Abstract
Background
Days alive and at home (DAH) is a patient centered outcome measureable in routinely collected health data. The validity and minimally important difference (MID) in hip fracture have not been evaluated.
Objective
We assessed construct and predictive validity and estimated a MID for the patient-centred outcome of DAH after hip fracture admission.
Methods
This is a cross-sectional observational study using linked health administrative data in Ontario, Canada. DAH was calculated as the number of days alive within 90 days of admission minus the number of days hospitalised or institutionalised. All hospital admissions (2012–2018) for hip fracture in adults aged >50 years were included. Construct validity analyses used Bayesian quantile regression to estimate the associations of postulated patient, admission and process-related variables with DAH. The predictive validity assessed was the correlation of DAH in 90 days with the value from 91 to 365 days; and the association and discrimination of DAH in 90 days predicting subsequent mortality. MID was estimated by averaging distribution-based and clinical anchor-based estimates.
Results
We identified 63 778 patients with hip fracture. The median number of DAH was 43 (range 0–87). In the 90 days after admission, 8050 (12.6%) people died; a further 6366 (10.0%) died from days 91 to 365. Associations between patient-level and admission-level factors with the median DAH (lower with greater age, frailty and comorbidity, lower if admitted to intensive care or having had a complication) supported construct validity. DAH in 90 days after admission was strongly correlated with DAH in 365 days after admission (r=0.922). An 11-day MID was estimated.
Conclusion
DAH has face, construct and predictive validity as a patient-centred outcome in patients with hip fracture, with an estimated MID of 11 days. Future research is required to include direct patient perspectives in confirming MID.
Keywords: comparative effectiveness research, evaluation methodology, health services research, patient-centred care, surgery
Background
Hip fractures are common among older people, with a yearly incidence of 10 per 1000 for women and 5 per 1000 for men >65 years of age.1 As populations age, hip fractures continue to be an important public health issue. One in four patients with hip fracture experience a serious in-hospital medical or surgical complication, one in four die within a year, and one in two either die or experience a new admission to long-term care.2 3 While preventing hip fractures must be a key focus in improving the health of older adults, improving care and outcomes for older people who do experience a hip fracture is also a top priority.
Given the large number of hip fractures that occur, population-level studies of hip fracture care and outcomes are common.4–9 While core outcome sets (ie, an agreed minimum set of outcomes that should be measured and reported in all clinical trials of a specific disease or trial population10) have been developed for patients with hip fracture, mortality is the only core outcome measure typically available in population-level data.11 12 Furthermore, other routinely available population-level outcomes (eg, length of stay) are not patient-centred. Patient-reported outcomes that reflect function, disability and quality of life are particularly important for older people; however, beyond limited examples in elective surgery,13 routinely collected data, such as health administrative records, do not reliably capture such measures.14 Recent advances have led to the development of patient-centred outcomes that can be measured in linked health administrative data. Specifically, days alive at home (DAH) has been identified as a patient-centred15 and high priority outcome for older people,16 which can also be accurately ascertained from population-based and health administrative data. DAH has recently been validated in elective surgery and other conditions.17–20 This outcome provides a count of days where an individual is both alive and not institutionalised (ie, in an acute care hospital, rehabilitation centre or nursing facility). Although distinct from a patient-reported outcome, which is a direct report from a patient about how they feel or function in relation to their health condition,21 DAH has distinct advantages over traditional measures derived from routinely collected data as it contains more information than binary measures (such as in-hospital mortality, non-home discharge or readmission), while incorporating the postdischarge trajectory, which simple hospital length of stay metrics cannot.20
While DAH has been validated in elective surgery,17 18 this outcome measure has not been evaluated in patients with hip fracture, who differ substantially from elective surgery patients in their baseline characteristics, hospitalisation courses and recovery trajectories.22 Furthermore, no minimally important difference (MID) in DAH has been proposed. Therefore, we conducted a population-based cross-sectional study to validate DAH as a patient-centred outcome in patients with hip fracture and estimate an MID.
Methods
Design and setting
This was a population-based cross-sectional study using linked health administrative data in Ontario, Canada. In Ontario, hospital, physician and postacute care services are provided through a universal health insurance plan that covers all residents. A protocol was preregistered at the Center for Open Science (osf.io/mnvx4/). Reporting follows the recommendations for observational research using routinely collected data, as well as for Bayesian analyses.23–26 Healthcare data in Ontario are collected using standardised methods and are stored at ICES (formerly known as the Institute for Clinical Evaluative Sciences), an independent research institute. For the current study, data were linked deterministically using encrypted, patient-specific identifiers across the following databases: Discharge Abstract Database (DAD; acute care hospitalisation details including diagnoses, procedures and length of stay); Ontario Health Insurance Plan (OHIP; physician service claims); National Ambulatory Care Reporting System (all emergency and outpatient care); Continuing Care Reporting System (long-term and respite care); National Rehabilitation Reporting System (designated rehabilitation hospitals/beds); Ontario Drug Benefits Database (ODB; prescription drug claims); and Registered Persons Database, which captures all death dates for residents of Ontario. The second author, an ICES analyst, accessed the data and performed all analyses.
Cohort
We identified all Ontario residents aged 50 and older on the day of their hip fracture admission using International Classification of Diseases-Tenth Edition (ICD-10-CA) hip fracture diagnostic code S72 (an age cut-off of 50 is often used as most hip fractures in older people are fragility-related instead of high-energy trauma-specific).4 27 Reabstraction studies demonstrate high levels of agreement when identifying patients with hip fracture (kappa 0.95; positive predictive value 0.95, 95% CI 0.94 to 0.97).28 We created a patient-level analytic data set by including the first fracture for each individual during our study period (1 April 2012–31 March 2018).
