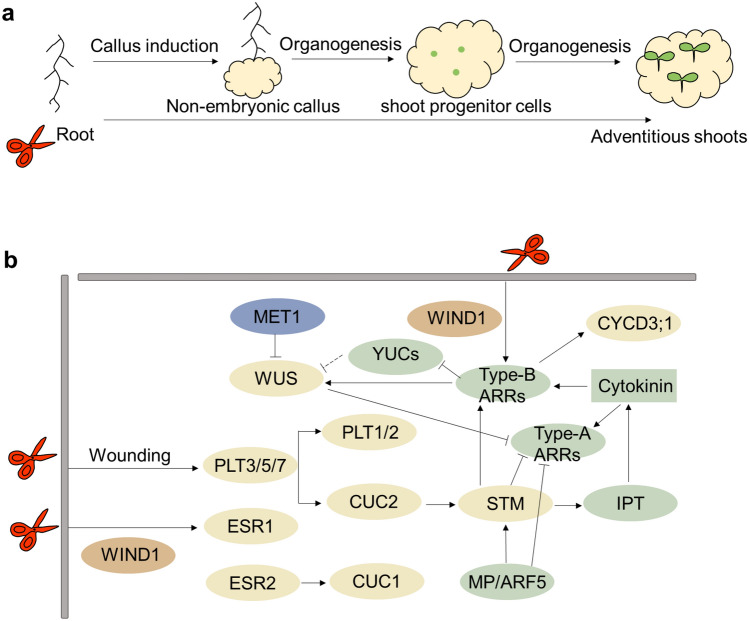
Fig. 4.
Schematic representation and molecular regulatory network of de novo shoot organogenesis. a Schematic representation of de novo shoot organogenesis. Adventitious shoots are directly or indirectly induced by de novo shoot organogenesis. In indirect induction, the non-embryonic callus is induced on the callus-inducing medium (CIM), and the callus then develops into adventitious shoots through two developmental processes: shoot progenitor cell regeneration and shoot formation after transferring to the shoot-inducing medium (SIM). b Molecular regulatory network of de novo shoot organogenesis. Wound-related genes are in brown; developmental regulatory genes are in yellow; hormone biosynthesis- and signaling-related genes are in green and epigenetic modification-related genes are in blue. Arrows and bar-head arrows represent activation and repression, respectively (color figure online)
De novo shoot organogenesis is also regulated by wounds, hormones, developmental regulatory genes, and epigenetic modifications. Studies showed an AP2/ERF transcription factor, WIND1 and its close homologs WIND2, WIND3, and WIND4 are rapidly induced by wounding, and these genes promote cell dedifferentiation and subsequent callus formation in Arabidopsis (Iwase et al. 2011a, b). WIND1 upregulates enhancer of shoot regeneration1/dornröschen (ESR1/DRN), encoding another AP2/ERF transcription factor, and promotes shoot regeneration in Arabidopsis (Banno et al. 2001; Iwase et al. 2017). In addition, WIND1 induces the B-type Arabidopsis response regulators (ARRs)-mediated cytokinin response (Iwase et al. 2011a). Double mutants of type-B ARRs (arr1-3arr12-1 and arr1-3arr10-5) display reduced callus formation. The expression of core cell cycle regulator Cyclin D3 (CYCD3;1) is downregulated in the arr1-3arr12-1 double mutant and triple loss-of-function mutants of cycd3;1–3 have low callus formation efficiency (Ikeuchi et al. 2017). Thus, these results suggest that wounding induces cytokinin signaling and then promotes cell cycle activation at wounded sites. Other AP2/ERF transcription factors, PLETHORA3 (PLT3), PLT5, and PLT7, are also induced after wounding, and plt357 triple mutants are less effective in callus formation (Ikeuchi et al. 2017). In addition, overexpression of PLT5 successfully obtained transgenic plants of Antirrhinum majus and Brassica rapa (Lian et al. 2022).
Induction of PLT3, PLT5, and PLT7 are among the earliest transcriptional responses induced by CIM and then the essential root meristem regulators PLT1 and PLT2 are activated (Aida et al. 2004; Galinha et al. 2007; Kareem et al. 2015). In addition, Cup-shaped cotyledon2 (CUC2), encoding a NAC family transcription factor, is also activated by PLT3, PLT5, and PLT7. Several pieces of evidence showed that CUC proteins are critical for shoot formation in the callus (Kareem et al. 2015). Overexpression of CUC1 or CUC2 enhances the adventitious shoot formation of calli derived from Arabidopsis hypocotyls (Daimon et al. 2003). Other transcription factors, such as ESR2, enhance shoot regeneration by directly regulating CUC1 transcription (Ikeda et al. 2006). After transferring to SIM, the essential shoot stem cell regulator WUS is induced (Gordon et al. 2007). The CUC2-expressing cells continue to proliferate and form promeristems in which PIN1 and a homeodomain transcription factor shoot meristemless (STM) are upregulated and further promote the formation of functional shoot meristems (Gordon et al. 2007). Overexpression of BnSTM induces type-B ARRs and represses type-A ARRs (Elhiti and Stasolla 2012). Activation of STM using an inducible system resulted in a rapid and dramatic increase of isopentenyl transferase 7 (IPT7), encoding a cytokinin biosynthesis gene (Yanai et al. 2005). In addition, MPΔ, an irrepressible variant of Monopteros (MP)/ARF5, promotes de novo shoot formation by activating the expression of STM and repressing the expression of ARRs-A (Ckurshumova et al. 2014; Krogan et al. 2012; Zhao et al. 2010).
WUS is essential for the maintenance of the stem cell niche in SAMs (Laux et al. 1996). Recent studies revealed that type-B ARRs activate the transcription of WUS. Type-B ARRs also inhibit auxin accumulation by repressing YUCs and indirectly inducing the expression of WUS (Meng et al. 2017). In addition, WUS directly represses the transcription of type-A ARRs (Leibfried et al. 2005). Like many other regeneration regulators, epigenetic marks modulate WUS expression during shoot regeneration (Li et al. 2011). Loss-of-function of a DNA methyltransferase 1 (MET1) led to increased WUS expression and accelerated developmental speed of in vitro shoot regeneration (Li et al. 2011) (Fig. 4B).
Application of developmental regulatory genes for genotype-independent plant transformation
Developmental regulatory genes that promote plant regeneration have been used to improve transformation efficiency and promote genotype-independent plant transformation. Ectopic expression of LEC1, L1L, or LEC2 in Arabidopsis (Lotan et al. 1998), Picea abies (Uddenberg et al. 2016), Citrus sinensis (Zhu et al. 2014) and Theobroma cacao (Shires et al. 2017) promotes embryo-like structure and somatic embryo formation but cannot obtain regenerated transgenic plants. However, inducible expression of LEC2 by β-estradiol could regenerate transgenic plants, though the regenerated plant displayed abnormal phenotypes (Rashid et al. 2007). AGL15 promotes the generation of secondary embryos from zygotic embryos and these secondary embryos maintain the potential for embryogenic development (Harding et al. 2003). Ectopic expression of AGL15 also enhances somatic embryo formation from the shoot apical meristem (Harding et al. 2003). Overexpression of GmAGL15, an ortholog of Arabidopsis AGL15, promotes somatic embryo development in Glycine max (Thakare et al. 2008). In G. hirsutum, overexpression of either GhAGL15-1, GhAGL15-3, or GhAGL15-4 promotes the embryogenic potential of transgenic calli (Yang et al. 2014).
Overexpression of TaWOX5 increases the transformation efficiency of multiple T. aestivum varieties without genotype dependency (Wang et al. 2022a). Ectopic expression of AtWOX2/8/9 led to a range of abnormal phenotypes in tobacco (Kyo et al. 2018). Overexpression of Z. mays homeobox gene knotted1 (Zmkn1) obtains a large number of transgenic calli and shoots on a hormone-free medium without antibiotic selection in tobacco. Under the same conditions, no callus or shoot was generated from explants that were infected with an Agrobacterium strain harboring the NPTII selection gene or uninfected controls. The use of 35S:ZmKn1 resulted in a three-fold increase in shoot organogenesis relative to the NPTII selection. These results suggest that ZmKn1 could be used as an effective selection marker with the potential to enhance plant transformation efficiency (Luo et al. 2006). Similarly, overexpression of ZmKn1 in transgenic citrus enhanced transformation efficiency by 3- to 15-fold (Hu et al. 2016). However, overexpression of Nicotiana tabacum homeobox (NTH) genes, knotted1-type homeobox genes, resulted in a range of abnormal leaf morphology. Transgenic plants overexpressing NTH1 or NTH9 displayed a relatively weak phenotype compared to NTH15 or NTH20 overexpression lines, which exhibited ectopic shoot formation on the leaf surface (Nishimura et al. 2000).
Overexpression of BBM enhances the spontaneous formation of somatic embryos in Arabidopsis and B. napus (Boutilier et al. 2002). BBM has also been used as an ectopic regulator in T. cacao (Florez et al. 2015) and tobacco (Srinivasan et al. 2007) genetic transformation. However, the BBM overexpression transgenic plants exhibited abnormal phenotypes. Thus, strategies that use inducible promoters or transgene excision to control the restricted spatiotemporal expression of BBM have been applied in tobacco (Srinivasan et al. 2007), Capsicum annuum (Heidmann et al. 2011) and Arabidopsis (Lutz et al. 2015). In Populus tomentosa genetic transformation, the generated transgenic plants are phenotypically normal when using a heat shock-inducible FRT/FLP system to excise BBM expression cassette from the callus stage (Deng et al. 2009). Transgenic plants generated by overexpression of WUS also exhibit negative pleiotropic phenotypes such as swollen hypocotyls, distorted leaves, and coiled root tips (Arroyo-Herrera et al. 2008; Bouchabke-Coussa et al. 2013; Rashid et al. 2007), suggesting that expression of WUS has to be strictly controlled.
