Abstract
Background
The triggering receptor expressed on myeloid cell 2 (TREM2) is a major regulator of neuroinflammatory processes in neurodegeneration. To date, the p.H157Y variant of TREM2 has been reported only in patients with Alzheimer’s disease. Here, we report three patients with frontotemporal dementia (FTD) from three unrelated families with heterozygous p.H157Y variant of TREM2: two patients from Colombian families (study 1) and a third Mexican origin case from the USA (study 2).
Methods
To determine if the p.H157Y variant might be associated with a specific FTD presentation, we compared in each study the cases with age-matched, sex-matched and education-matched groups—a healthy control group (HC) and a group with FTD with neither TREM2 mutations nor family antecedents (Ng-FTD and Ng-FTD-MND).
Results
The two Colombian cases presented with early behavioural changes, greater impairments in general cognition and executive function compared with both HC and Ng-FTD groups. These patients also exhibited brain atrophy in areas characteristic of FTD. Furthermore, TREM2 cases showed increased atrophy compared with Ng-FTD in frontal, temporal, parietal, precuneus, basal ganglia, parahippocampal/hippocampal and cerebellar regions. The Mexican case presented with FTD and motor neuron disease (MND), showing grey matter reduction in basal ganglia and thalamus, and extensive TDP-43 type B pathology.
Conclusion
In all TREM2 cases, multiple atrophy peaks overlapped with the maximum peaks of TREM2 gene expression in crucial brain regions including frontal, temporal, thalamic and basal ganglia areas. These results provide the first report of an FTD presentation potentially associated with the p.H157Y variant with exacerbated neurocognitive impairments.
Keywords: Genetics, Neurodegenerative Diseases, Neurology
What is already known on this topic
Genetic variants in TREM2 (triggering receptor expressed on myeloid cell 2) have been reported as risk factors for different neurodegenerative diseases, including Alzheimer’s disease and frontotemporal dementia.
What this study adds
We described for the first time new associations between the heterozygous p.H157Y variant in TREM2 with clinical presentations of the behavioural variant of frontotemporal dementia (bvFTD) and motor neuron disease-frontotemporal dementia (FTD-MND).
How this study might affect research, practice or policy
Our findings invite new research to further understand genetic-phenotype variability within subjects carrying new TREM2 variants and its impact on neurodegeneration.
Introduction
Emergent research points to the importance of the innate immune system and microglia in the pathogenesis of neurodegenerative diseases.1–3 The TREM2 gene (triggering receptor expressed on myeloid cells 2) encodes a transmembrane glycoprotein mainly expressed in cells of myeloid lineage.4–6 This receptor consists of an extracellular IgV domain, stalk domain, transmembrane domain and a short cytoplasmic tail.7 Recent studies have identified variants in TREM2 that are risk factors not only for Alzheimer’s disease (AD)8 9 but also for other neurodegenerative diseases such as frontotemporal dementia (FTD),10 Parkinson’s disease (PD)3 11 and amyotrophic lateral sclerosis (ALS).4 However, a complete understanding of the influence of these variants on clinical presentation, including the symptom profile, cognitive correlates and neuropathology, has not yet been established.
With the progress of genetic research in FTD, several TREM2 variants are associated with familial and sporadic FTD.7 10 12 13 The effect of these TREM2 variants in the central nervous system remains unknown, but most are located in exon 2, which encodes the IgV domain, a highly conserved region of the protein.14 Homozygous loss-of-function mutations in TREM2, such as p.Q33X, p.Y38C and p.T66M, are associated with either Nasu-Hakola disease with bone involvement15–17 or an early-onset behavioural variant frontotemporal dementia (bvFTD)-like phenotype lacking bone involvement.16 Interestingly, Guerreiro et al reported the first TREM2 compound heterozygous mutation (p.[(Y38C)]; [(D86V)]) in a Turkish family affected by FTD-like dementia.16 Most carriers of homozygous TREM2 variants in the IgV domain exhibit behavioural changes, frontal cognitive deterioration, seizures, akinetic-rigid syndrome and frontotemporal lobar atrophy without clinical or radiological evidence of bone involvement.16 18 Functional studies show that mutations such as p.Y38C and p.T66M strongly affect the uptake of certain apolipoproteins and lipoproteins.19 Apart from the literature identifying rare homozygous (and compound heterozygous) TREM2 variants in FTD-like syndromes, the heterozygous p.R47H variant of TREM2 has now been well established to increase the risk for late-onset Alzheimer’s disease (LOAD).8 9
New evidence suggests that the p.H157Y variant located in the stalk domain of TREM2 is involved in neurodegenerative pathogenesis.14 20 21 Jiang et al found that a heterozygous p.H157Y variant was strongly associated with LOAD risk in Han Chinese populations.5 22 Although potential mechanisms remain unclear, they might involve increased shedding and thus reduced cell surface levels of TREM2. To the best of our knowledge, no studies have reported associations between the heterozygous p.H157Y variant with clinical presentations related to bvFTD and motor neuron disease-frontotemporal dementia (FTD-MND).
Here, we report two patients from unrelated Colombian families and the third case from the USA of Mexican origin with a heterozygous TREM2 exon 3 variant (c.469C>T, p.H157Y, rs2234255). The two Colombian cases exhibited a bvFTD clinical profile and the third case presented with a motor neuron disease (MND). We performed exhaustive clinical, neuropsychological, neuroimaging and genetic assessments, including individualised gene atrophy mapping of these patients.
Materials and methods
All patients underwent clinical assessment by a multidisciplinary team composed of neurologists, psychiatrists, neuropsychologists, geneticists and geriatricians from the Memory Clinic at the Center for Memory and Cognition at Hospital San Ignacio in Bogotá, and the University of California, San Francisco Memory and Aging Center (UCSF MAC). Both clinical teams followed the Rascovsky criteria22 to diagnose bvFTD cases, Gorno-Tempini et al criteria23 to diagnose linguistic variants of FTD, and the Strong criteria to diagnose FTD-MND cases.24 After clinical assessment, blood samples were extracted to assess genetic characterisation of TREM2, Ng-FTD, Ng-FTD-MND, HC subjects. Finally, participants of all groups were scanned using structural MRI. All participants provided written informed consent in agreement with the Declaration of Helsinki.
Clinical and neuropsychological assessment
Our protocol included different instruments to evaluate the overall cognitive status (Montreal Cognitive Assessment (MoCA)25), executive function (Hayling B test,26 Ineco Frontal Screening battery27), behavioural symptoms (Frontal System Behavioural scale (FrSBe)28 and social cognition (Reading Mind in the Eyes test29; online supplemental information S1-S3). After clinical assessment, blood samples were extracted to assess genetic characterisation of TREM2, Ng-FTD, Ng-FTD-MND and HC participants (online supplemental information S1-S2).
jmg-2022-108627supp001.pdf (342.8KB, pdf)
Behavioural single-case analysis: Crawford method
This method allows for the comparison of single cases against a control sample score with different sizes. This method treats the control sample as statistics rather than parameters and is robust for non-normal distributions and small control samples (ie, n=5). Additionally, it presents low rates of type I error and has proven useful for single-case studies30–34 (online supplemental information S3).
Genetic analysis: study 1
Genomic DNA was extracted from blood samples of the bvFTD participants by the salting-out method.35 Targeted sequencing was done at the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania using a custom targeted multineurodegenerative disease sequencing panel (MiND-Seq), which covered the coding regions of 45 genes recognised as causative of AD, PD, FTLD and ALS, as well as several other genes identified as risk factors for other neurodegenerative diseases; risk factor genes include MAPT, GRN, VCP, TARDBP, CHMP2B, CSF1R, PSEN1 and TREM2.36 This panel employs next-generation sequencing technology using Haloplex Enrichment Custom Kit (Agilent) according to the manufacturer’s protocol; sequencing was conducted on Illumina Mi-Seq. All cases were tested for a C9orf72 hexanucleotide repeat expansion as previously described37 (online supplemental information S4).