Outcome
For each individual, we calculated the number of DAH in the 90 (primary outcome) and 365 (secondary outcome) days after their fracture (DAH90, DAH365). Because we anticipated that many patients with hip fracture would have hospital and institutional lengths of stay >30 days,6 we did not evaluate DAH30 as it is unlikely to be a plausibly responsive outcome metric in patients with hip fracture. DAH values were calculated by obtaining the number of days alive during each time window and then subtracting the number of days spent in an acute care hospital (index or readmission), rehabilitation or respite centre, or long-term care home. Individuals who died prior to discharge had DAH values of 0.
Covariates
We captured covariates that we postulated, based on clinical and epidemiological knowledge, may be associated with DAH.29 Demographics were identified from the DAD and from the Canadian Census. Standard methods were used to identify Elixhauser comorbidities using ICD-10 codes from the DAD in the 3 years preceding surgery.30 Preoperative residence in a long-term care facility was identified from the OHIP/ODB. A validated frailty index was calculated.31 Surgical procedures for hip fracture and a unique identifier for each hospital, surgeon and anaesthesiologist were recorded from the DAD. Details of anaesthesia care that may impact DAH (primary anaesthesia type, receipt of a peripheral nerve block) were also recorded. Any in-hospital complications32 33 or intensive care unit (ICU) admissions34 were also captured from the index hospitalisation’s DAD record.
Sample size
No sample size was prespecified. Instead, we included all available and eligible individuals during the study period. Based on experience with hip fracture data in Ontario,6 7 we expected to identify approximately 10 000 hip fractures per year, or approximately 50 000 over the duration of the study period.
Missing data
Our primary approach was a complete case analysis. As no covariate had >0.4% missing data, we did not pursue sensitivity analyses using multiple imputation, which were prespecified if >1.0% data were missing.
Analysis
Descriptive statistics were computed for subgroups defined by being below the median DAH90 value versus at the median or higher. Between-group differences in characteristics were compared using standardised differences, with values >0.10 being considered substantive.35 All regression analyses were conducted using the R programming language (R Foundation for Statistical Computing, Vienna, Austria) and the ‘brms’ package to allow a Bayesian framework for our analysis.36 In brms, quantile regression is performed using the asymmetric Laplace distribution and probabilities of non-null associations estimated using the hypothesis function. As we had no strong knowledge to inform our choice of prior distributions, weakly informative prior distributions were used for all fixed effects, which allowed more efficient propagation of Markov Chain Monte Carlo sampling by focusing estimation on plausible values without placing substantial influence on estimated posterior distributions.37 We tested the impact of prior distribution choice by repeating our main analysis with a non-informative prior distribution (ie, flat; normal distribution with a mean of 0 and SD of 106). A Bayesian approach allowed us to estimate 95% credible interval (CrI, which represents the range, based on our data and prior knowledge, with a 95% probability of containing the true value), as well as the probability of non-null associations between predictors and outcome.
Our validation analyses evaluated construct validity (how well an instrument measures a specific construct) and predictive validity (a submeasure of criterion validity), which reflects how well the instrument predicts future related outcomes.38
To assess construct validity, we estimated whether DAH measures differed in expected ways based on patient-level, clinician-level, intervention-level, admission-level and hospital-level factors available in our data. At the patient level, we estimated whether fewer DAH were experienced by older patients (with age expressed by decade), patients with greater frailty (with frailty expressed as a categorical variable: <0.10 (reference), >0.10–0.21, >0.21–0.45, >0.45), patients with multimorbidity (0 vs 1–2, vs ≥3 Elixhauser comorbidities30), patients with acute care hospitalisations in the year prior to fracture (vs none), patients with dementia,39 male versus female, rural versus urban residency, and lower versus higher quintile of neighbourhood income.40 At the clinician level, we estimated the extent that DAH measures varied by surgeon and anaesthesiologist (for those with surgical fixation). At the intervention level, we estimated whether DAH measures differed between operative and non-operative treatment, between types of surgical repair, between primary anaesthesia type (general, including combined general-neuraxial, vs isolated neuraxial), and by receipt versus non-receipt of a peripheral nerve block.6 41 At the admission level, we estimated whether DAH measures varied by surgical wait (in those who had surgical fixation: <1 day, 1–2 days, >2 days postadmission), whether any complication occurred and whether a patient was admitted to ICU. At the hospital level, we estimated whether DAH measures varied by total hip fracture volume quintile and the extent of variation between hospitals.
We computed differences using unadjusted and adjusted quantile regression with the median (ie, 0.5 quantile) specified, as DAH distributions were expected to be skewed.17 18 Three adjusted models were created. The first included terms for patient-level factors. The second combined patient-level, clinician-level, intervention-level, admission-level and hospital-level factors listed above with a random intercept for the index hospital. The third added surgeon and anaesthesiologist random intercepts to the second model but was limited to individuals who had surgical fixation (this allowed us to calculate intraclass correlation coefficients (ICC) for these random intercepts).
To assess predictive validity, we assessed the correlation between DAH90 and DAH365 using Spearman rank correlation (with this analysis limited to those who survived 90 days, as including those who did not survive to 90 days would upwardly bias the correlation coefficient estimate). Post-hoc, we calculated the correlation coefficient after subtracting the DAH90 value from the DAH365 value (as a high DAH90 value would upwardly bias the DAH365 value). Furthermore, for these individuals alive at the 90-day DAH ascertainment window, we calculated the ability of the quintile of DAH value in the 90-day period to discriminate subsequent mortality risk in the 365-day follow-up by calculating the c-statistic using Bayesian logistic regression. To test the impact of parameterising DAH90 as a quintile variable, we also repeated the predictive validity analysis with DAH90 as a restricted cubic spline with four knots.