A recent groundbreaking study showed that fine-tuning the expression of WUS and BBM enhanced the transformation efficiency of monocot plants (Lowe et al. 2016). Overexpression of ZmWUS2 driven by a strong callus promoter often causes callus necrosis. To solve this problem and to induce somatic embryogenesis in immature embryos, a relatively weak Agrobacterium-derived nopaline synthase promoter and a strong maize Ubiquitin promoter were used to drive ZmWUS2 (Nos:ZmWUS2) and ZmBBM (Ubi:ZmBBM) expression simultaneously (Lowe et al. 2016). Results showed that ectopic expression of ZmWUS2 and ZmBBM significantly enhanced callus transformation efficiency in Z. mays, Sorghum bicolor, O. sativa and Saccharum officinarum. However, the continuous expression of ZmWUS2 and ZmBBM leads to aberrant phenotypes, such as thick, short roots, stunted, twisted and sterile plants (Lowe et al. 2016). Thus, using desiccation-inducible promoter rab17 to activate CRE (a recombinase enzyme isolated from the P1 bacteriophage) expression, and remove the ZmWUS2, ZmBBM and CRE expression cassettes between two loxP sites in the transformed embryogenic calli generate healthy, fertile T0 transgenic plants (Lowe et al. 2016). This strategy could also obtain transgenic plants from previously non-transformable Z. mays and S. bicolor varieties (Mookkan et al. 2017, 2018). Another strategy for solving the phenotypic abnormalities is to select suitable endogenous promoters to trigger the required spatiotemporal expression of ZmWUS2 and ZmBBM. The promoter of a Z. mays phospholipid transferase protein gene (ZmPLTP) was selected to drive ZmBBM as ZmPLTP is highly expressed in leaves, embryos, and callus but has very low expression levels in roots, meristems, and reproductive tissues (Lowe et al. 2018). Somatic embryo formation was rapidly induced when ZmPLTP:ZmBBM and Nos:ZmWUS2 were co-transformed into Z. mays immature zygotic embryos, and these somatic embryos developed into healthy fertile plants without a callus phase (Lowe et al. 2018). However, T1 seeds continuously expressing Nos:ZmWUS2 showed poor germination. While replacing the Nos promoter with a Z. mays auxin-inducible promoter (ZmAxig1) and co-transformation of ZmPLTP:ZmBBM and ZmAxig1:ZmWUS2 stimulated somatic embryo formation and obtained phenotypically normal transgenic plants without excision ZmWUS2 and ZmBBM expression cassettes (Lowe et al. 2018). The callus-free transformation approach has been successfully tested in seven different Z. mays inbred lines (Lowe et al. 2018). Interestingly, a recent study showed that the ZmPLTP:ZmWUS2 alone was sufficient to promote rapid somatic embryo formation from Z. mays immature embryos in a noncell autonomous manner. When transforming Z. mays with two Agrobacterium strains, one containing ZmPLTP:ZmWUS2 and the other containing selectable and visual marker cassettes, the transformed Z. mays T0 plants expressed the selectable marker gene but without the integration of ZmWUS2 (Hoerster et al. 2020). This result suggests that transformed cells expressing ZmWUS2 could stimulate somatic embryogenesis of their neighboring cells. ZmPLTP:ZmWUS2 also significantly shortened the tissue culture time in S. bicolor by inducing direct somatic embryo formation and regeneration, and also bypassed genotype-dependent callus formation (Che et al. 2022). Similarly, using two strains with one containing ZmPLTP:ZmWUS2 and ZmPLTP:ZmBBM expression cassettes, and the other harboring a selectable marker expression cassette to transform S. bicolor, the transformed S. bicolor T0 plants expressed the selectable marker gene but without the integration of ZmWUS2 and ZmBBM (Aregawi et al. 2022). This strategy increases transformation efficiency and expands amenable genotypes of different monocot species. A recent study showed that Nos:ZmWUS2 and 3xENH–Ubi:ZmBBM (three consecutive viral enhancers including Figwart mosaic virus, Peanut chlorotic streak virus, and Mirabilis mosaic virus) were used to improve leaf transformation efficiency and obtain plants with Cas9-mediated gene dropouts and insertion in Z. mays and S. bicolor (Wang et al. 2023). Moreover, regenerated plants were successfully obtained by using Nos:ZmWUS2 and 3xEnh–Ubi:ZmBBM in Eragrostis tef, Panicum virgatum, Cenchrus americanus, Setaria italica, Secale cereale, Hordeum vulgare and O. sativa (Wang et al. 2023). These results suggest that this may be a universal method for genetic transformation and genome editing of the Poaceae. In addition, recent studies showed that using Nos:ZmWUS2, Ubi:IPT or Ubi:AtSTM enhanced organogenesis in aseptic seedling leaves of Arabidopsis, Nicotiana benthamiana, and Solanum lycopersicum, and in mature plants of N. benthamiana, Solanum tuberosum and Vitis vinifera (Cody et al. 2023; Maher et al. 2020). When Nos:ZmWUS2, Ubi:AtSTM or Ubi:IPT were co-transformed with Cas9/gRNA plasmids, gene-edited shoots were obtained without tissue culture. The tissue culture-free method has great potential to accelerate the breeding process for many plant species (Cody et al. 2023; Maher et al. 2020).
In contrast to the adverse effects of ectopic expression of ZmWUS2 and ZmBBM, overexpression of transcription factor encoding genes Growth-regulating factor (GRF) and/or its cofactor GRF-interacting factor1 (GIF) does not cause aberrant phenotypes in transgenic plants. In callus induction and plant regeneration, the GRF-GIF recruits SWITCH2/SUCROSE NONFERMENTING 2 chromatin remodeling complexes to confer the meristematic potential of the proliferative tissue during organogenesis. Accordingly, overexpression of AtGRF5 or GRF5 orthologs enhanced transformation efficiency in Beta vulgaris, B. napus, G. max, Helianthus annuusl and Z. mays (Kong et al. 2020). Furthermore, a fused GRF4-GIF1 chimeric protein increases transformation efficiency and accelerates the speed of regeneration in T. aestivum, O. sativa, and citrus (Debernardi et al. 2020). Compared with the control, the transformation efficiency with the chimeric GRF4-GIF1 protein expression was increased by 7.8-, 2.1- and 4.7- fold in T. aestivum, O. sativa, and citrus, respectively (Debernardi et al. 2020). Similarly, overexpression of GRF5, or GRF4 and GIF1 also achieved high transformation efficiency in Citrullus lanatus. AtGRF5, or ClGRF4 and ClGIF1 factors also facilitate efficient transformation and increase CRISPR/Cas9-based genome editing efficiency in C. lanatus (Feng et al. 2021; Pan et al. 2022b) (Table 1).
Table 1.
Summary of developmental regulatory genes applied to plant transformation
| No. | Gene expression cassette | Agrobacterium strains | Transformed species | Explant | Regeneration | Regenerated plant | Transform efficiency increases | Transgene excision | Abnormal phenotype | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35S:CcSERK1 | LBA4404 | Coffea canephora | Leaf | Embryogenesis | No | N.A. | No | No | Perez-Pascual et al. (2018) |
| 2 | 35S:AtSERK1 | C58C1 | Arabidopsis thaliana | Flower bud | Embryogenesis | No | N.A. | No | No | Hecht et al. (2001) |
| 3 | 35S:OsSERK1 | EHA105 | Oryza sativa | Callus | Embryogenesis | No | N.A. | No | No | Hu et al. (2005) |
| 4 | pER8:AtESR1 | EHA105 | Arabidopsis thaliana | Root | Organogenesis | Yes | ↑ | No | No | Banno et al. (2001) |
| 5 | 35S:AtPLT5 | GV3101 | Antirrhinum majus | Mature plant | Organogenesis | Yes | ↑ | No | Yes | Lian et al. (2022) |
| 35S:AtPLT5 | GV3101 | Brassica rapa | Cotyledon | Organogenesis | Yes | ↑ | No | Yes | ||
| 6 | 35S:AtCUC1, CUC2 | MP90 | Arabidopsis thaliana | Callus | Organogenesis | Yes | ↑ | No | Yes | Daimon et al. (2003) |
| 7 | pER10:AtESR2 | EHA105 | Arabidopsis thaliana | Flower bud and Root | Organogenesis | Yes | ↑ | No | No | Ikeda et al. (2006) |
| 8 | AtMP:AtMPΔ | N.A | Arabidopsis thaliana | Root, Cotyledon, Leaf and Petiole | Organogenesis | Yes | N.A. | No | Yes | Ckurshumova et al. (2014) and Krogan et al. (2012) |
| 9 | 35S:AtLEC1 | GV3101 | Arabidopsis thaliana | N.A | Embryogenesis | No | N.A. | No | No | Lotan et al. (1998) |
| 10 | pER8:PaHAP3A | C58C1 | Picea abies | Embryogenic culture | Embryogenesis | No | N.A. | No | No | Uddenberg et al. (2016) |
| 11 | 35S:CsL1L | EHA105 | Citrus sinensis | Epicotyl and embryogenic callus | Embryogenesis | No | N.A. | No | No | Zhu et al. (2014) |
| 12 | 35S:TcLEC2-GR | AGL1 | Theobroma cacao | Cotyledon | Embryogenesis | No | N.A. | No | No | Shires et al. (2017) |
| 13 | pER8:AtLEC22 | LBA4404 | Nicotiana tabacum | Leaf | Organogenesis | Yes | N.A. | No | Yes | Rashid et al. (2007) |
| 14 | 35S:AtAGL15 | N.A | Arabidopsis thaliana | Cotyledon | Embryogenesis | No | N.A. | No | No | Harding et al. (2003) |
| 15 | 35S:GmAGL15 | – | Glycine max | Cotyledon | Embryogenesis | Yes | N.A. | No | Yes | Thakare et al. (2008) |
| 16 | 35S:GhAGL15s | LBA4404 | Gossypium hirsutum | Hypocotyl | Embryogenesis | No | N.A. | No | No | Yang et al. (2014) |
| 17 | Ubi:TaWOX5 | C58C1 | Triticum aestivum and Zea mays | Immature embryo | Organogenesis | Yes | ↑ | No | No | Wang et al. (2022a) |
| 18 | pER8:AtWOX2/8 pER8:AtWOX2/9 | AGL1 | Nicotiana tabacum | Leaf | Organogenesis | Yes | N.A. | No | Yes | Kyo et al. (2018) |
| 19 | 35S:ZmKn1 | LBA4404 | Nicotiana tabacum | Leaf | Organogenesis | Yes | ↑threefold | No | Yes | Luo et al. (2006) |
| 20 | 35S:ZmKn1 | EHA105 | Citrus sinensis | Internodal stem | Organogenesis | Yes | ↑3–15 fold | No | Yes | Hu et al. (2016) |
