Abstract
Background
Polyphenolic antioxidants derived from plant foods may reduce oxidative stress and frailty, but the effect of the polyphenol subclass of dietary flavonoids and their subclasses on frailty is uncertain.
Objectives
To determine the association between dietary flavonoids, their subclasses, quercetin (a specific flavonol), and frailty onset in adults.
Methods
This prospective cohort study included individuals from the Framingham Heart Study with no frailty at baseline. Intake of total flavonoids, subclasses of flavonoids (flavonols, flavan-3-ols, flavonones, flavones, anthocyanins, and polymeric flavonoids), and quercetin were estimated via semi-quantitative FFQ along with frailty (Fried phenotype), and covariates at baseline (1998–2001). Frailty was re-evaluated in 2011–2014. Logistic regression estimated OR and 95% CIs for each flavonoid variable and frailty onset.
Results
Mean age was 58.4 y (SD ± 8.3, n = 1701; 55.5% women). The mean total flavonoid intake was 309 mg/d (SD ± 266). After 12.4 (SD ± 0.8) y, 224 (13.2%) individuals developed frailty. Although total flavonoid intake was not statistically associated with frailty onset (adjusted OR: 1.00; 95% CI: 0.99–1.01), each 10 mg/d of higher flavonol intake was linked with 20% lower odds of frailty onset (OR: 0.80; 95% CI: 0.67–0.96). Other subclasses showed no association (P values range: 0.12–0.99), but every 10 mg/d of higher quercetin intake was associated with 35% lower odds of frailty onset (OR: 0.65; 95% CI: 0.48–0.88).
Conclusions
Although no association was observed between total flavonoid intake and frailty onset in adults, a higher intake of flavonols was associated with lower odds of frailty onset, with a particularly strong association for quercetin. This hypothesis-generating study highlights the importance of assessing specific subclasses of flavonoids and the potential of dietary flavonols and quercetin as a strategy to prevent the development of frailty.
Keywords: frailty, flavonoids, quercetin, flavonols, older adults
Introduction
Frailty is a geriatric syndrome that afflicts 10–15% of older adults and is caused by multiple age-related physiological changes [1]. Individuals with frailty have a greater risk of falls, fractures, disability, hospitalization, and mortality [2], which results in an enormous personal and economic burden. As life expectancy increases, more individuals are predisposed to developing age-related conditions such as frailty and the associated health consequences, augmenting the societal and economic burden [3]. However, effective treatments for frailty are lacking, underscoring the importance of research in frailty prevention and treatment. Understanding risk factors that lead to frailty is necessary to develop interventions that delay, reverse, or prevent frailty.
Currently, in addition to physical exercise [4,5], poor nutrition (e.g., low protein intake) is considered to be an important risk factor for the development of frailty [6,7]. A recent meta-analysis of 13 studies reported that an overall healthy dietary pattern might decrease the odds of frailty onset by 50–70% [[10], [8], [9]]. Similarly, both Mediterranean-style and anti-inflammatory diets have been associated with frailty prevention over time [[11], [12], [13]]. Both dietary patterns encourage the intake of fruits and vegetables, which are rich in flavonoids, plant-derived compounds that are known for their anti-inflammatory and antioxidant properties [14]. In a cross-sectional study, higher urinary total polyphenols (a surrogate marker for polyphenols, including flavonoid intake and bioavailability) were associated with a 36% and 64% lower prevalence of prefrailty and frailty, respectively [15]. Supplementation with blueberries, a rich source of flavonoids, showed improvement in gait speed (1 of the components of the Fried frailty phenotype) in adults older than 60 y [16]. Given that flavonoids can mitigate the age-related accumulation of oxidative stress by reactive oxygen species, and some flavonoids even target the elimination of age-related senescent cells, they may have a role in reducing inflammation and consequent frailty development [14,17]. Together, the previous research suggests that flavonoid intake may have a role in frailty prevention.
Flavonoids contain several subclasses, including flavonols, flavan-3-ols, flavonones, flavones, anthocyanins, and polymeric flavonoids. Quercetin, a flavonol, increased lifespan and oxidative stress resistance in diverse organism models [18,19]. A recent review article suggested a therapeutic potential for quercetin to treat age-related diseases such as degenerative joint disorders, CVD, metabolic diseases, and neurodegenerative diseases through the clearance of senescent cell accumulation [20]. However, it is unclear if all subclasses of flavonoids have a role in frailty prevention or whether these associations are driven by specific subclasses (e.g., quercetin). Therefore, this study aims to determine the association of intake of total flavonoids, the individual flavonoid subclasses, and quercetin with frailty onset in middle-aged and older adults from the Framingham Heart Study (FHS). We hypothesize that higher dietary flavonoid intake, particularly quercetin, is associated with lower odds of frailty onset.
Methods
Subjects and study population
The FHS is a population-based cohort that began in 1948 by enrolling 5209 individuals to investigate CVD and familial risk factors [21]. In addition, from 1971–1975, 5124 offspring of the original participants in the FHS were enrolled to investigate the roles of family history and genetics in the development of CVD [22]. Detailed in-person examinations have been conducted every 4 y in each FHS cohort [23]. The current study is a prospective cohort study of middle-aged and older adults, including individuals without frailty from the FHS Offspring study, assessed for diet, frailty, and relevant covariates at baseline (1998–2001) and had a follow-up frailty assessment between 2011 and 2014. This study was approved by the institutional review board at Hebrew SeniorLife, Advarra (IRB protocol number Pro00046414). All participants gave their informed consent.
