Abstract
Background
Low birth weight (LBW) is associated with neonatal mortality and sequelae of lifelong health problems; prioritizing the most promising antenatal interventions may guide resource allocation and improve health outcomes.
Objective
We sought to identify the most promising interventions that are not yet included in the policy recommendations of the World Health Organization (WHO) but could complement antenatal care and reduce the prevalence of LBW and related adverse birth outcomes in low- and middle-income settings.
Methods
We utilized an adapted Child Health and Nutrition Research Initiative (CHNRI) prioritization method.
Results
In addition to procedures already recommended by WHO for the prevention of LBW, we identified six promising antenatal interventions that are not currently recommended by WHO with an indication for LBW prevention, namely: (1) provision of multiple micronutrients; (2) low-dose aspirin; (3) high-dose calcium; (4) prophylactic cervical cerclage; (5) psychosocial support for smoking cessation; and (6) other psychosocial support for targeted populations and settings. We also identified seven interventions for further implementation research and six interventions for efficacy research.
Conclusion
These promising interventions, coupled with increasing coverage of currently recommended antenatal care, could accelerate progress toward the global target of a 30% reduction in the number of LBW infants born in 2025 compared to 2006-10.
Keywords: Antenatal care (ANC), Child Health and Nutrition Research Initiative (CHNRI) method, Low birth weight (LBW), Preterm birth (PTB), Low- and middle-income countries (LMICs), Priority-setting
List of abbreviations used:
- ANC
antenatal care
- CHNRI
Child Health and Nutrition Research Initiative
- CI
confidence interval
- CIFF
The Children’s Investment Fund Foundation
- FGR
fetal growth restriction
- HIC
high-income country
- IFA
iron and folic acid
- IPD
Individual Participant Data
- IPS
intervention priority score
- IPTp
intermittent preventive malaria treatment in pregnancy
- IPTp-SP
intermittent preventive malaria treatment in pregnancy using sulfadoxine-pyrimethamine
- LMICs
low- and middle-income countries
- LBW
low birth weight
- LNS
lipid-based nutrient supplement
- MMN
multiple micronutrient
- MMS
multiple micronutrient supplementation
- PTB
preterm birth
- RCT
randomized controlled trial
- RR
relative risk
- SGA
small for gestational age
- WHO
World Health Organization
Introduction
Low birth weight (birth weight less than 2500 g, LBW) is a persistent global problem affecting approximately 15% of live births with the highest burden in southern Asia and sub-Saharan Africa [1,2]. LBW is a significant health concern because it sets into motion a cascade of early-life mortality and morbidity and is associated with numerous long-term adverse consequences [1,3]. Reducing its prevalence is a global priority and the World Health Assembly has agreed on a goal to reduce the annual number of infants born with LBW by 30% between 2010 and 2025 [4], which was recently extended to 2030 [5].
LBW may result from preterm birth (PTB, birth before 37 completed weeks of gestation), fetal growth restriction (FGR) that usually presents as the newborn being small for gestational age (SGA, weight below the 10th percentile for the gestational age and sex), or both [6,7]. There are many maternal, fetal and placental risk factors that can make infants susceptible to these conditions, including extremes of maternal age [8,9], multiple pregnancy [10], pregnancy complications and chronic maternal conditions [11,12], infections [13,14], nutritional deficiencies [15], harmful behaviors [16], psychosocial factors [17,18], and environmental exposures [19]. The World Health Organization (WHO) has published recommendations on interventions that can be delivered during the antenatal period that can reduce the risk of LBW and the related adverse birth outcomes of PTB, SGA, and stillbirth. Examples of such interventions include infection control in pregnancy [20] and education on energy and protein intake for pregnant women with undernutrition [21].
In recent years, there has been active research on interventions aimed at reducing the prevalence of LBW, expanding our knowledge of effectiveness specific to different strategies to prevent LBW [22]. However, the coverage of the currently recommended interventions has been inconsistent across different contexts [23,24], partially because of investment gaps. Some estimates suggest that even if the currently recommended antenatal care (ANC) interventions were to be scaled up to full coverage, the impact would be insufficient to meet the targets set to reduce LBW prevalence [25]. Therefore, novel strategies that address several core modifiable risk factors for PTB and FGR are needed to reduce the prevalence of LBW and associated ill health.
To identify promising antenatal interventions for the prevention of LBW in a systematic, transparent, and replicable way, it is necessary to: 1 know the current efficacy and effectiveness of potential antenatal interventions; 2 understand the feasibility, public health relevance, and potential for interventions to reduce health disparities; and 3 recognize gaps in the evidence base for which further implementation or efficacy research is required. Addressing these needs, we performed a prioritization exercise on the most promising antenatal interventions that could reduce the global prevalence of LBW by complementing the WHO-recommended compendium of ANC guidelines.
Methods
We carried out a priority-setting exercise through a series of meetings involving a multidisciplinary panel of international professionals. This exercise was informed by a large-scale systematic search and review of available evidence. We utilized an adapted Child Health and Nutrition Research Initiative (CHNRI) method established for setting priorities in global child health research. The method was originally designed for identifying research priorities but is adaptable for other needs and contexts [[26], [27], [28], [29], [30]].
To identify the most promising antenatal interventions, we merged the original 15 CHNRI steps [31] into four stages: project initiation, evidence synthesis, priority selection, and result consolidation. At the initiation stage, an international group of experts working in research, implementation and funding in maternal and child health in low- and middle-income countries (LMICs), hereafter called the “Author Group”, compiled the initial list of antenatal interventions to be studied. They also identified members of other groups in this review, allowing the groups to self-expand where relevant. The “Evidence Synthesis Group”, consisting of four researchers and 15 part-time research assistants, information specialists, and statisticians, reviewed the effectiveness of the interventions for selected birth outcomes.
At the priority selection stage, the “Scoring Group”, comprising professionals from academia, funding organizations, the United Nations, and governmental and nongovernmental organizations, conducted the preliminary ranking of the interventions and identified research priorities. The “Review Group” including some members who had also participated in the Scoring Group, involved experts in international maternal and child health. The purpose of this group was to review the results of the primary scoring of the priorities with the Author Group.
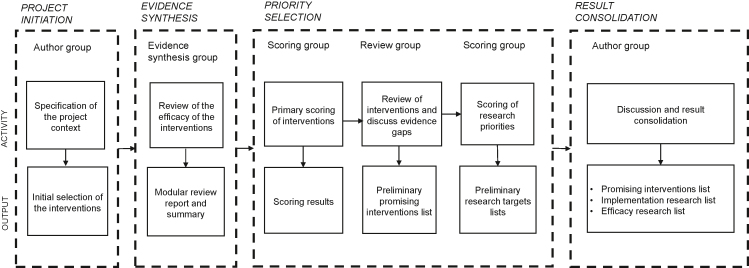
At the final stage, the Author Group consolidated the results of the exercise in the form of three separate lists on the most promising antenatal interventions and targets for further research (Figure 1). The first author (AK) was a member of the Evidence Synthesis Group, Scoring Group and Review Group and acted as a coordinator.
Figure 1.
Process stages, participants, activities, and outputs.
