Abstract
Background
Previous studies on calcium intake and lung cancer risk reported inconsistent associations, possibly due to the differences in intake amounts and contributing sources of calcium and smoking prevalence.
Objectives
We investigated the associations of lung cancer risk with intake of calcium from foods and/or supplements and major calcium-rich foods in 12 studies.
Methods
Data from 12 prospective cohort studies conducted in the United States, Europe, and Asia were pooled and harmonized. We applied the DRI to categorize calcium intake based on the recommendations and quintile distribution to categorize calcium-rich food intake. We ran multivariable Cox regression by each cohort and pooled risk estimates to compute overall HR (95% CI).
Results
Among 1,624,244 adult men and women, 21,513 incident lung cancer cases were ascertained during a mean follow-up of 9.9 y. Overall, the dietary calcium intake was not significantly associated with lung cancer risk; the HRs (95% CI) were 1.08 (0.98–1.18) for higher (>1.5 RDA) and 1.01 (0.95–1.07) for lower intake (<0.5 RDA) comparing with recommended intake (EAR to RDA). Milk and soy food intake were positively or inversely associated with lung cancer risk [HR (95% CI) = 1.07 (1.02–1.12) and 0.92 (0.84–1.00)], respectively. The positive association with milk intake was significant only in European and North American studies (P-interaction for region = 0.04). No significant association was observed for calcium supplements.
Conclusions
In this largest prospective investigation, overall, calcium intake was not associated with risk of lung cancer, but milk intake was associated with a higher risk. Our findings underscore the importance of considering food sources of calcium in studies of calcium intake.
Keywords: lung cancer, calcium, diet, supplements, milk, dairy products, soy products, pooled analysis, prospective studies
Introduction
Lung cancer is the leading cause of cancer deaths in the United States and worldwide [1,2]. Smoking is the major cause of lung cancer; however, the contribution from other modifiable risk factors for lung cancer is not well understood. Calcium, as a nutrient, plays a wide variety of roles and, in particular, cell-cycle regulation through apoptotic pathways and Wnt signaling pathways may be relevant for the effects of calcium on lung carcinogenesis [[3], [4], [5]]. In vitro studies reported that both inhibition and up-regulation of calcium-involved pathways lead to lung carcinogenesis through dysregulated lung tissue repair mechanisms and epithelial-mesenchymal transition, respectively [[3], [4], [5], [6], [7], [8], [9]]. Previously, we reported a statistically significant inverse association between dietary calcium intake and lung cancer risk among female never smokers in a large prospective cohort study, the Shanghai Women’s Health Study (SWHS) [10]. Findings from two other prospective cohort studies in the United States and Europe generally supported an inverse association, especially among women and current smokers [11,12]. In contrast, one United States case-control study reported a positive association of lung cancer risk with dietary calcium intake among men and current smokers [13], and other studies reported a null association [11,12]. A recent meta-analysis also reported a null association with risk of lung cancer [14]. These inconsistent results from different populations and subgroups may be explained by differences in calcium intake levels or contributing sources and smoking behavior [15,16]. Individual studies are typically limited by a relatively narrow distribution of calcium intake, with relatively higher calcium intake in countries where dairy food consumption and calcium fortification in foods are common than in countries where these are not as common. Moreover, except for sex and smoking status, few studies have investigated the associations of calcium and lung cancer risk by other potential effect modifiers such as race/ethnicity and region or by lung histology [13,17,18].
To address the limitations of previous investigations, we analyzed individual-level data pooled from 12 prospective cohort studies in the United States, Europe, and Asia to investigate associations of lung cancer risk with dietary and supplemental calcium intake and major food sources of calcium intake (for example, bovine milk). Furthermore, we evaluated possible effect modifications by sex, region, race, smoking status, and other lifestyle factors and differential associations by lung cancer histology.
Methods
Study population
Detailed descriptions of the 12 cohort studies included in our pooled analyses have been reported elsewhere [19]. All studies included in this pooling analysis met the following criteria: 1) collected comprehensive dietary intakes through a FFQ and detailed smoking history [for example, smoking status, duration, and intensity (the number of cigarettes smoked per day)]; 2) accrued ≥35 lung cancer cases among never smokers; and 3) had collected cancer incidence and total mortality information. Included are: the NIH-American Association of Retired Persons Diet and Health Study (NIH-AARP); Health Professionals Follow-up Study; Nurses’ Health Study; Iowa Women’s Health Study; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; Southern Community Cohort Study; Vitamins And Lifestyle Study and Women’s Health Initiative Observational Study conducted in the United States; Japan Public Health Center-based Prospective Study; Shanghai Men’s Health Study and SWHS conducted in Asia; and the European Prospective Investigation into Cancer and Nutrition conducted in Europe. Institutional review boards at respective institutions approved the original cohort studies, and institutional review board approval was also obtained at the Vanderbilt University Medical Center where the pooling project was conducted. Of the initial cohort participants, we excluded those who had a history of any cancer, or any cancer except non-melanoma skin cancer at cohort enrollment per cohort-specific protocol; had reported implausible total energy intake (pre-defined ranges by each cohort or beyond 3 standard deviations of the log-transformed mean energy intake based on the cohort- and sex-specific distribution) as we described previously [20], and/or who had no information on smoking status. To minimize the effect of reverse causation in our results, we further excluded the first 2 y of observation for all participants and lung cancer cases diagnosed within 2 y after enrollment (Supplemental Figure 1). The basic characteristics of the final analytic sample (n = 1,624,244) are summarized in Supplemental Table 1.
Diet and outcome assessment
Dietary data were collected at baseline using the cohort-specific validated FFQs that asked about habitual food consumption (for example, frequency and amount of foods commonly consumed in study populations) [19]. All studies used food composition databases specific to their region or country to estimate total energy, calcium, and other nutrient and food intakes to reflect potential regional differences in the nutrient contents of foods. In the current study, calcium intake from foods and/or supplements and food intake from major sources of calcium (that is, total dairy foods, milk, cheese, and soy products) were standardized to intake per 2000 kcal for women and 2500 kcal for men to approximate a daily intake. Calcium content in foods due to enrichment and fortification was counted toward food calcium intake. Supplemental calcium intake was assessed in eight United States cohorts; we estimated supplemental calcium intake based on frequency (from less than once per week to every day) and dose (from <200 mg/d to >1000 mg/d) of single calcium supplements and/or multivitamins in the preceding year [13]. All United States cohorts provided data for both calcium supplements and multivitamins, but the Southern Community Cohort Study collected data on the frequency of calcium supplements only. Hence, we estimated the amount of supplemental calcium intake based on the median dose of calcium supplements reported in other United States cohorts [13]. As to food sources of calcium, data on total dairy and milk intake were available in all studies. Since cheese intake was not available in SWHS and Shanghai Men’s Health Study and soy food intake was not available in NIH-AARP, those cohorts were excluded from the respective analysis.