Genetic analysis: study 2
The genomic DNA of patient with FTLD underwent Whole-genome sequencing (WGS) at the New York Genome Center (New York City, New York, USA) or HudsonAlpha Institute for Biotechnology (Huntsville, Alabama, USA) on an Illumina HiSeq-X, with 150 bp paired-end reads to obtain 30X sequencing coverage. Candidate variants were validated by Sanger sequencing at the UCLA Neuroscience Genomics Core (Los Angeles, California). The case was also tested for C9orf7237 (online supplemental information S4).
Structural brain imaging
All participants were scanned using structural MRI parameters with settings harmonised at post-recording (online supplemental information S7-S9). We analysed the grey matter (GM) volume of all participants and compared the atrophy pattern across groups (online supplemental information S7-S9).
Gene atrophy overlap
The overlap between atrophy and the TREM2 gene expression was implemented following previous procedures using the Allen Human Brain database38 (online supplemental information S8, online supplemental tables 4 and 5).
Results
Study 1: case 1
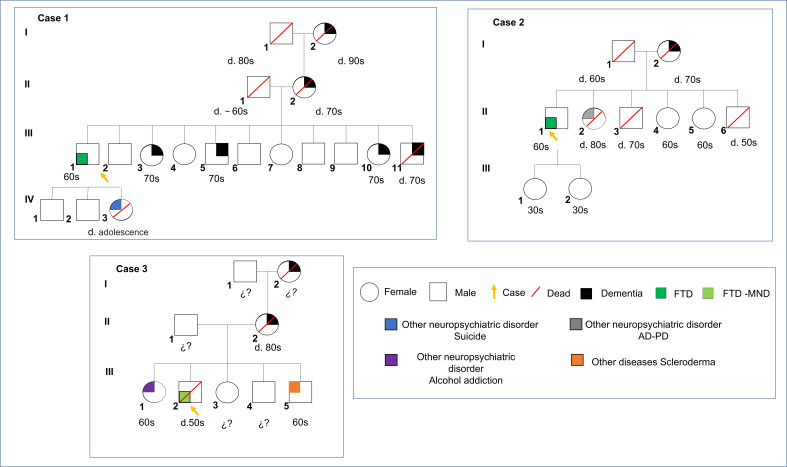
A male patient around 50s from Colombia with 9 years of formal education. He has exhibited behavioural symptoms, including apathy-related disinhibition, irritability, planning problems and violation of social norms, impacting his work and domestic spheres. Further, he presented episodes of affective exaltation, including sleep disturbances, increased loquacity and mistrust of caregivers with durations of up to 4 days. The motor assessment revealed unilateral myoclonus in upper limbs and trunks, echopraxia and marked apraxia of gait. Moreover, the patient exhibited neurological dysphagia (fluids and solids) and orolingual automatisms without dysphonia. Family history showed that his grandmother developed dementia at the age of 70s (I:1). His mother started exhibiting behavioural changes related to ‘hiding things’ at the age of 60s (II:2). One brother (III:11) died at the age of 70s due to neurodegenerative disease. In contrast, 3 siblings out of 10 (III:3, III:5, III:10) exhibited cognitive symptoms and behavioural disorders consisting of apathy and mutism. His daughter committed suicide at adolescence (IV:3) (figure 1).
Figure 1.
Pedigree of the two unrelated Colombian families (A, B) and one Mexican case in which the heterozygous mutation p.H157Y in TREM2 was found. Numbers following the letter ‘d’ represent the death age of the subject. The arrowhead indicates the proband with heterozygous p.H157Y variant. Black painting symbols represent affected subjects with dementia. White symbols represent unaffected family members. Green labels represent cases reported in study 1 (case 1 and case 2: FTD) and study 2 (case 3: FTD-MND). The red slash symbol was used to identify dead individuals. Blue, purple and grey colours indicate individual cases with neuropsychiatric disorders, including suicide, alcohol addiction and Parkinson’s disease (PD), respectively. Orange colour depicts a case with a somatic disease (scleroderma). FTD, frontotemporal dementia; FTD-MND, motor neuron disease-frontotemporal dementia.
Study 1: case 2
A male patient around 50s from Colombia with 8 years of formal education. He exhibited behavioural and affective symptoms. He showed disinhibition-behavioural symptoms, affective exaltation, impulsivity, risky financial behaviour, sexual disinhibition, logorrhoea, sleep disturbances and difficulties following social norms. He also exhibited executive dysfunction and an inability to perform self-care activities. The motor assessment presented frequent motor automatisms, neutral right flexor plantar response, left extensor and bilateral palmomental responses as positive neurological signs. His mother died at the age of 70s with behavioural and cognitive changes associated with dementia (I:2). Also, a sister was diagnosed with both PD and AD (II:2)(figure 1).
Study 2: case 3
A male around 50s with 14 years of education was diagnosed with bvFTD and MND after a clinical consensus review. He has exhibited inappropriate behaviours, disinhibition, irritability and impaired ability to follow social norms. Moreover, he developed verbal stereotypes, including expressions such as ‘yep’, ‘right’, ‘you got it’ and ‘that’s right’. MRI revealed essentially symmetric, moderate frontotemporal atrophy, as well as extensive, confluent bilateral white matter hyperintensities in the frontal regions, extending posteriorly and several frontal subcortical cysts. Autopsy revealed FTLD, TDP-43 inclusions type B, MND and other markers (see results in figure 2). His mother had an adult-onset behavioural syndrome and died of dementia around 80s (II:2). He had two brothers and two sisters in their early 60s. His oldest brother was diagnosed with an autoimmune disease (scleroderma)(III:5) around 60s. His younger sister had alcohol addiction (III:1) around 60s. The maternal grandmother died late of dementia (I:2). A cousin on the maternal side developed dementia in his 60s (figure 1).
Figure 2.
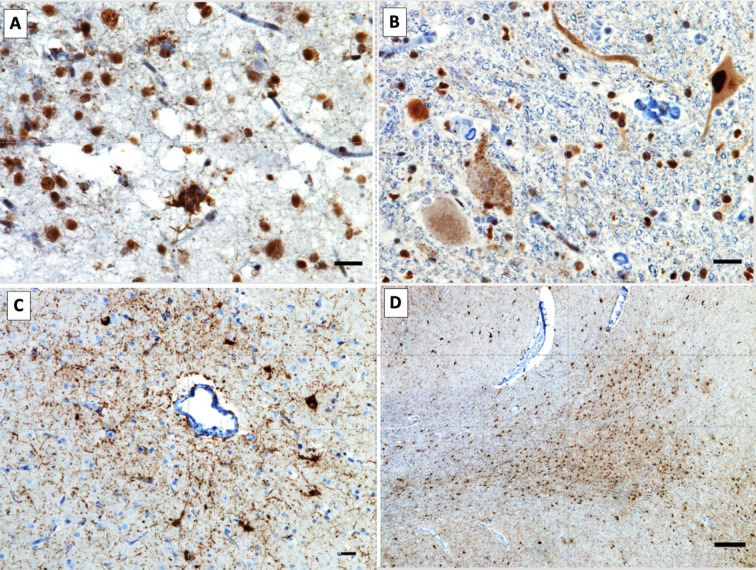
Neuropathological findings in the Mexican case. (A) TDP-43 mislocalisation is seen in neurons from the upper cortical layers of the middle frontal gyrus. Pathological TDP-43 is seen either as granular diffuse cytoplasmic inclusions in neurons not displaying the normal nuclear TDP-43 staining or as skein-like cytoplasmic inclusions. (B) Granular diffuse pathological cytoplasmic TDP-43 immunoreactivity in the lower motor neurons of the cervical cord. (C) Abundant perivascular neuronal and glial tau cytoplasmic aggregate in the cerebral cortex, the pathognomonic lesion of chronic traumatic encephalopathy. (D) Abundant argyrophilic thorny astrocytes in clusters (ATAC) at the juxtacortical white matter of the subgenual cingulate gyrus. FTD, frontotemporal dementia; TREM2, triggering receptor expressed on myeloid cell 2.
