Abstract
Background
A poor diet can result from adverse social determinants of health and increases the risk of adverse pregnancy outcomes.
Objective
We aimed to assess, using data from the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be prospective cohort, whether nulliparous pregnant individuals who lived in a food desert were more likely to experience poorer periconceptional diet quality compared with those who did not live in a food desert.
Methods
The exposure was living in a food desert based on a spatial overview of food access indicators by income and supermarket access per the Food Access Research Atlas. The outcome was periconceptional diet quality per the Healthy Eating Index (HEI)-2010, analyzed by quartile (Q) from the highest or best (Q4, reference) to the lowest or worst dietary quality (Q1); and secondarily, nonadherence (yes or no) to 12 key aspects of dietary quality.
Results
Among 7,956 assessed individuals, 24.9% lived in a food desert. The mean HEI-2010 score was 61.1 of 100 (SD: 12.5). Poorer periconceptional dietary quality was more common among those who lived in a food desert compared with those who did not live in a food desert (Q4: 19.8%, Q3: 23.6%, Q2: 26.5%, and Q1: 30.0% vs. Q4: 26.8%, Q3: 25.8%, Q2: 24.5%, and Q1: 22.9%; overall P < 0.001). Individuals living in a food desert were more likely to report a diet in lower quartiles of the HEI-2010 (i.e., poorer dietary quality) (aOR: 1.34 per quartile; 95% CI: 1.21, 1.49). They were more likely to be nonadherent to recommended standards for 5 adequacy components of the HEI-2010, including fruit, total vegetables, greens and beans, seafood and plant proteins, and fatty acids, and less likely to report excess intake of empty calories.
Conclusions
Nulliparous pregnant individuals living in a food desert were more likely to experience poorer periconceptional diet quality compared with those who did not live in a food desert.
Keywords: food insecurity, pregnancy, nutrition, diet quality
Introduction
Poor diet quality affects over 50% of pregnant individuals in the United States [1] and is an important risk factor for adverse pregnancy outcomes and abnormal gestational weight gain [[2], [3], [4]]. Dietary quality among pregnant individuals in the periconceptional period is suboptimal, frequently failing to meet all 12 of the recommended components of the Dietary Guidelines for Americans [5]. Nonpregnant individuals with household-level food insecurity are more likely to experience poor diet quality [6, 7]. Moreover, pregnant individuals with household-level food insecurity are more likely to experience adverse pregnancy outcomes [8], excessive or inadequate gestational weight gain [3], obesity [9], iron deficiency [10], and depressive symptoms and anxiety [11] than those with household food security. However, the relationship between living in a food desert and an individual-level diet during pregnancy is unclear [12].
Living in a food desert hereafter is defined as a lack of consistent and sustainable access to nutritionally adequate, culturally acceptable, and safe food in a specific geographic location. This designation is based on individuals’ income and access to food stores within a geographic region [13]. For example, this may include the lack of access to grocery stores or markets with affordable, nutritious food selections at the neighborhood level. Food deserts affect 20% of pregnant individuals in the United States [14], 33% of single mothers postpartum [4, 15, 16] and have increased in number during the COVID-19 pandemic [4]. Both food insecurity and poor diet may contribute to persistent disparities in adverse pregnancy outcomes and are potentially modifiable with the intervention [5, 17]. Food insecurity occurs at multiple levels and societal levels of influence (i.e., community), such that addressing household food security adequately requires consideration of broader structural factors, such as transportation and geographic access, community food-related norms and values, housing and employment policies, and systemic racism [18]. For example, even those without individual-level food insecurity may be affected by a built environment that does not allow ease of access to healthy food choices. Emerging data suggest that efforts to improve access to food in neighborhoods may positively influence an individual’s diet quality [[19], [20], [21]]. Nevertheless, the association between living in a food desert, regardless of individual-level food insecurity, and diet quality in pregnancy has not been assessed.
The objective of the current study was to assess whether nulliparous pregnant individuals (i.e., without a prior live birth), hereafter designated as nulliparas, who lived in a food desert were more likely to experience poorer individual periconceptional diet quality than those who did not live in a food desert.
Methods
Study setting
This is a secondary analysis of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a prospective cohort that was designed to evaluate maternal and environmental contributors to poor birth outcomes (ClinicalTrials.gov identifier NCT01322529) [22]. This study was conducted at 8 US medical centers (Case Western Reserve University, Columbia University, Indiana University, University of Pittsburgh, Northwestern University, University of California at Irvine, University of Pennsylvania, and the University of Utah) from October 2010 to September 2013. Data were centrally managed by the Data Coordinating and Analysis Center at RTI International. As previously described [22], enrolled individuals completed 3 study visits during pregnancy and 1 at delivery.
The current cross-sectional analysis used data from the first visit (completed 6–13 wk gestation,) during which study personnel administered structured questionnaires to ascertain data on demographic characteristics, medical history, dietary history (see below), and psychosocial factors. Trained personnel also abstracted data from the clinical chart. In addition, a Food Frequency Questionnaire (FFQ) about periconceptional dietary intake was self-administered, and each participant’s residential address was collected and geocoded. Each site’s institutional review board approved the study before initiation, and all participants gave written informed consent.
Participants
Enrollment criteria included pregnant individuals (regardless of gender identity) <14 wk’ gestation, no prior delivery at 20 wk gestation or later, a viable singleton pregnancy with estimated gestational age from 6 wk 0 d to 13 wk 6 d, and intention to deliver at a participating clinical site hospital. Those who were ineligible included age <13 y, history of ≥3 pregnancy losses, donor oocyte pregnancy, planned pregnancy termination, malformations likely to be lethal and aneuploidies known at enrollment, previous enrollment, and inability to provide informed consent.
Measures
At visit 1, individuals completed the modified Block 2005 FFQ, a semiquantitative assessment of usual dietary intake for the 3 mo around conception, which has been validated in pregnancy [23]. This questionnaire assessed 52 nutrients and 35 food groups from ∼120 food and beverage items, and included serial adjustment items to estimate portion size. Details of the Block questionnaire in the current study sample have been previously reported by Bodnar et al., [5] and Yee et al., [2]. Briefly, this listing of food groups was developed from the National Health and Nutrition Examination Survey 1999–2002 dietary recall data, and nutrients from the US Department of Agriculture Food and Nutrient Database for Dietary Studies version 1. Food groups were derived from the MyPyramid Equivalents Database, version 2.0. The questionnaire utilizes multiple adjustment questions to improve the estimation of fat and carbohydrate intake. In addition, portion size is asked for each food, and pictures are given to enhance accuracy.
