Abstract
Introduction
Oral corticosteroids (OCS) for asthma are associated with increased risks of developing adverse outcomes (adverse outcomes); no previous study has focused exclusively on intermittent OCS use.
Methods
This historical (2008–2019) UK cohort study using primary care medical records from two anonymised, real-life databases (OPCRD and CPRD) included patients aged≥4 years with asthma receiving only intermittent OCS. Patients were indexed on their first recorded intermittent OCS prescription for asthma and categorised by OCS prescribing patterns: one-off (single), less frequent (≥90 day gap) and frequent (<90 day gap). Non-OCS patients matched 1:1 on gender, age and index date served as controls. The association of OCS prescribing patterns with OCS-related AO risk was studied, stratified by age, Global Initiative for Asthma (GINA) 2020 treatment step, and pre index inhaled corticosteroid (ICS) and short-acting β2-agonist (SABA) prescriptions using a multivariable Cox-proportional hazard model.
Findings
Of 476 167 eligible patients, 41.7%, 26.8% and 31.6% had one-off, less frequent and frequent intermittent OCS prescribing patterns, respectively. Risk of any AO increased with increasingly frequent patterns of intermittent OCS versus non-OCS (HR; 95% CI: one-off 1.19 (1.18 to 1.20), less frequent 1.35 (1.34 to 1.36), frequent 1.42 (1.42 to 1.43)), and was consistent across age, GINA treatment step and ICS and SABA subgroups. The highest risks of individual OCS-related adverse outcomes with increasingly frequent OCS were for pneumonia and sleep apnoea.
Conclusion
A considerable proportion of patients with asthma receiving intermittent OCS experienced a frequent prescribing pattern. Increasingly frequent OCS prescribing patterns were associated with higher risk of OCS-related adverse outcomes. Mitigation strategies are needed to minimise intermittent OCS prescription in primary care.
Keywords: Asthma
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Although widely prescribed, particularly for acute exacerbations of asthma, both intermittent and long-term use of oral corticosteroids (OCS) are associated with an increased risk of developing acute and chronic adverse outcomes (adverse outcomes), and this risk increased with higher cumulative and mean daily systemic corticosteroid exposure. However, data on the adverse outcomes with patterns of intermittent OCS prescription are scarce.
WHAT THIS STUDY ADDS
Using two large, well-established UK databases (OPCRD and CPRD) with high data quality and granularity enabled us to study the specific association between patterns of intermittent OCS prescriptions and risk of different OCS-related adverse outcomes in almost half a million patients with asthma.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Increasingly frequent prescribing patterns of intermittent OCS were associated with a higher risk of individual OCS-related adverse outcomes, and this association remained consistent across levels of age, Global Initiative for Asthma treatment step and inhaled corticosteroid maintenance and short-acting β2-agonist reliever use. Our results therefore suggest that the use of intermittent OCS in the management of asthma should be minimised whenever possible.
Introduction
Despite the availability of novel and innovative treatments for asthma, oral corticosteroids (OCS) are still widely used.1 2 Both the British Thoracic Society/Scottish Intercollegiate Guidelines Network3 and the Global Initiative for Asthma (GINA) report1 recommend an intermittent OCS dose of 40–50 mg prednisolone daily for at least 5 days for the management of severe exacerbations, and in 2021, a Delphi study found consensus on a dose of 0.5 mg/kg/day for a short course of OCS.4 In a real-world study of OCS prescription patterns that included both intermittent and long-term OCS prescriptions in France, Germany, Italy and the UK from 2012 to 2017, 14%–44% of patients with asthma aged≥12 years were prescribed OCS and 6%–9% had high OCS use (defined as ≥450 mg within 90 days, ie, corresponding to an average of ≥5 mg per day) at some point during follow-up (median range 33–55 months).5 In a systematic review of studies from Europe, North America and Asia in patients with asthma aged>5 years, annual systemic corticosteroid (SCS) or OCS prescription was reported in ~50% of patients, with short-term use reported in up to 36% of patients; well above what might be expected for treatment according to guideline recommendations.6 Recent studies have shown a high prevalence of exacerbations treated with OCS across all severities of asthma,7 8 and exacerbations are increasingly being recognised as a major problem or as a risk factor for asthma death outside of severe asthma.
The use of SCS, even in the short-term, is associated with an increased risk of developing acute and chronic adverse outcomes (adverse outcomes).9 10 A study of both intermittent and long-term SCS prescriptions (parenteral or oral; median follow-up 7.4 years) showed that this risk increased with higher cumulative and mean daily SCS exposure.11 This is supported by another study of intermittent and long-term SCS prescriptions (parenteral or oral; median follow-up 7.1 years) in which increases in cumulative incidences of steroid-related adverse outcomes were dose dependent.12 Such OCS-related adverse outcomes can have a major impact on health‐related quality of life13–16 and are associated with increased healthcare resource use.17
Prior research on OCS-related adverse outcomes has focused mostly on overall OCS exposure, on cumulative or average daily OCS exposure from both intermittent and long-term prescriptions. There have been no longitudinal studies that have focused exclusively on examining the association between patterns of intermittent-only OCS prescriptions and risk of adverse outcomes. Previous attempts to classify OCS use as intermittent or long-term use were varied and based on aggregate measures over a specific time period, such as continuous OCS prescription,18 continuous OCS use without a gap,19 average number of days on OCS,20 titration patterns and cumulative daily dosage,17 21 none of which are perfect. As a result, it is challenging to separate out the adverse outcomes associated with intermittent use and long-term use given this absence of a clear definition of long-term use in secondary data. There is also a risk that, instead of identifying adverse outcomes related to intermittent OCS use, hidden historical cumulative OCS adverse outcomes are identified instead. Therefore, in this study, we specifically aimed to assess for the first time the association between longitudinal patterns of intermittent OCS prescriptions, cumulative OCS dosages and OCS-related adverse outcomes in a large cohort of UK primary care patients with asthma identified as treated with intermittent-only OCS.
