Abstract
Background
Although increasing evidence suggests that polyphenol helps regulate blood pressure (BP), evidence from large-scale and long-term population-based studies is still lacking.
Objectives
This study aimed to investigate the association between dietary polyphenol and hypertension risk in the China Health and Nutrition Survey (N = 11,056).
Methods
Food intake was assessed using 3-d, 24-h dietary recalls and household weighing method; polyphenol intake was calculated by multiplying consumption of each food and its polyphenol content. Hypertension was defined as BP ≥ 140/90 mmHg, physicians’ diagnosis, or taking antihypertension medications. HR and 95% CI were estimated using mixed-effects Cox models.
Results
During 91,561 person-years of follow-up, a total of 3866 participants developed hypertension (35%). The lowest multivariable-adjusted HR (95% CI) of hypertension risk occurred in the third quartile intake, which was 0.63 (0.57, 0.70) for total polyphenol, 0.61 (0.55, 0.68) for flavonoid, 0.62 (0.56, 0.69) for phenolic acid, 0.46 (0.42, 0.51) for lignan, and 0.58 (0.52, 0.64) for stilbene, compared with the lowest quartile. The polyphenol-hypertension associations were nonlinear (all Pnonlinearity < 0.001), and different patterns were observed. U-shaped relations with hypertension were observed for total polyphenol, flavonoid, and phenolic acid, whereas L-shaped associations were observed for lignan and stilbene. Moreover, higher fiber intake strengthened the polyphenol-hypertension association, especially for lignan (P-interaction = 0.002) and stilbene (P-interaction = 0.004). Polyphenol-containing food, particularly vegetables and fruits rich in lignan and stilbene, were significantly associated with lower hypertension risk.
Conclusions
This study demonstrated an inverse and nonlinear association between dietary polyphenol, especially lignan and stilbene, and hypertension risk. The findings provide implications for hypertension prevention.
Keywords: hypertension, polyphenol, plant-based food, fiber, cohort study, China
Introduction
Hypertension is a major health threat for humans, increasing risk of CVDs and mortality and affecting 1.39 billion adults globally [1,2]. Dietary modification is considered a promising strategy to prevent hypertension [3]. Several dietary patterns, such as DASH, Mediterranean, and Nordic diets, have been reported to help regulate hypertension [4]. The underlying mechanism explaining the hypotensive effect of these dietary patterns remains unclear, but almost all hypotensive dietary patterns emphasize the consumption of plant-based food.
Studies have suggested that phytochemicals might account for the hypotensive effect of plant-based food, among which polyphenol was suggested to be the most potent [5]. Evidence from animal and in vitro studies supporting the blood pressure (BP)–lowering effect of polyphenol has accumulated [6]. However, evidence from population-based studies is mixed. Although some cross-sectional studies demonstrated an inverse association between the intake of phenolic acid, lignan, and stilbene and hypertension incidence [[7], [8], [9]], some reported nonsignificant results [[10], [11], [12]]. Prospective studies on this topic are very limited and have inconsistent findings as well. One prospective study including 2725 Polish older individuals revealed that total polyphenol, flavonoid, and phenolic acid at different intake amounts reduced hypertension risk to varying extents in female participants [13]. In another prospective study of 1265 adults from Tehran, researchers found no difference in hypertension risk in relation to total and all polyphenol class intakes [14]. These findings suggest that different polyphenol classes at different intake amounts might have different effects on hypertension. Thus, it is essential to characterize the continuous relationship between different classes of polyphenol and hypertension and explore possibly nonlinear relationships to provide more general information.
Therefore, the purpose of this study is to examine the longitudinal relationship between different classes of dietary polyphenol and their source food with hypertension risk in the China Health and Nutrition Survey (CHNS), an 18-y Nationwide Cohort Study. The findings of this study will help clarify the relationship between polyphenol intake and hypertension risk and provide implications for hypertension prevention.
Methods
Study population
The CHNS is an ongoing, large-scale, household-based cohort launched in 1989 and follows up with participants every 2–4 y. Using multistage random-cluster sampling, the CHNS includes participants from 12 out of 31 provinces/autonomous cities in China [15]. The CHNS was approved by the Institutional Review Committees of the University of North Carolina at Chapel Hill, the National Institute for Nutrition and Health, and the Chinese Center for Disease Control and Prevention. Details of the CHNS have been described elsewhere [[15], [16], [17]].
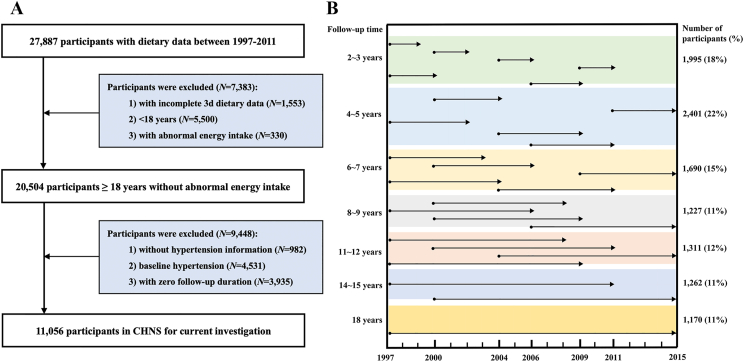
The current study used 7 rounds of the CHNS data from 1997 to 2015. A total of 27,887 participants with dietary data between 1997–2011 were included. Participants were excluded if they 1) were aged <18 y (N = 5500); 2) had incomplete 3-d dietary data (N = 1553); 3) reported implausible energy intake (<800 or >4200 kcal/d for men and <600 or >3500 kcal/d for women) (N = 330); 4) had missing hypertension information (N = 982); 5) had hypertension at baseline (N = 4531); 6) had no follow-up record (N = 3935). Finally, a total of 11,056 participants were included in the study (Figure 1A). The follow-up timeline of included participants is shown in Figure 1B.