The FFQ was completed on paper during the first study visit (mean: 11.5 wk gestation, SD: 1.51). Research staff checked all pages of the FFQ for completeness. Completed questionnaires were sent to Block Dietary Data Systems for optical scanning and nutrient analysis using National Cancer Institute software. For the nuMoM2b cohort, the FFQ was slightly modified to reflect a 3-mo recall period and to include more sources of marine n-3 fatty acids. To facilitate dietary recall of the 3 mo around conception, a trained study staff member labeled each participant’s FFQ with the months of interest.
Answers to the Block questionnaire were scored using the Healthy Eating Index-2010 (HEI-2010), with higher scores representing better adherence to national guidelines. For example, an ideal score was 100, and the mean HEI-2010 score for adult individuals in the United States in 2010 was 55 [24].
Exposure
The exposure was living in a food desert (yes or no) using criteria for both low income and low access as per the definition in the Food Access Research Atlas of the Economic Research Service of the US Department of Agriculture (USDA) [25]. This metric provided a spatial overview of food access indicators by income and access using measures of supermarket accessibility [26]. Residential addresses from the first visit were geocoded using ArcGIS, and then linked at the census-tract level to the Food Access Research Atlas. Low-income and -access status at the census level were first measured separately, and then the overlap between the 2 measures was assessed together [27, 28]. Low income at the community level was defined as a census-tract in which ≥20% of the population had a median family income at or below 80% of the metropolitan area or state median income. Low access to food at the community level was defined as the number (≥500) and proportion (≥33%) of individuals at the census-tract level who were >1 mile (urban) or 10 miles (rural) from the nearest food store per USDA recommendations (26).
Outcome
The primary outcome was periconceptional dietary quality per the 2010 Dietary Guidelines for Americans, measured using the HEI-2010 [29, 30]. The HEI-2010 assessed how well dietary intake aligned with key recommendations of the 2010 Dietary Guidelines for Americans using 12 key components of dietary quality, including adequacy of intake of specific food groups and moderation of intake of less nutritious dietary components [31]. Scores for each component increased as intake reached the recommended standard (i.e., higher intake for the adequacy components and lower intake for the moderation components). The usual intake of the 12 components was expressed relative to energy before calculating component and total scores.
The primary outcome of the total HEI-2010 score was analyzed by quartile (Q) from the highest or best (Q4, reference) to the lowest or worst dietary quality (Q1). Scores were analyzed in quartiles because such groupings reflected clinically relevant categories and were consistent with prior studies [2]. Secondary outcomes were adherence (yes or no) to each of the 12 dietary components of the HEI-2010. For 9 components, dietary intakes were considered to not meet the recommended standard (maximum cutoff score or, equivalently, the average daily intake measured as servings per 1,000 kilocalories) if below the following cutoffs: 1) fruit (<5 or <0.8 servings); 2) whole fruit (forms other than juice) (<5 or <0.4 servings); 3) total vegetables (<5 or <1.1 servings); 4) greens and beans (dark-green vegetables and beans and peas) (<5 or <0.2 servings); 5) whole grains (<10 or <1.5 servings); 6) dairy (all milk products and soy beverages) (<10 or <1.3 servings); 7) total protein foods (<5 or <2.5 servings); 8) seafood and plant proteins (<5 or <0.8 servings); and 9) fatty acids (ratio of poly- and monounsaturated fat to saturated fat; <10 or <1.2 servings). For the remaining 3 components (i.e., moderation components), dietary intake was considered to not meet the recommended standard (maximum cutoff score or, equivalently, the average daily intake measured as servings per 1,000 kilocalories) if above the following cutoffs: 10) refined grains (>10 or >1.8 servings); 11) sodium (>10 or >1.1 g); and 12) empty calories (all calories from solid fats and added sugars plus calories from alcohol beyond a moderate level; >20 or >19% of energy) [31].
Covariates
Baseline self-reported socio-demographic characteristics were defined to be consistent with prior analyses assessing dietary quality in this dataset [5] and included the highest level of educational attainment (high school or less; college inclusive of some college credit, but no degree; college graduate; and greater than college inclusive of a graduate degree); self-reported race or ethnicity assessed as a social determinant of health and proxy measure of racism (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian, and other); and gross income and size of the household classified relative to the 2013 federal poverty level (<130% [i.e., the income eligibility guidelines for participation in the Supplemental Nutrition Assistance Program, SNAP], 130% to 349%, or >350%) [32, 33]. Other self-reported data collected at the first visit included marital status, smoking before pregnancy, and medical insurance status. Community-level adverse social determinants of health were assessed by the Area Deprivation Index in quartiles [34]. Maternal weight and height measured at the first study visit were categorized by WHO cutoffs for early pregnancy body mass index (BMI) measured in kg/height in meters2.
Statistical analysis
We compared maternal socio-demographic and clinic characteristics between those who lived in a food desert compared with those who did not use chi-square and Student T-tests. We then compared these characteristics by the 4 quartiles of the HEI-2010 using chi-square and ANOVA tests. A multivariable proportional odds model was used to assess associations with the primary outcome (HEI-2010 quartile) because it was quantified as an ordinal variable [35]. Graphical summaries were used to qualitatively evaluate the proportional odds assumption, including a stacked bar plot with a sequential color scale with percentages for each category of the outcome by the exposure group [35, 36]. The ordinal model provided effect estimates comparing the outcome between HEI-2010 quartiles, from best (Q4, reference) to worst (Q1); an odds ratio > 1 indicated a greater likelihood that those in the exposed group (i.e., food desert) had poorer diet quality. Logistic regression was used to assess associations for binary secondary outcomes. Unadjusted and adjusted odds ratios (OR, aOR) with 95% confidence intervals (95% CI) were calculated. A directed acyclic graph was used to illustrate visually and conceptually the relationship between exposure, outcome, mediators, and confounding factors a priori [37], and models were adjusted for age, BMI, individual social determinants of health (income relative to the poverty level, educational attainment, and insurance status), and gestational age at assessment (Supplementary Figure 1). Imputation for missing data was performed for the following covariates (n = 30 imputations), and estimates were combined using Rubin’s rule for the following: age (1.7%), insurance (0.6%), education (<0.1%), BMI (1.3%), and income (16.5%). In secondary analyses, because of evidence for persistent and widening disparities in dietary quality by socio-demographic characteristics among US adults [38], including in this cohort [5], we assessed for effect modification in the adjusted model with an interaction term between each of 3 individual social determinants of health (race and ethnicity as a proxy for racism, educational attainment, and insurance status) and the main exposure of living in a food desert. All statistical analyses were performed using STATA (STATACORP, version 16.1, College Station, TX).
