Abstract
Objective
Assess mortality and neurodevelopmental outcomes at 2 years of corrected age in children who participated in the PlaNeT-2/MATISSE (Platelets for Neonatal Transfusion - 2/Management of Thrombocytopenia in Special Subgroup) study, which reported that a higher platelet transfusion threshold was associated with significantly increased mortality or major bleeding compared to a lower one.
Design
Randomised clinical trial, enrolling from June 2011 to August 2017. Follow-up was complete by January 2020. Caregivers were not blinded; however, outcome assessors were blinded to treatment group.
Setting
43 level II/III/IV neonatal intensive care units (NICUs) across UK, Netherlands and Ireland.
Patients
660 infants born at less than 34 weeks’ gestation with platelet counts less than 50×109/L.
Interventions
Infants were randomised to undergo a platelet transfusion at platelet count thresholds of 50×109/L (higher threshold group) or 25×109/L (lower threshold group).
Main outcomes measures
Our prespecified long-term follow-up outcome was a composite of death or neurodevelopmental impairment (developmental delay, cerebral palsy, seizure disorder, profound hearing or vision loss) at 2 years of corrected age.
Results
Follow-up data were available for 601 of 653 (92%) eligible participants. Of the 296 infants assigned to the higher threshold group, 147 (50%) died or survived with neurodevelopmental impairment, as compared with 120 (39%) of 305 infants assigned to the lower threshold group (OR 1.54, 95% CI 1.09 to 2.17, p=0.017).
Conclusions
Infants randomised to a higher platelet transfusion threshold of 50×109/L compared with 25×109/L had a higher rate of death or significant neurodevelopmental impairment at a corrected age of 2 years. This further supports evidence of harm caused by high prophylactic platelet transfusion thresholds in preterm infants.
Trial registration number
Keywords: Neonatology; Child Development; Intensive Care Units, Neonatal
WHAT IS ALREADY KNOWN ON THIS TOPIC
A higher prophylactic platelet transfusion threshold (50×109/L) in the preterm infant is associated with significantly increased mortality or major bleeding compared with a lower one (25×109/L).
WHAT THIS STUDY ADDS
Preterm infants randomised to a higher platelet transfusion threshold of 50×109/L compared with 25×109/L had a higher rate of death or significant neurodevelopmental impairment at a corrected age of 2 years.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This further supports evidence of harm caused by high prophylactic platelet transfusion thresholds in preterm infants.
Introduction
Fifteen million infants are born prematurely worldwide annually.1 Despite significant progress in neonatal care over the past decades and improved survival rates, extreme prematurity continues to be associated with long-term impairment, with a high risk of death and major brain injury at early gestations. More than 6% of very low birth weight infants (VLBWIs, birthweight <1500 g) develop cerebral palsy (CP), and 25%–44% have cognitive or behavioural deficits.2–4 Major bleeding, especially intraventricular haemorrhage (IVH), is associated with lifetime impairment. Clinicians’ concerns about increased risk of potential bleeding result in many infants with severe thrombocytopenia (platelets <50×109/L) receiving platelet transfusions, despite no evidence that this prevents bleeding and increasing concerns about potential harm.5 6
Until recently, the only evidence neonatologists had to support or refute prophylactic platelet transfusion in preterm infants was a single randomised controlled trial (RCT) of higher platelet transfusion thresholds in 19937 and a series of retrospective cohort analyses.6 8 From 2011, we conducted a large, international RCT of platelet transfusion thresholds to study short-term and long-term effects of higher, more liberal (50×109/L) versus lower, more restrictive (25×109/L) platelet transfusion thresholds. We found that those randomly assigned to undergo platelet transfusions at a platelet count threshold of 50×109/L had a significantly higher rate of death or major bleeding within 28 days after randomisation than those who received platelet transfusions at a platelet count threshold of 25×109/L.9 We also demonstrated that this risk was present irrespective of baseline risk of bleeding or death.10
Information on solely short-term outcomes is inadequate to assess the overall benefits and risks of neonatal interventions such as platelet transfusion, potentially affecting lifelong development. We now report results of prespecified longer-term follow-up outcomes in this trial. We aimed to determine whether a higher versus lower threshold for prophylactic platelet transfusion changed the composite rate of death or survival with significant neurodevelopmental impairment at the corrected age of 2 years.
Methods
The inclusion/exclusion criteria, randomisation procedures and short-term outcomes of the PlaNeT-2/MATISSE randomised trial investigating a liberal versus a restrictive platelet transfusion threshold have been reported previously.9 Infants born at <34 weeks’ gestation and without recent major bleeding in the previous 72 hours were eligible for the study if their platelet count dropped below 50×109/L. Between 2011 and 2017, 660 infants were enrolled at 43 centres in the UK, Ireland and The Netherlands and were randomly assigned to undergo platelet transfusions at a higher or lower platelet transfusion threshold. Randomisation was performed using gestational age (GA) and presence of intrauterine growth restriction (IUGR) as minimisation factors.
