Abstract
Background
Low 25-hydroxyvitamin D (25[OH]D) concentrations (<30 ng/mL [<50 nmol/L]) have been associated with muscle weakness and impaired physical performance in observational studies. However, the effect of vitamin D supplementation on changes in muscle strength and physical performance in randomized controlled trials has been mixed.
Objectives
To determine the effect of daily vitamin D supplementation on leg power, strength, and physical performance in low-functioning older adults with 25(OH)D concentrations of 18 to <30 ng/mL.
Methods
In this double-blind, randomized controlled trial, 136 low-functioning [Short Physical Performance Battery (SPPB) scores ≤10] adults aged 65–89 y with 25(OH)D concentrations of 18 to <30 ng/mL were randomly assigned to 2000 IU/d vitamin D3 or placebo for 12 mo. Lower-extremity leg power (primary outcome), leg and grip strength, SPPB, timed up and go (TUG), postural sway, and gait velocity and spatiotemporal parameters (secondary outcomes) were assessed at baseline, 4 and 12 mo. A subset (n = 37) also underwent a muscle biopsy at baseline and 4 mo and muscle fiber composition and contractile properties were assessed.
Results
Participants’ mean ± SD age and SPPB scores at baseline were 73.4 ± 6.3 y and 7.8 ± 1.8, respectively. Mean ± SD 25(OH)D concentrations at baseline and 12 mo were 19.4 ± 4.2 ng/mL and 28.6 ± 6.7 ng/mL in the vitamin D group and 19.9 ± 4.9 ng/mL and 20.2 ± 5.0 ng/mL in the placebo group for a mean ± SE difference of 9.1 ± 1.1 ng/mL (P < 0.0001). However, there were no differences in change in leg power, leg or grip strength, SPPB score, TUG, postural sway, or gait velocity and spatiotemporal parameters by intervention group over 12 mo or muscle fiber composition and contractile properties over 4 mo.
Conclusions
In low-functioning older adults with 25(OH)D concentrations of 18 to <30 ng/mL, randomization to 2000 IU/d vitamin D3 did not result in improvements in leg power, strength, or physical performance or muscle fiber composition and contractile properties.
This trial was registered at clinicaltrials.gov as NCT02015611.
Keywords: older adults, vitamin D, leg power, strength, physical performance
Introduction
Aging is associated with significant declines in muscle strength and physical performance, leading to disability, loss of independence, and adverse clinical outcomes[[1], [2], [3]]. Inadequate vitamin D status may contribute to these age-related declines through both direct effects on muscle function [4,5] and indirectly through its role in age-related conditions that frequently lead to declines in strength and physical performance [6]. However, there remains disagreement among the scientific community on the amount of vitamin D required for optimal muscle function. The most recent National Academy of Medicine report concluded that scientific evidence supports the role of vitamin D for bone health and 25(OH)D concentrations ≥20 ng/mL (≥50 nmol/L) were sufficient, but the evidence was lacking for nonbone health outcomes [7]. The Endocrine Society guidelines, however, recommend 25(OH)D concentrations ≥30 ng/mL (≥75 nmol/L) as beneficial for nonbone health outcomes, including muscle function [8]. The impact of these differing guidelines is relevant given that recent NHANES data suggest approximately one-fifth of older adults had 25(OH)D concentrations below 20 ng/mL, but another one-third had 25(OH)D concentrations between 20 and 30 ng/mL [9].
Observational studies have, in general, shown positive associations between 25(OH)D concentrations and muscle strength and physical performance in older adults [[10], [11], [12]]. Although some observational studies have suggested that muscle strength and physical function increases up to a threshold of ∼30 ng/mL [13,14], others have observed 25(OH)D thresholds closer to 20 ng/mL [[15], [16], [17]]. Trials of vitamin D supplementation and muscle strength and physical performance have been mixed [12,18] – possibly because of differences in trial duration, vitamin D dose, baseline 25(OH)D concentrations, muscle strength, and physical function. Moreover, vitamin D’s effects on the mechanisms underlying muscle function are not well understood. One possible mechanism is type 2 muscle fiber atrophy which has been reported in individuals with vitamin D deficiency [19].
The purpose of this double-blind, randomized controlled trial was to determine the effect of daily vitamin D supplementation with 2000 IU (50 ug) vitamin D3 over 12 mo on muscle power and strength and physical performance in low-functioning older adults with 25(OH)D concentrations of 18 to <30 ng/mL. A subset of participants also underwent a muscle biopsy to determine the effect of vitamin D supplementation on muscle fiber composition and contractile properties over the first 4 mo. We hypothesized that those randomly assigned to a vitamin D3 supplement would have better muscle power, strength, and physical performance at 12 mo and an increase in the proportion of type 2 muscle fibers, muscle fiber cross-sectional area (CSA), and single muscle fiber force at 4 mo compared to those randomly assigned to placebo.
Methods
Participants
Community-dwelling males and females aged 65–89 y were recruited from the Forsyth County, NC area using direct mailing and advertisements between December 2013 and August 2017. Interested individuals were initially screened by telephone for the following inclusion criteria: age 65–89 y; not taking >1000 IU/d (>25 ug/d) over-the-counter vitamin D3 or prescription vitamin D2; having some mobility difficulty; and BMI (in kg/m2) ≤40 kg/m2. Exclusion criteria included the following: ≥5% weight loss in the past 3 mo; insulin-dependent diabetes; class 3 or 4 congestive heart failure; cardiac event or stroke in the past 6 mo; chronic lung disease requiring oxygen; dependent on a walker; Parkinson’s, Alzheimer’s disease or some other serious neurological disorder; liver disease; kidney disease requiring dialysis; kidney stones in the past 5 y; treatment of cancer, except nonmelanoma skin cancer, in the past year; hip fracture or hip or knee replacement in the past 6 mo; sciatica, fibromyalgia, or severe back or leg pain; high-dose (>7.5 mg/d prednisone) oral corticosteroids for >3 mo; and hormone replacement therapy in females. Potentially eligible individuals were then screened in the clinic, which included vitals, medical history, medication review, Short Physical Performance Battery (SPPB), cognitive screen (Montreal Cognitive Assessment, MoCA), and blood draw. Individuals were excluded if SPPB score >10; MoCA score <18; blood pressure >200/110 mmHg; fasting glucose >200 mg/dL; estimated GFR <45 mL/min/1.73 m2 based on the Chronic Kidney Disease Epidemiology Collaboration equation [20]; serum calcium >10.6 mg/dL [21]; and serum 25(OH)D <18 or ≥30 ng/mL. All participants provided written informed consent to participate in the study according to the guidelines set forth by the Wake Forest University School of Medicine Institutional Review Board for Human Research.
Study design and intervention
Participants were randomly assigned to a daily vitamin D3 supplement or placebo in a 1:1 ratio using a web-based randomization scheme (developed by JAT) with stratification by sex and race (White, non-White). A subset of 38 participants was randomly selected from those who were not on anticoagulants to undergo a muscle biopsy of the vastus lateralis at baseline and 4 mo. The vitamin D3 (2000 IU) and placebo capsules were purchased from Tishcon Corp. Vitamin D and placebo capsules were dispensed at baseline and the 4-mo visit. At the 4-mo visit, serum 25(OH)D concentrations were measured in all participants at a clinical laboratory (LabCorp). Participants assigned to vitamin D supplement whose 25(OH)D concentrations were <30 ng/mL at the 4-mo visit were instructed to take an additional vitamin D supplement capsule (for a total of 4000 IU vitamin D3/d). To maintain blinding, for every participant in the vitamin D group that had to increase their vitamin D supplement dose to 2 capsules/d after the 4-mo follow-up visit, a random participant (selected by the unblinded biostatistician [JAT] using a random number generated in a SAS program) from the placebo group seen within the same time frame was also instructed to take 2 placebo capsules/d. Supplement adherence was assessed by a pill count of any unused capsules at the 4- and 12-mo follow-up visits. Monthly phone calls to participants were made to enhance supplement adherence and retention. Participants were asked not to start any new dietary supplements or change the frequency or dose of any dietary supplements they were taking at screening for the duration of the 12-mo intervention.