| 21 | 35S:NtNTH1, 9, 15, 20, 22 | LBA4404 | Nicotiana tabacum | Leaf | Organogenesis | Yes | N.A. | No | Yes | Nishimura et al. (2000) |
| 22 | 35S:BnBBM UBI:BnBBM | C58C1pMP90 | Arabidopsis thaliana | N.A | Embryogenesis | No | N.A. | No | Yes | Boutilier et al. (2002) |
| 35S:BnBBM UBI:BnBBM | C58C1pMP90 | Brassica napus | Haploid microspore | Embryogenesis | No | N.A. | No | Yes | ||
| 23 | 35S:TcBBM | AGL1 | Arabidopsis thaliana | Flower bud | Embryogenesis | No | N.A. | No | Yes | Florez et al. (2015) |
| 35S:TcBBM | AGL1 | Theobroma cacao | Cotyledon | Embryogenesis | No | N.A. | No | Yes | ||
| 24 | 35S:AtBBM 35S:BnBBM | C58C1 | Nicotiana tabacum | Leaf | Organogenesis | Yes | N.A. | No | Yes | Srinivasan et al. (2007) |
| 35S:AtBBM-GR 35S:BnBBM-GR | C58C1 | Nicotiana tabacum | Leaf | Organogenesis | Yes | N.A. | No | No | ||
| 25 | 35S:BnBBM-GR | GV3101 | Capsicum annuum | Cotyledon | Embryogenesis | Yes | ↑ | No | Yes | Heidmann et al. (2011) |
| 26 | 35S:BBM-GR | N.A | Arabidopsis thaliana | Flower bud | Organogenesis | Yes | ↑ | No | No | Lutz et al. (2015) |
| 27 | AtHSP18.2:FLP-35S:BcBBM | LBA4404 | Populus tomentosa | Leaf | Embryogenesis | Yes | ↑ | FRT/FLP | No | Deng et al. (2009) |
| 28 | pER10:AtWUS | C58C1 | Coffea canephora | Leaf | Embryogenesis | Yes | N.A. | No | Yes | Arroyo-Herrera et al. (2008) |
| 29 | 35S:AtWUS | N.A | Gossypium hirsutum | Hypocotyl | Embryogenesis | No | N.A. | No | Yes | Bouchabke-Coussa et al. (2013) |
| 30 | pER8:AtWUS | LBA4404 | Nicotiana tabacum | Leaf | Organogenesis | Yes | N.A. | No | Yes | Rashid et al. (2007) |
| 31 | RAB17:CRE-Nos:ZmWUS2-Ubi:ZmBBM | LBA4404 | Zea mays, Sorghum bicolor, Oryza sativa and Saccharum officinarum | Immature embryo, Mature Seed, Leaf and Callus | Embryogenesis | Yes | ↑ | Cre-loxP | No | Lowe et al. (2016) |
| 32 | RAB17:CRE-Nos:ZmWUS2-Ubi:ZmBBM | AGL1 EHA101 | Zea mays and Sorghum bicolor | Immature embryo | Embryogenesis | Yes | ↑ | Cre-loxP | No | Mookkan et al. (2018, 2017) |
| 33 | ZmPLTP:ZmBBM-ZmAxig1:ZmWUS2 | LBA4404 | Zea mays | Immature embryo | Embryogenesis | Yes | 8.7–96% | No | No | Lowe et al. (2018) |
| 34 | 3 × ENH-ZmPLTP:ZmWUS2-Nos:ZmCRC | LBA4404 | Zea mays and Sorghum bicolor | Immature embryo | Embryogenesis | Yes | ↑ | No | No | Che et al. (2022) and Hoerster et al. (2020) |
| 35 | ZmPLTP:ZmWUS2-ZmPLTP:ZmBBM | LBA4404 | Sorghum bicolor | Immature embryo | Embryogenesis | Yes | ↑ | No | No | Aregawi et al. (2022) |
| 36 | HSP17:CRE-Nos:ZmWUS2-3 × ENH -Ubi:ZmBBM | LBA4404 | Zea mays, Sorghum bicolor Eragrostis tef, Panicum virgatum, Cenchrus americanus, Setaria italica, Secale cereale, Hordeum vulgare and Oryza sativa | Leaf | Embryogenesis | Yes | ↑ | Cre-loxP | No | Wang et al. (2023) |
| 37 | Nos:ZmWUS2 Ubi:IPT | N.A | Arabidopsis thaliana, Nicotiana benthamiana and Solanum lycopersicum | Agro transient | Organogenesis | Yes | N.A. | No | No | Maher et al. (2020) |
| Nos:ZmWUS2 Ubi:STM | N.A | Nicotiana benthamiana, Solanum tuberosum and Vitis vinifera | Mature plant | Organogenesis | Yes | N.A. | No | No | ||
| 38 | 2×35S:AtGRF5 | AGL1 | Beta vulgaris | Leaf | Organogenesis | Yes | ↑sixfold | No | No | Kong et al. (2020) |
| 2×35S:BvGRF5-LIKE | ||||||||||
| PcUbi4-2:BnGRF5-LIKE | SHA001 | Brassica napus | Hypocotyl | Organogenesis | Yes | ↑ | No | No | ||
| PcUbi4-2:GmGRF5-LIKE | SHA017 | Glycine max | Primary node | Organogenesis | Yes | ↑ | No | No | ||
| 35S:AtGRF5 | EHA105 | Helianthus annuusl | Cotyledon | Organogenesis | Yes | ↑ | No | No | ||
| 35S:HaGRF5-LIKE | ||||||||||
| BdEF1:AtGRF5 | LBA4404 | Zea mays | Immature embryo | Organogenesis | Yes | ↑ | No | No | ||
| BdEF1:ZmGRF5-LIKE1 | ||||||||||
| BdEF1:ZmGRF5-LIKE2 | ||||||||||
| 39 | ZmUbi:GRF4-GIF1 | EHA105 | Triticum aestivum | Immature embryo | Organogenesis | Yes | ↑7.8-fold | No | No | Debernardi et al. (2020) |
| ZmUbi:GRF4-GIF1 | EHA105 | Oryza sativa | Callus | Organogenesis | Yes | ↑2.1-fold | No | No | ||
| 35S:GRF4-GIF1 | EHA105 | Citrus sinensis | Etiolated Epicotyl | Organogenesis | Yes | ↑4.7-fold | No | No | ||
| 40 | 35S:GRF4-GIF1 | EHA105 | Citrullus lanatus | Cotyledon | Organogenesis | Yes | ↑ninefold | No | No | Feng et al. (2021) |
| 41 | UBQ10:AtGRF5 | GV3101 | Citrullus lanatus | Cotyledon | Organogenesis | Yes | ↑40-fold | No | No | Pan et al. (2022b) |
N.A., not applicable; “↑” represent increased transform efficiency
Nanoparticle uptake and translocation in plant cells
Agrobacterium-mediated method is the most frequently used tool for gene delivery in plant transformation. However, this method usually requires regeneration from tissue culture and infects only some plant species. Furthermore, it is hard to use Agrobacterium for chloroplast or mitochondrion transformation. Nanoparticles (NPs), natural or manufactured ultradisperse objects ranging from 1 to 100 nm, are promising materials for exogenous biomolecule delivery because of their ability to traverse the plant cell without external force and their broad host applicability. The application of nanotechnology to plant cells requires understanding the interaction between NPs and plant cells, including the uptake and translocation of NPs.
Nanoparticle uptake in plant cells
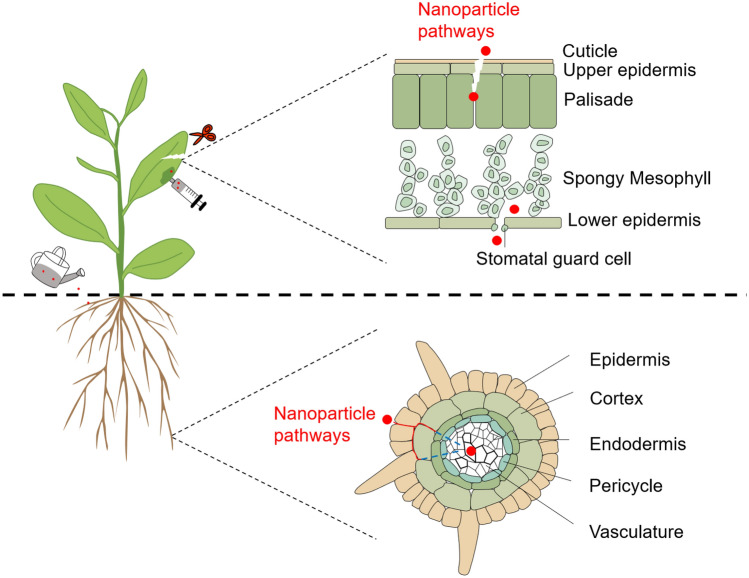
In plant science, NPs can be applied to roots and above-ground plant tissues especially leaves. Shoot surfaces are usually covered with a cuticle, which acts as a lipophilic barrier to protect primary organs of above-ground plants. NPs can enter the cell wall through natural openings, such as stomata pores (Eichert et al. 2008). Damages and wounds may also be feasible pathways for NP internalization in both aerial and hypogeal parts of plants (Al-Salim et al. 2011) (Fig. 5). In addition, delivery methods affect NP uptake efficiency in plants. A recent study showed that compared with the NP drop-cast method, the aerosol application help to improve NP uptake in C. lanatus (Raliya et al. 2016).
Fig. 5.
Schematic representation of uptake and translocation of nanoparticles (NPs) in plants. NPs can be applied to roots and leaves and uptaken into plants through damage or natural openings, such as stomata pores. Apoplastic and symplastic paths are the mobilization pathways of NPs after penetrating the outer protective layer of plants. Red solid lines indicate apoplastic paths, and blue dotted lines indicate symplastic paths (color figure online)
Nanoparticle translocation in plant cells
Once penetrate the outer protective layers of plants, mobilization of NPs in plants through apoplastic and symplastic paths. Apoplastic transport takes place outside the plasma membrane through the cell wall and extracellular spaces, while symplastic transport occurs between the cytoplasm of adjacent cells connected by plasmodesmata and sieve plate pores with the movement of water and solutes. Apoplastic transport has been demonstrated to facilitate the radial movement of NPs (Gonzalez-Melendi et al. 2008; Larue et al. 2014; Sun et al. 2014). However, the longitudinal Casparian strip composed of lignin-like structures prevents this radial movement in the root endodermis (Lv et al. 2015; Sun et al. 2014), and the symplastic path could bypass this barrier (Schwab et al. 2016) (Fig. 5). The cell wall is a multi-layered structure of pore diameter ranging from 5 to 20 nm (Fleischer et al. 1999; Fujino and Itoh 1998; Zemke-White et al. 2000). Recent studies demonstrated that different types of NPs with a mean diameter between 3 and 50 nm could easily pass through Arabidopsis and citrus cell walls (Etxeberria et al. 2016; Torney et al. 2007). When NPs penetrate the cell wall and reach the plasma membrane, they can enter cells through endocytosis. In addition, NPs can also cross the plasma membrane directly (Chang et al. 2013). Once NPs enter the cytoplasm, plasmodesmata promote the cell-to-cell movement of NPs. The transport of NPs of various sizes through plasmodesmata has been demonstrated in some plant species (Geisler-Lee et al. 2013; Lin et al. 2009; Zhai et al. 2014).
Application of nanoparticles in plant transformation
Over the past decade, NPs have been applied for plant delivery. Early studies showed NP-mediated plasmid DNA and protein delivery into plant cells with external force. For instance: (1) Gold-functionalized mesoporous silica nanoparticles (Au-MSNs)-mediated delivery of DNA (Torney et al. 2007) and proteins (Martin-Ortigosa et al. 2012, 2014) through the biolistic method. (2) Combined utilization of polyethylene glycol (PEG)-mediated transformation and polymeric dimethylaminoethyl metacrylate (DMAEM)-based polymers effectively deliver plasmid DNA into Ceratodon purpureus protoplasts and obtain stable transformants (Finiuk et al. 2017). (3) Polyethylenimine (PEI) nanoparticles deliver DNA into suspended cells of Crocus sativus through ultrasound, resulting in improved transfection efficiency (Firoozi et al. 2018). (4) Combined peptide-displaying micelle complexes (MCs) and cell wall-loosening zwitterionic liquid (ZIL) carry DNA into specific plant organelles through the vacuum/compression method (Miyamoto et al. 2022). Of these methods, cell-penetrating peptide-displaying MCs (CPP-MCs) was used to deliver DNA into nuclei while combined CPP-MCs and chloroplast-targeting peptide-displaying MCs (CPP/CTP-MCs) could be used for delivering DNA into chloroplasts (Table 2).