Next, we estimated an MID. There are a variety of methods described for estimating MID, with no one technique thought to be superior.42–44 As recommended we used multiple approaches (ie, anchor-based and distribution-based techniques) to estimate a final MID through averaging across techniques.44–46 Distribution-based techniques included estimation of 0.3 times the SD and our initial protocol specified a 5% of the score range (as we had a sample consisting of the full population under study, we did not include methods involving the SEM).47 However, post-hoc our team met to discuss the inconsistencies between 5% range estimates and all other anchor-based and distribution-based estimates. As range-based distribution estimates specify 5%–10%,47 we decided to proceed with 10% range (instead of a 5% range) as our second distribution-based estimate. For anchor-based techniques, our retrospective data did not contain patient preferences; therefore, we used what Guyatt and colleagues43 describe as the single-step, population-focused approach based on disease-related criteria (or what has alternatively been called a clinical anchor).48 With this approach, multiple anchors are required that should be interpretable and appreciably associated with the target outcome. For this study, we estimated the adjusted median difference in DAH at the group level between people who, during the index admission, were or were not admitted to the ICU (level 2 units: ie, high-dependency monitored areas with higher nurse to patient ratios, but no support for mechanical ventilation; or level 3 units: ie, providing full critical care functionalities, including mechanical ventilation), as well as the adjusted median difference in DAH at the group level between people who did or did not have a complication documented during the index admission. These were selected as each is validly captured in administrative data32 34 49 and each represents a meaningful departure from an uneventful clinical course.50–52 As MIDs could differ between relevant subgroups, we also estimated MIDs within prespecified groups (male vs female, frailty vs none (≤0.21 vs >0.21), long-term care prefracture versus home, and surgical versus non-surgical patients).
Because defining ‘home’ for an individual residing in a long-term care facility prefracture is not directly obvious, we performed sensitivity analyses to evaluate assumptions made regarding the definition of home for baseline long-term care residents. We recalculated our estimates of association between patient-level, clinician-level, intervention-level, admission-level and hospital-level factors and DAH measures after postdischarge long-term care days were redefined as ‘home’ for those living in long-term care at baseline. Our averaged MID was also re-estimated using this alternative definition of home for long-term care patients. Finally, based on post-hoc knowledge of the 5% range used to define our MID, and our subsequent decision that a 10% range was most applicable to this distribution and data, we also re-estimated our primary MID value using a 5% range criterion initially specified.
Results
We identified 63 778 patients with hip fracture between 2012 and 2018. The mean and median numbers of DAH90 in the study period were 38 days (SD 33) and 43 days (IQR 0–68); values ranged from 0 to 87 (see distributions in online supplemental appendix figure 1). In the first 90 days after admission, 8050 (12.6%) people died, while a further 6366 (10.0%) died from days 91 to 365 postadmission. Compared with those with DAH90 values above the median, those with DAH90 values below the median were substantially older, lived with greater frailty and comorbidity, and were more likely to have been admitted to a long-term care home (table 1).
Table 1.
Cohort characteristics
| Below median days alive at home* (n=31 713) | Above median days alive at home* (n=32 065) | Absolute standardised difference | |
| Age, mean (SD) | 85 (9) | 78 (11) | 0.68 |
| Female, n (%) | 21 815 (68.8) | 22 409 (69.9) | 0.02 |
| Rural, n (%) | 3824 (12.1) | 4533 (14.1) | 0.06 |
| Neighbourhood income quintile, n (%) | |||
| Lowest | 8151 (25.7) | 7436 (23.2) | 0.06 |
| 2 | 6917 (21.8) | 6922 (21.6) | 0.01 |
| 3 | 6046 (19.1) | 6163 (19.2) | 0.00 |
| 4 | 5369 (16.9) | 5671 (17.7) | 0.02 |
| Highest | 5103 (16.1) | 5757 (18.0) | 0.05 |
| Frailty index, mean (SD) | 0.26 (0.08) | 0.21 (0.07) | 0.72 |
| Number of comorbidities, median (IQR) | 2.00 (1.00–3.00) | 1.00 (0.00–2.00) | 0.44 |
| Dementia, n (%) | 8487 (26.8) | 2398 (7.5) | 0.53 |
| Acute hospitalisation in previous year, n (%) | 9702 (30.6) | 6338 (19.8) | 0.25 |
| Prefracture long-term care residence, n (%) | 9567 (30.2) | 658 (2.1) | 0.83 |
| Surgical fixation, n (%) | 28 336 (89.4) | 28 907 (90.2) | 0.03 |
| In-hospital complication, n (%) | 10 773 (34.0) | 4932 (15.4) | 0.44 |
| ICU admission, n (%) | 7847 (24.7) | 203 (0.6) | 0.78 |
| Hospital annual hip fracture volume, mean (SD) | 190 (91) | 190 (93) | 0.00 |
Median value was 43 days.
*All values represent the per cent of individuals above or below the median days alive at home value with the described characteristics, unless otherwise stated.
ICU, intensive care unit.
bmjqs-2021-013150supp001.pdf (279.3KB, pdf)
Validity of DAH90
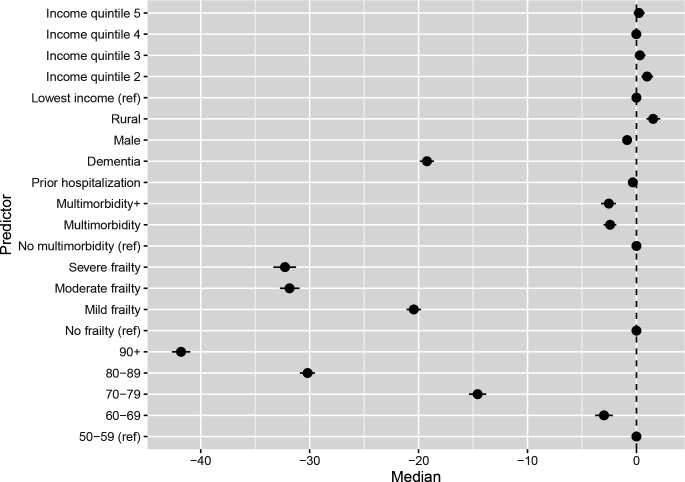
Unadjusted and multivariable adjusted analyses supported the construct validity of DAH90, as postulated patient-level predictors had strong evidence of association (ie, 95% CrIs excluding 0) with DAH90 in a directionally expected manner, and in a dose–response fashion for increasing categories of age, frailty and comorbidity (figure 1; unadjusted estimates in online supplemental appendix table 1; adjusted estimates in online supplemental appendix table 2; Bayesian model diagnostics in online supplemental appendix table 3).