Measurements
Participants came to the Wake Forest Geriatric Research Center at baseline (2 visits with randomization occurring at the end of the second visit), 4 mo (1 visit unless in the muscle biopsy subgroup and then 2 visits), and 12 mo (1 visit) with vitals measured, a fasting blood sample drawn, and muscle power and strength and physical performance measures assessed at each time point. All study outcome measures, procedures, and assays were collected by staff blinded to the intervention group.
Muscle power, strength, and physical performance
The primary outcome was a change in leg power over 12 mo assessed using the Nottingham Power Rig [22]. Participants unilaterally depressed a foot lever attached to a flywheel as hard and as fast as they could 5 times on each leg. The overall maximum leg power (from the right or left leg at baseline) in watts was used, and the same leg was used at 4- and 12-mo follow-up in all analyses. Relative leg power was calculated by taking the ratio of leg power to kilograms of body mass.
Secondary outcomes included change in muscle strength and physical performance over 12 mo. Knee extensor strength was assessed using an isokinetic dynamometer (Biodex) at 1 speed (60°/s) with the participant sitting and the hips and knee flexed at 90°. Two trials were done on each leg consisting of 4 repetitions each. The maximum knee extensor strength of the 4 repetitions from trial 2 for the dominant leg at baseline was used in analyses unless unable to test the dominant leg (i.e., knee replacement or knee pain), in which case the nondominant leg was used; the maximum knee extensor strength from the same leg was used at 4- and 12-mo follow-up in all analyses. Relative knee extensor strength was calculated by taking the ratio of knee extensor strength to kilograms of body mass. Handgrip strength was assessed using an isometric hydraulic hand dynamometer (Jamar). Two trials were performed in each hand (unless the participant reported hand pain or recent hand/wrist surgery and then only the unaffected side was tested), and the mean of each hand was calculated. The stronger hand at baseline was used in all analyses. Stair climbing, an indicator of knee extensor strength and functional capacity, was assessed by timing how long it took a participant to climb up 4 steps as quickly as possible [23]. Participants could hold onto the handrail if needed.
The SPPB was administered to assess lower-extremity physical performance [24]. The SPPB consisted of standing balance tasks (side-by-side, semi-, and full-tandem stands for 10 s each), a 4-meter walk to assess usual gait speed, and time to complete 5 repeated chair stands. Each of the 3 performance measures was assigned a score ranging from 0 (inability to perform the task) to 4 (the highest level of performance) and summed to create an SPPB score ranging from 0–12 (best). In addition, a modified physical performance battery, the Health ABC Physical Performance Battery (Health ABC PPB), was administered to minimize the ceiling effects of the SPPB [25]. The Health ABC PPB increased the holding time of the standing balance tasks to 30 s and added a single-leg stand and a narrow walk test of balance. Health ABC PPB scores are continuous and range from 0–4, with higher scores indicative of better performance. Physical performance was also assessed using the Timed Up and Go, which measures the time a person takes to stand up from a standard chair, walk 3 meters, turn, walk back to the chair, and sit down again [26].
Postural sway during quiet stance was assessed from Center-of-Pressure trajectory data collected at 100 Hz using an Advanced Mechanical Technology Incorporated AccuSway biomechanics force platform [27]. Participants were barefoot in an upright stance with arms relaxed comfortably at their sides and eyes open, feet abducted 10 degrees, and heels separated mediolaterally by 6 cm. Center-of-Pressure data was collected in a series of 10 30-s trials standing on the force plate alone (firm) followed by 5 30-s trials standing on the force plate with a 6.4 cm thick Airex foam pad placed on the top (foam). The 4 posturographic parameters assessed were maximum anteroposterior and mediolateral displacement, average sway velocity, and 95% confidence ellipse area from the average of the trials under each condition. Higher anteroposterior and mediolateral displacement, average sway velocity, and 95% confidence ellipse area indicate greater postural sway.
Gait velocity and other spatiotemporal parameters were measured using a 4.88-meter long instrumented carpet (GAITRite; CIR Systems Inc.) [28]. Participants made multiple passes over the carpet at either their usual (4 trials) or fast (4 trials) pace. GAITRite summary data was averaged over the 4 usual and 4 fast trials and included velocity (m/s), and the average of the left and right leg for double support (percent of the cycle), single support (percent of the cycle), stance (percent of the cycle), swing (percent of the cycle), stride length (cm), and stride width (cm).
Biochemical measurements
Blood was drawn in the morning after an overnight fast. At screening, 4 and 12 mo, serum total 25(OH)D was measured using an immunochemiluminometric assay in an independent clinical laboratory (DiaSorin LIAISON; LabCorp) in real-time. Kidney function (creatinine and estimated GFR) and serum calcium (colorimetric assay) were also assessed at screening, 4 and 12 mo at the clinical laboratory (LabCorp). Baseline, 4-mo, and 12-mo blood samples were stored at –80°C for analysis of 25(OH)D and 1,25-dihydroxyvitamin D (1,25[OH]2D) after the completion of the trial at Tufts Medical Center. Serum 25(OH)D was measured using liquid chromatography with tandem mass spectrometry (LC/MS/MS) (Waters Acquity ultra performance liquid chromatography with triple quadrupole mass spectrometer) and National Institute of Standards and Technology standards for assay calibration. 1,25[OH]2D was measured using the 5500 QTRAP® LC/MS/MS System (AB Sciex LLC) with Electrospray after immunoaffinity extraction, 4-phenyl-1,2,4-triazole-3,5-dione derivatization, and methylamine adduction [29]. The average intra-assay and inter-assay CVs were 7–8.5% and 9–10%.
Muscle biopsy, single-fiber histology, and physiology
A muscle biopsy of the vastus lateralis was taken at baseline and 4 mo after an overnight fast in a random subset of participants who were not taking anticoagulants. Participants were asked to refrain from taking aspirin, prescription and over-the-counter nonsteroidal anti-inflammatory drugs, or other compounds that may affect bleeding, platelets, or bruising for 5 d prior to and 3 d after the muscle biopsy and to refrain from strenuous activity for 36 h prior to and after the muscle biopsy. Muscle was obtained from the vastus lateralis using the percutaneous needle biopsy technique with a University College Hospital needle under local anesthesia with 1% lidocaine by a trained physician. Visible blood and connective tissue were removed from the muscle specimen, and the muscle portion was used for histochemical analysis. The muscle sample was oriented so that the fibers ran longitudinally, mounted perpendicularly on a plastic dish, partially embedded in Sakura optimal cutting temperature (OCT) compound (Fisher Scientific), thickened with infant powder, snap frozen in liquid nitrogen, and stored at –80°C until analysis [30].
Fiber-type histological analyses were performed following published procedures using the ATPase, pH 9.4 technique, combined with laminin immunostaining in 10 μm muscle sections and the absolute and relative number of type 1 and 2 fiber subtypes (the primary outcome for the muscle biopsy substudy) and their CSA quantified [30]. Vitamin D receptor (VDR) expression was measured by Western blot using the C20 antibody (sc-1008; Santa Cruz Biotechnology, Inc.) raised against a peptide mapping at the C terminus of VDR and normalized to actin used as a loading control. VDR band optical density (OD) was normalized to actin OD using ImageJ software version 1.52a (NIH). Single type 1 and type 2 muscle fiber contraction force, shortening velocity, and power were also examined as previously described (see Supplementary Methods) [31].
Other measurements
Demographic characteristics, including age, sex, race, and education, were assessed by self-report. BMI was calculated using measured weight and height at baseline. The season of the year in which visits occurred was recorded to account for seasonal effects on endogenous vitamin D synthesis and serum 25(OH)D concentrations. Calcium and vitamin D intake from diet and supplements over the past week was assessed at baseline, 4 and 12 mo using a short calcium and vitamin D intake questionnaire. Cognitive function was assessed during screening using the MoCA [32]. Depressed mood was assessed at baseline using the 20-item Center for Epidemiologic Studies Depression Scale [33]. Physical activity was assessed at baseline, 4 and 12 mo using the Community Healthy Activities Model Program for Seniors (CHAMPS) Physical Activity Questionnaire for Older Adults [34]. In addition, adverse events (AEs) were recorded during the monthly phone calls, at each study visit, and by the voluntary reporting of participants at any time during the study.