Table 2.
Summary of nanoparticles applied to plant transformation
| No. | Nanoparticles | Function modification | Delivery method | Cargo type | Target species/tissue | Expression | References |
|---|---|---|---|---|---|---|---|
| 1 | Mesoporous silica nanoparticles | Gold nanoparticles | Particle bombardment | Plasmid DNA and Chemicals | Nicotiana tabacum/Cotyledon and Zea mays/Immature embryo | Transient and stable expression | Torney et al. (2007) |
| 2 | Mesoporous silica nanoparticles | Gold nanoparticles | Particle bombardment | Protein | Zea mays/Immature embryo | Transient and stable expression | Martin-Ortigosa et al. (2014) |
| 3 | Mesoporous silica nanoparticles | Gold nanoparticles | Particle bombardment | Plasmid DNA and Protein | Nicotiana tabacum and Euchlaena mexicana/Leaf | Transient expression | Martin-Ortigosa et al. (2012) |
| 4 | Polymeric dimethylaminoethyl metacrylate-based polymers | – | Incubation and PEG-mediated transformation | Plasmid DNA | Nicotiana tabacum and Ceratodon purpureus/Protoplast | Transient and stable expression | Finiuk et al. (2017) |
| 5 | PEI nanoparticles | – | Ultrasound | DNA | Crocus sativus/Suspended cell | Transient expression | Firoozi et al. (2018) |
| 6 | Micelle complexes | Cell-penetrating peptide and chloroplast-targeting peptide | Vacuum/compression method | Plasmid DNA | Arabidopsis thaliana/Seedling and Leaf | Transient expression | Miyamoto et al. (2022) |
| 7 | Mesoporous silica nanoparticles | TMAPS, APTMS, THPMP | Infiltration | Plasmid DNA | Arabidopsis thaliana /Root | Transient expression | Chang et al. (2013) |
| 8 | Single-walled carbon nanotubes | Polyethylenimine | Infiltration and incubation | Plasmid DNA | Nicotiana benthamiana, Eruca sativa, Triticum aestivum and Gossypium hirsutum/Leaf and Eruca sativa/Protoplast | Transient expression | Demirer et al. (2019a, b) |
| 9 | Single-walled carbon nanotubes | Super-purified HiPCO SWNTs | Infiltration | Small interfering RNA | Nicotiana benthamiana/Leaf | Transient expression | Demirer et al. (2020) |
| 10 | Single-walled carbon nanotubes | Chitosan | Infiltration and incubation | Plasmid DNA | Eruca sativa, Nasturtium officinale, Spinacia oleracea and Nicotiana tabacum/Leaf and Arabidopsis thaliana/Protoplast | Transient expression | Kwak et al. (2019) |
| 11 | Carbon dots | Polyethylenimine | Spray | Small interfering RNA | Nicotiana benthamiana and Solanum lycopersicum/Leaf | Transient expression | Schwartz et al. (2020) |
| 12 | Carbon dots | Polyethylenimine | Infiltration | Plasmid DNA | Oryza Sativa, Triticum aestivum and Phaseolus radiatus/Leaf and Oryza Sativa/Root | Transient expression | Wang et al. (2020) |
| 13 | Gold nanoclusters | Polyethylenimine | Infiltration | Small interfering RNA | Nicotiana benthamiana/Leaf | Transient expression | Zhang et al. (2021a) |
| 14 | DNA nanostructures | – | Infiltration | Small interfering RNA | Nicotiana benthamiana/Leaf | Transient expression | Zhang et al. (2019) |
| 15 | Layered double hydroxide nanoparticles | – | Injection | Double-stranded RNA | Solanum lycopersicum/Flower pedicel | Transient expression | Molesini et al. (2022) |
| 16 | Graphene oxide nanoparticles | Polyethylenimine and polyethylene glycol | Infiltration | Small interfering RNA | Nicotiana benthamiana/Leaf | Transient expression | Li et al. (2022) |
| 17 | Chitosan nanoparticles | – | Incubation | Plasmid DNA | Paulownia tomentosa/Nodal segment | Stable expression | Hussien (2020) |
| 18 | Chitosan nanoparticles | – | Inoculation | Plasmid DNA | Allium cepa/Seedling | Stable expression | Hussien et al. (2022) |
| 19 | Magnetic nanoparticles | Polyethylenimine | Magnetic field | Plasmid DNA | Gossypium hirsutum, Capsicum annuum, Cucurbita moschata, Cucurbita pepo and Lilium brownii/Pollen | Stable expression | Zhao et al. (2017) |
| 20 | Magnetic nanoparticles | – | Magnetic field | Plasmid DNA | Zea mays/Pollen | Stable expression | Wang et al. (2022b) |
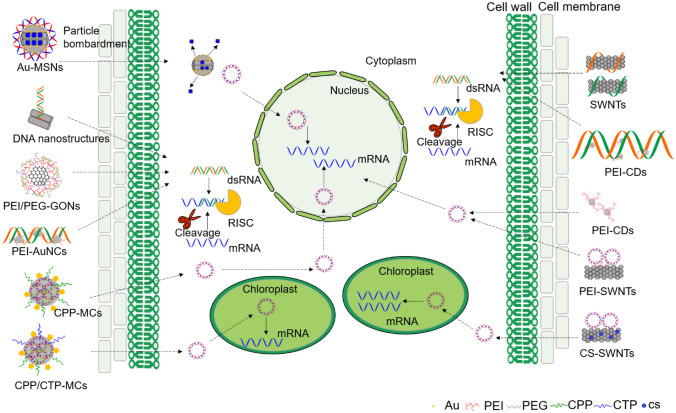
However, other studies have demonstrated that NPs can pass through plant cell walls without external force: (1) MSNs-mediated foreign DNA delivery into intact Arabidopsis roots without mechanical force (Chang et al. 2013). (2) Application of single-walled carbon nanotubes (SWNTs) for the delivery of small interfering RNA (siRNA) and plasmid DNA into a variety of plant species (Demirer et al. 2020, 2019a, b; Kwak et al. 2019). In addition, chitosan-complexed single-walled carbon nanotubes (CS-SWNTs) could deliver plasmid DNA into chloroplasts of mature Eruca sativa, Nasturtium officinale, Spinacia oleracea, tobacco plants and isolated Arabidopsis mesophyll protoplasts (Kwak et al. 2019). (3) Using PEI functionalized carbon dots (CDs) to efficiently deliver plasmid DNA or siRNA into intact plants (Schwartz et al. 2020; Wang et al. 2020). (4) DNA nanostructures and PEI functionalized gold nanoclusters (PEI-AuNCs) internalize into plant mature cells and deliver a siRNA to silence green fluorescent protein (GFP) expression in transgenic N. benthamiana plants (Zhang et al. 2021a; Zhang et al. 2019). Moreover, a recent study has reported that the double-stranded RNA (dsRNA) can be coupled to layered double hydroxides (LDH) nanoparticles, inducing gene silencing through injection into S. lycopersicum flower pedicel (Molesini et al. 2022). Another recent study used polymer-functionalized graphene oxide nanoparticles (GONs) to deliver siRNAs into intact N. benthamiana cells (Li et al. 2022). These successful applications indicate that NPs have great potential for plant delivery (Fig. 6, Table 2).
Fig. 6.
Schematic illustration of nanoparticle (NP) structures and NP-mediated nucleic acid and protein delivery into plant leaf cells. Au-MSNs gold-functionalized mesoporous silica nanoparticles, CPP-MCs cell-penetrating peptide-displaying micelle complexes, CPP/CTP-MCs combined CPP-MCs and chloroplast-targeting peptide-displaying MCs, CS-SWNTs chitosan-complexed single-walled carbon nanotubes, PEI-AuNCs Polyethylenimine-functionalized gold nanoclusters, PEI-CDs PEI-functionalized carbon dots, PEI/PEG-GONs PEI/polyethylene glycol (polymer)-functionalized graphene oxide nanoparticles, PEI-SWNTs PEI-functionalized single-walled carbon nanotubes, RISC RNA-induced silencing complex, SWNTs single-walled carbon nanotubes
To fully leverage NPs for plant genetic engineering, it is essential to achieve stable transformation enabling the generation of transgenic plants. Chitosan nanoparticles can deliver a thionin gene with antimicrobial properties into Allium cepa and Paulownia tomentosa cells, producing transgenic A. cepa and P. tomentosa resistance to black rot diseases and bacterial infection, respectively (Hussien 2020; Hussien et al. 2022). A groundbreaking study showed that stable genetic transformation had been successfully achieved in G. hirsutum plants using magnetic nanoparticles (MNPs) technology (Zhao et al. 2017). In this system, the BTΔα-CPTI gene-MNPs complex is delivered into G. hirsutum pollen under a magnetic field. Pollen magnetofection not only perfectly protects foreign DNA integrity, but also maintains pollen viability. Insect-resistant transformed plants are successfully generated through magnetofected pollen pollination. The exogenous gene was successfully integrated into the genome, effectively transcribed, and stably inherited into the offspring (Zhao et al. 2017). Y18 and SU12, two previously difficult-to-transform G. hirsutum varieties, are successfully transformed using this system. In addition, genetically modified C. annuum and Cucurbita moschata plants have also been successfully created (Zhao et al. 2017). A recent study reported that the MNPs system was also used to deliver exogenous genes to different Z. mays inbred lines and successfully obtained transgenic plants (Wang et al. 2022b). Further investigation found that transfection with a cool temperature pretreatment of pollen to open the germination aperture can improve the efficiency of DNA entry and maintain pollen viability (Wang et al. 2022b). As this method is genotype-independent, culture-free, and easy to handle, it has great potential to transform recalcitrant and genotype-dependent crops and thus accelerate the breeding process (Table 2).
Conclusions and perspectives
Many developmental regulatory genes have been shown to work effectively both in dicots and monocots (Table 1), and manipulation of these genes has great potential for developing genotype-independent genetic transformation methods in various crops. However, constitutive expression of developmental regulatory genes, such as ZmWUS2 and ZmBBM often interferes with normal plant development and leads to negative pleiotropic effects. So fine-tuning the expression of these genes is essential for applying them to plant transformation (Hoerster et al. 2020; Lowe et al. 2016, 2018; Mookkan et al. 2018, 2017). Overexpression of GRF and/or GIF can improve the transformation efficiency in a variety of crops but did not cause abnormal phenotypes in transgenic plants (Debernardi et al. 2020; Kong et al. 2020). This may be due to the post-transcriptional down-regulation of GRF by endougenous miRNA396 in T0 plants, which provides a built-in mechanism for alleviating pleiotropic problems (Debernardi et al. 2020; Li et al. 2021). There are many genes, such as ABI3 and LBDs, affecting plant regeneration have not been used for plant transformation. It is worth investigating whether fine-tuning the expression of these genes could facilitate the improvement of plant transformation. A recent study has established a versatile CRISPR-Combo platform for simultaneous genome editing and gene activation in plants (Pan et al. 2022a). This system can be applied to achieve plant regeneration by simultaneously activating BBM1 and editing the genome at Grain weight2 (GW2) and Grain number 1a (GN1a) loci without exogenous hormone application in O. sativa (Pan et al. 2022a). This system has promising application prospects in crop breeding.
