Abstract
Objective
To investigate relationships between serum neurofilament light chain (sNfL), ubiquitin C-terminal hydrolase L1 (sUCHL1), tau (sTau) and glial fibrillary acidic protein (sGFAP) levels and disease activity/disability in neuromyelitis optica spectrum disorder (NMOSD), and the effects of inebilizumab on these biomarkers in N-MOmentum.
Methods
N-MOmentum randomised participants to receive inebilizumab or placebo with a randomised controlled period (RCP) of 28 weeks and an open-label follow-up period of ≥2 years. The sNfL, sUCHL1, sTau and sGFAP were measured using single-molecule arrays in 1260 scheduled and attack-related samples from N-MOmentum participants (immunoglobulin G (IgG) autoantibodies to aquaporin-4-positive, myelin oligodendrocyte glycoprotein-IgG-positive or double autoantibody-negative) and two control groups (healthy donors and patients with relapsing–remitting multiple sclerosis).
Results
The concentration of all four biomarkers increased during NMOSD attacks. At attack, sNfL had the strongest correlation with disability worsening during attacks (Spearman R2=0.40; p=0.01) and prediction of disability worsening after attacks (sNfL cut-off 32 pg/mL; area under the curve 0.71 (95% CI 0.51 to 0.89); p=0.02), but only sGFAP predicted upcoming attacks. At RCP end, fewer inebilizumab-treated than placebo-treated participants had sNfL>16 pg/mL (22% vs 45%; OR 0.36 (95% CI 0.17 to 0.76); p=0.004).
Conclusions
Compared with sGFAP, sTau and sUCHL1, sNfL at attack was the strongest predictor of disability worsening at attack and follow-up, suggesting a role for identifying participants with NMOSD at risk of limited post-relapse recovery. Treatment with inebilizumab was associated with lower levels of sGFAP and sNfL than placebo.
Trial registration number
Keywords: CLINICAL NEUROLOGY, RANDOMISED TRIALS
WHAT IS ALREADY KNOWN ON THIS TOPIC
Neuromyelitis optica spectrum disorder (NMOSD) is characterised by incremental permanent disability; thus, availability of biomarkers as indicators of the presence and progression of NMOSD is highly desirable. The aim of this study was to investigate the potential relationship between four putative biomarkers and disease activity/disability in participants from the N-MOmentum study and to assess the impact of inebilizumab treatment on their observed concentrations.
WHAT THIS STUDY ADDS
Serum neurofilament light chain measured at attack was the best predictor among biomarkers studied for disability worsening during and after attacks but was inferior to serum glial fibrillary acidic protein in prediction of future attacks. Compared with placebo, treatment with inebilizumab attenuated the elevation of biomarkers during attacks and reduced levels of these biomarkers over time in the absence of adjudicated attacks.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings may help to inform progress towards assessment of clinical status, prognosis and treatment decisions for patients with NMOSD by means of routine measurement of biomarkers.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune inflammatory disease of the central nervous system (CNS) characterised by attacks of optic neuritis, transverse myelitis and, less commonly, brain or brainstem inflammation.1 Attacks are thought to be caused by astroglial injury resulting in secondary demyelination and substantial tissue injury.2 Incremental damage occurs in the context of acute attacks, which can be severe and cause permanent disability due to incomplete recovery; in contrast to multiple sclerosis (MS), progressive worsening of disability independent of attacks is rare.3
Immunoglobulin G (IgG) autoantibodies to aquaporin-4 (AQP4-IgG) are pathogenic; up to 90% of patients with NMOSD are AQP4-IgG seropositive (AQP4+).4 5 In the CNS, AQP4-IgG binds to the extracellular domain of AQP4 expressed in the astrocyte, resulting in astrocytic damage through complement-mediated and complement-independent mechanisms.6 7 Damaged astrocytes compromise trophic support for surrounding oligodendrocytes and neurons, presumably leading to secondary inflammation, demyelination and axonal loss.8 Experimental data also suggest that AQP4-IgG induces cytokine production, increasing blood–brain barrier permeability.9
Identifying biomarkers as indicators of the presence and course of disease promises to improve diagnosis and management of neurological conditions such as NMOSD.10 Soluble glial fibrillary acidic protein (GFAP), an abundant cytoskeletal intermediate filament in astrocytes, is released into cerebrospinal fluid (CSF) and serum following astroglial injury.11 Serum GFAP (sGFAP) was previously found to correlate strongly with NMOSD attacks,12–14 including attack prediction.14 Neurofilaments are structural proteins, exclusively expressed in neurons, forming an essential part of the cytoskeletal scaffold.15 Axonal damage leads to elevated CSF and serum concentrations of neurofilament light chain (NfL) in the CSF and serum.16 NfL was validated as a highly sensitive biomarker of neuroaxonal damage, regardless of cause,17 and serum NfL (sNfL) is a biomarker of neuronal injury in various neurodegenerative diseases, as well as in NMOSD.12 13 15 18 19
Ubiquitin C-terminal hydrolase L1 (UCHL1) is a highly abundant neuron-specific protein that forms part of the ubiquitin proteasome system of protein degradation.20 Tau forms an important part of the microtubule structure in the axonal cytoskeletion.21 Neuronal damage causes release of UCHL1 and tau into CSF and plasma, and elevated serum levels have been observed in several neurological and neurodegenerative diseases, including Alzheimer’s disease, epilepsy and MS.15 22 GFAP, NfL, tau and UCHL1 concentration detection was previously limited to CSF; however, development of highly sensitive, single-molecule array (SIMOA) technology (Quanterix; Lexington, Massachusetts, USA) has allowed for reliable serum measurement.
N-MOmentum was a randomised, placebo-controlled, double-blind, phase 2/3 trial assessing the efficacy and safety of inebilizumab, an anti-CD19, B-cell-depleting antibody, in participants with NMOSD.1 23 The aim of the current study was to investigate potential relationships between sGFAP, sNfL, serum tau (sTau) and serum UCHL1 (sUCHL1), and disease activity in N-MOmentum study participants and to assess the impact of inebilizumab on concentrations of these putative biomarkers.
Methods
Study design and participants
sGFAP, sNfL, sUCHL1 and sTau concentrations were assessed in N-MOmentum participants,23 a healthy donor reference cohort, and patients with relapsing–remitting MS (RRMS).24
Full details of N-MOmentum have been published previously.23 In brief, adults with AQP4+ or AQP4-IgG seronegative (AQP4−)25 NMOSD, an Expanded Disability Status Scale (EDSS) score of 8.0 or less and a recent history of NMOSD attack were eligible (see online supplemental methods).23
jnnp-2022-330412supp002.pdf (4.6MB, pdf)
Participants were randomly assigned (3:1) to receive intravenous inebilizumab 300 mg or placebo (saline) administered on days 1 and 15 of the randomised controlled period (RCP). The RCP lasted for 28 weeks or until adjudicated attack occurrence. Attacks, defined by protocol-defined criteria, were adjudicated based on neurological evaluations and MRI data (on a criteria-dependent basis) by an independent expert committee during the 17 days post-attack. Attack severity was graded according to a modified version of the Opticospinal Impairment Scale (OSIS) that characterises attacks as major or minor based on changes in domain-specific scores for neurological function.26–28 Attack recovery assessment was performed 28 days post-attack and graded according to change in the same domain-specific scores relative to the score at time of attack. Disability was assessed using the EDSS and modified Rankin Scale (mRS).28 Depending on baseline EDSS score, participants were considered to have a worsening EDSS score if they had a worsening of ≥2 points (baseline=0 points), ≥1 points (baseline=1–5 points) or ≥0.5 points (baseline≥5.5 points).
Two reference cohorts of individuals without NMOSD were used as controls: one comprising age-matched and sex-matched healthy donors (n=85) and another comprising untreated patients with moderate-to-severe RRMS (baseline EDSS score >3.5; n=23) from the USA and Europe.24
Standard protocol approvals, registrations and participant consent
Participants were screened at 99 outpatient specialty clinics or hospitals in 25 countries. Institutional review boards or ethics committees at study sites approved the protocol. The study is registered with ClinicalTrials.gov (NCT02200770), and was conducted in accordance with the provisions of the International Council for Harmonisation Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki in its currently applicable version.
Assessment of concentrations of biomarkers of CNS damage
Blood samples were collected from N-MOmentum participants during RCP study visits on days 1 (baseline), 15, 29, 57, 85, 113, 155 and 197, and during any assessment visit for new or worsening NMOSD symptoms. In the reference cohorts,24 participants were untreated and blood was collected at the single baseline visit. Ten healthy donors underwent repeated longitudinal sampling for serial biomarker measurements. As validated in serum samples from patients with MS or traumatic brain injury,29 30 biomarker concentrations were determined using the SIMOA assay. Quantification of sNfL, sTau and sUCHL1 was performed using ‘Neurology 4-plex B’ kits run on the SIMOA HD-X Analyzer, and also included sGFAP. In-depth analysis of the N-MOmentum sGFAP data set was recently reported, focusing on attack severity and prediction.14 This data set is also included in the current study for comparison with the other biomarkers that focus on long-term disability. Assays for all four biomarkers were undertaken in parallel on the same SIMOA assay plate. Elevated serum biomarker concentrations were defined as being more than two SDs above the healthy donor mean (sGFAP: >170 pg/mL, sNfL: >16 pg/mL, sTau: >1.3 pg/mL and sUCHL1: >52 pg/mL) according to established laboratory procedures31; two healthy donors were outliers (according to the 95% trend/mean SD rule) and excluded from analyses for all biomarkers measured.
Statistical analyses
The current analyses are exploratory and are for hypothesis generation only (see online supplemental methods for full details). In brief, the utility of biomarker concentrations at baseline and at time points during the RCP as a predictor of future attack risk was assessed using multivariate Cox proportional hazards regression. The Wilcoxon signed-rank test was used to evaluate statistical significance of increases of each biomarker from each time point to attack in paired samples.
A mixed-effects logistic regression model was used to evaluate statistical significance of elevated biomarker concentrations in attack samples versus samples drawn during scheduled visits; sensitivity analyses were conducted to assess the performance of the model across participants in different treatment arms and in those who did or did not experience attacks during the RCP.