Sample size and statistical analyses
The study was originally powered to detect a difference in leg power based on SD = 0.382 watts/kg and an effect size of 0.45 (0.17 watts/kg, based on 10% of the mean of 1.74 watts/kg) using a 2-group t-test at 12 mo, allowing for 20% drop out at a 2-sided alpha of 0.05. The mean (SD) was estimated from preliminary data from 17 older adults with measured 25(OH)D concentrations between 20 and 40 ng/mL and leg power measures. Upon recommendation of the trial’s NIH-appointed Data Safety Monitoring Board, we repowered the study using the SD from baseline (N = 116, SD = 0.447) and the correlation with baseline and 12-mo leg power (N = 78, r = 0.736) and adjusted our drop out assumption to 10%. A sample size of 120 participants (133 with adjustment for 10% dropout) provided 86% power for the 0.17 watts/kg difference in leg power using ANCOVA with adjustment for baseline leg power. The muscle biopsy substudy was powered to detect a difference between the 2 groups of a relative increase in type 2 fibers of 25% or more (SD = 23.7%) using a 2-group t-test at 80% power and a 2-sided alpha level of 0.05 with N = 18/group, based on 12% drop out. This was based on preliminary data that indicated a slope of a 2.35% relative increase in type 2 fibers over 4 mo/1 ng/mL increase in 25(OH)D. Extrapolated to our expected change in 25(OH)D of 14 ng/mL, we anticipated a change of 33% but based power on 25% because of the potential drop-in of the control group. Power was calculated using nQuery+nTerim version 4.0 (Statistical Solutions, Boston, MA) [35].
Descriptive statistics (means, SDs, and frequencies) were used to summarize baseline characteristics. Changes in serum 25(OH)D and 1,25(OH)2D were compared by group using 2 group t-tests, and Pearson correlations were used to describe relationships between screening and baseline 25(OH)D values. For the intervention group, “nonresponders” were compared to others using Fisher’s exact tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Muscle power, strength, and physical performance at baseline were analyzed using ANCOVA with adjustment for age, sex, race, and season at randomization (as stated a priori in the protocol). To model change in muscle power, strength, and physical performance, we used a mixed effects model with a constraint of a common baseline mean across treatment groups and an unstructured covariance matrix for baseline, 4 mo, and 12 mo, adjusted for age, sex, race, and season, termed a constrained longitudinal data analysis model [36]; linear contrasts were used to estimate the difference in change between the 2 groups between baseline and 12 mo. This analysis strategy was chosen as it is more efficient than the ANCOVA model when there are missing data at baseline or at the first follow-up time point [37] and was implemented as described in the SAS code provided by Lu [38]. To model differences in change in single-fiber CSA, force, normalized force, velocity, maximum velocity, power, and normalized power from baseline to 4 mo by randomization group, ANCOVA models adjusted for the baseline value, BMI, and season at randomization were used. Least squares mean, which are also referred to as estimated marginal means and are the estimates that would be expected if equal numbers were attainable or held at the mean value [39], are presented along with SEs. All analyses were conducted using SAS version 9.4 (SAS Institute), and a P value of 0.05 was considered statistically significant.
Results
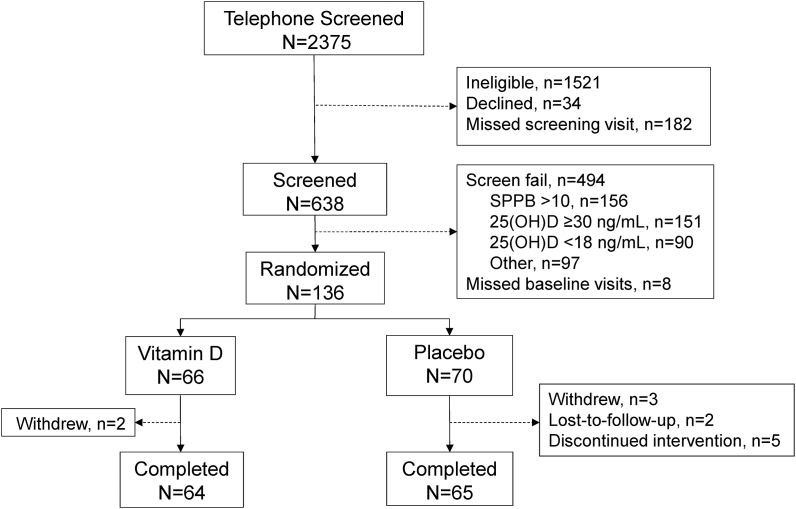
A total of 2375 individuals were screened by phone, 854 individuals were invited to come in for a clinical screening visit, and 638 individuals consented and completed a clinical screening visit (Figure 1). Of these, 144 individuals met all entry criteria, and 136 participants attended the randomization visit and were randomly assigned to either vitamin D3 (n = 66) or placebo (n = 70). Five participants discontinued treatment (0 in the vitamin D group and 5 in the placebo group), 5 participants withdrew (2 in the vitamin D group and 3 in the placebo group), and 2 participants were lost to follow-up (both in the placebo group) resulting in 64 participants in the vitamin D group and 65 participants in the placebo group completing the study.
FIGURE 1.
Consort diagram. SPPB, Short Physical Performance Battery; 25(OH)D, 25-hydroxyvitamin D.
At baseline, participants’ mean ± SD age and SPPB score were 73.4 ± 6.3 y and 7.8 ± 1.8, respectively; 49.3% were female, and 32.4% were African American. Participants in the vitamin D group and placebo group did not differ by age, sex, race, education, BMI, SPPB score, depressive symptoms, dietary or supplemental calcium or vitamin D intake, physical activity, chronic conditions, kidney function, or serum 25(OH)D concentrations at baseline; however, those in the vitamin D group had lower MoCA scores (Table 1).
TABLE 1.
Participant characteristics at baseline by intervention group1
| Vitamin D | Placebo | |
|---|---|---|
| N | 66 | 70 |
| Age, y | 73.7 ± 6.3 | 73.1 ± 6.3 |
| Female, n (%) | 32 (48.5%) | 35 (50.0%) |
| Race, n (%) | ||
| Caucasian | 45 (68.2%) | 47 (67.1%) |
| African American | 21 (31.8%) | 23 (32.9%) |
| ≤ High school education, n (%) | 20 (30.3%) | 14 (20.0%) |
| BMI, kg/m2 | 30.2 ± 4.3 | 30.4 ± 4.6 |
| SPPB score | 7.7 ± 1.9 | 7.8 ± 1.7 |
| MoCA score | 23.3 ± 3.2 | 24.4 ± 2.7 |
| CES-D score | 7.9 ± 6.7 | 7.1 ± 7.1 |
| Dietary intakes | ||
| Calcium, mg/d | 476.6 ± 302.2 | 472.1 ± 265.2 |
| Vitamin D, IU/d | 224.5 ± 147.2 | 186.9 ± 107.9 |
| Calcium supplement use, n (%) | 19 (28.8%) | 28 (40.0%) |
| Dose among users, mg/d | 363.7 ± 227.7 | 311.2 ± 190.3 |
| Vitamin D supplement use, n (%) | 30 (45.5%) | 37 (52.9%) |
| Dose among users, IU/d | 689.2 ± 286.3 | 727.5 ± 226.2 |
| Moderate-intensity physical activity, min/wk | 296.8 ± 345.2 | 289.9 ± 339.7 |
| Fallen in the past year, n(%) | ||
| No falls | 28 (42.4%) | 34 (48.6%) |
| 1 fall | 14 (21.2%) | 19 (27.1%) |
| 2+ falls | 24 (36.4%) | 17 (24.3%) |
| eGFR, mL/min/1.73m2 | 76.7 ± 14.0 | 74.7 ± 15.5 |
| Serum calcium, mg/dL | 9.4 ± 0.4 | 9.5 ± 0.4 |
| 25(OH)D, ng/mL | 19.4 ± 4.2 | 19.9 ± 4.9 |
| 1,25(OH)2D, pg/mL | 29.6 ± 9.7 | 30.5 ± 9.3 |
| Season, n (%) | ||
| Fall | 15 (22.7%) | 15 (21.4%) |
| Winter | 9 (13.6%) | 16 (22.9%) |
| Spring | 18 (27.3%) | 18 (25.7%) |
| Summer | 24 (36.4%) | 21 (30.0%) |
| Chronic conditions, n (%) | ||
| Hypertension | 48 (72.7%) | 45 (64.3%) |
| Diabetes | 15 (22.7%) | 17 (24.3%) |
| Heart disease | 3 (4.5%) | 7 (10.0%) |
| Chronic lung disease | 7 (10.6%) | 5 (7.1%) |
| History of stroke | 7 (10.6%) | 4 (5.7%) |
| Stage 3a kidney disease | 8 (12.1%) | 11 (15.7%) |
BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; MoCA, Montreal Cognitive Assessment; SD, standard deviation; SPPB, Short Physical Performance Battery; 1,25(OH)D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Stage 3a kidney disease, eGFR of 45 to <60 mL/min/1.73m2.