Flavonoids intake
Dietary intake was assessed at this study’s baseline examination (1998–2001) using a validated semi-quantitative, 126-item Willett FFQ [[24], [25], [26]]. The participants completed the self-administered FFQ based on their food intake over the previous year. Questionnaires were considered invalid if >12 food items were left blank or energy intake was <600 or >4000 kcal (women) and <600 or >4200 (men). Total flavonoid intake (mg/d) was calculated as the sum of intake of flavonoid subclasses (flavonols, flavones, flavan-3-ols, flavanones, anthocyanins, and polymeric flavonoids in mg/d, as defined by Cassidy et al. [27]) from the FFQ [24]. The FFQ was also used to estimate the intake of specific types of flavonoid subclasses (e.g., flavonols and quercetin, mg/d). Validation of this FFQ has been described in multiple studies [[24], [25], [26]]. Given that the total mean flavonoid intake in the United States has not changed significantly between 1999 and 2010 [28], and no significant differences have been found in the estimation of total flavonoid intake after several years of follow-up [28,29], baseline flavonoid intake was assumed to reflect mean intake over a 12 y follow-up.
Frailty assessment
Frailty was defined using a modification of Fried’s frailty phenotype [2], which characterizes frailty as a geriatric syndrome with the presence of 3 or more of the following outcomes: 1) Self-reported unintentional weight loss of >4.5 kg in the last year; 2) Self-reported exhaustion when answering “occasionally a moderate amount of time” or “most of the time” to 1 or more of either of the following Center for Epidemiologic Studies Depression Scale (CES-D) questions of “I could not get going” or “I felt that everything I did was an effort”; 3) Weak grip strength (kg); 4) Slow walking speed (m/s); 5) Low physical activity, defined as the lowest sex-specific quintile of the physical activity index (PAI). As previously described [11,12], the following amendments to the low physical activity and unintentional weight loss criteria were made to accommodate longitudinal frailty assessments. The maximum PAI in the lowest quintile at baseline was used to classify low physical activity. The same threshold was also used for the follow-up examination. If a participant had PAI below the threshold, they were considered as having low physical activity [11,12]. It may be unreasonable to expect a participant to lose 4.5 kg in the past year across several years of follow-up. Therefore, the unintentional weight loss definition was modified to fulfilling 1 of the following criteria [11,12]: 1) self-reported unintentional weight loss > 4.5 kg in the last year; 2) BMI (in kg/m2) lower than 18.5 kg/m2; 3) annual weight loss of > 4.5 kg between 2 measurements 4–8 y apart. Weight was measured with a standardized balance-beam scale wearing light clothing. Height was measured to the nearest quarter inch without shoes with a stadiometer.
Missing Fried frailty criteria
Missing information on Fried frailty criteria was handled as previously described [11,12]. When participants were missing the majority of the frailty criteria (e.g., missing ≥3), they were excluded. If participants did not meet the frailty status with ≥3 of the criteria, they were classified as nonfrail. However, when the participants were missing 2 out of the 5 criteria, and ≥1 of the criteria was in a different category from the other 2, they were excluded because, in theory, they could be either frail or nonfrail. Participants that were coded with missing gait speed or grip strength because of physical limitations were assumed to reach frailty status for these categories as such participants may be too frail to complete these measurements.
Measurement of covariates
Covariates included in the analysis were sex, age (y), energy intake (kcal/d), smoking (yes/no), depressive symptoms severity CES-D, diabetes (type 1 or type 2, yes/no), CVD (yes/no), and any form of nonskin cancer (except melanoma, yes/no). Energy intake was derived from the FFQ. Smoking was defined as current smoking (yes/no) and classified as having smoked cigarettes within the past 2 y or not at the baseline examination. CES-D scale (range 0–60 points with higher scores indicating more severe symptoms) was used to evaluate depressive symptom severity, and the continuous score was used as a covariate. Diabetes was defined as persons with a history of diabetes mellitus (type 1 or type 2), who used oral hypoglycemic medications or insulin, or who had a baseline fasting plasma glucose concentration >126 mg/dL (>7.0 mmol/L) or a baseline postoral glucose tolerance test plasma glucose concentration >200 mg/dL (>11.1 mmol/L). Prevalent CVD was defined as CHD (coronary death, MI, coronary insufficiency, and angina), cerebrovascular events (including ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral artery disease (intermittent claudication) and heart failure. Cancer was defined as a prevalence of nonskin cancer (except melanoma) at baseline examination.
Statistical analysis
Means and SD were calculated for continuous variables, whereas percentages were calculated for categorical variables. We further evaluated the means and SD of all variables among those who were either lost to follow-up or were not included because of missing information after follow-up. The study exposures were estimated intakes of total flavonoids (defined as the sum of intake of flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins, and polymeric flavonoids in mg/d), specific flavonoid subclasses (flavonols, flavones, flavan-3-ols, flavanones, anthocyanins, and polymeric flavonoids in mg/d), and the specific flavonols, quercetin (mg/d) [30]. Flavonoid variables were correlated with each other using Pearson’s (r). Each of these exposure variables was analyzed in separate models. For total flavonoid intake, the assumption of linearity for logistic models was checked using the Box-Tidwell test before implementing the models.