Neurodevelopmental outcome
Neurodevelopmental outcomes were assessed at all centres, with Bayley Scale of Infant Development III (BSID-III)11 and Griffiths Mental Development Scales–Extended Revised (GMDS-ER).12 We considered a score more than 2 SDs below the mean an unfavourable outcome. When these were not available, we contacted parents/guardians directly by phone or post to ask them to complete a parent reporting (Parent Report of Children’s Abilities–Revised (PARCA-R)) assessment.13 The PARCA-R13 is a parent-completed questionnaire used to assess children’s cognitive and language development at 24 months of age, corrected for gestation, translated into Dutch for infants in The Netherlands. Where formal assessment (BSID-III, GMDS-ER or PARCA-R) could not be obtained, all available information was used to deduce the outcome, including Schedule of Growing Skills II (SGS-II) reports. SGS-II is a screening tool that assesses child development, commonly used in the UK.14 Letters from hospital visits and information from hospital consultants, general practitioners, public health nurses, and early intervention services were also reviewed.
For the purposes of analysis, we dichotomised participants as having a favourable or unfavourable outcome, as used in other follow-up studies.15 Death was included as a composite with unfavourable neurodevelopmental outcomes to avoid bias due to different redirection of care practices in different neonatal units.16 A favourable outcome was given if we could ascertain that a child was alive at 2 years of age corrected for gestation and did not have any of the following: CP that impaired independent walking; global developmental delay assessed by healthcare professionals as >9 months behind expected for age, a pragmatic clinical equivalent of >2 SD below the mean; severe seizure disorder; hearing impairment not corrected by hearing aids; or bilateral visual impairment with no useful vision (light perception only). The assessing paediatricians (AC, AD’A and CMM) used all available information to report outcomes using the 2-year outcome form, a standardised classification of outcomes used in other UK studies.17 This pragmatic outcome was prespecified in the original trial protocol, aware of potential difficulties with obtaining formal follow-up on such a large group of children across a high number of sites, especially given moving across networks. When formal assessment scores were available, they were checked for concordance with other available data; however, letters from other healthcare professionals were not requested.
The favourable/unfavourable outcome was determined independently by three clinicians (AC, AD’A and CMM) who reviewed the data submitted by the local centres and were blinded to the treatment arm. In case of disagreement, the decision was made based on two of three assessors’ conclusions. Participants who could not be classified were excluded from the analysis, and reasons for non-classification were tabulated.
Statistical analysis
All prespecified and preplanned analyses were performed according to the intention-to-treat principle and were included all randomised participants with an available outcome (including those randomised in error). Data were analysed according to the trial arm to which the participant was randomised. The outcome for this follow-up study of death or adverse neurodevelopmental outcome was analysed using a mixed logistic regression model. All models were risk-adjusted for GA and IUGR as covariates (factors used for minimisation) and centre of recruitment as a random effect. All tests were two-sided; a p value of <0.05 was considered statistically significant. SAS software V.9.4 was used to conduct the analyses. This study was powered for the primary outcome of the original study—mortality or major bleed at 28 days post randomisation—not for death or adverse neurodevelopmental outcome at 2 years corrected for gestation.
Secondary outcomes
The component parts of the composite long-term neurodevelopmental outcome were analysed separately using a mixed logistic regression model.
Death or dependence on respiratory support at 2 years corrected
As we had found a significant difference in the proportion of participants with bronchopulmonary dysplasia (BPD, defined as respiratory support or oxygen dependence at 36 weeks’ postmenstrual age) between the arms in the main trial, respiratory status at 2 years was of particular interest. Therefore, post hoc exploratory analysis of the proportion of participants who had died or were dependent on oxygen or respiratory support at 2 years of age, corrected for GA, was analysed using a mixed logistic regression model, adjusted for GA and presence of IUGR as covariates, and the centre of recruitment as a random effect.
Results
Follow-up and baseline characteristics
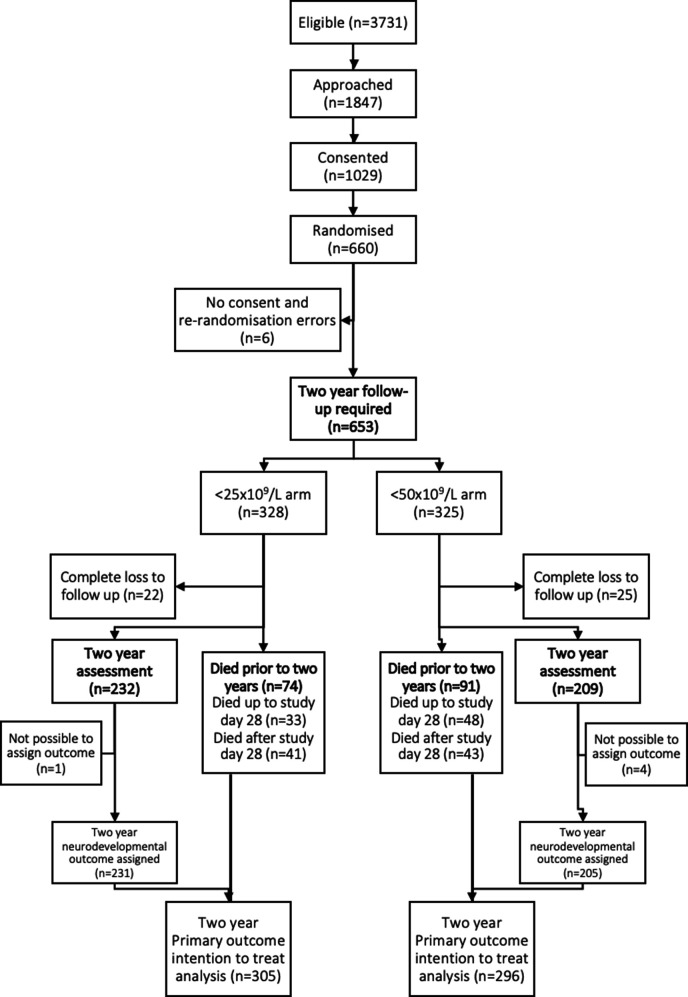
A total of 660 infants were enrolled between 2011 and 2017 at 43 centres in the UK, Ireland and The Netherlands. Of the 660 infants recruited to the PlaNet-2 trial, seven were not included in primary outcome analysis. This was due to lack of consent (n=3), rerandomisation errors (n=1) and missing data (no cranial ultrasound) in three infants receiving palliative care at the time of primary outcome assessment. These three infants subsequently died and were included in the 2-year follow-up. Three other infants were excluded as we did not have consent for 2-year follow-up, and 47 infants were completely lost to follow-up. Five children could not be included in our analysis as we had insufficient information to assign a neurodevelopmental outcome.