Project initiation
The Author Group developed a common framework for action to reduce the prevalence of LBW at a workshop in September 2019. The group initiated a priority-setting process on the best approaches to reduce LBW globally, with a focus on southern Asia and sub-Saharan Africa. Reflecting on the latest developments in their fields of expertise and on the existing WHO recommendations [20,21] (Supplementary Table 1), they selected interventions for evidence synthesis based on the potential efficacy of the interventions on selected birth outcomes. The group excluded antenatal single-nutrient supplementation from the analysis except for the nutrients already recommended by WHO. There was group consensus that global research and implementation focus was shifting from single-nutrient supplementation to multiple micronutrient supplementation (MMS) in pregnancy [32].
The impact of the interventions was measured using four specified adverse birth outcomes: LBW, PTB, SGA, and stillbirth. We included evidence on stillbirth at the synthesis stage because it was considered an extreme outcome of some of the pathways that limit fetal growth or shorten the duration of pregnancy. Because our interest was specifically in opportunities during ANC, interventions focusing on the preconception period, labor or postpartum period were excluded from the analysis.
Evidence synthesis
Utilizing a modular review methodology developed for this purpose [33], the Evidence Synthesis Group conducted a systematic search and review to synthesize evidence on the efficacy of the preselected antenatal interventions that may reduce the prevalence of LBW and related birth outcomes. Between March and June 2020, they performed a series of literature searches in five databases: MEDLINE (OvidSP), Embase (OvidSP), Cochrane Database of Systematic Reviews (Wiley Cochrane Library), Cochrane Central Register of Controlled Trials (Wiley Cochrane Library), and CINAHL Complete (EbscoHOST). They reported an effect size estimate for each intervention from the most recent examples of the highest level of evidence available (typically Cochrane review of RCTs) or, when not available, conducted a meta-analysis of RCTs to provide the estimate (Supplementary Table 2). They categorized the evidence based on its quantity and quality. The quantity referred to the number of studies contributing to the effect size estimate. The quality of evidence (reported as low, moderate, or high) was derived from the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) or an equivalent assessment for Cochrane reviews and from risk of bias assessment for de novo appraisal of RCTs.
The likely effect of evidence on the outcome was categorized into classes: positive effect, possible positive effect, no positive effect, and unknown effect. For an intervention to be categorized as likely to have a positive effect on an outcome, there needed to be consistent evidence from at least two high- or moderate-quality trials in which the 95% CI of the point estimate of the RR had to be entirely below 1. For an intervention to be categorized as having a possible positive effect on an outcome, evidence from at least two RCTs was required, where either the 95% CI of the point estimate of the RR was entirely below 1 but the quality of the evidence was low or the quality was moderate to high and the 90% CI of the point estimate of the RR was entirely below 1. Additionally, one moderate-to-high quality RCT, with a 95% CI of the point estimate of the RR entirely below 1, received this designation. The method is detailed in the Supplementary Methods with comprehensive definitions of the categories in Supplementary Table 3.
Priority selection
The Evidence Synthesis Group presented the modular review results, sent in advance, to the Scoring Group at a virtual meeting in 2020. The Scoring Group members were asked to score the performance of each intervention on four attributes: 1 efficacy; 2 practical and economic feasibility; 3 public health impact; and 4 potential to reduce health disparities. They were asked to provide the score based on their professional experience considering LMICs in sub-Saharan Africa and southern Asia. Based on the scoring, the interventions were given individual and combined intervention priority scores (IPSs).
In the second virtual meeting, the Author Group and the Review Group revisited the results of the primary scoring of the priorities in four small groups. They identified a maximum of ten priority interventions per group. To ensure that important interventions would not be missed, participants were given an opportunity to bring a “wild card”, i.e., up to two additional interventions outside the original list of scored interventions, into the discussion.
Next, the Scoring Group participated in an electronic follow-up survey to set further research priorities concerning the antenatal interventions ranked in the primary scoring of priorities. Using a Google Forms application, the group members were asked to select up to five interventions for which they would advise further implementation or efficacy research. Regarding implementation, they were encouraged to base their selection on the premise that further research would help service providers identify optimal platforms and modes of delivery for the interventions. Regarding efficacy, the premise was that further research could provide important new evidence and lead to a recommendation of the selected intervention to become a tool to prevent LBW, PTB, or SGA.
Results consolidation
This stage comprised four steps. First, the Author Group endorsed the antenatal interventions currently recommended in WHO guidelines for a positive pregnancy experience [21], focusing on the recommendations with an indication for the prevention of LBW, PTB or SGA in the actual recommendation sentence (Table 1). Second, the group identified the most promising additional interventions that could complement the ones already recommended. In compiling the consolidated list of promising antenatal interventions, the group therefore removed the LBW-indicated recommendations that were already part of the WHO compendium of ANC guidelines but left interventions that were not LBW-indicated in the recommendation sentence. Because malarial infection during pregnancy is a well-recognized determinant of LBW, the provision of intermittent preventive malaria treatment or insecticide-treated bed nets were also considered LBW-indicated interventions [20,34]. Since the purpose was to provide a limited number of priorities, all lists were restricted to a maximum of ten items: In practice, the lists included interventions that were prioritized by at least 20% of the experts who participated in the scoring. Third, the group identified implementation research targets, focusing on interventions that could benefit from further research for optimal implementation. These interventions could overlap with the WHO recommendations and the promising interventions list. To proceed to the promising interventions list, implementation research list or both, either the intervention was required to show efficacy in LMICs or the evidence from high-income countries (HICs) had to be applicable to the LMIC context.
Table 1.
Summary of antenatal interventions recommended by WHO, with an indication to the prevention of LBW, PTB, SGA, stillbirth, or malaria.
| Antenatal interventions recommended by WHO1 (Target condition) |
|---|
| 1. Dietary education for pregnant women with undernutrition (LBW) |
| 2. Provision of proteins and energy to pregnant women with undernutrition (SGA, stillbirth) |
| 3. Lowering daily caffeine intake of pregnant women with high daily caffeine intake (more than 300 mg per day) (LBW, pregnancy loss) |
| 4. Screening and treatment of asymptomatic bacteriuria in pregnancy (LBW, PTB) |
| 5. Provision of insecticide-treated bed nets during pregnancy (malaria) |
| 6. Provision of at least three doses of IPTp-SP (intermittent preventive malaria treatment in pregnancy using sulfadoxine-pyrimethamine) starting in the second trimester, and given at least one month apart (malaria) |
Includes interventions with an indication to LBW, PTB, SGA or stillbirth in the recommendation sentence, or recommendation on malaria control in pregnancy.
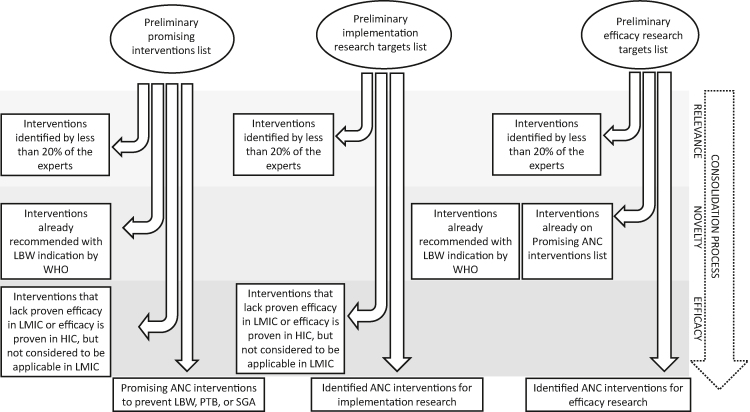
Finally, the Author Group identified efficacy research targets. The premise for this list was to include interventions believed to be efficacious but that had not been proven in LMICs, or alternatively the evidence from HICs was not considered directly applicable to the LMIC context. No overlap was allowed with WHO recommendations or the promising interventions list (Figure 2).