Mean ± SD or N (frequencies).
Serum 25(OH)D concentrations measured at screening using an immunochemiluminometric assay (LabCorp) were 18.0–29.7 ng/mL, and the mean ± SD serum 25(OH)D concentration was 24.5 ± 3.2 ng/mL. At the baseline visit, serum 25(OH)D concentrations measured using LC/MS/MS (Tufts) were 7.2–32.9 ng/mL, with a mean ± SD serum 25(OH)D concentration of 19.6 ± 4.6 ng/mL; 134 of the 136 participants had serum 25(OH)D concentrations <30 ng/mL (65 in the vitamin D group and 69 in the placebo group). There were 26 ± 14 d (mean ± SD) between participants’ screening and baseline study visits. The screening and baseline serum 25(OH)D concentrations were moderately correlated (Pearson r = 0.46, P < 0.0001). Mean ± SD screening 25(OH)D concentrations (LabCorp) were 4.8 ± 4.2 ng/mL higher than the baseline 25(OH)D concentrations (Tufts) (Supplementary Figure 1).
After 4 mo, 10 participants in the vitamin D group had serum 25(OH)D concentrations <30 ng/mL using the clinical laboratory 25(OH)D measure (LabCorp) and were given an additional 2000 IU vitamin D3/d. There were no significant differences between these 10 participants and the 56 participants whose 25(OH)D ≥30 ng/mL in regards to age, race, screening 25(OH)D, eGFR, BMI, vitamin D from food or supplements, or background vitamin D supplement use; however, they did have lower compliance to the vitamin D supplement based on the percent of pills consumed from pill counts than those whose 25(OH)D was ≥30 ng/mL at the 4-mo visit (87.1% compared with 93.6%, P = 0.03).
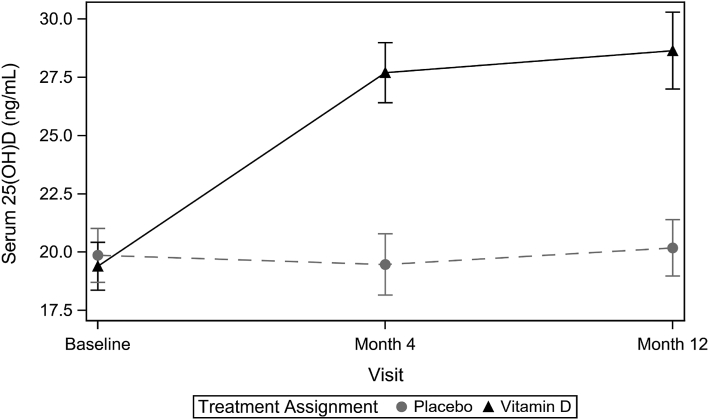
The mean ± SD serum 25(OH)D concentrations as measured by LC/MS/MS (Tufts) increased from 19.4 ± 4.2 ng/mL at baseline to 28.6 ± 6.7 ng/mL at 12-mo follow-up in the vitamin D group and from 19.9 ± 4.9 ng/mL to 20.2 ± 5.0 ng/mL in the placebo group for a mean ± SE difference of 9.1 ± 1.1 ng/mL (P < 0.0001; Figure 2). Baseline serum 1,25(OH)2D3 did not differ between the 2 groups (mean ± SD: 29.6 ± 9.7 compared with 30.5 ± 9.3 pg/mL in the vitamin D compared with the placebo group, respectively) and did not change significantly over the 12-mo follow-up (mean ± SD difference: 1.6 ± 9.8 compared with –0.1 ± 10.6 pg/mL in the vitamin D compared with the placebo group, respectively; P = 0.36). Supplement compliance (≥80% of supplement/placebo pills based on pill counts) was 90.3% in the vitamin D group and 87.7% in the placebo group at 12 mo. Although participants were asked to continue taking any multivitamin or other vitamin D-containing supplements they were consuming when they screened for the study for the duration of the 12-mo intervention, 6 participants reported stopping their background vitamin D-containing supplements during the study, 12 participants changed their background dose of supplemental vitamin D, and 6 participants reported starting a new vitamin D-containing supplement.
FIGURE 2.
Change in serum 25(OH)D concentrations as measured by LC/MS/MS over 12 mo by intervention group (n = 136 at baseline, n = 133 at 4 mo, and n = 129 at 12 mo). Solid line (triangles) is the vitamin D group; dashed line (circles) is the placebo group. 25(OH)D, 25-hydroxyvitamin D.
Muscle power and strength and physical performance
Leg power, leg and grip strength, and physical performance did not differ between the vitamin D and placebo group at baseline (Table 2). Change in leg power, leg and grip strength, and physical performance did not differ significantly between the vitamin D and placebo groups over 12 mo (Table 3); however, the placebo group had less decline in knee extensor strength over 12 mo than the vitamin D group, but the role of chance could not be ruled out (P = 0.08). Measures of postural sway and gait velocity and other spatiotemporal parameters also did not differ significantly between groups at baseline or over 12 mo. Unadjusted values (mean ± SD) for leg power, leg and grip strength, and physical performance at baseline, 4 mo, and 12 mo are shown in .Supplementary Table 1
TABLE 2.