Methods
Study design and data sources
This was a historical UK cohort study of patients≥4 years with asthma using primary care medical records from 2008 to 2019, where adverse outcomes in a cohort of patients with intermittent-only OCS use were compared with a matched patient cohort of never users of OCS. Data were sourced from the Optimum Patient Care Research Database (OPCRD)22 and Clinical Practice Research Datalink (CPRD) GOLD,23 two anonymised, real-life UK databases containing patient electronic medical records extracted from primary care and linked, in the case of CPRD, to a range of other health-related data to provide a longitudinal, representative UK population health dataset. CPRD contains data from primary care using Vision (Surgical Information Systems, Georgia, USA) software only; OPCRD contains data from a range of software providers, including EMIS (EMIS Health, Leeds, UK), iSoft (DXD Technology, Sydney, Australia) and Vision. The OPCRD comprises data extracted through the optimum patient care (OPC) clinical service evaluation and contains research-quality data for ~17 million patients across the UK.22 At the time of study, the CPRD GOLD database had coverage of over 11 million current patients from 674 practices in the UK, with data linked to Hospital Episode Statistics up to March 2019, allowing for identification of any hospital admission, including admissions with asthma as the primary diagnosis, as well as outpatient visits and emergency department attendances. To avoid overlap of the same primary care data between CPRD and OPCRD, Vision data were dropped from the OPCRD database prior to commencing the study.
Patients
Patients aged≥4 years with at least 12 months’ clinical data prior to index date receiving only intermittent OCS (OCS cohort) were matched 1:1 on gender, age and index date with patients with or without asthma who had received no exposure to OCS (‘never users’ or ‘non-OCS’ cohort). Patients who were receiving or suspected of receiving long-term OCS prescriptions were excluded, as per a previously defined algorithm.24 The index date for patients in the OCS cohort was the first intermittent OCS prescription within 3 months of an asthma event, which included asthma medication, consultation and/or diagnosis. Patients in the non-OCS cohort were indexed on the nearest general practitioner visit to the index date of the matched OCS patient.
OCS and non-OCS patients were also excluded if they had a diagnosis, ever, of one of the following chronic conditions commonly treated with OCS: ankylosing spondylitis, Sjögren’s syndrome, systemic lupus erythematosus, ulcerative colitis, rheumatoid and psoriatic arthritis, multiple sclerosis, and Crohn’s diseases. In order to ensure that the postindex adverse outcomes were incident events, patients with chronic adverse outcomes pre index were excluded, which led to different match sets for different individual adverse outcomes.
Study variables and outcomes
OCS exposures were converted into prednisolone equivalents using the defined daily dose (DDD) obtained from the Anatomical Therapeutic Chemical/DDD classification system.25 A patient’s cumulative dose was calculated as the sum of all prednisolone, or equivalent, doses (g) prescribed from index date up until the outcome event.
The methodology for classification of intermittent OCS prescribing patterns has been described separately.24 Briefly, OCS prescription patterns were evaluated across the entire follow-up period and classified as one-off, for patients with a single OCS prescription (as a proxy for OCS use), less frequent pattern, for patients with >1 prescription with any gaps>90 days but no gap<90 days, or frequent pattern, for patients with >1 prescription with <90 day gap(s).
Each patient was followed until first OCS-related AO and censored for that specific AO, or death or end of follow-up, but remained in the dataset for other conditions. The following adverse outcomes were recorded, defined according to International Classification of Diseases codes for diagnosis, and—for some adverse outcomes—other criteria, such as disease-specific medication prescription and/or physiological biomarkers: dyslipidaemia, behavioural disorders (diagnoses of distress, agitation, nervousness, emotional problems and irritable and abnormal behaviour for patients<18 years), renal impairment, osteoporosis/osteoporotic fractures, hypertension, peptic ulcer, cardiovascular disease, depression/anxiety, sleep disorders, cataracts, glaucoma, type 2 diabetes mellitus, pneumonia and sleep apnoea (online supplemental table S1).
thorax-2022-219642supp001.pdf (355.7KB, pdf)
Statistical analysis
Descriptive statistics were reported for the absolute and relative number of patients, mean, median, SD and IQR for continuous and categorical variables.
Patient demographic and clinical characteristics for the 12 months prior to index date (referred to as baseline) included age (4–<12, 12–<18, 18–<65 and ≥65 years), sex, body mass index (BMI; underweight, normal, overweight, obese and unknown), smoking status (never, current, former and unknown), short-acting β2-agonist (SABA) prescriptions (0, 1–2, 3–11 and ≥12 fills) and inhaled corticosteroid (ICS) prescriptions (0, 1–3, 4–6, 7–9, 10–12 and ≥13 fills). The SABA and ICS prescription categories were based on the distribution of the study data in the OCS cohort (online supplemental figure S1).