Figure 2.
Results consolidation process. ANC - antenatal care, HIC - high-income country, LMICs - low- and middle-income countries, LBW - low birth weight, PTB - preterm birth, SGA - small for gestational age, WHO - World Health Organization.
If two interventions were mutually conflicting, the intervention with lower IPS was removed. The target groups and the phrasing of the interventions were clarified in their final forms. Finally, interventions were organized in tables according to the intervention category (nutrition, infection, other) without consideration of their original IPS or rank.
Results
The prioritization exercise was conducted between September 2019 and August 2021. A total of 58 participants (35 females, 23 males) contributed to the process. The majority (41) represented academia or a research institution, and the remaining participants represented funding organizations (9), United Nations organizations (2), governmental institutions (2), nongovernmental organizations (2), and the private sector (2). Based on country of permanent residence, participants represented Europe (19), North America (17), Africa (15), Asia (6), and Middle East and North Africa (1). All participants reported significant professional experience in maternal and newborn health from sub-Saharan Africa, South Asia, Europe and Central Asia, North America, Latin America and the Caribbean, the Middle East and North Africa, and East Asia and the Pacific.
Project initiation
Of the 18 Author Group members, 16 participated in the workshop at the initiation stage. The Author Group compiled an initial list of 43 potential priority interventions to prevent LBW and related birth outcomes (Supplementary Table 4).
Evidence synthesis
We identified 61,279 articles on the effects of antenatal interventions on selected birth outcomes by literature search. After electronic removal of duplicates, we screened 35,244 articles by title/abstract. In total, we reviewed 6,272 full-text articles, resulting in 365 eligible articles. Of 46 interventions (43 from the initial list and three added by the Evidence Synthesis Group), the group deemed one infection-related intervention, four nutritional interventions, and three other interventions to have a positive effect, i.e., they are likely to reduce the risk of at least one of the birth outcomes (LBW, PTB, SGA, or stillbirth) (Table 2). The group deemed eight interventions to have possible positive effect, i.e., the intervention may reduce the risk of at least one of the selected outcomes (Table 3). Nineteen interventions were deemed likely not to reduce the risk of at least one of the outcomes (Supplementary Table 5). For most interventions, data were deemed insufficient to determine their impact on SGA and stillbirth (Supplementary Table 6 and Supplementary Table 7).
Table 2.
Evidence synthesis: Antenatal interventions that likely reduce the risk of LBW, PTB, SGA, or stillbirth.
| Positive effect1 | Birth outcome | Relative risk2 | Quality of evidence4 |
|---|---|---|---|
| Changing a two-dose IPTp regimen to more frequent IPTp dosing | LBW | 0.80 [0.69, 0.94] (N=6281)3 | Moderate |
| Replacement of IFA supplementation with MMN supplementation | LBW | 0.88 [0.85, 0.91] (N=68801)3 | High |
| SGA | 0.92 [0.88, 0.97] (N=57348)3 | Moderate | |
| Provision of proteins and energy to pregnant women with undernutrition | LBW | 0.68 [0.51, 0.92] (N=4196)3 | Moderate |
| SGA | 0.79 [0.69, 0.9] (N=4408)3 | ||
| stillbirth | 0.60 [0.39, 0.94] (N=3408)3 | ||
| Provision of lipid-based nutrient supplements instead of multiple micronutrients | LBW | 0.92 [0.86, 0.98] (N=2727)3 | Moderate |
| Supplementation with omega-3 fatty acids | LBW | 0.90 [0.82 0.99] (N=8449) | High |
| PTB | 0.89 [0.81, 0.97] (N=10304) | ||
| Provision of low-dose aspirin during pregnancy in women at high risk of preeclampsia | PTB | 0.67 [0.50, 0.90] (N=2391) | Moderate |
| SGA | 0.71 [0.58, 0.89] (N=2820) | ||
| stillbirth | 0.34 [0.19, 0.59] (N=2174) | ||
| Psychosocial interventions to reduce smoking in pregnancy | LBW | 0.83 [0.72, 0.94] (N=9420) | High |
| Prophylactic application of uterine cervical stitch (cerclage) in women at increased risk of PTB | PTB | 0.80 [0.69, 0.95] (N=2898) | High |
Positive effect: The intervention likely reduces the risk of the selected birth outcome: At least two moderate-to-high quality RCTs included in a meta-analysis or IPD analysis, 95% CI of the point estimate of the RR is entirely below 1.
Relative risk [95 % confidence interval] (number of participants).
The proportion of studies coming from sub-Saharan Africa or South Asia is 50% or higher.
The quality of evidence is based on GRADE or equivalent assessment for Cochrane reviews and on risk of bias assessment for de novo appraisal of RCTs, detailed in Supplementary Material, Section F: Assessment of quality of evidence.
Table 3.
Evidence synthesis: Antenatal interventions that may reduce the risk of LBW, PTB, SGA, or stillbirth.
| Possible positive effect1 | Birth outcome | Relative risk2 | Quality of evidence4 |
|---|---|---|---|
| Provision of insecticide-treated bed nets in pregnancy | LBW | 0.80 [0.64, 1.00] (N=3506)3 | Moderate |
| Treatment of documented periodontal disease during pregnancy | LBW | 0.67 [0.48, 0.95] (N=3470) | Low |
| Screening and treatment of asymptomatic bacteriuria in pregnancy | LBW | 0.64 [0.45, 0.93] (N=1437) | Low |
| PTB | 0.34 [0.13, 0.88] (N=327) | ||
| Dietary education of pregnant women with undernutrition | LBW | 0.46 [0.27, 0.79] (N=3440)3 | Low |
| Dietary supplementation with high dose calcium | LBW | 0.85 [0.72, 1.01] (N=14883) | Low |
| PTB | 0.76 [0.60, 0.97] (N=15275) | ||
| Professionally provided psychosocial support for women at risk of giving birth to LBW or preterm infant | PTB | 0.91 [0.83, 1.00] (N=11036) | Moderate |
| Progesterone supplementation for women at increased risk of PTB | PTB | 0.69 [0.53, 0.87] (N=3706) | Low |
| Bedrest among women at risk for preterm delivery | LBW | 0.92 [0.85, 1.00] (N=1837) 3 | Moderate |
Possible positive effect: The intervention may reduce the risk of selected birth outcome. a. At least two RCTs included in a meta-analysis or IPD analysis, 95% CI of the point estimate of the RR is entirely below 1, but there is concern about the quality of the data, or b. at least two moderate-to-high quality RCTs included in a meta-analysis or IPD analysis, 95% CI of the point estimate of the RR includes 1 but 90% CI of the point estimate of the RR is entirely below 1, or One moderate-to-high quality RCT, 95% CI of the point estimate of the RR is entirely below 1.
Relative risk [95 % confidence interval] (number of participants).
The proportion of studies coming from Sub-Saharan Africa or South Asia is 50% or higher.