Baseline muscle power and strength and physical performance by intervention group1
| Vitamin D |
Placebo |
|||
|---|---|---|---|---|
| N | LS means ± SE | N | LS means ± SE | |
| Muscle power and strength | ||||
| Leg power, watts | 66 | 117.3 ± 4.7 | 69 | 123.3 ± 4.5 |
| Leg power quality, watts/kg | 66 | 1.35 ± 0.05 | 69 | 1.41 ± 0.05 |
| Knee extensor strength, Nm | 55 | 101.9 ± 3.5 | 60 | 95.4 ± 3.2 |
| Knee extensor quality, Nm/kg | 55 | 1.19 ± 0.04 | 60 | 1.12 ± 0.04 |
| Grip strength, kg | 65 | 29.5 ± 0.9 | 69 | 30.6 ± 0.8 |
| Physical performance | ||||
| SPPB score (0–12) | 66 | 7.7 ± 0.2 | 70 | 7.7 ± 0.2 |
| Health ABC PPB score (0–4) | 62 | 1.62 ± 0.06 | 65 | 1.65 ± 0.06 |
| Balance time, s | 64 | 58.6 ± 3.0 | 67 | 60.5 ± 2.9 |
| 4-meter usual gait speed, meters/s | 65 | 0.77 ± 0.02 | 68 | 0.77 ± 0.02 |
| Chair stand times, s | 56 | 17.4 ± 0.7 | 59 | 16.5 ± 0.7 |
| Timed Up and Go, s | 66 | 12.3 ± 0.3 | 68 | 11.5 ± 0.3 |
| 4 stair climb, s | 63 | 3.0 ± 0.1 | 68 | 3.1 ± 0.1 |
| Force plate: firm base | ||||
| 95% confidence ellipse path, cm2 | 61 | 0.55 ± 0.06 | 68 | 0.55 ± 0.05 |
| Average sway velocity, cm/s | 61 | 0.85 ± 0.03 | 68 | 0.83 ± 0.03 |
| ML maximum displacement, cm | 61 | 0.74 ± 0.04 | 68 | 0.73 ± 0.04 |
| AP maximum displacement, cm | 61 | 1.00 ± 0.04 | 68 | 1.02 ± 0.04 |
| Force plate: foam base | ||||
| 95% confidence ellipse path, cm2 | 61 | 2.03 ± 0.16 | 68 | 1.93 ± 0.15 |
| Average sway velocity, cm/sec | 61 | 1.37 ± 0.05 | 68 | 1.34 ± 0.04 |
| ML maximum displacement, cm | 61 | 1.67 ± 0.07 | 68 | 1.62 ± 0.06 |
| AP maximum displacement, cm | 61 | 1.79 ± 0.07 | 68 | 1.79 ± 0.06 |
| GAITRite: usual pace | ||||
| Double support, % of cycle | 64 | 31.0 ± 0.5 | 69 | 30.7 ± 0.5 |
| Single support, % of cycle | 64 | 34.6 ± 0.3 | 69 | 34.7 ± 0.2 |
| Stance, % of cycle | 64 | 65.4 ± 0.3 | 69 | 65.3 ± 0.2 |
| Stride length, cm | 64 | 116.9 ± 2.2 | 69 | 117.6 ± 2.0 |
| Stride width, cm | 64 | 10.8 ± 0.4 | 69 | 10.8 ± 0.4 |
| Swing, % of cycle | 64 | 34.6 ± 0.3 | 69 | 34.7 ± 0.2 |
| Velocity, cm/s | 64 | 102.5 ± 2.5 | 69 | 102.3 ± 2.4 |
| GAITRite: fast pace | ||||
| Double support, % of cycle | 64 | 25.4 ± 0.6 | 69 | 25.0 ± 0.5 |
| Single support, % of cycle | 64 | 37.4 ± 0.3 | 69 | 37.7 ± 0.3 |
| Stance, % of cycle | 64 | 62.6 ± 0.3 | 69 | 62.3 ± 0.3 |
| Stride length, cm | 64 | 137.9 ± 2.6 | 69 | 141.3 ± 2.4 |
| Stride width, cm | 64 | 10.7 ± 0.4 | 69 | 10.6 ± 0.4 |
| Swing, % of cycle | 64 | 37.4 ± 0.3 | 69 | 37.7 ± 0.3 |
| Velocity, cm/s | 64 | 152.8 ± 4.0 | 69 | 154.7 ± 3.7 |
AP, anterior-posterior; Health ABC PPB, Health ABC Physical Performance Battery; LS means, least squares means; ML, medial-lateral; SE, standard error; SPPB, Short Physical Performance Battery.
LS means ± SE adjusted for age, sex, race, and season.
TABLE 3.
Adjusted change in muscle power and strength and physical performance over 12 mo by intervention group and the difference in 12-mo change between intervention groups1
| Vitamin D |
Placebo |
Difference in change (vitamin D – placebo) |
P value for difference in change by group | |||
|---|---|---|---|---|---|---|
| N2 | LS Means ± SE | N2 | LS Means ± SE | LS Means ± SE | ||
| Muscle power and strength | ||||||
| Leg power, watts | 61 | –11.96 ± 3.75 | 61 | –7.97 ± 3.77 | –4.00 ± 5.26 | 0.45 |
| Leg power quality, watts/kg | 61 | –0.13 ± 0.04 | 61 | –0.10 ± 0.04 | –0.03 ± 0.06 | 0.63 |
| Knee extensor strength, Nm | 45 | –8.09 ± 1.75 | 50 | –3.84 ± 1.66 | –4.25 ± 2.37 | 0.08 |
| Knee extensor quality, Nm/kg | 45 | –0.09 ± 0.02 | 50 | –0.04 ± 0.02 | –0.04 ± 0.03 | 0.15 |
| Grip strength, kg | 56 | –1.54 ± 0.50 | 64 | –1.39 ± 0.48 | –0.15 ± 0.69 | 0.82 |
| Physical performance | ||||||
| SPPB score (0–12) | 60 | 1.64 ± 0.22 | 63 | 1.83 ± 0.22 | –0.18 ± 0.29 | 0.53 |
| Health ABC PPB score (0–4) | 54 | 0.19 ± 0.04 | 51 | 0.17 ± 0.04 | 0.02 ± 0.06 | 0.75 |
| Balance time, s | 59 | 1.32 ± 1.98 | 57 | 3.06 ± 2.02 | –1.74 ± 2.58 | 0.50 |
| 4-meter usual gait speed, meters/s | 60 | 0.07 ± 0.02 | 61 | 0.08 ± 0.02 | 0.00 ± 0.02 | 0.83 |
| Chair stand times, s | 56 | –3.12 ± 0.53 | 54 | –3.04 ± 0.53 | –0.08 ± 0.59 | 0.89 |
| Timed Up and Go, s | 58 | 1.01 ± 0.21 | 59 | 0.98 ± 0.21 | 0.03 ± 0.27 | 0.92 |
| 4 stair climb, s | 60 | 0.41 ± 0.07 | 61 | 0.32 ± 0.07 | 0.09 ± 0.10 | 0.35 |
| Force plate: firm base | ||||||
| 95% confidence ellipse path, cm2 | 54 | 0.03 ± 0.04 | 51 | 0.00 ± 0.04 | 0.03 ± 0.06 | 0.61 |
| Average sway velocity, cm/s | 54 | 0.01 ± 0.01 | 51 | 0.01 ± 0.01 | 0.00 ± 0.02 | 0.94 |
| ML maximum displacement, cm | 54 | 0.06 ± 0.04 | 51 | –0.01 ± 0.04 | 0.07 ± 0.05 | 0.21 |
| AP maximum displacement, cm | 54 | 0.02 ± 0.03 | 51 | 0.00 ± 0.03 | 0.02 ± 0.04 | 0.59 |
| Force plate: foam base | ||||||
| 95% confidence ellipse path, cm2 | 53 | –0.16 ± 0.09 | 55 | –0.05 ± 0.09 | –0.11 ± 0.13 | 0.39 |
| Average sway velocity, cm/sec | 53 | 0.04 ± 0.03 | 55 | 0.00 ± 0.03 | 0.04 ± 0.04 | 0.37 |
| ML maximum displacement, cm | 53 | –0.04 ± 0.05 | 55 | –0.06 ± 0.05 | 0.01 ± 0.07 | 0.86 |
| AP maximum displacement, cm | 53 | –0.02 ± 0.05 | 55 | –0.07 ± 0.04 | 0.04 ± 0.06 | 0.49 |
| GAITRite: usual pace | ||||||
| Double support, % of cycle | 54 | 0.03 ± 0.27 | 58 | 0.33 ± 0.26 | –0.30 ± 0.37 | 0.41 |
| Single support, % of cycle | 54 | 0.00 ± 0.14 | 58 | –0.11 ± 0.13 | 0.11 ± 0.19 | 0.56 |
| Stance, % of cycle | 54 | 0.00 ± 0.14 | 58 | 0.11 ± 0.13 | –0.11 ± 0.19 | 0.58 |
| Stride length, cm | 54 | –0.53 ± 1.00 | 58 | –1.02 ± 0.98 | 0.49 ± 1.38 | 0.72 |
| Stride width, cm | 54 | 0.57 ± 0.21 | 58 | 0.13 ± 0.21 | 0.45 ± 0.29 | 0.13 |
| Swing, % of cycle | 54 | 0.01 ± 0.14 | 58 | –0.10 ± 0.13 | 0.11 ± 0.19 | 0.57 |
| Velocity, cm/s | 54 | 0.42 ± 1.49 | 58 | –0.07 ± 1.45 | 0.49 ± 2.05 | 0.81 |
| GAITRite: fast pace | ||||||
| Double support, % of cycle | 55 | 1.06 ± 0.33 | 60 | 0.73 ± 0.32 | 0.33 ± 0.45 | 0.46 |
| Single support, % of cycle | 55 | –0.50 ± 0.17 | 60 | –0.33 ± 0.16 | –0.16 ± 0.23 | 0.48 |
| Stance, % of cycle | 55 | 0.50 ± 0.17 | 60 | 0.33 ± 0.16 | 0.18 ± 0.23 | 0.44 |
| Stride length, cm | 55 | –4.21 ± 1.12 | 60 | –4.49 ± 1.09 | 0.28 ± 1.53 | 0.85 |
| Stride width, cm | 55 | 0.00 ± 0.20 | 60 | 0.14 ± 0.20 | –0.15 ± 0.28 | 0.60 |
| Swing, % of cycle | 55 | –0.49 ± 0.17 | 60 | –0.33 ± 0.16 | –0.16 ± 0.23 | 0.48 |
| Velocity, cm/s | 55 | –11.03 ± 2.13 | 60 | –6.65 ± 2.06 | –4.37 ± 2.88 | 0.13 |
AP, anterior-posterior; Health ABC PPB, Health ABC Physical Performance Battery; LS means, least squares means; ML, medial-lateral; SE, standard error; SPPB, Short Physical Performance Battery.