The risk of developing an AO was analysed using survival analysis methods. Patients with prior diagnosis of an AO, and which was classed as chronic, were excluded from the corresponding analysis in order to specifically assess incident-only adverse outcomes, meaning that the sample size for analysis of individual adverse outcomes or a set of any adverse outcomes differed from one to another. Kaplan-Meier curves were used to describe the overall risk profile of each AO. To assess the association between intermittent OCS (vs non-OCS) prescription and risk of adverse outcomes, a multivariable Cox proportional hazard model was used to calculate HR and 95% CI for each AO. The Cox models were adjusted for age, sex, BMI, smoking status and time-varying OCS prescriptions, defined a priori. The multivariable analysis was further stratified by age, GINA 2020 treatment step and prescriptions for ICS and SABA in the 12 months pre index.
To assess the relation between cumulative OCS dose and each AO, adjusted incidence rate ratios and 95% CI were calculated for each AO, comparing the OCS categories (>0 to <0.5 g, 0.5 to <1.0 g, 1.0 to <2.5 g, 2.5 to <5.0 g, 5.0 to <10.0 g and ≥10.0 g) to the non-OCS group.
Analyses were conducted using Stata SE V.14.2 (StataCorp, College Station, Texas, USA) and statistical significance was defined at two-sided p<0.05.
Results
Patient characteristics
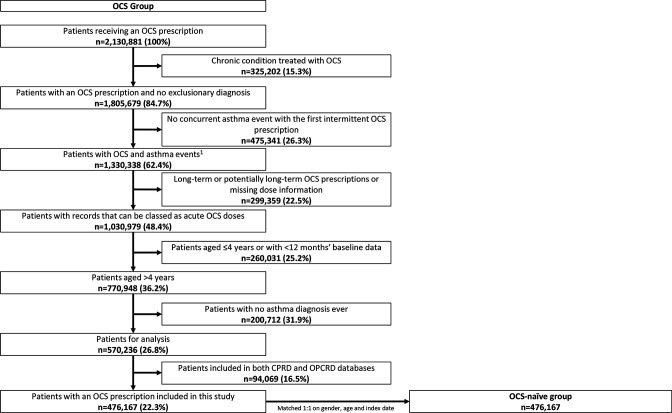
Of 2 130 881 patients receiving an OCS prescription for any condition (CPRD and OPCRD) between 1985 and 2019, 476 167 met the eligibility criteria (figure 1); records from 476 167 matched non-OCS patients were included, 16 716 (3.5%) of whom had an asthma diagnosis or asthma treatment ever (0.8% and 1.1% of non-OCS patients had ≥1 ICS and ≥1 SABA prescription, respectively, in the 12 months before the index date). The median follow-up (IQR) duration was 8.3 (4.2–13.7) years for the OCS cohort and 9.0 (4.7–14.7) years for the non-OCS cohort; length of time in database preindex was 17.0 (7.5–31.1) and 16.8 (7.8–30.4) years, respectively. The mean (SD) age and sex distribution at index date for patients receiving OCS prescriptions and matched non-OCS was 38.1 (22.4) years and 55.7% were female in both cohorts. There were more obese patients in the OCS cohort than in the non-OCS cohort (table 1).
Figure 1.
Flow chart of UK patients with asthma receiving intermittent OCS prescriptions in this study. 1Asthma events include asthma medication, asthma review or asthma diagnosis. CPRD, Clinical Practice Research Datalink; OCS, oral corticosteroid; OPCRD, Optimum Patient Care Research Database.
Table 1.
Baseline demographics and clinical characteristics of UK patients with asthma receiving intermittent OCS
| Non-OCS (never users) n=476 167* | Any OCS n=476 167 (100%) | One-off OCS n=198 422 (41.7%) | Less frequent OCS† n=127 419 (26.8%) | Frequent OCS‡ n=150 326 (31.6%) | |
| Age, mean, years (SD) | 38.1 (22.4) | 38.1 (22.4) | 35.6 (22.3) | 36.9 (22.1) | 42.4 (22.2) |
| Age category, n (%) | |||||
| 4–<12 years | 76 859 (16.1) | 77.131 (16.2) | 35 478 (17.9) | 22 568 (17.7) | 19 085 (12.7) |
| 12–<18 years | 36 329 (7.6) | 36 262 (7.6) | 19 336 (9.7) | 9518 (7.5) | 7408 (4.9) |
| 18–<65 years | 292 846 (61.5) | 292 778 (61.5) | 118 163 (59.6) | 78 572 (61.7) | 96 043 (63.9) |
| ≥65 years | 70 133 (14.7) | 69 996 (14.7) | 25 445 (12.8) | 16 761 (13.2) | 27 790 (18.5) |
| Female, % | 55.7 | 55.7 | 51.8 | 56.3 | 60.2 |
| Follow-up, median, years (IQR) | 9.0 (4.7, 14.7) | 8.3 (4.2, 13.7) | 6.4 (3.1, 11.5) | 9.8 (5.6, 14.9) | 9.6 (5.1, 15.2) |
| Time in database pre index, median, years (IQR) | 16.8 (7.8, 30.4) | 17.0 (7.5, 31.1) | 15.1 (6.9, 28.3) | 16.2 (7.2, 30.1) | 20.6 (9.1, 34.9) |
| BMI | |||||
| Underweight, n (%) | 23 904 (5.0) | 35 998 (7.6) | 15 159 (7.6) | 10 257 (8.0) | 10 553 (7.0) |
| Normal, n (%) | 144 517 (30.4) | 138 469 (29.1) | 56 728 (28.6) | 37 665 (29.6) | 44 061 (29.3) |