Figure 1.
The forest plot depicts the median adjusted difference in days alive at home after hip fracture admission and the associated 95% credible intervals based on the highest probability density interval from the posterior distribution, with results adjusted for patient-level factors.
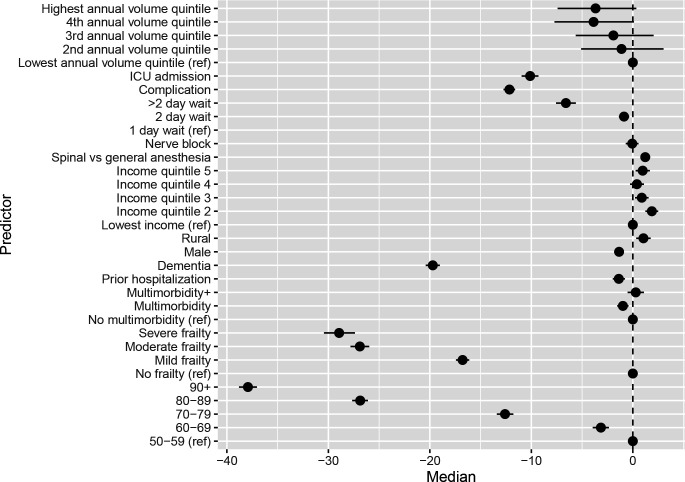
Adjusting for patient factors and clustering within hospitals, intervention-level factors were also associated with DAH90 in a directionally expected manner (eg, increased with surgical fixation, decreased with ICU admission and documentation of a complication). At the admission level, decreasing hospital volume of hip fracture care was associated with decreasing DAH90 (see all associations in figure 2 and online supplemental appendix table 4). Approximately 13% of variations in DAH90 were attributable to the index hospital (ICCHospital 13.2%, 95% CrI 9.8% to 17.4%). In patients who had surgical fixation, the index hospital continued to explain approximately 13% of outcome variations (ICCHospital 13.0%, 95% CrI 9.4% to 17.9%), with minimal variation attributable to surgeons or anaesthesiologists (ICCSurgeon 3.8%, 95% CrI 2.8% to 4.7%; ICCAnaesthesiologist 3.7%, 95% CrI 2.7% to 4.5%). Results using a non-informative prior were essentially unchanged (online supplemental appendix table 5).
Figure 2.
The forest plot depicts the median adjusted difference in days alive at home after hip fracture admission and the associated 95% credible intervals based on the highest probability density interval from the posterior distribution, adjusted for admission-level and procedure-level factors in those with surgical fixation. ICU, intensive care unit.
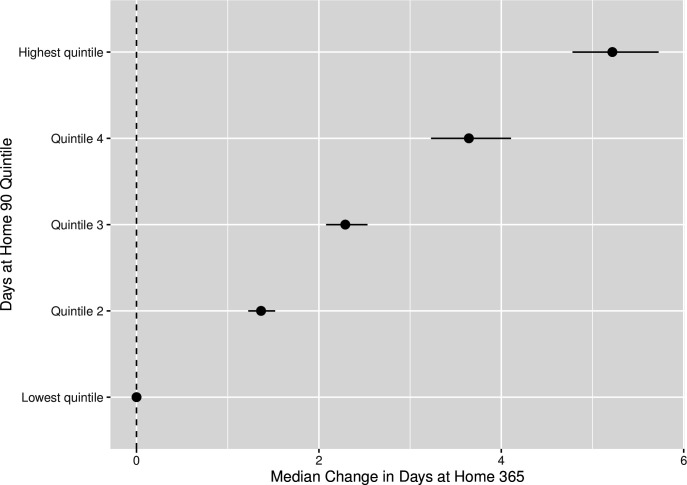
When evaluating predictive validity, we found that DAH90 was highly correlated with DAH365 (correlation coefficient=0.922). The correlation was still strong, but attenuated, between the DAH90 and the DAH365−90 value (correlation coefficient=0.746). For patients who survived the first 90 days after admission, their quintile of DAH90 was strongly associated with subsequent death between 90 and 365 days after admission in a dose–response fashion (figure 3), although the quintile of DAH90 was not strongly discriminative of mortality in that time frame (c-statistic=0.68). Using a four-knot restricted cubic spline instead of a quintile parameterisation led to no change in discrimination (c-statistic=0.68).
Figure 3.
The forest plot depicts the median adjusted difference in days alive at home in the 365 days after hip fracture admission and the associated 95% credible intervals (based on the highest probability density interval from the posterior distribution) among patients with hip fracture who survived 90 days after admission, with the quintile of days alive at home in 90 days as the predictor.
Minimally important difference
Distribution-based techniques suggested an MID of 11 days based on 0.3 times the SD and 9 days based on 10% of the range of DAH90 values. Using clinical anchor-based techniques suggested an MID of 10 days based on requiring an ICU admission and 12 days based on experiencing a complication during the index hospitalisation; both anchors were appreciably associated with outcome (>99% probability of non-zero association). When averaged, our data supported a final MID of 11 days (see table 2; all values have been rounded to the nearest day to avoid exaggerated accuracy as DAH was measured only to the unit). The prespecified, but inconsistent with other data, estimation using a 5% range resulted in an estimated MID of 9 days (see full estimates using 5% in online supplemental appendix table 7).