Baselines characteristics of these 601 children were similar in the two randomised groups at birth (birth weight, GA, antenatal corticosteroids, presence of chorioamnionitis) and at the time of randomisation (incidence of major bleeding including IVH prior to study entry and incidence of sepsis or necrotising enterocolitis (NEC)).9
Primary outcome: death or neurodevelopmental outcome at 2 years of age
Of 653 children, 601 (92%) were available for analysis of the primary composite outcome of death or neurodevelopmental impairment (figure 1). Mortality data were available for 606 of the 653 (93%) infants: 81 infants died within 28 days of recruitment,9 and an additional 84 children died before 2 years of corrected age. Five of the surviving children had insufficient data to assign a neurodevelopmental outcome, leaving 436 children available for full neurodevelopmental analysis.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram.
Of 436 children, 177 (41%) had a formal neurodevelopmental assessment, either BSID (158), GMDS (21) or both performed. Of 158 BSID assessments, 134 were sufficient to assign outcome; 15 were incomplete; and 8 were carried out outside of the protocol age range. Of 21 GMDS-ER assessments, 18 met the criteria for assigning an outcome. Of 26 PARCA-R assessments, 3 were sufficient to assign outcome; 4 were incomplete; and 19 were out of the age range. Forty-two children had SGS-II assessment performed. These results are available in online supplemental appendix 1.
fetalneonatal-2022-324915supp001.pdf (163.3KB, pdf)
Infants randomised to a higher platelet transfusion threshold of 50×109/L compared with 25×109/L had a higher rate of death or significant neurodevelopmental impairment. Of 296 (50%) assigned to the higher threshold group, 147 died or survived with an unfavourable outcome, as compared with 120 of 305 (39%) assigned to the lower threshold group (OR 1.54, adjusted for GA and the presence of IUGR as covariates and centre as a random effect; 95% CI 1.09 to.17; p=0.017), see table 1. The number of infants who would need to be assigned to a lower transfusion threshold to prevent one unfavourable neurodevelopmental outcome was nine (95% CI 5 to 48), based on the risk-adjusted OR and corresponding CI.
Table 1.
Outcomes
| Low threshold group (n=328) | High threshold group (n=325) | P value | |
| Primary outcome | |||
| Death up to 2 years or unfavourable outcome | 120/305 (39) | 147/296 (50) | |
| OR (<50 vs <25) (95% CI) | 1.54 (1.09 to 2.17)* | 0.0167 | |
| Secondary outcomes | |||
| Deaths up to 2 years, n/total n(%)† | 74/306 (24) | 91/300 (30) | |
| OR (<50 vs <25) (95% CI) | 1.36 (0.93 to 1.99) | 0.1067 | |
| Unfavourable outcome (excluding deaths), n/total n (%) | 46/231 (20) | 56/205 (27) | |
| OR (<50 vs <25) (95% CI) | 1.53 (0.95 to 2.46) | 0.0788 | |
| Participants who have cerebral palsy that impaired independent walking, n/total n (%)‡ | 23/229 (10) | 26/203 (13) | |
| Participants who have global development delay, n/total n (%)‡ | 33/228 (14) | 44/204 (22) | |
| Participants who have a severe seizure disorder requiring treatment, n/total n (%)‡ | 4/225 (2) | 7/200 (4) | |
| Participants who have hearing impairment not corrected by hearing aids, n/total n (%)‡ | 3/227 (1) | 5/203 (2) | |
| Participants who have bilateral cortical visual impairment with no useful vision, n/total n (%)‡ | 2/225 (1) | 3/200 (2) | |
| Participants who died or required respiratory support at 2 years of corrected age | 83/301 (28) | 113/296 (38) | |
| OR (<50 vs <25) (95% CI) | 1.62 (1.12 to 2.34) | ||
| Participants who were dependent on oxygen or respiratory support at 2 years of corrected age | 9/228 (4) | 22/207(11) | |
| OR (<50 vs <25) (95% CI) | 2.86 (1.25 to 6.51) | ||
All ORs have been adjusted for GA and presence of IUGR as covariates and centre has been adjusted for using a random effect. Marginal effects are reported.
*The OR has been adjusted for GA and presence of IUGR as covariates and centre has been adjusted for using a random effect. Outcome data were missing for 52 participants (23 in the <25 arm and 29 in the <50 arm). The OR is presented as <50 arm vs <25 arm. Marginal effects are reported.