The quality of evidence is based on GRADE or equivalent assessment for Cochrane reviews and on risk of bias assessment for de novo appraisal of RCTs, detailed in Supplementary Material, Section F: Assessment of quality of evidence
Priority selection
Of the 65 invited Scoring Group members, 43 members (66%) accepted the invitation and participated in the primary scoring of the interventions against the four attributes (efficacy, impact, feasibility, equity). In general, nutritional interventions received higher combined IPSs, whereas the IPSs for the infection-related and other interventions were more widely dispersed across the range (Supplementary Table 8).
Out of the 32 invited members of the Review and Author Groups, 27 ranked the primary scoring results into four small groups. Combining the top 10 prioritized interventions of each group resulted in a list of 16 interventions. Of these interventions, 13 matched with the 13 interventions that received the highest IPS by the Scoring Group (Supplementary Tables 9 and 10). No “wild cards”, i.e., interventions outside the original list, were proposed.
In the last step, the Scoring Group scored the 46 interventions according to the need for further implementation or efficacy research. Of the 65 invited members, 30 participated (46%). Eight interventions (four nutrition-related interventions, three infection-related infections, and one other intervention) were identified by at least 20% of the experts as targets for further implementation research (Supplementary Table 11). For further efficacy research, the experts identified nine interventions (four nutrition-related interventions, one infection-related intervention, and four other interventions) (Supplementary Table 12).
Result consolidation
Of the 13 interventions ranked highest by the Scoring Group, six were selected by the Author Group for inclusion on the list of promising interventions to prevent LBW, PTB, or SGA (Table 4). Of these, two interventions addressed maternal nutrition, none addressed maternal infection, and four addressed other conditions among at-risk populations. Two interventions (prophylactic cervical cerclage, and professionally provided psychosocial support) were not previously recommended by WHO whereas replacement of iron and folic acid (IFA) supplementation with MMS was recommended in the context of rigorous research and high-dose calcium supplementation, low-dose aspirin provision, and screening of maternal tobacco use were already recommended by WHO, although not specifically with an indication to prevent LBW.
Table 4.
Promising antenatal interventions to prevent LBW, PTB, or SGA.
| Antenatal interventions currently not recommended by WHO | Intervention category |
|---|---|
| Replacement of IFA supplementation for pregnant women with MMN supplementation1 | Nutrition |
| Prophylactic application of uterine cervical stitch (cerclage) for women at increased risk of PTB | Other |
| Professionally provided psychosocial support for women at risk of giving birth to a LBW or preterm infant | Other |
| Antenatal interventions recommended by WHO, for another indication2 | Intervention category |
|---|---|
| Provision of high-dose calcium supplements (>1 g / day) to pregnant women in areas with low dietary calcium3 | Nutrition |
| Provision of low-dose aspirin during pregnancy to women at increased risk of preeclampsia4 | Other |
| Psychosocial interventions to reduce smoking in pregnancy5 | Other |
WHO recommendation sentence: Antenatal multiple micronutrient supplements that include iron and folic acid are recommended in the context of rigorous research.
Includes interventions without an indication to LBW, PTB, SGA or stillbirth in the recommendation sentence, or recommendation on malaria control in pregnancy
WHO recommendation sentence: In populations with low dietary calcium intake, daily calcium supplementation (1.5–2.0 g oral elemental calcium) is recommended for pregnant women to reduce the risk of pre-eclampsia.
WHO recommendation sentence: Low-dose acetylsalicylic acid (aspirin, 75 mg per day) is recommended for the prevention of pre-eclampsia in women at moderate or high risk of developing the condition.
WHO recommendation sentence: Health-care providers should ask all pregnant women about their tobacco use (past and present) and exposure to second-hand smoke as early as possible in the pregnancy and at every antenatal care visit.
Of the seven excluded interventions, five were left out because they are already recommended by WHO with an indication for preventing LBW. The provision of lipid-based nutrient supplements (LNSs) (instead of MMS) was excluded because the evidence synthesis had combined data from trials providing small-quantity, medium-quantity, or large-quantity LNS to pregnant women. During the consolidation of the results, the authors agreed that these three interventions should be considered separately. They also noted that none of the trials was conclusive alone and that an earlier meta-analysis combining data only from the two trials involving small-quantity LNS was also inconclusive [35,36]. Given these findings, the authors concluded that there was insufficient data to include any type of LNS on the prioritized interventions lists. Provision of omega-3 fatty acids to pregnant women with undernutrition was removed because most of the evidence came from HICs, and this evidence was not considered to be directly applicable to the LMIC context.
Seven of the eight interventions identified by the Scoring Group as requiring further implementation research were included in the final implementation research priority list (Table 5). Two of the included interventions (replacement of IFA supplementation with MMS and provision of low-dose aspirin) were also on the promising interventions list and had not been previously recommended by WHO with an indication for LBW, and five (provision of proteins and energy, dietary education, provision of insecticide-treated bed nets, screening and treatment of asymptomatic bacteriuria, and changing a two-dose IPTp (intermittent preventive malaria treatment in pregnancy) regimen to more frequent IPTp dosing) were current LBW- or malaria-indicated recommendations. In line with the promising interventions list, the provision of omega-3 fatty acids to pregnant women with undernutrition was excluded.
Table 5.
Identified antenatal interventions for implementation research.
| Antenatal interventions for implementation research: interventions currently not recommended by WHO1 | Intervention category |
|---|---|
| Replacement of IFA supplementation for pregnant women with MMN supplementation2 | Nutrition |
| Antenatal interventions for implementation research: interventions currently recommended by WHO3 | Intervention category |
|---|---|
| Provision of proteins and energy to pregnant women with undernutrition4 | Nutrition |
| Dietary education for pregnant women with undernutrition 5 | Nutrition |
| Provision of insecticide-treated bed nets during pregnancy6 | Infection |
| Screening and treatment of asymptomatic bacteriuria in pregnancy7 | Infection |
| Changing a two-dose IPTp (intermittent preventive malaria treatment in pregnancy) regimen to more frequent IPTp dosing8 | Infection |
| Antenatal interventions recommended by the WHO, for another indication2 | Intervention category |
|---|---|
| Provision of low-dose aspirin during pregnancy to women at increased risk of preeclampsia9 | Other |
Includes interventions that are either not recommend or are recommended in the context of research.
WHO recommendation sentence: Antenatal multiple micronutrient supplements that include iron and folic acid are recommended in the context of rigorous research.
Includes interventions with an indication to LBW, PTB, SGA or stillbirth in the recommendation sentence, or recommendation on malaria control in pregnancy.
WHO recommendation sentence: In undernourished populations, balanced energy and protein dietary supplementation is recommended or pregnant women to reduce the risk of stillbirths and small-for-gestational-age neonates.
WHO recommendation sentence: In undernourished populations, nutrition education on increasing daily energy and protein intake is recommended for pregnant women to reduce the risk of low-birth-weight neonates.
WHO recommendation footnote: Integrated from the WHO publication Guidelines for the treatment of malaria (2015), [20] which also states: “WHO recommends that, in areas of moderate-to-high malaria transmission of Africa, IPTp-SP be given to all pregnant women at each scheduled ANC visit, starting as early as possible in the second trimester, provided that the doses of SP are given at least 1 month apart. WHO recommends a package of interventions for preventing malaria during pregnancy, which includes promotion and use of insecticide-treated nets, as well as IPTp-SP”. To ensure that pregnant women in endemic areas start IPTp-SP as early as possible in the second trimester, policy-makers should ensure health system contact with women at 13 weeks of gestation.