FIGURE 1.
The study population (A) and follow-up timeline (B) in the CHNS study (N = 11,056). The height of the colored bar represents the percentage of the population in that follow-up duration in the timeline figure. CHNS, China Health and Nutrition Survey.
Assessment of dietary polyphenol and nutrient intake
In each survey, diet was assessed using individual 3-d, 24-h dietary recalls (24-HDRs) and household food inventory [15]. Beverage intake was assessed using a self-reported consumption frequency questionnaire [18].
A composition database for assessing polyphenol intake was derived from the polyphenol content in the China Food Composition Table (CFCT), Phenol-Explorer [19], and the US Department of Agriculture Database [20]. To combine different databases, polyphenol content was converted into aglycone content using molecular weights [21]. The database included 5 major polyphenol classes—flavonoid, phenolic acid, lignan, stilbene, and other polyphenol. Polyphenol intake was calculated by multiplying the consumption of each food by its polyphenol content. Then, effects of cooking on polyphenol content were considered by applying retention factors [22]. Polyphenol intake was adjusted for total energy intake using the residual method.
Nutrient intake was calculated using updated versions of the CFCT for each round and included total energy, fiber, sodium, potassium, calcium, magnesium, vitamin C, vitamin E, SFA, PUFA, and MUFA. Cumulative mean intake of polyphenol and nutrients was calculated from all available rounds from baseline to the last round before hypertension diagnosis, death, or end of follow-up to represent participants’ long-term diet.
BP assessment and hypertension diagnosis
BP was measured with calibrated mercury sphygmomanometers using the same standard procedures in each wave. After a 5-min rest, the BP of each participant was measured from the right arm in the sitting position by certified health workers or nurses. Three measurements of BP were taken and averaged to derive the BP in each wave [23].
Hypertension was diagnosed if any of the following criteria were met: 1) an average of 3 systolic BP (SBP) measurements ≥140 mmHg; 2) an average of 3 diastolic BP (DBP) measurements ≥90 mmHg; 3) the participant reported a physician diagnosis of hypertension; or 4) the participant was currently taking antihypertensive medication.
Covariates
To control for potential confounding between polyphenol intake and hypertension risk, we included the following covariates in the analysis—age; sex (male or female); district (rural or urban); region (north or south); education (never, ≤9 y, >9 y, or unknown); income (quartile); physical activity (very light, light, moderate, heavy, very heavy, no working ability, or unknown); smoking (nonsmoker, former smoker, current smoker, or unknown); current drinking status (no, yes, or unknown); BMI (in kg/m2; <18.5, 18.5–24, 24–28, ≥28, or unknown); abdominal obesity (no, yes, or unknown); baseline SBP; diabetes (yes, no, or unknown); stroke (yes, no, or unknown); MI (yes, no, or unknown); and dietary intakes of total energy, sodium, potassium, fiber, and PUFA (all in quartiles).
Statistical analysis
Person-years were calculated from the time of the baseline survey to the date of hypertension diagnosis, death, or end of follow-up, whichever came first. Mixed-effects Cox proportional hazards models with a random intercept for household (to account for the similar dietary habits in clustering of family) were used to estimate HR and 95% CI of hypertension risk according to polyphenol intake quartile, adjusting for the abovementioned covariates. The proportional hazards assumption was checked using the Schoenfeld residuals method with no evidence of deviation. The linear trend was tested by assigning a median value to each quartile of the polyphenol intake. We constructed restricted cubic spline mixed-effects Cox regression models with 4 knots (the fifth, 35th, 65th, and 95th percentiles) to explore the potential nonlinear relationship between polyphenol intake and hypertension risk.
In food-based analyses, HRs were estimated using the mixed-effects Cox model, adjusting for the abovementioned nondietary factors and intake of energy, salts, red meat, and other polyphenol-derived food groups. Spearman’s correlation analysis was conducted to examine the correlations among total and polyphenol class, fiber, potassium, calcium, magnesium, vitamin C, and vitamin E.
Analyses were further stratified by sex, age, BMI, physical activity, smoking status, and drinking status. P values for interaction were calculated using likelihood ratio tests comparing models with and without interaction between stratified variables and polyphenol intake. To evaluate the potential interaction between fiber and polyphenol intake on hypertension risk, we first dichotomized the polyphenol intake into 2 halves at the median cutoff and then calculated HRs comparing the second and first half of polyphenol intake by a median of fiber intake.
We conducted several sensitivity analyses to test the robustness of the primary findings. First, calcium, magnesium, vitamin C, vitamin E, SFA, and MUFA intake were further adjusted to examine if the associations were independent of other dietary confounders. Second, 5 classes of polyphenols were mutually adjusted in one model to examine whether the effect of polyphenol classes was independent of each other. Third, pregnant women, participants with other chronic diseases (diabetes, MI, and stroke) at baseline or during follow-up, and participants who developed hypertension in the first 2 y of follow-up were excluded. Fourth, the analyses were restricted to the participants without missing covariates, and multiple imputations of missing covariates by chained equation was performed. Fifth, the analyses were restricted to participants participating in all waves of the CHNS survey. Sixth, participants with or without family members having CVDs/hypertension was further adjusted, and subgroup analysis by this variable was performed. Seventh, a restricted cubic spline with 5 knots (the fifth, 27.5th, 50th, 72.5th, and 95th percentiles) was constructed to test the robustness of the relationship. All analyses were performed using R (version 4.2.2) and SPSS 25. A 2-tailed P < 0.05 was considered statistically significant.