Correlations between changes in EDSS scores, EDSS component scores and biomarker concentrations from baseline to attack were evaluated using Spearman’s Rho. Multiple linear regression was used to assess independent correlation of each biomarker with EDSS score change at attack and proceeding attack after controlling for baseline EDSS score and age. The Mann-Whitney U test was used to further evaluate statistical significance of biomarker changes and the occurrence/absence of protocol-defined EDSS score worsening.
The significance of changes in biomarker concentrations from baseline to attack was evaluated in both RCP treatment groups using the Wilcoxon signed-rank test. Fold changes from baseline between treatment groups and in participants who did or did not experience attacks were assessed using the Mann-Whitney U test. Significance of changes in sNfL concentrations between treatment groups was also assessed using a mixed linear model including baseline sNfL, EDSS score and age as covariates and a per-subject random intercept term. All statistical analysis was performed in R V.4.1.3.
Data availability
Study data will be made available to others (see online supplemental methods). For more information, or to submit a request, please email: medicalinformation@horizontherapeutics.com.
Results
Study participants
The trial profile for N-MOmentum and full details of participant demographics who provided biomarker samples were reported.14 23 In total, 215 participants provided 1260 serial and NMOSD attack-related samples for biomarker concentration analysis. Most participants were women (194/215 (90.2%)) and approximately half were white (110/215 (51.2%)). At baseline, 198 participants (92.1%) were AQP4+, 7 (3.3%) were seropositive for myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG+), and 10 (4.7%) had no detectable autoantibodies for AQP4 or myelin oligodendrocyte glycoprotein (MOG) (double-negative participants). Demographics were similar in the inebilizumab (n=164) and placebo (n=51) groups.14
CNS damage biomarker concentrations increase during NMOSD attacks
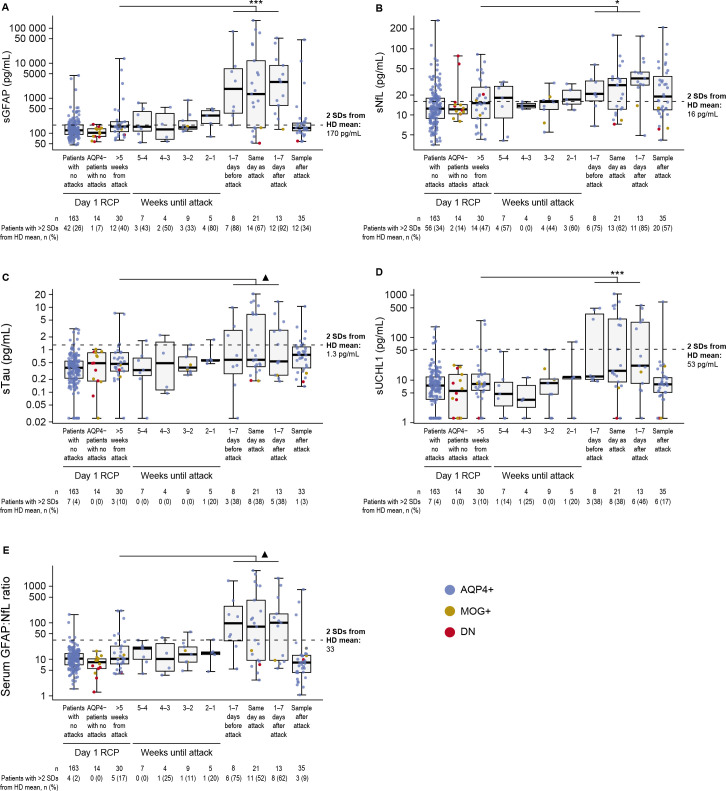
Significant increases in all four biomarkers were observed in serum samples taken from the whole cohort in the week before, on the day of and during the week following adjudicated attacks (figure 1); every participant had at least one EDSS assessment after an attack therefore guarding against bias introduced by informative censoring of participants who withdrew from the trial following attack. Mean sGFAP and sNfL concentrations increased above the defined elevated concentration (ie, >2 SDs from healthy donor means) during the week following clinical attack onset, while mean sTau and sUCHL1 concentrations remained below elevated levels at all time points assessed. Mean sNfL levels remained elevated in samples taken after (>7 days) attack onset, in contrast to sGFAP, which returned to baseline levels (figure 1A–B). Locally estimated scatterplot smoothing regression lineplots of biomarker changes in relation to time of attack show similar results (online supplemental efigure 1). These attack-related biomarker patterns were obvious for AQP4+ participants, but not for MOG+ or double-negative participants.
Figure 1.
Boxplots displaying (A) sGFAP, (B) sNfL, (C) sTau, (D) sUCHL1 and (E) serum GFAP:NfL ratio in samples drawn during the lead-up to an NMOSD attack and in day1/RCP samples drawn >5 weeks from the attack in participants who experienced an NMOSD attack during the RCP. Horizontal dotted lines show 2 SDs from the mean of the HD control cohort; sGFAP, 170 pg/mL; sNfL, 16 pg/mL; sTau, 1.3 pg/mL; sUCHL1, 53 pg/mL; sGFAP:sNfL ratio, 33. Note the differing y-axis scales, particularly the logarithmic scale for sGFAP. Median (IQR) attack follow-up was at 108 (27–124) days. ▲p<0.10; *p<0.05; **p<0.01; ***p<0.001, Wilcoxon signed-rank test, samples drawn at baseline versus 1 week until attack. AQP4+, seropositive for immunoglobulin G autoantibodies to aquaporin-4; AQP4−, seronegative for immunoglobulin G autoantibodies to aquaporin-4; DN, double-negative; GFAP, glial fibrillary acidic protein; HD, healthy donor; MOG+, seropositive for myelin oligodendrocyte glycoprotein-immunoglobulin G; NfL, neurofilament light chain; NMOSD, neuromyelitis optica spectrum disorder; RCP, randomised controlled period; sGFAP, serum glial fibrillary acidic protein; sNfL, serum neurofilament light chain; sTau, serum tau; sUCHL1, serum ubiquitin C-terminal hydrolase L1.
Among 198 AQP4+ participants with available biomarker data, there were 32 adjudicated attacks. Twelve participants had a more than twofold change in sNfL (3 had a more than twofold change in sNfL but not in sGFAP), 20 had a more than twofold increase in sGFAP (11 had a more than twofold increase in sGFAP but not in sNfL) and 9 did not have a corresponding increase of more than twofold in sGFAP or sNfL. Additionally, outside of attacks, 4 participants had a more than twofold change in sNfL alone, 15 in sGFAP alone and 3 in both sGFAP and sNfL. As a sensitivity analysis, a mixed-effects model for logistic regression was used to account for relevant variables and recurrent measurements in one model. The results were similar, with sGFAP, sNfL and sTau showing statistically significant associations with attacks (online supplemental eTable 1).
Biomarkers of CNS damage are elevated in patients with NMOSD and may be predictive of upcoming attacks
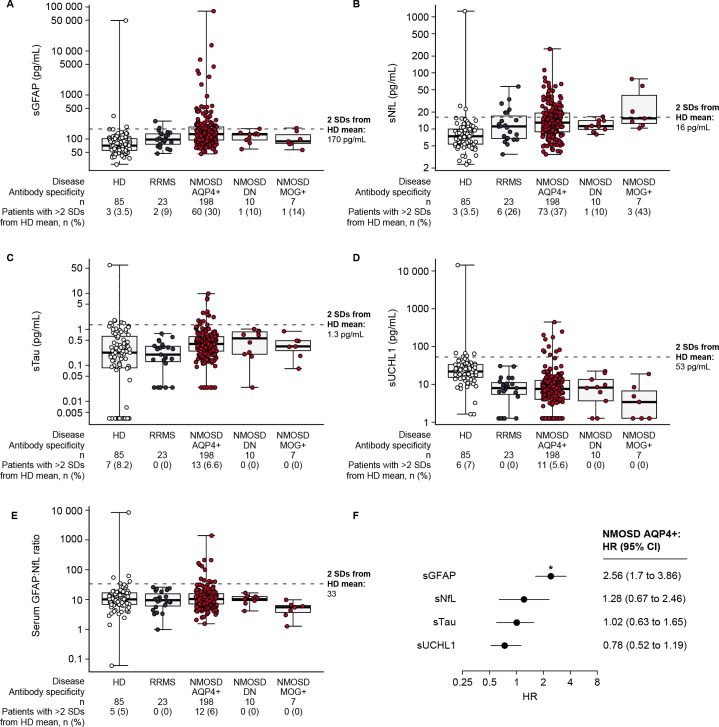
In participants with NMOSD, sGFAP concentrations were elevated at baseline versus healthy donors and patients with RRMS (30% of AQP4+ participants had sGFAP concentrations >2 SDs above healthy donor means vs 14% of MOG+ patients, 10% of double-negative patients, 9% of those with RRMS and 3.5% of healthy donors) (figure 2A). Similarly, elevated sNfL levels were observed in 37% of AQP4+ patients, 43% of MOG+ participants, 10% of double-negative patients, 26% of those with RRMS and 3.5% of healthy donors (figure 2B). The proportions of participants with elevated baseline sTau and sUCHL1 concentrations, or with increased serum GFAP:NfL ratios, were similar in the three N-MOmentum populations (figure 2C–E).
Figure 2.
Boxplots of day 1/RCP serum CNS damage biomarkers relative to HDs. (A) sGFAP, (B) sNfL, (C) sTau, (D) sUCHL1 and (E) serum GFAP:NfL ratio. Patients were determined to be ‘high’ for each cut-off based on levels being higher than two SDs from the mean of the HD cohort. Horizontal dotted line in each case represents the threshold for 2 SDs from the HD mean; sGFAP, 170 pg/mL; sNfL, 16 pg/mL; sTau, 1.3 pg/mL; sUCHL1, 53 pg/mL; sGFAP:sNfL ratio, 33. (F) Forest plot of Cox regression coefficients from model fit using day 1/RCP CNS damage biomarker concentrations as predictors of RCP attack risk in AQP4+ participants. Note the differing logarithmic y-axis scales, particularly the scale for sGFAP. HR for sGFAP, *p<0.05. AB, antibody; AQP4+, seropositive for immunoglobulin G autoantibodies to aquaporin-4; CNS, central nervous system; DN, double-negative; GFAP, glial fibrillary acidic protein; HD, healthy donor; MOG+, seropositive for myelin oligodendrocyte glycoprotein-immunoglobulin G; NfL, neurofilament light chain; NMOSD, neuromyelitis optica spectrum disorder; RCP, randomised controlled period; RRMS, relapsing–remitting multiple sclerosis; sGFAP, serum glial fibrillary acidic protein; sNfL, serum neurofilament light chain; sTau, serum tau; sUCHL1, serum ubiquitin C-terminal hydrolase L1.