WHO recommendation sentence: A seven-day antibiotic regimen is recommended for all pregnant women with asymptomatic bacteriuria to prevent persistent bacteriuria, preterm birth and low birth weight.
WHO recommendation sentence: In malaria-endemic areas in Africa, intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP) is recommended for all pregnant women. Dosing should start in the second trimester, and doses should be given at least one month apart, with the objective of ensuring that at least three doses are received.
WHO recommendation sentence: Low-dose acetylsalicylic acid (aspirin, 75 mg per day) is recommended for the prevention of pre-eclampsia in women at moderate or high risk of developing the condition [51].
Six of the nine identified interventions for further efficacy research were included in the final efficacy research target list (Table 6). Of these six interventions, five (provision of low-dose calcium; screening of maternal weight gain followed, if indicated, by dietary or other intervention; provision of omega-3 fatty acids; water, sanitation, and hygiene (WASH) interventions; and reduction of indoor air pollution) were not currently recommended by WHO. One intervention (intimate partner violence prevention) aligned with an existing WHO recommendation with a different indication. Of the three excluded interventions, two overlapped with WHO recommendations (screening and treatment of asymptomatic bacteriuria in pregnancy and provision of proteins and energy to pregnant women with undernutrition), and one overlapped with the promising interventions list (provision of low-dose aspirin during pregnancy in women at high risk of preeclampsia).
Table 6.
Identified antenatal interventions for efficacy research.
| Antenatal interventions for efficacy research: interventions currently not recommended by WHO | Intervention category |
|---|---|
| Provision of low-dose calcium supplements (<1 g / day) to pregnant women | Nutrition |
| Regular screening of maternal weight gain followed, if indicated, by dietary supplementation or other intervention | Nutrition |
| Provision of omega-3 fatty acids to pregnant women with undernutrition | Nutrition |
| Water, sanitation, and hygiene (WASH) interventions in pregnancy | Infection |
| Reduction of indoor air pollution | Other |
| Antenatal interventions for efficacy research: interventions recommended by WHO1 | Intervention category |
|---|---|
| Intimate partner violence prevention interventions2 | Other |
Includes interventions with and without an indication to LBW, PTB, SGA or stillbirth in the recommendation sentence, or recommendation on malaria control in pregnancy.
WHO recommendation sentence: Clinical enquiry about the possibility of intimate partner violence should be strongly considered at antenatal care visits when assessing conditions that may be caused or complicated by intimate partner violence in order to improve clinical diagnosis and subsequent care, where there is the capacity to provide a supportive response (including referral where appropriate) and where the WHO minimum requirements are met.
Discussion
This priority-setting exercise aimed to fill a critical gap by producing a consolidated view on promising antenatal interventions that could complement the globally recommended standard of care for pregnant women to reduce the major global burden of LBW and related adverse birth outcomes. Informed by a systematic literature search and review on the intervention efficacy [[37], [38], [39], [40]], we ranked interventions, focusing particularly on those that would reduce the burden of LBW in LMIC settings, and identified urgent gaps to fill in knowledge. We identified six promising antenatal interventions that address maternal nutrition in both general and targeted populations in LMICs, as well as other conditions among at-risk groups globally. We also identified antenatal interventions that would benefit from further implementation research or efficacy research.
Strengths and limitations
We drew on the intrinsic flexibility of the CHNRI method and adapted it for this purpose. We used the advantages of CHNRI, including a systematic and transparent process [31] and multistakeholder engagement, which helps make the final priorities more acceptable to stakeholders [41]. Having participants with various geographical and professional backgrounds and a ratio of 3:2 female to male participants further enhanced the credibility of the results.
Our exercise had some potential shortcomings typical of the CHNRI method, such as a limited number of participants, which may affect the representativeness of the findings, spectrum of research topics or response rates [26,42]. Although the invitation was shared extensively and via various means, participation may have been reduced by the restriction to English language and the snowballing approach that may more easily identify well-networked stakeholders. Hence, academic experts formed the majority of the respondents, whereas LMIC government experts and those with nongovernmental organization backgrounds or recent field-level experience were underrepresented, and only one-third of the participants permanently resided in Africa or Asia. However, there was substantial diversity and technical expertise among the respondents and most of those currently residing in HICs had significant experience in low-income settings. Finally, the incorporation of the “wild card” function into the method provided a means to increase the diversity of considered topics. Therefore, although a prioritization list is always subjective, we argue that the presented lists reflect priorities for a professionally and geographically diverse group of stakeholders.
Our demarcation between explicitly LBW-indicated and other interventions could be regarded as arbitrary. We considered explicit LBW indication in the actual WHO recommendation sentence to be important, cognizant that many of the WHO recommendations that lack specified indication still provide a summary of evidence or refer to other documents that summarize evidence, and these sections often describe a likely effect of the intervention on LBW and related birth outcomes. Despite the limitations, this approach provided a framework to discuss the degree to which the interventions had been previously recognized, particularly as LBW prevention strategies, by the global community.
The response rates of the surveys for the primary scoring of the interventions (66%) and for scoring the research priorities (46%) can be considered slightly low. Therefore, nonresponse bias cannot be ruled out. However, our results were consistent with the issues raised in the evidence synthesis, suggesting strong internal validity of our findings and supporting the argument that many stakeholders beyond this exercise would likely find these topics important. Considering the above, our exercise based on the collective knowledge of the participants can be seen as a valid analysis of current key priorities.
Study findings in the context of existing research
Among other topics, previous studies have utilized the CHNRI method to prioritize research ideas to reduce global mortality from LBW and PTB [43], prevent stillbirth and improve newborn health [42], reduce PTB and stillbirth through community-level implementation research [44], and, more recently, support the implementation of MMS in pregnancy [45]. While the original “long lists” of the earlier CHNRI exercises [42,43] included questions relevant to the antenatal period, their prioritized top ten lists included solely intrapartum and postnatal interventions, such as timely identification of LBW infants born at home [43] and improving the delivery of known neonatal interventions [42]. The prioritized community-level interventions by the Global Alliance to Prevent Prematurity and Stillbirth highlighted the equity aspect: their highest-ranking question focused on the reduction of financial barriers to facility births through, for instance, conditional cash transfers [44], which were considered in the current exercise but were not selected in the consolidated list. The highest-ranking questions in the exercise on MMS in pregnancy included strategies to increase ANC attendance and adherence to MMS [45], which aligns with the listing of MMS as an implementation research target in the current exercise.
The current exercise differs from the previous CHNRI exercises in LBW and PTB prevention in two aspects. First, it focused exclusively on the antenatal period as a crucial window of opportunity to prevent or treat conditions that can contribute to LBW. Second, it identified interventions separately as usable, recommendable action points as well as research targets to be subjected to further academic inquiry. In contrast to earlier studies, which in the context of the Millennium Development Goals were geared toward rapid progress in mortality reduction, the current project aimed to identify sustainable upstream solutions to LBW prevention. By expanding the focus on prevention and discussing the interventions as new recommendations in the context of existing global ANC guidance, the current exercise builds upon and broadens the evidence base for the pathways to continuous reduction in the prevalence of LBW and subsequent mortality and morbidity.