Results
Characteristics
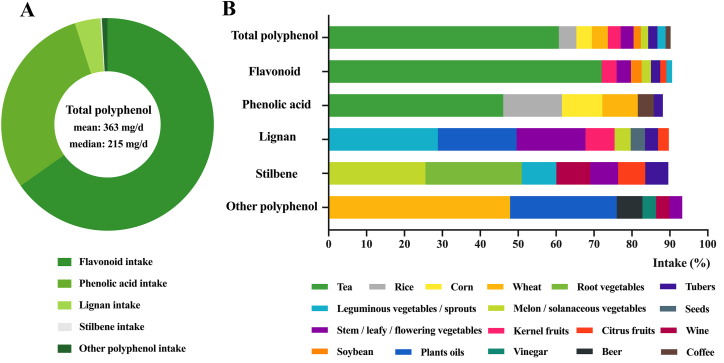
The mean (SD) age of the 11,056 participants free of hypertension at baseline was 41.5 (14.1) y. Most participants were female (54.2%). The median follow-up time was 7 y (range: 2–18 y). The median total polyphenol intake was 214.5 (111.1–473.3) mg/d (Supplemental Table 1). Flavonoid and phenolic acid were the major contributors to total polyphenol intake, accounting for 65% and 30% of the total consumption, respectively. Lignan provided 4% of the total intake. About 1% was from stilbene and other polyphenol (Figure 2A, Supplemental Table 1). Tea was the top dietary contributor to flavonoid and phenolic acid (72% and 42%, respectively). Leguminous vegetables/sprouts, plant oils, stem/leafy/flowering vegetables, and kernel fruits were predominant food contributors to lignan. Melon/solanaceous vegetables, root vegetables, and leguminous vegetables/sprouts were the main sources of stilbene (Figure 2B). Intake of all dietary nutrients, excepted for sodium intake, increased across polyphenol quartiles. Participants with higher polyphenol intake were slightly older; more likely to be male, urban residents, smokers, and drinkers; and had higher education and income (Supplemental Table 2).
FIGURE 2.
Constituents of polyphenol intake and major food source (N = 11,056).
Polyphenol intake and incident hypertension
During the 91,561 person-y of follow-up, 3866 out of 11,056 participants (35%) developed hypertension. The lowest multivariable-adjusted HR (95% CI) of hypertension risk occurred in the third quartile intake, which was 0.63 (0.57, 0.70) for total polyphenol, 0.61 (0.55, 0.68) for flavonoid, 0.62 (0.56, 0.69) for phenolic acid, 0.46 (0.42, 0.51) for lignan, and 0.58 (0.52, 0.64) for stilbene, compared with the lowest quartile (Table 1).
TABLE 1.
Associations between polyphenol intake and hypertension risk (N = 11,056)1
| Types of polyphenol | Quartile |
P-trend2 | |||
|---|---|---|---|---|---|
| Q1 (N = 2764) | Q2 (N = 2764) | Q3 (N = 2764) | Q4 (N = 2764) | ||
| Total polyphenol | |||||
| Intake (mg/d)3 | 79 [10–113] | 155 [113–215] | 305 [215–456] | 728 [456–7556] | |
| Number of cases/person-y | 1085/20,942 | 907/24,448 | 886/24,169 | 988/22,002 | |
| Model 14 | 1.00 | 0.66 (0.60, 0.73) | 0.62 (0.57, 0.69) | 0.68 (0.61, 0.75) | <0.001 |
| Model 25 | 1.00 | 0.64 (0.58, 0.71) | 0.63 (0.57, 0.70) | 0.69 (0.62, 0.76) | <0.001 |
| Flavonoid | |||||
| Intake (mg/d)3 | 34 [3–50] | 75 [50–117] | 186 [117–304] | 509 [304–5708] | |
| Number of cases/person-y | 1105/20,638 | 921/24,430 | 831/24,213 | 1009/22,280 | |
| Model 14 | 1.00 | 0.66 (0.60, 0.73) | 0.56 (0.51, 0.62) | 0.66 (0.60, 0.73) | <0.001 |
| Model 25 | 1.00 | 0.70 (0.63, 0.77) | 0.61 (0.55, 0.68) | 0.69 (0.63, 0.77) | <0.001 |
| Phenolic acid | |||||
| Intake (mg/d)3 | 30 [4–39] | 51 [39–69] | 94 [69–132] | 198 [132–1832] | |
| Number of cases/person-y | 1042/20,838 | 914/23,992 | 903/24,762 | 1007/21,969 | |
| Model 14 | 1.00 | 0.70 (0.64, 0.78) | 0.64 (0.58, 0.70) | 0.74 (0.67, 0.81) | 0.021 |
| Model 25 | 1.00 | 0.65 (0.58, 0.72) | 0.62 (0.56, 0.69) | 0.71 (0.64, 0.78) | 0.073 |
| Lignan | |||||
| Intake (mg/d)3 | 1 [0–2.5] | 4.5 [2.5–7.2] | 10.9 [7.2–17.1] | 28 [17.1–1278.3] | |
| Number of cases/person-y | 1279/19,752 | 927/24,905 | 835/25,059 | 825/21,845 | |
| Model 14 | 1.00 | 0.51 (0.46, 0.56) | 0.46 (0.41, 0.50) | 0.54 (0.49, 0.59) | <0.001 |
| Model 25 | 1.00 | 0.54 (0.49, 0.60) | 0.46 (0.42, 0.51) | 0.48 (0.43, 0.54) | <0.001 |
| Stilbene | |||||
| Intake (mg/d)3 | 0.16 [0–0.28] | 0.39 [0.28–0.53] | 0.69 [0.53–0.91] | 1.38 [0.91–45.75] | |
| Number of cases/person-y | 1175/19,670 | 939/24,287 | 847/24,773 | 905/22,831 | |
| Model 14 | 1.00 | 0.62 (0.56, 0.68) | 0.51 (0.46, 0.57) | 0.59 (0.54, 0.66) | <0.001 |
| Model 25 | 1.00 | 0.66 (0.59, 0.72) | 0.58 (0.52, 0.64) | 0.68 (0.61, 0.76) | <0.001 |
| Other polyphenol | |||||
| Intake (mg/d)3 | 0.47 [0–1.04] | 1.67 [1.04–2.37] | 3.14 [2.37–4.03] | 5.27 [4.03–409.84] | |
| Number of cases/person-y | 1044/22,504 | 901/23,724 | 954/23,544 | 967/21,789 | |
| Model 14 | 1.00 | 0.84 (0.76, 0.92) | 0.87 (0.79, 0.96) | 0.96 (0.87, 1.06) | 0.381 |
| Model 25 | 1.00 | 0.80 (0.72, 0.88) | 0.81 (0.73, 0.89) | 0.85 (0.76, 0.95) | 0.085 |
Values are HRs (95% CIs) based on mixed-effects Cox proportional hazards models with a random intercept for household.