Regression analysis performed in the AQP4+ participants demonstrated that elevated biomarker concentrations in the day 1 RCP sample equated to HRs for an adjudicated NMOSD attack during the RCP of 0.78–2.56, although only the highest value, obtained for sGFAP, was significant (figure 2F); time between day 1 of the RCP and last attack ranged from 3 to 199 days. Similarly, the likelihood ratio test on nested Cox regression models (sGFAP vs all four biomarkers) lacked statistical significance (χ2=5.22; p=0.16), indicating that biomarkers other than sGFAP did not contribute added value as predictors of upcoming attack risk (online supplemental eTable 2).
When AQP4+ participants in the two treatment arms of N-MOmentum were stratified according to respective cut-off values for sGFAP, sNfL, sTau and sUCHL1, those with elevated baseline values in the inebilizumab group had a significantly higher likelihood of experiencing an attack during the RCP than those without elevations. In the placebo group, a similar trend in AQP4+ participants was observed for sGFAP (p=0.067); however, differences in the Kaplan-Meier curves for all four biomarkers lacked statistical significance (online supplemental efigure 2). For all AQP4+ placebo- and inebilizumab-treated participants, broadly similar findings were observed for sNfL, sTau and sUCHL1 in heat maps of day 1 CNS biomarker concentrations in the subsets of participants who experienced attacks during the RCP (online supplemental efigure 3).
Biomarkers other than sGFAP do not add value in identifying attacks
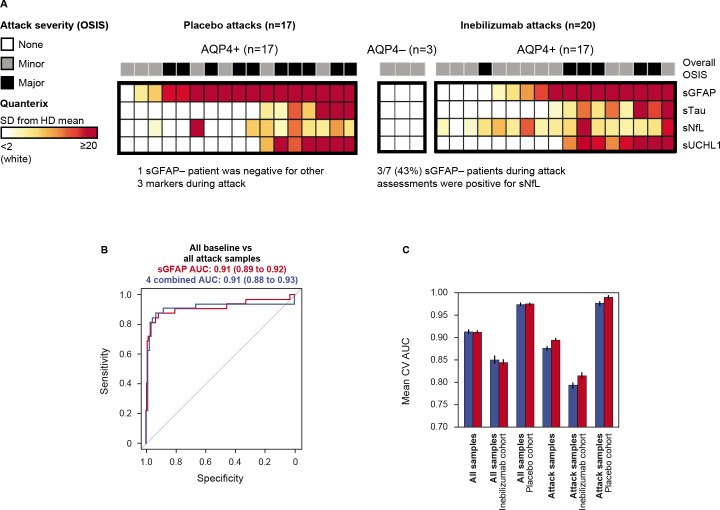
Assessment of biomarker concentrations in the context of attacks and attack severity demonstrated that sNfL, sTau and sUCHL1 were not as sensitive in predicting attacks or attack severity as was sGFAP. Notably, 37.5% of participants (3/8) without elevated sGFAP during an attack had elevated sNfL; sTau and sUCHL1 elevations did not occur in the absence of increased sGFAP (figure 3A). Logistic regression analysis demonstrated that the predictive capability of the biomarkers assessed was equal to or worse than the predictive capability of sGFAP alone in predicting attacks in AQP4+ participants (figure 3B and C). These findings were not observed in AQP4− participants (figure 3A).
Figure 3.
(A) Heatmap displaying concentration of CNS damage markers relative to HD reference cohort in samples drawn most proximal (+/− 7 days) to NMOSD attack in placebo-treated participants (left) and inebilizumab-treated participants (right). Heatmaps ordered by sGFAP concentration. (B) Mean ROC curves for out-of-fold predictions from 10 iterations of fivefold cross validation for mixed-effect logistic regression model fit to identify 37 attack samples versus remaining samples drawn during the study using sGFAP alone (red) versus all four CNS markers (blue) as predictors. (C) Sensitivity analysis displaying mean (+/− SEM) AUC from out-of-fold predictions for both models for all samples, samples drawn from the inebilizumab cohort and placebo cohort separately, then for those samples drawn from participants who experienced attacks during the RCP. One participant had a missing attack sample. AQP4+, seropositive for immunoglobulin G autoantibodies to aquaporin-4; AQP4−, seronegative for immunoglobulin G autoantibodies to aquaporin-4; AUC, area under the curve; CNS, central nervous system; CV, coefficient of variation; HD, healthy donor; NMOSD, neuromyelitis optica spectrum disorder; OSIS, Opticospinal Impairment Scale; RCP, randomised controlled period; ROC, receiver operator curve; sGFAP, serum glial fibrillary acidic protein; sNfL, serum neurofilament light chain; sTau, serum tau; sUCHL1, serum ubiquitin C-terminal hydrolase L1.
sNfL is the strongest correlate of changes in disability
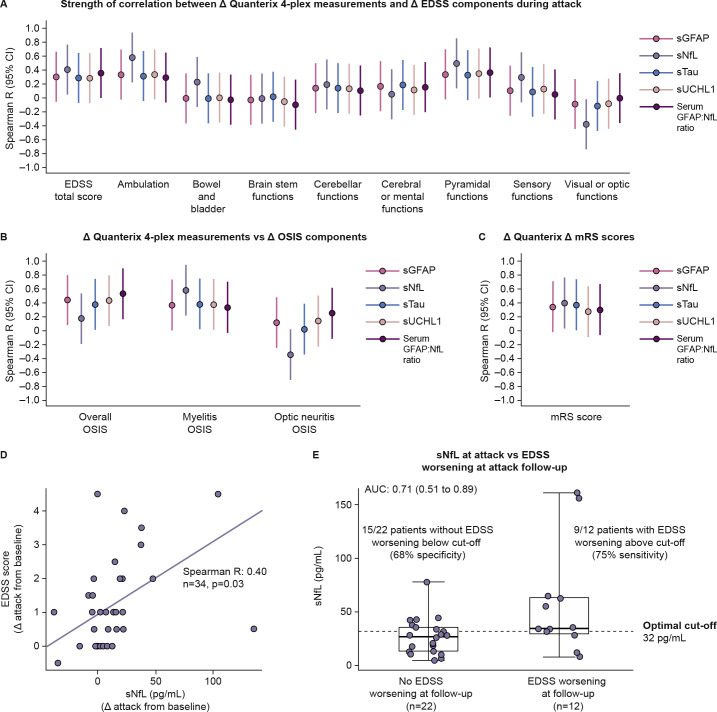
A correlation analysis between biomarker concentrations and three measures of disability in the N-MOmentum study (EDSS, OSIS26 27 and mRS28) was undertaken in AQP4+ participants. sNfL correlated significantly with changes in ambulation subscale scores (Spearman R=0.58 (95% CI 0.24 to 0.92)) and pyramidal functional system scores (Spearman R=0.46 (95% CI 0.12 to 0.80)) of the EDSS during attack assessments (figure 4A). Increased biomarker concentrations generally correlated with OSIS scores (figure 4B). Compared with the other biomarkers, sNfL was more sensitive for myelitis events but less sensitive for optic neuritis events. sNfL was the only biomarker to correlate with changes in mRS scores (figure 4C). Correlation analysis revealed that, of the four biomarkers, sNfL was the strongest correlate of EDSS scores (Spearman R=0.40 (95% CI 0.06 to 0.74)) (figure 4D, online supplemental eTable 3).
Figure 4.
(A-C) Forest plots displaying strength of correlation between change from baseline in concentration of CNS damage biomarkers and change from baseline in (A) EDSS component scores, (B) the OSIS components scores and (C) mRS scores in AQP4+ participants. (D) Scatterplot displaying correlation between changes from baseline to attack in sNfL and EDSS scores during attack assessments. (E) Box and whisker plot displaying distribution of sNfL changes from baseline in participants who displayed EDSS score worsening at attack follow-up versus those who did not experience EDSS score change at follow-up. Optimal cut-off point determined using Youden’s index. Attack follow-up was (median (±IQR)) 108 (27–124) days. AUC, area under the curve; CNS, central nervous system; EDSS, Expanded Disability Status Scale; GFAP, glial fibrillary acidic protein; mRS, modified Rankin Scale; OSIS, Opticospinal Impairment Scale; sGFAP, serum glial fibrillary acidic protein; sNfL, serum neurofilament light chain; sTau, serum tau; sUCHL1, serum ubiquitin C-terminal hydrolase L1.
We explored whether elevated sNfL at attack predicted EDSS score worsening at follow-up assessments in AQP4+ participants, because elevated sNfL during attacks correlated with EDSS score changes during attack assessments. All participants were followed up at week 26 of the open-label period (OLP) (median (±IQR): 108 (27–124) days). Participants who displayed EDSS score worsening at follow-up had elevated sNfL (area under the curve: 0.71 (95% CI 0.51 to 0.89)). A cut-off of sNfL 32 pg/mL at attack optimally distinguished those with EDSS score worsening from those without at follow-up (figure 4E). We then assessed whether sNfL concentrations at attack were indicative of EDSS score worsening in the longer term. Median (IQR) sNfL concentration was higher in placebo-treated participants with EDSS score worsening at attack follow-up and confirmed at 3 months (3-month confirmed disability progression (CDP); n=5, 55.3 (34.1–62.7) pg/mL) compared with the one participant with EDSS score worsening at attack follow-up but not confirmed at 3 months (35.6 pg/mL) or those with no EDSS score worsening at attack follow-up (n=11, 13.50 (10.31–26.10) pg/mL). In inebilizumab-treated participants, only one had EDSS score worsening with 3-month CDP. No statistical analysis was possible owing to the low sample size. Other CNS biomarkers were not significantly associated with EDSS score changes during attack or follow-up after controlling for sNfL changes in a multiple regression model. A mixed linear model of sNfL concentration versus EDSS score at attack and at attack follow-up was run as a sensitivity analysis and confirmed the correlation between these two variables after controlling for age and baseline disability score (online supplemental eTable 4).