LS means ± SE adjusted for age, sex, race, and season (time-varying).
Number of participants with measures at 12-mo visit.
Muscle biopsy substudy
In the muscle biopsy substudy (n = 38 randomly assigned; n = 37 with baseline muscle biopsy because of 1 refusal), the participants’ mean ± SD age was 72.8 ± 7.0, 51% were female, and 35% were African American. Participants in the vitamin D group (n = 19) and placebo group (n = 18) did not differ by demographics, leg power, leg and grip strength, physical performance measures, or serum 25(OH)D concentrations at baseline; however, those in the placebo group had a higher mean BMI and were more likely to be randomly assigned during the Winter/Spring months (Supplementary Table 2). Two participants experienced an adverse event to the muscle biopsy procedure (1 participant in the placebo group and 1 participant in the vitamin D group) and did not have the procedure repeated at the 4-mo follow-up visit. In addition, another participant (in the placebo group) withdrew from the study prior to the 4-mo follow-up visit, and another participant’s baseline muscle sample was not usable (in the placebo group).
The mean ± SD serum 25(OH)D concentrations as measured by LC/MS/MS (Tufts) in the muscle biopsy substudy increased from 20.0 ± 2.8 ng/mL at baseline to 27.7 ± 5.7 ng/mL at 4-mo follow-up in the vitamin D group and from 20.6 ± 3.8 ng/mL to 20.8 ± 4.7 ng/mL in the placebo group for a mean ± SE difference of 8.1 ± 4.2 ng/mL (P < 0.0001; Supplementary Figure 2). There were no differences between the vitamin D and placebo group at baseline in the relative number of type 1 to type 2 fiber subtypes (mean ± SD: 31 ± 12% and 69 ± 12% compared with 38 ± 17% and 62 ± 17%, respectively, in the vitamin D compared with the placebo group) or their CSA (mean ± SD: 5836 ± 2156 compared with 5928 ± 1431 um2 for type 1 subtype and 3771 ± 1921 compared with 4069 ± 2164 um2 for type 2 subtype in the vitamin D compared with the placebo group, respectively; Supplementary Table 3). At 4-mo follow-up, there was a decrease in the relative number of type 2 fiber subtypes in both the vitamin D group (mean ± SD: –3.4 ± 3.0%, n = 18) and placebo group (–2.8 ± 3.5%, n = 14; P = 0.91 between groups), as well as a decrease in the CSA for both type 1 (mean ± SD: –566 ± 403 compared with –492 ± 465 μm2 in the vitamin D compared with the placebo group, respectively; P = 0.91) and type 2 (mean ± SD: –245 ± 385 compared with –616 ± 445 μm2 in the vitamin D compared with the placebo group, respectively; P = 0.57) fiber subtypes (Supplementary Table 4). There was no difference in VDR expression at baseline by intervention group (mean ± SD: 1.9 ± 0.6 compared with 2.2 ± 1.1 in the vitamin D and placebo group, respectively). At 4-mo follow-up, there was a decrease in VDR expression in both groups (mean ± SD: –0.11 ± 0.15, n = 18 compared with –0.33 ± 0.17, n = 15, for vitamin D and placebo groups, respectively; P = 0.37 between groups; Supplementary Table 4). There were also no differences in baseline or 4-mo change in single-fiber contraction force, shortening velocity, or power by intervention group (see Supplementary Tables 3 and 4). Results for change in fiber type, VDR, and single-fiber contractility were similar in unadjusted models (Supplementary Table 5).
AEs
There were 25 serious AEs reported during the trial (10 events in the vitamin D group and 15 in the placebo group) – none of which were related to the study intervention or procedures. During the trial, there were 165 nonserious AEs (72 events in the vitamin D group and 93 in the placebo group). There were 2 AEs that were related to the study procedures – 2 participants experienced an AE during the muscle biopsy procedure (1 participant in the placebo group had a vasovagal response during the muscle biopsy; 1 participant in the vitamin D group had numbness with paresthesia in an area distal to the incision following the muscle biopsy). Sixteen falls were reported as AEs – 6 in the vitamin D group and 10 in the placebo group. No participants experienced elevated serum calcium (>10.6 mg/dL) over the 12-mo intervention. Three participants (1 in the placebo group and 2 in the vitamin D group) were prescribed high-dose corticosteroids (40–50 mg/d) for short durations (3–8 d) during the trial.
Discussion
In this 12-mo double-blind, randomized controlled trial of low-functioning older adults with 25(OH)D concentrations between 18 and <30 ng/mL, daily supplementation with 2000 IU vitamin D3 improved 25(OH)D concentrations by ∼9 ng/mL; however, change in leg power, leg and grip strength, and physical performance did not differ by intervention group. There was also no effect of vitamin D3 supplementation on the underlying physiological mechanisms, including muscle fiber composition, contraction force, shortening velocity, and power over the first 4 mo.
Previous vitamin D supplementation trials on muscle strength and physical performance in older adults have been mixed [12,18]. The discrepancy in findings in vitamin D supplementation trials has been hypothesized to be due in part to differences in baseline sample characteristics, such as including those with sufficient 25(OH)D concentrations and those who are well-functioning, as well as differences in trial duration and vitamin D dose. Similar to our study, several other recent randomized controlled trials found no effect of vitamin D supplementation on leg power [40], leg strength [40,41], grip strength [[40], [41], [42], [43], [44]], SPPB scores [[40], [41], [42], [43], [44], [45]], or Timed Up and Go [41,43,44,46]. Several of these studies included older adults with low serum 25(OH)D concentrations (25(OH)D <30 ng/mL) hypothesized to benefit from vitamin D supplementation [[40], [41], [42], [43], [44]]. However, even in the studies limited to older adults with serum 25(OH)D concentrations <20 ng/mL [40,41], there was no effect of vitamin D supplementation on muscle strength or physical performance in these studies even though the achieved serum 25(OH)D concentrations were generally >30 ng/mL. Several studies also included older adults who were low-functioning [[41], [42], [43]] or at elevated fall risk [44] in whom increasing serum 25(OH)D concentrations would be hypothesized to benefit muscle strength and physical performance; however, there was no effect of vitamin D supplementation on muscle strength or physical performance. Most studies were 12 mo or longer [40,[43], [44], [45]], a sufficient length of time to observe the beneficial effect of vitamin D supplementation on muscle strength and physical performance. Although vitamin D3 supplement doses ranged from 800–4000 IU/d, even those studies with lower vitamin D3 doses achieved serum 25(OH)D concentrations close to or above 30 ng/mL and did not observe a beneficial effect of vitamin D supplementation on muscle strength or physical performance [40,41]. Studies using different vitamin D dosing regimens (weekly, monthly, etc.) and formulations over 4–12 mo also found no effect on muscle strength or physical performance [[47], [48], [49], [50]]. Thus, our findings are consistent with these trials showing no beneficial effect of vitamin D supplementation on muscle strength and physical performance in older adults. Although Pfeifer et al. [51,52] observed a decrease in postural sway with vitamin D supplementation in older adults over both 8 wk and 20 mo, we found no effect of vitamin D3 supplementation on postural sway.