Results
Among 10,038 nulliparas enrolled in this prospective cohort, we excluded 2082 (20.7%) individuals for missing either the exposure (food desert status, n = 450) or the outcome (HEI-2010, n = 1779), resulting in a final analytic sample of 7956 (79.3%) (Supplementary Figure 2). Excluded individuals were more likely to be younger, unmarried, self-identify as a race or ethnicity other than non-Hispanic White, have lower educational attainment, higher BMI, lower household income, and higher Area Deprivation Index (P < 0.05 for all) (Supplementary Table 1).
Participant characteristics
Of 7956 nulliparas in the analytic population, the median maternal age was 28.0 y (IQR: 23.0, 32.0), and 43% were classified as overweight or obese (Table 1). Over two-thirds (64.2%) had either a college or graduate degree, 18.6% reported a household income at 130% or below the federal poverty level, and 11.1% and 16.4% self-identified as non-Hispanic Black and Hispanic, respectively.
TABLE 1.
Maternal characteristics overall and by living in a food desert
| Variable | Overall n = 7,956 | Living in a food desert |
||
|---|---|---|---|---|
| No n = 5,971 | Yes n = 1,985 | P-value1 | ||
| Age, y, median (IQR) (n = 7,818) | 28.0 (23.0, 31.0) | 28.0 (23.0, 32.0) | 26.0 (23.0, 30.0) | <0.001 |
| Body mass index, kg/m2 (n = 7,853) | ||||
| Underweight | 185 (2.4) | 147 (2.5) | 38 (1.9) | <0.001 |
| Normal weight | 4,052 (51.6) | 3,107 (52.8) | 945 (47.9) | |
| Overweight | 1,955 (24.9) | 1,437 (24.4) | 518 (26.3) | |
| Obesity | 913 (11.6) | 652 (11.1) | 261 (13.2) | |
| Severe obesity | 748 (9.5) | 537 (9.1) | 211 (10.7) | |
| Married (n = 7,546) | 5,038 (66.8) | 3,747 (66.6) | 1,291 (67.4) | 0.03 |
| Education (n = 7,955) | ||||
| High school or less | 528 (6.6) | 392 (6.6) | 136 (6.9) | <0.001 |
| Some college | 2,323 (29.2) | 1,688 (28.3) | 635 (32.0) | |
| College graduate | 3,165 (39.8) | 2,289 (38.3) | 876 (44.1) | |
| Graduate degree | 1,939 (24.4) | 1,601 (26.8) | 338 (17.0) | |
| Household income and size relative to the US poverty level (n = 6,645) | ||||
| <130% | 1,236 (18.6) | 926 (18.6) | 310 (18.6) | <0.001 |
| 130%–350% | 1,958 (29.5) | 1,385 (27.8) | 573 (34.4) | |
| >350% | 3,451 (51.9) | 2,666 (53.6) | 785 (47.1) | |
| Race and ethnicity | ||||
| Non-Hispanic White | 5,064 (63.7) | 3,568 (59.8) | 1,496 (75.4) | <0.001 |
| Non-Hispanic Black | 884 (11.1) | 689 (11.5) | 195 (9.8) | |
| Hispanic | 1,303 (16.4) | 1,140 (19.1) | 163 (8.2) | |
| Non-Hispanic Asian | 327 (4.1) | 284 (4.8) | 43 (2.2) | |
| Other | 378 (4.8) | 290 (4.9) | 88 (4.4) | |
| Smoked during the 3 mo before pregnancy (n = 7,950) | 1,333 (16.8) | 937 (15.7) | 396 (20.0) | <0.001 |
| Area deprivation index | ||||
| Quartile 1 | 1,956 (24.6) | 1,630 (27.3) | 326 (16.4) | <0.001 |
| Quartile 2 | 2,143 (26.9) | 1,394 (23.3) | 749 (37.7) | |
| Quartile 3 | 2,015 (25.3) | 1,436 (24.0) | 579 (29.2) | |
| Quartile 4 | 1,842 (23.2) | 1,511 (25.3) | 331 (16.7) | |
| Diabetes in pregnancy (n = 7,709) | ||||
| Pregestational diabetes | 108 (1.4) | 78 (1.4) | 30 (1.6) | 0.2 |
| Gestational diabetes | 313 (4.1) | 223 (3.9) | 90 (4.7) | |
| Chronic hypertension (n = 7,643) | 177 (2.3) | 125 (2.2) | 52 (2.7) | 0.2 |
Chi-square test was used to compare categorical variables and the Wilcoxon rank sum test for continuous variables.
A total of 1985 (24.9%) individuals lived in a food desert. These individuals were more likely to be of younger age, have a higher BMI, be married, report lower educational attainment, report a lower household income, self-identify as non-Hispanic White, report smoking, and live in a community with a higher Area Deprivation Index score than those not living in a food desert (P < 0.05 for all) (Table 1).