†Includes five participants who were known to be alive at 2 years, but it was not possible to establish their neurodevelopmental outcome due to insufficient information.
‡Note differing denominators for component parts of neurodevelopmental outcome due to missing data, but unfavourable outcome could still be assigned based on the presence of another adverse factor.
GA, gestational age; IUGR, intrauterine growth restriction.
The higher rates of neurodevelopmental impairment at 2 years consist of a combination of impairments, with global developmental delay and CP being the most common. Fourteen per cent of the lower transfusion threshold and 22% of the higher transfusion threshold group had developmental delay (table 1). CP rates were 10% in the lower threshold group compared with 13% in the higher group. The individual rates of seizure disorder, deafness and blindness at follow-up were similar.
Secondary outcomes: respiratory
Four of 300 children in the higher threshold group and 5 of 306 children in the lower threshold group had missing data on oxygen dependence at 2 years. Of 296 children assigned to the higher threshold group, 113 (38%) died or required respiratory support at 2 years of age, as compared with 83 (28%) of 301 children assigned to the lower threshold group (OR=1.62, 95% CI 1.12 to 2.34). Twenty-two children in the higher threshold group required respiratory support at 2 years of corrected age compared with 9 children in the lower threshold group. We carried out an additional post hoc analysis to calculate the OR for the participants who were dependent on oxygen or respiratory support at 2 years of corrected age, excluding infants who had died. A statistically significant difference between the trial arms was found in favour of the lower treatment threshold group (OR=2.86, 95% CI 1.25 to 6.51).
Discussion
We reported previously that a higher threshold for transfusion of 50×109/L increased the rate of mortality or major bleeding 28 days after randomisation in infants born less than 34 weeks’ gestation.9 Our finding of potential harm from transfusions is consistent with the results of many observational studies.5 6 18 We now report that infants randomised to a higher platelet transfusion threshold of 50×109/L compared with 25×109/L also had a higher composite rate of death or adverse neurodevelopmental outcome at a corrected age of 2 years. Although neither rates of death nor adverse neurological outcome alone differed significantly between trial arms, they both favoured the lower platelet threshold arm. Despite increased survival in the lower threshold cohort, we did not see increased impairment in survivors.
The mechanisms by which platelet transfusions could mediate harmful short-term and long-term effects in the ex-preterm infant remain unknown. It is biologically plausible that platelets could be causing harm through effects on haemodynamics, immunity, inflammation, haemostasis or angiogenesis.19–22 These could explain the increase in mortality or affect neurodevelopment. In addition, these mechanisms could potentially cause or aggravate several pre-existing conditions unique to preterm infants including retinopathy of prematurity (ROP), IVH, NEC and BPD, all of which can impact long-term outcomes.9 18 23–27 In our study, we identified an increase in IVH and BPD, but not NEC and ROP.9
Higher rates of adverse neurodevelopmental and mortality outcomes in the higher-threshold group may be explained by the trend towards an increased rate of severe bleeding up to 28 days and BPD up to 2 years of age. Severe IVH occurred in 11 infants in the higher versus 5 in the lower threshold study arm.9 Severe IVH is associated with a high probability of death or impairment, predicting CP and abnormal neurodevelopmental outcome in almost half of infants affected.28 BPD is an important cause of mortality beyond the first few months of life29 and a very significant predictor of future neurodevelopmental impairment in infancy, childhood and adolescence.30 31 There was a trend towards increased rate of death or BPD in the higher threshold group in our study, persisting at 2 years of corrected age.9 The mechanisms through which platelet transfusion strategies could cause BPD are unknown but could include inflammation, platelet-neutrophil interactions or neovascularisation due to platelet-derived growth factors.32–34 Although BPD occurs in almost one-third of VLBWIs, dependence on respiratory support or oxygen at 2 years of corrected age is uncommon, indicating severe ongoing lung disease.29
Infants with NEC and sepsis are frequently thrombocytopenic and are among the highest users of platelet transfusions in the neonatal ICU.35 Many of the neonates in our original cohort presented with these conditions causing the thrombocytopenia that led to their inclusion in the study. Platelet transfusion may have proinflammatory effects,19 potentially aggravating pre-existing inflammation or need for surgery, thus potentially worsening long-term outcomes in the high threshold group.9 36–40
Strengths and limitations
Few neonatal studies demonstrate significant positive effects on longer-term outcome.41 This is the first study assessing long-term effects of platelet transfusion thresholds on neurodevelopmental outcomes in children who were born preterm. We achieved 92% follow-up in our cohort, comparable to other neonatal studies.42 43 We chose a pragmatic approach, with crude outcome definition, so that it could be assigned by local paediatricians using a standardised assessment form without formal assessment (if unavailable).17 We used standard definitions used in other follow-up studies.44 The advantage is that it enabled us to reach high follow-up rates and to assess severe and clinically meaningful outcome data. However, as only 41% of children had formal neurodevelopmental assessment, it was not possible to detect subtle outcome differences. Another limitation is that our study was powered to assess the primary outcome of mortality or major bleed at 28 days post randomisation and not neurodevelopmental or respiratory outcomes at 2 years corrected.