Over the past decade, NPs have been widely used to deliver genes and proteins into plant cells (Demirer et al. 2019a; Kwak et al. 2019; Martin-Ortigosa et al. 2014). However, most of them are transient transformations of foreign genes. To fully leverage NPs for plant genetic engineering, transgenes have to be stably inherited to the next generation. Currently, MNPs have successfully achieved stable genetic transformation in G. hirsutum, C. annuum, C. moschata, and Z. mays (Wang et al. 2022b; Zhao et al. 2017). However, a recent study reported that the transfection of Lilium brownii, S. bicolor, and Z. mays pollens by MNPs was unsuccessful (Vejlupkova et al. 2020), possibly due to the structure of the single aperture on the pollen wall and the entry of exogenous DNA is blocked when the aperture is covered by wall material or the operculum. Indeed, promoting aperture open by pretreating maize pollens at cool temperatures facilitates exogenous DNA entry and expression (Wang et al. 2022b), which opens a window for applying NP-mediated plant transformation in troublesome plant species. Overall, either manipulation of developmental regulatory genes or nanotechnology facilitates genotype-independent plant genetic transformation and further promotes functional genome research and crop breeding.
Abbreviations
- ABI
Abscisic acid insensitive
- AGL
Agamous-like
- ARF
Auxin response factor
- ARR
Arabidopsis response regulator
- ATP
Adenosine triphosphate
- ATXR
Arabidopsis trithorax-related
- Au-MSN
Gold-functionalized mesoporous silica nanoparticle
- AuNC
Gold nanocluster
- AuxRE
Auxin response element
- BBM
Baby boom
- CD
Carbon dot
- CIM
Callus-inducing medium
- CPP
Cell-penetrating peptide
- CTP
Chloroplast-targeting peptide
- CUC
Cup-shaped cotyledon
- CYCD
Cyclin D
- DMAEM
Dimethylaminoethyl metacrylate
- DRN
Dornröschen
- dsRNA
Double-stranded RNA
- ESR
Enhancer of shoot regeneration
- FUS
FUSCA
- GFP
Green fluorescent protein
- GIF
GRF-interacting factor
- GN
Grain number
- GON
Graphene oxide nanoparticle
- GRF
Growth-regulating factor
- GW
Grain weight
- HDAC
Histone deacetylases
- H3K27me3
Histone H3 lysine 27 trimethylation
- H3K36me3
Histone H3 lysine 36 trimethylation
- iPB
in planta Particle bombardment
- IPT
Isopentenyl transferase
- JMJ
JUMONJI C domain-containing protein
- LBD
Lateral organ boundaries domain
- LDH
Layered double hydroxide
- LEC
Leafy cotyledon
- LRR
Leu-rich repeat
- MC
Micelle complex
- MET
Methyltransferase
- MNP
Magnetic nanoparticle
- MP
Monopteros
- NM
Nanomaterial
- NP
Nanoparticle
- NTH
Nicotiana tabacum Homeobox
- PEG
Polyethylene glycol
- PEI
Polyethylenimine
- PIN
PIN-PORMED
- PLK
Receptor-like kinase
- PLT
PLETHORA
- PRC
POLYCOMB REPRESSIVE COMPLEX
- RIM
Root-inducing medium
- SAM
Shoot apical meristem
- SERK
Somatic embryogenesis receptor-like kinase
- SIM
Shoot-inducing medium
- siRNA
Small interfering RNA
- STM
Shoot meristemless
- SWNT
Single-walled carbon nanotube
- TSA
Trichostatin A
- Vir
Virulence
- WIND
Wound-induced dedifferentiation
- WOX
Wuschel related homeobox
- WUS
WUSCHEL
- YUC
YUCCA
- ZIL
Zwitterionic liquid
Author contributions
QH and XWan conceived and designed the review. TY, XWei and AP contributed to collecting and analyzing the literature of original studies. TY and YQ contributed to figure preparation. TY and QH wrote the manuscript. SW, XA and XWan revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFF1003500 and 2022YFF1002400), the National Natural Science Foundation of China (31900610 and 31871702) and the Beijing Nova Program (Z201100006820114).
Data availability
This manuscript is a review, no data was used for the research described in the article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tingwei Yan and Quancan Hou contributed equally to this article.
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Al-Salim N, Barraclough E, Burgess E, Clothier B, Deurer M, Green S, Malone L, Weir G. Quantum dot transport in soil, plants, and insects. Sci Total Environ. 2011;409:3237–3248. doi: 10.1016/j.scitotenv.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Altpeter F, Springer NM, Bartley LE, Blechl AE, Brutnell TP, Citovsky V, Conrad LJ, Gelvin SB, Jackson DP, Kausch AP, Lemaux PG, Medford JI, Orozco-Cardenas ML, Tricoli DM, Van Eck J, Voytas DF, Walbot V, Wang K, Zhang ZJ, Stewart CN., Jr Advancing crop transformation in the era of genome editing. Plant Cell. 2016;28:1510–1520. doi: 10.1105/tpc.16.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Dong Z, Tian Y, Xie K, Wu S, Zhu T, Zhang D, Zhou Y, Niu C, Ma B, Hou Q, Bao J, Zhang S, Li Z, Wang Y, Yan T, Sun X, Zhang Y, Li J, Wan X. ZmMs30 encoding a novel GDSL lipase is essential for male fertility and valuable for hybrid breeding in maize. Mol Plant. 2019;12:343–359. doi: 10.1016/j.molp.2019.01.011. [DOI] [PubMed] [Google Scholar]
- An X, Ma B, Duan M, Dong Z, Liu R, Yuan D, Hou Q, Wu S, Zhang D, Liu D, Yu D, Zhang Y, Xie K, Zhu T, Li Z, Zhang S, Tian Y, Liu C, Li J, Yuan L, Wan X. Molecular regulation of ZmMs7 required for maize male fertility and development of a dominant male-sterility system in multiple species. Proc Natl Acad Sci USA. 2020;117:23499–23509. doi: 10.1073/pnas.2010255117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aregawi K, Shen J, Pierroz G, Sharma MK, Dahlberg J, Owiti J, Lemaux PG. Morphogene-assisted transformation of Sorghum bicolor allows more efficient genome editing. Plant Biotechnol J. 2022;20:748–760. doi: 10.1111/pbi.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Herrera A, Ku Gonzalez A, Canche Moo R, Quiroz-Figueroa FR, Loyola-Vargas VM, Rodriguez-Zapata LC, Burgeff D’Hondt C, Suárez-Solís VM, Castaño E. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Culture. 2008;94:171–180. doi: 10.1007/s11240-008-9401-1. [DOI] [Google Scholar]
- Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc'h A, Carnero E, Giraudat-Pautot V, Rech P, Chriqui D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009;57:626–644. doi: 10.1111/j.1365-313X.2008.03715.x. [DOI] [PubMed] [Google Scholar]
- Banno H, Ikeda Y, Niu QW, Chua NH. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell. 2001;13:2609–2618. doi: 10.1105/tpc.010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, De Smet I. Pericycle. Curr Biol. 2014;24:R378–379. doi: 10.1016/j.cub.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Bishop PD, Makus DJ, Pearce G, Ryan CA. Proteinase inhibitorinducing factor activity in tomato leaves resides in oligosaccharides enzymically released from cell walls. Proc Natl Acad Sci USA. 1981;78:3536–3540. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchabke-Coussa O, Obellianne M, Linderme D, Montes E, Maia-Grondard A, Vilaine F, Pannetier C. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutumL.) tissues cultured in vitro. Plant Cell Rep. 2013;32:675–686. doi: 10.1007/s00299-013-1402-9. [DOI] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JB, van Lookeren Campagne MM. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook SA, Stone SL, Park S, Bui AQ, Le BH, Fischer RL, Goldberg RB, Harada JJ. Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci USA. 2006;103:3468–3473. doi: 10.1073/pnas.0511331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MT, Chang HH, Ho SL, Tong WF, Yu SM. Agrobacterium-mediated production of transgenic rice plants expressing a chimeric α-amylase promoter/β-glncuronidase gene. Plant Mol Biol. 1993;22:491–506. doi: 10.1007/BF00015978. [DOI] [PubMed] [Google Scholar]
- Chang FP, Kuang LY, Huang CA, Jane WN, Hung Y, Hsing YC, Mou CY. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J Mater Chem B. 2013;1:5279–5287. doi: 10.1039/c3tb20529k. [DOI] [PubMed] [Google Scholar]
- Che P, Lall S, Howell SH. Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta. 2007;226:1183–1194. doi: 10.1007/s00425-007-0565-4. [DOI] [PubMed] [Google Scholar]
- Che P, Chang S, Simon MK, Zhang Z, Shaharyar A, Ourada J, O'Neill D, Torres-Mendoza M, Guo Y, Marasigan KM, Vielle-Calzada JP, Ozias-Akins P, Albertsen MC, Jones TJ. Developing a rapid and highly efficient cowpea regeneration, transformation and genome editing system using embryonic axis explants. Plant J. 2021;106:817–830. doi: 10.1111/tpj.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che P, Wu E, Simon MK, Anand A, Lowe K, Gao H, Sigmund AL, Yang M, Albertsen MC, Gordon-Kamm W, Jones TJ. Wuschel2 enables highly efficient CRISPR/Cas-targeted genome editing during rapid de novo shoot regeneration in sorghum. Commun Biol. 2022;5:344. doi: 10.1038/s42003-022-03308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet. 2014;15:93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tong J, Xiao L, Ruan Y, Liu J, Zeng M, Huang H, Wang JW, Xu L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J Exp Bot. 2016;67:4273–4284. doi: 10.1093/jxb/erw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G. Identification of a plant receptor for extracellular ATP. Science. 2014;343:290–294. doi: 10.1126/science.343.6168.290. [DOI] [PubMed] [Google Scholar]
- Ckurshumova W, Smirnova T, Marcos D, Zayed Y, Berleth T. Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytol. 2014;204:556–566. doi: 10.1111/nph.13014. [DOI] [PubMed] [Google Scholar]
- Cody JP, Maher MF, Nasti RA, Starker CG, Chamness JC, Voytas DF. Direct delivery and fast-treated Agrobacterium co-culture (Fast-TrACC) plant transformation methods for Nicotiana benthamiana. Nat Protoc. 2023;18:81–107. doi: 10.1038/s41596-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curaba J, Moritz T, Blervaque R, Parcy F, Raz V, Herzog M, Vachon G. AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol. 2004;136:3660–3669. doi: 10.1104/pp.104.047266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimon Y, Takabe K, Tasaka M. The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on calli. Plant Cell Physiol. 2003;44:113–121. doi: 10.1093/pcp/pcg038. [DOI] [PubMed] [Google Scholar]
- Debernardi JM, Tricoli DM, Ercoli MF, Hayta S, Ronald P, Palatnik JF, Dubcovsky J. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat Biotechnol. 2020;38:1274–1279. doi: 10.1038/s41587-020-0703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirer GS, Zhang H, Goh NS, Gonzalez-Grandio E, Landry MP. Carbon nanotube-mediated DNA delivery without transgene integration in intact plants. Nat Protoc. 2019;14:2954–2971. doi: 10.1038/s41596-019-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirer GS, Zhang H, Matos JL, Goh NS, Cunningham FJ, Sung YH, Chang R, Aditham AJ, Chio L, Cho MJ, Staskawicz B, Landry MP. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat Nanotechnol. 2019;14:456–464. doi: 10.1038/s41565-019-0382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirer GS, Zhang H, Goh NS, Pinals RL, Chang R, Landry MP. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci Adv. 2020;6:eaaz0495. doi: 10.1126/sciadv.aaz0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Luo KM, Li ZG, Yang YW. A novel method for induction of plant regeneration via somatic embryogenesis. Plant Sci. 2009;177:43–48. doi: 10.1016/j.plantsci.2009.03.009. [DOI] [Google Scholar]