HEI-2010 scores
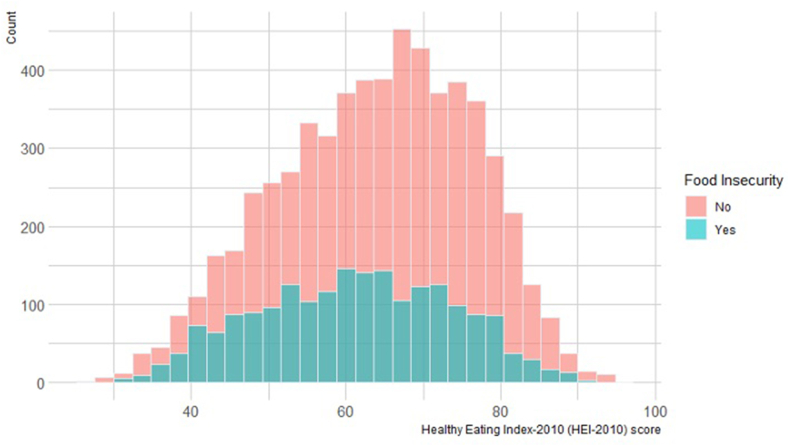
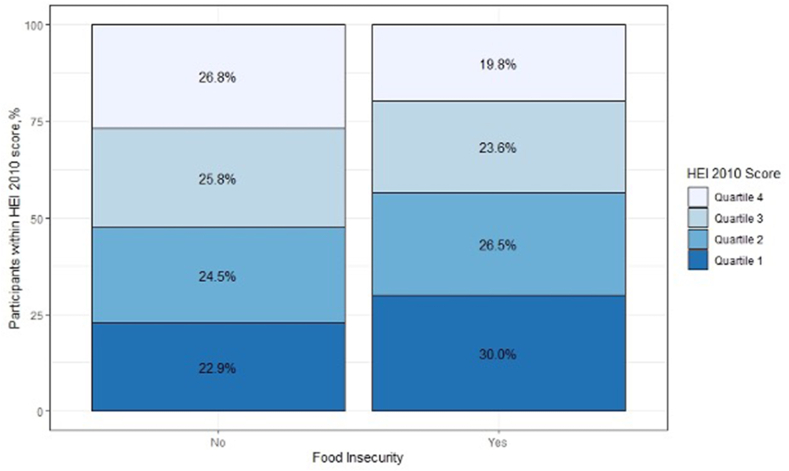
The overall mean HEI-2010 score was 61.1 (SD: 12.5) out of 100 possible points (Figure 1), which was lower (i.e., poorer dietary quality) for individuals living in a food desert than in those not living in a food desert (mean ± SD: 61.1 ± 12.5 vs. 63.7 ± 12.5; P < 0.001). Similarly, individuals living in a food desert were more likely to report poorer periconceptional dietary quality when HEI-2010 scores were evaluated by quartile (Q1: 30.0%, Q2: 26.5%, and Q3: 23.6%, and Q4: 19.8%) than in those not living in a food desert (Q1: 22.9%, Q2: 24.5%, and Q3: 25.8%, and Q4: 26.8%; overall P < 0.001) (Figure 2). As previously reported,5 individuals classified at lower quartiles of the HEI-2010 (i.e., poorer dietary quality) were more likely to be of younger age, unmarried, identify as Non-Hispanic Black or Hispanic, have lower educational attainment, to smoke, have a higher BMI, have a lower income, and live in a community with a higher Area Deprivation Index score than those at higher quartiles of the HEI-2010 (P < 0.05 for all) (Supplementary Table 2).
FIGURE 1.
Healthy Eating Index-2010 score distribution by living in a food desert
FIGURE 2.
Percentage of Healthy Eating Index-2010 scores by living in a food desert
Multivariable analysis
Individuals living in a food desert were increasingly more likely to report poorer dietary quality, as represented by lower HEI-2010 quartiles than those not living in a food desert (aOR: 1.34; 95% CI: 1.21, 1.49) (Table 2). Also, individuals living in a food desert were more likely not to meet recommended standards for 5 of 9 adequacy components of the HEI-2010, specifically total fruit (aOR: 1.24; 95% CI: 1.12, 1.39), total vegetables (aOR: 1.18; 95% CI: 1.03, 1.34), greens and beans (aOR: 1.17; 95% CI: 1.03, 1.32), seafood and plant proteins (aOR: 1.33; 95% CI: 1.18, 1.51), and fatty acids (aOR: 1.46; 95% CI: 1.19, 1.80) (Table 3). However, individuals living in a food desert were more likely to meet recommended standards for dairy (aOR: 0.76; 95% CI: 0.65, 0.88) compared with those not living in a food desert. No statistically significant difference by food desert status was observed for meeting recommended intakes of whole grains, whole fruit, or total protein. With regards to HEI-2010 moderation components, individuals living in a food desert were less likely to exceed the recommended threshold of empty calorie consumption (aOR: 0.49; 95% CI: 0.12, 0.86). No statistically significant difference was observed for excess refined grains and sodium intake based on whether an individual lived in a food desert status.
TABLE 2.
Association between living in a food desert and poorer periconceptional dietary intake per the Healthy Eating Index-2010 (HEI-2010)
| Living in a food desert (column percentage) |
Unadjusted odds ratio OR (95% CI)2 | Adjusted odds ratio a OR (95% CI)1,2,3 | |||
|---|---|---|---|---|---|
| Overall n | No | Yes | |||
| Quartile 1 | 1,962 | 1,366 (22.9) | 596 (30.0) | ||
| Quartile 2 | 1,991 | 1,464 (24.5) | 527 (26.5) | ||
| Quartile 3 | 2,011 | 1,542 (25.8) | 469 (23.6) | 1.45 (1.33,1.59)∗ | 1.34 (1.21, 1.49)∗ |
| Quartile 4 (reference)4 | 1,992 | 1,599 (26.8) | 393 (19.8) | 1.00 | 1.00 |
Model adjusted for: age, insurance status, education, body mass index, income, and gestational age at assessment.
Ordinal logistic regression within multiple imputation was used for nonbinary outcome (Quartile 4 = reference).
N in adjusted model = 7,956 with imputation for missing covariates.
The outcome was analyzed by quartile (Q) from the highest or best (Q4, reference) to the lowest or worst dietary quality (Q1).
Statistically significant finding with P-value < 0.05.
TABLE 3.