Table 2.
Minimally important difference estimates
| Group | Distribution estimates | Anchor estimates | Average | ||
| SD×0.3 | Range×0.10 | ICU admission | Complication | ||
| Overall | 11 | 9 | 10 | 12 | 11 |
| Subgroups | |||||
| Male | 10 | 9 | 10 | 14 | 11 |
| Female | 11 | 9 | 10 | 11 | 10 |
| No frailty | 10 | 9 | 17 | 18 | 14 |
| With frailty | 10 | 9 | 10 | 9 | 10 |
| Prefracture institutionalisation | 6 | 9 | 1 | 1 | 4 |
| No prefracture institutionalisation | 10 | 9 | 13 | 20 | 13 |
| Surgical fixation | 11 | 9 | 16 | 14 | 13 |
| No surgical fixation | 10 | 9 | 10 | 12 | 10 |
Frailty: frailty index >0.21.
ICU, intensive care unit.
Table 2 provides distribution-based, anchor-based and averaged estimates of DAH90 for each prespecified subgroup. The subgroup MID estimates varied from 10 to 14 days, except for those who were in long-term care prior to admission, where the MID was estimated at 4 days.
Long-term care resident sensitivity analysis
When analyses were repeated with postadmission days in long-term care counted as DAH for individuals residing in long-term care preadmission, the median DAH90 value was 58. Associations supporting construct validity were directionally unchanged (online supplemental appendix table 8). The MID estimate was 14 days (online supplemental appendix table 7).
Discussion
In this population-based cross-sectional analysis of linked health administrative data, we found that the number of days alive and at home after hip fracture admission had construct and predictive validity as a patient-centred outcome that can be captured from routinely collected electronic data sources. We further estimated that a difference of 11 days may represent an MID in DAH90 for patients with hip fracture, although this value may vary in certain clinically relevant subgroups and should be further evaluated with direct patient engagement. Overall, DAH90 may represent an important outcome for reporting, quality improvement and future registry-linked pragmatic trials in the growing population of older people with hip fractures.
Based on our findings, DAH90 could be an important outcome to guide care and inform trials in hip fracture populations as it can be routinely calculated using valid indicators in linked health administrative data. In other words, unlike codes for physical or cognitive function, codes for mortality, length of stay, discharge and readmission (which are combined to calculate DAH) are typically accurately captured in health data.28 Furthermore, DAH incorporates the impact of complications, poor recovery and postdischarge events, making it patient and system relevant, while further supporting its face validity.17 This may make DAH a particularly relevant outcome when evaluating the impact of care bundles or process change that may act through a variety of postulated mechanisms. DAH also likely has particular relevance in pragmatic and registry-linked trials, which can gain substantial efficiency through evaluation of interventions in real-world settings using outcomes that are routinely collected.53–55 Patients, family members and caregivers may also benefit from knowing anticipated values of DAH at key points in the hip fracture admission. Older people have expressed substantial concerns about recovery time after hip fracture,56 while caregivers often experience substantial burden during the transitional period after hip fracture.57 58 Unfortunately, DAH may not be ascertainable in all sources of electronic and routinely collected data. In particular, health systems that lack linkage across different data sources (eg, between acute and long-term care) may not be able to obtain the variables necessary to compute DAH. Furthermore, registry data, such as those collected by the National Surgical Quality Improvement Program (NSQIP), do not extend beyond 30 postoperative days and do not include postdischarge place of residence.59 Fortunately, data from the NSQIP geriatric pilot programme, which do include postdischarge residence, could facilitate incorporation of DAH as a routinely collected outcome in the future.60 61
Our findings should be considered in the context of previous validation studies of DAH in relation to perioperative settings, which have been conducted in a single-centre mixed surgery and urgency cohort,17 and a population-based, elective non-cardiac surgery cohort.18 First, unlike elective surgery where DAH has been defined within the first 30 days, we measured DAH values at 90 days, as almost 50% of patients with hip fracture were (expectedly) deceased or still institutionalised 1 month after admission. However, Jerath and colleagues18 report of DAH90 values as a secondary outcome highlights the difference in postoperative trajectory between elective surgical and hip fracture patients (median DAH90 in elective surgery 86 vs 43 in hip fracture). Despite the differing temporal ascertainment windows, general trends in the association of patient characteristics are similar across DAH validation studies. For example, men typically experience fewer DAH than women, while those with higher comorbidity burden and older ages also have reduced DAH. Furthermore, similar to findings in elective surgery, a lower DAH value among survivors in a proximal ascertainment window (ie, DAH90) was strongly correlated with more distal DAH values (ie, DAH365) and was associated with higher risk of death in the subsequent follow-up period.18 While we are not aware of previous estimates of variation in DAH at a hospital and provider level, our estimates of variation (13% at the index hospital, <4% attributable to providers) are similar to variation in mortality rates after complex elective surgery,62 suggesting that most variations in these acute care outcomes are related to patient factors.