In conclusion, our study found that a higher platelet count threshold of 50×109/L for prophylactic transfusion in preterm infants less than 34 weeks’ gestation at birth increased the rate of death or neurodevelopmental impairment at 2 years of corrected age. There is no evidence to support benefit for high prophylactic platelet transfusion thresholds in the preterm infant. There is increasing evidence of harm persisting into childhood. As clinicians, we need to question whether a liberal approach to platelet transfusion can be justified on clinical or ethical grounds.
Acknowledgments
We thank all the hospital staff and research teams who helped conduct this trial (for a complete list, see the online supplemental appendix), all the families who agreed to participate; Dr Shobha Cherian, consultant neonatologist and principal investigator at University Hospital of Wales, Cardiff, and Anna Hendrickson, research nurse at St. Mary’s Hospital, Manchester, both of whom died in 2015 and 2016, respectively, for their valued contributions to and enthusiasm in the success of the trial; the National Health Service Blood and Transplant Clinical Trials Unit team, including Anna Sidders and Heather Smethurst (trial coordinators), Nikki Dallas (database manager), Louise Choo, Brennan Kahan, Helen Thomas, Laura Pankhurst and Agne Zarankaite (trial statisticians), and Colin Morley, Sandy Calvert and Anthony Emmerson (trial steering committee) for their support; and Adrian Newland, Henry Halliday, Paul White, Gavin Murphy, Michael Greaves, Keith Wheatley and Marc Turner (the data and safety monitoring committee).
Footnotes
Collaborators: We wish to acknowledge the contribution of the clinical sites involved in the trial: Timothy Watts, Helen Broomfield, Beatriz Lopez Santamaria, Evelina London Children’s Hospital, Guy’s Anna Curley, Vidheya Venkatesh, Rizwan Khan, Gusztav Belteki, Heather Smethurst, Rosie Maternity Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; Ajit Mahaveer, Nicola Booth, KarenDockery, Imelda Mayor, Anna Hendrickson, Clare Clifford, St Mary’s Hospital Manchester University NHS Foundation Trust, Manchester, UK; Porus Bustani, Pauline Bayliss, Sheffield Teaching Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK; Paul Clarke, Priya Muthukumar, Karen Few, Katherine Lloyd, Norfolk and Norwich Hospital, Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK; Zoltan Molnar, Sheula Barlow, Sharon Baugh, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, UK; Enrico Lopriore, Romy Berkhout, Division of Neonatology, Department of Pediatrics, Leiden University Medical Center, Leiden, The Netherlands; Helen McElroy, Helen Harizaj, Medway Maritime Hospital, Medway NHS Foundation Trust, UK; Manobi Borooah, Rachel Jackson, Heather Barrow, Elizabeth Simcox, Department of Neonatology, Birmingham Women’s and Children’s NHS Foundation Trust Birmingham, UK; Sunit Godambe, Sara Barnett, Imperial College Healthcare NHS Trust London, UK; Anton van Kaam, Wes Onland, Debbie Nuytemans, Department of Neonatology, Emma Children’s Hospital, Academic Medical Centre, Amsterdam, The Netherlands; Simon Power, Claire Abbott, Patsy Graham, Royal BoltonHospital, Bolton NHS Foundation Trust, Bolton, UK; Oliver Rackham, Joanne Mullen, Sharon Hughes, Lucy Lewis, Arrowe Park Hospital, Wirral University Teaching Hospital NHS Foundation Trust, Wirral, UK; Sundeep Harigopal, Linda Smith, Julie Groombridge, Tracey Downes, Royal Victoria Infirmary, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Charlotte Huddy, Naomi Hayward, Vana Wardley, St George’s Hospital, St George’s Healthcare NHS Trust, London, UK; ShobhaCherian, Mallinath Chakraborty, University Hospital of Wales, Cardiff and Vale University Health Board, Cardiff, Wales; Sarah McCullough, Louise Woodhead, Joanne Reed, Chloe Rishton, Grainne O’Connor, The Royal Oldham Hospital, Pennine Acute Hospitals NHSTrust, Oldham, UK; Anna Curley, Rebekah Prabharan, National Maternity Hospital, Dublin, Ireland; Raju Narasimhan, Claire Lodge, Nikki Childs, Kylie Reid, Joanna Lees, Natalie Mattos-Harris, Royal Preston Hospital, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK; Mark van der Hoeven, Neonatal Intensive Care Unit, MUMC, Maastricht, The Netherlands; C Harikumar, Wendy Cheadle, Alex Ramshaw, Julie Colarossi, Sharon Clarke, University Hospital of North Tees, North Tees and Hartlepool NHS Foundation Trust, North Tees, UK; Anitha James, Royal Gwent Hospital, Aneurin Bevan University Health Board, Newport, Wales; Mithilesh Lal, Amanda Forster, Helena Smith,The James Cook University Hospital, South Tees Hospitals NHS Foundation Trust, South Tees, UK; Andre Kroon, Annelies Bos, Division of Neonatology and Elise Huijssen Huisman, Department of Pediatric Hematology, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands; Melanie Sutcliffe, Sharon Kempson, Nick Denyer, Lyndsay Bibb, New Cross Hospital, The Royal Wolverhampton NHS Trust, Wolverhampton, UK; Andy Cox, Heather Collier, Emily Andrews, Burnley General Hospital, East Lancashire Hospitals NHS Foundation Trust, Burnley, UK; Vimal Vasu, Shermi George, Claire Moloney, William Harvey Hospital, East Kent Hospitals University NHS Foundation Trust, Ashford, UK; Esther d’Haens, Liesbeth Groot Jebbink-Akkerman, Princess Amalia Department of Pediatrics, Department of Neonatology, Isala Clinics, Zwolle, The Netherlands; Paul Munyard, Barbara Bromage, Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust, Cornwall, UK; Tim Scorrer, Tamsyn