Neuropsychological assessment
Study 1
General cognitive state
In the MoCA, case 1 and case 2 exhibited significantly lower scores than both HCs (case 1 t=−5.903, p=0.0003, zcc=−6.26; case 2 t=−2.983, p=0.042, zcc=−4.12) and the Ng-FTD group (t=−3.32, p=0.001, zcc=−4.41). Additionally, Ng-FTD presented significantly lower scores than HC in MoCA scores (t=−4.572, p=0.001, zcc=−4.84) (table 1).
Table 1.
Demographic and neuropsychological data comparison between TREM2 cases, Ng-FTD controls and HC
| TREM2 | TREM2 | TREM2 | Ng-FTD | HC | Ng-FTD-MND | |
| Case 1 | Case 2 | Case 3 | Controls (n=5) |
(n=5) | Controls (n=11) |
|
| Demographic data | ||||||
| Age (years), SD | 63 | 61 | 56 | 62.8, SD (6.1) | 61.3, SD (3.9) | 61.16, SD (11.6) |
| Education (years), SD | 9 | 8 | 14 | 10.6, SD (3.7) | 11.3, SD (2.9) | 15.5, SD (2.0) |
| Neuropsychological data | ||||||
| General cognitive assessment | ||||||
| MoCA | 12 | 9 | 20 | 15.9, SD (1.9) | 26.5, SD(2.1) | 21.5, SD(12.5) |
| Executive function | ||||||
| Total IFS score | 9 | 5 | 16.3, SD (2.6) | 22.1, SD (1.9) | 22.1, SD (1.9) | |
| Hayling test | 16 | 18 | 23.5, SD (9.6) | 10.6, SD (2.6) | 10.6, SD (2.6) | |
| Stroop test | 55 | 38.7, SD (34.9) | ||||
| Social cognition | ||||||
| RMET | 8 | 6 | 13.3, SD (2.1) | 14.3, SD (1.1) | 14.3, SD (1.1) | |
| Social changes in NPI | 5 | 2.7, SD (3.7) | ||||
| Behavioural changes | ||||||
| FrSBe Total | 134 | 188 | 115, SD (33.5) | 9.5, SD (1.1) | 9.5, SD (1.1) | |
| FrSBe Apathy | 22 | 18 | 38.5, SD (10.8) | 3.1, SD (.6) | 3.1, SD (.6) | |
| FrSBe Disinhibition | 55 | 58 | 28.6, SD (11.7) | 3.1, SD (.6) | 3.1, SD (.6) | |
| FrSBe Executive functions | 49 | 77 | 47.6, SD(12.4) | 3.3, SD(.4) | 3.3, SD(.4) | |
| NPI Total scores | 73 | 21.9, SD (21.7) |
Data shown as mean with SD reported.
FrSBe, Frontal Systems Behavioural Scale; HC, healthy control; IFS, Ineco Frontal Screening; MoCA, Montreal Cognitive Assessment; NPI, Neuropsychiatric Inventory; RMET, Reading Mind in the Eyes Test.
Executive functions
Both TREM2 cases showed lower scores on total IFS (Ineco Frontal Screening) than HC (case 1 t=−8.16, p=0.0009, zcc=−5.12; case 2 t=−6.24, p=0.0001, zcc=−5.75) and Ng-FTD group (case 1 t=−3.86, p=0.009, zcc=−3.11; case 2 t=−2.45, p<0.05, zcc=−2.92). In addition, TREM2 cases exhibited lower Hayling scores than HC (case 1 t = −2,65; p<0.05, zcc=−4.11; case 2 t = −2,89; p<0.05, zcc=−4.22, table 1).
Behavioural changes
TREM2 cases showed significantly higher scores in total FrSBe (Frontal Systems Behavioural Scale) than HC (case 1 t=48.78, p=0.00009, zcc=6.34; case 2 t=53.18, p=0.00009, zcc=−7.46). Case 2 exhibited lower total FrSBe scores than Ng-FTD group (t=2.29, p<0.05, zcc=2.11). Individualised analyses on each FrSBe subfactor revealed higher scores for TREM2 cases compared with HC (table 1).
Social cognition
Both TREM2 cases attained significantly lower scores for social cognition (RMET) compared with HC (case 1 t=−2.78, p<0.05, zcc=−3.34; case 2 t=−3.18, p<0.05, zcc=−4.13). Complementary results are provided in online supplemental information S9 and table 1.
Study 2
General cognitive state
In the MoCA, no differences when compared with Ng-FTD-MND nor with the Ng-FTD group were observed (online supplemental information S9 and table 1).
Executive functions
Case 3 showed lower scores on the total of correct trials in Stroop task compared with Ng-FTD-MND cases (case 3 t=3.99, p<0.05, zcc=−4.11, table 1).
Behavioural changes
Case 3 showed significantly higher scores on the total scores of the NPI than Ng-FTD-MND cases (case 3 t=3.83, p<0.05, zcc=−3.71). The patient exhibited worst scores of agitation, apathy, disinhibition, motor problems, sleep disturbances and eating habits (table 1).
Social changes
Case 3 showed significantly higher scores on the items tracking social norms (NPI disinhibition score) than Ng-FTD-MND cases (case 3 t=3.07, p<0.05, zcc=−3.22) (table 1). Complementary results are provided in online supplemental information S9and table 1.
Genetic findings
The most plausible candidate to explain the phenotype exhibited by the three cases was a heterozygous missense in exon 3 of the TREM2 gene (p.H157Y). Targeted sequencing confirmed this variant in the three cases. Diverse prediction programmes were used as a filter to predict how the amino acid exchange found would affect protein function, considering the physical properties of amino acids, sequence homology, domain involvement and allelic frequency. From the analysis of these predictors, Pholyphen and Proven scores indicated a potentially deleterious effect of the p.H157Y variant. The conservation score calculated was 3.6–4.7. Other variants were found among these cases. Patient 1 (III:9), another non-synonymous missense variant in TREM2 (p.R62H), was identified. It was not possible to differentiate between the possibilities of co-inheritance or compound heterozygosity of these variants based on the available data. Patient 2 (II:1), two additional gene variants were found: (1) a synonymous variant in the SNCA gene (p.Thr72=) and (2) a variant in OPTN gene (p.M447V) that maps to the region of interaction with huntingtin. Patient 3 (III:2), a non-synonymous variant in MSFD8 (p.Gly385Arg), was also detected. While this variant is not rare in Latino/Admixed American controls in gnomAD (allele frequency 0.063), rare coding variants in MFSD8 have been implicated in FTLD 27. Thus, we cannot exclude the possibility that the MFSD8 variant also contributes to disease in this individual (table 2).
Table 2.