| Overweight, n (%) | 102 328 (21.5) | 109 042 (22.9) | 42 521 (21.4) | 28 899 (22.7) | 37 612 (25.0) |
| Obese, n (%) | 66 473 (14.0) | 100 995 (21.2) | 36 410 (18.3) | 26 529 (20.8) | 38 048 (25.3) |
| Unknown, n (%) | 138 946 (29.2) | 91 710 (19.3) | 47 601 (24.0) | 24 057 (18.9) | 20 038 (13.3) |
| Mean (SD) | 25.8 (5.9) | 26.6 (7.0) | 26.2 (6.8) | 26.5 (6.9) | 27.2 (7.1) |
| Smoking status, n (%§) | |||||
| Never | 135 708 (28.5) | 141 231 (29.7) | 60 921 (30.7) | 37 512 (29.4) | 42 813 (28.5) |
| Current | 117 089 (24.6) | 131 851 (27.7) | 53 508 (27.0) | 34 951 (27.4) | 43 384 (28.9) |
| Ex | 67 711 (14.2) | 84 377 (17.7) | 33 951 (17.1) | 21 075 (16.5) | 29 375 (19.5) |
| Unknown | 155 659 (32.7) | 118 708 (24.9) | 50 042 (25.2) | 33 881 (26.6) | 34 754 (23.1) |
| SABA prescriptions; n (%) | |||||
| 0 | 470 929 (98.9) | 113 262 (23.8) | 44 766 (22.6) | 30 987 (24.3) | 37 507 (25.0) |
| 1–2 | 3524 (0.7) | 213 594 (44.9) | 96 751 (48.8) | 55 660 (43.7) | 61 183 (40.7) |
| 3–11 | 1476 (0.3) | 133 255 (28.0) | 51 519 (26.0) | 36 602 (28.7) | 45 134 (30.0) |
| ≥12 | 238 (0.0) | 16 056 (3.4) | 5384 (2.7) | 4170 (3.3) | 6502 (4.3) |
| ICS prescriptions; n (%) | |||||
| 0 | 472 462 (99.2) | 191 141 (40.1) | 80 403 (40.5) | 50 177 (39.4) | 60 560 (40.3) |
| 1–3 | 2354 (0.5) | 183 159 (38.5) | 79 561 (40.1) | 50 015 (39.2) | 53 583 (35.6) |
| 4–6 | 747 (0.2) | 59 230 (12.4) | 22 712 (11.4) | 16 260 (12.8) | 20 258 (13.5) |
| 7–9 | 310 (0.1) | 23 952 (5.0) | 8874 (4.5) | 6315 (4.9) | 8763 (5.8) |
| 10–12 | 179 (0.0) | 11 968 (2.5) | 4393 (2.2) | 3043 (2.4) | 4532 (3.0) |
| ≥13 | 115 (0.0) | 6718 (1.4) | 2479 (1.2) | 1609 (1.3) | 2630 (1.7) |
*Non-OCS patients were matched with all patients receiving OCS prescriptions according to 1:1 ratio.
†Patients who received all OCS prescriptions with a gap of ≥90 days.
‡Patients who received at least some OCS prescriptions with a gap of <90 days, allowing for other prescription gaps to be ≥90 days.
§Only the percentages of patient with known smoking status were calculated.
BMI, body mass index; ICS, inhaled corticosteroid; OCS, oral corticosteroid; SABA, short-acting β2 agonist.
Of the study patients, 198 422 (41.7%), 127 419 (26.8%) and 150 326 (31.6%) had one-off, less frequent and frequent patterns of intermittent OCS prescription during the follow-up, respectively. The mean (median (IQR)) cumulative OCS dose was 176 (150 (150–200)) mg for one-off, 510 (420 (300–600)) mg for less frequent and 2357 (940 (540–1800)) mg for frequent patterns. Patients who had more frequent patterns of intermittent OCS prescriptions were older and more likely to be female. Those who had a frequent pattern of intermittent OCS prescriptions were more likely to be obese and to be current or ex-smokers compared with the one-off and less frequent OCS groups (table 1).
Across all patterns of intermittent OCS prescriptions, in the 12 months prior to initial OCS prescriptions, patients were most commonly receiving 1–2 SABA fills and ≤3 ICS fills. The proportion of patients receiving≥3 SABA and≥4 ICS fills at baseline increased with increasingly frequent patterns of OCS prescription (table 1).
Intermittent OCS prescribing patterns and risk of OCS-related adverse outcomes
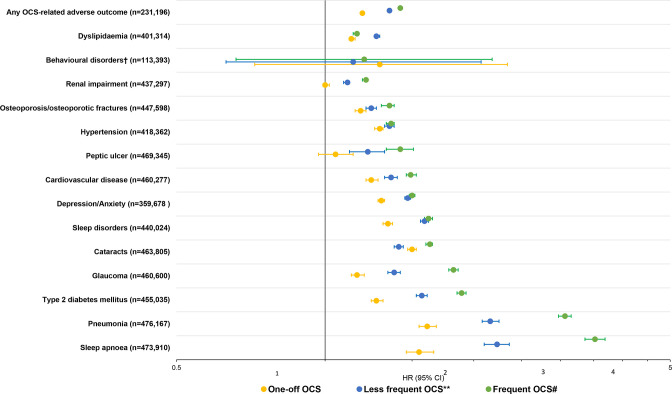
The overall analysis of the association between patterns of intermittent OCS prescriptions and any OCS-related adverse outcomes included 231 196 patients, due to the exclusion of those with prevalent conditions. Compared with non-OCS patients, the risk of experiencing any adverse outcomes was elevated for one-off OCS and increased with increasing frequent pattern of intermittent OCS prescribing. The HR (95% CI) of experiencing any adverse outcomes versus non-OCS patients was 1.19 (1.18 to 1.20) for one-off OCS, 1.35 (1.34 to 1.36) for less frequent OCS and 1.42 (1.42 to 1.43) for frequent pattern of OCS prescriptions.