Health and vital status were assessed regularly for all study participants according to each study’s protocol. Incident cancer cases and deaths were ascertained by linkage to cancer or death registries, active surveys, and medical record review, or in a combination of these methods. We identified primary lung cancer cases based on the ICD, 9th and 10th Revisions, codes 162 and C34, respectively. Lung cancer was further classified by histologic types such as adenocarcinoma, squamous cell carcinoma, other non–small-cell lung cancer, small-cell carcinoma, and others/unknown. Follow-up years were calculated from 2 y after the date of enrollment to the date of any cancer diagnosis, death, loss to follow-up, or the latest follow-up, whichever occurred first.
All cohort study data were sent to the Vanderbilt University Medical Center, where data were harmonized as described in detail elsewhere [19].
Statistical analysis
The characteristics of the study populations were described by sex and dietary calcium intake, and also by sex and cohort. For calcium intake from food (“dietary calcium intake”), we applied the project-wide cut-offs based on the dietary recommendations in the United States (DRI) [21]: <0.5 RDA, 0.5 RDA to estimated average requirement (EAR), EAR to RDA, RDA to 1.5 RDA, or >1.5 RDA appropriate for their sex and age at the time of dietary assessment, as described below. We used the United States DRI given that the comprehensive set of recommended values (for example, EAR and RDA) is available by specific sex and age group (separating adults into multiple groups) of our study participants; similar recommended values were used by the WHO, Europe, and China [22,23]; and that the United States represented >58% of the participants in our analysis. In the Cox model, the middle category (EAR to RDA) was treated as the reference. The EAR was 800 mg/d for men aged 19–70 y and women aged 19–50 y, and 1000 mg/d for men aged >70 y and women aged >50 y. The RDA was 1000 mg/d for men aged 19 to 70 y and women aged 19 to 50 y, and 1200 mg/d for men aged >70 y and women aged >50 y. Supplemental calcium intake information was only available for United States cohorts (n = 940,728) and the following cut-offs were applied: none (reference), >0–200, >200–500, >500–1000, and >1000 mg/d. Given the large difference in the amount of major food sources of calcium by geographic regions and across studies, we adopted the cohort- and sex-specific quintiles (g/d) of total dairy foods, milk, and cheese intakes, using the middle category (quintile 3) as the reference. Considering that soy food intake was uncommon in the United States and Europe, many participants reported no soy food consumption. For the United States and European cohorts, we categorized soy food intakes into five groups: no consumption group (0 g/d) and four consumption groups subclassified by cohort- and sex-specific quartile distributions among soy food consumers. For the Asian cohorts, we applied the cohort- and sex-specific quintiles the same as other calcium-containing foods. For soy food intake analysis, the lowest category of soy food intake (no consumption for United States and European cohorts and the lowest quintile for Asian cohorts) was used as the reference.
To estimate lung cancer risk associated with dietary and supplemental calcium and calcium-rich food intakes, multivariable-adjusted HRs were obtained in two steps: cohort-specific Cox regression analysis and random-effects meta-analysis. In the first step of cohort-specific analyses, Cox models were stratified by calendar year of dietary assessment (<1985, 1985–1990, 1991–1995, 1996–2000, 2001–2004, and ≥2005, where applicable) and birth year (5-y intervals from <1925 to ≥1960). Follow-up years were treated as the time scale. Covariates included in the Cox models are: age at time of dietary assessment (continuous); sex; smoking status (current, former, or never smokers); pack-years of smoking (continuous); total energy intake (continuous); race (Whites, Blacks, Asians, or others); education (less than high school, high school graduate, professional or vocational education, some college education, college graduate, or graduate education); alcohol consumption [0 g/d of ethanol intake in both men and women (non-drinkers), ≤28 or 14 g/d of ethanol intake in men and women, respectively (moderate drinkers), or >28 or 14 g/d of ethanol intake in men and women, respectively (heavy drinkers)]; family history of lung cancer (yes or no); history of diabetes (yes or no); BMI category (underweight: < 18.5 kg/m2; normal weight:18.5–24.9 kg/m2; overweight: 25–29.9 kg/m2; and obese: ≥30 kg/m2); physical activity (tertiles of total physical activity hours based on metabolic equivalent hours); and among women, hormone therapy use (yes or no) and menopausal status (pre or post). Cox models were further adjusted for dietary intakes that were significantly associated with lung cancer risk in our study population (that is, saturated fat, polyunsaturated fat, and dietary fiber) [20,24] and for magnesium, phosphorus, and vitamin D intakes given the interplay of these nutrients with calcium absorption and activity. Multiple imputations were conducted in each cohort for missing covariates due to no participant response, data not collected for a subset of the cohort participants, or any other reasons. Missing values of ≥3% were imputed using a multivariate imputation based on covariates such as smoking status and sex, using fully conditional specification methods in the SAS PROC MI procedure. Missing values of <3% were replaced with the median value or most frequent category specific to the cohort and sex. In the second step, after obtaining multivariable-adjusted HRs from each cohort separately, we combined cohort-specific log HRs through the random-effects meta-analysis approach. The random-effects model was chosen to allow for the potential heterogeneity across the cohorts.
To evaluate the potential heterogeneity of associations between calcium intake and lung cancer risk, we performed stratified analyses by sex, region (North America, Asia, or Europe), race (Whites, Blacks, or Asians), smoking status (current, former, or never smokers), alcohol consumption (non-drinkers, moderate, or heavy drinkers), BMI category (underweight/normal weight, overweight, or obese), calcium supplement use (yes or no) and by lung cancer histology (adenocarcinoma, squamous cell carcinoma, or small-cell carcinoma) and among women, hormone therapy use (yes or no), and menopausal status (pre or post). Interaction effects were assessed in each cohort by entering the interaction terms; then, the estimates were combined by random-effects meta-analysis. Potential non-linear associations were tested using restricted cubic spline regression by applying three knots at the 5th, 50th, and 95th percentiles. Along with adjustment for all potential confounders, spline regression was conducted using a single pooled dataset in which participants with the top 1% of calcium or calcium-containing food intakes were excluded to minimize the potential effects of extreme values.