The rates are the percentage of mutation carriers in the Colombian and global population, using the 1000 Genomes Project
| Cases | Gene | Position at cDNA | Protein change | rs | Variant type | GMAF | Colombian rate |
| Case 1 | TREM2 | c.469C>T | p.H157Y | rs2234255 | Missense | 0.0028 | 0.020 |
| TREM2 | c.185G>A | p.R62H | rs143332484 | Missense | 0.0049 | 0.016 | |
| VAPB | c.510G>A | p.M170I | rs143144050 | Missense | 0.0010 | 0.000 | |
| DCTN1 | c.1998G>A | p.T666= | rs149900553 | Synonymous | 0.0008 | 1000 | |
| SETX | c.6507G>A | p.G2169= | rs34073320 | Synonymous | 0.0098 | 0.037 | |
| LRRK2 | c.4624C>T | p.P1542S | rs33958906 | Missense | 0.0132 | 0.069 | |
| Case 2 | TREM2 | c.469C>T | p.H157Y | rs2234255 | Missense | 0.0028 | 0.020 |
| OPTN | c.1339ª>G | p.M447V | Missense | ND | ND | ||
| SNCA | c.216G>A | p.Thr72= | rs144758871 | Synonymous | ND | ND | |
| Case 3 | TREM2 | c.469C>T | p.H157Y | rs2234255 | Missense | 0.0028 | 0.020 |
| MFSD8 | c.1153G>C | p.G385R | rs11098943 | Missense | ND | ND |
DCTN1, dynactin subunit 1; GMAF, global minor allele frequency; LRRK2, leucine-rich repeat kinase 2; MSFD8, major facilitator superfamily domain-containing protein 8; ND, no data; OPTN, optineurin; SETX, senataxin; SNCA, synuclein alpha; TREM2, triggering receptor expressed on myeloid cells 2; VAPB, VAMP-associated protein B and C.
Neuroimaging assessment
Study 1
Global atrophy of cases compared with healthy controls
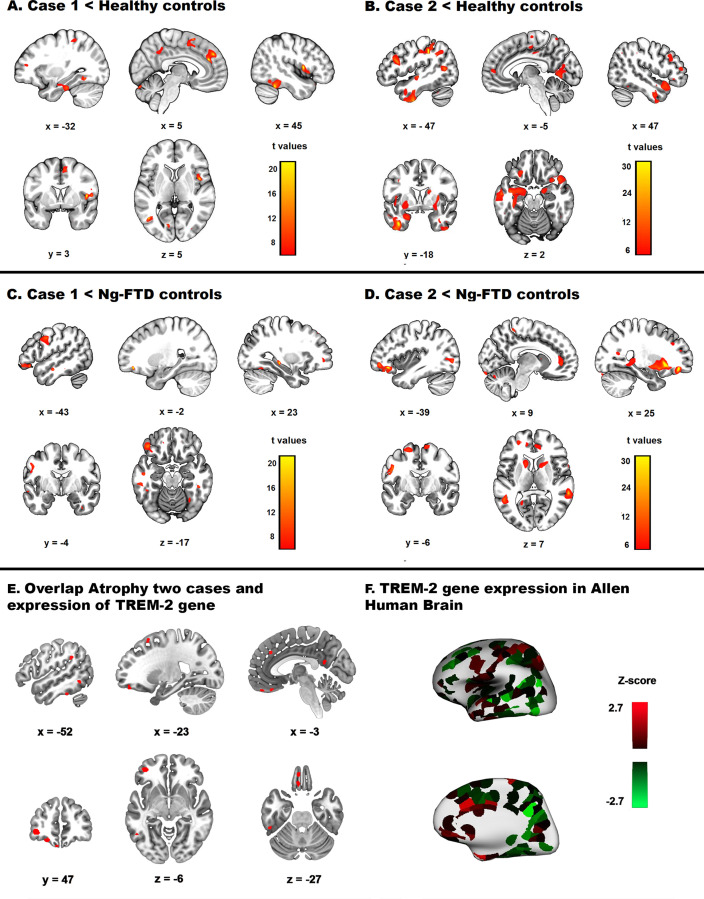
Compared with HC, both cases exhibited significant atrophy in the frontal, temporal, parietal and cerebellar regions. In addition, case 1 showed significant GM reduction in the right supplementary motor area, right rolandic operculum, left supramarginal gyrus, left precuneus and left paracentral lobule (online supplemental table 1, figure 3A). Case 2 showed significant atrophy in the left parietal inferior lobule, left precentral gyrus, right superior temporal gyrus, right angular gyrus, right inferior frontal gyrus, left precuneus and bilateral superior frontal gyrus (online supplemental table 1, figure 3B).
Figure 3.
Brain atrophy pattern and gene expression overlap in study 1. (A) Atrophy pattern of case 1 compared with healthy controls. (B) Atrophy pattern of case 2 compared with HC. (C) Atrophy pattern of case 1 compared with Ng-FTD controls. (D) Atrophy pattern of case 2 compared with Ng-FTD controls. All brain areas reported reached p<0.001, uncorrected, and had an extent threshold of 50 voxels. (E) Overlap between atrophied regions in cases 1 or 2 and sites of expression of the TREM2 gene. (F Three-dimensional surface rendering and medial view of TREM2 gene expression in donor H0351.1009 (Allen Human Brain). FTD, frontotemporal dementia.
Global atrophy of cases compared with the Ng-FTD group
Compared with the Ng-FTD group, both patients showed significant atrophy in the middle frontal gyrus, precentral gyrus and fusiform gyrus (online supplementary tables 1 and 2; figure 3C D). In addition, case 2 exhibited significant GM reduction in other frontal, temporal, parietal, basal ganglia, hippocampal and parahippocampal, and cerebellar regions (online supplemental information and tables 1 and 2, figure 3D).
Gene atrophy overlapping
We found a group of atrophy peaks in TREM2 variant cases that overlapped with an expression of the TREM2 gene in the Allen database. These coordinates correspond to the left superior frontal, orbital, middle frontal, precentral, inferior temporal, fusiform and supramarginal gyri, left precuneus and inferior parietal lobule (online supplemental table 4, figure 3E F).
Study 2
Global atrophy of cases compared with the Ng-FTD-MND and Ng-FTD groups
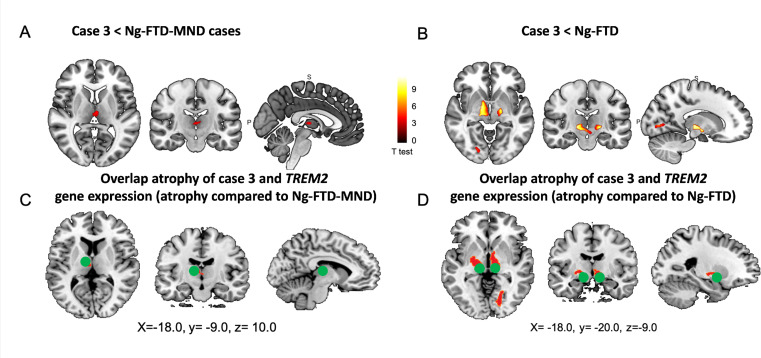
We compared case 3 with an Ng-FTD-MND group and with an Ng-FTD group. Compared with the Ng-FTD-MND group, case 3 exhibited significant atrophy in bilateral basal ganglia (mainly in the pallidum) and bilateral thalamus. Compared with the Ng-FTD group, case 3 showed GM reduction in the bilateral basal ganglia (pallidum), bilateral thalamus and left inferior temporal gyrus (online supplemental table 3, figure 4).
Figure 4.
Neuropathological findings in the Mexican case. A) TDP-43 mislocalization is seen in neurons from the upper cortical layers of the middle frontal gyrus. Pathological TDP-43 is seen either as granular diffuse cytoplasmic inclusions in neurons not displaying the normal nuclear TDP-43 staining or as skein-like cytoplasmic inclusions. B) Granular diffuse pathological cytoplasmic TDP-43 immunoreactivity in the lower motor neurons of the cervical cord. C) Abundant perivascular neuronal and glial tau cytoplasmic aggregate in the cerebral cortex, the pathognomonic lesion of chronic traumatic encephalopathy. D) abundant argyrophilic thorny astrocytes in clusters (ATAC) at the juxtacortical white matter of the subgenual cingulate gyrus.