Logistic regression was used for total flavonoids, flavonoids subclass, and quercetin to estimate OR and 95% CI per unit higher intake of total flavonoids (1 unit = 50 mg/d) or flavonoids subclasses 1 unit = 10 mg/d, except for flavones for which 1 unit = 1 mg/d because of lower mean daily intake. These increments for 1 unit of flavonoid exposure variables were chosen based on the mean intake of these flavonoid variables in our study population, which is relatively consistent with estimates from middle-aged and older Americans [28]. Model 1 adjusted for age and sex, and model 2 further adjusted for energy intake, current smoking, depressive symptoms, and disease indicators (i.e., cancer, CVD, and diabetes). The prevalence of frailty is known to be higher in older adults, particularly in women [31,32]. Given this and the wide age range of the study population, we determined whether the association of flavonoid exposures with frailty onset was modified by age or sex. Interactions were tested between each of the flavonoid exposure variables with age and sex (separately) using model 2. If the interaction term was significant (P < 0.05), then stratification by age groups (≥60 y compared to <60 y) or sex was performed. Given that we considered the study to be hypothesis-generating, no adjustments for multiple comparisons were made. All analyses were conducted using SAS software version 9.3 (SAS Institute Inc.).
Results
Study population
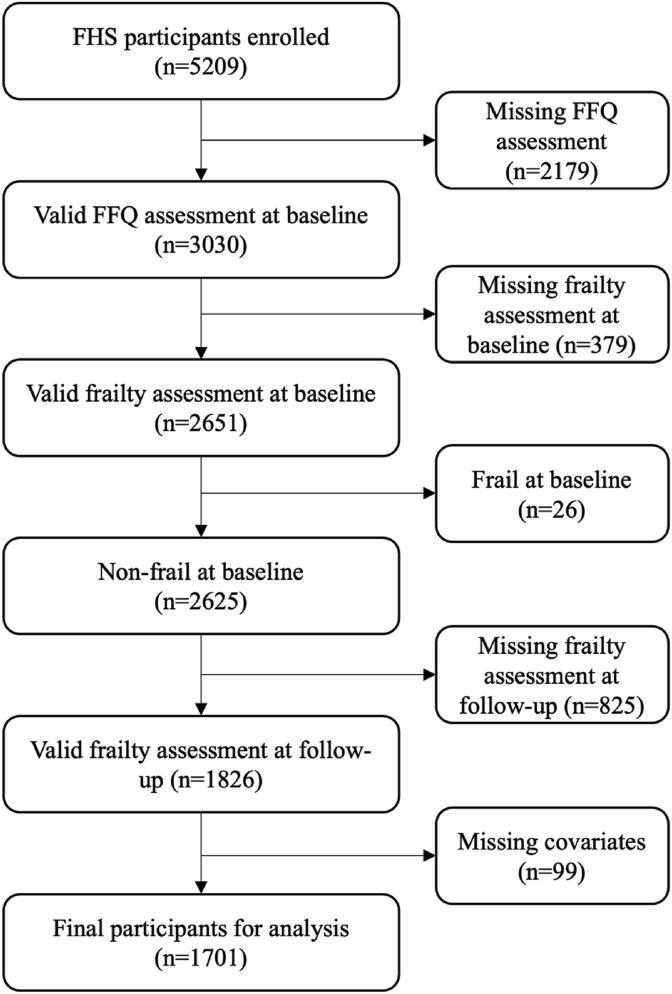
Of the 5124 participants enrolled in the FHS, 3030 completed the dietary assessment at baseline. Of these, 1826 had frailty assessments at baseline and follow-up examinations. Participants categorized as frail at baseline were excluded (n = 26), along with 99 participants missing covariates at baseline (88 missing data on depressive symptoms and 11 missing diabetes information). The final sample for analysis included 1701 individuals (Figure 1), of which 55.5% were women, with a mean age of 58.4 y (SD = 8.3, Table 1). The mean total flavonoid intake was 309 mg/d (SD = 266). Compared to participants included in this study (Table 1), those who were lost to follow-up or were not included in the analysis because of missing information at the baseline or follow-up (n = 783) tended to be older [mean age: 66.0 y (SD = 9.2)], males (49%) with higher prevalence of diabetes (18%) and CVD (19%).
Figure 1.
Inclusion and exclusion criteria for Framingham Heart Study - Offpsring Cohort participants included in this analysis. FFQ, Food Frequency Questionnaire; FHS, Framingham Heart Study.
TABLE 1.
Baseline characteristics of the men and women from the Framingham Heart Study - Offspring Cohort
| Characteristics | Study participants (n = 1701) | Participants with incomplete data at follow-up or lost to follow-up (n = 783) |
|---|---|---|
| Age, y (range: 33–85) | 58.4 ± 8.3 | 66.0 ± 9.2 |
| Sex, n (% male) | 758 (44.5) | 384 (49.0) |
| BMI, mg/kg2 | 27.9 ± 5.1 | 28.5 ± 5.5 |
| Total flavonoid intake, mg/d | 309.4 ± 265.9 | 301.7 ± 264.2 |
| Energy intake, kcal/d | 1859 ± 600 | 1792 ± 593 |
| Physical activity index (range 24–120) | 38.2 ± 5.9 | 39.4 ± 6.8 |
| CES-D score (range 0–60) | 4.6 ± 5.6 | 4.7 ± 5.8 |
| Current smokers, n (%) | 170 (10) | 118 (5.1) |
| CVD, n (%) | 137 (8.1) | 150 (19.2) |
| Cancer, n (%) | 42 (2.5) | 33 (4.2) |
| Diabetes n (%) | 110 (6.5) | 137 (17.9) |
BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; CVD, cardiovascular disease; SD, standard deviation.