Inebilizumab-treated participants had lower CNS damage biomarker levels than placebo-treated participants
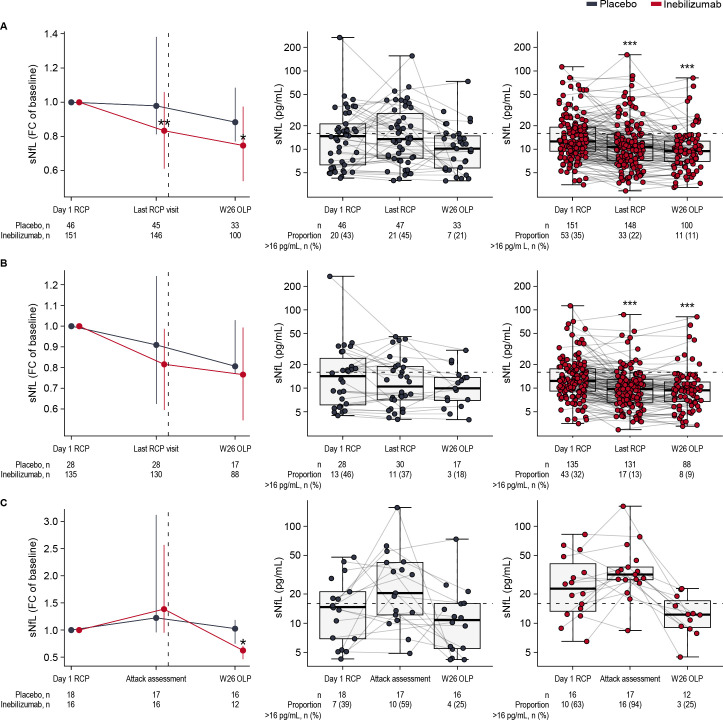
sNfL levels were significantly lower in inebilizumab-treated than in placebo-treated AQP4+ participants (figure 5A). At the end of the RCP, fewer participants receiving inebilizumab than placebo (22% vs 45%; OR 0.36 (95% CI 0.17 to 0.76), p=0.004, Fisher’s exact test) had sNfL values above 16 pg/mL.
Figure 5.
(A) Median (+/− IQR) fold change from baseline of sNfL in inebilizumab-treated and placebo-treated participants. Significance of changes between treatment groups assessed using Mann-Whitney U test. Boxplots of sNfL concentrations in (B) participants who did not experience RCP attacks and (C) those who did experience RCP attacks. Dashed vertical lines show when all participants received inebilizumab therapy at the end of the RCP and start of the OLP. Dashed horizontal lines show the threshold for elevated sNfL concentrations. Significance of changes from baseline in each treatment group was assessed using the Wilcoxon-signed rank test; *p<0.05; **p<0.01; ***p<0.001. FC, fold change; OLP, open-label period; RCP, randomised controlled period; sNfL, serum neurofilament light chain; W, week.
At the end of the RCP, inebilizumab-treated attack-free participants had significantly lower sNfL levels than attack-free participants receiving placebo (figure 5B, online supplemental file 2 eTable 5). Levels of the other three biomarkers in attack-free participants were also numerically lower after inebilizumab (online supplemental efigure 4).
Finally, in participants who experienced an attack during the RCP (figure 5C), sNfL levels were lower in inebilizumab-treated than in placebo-treated patients after the end of the RCP (week 26 OLP, p=0.03 for median fold change from baseline between the two treatment groups). Elevated levels of the other three biomarkers were observed at the time of attack, with sGFAP reaching statistical significance (p=0.037 for median fold change from baseline between the two treatment groups) (online supplemental efigure 5).
Analysis using a mixed-effects linear model to assess the impact of inebilizumab on sNfL concentrations similarly showed significant decreases at week 28 of the RCP and during attack assessments in inebilizumab-treated participants (online supplemental eTable 5).
Discussion
Serum levels of CNS tissue injury biomarkers (GFAP, NfL, tau and UCHL1) increased during NMOSD attacks, reflecting CNS damage associated with the clinical event. Concentrations of the biomarkers (especially sGFAP, sNfL and sUCHL1) increased during the days before the attack, which is consistent with the hypothesis that attacks are caused by accumulation of underlying tissue damage, ultimately culminating in clinical manifestations.
The correlation between sNfL levels and EDSS scores at attack support a direct link between neuroaxonal loss and disability worsening in NMOSD attacks. Assessment of sNfL during an attack could help to inform clinicians about attack severity and the likelihood of post-attack residual disability, and therefore may have the potential to guide therapeutic interventions at the time of attack.32–35
Follow-up studies could assess potential clinical benefits of triaging patients with high sNfL at attack onset for immediate plasmapheresis rather than awaiting the outcome of standard treatment with corticosteroids alone. Determining the clinical utility of sNfL for NMOSD attack management requires further investigation.
sGFAP levels have been linked to attack severity, inflammatory damage and disability worsening in NMOSD.14 The results presented here, however, indicate that sNfL is a stronger biomarker of NMOSD-related disability than sGFAP. Additionally, the correlation between sNfL levels at attack and disability worsening at follow-up may reflect attack severity, with more severe attacks associated with higher sNfL levels and increased risk of residual disability. Another interpretation is that neuronal damage may not be limited solely to acute attacks but may also occur during apparent clinical remission. Limited data are available from the literature to support or refute this hypothesis. Studies longitudinally assessing the visual pathway in patients with NMOSD observed attack-independent deterioration, suggesting neurodegeneration.36 37 Cross-sectional sample collection studies have demonstrated a lack of significant reduction in sNfL levels between relapse and remission phases,12 and a slower reduction in sNfL levels during remission phases in NMOSD versus MS.13 In a recent small longitudinal Korean study (n=20), however, inter-attack sNfL elevations were not observed in 14 patients whose disease was clinically stable for at least 24 months following rituximab treatment.38
The N-MOmentum data and those of other groups12 13 contrast with other recent study results of sGFAP and sNfL in patients with AQP4+ NMOSD. In a prospective cohort of 33 patients, median baseline sGFAP was not elevated compared with MOG+ patients and healthy donors, and baseline sNfL levels did not differ substantially between groups. In contrast to N-MOmentum findings, only elevated sGFAP, and not elevated sNfL, predicted a future attack and was significantly associated with worse clinical disability scores, including EDSS scores.39 These discordant findings may be explained by the time period between the most recent clinical attack and blood sampling (median: 2639 vs 4 months13), patients with quiescent versus active disease, and different immunotherapies included in each study.13 14 39
Compared with placebo, participants treated with inebilizumab had smaller biomarker elevations during attacks, likely reflecting overall less severe attacks, as previously described for inebilizumab.23 At the end of the RCP, sNfL levels were significantly lower with inebilizumab than with placebo. Furthermore, in the absence of attack, sGFAP levels were significantly lower with treatment. This evidence for a putative effect of inebilizumab on disease severity is reinforced by previous results that showed more favourable disability outcomes with inebilizumab than with placebo.28
In approximately one-third of adjudicated attacks in AQP4+ inebilizumab-treated participants, no sNfL or sGFAP elevations were reported, which may be because of inebilizumab decreasing NMOSD attack severity and therefore lowering levels of these biomarkers. Conversely, some participants did not experience attacks but had elevated sNfL or sGFAP levels, which could potentially reflect subclinical disease activity previously described with other paraclinical modalities.36 Further investigations are underway to determine whether these findings have clinical significance.
Several strengths and limitations deserve mention. N-MOmentum was a large, randomised, placebo-controlled trial in which attacks were confirmed by independent expert committees using prespecified criteria.1 Availability of data from inebilizumab-treated and placebo-treated participants provided the potential to reveal effects of therapy on CNS damage biomarkers. Reference cohorts of healthy donors and patients with RRMS were also included to assess the specific utility of biomarker elevation in participants with NMOSD, but sampling serum and CSF from healthy controls can be challenging. Then again, the biomarker assessment was exploratory, and N-MOmentum was not specifically designed to have adequate statistical power for biomarker analysis. Moreover, although an analysis on the relationship between sNfL concentrations at attack and 3-month CDP was performed, there was no definitive confirmation that sNfL concentrations predict the durability of the EDSS score worsening, probably owing to the small sample size. Further study focusing on EDSS scores over longer intervals in a larger population is needed to improve understanding of the implications of the data presented here.40
In conclusion, sNfL, a biomarker of axonal damage, measured at attack, is the best predictor among the CNS damage biomarkers studied here for disability worsening during and after attacks. However, sNfL is inferior to sGFAP in predicting future attacks, consistent with the current concept of NMOSD being a primary astrocytopathy. Compared with placebo, inebilizumab appears to attenuate biomarker elevation during attacks and to reduce biomarker levels over time in the absence of adjudicated attacks. These findings may help to inform progress towards assessment of clinical status, prognosis and treatment decisions for patients with NMOSD by routinely measuring easily accessible serum biomarkers.
jnnp-2022-330412supp001.pdf (8.5MB, pdf)
Acknowledgments
The authors would like to thank the participants of the N-MOmentum trial.