Implications for policy and research
The six promising interventions for pregnant women included replacement of IFA supplementation with MMS in the general population in LMIC settings and the provision of selected interventions to specific risk groups globally: high-dose calcium supplements to women in areas with low dietary calcium, low-dose aspirin to women at increased risk for preeclampsia, prophylactic application of cervical cerclage for women at risk for spontaneous PTB, professionally provided psychosocial support for women at risk of PTB or LBW, and psychosocial smoking cessation interventions for those smoking during pregnancy. While we identified these interventions as evidence-based strategies that can be used to address core modifiable risk factors in pregnant women, the potential of these strategies is highly dependent on the burden and variation of risk factors in the target settings and the acceptability of the interventions to the target populations. Moreover, it is reliant on the implementation capacity of the health systems to deliver the interventions efficiently, which may be limited regarding the already established and well-known interventions. Thus, the potential of these interventions should always be considered contextually. Furthermore, they should be considered complementary, not alternative to the antenatal interventions already recommended by WHO [20,21]. For the best outcomes, we recommend that these interventions be integrated into ANC in a comprehensive manner, avoiding vertical and siloed approaches.
Regarding the replacement of IFA supplementation with MMS for pregnant women, the judgment was affected by the evidence synthesis indicating the intervention’s effectiveness in reducing the risk of LBW and SGA and the accumulation of evidence in recent years. The global guidance on MMS has been in flux: it is currently “recommended in the context of rigorous research” by WHO [46]. The high ranking of this intervention in the present exercise provides a premise for MMS becoming an established part of cost-effective and comprehensive health care and nutritional support for pregnant women in LMIC settings. High-dose calcium supplementation in areas with low dietary calcium is another WHO-recommended antenatal context-specific intervention that has not been extensively implemented, partly because of the cost, adherence and logistical issues related to the large dose and dosing schedule [47], and thus deserves renewed focus.
Based on the available evidence and our CHNRI exercise, we propose the use of the prophylactic application of uterine cervical stitch (cerclage) for women at increased risk of spontaneous PTB based on, e.g., prior PTB or short cervix [48]. However, while high-quality evidence has shown that cerclage is effective in reducing the risk of PTB, it is also a surgical procedure that requires skilled health personnel and at least regional anesthesia [48]. Contextual factors should therefore be taken into consideration in implementing this intervention. Conversely, low-dose aspirin for women who are at increased risk for preeclampsia may have fewer implementation issues; lack of need for complex changes in clinical routines, low cost, and the demonstrated tolerability of aspirin suggest that this intervention could be safely and readily adopted in different settings [49].
We also identified two psychosocial interventions that could be included in the care of targeted population groups: psychosocial interventions to reduce smoking in pregnancy and professionally provided psychosocial support for women who are at risk of giving birth to a LBW or preterm infant due to, e.g., social or obstetric risk. These low-risk, relatively low-cost, yet effective interventions are consistent with the WHO recommendations for a positive pregnancy experience and underscore the importance of effective communication and social, cultural, emotional, and psychological support for pregnant women [21].
The Author Group highlighted the need for additional efficacy, effectiveness and implementation data in connection with almost all of the interventions that were either recommended already by WHO or identified in our exercise as promising. Participants noted that while the efficacy in ideal conditions was proven for many interventions, practical implementation across contexts can be difficult. Supplementation with omega-3 fatty acids was considered to lack proven efficacy in low-income settings. For the treatment of asymptomatic bacteriuria in pregnancy with antibiotics to prevent persistent bacteriuria, PTB and LBW [21], there was also concern that the evidence on the effect of the intervention on LBW and PTB was old and of low certainty. The studies did not adequately assess the adverse effects of antibiotic treatment for the mother or the baby, and some studies used antibiotics that are no longer recommended for use in pregnancy [50].
The evidence we synthesized was primarily based on reviews of RCTs to indicate the presence of an intervention effect. While we used the synthesis to provide a starting point for the discussion, other research designs such as cohort studies could in some cases also be informative in assessing the impact and the amount of evidence on some of the environmental and community-based interventions. We also acknowledge that an intervention that works in controlled research contexts in HICs may not work in real-life LMIC settings. The obverse is also possible; interventions administered in high-income settings may reveal only marginal benefit because birth outcomes may be closer to optimal in the underlying population of pregnant women. Moreover, a poorly designed intervention may not reduce the risk of adverse outcomes in the synthesis of evidence, particularly if the number of included studies or sample sizes are small, while a better-designed version of the same intervention could be more accepted and used. Finally, when discussing the synthesis results, it is always important to make a distinction between the absence of impact and the absence of evidence.
The principle that prevention is better than a cure is particularly crucial for LBW, PTB, and SGA. At the time of birth, the damage has already been done and can only be partially mitigated by postnatal interventions. By identifying promising nutritional, medical and other interventions for pregnant women, our exercise outlines an agenda for improving primary prevention of LBW, an achievement that will decrease the burden of lifelong adverse health consequences, lower the cost to the health care system, and reduce neonatal and child mortality globally. Alongside the existing WHO guidelines, we call for these promising interventions to be considered as part of the efforts to reach the global target for LBW reduction by 30% by 2025.
Acknowledgements
We are grateful for the Children’s Investment Fund Foundation (CIFF) for providing us financial support. We gratefully acknowledge those who supported us in conducting the Modular Review. Specifically, we thank Patricia Hunter and Yvonne Muthiani in search, review and analysis of antenatal interventions, Otto Heimonen and Juho Luoma for statistical support; Jaana Isojärvi, Pia Pörtfors, Taina Peltonen, Päivi Lukin and Heather Chesters for support in information retrieval; and Kalpana Bastola, Maryam Hadji, Meeri Salenius, Viivi Kajander, Raija Vimpeli and Leon Csonka for research assistance. The authors’ responsibilities were as follows:
SA, NB, RB, RMC, KD, CPD, NK, SK, JL, KM, PNG, MS, KS, UA and PA participated in project conception and research design. AK, TH, PA and UA conducted the research and analyzed the data. AK, TH, UA and PA wrote the paper. AK had primary responsibility for final content. All authors, including corporate authors, have read and approved the final manuscript.
Disclaimers
Christopher P. Duggan is Editor-in-Chief of the American Journal of Clinical Nutrition but played no role in the editorial handling of the manuscript.
Sources of support for the work
The study was funded by the Children’s Investment Fund Foundation (CIFF), Grant Reference Number 1808-02973. Dr. Duggan was supported in part by the National Institutes of Health (K24DK104676 and 2P30 DK040561).
Conflict of Interest and Funding Disclosure
Annariina M. Koivu - No conflict of interest
Tiia Haapaniemi - No conflict of interest
Sufia Askari - No conflict of interest
Nita Bhandari - No conflict of interest
Robert E. Black - No conflict of interest
R. Matthew Chico - No conflict of interest
Kathryn G. Dewey - No conflict of interest
Christopher P. Duggan - No conflict of interest
Nigel Klein - No conflict of interest
Somesh Kumar - No conflict of interest
Joy E. Lawn - No conflict of interest
Karim Manji - No conflict of interest
Pieta Näsänen-Gilmore - No conflict of interest
Mihretab Salasibew - No conflict of interest
Katherine E.A. Semrau - No conflict of interest
Ulla Ashorn - No conflict of interest
Per Ashorn - No conflict of interest
Data Availability
Data described in the manuscript will be made available upon request pending application to and approval by the Author Group of the LBW priority-setting exercise.