VDRs have been identified in skeletal muscle [53]. Vitamin D metabolites affect muscle metabolism through both genomic and nongenomic pathways to influence calcium uptake, phosphate transport across the cell membrane, phospholipid metabolism, initiation of myogenesis, and muscle cell proliferation, differentiation, and apoptosis [5]. Type 2 muscle fiber atrophy is found in patients with severe vitamin D deficiency [19]. Among low-functioning (SPPB < 10) older females at risk for disability with 25(OH)D concentrations between 9 and 24 ng/mL (n = 21), 4000 IU/d vitamin D3 increased intramyonuclear VDR concentration and total muscle fiber (combined type 1 and 2) CSA over 4 mo; however, similar to our results, change in the relative proportion of type 1 and type 2 fibers and type 1- and type 2-specific CSA did not differ between the vitamin D and placebo groups [54]. Among older females with osteopenia and 25(OH)D concentrations <30 ng/mL (n = 25), 3200 IU/d of vitamin D3 for 6 mo increased type 1 fiber CSA compared to placebo; however, there was no difference in the relative proportion of type 1 and type 2 fibers between groups [55]. There were also no differences in muscle fiber-type distribution or CSA with 800 IU/d vitamin D3 in older frail and prefrail adults with 25(OH)D concentrations <20 ng/mL (n = 35) over 6 mo [41].
Strengths of this study include the double-blind, randomly assigned, placebo-controlled design and inclusion of low-functioning older adults with 25(OH)D concentrations between 18 to <30 ng/mL at screening. A vitamin D3 dose expected to increase 25(OH)D concentrations above 30 ng/mL was used, and compliance with the vitamin D3 supplement was excellent (>90%). All enrolled participants had screening 25(OH)D concentrations between 18 and <30 ng/mL based on the clinical laboratory (LabCorp); however, 36% had baseline 25(OH)D concentrations <18 ng/mL based on the Tufts research laboratory. Furthermore, only 45% of those randomly assigned to vitamin D3 achieved 25(OH)D concentrations >30 ng/mL based on the Tufts research laboratory, possibly because of lower than the planned baseline 25(OH)D concentrations. Differences in the 25(OH)D concentrations by the clinical laboratory in real-time (DiaSorin LIAISON) and the 25(OH)D concentrations in stored blood samples analyzed at the completion of the trial at Tufts (LC/MS/MS) may have also contributed to fewer participants in the vitamin D group having adjustments made to the initial vitamin D3 dose to achieve a 25(OH)D concentration of 30 ng/mL. Participants were allowed to continue taking their baseline vitamin D supplements (<1000 IU/d); however, some participants changed the dose or stopped taking their vitamin D supplements, whereas others started new vitamin D supplements, which may have impacted their 25(OH)D concentrations. Furthermore, the vitamin D supplement dose used in the trial was not independently verified. Participant retention in the main study was excellent, with >90% follow-up overall; however, dropout in the placebo group was higher than anticipated in the muscle biopsy substudy (resulting in a sample size of 15 compared with the 16 anticipated), and we were unable to obtain single-fiber contractility measures, particularly for type 2 fibers, in a large proportion of participants which reduced our power to compare groups. Unlike many prior studies that have typically been conducted in majority White female populations, the study participants were approximately half male and one-third African American. The underlying physiological mechanisms were examined in a substudy over 4 mo; however, there are seasonal variations in 25(OH)D concentrations that may have impacted the achieved 25(OH)D concentrations among substudy participants over the 4-mo period. The primary clinical outcomes were assessed over a 12-mo period, thus, reducing any seasonal effects on the results. These findings may not generalize to older adults who are not low-functioning or those with vitamin D deficiency.
In conclusion, although vitamin D supplementation did improve 25(OH)D concentrations by ∼9 ng/mL, there was no effect of vitamin D3 supplementation on muscle power and strength and physical performance measures or underlying muscle physiology in low-functioning older adults with 25(OH)D concentrations between 18 and <30 ng/mL. Results of this and other recent trials suggest that vitamin D supplementation does not improve muscle power, strength, and physical performance in older adults with 25(OH)D concentrations of 18 to <30 ng/mL.
Author contributions
The authors’ responsibilities were as follows– DKH, APM, SBK, OD, and JAT: designed the trial; DKH: implemented the trial; JLD: conducted the muscle biopsies and provided medical oversight of the trial; OD: provided oversight of the substudy muscle physiology and histology measurements; RHN and JAT: analyzed the data; APM and SBK: provided methodological advice; DKH drafted the manuscript; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This trial was supported by the National Institute on Aging (R01 AG042411) and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30 AG021332).
Data availability
The data described in the manuscript, codebook, and analytic code will be made available upon request, pending application and approval from the Wake Forest University School of Medicine Institutional Review Board.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.04.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., Wallace R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen I., Heymsfield S.B., Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 3.Visser M., Goodpaster B.H., Kritchevsky S.B., Newman A.B., Nevitt M., Rubin S.M., et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. Am. J. Clin. Nutr. 2008;88(2):537S–540S. doi: 10.1093/ajcn/88.2.537S. [DOI] [PubMed] [Google Scholar]
- 5.Ceglia L., Harris S.S. Vitamin D and its role in skeletal muscle, Calcif. Tissue Int. 2013;92(2):151–162. doi: 10.1007/s00223-012-9645-y. [DOI] [PubMed] [Google Scholar]
- 6.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board . The National Academies Press; 2011. Dietary Reference Intakes for Calcium and Vitamin D. [Google Scholar]
- 8.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 9.Schleicher R.L., Sternberg M.R., Lacher D.A., Sempos C.T., Looker A.C., Durazo-Arvizu R.A., et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am. J. Clin. Nutr. 2016;104(2):454–461. doi: 10.3945/ajcn.115.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler C., Schott A.M., Berrut G., Fantino B., Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J. Nutr. Health Aging. 2009;13(10):893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 11.Dhaliwal R., Aloia J.F. Effect of vitamin D on falls and physical performance. Endocrinol. Metab. Clin. North Am. 2017;46(4):919–933. doi: 10.1016/j.ecl.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Dawson-Hughes B. Vitamin D and muscle function. J. Steroid Biochem. Mol. Biol. 2017;173:313–316. doi: 10.1016/j.jsbmb.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Dam T.T., von Mühlen D., Barrett-Connor E.L. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos. Int. 2009;20(5):751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houston D.K., Tooze J.A., Neiberg R.H., Hausman D.B., Johnson M.A., Cauley J.A., et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: the Health, Aging, and Body Composition Study. Am. J. Epidemiol. 2012;176(11):1025–1034. doi: 10.1093/aje/kws147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicherts I.S., van Schoor N.M., Boeke A.J., Visser M., Deeg D.J., Smit J., et al. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007;92(6):2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 16.Sohl E., de Jongh R.T., Heymans M.W., van Schoor N.M., Lips P. Thresholds for Serum 25(OH)D concentrations with respect to different outcomes. J. Clin. Endocrinol. Metab. 2015;100(6):2480–2488. doi: 10.1210/jc.2015-1353. [DOI] [PubMed] [Google Scholar]
- 17.Shardell M., Cappola A.R., Guralnik J.M., Hicks G.E., Kritchevsky S.B., Simonsick E.M., et al. Sex-specific 25-hydroxyvitamin D threshold concentrations for functional outcomes in older adults: Project on Optimal VItamin D in Older adults (PROVIDO) Am. J. Clin. Nutr. 2021;114(1):16–28. doi: 10.1093/ajcn/nqab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bislev L.S., Grove-Laugesen D., Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials. J. Bone Miner. Res. 2021;36(9):1651–1660. doi: 10.1002/jbmr.4412. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa S., Nakamura T., Tanabe H., Imamura T. Osteomalacic myopathy. Endocrinol. Jpn. 1979;26(suppl):65–72. doi: 10.1507/endocrj1954.26.supplement_65. [DOI] [PubMed] [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goltzman D, Hypercalcemia Endotext [internet], K.R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, W.W. de Herder, K. Dhatariya, K. Dungan, J.M. Hershman, J. Hofland, S. Kalra, et al., MDText.com, Inc., South Dartmouth, MA, 2000–2016.