P-trend was tested by assigning a median value to each quartile of the polyphenol intake.
Median [range]. Polyphenol intake was adjusted for total energy intake using the residual method.
Model 1, adjusted for age and sex.
Model 2, further adjusted for district, region, education, income, physical activity, smoking status, drinking status, BMI, abdominal obesity, baseline systolic blood pressure, diabetes, MI, stroke, intakes of total energy, sodium, potassium, fiber, and PUFA.
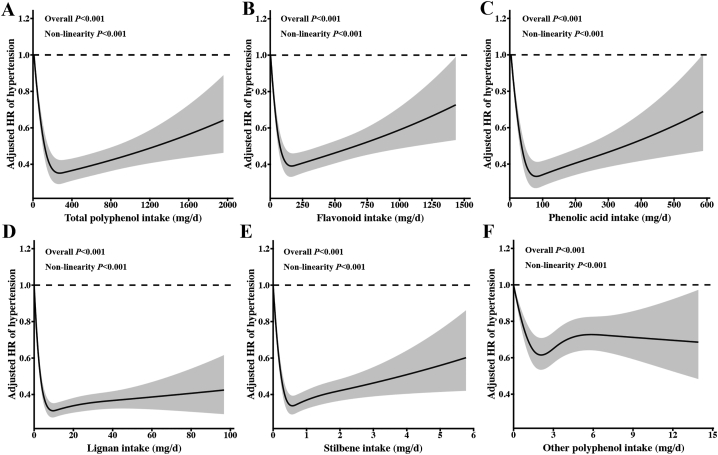
In the spline regression models, the nonlinearity test was significant for all polyphenol classes (Pnonlinearity < 0.001) (Figure 3). Slightly U-shaped associations were observed between total polyphenol, flavonoid, and phenolic acid with hypertension risk; the lowest risk exited at ∼300, 170, and 85 mg/d, respectively (Figure 3A–C). Lignan and stilbene showed stronger L-shaped relations with hypertension, and risk reduction plateaued at 10 and 0.6 mg/d, respectively (Figure 3D, E).
FIGURE 3.
Cubic spline curves for the association between polyphenol intake and hypertension risk (N = 11,056). The associations between intakes of total polyphenol (A), flavonoid (B), phenolic acid (C), lignan (D), stilbene (E), other polyphenol (F) with hypertension risk. HR (95% CI) based on mixed-effects Cox proportional hazards models with a random intercept for household, adjusted for age, sex, district, region, education, income, physical activity, smoking status, drinking status, BMI, abdominal obesity, baseline systolic blood pressure, diabetes, MI, stroke, intakes of total energy, sodium, potassium, fiber, and PUFA. Polyphenol intake was adjusted for total energy intake using the residual method. Data were truncated at the 99th percentiles of polyphenol intake to limit the impact of extreme values. Poverall and Pnonlinearity were calculated using the likelihood ratio test.
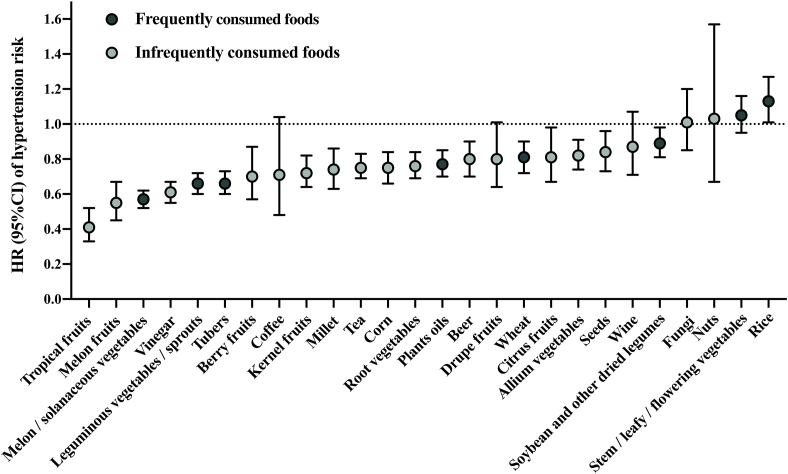
Many types of polyphenol-containing food were significantly associated with a lower risk of hypertension (Figure 4). Using stepwise regression, lignan- and stilbene-rich food, including leguminous vegetables/sprouts, melon/solanaceous vegetables, root vegetables, seeds, and plant oils, flavonoid- and phenolic acid-rich food, including tea, corn, tropical fruits, melon fruits, tubers, and vinegar, were selected as independent factors of hypertension risk. Generally, polyphenol content did not correlate with other nutrient content within food (Supplemental Figure 1).