Characteristics are presented as mean value ± SD or n (%).
Flavonoid intake and frailty
The mean intake of flavonols was 13.6 mg/d (SD = 9.1), and that of quercetin was 9 mg/d (SD = 5, Table 2). Over an average of 12 y (SD = 1) of follow-up, 224 (13.2%) individuals exhibited frailty. In age and sex-adjusted model 1, every 50 mg/d of higher total flavonoid intake was associated with 3% reduced odds of frailty onset (OR: 0.97; 95% CI: 0.94–1.00, P = 0.05, Table 2). Further adjustment for energy intake, current smoking, CES-D, cancer, CVD, and diabetes in model 2 did not meaningfully change the magnitude of the association, but the association became nonsignificant (OR: 0.98; 95% CI: 0.95–1.01, P = 0.12). We observed no significant interactions between total flavonoids and age (P = 0.11) or sex (P = 0.09) in model 2. The correlation between the flavonoid subclasses ranged from 0.23–0.95 (P range: <0.001 – 0.97, Supplementary Table 1).
TABLE 2.
Mean ± SD intake of total flavonoids, flavonoid subclasses, and quercetin and their association with odds of frailty onset in men and women
| Exposure variables (mg/d) | Mean ± SD |
Model 1 |
P value | Model 2 |
P value |
|---|---|---|---|---|---|
| (mg/d) | OR (95% CI)1 | OR (95% CI)1 | |||
| Total flavonoids | 309.4 ± 265.9 | 0.99 (0.98–1.00) | 0.05 | 1.00 (0.99–1.01) | 0.12 |
| Flavonols | 13.6 ± 9.1 | 0.80 (0.67–0.96) | 0.02 | 0.81 (0.67–0.97) | 0.03 |
| Quercetin | 9.0 ± 5.8 | 0.65 (0.48–0.88) | 0.01 | 0.66 (0.48–0.89) | 0.01 |
| Flavones | 2.2 ± 1.8 | 0.91 (0.82–1.01) | 0.07 | 0.94 (0.85–1.04) | 0.25 |
| Flavanones | 45.1 ± 41.6 | 0.99 (0.95–1.03) | 0.56 | 1.00 (0.96–1.04) | 0.99 |
| Flavan-3-ols | 44.3 ± 54.7 | 0.98 (0.95–1.01) | 0.13 | 0.98 (0.96–1.01) | 0.27 |
| Anthocyanins | 16.6 ± 21.3 | 0.95 (0.87–1.03) | 0.19 | 0.95 (0.87–1.03) | 0.21 |
| Polymeric flavonoids | 187.7 ± 191.1 | 0.99 (0.98–1.00) | 0.06 | 0.99 (0.98–1.00) | 0.12 |
Model 1 adjusted for age and sex. Model 2 additionally adjusts for energy intake, current smoking, depressive symptoms severity, cardiovascular disease, type 1 or 2 diabetes, and nonskin cancer.
CI, confidence interval; OR, odds ratio; SD, standard deviation.
OR (95% CI) presented per 50 mg (total flavonoids), per 10 mg (flavonols, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids and quercetin) and per 1 mg for flavones. P < 0.05 was considered significant.
Subclasses of flavonoids
In the analysis of individual subclasses of flavonoids, each 10 mg/d higher flavonols intake was associated with 20% lower odds of becoming frail (OR: 0.80; 95% CI: 0.67–0.96, P = 0.02, Table 2). These associations did not change meaningfully after adjustment for other covariates in model 2. No significant associations were observed for flavones, flavanones, flavan-3-ols, anthocyanin, or polymeric flavonoids in model 2 (P values range: 0.12–0.99).
Quercetin intake and frailty
Every 10 mg/d higher quercetin intake was associated with 35% lower odds of frailty onset (OR: 0.65; 95% CI: 0.48–0.88, P = 0.01, Table 2) over 12 y. These associations did not change meaningfully after further adjustment in model 2. No interactions between quercetin intake and age and sex were observed in model 2 (P value range: 0.11–0.46).
Interaction by age and sex
A significant interaction was observed between age and anthocyanins in model 2 (P < 0.001). Characteristics of participants by age groups (≥60 y compared to <60 y) is described in Table 3. Among individuals younger than 60 y (n = 985), each 10 mg/d higher intake of anthocyanins was associated with 52% lower odds of frailty onset (OR: 0.48; 95% CI: 0.32–0.71, P < 0.01, model 2, Table 4). For participants aged 60 y and older (n = 716), no significant association between anthocyanin intake and frailty onset existed (OR: 1.02; 95% CI: 0.94–1.12, P = 0.61, model 2, Table 4). No significant interactions between other subclasses of flavonoids and either age or sex were observed in model 2 (P values range: 0.07–0.90).
TABLE 3.