Footnotes
Collaborators: The N-MOmentum study investigators. Australia: Neil Shuey (Melbourne, VIC); Bulgaria: Ivan Milanov (Sofia), Ara Kaprelyan (Varna), Ivaylo Tarnev (Sofia), Lyubomir Haralanov (Sofia); Canada: Robert Carruthers (Vancouver, BC); Colombia: Mario Muñoz (Bogotá), Jairo Quiñones (Cali), Jose Vargas (Barranquilla), Jesus Rodriguez (Bogotá); Czech Republic: Petra Nytrova (Praha), Marta Vachova (Teplice), Jan Mares (Olomouc); Estonia: Sulev Haldre (Tartu), Katrin Gross-Paju (Tallinn); Germany: Tjalf Ziemssen (Dresden), Uwe Klaus Zettl (Rostock), Orhan Aktas (Düsseldorf), Friedemann Paul (Berlin), Luisa Klotz (Münster), Florian Then Bergh (Leipzig); Hong Kong: Alexander Lau (Sha Tin); Hungary: Peter Dioszeghy (Nyiregyhaza), Mária Sátori (Esztergom), László Vécsei (Szeged); Israel: Anat Achiron (Ramat-Gan), Arnon Karni (Tel Aviv), Adi Vaknin-Dembinsky (Jerusalem); Japan: Takahiko Saida (Kyoto), Tatsuro Misu (Sendai), Masayuki Baba (Aomori), Akira Tamaoka (Tsukuba City), Chiyoko Nohara (Tokyo), Kazumasa Yokoyama (Bunkyo); Mexico: Freddy Castro Farfan (Mexico City), Daniel San Juan Orta (Tlalnepantla De Baz), Ildefonso Rodríguez (San Luis Potosi), Juan Gongora Rivera (Monterrey); Moldova: Olesea Odainic (Chisinau); New Zealand: Ernest Willoughby (Auckland); Peru: Edwin Pretell Alva (Callao), Julio Perez Villegas (Lima); Poland: Anna Czlonkowska (Warszawa), Krzysztof Selmaj (Lodz), Andrzej Tutaj (Olsztyn), Stanislaw Rusek (Kraków), Beata Zakrzewska-Pniewska (Warszawa), Maciej Maciejowski (Katowice), Konrad Rejdak (Lublin); Russia: Anna Belova (Nizhny Novgorod), Denis Sazonov (Novosibirsk), Farit Khabirov (Kazan), Klara Bakhtiyarova (Ufa), Ekaterina Kairbekova (St Petersburg), Tatiana Shcherbоnosova (Khabarovsk), Zhanna Chefranova (Belgorod), Alexey Boyko (Moscow), Alexey Rozhdestvenskiy (Omsk), Dmitry Pokhabov (Krasnoyarsk), Maria Zakharova (Moscow); Serbia: Jelena Drulovic (Belgrade); South Africa: Edward Bernard Leepan (Cape Town), Franclo Henning (Cape Town); South Korea: Ho Jin Kim (Goyang), Byoung Joon Kim (Seoul), Sung Min Kim (Seoul), Jee Young Oh (Seoul); Spain: Celia Oreja-Guevara (Madrid); Taiwan: Chou-Ching Lin (Tainan City), Shey-Lin Wu (Changhua City), An-Bang Liu (Hualien City); Thailand: Somsak Tiamkao (Khon Kaen), Surat Tanprawate (Chiang Mai), Naraporn Prayoonwiwat (Bangkok); Turkey: Aksel Siva (Istanbul), Kadriye Agan Yildirim (Istanbul), Muhtesem Gedizlioglu (Izmir), Murat Terzi (Samsun), Aysun Soysal (Istanbul); USA: Michael Levy (Baltimore, MD), Adil Javed (Chicago, IL), Benjamin Greenberg (Dallas, TX), Bruce Cree (San Francisco, CA), Jeffrey Bennett (Aurora, CO), Evanthia Bernitsas (Detroit, MI), George Hutton (Houston, TX), Mark Tullman (St Louis, MO), William Honeycutt (Maitland, FL), John Scagnelli (Raleigh, NC), Michelle Apperson (Sacramento, CA), Sharon Lynch (Kansas City, MO), Khurram Bashir (Birmingham, AL), Mary Rensel (Cleveland, OH), John Lindsey (Houston, TX), Sarah Wesley (North Haven, CT), Eoin Flanagan (Rochester, MN), Aram Zabeti (Cincinnati, OH), Geoffrey Eubank (Columbus, OH), Warren Felton, III (Richmond, VA).
Contributors: *OA: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. H-PH: Conceptualisation; Methodology; Investigation, Writing—Review and Editing. MAS: Conceptualisation; Methodology; Investigation, Formal analysis; Data Curation; Writing—Review and Editing. WAR: Conceptualisation; Methodology; Investigation; Formal analysis; Data Curation; Writing—Review and Editing. KF: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. FP: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. RM: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. JLB: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. HJK: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. BGW: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. SJP: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. DMW: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. GC: Formal analysis; Data Curation; Writing—Review and Editing. DS: Conceptualisation; Methodology; Investigation; Formal analysis; Data Curation; Writing—Review and Editing. MG: Methodology; Investigation; Data Curation; Writing—Review and Editing. DC: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. EK: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. *BAC: Conceptualisation; Methodology; Investigation; Writing—Review and Editing. *Corresponding authors and guarantors. Writing and editing assistance was provided by Dr Ian M. Williams of Oxford PharmaGenesis, who supported the development of the manuscript according to the direction of the authors. This manuscript was submitted on behalf of the principal investigators of the N-MOmentum study group, who administered the clinical trial.
Funding: This study was funded by Horizon Therapeutics (formerly by Viela Bio/MedImmune). Horizon Therapeutics supported the development of this manuscript, provided data analyses according to the direction of the authors and paid for medical writing support, provided by Oxford PharmaGenesis.
Competing interests: OA reports grants from the German Ministry of Education and Research (BMBF) and the German Research Foundation (DFG); grants and personal fees from Biogen and Novartis; and travel support and personal fees from Alexion, Almirall, MedImmune, Merck Serono, Roche, Sanofi, Viela Bio/Horizon Therapeutics and Zambon. OA is a member of the European Reference Network for Rare Eye Diseases (ERN-EYE), co-funded by the Health Program of the European Union under the Framework Partnership Agreement No 739534 'ERN-EYE. H-PH has received fees for consulting, speaking and serving on steering committees from Bayer Healthcare, Biogen Idec, Celgene Receptos, CSL Behring, GeNeuro, Genzyme, MedDay, MedImmune, Merck Serono, Novartis, Roche, Sanofi, TG Therapeutics and Viela Bio/Horizon Therapeutics, with approval by the Rector of Heinrich Heine University Düsseldorf. KF has received fees for consulting, speaking and serving on steering committees from AbbVie, Alexion, Asahi Kasei Medical, Biogen, Chugai/Roche, Eisai, Japan Tobacco, MedImmune/Viela Bio, Merck, Merck Biopharma, Mitsubishi-Tanabe, Novartis, Takeda, Teijin and UCB, and has received Grant-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labor of Japan. FP has received research support, speaker fees and travel reimbursement from Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme and Teva; is supported by the German Competence Network for Multiple Sclerosis and the German Research Council (DFG Exc 257); has received travel reimbursement from the Guthy–Jackson Charitable Foundation; and serves on the steering committee of the OCTIMS study sponsored by Novartis. RM serves on scientific advisory boards for Alexion, Roche and Viela Bio/Horizon Therapeutics; and has received funding for travel and fees from Alexion, Biogen, Merck, Novartis, Roche and Viela Bio/Horizon Therapeutics. JLB reports payment for study design/consultation from MedImmune/Viela Bio/Horizon Therapeutics; reports personal fees from AbbVie, Alexion, Beigene, Chugai, Clene Nanomedicine, Genentech, Genzyme, Mitsubishi Tanabe Pharma, Reistone Biopharma and Roche; reports grants and personal fees from EMD Serono and Novartis; reports grants from Alexion, Mallinckrodt and the National Institutes of Health; and has a patent for Aquaporumab issued. HJK has received a grant from the National Research Foundation of Korea; consultancy/speaker fees or research support from Alexion, AprilBio, ALTOS Biologics, Biogen, Celltrion, Daewoong, Eisai, GC Pharma, HanAll BioPharma, Handok, Horizon Therapeutics (formerly Viela Bio), Kolon Life Science, MDimune, Mitsubishi Tanabe Pharma, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok and UCB; and is a co-editor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology. BGW receives payments for serving as chair of attack adjudication committees for clinical trials in NMOSD for Alexion, MedImmune, UCB Bioscience and Viela Bio/Horizon Therapeutics; has consulted with Chugai, Genentech, Horizon Pharmaceuticals, Mitsubishi Tanabe Pharma and Roche; has received payments for speaking for Genentech and Roche; and has a patent for NMO-IgG for diagnosis of neuromyelitis optica, with royalties paid by Hospices Civils de Lyon, MVZ Labor PD Dr Volkmann und Kollegen GbR, RSR and the University of Oxford. SJP has received personal compensation for serving as a consultant for Astellas, Genentech and Sage Therapeutics; has received personal compensation for serving on scientific advisory boards or data safety monitoring boards for F. Hoffman-La Roche AG, Genentech and UCB; has received research support from Alexion, Roche/Genentech and Viela Bio/MedImmune/Horizon; has a Patent# 8,889,102 (Application#12-678350, Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia)—issued; has a patent, Patent# 9,891,219B2 (Application#12-573942, Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive)—issued; and his institution has received compensation for serving as a consultant for Alexion, Astellas and Viela Bio/MedImmune/Horizon. DMW reports personal fees from Biogen, Genentech, Horizon, Mitsubishi Tanabe, Roche, UCB Pharma and Viela Bio; and research support paid to Mayo Clinic by Alexion Pharmaceuticals. GC has received personal fees for participation on data and safety monitoring boards from AI Therapeutics, AMO Pharma, AstraZeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Karuna Therapeutics, Mapi Pharmaceuticals, Merck, Mitsubishi Tanabe Pharma Holdings, NHLBI (Protocol Review Committee), Novartis, Opko Biologics, Prothena Biosciences, Regeneron, Sanofi-Aventis, Reata Pharmaceuticals, University of Texas Southwestern, University of Pennsylvania and Visioneering Technologies; has received personal fees for consulting or advisory board participation from Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions, Entelexo Biotherapeutics, Genentech, Genzyme, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Merck/Serono, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics, Recursion/Cerexis Pharmaceuticals, Regeneron, Roche, SAB Biotherapeutics; and is employed by the University of Alabama at Birmingham and President of Pythagoras, a private consulting company located in Birmingham, Alabama. BAC reports personal compensation for consulting from Alexion, Atara Biotherapeutics, Autobahn, Avotres, Biogen, EMD Serono, Gossamer Bio, Horizon, Neuron23, Novartis, Sanofi, TG Therapeutics and Therini Bio; and has received research support from Genentech. MAS, WAR, DS and DC are employees of Horizon Therapeutics and own stock. MG and EK are former employees of Horizon Therapeutics and own stock.