Similar to the risk of any OCS-related adverse outcomes, the risk of all individual OCS-related adverse outcomes except behavioural disorders, renal impairment and peptic ulcer was already detectable in one-off OCS, and increased with increasingly frequent patterns of intermittent OCS prescriptions (figure 2). The highest risk (HR>2) of OCS-related individual adverse outcomes with increasingly frequent OCS prescribing were for pneumonia and sleep apnoea (figure 2). Kaplan-Meier curves for the relation between patterns of intermittent OCS prescriptions and individual adverse outcomes are shown in online supplemental figure S2.
Figure 2.
HRs‡ of adverse outcomes in UK patients with asthma receiving intermittent OCS prescriptions versus non-OCS patients. OCS, oral corticosteroid. Non-OCS patients were matched with all patients receiving OCS prescriptions according to a 1:1 ratio. **Patients with less frequent OCS use received all OCS prescriptions with a gap of ≥90 days. #Patients with frequent OCS use received at least some OCS prescriptions with a gap of <90 days, allowing for other prescription gaps to be ≥90 day. ‡HRs were calculated using Cox regression analysis, adjusted for age, gender, body mass index, smoking and time-varying OCS prescriptions. *Behavioural disorders include diagnoses for distress, agitation, nervousness, emotional problems, irritable and abnormal behaviour among patients <18 years.
The association between patterns of intermittent OCS prescription and risk of OCS-related adverse outcomes remained consistent when stratified by age, GINA treatment step and ICS or SABA prescriptions (table 2).
Table 2.
HRs for any adverse outcomes in UK patients with asthma receiving intermittent OCS stratified by age, GINA treatment step, ICS and SABA prescriptions versus all non-OCS patients
| HR (95% CI) by category | One-off OCS n=198 422 (41.7%) | Less frequent OCS* n=127 419 (26.8%) | Frequent OCS† n=150 326 (31.6%) |
| Age | |||
| 4–<12 years | 1.11 (1.08, 1.14) | 1.21 (1.17, 1.24) | 1.32 (1.29, 1.36) |
| 12–<18 years | 1.17 (1.14, 1.20) | 1.33 (1.29, 1.37) | 1.45 (1.40, 1.50) |
| 18–<65 years | 1.24 (1.23, 1.26) | 1.39 (1.38, 1.41) | 1.48 (1.47, 1.50) |
| ≥65 years | 1.20 (1.18, 1.23) | 1.35 (1.33, 1.38) | 1.35 (1.33, 1.37) |
| GINA | |||
| Step 0 | 1.25 (1.22, 1.28) | 1.43 (1.40, 1.47) | 1.46 (1.43, 1.49) |
| Step 1 | 1.21 (1.19, 1.23) | 1.35 (1.33, 1.38) | 1.40 (1.38, 1.43) |
| Step 2 | 1.16 (1.15, 1.18) | 1.32 (1.30, 1.34) | 1.38 (1.36, 1.41) |
| Step 3 | 1.19 (1.17, 1.21) | 1.38 (1.35, 1.41) | 1.46 (1.43, 1.49) |
| Step 4 | 1.28 (1.24, 1.32) | 1.47 (1.43, 1.52) | 1.58 (1.54, 1.63) |
| Step 5 | 1.33 (1.24, 1.43) | 1.59 (1.49, 1.70) | 1.89 (1.80, 1.99) |
| ICS prescriptions | |||
| 0 | 1.22 (1.20, 1.23) | 1.38 (1.36, 1.40) | 1.42 (1.40, 1.44) |
| 1–3 | 1.18 (1.17, 1.20) | 1.34 (1.32, 1.36) | 1.45 (1.43, 1.47) |
| 4–6 | 1.21 (1.17, 1.24) | 1.41 (1.37, 1.46) | 1.49 (1.45, 1.53) |
| 7–9 | 1.20 (1.17, 1.20) | 1.38 (1.35, 1.41) | 1.44 (1.41, 1.47) |
| 10–12 | 1.25 (1.18, 1.32) | 1.44 (1.36, 1.52) | 1.56 (1.49, 1.64) |
| ≥13 | 1.26 (1.18, 1.36) | 1.49 (1.38, 1.60) | 1.65 (1.56, 1.76) |
| SABA prescriptions | |||
| 0 | 1.25 (1.23, 1.27) | 1.42 (1.40, 1.45) | 1.46 (1.44, 1.48) |
| 1–2 | 1.21 (1.19, 1.23) | 1.37 (1.34, 1.39) | 1.43 (1.40, 1.45) |
| 3–11 | 1.18 (1.17, 1.20) | 1.34 (1.32, 1.36) | 1.44 (1.42, 1.46) |
| ≥12 | 1.08 (1.03,1.13) | 1.41 (1.35, 1.48) | 1.45 (1.40, 1.51) |
HR were calculated using Cox regression analysis, adjusted for age, gender, body mass index, smoking and time-varying OCS prescriptions; HR=1 for non-OCS patients.
*Patients who received all OCS prescriptions with a gap of ≥90 days.
†Patients who received at least some OCS prescriptions with a gap of <90 days, allowing for other prescription gaps to be ≥90 days.
GINA, The Global Initiative for Asthma; ICS, inhaled corticosteroid; OCS, oral corticosteroid; SABA, short-acting β2 agonist.