All analyses were carried out using the SAS Enterprise Guide, version 7.1 (SAS Institute Inc), or Stata, version 12 (StataCorp). Two-sided P < 0.05 were considered statistically significant.
Results
This pooled analysis included a total of 1,624,244 participants comprising 672,258 men and 951,986 women, and included a total of 21,513 lung cancer cases (Supplemental Table 1). A wide range of calcium intakes across the cohorts was observed. The mean intake of calcium from food among all participants was 947 mg/d, which ranged from 554 mg/d among women in SWHS to 1149 mg/d among men in Vitamins And Lifestyle Study. Among the United States cohorts, about half of the participants used calcium supplements, among whom the mean intake was 682 mg/d.
Participants with higher dietary calcium intake were more likely to be White, have a university degree, drink less alcohol, and be physically active among both men and women (Table 1). For smoking status, men with higher calcium intake were more likely to be never smokers, whereas the opposite trend was observed among women.
TABLE 1.
Baseline characteristics of the study population by dietary calcium intake
| Characteristics | Dietary calcium intake |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 672,258) |
Women (n = 951,986) |
|||||||||
| ≤0.5 RDA | 0.5 RDA to EAR | EAR to RDA | RDA to 1.5 RDA | >1.5 RDA | ≤0.5 RDA | 0.5 RDA to EAR | EAR to RDA | RDA to 1.5 RDA | >1.5 RDA | |
| Population, n | 48,805 | 194,869 | 156,212 | 203,497 | 68,875 | 144,004 | 411,030 | 171,389 | 193,227 | 32,336 |
| Age, y | 54.2 | 57.0 | 57.9 | 58.0 | 58.9 | 56.6 | 56.4 | 54.1 | 53.9 | 55.0 |
| Race, % | ||||||||||
| Whites | 28.0 | 68.5 | 81.3 | 90.0 | 95.0 | 48.5 | 78.7 | 90.6 | 95.4 | 95.8 |
| Blacks | 4.1 | 7.7 | 4.4 | 2.0 | 1.3 | 8.8 | 6.6 | 2.5 | 1.5 | 1.5 |
| Asians | 67.0 | 22.3 | 12.9 | 6.7 | 2.3 | 41.3 | 13.2 | 5.8 | 2.0 | 1.3 |
| Others | 0.9 | 1.5 | 1.4 | 1.3 | 1.4 | 1.4 | 1.5 | 1.1 | 1.1 | 1.4 |
| University degrees or higher, % | 15.8 | 34.2 | 41.2 | 43.7 | 46.6 | 14.0 | 23.0 | 27.6 | 31.7 | 34.8 |
| Family history of lung cancer, % | 2.4 | 2.3 | 2.5 | 2.1 | 1.8 | 2.8 | 2.3 | 1.5 | 1.3 | 1.6 |
| Smoking status, % | ||||||||||
| Never | 21.1 | 26.0 | 29.6 | 32.8 | 34.5 | 65.7 | 57.7 | 56.5 | 55.6 | 54.2 |
| Former | 29.0 | 45.1 | 49.7 | 50.2 | 51.8 | 18.5 | 27.0 | 28.4 | 29.9 | 31.6 |
| Current | 49.9 | 28.9 | 20.7 | 17.0 | 13.7 | 15.8 | 15.3 | 15.1 | 14.5 | 14.3 |
| Smoking pack-years, among smokers | 33.8 | 32.3 | 31.5 | 30.9 | 32.7 | 26.3 | 22.0 | 18.5 | 17.9 | 19.3 |
| Alcohol intake, (g/d) | 47.2 | 22.4 | 14.7 | 11.9 | 8.3 | 7.2 | 5.9 | 5.8 | 5.3 | 3.8 |
| Low-level physical activity1, % | 40.9 | 39.6 | 33.8 | 30.0 | 28.4 | 43.8 | 34.4 | 30.2 | 26.5 | 24.6 |
| BMI (kg/m2) | 24.7 | 26.4 | 26.7 | 26.7 | 26.9 | 25.9 | 26.2 | 25.8 | 25.6 | 25.7 |
| History of diabetes, % | 4.7 | 6.3 | 7.8 | 8.7 | 10.6 | 4.7 | 5.5 | 4.5 | 4.3 | 4.8 |
| Menopause, % among women | - | - | - | - | - | 76.7 | 74.9 | 63.0 | 62.9 | 66.9 |
| Hormone therapy use, % among women | - | - | - | - | - | 29.6 | 40.1 | 37.4 | 38.1 | 41.6 |
| Dietary intakes | ||||||||||
| Energy, kcal/d | 2203 | 2228 | 2151 | 2111 | 2049 | 1650 | 1749 | 1784 | 1748 | 1625 |
| Dietary calcium2, (mg/d) | 390 | 670 | 896 | 1200 | 1861 | 464 | 764 | 1018 | 1328 | 1989 |
| Supplemental calcium2,3 (mg/d) | 353 | 406 | 423 | 437 | 442 | 810 | 837 | 862 | 875 | 928 |
| Total dairy foods2 (g/d) | 40 | 123 | 234 | 423 | 949 | 65 | 219 | 371 | 578 | 1026 |
| Milk2, (g/d) | 27 | 82 | 172 | 343 | 880 | 34 | 138 | 248 | 427 | 853 |
| Cheese2, (g/d) | 3 | 10 | 17 | 24 | 25 | 10 | 24 | 25 | 42 | 46 |
| Soy products2, (g/d) | 41 | 19 | 14 | 9 | 5 | 21 | 11 | 8 | 5 | 5 |
| Lung cancer cases, n | ||||||||||
| Adenocarcinoma | 357 | 1191 | 795 | 942 | 318 | 1012 | 2113 | 658 | 726 | 132 |
| Squamous cell carcinoma | 296 | 721 | 504 | 600 | 197 | 263 | 670 | 218 | 213 | 37 |
| Small-cell carcinoma | 159 | 427 | 265 | 378 | 154 | 239 | 328 | 208 | 259 | 42 |
| All others | 375 | 1136 | 738 | 964 | 345 | 745 | 1424 | 442 | 506 | 116 |
Data are proportion (%) or mean. All differences across groups of dietary calcium intake were statistically significant (P < 0.05). EAR, estimated average requirement; n, number.
The lowest cohort- and sex-specific tertile of total physical activity was measured by hours or metabolic equivalent hours.
Adjusted for total energy intake and standardized to intakes per 2500 kcal for men and per 2000 kcal for women.
Estimated among current calcium supplement users.