Finally, along with validating DAH90 in patients with hip fracture, our data also provide an MID estimate for the DAH90 outcome in this population. Using averaged anchor-based and distribution-based techniques, we estimated that an 11-day difference may represent an MID in patients with hip fracture. Use of a 10% range criterion (as opposed to the 5% that we prespecified) was thought to be more appropriate for patients with hip fracture, as 10% of the range of measured values (9 days) was more consistent with other prespecified anchor and distributional estimates than the 4 days estimated using a 5% range (table 2). Fortunately, the averaged estimates of MID (9 days with a 5% range; 11 days with a 10% range) are not substantively different and support the need to estimate MIDs using multiple approaches. While previous validation studies have not investigated DAH MID values, available data suggest that values would likely differ substantially in elective surgery measured at 30 days. For example, averaging anchor values reported by Myles and colleagues17 (eg, readmission (6 days), complication (~3.5 days)) with a 5% range distributional criterion (1.5 days) would suggest a value of approximately 3.5 days being minimally important. Finally, our MID estimates in clinically relevant subgroups were qualitatively consistent (10–14 days), except for individuals who resided in long-term care before admission. This is an important group who present substantial challenges in assigning days at home, as estimates will vary greatly depending on assignment of the institutional residence as ‘home’ after discharge. Based on our analysis, assigning days back in an institutional setting as being alive and at home may be the preferred approach, as the estimated MID (14) was more consistent with the overall and other subgroup estimates compared with treating such days as non-home (which led to an MID estimate of 4). Furthermore, anchor values informing the MID for those institutionalised before their fracture suggest that their trajectory may differ substantially, as complications and ICU admission resulted in only one fewer day alive at home, compared with 10 or more days away from home for those in the community before their fracture. Ultimately, the growing recognition of the value of patient engagement63 speaks to the need for patient and caregiver perspectives to be added to statistical validation and data-based estimates of MID values. While reports exist of days spent at home having intrinsic value to patients,15 direct patient engagement including quantitative and qualitative evaluation of patient preferences of DAH as an outcome compared with measures like short-term survival and length of stay is still required to understand its full value. Furthermore, patient-reported outcome scales included in current hip fracture core outcome sets (eg, mobility, activities of daily living, quality of life) could be considered as relevant anchors in future prospective MID estimations.11
Strengths and limitations
This study should be appraised considering its strengths and limitations. First, we followed a preregistered and prespecified protocol. We also identified our cohort and outcome using well-validated fields in our data sources. However, as health administrative data are not initially collected for research purposes, misclassification bias is possible. Established and valid methods were also used to identify patient-level and admission-level factors used in our construct validity analysis, but generalisability in structurally different health systems cannot be confirmed. Comorbidities were ascertained using a 3-year lookback; however, comorbidity ascertainment in administrative data can differ by lookback period.64–67 As recommended, we estimated our MID value using the average of a variety of measurement techniques. However, our anchor-based methods could not incorporate direct patient-reported outcome measures, and substantial differences were present between two distributional inputs (4 days for 5% range, 11 days for 0.3 of the SD; although the impact on the averaged MID was not substantial). This further reflects the lack of patient participation in our study and the clear need to use these data as a starting point for future patient input into establishing an MID for DAH90 in hip fracture populations.
Conclusions
In a population-based cross-sectional analysis of patients with hip fracture, DAH in the 90 days after admission was found to have construct and predictive validity as a patient-centred outcome measure. Furthermore, an MID in this outcome was estimated to be 11 days. DAH may represent a useful and important outcome in future quality, research and reporting efforts aimed at improving hip fracture care and outcomes. However, direct patient engagement would help to clarify the importance and role and solidify MID estimates for older adults with hip fracture.
Acknowledgments
DIM receives salary support from The Ottawa Hospital Department of Anesthesiology Alternate Funds Association and a Research Chair from the University of Ottawa Faculty of Medicine (Ottawa, Ontario, Canada). DNW is supported in part by a Merit Award from the Department of Anesthesiology and Pain Medicine at the University of Toronto, and the Endowed Chair in Translational Anesthesiology Research at St. Michael’s Hospital and the University of Toronto. This study was also supported by ICES (Toronto, Ontario, Canada), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (Toronto, Ontario, Canada). No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. These data sets were held securely in a linked, de-identified form and analysed at ICES. We thank IMS Brogan Inc. for use of their Drug Information Database.
Footnotes
Twitter: @mcisaac_d
Contributors: DIM, RT, AJ and DNW contributed to conception and design of the study. DIM and RT contributed to acquisition and analysis of data. DIM, RT, AJ and DNW contributed to interpretation of data, drafting the work and gave final approval for publication. DIM, RT, AJ and DNW agree to be accountable for all aspects of the work.
Funding: The study received funding from the Institute of Health Services and Policy Research (PJT-165805), which is part of the Canadian Institutes of Health Research.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. All data used are held as de-identified data at ICES, whose governing privacy legislation does not permit data sharing.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
As all data used were routinely collected and de-identified, this study was legally exempt from research ethics review.
References
- 1. Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA 2009;302:1573–9. 10.1001/jama.2009.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eastwood EA, Magaziner J, Wang J, et al. Patients with hip fracture: subgroups and their outcomes. J Am Geriatr Soc 2002;50:1240–9 http://www.ncbi.nlm.nih.gov/pubmed/12133019 10.1046/j.1532-5415.2002.50311.x [DOI] [PubMed] [Google Scholar]
- 3. Hannan EL, Magaziner J, Wang JJ, et al. Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes. JAMA 2001;285:2736–42. 10.1001/jama.285.21.2736 [DOI] [PubMed] [Google Scholar]
- 4. Neuman MD, Rosenbaum PR, Ludwig JM, et al. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA 2014;311:2508–17. 10.1001/jama.2014.6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med 2014;174:1273. 10.1001/jamainternmed.2014.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamilton GM, Lalu MM, Ramlogan R, et al. A population-based comparative effectiveness study of peripheral nerve blocks for hip fracture surgery. Anesthesiology 2019;131:1025–35. 10.1097/ALN.0000000000002947 [DOI] [PubMed] [Google Scholar]
- 7. McIsaac DI, Wijeysundera DN, Bryson GL, et al. Hospital-, Anesthesiologist-, and patient-level variation in primary anesthesia type for hip fracture surgery: a population-based cross-sectional analysis. Anesthesiology 2018;129:1121–31. 10.1097/ALN.0000000000002453 [DOI] [PubMed] [Google Scholar]
- 8. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ 2010;182:1609–16. 10.1503/cmaj.092220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA 2017;318:1994–2003. 10.1001/jama.2017.17606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prinsen CAC, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a "Core Outcome Set" - a practical guideline. Trials 2016;17:449. 10.1186/s13063-016-1555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haywood KL, Griffin XL, Achten J, et al. Developing a core outcome set for hip fracture trials. Bone Joint J 2014;96-B:1016–23. 10.1302/0301-620X.96B8.33766 [DOI] [PubMed] [Google Scholar]
- 12. Akpan A, Roberts C, Bandeen-Roche K, et al. Standard set of health outcome measures for older persons. BMC Geriatr 2018;18:36. 10.1186/s12877-017-0701-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patient reported outcome measures (PROMs). Available: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/patient-reported-outcome-measures-proms [Accessed 31 May 2021].