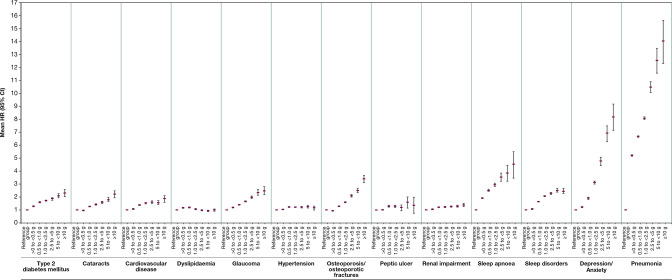
When examining the dose–response relation between annual cumulative OCS exposure and risk of adverse outcomes, the risk increased in a dose–response fashion with greater annual cumulative OCS dose. Importantly, compared with the non-OCS group, risks started to increase at 0.5–1.0 g for all adverse outcomes except dyslipidaemia, with modest increases for hypertension and renal impairment (figure 3).
Figure 3.
HR (95% CI) for each adverse outcome by overall, cumulative OCS exposures, compared with the reference category of non-OCS patients in UK patients with asthma. OCS, oral corticosteroid.
Discussion
In this historical UK cohort study of patients with asthma, we found that almost a third of the patients with intermittent OCS use had a frequent pattern of use (ie, more than one prescription with less than 90-day gaps) at some point during the follow-up period. In those patients with intermittent-only OCS use, compared with matched individuals who were non-users of OCS, the risk of experiencing any OCS-related adverse outcomes increased with increasing frequent pattern of intermittent OCS prescriptions for most adverse outcomes studied, particularly pneumonia and sleep apnoea. The elevated risk of adverse outcomes was observed even for patients with one-off OCS prescriptions. The association between patterns of intermittent OCS prescriptions and risk of OCS-related adverse outcomes was relatively consistent across age categories, GINA treatment steps and prior asthma medication use.
To our knowledge, this study is the largest (with almost half a million patients), has the longest patient follow-up (34 years, with a median study period of over 25 years (pre-first and post-first OCS exposure)) and is the most definitive study yet to identify intermittent OCS use and assess the association of patterns of intermittent OCS use with risk of developing OCS-related adverse outcomes. Our study overcame the issues associated with mixed reporting of long-term and intermittent OCS prescriptions and related adverse outcomes by focusing exclusively on intermittent-only OCS prescriptions24; it also fills a need for a longitudinal study using objective methods to collect data on intermittent OCS-related adverse outcomes, increasing the specificity of the findings by minimising the risk of bias.5
Despite the extensive research on adverse outcomes associated with OCS, the use of OCS remains widespread5 and recommended in certain instances.1 3 Our finding that increasingly frequent patterns of intermittent OCS prescription were associated with an increased risk for adverse outcomes is consistent with previous studies reporting a high risk of corticosteroid-related adverse outcomes with OCS prescriptions,1 12 26 and with studies in adult patients with severe asthma, showing that long-term high-dose use of OCS (defined as >10 mg/day) resulted in a higher risk of developing OCS-related complications than medium OCS use (defined as 5–10 mg/day), when both were compared with low OCS use (defined as <5 mg/day) and non-OCS patients.13 27 The fact that the risk of an OCS-related AO was highest for pneumonia in our study is not unexpected, as this is one of the most well-recognised adverse effects of even short-term OCS use.6 11 28 The elevated risk of sleep apnoea was more surprising, as this is not as commonly associated with OCS use. However, a link with sleep apnoea has previously been shown in patients with severe asthma receiving SCS29 and in patients with difficult-to-control asthma receiving long-term or frequent bursts of OCS,30 as well as in a broader population of patients with active asthma of all severities receiving SCS.11 Furthermore, as our analysis was adjusted for BMI, our findings suggest that the association with OCS use may be independent of obesity—one of the best-known risk factors for sleep apnoea31 and also a common adverse effect of OCS use2—and is instead likely mediated by other mechanisms. Possible explanations include fat deposition in the upper airway or airway dilator muscle myopathy, as has been suggested for ICS,32 33 or an increase in airway collapsibility.30
Our results demonstrating increased risks with even a one-off OCS prescription are also supported by previous research showing that short bursts of OCS can be associated with certain adverse outcomes and that each OCS prescription contributes to a cumulative burden, regardless of the dose and duration.9 Given that the median cumulative OCS dose is higher in this study in patients with a frequent pattern of prescription than the less frequent and one-off patterns, there may be correlation between frequent patterns of intermittent prescriptions and total exposure. This observation gives physicians another tool to gauge the risk of adverse outcomes in their patients, that is, future risk is higher if patients have received OCS with smaller gaps between prescriptions. Our finding that risks started to increase at dose levels of 0.5–1.0 g also confirms the importance of minimising total exposure, and the reasons behind persistent prescribing of OCS should be further investigated. Therefore, all patients, including those with mild disease and/or receiving regular ICS and SABA treatment, could potentially benefit from being monitored for frequent patterns of OCS prescription.
Our results also showed that over 30% of patients with intermittent-only OCS prescriptions had ≥3 SABA fills in the 12-month baseline period, and those with a more frequent pattern of intermittent OCS prescriptions had more prescriptions for SABA. It has been previously shown that increased SABA exposure is associated with severe exacerbation risk,34 which is reflected in this study with more frequent pattern of intermittent OCS use among patients with a higher number of SABA prescriptions. This supports the move away from SABA-only treatment at GINA step 1. Similarly to the findings for SABA prescriptions, more ICS prescriptions were also observed in those with a more frequent pattern of OCS prescription, although the number of ICS prescriptions was low. On that note, the fact that 40% of the OCS cohort had no ICS prescriptions during the 12-month baseline period suggests that some patients might have received their first OCS prescriptions at the same time as their first attendance in primary care with asthma, and there might also be a population of patients with asthma who are underusing ICS. It is worth noting the population in this study generally had milder disease as a result of the exclusion of long-term OCS use.