Overall, calcium intake from foods and/or supplements was not statistically significantly associated with lung cancer risk (Table 2). After adjustment for demographic and lifestyle factors, higher dietary calcium intake (>1.5 RDA) was associated with a higher risk of incident lung cancer than the recommended calcium intake (EAR to RDA) [HR (95% CI) = 1.08 (0.98–1.18)], which has almost reached statistical significance. Similar associations were observed after further adjustment for saturated and polyunsaturated fat and dietary fiber intakes [1.05 (0.96–1.15)] or intakes of magnesium, phosphorus, and vitamin D [1.07 (0.98–1.07)] (data not shown). Restricted cubic spline regression supported a non-linear association of dietary calcium; the lowest risk of lung cancer was observed for the range of recommended calcium intake, whereas very low or very high intakes were associated with a higher risk (P = 0.02) (Supplemental Figure 2). An additional analysis of the joint association between dietary and supplemental calcium intake on lung cancer risk demonstrated no significant association (Figure 1 and Supplemental Table 2).
TABLE 2.
Association1 of dietary calcium intake and calcium supplement use with lung cancer risk
| Calcium intake, (mg/d)2 | Number of cases | HR (95% CI)3 |
|---|---|---|
| Dietary calcium intake | ||
| ≤500 or ≤600 | 3446 | 1.01 (0.95–1.07) |
| >500–800 or >600–1000 | 8310 | 1.02 (0.97–1.07) |
| >800–1000 or >1000–1200 | 3828 | 1 (ref.) |
| >1000–1500 or >1200–1800 | 4588 | 1.04 (0.99–1.08) |
| >1500 or >1800 | 1341 | 1.08 (0.98–1.18) |
| Supplemental calcium intake4 | ||
| None | 8466 | 1 (ref.) |
| >0–200 | 2018 | 1.00 (0.95–1.05) |
| >200–500 | 2216 | 0.97 (0.93–1.02) |
| >500–1000 | 1625 | 0.99 (0.91–1.07) |
| >1000 | 1562 | 0.98 (0.92–1.04) |
| Dietary and supplemental calcium intake4 | ||
| ≤500 or ≤600 | 1112 | 0.97 (0.90–1.05) |
| >500–800 or >600–1000 | 4378 | 0.99 (0.93–1.05) |
| >800–1000 or >1000–1200 | 2622 | 1 (ref.) |
| >1000–1500 or >1200–1800 | 4417 | 1.00 (0.95–1.05) |
| >1500 or >1800 | 3358 | 0.98 (0.90–1.06) |
ref., reference.
Estimated by random-effects meta-analysis.
Standardized to intakes per 2500 kcal for men and per 2000 kcal for women. For men <70 years and women <50 years, RDA for calcium is 1,000 mg/d and EAR is 800 mg/d. For men >70 years and women >50 years, RDA is 1,200 mg/d and EAR is 1,000 mg/d. Calcium intakes were categorized into five groups: <0.5 RDA; 0.5 RDA to EAR; EAR to RDA; RDA to 1.5 RDA; and >1.5 RDA.
Adjusted for age, sex, smoking status, smoking pack-years, and total energy, race, education, alcohol consumption, family history of lung cancer, history of diabetes, physical activity level, obesity status, and hormone therapy and menopausal status in women, and stratified by birth year and enrollment year.
Data were only available in the United States cohorts (n = 940,728).
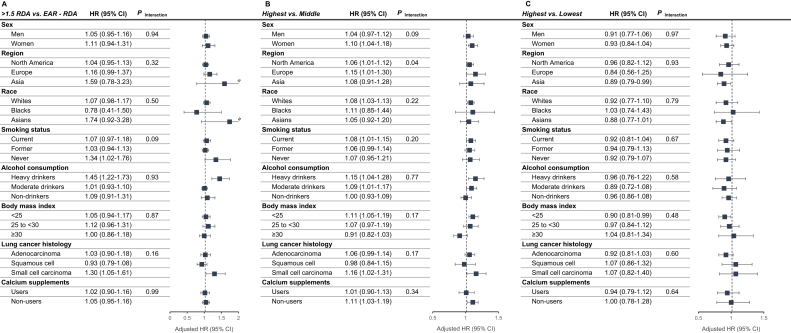
FIGURE 1.
Association of high consumption of dietary calcium, milk, and soy with lung cancer risk in subgroups of participants. (A) dietary calcium intake, (B) milk intake, and (C) soy intake. HRs and 95% CIs were estimated by random-effects meta-analysis. Intakes were standardized to intakes per 2500 kcal for men and per 2000 kcal for women. All estimates were adjusted for age, sex, smoking status, smoking pack-years, total energy, race, education, alcohol consumption, family history of lung cancer, history of diabetes, physical activity level, obesity status, and hormone therapy and menopausal status in women, except for the stratifying variable used to define each subgroup; and stratified by cohort, birth year, and enrollment year. P-interaction for country and race were estimated in a single pooled dataset. Overall heterogeneity across histological types is presented. Data on calcium supplements were only available in the United States cohorts (n = 940,728).
In our analyses of major food sources of calcium, milk intake was modestly positively associated with risk of lung cancer (Table 3). After adjustment for smoking variables and other risk factors, the highest quintile of milk intake was associated with a 7% higher risk of lung cancer than the middle quintile [HR (95% CI) = 1.07 (1.02–1.12)]. The positive association remained statistically significant when further controlling for other dietary factors, such as saturated and polyunsaturated fat and dietary fiber intakes [1.05 (1.01–1.10)] or intakes of magnesium, phosphorus, and vitamin D [1.10 (1.04–1.17)] (data not shown). In our restricted cubic spline regression analysis, a non-linear association was observed with a tendency of a higher risk of lung cancer at very high or low intakes of milk (P = 0.03) (Supplemental Figure 2). No association was found for total dairy food and cheese. Meanwhile, as the main non-dairy food source for calcium, a higher intake of soy products was associated with an 8% lower risk of lung cancer after adjustment for smoking and other risk factors [0.92 (0.84–1.00)]. When further controlling for the intake of saturated and polyunsaturated fats and dietary fiber, the association was no longer statistically significant (data not shown). Further, restricted cubic spline regression analysis suggested a non-linear association; a high intake of soy products (P = 0.05) was associated with a low risk of lung cancer, which seemingly leveled off after 50 g/d (Supplemental Figure 2).
TABLE 3.