Association between living in a food desert and NOT meeting recommended standards per components of the Healthy Eating Index-2010 (HEI-2010)
| HEI-2010 components4 | Overall n | Living in a food desert (column percentage) |
Unadjusted odds ratio OR (95% CI)2 | Adjusted odds ratio aOR (95% CI)1,2,3 | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Adequacy components below the recommended cutoff | |||||
| Total fruit | |||||
| >5 | 3,573 | 2,794 (46.8) | 779 (39.2) | 1.00 | 1.00 |
| <5 | 4,383 | 3,177 (53.2) | 1,206 (60.8) | 1.36 (1.23, 1.51)∗ | 1.24 (1.10, 1.39)∗ |
| Whole fruit | |||||
| >5 | 4,329 | 3,324 (55.7) | 1,005 (50.6) | 1.00 | 1.00 |
| <5 | 3,627 | 2,647 (44.3) | 980 (49.4) | 1.22 (1.11, 1.36)∗ | 1.12 (0.99, 1.26) |
| Total vegetables | |||||
| >5 | 2,393 | 1,874 (31.4) | 519 (26.1) | 1.00 | 1.00 |
| <5 | 5,563 | 4,097 (68.6) | 1,466 (73.9) | 1.29 (1.15, 1.45)∗ | 1.18 (1.03, 1.34)∗ |
| Greens and beans | |||||
| >5 | 3,629 | 2,815 (47.1) | 814 (41.0) | 1.00 | 1.00 |
| <5 | 4,327 | 3,156 (52.9) | 1,171 (59.0) | 1.28 (1.16, 1.42)∗ | 1.17 (1.03, 1.32)∗ |
| Whole grains | |||||
| >10 | 366 | 272 (4.6) | 94 (4.7) | 1.00 | 1.00 |
| <10 | 7,590 | 5,699 (95.4) | 1,891 (95.3) | 0.96 (0.76, 1.23) | 1.01 (0.78, 1.31) |
| Dairy | |||||
| >10 | 1,207 | 849 (14.2) | 358 (18.0) | 1.00 | 1.00 |
| <10 | 6,749 | 5,122 (85.8) | 1,627 (82.0) | 0.75 (0.66, 0.86)∗ | 0.76 (0.65, 0.88)∗ |
| Total protein | |||||
| >5 | 3,523 | 2,678 (44.9) | 845 (42.6) | 1.00 | 1.00 |
| <5 | 4,433 | 3,293 (55.1) | 1,140 (57.4) | 1.10 (0.99, 1.22) | 1.06 (0.94, 1.19) |
| Seafood and plant protein | |||||
| >5 | 3,188 | 2,533 (42.4) | 655 (33.0) | 1.00 | 1.00 |
| <5 | 4,768 | 3,438 (57.6) | 1,330 (67.0) | 1.50 (1.35, 1.67)∗ | 1.33 (1.18, 1.51)∗ |
| Fatty acids | |||||
| >10 | 813 | 680 (11.4) | 133 (6.7) | 1.00 | 1.00 |
| <10 | 7,143 | 5,291 (88.6) | 1,852 (93.3) | 1.79 (1.48, 2.18)∗ | 1.46 (1.19, 1.80)∗ |
| Moderation components above the recommended cutoff | |||||
| Refined grains | |||||
| <10 | 5,699 | 4,239 (71.0) | 1,460 (73.6) | 1.00 | 1.00 |
| >10 | 2,257 | 1,732 (29.0) | 525 (26.4) | 0.88 (0.78, 0.99)∗ | 0.91 (0.80, 1.03) |
| Sodium | |||||
| <10 | 7,745 | 5,810 (97.3) | 1,935 (97.5) | 1.00 | 1.00 |
| >10 | 211 | 161 (2.7) | 50 (2.5) | 0.93 (0.67, 1.28) | 0.73 (0.48, 1.12) |
| Empty calories | |||||
| <20 | 7,672 | 5,723 (95.8) | 1,949 (98.2) | 1.00 | 1.00 |
| >20 | 284 | 248 (4.2) | 36 (1.8) | 0.43 (0.29, 0.60)∗ | 0.49 (0.34, 0.72)∗ |
Model adjusted for age, insurance status, education, body mass index, income, and gestational age at assessment.
Logistic regression within multiple imputation was used for binary outcomes.
N in adjusted model = 7,956 with imputation for missing covariates.
Intake between the minimum and maximum standards are scored proportionately.
Statistically significant finding with P-value < 0.05.
Secondary analyses for interaction effects
Statistical interactions between self-reported race and ethnicity (P = 0.4), educational attainment (P = 0.8), and insurance status (P = 0.5) and the main exposure of living in a food desert were not significant in the above-adjusted model, and hence, we did not further present additional stratified analyses.
Discussion
Pregnant individuals living in a food desert were more likely to report poorer periconceptional dietary quality, as represented by lower HEI-2010 scores, compared with those not living in a food desert. Individuals living in a food desert were more likely not to meet recommended standards for 5 of 9 adequacy components of the HEI-2010, which included some components related to fruit and vegetable intake as well as seafood and plant-based protein foods. Interestingly, they were less likely to exceed recommendations for empty calories (i.e., intake of added sugars and solid fats) or to fall short of the recommendations for dairy intake, which may suggest that access to food does not necessarily ensure consumption of more nutritious food [12].
The findings of the current study among nulliparas are consistent with studies among nonpregnant populations that have shown that individuals primarily living with an individual- or household-level food insecurity experience poorer diet quality and consequent health outcomes, albeit the mean difference in HEI scores by living in a food desert was only 2.6 points in the current study [7]. Living in a food desert, as opposed to having individual-level food insecurity, has been assessed less frequently in these nonpregnant populations [39, 40]. In pregnancy, prior studies have not consistently demonstrated an association between individual food insecurity and dietary quality [41, 42], and the impact of food deserts was not assessed.
When assessing for statistical interaction, the association between the primary exposure of living in a food desert and the outcome of poor dietary quality did not vary by self-identified race and ethnicity, educational attainment, or insurance status. It is possible that the higher frequency of poorer diet quality among different groups, then, may be related to living in a food desert in addition to individual-level social determinants of health [43]. These findings provide insight into the structural factors that may contribute to dietary quality among different groups of individuals [5].