FIGURE 4.
Associations between polyphenol-containing foods and hypertension risk (N = 11,056). For frequently consumed foods, HR was the highest tertile group compared with the lowest tertile group; for infrequently consumed foods, HR was the high consumption group compared with the nonconsumption group. HR (95% CI) based on mixed-effects Cox proportional hazards models with a random intercept for household, adjusted for age, sex, district, region, education, income, physical activity, smoking status, drinking status, BMI, abdominal obesity, baseline systolic blood pressure, diabetes, MI, stroke, dietary intakes of total energy, salts, red meat, and other polyphenol food groups.
Stratified analysis of sex, age, BMI, smoking, drinking status, physical activity, and fiber intake
Consistent results were observed across sex, age, BMI, and drinking status. The stronger negative polyphenol-hypertension association was observed among participants with light physical activity (P-interaction < 0.001) or nonsmokers (P-interaction = 0.048) (Supplemental Figure 2). The inverse association between total polyphenol intake and hypertension was more apparent among individuals with higher fiber intake, but the difference did not reach statistical significance. The inverse association of lignan and stilbene with hypertension risk was significantly strengthened by higher fiber intake (P-interaction = 0.002 for lignan, P-interaction = 0.004 for stilbene) (Table 2).
TABLE 2.
Associations between polyphenol intake and hypertension risk by median of fiber intake (N = 11,056)1
| Fiber intake | Higher vs. lower median of polyphenol intake2 |
|||||
|---|---|---|---|---|---|---|
| Total polyphenol | Flavonoid | Phenolic acid | Lignan | Stilbene | Other polyphenol | |
| Low | 0.87 (0.78, 0.97) | 0.81 (0.73, 0.90) | 0.86 (0.78, 0.96) | 0.71 (0.64, 0.80) | 0.87 (0.78, 0.97) | 0.87 (0.78, 0.98) |
| High | 0.79 (0.71, 0.88) | 0.75 (0.68, 0.84) | 0.80 (0.72, 0.88) | 0.59 (0.53, 0.65) | 0.69 (0.62, 0.77) | 1.00 (0.90, 1.12) |
| P-interaction3 | 0.171 | 0.385 | 0.294 | 0.002 | 0.004 | 0.082 |
Values are HRs (95% CIs) based on mixed-effects Cox proportional hazards models with a random intercept for household, adjusted for age, sex, district, region, education, income, physical activity, smoking status, drinking status, BMI, abdominal obesity, baseline systolic blood pressure, diabetes, MI, stroke, intakes of total energy, sodium, potassium, and PUFA.
Polyphenol intake was adjusted for total energy intake using the residual method.
P-interaction were calculated using likelihood ratio tests comparing models with and without interaction between fiber intake and polyphenol intake.
Sensitivity analysis
In the sensitivity analysis of further adjustment for other potential dietary factors, similar results were observed (Supplemental Table 3). When mutually adjusting for the 5 polyphenol classes, no meaningful difference was found for most classes, except for flavonoid, of which the inverse association was attenuated (Supplemental Table 3). After excluding participants who were pregnant, had chronic diseases, or developed hypertension in the first 2 y of follow-up, the results were materially unchanged (Supplemental Table 3). After multiple imputations of missing covariates or restricting participants without missing covariates, the results remained unchanged. Similar results were found in participants participating in all waves (Supplemental Table 3). Further adjustment for participants with or without family members having CVDs/hypertension did not change the results (Supplemental Table 3), and the estimates were similar in subgroup analysis (Supplemental Table 4). The general shape, trend, and dose-effect point of the 5-knots cubic spline was similar to the 4-knots spline (Supplemental Figure 3).
Discussion
In this 18-y cohort study of 11,056 participants, the intake of total polyphenol and 4 main polyphenol classes as well as many polyphenol-containing foods was associated with lower hypertension risk, with lignan and stilbene showing the strongest protective effests despite the lowest consumption. L-shaped associations were documented for lignan and stilbene with hypertension risk whereas U-shaped associations were observed for total polyphenol and its major class flavonoid and phenolic acid. Furthermore, potential synergistic interactions were found between polyphenol and fiber intake, especially for lignan and stilbene.
Lignan and stilbene, comprising <5% of total polyphenol intake, showed the strongest associations with hypertension. L-shaped curves that plateaued at 10 and 0.6 mg/d, respectively, were documented in the spline. The effective dose of lignan and stilbene is relatively high in comparison with previous studies [11,13]. Several factors may contribute to this phenomenon. First, vegetable intake in China ranks top 5 globally (357 g/capita/d) [24], with per capita legume intake 3 times that of Europe/United States [25]. Melon/cucurbit crops consumption is the largest worldly, and root vegetables, represented by radish, are the second largest consumed vegetable in China [26,27], contributing to the high lignan/stilbene intake. Second, most previous studies assessed 2 to 8 individual lignans using earlier polyphenol databases. We quantified 27 individual lignans and 5 individual stilbenes with expanded databases, which inferred broader dose-response relationships. Last, we utilized 3-d 24-HDRs and household weighing methods and included comprehensive polyphenol-source food, which captured more potential sources of polyphenol than food frequency questionnaires.