Association1 of calcium-rich food consumption with lung cancer risk
| Calcium-rich food2 | Number of cases | HR (95% CI)3 |
|---|---|---|
| Total dairy foods | ||
| Quintile 1 | 5322 | 1.03 (0.99–1.07) |
| Quintile 2 | 4119 | 0.99 (0.95–1.04) |
| Quintile 3 | 3948 | 1 (ref.) |
| Quintile 4 | 3924 | 0.99 (0.95–1.04) |
| Quintile 5 | 4194 | 1.04 (0.99–1.08) |
| Milk | ||
| Quintile 1 | 5160 | 1.02 (0.97–1.07) |
| Quintile 2 | 4180 | 1.02 (0.98–1.07) |
| Quintile 3 | 3845 | 1 (ref.) |
| Quintile 4 | 3981 | 1.02 (0.97–1.07) |
| Quintile 5 | 4329 | 1.07 (1.02–1.12) |
| Cheese4 | ||
| Quintile 1 | 4218 | 0.99 (0.95–1.04) |
| Quintile 2 | 3794 | 0.97 (0.93–1.02) |
| Quintile 3 | 3861 | 1 (ref.) |
| Quintile 4 | 3663 | 0.95 (0.91–1.00) |
| Quintile 5 | 3778 | 0.97 (0.93–1.02) |
| Soy products5 | ||
| Quintile 1 | 8756 | 1 (ref.) |
| Quintile 2 | 1223 | 1.04 (0.91–1.19) |
| Quintile 3 | 1160 | 0.95 (0.84–1.08) |
| Quintile 4 | 1150 | 0.93 (0.86–1.01) |
| Quintile 5 | 1108 | 0.92 (0.84–1.00) |
ref., reference.
Estimated by random-effects meta-analysis.
The cohort- and sex-specific quintiles standardized to intakes per 2500 kcal for men and per 2000 kcal for women.
Adjusted for age, sex, smoking status, smoking pack-years, total energy, race, education, alcohol consumption, family history of lung cancer, history of diabetes, physical activity level, obesity status, and hormone therapy and menopausal status in women, and stratified by birth year and enrollment year.
No data were available in the Shanghai Men’s Health Study and SWHS.
No data were available in NIH-American Association of Retired Persons Diet and Health Study.
In the stratified analyses (Figure 1 and Supplemental Tables 2–4), dietary calcium intake 1.5 times higher than the recommended was statistically significantly associated with higher lung cancer risk among never smokers [HR (95% CI) for >1.5 RDA compared with EAR to RDA = 1.34 (1.02–1.76)] and among heavy drinkers [1.45 (1.22–1.73)], as well as with small-cell carcinoma [1.30 (1.05–1.61)]. However, multiplicative interaction tests for smoking status, alcohol consumption, and lung histology were not statistically significant (P-interaction = 0.09, 0.93, and 0.16, respectively). Additionally, when we assessed associations for intake above the tolerable upper-level intake (UL: >2000 mg/d), there were generally no statistically significantly higher risks (Supplemental Table 5). The highest quintile of milk intake relative to the middle quintile was statistically significantly associated with a higher risk of lung cancer in studies conducted in the North America [1.06 (1.01–1.12)] and Europe [1.15 (1.01–1.30)], but not in Asia [1.08 (0.91–1.28) (P-interaction = 0.04)]. The statistically significant positive association for milk intake was also observed among women [1.10 (1.04–1.18)], Whites [1.08 (1.03–1.13)], current smokers [1.08 (1.01–1.15)], heavy alcohol drinkers [1.15 (1.04–1.28)], moderate alcohol drinkers [1.09 (1.01–1.17)], and underweight/normal-weight participants [1.11 (1.05–1.19)] and for small-cell carcinoma [1.16 (1.02–1.31)]; none of the multiplicative interaction tests was significant (P-interaction = 0.09–0.77). Meanwhile, the inverse association between higher soy food intake and lung cancer risk was mainly observed in Asia [0.89 (0.79–0.99)] and among underweight/normal-weight participants [0.90 (0.81–0.99)], although tests for multiplicative interactions were not statistically significant (P-interaction = 0.93 and 0.48, respectively). For the rest of stratified analyses on calcium or major food sources of calcium, association patterns did not differ across strata, and interaction effects were not statistically significant.
Discussion
In this pooled analysis of 12 prospective cohort studies comprising almost 1.6 million adult men and women from the United States, Europe, and Asia, we found no overall association between calcium intake from foods and/or supplements with risk of lung cancer among all participants. In the stratified analyses, however, compared with the recommended intake of dietary calcium, intakes higher than the recommended values were associated with a higher risk of lung cancer among never smokers and heavy drinkers, with a higher risk of small-cell carcinoma. However, tests of multiplicative interactions did not reach statistical significance. Regarding major food sources of calcium, we found a moderate positive association of milk intake and a moderate inverse association of soy food intake with lung cancer risk. Interestingly, the milk-lung cancer association was mainly observed in the North America and Europe with a significant interaction effect with region, whereas the soy-lung cancer association was observed only in Asian populations, without a significant interaction effect with region. This may suggest that lung cancer risk associated with dietary calcium intake may differ across countries and populations owing to the differences in the major food sources of calcium as well as the amount of calcium intake.
In our study, we assessed associations of lung cancer risk with calcium intake based on recommended intakes and observed no overall statistically significant associations. These findings are consistent with two previous studies based on quantile analyses [11,12], but not with other studies that reported a statistically significant inverse or positive association [10,13]. Another source of calcium intake, supplemental calcium intake, was also not associated with lung cancer risk in our study. This finding is consistent with a previous study that reported no association for calcium and vitamin D supplementation among postmenopausal women in the Women’s Health Initiative Observational Study [25]. Although a multiplicative interaction effect by smoking status was not statistically significant, we found that higher than the recommended intake of dietary calcium compared with the recommended intake was associated with a higher risk of lung cancer among never smokers. This finding contrasts with our previous findings from SWHS, a population with relatively low calcium intake [10] (the mean intake of 554 mg/d in SWHS compared with 947 mg/d in 12 cohorts in this current study, Supplemental Table 1). This finding might indicate that caution is needed at intakes higher than the recommended calcium intake levels, even at levels lower than UL. However, we found no statistically significantly higher risk with intake above the UL and no associations with supplemental calcium intake among never smokers. Hence, the nature of this association is unclear. Similarly, compared with the recommended intake, we observed a moderately increased risk of lung cancer associated with intakes higher than the recommended values among heavy drinkers and with small-cell carcinoma, without significant interaction effects. To our knowledge, our study is the first to report a positive association between dietary calcium intake and lung cancer risk among never smokers and heavy drinkers and with small-cell carcinoma. These findings are inconsistent with the current evidence on the molecular mechanism of calcium on lung carcinogenesis and calcium bioavailability. First, calcium’s involvement in the Wnt and cell-cycle signaling pathways is more relevant to non–small-cell carcinoma than small-cell carcinoma [4,5], but our study found no association with non–small-cell carcinoma. Second, alcohol was not reported to be involved in these pathways for lung carcinogenesis, and heavy drinkers tend to have lower bioavailability of calcium than non-drinkers [26], among whom we found no significant associations in our study. Third, regarding never smokers, as mentioned above, no significant association was observed at intake higher than UL. Hence, there is no known molecular mechanism that specifically explains our findings on never smokers, heavy drinkers, and small-cell carcinoma, which needs to be clarified in future studies.