Gene atrophy overlapping
We found two atrophy peaks in case 3 that overlapped with an expression of the TREM2 gene in the Allen database. These coordinates correspond to the bilateral basal ganglia and bilateral thalamus (online supplemental table 5, figure 4).
Postmortem pathological findings
The autopsy revealed FTLD with TDP-43 inclusions type B and MND, which was felt like the primary neuropathological diagnosis. In addition, comorbid chronic traumatic encephalopathy (CTE) stage 4 and additional tauopathies such as ageing-related tau astrogliopathy, argyrophilic thorny astrocytes in clusters and limbic argyrophilic grain disease. Beta-amyloid immunohistochemistry was negative, while the neurofibrillary tangles Braak stage of AD could not be ascertained because of the confounding effect caused by the high severity of CTE (figure 2).
Discussion
Here we analysed a possible association of the p.H157Y variant in the TREM2 gene in two unrelated familial cases from Colombia and in one case from Mexican with a bvFTD and FTD-MND-compatible profile, respectively. Neuropsychological results revealed that the patients of the first study exhibited significantly impaired scores compared with Ng-FTD and HC groups in general cognitive and executive function assessments, social cognition and behavioural symptoms. In addition, the patient of study 2 did not exhibit significant differences in cognitive functioning but showed clinical motor neuron impairments associated with brain atrophy in classical motor-related areas including bilateral basal ganglia in comparison to the Ng-FTD-MND group. On the other hand, atrophy and gene expression-level association study revealed diverse atrophy peaks that overlapped with TREM2 gene expression in regions corresponding to the left superior frontal, orbital, middle frontal, precentral, inferior temporal, fusiform and supramarginal gyri, left precuneus, inferior parietal lobule, bilateral basal ganglia and bilateral thalamus. Our findings question a plausible association of the p.H157Y variant in bvFTD and FTD-MND disease progression.
Previous studies reported associations between TREM2 variants and neurodegeneration, including the Nasu-Hakola syndrome,39–41 early-onset42 and late-onset AD,8 9 and PD.43 Moreover, different TREM2 variants have been associated with increased risk of FTD-like syndrome,10 44–47 FTLD18 and bvFTD34 48 without bone cysts disease. Different reports have shown that TREM2 can increase the genetic risk of neurodegenerative disorders via dysregulation of the immune system.8 9 49
The association between TREM2 mutations and FTD-like syndromes without bone cysts is population specific. TREM2 mutations have been associated with FTD in Asian,44 45 50 European,16 46 North American samples,7 and in non-stereotypical samples from Latin America-Bolivian39 and Colombian.18 34 51 The specific TREM2 variant observed in our study (TREM2 c.469C>T (p.His157Tyr)) has been has been previously associated with neurodegeneration in European,7 Asian,5 8 Colombian34 51 and Mexican52 populations. The p.H157Y missense variant resides in a conserved coding region where the histidine-to-tyrosine exchange at amino acid 157 leads to enhanced shedding of TREM2 from the cell surface, as well as altered cleavage of the TREM2 ectodomain during its transport from the ER to the plasma membrane. As a result, reduced levels of TREM2 on the plasma membrane have been observed for this variant.53 Different reports have shown that TREM2 is an AD genetic risk factor involved in dysregulation of the immune system.8 9 49
Although the TREM2 pH157Y variant presents high allele frequency in Latino populations, two out of three algorithms classified this variant as definitely pathogenic.51 Moreover, a meta-analysis revealed that this variant is associated with neurodegeneration in different populations with an OR=3.65.5 The potential pathogenicity of this variant in Latin American populations can vary due to mixed ancestries. Recent reports have shown that in non-stereotypical samples, different genotype-phenotype interactions are often observed.54
The potential pathogenicity of the TREM2 pH157Y variant has been recently supported in Latin American studies. Hundred Colombian individuals with neurodegenerative diseases have the p.H157Y variant, with almost 50 heterozygous and seven homozygous.51 The p.H157Y variant was found in a Native American haplotype, and it has been estimated that this allele emerged around 1265 generations ago (95% CI of 1108.5 to 1430.9). Past evidence has shown that the disease burden in Latin American populations is affected not only by the recent admixture processes but also by ancestral migrations.51 55
Thus, the variant reported in our study has been previously found to be pathogenic in under-represented populations and is associated with neurodegeneration in Asian, European and Latin American samples. Current results add new evidence by showing that this variant can also be associated with FTD syndromes in non-stereotypical populations. Moreover, our pathological findings suggest additional insights. TREM2 deficiency impairs clearance of pathological TDP-43 and enhances neuronal damage56; and TDP-43 and TREM2 interact in neurodegenerative tissues from patients with FTD-MND.56 Case 3 pathological results support these links between TREM2, TDP-43 and FTD motor phenotype. Allostatic overload may be an important factor combining genetic and fundamental factors contributing to TDP-43 aggregation associated with FTD.57
Functions of TREM2 such as binding to ApoE and amyloid-beta 42, microglial activation, expression of proinflammatory cytokines and phagocytosis are decreased.5 14 Interestingly, each case has shown additional variants besides heterozygous variant p.H157Y, presenting a specific FTD clinical profile to consider. The first case has shown a second heterozygous TREM2 variant (p.R62H), which alters the flanking consensus sequence of ligand binding but does not alter protein conformation.58 Yeh et al showed that p.R62H modestly but significantly reduces uptake of Aβ-LDL complexes in human monocyte-derived macrophages.19 In this case 1, we could not possibly differentiate between the possibilities of co-inheritance or compound heterozygosity of these variants. In case 2, two additional variants were revealed: a variant in the OPTN gene (p.M447V) and a second synonymous silent polymorphic SNVs in the SNCA gene (p.Thr72=). Despite silent SNVs could have biologically important effects, the low allelic frequency (0.00005645) in Latino/Admixed American population does not allow any conclusion on whether they might modulate bvFTD phenotype. Finally, in case 3, a non-synonymous variant in MSFD8 (p.G385R) was also detected. This variant is presented with an allele frequency of 0.063 in Latino/Admixed Americans; however, some studies suggest that rare coding variants in MFSD8 could contribute to FTLD pathology.1 Thus, we cannot exclude the possibility that the MFSD8 variant also could contribute to disease in this individual.