This study had several strengths including the use of an algorithm that exclusively included patients on intermittent OCS and took a longitudinal view on the patterns of intermittent OCS use, resulting in a highly specific representation of patients receiving intermittent OCS only. Furthermore, the real-world design of this study allows for generalisation of the results to patients managed in all healthcare settings. Using two well-established databases (OPCRD and CPRD) with large size and high data quality and granularity allowed us to study the association between intermittent OCS prescriptions and associated risk of multiple different adverse outcomes.
Limitations of the study included the fact that, despite using a sophisticated algorithm that was reviewed by experts familiar with the clinical implications of our study, some of the intermittent and long-term OCS prescriptions might have been misclassified, thereby underestimating the strengths of the effect estimates. The validity and completeness of individual patient records could not be assessed because the datasets represent information collected for clinical and routine use rather than specifically for research purposes. As a result, there were some missing data, especially for BMI and smoking status, which could potentially affect how confounding by these factors is adjusted for. Furthermore, since the multivariable Cox proportional hazard model was not adjusted for obesity but for BMI, with some missing values, there could have been some residual confounding. Although our study included patients of all ages, the only children-specific and adolescent-specific adverse outcomes to be analysed were behavioural disorders (including diagnoses of distress, agitation, nervousness, emotional problems, irritability and abnormal behaviour). Such diagnoses are difficult to capture and of varying origin, hence a possible reason for not detecting associations with this outcome. However, analysis of all adverse outcomes stratified by the different age categories also showed an increased risk with higher frequency of prescribing patterns of intermittent OCS for the youngest patients. In patients with one-off OCS use, there could be additional bursts that were not captured, which might explain the association detected between one-off OCS prescription and developing adverse outcomes. In the context of a chest infection, prescription of prednisolone alongside antibiotics in a patient with asthma, coupled with the potential for mislabelling of a chest infection as pneumonia, may have contributed to an association between the two. Lastly, OCS prescriptions may not be directly linked to OCS use, as there is no guarantee that patients used the prescriptions that they received.
Conclusions
In conclusion, our study found that a considerable proportion of patients with asthma who are prescribed OCS intermittently have a frequent pattern of use at some point. Increasingly frequent prescribing patterns of intermittent OCS were associated with a higher risk of individual OCS-related adverse outcomes, and this association remained consistent across levels of age, GINA treatment step and ICS maintenance and SABA reliever use. The increase in risk occurred early, even with one-off prescriptions and with doses as low as 0.5–1.0 g. Our results suggest that the use of OCS, even intermittently, in the management of asthma should be minimised whenever possible.
Acknowledgments
We would like to thank Sam Hijazi and Stefan Courtney of inScience Communications, Springer Healthcare, UK, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3 http://www.ismpp.org/gpp3).
Footnotes
Twitter: @JeffreyChansky, @OPRI_SG
Correction notice: This article has been corrected since it published Online to reflect the correct author name: David J Jackson.
Contributors: All authors contributed to the writing of the manuscript. In addition, the following authors participated in research design and performed data analysis: HH, TNT, EM, DS, VC, CA, JH and DBP. DBP had full access to the data, had final responsibility to submit for publication, and accepts full responsibility for the work
Funding: AstraZeneca funded the study (grant/award number: not applicable). AstraZeneca and OPRI had a role in study design, data collection, data analysis, data interpretation and writing of the report.
Competing interests: HH, DS, JSKC, CA and VC are employees of Observational and Pragmatic Research Institute Singapore who conducted this study, funded by AstraZeneca. JC, EM and TNT are employees of, and own stock in, AstraZeneca. AB has received consultancy fees and speakers’ fees from of AstraZeneca, Amgen, Boehringer Ingelheim, Novartis, GlaxoSmithKline, Sanofi Regeneron and Chiesi, and research grants from GlaxoSmithKline, Boehringer Ingelheim and AstraZeneca. AM-G has received grants, advisory board fees, lecture fees and consulting fees from AstraZeneca; advisory board fees from GlaxoSmithKline; advisory board fees and lecture fees from Novartis; advisory board fees, lecture fees and travel expenses from Teva; advisory board fees from Regeneron; advisory board fees, lecture fees and consulting fees from Sanofi. DJJ has received consultancy fees and speakers’ fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Sanofi Regeneron and Chiesi, and research grants from AstraZeneca. JH reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Circassia and Teva unrelated to the conduct of the study. DP has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Thermofisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study adhered to standards recommended for observational research.24 The OPCRD is maintained by Optimum Patient Care and has ethical approval from the National Health Service Research Authority (Research Ethics Committee reference: 15/EM/0150). This study was approved by the Anonymised Data Ethics & Protocol Transparency (ADEPT) Committee25 (OPCRD; ADEPT1120), and the Independent Scientific Advisory Committee (CPRD; ISAC 20_000071). The study is registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (registration number: EUPAS37065). No patient-identifying information was accessible during the study.