Alternatively, major food sources of calcium, but not calcium per se, might have played a role in lung cancer risk in our study. In North America and some European countries, milk and dairy products were estimated to contribute 90% of dietary calcium intake [27]. In contrast, in China, the major food sources of calcium are soy products and vegetables [10]. To date, only two previous large prospective cohort studies in the United States and Japan investigated and reported null associations of milk intake with lung cancer mortality, but no study has investigated lung cancer incidence [28,29]. In the United States study, the relative risk (RR) (95% CI) comparing higher than the median intake with no intake were 0.7 (0.3–1.7) for whole milk, 0.5 (0.2–1.4) for 2% fat milk, and 0.8 (0.4–1.6) for ≤1% fat milk, respectively [28]. In the Japanese study, the corresponding HR (95% CI) compared consuming milk almost every day with scarcely any were 0.87 (0.67–1.14) among men and 0.89 (0.50–1.59) among women [29]. Contrary to these two previous cohort studies on lung cancer mortality, we found a higher risk of lung cancer for participants with the highest quintile of milk intake than those with the middle quintile intake, especially in the North American and European populations. Notably, our study is the first prospective study to investigate and report a positive association between milk intake and risk of incident lung cancer. We have previously reported an inverse association for yogurt intake in our study population [24]. Altogether, we found a positive association with milk intake, especially among smokers, whereas an inverse association for yogurt, especially among never smokers. These differential associations by dairy foods may explain no overall association with dietary calcium intake in our study and also suggest that nutrients or bioactive compounds in dairy products other than calcium may play a role in lung cancer prevention.
It is possible that, in our study, other nutrients and compounds in foods high in calcium rather than calcium per se might have driven the associations we observed. This is due to the fact that associations with lung cancer risk differed by dairy product types and calcium intake from supplements was not associated with lung cancer risk in our study, as well as that circulating calcium concentrations are well maintained in adults [30]. Dairy products contain many nutrients and compounds that were suggested to inhibit or promote carcinogenesis. Vitamins A and D, both of which were often fortified in milk, and conjugated linoleic acids were suggested to have anticarcinogenic, anti-inflammatory, and/or antioxidative effects [31,32]. In contrast, saturated fat content, which is the main fat subtype found in dairy products, has been suggested to promote carcinogenesis through increasing DNA damage and inducing cyclooxygenase-2 activity and proinflammatory responses [33,34], especially in the presence of nicotine-derived nitrosamine ketone, a tobacco carcinogen. In our study population, we have previously reported a positive association with saturated fat intake, which was stronger for smokers and small-cell carcinoma [20]. Hence, our findings of the positive association with milk intake for smokers and small-cell carcinoma are consistent with our previous findings. In other study populations, only two previous case-control studies, but no cohort study, investigated associations with small-cell carcinoma and reported no association for milk/dairy product intakes among 225 female never smokers in Hong Kong [RR of 0.84 for no intake and 1.67 for consuming less than monthly, compared with consuming at least once a month, with non-significant 95% CIs (values not provided)] [35] and for milk intake in the Czech Republic [ORs (95% CI) of 1.12 (0.54–2.34) among 1297 men and 0.60 (0.31–1.15) among 2765 women for comparing daily or more consumption with less than daily consumption] [17]. In addition, correlates of smoking behaviors, such as dietary pattern/quality, might have played a role in these findings, as dietary quality tends to be poor among smokers [36]. Regarding yogurt, as we previously reported, some probiotic strains were linked to lung carcinogenesis and inflammatory and immune responses [37]. For soy products, isoflavones were postulated to exert anticarcinogenic and antioxidative effects [38]. In our previous report from SWHS, we reported an inverse association of lung cancer risk with soy food intake [39]. Altogether, the associations of lung cancer risk varied with different food sources of calcium.
The strengths of this pooled analysis based on individual-level data are, by far, the largest sample size, the wide variation in calcium intake, food sources of calcium, smoking prevalence, and lung cancer incidence. Although individuals who have never smoked are an ideal population for elucidating dietary effects on the lung cancer risk due to a strong residual confounding by smoking, previous studies have been limited in conducting such analyses among never smokers due to few lung cancer cases accrued among never smokers [40,41]. Therefore, our study represented a unique opportunity to investigate calcium intake with lung cancer not only among never smokers but also among other subgroups defined by lung histology and geographic region. There are also limitations in our study. The observational study design precludes causal inferences. Dietary intakes were based on self-reported intakes, which have inherent measurement errors. All self-reported intakes, however, were collected using validated FFQs. We applied energy-adjusted approaches as recommended previously to standardize intake levels across studies by categorizing calcium intake according to the DRI values and to minimize the influence of over/under-reporting in dietary assessments [42]. Because of the varied distributions of soy product intake across studies, we used study-specific cut-offs in our analysis, which limits the interpretation of specific amounts of intake. Due to the data availability, we only included a single one-time dietary measure in our analyses. Changes in dietary habits may affect the calcium and lung cancer associations, although previous studies of diet and lung cancer or calcium and other cancer reported no substantial changes in associations using multiple-time dietary measures [39,43,44]. Our findings need to be followed up in future studies. Vitamin D status is closely related to calcium; however, data on circulating vitamin D concentrations were not available. Hence, we used factors associated with vitamin D concentrations such as race and physical activity level [45] as a surrogate and did not find major effect modification of these variables. We were not able to adjust for the overall diet quality in our analyses due to the lack of relevant information, which needs to be addressed in future studies. Air pollution and other environmental exposures are established/suspected risk factors for lung cancer. However, we did not have specific exposure information and attempted to account for them by calculating cohort-specific risk estimates because each cohort is a proxy for geographic location. As is often the case for observational studies for smoking-related cancers, residual confounding on smoking cannot be fully excluded. Finally, as multiple outcomes and subgroups were examined, the nominal P values and 95% CI should be interpreted with caution.