Having detected these additional variants in all cases, we have found interesting neurocognitive outcomes in our series. The neuropsychological presentation consisted of insidious onset with personality changes, disinhibited behaviour, executive dysfunction and emotional disorders, resembling bvFTD. Similar findings were obtained by Samanci et al, who revealed bvFTD-like clinical features in 14 patients carrying several pathogenic TREM2 variants.59 Furthermore, Guerreiro et al reported compound heterozygous missense mutations (p.Y38C and p.D86V) associated with personality and behavioural changes at an early age in a Turkish family. This case showed cortical atrophy with periventricular white matter disease, culminating in severe dementia.16 Giraldo et al reported a novel homozygous nonsense mutation (p.W198X) in a Colombian family with an autosomal recessive pattern of inheritance of FTD without bone cysts and with behavioural and personality changes around the ages of 45–50.18 Although no studies have reported cases of heterozygous p.H157Y variants in patients with FTD, Jiang et al showed a similarly increased frequency of this variant is strongly associated with LOAD.5
Cases 1 and 2 have shown impairments in general cognition assessment, executive dysfunctions disinhibition compared with HC and the Ng-FTD group. These results suggest a pattern of multidomain, intertwined deficist triggered by FTD.60 Furthermore, compared with HC, TREM2 cases exhibited major apathy and impaired social cognitive skills. Although disinhibition is one of the most typical manifestations of bvFTD,61 our results revealed increased disinhibition in TREM2 cases (relative to the Ng-FTD group). This cognitive pattern of alterations was associated with typical atrophy in frontal, temporal, parietal and cerebellar regions.22 62–64 Crucially, case 1 and case 2 showed increased atrophy in the orbitofrontal cortices, anterior cingulate, right superior frontal gyrus, right medial frontal gyrus, precentral gyrus and fusiform gyrus when compared with Ng-FTD cases. Additionally, case 2 exhibited frontal and temporal atrophy but also reduced GM in the basal ganglia, cerebellum, parahippocampus, hippocampus, precuneus and supramarginal gyrus. These findings suggest more severe atrophy progression in cases 1 and 2 that compromise classical areas but also extend to other areas. On the other hand, in study 2, case 3 had a worse GM disease in basal ganglia and thalamus than the Ng-FTD-MND group. Although in this case, preserved general cognitive skills were observed in comparison to the Ng-FTD-MND group, worse executive functioning, significant behavioural changes (apathy, disinhibition, motor problems, sleep and eating disturbances) and loss of social norms were identified. Previous studies have shown a clinical association in 5% of FTD cases and a deficit in executive function that overlaps with MND.65
Although the observed effects of the p.H157Y mutation are similar to those generated by most of the mutations in the IgV domain, the biochemical and cellular mechanisms underlying its effects have been little described.13 66 In assessing TREM2 gene expression profiles of brain regions in p.H157Y mutation carriers, we observed a high spatial correspondence between their expression throughout the brain and the atrophy pattern in each case, including frontal (orbitofrontal and cingulate cortex), inferior medial temporal, basal ganglia, precuneus and inferior parietal regions. These brain areas were associated with the cognitive and social cognition deficits characteristic of bvFTD.67–74 Similarly, this pattern of brain atrophy associated with TREM2 variants coincides with other reports.3 Moreover, similar relationships have been found in MRI studies of progressive non-fluent aphasia cases which suggest that the motor impairments are due to atrophy in regions within a left front-insular-basal ganglia network.75 76 In this work, we have observed an interesting association between two regions involved in executive function (basal ganglia and thalamic regions) and TREM2 gene expression levels. Several authors highlight that activated microglia in the vicinity of degenerating motoneurons and the presence of immunologically active macrophages are pathological features of ALS.77 78 However, the mechanism underlying which such activation impacts the disease process remains unclear.
The postmortem neuropathological findings of case 3 revealed MND with TDP-43 type B inclusions and severe cystic degeneration of white matter and split septum pellucidum, consistent with comorbid CTE pathology. Moreover, perivascular neuronal and glial tau cytoplasmic aggregate were observed in the cerebral cortex, which is considered a pathognomonic sign of chronic traumatic encephalopathy. The pattern of TDP-43 pathological findings supports the notion of neurodegeneration due to TREM2 variants. However, the presence of glial tau lesions is suggestive of traumatic encephalopathy and mixed pathology. Although some studies have shown associations between TDP-43 inclusions and brain injury,79 most cases have been associated with risk variants in causative genes.80 Other reports also found associations between TDP-43 type B inclusions and TREM2 risk variants.56 Future studies should assess the specific changes associated with brain injury in individuals with FTD genotypes.
Moreover, TREM2 deficiency impairs clearance of pathological TDP-43 and enhances neuronal damage56; and TDP-43 and TREM2 interacts in neurodegenerative tissues from patients with FTD-MND.56 Case 3 pathological results support these links between TREM2, TDP-43 and the FTD motor phenotype.
Limitations and future directions
Our results have important limitations. First, our study is limited by a small sample size, with three cases reported harbouring the p.H157Y variant in two Colombian families with bvFTD and one from Mexico. One future source of replication comes from genome sequencing studies including FTD cases.81 Case 3 had Latin American ancestry, and ancestry-related genetic analysis may require further investigation. Second, the precise molecular mechanisms potentially connecting the effects of the p.H157Y variant to the pathogenesis of FTD are still unknown. Third, although the TREM2 gene has been reported to be an intermediate risk gene for AD and FTD, the p.H157Y variant was present with a high allelic frequency in several Latin American cohorts. Here, we have shown an interesting association between TREM gene expression and the clinical phenotype of FTD; however, further functional studies should be included to confirm this association. On the other hand, our study lacks homogeneity in measurements of cognitive functioning across cases from Colombia and Mexico. Although the assessment of executive functioning and other behavioural symptoms were different between Colombian and Mexican cases, all patients were assessed with conventional measures for tracking those cognitive functions. Future studies should homologate the cognitive and behavioural assessments of samples of patients with genetic antecedents. Finally, sample patients were identified based on genetic diagnosis by targeted sequencing techniques using a gene panel established previously and outside of this study. In future investigations, circulating cell-free mRNA analysis in plasma levels may be considered to predict the functional effects of this variant on phenotype clinical.
Conclusions
This study offers a suggestive association that the p.H157Y variant could contribute to bvFTD and FTD-MND clinical phenotype. TREM2 variants exhibit pleiotropic effects producing a wide spectrum of disorders with different clinical phenotypes. In three cases of this report, we found a bvFTD presentation with classical atrophy extending to other brain areas, accompanied by major disinhibition with impairments in general cognition, executive functions and social cognition. In addition, there is strong evidence to alter motor neurons in one case. We found an interesting association between atrophy peaks and TREM2 gene expression to the susceptibility of FTD. These findings highlight the marked phenotypic variability possible within subjects carrying the same TREM2 variant.
Footnotes
Twitter: @AgustinMIBanez
NO, HS-G, SB and AL contributed equally.
Contributors: NO, HS-G and AI developed the study concept and the study design. NO, HS-G, SB, A Lopez, PR, A Laserna, PA, EG, FS, IZ and JMS-E performed testing and data collection. NO, HS-G, SB, A Lopez and A Laserna performed data analysis and interpretation under the supervision of AI. NO, HS-G, SB, A Lopez, and AI drafted the manuscript. SS provided the pathological findings. DM, FS, IZ, PR, JY, BM, KK, DS, NC and MK provided critical revisions. HG-G and AI are responsible for the overall content. All authors have participated sufficiently in the work and approved he final version of the manuscript for submission.
Funding: AI is partially supported by grants from CONICET; ANID/FONDECYT Regular (1210195, 1210176 and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; FONDEF ID20I10152, ID22I10029; Takeda CW2680521 and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA (ReDLat, supported by National Institutes of Health, National Institutes of Aging (R01 AG057234), Alzheimer’s Association (SG-20-725707), Rainwater Charitable foundation – Tau Consortium, and Global Brain Health Institute). The contents of this publication are solely the responsibility of the authors and do not represent the official views of these Institutions.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
Ethics approval
The Institutional Review Board (IRB) of Pontificia Universidad Javeriana approved the study.