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Institutional review boards or ethics committees at each study site approved the protocol. Full list of institutional ethics committees or institutional review boards for N-MOmentum. (1) Central institutional ethics committees or institutional review boards (Region; Country; Site number; Agency type; Agency) Asia Pacific New Zealand Ethics - Central Southern Health and Disability Ethics Committee EMEA Bulgaria Ethics - Central Ethics Committee for Clinical Trials EMEA Czech Republic Ethics - Central/Local Eticka komise Vseobecne fakultni nemocnice v Praze EMEA Estonia Ethics - Central Research Ethics Committee of the University of Tartu EMEA Germany Ethics - Central/Local Ethikkommission an der medizinischen Fakultät der Heinrich-Heine-Universität EMEA Greece Ethics - Central National Ethics Committee EMEA Hungary Ethics - Central Egeszsegugyi Tudomanyos Tanacs Klinikai Farmakologiai Etikai Bizottsaga EMEA Moldova, Republic of Ethics - Central The National Committee for Ethical Review of Clinical Trials EMEA Netherlands Ethics - Central METC Erasmus MC EMEA Poland Ethics - Central/Local Komisja Bioetyczna przy Uniwersytecie Medycznym w Lodzi EMEA Portugal Ethics - Central Comissão de Ética para a Investigação Clínica - CEIC EMEA Russian Federation Ethics - Central The RF MoH, Department of State Regulation of Circulation of Medicines, Ethics Council EMEA Spain Ethics - Central/Local CEIC Hospital Clinico San Carlos EMEA Turkey Ethics - Central Istanbul Universitesi Cerrahpasa Tip Fakultesi Klinik Arastirmalar Etik KuruluLatin America Brazil Ethics - Central Comissao Nacional de Etica em Pesquisa - CONEP North America Canada Ethics - Central/Local Quorum Review IRB North America USA Ethics - Central/Local Quorum Review IRB2) Local institutional ethics committees or institutional review boards (Region; Country; Site number; Agency type; Agency) Asia Pacific Hong Kong 2000594 Ethics - Local Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Commit Asia Pacific Hong Kong 2000595 Ethics - Local The Unviersity of Hong Kong/Hospital Authority Hong Kong West Cluster Institutional Review Board Asia Pacific Hong Kong 2000597 Ethics - Local Kowloon Central Cluster REC/Kowloon East Cluster REC Asia Pacific China 2003693 Ethics - Local The Third Affiliated Hospital, Sun Yat-Sen University - EC Asia Pacific China 2003732 Ethics - Local Tianjin Medical University-General Hospital EC office Asia Pacific China 2003755 Ethics - Local Xuanwu Hospital Capital Medical University EC Asia Pacific India 2003588 Ethics - Local Ethics Committee, Sir Ganga Ram Hospital Asia Pacific India 2003599 Ethics - Local Central Ethics Committee, Nitte University, University Enclave Asia Pacific India 2003609 Ethics - Local Institutional Ethics Committee, Amrita Institute of Medical Sciences and Research CentreAsia Pacific India 2003731 Ethics - Local Institutional Ethics Committee I, Seth GS Medical College and KEM HospitalAsia Pacific India 2003737 Ethics - Local Institutional Ethics Committee Sree Chitra Tirunal Institute Asia Pacific India 2003744 Ethics - Local NIMS Institutional Ethics Committee Asia Pacific Japan 2000669 Ethics - Local Kyoto Miniren Hospital Institutional Review Board Asia Pacific Japan 2000669 Ethics - Local Kyoto University Hospital Institutional Review Board Asia Pacific Japan 2000711 Ethics - Local Tokyo Women’s Medical University Hospital Institutional Review Board Asia Pacific Japan 2000846 Ethics - Local Yamaguchi University Hospital IRB Asia Pacific Japan 2001081 Ethics - Local National University Corporation Tohoku University Tohoku University Hospital IRB Asia Pacific Japan 2001128 Ethics - Local Aomori Prefectural Central Hospital Institutional Review Board Asia Pacific Japan 2001230 Ethics - Local University of Tsukuba Hospital Institutional Review Board Asia Pacific Japan 2002885 Ethics - Local Ebara Hospital Institutional Review Board Asia Pacific Japan 2003213 Ethics - Local Juntendo University Hospital IRB Asia Pacific Japan 2003552 Ethics - Local National Hospital Organization Hokkaido Medical Center Institutional Review Board Asia Pacific Japan 2003605 Ethics - Local Southern TOHOKU Research Institute for Neuroscience Southern TOHOKU General Hospital IRB Asia Pacific Korea, Republic of 2000601 Ethics - Local National Cancer Center IRB Asia Pacific Korea, Republic of 2000602 Ethics - Local Samsung Medical Center Institutional Review Board Asia Pacific Korea, Republic of 2000603 Ethics - Local Seoul National University Hospital IRB Asia Pacific Korea, Republic of 2000604 Ethics - Local Keimyung University Dongsan Hospital IRB Asia Pacific Korea, Republic of 2000605 Ethics - Local Konkuk University Medical Center Institutional Review Board Asia Pacific Taiwan, Province of China 2000643 Ethics - Local Institutional Review Board of National Cheng Kung University Hospital Asia Pacific Taiwan, Province of China 2000644 Ethics - Local Cheng-Hsin General Hospital Institutional Review Board Asia Pacific Taiwan, Province of China 2000645 Ethics - Local Institutional Review Board, Changhua Christian Hospital Asia Pacific Taiwan, Province of China 2000646 Ethics - Local Tzu Chi General Hospital Research Ethics Committee Asia Pacific Thailand 2000647 Ethics - Local Office of The Khon Kaen University Ethics Committee in Human Research Asia Pacific Thailand 2000648 Ethics - Local Research Ethics Committee, Faculty of Medicine, Chiang Mai University Asia Pacific Thailand 2000650 Ethics - Local The Institutional Review Board of the Faculty of Medicine, Chulalongkorn University Asia Pacific Thailand 2000651 Ethics - Local Siriraj Institutional Review Board EMEA Czech Republic 2000585 Ethics - Local Eticka komise Krajska Zdravotni As Nemocnice Teplice Oz EMEA Israel 2000598 Ethics - Local The Chaim Sheba Medical Center EC EMEA Israel 2000600 Ethics - Local Barzilai Medical Center Local EC EMEA Israel 2002785 Ethics - Local Tel Aviv Sourasky EC EMEA Israel 2002786 Ethics - Local Hadassah University Hospital Local EC EMEA Russian Federation 2000632 Ethics - Local Ethics Committee at City Clinical Hospital #3 EMEA Russian Federation 2000633 Ethics - Local Ethics Committee at Siberian Regional Medical Centre EMEA Russian Federation 2000634 Ethics - Local Ethics Committee at Republic Clinical Hospital for Rehabilitation Treatment EMEA Russian Federation 2000635 Ethics - Local Ethics Committee at Bashkiria State Medical University EMEA Russian Federation 2000636 Ethics - Local Ethics Committee at City Clinical Hospital #31 EMEA Russian Federation 2000639 Ethics - Local Ethics Committee at City Clinical Hospital #11 EMEA Russian Federation 2000640 Ethics - Local Ethics Committee at Omsk State Medical University EMEA Russian Federation 2003146 Ethics - Local Ethics Committee at Siberian Regional Medical Centre EMEA Russian Federation 2003147 Ethics - Local Ethics Committee at Research Center of Neurology of RAMS EMEA Russian Federation 2003745 Ethics - Local Ethics Committee at Kirov City Hospital # 4 EMEA Russian Federation 2003746 Ethics - Local Ethics Committee at City Clinical Hospital a.n. Buyanov V. M. EMEA Serbia 2003142 Ethics - Local LEC Clinical Centre Serbia EMEA Serbia 2003144 Ethics - Local LEC Clinical Centre Nis EMEA Serbia 2003145 Ethics - Local LEC General Hospital Uzice EMEA South Africa 2000641 Ethics - Local University of Cape Town, Faculty of Health Sciences Human Research Ethics Committee EMEA South Africa 2000642 Ethics - Local University of Stellenbosch, Health Research Ethics Committee EMEA Ukraine 2003736 Ethics - Local CEQ of Branch Medical Center Cyber Clinic Spizhenko EMEA Ukraine 1-3PJSOU Ethics - Local Comission on Ethics Questions of Kyiv City Clinical Hospital #4 EMEA Ukraine 1-GB3UGZ Ethics - Local Commission on Ethics Questions of Municipal Institution Odesa Regional Clinical Hospital Latin America Argentina 2000519 Ethics - Local Comité Independiente de Etica en investigación clínica "Dr. Carlos A. BarclayLatin America Argentina 2000520 Ethics - Local Comisión Conjunta de Investigación en Salud (CCIS) Latin America Argentina 2000520 Ethics - Local Comité Institucional de Evaluación de la Facultad de Ciencias Biomédicas de la Universidad Austral Latin America Brazil 2000523 Ethics - Local Comitê de Ética em Pesquisa em Seres Humanos da Universidade de Passo Fundo Latin America Brazil 2000524 Ethics - Local Comite de Etica em Pesquisa em do Hospital Moinhos de Vento/RS Latin America Brazil 2000525 Ethics - Local Comitê de Ética em Pesquisa em Seres Humanos da Universidade de Passo Fundo Latin America Brazil 2000745 Ethics - Local Comitê de Ética em Pesquisa em Seres Humanos da Universidade Federal de Minas Gerais Latin America Colombia 2000533 Ethics - Local Comite de Etica en Investigacion Colegio Mayor de Nuestra Senora del Rosario Latin America Colombia 2000537 Ethics - Local Comite de Etica e Investigacion Clinica de la Fundacion Cardioinfantil Latin America Mexico 2000606 Ethics - Local Comité de Etica en Investigacion de Medica Sur Sociedad Anónima Bursátil de Capital Variable Latin America Mexico 2000607 Ethics - Local Comite de Etica en Investigacion del Grupo Medico Camino SC Latin America Mexico 2000608 Ethics - Local Comité de Ética en Investigación de Christus Muguerza del Parque S.A. de C.V. Latin America Mexico 2000609 Ethics - Local Comite Institucional de Etica CRI y Comite Institucional de Investigacion CRI Latin America Mexico 2000610 Ethics - Local Comite de ética del Antiguo Hospital Civil de Guadalajara Fray Antonio Alcalde Latin America Mexico 2000611 Ethics - Local Comite de Etica en Investigacion del Hospital Central Doctor Ignacio Morones Prieto Latin America Mexico 2000612 Ethics - Local Comite de Etica en Investigacion Winsett Rethman SA de CV Latin America Mexico 2000613 Ethics - Local Comite de Etica de la Facultad de Medicina de la UANL y Hosp. Univ. Dr. Jose Eleuterio González Latin America Peru 2000618 Ethics - Local Comite de Bioetica de la Red Asistencial Sabogal - EsSalud Latin America Peru 2000621 Ethics - Local Comite Institucional de Etica en la Investigacion del Hospital Nacional Cayetano Heredia Latin America Peru 2000622 Ethics - Local Comité de Etica en Investigación - Arequipa North America Canada 2000517 Ethics - Local McGill University Health Center Montreal Hospital North America Canada 2000518 Ethics - Local Ottawa Hospital Research Ethics Board North America Canada 2000662 Ethics - Local UBC Clinical Research Ethics Board North America USA 2000491 Ethics - Local Henry Ford Health System Institutional