Characteristics of men and women from the Framingham Heart Study - Offspring Cohort stratified by age-stratified groups (age <60 compared with ≥60 y)
| Variables | Age <60 y |
Age ≥60 y |
|---|---|---|
| (n = 985) | (n = 716) | |
| Anthocyanin intake (mg/d) | 16.4 ± 21.4 | 16.9 ± 21.2 |
| Age (y, range: 33–85) | 52.6 ± 4.8 | 66.3 ± 4.8 |
| Energy intake (kcal/d) | 1887 ± 637 | 1821 ± 545 |
| Grip strength (kg) | 36.5 ± 13.2 | 32.0 ± 12.0 |
| Gait speed (m/s) | 1.27 ± 0.25 | 1.16 ± 0.24 |
| BMI (kg/m2) | 27.8 ± 5.5 | 27.9 ± 4.6 |
| Physical activity index, range 24–120 | 37.5 ± 5.7 | 39.0 ± 6.0 |
| CES-D score, range 0–60 | 5.1 ± 5.8 | 3.8 ± 5.3 |
Characteristics are presented as mean value ± SD or n (%).
BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; SD, standard deviation.
TABLE 4.
Associations between dietary anthocyanins (per 10 mg/d higher intake) and odds of frailty onset stratified by age (<60 y compared with >60 y)
| n | Model 2 OR (95% CI) | P value | |
|---|---|---|---|
| All participants | 1701 | 0.95 (0.87–1.03) | 0.21 |
| <60 y | 985 | 0.48 (0.32–0.71) | 0.0002 |
| ≥60 y | 716 | 1.02 (0.94–1.12) | 0.61 |
Adjusted for age and sex, energy intake, current smoking, depressive symptoms severity, cardiovascular disease, diabetes 1 or 2, and nonskin cancer. P<0.05 was considered significant
CI, confidence interval; OR, odds ratio.
Discussion
In this prospective cohort study of middle-aged and older adults, total flavonoid intake was not significantly associated with odds of frailty onset over 12 y, but each 10 mg/d higher intake of flavonols was significantly associated with 20% lower odds of frailty onset after adjustment for potential confounders. Additionally, each 10 mg/d higher quercetin intake (a specific flavonol) was significantly associated with a 35% lower odds of frailty onset. Age significantly modified the association of anthocyanin intake with frailty onset such that each 10 mg/d higher anthocyanin intake was associated with 52% lower odds of frailty onset among participants aged <60 y, although no significant association was observed in those aged 60 y and above.
Previous studies have focused on age-related declines in mobility in association with the Mediterranean diet (rich in dietary flavonoids) [8], flavonoid supplementation [33], or total dietary polyphenols in humans [15,34,35] or murine models [36,37]. Lifestyle modifications such as diet-based interventions may protect against frailty [4,5], but information about dietary flavonoids is limited. Although research on flavonoids and frailty remains limited, several studies have evaluated the utility of flavonoids on specific aspects of frailty. A 2-phase randomized controlled trial assigned 60 men and women (aged 55–70 y) to a daily intake of flavonoid-rich (179 mg/d) cocoa beverage, non-flavonoid cocoa beverage, or placebo for 12 wks. Participants randomly assigned to the flavonoid-rich group demonstrated improvement in their skeletal muscle index, walking speed, and balance test compared to the other 2 groups [33]. A follow-up randomized controlled trial in 74 older adults (aged 65–90 y) assigned participants to a daily intake of flavonoid-rich or non-flavonoid cocoa beverages for 8 wks. This study showed that a flavonoid-rich beverage significantly improved gait speed and grip speed and reduced prefrailty by 29% compared to the non-flavonoid cocoa beverage [33]. We did not observe a similarly strong association between high flavonoid intake and odds of frailty onset in the current study. However, the current study extends the knowledge base by providing information on subclasses of flavonoids such that higher flavonols intake was significantly associated with reduced frailty onset. Higher urinary isoflavone metabolite concentrations were associated with a 24% frailty reduction in 600 women (median age 66.5 y) in a cross-sectional study [34]. Similar results were reported by 2 other prospective cohorts in 807 and 811 participants, respectively, mean age of 74.3 ± 6.9 y for both. They reported no association between total dietary polyphenols and frailty onset; however, they reported a significantly reduced association with higher urinary total polyphenol concentrations [15,35]. This suggests that more research is needed to isolate the role of flavonoid subclasses and their role in frailty prevention and treatment. The benefits of polyphenols are dependent on the quantity consumed and their bioavailability, which varies between the different subclasses [38].
Furthermore, we showed that quercetin is associated with a lower risk of frailty when consumed in the diet. Mechanistically, quercetin in combination with the tyrosine kinase inhibitor, Dasatinib, has been shown in mice [39] and humans to reduce the accumulation of senescent cells that no longer divide, but develop a secretory phenotype, generating increased inflammatory cytokines, extracellular matrix-modifying proteases, and reactive oxygen, which impair neighboring cell function and alter tissue structure [40,41]. Since senescence is an important predictor of sarcopenia and frailty [41], this mechanism might be pivotal in developing frailty, but data on this topic is lacking. Supplementation with 500 mg/d of quercetin in combination with Dasatinib over 3 d showed a decrease in senescent cells in humans [42]. Supplementation with 250 mg/kg body weight of quercetin in cancer-induced cachectic mice led to limited body weight loss and muscle wasting [43]. For reference, 10 mg of quercetin can be obtained from 1 apple or 100 g of kale [44]. Other quercetin-rich foods include blueberries, brassica vegetables, onions, and tea [44]. Subclasses of flavonoids are present in many different foods (Table 5) [45,46]. It should be noted that in animal studies, quercetin dosages were considerably higher and should therefore be carefully compared to analyses with human dietary intake [43]. Interestingly, the mean intake of quercetin in our study (mean = 9.0, SD = 5.8 mg/d) is notably lower than the current mean intake in the United States (14–22 mg/d) [29,44]. Comparatively, previous intervention studies have used therapeutic doses that were much higher to study the acute health benefits of quercetin. Chronic low doses may also be physiologically relevant as compared to high acute doses. It is also possible that these findings could be influenced by other factors that coincide with quercetin intakes, such as other dietary antioxidants and overall healthier dietary patterns. Therefore, additional studies are needed to confirm these findings. Furthermore, because of increased intestinal permeability and altered gut microbiome, there is likely an impact on the bioavailability of flavonoids and their metabolites with advancing age [47,48]; therefore, additional studies are needed to address these age-related changes in the absorption and metabolism of flavonoids and determine the physiological relevance of chronic compared with acute intake.