In summary, in this large pooled analysis of 12 cohort studies, there was no overall association between dietary calcium and lung cancer risk. After stratification, compared with the recommended intake, intakes higher than the recommended intakes of dietary calcium were associated with a moderately higher risk of small-cell lung cancer, and all types of lung cancer among never smokers and heavy alcohol drinkers, with no clear molecular mechanism to support these associations. There was no association between supplemental calcium intake and lung cancer risk. Milk intake was associated with a modestly higher risk of lung cancer in studies conducted in the North America and Europe, whereas soy food intake was associated with a lower risk in Asia. Notably, our study based on populations with different food intake patterns observed differential associations by food sources of calcium, reflective of regional differences in major food sources of calcium. It is possible that nutrients and bioactive components in these foods other than calcium might have contributed to the observed associations, and future studies may need to consider food sources of calcium as well as calcium intake.
Acknowledgments
The data used for this study were contributed by the National Cancer Institute Cohort Consortium. We would like to thank the staff, investigators, and participants of the contributing cohorts for their dedicated time and efforts and, in particular, the National Institute for Public Health and the Environment, Bilthoven, The Netherlands, for their contribution and ongoing support to the European Prospective Investigation into Cancer and Nutrition Study; and Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis of the NIH- American Association of Retired Persons Diet and Health Study. We would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, and Wyoming.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.03.011.
Author contribution
The authors’ responsibilities were as follows–YT, JJY, DY, and X-OS: designed the research; SAS-W, WJB, EW, KR, AP, NS, YP, Y-TG, QC, AA, SP, BB-M, LML, ST, RTC, THN, MBS, MJ, WZ, and X-OS: conducted/oversaw the data collection and provided the data; YT, JJY, DY, and X-OS: analyzed data; YT, JJY, and DY: wrote the original draft of the manuscript; YT and X-OS: had primary responsibility for the final content; and all authors: provided critical review and read and approved the final manuscript. YT, JJY and DY contributed equally to this work.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval of principle investigators of the participating cohorts.
Funding
This work was supported partially by a grant from the United States NIH (R03 CA183021) and the Ingram Cancer Professorship fund. Contributing cohorts were supported by grants from the NIH (UM1 CA182910, U01 CA202979, UM1 CA173640, UM1 CA186107, P01 CA87969, U01 CA167552, and R01 CA39742); the Intramural Research Program of the United States NIH/NCI; the Japan National Cancer Center Research and Development Fund; the Ministry of Health, Labor and Welfare of Japan; IARC; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, United Kingdom; and the United Kingdom National Institute for Health and Care Research Imperial Biomedical Research Centre.
Author disclosures
KW is currently employed by Vertex Pharmaceuticals since August 2022 and holds stocks in Vertex Pharmaceuticals. She continues the affiliation with Harvard University and this project was carried out while she was employed by Harvard University and was not funded by this company. All other authors report no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. C.A. Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. C.A. Cancer J. Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe L.F. A calcium-based theory of carcinogenesis. Adv. Cancer Res. 2005;94:231–263. doi: 10.1016/S0065-230X(05)94006-2. [DOI] [PubMed] [Google Scholar]
- 4.Pongracz J.E., Stockley R.A. Wnt signalling in lung development and diseases. Respir. Res. 2006;7(1):15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapp J., Jaromi L., Kvell K., Miskei G., Pongracz J.E. WNT signalling-lung cancer is no exception. Respir. Res. 2017;18(1):167. doi: 10.1186/s12931-017-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergner A., Kellner J., Tufman A., Huber R.M. Endoplasmic reticulum Ca2+-homeostasis is altered in small and non-small-cell lung cancer cell lines. J. Exp. Clin. Cancer Res. 2009;28:25. doi: 10.1186/1756-9966-28-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartis D., Mise N., Mahida R.Y., Eickelberg O., Thickett D.R. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69(8):760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 8.Carboni G.L., Gao B., Nishizaki M., Xu K., Minna J.D., Roth J.A., et al. CACNA2D2-mediated apoptosis in NSCLC cells is associated with alterations of the intracellular calcium signaling and disruption of mitochondria membrane integrity. Oncogene. 2003;22(4):615–626. doi: 10.1038/sj.onc.1206134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren C.F.A., Wong-Brown M.W., Bowden N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10(3):177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takata Y., Shu X.O., Yang G., Li H., Dai Q., Gao J., et al. Calcium intake and lung cancer risk among female nonsmokers: a report from the Shanghai Women’s Health Study. Cancer Epidemiol. Biomarkers Prev. 2013;22(1):50–57. doi: 10.1158/1055-9965.EPI-12-0915-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y., Leitzmann M.F., Subar A.F., Hollenbeck A., Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2009;169(4):391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li K., Kaaks R., Linseisen J., Rohrmann S. Dietary calcium and magnesium intake in relation to cancer incidence and mortality in a German prospective cohort (EPIC-Heidelberg) Cancer Causes Control. 2011;22(10):1375–1382. doi: 10.1007/s10552-011-9810-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W., Park S., Liu G., Miller D.P., Wang L.I., Pothier L., et al. Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology. 2005;16(6):772–779. doi: 10.1097/01.ede.0000181311.11585.59. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Wang X., Yao Q., Qin L., Xu C. Dairy product, calcium intake and lung cancer risk: a systematic review with meta-analysis. Sci Rep. 2016;6:20624. doi: 10.1038/srep20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel J.D., Bach P.B., Kris M.G. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291(14):1763–1768. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 16.Kiyohara C., Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend. Med. 2010;7(5):381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Koo L.C. Dietary habits and lung cancer risk among Chinese females in Hong Kong who never smoked. Nutr. Cancer. 1988;11(3):155–172. doi: 10.1080/01635588809513983. [DOI] [PubMed] [Google Scholar]