- Eichert T, Kurtz A, Steiner U, Goldbach HE. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol Plant. 2008;134:151–160. doi: 10.1111/j.1399-3054.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Elhiti M, Stasolla C. In vitro shoot organogenesis and hormone response are affected by the altered levels of Brassica napus meristem genes. Plant Sci. 2012;190:40–51. doi: 10.1016/j.plantsci.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Etxeberria E, Gonzalez P, Bhattacharya P, Sharma P, Ke PC. Determining the size exclusion for nanoparticles in citrus leaves. HortScience. 2016;51:732–737. doi: 10.21273/HORTSCI.51.6.732. [DOI] [Google Scholar]
- Fan M, Xu C, Xu K, Hu Y. LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 2012;22:1169–1180. doi: 10.1038/cr.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Wu S, Niu C, Hou Q, An X, Wei X, Zhao L, Jiang Y, Liu X, Wan X. Triphasic regulation of ZmMs13 encoding an ABCG transporter is sequentially required for callose dissolution, pollen exine and anther cuticle formation in maize. J Adv Res. 2022 doi: 10.1016/j.jare.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher A. Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Front Plant Sci. 2019;10:536. doi: 10.3389/fpls.2019.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Xiao L, He Y, Liu M, Wang J, Tian S, Zhang X, Yuan L. Highly efficient, genotype-independent transformation and gene editing in watermelon (Citrulluslanatus) using a chimeric ClGRF4-GIF1 gene. J Integr Plant Biol. 2021;63:2038–2042. doi: 10.1111/jipb.13199. [DOI] [PubMed] [Google Scholar]
- Finiuk N, Buziashvili A, Burlaka O, Zaichenko A, Mitina N, Miagkota O, Lobachevska O, Stoika R, Blume Y, Yemets A. Investigation of novel oligoelectrolyte polymer carriers for their capacity of DNA delivery into plant cells. Plant Cell Tissue Organ Culture (PCTOC) 2017;131:27–39. doi: 10.1007/s11240-017-1259-7. [DOI] [Google Scholar]
- Firoozi B, Nasser Z, Sofalian O, Sheikhzade-Mosadegh P. Enhancement of the transfection efficiency of DNA into Crocus sativus L. cells via PEI nanoparticles. J Integr Agric. 2018;17:1768–1778. doi: 10.1016/S2095-3119(18)61985-9. [DOI] [Google Scholar]
- Fleischer A, O’Neill MA, Ehwald R. The pore size of nongraminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez SL, Erwin RL, Maximova SN, Guiltinan MJ, Curtis WR. Enhanced somatic embryogenesis in Theobroma cacao using the homologous BABY BOOM transcription factor. BMC Plant Biol. 2015;15:121. doi: 10.1186/s12870-015-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML, Brand LA, Fink CL, Fry JS, Galluppi GR, Goldberg SB, Hoffmann NL, Woo SC. Expression of bacterial genes in plant cells. Proc Natl Acad Sci USA. 1983;80:4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Taylor LP, Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci USA. 1985;82:5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Itoh T. Changes in pectin structure during epidermal cell elongation in pea (Pisum sativum) and its implications for cell wall architecture. Plant Cell Physiol. 1998;39:1315–1323. doi: 10.1093/oxfordjournals.pcp.a029336. [DOI] [Google Scholar]
- Gaillochet C, Lohmann JU. The never-ending story: from pluripotency to plant developmental plasticity. Development. 2015;142:2237–2249. doi: 10.1242/dev.117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- Ge X, Xu J, Yang Z, Yang X, Wang Y, Chen Y, Wang P, Li F. Efficient genotype-independent cotton genetic transformation and genome editing. J Integr Plant Biol. 2023;65:907–917. doi: 10.1111/jipb.13427. [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, Wang Q, Yao Y, Zhang W, Geisler M, Li K, Huang Y, Chen Y, Kolmakov A, Ma X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology. 2013;7:323–337. doi: 10.3109/17435390.2012.658094. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Melendi P, Fernandez-Pacheco R, Coronado MJ, Corredor E, Testillano PS, Risueno MC, Marquina C, Ibarra MR, Rubiales D, Perez-de-Luque A. Nanoparticles as smart treatment-delivery systems in plants: assessment of different techniques of microscopy for their visualization in plant tissues. Ann Bot. 2008;101:187–195. doi: 10.1093/aob/mcm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 2007;134:3539–3548. doi: 10.1242/dev.010298. [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Daines RJ, Start WG, O’Brien JV, Chambers SA, Adams WR, Willetts NG, Rice TB, Mackey CJ, Krueger RW, Kausch AP, Lemaux PG. Transformation of Maize cells and regeneration of fertile transgenic plants. Plant Cell. 1990;2:603–618. doi: 10.2307/3869124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Liu Y, Nagira Y, Miki R, Taoka N, Imai R. Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci Rep. 2018;8:14422. doi: 10.1038/s41598-018-32714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol. 2003;133:653–663. doi: 10.1104/pp.103.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Chen X, Huang H, Xu L. Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genet. 2012;8:e1002911. doi: 10.1371/journal.pgen.1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC. The Arabidopsis somatic embryogenesis receptor kinase 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001;127:803–816. doi: 10.1104/pp.010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann I, de Lange B, Lambalk J, Angenent GC, Boutilier K. Efficient sweet pepper transformation mediated by the BABY BOOM transcription factor. Plant Cell Rep. 2011;30:1107–1115. doi: 10.1007/s00299-011-1018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L, Depicker AV, Montagu M, Schell J. Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature. 1983;303:209–213. doi: 10.1038/303209a0. [DOI] [PubMed] [Google Scholar]
- Hoerster G, Wang N, Ryan L, Wu E, Anand A, McBride K, Lowe K, Jones T, Gordon-Kamm B. Use of non-integrating Zm-Wus2 vectors to enhance maize transformation. In Vitro Cell Dev Biol Plant. 2020;56:265–279. doi: 10.1007/s11627-019-10042-2. [DOI] [Google Scholar]
- Holec S, Berger F. Polycomb group complexes mediate developmental transitions in plants. Plant Physiol. 2012;158:35–43. doi: 10.1104/pp.111.186445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method of transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Horstman A, Li M, Heidmann I, Weemen M, Chen B, Muino JM, Angenent GC, Boutilier K. The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol. 2017;175:848–857. doi: 10.1104/pp.17.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Wan X. Epigenome and epitranscriptome: potential resources for crop improvement. Int J Mol Sci. 2021;22:12912. doi: 10.3390/ijms222312912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Zhang T, Qi Y, Dong Z, Wan X. Epigenetic dynamics and regulation of plant male reproduction. Int J Mol Sci. 2022;23:10420. doi: 10.3390/ijms231810420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Zhang T, Sun K, Yan T, Wang L, Lu L, Zhao W, Qi Y, Wei X, Wan X. Mining of potential gene resources for breeding nutritionally improved maize. Plants. 2022;11:627. doi: 10.3390/plants11050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 2005;222:107–117. doi: 10.1007/s00425-005-1534-4. [DOI] [PubMed] [Google Scholar]
- Hu W, Li W, Xie SX, Fagundez S, McAvoy R, Deng ZN, Li Y. Kn1 gene overexpression drastically improves genetic transformation efficiencies of citrus cultivars. Plant Cell Tissue Organ Culture (PCTOC) 2016;125:81–91. doi: 10.1007/s11240-015-0931-z. [DOI] [Google Scholar]
- Hussien ET. Production of transgenic Paulownia tomentosa (Thunb.) steud. using chitosan nanoparticles to express antimicrobial genes resistant to bacterial infection. Mol Biol Res Commun. 2020;9:55–62. doi: 10.22099/mbrc.2019.35331.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien ET, Hammad IBA. Production of transgenic allium cepa by nanoparticles to resist Aspergillus Niger infection. Mol Biol Rep. 2022;49:1783–1790. doi: 10.1007/s11033-021-06988-5. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Banno H, Niu QW, Howell SH, Chua NH. The ENHANCER OF SHOOT REGENERATION 2 gene in Arabidopsis regulates CUP-SHAPED COTYLEDON 1 at the transcriptional level and controls cotyledon development. Plant Cell Physiol. 2006;47:1443–1456. doi: 10.1093/pcp/pcl023. [DOI] [PubMed] [Google Scholar]
- Ikeda-Iwai M, Satoh S, Kamada H. Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. J Exp Bot. 2002;53:1575–1580. doi: 10.1093/jxb/erf006. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25:3159–3173. doi: 10.1105/tpc.113.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Rymen B, Harashima H, Shibata M, Ohnuma M, Breuer C, Morao AK, de Lucas M, De Veylder L, Goodrich J, Brady SM, Roudier F, Sugimoto K. PRC2 represses dedifferentiation of mature somatic cells in Arabidopsis. Nat Plants. 2015;1:15089. doi: 10.1038/nplants.2015.89. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Sugimoto K. Control of plant cell differentiation by histone modification and DNA methylation. Curr Opin Plant Biol. 2015;28:60–67. doi: 10.1016/j.pbi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K. Plant regeneration: cellular origins and molecular mechanisms. Development. 2016;143:1442–1451. doi: 10.1242/dev.134668. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Rymen B, Lambolez A, Kojima M, Takebayashi Y, Heyman J, Watanabe S, Seo M, De Veylder L, Sakakibara H, Sugimoto K. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017;175:1158–1174. doi: 10.1104/pp.17.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, Ohme-Takagi M. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol. 2011;21:508–514. doi: 10.1016/j.cub.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Iwase A, Ohme-Takagi M, Sugimoto K. WIND1: a key molecular switch for plant cell dedifferentiation. Plant Signal Behav. 2011;6:1943–1945. doi: 10.4161/psb.6.12.18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Harashima H, Ikeuchi M, Rymen B, Ohnuma M, Komaki S, Morohashi K, Kurata T, Nakata M, Ohme-Takagi M, Grotewold E, Sugimoto K. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell. 2017;29:54–69. doi: 10.1105/tpc.16.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, An X, Li Z, Yan T, Zhu T, Xie K, Liu S, Hou Q, Zhao L, Wu S, Liu X, Zhang S, He W, Li F, Li J, Wan X. CRISPR/Cas9-based discovery of maize transcription factors regulating male sterility and their functional conservation in plants. Plant Biotechnol J. 2021;19:1769–1784. doi: 10.1111/pbi.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker A, Monke G, Rutten T, Keilwagen J, Seifert M, Thi TM, Renou JP, Balzergue S, Viehover P, Hahnel U, Ludwig-Muller J, Altschmied L, Conrad U, Weisshaar B, Baumlein H. Elongation-related functions of LEAFY COTYLEDON1 during the development of Arabidopsis thaliana. Plant J. 2012;71:427–442. doi: 10.1111/j.1365-313X.2012.04999.x. [DOI] [PubMed] [Google Scholar]