TABLE 5.
Examples of the 6 subclasses of flavonoids and typical foods containing these flavonoids1
| Flavonoid subclass | Typical food sources |
|---|---|
| Flavonol | Green brewed tea and wine |
| Flavones | Green leafy spices, e.g., parsley |
| Flavan-3-ols | Apple, tea, and dark chocolate |
| Flavanones | Citrus foods |
| Anthocyanins | Red, purple, and blueberries |
| Polymeric flavonoids | Black tea, coffee, and wine |
Frailty is an age-related condition, with older individuals at greater risk of frailty development. The current study showed that the association between anthocyanin intake and odds of frailty onset was stronger among individuals below the age of 60 y compared to relatively older individuals. As expected, the participants younger than 60 y tended to have a lower prevalence of the disease indicators, but they also appeared to have worse lifestyle habits, such as higher current smoking and less physical activity. Similar age-related effect modification was previously seen in this cohort with a pro-inflammatory diet and frailty [12]. Therefore, it is possible that the anti-oxidative compounds may be more beneficial for those with more lifestyle-induced oxidative stress [49].
This is one of the first studies to comprehensively investigate the association between dietary flavonoids, subclasses of flavonoids, and quercetin with frailty onset over a long-term follow-up of ∼12 y in both men and women. Frailty assessments were conducted longitudinally over a long-term follow-up. Dietary data was sourced from the FFQ, which had been previously validated in this cohort and other studies [11,12,[24], [25], [26]]. Limitations of the study include using an FFQ, which may be affected by misclassification and recall bias. In total, 32% (n = 783) of the individuals were lost to follow-up. Those lost to follow-up tended to be older with a higher prevalence of certain diseases; therefore, healthy survivor bias might have occurred such that the findings of this study are perhaps generalizable to relatively healthier older adults. Despite adjusting for many potential confounders, residual confounding may occur. Additionally, since our study included mostly participants of self-reported European ancestry (∼99.4%), the results may not be generalizable to other races. In addition, foods with higher flavonoid content (fruits and vegetables) tend to have higher dietary fiber and antioxidants [50], known nutritional factors that have been previously associated with frailty [11,17]. Therefore, overall diet quality may be a potentially confounding variable. Given that diet quality is likely to be highly correlated with the exposure of interest, adjustment for this variable poses additional challenges. Furthermore, we did not account for changes in diet over time. However, previous studies show that older adults tend to have stable diets over time [29]. The total mean flavonoid intake in the United States has not changed significantly between 1999 and 2010 [28] and, therefore, is unlikely to impact the study results meaningfully. The age range of the participants was wide and included individuals aged <60 y of age at baseline; however, this issue was addressed by testing for age interactions and stratification by age for select nutrition exposures. Finally, especially of importance for quercetin, no senescent biomarkers were included in these exploratory analyses. Current results should be interpreted as hypothesis generation for future research in this area.
In conculusion, this prospective cohort study found that flavonoid intake was not significantly associated with the odds of frailty onset in middle-aged and older adults, however, higher intake of the flavonoid subclass, flavonols, was associated with reduced odds of frailty onset, which appeared to be driven by the specific flavonol, quercetin. The protective association between the flavonoid subclass anthocyanins and frailty onset was primarily seen in participants below the age of 60 y. Although hypothesis-generating, this study highlights the potential of dietary flavonols and quercetin as a strategy to prevent frailty onset. Future research should focus on dietary interventions of flavonols or quercetin for treating frailty.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.04.013.
Author contributions
The authors’ responsibilities were as follows– CLM, MTH, and SS: designed the research; SS: had primary responsibility for the final content; DPK, MTH, and SS: provided access to the data; CLM: analyzed the data with critical input from all coauthors; SO, CLM, and TNN: wrote the manuscript with SO and CLM serving as co-first authors; and all authors provided edits to the manuscript and read and approved the final manuscripts.
Conflict of interest
DPK has received grant funding from Amgen and Solarea Bio. He serves on the scientific advisory board of Solarea Bio Inc., Reneo, and Pfizer, and a Data Monitoring Committee for Agnovos, and receives royalties for publication by Wolters Kluwer. LAL is the Editor-in-Chief of the Journal of Gerontology Medical Sciences. MTH has received funding to her institution from Amgen, Inc. for work unrelated to this project. KJM and SS have received grant funding from the United States Highbush Blueberry Council for research unrelated to this project. SS further reports institutional grants from Dairy Management Inc. (ended September 2022) and Solarea Bio Inc. (ended March 2022), has reviewed grants for the American Egg Board’s Egg Nutrition Center and National Dairy Council. All other authors report no conflicts of interest.