Consistent with the L-shape relationship of lignan and stilbene with hypertension risk identified in our study, human intervention trials have shown that the flaxseed lignan complex or resveratrol supplement significantly decreases BP at high doses [28,29]. In comparison, other studies examining lower intake levels found no significant risk reductions [11,13]. Interestingly, a study reported that lignan intake at 2.21 mg/d was associated with lower hypertension risk (OR: 0.41; 95% CI: 0.22, 0.76) compared with that at 0.75 mg/d in 301 Dutch postmenopausal women [7]. One possible explanation is that lignan and stilbene are structurally similar to estradiol and may act as a phytoestrogen [30], which offers better protection to women experiencing a steep decline of estrogen, even at a low level of consumption.
Our study showed a U-shaped relationship of flavonoid and phenolic acid with hypertension risk, which is consistent with an Australian cohort showing a U-shaped relationship between quintile flavonoid intake and hypertension in young women, and the maximal risk reduction at 169.6 mg/d in middle-aged women was close to our dose-effect point (170 mg/d) [31]. The U-shaped relationship partly explained the inconsistent results from previous epidemiological studies. Cassidy et al. [32] found a significant 6% risk reduction in hypertension in the third quintile (358 mg/d) during a 14-y follow-up in the Nurses’ Health Study I. A study of 550 Brazilians, with the highest flavonoid intake tertile (41.5 mg/d) far lower than the dose-effect point of our curve, reported nonsignificant hypertension risk reductions [9], and another study of 2618 adults from Iran, with the lowest intake group (549 mg/d) at the right segment of our U-curve, also showed a nonsignificant effect [12].
For total polyphenol intake level, the dose-effect is much more complicated because the composition varied in different studies. Existing evidence supports that the biological activities of polyphenol do not follow a typically linear dose-response relationship, and a threshold probably exists [33,34]. Researchers have found that most polyphenols cannot be well-absorbed in high doses from food [35]. Consistent with our results, a cross-sectional study in adults from Brazil indicated a U-shaped polyphenol-hypertension relationship in which the second tertile (360.58 mg/d) achieved the greatest risk reduction (OR: 0.36; 95% CI: 0.19, 0.69) [9]. Another 4-y follow-up study in 2725 Polish older individuals showed significantly inverse polyphenol-hypertension associations in women [13]. These findings are consistent with our study. However, a cohort of 1265 adults with the lowest total polyphenol tertile (827 mg/d) at the right side of our U-shaped curve, reported no significant associations with elevated BP (SBP/DBP ≥ 130/85 mmHg) during a 6-y follow-up [14]. We guess that the difference in risk estimates may stem from different polyphenol composition because phenolic acid was the main contributor to their total intake whereas flavonoid contributes most in our study. Meanwhile, the difference in the hypertension diagnosis (SBP/DBP ≥ 140/90 mmHg compared with SBP/DBP ≥ 130/85 mmHg) may also account for the discrepancy.
In addition to the dietary intake amounts, the final biological effects of polyphenols greatly depend on gut microbial metabolism [30]. For example, lignans require gut microbiota to convert them into enterolignans, which can then be absorbed by the human body and exhibit stronger estrogenic and antioxidant activity compared with their precursors [36]. Dietary fiber, another important component in plant food, functions as a prebiotic and increases gut microbial diversity [37]. An in vitro study demonstrated that fecal bacteria suspensions incubating flavanone with fiber significantly increased the abundance of Lactobacillus and Clostridium leptum, which promoted more flavanone metabolite production [38]. An animal study showed that dogs fed with more fiber produced more fecal lignan metabolites [39]. For the first time, we confirmed the synergistic BP-lowering effect of fiber and polyphenol, specifically lignan and stilbene, in a population-based study.
The association with hypertension appeared to be class dependent. The large variety of individual polyphenols, varying structures and bioactivities, potential interaction with other food components, and the distinct absorption and metabolism process may influence the effects of different polyphenol classes [6]. Thus, it makes this study a unique contribution to the literature. To our knowledge, this is the first study investigating the nonlinear relationship between dietary polyphenol and different classes with hypertension risk in a nationwide cohort; this study is also the first population-based study evaluating the role of fiber-polyphenol interaction in hypertension risk. Our findings offer new evidence for dietary hypertension prevention, highlighting the importance of incorporating polyphenol-rich foods, particularly those rich in lignan and stilbene, into the daily diet. The protective threshold is achievable in a daily diet—50 g of legumes or half a pear provides ∼10 mg lignan, whereas 50 g of melon vegetables or 30 g of carrots contains ∼0.6 mg stilbene. However, several limitations should be noted. First, polyphenol content in each food varies based on plant species, growing condition, storage, and processing; thus, it is hard to assess it accurately. We utilized the average polyphenol content of each food, which is commonly applied in large epidemiologic studies because participants likely consumed various food from different sources. We used the most recent database in China combined with 2 of the most comprehensive databases worldwide to maximize accuracy. Second, dietary measurement error is inevitable. Detailed intakes of highly diverse polyphenol-containing food are obtained using 24-HDRs and averaging multiple dietary assessments during follow-up to reduce random error and best reflect long-term diet.
In conclusion, evidence from this 18-y nationwide study suggests that higher polyphenol intake is significantly associated with a lower hypertension risk. Lignan and stilbene showed the strongest associations with hypertension, and risk reduction plateaued at 10 and 0.6 mg/d of intake, respectively. This study provides important evidence for developing strategies for hypertension prevention.
Acknowledgments
We thank Professor Gang Liu for his helpful comments and guidance in this work. We thank Qiao Huang and Pengfei Xia for their support and guidance on data analysis. This research uses data from China Health and Nutrition Survey (CHNS). We are grateful for research grant funding from the NIH; the Eunice Kennedy Shriver NICHD for R01 HD30880 and R01 HD38700; the NIA for R01 AG065357; the NIDDK for R01 DK104371 and P30 DK056350; the NHLBI for R01 HL108427; the NIH Fogarty grant D43 TW009077; the Carolina Population Center for P2 CHD050924 and P30 AG066615 since 1989; and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011. We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Beijing Municipal Center for Disease Control and Prevention, and the Chinese National Human Genome Center in Shanghai.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.05.001.