- Kadosh D, Struhl K. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin invivo. Mol Cell Biol. 1998;18:5121–5127. doi: 10.1128/MCB.18.9.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppler HF, Gu W, Somers DA, Rines HW, Cockburn AF. Silicon carbide fiber-mediated DNA delivery into plant cells. Plant Cell Rep. 1990;9:415–418. doi: 10.1007/BF00232262. [DOI] [PubMed] [Google Scholar]
- Kareem A, Durgaprasad K, Sugimoto K, Du Y, Pulianmackal AJ, Trivedi ZB, Abhayadev PV, Pinon V, Meyerowitz EM, Scheres B, Prasad K. PLETHORA genes control regeneration by a two-step mechanism. Curr Biol. 2015;25:1017–1030. doi: 10.1016/j.cub.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TM, Wolf ED, Wu R, Sanford JC. High-velocity microprojectiles for delivering nucleic-acids into living cells. Nature. 1987;327:70–73. doi: 10.1038/327070a0. [DOI] [PubMed] [Google Scholar]
- Kong J, Martin-Ortigosa S, Finer J, Orchard N, Gunadi A, Batts LA, Thakare D, Rush B, Schmitz O, Stuiver M, Olhoft P, Pacheco-Villalobos D. Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front Plant Sci. 2020;11:572319. doi: 10.3389/fpls.2020.572319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NT, Ckurshumova W, Marcos D, Caragea AE, Berleth T. Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol. 2012;194:391–401. doi: 10.1111/j.1469-8137.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- Kwak SY, Lew TTS, Sweeney CJ, Koman VB, Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua NH, Strano MS. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat Nanotechnol. 2019;14:447–455. doi: 10.1038/s41565-019-0375-4. [DOI] [PubMed] [Google Scholar]
- Kyo M, Maida K, Nishioka Y, Matsui K. Coexpression of WUSCHEL related homeobox (WOX) 2 with WOX8 or WOX9 promotes regeneration from leaf segments and free cells in NicotianatabacumL. Plant Biotechnol (tokyo) 2018;35:23–30. doi: 10.5511/plantbiotechnology.18.0126a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue C, Castillo-Michel H, Sobanska S, Cecillon L, Bureau S, Barthes V, Ouerdane L, Carriere M, Sarret G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: evidence for internalization and changes in Ag speciation. J Hazard Mater. 2014;264:98–106. doi: 10.1016/j.jhazmat.2013.10.053. [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KFX, Berger J, Jurgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122:87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- Lee K, Seo PJ. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 2018;23:235–247. doi: 10.1016/j.tplants.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Lee K, Park OS, Seo PJ. Arabidopsis ATXR2 deposits H3K36me3 at the promoters of LBD genes to facilitate cellular dedifferentiation. Sci Signal. 2017;10:eaan0316. doi: 10.1126/scisignal.aan0316. [DOI] [PubMed] [Google Scholar]
- Lee K, Park OS, Seo PJ. JMJ30-mediated demethylation of H3K9me3 drives tissue identity changes to promote callus formation in Arabidopsis. Plant J. 2018;95:961–975. doi: 10.1111/tpj.14002. [DOI] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- Li W, Liu H, Cheng ZJ, Su YH, Han HN, Zhang Y, Zhang XS. DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. 2011;7:e1002243. doi: 10.1371/journal.pgen.1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Soriano M, Cordewener J, Muino JM, Riksen T, Fukuoka H, Angenent GC, Boutilier K. The histone deacetylase inhibitor trichostatin a promotes totipotency in the male gametophyte. Plant Cell. 2014;26:195–209. doi: 10.1105/tpc.113.116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhu T, Liu S, Jiang Y, Liu H, Zhang Y, Xie K, Li J, An X, Wan X. Genome-wide analyses on transcription factors and their potential microRNA regulators involved in maize male fertility. Crop J. 2021;9:1248–1262. doi: 10.1016/j.cj.2021.03.016. [DOI] [Google Scholar]
- Li S, Li J, Du M, Deng G, Song Z, Han H. Efficient gene silencing in intact plant cells using siRNA delivered by functional graphene oxide nanoparticles. Angew Chem Int Ed Engl. 2022;61:e202210014. doi: 10.1002/anie.202210014. [DOI] [PubMed] [Google Scholar]
- Lian Z, Nguyen CD, Liu L, Wang G, Chen J, Wang S, Yi G, Wilson S, Ozias-Akins P, Gong H, Huo H. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol J. 2022;20:1622–1635. doi: 10.1111/pbi.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small. 2009;5:1128–1132. doi: 10.1002/smll.200801556. [DOI] [PubMed] [Google Scholar]
- Liu CM, Xu ZH, Chua NH. Auxin Polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993;5:621–630. doi: 10.2307/3869805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sheng L, Xu Y, Li J, Yang Z, Huang H, Xu L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014;26:1081–1093. doi: 10.1105/tpc.114.122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang Y, Wu S, Wang J, Fang C, Zhang S, Xie R, Zhao L, An X, Wan X. The ZmMYB84-ZmPKSB regulatory module controls male fertility through modulating anther cuticle-pollen exine trade-off in maize anthers. Plant Biotechnol J. 2022;20:2342–2356. doi: 10.1111/pbi.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang S, Jiang Y, Yan T, Fang C, Hou Q, Wu S, Xie K, An X, Wan X. Use of CRISPR/Cas9-based gene editing to simultaneously mutate multiple homologous genes required for pollen development and male fertility in maize. Cells. 2022;11:439. doi: 10.3390/cells11030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Ohto M, Yee KM, West MAL, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/S0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho MJ, Scelonge C, Lenderts B, Chamberlin M, Cushatt J, Wang L, Ryan L, Khan T, Chow-Yiu J, Hua W, Yu M, Banh J, Bao Z, Brink K, Igo E, Rudrappa B, Shamseer PM, Bruce W, Newman L, Shen B, Zheng P, Bidney D, Falco C, Register J, Zhao ZY, Xu D, Jones T, Gordon-Kamm W. Morphogenic regulators baby boom and Wuschel improve monocot transformation. Plant Cell. 2016;28:1998–2015. doi: 10.1105/tpc.16.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K, La Rota M, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W. Rapid genotype "independent" Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell Dev Biol Plant. 2018;54:240–252. doi: 10.1007/s11627-018-9905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Vasil V, Vasil IK. Improved efficiency of somatic embryogenesis and plant regeneration in tissue cultures of maize (Zea mays L.) Theor Appl Genet. 1983;66:285–289. doi: 10.1007/BF00251161. [DOI] [PubMed] [Google Scholar]
- Luo K, Zheng X, Chen Y, Xiao Y, Zhao D, McAvoy R, Pei Y, Li Y. The maize Knotted1 gene is an effective positive selectable marker gene for Agrobacterium-mediated tobacco transformation. Plant Cell Rep. 2006;25:403–409. doi: 10.1007/s00299-005-0051-z. [DOI] [PubMed] [Google Scholar]
- Lutz KA, Martin C, Khairzada S, Maliga P. Steroid-inducible BABY BOOM system for development of fertile Arabidopsis thaliana plants after prolonged tissue culture. Plant Cell Rep. 2015;34:1849–1856. doi: 10.1007/s00299-015-1832-7. [DOI] [PubMed] [Google Scholar]
- Lv J, Zhang SZ, Luo L, Zhang J, Yang K, Christie P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ Sci Nano. 2015;2:68–77. doi: 10.1039/C4EN00064A. [DOI] [Google Scholar]
- Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF. Plant gene editing through de novo induction of meristems. Nat Biotechnol. 2020;38:84–89. doi: 10.1038/s41587-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ortigosa S, Valenstein JS, Lin VSY, Trewyn BG, Wang K. Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv Func Mater. 2012;22:3576–3582. doi: 10.1002/adfm.201200359. [DOI] [Google Scholar]
- Martin-Ortigosa S, Peterson DJ, Valenstein JS, Lin VSY, Trewyn BG, Lyznik LA, Wang K. Mesoporous silica nanoparticle-mediated intracellular cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014;164:537–547. doi: 10.1104/pp.113.233650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng WJ, Cheng ZJ, Sang YL, Zhang MM, Rong XF, Wang ZW, Tang YY, Zhang XS. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem Cell Niche by dual regulation of WUSCHEL. Plant Cell. 2017;29:1357–1372. doi: 10.1105/tpc.16.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Tsuchiya K, Toyooka K, Goto Y, Tateishi A, Numata K. Relaxation of the plant cell wall barrier via zwitterionic liquid pretreatment for micelle-complex-mediated DNA delivery to specific plant organelles. Angew Chem Int Ed Engl. 2022;61:e202204234. doi: 10.1002/anie.202204234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesini B, Pennisi F, Cressoni C, Vitulo N, Dusi V, Speghini A, Pandolfini T. Nanovector-mediated exogenous delivery of dsRNA induces silencing of target genes in very young tomato flower buds. Nanoscale Adv. 2022;4:4542–4553. doi: 10.1039/D2NA00478J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookkan M, Nelson-Vasilchik K, Hague J, Zhang ZJ, Kausch AP. Selectable marker independent transformation of recalcitrant maize inbred B73 and sorghum P898012 mediated by morphogenic regulators BABY BOOM and WUSCHEL2. Plant Cell Rep. 2017;36:1477–1491. doi: 10.1007/s00299-017-2169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookkan M, Nelson-Vasilchik K, Hague J, Kausch A, Zhang ZJ. Morphogenic regulator-mediated transformation of maize inbred B73. Curr Protoc Plant Biol. 2018;3:e20075. doi: 10.1002/cppb.20075. [DOI] [PubMed] [Google Scholar]
- Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–426. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Tamaoki M, Sakamoto T, Matsuoka M. Over-expression of tobacco knotted1-type class1 homeobox genes alters various leaf morphology. Plant Cell Physiol. 2000;41:583–590. doi: 10.1093/pcp/41.5.583. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Yasutani I, Fukuda H, Komamine A, Sugiyama M. Organogenic responses in tissue culture of srd mutants of Arabidopsis thaliana. Development. 1998;125:135–142. doi: 10.1242/dev.125.1.135. [DOI] [PubMed] [Google Scholar]
- Pan C, Li G, Malzahn AA, Cheng Y, Leyson B, Sretenovic S, Gurel F, Coleman GD, Qi Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat Plants. 2022;8:513–525. doi: 10.1038/s41477-022-01151-9. [DOI] [PubMed] [Google Scholar]