Wilson, Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust, Portsmouth, UK; Ellen de Kort, Tinneke Jonckers, Marieke Vervoorn, Department of Pediatrics and Neonatology, Máxima Medical Center, Veldhoven, The Netherlands; Dr David Sweet, Patricia McCreesh, Sonia McKenna, Muriel Millar. Royal Jubilee Maternity Hospital, Belfast Health and Social Care Trust. Belfast, UK; David Grier, Mike Smith, Sara Gilpin, Judith Ratcliffe, Craigavon Area Hospital, Southern Health and Social Care Trust, Craigavon,UK; Damien Armstrong, Julie Brown. Altnagelvin Area Hospital, Western Health and Social Trust, Derry/Londonderry, UK; Dr Conor O’Neill. Antrim Area Hospital, Northern Health and Social Care Trust Antrim, UK; Willem de Boode, Wendy Jansen, Department of Neonatology, Radboud University Medical Center, Radboud Institute for Health Sciences, Amalia Children’sHospital, Nijmegen, The Netherlands; Christopher Knight, Nicki Thorne, Kirsty O Brien, Musgrove Park Hospital, Taunton Susan Chatfield, Rachel Wane, Elizabeth Ingram, Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK; Christian Hulzebos, Annelies Olthuis, Department of Pediatrics, Beatrix Children Hospital, University Medical Center Groningen,University of Groningen, Groningen, The Netherlands; Anthony Ryan, Siobhan Foley, Carol Anne O’Shea, Cork University Maternity Hospital, Cork, Ireland; Karen Luyt, Joanne Innoles, St Michael’s Hospital University Hospitals Bristol NHS Foundation Trust, Bristol, UK; Osama Hamud, Imran Ahmed, Natalie Talbot, Sunderland Royal Hospital, City Hospitals Sunderland NHS Foundation Trust, Sunderland, UK; Nicola Pritchard, Susan Hallett, Royal Berkshire Hospital, Royal Berkshire Hospital NHS Foundation Trust, Reading, UK; Poonam Belani, Claudia Chetcutiganado, Yvonne Miller, Luton
Contributors: CMM and AD'A contributed equally to this paper. CH, AN, AD, RH, VH, AM and CL: oversight, administration, statistical and data support. AC, SS and AD'A conceptualised the study. CMM, AD'A and AC assessed the outcomes. CMM and AC wrote the original draft. CM, AD'A, AC, SS, SFG, TW, EL, PC, HVN, KF, WO, RK, VV, BLS and KW: contributed to methodology, reviewed and edited. All authors have reviewed this article. AC: guarantor.
Funding: This work was supported by the National Health Service Blood and Transplant Research and Development Committee (reference number BS06/1); Sanquin Research, Amsterdam (grant PPOC-12-012027); Addenbrooke’s Charitable Trust; the Neonatal Breath of Life Fund 9145; the National Institute for Health Research Clinical Research Network; the National Maternity Hospital Foundation and the Health Service Executive.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
for the PlaNeT2 MATISSE:
Timothy Watts, Helen Broomfield, Beatriz Lopez Santamaria, Guy’s Anna Curley, Vidheya Venkatesh, Rizwan Khan, Gusztav Belteki, Heather Smethurst, Ajit Mahaveer, Nicola Booth, Imelda Mayor KarenDockery, Anna Hendrickson, Clare Clifford, Porus Bustani, Pauline Bayliss, Paul Clarke, Priya Muthukumar, Karen Few, Katherine Lloyd, Zoltan Molnar, Sheula Barlow, Sharon Baugh, Enrico Lopriore, Romy Berkhout, Helen McElroy, Helen Harizaj, Manobi Borooah, Rachel Jackson, Heather Barrow, Elizabeth Simcox, Sunit Godambe, Sara Barnett, Anton van Kaam, Wes Onland, Debbie Nuytemans, Simon Power, Claire Abbott, Patsy Graham, Oliver Rackham, Joanne Mullen, Sharon Hughes, Lucy Lewis, Sundeep Harigopal, Linda Smith, Julie Groombridge, Tracey Downes, Charlotte Huddy, Naomi Hayward, ShobhaCherian Vana Wardley, Mallinath Chakraborty, Sarah McCullough, Louise Woodhead, Joanne Reed, Chloe Rishton, Grainne O’Connor, Anna Curley, Rebekah Prabharan, Raju Narasimhan, Claire Lodge, Nikki Childs, Kylie Reid, Joanna Lees, Natalie Mattos-Harris, Mark van der Hoeven, C Harikumar, Wendy Cheadle, Alex Ramshaw, Julie Colarossi, Sharon Clarke, Anitha James, Mithilesh Lal, Amanda Forster, Helena Smith, Andre Kroon, Annelies Bos, Melanie Sutcliffe, Sharon Kempson, Nick Denyer, Lyndsay Bibb, Andy Cox, Heather Collier, Emily Andrews, Vimal Vasu, Shermi George, Claire Moloney, Esther d’Haens, Liesbeth Groot Jebbink-Akkerman, Paul Munyard, Barbara Bromage, Tim Scorrer, Tamsyn Wilson, Ellen de Kort, Tinneke Jonckers, Marieke Vervoorn, Dr David Sweet, Patricia McCreesh, Sonia McKenna, Muriel Millar, David Grier, Mike Smith, Sara Gilpin, Judith Ratcliffe, Damien Armstrong, Julie Brown, Dr Conor O’Neill, Willem de Boode, Wendy Jansen, Christopher Knight, Nicki Thorne, Kirsty O Brien, Taunton Susan Chatfield, Rachel Wane, Elizabeth Ingram, Christian Hulzebos, Annelies Olthuis, Anthony Ryan, Siobhan Foley, Carol Anne O’Shea, Karen Luyt, Joanne Innoles, Osama Hamud, Imran Ahmed, Natalie Talbot, Nicola Pritchard, Susan Hallett, Poonam Belani, Claudia Chetcutiganado, and Yvonne Miller
Data availability statement
Data are available upon reasonable request. Data will be available upon reasonable request, with approval of the trial group.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. Research ethics boards of all clinical centres/countries approved the protocol for the trial and follow-up of participants. UK ethical approval was granted by East of England-Cambridge (10/H0306 /H0306/61). Written informed consent for study and follow-up was obtained from infants’ parent/guardians. An independent data and safety monitoring committee reviewed interim data analysis and monitored patient safety. The PlaNeT-2/MATISSE trial was undertaken in accordance with Declaration of Helsinki and Good Clinical Practice guidelines.