Contributor Information
Chong Tian, Email: tianchong0826@hust.edu.cn.
Chenjiang Ying, Email: yingcj@hust.edu.cn.
Authors’ contributions
The authors’ responsibilities were as follows—CT and CY: designed the research; XL: performed statistical analyses and drafted the initial manuscript; JZ, QX, XG, and LL: performed data management and statistical analyses; SH, JW, and FP: searched for literature; SG, HL, YF, XZ, CT, and CY: revised it critically for important intellectual content; and all authors: revised and approved the final manuscript.
Conflicts of interest
The authors report no conflicts of interest.
Funding
The study was supported by the National Natural Science Foundation of China (82173522, 81673161).
Data availability
The datasets generated and/or analyzed during the current study are available in the China Health and Nutrition Survey (https://www.cpc.unc.edu/projects/china).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Risk Factors Collaborators, Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelong H., Blacher J., Baudry J., Adriouch S., Galan P., Fezeu L., et al. Individual and combined effects of dietary factors on risk of incident hypertension: prospective analysis from the NutriNet-Sante cohort. Hypertension. 2017;70(4):712–720. doi: 10.1161/HYPERTENSIONAHA.117.09622. [DOI] [PubMed] [Google Scholar]
- 4.Sukhato K., Akksilp K., Dellow A., Vathesatogkit P., Anothaisintawee T. Efficacy of different dietary patterns on lowering of blood pressure level: an umbrella review. Am. J. Clin. Nutr. 2020;112(6):1584–1598. doi: 10.1093/ajcn/nqaa252. [DOI] [PubMed] [Google Scholar]
- 5.Silva A.S., Reboredo-Rodríguez P., Süntar I., Sureda A., Belwal T., Loizzo M.R., et al. Evaluation of the status quo of polyphenols analysis: part I-phytochemistry, bioactivity, interactions, and industrial uses. Compr. Rev. Food Sci. Food Saf. 2020;19(6):3191–3218. doi: 10.1111/1541-4337.12629. [DOI] [PubMed] [Google Scholar]
- 6.Grosso G., Godos J., Currenti W., Micek A., Falzone L., Libra M., et al. The effect of dietary polyphenols on vascular health and hypertension: current evidence and mechanisms of action. Nutrients. 2022;14(3):545. doi: 10.3390/nu14030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreijkamp-Kaspers S., Kok L., Bots M.L., Grobbee D.E., van der Schouw Y.T. Dietary phytoestrogens and vascular function in postmenopausal women: a cross-sectional study. J. Hypertens. 2004;22(7):1381–1388. doi: 10.1097/01.hjh.0000125435.28861.d2. [DOI] [PubMed] [Google Scholar]
- 8.Godos J., Sinatra D., Blanco I., Mulè S., La Verde M., Marranzano M. Association between dietary phenolic acids and hypertension in a Mediterranean cohort. Nutrients. 2017;9(10):1069. doi: 10.3390/nu9101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miranda A.M., Steluti J., Fisberg R.M., Marchioni D.M. Association between polyphenol intake and hypertension in adults and older adults: a population-based study in Brazil. PLOS ONE. 2016;11(10) doi: 10.1371/journal.pone.0165791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godos J., Bergante S., Satriano A., Pluchinotta F.R., Marranzano M. Dietary phytoestrogen intake is inversely associated with hypertension in a cohort of adults living in the Mediterranean area. Molecules. 2018;23(2):368. doi: 10.3390/molecules23020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creus-Cuadros A., Tresserra-Rimbau A., Quifer-Rada P., Martínez-González M.A., Corella D., Salas-Salvadó J., et al. Associations between both lignan and yogurt consumption and cardiovascular risk parameters in an elderly population: observations from a cross-sectional approach in the PREDIMED study. J. Acad. Nutr. Diet. 2017;117(4):609–622.e1. doi: 10.1016/j.jand.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Sohrab G., Hosseinpour-Niazi S., Hejazi J., Yuzbashian E., Mirmiran P., Azizi F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int. J. Food Sci. Nutr. 2013;64(6):661–667. doi: 10.3109/09637486.2013.787397. [DOI] [PubMed] [Google Scholar]
- 13.Grosso G., Stepaniak U., Micek A., Kozela M., Stefler D., Bobak M., et al. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur. J. Nutr. 2018;57(4):1535–1544. doi: 10.1007/s00394-017-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohrab G., Ebrahimof S., Hosseinpour-Niazi S., Yuzbashian E., Mirmiran P., Azizi F. Association of dietary intakes of total polyphenol and its subclasses with the risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metab. Syndr. Relat. Disord. 2018;16(6):274–281. doi: 10.1089/met.2017.0140. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B., Zhai F.Y., Du S.F., Popkin B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014;15(Suppl 1):2–7. doi: 10.1111/obr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C., Wu Q., Ye Z., Liu M., Zhang Z., Zhang Y., et al. Inverse association between variety of proteins with appropriate quantity from different food sources and new-onset hypertension. Hypertension. 2022;79(5):1017–1027. doi: 10.1161/HYPERTENSIONAHA.121.18222. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Yang Q., An R., Sesso H.D., Zhong V.W., Chan K.H.K., et al. Famine and trajectories of body mass index, waist circumference, and blood pressure in two generations: results from the CHNS from 1993–2015. Hypertension. 2022;79(3):518–531. doi: 10.1161/HYPERTENSIONAHA.121.18022. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y.H., Wang Z., Chiang T.C., Liu C.T. Beverage intake, smoking behavior, and alcohol consumption in contemporary China-a cross-sectional analysis from the 2011 China Health and Nutrition Survey. Int. J. Environ. Res. Public Health. 2017;14(5):493. doi: 10.3390/ijerph14050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neveu V., Perez-Jiménez J., Vos F., Crespy V., du Chaffaut L., Mennen L., et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010 doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhagwat S., Haytowitz D.B. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA; March 2018. USDA database for the flavonoid content of selected foods, Release 3.3.https://data.nal.usda.gov/dataset/usda-database-flavonoid-content-selected-foods-release-33-march-2018 [Internet] [date updated 2022-06-11; date cited 2019-06-14]. Available from: [Google Scholar]