- 18.Mahabir S., Forman M.R., Dong Y.Q., Park Y., Hollenbeck A., Schatzkin A. Mineral intake and lung cancer risk in the NIH-American Association of Retired Persons Diet and Health study. Cancer Epidemiol. Biomarkers Prev. 2010;19(8):1976–1983. doi: 10.1158/1055-9965.EPI-10-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu D., Takata Y., Smith-Warner S.A., Blot W., Sawada N., White E., et al. Prediagnostic calcium intake and lung cancer survival: a pooled analysis of 12 cohort studies. Cancer Epidemiol. Biomarkers Prev. 2017;26(7):1060–1070. doi: 10.1158/1055-9965.EPI-16-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J.J., Yu D., Takata Y., Smith-Warner S.A., Blot W., White E., et al. Dietary fat intake and lung cancer risk: a pooled analysis. J. Clin. Oncol. 2017;35(26):3055–3064. doi: 10.1200/JCO.2017.73.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Institute of Medicine (US) 2011. Committee to review dietary reference intakes for vitamin D and calcium. Dietary reference intakes for calcium and vitamin D. Washington, DC. [Google Scholar]
- 22.EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies) Scientific opinion on dietary reference values for calcium by the European Food Safety Authority Panel on dietetic products, nutrition and Allergies. E.F.S.A. J. 2015;13(5) [Google Scholar]
- 23.Zhang J., Wang H.J., Wang Z.H., Zhang J.G., Du W.W., Su C., et al. [Trend in dietary calcium intake among Chinese elderly aged 50 years and over in nine provinces, from 1991 to 2009] Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33(11):1119–1122. [PubMed] [Google Scholar]
- 24.Yang J.J., Yu D., Xiang Y.B., Blot W., White E., Robien K., et al. Association of dietary fiber and yogurt consumption with lung cancer risk: a pooled analysis. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2019.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao M.H., Dai Q., Chen S., Freudenheim J.L., Rohan T., Wakelee H., et al. Calcium plus vitamin D supplementation and lung cancer incidence among postmenopausal women in the Women’s Health Initiative. Lung Cancer. 2017;110:42–47. doi: 10.1016/j.lungcan.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baj J., Flieger W., Teresiński G., Buszewicz G., Sitarz R., Forma A., et al. Magnesium, calcium, potassium, sodium, phosphorus, selenium, zinc, and chromium levels in alcohol use disorder: a review. J. Clin. Med. 2020;9(6) doi: 10.3390/jcm9061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garland C.F., Garland F.C., Gorham E.D. Calcium and vitamin D. Their potential roles in colon and breast cancer prevention. Ann. N. Y. Acad. Sci. 1999;889:107–119. doi: 10.1111/j.1749-6632.1999.tb08728.x. [DOI] [PubMed] [Google Scholar]
- 28.Breslow R.A., Graubard B.I., Sinha R., Subar A.F. Diet and lung cancer mortality: a 1987 National Health Interview Survey cohort study. Cancer Causes Control. 2000;11(5):419–431. doi: 10.1023/a:1008996208313. [DOI] [PubMed] [Google Scholar]
- 29.Ozasa K., Watanabe Y., Ito Y., Suzuki K., Tamakoshi A., Seki N., et al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC Study) in Japan by sex and smoking habits. Jpn. J. Cancer Res. 2001;92(12):1259–1269. doi: 10.1111/j.1349-7006.2001.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boden S.D., Kaplan F.S. Calcium homeostasis. Orthop. Clin. North Am. 1990;21(1):31–42. doi: 10.1016/S0030-5898(20)31563-7. [DOI] [PubMed] [Google Scholar]
- 31.Lampe J.W. Dairy products and cancer. J. Am. Coll. Nutr. 2011;30(Suppl 1):464S–470S. doi: 10.1080/07315724.2011.10719991. [DOI] [PubMed] [Google Scholar]
- 32.Epstein K.R. The role of carotenoids on the risk of lung cancer. Semin. Oncol. 2003;30(1):86–93. doi: 10.1053/sonc.2003.50020. [DOI] [PubMed] [Google Scholar]
- 33.Zeng L., Wu G.Z., Goh K.J., Lee Y.M., Ng C.C., You A.B., et al. Saturated fatty acids modulate cell response to DNA damage: implication for their role in tumorigenesis. PLOS ONE. 2008;3(6):e2329. doi: 10.1371/journal.pone.0002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Bayoumy K., Iatropoulos M., Amin S., Hoffmann D., Wynder E.L. Increased expression of cyclooxygenase-2 in rat lung tumors induced by the tobacco-specific nitrosamine 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone: the impact of a high-fat diet. Cancer Res. 1999;59(7):1400–1403. [PubMed] [Google Scholar]
- 35.Kubik A., Zatloukal P., Tomasek L., Dolezal J., Syllabova L., Kara J., et al. A case-control study of lifestyle and lung cancer associations by histological types. Neoplasma. 2008;55(3):192–199. [PubMed] [Google Scholar]
- 36.Dallongeville J., Marécaux N., Fruchart J.C., Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J. Nutr. 1998;128(9):1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 37.Pizzo F., Maroccia Z., Hammarberg Ferri I., Fiorentini C. Role of the microbiota in lung cancer: insights on prevention and treatment. Int. J. Mol. Sci. 2022;23(11) doi: 10.3390/ijms23116138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor C.K., Levy R.M., Elliott J.C., Burnett B.P. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009;67(7):398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang G., Shu X.O., Chow W.H., Zhang X., Li H.L., Ji B.T., et al. Soy food intake and risk of lung cancer: evidence from the Shanghai Women’s Health Study and a meta-analysis. Am. J. Epidemiol. 2012;176(10):846–855. doi: 10.1093/aje/kws168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Key T.J. Fruit and vegetables and cancer risks. Br. J. Cancer. 2011;104(1):6–11. doi: 10.1038/sj.bjc.6606032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stram D.O., Huberman M., Wu A.H. Is residual confounding a reasonable explanation for the apparent protective effects of beta-carotene found in epidemiologic studies of lung cancer in smokers? Am. J. Epidemiol. 2002;155(7):622–628. doi: 10.1093/aje/155.7.622. [DOI] [PubMed] [Google Scholar]
- 42.Subar A.F., Freedman L.S., Tooze J.A., Kirkpatrick S.I., Boushey C., Neuhouser M.L., et al. Addressing current criticism regarding the value of self-report dietary data. J. Nutr. 2015;145(12):2639–2645. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michaud D.S., Feskanich D., Rimm E.B., Colditz G.A., Speizer F.E., Willett W.C., et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000;72(4):990–997. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 44.Wu K., Willett W.C., Fuchs C.S., Colditz G.A., Giovannucci E.L. Calcium intake and risk of colon cancer in women and men. J. Natl. Cancer Inst. 2002;94(6):437–446. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 45.McCullough M.L., Weinstein S.J., Freedman D.M., Helzlsouer K., Flanders W.D., Koenig K., et al. Correlates of circulating 25-hydroxyvitamin D: cohort consortium vitamin D pooling project of rarer cancers. Am. J. Epidemiol. 2010;172(1):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval of principle investigators of the participating cohorts.