- Pan W, Cheng Z, Han Z, Yang H, Zhang W, Zhang H. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing of watermelon assisted by genes encoding developmental regulators. J Zhejiang Univ Sci B. 2022;23:339–344. doi: 10.1631/jzus.B2200119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pascual D, Jimenez-Guillen D, Villanueva-Alonzo H, Souza-Perera R, Godoy-Hernandez G, Zuniga-Aguilar JJ. Ectopic expression of the Coffea canephora SERK1 homolog-induced differential transcription of genes involved in auxin metabolism and in the developmental control of embryogenesis. Physiol Plant. 2018;163:530–551. doi: 10.1111/ppl.12709. [DOI] [PubMed] [Google Scholar]
- Raliya R, Franke C, Chavalmane S, Nair R, Reed N, Biswas P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front Plant Sci. 2016;7:1288. doi: 10.3389/fpls.2016.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid SZ, Yamaji N, Kyo M. Shoot formation from root tip region: a developmental alteration by WUS in transgenic tobacco. Plant Cell Rep. 2007;26:1449–1455. doi: 10.1007/s00299-007-0342-7. [DOI] [PubMed] [Google Scholar]
- Rundlett SE, Carmen AA, Suka N, Turner BM, Grunstein M. Transcription repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392:831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, Wiesner MR. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology. 2016;10:257–278. doi: 10.3109/17435390.2015.1048326. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Hendrix B, Hoffer P, Sanders RA, Zheng W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020;184:647–657. doi: 10.1104/pp.20.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires ME, Florez SL, Lai TS, Curtis WR. Inducible somatic embryogenesis in Theobroma cacao achieved using the DEX-activatable transcription factor-glucocorticoid receptor fusion. Biotechnol Lett. 2017;39:1747–1755. doi: 10.1007/s10529-017-2404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan C, Liu Z, Heidmann I, Supena ED, Fukuoka H, Joosen R, Lambalk J, Angenent G, Scorza R, Custers JB, Boutilier K. Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (NicotianatabacumL.) Planta. 2007;225:341–351. doi: 10.1007/s00425-006-0358-1. [DOI] [PubMed] [Google Scholar]
- Stachel SE, Messens E, Van Marc M, Zambryski P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature. 1985;318:624–629. doi: 10.1038/318624a0. [DOI] [Google Scholar]
- Steward FC, Mapes MO, Mears K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am J Bot. 1958;45:705–708. doi: 10.1002/j.1537-2197.1958.tb10599.x. [DOI] [Google Scholar]
- Stone SL, Braybrook SA, Paula SL, Kwong LW, Meuser J, Pelletier J, Hsieh T-F, Fischer RL, Goldberg RB, Harada JJ. Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: implications for somatic embryogenesis. Proc Natl Acad Sci USA. 2008;105:3151–3156. doi: 10.1073/pnas.0712364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, Zhao XY, Liu YB, Zhang CL, O'Neill SD, Zhang XS. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009;59:448–460. doi: 10.1111/j.1365-313X.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011;21:212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Sun D, Hussain HI, Yi Z, Siegele R, Cresswell T, Kong L, Cahill DM. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014;33:1389–1402. doi: 10.1007/s00299-014-1624-5. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kikuchi A, Kamada H. The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiol. 2008;146:149–161. doi: 10.1104/pp.107.111674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Choi J, Cao Y, Stacey G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front Plant Sci. 2014;5:446. doi: 10.3389/fpls.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakare D, Tang W, Hill K, Perry SE. The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol. 2008;146:1663–1672. doi: 10.1104/pp.108.115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torney F, Trewyn BG, Lin VS, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol. 2007;2:295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- Uddenberg D, Abrahamsson M, Arnold SV. Overexpression of PaHAP3A stimulates differentiation of ectopic embryos from maturing somatic embryos of Norway spruce. Tree Genet Genomes. 2016;12:18. doi: 10.1007/s11295-016-0974-2. [DOI] [Google Scholar]
- Valvekens D, Montagu MV, Lusebettens MV. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejlupkova Z, Warman C, Sharma R, Scheller HV, Mortimer JC, Fowler JE. No evidence for transient transformation via pollen magnetofection in several monocot species. Nat Plants. 2020;6:1323–1324. doi: 10.1038/s41477-020-00798-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Caruso LV, Downie AB, Perry SE. The embryo MADS domain protein AGAMOUS-Like 15 directly regulates expression of a gene encoding an enzyme involved in gibberellin metabolism. Plant Cell. 2004;16:1206–1219. doi: 10.1105/tpc.021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Huang J, Zhang M, Wang Y, Wang H, Ma Y, Zhao X, Wang X, Liu C, Huang H, Liu Y, Lu F, Yu H, Shao M, Kang Z. Carbon dots enable efficient delivery of functional DNA in plants. ACS Appl Bio Mater. 2020;3:8857–8864. doi: 10.1021/acsabm.0c01170. [DOI] [PubMed] [Google Scholar]
- Wang K, Shi L, Liang X, Zhao P, Wang W, Liu J, Chang Y, Hiei Y, Yanagihara C, Du L, Ishida Y, Ye X. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat Plants. 2022;8:110–117. doi: 10.1038/s41477-021-01085-8. [DOI] [PubMed] [Google Scholar]
- Wang ZP, Zhang ZB, Zheng DY, Zhang TT, Li XL, Zhang C, Yu R, Wei JH, Wu ZY. Efficient and genotype independent maize transformation using pollen transfected by DNA-coated magnetic nanoparticles. J Integr Plant Biol. 2022;64:1145–1156. doi: 10.1111/jipb.13263. [DOI] [PubMed] [Google Scholar]
- Wang N, Ryan L, Sardesai N, Wu E, Lenderts B, Lowe K, Che P, Anand A, Worden A, van Dyk D, Barone P, Svitashev S, Jones T, Gordon-Kamm W. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat Plants. 2023;9:255–270. doi: 10.1038/s41477-022-01338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Pu A, Liu Q, Hou Q, Zhang Y, An X, Long Y, Jiang Y, Dong Z, Wu S, Wan X. The bibliometric landscape of gene editing innovation and regulation in the worldwide. Cells. 2022;11:2682. doi: 10.3390/cells11172682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Long Y, Yi C, Pu A, Hou Q, Liu C, Jiang Y, Wu S, Wan X. Bibliometric analysis of functional crops and nutritional quality: identification of gene resources to improve crop nutritional quality through gene editing technology. Nutrients. 2023;15:373. doi: 10.3390/nu15020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke W, Brettell R. Somatic embryogenesis from Sorghum bicolor leaves. Nature. 1980;287:138–139. doi: 10.1038/287138a0. [DOI] [Google Scholar]
- Xu C, Hu Y. The molecular regulation of cell pluripotency in plants. aBIOTECH. 2020;1:169–177. doi: 10.1007/s42994-020-00028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol. 2005;15:1566–1571. doi: 10.1016/j.cub.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Yang Z, Li C, Wang Y, Zhang C, Wu Z, Zhang X, Liu C, Li F. GhAGL15s, preferentially expressed during somatic embryogenesis, promote embryogenic callus formation in cotton (GossypiumhirsutumL.) Mol Genet Genomics. 2014;289:873–883. doi: 10.1007/s00438-014-0856-y. [DOI] [PubMed] [Google Scholar]
- Zemke-White WL, Clements KD, Harris PJ. Acid lysis of macroalgae by marine herbivorous fishes: effects of acid pH on cell wall porosity. J Exp Mar Bio Ecol. 2000;245:57–68. doi: 10.1016/S0022-0981(99)00151-3. [DOI] [Google Scholar]
- Zhai G, Walters KS, Peate DW, Alvarez PJ, Schnoor JL. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ Sci Technol Lett. 2014;1:146–151. doi: 10.1021/ez400202b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wu S, An X, Xie K, Dong Z, Zhou Y, Xu L, Fang W, Liu S, Liu S, Zhu T, Li J, Rao L, Zhao J, Wan X. Construction of a multicontrol sterility system for a maize male-sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD-finger transcription factor. Plant Biotechnol J. 2018;16:459–471. doi: 10.1111/pbi.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Demirer GS, Zhang H, Ye T, Goh NS, Aditham AJ, Cunningham FJ, Fan C, Landry MP. DNA nanostructures coordinate gene silencing in mature plants. Proc Natl Acad Sci USA. 2019;116:7543–7548. doi: 10.1073/pnas.1818290116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cao Y, Xu D, Goh NS, Demirer GS, Cestellos-Blanco S, Chen Y, Landry MP, Yang P. Gold-nanocluster-mediated delivery of siRNA to intact plant cells for efficient gene knockdown. Nano Lett. 2021;21:5859–5866. doi: 10.1021/acs.nanolett.1c01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wu S, Niu C, Liu D, Yan T, Tian Y, Liu S, Xie K, Li Z, Wang Y, Zhao W, Dong Z, Zhu T, Hou Q, Ma B, An X, Li J, Wan X. ZmMs25 encoding a plastid-localized fatty acyl reductase is critical for anther and pollen development in maize. J Exp Bot. 2021;72:4298–4318. doi: 10.1093/jxb/erab142. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU. Hormonal control of the shoot stem-cell niche. Nature. 2010;465:1089–1092. doi: 10.1038/nature09126. [DOI] [PubMed] [Google Scholar]
- Zhao X, Meng Z, Wang Y, Chen W, Sun C, Cui B, Cui J, Yu M, Zeng Z, Guo S, Luo D, Cheng JQ, Zhang R, Cui H. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat Plants. 2017;3:956–964. doi: 10.1038/s41477-017-0063-z. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ren N, Wang H, Stromberg AJ, Perry SE. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-Like15. Plant Cell. 2009;21:2563–2577. doi: 10.1105/tpc.109.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GY, Weng J, Zeng Y, Huang J, Qian S, Liu G. Introduction of exogenous DNA into cotton embryos. Methods Enzymol. 1983;101:433–481. doi: 10.1016/0076-6879(83)01032-0. [DOI] [PubMed] [Google Scholar]
- Zhu SP, Wang J, Ye JL, Zhu AD, Guo WW, Deng XX. Isolation and characterization of LEAFY COTYLEDON 1-LIKE gene related to embryogenic competence in Citrussinensis. Plant Cell Tissue Organ Culture (PCTOC) 2014;119:1–13. doi: 10.1007/s11240-014-0509-1. [DOI] [Google Scholar]
- Zhu T, Li Z, An X, Long Y, Xue X, Xie K, Ma B, Zhang D, Guan Y, Niu C, Dong Z, Hou Q, Zhao L, Wu S, Li J, Jin W, Wan X. Normal structure and function of endothecium chloroplasts maintained by ZmMs33-mediated lipid biosynthesis in tapetal cells are critical for Anther development in maize. Mol Plant. 2020;13:1624–1643. doi: 10.1016/j.molp.2020.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript is a review, no data was used for the research described in the article.