This study suggests that nonadherence to nutrition recommendations was not universally poorer among individuals in food-insecure communities. Individuals living in a food desert were less likely to exceed recommendations for empty calories. However, data increasingly show that low-income pregnant individuals are more likely to report consumption of ultra-processed foods [44]. In addition, individuals living in a food desert had a higher dairy intake, which may reflect specific assistance from federal programs aimed at addressing food security and nutrition [45], including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and SNAP. We did not assess the effect of these federal programs as these data were not available in this dataset. Most nulliparas would not have registered for these programs in the periconceptional period, as only ∼50% of WIC-eligible individuals enroll during pregnancy, and fewer are eligible in the periconceptional period [46]. Whereas WIC benefits generally include the provision to purchase a set of approved food items by category, SNAP provides a monthly direct cash transfer to buy food. Yet, concurrent enrollment in these programs by eligible pregnant individuals is reported to be low (<10%) [47]. In the current study, we did not identify differences by Medicaid insurance status, and Medicaid recipients are typically eligible for WIC benefits. It is also possible that some of these programs may enable the consumption of inexpensive foods that are readily available to individuals who live in low-income neighborhoods [39].
These findings suggest more research is needed to identify and address barriers to access, affordability, and preparation of nutritious food to support a healthy diet among pregnant individuals who live in a food desert. Those identified as living in food desert could be assessed for dietary adequacy, referred to community-based resources [12], and receive resources for related social needs (e.g., transportation) that affect logistic barriers to obtaining healthy foods [48]. Structural interventions for pregnant individuals with greater socioeconomic or neighborhood barriers to obtaining healthy and nutritious food, and its impact on improving perinatal outcomes, should be considered.[49] For example, a recent National Institutes of Health “State of the Science” symposium identified several approaches to change the neighborhood food environment, including placing new grocery stores in existing food deserts, providing choice pantries, and improving access to healthier food in small retail food sources (e.g., corner stores, bodegas, convenience stores) [50]. In addition, the White House has recently released a National Strategy to address structural determinants of hunger and nutrition [51].
This study should be considered in light of its limitations. First, we conducted a cross-sectional assessment of data collected in the first trimester about the periconception period, which precludes making causal inferences about food insecurity and diet quality. Although diet recall was over a period of 3 mo, it is possible that dietary patterns and recall in the first trimester may be affected by new symptoms of pregnancy-related nausea and gastrointestinal distress. Nevertheless, there is no evidence that recall or symptoms should differ by residential location. As such, any bias introduced by this phenomenon should be similarly present and biased toward the null. Whether these differences would be sustained over time or contribute to differences in health outcomes requires further study, including the relative impact of community- and individual-level food insecurity and individual-level dietary quality on adverse pregnancy outcomes. Second, all dietary quality data were self-reported, which can lead to social desirability or recall bias. Third, not all individuals living in a food desert may experience food insecurity at a household or individual level. Nevertheless, they may still be affected by a lack of proximate access to food choices. Further data are needed to understand the relationship between living in a food desert and individual-level food insecurity, including scales that directly measure the perception and experience of food insecurity and hunger at the household level as well as enrollment in specific programs such as WIC and SNAP. Fourth, the primary reason for exclusion from the current analysis was missing HEI-2010 data, and these excluded individuals were more likely to experience individual adverse social determinants of health. It is possible the frequency of food insecurity and poor dietary quality may have been underestimated in this analysis. Although data are now nearly a decade old, there is no reason to believe that the underlying social conditions that result in food and nutritional insecurity are dependent upon or specific to that particular time. Finally, these results may generalize only to nulliparas receiving care at larger medical centers. Participants were more likely to be older, married, college-educated, and non-Hispanic White compared with the larger population of US pregnant individuals. This may explain why the mean HEI-2010 score was higher than the United States average for adults (61 vs. 55). Generalizability may also be limited because participants entered prenatal care in the first trimester and were enrolled in a longitudinal study.
Strengths of this analysis include a detailed assessment of dietary quality using direct questioning via the FFQ, which is a validated measure to assess diet in pregnancy among a large, diverse cohort of US individuals. This analysis utilized a standardized community measure of food insecurity, the Food Access Research Atlas, which accounts for structural and environmental factors that impact health outcomes and can be easily accessed via a web-based portal [25].
In conclusion, pregnant individuals living in a food desert were more likely to experience poorer periconceptional diet quality compared with those not living in a food desert. Dietary quality and food insecurity are public health issues that contribute to adverse pregnancy outcomes. These findings emphasize the relationship between living in an environment with reduced access to food and individual dietary quality in pregnancy [39]. The systems-level changes that will promote healthy food availability and that will allow achieving better dietary quality in pregnancy within communities that experience food insecurity require further study.
Authors’ disclosures
Dr. Venkatesh was supported by the Care Innovation and Community Improvement Program at The Ohio State University. Support for the nuMoM2b study was provided by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: RTI International grant U10 HD063036; Case Western Reserve University grant U10 HD063072; Columbia University grant U10 HD063047; Indiana University grant U10 HD063037; University of Pittsburgh grant U10HD063041; Northwestern University grant U10 HD063020; University of California, Irvine grant U10 HD063046; University of Pennsylvania grant U10 HD063048; and University of Utah grant U10 HD063053. In addition, support was provided by respective Clinical and Translational Science Institutes to Indiana University (grant UL1TR001108) and the University of California, Irvine (grant UL1TR000153). The rest of the authors have no conflicts of interest to disclose.
Author Contribution
The authors’ responsibilities were as follows—conceptualization: KKV, DMW, JG, WAG; data curation: JW, BM; formal analysis: KKV, LMY, JW, WAG; funding acquisition: DMH, BM, UR, RS, RW, GS, SP, HS, WAG; investigation: DMH, BM, UR, RS, RW, GS, SP, HS, WAG; methodology: KKV, JG, DMW, KL, WAG; manuscript writing: KKV, DMW, LMY, JG, KL, WAG; manuscript review and editing: DMH, BM, UR, RS, RW, GS, SP, HS, BL; and all authors: read and approved the final manuscript.
Data availability statement
Data described in the manuscript, code book, and analytic code will not be made available because this analysis includes data based on geocoding of individual patient addresses or residential locations, and deductive identification may be possible with the full dataset.