Footnotes
This article is published as part of a supplement sponsored by Tampere University, Faculty of Medicine and Health Technology with grant support from the Children’s Investment Fund Foundation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2022.10.022.
Contributor Information
Annariina M. Koivu, Email: annariina.koivu@tuni.fi.
The LBW prevention prioritization working group:
Toluwalase Awoyemi, Adejumoke I. Ayede, Kalpana Bastola, Zulfiqar A. Bhutta, Hannah Blencowe, Parul Christian, Anna David, Patricia Hunter, Jaana Isojärvi, Joanne Katz, Ayesha De Costa, Daniel J. Erchick, Sarah Gibson, Bronner P. Goncalves, Michael G. Gravett, Maryam Hadji, Elizabeth Hazel, G Justus Hofmeyr, Naoko Kozuki, Anne CC. Lee, Hema Magge, Albert Manasyan, Abdulrahman Mohiddin, Melissa Morrison, Yvonne Muthiani, Helen Nabwera, Annettee Nakimuli, Pius Okong, Andrew J. Prendergast, Jonathon Simon, Marleen Temmerman, and Jian Yan
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Blencowe H., Krasevec J., de Onis M., Black R.E., An X., Stevens G.A., Borghi E., Hayashi C., Estevez D., Cegolon L., et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7:e849–e860. doi: 10.1016/S2214-109X(18)30565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Children’s Fund (UNICEF), World Health Organization (WHO) World Health Organization; Geneva: 2019. UNICEF-WHO Low birthweight estimates: Levels and trends 2000–2015. [Google Scholar]
- 3.A K.C., Basel P.L., Singh S. Low birth weight and its associated risk factors: Health facility-based case-control study. PloS One. 2020;15 doi: 10.1371/journal.pone.0234907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Assembly . Sixty-fifth World Health Assembly Geneva. World Health Organization; Geneva: 2012. Resolution WHA65.6. Comprehensive implementation plan on maternal, infant and young child nutrition. May 21–26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO), United Nations Children’s Fund (UNICEF) WHO-UNICEF; 2019. Discussion paper on the extension of the 2025 Maternal, Infant and Young Child nutrition targets to 2030. [Google Scholar]
- 6.World Health Organization, United Nations Children’s Fund (UNICEF) World Health Organization; UNICEF; Geneva : New York: 2004. Low birthweight: country, regional and global estimates; p. 31. [Google Scholar]
- 7.Katz J., Lee A.C., Kozuki N., Lawn J.E., Cousens S., Blencowe H., Ezzati M., Bhutta Z.A., Marchant T., Willey B.A., et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet Lond Engl. 2013;382:417–425. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Althabe F., Moore J.L., Gibbons L., Berrueta M., Goudar S.S., Chomba E., Derman R.J., Patel A., Saleem S., Pasha O., et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network’s Maternal Newborn Health Registry study. Reprod Health. 2015;12(Suppl 2):S8. doi: 10.1186/1742-4755-12-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lean S.C., Derricott H., Jones R.L., Heazell A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PloS One. 2017;12 doi: 10.1371/journal.pone.0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray S.R., Stock S.J., Cowan S., Cooper E.S., Norman J.E. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynaecol J Contin Prof Dev R Coll Obstet Gynaecol. 2018;20:57–63. doi: 10.1111/tog.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F., Wang T., Chen L., Zhang S., Chen L., Qin J. Adverse pregnancy outcomes among mothers with hypertensive disorders in pregnancy: A meta-analysis of cohort studies. Pregnancy Hypertens. 2021;24:107–117. doi: 10.1016/j.preghy.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Erez O., Novack L., Klaitman V., Erez-Weiss I., Beer-Weisel R., Dukler D., Mazor M. Early preterm delivery due to placenta previa is an independent risk factor for a subsequent spontaneous preterm birth. BMC Pregnancy Childbirth. 2012;12:82. doi: 10.1186/1471-2393-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakken L., Iversen P.O. The impact of malaria during pregnancy on low birth weight in East-Africa: a topical review. Malar J. 2021;20:348. doi: 10.1186/s12936-021-03883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao P.-L., Zhou Y.-B., Chen Y., Yang M.-X., Song X.-X., Shi Y., Jiang Q.-W. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth. 2015;15:246. doi: 10.1186/s12884-015-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kheirouri S., Alizadeh M. Maternal dietary diversity during pregnancy and risk of low birth weight in newborns: a systematic review. Public Health Nutr. 2021;24:4671–4681. doi: 10.1017/S1368980021000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hines L.A., Spry E.A., Moreno-Betancur M., Mohamad Husin H., Becker D., Middleton M., Craig J.M., Doyle L.W., Olsson C.A., Patton G. Cannabis and tobacco use prior to pregnancy and subsequent offspring birth outcomes: a 20-year intergenerational prospective cohort study. Sci Rep. 2021;11 doi: 10.1038/s41598-021-95460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah P.S., Shah J. Knowledge Synthesis Group on Determinants of Preterm/LBW Births. Maternal exposure to domestic violence and pregnancy and birth outcomes: a systematic review and meta-analyses. J Womens Health. 2002 2010;19:2017–2031. doi: 10.1089/jwh.2010.2051. [DOI] [PubMed] [Google Scholar]
- 18.Staneva A., Bogossian F., Pritchard M., Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth J Aust Coll Midwives. 2015;28:179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Amegah A.K., Quansah R., Jaakkola J.J.K. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: a systematic review and meta-analysis of the empirical evidence. PloS One. 2014;9 doi: 10.1371/journal.pone.0113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . 3rd ed. World Health Organization; 2015. Guidelines for the treatment of malaria. [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva: 2016. WHO recommendations on antenatal care for a positive pregnancy experience; p. 196. [PubMed] [Google Scholar]
- 22.World Health Organization . World Health Organization; Geneva: 2014. Global nutrition targets 2025: low birth weight policy brief. WHO/NMH/NHD/14.5) [Google Scholar]
- 23.Moller A.-B., Petzold M., Chou D., Say L. Early antenatal care visit: a systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob Health. 2017;5:e977–e983. doi: 10.1016/S2214-109X(17)30325-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darteh E.K.M., Dickson K.S., Ahinkorah B.O., Owusu B.A., Okyere J., Salihu T., Bio Bediako V., Budu E., Agbemavi W., Edjah J.O., et al. Factors influencing the uptake of intermittent preventive treatment among pregnant women in sub-Saharan Africa: a multilevel analysis. Arch Public Health Arch Belg Sante Publique. 2021;79:182. doi: 10.1186/s13690-021-00707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang H.H., Larson J., Blencowe H., Spong C.Y., Howson C.P., Cairns-Smith S., Lackritz E.M., Lee S.K., Mason E., Serazin A.C., et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. The Lancet. 2013;381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudan I., Yoshida S., Chan K.Y., Sridhar D., Wazny K., Nair H., Sheikh A., Tomlinson M., Lawn J.E., Bhutta Z.A., et al. Setting health research priorities using the CHNRI method: VII. A review of the first 50 applications of the CHNRI method. J Glob Health. 2017;7 doi: 10.7189/jogh.07.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudan I., El Arifeen S., Bhutta Z.A., Black R.E., Brooks A., Chan K.Y., Chopra M., Duke T., Marsh D., Pio A., et al. Setting research priorities to reduce global mortality from childhood pneumonia by 2015. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wazny K., Zipursky A., Black R., Curtis V., Duggan C., Guerrant R., Levine M., Petri W.A., Santosham M., Scharf R., et al. Setting research priorities to reduce mortality and morbidity of childhood diarrhoeal disease in the next 15 years. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frison S., Angood C., Khara T., Bahwere P., Black R.E., Briend A., Connell N., Fenn B., Isanaka S., James P., et al. Prevention of child wasting: Results of a Child Health & Nutrition Research Initiative (CHNRI) prioritisation exercise. Masquelier B, editor. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0228151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans K., Janiszewski H., Evans C., Spiby H. Establishing information needs and research priorities in response to the Covid-19 pandemic in the local maternity setting. Midwifery. 2021;95 doi: 10.1016/j.midw.2021.102922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudan I., Gibson J.L., Ameratunga S., El Arifeen S., Bhutta Z.A., Black M., Black R.E., Brown K.H., Campbell H., Carneiro I., et al. Setting Priorities in Global Child Health Research Investments: Guidelines for Implementation of the CHNRI Method. Croat Med J. 2008;49:720–733. doi: 10.3325/cmj.2008.49.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haider B.A., Bhutta Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2017;4:CD004905. doi: 10.1002/14651858.CD004905.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koivu A.M., Hunter P.J., Näsänen-Gilmore P., Muthiani Y., Isojärvi J., Pörtfors P., Ashorn U., Ashorn P. Modular literature review: a novel systematic search and review method to support priority setting in health policy and practice. BMC Med Res Methodol. 2021;21:268. doi: 10.1186/s12874-021-01463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisele T.P., Larsen D.A., Anglewicz P.A., Keating J., Yukich J., Bennett A., Hutchinson P., Steketee R.W. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis. 2012;12:942–949. doi: 10.1016/S1473-3099(12)70222-0. [DOI] [PubMed] [Google Scholar]
- 35.Ashorn P., Alho L., Ashorn U., Cheung Y.B., Dewey K.G., Harjunmaa U., Lartey A., Nkhoma M., Phiri N., Phuka J., et al. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. Am J Clin Nutr. 2015;101:387–397. doi: 10.3945/ajcn.114.088617. [DOI] [PubMed] [Google Scholar]
- 36.Adu-Afarwuah S., Lartey A., Okronipa H., Ashorn P., Zeilani M., Peerson J.M., Arimond M., Vosti S., Dewey K.G. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr. 2015;101:835–846. doi: 10.3945/ajcn.114.091546. [DOI] [PubMed] [Google Scholar]
- 37.Koivu A.M., Nasanen-Gilmore P.K., Hunter P.J., Muthiani Y., Isojarvi J., Heimonen O., Bastola K., Csonka L., Ashorn P., Ashorn U. Antenatal interventions to address harmful behaviors and psychosocial risk factors in the prevention of low birth weight. Am J Clin Nutr. 2023;117:S148–S159. doi: 10.1016/j.ajcnut.2022.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Muthiani Y., Hunter P.J., Nasanen-Gilmore P.K., Koivu A.M., Isojarvi J., Luoma J., Salenius M., Hadji M., Ashorn U., Ashorn P. Antenatal interventions to reduce risk of low birth weight related to maternal infections during pregnancy. Am J Clin Nutr. 2023;117:S118–S133. doi: 10.1016/j.ajcnut.2023.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Nasanen-Gilmore P.K., Koivu A.M., Hunter P.J., Muthiani Y., Portfors P., Heimonen O., Kajander V., Ashorn P., Ashorn U. A modular systematic review of antenatal interventions targeting modifiable environmental exposures in improving low birth weight. Am J Clin Nutr. 2023;117:S160–S169. doi: 10.1016/j.ajcnut.2022.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Hunter P.J., Muthiani Y., Nasanen-Gilmore P.K., Koivu A.M., Portfors P., Bastola K., Vimpeli R., Luoma J., Ashorn U., Ashorn P. A modular systematic review of antenatal interventions to address undernutrition during pregnancy in the prevention of low birth weight. Am J Clin Nutr. 2023;117:S134–S147. doi: 10.1016/j.ajcnut.2023.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Kapiriri L., Chanda-Kapata P. The quest for a framework for sustainable and institutionalised priority-setting for health research in a low-resource setting: the case of Zambia. Health Res Policy Syst. 2018;16:11. doi: 10.1186/s12961-017-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida S., Martines J., Lawn J.E., Wall S., Souza J.P., Rudan I., Cousens S. neonatal health research priority setting group, Aaby P, Adam I, et al. Setting research priorities to improve global newborn health and prevent stillbirths by 2025. J Glob Health. 2016;6 doi: 10.7189/jogh.06.010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahl R., Martines J., Bhandari N., Biloglav Z., Edmond K., Iyengar S., Kramer M., Lawn J.E., Manandhar D.S., Mori R., et al. Setting research priorities to reduce global mortality from preterm birth and low birth weight by 2015. J Glob Health. 2012;2 doi: 10.7189/jogh.02-010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.GAPPS Expert Group on Community Based Strategies and Constraints. George A., Young M., Bang A., Chan K.Y., Rudan I., Victora C.G., Chopra M., Rubens C. Setting implementation research priorities to reduce preterm births and stillbirths at the community level. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes F., Bourassa M.W., Adu-Afarwuah S., Ajello C., Bhutta Z.A., Black R., Catarino E., Chowdhury R., Dalmiya N., Dwarkanath P., et al. Setting research priorities on multiple micronutrient supplementation in pregnancy. Ann N Y Acad Sci. 2020;1465:76–88. doi: 10.1111/nyas.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization . 2020. WHO antenatal care recommendations for a positive pregnancy experience: nutritional interventions update : multiple micronutrient supplements during pregnancy. [PubMed] [Google Scholar]
- 47.Gomes F., Ashorn P., Askari S., Belizan J.M., Boy E., Cormick G., Dickin K.L., Driller-Colangelo A.R., Fawzi W., Hofmeyr G.J., et al. Calcium supplementation for the prevention of hypertensive disorders of pregnancy: current evidence and programmatic considerations. Ann N Y Acad Sci. 2022;1510:52–67. doi: 10.1111/nyas.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alfirevic Z., Stampalija T., Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Pregnancy and Childbirth Group. 2017 doi: 10.1002/14651858.CD008991.pub3. Cochrane Database Syst Rev [Internet] 2017 [cited 2021 Nov 10] Available from: https://doi.wiley.com/10.1002/14651858.CD008991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Short V.L., Hoffman M., Metgud M., Kavi A., Goudar S.S., Okitawutshu J., Tshefu A., Bose C.L., Mwenechanya M., Chomba E., et al. Safety of daily low-dose aspirin use during pregnancy in low-income and middle-income countries. AJOG Glob Rep. 2021;1 doi: 10.1016/j.xagr.2021.100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smaill F.M., Vazquez J.C. Cochrane Pregnancy and Childbirth Group; 2019. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev [Internet] [cited 2021 Oct 29]; Available from: https://doi.wiley.com/10.1002/14651858.CD000490.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization . World Health Organization; Geneva: 2021. WHO recommendations on antiplatelet agents for the prevention of pre-eclampsia. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request pending application to and approval by the Author Group of the LBW priority-setting exercise.