Funding
This study was supported by the National Institute on Aging (NIA) grant number R01 AG051728, the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract numbers HHSN268201500001I and N01-HC 25195). CLM was supported by the NIA and the National Institute of Health (NIH) T32-AG023480 and the Beth and Richard Applebaum Research Fund. TNN was supported by the NIA through the MSTAR (Medical Student Training in Aging Research) Program. DPK was funded by R01 AR041398 and R01 AR061445. LAL was supported by R21 AG073886 from the NIA, and he holds the Irving and Edyth S. Usen Chair in geriatric medicine at Hebrew SeniorLife. KJM was supported by K24 AG065525.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application to and approval by the Framingham Heart Study.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the official views of the National Institutes of Health.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yannakoulia M., Ntanasi E., Anastasiou C.A., Scarmeas N. Frailty and nutrition: from epidemiological and clinical evidence to potential mechanisms. Metabolism. 2017;68:64–76. doi: 10.1016/j.metabol.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Kojima G., Liljas A.E.M., Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag. Healthc. Policy. 2019;12:23–30. doi: 10.2147/RMHP.S168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giné-Garriga M., Roqué-Fíguls M., Coll-Planas L., Sitjà-Rabert M., Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014;95(4):753–769.e3. doi: 10.1016/j.apmr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Tarazona-Santabalbina F.J., Gómez-Cabrera M.C., Pérez-Ros P., Martinez-Arnau F.M., Cabo H., Tsaparas K., et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J. Am. Med. Dir. Assoc. 2016;17(5):426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Coelho-Júnior H.J., Rodrigues B., Uchida M., Marzetti E. Low protein intake is associated with frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10(9) doi: 10.3390/nu10091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coelho-Junior H.J., Marzetti E., Picca A., Cesari M., Uchida M.C., Calvani R. Protein intake and frailty: a matter of quantity, quality, and timing. Nutrients. 2020;12(10) doi: 10.3390/nu12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capurso C., Bellanti F., Lo Buglio A., Vendemiale G. The Mediterranean diet slows down the progression of aging and helps to prevent the onset of frailty: a narrative review. Nutrients. 2019;12(1) doi: 10.3390/nu12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashidi Pour Fard N., Amirabdollahian F., Haghighatdoost F. Dietary patterns and frailty: a systematic review and meta-analysis. Nutr. Rev. 2019;77(7):498–513. doi: 10.1093/nutrit/nuz007. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Hao Q., Su L., Liu Y., Liu S., Dong B. Adherence to the Mediterranean diet and the risk of frailty in old people: a systematic review and meta-analysis. J. Nutr. Health Aging. 2018;22(5):613–618. doi: 10.1007/s12603-018-1020-x. [DOI] [PubMed] [Google Scholar]
- 11.Millar C.L., Costa E., Jacques P.F., Dufour A.B., Kiel D.P., Hannan M.T., et al. Adherence to the Mediterranean-style diet and high intake of total carotenoids reduces the odds of frailty over 11 years in older adults: results from the Framingham Offspring Study. Am. J. Clin. Nutr. 2022;116(3):630–639. doi: 10.1093/ajcn/nqac130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar C.L., Dufour A.B., Shivappa N., Habtemariam D., Murabito J.M., Benjamin E.J., et al. A proinflammatory diet is associated with increased odds of frailty after 12-year follow-up in a cohort of adults. Am. J. Clin. Nutr. 2022;115(2):334–343. doi: 10.1093/ajcn/nqab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Khayri J.M., Sahana G.R., Nagella P., Joseph B.V., Alessa F.M., Al-Mssallem M.Q. Flavonoids as potential anti-inflammatory molecules: a review. Molecules. 2022;27(9) doi: 10.3390/molecules27092901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urpi-Sarda M., Andres-Lacueva C., Rabassa M., Ruggiero C., Zamora-Ros R., Bandinelli S., et al. The relationship between urinary total polyphenols and the frailty phenotype in a community-dwelling older population: the InCHIANTI Study. J Gerontol. A. Biol. Sci. Med. Sci. 2015;70(9):1141–1147. doi: 10.1093/gerona/glv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrager M.A., Hilton J., Gould R., Kelly V.E. Effects of blueberry supplementation on measures of functional mobility in older adults. Appl. Physiol. Nutr. Metab. 2015;40(6):543–549. doi: 10.1139/apnm-2014-0247. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Satta M., Berna-Erro A., Carrasco-Garcia E., Alberro A., Saenz-Antonanzas A., Vergara I., et al. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging (Albany NY) 2020;12(10):9982–9999. doi: 10.18632/aging.103295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proshkina E., Lashmanova E., Dobrovolskaya E., Zemskaya N., Kudryavtseva A., Shaposhnikov M., et al. Geroprotective and radioprotective activity of quercetin, (–)–epicatechin, and ibuprofen in Drosophila melanogaster. Front Pharmacol. 2016;7:505. doi: 10.3389/fphar.2016.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkel C., Cacan E. A collective analysis of lifespan-extending compounds in diverse model organisms, and of species whose lifespan can be extended the most by the application of compounds. Biogerontology. 2021;22(6):639–653. doi: 10.1007/s10522-021-09941-y. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Hamidu S., Yang X., Yan Y., Wang Q., Li L., et al. Dietary supplements and natural products: an update on their clinical effectiveness and molecular mechanisms of action during accelerated biological aging. Front Genet. 2022;13 doi: 10.3389/fgene.2022.880421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannel W.B., Feinleib M., McNamara P.M., Garrison R.J., Castelli W.P. An investigation of coronary heart disease in families. The Framingham offspring study. Am. J. Epidemiol. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 22.Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao C.W., Vasan R.S. Cohort Profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol. 2015;44(6):1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shishtar E., Rogers G.T., Blumberg J.B., Au R., DeCarli C., Jacques P.F. Flavonoid intake and MRI markers of brain health in the Framingham offspring cohort. J. Nutr. 2020;150(6):1545–1553. doi: 10.1093/jn/nxaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feskanich D., Rimm E.B., Giovannucci E.L., Colditz G.A., Stampfer M.J., Litin L.B., et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J. Am. Diet. Assoc. 1992;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 26.Willett W.C., Reynolds R.D., Cottrell-Hoehner S., Sampson L., Browne M.L. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J. Am. Diet. Assoc. 1987;87(1):43–47. doi: 10.1016/S0002-8223(21)03057-1. [DOI] [PubMed] [Google Scholar]
- 27.Cassidy A., O’Reilly É.J., Kay C., Sampson L., Franz M., Forman J.P., et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011;93(2):338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K., Vance T.M., Chun O.K. Estimated intake and major food sources of flavonoids among US adults: changes between 1999-2002 and 2007-2010 in NHANES. Eur. J. Nutr. 2016;55(2):833–843. doi: 10.1007/s00394-015-0942-x. [DOI] [PubMed] [Google Scholar]
- 29.Escobar-Cévoli R., Castro-Espin C., Béraud V., Buckland G., Zamora-Ros R. 2017. An Overview of Global Flavonoid Intake and its Food Sources. Edtion ed. Flavonoids - From Biosynthesis to Human Health. Rijeka: IntechOpen. [DOI] [Google Scholar]
- 30.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997;65(4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. suppl. discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Zepeda M.U., Ávila-Funes J.A., Gutiérrez-Robledo L.M., García-Peña C. Frailty across age groups. J. Frailty. Aging. 2016;5(1):15–19. doi: 10.14283/jfa.2016.77. [DOI] [PubMed] [Google Scholar]
- 32.Gordon E.H., Peel N.M., Samanta M., Theou O., Howlett S.E., Hubbard R.E. Sex differences in frailty: a systematic review and meta-analysis. Exp. Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Munguia L., Rubio-Gayosso I., Ramirez-Sanchez I., Ortiz A., Hidalgo I., Gonzalez C., et al. High flavonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: a double-blind randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(10):1620–1627. doi: 10.1093/gerona/glz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichholzer M., Richard A., Walser-Domjan E., Linseisen J., Rohrmann S. Urinary phytoestrogen levels and frailty in older American women of the National Health and Nutrition Examination Survey (NHANES) 1999-2002: a cross-sectional study. Ann. Nutr. Metab. 2013;63(4):269–276. doi: 10.1159/000356453. [DOI] [PubMed] [Google Scholar]
- 35.Zamora-Ros R., Rabassa M., Cherubini A., Urpi-Sarda M., Bandinelli S., Ferrucci L L., et al. High concentrations of a urinary biomarker of polyphenol intake are associated with decreased mortality in older adults. J. Nutr. 2013;143(9):1445–1450. doi: 10.3945/jn.113.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira L., Ramirez-Sanchez I., Perkins G.A., Murphy A., Taub P.R., Ceballos G., et al. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011;589(18):4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si H., Wang X., Zhang L., Parnell L.D., Admed B., LeRoith T., et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 2019;33(1):965–977. doi: 10.1096/fj.201800554RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81(1):230S–242S. doi: 10.1093/ajcn/81.1.230S. suppl. [DOI] [PubMed] [Google Scholar]
- 39.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLOS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng S., Shen W.H., Liu L. Senescence and cancer, Cancer Transl. Med. 2018;4(3):70–74. doi: 10.4103/ctm.ctm_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hickson L.J., Langhi Prata L.G.P., Bobart S.A., Evans T.K., Giorgadze N., Hashmi S.K., et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBiomedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levolger S., van den Engel S., Ambagtsheer G., Ijzermans J.N.M., de Bruin R.W.F. Quercetin supplementation attenuates muscle wasting in cancer-associated cachexia in mice. Nutr. Healthy Aging. 2021;6(1):35–47. doi: 10.3233/NHA-200084. [DOI] [Google Scholar]
- 44.Sampson L., Rimm E., Hollman P.C., de Vries J.H., Katan M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002;102(10):1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 45.Beecher G.R. Overview of dietary flavonoids: nomenclature, occurrence and intake. J. Nutr. 2003;133(10):3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 46.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 47.Hidalgo-Liberona N., Gonzalez-Dominguez R., Vegas E., Riso P., Del Bo C., Bernardi S., et al. Increased intestinal permeability in older subjects impacts the beneficial effects of dietary polyphenols by modulating their bioavailability. J. Agric. Food Chem. 2020;68(44):12476–12484. doi: 10.1021/acs.jafc.0c04976. [DOI] [PubMed] [Google Scholar]
- 48.Visvanathan R., Williamson G. Review of factors affecting citrus polyphenol bioavailability and their importance in designing in vitro, animal, and intervention studies. Compr. Rev. Food Sci. Food Saf. 2022;21(6):4509–4545. doi: 10.1111/1541-4337.13057. [DOI] [PubMed] [Google Scholar]
- 49.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slavin J.L., Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3(4):506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application to and approval by the Framingham Heart Study.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the official views of the National Institutes of Health.