Acknowledgments
KV, DM, JG, LY, and WG wrote the manuscript. KV, DM, LY, and BW conceptualized the study question. KV, JW, and WG conducted all statistical analyses. KV, DM, JG, and WG wrote the manuscript. BM, DH, BM, UR, RS, RW, GS, SP, KS, and WG led the study, collected the data, and contributed to designing the study and interpreting the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.06.032.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bailey R., Pac S.G., Fulgoni V.L., 3rd, Reidy K.C., Catalano P.M. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Open Network. 2019;2 doi: 10.1001/jamanetworkopen.2019.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yee L.M., Silver R.M., Haas D.M., Parry S., Mercer B.M., Iams J., et al. Quality of periconceptional dietary intake and maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2020;223:121.e1–121.e8. doi: 10.1016/j.ajog.2020.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheu L.A., Yee L.M., Kominiarek M.A. Food insecurity during pregnancy and gestational weight gain. Am. J. Obstet. Gynecol. MFM. 2020;2:100068. doi: 10.1016/j.ajogmf.2019.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolin C.D., Compher C.C., Oh J.K., Durnwald C.P. Pregnant and hungry: addressing food insecurity in pregnant women during the COVID-19 pandemic in the United States. Am. J. Obstet. Gynecol. MFM. 2021;3:100378. doi: 10.1016/j.ajogmf.2021.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar L.M., Simhan H.N., Parker C.B., Meier H., Mercer B.M., Grobman W.A., et al. Racial or ethnic and socioeconomic inequalities in adherence to national dietary guidance in a large cohort of US pregnant women. J. Acad. Nutr. Diet. 2017;117:867–877.e3. doi: 10.1016/j.jand.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung C.W., Epel E.S., Ritchie L.D., Crawford P.B.B.A., Laraia B.A. Food insecurity is inversely associated with diet quality of lower-income adults. J. Acad. Nutr. Diet. 2014;114:1943–1953.e2. doi: 10.1016/j.jand.2014.06.353. [DOI] [PubMed] [Google Scholar]
- 7.Hanson K.L., Connor L.M. Food insecurity and dietary quality in US adults and children: a systematic review. Am. J. Clin. Nutr. 2014;100:684–692. doi: 10.3945/ajcn.114.084525. [DOI] [PubMed] [Google Scholar]
- 8.Tipton M.J., Wagner S.A., Dixon A., Westbay L., Darji H., Graziano S. Association of living in a food desert with pregnancy morbidity, Obstet. Gynecol. 2020;136:140–145. doi: 10.1097/AOG.0000000000003868. [DOI] [PubMed] [Google Scholar]
- 9.Laraia B.A., Siega-Riz A.M., Gundersen C. Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. J. Am. Diet. Assoc. 2010;110:692–701. doi: 10.1016/j.jada.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park C.Y., Eicher-Miller H.A. Iron deficiency is associated with food insecurity in pregnant females in the United States: national health and nutrition examination survey 1999–2010. J. Acad. Nutr. Diet. 2014;114:1967–1973. doi: 10.1016/j.jand.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Tarasuk V., Gundersen C., Wang X., Roth D.E., Urquia M.L. Maternal food insecurity is positively associated with postpartum mental disorders in Ontario, Canada. J. Nutr. 2020;150:3033–3040. doi: 10.1093/jn/nxaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez H., DiTosto J.D., Niznik C.M., Yee L.M. Understanding food security as a social determinant of diabetes-related health during pregnancy. Am. J. Perinatol. 2023;40:825–832. doi: 10.1055/s-0041-1740194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamm M.W., Bellows A.C. Community food security: background and future directions. J. Nutr. Educ. Behav. 2003;35:37–43. doi: 10.1016/s1499-4046(06)60325-4. [DOI] [PubMed] [Google Scholar]
- 14.Richards M., Weigel M., Li M., Rosenberg M., Ludema C. Food insecurity, gestational weight gain and gestational diabetes in the National children’s study, 2009–2014. J. Public Health (Oxf) 2021;43:558–566. doi: 10.1093/pubmed/fdaa093. [DOI] [PubMed] [Google Scholar]
- 15.Cukrowska-Torzewska E., Matysiak A. The motherhood wage penalty: a meta-analysis. Soc. Sci. Res. 2020;88–89:102416. doi: 10.1016/j.ssresearch.2020.102416. [DOI] [PubMed] [Google Scholar]
- 16.Coleman-Jensen A., Rabbitt M.P., Gregory C.A., Singh A. US Department of Agriculture; 2020. Household food security in the United States in 2019: economic research service.https://www.ers.usda.gov/publications/pub-details/?pubid=99281 Available from: [Google Scholar]
- 17.Rehm C.D., Peñalvo J.L., Afshin A., Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315:2542–2553. doi: 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger M., Sartain C., Klassen A.C. Addressing community needs through a participatory food security assessment. J. Hunger Environ. Nutr. 2022;17:170–190. doi: 10.1080/19320248.2021.1903643. [DOI] [Google Scholar]
- 19.Rummo P., Sze J., Elbel B. Association between a policy to subsidize supermarkets in underserved areas and childhood obesity risk. JAMA Pediatr. 2022;176:646–653. doi: 10.1001/jamapediatrics.2022.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson A.S., Collins R.L., Ghosh-Dastidar B., Ye F., Hunter G.P., Baird M.D., et al. Improvements in neighborhood socioeconomic conditions may improve resident diet. Am. J. Epidemiol. 2021;190:798–806. doi: 10.1093/aje/kwaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross R., Edmunds M., Schwartz P. American Public Health Association; 2021. Food Security: A community driver of health.https://www.apha.org/-/media/Files/PDF/pubs/Food_Security_Health_Community_Driver.ashx Available from: [Google Scholar]
- 22.Haas D.M., Parker C.B., Wing D.A., Parry S., Grobman W.A., Mercer B.M., et al. A description of the methods of the nulliparous pregnancy outcomes study: monitoring mothers-to-be (nuMoM2b) Am. J. Obstet. Gynecol. 2015;212:539.e1–539.e24. doi: 10.1016/j.ajog.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson B.A., Herring A.H., Ibrahim J.G., Siega-Riz A.M. Structured measurement error in nutritional epidemiology: applications in the pregnancy, infection, and nutrition (PIN) study. J. Am. Stat. Assoc. 2007;102:856–866. doi: 10.1198/016214506000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller P.E., Reedy J., Kirkpatrick S.I., Krebs-Smith S.M. The United States food supply is not consistent with dietary guidance: evidence from an evaluation using the healthy eating index-2010. J. Acad. Nutr. Diet. 2015;115:95–100. doi: 10.1016/j.jand.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Economic Research Service (ERS) 2022. Food access research atlas USDA.https://www.ers.usda.gov/data-products/food-access-research-atlas/go-to-the-atlas/ Available from: [Google Scholar]