References
- 1. Global Initiative for Asthma . Global strategy for asthma management and prevention, 2022. Available: www.ginasthma.org
- 2. Sullivan PW, Ghushchyan VH, Globe G, et al. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol 2018;141:110–6. 10.1016/j.jaci.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 3. British Thoracic Society, Scottish Intercollegiate Guidelines Network . British guideline on the management of asthma, 2019. Available: https://www.brit-thoracic.org.uk/document-library/guidelines/asthma/btssign-guideline-for-the-management-of-asthma-2019/
- 4. Suehs CM, Menzies-Gow A, Price D, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma. A Delphi study. Am J Respir Crit Care Med 2021;203:871–81. 10.1164/rccm.202007-2721OC [DOI] [PubMed] [Google Scholar]
- 5. Tran TN, King E, Sarkar R, et al. Oral corticosteroid prescription patterns for asthma in France, Germany, Italy and the UK. Eur Respir J 2020;55:1902363. 10.1183/13993003.02363-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med 2020;201:276–93. 10.1164/rccm.201904-0903SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryan D, Heatley H, Heaney LG, et al. Potential severe asthma hidden in UK primary care. J Allergy Clin Immunol Pract 2021;9:1612–23. 10.1016/j.jaip.2020.11.053 [DOI] [PubMed] [Google Scholar]
- 8. Hancock KL, Bosnic-Anticevich S, Blakey JD, et al. Characterisation of the Australian adult population living with Asthma: severe - exacerbation frequency, long-term OCS use and adverse effects. Pragmat Obs Res 2022;13:43–58. 10.2147/POR.S360044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ekström M, Nwaru BI, Hasvold P, et al. Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy 2019;74:2181–90. 10.1111/all.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357:j1415. 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018;11:193–204. 10.2147/JAA.S176026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voorham J, Xu X, Price DB, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy 2019;74:273–83. 10.1111/all.13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung LP, Upham JW, Bardin PG, et al. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology 2020;25:161–72. 10.1111/resp.13730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hyland ME, Whalley B, Jones RC, et al. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res 2015;24:631–9. 10.1007/s11136-014-0801-x [DOI] [PubMed] [Google Scholar]
- 15. Sweeney J, Brightling CE, Menzies-Gow A, et al. Clinical management and outcome of refractory asthma in the UK from the British Thoracic Society difficult asthma registry. Thorax 2012;67:754–6. 10.1136/thoraxjnl-2012-201869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson PJ, Salvi S, Lin J, et al. Insights, attitudes and perceptions about asthma and its treatment: findings from a multinational survey of patients from 8 Asia-Pacific countries and Hong Kong. Respirology 2013;18:957–67. 10.1111/resp.12137 [DOI] [PubMed] [Google Scholar]
- 17. Zeiger RS, Schatz M, Li Q, et al. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol Pract 2017;5:1050–60. 10.1016/j.jaip.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 18. Bengtson LGS, Yu Y, Wang W, et al. Inhaled corticosteroid-containing treatment escalation and outcomes for patients with asthma in a U.S. health care organization. J Manag Care Spec Pharm 2017;23:1149–59. 10.18553/jmcp.2017.23.11.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lefebvre P, Duh MS, Lafeuille M-H, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin 2017;33:57–65. 10.1080/03007995.2016.1233101 [DOI] [PubMed] [Google Scholar]
- 20. Moore WC, Evans MD, Bleecker ER, et al. Safety of investigative bronchoscopy in the severe asthma research program. J Allergy Clin Immunol 2011;128:328–36. 10.1016/j.jaci.2011.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sá-Sousa A, Almeida R, Vicente R, et al. High oral corticosteroid exposure and overuse of short-acting beta-2-agonists were associated with insufficient prescribing of controller medication: a nationwide electronic prescribing and dispensing database analysis. Clin Transl Allergy 2019;9:47. 10.1186/s13601-019-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Optimum Patient Care . Optimum patient care UK, 2022. Available: https://optimumpatientcare.org/
- 23. Kousoulis AA, Rafi I, de Lusignan S. The CPRD and the RCGP: building on research success by enhancing benefits for patients and practices. Br J Gen Pract 2015;65:54–5. 10.3399/bjgp15X683353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heatley H, Tran T, Bourdin A, et al. The burden of intermittent oral corticosteroid use in asthma. Eur Respir J 2021;58:OA1491. [Google Scholar]
- 25. World Health Organization . Atc/ddd index, 2022. Available: https://www.whocc.no/atc_ddd_index/
- 26. Rice JB, White AG, Scarpati LM, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther 2017;39:2216–29. 10.1016/j.clinthera.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 27. Volmer T, Effenberger T, Trautner C, et al. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J 2018;52:1800703. 10.1183/13993003.00703-2018 [DOI] [PubMed] [Google Scholar]
- 28. Yao T-C, Wang J-Y, Chang S-M, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr 2021;175:723–9. 10.1001/jamapediatrics.2021.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016;71:339–46. 10.1136/thoraxjnl-2015-207630 [DOI] [PubMed] [Google Scholar]
- 30. Yigla M, Tov N, Solomonov A, et al. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003;40:865–71. 10.1081/JAS-120023577 [DOI] [PubMed] [Google Scholar]
- 31. Jehan S, Zizi F, Pandi-Perumal SR, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord 2017;1. [Epub ahead of print: 12 12 2017]. [PMC free article] [PubMed] [Google Scholar]
- 32. Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med 2014;10:183–93. 10.5664/jcsm.3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiao Y-X, Xiao Y. Asthma and obstructive sleep apnea. Chin Med J 2015;128:2798–804. 10.4103/0366-6999.167361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quint JK, Arnetorp S, Kocks JWH, et al. Short-acting beta-2-agonist exposure and severe asthma exacerbations: SABINA findings from Europe and North America. J Allergy Clin Immunol Pract 2022;10:2297–309. 10.1016/j.jaip.2022.02.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thorax-2022-219642supp001.pdf (355.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request.