References
- 1. Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol 2017;41:387–91. 10.1053/j.semperi.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 2. Pascal A, Govaert P, Oostra A, et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol 2018;60:342–55. 10.1111/dmcn.13675 [DOI] [PubMed] [Google Scholar]
- 3. Reijneveld SA, de Kleine MJK, van Baar AL, et al. Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Arch Dis Child Fetal Neonatal Ed 2006;91:F423–8. 10.1136/adc.2006.093674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayes B, Sharif F. Behavioural and emotional outcome of very low birth weight infants -- literature review. J Matern Fetal Neonatal Med 2009;22:849–56. 10.1080/14767050902994507 [DOI] [PubMed] [Google Scholar]
- 5. Sparger KA, Assmann SF, Granger S, et al. Platelet transfusion practices among very-low-birth-weight infants. JAMA Pediatr 2016;170:687–94. 10.1001/jamapediatrics.2016.0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baer VL, Lambert DK, Henry E, et al. Severe thrombocytopenia in the NICU. Pediatrics 2009;124:e1095–100. 10.1542/peds.2009-0582 [DOI] [PubMed] [Google Scholar]
- 7. Andrew M, Vegh P, Caco C, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr 1993;123:285–91. 10.1016/s0022-3476(05)81705-6 [DOI] [PubMed] [Google Scholar]
- 8. Baer VL, Lambert DK, Henry E, et al. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. J Perinatol 2007;27:790–6. 10.1038/sj.jp.7211833 [DOI] [PubMed] [Google Scholar]
- 9. Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 2019;380:242–51. 10.1056/NEJMoa1807320 [DOI] [PubMed] [Google Scholar]
- 10. Fustolo-Gunnink SF, Fijnvandraat K, van Klaveren D, et al. Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood 2019;134:2354–60. 10.1182/blood.2019000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albers CA, Grieve AJ. Test review: bayley, N. (2006). Bayley scales of infant and toddler development– third edition. San antonio, TX: harcourt assessment. J Psychoeduc Assess 2007;25:180–90. 10.1177/0734282906297199 [DOI] [Google Scholar]
- 12. Luiz D, Barnard A, Knoesen N, et al. Griffiths mental development scales–extended revised. Two to eight years. administration manual. UK: Hogrefe, Oxford, 2006. [Google Scholar]
- 13. Johnson S, Bountziouka V, Brocklehurst P, et al. Standardisation of the parent report of children’s abilities-revised (PARCA-R): a norm-referenced assessment of cognitive and language development at age 2 years. Lancet Child Adolesc Health 2019;3:705–12. 10.1016/S2352-4642(19)30189-0 [DOI] [PubMed] [Google Scholar]
- 14. Bellman M, Lingam S, Aukett A. Schedule of growing skills II: NFER-nelson; 1987.