- 21.Witkowska A.M., Zujko M.E., Waśkiewicz A., Terlikowska K.M., Piotrowski W. Comparison of various databases for estimation of dietary polyphenol intake in the population of Polish adults. Nutrients. 2015;7(11):9299–9308. doi: 10.3390/nu7115464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothwell J.A., Medina-Remón A., Pérez-Jiménez J., Neveu V., Knaze V., Slimani N., et al. Effects of food processing on polyphenol contents: a systematic analysis using phenol-explorer data. Mol. Nutr. Food Res. 2015;59(1):160–170. doi: 10.1002/mnfr.201400494. [DOI] [PubMed] [Google Scholar]
- 23.Kalmpourtzidou A., Eilander A., Talsma E.F. Global vegetable intake and supply compared to recommendations: a systematic review. Nutrients. 2020;12(6):1558. doi: 10.3390/nu12061558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin B., Viera A.J., Muntner P., Plassman B.L., Edwards L.J., Adair L.S., et al. Visit-to-visit variability in blood pressure is related to late-life cognitive decline. Hypertension. 2016;68(1):106–113. doi: 10.1161/HYPERTENSIONAHA.116.07494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo F., Zhang Q., Yin Y., Liu Y., Jiang H., Yan N., et al. Legume consumption and risk of hypertension in a prospective cohort of Chinese men and women. Br. J. Nutr. 2020;123(5):564–573. doi: 10.1017/S0007114519002812. [DOI] [PubMed] [Google Scholar]
- 26.Li Z.G., Nong Y., Farooq T., Tang Y.F., She X.M., Yu L., et al. Small RNA deep sequencing reveals the presence of multiple viral infections in cucurbit crops in Guangdong, China. J. Integr. Agric. 2022;21(5):1389–1400. doi: 10.1016/s2095-3119(21)63661-4. [DOI] [Google Scholar]
- 27.Zhang J., He W., Smith W.N., Grant B.B., Ding W., Jiang R., et al. Exploring management strategies to improve yield and mitigate nitrate leaching in a typical radish field in northern China. J. Environ. Manage. 2021;290 doi: 10.1016/j.jenvman.2021.112640. [DOI] [PubMed] [Google Scholar]
- 28.Peterson J., Dwyer J., Adlercreutz H., Scalbert A., Jacques P., McCullough M.L. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010;68(10):571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Ma W., Zhang P., He S., Huang D. Effect of resveratrol on blood pressure: a meta-analysis of randomized controlled trials. Clin. Nutr. 2015;34(1):27–34. doi: 10.1016/j.clnu.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Seyed Hameed A.S., Rawat P.S., Meng X., Liu W. Biotransformation of dietary phytoestrogens by gut microbes: a review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020;43 doi: 10.1016/j.biotechadv.2020.107576. [DOI] [PubMed] [Google Scholar]
- 31.do Rosario V.A., Schoenaker D.A.J.M., Kent K., Weston-Green K., Charlton K. Association between flavonoid intake and risk of hypertension in two cohorts of Australian women: a longitudinal study. Eur. J. Nutr. 2021;60(5):2507–2519. doi: 10.1007/s00394-020-02424-9. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy A., O’Reilly É.J., Kay C., Sampson L., Franz M., Forman J.P., et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011;93(2):338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bondonno N.P., Dalgaard F., Kyrø C., Murray K., Bondonno C.P., Lewis J.R., et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 2019;10(1):3651. doi: 10.1038/s41467-019-11622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y., Li Y., Sampson L., Wang M., Manson J.E., Rimm E., et al. Lignan intake and risk of coronary heart disease. J. Am. Coll. Cardiol. 2021;78(7):666–678. doi: 10.1016/j.jacc.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark J.L., Zahradka P., Taylor C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015;73(12):799–822. doi: 10.1093/nutrit/nuv048. [DOI] [PubMed] [Google Scholar]
- 36.Landete J.M., Arqués J., Medina M., Gaya P., de Las Rivas B., Muñoz R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit. Rev. Food Sci. Nutr. 2016;56(11):1826–1843. doi: 10.1080/10408398.2013.789823. [DOI] [PubMed] [Google Scholar]
- 37.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang R., Yu H., Ruan Z., Zhang L., Xue Y., Yuan X., et al. Effects of food matrix elements (dietary fibres) on grapefruit peel flavanone profile and on faecal microbiota during in vitro fermentation. Food Chem. 2022;371 doi: 10.1016/j.foodchem.2021.131065. [DOI] [PubMed] [Google Scholar]
- 39.Ephraim E., Jewell D.E. Effect of added dietary betaine and soluble fiber on metabolites and fecal microbiome in dogs with early renal disease. Metabolites. 2020;10(9):370. doi: 10.3390/metabo10090370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the China Health and Nutrition Survey (https://www.cpc.unc.edu/projects/china).