References
- 1. Geier EG, Bourdenx M, Storm NJ, et al. Rare variants in the neuronal ceroid lipofuscinosis gene MFSD8 are candidate risk factors for frontotemporal dementia. Acta Neuropathol 2019;137:71–88. 10.1007/s00401-018-1925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hickman S, Izzy S, Sen P, et al. Microglia in neurodegeneration. Nat Neurosci 2018;21:1359–69. 10.1038/s41593-018-0242-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jay TR, von Saucken VE, Landreth GE. Trem2 in neurodegenerative diseases. Mol Neurodegener 2017;12:56. 10.1186/s13024-017-0197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmona S, Zahs K, Wu E, et al. The role of TREM2 in alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol 2018;17:721–30. 10.1016/S1474-4422(18)30232-1 [DOI] [PubMed] [Google Scholar]
- 5. Jiang T, Hou J-K, Gao Q, et al. Trem2 p.H157Y variant and the risk of Alzheimer’s disease: a meta-analysis involving 14,510 subjects. Curr Neurovasc Res 2016;13:318–20. 10.2174/1567202613666160808095530 [DOI] [PubMed] [Google Scholar]
- 6. Ulrich JD, Ulland TK, Colonna M, et al. Elucidating the role of TREM2 in alzheimer’s disease. Neuron 2017;94:237–48. 10.1016/j.neuron.2017.02.042 [DOI] [PubMed] [Google Scholar]
- 7. Zhou S-L, Tan C-C, Hou X-H, et al. Trem2 variants and neurodegenerative diseases: a systematic review and meta-analysis. J Alzheimers Dis 2019;68:1171–84. 10.3233/JAD-181038 [DOI] [PubMed] [Google Scholar]
- 8. Guerreiro R, Wojtas A, Bras J, et al. Trem2 variants in Alzheimer’s disease. N Engl J Med 2013;368:117–27. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 2013;368:107–16. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thelen M, Razquin C, Hernández I, et al. Investigation of the role of rare TREM2 variants in frontotemporal dementia subtypes. Neurobiol Aging 2014;35:2657. 10.1016/j.neurobiolaging.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 11. Yeh FL, Hansen DV, Sheng M. TREM2, microglia, and neurodegenerative diseases. Trends Mol Med 2017;23:512–33. 10.1016/j.molmed.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 12. Sheng L, Chen M, Cai K, et al. Microglial TREM2 induces synaptic impairment at early stage and prevents amyloidosis at late stage in APP/PS1 mice. FASEB J 2019;33:10425–42. 10.1096/fj.201900527R [DOI] [PubMed] [Google Scholar]
- 13. Song W, Hooli B, Mullin K, et al. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement 2017;13:381–7. 10.1016/j.jalz.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlepckow K, Kleinberger G, Fukumori A, et al. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol Med 2017;9:1356–65. 10.15252/emmm.201707672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bianchin MM, Lima JE, Natel J, et al. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology 2006;66:615–6; 10.1212/01.wnl.0000216105.11788.0f [DOI] [PubMed] [Google Scholar]
- 16. Guerreiro R, Bilgic B, Guven G, et al. Novel compound heterozygous mutation in TREM2 found in a turkish frontotemporal dementia-like family. Neurobiol Aging 2013;34:2890. 10.1016/j.neurobiolaging.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paloneva J, Mandelin J, Kiialainen A, et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med 2003;198:669–75. 10.1084/jem.20030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giraldo M, Lopera F, Siniard AL, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and alzheimer’s disease. Neurobiol Aging 2013;34:2077. 10.1016/j.neurobiolaging.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeh FL, Wang Y, Tom I, et al. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 2016;91:328–40. 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 20. Feuerbach D, Schindler P, Barske C, et al. ADAM17 is the main sheddase for the generation of human triggering receptor expressed in myeloid cells (htrem2) ectodomain and cleaves TREM2 after histidine 157. Neurosci Lett 2017;660:109–14. 10.1016/j.neulet.2017.09.034 [DOI] [PubMed] [Google Scholar]
- 21. Thornton P, Sevalle J, Deery MJ, et al. TREM2 shedding by cleavage at the H157-S158 bond is accelerated for the alzheimer’s disease-associated H157Y variant. EMBO Mol Med 2017;9:1366–78. 10.15252/emmm.201707673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456–77. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–14. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:153–74. 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 26. Bouquet CA, Bonnaud V, Gil R. Investigation of supervisory attentional system functions in patients with Parkinson’s disease using the hayling task. J Clin Exp Neuropsychol 2003;25:751–60. 10.1076/jcen.25.6.751.16478 [DOI] [PubMed] [Google Scholar]
- 27. Torralva T, Roca M, Gleichgerrcht E, et al. INECO frontal screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc 2009;15:777–86. 10.1017/S1355617709990415 [DOI] [PubMed] [Google Scholar]
- 28. Carvalho JO, Ready RE, Malloy P, et al. Confirmatory factor analysis of the frontal systems behavior scale (frsbe). Assessment 2013;20:632–41. 10.1177/1073191113492845 [DOI] [PubMed] [Google Scholar]
- 29. Baron-Cohen S, Jolliffe T, Mortimore C, et al. Another advanced test of theory of mind: evidence from very high functioning adults with autism or Asperger syndrome. J Child Psychol Psychiatry 1997;38:813–22. 10.1111/j.1469-7610.1997.tb01599.x [DOI] [PubMed] [Google Scholar]
- 30. Couto B, Salles A, Sedeño L, et al. The man who feels two hearts: the different pathways of interoception. Soc Cogn Affect Neurosci 2014;9:1253–60. 10.1093/scan/nst108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. García AM, Sedeño L, Herrera Murcia E, et al. A lesion-proof brain? multidimensional sensorimotor, cognitive, and socio-affective preservation despite extensive damage in a stroke patient. Front Aging Neurosci 2016;8:335. 10.3389/fnagi.2016.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crawford JR, Garthwaite PH, Ryan K. Comparing a single case to a control sample: testing for neuropsychological deficits and dissociations in the presence of covariates. Cortex 2011;47:1166–78. 10.1016/j.cortex.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 33. García AM, Abrevaya S, Kozono G, et al. The cerebellum and embodied semantics: evidence from a case of genetic ataxia due to STUB1 mutations. J Med Genet 2017;54:114–24. 10.1136/jmedgenet-2016-104148 [DOI] [PubMed] [Google Scholar]
- 34. Santamaría-García H, Ogonowsky N, Baez S, et al. Neurocognitive patterns across genetic levels in behavioral variant frontotemporal dementia: a multiple single cases study. BMC Neurol 2022;22:454. 10.1186/s12883-022-02954-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. 10.1093/nar/16.3.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toledo JB, Brettschneider J, Grossman M, et al. Csf biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol 2012;124:23–35. 10.1007/s00401-012-0983-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Blitterswijk M, van Es MA, Koppers M, et al. VAPB and c9orf72 mutations in 1 familial amyotrophic lateral sclerosis patient. Neurobiol Aging 2012;33:2950. 10.1016/j.neurobiolaging.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 38. Jones AR, Overly CC, Sunkin SM. The Allen brain atlas: 5 years and beyond. Nat Rev Neurosci 2009;10:821–8. 10.1038/nrn2722 [DOI] [PubMed] [Google Scholar]
- 39. Paloneva J, Manninen T, Christman G, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 2002;71:656–62. 10.1086/342259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bianchin MM, Capella HM, Chaves DL, et al. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy -- PLOSL): a dementia associated with bone cystic lesions. from clinical to genetic and molecular aspects. Cell Mol Neurobiol 2004;24:1–24. 10.1023/b:cemn.0000012721.08168.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Numasawa Y, Yamaura C, Ishihara S, et al. Nasu-Hakola disease with a splicing mutation of TREM2 in a Japanese family. Eur J Neurol 2011;18:1179–83. 10.1111/j.1468-1331.2010.03311.x [DOI] [PubMed] [Google Scholar]
- 42. Pottier C, Wallon D, Rousseau S, et al. Trem2 R47H variant as a risk factor for early-onset Alzheimer’s disease. J Alzheimers Dis 2013;35:45–9. 10.3233/JAD-122311 [DOI] [PubMed] [Google Scholar]
- 43. Guerreiro R, Hardy J. Trem2 and neurodegenerative disease. N Engl J Med 2013;369:1569–70. 10.1056/NEJMc1306509 [DOI] [PubMed] [Google Scholar]