- 15. Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol 2016;33:318–28. 10.1055/s-0035-1571202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuttini M, Nadai M, Kaminski M, et al. End-of-life decisions in neonatal intensive care: physicians’ self-reported practices in seven european countries. euronic study group. Lancet 2000;355:2112–8. 10.1016/s0140-6736(00)02378-3 [DOI] [PubMed] [Google Scholar]
- 17. Jones HP, Guildea ZES, Stewart JH, et al. The health status questionnaire: achieving concordance with published disability criteria. Arch Dis Child 2002;86:15–20. 10.1136/adc.86.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenton AB, Hegemier S, Smith EO, et al. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol 2005;25:173–7. 10.1038/sj.jp.7211237 [DOI] [PubMed] [Google Scholar]
- 19. McFadyen JD, Kaplan ZS. Platelets are not just for clots. Transfus Med Rev 2015;29:110–9. 10.1016/j.tmrv.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 20. Ferrer-Marin F, Chavda C, Lampa M, et al. Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost 2011;9:1020–8. 10.1111/j.1538-7836.2011.04233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weyrich AS. Platelets: more than a sack of glue. Hematology Am Soc Hematol Educ Program 2014;2014:400–3. 10.1182/asheducation-2014.1.400 [DOI] [PubMed] [Google Scholar]
- 22. Sola-Visner MC. Platelet transfusions in neonates-less is more. N Engl J Med 2019;380:287–8. 10.1056/NEJMe1813419 [DOI] [PubMed] [Google Scholar]
- 23. Kumar J, Dutta S, Sundaram V, et al. Platelet transfusion for PDA closure in preterm infants: a randomized controlled trial. Pediatrics 2019;143. 10.1542/peds.2018-2565 [DOI] [PubMed] [Google Scholar]
- 24. Bazacliu C, Neu J. Pathophysiology of necrotizing enterocolitis: an update. Curr Pediatr Rev 2019;15:68–87. 10.2174/1573396314666181102123030 [DOI] [PubMed] [Google Scholar]
- 25. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med 2017;132:170–7. 10.1016/j.rmed.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savani RC. Modulators of inflammation in bronchopulmonary dysplasia. Semin Perinatol 2018;42:459–70. 10.1053/j.semperi.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang Q, Zhang L, Li H, et al. The fetal inflammation response syndrome and adverse neonatal outcomes: a meta-analysis. J Matern Fetal Neonatal Med 2021;34:3902–14. 10.1080/14767058.2019.1702942 [DOI] [PubMed] [Google Scholar]
- 28. Maitre NL, Marshall DD, Price WA, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics 2009;124:e1153–60. 10.1542/peds.2009-0953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeMauro SB, Jensen EA, Bann CM, et al. Home oxygen and 2-year outcomes of preterm infants with bronchopulmonary dysplasia. Pediatrics 2019;143:e20182956. 10.1542/peds.2018-2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han YS, Kim SH, Sung TJ. Impact of the definition of bronchopulmonary dysplasia on neurodevelopmental outcomes. Sci Rep 2021;11:22589. 10.1038/s41598-021-01219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malavolti AM, Bassler D, Arlettaz-Mieth R, et al. Bronchopulmonary dysplasia-impact of severity and timing of diagnosis on neurodevelopment of preterm infants: a retrospective cohort study. BMJ Paediatr Open 2018;2:e000165. 10.1136/bmjpo-2017-000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Callahan R, Kieran MW, Baird CW, et al. Adjunct targeted biologic inhibition agents to treat aggressive multivessel intraluminal pediatric pulmonary vein stenosis. J Pediatr 2018;198:29–35. 10.1016/j.jpeds.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 33. Cui TX, Brady AE, Fulton CT, et al. CCR2 mediates chronic LPS-induced pulmonary inflammation and hypoalveolarization in a murine model of bronchopulmonary dysplasia. Front Immunol 2020;11:579628. 10.3389/fimmu.2020.579628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hikino S, Ohga S, Kinjo T, et al. Tracheal aspirate gene expression in preterm newborns and development of bronchopulmonary dysplasia. Pediatr Int 2012;54:208–14. 10.1111/j.1442-200X.2011.03510.x [DOI] [PubMed] [Google Scholar]
- 35. Dohner ML, Wiedmeier SE, Stoddard RA, et al. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion 2009;49:869–72. 10.1111/j.1537-2995.2008.02074.x [DOI] [PubMed] [Google Scholar]
- 36. Mondal A, Misra D, Al-Jabir A, et al. Necrotizing enterocolitis in neonates: has the brain taken a hit 10 years later? J Pediatr Neurosci 2021;16:30–4. 10.4103/jpn.JPN_41_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Humberg A, Spiegler J, Fortmann MI, et al. Surgical necrotizing enterocolitis but not spontaneous intestinal perforation is associated with adverse neurological outcome at school age. Sci Rep 2020;10:2373. 10.1038/s41598-020-58761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allendorf A, Dewitz R, Weber J, et al. Necrotizing enterocolitis as a prognostic factor for the neurodevelopmental outcome of preterm infants-match control study after 2years. J Pediatr Surg 2018;53:1573–7. 10.1016/j.jpedsurg.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 39. Adesanya OA, O’Shea TM, Turner CS, et al. Intestinal perforation in very low birth weight infants: growth and neurodevelopment at 1 year of age. J Perinatol 2005;25:583–9. 10.1038/sj.jp.7211360 [DOI] [PubMed] [Google Scholar]
- 40. Yeh TC, Chang JH, Kao HA, et al. Necrotizing enterocolitis in infants: clinical outcome and influence on growth and neurodevelopment. J Formos Med Assoc 2004;103:761–6. [PubMed] [Google Scholar]
- 41. Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006;354:2112–21. 10.1056/NEJMoa054065 [DOI] [PubMed] [Google Scholar]
- 42. Kirpalani H, Bell EF, Hintz SR, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med 2020;383:2639–51. 10.1056/NEJMoa2020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Franz AR, Engel C, Bassler D, et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA 2020;324:560–70. 10.1001/jama.2020.10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marlow N, Abbott J, Field D, et al. Report of a BAPM/RCPCH working group: classification of health status at 2 years as a perinatal outcome. London, UK: Br Assoc Perinatol Med; 2008. 23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fetalneonatal-2022-324915supp001.pdf (163.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data will be available upon reasonable request, with approval of the trial group.