- 26.Rhone A., Ver Ploeg M., Dicken C., Williams R., Breneman V. Low-income and low-supermarket-access census tracts, 2010–2015. Econom. Inform. Bulletin. 2015;165:1–15. [Google Scholar]
- 27.USDA . 2022. Introduction to the food research atlas.https://gisportal.ers.usda.gov/portal/apps/experiencebuilder/experience/?id=a53ebd7396cd4ac3a3ed09137676fd40&page=page_0 Available from. [Google Scholar]
- 28.Economic Research Service (ERS) 2021. US Department of Agriculture (USDA), Food access research atlas.https://www.ers.usda.gov/data-products/food-access-research-atlas/ Available from: [Google Scholar]
- 29.Food and Nutrition Service . 2019. Healthy eating index (HEI)https://www.fns.usda.gov/resource/healthy-eating-index-hei Available from: [Google Scholar]
- 30.Guenther P.M., Kirkpatrick S.I., Reedy J., Krebs-Smith S.M., Buckman D.W., Dodd K.W., et al. The healthy eating index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for Americans. J. Nutr. 2014;144:399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guenther P.M., Casavale K.O., Reedy J., Kirkpatrick S.I., Hiza H.A.B., Kuczynski K.J., et al. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Diet. 2013;113:569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poverty guidelines 2013. https://aspe.hhs.gov/2013-poverty-guidelines Available from:
- 33.Supplemental Nutrition Assistance Program (SNAP) 2013. https://aspe.hhs.gov/2013-poverty-guidelines Available from:
- 34.Kind A.J.H., Buckingham W.R. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N. Engl. J. Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.French B., Shotwell M.S. Regression models for ordinal outcomes. JAMA. 2022;328:772–773. doi: 10.1001/jama.2022.12104. [DOI] [PubMed] [Google Scholar]
- 36.Harrel F. 2022. Assessing the proportional odds assumption and its impact. Statistical thinking blog.https://www.fharrell.com/post/impactpo Available from. [Google Scholar]
- 37.Fleischer N.L., Diez Roux A.V. Using directed acyclic graphs to guide analyses of neighbourhood health effects: an introduction. J. Epidemiol. Community Health. 2008;62:842–846. doi: 10.1136/jech.2007.067371. [DOI] [PubMed] [Google Scholar]
- 38.Wang D.D., Leung C.W., Li Y., Ding E.L., Chiuve S.E., Hu F.B., et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern. Med. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zenk S.N., Tabak L.A., Pérez-Stable E.J. Research opportunities to address nutrition insecurity and disparities. JAMA. 2022;327:1953–1954. doi: 10.1001/jama.2022.7159. [DOI] [PubMed] [Google Scholar]
- 40.Malambo P., Kengne A.P., De Villiers A., Lambert E.V., Puoane T. Built environment, selected risk factors and major cardiovascular disease outcomes: a systematic review. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamba R., Leung C.W., Guendelman S., Lahiff M., Laraia B.A. Household food insecurity is not associated with overall diet quality among pregnant women in NHANES 1999–2008, Matern. Child Health J. 2016;20:2348–2356. doi: 10.1007/s10995-016-2058-1. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Nahm S., Østbye T., Hoyo C., Kravitz R.M., Benjamin-Neelon S.E. Associations among food security, diet quality, and dietary intake during pregnancy in a predominantly African American group of women from North Carolina. J. Acad. Nutr. Diet. 2022;122:565–572. doi: 10.1016/j.jand.2021.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger N., Van Wye G., Huynh M., Waterman P.D., Maduro G., Li W., et al. Structural racism, historical redlining, and risk of preterm birth in New York City, 2013–2017. Am. J. Public Health. 2020;110:1046–1053. doi: 10.2105/AJPH.2020.305656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paula W.O., Patriota E.S.O., Gonçalves V.S.S., Pizato N. Maternal consumption of ultra-processed foods-rich diet and perinatal outcomes: a systematic review and meta-analysis. Nutrients. 2022;14:3242. doi: 10.3390/nu14153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Litvak J., Parekh N., Juul F., Deierlein A. Food assistance programs and income are associated with the diet quality of grocery purchases for households consisting of women of reproductive age or young children. Prev. Med. 2020;138:106149. doi: 10.1016/j.ypmed.2020.106149. [DOI] [PubMed] [Google Scholar]
- 46.Soneji S., Beltrán-Sánchez H. Association of special supplemental nutrition program for women, infants, and children with preterm birth and infant mortality. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert D., Nanda J., Paige D. Securing the safety net: concurrent participation in income eligible assistance programs. Matern. Child Health J. 2014;18:604–612. doi: 10.1007/s10995-013-1281-2. [DOI] [PubMed] [Google Scholar]
- 48.Waselewski M., Plegue M., Sonneville K., Resnicow K., Ghumman A., Ebbeling C., et al. Grocery delivery to support healthy weight gain among pregnant young women with low income: protocol for a randomized controlled trial. JMIR Res. Protoc. 2022;11 doi: 10.2196/40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brownell K.D., Kersh R., Ludwig D.S., Post R.C., Puhl R.M., Schwartz M.B., Willett W.C. Personal responsibility and obesity: a constructive approach to a controversial issue. Health Aff. (Millwood) 2010;29:379–387. doi: 10.1377/hlthaff.2009.0739. [DOI] [PubMed] [Google Scholar]
- 50.National Heart Lung and Blood Institute Food insecurity, neighborhood food environment, and nutrition health disparities: state of the science. Virtual workshop held on September 21-23, 2021. 2022 https://www.nhlbi.nih.gov/events/2021/food-insecurity-neighborhoodfood-environment-and-nutrition-health-disparities Available from: [Google Scholar]
- 51.Benjamin S., Au T.Y., Assavarittirong C. Lack of supplement regulation: a potential for ethical and physiological repercussions. Nutr. Health. White House Conference on Hunger, Nutrition, and Health. 2022 doi: 10.1177/02601060221108145. https://health.gov/our-work/nutrition-physical-activity/white-house-conference-hunger-nutrition-and-health Available from: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available because this analysis includes data based on geocoding of individual patient addresses or residential locations, and deductive identification may be possible with the full dataset.