Review Board North America USA 2000492 Ethics - Local Johns Hopkins Medicine Office of Human Subjects Research Institutional Review Board North America USA 2000493 Ethics - Local University of Chicago Hospitals Institutional Review Board North America USA 2000494 Ethics - Local University of Maryland Human Research Protections Office North America USA 2000495 Ethics - Local UT Southwestern IRB North America USA 2000496 Ethics - Local UCSF Human Research Protection Program North America USA 2000498 Ethics - Local Wayne State University IRB North America USA 2000501 Ethics - Local Baylor College of Medicine IRB North America USA 2000502 Ethics - Local Missouri Baptist Medical Center Institutional Review Board North America USA 2000509 Ethics - Local UCDHS IRB North America USA 2000510 Ethics - Local Kansas City Veterans Administration Medical Center North America USA 2000511 Ethics - Local Vanderbilt Institutional Review Board North America USA 2000513 Ethics - Local LSU-HSC-S IRB North America USA 2000514 Ethics - Local Weill Cornell Medical College Institutional Review Board North America USA 2000515 Ethics - Local Cleveland Clinic Institutional Review Board North America USA 2000516 Ethics - Local University of Texas MD Anderson Cancer Center Institutional Review Board North America USA 2003421 Ethics - Local University of California Irvine Institutional Review Board North America USA 2003451 Ethics - Local Mayo Clinic Institutional Review Board North America USA 2003524 Ethics - Local University of Pennsylvania Institutional Review Board North America USA 2003601 Ethics - Local SUNY Buffalo Asia Pacific Japan 2000669 Ethics - Local Kyoto University Hospital Institutional Review Board Asia Pacific Japan 2000711 Ethics - Local Tokyo Women’s Medical University Hospital Institutional Review Board Asia Pacific Japan 2000846 Ethics - Local Yamaguchi University Hospital IRB Asia Pacific Japan 2001081 Ethics - Local National University Corporation Tohoku University Tohoku University Hospital IRB Asia Pacific Japan 2001128 Ethics - Local Aomori Prefectural Central Hospital Institutional Review Board Asia Pacific Japan 2001230 Ethics - Local University of Tsukuba Hospital Institutional Review Board Asia Pacific Japan 2002885 Ethics - Local Ebara Hospital Institutional Review Board Asia Pacific Japan 2003213 Ethics - Local Juntendo University Hospital IRB Asia Pacific Japan 2003552 Ethics - Local National Hospital Organization Hokkaido Medical Center Institutional Review Board Asia Pacific Japan 2003605 Ethics - Local Southern TOHOKU Research Institute for Neuroscience Southern TOHOKU General Hospital IRB Asia Pacific Korea, Republic of 2000601 Ethics - Local National Cancer Center IRB Asia Pacific Korea, Republic of 2000602 Ethics - Local Samsung Medical Center Institutional Review Board Asia Pacific Korea, Republic of 2000603 Ethics - Local Seoul National University Hospital IRB Asia Pacific Korea, Republic of 2000604 Ethics - Local Keimyung University Dongsan Hospital IRB Asia Pacific Korea, Republic of 2000605 Ethics - Local Konkuk University Medical Center Institutional Review Board Asia Pacific Taiwan, Province of China 2000643 Ethics - Local Institutional Review Board of National Cheng Kung University Hospital Asia Pacific Taiwan, Province of China 2000644 Ethics - Local Cheng-Hsin General Hospital Institutional Review Board Asia Pacific Taiwan, Province of China 2000645 Ethics - Local Institutional Review Board, Changhua Christian Hospital Asia Pacific Taiwan, Province of China 2000646 Ethics - Local Tzu Chi General Hospital Research Ethics Committee Asia Pacific Thailand 2000647 Ethics - Local Office of The Khon Kaen University Ethics Committee in Human Research Asia Pacific Thailand 2000648 Ethics - Local Research Ethics Committee, Faculty of Medicine, Chiang Mai University Asia Pacific Thailand 2000650 Ethics - Local The Institutional Review Board of the Faculty of Medicine, Chulalongkorn University Asia Pacific Thailand 2000651 Ethics - Local Siriraj Institutional Review Board EMEA Czech Republic 2000585 Ethics - Local Eticka komise Krajska Zdravotni As Nemocnice Teplice OzEMEA Israel 2000598 Ethics - Local The Chaim Sheba Medical Center ECEMEA Israel 2000600 Ethics - Local Barzilai Medical Center Local ECEMEA Israel 2002785 Ethics - Local Tel Aviv Sourasky ECEMEA Israel 2002786 Ethics - Local Hadassah University Hospital Local EC EMEA Russian Federation 2000632 Ethics - Local Ethics Committee at City Clinical Hospital #3 EMEA Russian Federation 2000633 Ethics - Local Ethics Committee at Siberian Regional Medical Centre EMEA Russian Federation 2000634 Ethics - Local Ethics Committee at Republic Clinical Hospital for Rehabilitation Treatment EMEA Russian Federation 2000635 Ethics - Local Ethics Committee at Bashkiria State Medical University EMEA Russian Federation 2000636 Ethics - Local Ethics Committee at City Clinical Hospital #31 EMEA Russian Federation 2000639 Ethics - Local Ethics Committee at City Clinical Hospital #11 EMEA Russian Federation 2000640 Ethics - Local Ethics Committee at Omsk State Medical University EMEA Russian Federation 2003146 Ethics - Local Ethics Committee at Siberian Regional Medical Centre EMEA Russian Federation 2003147 Ethics - Local Ethics Committee at Research Center of Neurology of RAMS EMEA Russian Federation 2003745 Ethics - Local Ethics Committee at Kirov City Hospital # 4 EMEA Russian Federation 2003746 Ethics - Local Ethics Committee at City Clinical Hospital a.n. Buyanov V. M. EMEA Serbia 2003142 Ethics - Local LEC Clinical Centre Serbia EMEA Serbia 2003144 Ethics - Local LEC Clinical Centre Nis EMEA Serbia 2003145 Ethics - Local LEC General Hospital Uzice EMEA South Africa 2000641 Ethics - Local University of Cape Town, Faculty of Health Sciences Human Research Ethics Committee EMEA South Africa 2000642 Ethics - Local University of Stellenbosch, Health Research Ethics Committee EMEA Ukraine 2003736 Ethics - Local CEQ of Branch Medical Center Cyber Clinic Spizhenko EMEA Ukraine 1-3PJSOU Ethics - Local Comission on Ethics Questions of Kyiv City Clinical Hospital #4 EMEA Ukraine 1-GB3UGZ Ethics - Local Commission on Ethics Questions of Municipal Institution Odesa Regional Clinical Hospital Latin America Argentina 2000519 Ethics - Local Comité Independiente de Etica en investigación clínica "Dr. Carlos A. BarclayLatin America Argentina 2000520 Ethics - Local Comisión Conjunta de Investigación en Salud (CCIS) Latin America Argentina 2000520 Ethics - Local Comité Institucional de Evaluación de la Facultad de Ciencias Biomédicas de la Universidad Austral Latin America Brazil 2000523 Ethics - Local Comitê de Ética em Pesquisa em Seres Humanos da Universidade de Passo Fundo Latin America Brazil 2000524 Ethics - Local Comite de Etica em Pesquisa em do Hospital Moinhos de Vento/RS Latin America Brazil 2000525 Ethics - Local Comitê de Ética em Pesquisa em Seres Humanos da Universidade de Passo Fundo Latin America Brazil 2000745 Ethics - Local Comitê de Ética em Pesquisa em Seres Humanos da Universidade Federal de Minas Gerais Latin America Colombia 2000533 Ethics - Local Comite de Etica en Investigacion Colegio Mayor de Nuestra Senora del Rosario Latin America Colombia 2000537 Ethics - Local Comite de Etica e Investigacion Clinica de la Fundacion Cardioinfantil Latin America Mexico 2000606 Ethics - Local Comité de Etica en Investigacion de Medica Sur Sociedad Anónima Bursátil de Capital Variable Latin America Mexico 2000607 Ethics - Local Comite de Etica en Investigacion del Grupo Medico Camino SC Latin America Mexico 2000608 Ethics - Local Comité de Ética en Investigación de Christus Muguerza del Parque S.A. de C.V. Latin America Mexico 2000609 Ethics - Local Comite Institucional de Etica CRI y Comite Institucional de Investigacion CRI Latin America Mexico 2000610 Ethics - Local Comite de ética del Antiguo Hospital Civil de Guadalajara Fray Antonio Alcalde Latin America Mexico 2000611 Ethics - Local Comite de Etica en Investigacion del Hospital Central Doctor Ignacio Morones Prieto Latin America Mexico 2000612 Ethics - Local Comite de Etica en Investigacion Winsett Rethman SA de CV Latin America Mexico 2000613 Ethics - Local Comite de Etica de la Facultad de Medicina de la UANL y Hosp. Univ. Dr. Jose Eleuterio González Latin America Peru 2000618 Ethics - Local Comite de Bioetica de la Red Asistencial Sabogal - EsSalud Latin America Peru 2000621 Ethics - Local Comite Institucional de Etica en la Investigacion del Hospital Nacional Cayetano Heredia Latin America Peru 2000622 Ethics - Local Comité de Etica en Investigación - Arequipa North America Canada 2000517 Ethics - Local McGill University Health Center Montreal Hospital North America Canada 2000518 Ethics - Local Ottawa Hospital Research Ethics Board North America Canada 2000662 Ethics - Local UBC Clinical Research Ethics Board North America USA 2000491 Ethics - Local Henry Ford Health System Institutional Review Board North America USA 2000492 Ethics - Local Johns Hopkins Medicine Office of Human Subjects Research Institutional Review Board North America USA 2000493 Ethics - Local University of Chicago Hospitals Institutional Review Board North America USA 2000494 Ethics - Local University of Maryland Human Research Protections Office North America USA 2000495 Ethics - Local UT Southwestern IRB North America USA 2000496 Ethics - Local UCSF Human Research Protection Program North America USA 2000498 Ethics - Local Wayne State University IRB North America USA 2000501 Ethics - Local Baylor College of Medicine IRB North America USA 2000502 Ethics - Local Missouri Baptist Medical Center Institutional Review Board North America USA 2000509 Ethics - Local UCDHS IRB North America USA 2000510 Ethics - Local Kansas City Veterans Administration Medical Center North America USA 2000511 Ethics - Local Vanderbilt Institutional Review Board North America USA 2000513 Ethics - Local LSU-HSC-S IRB North America USA 2000514 Ethics - Local Weill Cornell Medical College Institutional Review Board North America USA 2000515 Ethics - Local Cleveland Clinic Institutional Review Board North America USA 2000516 Ethics - Local University of Texas MD Anderson Cancer Center Institutional Review Board North America USA 2003421 Ethics - Local University of California Irvine Institutional Review Board North America USA 2003451 Ethics - Local Mayo Clinic Institutional Review Board North America USA 2003524 Ethics - Local University of Pennsylvania Institutional Review Board North America USA 2003601 Ethics - Local SUNY Buffalo. Participants gave informed consent to participate in the study before taking part.