- 14. Ladha KS, Wijeysundera DN. Role of patient-centred outcomes after hospital discharge: a state-of-the-art review. Anaesthesia 2020;75(Suppl 1):e151–7. 10.1111/anae.14903 [DOI] [PubMed] [Google Scholar]
- 15. Groff AC, Colla CH, Lee TH. Days spent at home - a patient-centered goal and outcome. N Engl J Med 2016;375:1610–2. 10.1056/NEJMp1607206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sayer C. “Time spent at home” — a patient-defined outcome. NEJM Catalyst 2016. 10.1056/CAT.16.0854 [DOI] [Google Scholar]
- 17. Myles PS, Shulman MA, Heritier S, et al. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. BMJ Open 2017;7:e015828. 10.1136/bmjopen-2017-015828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jerath A, Austin PC, Wijeysundera DN. Days alive and out of hospital: validation of a patient-centered outcome for perioperative medicine. Anesthesiology 2019;131:84–93. 10.1097/ALN.0000000000002701 [DOI] [PubMed] [Google Scholar]
- 19. Fernando SM, Tran A, Cheng W, et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ 2019;367:l6373. 10.1136/bmj.l6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ariti CA, Cleland JGF, Pocock SJ, et al. Days alive and out of hospital and the patient journey in patients with heart failure: insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J 2011;162:900–6. 10.1016/j.ahj.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 21. Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–9. 10.1093/eurheartj/ehu205 [DOI] [PubMed] [Google Scholar]
- 22. Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA 2015;314:1159–66. 10.1001/jama.2015.10842 [DOI] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (record) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Depaoli S, van de Schoot R. Improving transparency and replication in Bayesian statistics: the WAMBS-Checklist. Psychol Methods 2017;22:240–61. 10.1037/met0000065 [DOI] [PubMed] [Google Scholar]
- 26. Sung L, Hayden J, Greenberg ML, et al. Seven items were identified for inclusion when reporting a Bayesian analysis of a clinical study. J Clin Epidemiol 2005;58:261–8. 10.1016/j.jclinepi.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 27. Neuman MD, Ellenberg SS, Sieber FE, et al. Regional versus general anesthesia for promoting independence after hip fracture (regain): protocol for a pragmatic, international multicentre trial. BMJ Open 2016;6:e013473. 10.1136/bmjopen-2016-013473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Juurlink DN, Croxford R. Canadian institute for health information discharge abstract database : a validation study ICES investigative report June 2006; 2006. https://www.ices.on.ca/Publications/Atlases-and-Reports/2006/Canadian-Institute-for-Health-Information
- 29. Marufu TC, Mannings A, Moppett IK. Risk scoring models for predicting peri-operative morbidity and mortality in people with fragility hip fractures: qualitative systematic review. Injury 2015;46:2325–34. 10.1016/j.injury.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 30. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 31. McIsaac DI, Wong CA, Huang A, et al. Derivation and validation of a generalizable preoperative frailty index using population-based health administrative data. Ann Surg 2019;270:102–8. 10.1097/SLA.0000000000002769 [DOI] [PubMed] [Google Scholar]
- 32. Southern DA, Burnand B, Droesler SE, et al. Deriving ICD-10 codes for patient safety indicators for large-scale surveillance using administrative hospital data. Med Care 2017;55:252–60. 10.1097/MLR.0000000000000649 [DOI] [PubMed] [Google Scholar]
- 33. McIsaac DI, Hamilton GM, Abdulla K, et al. Validation of new ICD-10-based patient safety indicators for identification of in-hospital complications in surgical patients: a study of diagnostic accuracy. BMJ Qual Saf 2020;29:209–16. 10.1136/bmjqs-2018-008852 [DOI] [PubMed] [Google Scholar]
- 34. Garland A, Yogendran M, Olafson K, et al. The accuracy of administrative data for identifying the presence and timing of admission to intensive care units in a Canadian province. Med Care 2012;50:e1–6. 10.1097/MLR.0b013e318245a754 [DOI] [PubMed] [Google Scholar]
- 35. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–34. 10.1080/03610910902859574 [DOI] [Google Scholar]
- 36. Bürkner P-C. Brms : an R package for bayesian multilevel models using stan. J Stat Softw 2017;80:1–27. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- 37. Gelman A, Jakulin A, Pittau MG, et al. A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat 2008;2:1360–83. 10.1214/08-AOAS191 [DOI] [Google Scholar]
- 38. De Vet H, Terwee C, Mokkink L, et al. Measurement in medicine - a practical guide. Cambridge University Press, 2011. [Google Scholar]
- 39. Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of Physician-Diagnosed Alzheimer's disease and related dementias in population-based administrative data: a validation study using family physicians' electronic medical records. J Alzheimers Dis 2016;54:337–49. 10.3233/JAD-160105 [DOI] [PubMed] [Google Scholar]
- 40. Jerath A, Austin PC, Ko DT, et al. Socioeconomic status and days alive and out of hospital after major elective noncardiac surgery: a population-based cohort study. Anesthesiology 2020;132:713–22. 10.1097/ALN.0000000000003123 [DOI] [PubMed] [Google Scholar]