- 22.Bassey E.J., Short A.H. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur. J. Appl. Physiol. Occup. Physiol. 1990;60(5):385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 23.Rejeski W.J., Ettinger W.H., Jr., Schumaker S., James P., Burns R., Elam J.T. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthr. Cartil. 1995;3(3):157–167. doi: 10.1016/s1063-4584(05)80050-0. 1995. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Simonsick E.M., Newman A.B., Nevitt M.C., Kritchevsky S.B., Ferrucci L., Guralnik J.M., et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 26.Podsiadlo D., Richardson S. The timed “Up and Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 27.Swanenburg J., de Bruin E.D., Favero K., Uebelhart D., Mulder T. The reliability of postural balance measures in single and dual tasking in elderly fallers and non-fallers. BMC Musculoskelet Disord. 2008;9:162. doi: 10.1186/1471-2474-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilney B., Morris M., Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait. Posture. 2003;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 29.Stanley B.A., Dai J.P., Xu A., Battaglioli E.J., Wilson R.T. 2015. A sensitive and cost-effective LC-MS-MS method for determination of 1,25-dihydroxyvitamin D3 in human plasma, Chromatogr Online.https://www.chromatographyonline.com/view/sensitive-and-cost-effective-lc-ms-ms-method-determination-1-25-dihydroxyvitamin-d3-human-plasma-0 Available from: [Google Scholar]
- 30.Messi M.L., Li T., Wang Z.M., Marsh A.P., Nicklas B., Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2016;71(10):1273–1280. doi: 10.1093/gerona/glv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi S.J., Shively C.A., Register T.C., Feng X., Stehle J., High K., et al. Force-generation capacity of single vastus lateralis muscle fibers and physical function decline with age in African green vervet monkeys. J. Gerontol. A. Biol. Sci. Med. Sci. 2013;68(3):258–267. doi: 10.1093/gerona/gls143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 33.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385–401. [Google Scholar]
- 34.Stewart A.L., Mills K.M., King A.C., Haskell W.L., Gillis D., Ritter P.L. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien R., Muller K. Marcel Dekker; 1983. Applied Analysis of Variance in Behavioral Science. [Google Scholar]
- 36.Liang K.-Y., Zeger S.L. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā: The Indian Journal of Statistics (Series B). 2000;62(1):134–148. [Google Scholar]
- 37.Liu G.F., Lu K., Mogg R., Mallick M., Mehrotra D.V. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat. Med. 2009;28(20):2509–2530. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 38.Lu K. On efficiency of constrained longitudinal data analysis versus longitudinal analysis of covariance. Biometrics. 2010;66(3):891–896. doi: 10.1111/j.1541-0420.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 39.Harvey W.R. United States Department of Agriculture; 1960. Least-Squares Analysis of Data with Unequal Subclass Numbers, Agricultural Research Service. [Google Scholar]
- 40.Shea M.K., Fielding R.A., Dawson-Hughes B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: a randomized controlled trial. Am. J. Clin. Nutr. 2019;109(2):369–379. doi: 10.1093/ajcn/nqy290. [DOI] [PubMed] [Google Scholar]
- 41.Vaes A.M.M., Tieland M., Toussaint N., Nilwik R., Verdijk L.B., van Loon L.J.C., et al. Cholecalciferol or 25-hydroxycholecalciferol supplementation does not affect muscle strength and physical performance in prefrail and frail older adults. J. Nutr. 2018;148(5):712–720. doi: 10.1093/jn/nxy024. [DOI] [PubMed] [Google Scholar]
- 42.Levis S., Gómez-Marín O. Vitamin D and physical function in sedentary older men. J. Am. Geriatr. Soc. 2017;65(2):323–331. doi: 10.1111/jgs.14510. [DOI] [PubMed] [Google Scholar]
- 43.de Koning E.J., Lips P., Penninx B., Elders P.J.M., Heijboer A.C., den Heijer M., et al. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am. J. Clin. Nutr. 2019;110(5):1119–1130. doi: 10.1093/ajcn/nqz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guralnik J.M., Sternberg A.L., Mitchell C.M., Blackford A.L., Schrack J., Wanigatunga AA A.A., et al. Effects of vitamin D on physical function: results from the STURDY trial. J. Gerontol. A. Biol. Sci. Med. Sci. 2022;77(8):1585–1592. doi: 10.1093/gerona/glab379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bischoff-Ferrari H.A., Vellas B., Rizzoli R., Kressig R.W., da Silva J.A.P., Blauth M., et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen K.E., Johnson R.E., Chambers K.R., Johnson M.G., Lemon C.C., Vo T.N., et al. Treatment of vitamin D insufficiency in postmenopausal women: a randomized clinical trial. JAMA Intern. Med. 2015;175(10):1612–1621. doi: 10.1001/jamainternmed.2015.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lips P., Binkley N., Pfeifer M., Recker R., Samanta S., Cohn D.A., et al. Once-weekly dose of 8400-IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am. J. Clin. Nutr. 2010;91(4):985–991. doi: 10.3945/ajcn.2009.28113. [DOI] [PubMed] [Google Scholar]
- 48.Glendenning P., Zhu K., Inderjeeth C., Howat P., Lewis J.R., Prince R.L. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J. Bone Miner. Res. 2012;27(1):170–176. doi: 10.1002/jbmr.524. [DOI] [PubMed] [Google Scholar]
- 49.Bischoff-Ferrari H.A., Dawson-Hughes B., Orav E.J., Staehelin H.B., Meyer O.W., Theiler R., et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern. Med. 2016;176(2):175–183. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 50.Ranathunga R., Hill T.R., Mathers J.C., Francis R.M., Prentice A., Schoenmakers I., et al. No effect of monthly supplementation with 12000 IU, 24000 IU or 48000-IU vitamin D3 for one year on muscle function: the vitamin D in older people study. J. Steroid. Biochem. Mol. Biol. 2019;190:256–262. doi: 10.1016/j.jsbmb.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer M., Begerow B., Minne H.W., Abrams C., Nachtigall D., Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J. Bone Miner. Res. 2000;15(6):1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 52.Pfeifer M., Begerow B., Minne H.W., Suppan K., Fahrleitner-Pammer A., Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int. 2009;20(2):315–322. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 53.Bischoff-Ferrari H.A. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord. 2012;13(1):71–77. doi: 10.1007/s11154-011-9200-6. [DOI] [PubMed] [Google Scholar]
- 54.Ceglia L., Niramitmahapanya S., da Silva M.M., Rivas D.A., Harris S.S., Bischoff-Ferrari H., et al. A randomized study on the effect of vitamin D(3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 2013;98(12):E1927–E1935. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ceglia L., Rivas D.A., Schlögl M., Fielding G.B., Egli A., Bischoff-Ferrari H.A., et al. Effect of vitamin D(3) vs. calcifediol on VDR concentration and fiber size in skeletal muscle. J. Bone Miner. Metab. 2023;41(1):41–51. doi: 10.1007/s00774-022-01374-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript, codebook, and analytic code will be made available upon request, pending application and approval from the Wake Forest University School of Medicine Institutional Review Board.