References
- 1. Cree BA, Bennett JL, Sheehan M, et al. Placebo-controlled study in neuromyelitis optica—ethical and design considerations. Mult Scler 2016;22:862–72. 10.1177/1352458515620934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujihara K, Misu T, Nakashima I, et al. Neuromyelitis optica should be classified as an astrocytopathic disease rather than a demyelinating disease. Clin Exp Neuroimmunol 2012;3:58–73. 10.1111/j.1759-1961.2012.00030.x Available: http://doi.wiley.com/10.1111/cen3.2012.3.issue-2 [DOI] [Google Scholar]
- 3. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 2007;68:603–5. 10.1212/01.wnl.0000254502.87233.9a [DOI] [PubMed] [Google Scholar]
- 4. Jarius S, Paul F, Weinshenker BG, et al. Neuromyelitis optica. Nat Rev Dis Primers 2020;6:85. 10.1038/s41572-020-0214-9 [DOI] [PubMed] [Google Scholar]
- 5. Li J, Bazzi SA, Schmitz F, et al. Molecular level characterization of circulating aquaporin-4 antibodies in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm 2021;8:e1034. 10.1212/NXI.0000000000001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duan T, Smith AJ, Verkman AS. Complement-independent bystander injury in AQP4-igG seropositive neuromyelitis optica produced by antibody-dependent cellular cytotoxicity. Acta Neuropathol Commun 2019;7:112. 10.1186/s40478-019-0766-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herwerth M, Kenet S, Schifferer M, et al. A new form of axonal pathology in a spinal model of neuromyelitis optica. Brain 2022;145:1726–42. 10.1093/brain/awac079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yick L-W, Ma OK-F, Ng RC-L, et al. Aquaporin-4 autoantibodies from neuromyelitis optica spectrum disorder patients induce complement-independent immunopathologies in mice. Front Immunol 2018;9:1438.:. 10.3389/fimmu.2018.01438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeshita Y, Fujikawa S, Serizawa K, et al. New BBB model reveals that IL-6 blockade suppressed the BBB disorder, preventing onset of NMOSD. Neurol Neuroimmunol Neuroinflamm 2021;8:e1076. 10.1212/NXI.0000000000001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polivka J, Polivka J Jr, Krakorova K, et al. Current status of biomarker research in neurology. EPMA J 2016;7:14. 10.1186/s13167-016-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Misu T, Takano R, Fujihara K, et al. Marked increase in cerebrospinal fluid glial fibrillar acidic protein in neuromyelitis optica: an astrocytic damage marker. J Neurol Neurosurg Psychiatry 2009;80:575–7. 10.1136/jnnp.2008.150698 [DOI] [PubMed] [Google Scholar]
- 12. Chang X, Huang W, Wang L, et al. Serum neurofilament light and GFAP are associated with disease severity in inflammatory disorders with aquaporin-4 or myelin oligodendrocyte glycoprotein antibodies. Front Immunol 2021;12:647618. 10.3389/fimmu.2021.647618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watanabe M, Nakamura Y, Michalak Z, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology 2019;93:e1299–311. 10.1212/WNL.0000000000008160 [DOI] [PubMed] [Google Scholar]
- 14. Aktas O, Smith MA, Rees WA, et al. Serum glial fibrillary acidic protein: a neuromyelitis optica spectrum disorder biomarker. Ann Neurol 2021;89:895–910. 10.1002/ana.26067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lycke JN, Karlsson JE, Andersen O, et al. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998;64:402–4. 10.1136/jnnp.64.3.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forgrave LM, Ma M, Best JR, et al. The diagnostic performance of neurofilament light chain in CSF and blood for alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Alzheimers Dement (Amst) 2019;11:730–43. 10.1016/j.dadm.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bäckström D, Linder J, Jakobson Mo S, et al. Nfl as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 2020;95:e827–38. 10.1212/WNL.0000000000010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019;90:870–81. 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 19. Lin T-Y, Vitkova V, Asseyer S, et al. Increased serum neurofilament light and thin ganglion cell-inner plexiform layer are additive risk factors for disease activity in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2021;8:e1051. 10.1212/NXI.0000000000001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hallengren J, Chen PC, Wilson SM. Neuronal ubiquitin homeostasis. Cell Biochem Biophys 2013;67:67–73. 10.1007/s12013-013-9634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbier P, Zejneli O, Martinho M, et al. Role of tau as a microtubule-associated protein: structural and functional aspects. Front Aging Neurosci 2019;11:204. 10.3389/fnagi.2019.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mondello S, Palmio J, Streeter J, et al. Ubiquitin carboxy-terminal hydrolase l1 (uch-l1) is increased in cerebrospinal fluid and plasma of patients after epileptic seizure. BMC Neurol 2012;12:85. 10.1186/1471-2377-12-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019;394:1352–63. 10.1016/S0140-6736(19)31817-3 [DOI] [PubMed] [Google Scholar]
- 24. Agius MA, Klodowska-Duda G, Maciejowski M, et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler 2019;25:235–45. 10.1177/1352458517740641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sechi E, Buciuc M, Pittock SJ, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol 2021;78:741–6. 10.1001/jamaneurol.2021.0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999;53:1107–14. 10.1212/wnl.53.5.1107 [DOI] [PubMed] [Google Scholar]
- 27. Pittock SJ, Lennon VA, McKeon A, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol 2013;12:554–62. 10.1016/S1474-4422(13)70076-0 [DOI] [PubMed] [Google Scholar]
- 28. Marignier R, Bennett JL, Kim HJ, et al. Disability outcomes in the N-MOmentum trial of inebilizumab in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm 2021;8:e978. 10.1212/NXI.0000000000000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdelhak A, Huss A, Kassubek J, et al. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 2018;8:14798. 10.1038/s41598-018-33158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Czeiter E, Amrein K, Gravesteijn BY, et al. Blood biomarkers on admission in acute traumatic brain injury: relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine 2020;56:102785. 10.1016/j.ebiom.2020.102785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marshall WJ, Bangert SK. Clinical biochemistry: metabolic and clinical aspects. 2nd edition. Churchill Livingstone, 2008. [Google Scholar]
- 32. Kimbrough DJ, Fujihara K, Jacob A, et al. Treatment of neuromyelitis optica: review and recommendations. Mult Scler Relat Disord 2012;1:180–7. 10.1016/j.msard.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 2016;79:206–16. 10.1002/ana.24554 [DOI] [PubMed] [Google Scholar]
- 34. Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of Neuromyelitis Optica: recommendations of the neuromyelitis optica Study Group (NEMOS). J Neurol 2014;261:1–16. 10.1007/s00415-013-7169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kleiter I, Gahlen A, Borisow N, et al. Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm 2018;5:e504. 10.1212/NXI.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oertel FC, Havla J, Roca-Fernández A, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry 2018;89:1259–65. 10.1136/jnnp-2018-318382 [DOI] [PubMed] [Google Scholar]
- 37. Ringelstein M, Harmel J, Zimmermann H, et al. Longitudinal optic neuritis-unrelated visual evoked potential changes in NMO spectrum disorders. Neurology 2020;94:e407–18. 10.1212/WNL.0000000000008684 [DOI] [PubMed] [Google Scholar]
- 38. Hyun J-W, Kim Y, Kim SY, et al. Investigating the presence of interattack astrocyte damage in neuromyelitis optica spectrum disorder: longitudinal analysis of serum glial fibrillary acidic protein. Neurol Neuroimmunol Neuroinflamm 2021;8:e965. 10.1212/NXI.0000000000000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schindler P, Grittner U, Oechtering J, et al. Serum GFAP and NFL as disease severity and prognostic biomarkers in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder. J Neuroinflammation 2021;18:105. 10.1186/s12974-021-02138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barro C, Healy BC, Liu Y, et al. Serum GFAP and NFL levels differentiate subsequent progression and disease activity in patients with progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2023;10:e200052. 10.1212/NXI.0000000000200052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330412supp002.pdf (4.6MB, pdf)
jnnp-2022-330412supp001.pdf (8.5MB, pdf)
Data Availability Statement
Study data will be made available to others (see online supplemental methods). For more information, or to submit a request, please email: medicalinformation@horizontherapeutics.com.
Data are available upon reasonable request.