- 41. Hamilton GM, Ramlogan R, Lui A, et al. Association of peripheral nerve blocks with postoperative outcomes in ambulatory shoulder surgery patients: a single-centre matched-cohort study. Can J Anaesth 2019;66:63–74. 10.1007/s12630-018-1234-8 [DOI] [PubMed] [Google Scholar]
- 42. Wijeysundera DN, Johnson SR. How much better is good enough? Anesthesiology 2016;125:7–10. 10.1097/ALN.0000000000001159 [DOI] [PubMed] [Google Scholar]
- 43. Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002;77:371–83. 10.4065/77.4.371 [DOI] [PubMed] [Google Scholar]
- 44. Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102–9. 10.1016/j.jclinepi.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 45. Wells G, Beaton D, Shea B, et al. Minimal clinically important differences: review of methods. J Rheumatol 2001;28:406–12. [PubMed] [Google Scholar]
- 46. Shulman MA, Kasza J, Myles PS. Defining the minimal clinically important difference and patient-acceptable symptom state score for disability assessment in surgical patients. Anesthesiology 2020;132:1362–70. 10.1097/ALN.0000000000003240 [DOI] [PubMed] [Google Scholar]
- 47. Myles PS, Myles DB, Galagher W, et al. Minimal clinically important difference for three quality of recovery scales. Anesthesiology 2016;125:39–45. 10.1097/ALN.0000000000001158 [DOI] [PubMed] [Google Scholar]
- 48. King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res 2011;11:171–84. 10.1586/erp.11.9 [DOI] [PubMed] [Google Scholar]
- 49. McIsaac DI, Hamilton GM, Abdulla K, et al. Validation of new ICD-10-based patient safety indicators for identification of in-hospital complications in surgical patients: a study of diagnostic accuracy. BMJ Qual Saf 2020;29:209–16. 10.1136/bmjqs-2018-008852 [DOI] [PubMed] [Google Scholar]
- 50. Beattie WS, Lalu M, Bocock M, et al. Systematic review and consensus definitions for the standardized endpoints in perioperative medicine (step) initiative: cardiovascular outcomes. Br J Anaesth 2021;126:56–66. 10.1016/j.bja.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 51. Barnes J, Hunter J, Harris S, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (StEP) initiative: infection and sepsis. Br J Anaesth 2019;122:500–8. 10.1016/j.bja.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haller G, Bampoe S, Cook T, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine initiative: clinical indicators. Br J Anaesth 2019;123:228–37. 10.1016/j.bja.2019.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Can Med Assoc J 2009;180:E47–57. 10.1503/cmaj.090523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 55. Lauer MS, D'Agostino RB. The randomized registry trial--the next disruptive technology in clinical research? N Engl J Med 2013;369:1579–81. 10.1056/NEJMp1310102 [DOI] [PubMed] [Google Scholar]
- 56. Neuman MD, Ibrahim SA, Barg FA, et al. Race and patient preferences for hip fracture care. J Am Geriatr Soc 2013;61:468–70. 10.1111/jgs.12082 [DOI] [PubMed] [Google Scholar]
- 57. Ariza-Vega P, Ortiz-Piña M, Kristensen MT, et al. High perceived caregiver burden for relatives of patients following hip fracture surgery. Disabil Rehabil 2019;41:311–8. 10.1080/09638288.2017.1390612 [DOI] [PubMed] [Google Scholar]
- 58. Parry JA, Langford JR, Koval KJ. Caregivers of hip fracture patients: the forgotten victims? Injury 2019;50:2259–62. 10.1016/j.injury.2019.09.030 [DOI] [PubMed] [Google Scholar]
- 59. User guide for the ACS NSQIP participant use data file, 2014. Available: https://www.facs.org/quality-programs/acs-nsqip/participant-use
- 60. Geriatric surgery ACS NSQIP collaborative. Available: https://www.facs.org/quality-programs/acs-nsqip/about/participants/geriatric-pilot [Accessed 28 Nov 2020].
- 61. Ma C JG, Hou P, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV 2020. 10.1101/2020.03.17.20037572 [DOI] [Google Scholar]
- 62. Hallet J, Jerath A, Turgeon AF, et al. Association between anesthesiologist volume and short-term outcomes in complex gastrointestinal cancer surgery. JAMA Surg 2021;156:479. 10.1001/jamasurg.2021.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Strategy for patient oriented research: putting patients first, 2014. Available: http://www.cihr-irsc.gc.ca/e/documents/spor_framework-en.pdf
- 64. Maringe C, Fowler H, Rachet B, et al. Reproducibility, reliability and validity of population-based administrative health data for the assessment of cancer non-related comorbidities. PLoS One 2017;12:e0172814. 10.1371/journal.pone.0172814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang JX, Iwashyna TJ, Christakis NA. Tthe performance of different lookback periods and sources of information for charlson comorbidity adjustment in medicare. Med Care 1999;37:1128–39. 10.1097/00005650-199911000-00005 [DOI] [PubMed] [Google Scholar]
- 66. Preen DB, Holman C D'Arcy J, Spilsbury K, et al. Length of comorbidity lookback period affected regression model performance of administrative health data. J Clin Epidemiol 2006;59:940–6. 10.1016/j.jclinepi.2005.12.013 [DOI] [PubMed] [Google Scholar]
- 67. Chen G, Lix L, Tu K, et al. Influence of using different databases and 'look back' intervals to define comorbidity profiles for patients with newly diagnosed hypertension: implications for health services researchers. PLoS One 2016;11:e0162074. 10.1371/journal.pone.0162074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2021-013150supp001.pdf (279.3KB, pdf)
Data Availability Statement
No data are available. All data used are held as de-identified data at ICES, whose governing privacy legislation does not permit data sharing.