- 44. Chouery E, Delague V, Bergougnoux A, et al. Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat 2008;29:E194–204. 10.1002/humu.20836 [DOI] [PubMed] [Google Scholar]
- 45. Guerreiro RJ, Lohmann E, Brás JM, et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol 2013;70:78–84. 10.1001/jamaneurol.2013.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Ber I, De Septenville A, Guerreiro R, et al. Homozygous TREM2 mutation in a family with atypical frontotemporal dementia. Neurobiol Aging 2014;35:2419. 10.1016/j.neurobiolaging.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuyvers E, Bettens K, Philtjens S, et al. Investigating the role of rare heterozygous TREM2 variants in alzheimer’s disease and frontotemporal dementia. Neurobiol Aging 2014;35:726. 10.1016/j.neurobiolaging.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 48. Luis EO, Ortega-Cubero S, Lamet I, et al. Frontobasal gray matter loss is associated with the TREM2 p.r47h variant. Neurobiol Aging 2014;35:2681–90. 10.1016/j.neurobiolaging.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in alzheimer’s disease. Lancet Neurol 2015;14:388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peplonska B, Berdynski M, Mandecka M, et al. Trem2 variants in neurodegenerative disorders in the Polish population. homozygosity and compound heterozygosity in FTD patients. Amyotroph Lateral Scler Frontotemporal Degener 2018;19:407–12. 10.1080/21678421.2018.1451894 [DOI] [PubMed] [Google Scholar]
- 51. Acosta-Uribe J, Aguillón D, Cochran JN, et al. A neurodegenerative disease landscape of rare mutations in Colombia due to founder effects. Genome Med 2022;14:27. 10.1186/s13073-022-01035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Genis-Mendoza AD, Martínez-Magaña JJ, Téllez-Martínez JA, et al. Identificación de variantes de alto impacto en TREM2 y ABCA7 en individuos mexicanos diagnosticados con enfermedad de alzheimer. Rev Mex Psiq 2020;1:224–9. [Google Scholar]
- 53. Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol 2003;3:445–53. 10.1038/nri1106 [DOI] [PubMed] [Google Scholar]
- 54. Greene AS, Shen X, Noble S, et al. Brain-phenotype models fail for individuals who defy sample stereotypes. Nature 2022;609:109–18. 10.1038/s41586-022-05118-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Llibre-Guerra JJ, Behrens MI, Hosogi ML, et al. Frontotemporal dementias in latin america: history. Epidemiology, Genetics, and Clinical Research Frontiers in Neurology 2021;12:710332. 10.3389/fneur.2021.710332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xie M, Liu YU, Zhao S, et al. Trem2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration. Nat Neurosci 2022;25:26–38. 10.1038/s41593-021-00975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Migeot JA, Duran-Aniotz CA, Signorelli CM. A predictive coding framework of allostatic–interoceptive overload in frontotemporal dementia. Trends in Neurosciences 2022. 10.1016/j.tins.2022.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kober DL, Alexander-Brett JM, Karch CM, et al. Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. Elife 2016;5:e20391. 10.7554/eLife.20391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Samanci B, Bilgiç B, Gelişin Ö, et al. Trem2 variants as a possible cause of frontotemporal dementia with distinct neuroimaging features. Eur J Neurol 2021;28:2603–13. 10.1111/ene.14908 [DOI] [PubMed] [Google Scholar]
- 60. Ibanez A. The mind’s golden cage and cognition in the wild. Trends Cogn Sci 2022;26:1031–4. 10.1016/j.tics.2022.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet 2015;386:1672–82. 10.1016/S0140-6736(15)00461-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cajanus A, Solje E, Koikkalainen J, et al. The association between distinct frontal brain volumes and behavioral symptoms in mild cognitive impairment, alzheimer’s disease, and frontotemporal dementia. Front Neurol 2019;10:1059. 10.3389/fneur.2019.01059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pan PL, Song W, Yang J, et al. Gray matter atrophy in behavioral variant frontotemporal dementia: a meta-analysis of voxel-based morphometry studies. Dement Geriatr Cogn Disord 2012;33:141–8. 10.1159/000338176 [DOI] [PubMed] [Google Scholar]
- 64. Schroeter ML, Raczka K, Neumann J, et al. Towards a nosology for frontotemporal lobar degenerations-a meta-analysis involving 267 subjects. Neuroimage 2007;36:497–510. 10.1016/j.neuroimage.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 65. Rabinovici GD, Stephens ML, Possin KL. Executive dysfunction. Continuum (Minneap Minn) 2015;21(3 Behavioral Neurology and Neuropsychiatry):646–59. 10.1212/01.CON.0000466658.05156.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang T, Tan L, Chen Q, et al. A rare coding variant in TREM2 increases risk for alzheimer’s disease in han chinese. Neurobiol Aging 2016;42:217. 10.1016/j.neurobiolaging.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 67. Donnelly-Kehoe PA, Pascariello GO, García AM, et al. Robust automated computational approach for classifying frontotemporal neurodegeneration: multimodal/multicenter neuroimaging. Alzheimers Dement (Amst) 2019;11:588–98. 10.1016/j.dadm.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frith U, Frith C. The social brain: allowing humans to boldly go where no other species has been. Philos Trans R Soc Lond B Biol Sci 2010;365:165–76. 10.1098/rstb.2009.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 2012;78:1354–62. 10.1212/WNL.0b013e3182518375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maito MA, Santamaría-García H, Moguilner S, et al. Classification of alzheimer’s disease and frontotemporal dementia using routine clinical and cognitive measures across multicentric underrepresented samples: A cross sectional observational study. Lancet Reg Health Am 2023;17:100387. 10.1016/j.lana.2022.100387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Birba A, Santamaría-García H, Prado P, et al. Allostatic-interoceptive overload in frontotemporal dementia. Biol Psychiatry 2022;92:54–67. 10.1016/j.biopsych.2022.02.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moguilner S, Birba A, Fittipaldi S, et al. Multi-feature computational framework for combined signatures of dementia in underrepresented settings. J Neural Eng 2022;19. 10.1088/1741-2552/ac87d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Salamone PC, Legaz A, Sedeño L, et al. Interoception primes emotional processing: multimodal evidence from neurodegeneration. J Neurosci 2021;41:4276–92. 10.1523/JNEUROSCI.2578-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Legaz A, Abrevaya S, Dottori M, et al. Multimodal mechanisms of human socially reinforced learning across neurodegenerative diseases. Brain 2022;145:1052–68. 10.1093/brain/awab345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eslinger PJ, Moore P, Antani S, et al. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol 2012;25:127–36. 10.3233/BEN-2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ogar JM, Dronkers NF, Brambati SM, et al. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 2007;21:S23–30. 10.1097/WAD.0b013e31815d19fe [DOI] [PubMed] [Google Scholar]
- 77. Cady J, Koval ED, Benitez BA, et al. Trem2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol 2014;71:449–53. 10.1001/jamaneurol.2013.6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Engelhardt JI, Appel SH. Igg reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol 1990;47:1210–6. 10.1001/archneur.1990.00530110068019 [DOI] [PubMed] [Google Scholar]
- 79. Kawakami I, Arai T, Hasegawa M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol 2019;138:751–70. 10.1007/s00401-019-02077-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Seelaar H, Schelhaas HJ, Azmani A, et al. Tdp-43 pathology in familial frontotemporal dementia and motor neuron disease without progranulin mutations. Brain 2007;130(Pt 5):1375–85. 10.1093/brain/awm024 [DOI] [PubMed] [Google Scholar]
- 81. Cochran JN, Geier EG, Bonham LW, et al. Non-coding and loss-of-function coding variants in TET2 are associated with multiple neurodegenerative diseases. Am J Hum Genet 2020;106:632–45. 10.1016/j.ajhg.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jmg-2022-108627supp001.pdf (342.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request.